Abstract
Notch refers to a set of genes encoding a family of transmembrane receptors in mammalian cells. Previous evidence indicated that Notch receptors were implicated in the onset of gastric cancer. However, there is little evidence on the different genetic expression patterns of the four Notch receptors and their values for patient prognosis. Most recently, we investigated the transcriptional data of Notch receptors and related patient survival in patients with GC based on several databases, including ONCOMINE, GEPIA, Kaplan–Meier Plotter, cBioPortal and TIMER. According to our findings, gastric cancer tissues, compared with adjacent normal tissues presented a higher level of expression of Notch1/2/3. We also performed a survival analysis and noted that gastric cancer patients with high transcription levels of Notch1/2/3/4 had a low relapse-free survival. In gastric cancer patients, higher levels of infiltration in their CD4+ T cells, macrophages, neutrophils, and dendritic cells were positive associated with the expression of Notch receptors. Notch expression had significant association with diverse immune marker sets in gastric cancer. Overall, this study provides evidence that Notch1/2/3/4 could become the potential targets for precision treatment and new biomarkers in the prognosis of gastric cancer.
Keywords: Notch, gastric cancer, prognosis, immune infiltrates
INTRODUCTION
Gastric cancer (GC) is a very common disease worldwide and has the second highest mortality rate among all cancers. In the past decade, researchers have found that the deregulated expression of specific genes can increase the risk of GC. According to microarray analysis, previous studies revealed that in GC tissues the expression of specific genes is different from that in adjacent normal tissue. Although significant progress has been achieved in GC diagnosis and treatment, the five-year survival of patients is still unsatisfactory [1]. Researchers have identified epigenetic and genetic alterations as some of the main factors inducing GC. However, the underlying molecular pathogenic mechanisms on molecular level are still obscure. Hence, it is important to identify prognostic markers and potential drug targets to enhance prognosis and individualized treatments.
The Notch signaling pathway is highly conserved among various species. In mammals, four type I transmembrane Notch receptors (Notch 1-4) are synthesized, all of them with unique roles during the generation of immunocytes [2, 3]. Notch signaling also exerts important function in the development and tissue homeostasis of various organ [4, 5]. Provided the importance of Notch signaling in regulating cellular behavior, it is perhaps not surprising that Notch also has an important role in many types of cancer, particularly due to its importance in the regulation of stem and progenitor cells. Several mechanisms such as epigenetic regulation, posttranslational, modifications, gene overexpression and mutations, may lead to the dysregulation of the Notch pathway [2]. Interestingly, Notch activity is associated with oncogenic and tumor-suppressive functions [6, 7]. It is involved in cell survival, cell death pathways, proliferation and growth arrest, as well as cell differentiation into terminally differentiated cells versus cancer cell “stemness” [8]. These functions provide evidence of a context-dependent nature of Notch-induced cellular reactions.
The onset of GC can be described as the result of interactions between a series of factors concerning genetics, epigenetics and the external environment, which jointly lead to the deregulation of the signaling pathways that may induce the onset of cancer [9, 10]. Further, there has been a general belief that it is the dysfunctional oncogenic pathways that induce the onset of GC, which may include the epidermal growth factor receptor (EGFR), Notch, Hedgehog, nuclear factor-κB and Wnt/β-catenin pathway [11]. Among these pathways, Notch signaling is involved in direct cell-cell communication, cell differentiation, proliferation and apoptosis [12].
The balance between immune effector cells in the tumor microenvironment helps the malignant cells escape from the immune response. Tumor infiltrations of tumor associated macrophages, neutrophils, regulatory T cells are correlated with poor prognosis [13–15]. Tumor infiltrating CD8+ cytotoxic T lymphocytes and DCs are generally associated with favorable outcome of GC [16, 17], although some subsets of these immune cells have inverse prognosis prediction values. High ratios of Foxp3+/CD4+ and Foxp3+/CD8+ in tumors are associated with a poor prognosis [18, 19]; whereas high Th1/Th2 ratio in tumors predicts a good prognosis [20].
In previous studies, researchers have investigated the consequences of dysregulation of the Notch pathway and how it relates to clinicopathological features and prognosis in human GC. Nevertheless, the role of Notch family members in the development and progression of GC remains unknown. This study aims to address this question through in-depth analysis of the mutational activation and expression of Notch family members and their link with prognosis and immune infiltrates in GC patients.
RESULTS
Transcriptional levels of Notch in patients with GC
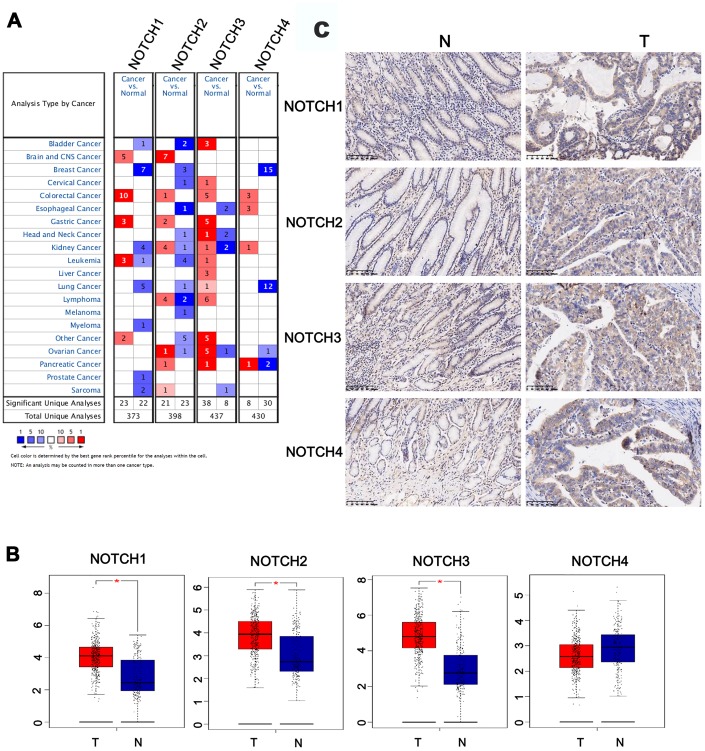
Using the ONCOMINE databases, a comparative analysis investigating transcription levels of Notch receptors was performed on cancer tissues and adjacent normal tissues (Figure 1A). According to the information from five datasets, a significant upregulation of Notch3 mRNA expression was detected in GC patients. In Chen’s dataset [21], the expression of Notch3 in gastric adenocarcinoma was 1.594 and 1.871 times respectively of that in the samples of normal tissue (Table 1). In Wang’s dataset [5], the expression of Notch3 in GC tissue was 2.549 times of that in normal tissue. In DErrico’s dataset [22] the expression of Notch3 in gastric intestinal type adenocarcinoma was 2.869 times of that in normal tissues. In Cho’s dataset [23] the expression of Notch3 in GC tissue was 1.630 times of that in normal tissue. Chen [21] showed that the expression of Notch1 was also higher in cancer tissues, since the expression in gastric intestinal type adenocarcinoma and diffuse gastric adenocarcinoma was 1.920 and 1.733 times respectively of that in patients with normal gastric tissue, respectively (Table 1). Based on the dataset of DErrico, the expression of Notch1 in gastric intestinal type adenocarcinoma was also 2.247 times of that in normal tissue [22]. In comparison with normal tissue, GC exhibited 1.625 [5] and 1.832 [22] times expression levels of Notch2, respectively.
Figure 1.
The expression of Notch in different tissues. (A) The transcription levels of Notch receptors in different types of cancers. (B) The expression of Notch receptors in GC. (C) The expression of Notch protein in GC. Students’t-test was used in comparative analysis of Notch expression. T: tumor, N: normal tissue, number (T) = 415, number (N) = 211.
Table 1. The significant changes of Notch expression in mRNA level between different types of GC and stomach tissues.
| Type of GC VS. Stomach | Fold Change | P value | t-test | Ref | |
| NOTCH1 | Gastric intestinal type adenocarcinoma vs. Normal | 2.247 | 1.04E-13 | 9.844 | DErrico [14] |
| Diffuse gastric adenocarcinoma vs. Normal | 1.733 | 6.14E-8 | 6.700 | Chen [13] | |
| Gastric intestinal type adenocarcinoma vs. Normal | 1.920 | 9.17E-11 | 7.739 | Chen [13] | |
| NOTCH2 | Gastric cancer vs. Normal | 1.625 | 5.76E-5 | 5.128 | Wang [5] |
| Gastric intestinal type adenocarcinoma vs. Normal | 1.832 | 3.15E-8 | 6.617 | DErrico [14] | |
| NOTCH3 | Gastric cancer vs. Normal | 2.549 | 1.42E-6 | 6.232 | Wang [5] |
| Diffuse gastric adenocarcinoma vs. Normal | 1.871 | 4.15E-8 | 7.496 | Chen [13] | |
| Gastric intestinal type adenocarcinoma vs. Normal | 1.594 | 4.15E-10 | 7.175 | Chen [13] | |
| Gastric intestinal type adenocarcinoma vs. Normal | 2.869 | 1.14E-9 | 7.723 | DErrico [14] | |
| Diffuse gastric adenocarcinoma vs. Normal | 1.630 | 7.15E-5 | 4.142 | Cho [15] | |
| NOTCH4 | NA | NA | NA | NA | NA |
Ref: reference
Expression levels of Notch in GC patients
The comparative investigation was conducted between the Notch mRNA expression in normal and gastric adenocarcinoma tissues based on the information provided by the GEPIA (Gene Expression Profiling Interactive Analysis) dataset. Based on the findings, in comparison with normal gastric tissues, gastric adenocarcinoma tissues presented higher expression levels of Notch1/2/3 (Figure 1B). The expression of Notch1, Notch2, Notch3 and Notch4 proteins, which were examined by immunohistochemistry, were higher in the gastric adenocarcinoma tissues than that in the normal gastric tissues (Figure 1C).
Association of Notch1/2/3/4 mRNA expression with the prognosis of GC patients
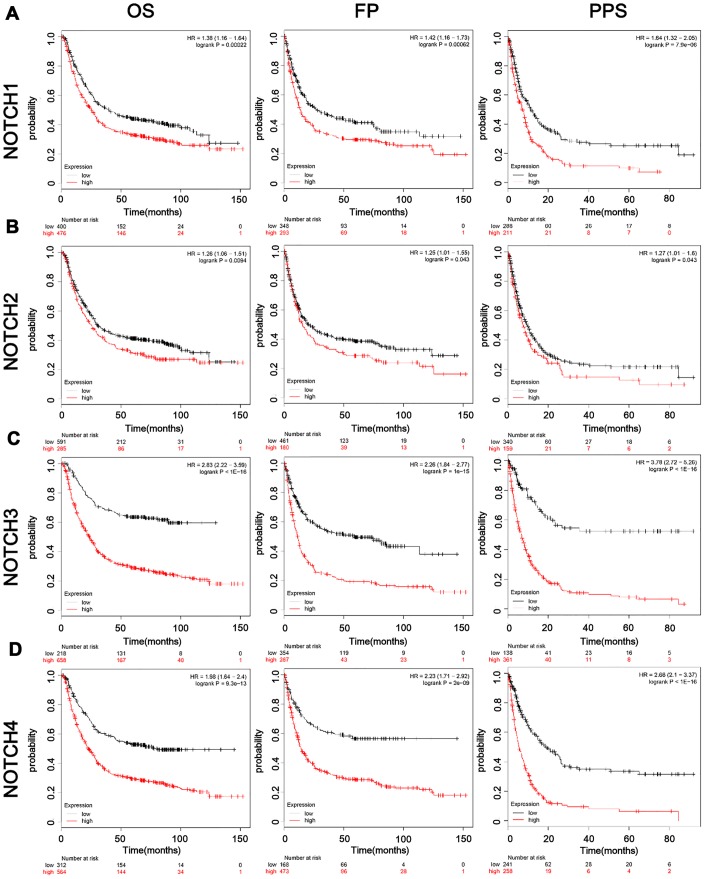
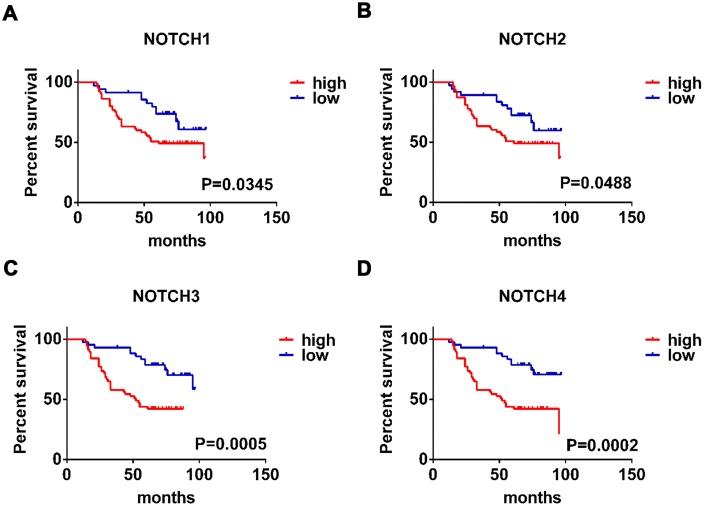
In this study we also conducted further research to investigate how the survival of GC patients was affected by Notch. Kaplan–Meier Plotter tools were applied to analyze the correlation between Notch mRNA levels and the survival of patients with GC in 882 gastric tumors. According to the results of analysis, all the sampled GC patients were negative correlated with mRNA expression of all the four types of Notch in terms of post-progression survival (PPS), progression-free survival (FP), and overall survival (OS) (p < 0.05) (Figure 2A–2D). We further validated these results through the analysis of samples from gastric adenocarcinoma patients with immunohistochemistry (Figure 1C) and survival analysis. The patients with higher Notch1/2/3/4 expression levels had worse overall survival than those with lower Notch1/2/3/4 expression levels (Figure 3A–3D). Generally, lower Notch mRNA expression levels in GC patients indicated higher PPS, FP, and OS.
Figure 2.
The prognostic value of mRNA level of Notch receptors in GC patients. (A–D). Logrank test was used in analysis of OS/FP/PPS.
Figure 3.
The prognostic value of expression level of Notch receptors in gastric adenocarcinoma patients. (A–D). Logrank test was used in analysis of overall survival. The numbers of high Notch expression group and low Notch expression group are 50, respectively.
Cellular functions, pathways and frequently altered neighbor genes predicted to be affected by changes in Notch receptors in patients with GC
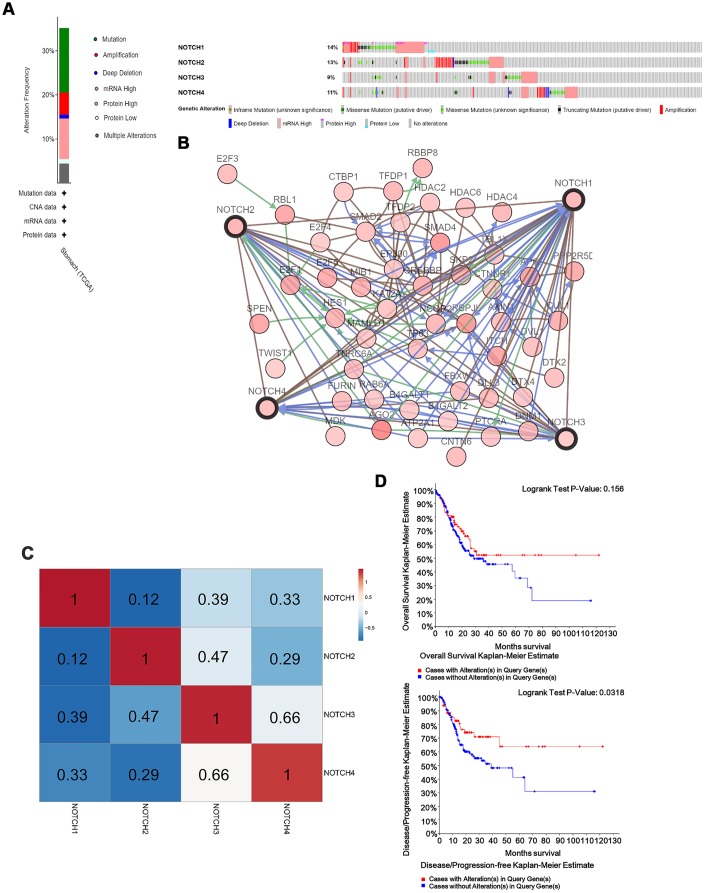
In this study, the cBioPortal online tool was used for analysis of Notch alterations, correlation, and networks. Notch receptors were altered in 223 samples out of 478 patients with stomach adenocarcinoma (47%). Two or more alterations were detected in 51 samples (10%) (Figure 4A). Subsequently, a Notch network was built up using the 50 most frequently altered neighbor genes. According to the results, there was a close correlation between the alterations of Notch and cell cycle-related genes, including E2F1, E2F3, E2F4 and E2F5, as well as histone acetylation-related genes, including HDAC2, HDAC4 and HDAC6 (Figure 4B). The analysis of mRNA was used to assess the correlation between Notch receptors with Pearson’s test. Results showed the following Notch members to be significantly positively correlated: Notch1 with Notch2, Notch3, and Notch4; Notch2 with Notch1, Notch3, and Notch4; Notch3 with Notch1, Notch2 and Notch4; Notch4 with Notch1, Notch2 and Notch3 (Figure 4C). Furthermore, a Kaplan-Meier plot and log-rank test demonstrated no significant effect of Notch genetic alterations on OS (Figure 4D, p=0.156) but were associated with disease free survival (DFS) (Figure 4D, p=0.0318) of gastric adenocarcinoma patients.
Figure 4.
Notch genes expression and mutation analysis in GC. (A) Notch1, Notch2, Notch3 and Notch4 mutation rates were 14%, 13%, 9% and 11%, respectively. (B) Network for 4 Notch receptors and the 50 most frequently altered neighbor genes. (C) Genetic alterations in Notch receptor were correlated with longer DFS in gastric adenocarcinoma patients. (D) The relationship between genetic alterations in Notch and OS/DFS. Logrank test was used in analysis of OS/DFS.
Based on the analysis of Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) using the Database for Annotation, Visualization, and Integrated Discovery (DAVID), we predicted a significant correlation between Notch alterations and functional activities. The function of target host genes were predicted via GO enrichment analysis in terms of three aspects, namely, biological processes, cellular components, and molecular functions. Enrichment analysis was applied to investigate the biological functions of these target genes. According to the enriched gene ontology (GO) cell component (CC) analysis, there was significant enrichment for genes related to the Golgi membrane, endoplasmic reticulum membrane, and receptor complex (Supplementary Figure 1A). For biological processes (BP), there was an enrichment in receptor activity, calcium ion binding and enzyme binding (Supplementary Figure 1B). In addition, in terms of molecular function (MF) results were mainly associated with the Notch signaling pathway and transcription initiation from RNA polymerase II promoter (Supplementary Figure 1C). The KEGG disease terms were mainly enriched in cancers, cancers of the digestive system and musculoskeletal diseases (Supplementary Figure 1D). We also observed significant enrichment in KEGG pathways (Supplementary Figure 1E). Among these pathways, cell cycle, TGF-β signaling pathway, Notch signaling pathways, microRNA in cancer, and Wnt signaling pathway were involved in the tumorigenesis and pathogenesis of GC (Supplementary Figures 2A and 2B).
Notch expression is correlated with immune infiltration levels in gastric cancer
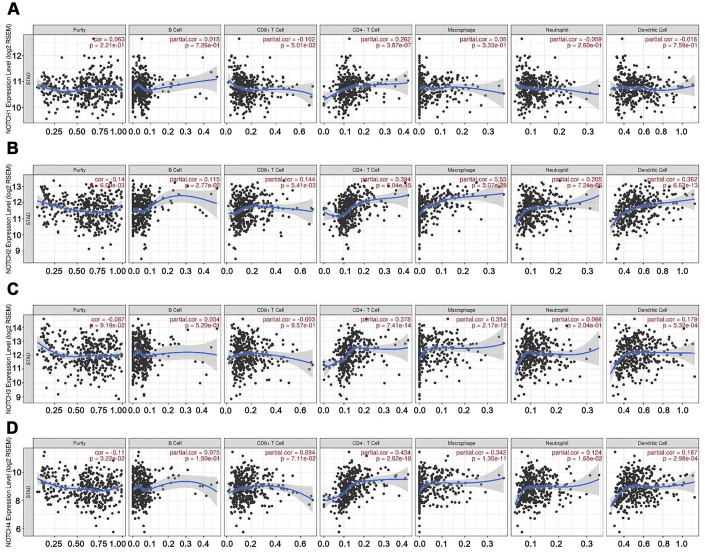
Tumor-infiltrating lymphocytes have been used to predict sentinel lymph node status and survival in cancers [24]. Hence, we explored the correlation between the levels of immune infiltration and the expression of Notch in gastric adenocarcinoma patients based on TIMER. Interestingly, we found high levels of Notch mRNA expression to be associated with high immune indiltration in gastric adenocarcinoma. Notch1 mRNA expression level was significantly positively correlated with infiltrating levels of CD4+ T cells (r = 0.262, P = 3.87e-07) (Figure 5A). Notch2 mRNA expression level was significantly positively correlated with infiltrating levels of CD4+ T cells (r = 0.394, P = 5.04e-15), macrophages (r = 0.53, P = 3.07e-28), neutrophils (r = 0.205, P = 7.24e-05) and dendritic cells (DCs) (r = 0.362, P = 6.62e-13) (Figure 5B). Notch3 mRNA expression level was significantly positively correlated with infiltrating levels of CD4+ T cells (r = 0.378, P = 7.41e-14) and macrophages (r = 0.354, P = 2.17e-12) (Figure 5C). Similarly, Notch4 mRNA expression was positively correlated with infiltrating levels of CD4+ T cells (r = 0.434, P = 2.82e-18) and macrophages (r = 0.342, P = 1.30e-11) (Figure 5D). In addition, this study did not find significant correlations between the expression of Notch and infiltrating levels of B cells and CD8+ T cells in gastric adenocarcinoma. These findings strongly indicated that Notch played an important role in immune infiltration in gastric adenocarcinoma, particularly for CD4+ T cells and macrophages.
Figure 5.
Correlation of Notch expression with immune infiltration level in GC. (A) Notch1 expression had significant positive correlations with infiltrating levels of CD4+ T cells (n = 415). (B) Notch2 expression had significant correlations with infiltrating levels of CD4+ T cells, macrophages, neutrophils, and dendritic cells in GC (n = 415). (C) Notch3 expression was significantly related to infiltrating levels of CD4+ T cells and macrophages in GC but no significant correlation with infiltrating level of B cells (n = 415). (D) Notch4 expression had significant positive correlations with infiltrating levels of CD4+ T cells and macrophages in GC (n = 415). Spearman’s correlation was used to analyze correlation between Notch receptors and immune cells.
Correlation analysis between Notch expression and immune markers
In order to detect the relationship between Notch and the diverse immune infiltrating cells, we concentrated particularly on the correlations between Notch and immune markers of various immune cells in gastric adenocarcinoma using the TIMER databases. We analyzed different immune cells including tumor-associated macrophages (TAMs), neutrophils and DCs in gastric adenocarcinoma (Table 2). Meanwhile, Th1 cells, Th2 cells and Tregs were also analyzed. After adjusting the correlation for tumor purity, results demonstrated that the Notch mRNA expression level had significant correlations with TAMs, DCs, CD4+ T cells and neutrophils in gastric adenocarcinoma (Table 2). Specifically, we showed chemokine (C-C motif) ligand (CCL)-2, IL-10, CD163, CSF1R and FCGR2A of TAMs, ITGAX, CD1C, NRP1 and THBD of DCs, CCR7, ITGAM of neutrophils, STAT4 of Th1 cells, GATA3, CXCR4, CCR4 and CCR8 of Th2 cells, STAT5B and TGFB1 of Tregs to be significantly correlated with the expression of Notch2 in gastric adenocarcinoma (Table 2). High Notch3/4 expression was associated with high levels of infiltration of macrophage and CD4+ T cells in gastric adenocarcinoma. TAM markers such as CCL2 and CSF1R, DCs markers such as ITGAX, NRP1 and THBD, neutrophils markers such as CCR7 and ITGAM, Th2 markers such as GATA3, CXCR4, CCR4 and CCR8 and Treg markers such as STAT5B and TGFB1 showed significant correlation with Notch3 expression. TAM markers such as CCL2 and CSF1R, DCs markers such as ITGAX, CD1C, NRP1 and THBD, neutrophils markers such as CCR7 and ITGAM, Th2 markers such as GATA3, CXCR4, CCR4, Treg markers such as FOXP3, STAT5B, TGFB1 were also significantly correlated the expression of Notch4. Based on all the findings above, we provide evidence that infiltration of TAMs/Th2/Tregs is increased by expression of Notch3/4. Additionally, Notch1 had a positive correlation with CD4, GATA3 and STAT5B and a negative correlation with CD59 in gastric adenocarcinoma. Overall, these results further confirm the critical role of Notch in inhibiting immune activities in the gastric adenocarcinoma microenvironment.
Table 2. Correlation analysis between Notch and related markers of immune cells.
| Description | Gene marker | NOTCH1 | NOTCH2 | NOTCH3 | NOTCH4 | ||||
| Cor | P | Cor | P | Cor | P | Cor | P | ||
| TAM | CCL2 | 0.052 | 0.313 | 0.264 | *** | 0.239 | *** | 0.27 | *** |
| IL10 | 0.081 | 0.117 | 0.27 | *** | 0.171 | *** | 0.199 | *** | |
| CD163 | 0.097 | 0.058 | 0.454 | *** | 0.193 | *** | 0.163 | ** | |
| VSIG4 | 0.019 | 0.711 | 0.402 | *** | 0.177 | *** | 0.099 | 0.053 | |
| CSF1R | 0.066 | 0.198 | 0.466 | *** | 0.251 | *** | 0.235 | *** | |
| FCGR2A | -0.012 | 0.22 | 0.337 | *** | 0.168 | ** | 0.125 | * | |
| FCER2 | 0.141 | ** | 0.249 | *** | 0.197 | *** | 0.327 | *** | |
| DCs | ITGAX | 0.111 | * | 0.327 | *** | 0.252 | *** | 0.278 | *** |
| CD1C | 0.031 | 0.55 | 0.396 | *** | 0.182 | *** | 0.287 | *** | |
| NRP1 | 0.135 | ** | 0.483 | *** | 0.461 | *** | 0.507 | *** | |
| THBD | 0.164 | ** | 0.453 | *** | 0.518 | *** | 0.598 | *** | |
| Neutrophils | CCR7 | 0.15 | ** | 0.324 | *** | 0.209 | *** | 0.338 | *** |
| ITGAM | 0.105 | * | 0.479 | *** | 0.286 | *** | 0.28 | *** | |
| CD59 | -0.323 | *** | 0.311 | *** | 0.152 | ** | 0.153 | ** | |
| Th1 | STAT4 | 0.047 | 0.363 | 0.316 | *** | 0.075 | 0.146 | 0.152 | ** |
| TBX21 | 0.149 | ** | 0.185 | *** | 0.088 | 0.088 | 0.148 | ** | |
| CD4 | 0.324 | *** | 0.178 | *** | 0.324 | *** | 0.197 | *** | |
| Th2 | GATA3 | 0.22 | *** | 0.173 | *** | 0.22 | *** | 0.2 | *** |
| CXCR4 | 0.097 | 0.059 | 0.375 | *** | 0.213 | *** | 0.288 | *** | |
| CCR4 | 0.169 | *** | 0.408 | *** | 0.24 | *** | 0.3 | *** | |
| CCR8 | 0.137 | ** | 0.347 | *** | 0.216 | *** | 0.198 | *** | |
| Treg | FOXP3 | 0.136 | ** | 0.173 | *** | 0.176 | *** | 0.204 | *** |
| STAT5B | 0.351 | *** | 0.584 | *** | 0.399 | *** | 0.414 | *** | |
| TGFB1 | 0.152 | ** | 0.373 | *** | 0.532 | *** | 0.384 | *** | |
*P<0.05, **P<0.01, ***P<0.001
DISCUSSION
The importance and effect of the Notch signaling pathway on cellular activities and in cancer with either oncogenic or tumor-suppressive functions have been widely recognized [2, 25, 26]. Notch signaling functions as juxtacrine signaling between cells. This type of signaling allows Notch to regulate heterotypic interactions between stroma and tumor. These interactions are known to be of importance in various aspects of tumor biology, such as angiogenesis, cancer stem cell maintenance, immune infiltration, and resistance to therapy. Despite the already confirmed effects of Notch signaling on GC, the functions of different Notch family members in GC remain to be elucidated. In this study, we conducted detailed analyses on various members of Notch referring to mutation, expression and prognostic values in GC patients.
Results from our study showed that over-expression of mRNA and protein were found in Notch family members. Also, higher mRNA expressions of Notch1/2/3/4 were significantly associated with shorter OS in GC patients. Moreover, a high Notch mutation rate (47%) was observed in gastric adenocarcinoma patients. Further, genetic alteration of Notch receptors was associated with shorter DFS in gastric adenocarcinoma patients. Finally, the functions and pathways affected by mutations in Notch receptors as well as the most significant 50 frequently altered neighbor genes in gastric adenocarcinoma patients were analyzed. Results showed that cell cycle-related genes, including E2F1, E2F3, E2F4 and E2F5, and histone acetylation-related genes, including HDAC2, HDAC4 and HDAC6 were significantly affected by mutations in Notch receptors. Results also showed the following to be remarkably regulated by Notch mutation in GC: biological processes such as receptor activity, calcium ion binding and enzyme binding; cellular components such as Golgi membrane, endoplasmic reticulum membrane, and receptor complex; molecular functions such as Notch signaling pathway and transcription initiation from RNA polymerase II promoter; KEGG disease terms such as cancers, cancers of the digestive system and musculoskeletal diseases; KEGG pathways such as cell cycle, Notch signaling pathways and TGF-β signaling pathway.
Of note, Notch1 played a critical role in regulating the senescence secretome in fibroblasts [27], which was perhaps a part of its function in the regulation of stromal activation in the process of tumorigenesis. In breast cancer models, Notch1 was induced by fibroblast-derived CCL2 to maintain a stem cell phenotype and had a possible oncogenic role [17, 28]. Importantly, Notch1 activation enabled primary melanoma cells to acquire metastatic capabilities [29]. In gastric cancer, the AKT1/NF-kB/Notch1/PTEN axis had a significant role in the development of chemoresistance [30]. Notch1 activation also showed correlation with GC progression and was defined as an independent prognostic factor [31]. In B cell lymphoma, Jag1 induced the expression of FGF4 which in turn activated Notch2 in lymphoma cells [32]. Notch ligand Jag1 interacted with Notch3 to regulate the resistance [33]. Moreover, Notch3 triggered by stromal cell-derived exosomes activated antiviral signaling depending on STAT1 in cancer cells [33]. Induction of Notch3 by CAFs led to an increase in proliferation of cancer stem cells [34]. Meanwhile, the renewal of cancer stem cell could be induced by IL-6 through Notch3 in breast cancer [35]. Notch4 had a causative role in the tumorigenesis [36] and might affect the development of fibroblasts [37]. Recent data also showed that Notch is involved in liver glucose and lipid homeostasis [38, 39].
Another important aspect of this study was that Notch expression was correlated with diverse immune infiltration levels in gastric adenocarcinoma. Notch1 mRNA expression level was significantly positively correlated with infiltrating levels of CD4+ T cells. Notch2 mRNA expression level was significantly positively correlated with infiltrating levels of CD4+ T cells, macrophages, neutrophils and DCs. Notch3 mRNA expression level was significantly positively correlated with infiltrating levels of CD4+ T cells and macrophages. Moreover, the correlation between Notch expression and the marker genes of immune cells imply the role of Notch in regulating tumor immunology in gastric adenocarcinoma. The M2 macrophage markers such as CD163, VSIG4, and CSF1R showed correlations with Notch2 expression. These results indicated the potential role of Notch2 in regulation of TAMs polarization. Furthermore, our results revealed that Notch had the potential to activate Tregs. The increase in Notch expression positively correlates with the expression of Treg markers (FOXP3, CCR8, STAT5B in Table 2). In addition, there were significant correlations between Notch expression and several markers of T helper cells (Th1, Th2), DCs and neutrophils in gastric adenocarcinoma. These correlations could be indicative of a potential mechanism that Notch regulated immune cells in gastric adenocarcinoma. Together these findings suggested that Notch play a significant role in recruitment and regulation of immune infiltrating cells in gastric adenocarcinoma.
In this study, a systemic analysis was performed on Notch receptors expression, mutation and GC patients’ prognosis, which provided a further understanding of the biomolecular properties of GC. Our results demonstrated that the high expression of Notch1/2/3/4 in GC tissues might exert a significant function in GC tumorigenesis. High Notch1/2/3/4 expression could also act as molecular markers to categorized high-risk subgroups of GC patients. According to the present study, Notch1/2/3/4 could be potential therapeutic targets for GC, and transcription levels of Notch1/2/3/4 could be potential prognostic markers overall promoting GC survival and prognostic accuracy.
MATERIALS AND METHODS
ONCOMINE analysis
Using the online cancer microarray database, ONCOMINE (https://www.oncomine.org/), we analyzed the transcription levels of Notch in various cancers tissues. A students’ t-test was performed to conduct a comparative analysis of the different Notch expression in normal tissues versus cancer tissue samples. The cut-off value for a significant fold change was 1.5, while the cut-off value for significant p value was 0.0001.
GEPIA dataset
GEPIA (http://gepia.cancer-pku.cn/), a web server for analyzing the sequencing expression data of RNA based on 9,736 tumors and 8,587 normal samples from the cancer genome atlas (TCGA) and the GTEx projects [40], was used for analysis of differential expression.
The Kaplan-Meier plotter
Kaplan-Meier Plotter (https://kmplot.com/) was applied to the assessment the prognostic value of Notch. This online database provides gene expression data and information on patients’ survival performance based on 882 clinical GC cases [41]. All the sampled patients were categorized into groups based on median expression of Notch (high vs. low expression) to analyze the overall survival (OS), progression-free survival (FP), and post progression survival (PPS) of GC patients using a Kaplan-Meier survival plot. We only chose the best JetSet probe set of Notch to obtain Kaplan-Meier plots.
TCGA data and cBioPortal
TCGA provides sequencing and pathology data for over 30 different cancers [42]. We used the dataset for stomach adenocarcinoma, which included 478 cases with pathology reports to further analyze Notch expression using cBioPortal (https://www.cbioportal.org/) [43]. From the genomic profiles, we obtained data regarding protein expression Z-scores (RPPA), mRNA expression z-scores, putative copy-number alterations (CNA) from GISTIC and mutations. Co-expression and network analysis were conducted following online instructions of cBioPortal.
Immunohistochemistry
Tissue samples were fixed in 4% paraformaldehyde and embedded in paraffin. Anti-Notch1 (1:200; CST, Cat#: 3608, Danvers, MA, USA), anti-Notch2 (1:200; CST, Cat#: 5732), anti-Notch3 (1:200; Abcam, Cat#: ab23426, Cambridge, MA, USA) and anti-Notch4 (1:200; Abcam, Cat#: ab222400) were used as primary antibodies. Immunohistochemistry was performed according to the method previously described [44–46]. Gastric cancer tissues and adjacent normal gastric tissues were obtained from the Huashan Hospital (Shanghai, China).
Immune infiltrates analysis in TIMER database
TIMER is a comprehensive tool established for analyzing immune infiltrates across different types of cancer (https://cistrome.shinyapps.io/timer/) [47]. We analyzed Notch expression in different cancer types and the correlation of Notch expression with the abundance of immune infiltrates. Meanwhile, correlations between Notch expression and gene markers of tumor-infiltrating immune cells were also explored.
Supplementary Material
Footnotes
AUTHOR CONTRIBUTIONS: Conceptualization, Ziqiang Zhang; Investigation, Jian Hu, Jianghong Yu; Writing – Original Draft, Jian Hu, Jianghong Yu; Writing – Review & Editing, Ziqiang Zhang, Jun Gan, Ning Song, Liubin Shi, Jie Liu, Jianjun Du; Visualization, Ziqiang Zhang; Supervision, Jie Liu, Jianjun Du; Funding Acquisition, Jianjun Du.
CONFLICTS OF INTEREST: The authors declare that they have no competing interests.
FUNDING: This study was supported by the National Natural Science Foundation of China (grant No. 81270440).
REFERENCES
- 1.Luebeck EG, Curtius K, Jeon J, Hazelton WD. Impact of tumor progression on cancer incidence curves. Cancer Res. 2013; 73:1086–96. 10.1158/0008-5472.CAN-12-2198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ntziachristos P, Lim JS, Sage J, Aifantis I. From fly wings to targeted cancer therapies: a centennial for notch signaling. Cancer Cell. 2014; 25:318–34. 10.1016/j.ccr.2014.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Espinoza I, Miele L. Notch inhibitors for cancer treatment. Pharmacol Ther. 2013; 139:95–110. 10.1016/j.pharmthera.2013.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilson A, Radtke F. Multiple functions of Notch signaling in self-renewing organs and cancer. FEBS Lett. 2006; 580:2860–68. 10.1016/j.febslet.2006.03.024 [DOI] [PubMed] [Google Scholar]
- 5.Wang Q, Wen YG, Li DP, Xia J, Zhou CZ, Yan DW, Tang HM, Peng ZH. Upregulated INHBA expression is associated with poor survival in gastric cancer. Med Oncol. 2012; 29:77–83. 10.1007/s12032-010-9766-y [DOI] [PubMed] [Google Scholar]
- 6.Koch U, Radtke F. Notch and cancer: a double-edged sword. Cell Mol Life Sci. 2007; 64:2746–62. 10.1007/s00018-007-7164-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Radtke F, Raj K. The role of Notch in tumorigenesis: oncogene or tumour suppressor? Nat Rev Cancer. 2003; 3:756–67. 10.1038/nrc1186 [DOI] [PubMed] [Google Scholar]
- 8.South AP, Cho RJ, Aster JC. The double-edged sword of Notch signaling in cancer. Semin Cell Dev Biol. 2012; 23:458–64. 10.1016/j.semcdb.2012.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brose K, Tessier-Lavigne M. Slit proteins: key regulators of axon guidance, axonal branching, and cell migration. Curr Opin Neurobiol. 2000; 10:95–102. 10.1016/S0959-4388(99)00066-5 [DOI] [PubMed] [Google Scholar]
- 10.Houghton J, Wang TC. Helicobacter pylori and gastric cancer: a new paradigm for inflammation-associated epithelial cancers. Gastroenterology. 2005; 128:1567–78. 10.1053/j.gastro.2005.03.037 [DOI] [PubMed] [Google Scholar]
- 11.Wu WK, Cho CH, Lee CW, Fan D, Wu K, Yu J, Sung JJ. Dysregulation of cellular signaling in gastric cancer. Cancer Lett. 2010; 295:144–53. 10.1016/j.canlet.2010.04.025 [DOI] [PubMed] [Google Scholar]
- 12.Previs RA, Coleman RL, Harris AL, Sood AK. Molecular pathways: translational and therapeutic implications of the Notch signaling pathway in cancer. Clin Cancer Res. 2015; 21:955–61. 10.1158/1078-0432.CCR-14-0809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang QW, Liu L, Gong CY, Shi HS, Zeng YH, Wang XZ, Zhao YW, Wei YQ. Prognostic significance of tumor-associated macrophages in solid tumor: a meta-analysis of the literature. PLoS One. 2012; 7:e50946. 10.1371/journal.pone.0050946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kashimura S, Saze Z, Terashima M, Soeta N, Ohtani S, Osuka F, Kogure M, Gotoh M. CD83(+) dendritic cells and Foxp3(+) regulatory T cells in primary lesions and regional lymph nodes are inversely correlated with prognosis of gastric cancer. Gastric Cancer. 2012; 15:144–53. 10.1007/s10120-011-0090-9 [DOI] [PubMed] [Google Scholar]
- 15.Zhao JJ, Pan K, Wang W, Chen JG, Wu YH, Lv L, Li JJ, Chen YB, Wang DD, Pan QZ, Li XD, Xia JC. The prognostic value of tumor-infiltrating neutrophils in gastric adenocarcinoma after resection. PLoS One. 2012; 7:e33655. 10.1371/journal.pone.0033655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee HE, Chae SW, Lee YJ, Kim MA, Lee HS, Lee BL, Kim WH. Prognostic implications of type and density of tumour-infiltrating lymphocytes in gastric cancer. Br J Cancer. 2008; 99:1704–11. 10.1038/sj.bjc.6604738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsukayama S, Omura K, Yoshida K, Tanaka Y, Watanabe G. Prognostic value of CD83-positive mature dendritic cells and their relation to vascular endothelial growth factor in advanced human gastric cancer. Oncol Rep. 2005; 14:369–75. 10.3892/or.14.2.369 [DOI] [PubMed] [Google Scholar]
- 18.Kim HI, Kim H, Cho HW, Kim SY, Song KJ, Hyung WJ, Park CG, Kim CB. The ratio of intra-tumoral regulatory T cells (Foxp3+)/helper T cells (CD4+) is a prognostic factor and associated with recurrence pattern in gastric cardia cancer. J Surg Oncol. 2011; 104:728–33. 10.1002/jso.22038 [DOI] [PubMed] [Google Scholar]
- 19.Shen Z, Zhou S, Wang Y, Li RL, Zhong C, Liang C, Sun Y. Higher intratumoral infiltrated Foxp3+ Treg numbers and Foxp3+/CD8+ ratio are associated with adverse prognosis in resectable gastric cancer. J Cancer Res Clin Oncol. 2010; 136:1585–95. 10.1007/s00432-010-0816-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ubukata H, Motohashi G, Tabuchi T, Nagata H, Konishi S, Tabuchi T. Evaluations of interferon-γ/interleukin-4 ratio and neutrophil/lymphocyte ratio as prognostic indicators in gastric cancer patients. J Surg Oncol. 2010; 102:742–47. 10.1002/jso.21725 [DOI] [PubMed] [Google Scholar]
- 21.Chen X, Leung SY, Yuen ST, Chu KM, Ji J, Li R, Chan AS, Law S, Troyanskaya OG, Wong J, So S, Botstein D, Brown PO. Variation in gene expression patterns in human gastric cancers. Mol Biol Cell. 2003; 14:3208–15. 10.1091/mbc.e02-12-0833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D’Errico M, de Rinaldis E, Blasi MF, Viti V, Falchetti M, Calcagnile A, Sera F, Saieva C, Ottini L, Palli D, Palombo F, Giuliani A, Dogliotti E. Genome-wide expression profile of sporadic gastric cancers with microsatellite instability. Eur J Cancer. 2009; 45:461–69. 10.1016/j.ejca.2008.10.032 [DOI] [PubMed] [Google Scholar]
- 23.Cho JY, Lim JY, Cheong JH, Park YY, Yoon SL, Kim SM, Kim SB, Kim H, Hong SW, Park YN, Noh SH, Park ES, Chu IS, Hong WK, Ajani JA, Lee JS. Gene expression signature-based prognostic risk score in gastric cancer. Clin Cancer Res. 2011; 17:1850–7. 10.1158/1078-0432.CCR-10-2180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Azimi F, Scolyer RA, Rumcheva P, Moncrieff M, Murali R, McCarthy SW, Saw RP, Thompson JF. Tumor-infiltrating lymphocyte grade is an independent predictor of sentinel lymph node status and survival in patients with cutaneous melanoma. J Clin Oncol. 2012; 30:2678–83. 10.1200/JCO.2011.37.8539 [DOI] [PubMed] [Google Scholar]
- 25.Aster JC, Pear WS, Blacklow SC. The Varied Roles of Notch in Cancer. Annu Rev Pathol. 2017; 12:245–75. 10.1146/annurev-pathol-052016-100127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nowell CS, Radtke F. Notch as a tumour suppressor. Nat Rev Cancer. 2017; 17:145–59. 10.1038/nrc.2016.145 [DOI] [PubMed] [Google Scholar]
- 27.Hoare M, Ito Y, Kang TW, Weekes MP, Matheson NJ, Patten DA, Shetty S, Parry AJ, Menon S, Salama R, Antrobus R, Tomimatsu K, Howat W, et al. NOTCH1 mediates a switch between two distinct secretomes during senescence. Nat Cell Biol. 2016; 18:979–92. 10.1038/ncb3397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weijzen S, Rizzo P, Braid M, Vaishnav R, Jonkheer SM, Zlobin A, Osborne BA, Gottipati S, Aster JC, Hahn WC, Rudolf M, Siziopikou K, Kast WM, Miele L. Activation of Notch-1 signaling maintains the neoplastic phenotype in human Ras-transformed cells. Nat Med. 2002; 8:979–86. 10.1038/nm754 [DOI] [PubMed] [Google Scholar]
- 29.Balint K, Xiao M, Pinnix CC, Soma A, Veres I, Juhasz I, Brown EJ, Capobianco AJ, Herlyn M, Liu ZJ. Activation of Notch1 signaling is required for beta-catenin-mediated human primary melanoma progression. J Clin Invest. 2005; 115:3166–76. 10.1172/JCI25001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou W, Fu XQ, Zhang LL, Zhang J, Huang X, Lu XH, Shen L, Liu BN, Liu J, Luo HS, Yu JP, Yu HG. The AKT1/NF-kappaB/Notch1/PTEN axis has an important role in chemoresistance of gastric cancer cells. Cell Death Dis. 2013; 4:e847. 10.1038/cddis.2013.375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang H, Wang X, Xu J, Sun Y. Notch1 activation is a poor prognostic factor in patients with gastric cancer. Br J Cancer. 2014; 110:2283–90. 10.1038/bjc.2014.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cao Z, Ding BS, Guo P, Lee SB, Butler JM, Casey SC, Simons M, Tam W, Felsher DW, Shido K, Rafii A, Scandura JM, Rafii S. Angiocrine factors deployed by tumor vascular niche induce B cell lymphoma invasiveness and chemoresistance. Cancer Cell. 2014; 25:350–65. 10.1016/j.ccr.2014.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boelens MC, Wu TJ, Nabet BY, Xu B, Qiu Y, Yoon T, Azzam DJ, Twyman-Saint Victor C, Wiemann BZ, Ishwaran H, Ter Brugge PJ, Jonkers J, Slingerland J, Minn AJ. Exosome transfer from stromal to breast cancer cells regulates therapy resistance pathways. Cell. 2014; 159:499–513. 10.1016/j.cell.2014.09.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu C, Liu L, Chen X, Cheng J, Zhang H, Zhang C, Shan J, Shen J, Qian C. LSD1 Stimulates Cancer-Associated Fibroblasts to Drive Notch3-Dependent Self-Renewal of Liver Cancer Stem-like Cells. Cancer Res. 2018; 78:938–49. 10.1158/0008-5472.CAN-17-1236 [DOI] [PubMed] [Google Scholar]
- 35.Sansone P, Storci G, Tavolari S, Guarnieri T, Giovannini C, Taffurelli M, Ceccarelli C, Santini D, Paterini P, Marcu KB, Chieco P, Bonafè M. IL-6 triggers malignant features in mammospheres from human ductal breast carcinoma and normal mammary gland. J Clin Invest. 2007; 117:3988–4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raafat A, Bargo S, Anver MR, Callahan R. Mammary development and tumorigenesis in mice expressing a truncated human Notch4/Int3 intracellular domain (h-Int3sh). Oncogene. 2004; 23:9401–07. 10.1038/sj.onc.1208187 [DOI] [PubMed] [Google Scholar]
- 37.Hu MS, Borrelli MR, Januszyk M, Luan A, Malhotra S, Walmsley GG, Hong WX, Tevlin R, Gurtner GC, Longaker MT, Lorenz HP. Pathway Analysis of Gene Expression of E14 Versus E18 Fetal Fibroblasts. Adv Wound Care (New Rochelle). 2018; 7:1–10. 10.1089/wound.2017.0763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pajvani UB, Shawber CJ, Samuel VT, Birkenfeld AL, Shulman GI, Kitajewski J, Accili D. Inhibition of Notch signaling ameliorates insulin resistance in a FoxO1-dependent manner. Nat Med. 2011; 17:961–67. 10.1038/nm.2378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pajvani UB, Qiang L, Kangsamaksin T, Kitajewski J, Ginsberg HN, Accili D. Inhibition of Notch uncouples Akt activation from hepatic lipid accumulation by decreasing mTorc1 stability. Nat Med. 2013; 19:1054–60. 10.1038/nm.3259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017; 45:W98–102. 10.1093/nar/gkx247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Szász AM, Lánczky A, Nagy Á, Förster S, Hark K, Green JE, Boussioutas A, Busuttil R, Szabó A, Győrffy B. Cross-validation of survival associated biomarkers in gastric cancer using transcriptomic data of 1,065 patients. Oncotarget. 2016; 7:49322–33. 10.18632/oncotarget.10337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tomczak K, Czerwińska P, Wiznerowicz M. The Cancer Genome Atlas (TCGA): an immeasurable source of knowledge. Contemp Oncol (Pozn). 2015; 19:A68–77. 10.5114/wo.2014.47136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, Cerami E, Sander C, Schultz N. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013; 6:pl1. 10.1126/scisignal.2004088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wise LM, Stuart GS, Real NC, Fleming SB, Mercer AA. VEGF Receptor-2 Activation Mediated by VEGF-E Limits Scar Tissue Formation Following Cutaneous Injury. Adv Wound Care (New Rochelle). 2018; 7:283–97. 10.1089/wound.2016.0721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McLaughlin PJ, Cain JD, Titunick MB, Sassani JW, Zagon IS. Topical Naltrexone Is a Safe and Effective Alternative to Standard Treatment of Diabetic Wounds. Adv Wound Care (New Rochelle). 2017; 6:279–88. 10.1089/wound.2016.0725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lei J, Priddy LB, Lim JJ, Massee M, Koob TJ. Identification of Extracellular Matrix Components and Biological Factors in Micronized Dehydrated Human Amnion/Chorion Membrane. Adv Wound Care (New Rochelle). 2017; 6:43–53. 10.1089/wound.2016.0699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li T, Fan J, Wang B, Traugh N, Chen Q, Liu JS, Li B, Liu XS. TIMER: A Web Server for Comprehensive Analysis of Tumor-Infiltrating Immune Cells. Cancer Res. 2017; 77:e108–10. 10.1158/0008-5472.CAN-17-0307 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.