Abstract
During aging, deterioration in cardiac structure and function leads to increased susceptibility to heart failure. The need for interventions to combat this age-related cardiac decline is becoming increasingly urgent as the elderly population continues to grow. Our understanding of cardiac aging, and aging in general, is limited. However, recent studies of age-related decline and its prevention through interventions like exercise have revealed novel pathological and cardioprotective pathways. In this review, we summarize recent findings concerning the molecular mechanisms of age-related heart failure, and highlight exercise as a valuable experimental platform for the discovery of much-needed novel therapeutic targets in this chronic disease.
Keywords: Heart failure, Aging, Senescence, Epigenetics, Exercise
INTRODUCTION
A major risk factor for heart failure (HF) and overall cardiovascular disease is age. Approximately 1% of individuals aged over 50 years are affected by HF and this number doubles with each decade of life,1 making HF the major cause of mortality in the elderly.2 This is a matter of increasing concern in the United States, where the population aged 65 and over increased from 40 million in 2007 to 51 million in 2017 and is projected to reach 95 million in 2060.2 Given this dramatic growth in the aged population, age-related HF represents one of the greatest challenges confronting global healthcare today.
Undoubtedly, an important part of the explanation for increased HF with increasing age lies in greater time for exposure to injurious stimuli, such as hypertension, metabolic stress, or ischemic injury. The heart’s limited endogenous capacity for repair or regeneration implies that heart function at any specific time reflects the cumulative burden of prior insults. Thus, it makes sense that older patients would have a greater impairment of cardiac reserves and elevated HF risk.
However, even in the absence of overt injury, structural and functional changes occur in the heart as it ages, which appear to contribute to the increased susceptibility to HF in older adults.3, 4 Normal aging is generally accompanied by a thickening and stiffening of the left ventricular walls, particularly the interventricular septum, an increase in left atrial dilation, and an overall increase in cardiac fibrosis.5 Although resting cardiac function is not markedly impaired in the aged heart, both subclinical diastolic and systolic dysfunction are present.6, 7 However, the most notable functional change observed in the aged heart is the progressive decline in cardiac reserves, which not only contributes to the age-related decline in exercise capacity,8-10 but interestingly is also a major pathophysiological feature of HF with preserved ejection fraction (HFpEF), the most common form of HF in older adults.11, 12
Although aging has long been considered an unmodifiable consequence of passage of time (chronological aging),13 the observation that the rate of age-related deterioration (biological aging) differs substantially across species, individuals, and organs,14 has led to a more nuanced understanding of biological aging as mutable and potentially amenable to manipulation. Thus, we and others have proposed that understanding the underlying biology of cardiac aging could potentially lead to the discovery of novel therapeutic targets for age-related cardiovascular diseases. Indeed, accumulating evidence suggests that it may be possible to target age-related pathways to counteract and even reverse some of the structural and functional changes that drive age-related HF.15, 16 Epidemiological data support the idea that age-related cardiac decline can also be modulated by lifestyle factors, such as diet and exercise, and experimental studies in animals suggest that behavioral, pharmacological and genetic interventions can slow or accelerate age-related changes and the development of HF.15-17 These studies suggest protective physiological pathways can counteract the effects of aging and prolong cardiac health.
One of the most striking behavioral modulators of cardiac aging is physical activity, which appears to prevent or mitigate cardiovascular disease in older adults and the elderly.18-21 There is growing evidence that exercise is associated with lower HF risk and attenuation of age-associated intermediate phenotypes such as cellular senescence, telomere length, and cell survival signaling.22-25 Although much of the human data suggesting that exercise can attenuate cardiac aging is cross-sectional and observational in nature, multiple prospective studies suggest exercise is capable of mitigating or even partially reversing at least some of cardiac aging phenotypes associated with HF.26-29 Thus, investigation into the physiological effects of exercise has the potential to complement therapeutic insights derived from investigation of the mechanisms of cardiac decline. Deciphering the mechanisms underlying the beneficial effects of exercise on cardiac aging could be helpful in the development of therapeutic interventions to curtail or even reverse age-related functional decline in the heart.
This review summarizes the pathophysiological changes in the aging heart, recent advances in our understanding of the underlying molecular mechanisms, and the potential therapeutic implications. The cardioprotective effects of exercise will also be discussed, with emphasis on the potential value of exercise as an experimental paradigm for discovering cardioprotective pathways that can be exploited for prevention and treatment of aged-related HF.
PATHOPHYSIOLOGICAL PROCESSES DRIVING CARDIAC AGING
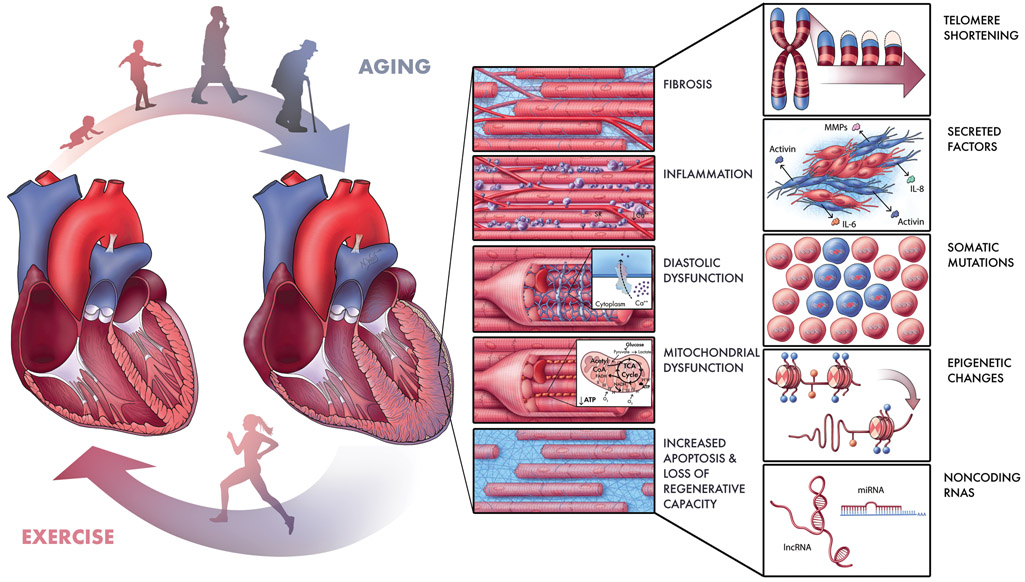
Both systemic and cardiac-specific changes in cellular physiology likely contribute to age-related alterations in heart structure and function (Figure 1). Although there is an increase in left ventricular wall thickness with age, not number, of cardiomyocytes. In fact, aging is associated with a decrease in regenerative capacity, which may be compounded by an increase in cell death. This in turn may be related to an age-dependent decline in mitochondrial function and accumulation of senescent cells. At the same time, elevated inflammatory activity likely drives the increase in myocardial fibrosis with age. The following sections will discuss new insights into the cellular processes that contribute to cardiac deterioration with aging. While some of these are systemic processes that affect a range of organ systems, we will concentrate on the cardiac manifestations of these systemic processes.
Figure 1. Schematic of Processes and Pathways Contributing to Age-Related Cardiac Disease.
As we age, multiple processes likely contribute to cardiac dysfunction, including fibrosis, inflammation, mechanical stiffening and diastolic dysfunction, mitochondrial dysfunction, and a growing imbalance between loss and birth of cardiomyocytes (Center). These processes are driven by molecular mechanisms (some of which are depicted at right) such as telomere shortening, senescence-associated secreted factors, accumulation of somatic mutations, epigenetic changes, and alterations in noncoding RNAs regulating gene expression. Some of these may represent targets for new therapeutic strategies to mitigate both age-related and other forms of heart failure. Since exercise mitigates many effects of aging (Left, bottom), it may provide a useful tool by enabling us to prioritize candidate cardiac pathways exacerbated by aging and mitigated by exercise. Illustration by Nicole Wolf, MS, ©2019. Printed with permission.
Cardiomyocyte Death and Regeneration
While neonatal mammals can regenerate myocardial tissue following damage, the adult mammalian heart does not regenerate after injury and has traditionally been understood to lack the capacity for cardiomyogenesis.30 However, studies in mice and humans have revealed that adult cardiomyocytes renew at a rate of 0.5-2% per year, indicating that the adult heart has some, albeit limited, endogenous regenerative potential.31-33 This renewal was found to decline with age implying a diminished ability to compensate for cardiomyocyte loss.31-33 The consequences of this decline in regenerative potential could be serious as even very low levels of experimentally-induced cardiomyocyte loss have been shown to result in cardiomyopathy and death.34 Elevated rates of cardiomyocyte apoptosis may pose an additional challenge to cardiac homeostasis,35 although the evidence for this is mixed, with recent human studies failing to detect a correlation.36 Given the aging heart’s declining regenerative potential, therapeutic interventions to promote endogenous regenerative capacity may be able to favorably tilt the homeostatic balance and improve performance in aging hearts.
Adult cardiomyocyte regeneration has been the subject of intense investigation and debate. Although there have been reports of cardiomyogenesis from bone marrow-derived and tissue resident putative cardiac progenitors, many of the original reports have been discredited and some retracted.37-39 In particular, cell fate-mapping studies have demonstrated that cells expressing the hematopoietic stem cell marker c-kit, once proposed as resident cardiac stem cells, give rise to remarkably few cardiomyocytes and do not appear to contribute to myocardial regeneration in a meaningful way.40-43 Even these small numbers of labeled cardiomyocytes were further determined to be present independent of the ability of c-kit+ cell progeny to adopt a cardiomyocyte fate, reflecting the fusion of pre-existing cardiomyocytes with labeled leukocytes, but not cardiomyogenesis.44 The consensus that has emerged from these studies is that cardiomyogenesis in the adult heart results from division of preexisting cardiomyocytes and not from differentiation of stem cells.31, 37, 45, 46 Investigation into the cardiomyocyte populations mediating endogenous cardiomyocyte renewal and the factors regulating their proliferative activity over time will likely lead to new therapeutic targets relevant to aging. Indeed, several molecular mechanisms implicated in regulation of cardiomyocyte proliferation are now being investigated as potential therapeutic targets, some of which will be discussed in later sections. Interestingly, mechanical strain induced by hemodynamic forces has been shown to modulate the activity of yes-associated protein (YAP) and transcriptional co-activator with PDZ-binding motif (TAZ), transcriptional co-activators implicated in proliferation, with implications for the development of atherosclerosis.47 The potential impact of this mechanosignaling pathway in the age-related decline in cardiomyocyte proliferation is an intriguing topic for future investigation.
Mitochondrial Dysfunction
Mitochondria are not only the primary source of energy in the heart, in the form of ATP, but are also key regulators of cardiomyocyte survival. Mitochondrial dysfunction is a core feature of cardiac aging and is at the crossroads of multiple key pathways related to senescence.48 Cardiac aging is accompanied by a general decline in mitochondrial function and the resulting increase in reactive oxygen species (ROS) production appears to be a major contributing factor in HF (Figure 1).
Recent studies of mitochondrial dysfunction in the aging heart are consistent with the oxidative stress hypothesis of aging, which posits that oxidative stress induced by excessive mitochondrial ROS generation damages mitochondrial DNA (mtDNA) and redox-sensitive mitochondrial proteins, leading to mitochondrial dysfunction and further increasing ROS production. This self-perpetuating cycle of oxidative damage is hypothesized to result in cellular and organ functional decline, limiting lifespan and healthspan. The direct role of mitochondrial oxidative damage in cardiac aging has been demonstrated using mitochondria-specific overexpression of human catalase, an antioxidant enzyme, in mice (mCAT). mCAT mice exhibited prolonged lifespan and displayed attenuated cardiac aging phenotypes including reduced cardiac hypertrophy, improved diastolic function, and improved myocardial performance. On the molecular level, these improvements were accompanied by reduced mitochondrial protein oxidative damage, mtDNA mutations, and deletion frequencies.49, 50
Mitochondrial dysfunction is also sufficient to induce deterioration of cardiac function. In mice, knock-out of proliferator-activated receptor γ coactivator 1α (PGC-1α), a key regulator of mitochondrial biogenesis, resulted in suppression of mitochondrial gene expression within the heart and development of cardiac dysfunction at 7-8 months old.51 Notably, the same PGC-1α knockout mouse line exhibited accelerated cardiac dysfunction when subjected to transverse aortic constriction (TAC).52 This indicates a protective role of PGC-1α in cardiac function and highlights the increased vulnerability of the heart to insult once mitochondrial biogenesis is compromised.
Taken together, these findings suggest that mitochondrial dysfunction and the resulting ROS generation could contribute to age-associated cardiac dysfunction. Consequently, mitochondrial-targeted antioxidant therapies may have therapeutic value. However, the effects of mitochondrial ROS appear to be context-dependent, limiting the therapeutic potential of antioxidant therapy. In healthy young men, a 4-week intervention of physical exercise improved insulin sensitivity and induced endogenous ROS defenses as well as PGC-1α, but counterintuitively, these beneficial effects were blocked by antioxidant supplementation, demonstrating that under some conditions ROS production can be beneficial perhaps through induction of adaptive responses that mediate chronic benefit.53 Indeed, small increases in mitochondrial ROS have been shown to increase cellular defenses54 and in some cases extend lifespan.55 Further evidence for the context-dependent effects of mitochondrial manipulations comes from experiments showing that upregulating mitochondrial biogenesis through cardiac-specific overexpression of PGC-1α has protective effects in young mice but accelerates cardiac aging in old mice.56 Perhaps because of these complexities, multiple attempts to exploit antioxidant treatments for a range of cardiovascular diseases have thus far failed to demonstrate clinical benefits.
Recent research has suggested other possible approaches to targeting mitochondrial dysfunction and/or metabolic remodeling in heart failure. For example, complementary metabolomic and proteomic analyses in advanced human heart failure and animal models, respectively, suggest a reduction in fatty acid oxidation and an increased use of ketones and ketone bodies as fuel in heart failure.57, 58 While the pathophysiological consequences of this shift in substrate utilization remain incompletely understood,59, 60 prior work in murine models suggests ketone metabolism may be important in mitigating cardiac dysfunction.61 In these studies, lineage-specific deletion of succinyl-CoA:3-oxoacid CoA transferase (SCOT), the enzyme mediating terminal oxidation of ketone bodies, in cardiomyocytes accelerated adverse remodeling and increased ROS production after TAC.61 Collectively these studies raise the intriguing hypothesis that altering substrate availability through dietary interventions in patients could improve energy homeostasis in the failing heart.
Another approach to metabolic remodeling has focused on the NADH/NAD(+) ratio, as a critical determinant of electron transport, oxidative stress, and intracellular signaling.62 In both experimental pathological hypertrophy and failing human hearts, NADH/NAD(+) ratios were increased62. Experimental models suggest NAD(+) may exert its functional effects in part through mitochondrial NAD(+)-dependent deacetylases Sirtuin 3 (Sirt3) and Sirt4, which regulate cell survival and the mitochondrial permeability transition pore (mPTP), important determinants of cardiomyocyte survival and function.63, 64 Importantly, normalizing NADH/NAD(+) ratios through either genetic or pharmacological interventions had important metabolic and functional benefits in murine models62. Since NADH/NAD(+) ratios can be modified through dietary nicotinamide riboside supplementation65, translation of preclinical findings to clinical trials to test this hypothesis in humans appears feasible.
Cellular Senescence and Inflammation
Cellular senescence is a state triggered by telomere attrition due to repeated replication,66 or by other forms of cellular stress,67 in which cells undergo permanent cell cycle arrest, functional decline, and take on a pro-inflammatory phenotype. Senescent cells progressively accumulate in tissues during life and confer deleterious paracrine effects on neighboring cells and systemic effects on other tissues/organs. These effects are mediated by secretion of pro-inflammatory cytokines, proteases and insoluble extracellular matrix components, known as the senescence-associated secretory phenotype (SASP).67 SASP signaling promotes inflammation as well as cell death and senescence in other cells, and growing evidence suggests that senescent cells contribute to cardiac remodeling and dysfunction during aging (Figure 1).68, 69
Consistent with a role for cellular senescence in cardiac aging, there is evidence that senescent cells in the heart contribute to functional decline including decreased contractility and impaired mitochondrial function.70, 71 Furthermore, promoting senescence can accelerate the development of cardiac aging phenotypes. Cardiomyocyte death, hypertrophy, and HF were increased in mice with accelerated senescence resulting from a deficiency of telomerase, an enzyme that prevents senescence by combating the loss of telomeres during replication.72 Similarly, in senescence-accelerated mice fed a high-fat, high-salt diet for 24 weeks, senescent endothelial cells were increased in the heart and this was associated with diastolic dysfunction and left ventricular hypertrophy,73 suggesting a role for endothelial cell senescence in cardiac dysfunction in aged hearts.
Complementary findings suggest that reducing senescence can have cardioprotective effects. Elimination of cells expressing the senescence marker p16Ink4a inhibited aging phenotypes in the heart (e.g. reduced cardiomyocytes size, preserved cardioprotective KATP channels, reduced cardiac fibrosis) and extended lifespan.74 Similarly, clearance of p16Ink4a positive senescent cells in aged mice reduced cardiac hypertrophy and fibrosis, and induced cardiomyocyte proliferation manifested as elevated EdU and Ki-67 positive cardiomyocytes.75, 76
The beneficial effects of eliminating senescent cells suggest that these cells may be actively suppressing the function of otherwise healthy heart cells. In line with this, Anderson et al showed that during aging, human and murine cardiomyocytes exhibited a senescent-like phenotype, including secretion of non-typical SASP signals (endothelin 3, Edn3; transforming growth factor β 2, Tgfb2; and growth differentiation factor-15, GDF15), and incubation of neonatal fibroblasts with conditioned culture medium from cardiomyocytes isolated from old mice resulted in fibroblast activation and senescence. This implies an interaction between senescent cardiomyocytes and fibroblasts during cardiac aging and dysfunction.70
These observations support a model in which, during aging, cellular stress induced by oxidative stress and inflammation leads to cardiomyocyte senescence. These senescent cardiomyocytes undergo functional decline, including decreased contractility, increased cell size, and mitochondrial dysfunction, negatively affecting cardiac performance. As aging progresses, senescent cardiomyocytes accumulate, interfere with intercellular communication, compromise cardiac function, and further induce chronic inflammation, resulting in cell death, and eventual cardiac dysfunction.77
Our current understanding of what drives senescence may not be sufficient to enable therapeutic strategies to prevent it. However, it may be possible to intervene further downstream. The dramatic beneficial effects seen with genetic elimination of senescent cells, described above, have prompted efforts to develop senolytics, small molecules capable of reducing or eradicating these cells.78-81 While these approaches are novel and exciting, the clinical benefits will obviously need to be demonstrated in realistic clinical contexts where multiple cardiometabolic co-morbidities co-exist. A complementary strategy would be to target the downstream effectors mediating the adverse consequences of senescent cells. As noted, secreted proteins likely contribute to these adverse effects in many settings and in cases where the principal culprits can be identified, a variety of strategies could be used to mitigate the effects of these secreted proteins. Activin and the closely related family of Growth and Differentiation Proteins (GDFs) are senescence-associated secreted proteins that provide one example of this strategy. As detailed below, we recently described an increase in activin signaling in age-related heart failure that could be dramatically mitigated by either antibodies directed to the dominant activin receptors or a soluble-receptor acting as a ligand trap.15
Another consideration is that, although the findings described strongly indicate a mechanistic role of cellular senescence in the development of cardiac disease during aging, cellular senescence in specific cell lineages and conditions may actually be beneficial to the heart. In neonatal mice subjected to apical resection, a model for cardiac regeneration, senescent cells were identified in the peri-resected regions at several time points after apical resection but disappeared when the hearts were fully restored. Elimination of the senescent fibroblasts by fibroblast-targeted genetic deletion of tumor protein 53 (Trp53) inhibited regeneration after apical resection, indicating that senescent fibroblasts promote neonatal heart regeneration.82 The regenerative activity of senescent fibroblasts is not limited to the neonatal heart. Meyer et al. found that senescent fibroblasts accumulated in fibrotic regions of hearts subjected to TAC, and inactivation of cardiac senescent cells by double knockout of Trp53 and cyclin-dependent kinase inhibitor 2a (Cdkn2a) genes further increased fibrosis and fibroblast proliferation leading to severe cardiac dysfunction. In contrast, activation of premature senescence by cardiac-specific overexpression of cellular communication network factor 1 (CCN1) reduced perivascular fibrosis after TAC and improved cardiac function.75 These results indicate that, in contrast to the deleterious effects of senescence in the aging heart, fibroblast senescence in the apically resected neonatal heart and in TAC is actually beneficial. Thus, while there is strong evidence implicating cellular senescence in age-related cardiac deterioration, the effect of senescence on cardiac regeneration and disease may be complex and context-specific, perhaps varying depending on cell type, age, health and other factors.
Exercise Counteracts Age-related Pathophysiological Changes
Exercise is associated with improved cardiac function and may even partially reverse pathological cardiac remodeling in the elderly (Figure 1).21 In one study, for instance, three to six months’ aerobic exercise training improved peak oxygen consumption and exercise efficiency in elderly subjects (65-79 years).83 While these benefits likely derive in part from peripheral conditioning, there is also evidence of cardiac remodeling through exercise training in the elderly. For example, one year of progressive and vigorous endurance exercise training in previously inactive individuals over age 65 induced physiological left ventricular remodeling, increasing left ventricular mass without affecting left ventricular mass-volume ratio.26 These cardiovascular benefits have been attributed, in part, to antioxidant effects. Consistent with this, a six-month exercise training regimen increased the activity of ROS scavenging enzymes in the skeletal muscle of HF patients relative to sedentary controls in a randomized controlled study.84
In line with its reported therapeutic effects in humans, experiments in animal models show that exercise has the potential to counteract many pathological processes thought to contribute to age-related HF, including senescence, inflammation, mitochondrial dysfunction and declining cardiomyocyte regeneration (Figure 3). In mice, voluntary running for 21 days down-regulated senescence gene markers in the heart including cell-cycle-checkpoint kinase 2 (Chk2), p53, p16, and up-regulated cardiac telomerase activity by 2-fold.22, 23 These effects were abolished by in telomerase reverse transcriptase (TERT) deficiency mice, suggesting an anti-senescent effect of exercise on the heart. In line with this, exercise reduced age-related cardiac inflammation and fibrosis.85 Exercise also increased left ventricular mitochondrial number and volume,86 and induced expression of genes involved in mitochondrial biogenesis and antioxidant response.87, 88 Exercise also reduced cardiomyocyte apoptosis in aging animals,89 and in both young and old animals subjected to ischemia-reperfusion injury.90 Furthermore, we demonstrated that exercise can promote cardiomyogenesis in young adult mice. We found that two weeks of swimming induced cell proliferation markers (e.g. BrdU, phosphorylated histone H3, Aurora B kinase, etc,) in young (12 weeks old) adult mouse hearts compared to sedentary controls.91 Exercise also induced cell proliferation in adult cardiomyocytes, measured with multiphoton ionization mass spectrometry (MIMS92)-based imaging using thymidine labeled with the stable isotope,93 and expanded the proliferative zone after myocardial ischemia reperfusion injury.94
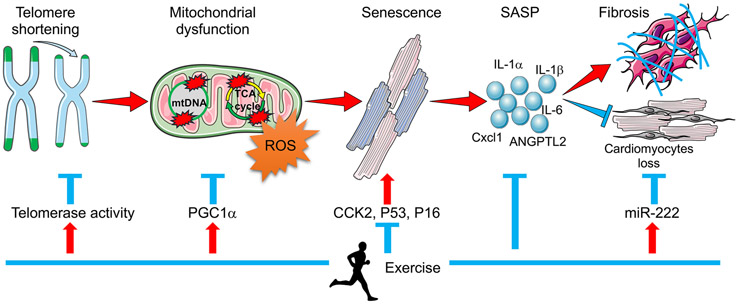
Figure 3. Schematic of Telomere Shortening Contributing to Age-related Cardiac Disease.
Telomeres shorten with cell division and aging is associated with reduced telomere length. Mitochondria are particularly vulnerable. Critically short telomeres lead to mitochondrial DNA damage, ultimately disrupting mitochondrial function and inducing senescence. Through secretion of senescence-associated secretory phenotype (SASP) factors, senescent cells induce fibrosis and promote cardiomyocyte loss, eventually leading to cardiac dysfunction. Exercise has been shown to protect the heart against cardiac aging and cardiac dysfunction by modulating each step in this sequence.
Interestingly, the cardiac response to exercise and pathological growth stimuli appear to involve distinct molecular pathways.91 Thus, the study of exercise models is likely to yield different candidates for intervention than those identified through studies of disease models. Moreover, with remarkable consistency, pathway identified as functionally important in the cardiac response to exercise also protect the heart against pathological stress.21, 95 Examples include PI3-kinase,96, 97 Akt1,98-100 eNOS,101 PGC1α,52, 102 C/EBPβ,91 CITED4,103 and miR-222.94 Thus, we suggest that exercise models provide platforms for discovery of new therapeutic target candidates likely to complement those gleaned from disease models. Below we will discuss recent developments in our understanding of the molecular mechanisms underlying cardiac aging, and the cardioprotective effects of exercise.
MOLECULAR MECHANISMS OF HEART FAILURE DURING AGING
Our understanding of the fundamental mechanisms underlying cardiac aging, and aging in general, is limited. However, recent studies have begun to elucidate the molecular basis for cardiac decline as well as potentially protective molecular pathways. Molecular mechanisms underlying cardiac aging are multifactorial and interactive. Here, we will discuss important findings in recent years concerning the roles of telomere shortening and damage, circulating factors, epigenetic alterations, noncoding RNAs, and somatic mutations in cardiac aging, and insights into the protective effects of exercise (Figure 1).
Telomere Shortening and Damage
Telomeres are DNA repeats serving as protective caps at the end of chromosomes. Telomeric DNA is bound by the shelterin protein complex, which is essential for telomere structure maintenance. Telomeres are synthesized by telomerase, an enzyme composed of an RNA component (TERC) and a catalytic subunit (TERT).104 Telomeres shorten with cell division and aging, or in response to stressors such as inflammation and oxidative stress. Critically short telomeres destabilize the shelterin complex, thereby disrupting the telomeric DNA structure, leading to DNA damage, cell cycle arrest, cellular senescence, and cell death.105
Telomere length is perhaps the best-known cellular marker of aging. Although rates of cardiomyocyte division are extremely low in adults,33 resulting in minimal end-replication-associated telomere shortening, animal studies have consistently pointed to a key role for telomere shortening in cardiac aging and disease.106 The wild mouse strain Mus musculus castaneus (CAST), which bears short telomeres from birth, shows a premature cardiac aging phenotype.107 Similarly, Wong et al showed that breeding multiple generations of TERC-deficient mice induced critical telomere shortening, leading to cardiac dysfunction and myocardial remodeling, similar to the effects of aging.108 In cardiomyocytes from TERC-deficient mice, Aix et al showed that critically short telomeres marked by γH2AX, a DNA double strand break biomarker, induced p21-dependent cardiomyocyte cell cycle arrest, consistent with senescence and an aging and diseased cardiac profile.109 In addition, accelerated telomere shortening has been linked to several hereditary cardiomyopathies in humans, including Duchenne Muscular Dystrophy (DMD),110 with animal models supporting a causal relationship.111 Together, these results suggest that accelerated telomere shortening phenocopies the effects of aging on the heart.
Conversely, slowing telomere shortening can protect the heart against pathological stress. Biomechanical stress induced by partial aortic constriction in mice caused reduction in TRF2, one of the shelterin proteins, leading to activation of the DNA damage response protein Chk2, a reduction in telomere length and cardiomyocyte apoptosis, and these changes were attenuated by cardiac-specific overexpression of telomerase reverse transcriptase (TERT) or overexpression of telomeric repeat-binding factor 2 (TRF2).112 Cardiac-specific overexpression of telomerase also decreased cardiomyocyte apoptosis during myocardial ischemia reperfusion in vivo or serum-free insulin-free challenge in vitro.113 Telomere shortening may affect the heart in part by triggering mitochondrial dysfunction. In the third generation TERC−/− telomerase-deficient premature aging mouse model, accelerated cardiac aging is associated with p53 mediated suppression of PGC-1α, leading to mitochondrial dysfunction. Furthermore, cardiac-specific overexpresssion of PGC-1α in TERC−/− mice partially rescued cardiac function and delayed the onset of age-related cardiac symptoms and extend healthspan.56 It is worth noting, however, that the value of TERC deficient mice as a model for telomere-associated cardiac aging has been questioned as the degree of telomere shortening in late-generation TERC mice far exceeds the shortening experienced during normal aging.114 Although there are some caveats to consider, overall results from animal models point to a critical role for telomere length in cardiac cells in mitochondrial function, cardiac aging and HF.
The role of telomere shortening in human cardiac aging and HF, however, is controversial. Terai et al reported that human myocardial telomere length is reduced at a rate of 20 base pairs per year.115 Compared with cardiac tissues from age- and sex- matched normal or hypertrophic obstructive cardiomyopathy patients without pump failure, cardiac tissues from end-stage HF patients showed shorter telomeres and less TRF2, associated with increased cardiac apoptosis.112 Similar results were observed in cardiac tissue from end-stage genetic hypertrophic cardiomyopathy and dilated cardiomyopathy patients as well as cardiomyocytes derived from patient-induced pluripotent stem cells.110 These results were confirmed by a recent study showing that, compared with nonfailing controls, HF patients exhibited shorter telomeres specifically in cardiomyocytes but not in cardiac smooth muscle cells from the same hearts.116 Further, this telomere shortening in HF patients was associated with extensive DNA damage in cardiomyocytes.116 However, the authors did not observe any difference in cardiomyocyte telomere length between young and old nonfailing controls.116 These results not only highlight the important role of telomere length in HF but also raise the possibility that telomere shortening does not reflect normal (physiological) cardiac aging. Indeed, most recently, Anderson et al demonstrated that in response to oxidative stress induced by mitochondrial dysfunction during cardiac aging, persistent telomere-associated foci (TAF), which contain DNA damage response proteins and trigger cellular senescence and cell cycle arrest, increased in cardiomyocytes but not in other cell types. This suggests telomere damage in cardiomyocytes occurs independently of telomere length during cardiac aging.70
Secreted Factors
Given the progressive functional decline in multiple organ systems seen with aging, it has long been hypothesized that secreted or circulating factors might contribute to the systemic phenotypes seen with aging. The role of pro-inflammatory SASP factors, such as IL-1α, IL-1β, IL-6, Cxcl1, have been extensively studied in the context of chronic systemic inflammation leading to HF, but an increasing number of secreted proteins or RNAs other than traditional SASP factors are now also being found to contribute to the development of aging-related disease (Figure 2).117 An interesting experimental approach to explore the role of circulating factors in aging phenotypes has been the use of the heterochronic parabiosis model, in which animals of different chronological ages are surgically sewn together to develop a shared circulatory system.118 Indeed, a recent series of studies utilizing this approach in mice found that introducing youthful blood into the circulation of old animals improved age-related neurologic, skeletal muscle, and cardiac phenotypes.119-121 Aptamer-based proteomic analyses of plasma samples from these mice identified 13 differentially expressed proteins, of which circulating levels of growth differentiation factor-11 (GDF11), a secreted member of the TGFβ superfamily, was reported to be significantly lower in aged mice. Subsequent experiments suggested that increasing circulating levels of GDF11 was sufficient to improve neurogenesis, skeletal muscle regeneration, cardiac hypertrophy regression, and overall fitness in old mice, suggesting the exciting possibility that an age-related decline in a secretory factor like GDF11 might be causal in the systemic functional decline seen in aging.
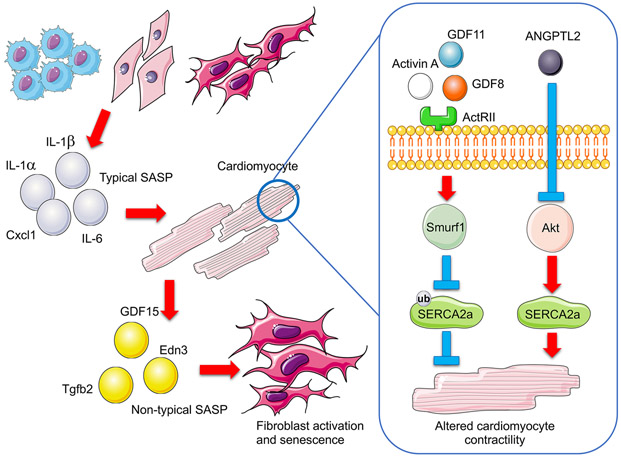
Figure 2. Schematic of Secreted Factors Contributing to Age-related Cardiac Disease.
Accumulating senescent cells adversely affect other cells (e.g. cardiomyocytes and fibroblasts) through a senescence-associated secretory phenotype (SASP) which promotes inflammation, cell death and senescence. While systemic increases in senescent cells lead to increasing secretion of typical SASP proteins with age (e.g., IL-1α, IL-1β, IL-6, Cxcl1), senescent cardiomyocytes secrete non-typical SASP (e.g., GDF15, Tgfb2, and Edn3). Circulating activin and ANGPTL2 are also increased with age. Elevated activin, through binding to its receptor ActRIIb, triggers Smurf1-mediated ubiquitination and subsequent degradation of SERCA2a, while increased ANGPTL2 inactives Akt and thereby degrades SERCA2a. All of these processes contribute to cardiac aging and cardiac dysfunction.
Numerous follow-up studies, however, have questioned some of these results. More extensive validation of the GDF11 aptamer used in the proteomics analyses revealed that it recognizes not only GDF11, but also the closely homologous protein GDF8 (also known as myostatin), which is likely responsible for the decrease reported in the initial studies.122 Of note, GDF8 (myostatin) knockout mice have better preservation of cardiac function and less cardiac fibrosis as they age, arguing against an age-related reduction in myostatin being detrimental.123 Interestingly, myostatin is a catabolic protein best known for inducing muscle atrophy and dysfunction, and subsequent work investigating the role of GDF11 overexpression in aged animals demonstrated that both GDF11 and myostatin have similar effects in the skeletal muscle and the heart.15, 124, 125 This has raised a number of important questions, including whether this secretory pathway changes with age, and if so, what its functional effects are in aged cardiac and skeletal muscle. Most recently, our group conducted an extensive investigation of this pathway in humans and animal models of aging and HF.15 We confirmed that circulating GDF8 levels indeed decline with age. However, we found that overall systemic activation of this secreted pathway is actually upregulated in aging and HF, and that its activation appears to be largely driven by an age-related increase in secreted Activins, another TGFβ family member that binds the same receptors as GDF8 and GDF11. We further showed that activation of the ActRIIb receptor downstream of activins triggers Smurf1-mediated ubiquitination and subsequent degradation of the SERCA2a, a Ca2+ ATPase critical for normal cardiomyocytes contractility (Figure 2). Importantly, targeted inhibition of ActRIIb receptor signaling improved the function and adverse remodeling of aged or failing hearts, confirming that these secreted factors likely play important roles in cardiac aging and HF.
Angiopoietin-like protein 2 (ANGPTL2) is a glycoprotein that has recently been shown to be secreted by cardiomyocytes and adipocytes and to play a role in age-related HF in animal models. Cardiac ANGPTL2 is increased in mice subjected to TAC, angiotensin II-induced HF, and in HF patients. ANGPTL2 is also increased in hearts from aged mice compared with young mice,126 and in senescent fibroblasts from patients with adult progeria Werner syndrome.127 Overexpression of ANGPTL2 in the mouse heart induced inactivation of Akt and sarcoplasmic reticulum Ca2+-ATPase signaling, leading to cardiac dysfunction, while, knockdown of ANGPTL2 protected the heart against HF.126 Interestingly, the same group further showed that, while circulating ANGPTL2 was increased both in dilated cardiomyopathy patients and in mice subjected to TAC-induced HF, experimentally increasing circulating ANGPTL2 did not induce cardiac dysfunction. Thus, cardiomyocyte-secreted ANGPTL2 may require other signaling mechanism to induce HF or could work through an autocrine/paracrine signaling mechanism not recapitulated with ectopic expression.128 Interestingly, ANGPTL2 was decreased in exercised hearts,126 indicating that exercise may protect against HF in part through lowering ANGPTL2 (Figure 3).
In non-purified cardiomyocytes isolated from old mice compared with those from young mice, significant differences in gene expression of traditional SASP components (e.g., IL-6 and Cxcl1) were observed. However, such differences in SASP were not observed in purified cardiomyocytes isolated from old and young mice, indicating that traditional SASP may be secreted from non-cardiomyocytes.70 Interestingly, in the purified cardiomyocytes isolated from old mice, three non-typical SASP signals were identified including Edn3, Tgfb2 and GDF15. Induction of Edn3, Tgfb2 and GDF15 induced neonatal fibroblast activation and senescence, while induction of Edn3 or Tgfb2 but not GDF15 increased neonatal cardiomyocyte size,70 suggesting that senescent cardiomyocytes can induce senescence in neighboring cells through secretion of non-typical SASP. While these studies provide compelling evidence for a role of secreted factors in aging, further investigations are needed to better define this group of secreted factors and determine how to best target them therapeutically.
Given the antioxidant and anti-inflammatory effects of exercise, it is likely that exercise counteracts the age-related upregulation of some or all of these secretory signaling pathways. Consistent with this, twelve weeks of exercise training resulted in a reduction of myostatin levels in skeletal muscle of chronic HF patients.129 In rats with chronic HF induced by coronary ligation, four weeks of treadmill training also reduced myostatin protein expression both in the skeletal muscle and the myocardium, and this was associated with improvement of cardiac function.130 These data are consistent with our observation that cardiac structure and function are better preserved in aged myostatin knockout mice.123 Other ligands in this family, including GDF11 and activin, were not investigated in these studies but would be of great interest given more recent findings cited above. Future investigation of the effects of exercise on age-related secreted secretory signaling pathways may suggest new therapeutic approaches.
Epigenetic Alterations
Epigenetics refers to somatically-acquired and/or inherited modifications in gene function that occur, not as a consequence of changes in DNA sequence, but rather as a result of changes in DNA methylation, histone modifications, or chromatin remodeling.131 Epigenetic changes are a hallmark of aging,106 and likely contribute to cardiac aging and cardiovascular disease more generally.132
DNA methylation, which mainly occurs through attachment of methyl groups to the carbon-5 position in CpG dinucleotide sequences, generally represses gene activity by preventing transcription factors from binding to gene promoters, favoring instead the recruitment of chromatin modifying enzymes.133 DNA methylation patterns are maintained by DNA methyltransferase (DNMT) 1, while de novo DNA methylation is typically mediated by DNMT3a and DNMT3b.133 Global differences in DNA methylation patterns were observed in patients with two cardiomyopathy-prone premature aging syndromes, Hutchinson-Gilford Progeria and Werner syndrome.134 DNA methylation increases with age and is negatively correlated with lifespan in mice and humans, and the gradual accumulation of this difference in DNA methylation with age constitutes an “epigenetic clock”, serving as a biomarker for chronological age, or even biological age.135
DNA methylation also appears to play a role in HF. In a community-based study involving 1568 residents over the age of 65, significant differences in methylation state at CpG sites were observed in peripheral blood from patients with left ventricular HFpEF compared to controls.136 Differential methylation patterns were also found in large genomic regions in cardiomyocytes from neonatal, adult heathy, and adult failing hearts.137 Similarly, genome-wide DNA methylation profiling revealed significant differences in promoter CpG islands, intragenic CpG islands, and gene bodies in left ventricle tissue from HF patients compared with healthy controls. Among these, the DUX4 locus was associated with differential DNA methylation, which was responsive to DNA methylation inhibition. Gene knockdown of DUX4 in a mouse HL1 cardiac cell line led to a decrease in cell viability, suggesting a possible causal role for DUX4 methylation in cardiac dysfunction.138 Further supporting a causal role for DNA hypermethylation in HF, hypermethylation was found in hearts from rats with norepinephrine-induced cardiac hypertrophy, associated with elevated ROS production in the heart. Inhibition of DNMT reduced norepinephrine-induced cardiac ROS level and reversed both norepinephrine-induced cardiac hypertrophy and HF.139 Most recently, Dorn et al showed that enhancement of METTL3-mediated N6-Methyladenosine methylation induced compensated cardiac hypertrophy, whereas its diminishment induced cardiac dysfunction,140 pointing to the importance of methylation in cardiac disease.
Histone proteins are responsible for maintaining chromatin structure and mediating dynamic and long-term gene regulation. Reduction of histone expression, maturation or deposition during replication leads to histone depletion, a feature of cellular aging.141 Telomere attrition-induced DNA damage triggers global histone depletion, chromatin disorganization and genomic instability, thereby inducing cellular senescence.142 This, along with the fact that telomere attrition is a hallmark of aging that may contribute to HF in aging hearts, suggests a possible link between histone levels and HF during aging.
In addition to histone levels, post-translational histone modifications, particularly acetylation, are also implicated in the regulation of chromatin structure and cardiac function in aging. Histones are acetylated by histone acetyltransferases (HAT) and deacetylated by histone deacetylases (HDACs). The roles of HDACs in regulating cardiac homeostasis and longevity have been examined in various HDAC deletion or overexpression mouse models, which have led to the conclusion that loss of specific HDAC isoforms promotes cardiac hypertrophy, dysfunction and vulnerability to cardiac injury, in some cases mimicking the effects of aging.143-145 Cardiac-specific knockout of Sirt1 (an NAD(+)-dependent histone deacetylase) in young mice (4-6 months) recapitulates the exacerbated response to ischemia reperfusion normally observed in older mice (24-26 months), including cardiac dysfunction, cardiac metabolic phenotypes and mitochondrial dysfunction (increase in mitochondria fission, reduction in mitochondrial density, and increase in mitochondrial DNA damage) in response to ischemia reperfusion.146 Mechanistically, cardiac specific Sirt1 knockout leads to mitochondrial biogenesis defects in which liver kinase B1 (LKB1) hyperacetylation compromises AMPK activation and acetyl-CoA carboxylase phosphorylation, resulting in cardiac dysfunction that can be reversed by Sirt1 overexpression.146 Age-related chromatin modifications have also been linked to changes in DNA damage repair, potentially contributing to senescence and loss of functional cardiomyocytes. In aged mice, loss of histone 4 lysine 20 trimethylation (H4K20me3) disrupts DNA damage repair and causes TGF-β/miR-29-induced cardiac dysfunction, whereas disruption of TGF-β signaling restores H4K20me3 and improves cardiac function. These findings are consistent with a role for histone modification in cardiac dysfunction during aging.147
Although loss of specific HDACs causes cardiac dysfunction in animal models, pharmacological HDAC inhibition appears to protect against age-related heart dysfunction. While cardiac diastolic function progressively declined in aged mice fed with normal chow, it was preserved out to 20 months of age in aged mice fed with ITF2357, an HDACs inhibitor currently in a phase 3 trial for treating patients with Duchenne muscular dystrophy, and this was associated with improved myofibril relaxiation.16 The apparently conflicting outcomes of targeted elimination of specific HDACs versus pharmacological HDAC inhibition likely reflects the broad isoform specificity of pharmacological intervention. Interestingly, studies of exercise have revealed dynamic regulation of specific HDAC isoforms in the heart. High volume swimming exercise in rats induced physiological cardiac growth that was associated with down-regulated HDAC3 and 4 but up-regulated HDAC1.148 Moreover, in aged mice (18-month-old) with myocardial infarction, eight weeks of swimming training improved cardiac function and mitochondrial biogenesis, which was associated with increased cardiac Sirt3.149 Gain- and loss-of-function studies have yielded some insight into the specific mechanisms of action of different HDACs in the heart.150 However, further investigation is needed to clarify their functional roles in exercise-mediated beneficial effects on the aging heart.
Bromodomain and extra-terminal (BET) proteins are histone acetylation “readers” that recognize acetyl-lysine labels on histones and non-histone proteins and serve to interpret and transmit the signals initiated by histone acetylation.151 The BET family consist of BRD2, BRD3, BRD4, and testis-specific BRDT, with BRD4 being the most abundant isoform in the heart.152 BET inhibition by the selective inhibitor JQ1 or siRNA reduced cardiac fibrosis and apoptosis and attenuated cardiac hypertrophy and pathological gene induction in phenylephrine-treated neonatal rat cardiomyocytes in vitro or in mice subjected to TAC or phenylephrine infusion in vivo. Further molecular analysis showed that BETs serve as co-activators for transcription factors implicated in HF, including NFAT (nuclear factor of activated T cells); NF-κB (nuclear factor-κB); and GATA4.152 This effect of BET inhibition was confirmed by the same group in human induced pluripotent stem cell-derived cardiomyocytes, where the BET inhibitor JQ1 blocked agonist-induced hypertrophy.153 They further showed that BET inhibition confers this protective effect by blocking NF-κB and TGF-β-mediated innate inflammatory and profibrotic gene networks. Given the contributions of inflammatory and profibrotic genes to the pathogenesis of age-related HF, it is possible that pharmacological BET inhibition could also protect against cardiac aging phenotypes and age-related HF.
Noncoding RNAs
Noncoding RNAs (ncRNAs) are functional RNA molecules that are transcribed from DNA but not translated into proteins. In general, ncRNAs can be classified into two categories based on their length as small ncRNAs (< 200 nucleotides) and long ncRNAs (lncRNAs). ncRNAs have been increasingly recognized as important regulators and biomarkers of cardiac aging and disease.
MicroRNAs (miRNAs) are endogenous small ncRNAs, approximately 22 nucleotides long that work as post-transcriptional regulators by binding to complementary sequences of messenger RNAs (mRNAs) to inhibit mRNA translation or to promote mRNA degradation.154 A number of miRNAs have been found to have pathophysiological roles in HF. In a screen for 380 miRNAs in cardiomyocytes, miRNA (miR)-22 was identified as an abundant and strong inhibitor of cardiac autophagy, whose expression level increased during aging in mice in vivo and in cardiomyocytes in vitro by a p53-dependent mechanism.155 Pharmacological inhibition of miR-22 in mice prevented post-infarction cardiac remodeling and improved cardiac function by activating autophagy in old mice. Moreover, circulating miR-22 was increased in patients with systolic HF associated with early mortality.155 miR-34a is predominantly expressed in cardiomyocytes, and is upregulated in aging mouse hearts and in biopsies from aging human hearts. Silencing of miR-34a reduced cardiomyocyte cell death in aged hearts and improved recovery of cardiac infarction. Phosphatase-1 nuclear targeting subunit (PNUTS) was identified as downstream target of miR-34a.156 More recently, Lyu et al demonstrated that miR-29a and −29c mediated TGF-β/Smad-induced senescence by suppressing Suv4-20h. This reduces H4K20me3, which compromises DNA damage repair and genome maintenance, contributing to cardiac aging and cardiac dysfunction.147 Moreover, miR-128 has been showed to increase progressively in cardiomyocytes during the postnatal switching from proliferation to terminal differentiation. Deletion of miR-128 promoted cell cycle re-entry in adult cardiomyocytes, reduced cardiac fibrosis and improved cardiac function after myocardial infarction.157 Identification of noncoding RNA pathways where inhibition has cardiac benefits could have important practical implications given that modified oligonucleotide antisense approaches appear highly effective in both animal models and clinical trials.158, 159
In contrast to these pathological miRNA-dependent pathways, other miRNAs promote cardiac health. The miRNAs miR-18 and miR-19 were down-regulated in HF-prone but up-regulated in HF-resistant aged hearts, where they regulated aging cardiomyopathy through targeting profibrotic pathways involving TGF-β and thrombospondin-1 signaling.160 Eulalio et al found that induction of miR-199 and miR-590 promoted cell cycle re-entry of adult cardiomyocytes ex vivo, and that overexpression of these miRNAs in adult mice undergoing myocardial ischemia reperfusion can induce cardiac regeneration and improve cardiac function.161 .
We and others have also identified miRNAs that act as important mediators of exercise-induced cardiac growth and cardiomyogenesis, further expanding the repertoire of potential therapeutic targets of cardiac aging and cardiac disease. Cardiac miR-222 is upregulated in mice after swimming or voluntary wheel running exercise training, and this was associated with physiological cardiac growth and birth of new cardiomyocytes.93, 94 This effect was abolished by inhibition of miR-222, indicating miR-222 is necessary for exercise-induced physiological cardiac growth (Figure 3). Molecular studies further indicated that miR-222 acts through down-regulation of HIPK1, p27, and HMBOX1.93, 94 Interestingly, the exercise-induced downregulation of the pathophysiological secreted signaling protein ANGPTL2 was also blocked by miR-222 inhibition.126 An area of growing interest concerns the role of exercise-induced exosomes in cardiac health. Exosomal miR-342-5p was increased in plasma from volunteers who had been on a rowing team for over 1 year and in rats with 4 weeks of swimming training. Increase miR-342-5p protected cardiomyocytes against hypoxia reoxygenation-induced cell apoptosis via Jnk2/Caspase 9 and Ppm1f/Akt signaling.162 All of these data point to miRNAs as important mediators of the beneficial effects of exercise on the heart. However, whether or not miRNAs play a role in the beneficial effects of exercise in the aging heart needs further investigation. Moreover, given that a single miRNA may have over 100 predicted targets, additional studies delineating miRNA biology, and identifying the relevant downstream targets in cardiac aging, are needed.
Unlike miRNAs, lncRNAs are poorly conserved across species, and most known lncRNAs are tissue- and developmental stage-specific. lncRNAs regulate cardiac development, cardiac aging, and disease through multiple mechanisms including epigenetic regulation, transcriptional regulation, nuclear compartmentalization, and post-transcriptional gene regulation, and by acting as competing RNA sponges. Zhang and colleagues found that lncRNA H19 is down-regulated in D-galactose-induced senescence in neonatal rat cardiomyocytes associated with loss of cardioprotection of ischemic postconditioning. Furthermore, H19 gene silencing exacerbated post-hypoxic cell injury in senescent cardiomyocytes by releasing miR-29b-3p, thereby down-regulating cIAP1.163 lncRNA metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) is decreased in aged hearts and in senescent heats induced by D-galactose. Infusion of umbilical cord mesenchymal stem cells (UMSC)-derived exosomes from young mice into D-galactose-induced senescent hearts improved cardiac function and increased telomere length by downregulating TNFα and p65, while these effects were reduced by silencing MALAT1 in UMSC-derived exosome. This suggests that the age-related decline in exosomal MALAT1 may contribute to cardiac dysfunction in aged hearts, and that combating this decline could have beneficial effects.164 By microarray screening, lncRNA ENSMUST00000134285 was found to be upregulated in aged mouse hearts (18 months) compared with young mice (8 weeks). Interestingly, lncRNA ENSMUST00000134285 knockdown increased while overexpression decreased post-hypoxic cell apoptosis in neonatal mice cardiomyocytes through miR-760/MAPK11 signaling.165 This suggests a protective role for the aging-dependent increase in lncRNA ENSMUST00000134285, which is somewhat counterintuitive given the evidence of increased cardiomyocyte loss in aging.
Circular RNAs (circRNAs) are a newly identified subtype of lncRNAs, which are generated from back-splicing of pre-mRNA. Specific circRNAs have recently been shown to have a role in cardiac regenerative repair. circNifx was upregulated in adult heart under the regulation of a super enhancer. Knockout of circNfix promoted cardiomyocyte proliferation and angiogenesis and improved post-infarction cardiac functional recovery, through suppression of Ybx1 ubiquitin-dependent degradation and enhanced miR-214 activity.166 In separate studies, cardiac circFndc3b was found to decrease after ischemia-reperfusion injury in animals and in ischemic cardiomyopathy patients. Induction of circFndc3b through FUS/VEGF-A axis promoted cardiac repair after ischemia reperfusion injury.167 Interestingly, circFndc3b was also downregulated in hearts from 22-month-old as compared to 1-month-old mice,168 raising the possibility that downregulation of circFndc3b contributes to cardiac aging and that overexpression of circFndc3b may provide cardiac protective effects in aging hearts. However, research determining the role of lncRNA in cardiac aging is still in its infancy. Further investigation is needed to unveil fully the role of lncRNAs in cardiac aging.
Somatic DNA Mutations
Somatic DNA mutations accumulate over time in many tissues, representing not only a hallmark of the aging process but also a potential cause of cardiac aging and diseases.169, 170 Due to the high rate of mutation and limited repair capacity of mitochondrial DNA, mtDNA mutation is a common feature in cellular aging and cardiac disease.170 Mitochondrial DNA mutations compromise the integrity of the mitochondrial genome, thereby impairing mitochondrial biogenesis and increasing mitochondrial ROS production, and are perhaps the best-studied somatic DNA mutations in cardiac aging and disease.104 The direct roles of mtDNA mutations in cardiac aging and disease have been supported by evidence from several mouse models. In “mutator” mice with homozygous mutation of mitochondrial polymerase gamma (Polgm/m), and in mice with disruption of the mitochondrial DNA helicase Twinkle (Twnk), mitochondrial function was compromised, leading to oxidative damage as well as accelerated cardiac aging and cardiac dysfunction.171-173 As early as 8 weeks old, the accumulation rate of mtDNA mutations is 3 to 5 times higher in mutator mice compared to wildtype controls in multiple tissues including the heart. By 6 months, mutator mice develop premature aging phenotypes. Within the first year after birth, mutator mice exhibit cardiac hypertrophy and dilation cardiomyopathy as well as cardiac fibrosis, with an average lifespan of 12 months.171, 173 Although ROS markers were normal in Polgm/m mice, the accelerated cardiac aging and HF phenotypes in these animals were partially rescued by crossing with mCAT antioxidant overexpressing mice, suggesting a role for oxidative damage.174-176 Similarly, in mice overexpressing dominant-negative Twnk helicase in the myocardium, mtDNA deletions accumulated at an accelerated rate in cardiomyocytes, and this was paralleled by mitochondrial deficiency and the development of arrhythmias, a common correlate of aging.172 In contrast, mice with targeted mutation of the p66Shc gene involved in mitochondrial ROS production display prolonged lifespan, reduced ROS production and resistance to ROS-mediated apoptosis.177 Moreover, disruption of p66Shc protected against angiotensin-II induced cardiac hypertrophy and cardiomyocyte apoptosis as well as reducing oxidative damage in multiple cardiovascular lineages in diabetic mice.178-180 All of these observations highlight the importance of mtDNA mutations in cardiac aging and diseases. Notably, in mutator mice, 6 months of treadmill exercise training reduced mtDNA mutation, attenuated mitochondrial dysfunction and extended lifespan,181 indicating a protective role of exercise in mtDNA repair during aging.
In another example of the detrimental effects of somatic mutations, using whole-genome sequencing of blood-derived DNA in humans, Jaiswal and colleagues demonstrated that clonal expansion of hematopoietic cells with somatic mutations in leukemogenic genes increased with age and correlated with increased mortality. They termed this phenomenon clonal hematopoiesis of indeterminate potential (CHIP).182 They further showed that CHIP in peripheral-blood cells was associated with a nearly doubled risk of coronary heart disease or early-onset myocardial infarction.183 This was supported by mouse models with CRISPR-mediated inactivation of ten-eleven translocation enzymes 2 (Tet2) or DNA Methyltransferase 3a (Dnmt3a), the two commonly mutated genes in CHIP. Inactivation of Tet2 or Dnmt3a in hematopoietic stem/progenitor cells also worsened cardiac function in mice infused with angiotensin-II, and this was mediated by elevated inflammation.184 Similar effects of Tet2 inactivation in hematopoietic cells were observed in two other heart failure mice models. Sano et al showed that Tet2 inactivation in hematopoietic cells worsened late-stage cardiac remodeling through effects on IL-1β/NLRP3 inflammasome activity, and reduced cardiac function both in mice with HF induced by permanent left anterior descending artery ligation or TAC.185 Given that CHIP has been found to be relatively common in asymptomatic, cancer-free older individuals,183, 186 the association between CHIP and cardiac disease in the elderly provides a potential causal link between somatic mutation and aging-related cardiovascular disease. CHIP-associated mutations may provide useful markers for assessing cardiovascular risk in aging populations independent of traditional cardiometabolic risk factors. Moreover the link to inflammatory signaling raises the possibility that therapies directed at specific mediators of inflammation, such as antibodies to IL-1β,187 might be particularly beneficial in subjects with a high burden of CHIP.
While all organs are subject to accumulation of somatic mutations over time, the cells of the cardiovascular system may be particularly prone to DNA damage due to the hemodynamic forces and mechanical strain these cells experience. In a process known as mechanosignaling, physical stresses can modulate intracellular signaling pathways, including pathways that protect the cell from DNA damage, and the activity of these mechanosensitive protective pathways may change over time. Lamin A is a mechanosensitive nuclear structural protein that promotes DNA damage control in cardiomyocytes by preventing strain-induced breaches in the nuclear membrane.188 Genetic perturbations of lamin A in human patients result in dilated cardiomyopathy, likely reflecting excessive activation of the platelet-derived growth factor (PDGF) pathway, and are also associated with a premature aging phenotype similar to that resulting from disruption of DNA repair enzymes.189, 190 Lamin A levels are greatest in cell types that experience a high degree of physical strain, and physical strain is required to maintain lamin A levels in cardiomyocytes by blocking its MMP-dependent degradation.190 Notably, accumulation of the lamin A-precursor, prelamin A, increases with age in arterial vascular smooth muscle cells, and results in DNA damage and senescence in these cells, suggesting that this protective mechanosignaling mechanism may become pathologically dysregulated in the aging cardiovascular system.191 In addition to its protective structural role, lamin A levels also regulate histone methylation in lamin A-associated domains (LADs) near the nuclear membrane in iPSC-derived cardiomyocytes, raising the interesting possibility that declining lamin A may also contribute to epigenetic changes observed with age.190
CONCLUSIONS AND PERSPECTIVES
Given the aging of populations around the world and the associated increase in HF prevalence, there is a compelling need to develop therapeutic interventions that mitigate age-related HF. New candidates for intervention have emerged as our knowledge of the biology of aging and HF has progressed in recent years. Moreover, exercise studies have provided complementary insights in the search for new targets.21, 52, 91, 94, 96-100, 102, 103, 192-194 We can anticipate that as a deeper understanding of the fundamental biology of aging develops, there will be additional opportunities for translation in the future. Since biological aging and HF are complex and multifactorial systemic processes, it seems unlikely that any single pathway or intervention will fully mitigate age-related cardiac phenotypes. However, we are optimistic that insights gained through studies of aging biology and its interplay with cardiovascular pathophysiology will lay a foundation for new therapeutic strategies that can at least mitigate some features of age-related heart disease and may even be generalizable to other forms of heart failure.
Supplementary Material
Acknowledgments
SOURCES OF FUNDING
This work was supported by the NIH (AG061034, HL122987, HL135886, K08HL140200, K76AG064328), AHA (16SFRN31720000, 16FTF29630016, 14FTF20440012), the Fred and Ines Yeatts Fund for Innovative Research, the Foundation for Anesthesia Education and Research (FAER), and the Sarnoff Cardiovascular Research Foundation Fellowship.
NON-STANDARD ABBREVIATIONS AND ACRONYMS
- HF
Heart failure
- ROS
reactive oxygen species
- mtDNA
mitochondrial DNA
- PGC-1α
proliferator-activated receptor γ coactivator 1α
- TAC
transverse aortic constriction
- SCOT
succinyl-CoA:3-oxoacid CoA transferase
- SASP
senescence-associated secretory phenotype
- GDFs
growth and differentiation proteins
- MIMS
multiphoton ionization mass spectrometry
- DMD
Duchenne Muscular Dystrophy
- TERT
telomerase reverse transcriptase
- TRF2
telomeric repeat-binding factor 2
- TAF
telomere-associated foci
- ANGPTL2
Angiopoietin-like protein 2
- DNMT
DNA methyltransferase
- HAT
histone acetyltransferases
- HDACs
histone deacetylases
- H4K20me3
histone 4 lysine 20 trimethylation
- BET
bromodomain and extra-terminal
- ncRNAs
noncoding RNAs
- lncRNA
long noncoding RNA
- MALAT1
metastasis-associated lung adenocarcinoma transcript 1
- UMSC
umbilical cord mesenchymal stem cells
- circRNAs
circular RNAs
- Polg
polymerase gamma
- Twnk
Twinkle
- CHIP
clonal hematopoiesis of indeterminate potential
Footnotes
DISCLOSURES
J.D.R. and A.R. are inventors on a pending patent (PCT/US2018/023390; Methods for Preventing and Treating Heart Disease; WIPO) submitted by BIDMC and Novartis that covers methods for preventing and treating heart disease with activin receptor inhibition.
REFERENCES
- 1.Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart. 2007;93:1137–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Jordan LC, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, O’Flaherty M, Pandey A, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Spartano NL, Stokes A, Tirschwell DL, Tsao CW, Turakhia MP, VanWagner LB, Wilkins JT, Wong SS, Virani SS, American Heart Association Council on E, Prevention Statistics C, Stroke Statistics S. Heart disease and stroke statistics-2019 update: A report from the american heart association. Circulation. 2019;139:e56–e528 [DOI] [PubMed] [Google Scholar]
- 3.Fleg JL, Cooper LS, Borlaug BA, Haykowsky MJ, Kraus WE, Levine BD, Pfeffer MA, Pina IL, Poole DC, Reeves GR, Whellan DJ, Kitzman DW, National Heart L, Blood Institute Working G. Exercise training as therapy for heart failure: Current status and future directions. Circ Heart Fail. 2015;8:209–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lakatta EG. So! What’s aging? Is cardiovascular aging a disease? J Mol Cell Cardiol. 2015;83:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lakatta EG, Levy D. Arterial and cardiac aging: Major shareholders in cardiovascular disease enterprises: Part i: Aging arteries: A “set up” for vascular disease. Circulation. 2003;107:139–146 [DOI] [PubMed] [Google Scholar]
- 6.Vasan RS, Xanthakis V, Lyass A, Andersson C, Tsao C, Cheng S, Aragam J, Benjamin EJ, Larson MG. Epidemiology of left ventricular systolic dysfunction and heart failure in the framingham study: An echocardiographic study over 3 decades. JACC Cardiovasc Imaging. 2018;11:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pfeffer MA, Shah AM, Borlaug BA. Heart failure with preserved ejection fraction in perspective. Circ Res. 2019;124:1598–1617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pandey A, Kraus WE, Brubaker PH, Kitzman DW. Healthy aging and cardiovascular function: Invasive hemodynamics during rest and exercise in 104 healthy volunteers. JACC Heart Fail. 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fleg JL, Morrell CH, Bos AG, Brant LJ, Talbot LA, Wright JG, Lakatta EG. Accelerated longitudinal decline of aerobic capacity in healthy older adults. Circulation. 2005;112:674–682 [DOI] [PubMed] [Google Scholar]
- 10.Fleg JL, O’Connor F, Gerstenblith G, Becker LC, Clulow J, Schulman SP, Lakatta EG. Impact of age on the cardiovascular response to dynamic upright exercise in healthy men and women. J Appl Physiol (1985). 1995;78:890–900 [DOI] [PubMed] [Google Scholar]
- 11.Borlaug BA, Olson TP, Lam CS, Flood KS, Lerman A, Johnson BD, Redfield MM. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2010;56:845–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Upadhya B, Rocco M, Lewis CE, Oparil S, Lovato LC, Cushman WC, Bates JT, Bello NA, Aurigemma G, Fine LJ, Johnson KC, Rodriguez CJ, Raj DS, Rastogi A, Tamariz L, Wiggers A, Kitzman DW, Group SR. Effect of intensive blood pressure treatment on heart failure events in the systolic blood pressure reduction intervention trial. Circ Heart Fail. 2017;10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aviv A Chronology versus biology: Telomeres, essential hypertension, and vascular aging. Hypertension. 2002;40:229–232 [DOI] [PubMed] [Google Scholar]
- 14.Campisi J, Kapahi P, Lithgow GJ, Melov S, Newman JC, Verdin E. From discoveries in ageing research to therapeutics for healthy ageing. Nature. 2019;571:183–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roh JD, Hobson R, Chaudhari V, Quintero P, Yeri A, Benson M, Xiao C, Zlotoff D, Bezzerides V, Houstis N, Platt C, Damilano F, Lindman BR, Elmariah S, Biersmith M, Lee SJ, Seidman CE, Seidman JG, Gerszten RE, Lach-Trifilieff E, Glass DJ, Rosenzweig A. Activin type ii receptor signaling in cardiac aging and heart failure. Sci Transl Med. 2019;11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeong MY, Lin YH, Wennersten SA, Demos-Davies KM, Cavasin MA, Mahaffey JH, Monzani V, Saripalli C, Mascagni P, Reece TB, Ambardekar AV, Granzier HL, Dinarello CA, McKinsey TA. Histone deacetylase activity governs diastolic dysfunction through a nongenomic mechanism. Sci Transl Med. 2018;10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Safdar A, Annis S, Kraytsberg Y, Laverack C, Saleem A, Popadin K, Woods DC, Tilly JL, Khrapko K. Amelioration of premature aging in mtdna mutator mouse by exercise: The interplay of oxidative stress, pgc-1alpha, p53, and DNA damage. A hypothesis. Curr Opin Genet Dev. 2016;38:127–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hammill BG, Curtis LH, Schulman KA, Whellan DJ. Relationship between cardiac rehabilitation and long-term risks of death and myocardial infarction among elderly medicare beneficiaries. Circulation. 2010;121:63–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kitzman DW, Brubaker P, Morgan T, Haykowsky M, Hundley G, Kraus WE, Eggebeen J, Nicklas BJ. Effect of caloric restriction or aerobic exercise training on peak oxygen consumption and quality of life in obese older patients with heart failure with preserved ejection fraction: A randomized clinical trial. JAMA. 2016;315:36–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, Leifer ES, Kraus WE, Kitzman DW, Blumenthal JA, Rendall DS, Miller NH, Fleg JL, Schulman KA, McKelvie RS, Zannad F, Pina IL, Investigators H-A. Efficacy and safety of exercise training in patients with chronic heart failure: Hf-action randomized controlled trial. JAMA. 2009;301:1439–1450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roh J, Rhee J, Chaudhari V, Rosenzweig A. The role of exercise in cardiac aging: From physiology to molecular mechanisms. Circ Res. 2016;118:279–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Werner C, Hanhoun M, Widmann T, Kazakov A, Semenov A, Poss J, Bauersachs J, Thum T, Pfreundschuh M, Muller P, Haendeler J, Bohm M, Laufs U. Effects of physical exercise on myocardial telomere-regulating proteins, survival pathways, and apoptosis. J Am Coll Cardiol. 2008;52:470–482 [DOI] [PubMed] [Google Scholar]
- 23.Werner C, Furster T, Widmann T, Poss J, Roggia C, Hanhoun M, Scharhag J, Buchner N, Meyer T, Kindermann W, Haendeler J, Bohm M, Laufs U. Physical exercise prevents cellular senescence in circulating leukocytes and in the vessel wall. Circulation. 2009;120:2438–2447 [DOI] [PubMed] [Google Scholar]
- 24.Kraigher-Krainer E, Lyass A, Massaro JM, Lee DS, Ho JE, Levy D, Kannel WB, Vasan RS. Association of physical activity and heart failure with preserved vs. Reduced ejection fraction in the elderly: The framingham heart study. Eur J Heart Fail. 2013;15:742–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pandey A, Patel M, Gao A, Willis BL, Das SR, Leonard D, Drazner MH, de Lemos JA, DeFina L, Berry JD. Changes in mid-life fitness predicts heart failure risk at a later age independent of interval development of cardiac and noncardiac risk factors: The cooper center longitudinal study. Am Heart J. 2015;169:290–297 e291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fujimoto N, Prasad A, Hastings JL, Arbab-Zadeh A, Bhella PS, Shibata S, Palmer D, Levine BD. Cardiovascular effects of 1 year of progressive and vigorous exercise training in previously sedentary individuals older than 65 years of age. Circulation. 2010;122:1797–1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spina RJ, Ogawa T, Kohrt WM, Martin WH 3rd, Holloszy JO, Ehsani AA. Differences in cardiovascular adaptations to endurance exercise training between older men and women. J Appl Physiol (1985). 1993;75:849–855 [DOI] [PubMed] [Google Scholar]
- 28.Kohrt WM, Malley MT, Coggan AR, Spina RJ, Ogawa T, Ehsani AA, Bourey RE, Martin WH 3rd, Holloszy JO. Effects of gender, age, and fitness level on response of vo2max to training in 60-71 yr olds. J Appl Physiol (1985). 1991;71:2004–2011 [DOI] [PubMed] [Google Scholar]
- 29.Stratton JR, Levy WC, Cerqueira MD, Schwartz RS, Abrass IB. Cardiovascular responses to exercise. Effects of aging and exercise training in healthy men. Circulation. 1994;89:1648–1655 [DOI] [PubMed] [Google Scholar]
- 30.Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, Sadek HA. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331:1078–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Senyo SE, Steinhauser ML, Pizzimenti CL, Yang VK, Cai L, Wang M, Wu TD, Guerquin-Kern JL, Lechene CP, Lee RT. Mammalian heart renewal by pre-existing cardiomyocytes. Nature. 2013;493:433–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bergmann O, Zdunek S, Felker A, Salehpour M, Alkass K, Bernard S, Sjostrom SL, Szewczykowska M, Jackowska T, Dos Remedios C, Malm T, Andra M, Jashari R, Nyengaard JR, Possnert G, Jovinge S, Druid H, Frisen J. Dynamics of cell generation and turnover in the human heart. Cell. 2015;161:1566–1575 [DOI] [PubMed] [Google Scholar]
- 33.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisen J. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wencker D, Chandra M, Nguyen K, Miao W, Garantziotis S, Factor SM, Shirani J, Armstrong RC, Kitsis RN. A mechanistic role for cardiac myocyte apoptosis in heart failure. Journal Of Clinical Investigation. 2003;111:1497–1504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.James TN. Normal and abnormal consequences of apoptosis in the human heart. From postnatal morphogenesis to paroxysmal arrhythmias. Circulation. 1994;90:556–573 [PubMed] [Google Scholar]
- 36.Mallat Z, Fornes P, Costagliola R, Esposito B, Belmin J, Lecomte D, Tedgui A. Age and gender effects on cardiomyocyte apoptosis in the normal human heart. J Gerontol A Biol Sci Med Sci. 2001;56:M719–723 [DOI] [PubMed] [Google Scholar]
- 37.Eschenhagen T, Bolli R, Braun T, Field LJ, Fleischmann BK, Frisen J, Giacca M, Hare JM, Houser S, Lee RT, Marban E, Martin JF, Molkentin JD, Murry CE, Riley PR, Ruiz-Lozano P, Sadek HA, Sussman MA, Hill JA. Cardiomyocyte regeneration: A consensus statement. Circulation. 2017;136:680–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vicinanza C, Aquila I, Scalise M, Cristiano F, Marino F, Cianflone E, Mancuso T, Marotta P, Sacco W, Lewis FC, Couch L, Shone V, Gritti G, Torella A, Smith AJ, Terracciano CM, Britti D, Veltri P, Indolfi C, Nadal-Ginard B, Ellison-Hughes GM, Torella D. Adult cardiac stem cells are multipotent and robustly myogenic: C-kit expression is necessary but not sufficient for their identification. Cell Death Differ. 2017;24:2101–2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, Pasumarthi KB, Virag JI, Bartelmez SH, Poppa V, Bradford G, Dowell JD, Williams DA, Field LJ. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664–668 [DOI] [PubMed] [Google Scholar]
- 40.Liu Q, Yang R, Huang X, Zhang H, He L, Zhang L, Tian X, Nie Y, Hu S, Yan Y, Zhang L, Qiao Z, Wang QD, Lui KO, Zhou B. Genetic lineage tracing identifies in situ kit-expressing cardiomyocytes. Cell Res. 2016;26:119–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sultana N, Zhang L, Yan J, Chen J, Cai W, Razzaque S, Jeong D, Sheng W, Bu L, Xu M, Huang GY, Hajjar RJ, Zhou B, Moon A, Cai CL. Resident c-kit(+) cells in the heart are not cardiac stem cells. Nature communications. 2015;6:8701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Berlo JH, Kanisicak O, Maillet M, Vagnozzi RJ, Karch J, Lin SC, Middleton RC, Marban E, Molkentin JD. C-kit+ cells minimally contribute cardiomyocytes to the heart. Nature. 2014;509:337–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.He L, Li Y, Li Y, Pu W, Huang X, Tian X, Wang Y, Zhang H, Liu Q, Zhang L, Zhao H, Tang J, Ji H, Cai D, Han Z, Han Z, Nie Y, Hu S, Wang QD, Sun R, Fei J, Wang F, Chen T, Yan Y, Huang H, Pu WT, Zhou B. Enhancing the precision of genetic lineage tracing using dual recombinases. Nat Med. 2017;23:1488–1498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maliken BD, Kanisicak O, Karch J, Khalil H, Fu X, Boyer JG, Prasad V, Zheng Y, Molkentin JD. Gata4-dependent differentiation of c-kit(+)-derived endothelial cells underlies artefactual cardiomyocyte regeneration in the heart. Circulation. 2018;138:1012–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ali SR, Hippenmeyer S, Saadat LV, Luo L, Weissman IL, Ardehali R. Existing cardiomyocytes generate cardiomyocytes at a low rate after birth in mice. Proc Natl Acad Sci U S A. 2014;111:8850–8855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hsieh PC, Segers VF, Davis ME, MacGillivray C, Gannon J, Molkentin JD, Robbins J, Lee RT. Evidence from a genetic fate-mapping study that stem cells refresh adult mammalian cardiomyocytes after injury. Nat Med. 2007;13:970–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang L, Luo JY, Li B, Tian XY, Chen LJ, Huang Y, Liu J, Deng D, Lau CW, Wan S, Ai D, Mak KK, Tong KK, Kwan KM, Wang N, Chiu JJ, Zhu Y, Huang Y. Integrin-yap/taz-jnk cascade mediates atheroprotective effect of unidirectional shear flow. Nature. 2016;540:579–582 [DOI] [PubMed] [Google Scholar]
- 48.Picca A, Mankowski RT, Burman JL, Donisi L, Kim JS, Marzetti E, Leeuwenburgh C. Mitochondrial quality control mechanisms as molecular targets in cardiac ageing. Nat Rev Cardiol. 2018;15:543–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, Coskun PE, Ladiges W, Wolf N, Van Remmen H, Wallace DC, Rabinovitch PS. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science. 2005;308:1909–1911 [DOI] [PubMed] [Google Scholar]
- 50.Dai DF, Santana LF, Vermulst M, Tomazela DM, Emond MJ, MacCoss MJ, Gollahon K, Martin GM, Loeb LA, Ladiges WC, Rabinovitch PS. Overexpression of catalase targeted to mitochondria attenuates murine cardiac aging. Circulation. 2009;119:2789–2797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arany Z, He H, Lin J, Hoyer K, Handschin C, Toka O, Ahmad F, Matsui T, Chin S, Wu PH, Rybkin II, Shelton JM, Manieri M, Cinti S, Schoen FJ, Bassel-Duby R, Rosenzweig A, Ingwall JS, Spiegelman BM. Transcriptional coactivator pgc-1 alpha controls the energy state and contractile function of cardiac muscle. Cell Metab. 2005;1:259–271 [DOI] [PubMed] [Google Scholar]
- 52.Arany Z, Novikov M, Chin S, Ma Y, Rosenzweig A, Spiegelman BM. Transverse aortic constriction leads to accelerated heart failure in mice lacking ppar-gamma coactivator 1alpha. Proceedings Of The National Academy Of Sciences Of The United States Of America. 2006;103:10086–10091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ristow M, Zarse K, Oberbach A, Kloting N, Birringer M, Kiehntopf M, Stumvoll M, Kahn CR, Bluher M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci U S A. 2009;106:8665–8670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yun J, Finkel T. Mitohormesis. Cell Metab. 2014;19:757–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ristow M, Schmeisser S. Extending life span by increasing oxidative stress. Free radical biology & medicine. 2011;51:327–336 [DOI] [PubMed] [Google Scholar]
- 56.Zhu X, Shen W, Yao K, Wang H, Liu B, Li T, Song L, Diao D, Mao G, Huang P, Li C, Zhang H, Zou Y, Qiu Y, Zhao Y, Wang W, Yang Y, Hu Z, Auwerx J, Loscalzo J, Zhou Y, Ju Z. Fine-tuning of pgc1alpha expression regulates cardiac function and longevity. Circ Res. 2019;125:707–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bedi KC Jr., Snyder NW, Brandimarto J, Aziz M, Mesaros C, Worth AJ, Wang LL, Javaheri A, Blair IA, Margulies KB, Rame JE. Evidence for intramyocardial disruption of lipid metabolism and increased myocardial ketone utilization in advanced human heart failure. Circulation. 2016;133:706–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aubert G, Martin OJ, Horton JL, Lai L, Vega RB, Leone TC, Koves T, Gardell SJ, Kruger M, Hoppel CL, Lewandowski ED, Crawford PA, Muoio DM, Kelly DP. The failing heart relies on ketone bodies as a fuel. Circulation. 2016;133:698–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kolwicz SC Jr., Airhart S, Tian R. Ketones step to the plate: A game changer for metabolic remodeling in heart failure? Circulation. 2016;133:689–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Taegtmeyer H Failing heart and starving brain: Ketone bodies to the rescue. Circulation. 2016;134:265–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schugar RC, Moll AR, Andre d’Avignon D, Weinheimer CJ, Kovacs A, Crawford PA. Cardiomyocyte-specific deficiency of ketone body metabolism promotes accelerated pathological remodeling. Mol Metab. 2014;3:754–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee CF, Chavez JD, Garcia-Menendez L, Choi Y, Roe ND, Chiao YA, Edgar JS, Goo YA, Goodlett DR, Bruce JE, Tian R. Normalization of nad+ redox balance as a therapy for heart failure. Circulation. 2016;134:883–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hafner AV, Dai J, Gomes AP, Xiao CY, Palmeira CM, Rosenzweig A, Sinclair DA. Regulation of the mptp by sirt3-mediated deacetylation of cypd at lysine 166 suppresses age-related cardiac hypertrophy. Aging (Albany NY). 2010;2:914–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang H, Yang T, Baur JA, Perez E, Matsui T, Carmona JJ, Lamming DW, Souza-Pinto NC, Bohr VA, Rosenzweig A, de Cabo R, Sauve AA, Sinclair DA. Nutrient-sensitive mitochondrial nad+ levels dictate cell survival. Cell. 2007;130:1095–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Airhart SE, Shireman LM, Risler LJ, Anderson GD, Nagana Gowda GA, Raftery D, Tian R, Shen DD, O’Brien KD. An open-label, non-randomized study of the pharmacokinetics of the nutritional supplement nicotinamide riboside (nr) and its effects on blood nad+ levels in healthy volunteers. PLoS One. 2017;12:e0186459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hayflick L The limited in vitro lifetime of human diploid cell strains. Exp Cell Res. 1965;37:614–636 [DOI] [PubMed] [Google Scholar]
- 67.Yun MH. Cellular senescence in tissue repair: Every cloud has a silver lining. Int J Dev Biol. 2018;62:591–604 [DOI] [PubMed] [Google Scholar]
- 68.Wang M, Shah AM. Age-associated pro-inflammatory remodeling and functional phenotype in the heart and large arteries. J Mol Cell Cardiol. 2015;83:101–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhu Y, Tchkonia T, Pirtskhalava T, Gower AC, Ding H, Giorgadze N, Palmer AK, Ikeno Y, Hubbard GB, Lenburg M, O’Hara SP, LaRusso NF, Miller JD, Roos CM, Verzosa GC, LeBrasseur NK, Wren JD, Farr JN, Khosla S, Stout MB, McGowan SJ, Fuhrmann-Stroissnigg H, Gurkar AU, Zhao J, Colangelo D, Dorronsoro A, Ling YY, Barghouthy AS, Navarro DC, Sano T, Robbins PD, Niedernhofer LJ, Kirkland JL. The achilles’ heel of senescent cells: From transcriptome to senolytic drugs. Aging Cell. 2015;14:644–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Anderson R, Lagnado A, Maggiorani D, Walaszczyk A, Dookun E, Chapman J, Birch J, Salmonowicz H, Ogrodnik M, Jurk D, Proctor C, Correia-Melo C, Victorelli S, Fielder E, Berlinguer-Palmini R, Owens A, Greaves LC, Kolsky KL, Parini A, Douin-Echinard V, LeBrasseur NK, Arthur HM, Tual-Chalot S, Schafer MJ, Roos CM, Miller JD, Robertson N, Mann J, Adams PD, Tchkonia T, Kirkland JL, Mialet-Perez J, Richardson GD, Passos JF. Length-independent telomere damage drives post-mitotic cardiomyocyte senescence. EMBO J. 2019;38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shimizu I, Minamino T. Cellular senescence in cardiac diseases. J Cardiol. 2019;74:313–319 [DOI] [PubMed] [Google Scholar]
- 72.Leri A, Franco S, Zacheo A, Barlucchi L, Chimenti S, Limana F, Nadal-Ginard B, Kajstura J, Anversa P, Blasco MA. Ablation of telomerase and telomere loss leads to cardiac dilatation and heart failure associated with p53 upregulation. EMBO J. 2003;22:131–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gevaert AB, Shakeri H, Leloup AJ, Van Hove CE, De Meyer GRY, Vrints CJ, Lemmens K, Van Craenenbroeck EM. Endothelial senescence contributes to heart failure with preserved ejection fraction in an aging mouse model. Circ Heart Fail. 2017;10 [DOI] [PubMed] [Google Scholar]
- 74.Baker DJ, Childs BG, Durik M, Wijers ME, Sieben CJ, Zhong J, Saltness RA, Jeganathan KB, Verzosa GC, Pezeshki A, Khazaie K, Miller JD, van Deursen JM. Naturally occurring p16(ink4a)-positive cells shorten healthy lifespan. Nature. 2016;530:184–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Meyer K, Hodwin B, Ramanujam D, Engelhardt S, Sarikas A. Essential role for premature senescence of myofibroblasts in myocardial fibrosis. J Am Coll Cardiol. 2016;67:2018–2028 [DOI] [PubMed] [Google Scholar]
- 76.Lewis-McDougall FC, Ruchaya PJ, Domenjo-Vila E, Shin Teoh T, Prata L, Cottle BJ, Clark JE, Punjabi PP, Awad W, Torella D, Tchkonia T, Kirkland JL, Ellison-Hughes GM. Aged-senescent cells contribute to impaired heart regeneration. Aging Cell. 2019;18:e12931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Campisi J Cellular senescence: Putting the paradoxes in perspective. Curr Opin Genet Dev. 2011;21:107–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Walaszczyk A, Dookun E, Redgrave R, Tual-Chalot S, Victorelli S, Spyridopoulos I, Owens A, Arthur HM, Passos JF, Richardson GD. Pharmacological clearance of senescent cells improves survival and recovery in aged mice following acute myocardial infarction. Aging Cell. 2019;18:e12945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Parikh P, Wicher S, Khandalavala K, Pabelick CM, Britt RD Jr., Prakash YS. Cellular senescence in the lung across the age spectrum. Am J Physiol Lung Cell Mol Physiol. 2019;316:L826–L842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Triana-Martinez F, Picallos-Rabina P, Da Silva-Alvarez S, Pietrocola F, Llanos S, Rodilla V, Soprano E, Pedrosa P, Ferreiros A, Barradas M, Hernandez-Gonzalez F, Lalinde M, Prats N, Bernado C, Gonzalez P, Gomez M, Ikonomopoulou MP, Fernandez-Marcos PJ, Garcia-Caballero T, Del Pino P, Arribas J, Vidal A, Gonzalez-Barcia M, Serrano M, Loza MI, Dominguez E, Collado M. Identification and characterization of cardiac glycosides as senolytic compounds. Nat Commun. 2019;10:4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Prata L, Ovsyannikova IG, Tchkonia T, Kirkland JL. Senescent cell clearance by the immune system: Emerging therapeutic opportunities. Semin Immunol. 2019:101275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Feng T, Meng J, Kou S, Jiang Z, Huang X, Lu Z, Zhao H, Lau LF, Zhou B, Zhang H. Ccn1-induced cellular senescence promotes heart regeneration. Circulation. 2019;139:2495–2498 [DOI] [PubMed] [Google Scholar]
- 83.Woo JS, Derleth C, Stratton JR, Levy WC. The influence of age, gender, and training on exercise efficiency. J Am Coll Cardiol. 2006;47:1049–1057 [DOI] [PubMed] [Google Scholar]
- 84.Linke A, Adams V, Schulze PC, Erbs S, Gielen S, Fiehn E, Mobius-Winkler S, Schubert A, Schuler G, Hambrecht R. Antioxidative effects of exercise training in patients with chronic heart failure: Increase in radical scavenger enzyme activity in skeletal muscle. Circulation. 2005;111:1763–1770 [DOI] [PubMed] [Google Scholar]
- 85.Liao PH, Hsieh DJ, Kuo CH, Day CH, Shen CY, Lai CH, Chen RJ, Padma VV, Kuo WW, Huang CY. Moderate exercise training attenuates aging-induced cardiac inflammation, hypertrophy and fibrosis injuries of rat hearts. Oncotarget. 2015;6:35383–35394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Eisele JC, Schaefer IM, Randel Nyengaard J, Post H, Liebetanz D, Bruel A, Muhlfeld C. Effect of voluntary exercise on number and volume of cardiomyocytes and their mitochondria in the mouse left ventricle. Basic Res Cardiol. 2008;103:12–21 [DOI] [PubMed] [Google Scholar]
- 87.Safdar A, Little JP, Stokl AJ, Hettinga BP, Akhtar M, Tarnopolsky MA. Exercise increases mitochondrial pgc-1alpha content and promotes nuclear-mitochondrial cross-talk to coordinate mitochondrial biogenesis. J Biol Chem. 2011;286:10605–10617 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 88.Kang KW, Lee SJ, Kim SG. Molecular mechanism of nrf2 activation by oxidative stress. Antioxid Redox Signal. 2005;7:1664–1673 [DOI] [PubMed] [Google Scholar]
- 89.Kwak HB, Song W, Lawler JM. Exercise training attenuates age-induced elevation in bax/bcl-2 ratio, apoptosis, and remodeling in the rat heart. FASEB J. 2006;20:791–793 [DOI] [PubMed] [Google Scholar]
- 90.Quindry J, French J, Hamilton K, Lee Y, Mehta JL, Powers S. Exercise training provides cardioprotection against ischemia-reperfusion induced apoptosis in young and old animals. Exp Gerontol. 2005;40:416–425 [DOI] [PubMed] [Google Scholar]
- 91.Bostrom P, Mann N, Wu J, Quintero PA, Plovie ER, Panakova D, Gupta RK, Xiao C, MacRae CA, Rosenzweig A, Spiegelman BM. C/ebpbeta controls exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell. 2010;143:1072–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Steinhauser ML, Bailey AP, Senyo SE, Guillermier C, Perlstein TS, Gould AP, Lee RT, Lechene CP. Multi-isotope imaging mass spectrometry quantifies stem cell division and metabolism. Nature. 2012;481:516–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vujic A, Lerchenmüller C, Wu T-D, Guillermier C, Rabolli CP, Gonzalez E, Senyo SE, Liu X, Guerquin-Kern J-L, Steinhauser ML, Lee RT, Rosenzweig A. Exercise induces new cardiomyocyte generation in the adult mammalian heart. Nature communications. 2018;9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu X, Xiao J, Zhu H, Wei X, Platt C, Damilano F, Xiao C, Bezzerides V, Bostrom P, Che L, Zhang C, Spiegelman BM, Rosenzweig A. Mir-222 is necessary for exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell Metab. 2015;21:584–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wei X, Liu X, Rosenzweig A. What do we know about the cardiac benefits of exercise? Trends Cardiovasc Med. 2015;25:529–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.McMullen JR, Shioi T, Zhang L, Tarnavski O, Sherwood MC, Kang PM, Izumo S. Phosphoinositide 3-kinase(p110alpha) plays a critical role for the induction of physiological, but not pathological, cardiac hypertrophy. Proceedings Of The National Academy Of Sciences Of The United States Of America. 2003;100:12355–12360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Weeks KL, Gao X, Du XJ, Boey EJ, Matsumoto A, Bernardo BC, Kiriazis H, Cemerlang N, Tan JW, Tham YK, Franke TF, Qian H, Bogoyevitch MA, Woodcock EA, Febbraio MA, Gregorevic P, McMullen JR. Phosphoinositide 3-kinase p110alpha is a master regulator of exercise-induced cardioprotection and pi3k gene therapy rescues cardiac dysfunction. Circ Heart Fail. 2012;5:523–534 [DOI] [PubMed] [Google Scholar]
- 98.Matsui T, Li L, Monte F d, Fukui Y, Franke TF, Hajjar RJ, Rosenzweig A. Adenoviral gene transfer of activated phosphatidylinositol 3’-kinase and akt inhibits apoptosis of hypoxic cardiomyocytes in vitro. Circulation. 1999;100:2373–2379 [DOI] [PubMed] [Google Scholar]
- 99.Matsui T, Tao J, del Monte F, Lee K-H, Li L, Picard M, Force TL, Franke TF, Hajjar RJ, Rosenzweig A. Akt activation preserves cardiac function and prevents injury after transient cardiac ischemia in vivo. Circulation. 2001;104:330–335 [DOI] [PubMed] [Google Scholar]
- 100.DeBosch B, Treskov I, Lupu TS, Weinheimer C, Kovacs A, Courtois M, Muslin AJ. Akt1 is required for physiological cardiac growth. Circulation. 2006;113:2097–2104 [DOI] [PubMed] [Google Scholar]
- 101.Calvert JW, Condit ME, Aragon JP, Nicholson CK, Moody BF, Hood RL, Sindler AL, Gundewar S, Seals DR, Barouch LA, Lefer DJ. Exercise protects against myocardial ischemia-reperfusion injury via stimulation of beta(3)-adrenergic receptors and increased nitric oxide signaling: Role of nitrite and nitrosothiols. Circ Res. 2011;108:1448–1458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Arany Z, Foo SY, Ma Y, Ruas JL, Bommi-Reddy A, Girnun G, Cooper M, Laznik D, Chinsomboon J, Rangwala SM, Baek KH, Rosenzweig A, Spiegelman BM. Hif-independent regulation of vegf and angiogenesis by the transcriptional coactivator pgc-1alpha. Nature. 2008;451:1008–1012 [DOI] [PubMed] [Google Scholar]
- 103.Bezzerides VJ, Platt C, Lerchenmuller C, Paruchuri K, Oh NL, Xiao C, Cao Y, Mann N, Spiegelman BM, Rosenzweig A. Cited4 induces physiologic hypertrophy and promotes functional recovery after ischemic injury. JCI Insight. 2016;1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Martinez P, Blasco MA. Heart-breaking telomeres. Circ Res. 2018;123:787–802 [DOI] [PubMed] [Google Scholar]
- 105.Blackburn EH, Epel ES, Lin J. Human telomere biology: A contributory and interactive factor in aging, disease risks, and protection. Science. 2015;350:1193–1198 [DOI] [PubMed] [Google Scholar]
- 106.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Matsumoto C, Jiang Y, Emathinger J, Quijada P, Nguyen N, De La Torre A, Moshref M, Nguyen J, Levinson AB, Shin M, Sussman MA, Hariharan N. Short telomeres induce p53 and autophagy and modulate age-associated changes in cardiac progenitor cell fate. Stem Cells. 2018;36:868–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wong LS, Oeseburg H, de Boer RA, van Gilst WH, van Veldhuisen DJ, van der Harst P. Telomere biology in cardiovascular disease: The terc−/− mouse as a model for heart failure and ageing. Cardiovasc Res. 2009;81:244–252 [DOI] [PubMed] [Google Scholar]
- 109.Aix E, Gutierrez-Gutierrez O, Sanchez-Ferrer C, Aguado T, Flores I. Postnatal telomere dysfunction induces cardiomyocyte cell-cycle arrest through p21 activation. J Cell Biol. 2016;213:571–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chang ACY, Chang ACH, Kirillova A, Sasagawa K, Su W, Weber G, Lin J, Termglinchan V, Karakikes I, Seeger T, Dainis AM, Hinson JT, Seidman J, Seidman CE, Day JW, Ashley E, Wu JC, Blau HM. Telomere shortening is a hallmark of genetic cardiomyopathies. Proc Natl Acad Sci U S A. 2018;115:9276–9281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mourkioti F, Kustan J, Kraft P, Day JW, Zhao MM, Kost-Alimova M, Protopopov A, DePinho RA, Bernstein D, Meeker AK, Blau HM. Role of telomere dysfunction in cardiac failure in duchenne muscular dystrophy. Nat Cell Biol. 2013;15:895–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Oh H, Wang SC, Prahash A, Sano M, Moravec CS, Taffet GE, Michael LH, Youker KA, Entman ML, Schneider MD. Telomere attrition and chk2 activation in human heart failure. Proc Natl Acad Sci U S A. 2003;100:5378–5383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Oh H, Taffet GE, Youker KA, Entman ML, Overbeek PA, Michael LH, Schneider MD. Telomerase reverse transcriptase promotes cardiac muscle cell proliferation, hypertrophy, and survival. Proc Natl Acad Sci U S A. 2001;98:10308–10313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rudolph KL, Chang S, Lee HW, Blasco M, Gottlieb GJ, Greider C, DePinho RA. Longevity, stress response, and cancer in aging telomerase-deficient mice. Cell. 1999;96:701–712 [DOI] [PubMed] [Google Scholar]
- 115.Terai M, Izumiyama-Shimomura N, Aida J, Ishikawa N, Sawabe M, Arai T, Fujiwara M, Ishii A, Nakamura K, Takubo K. Association of telomere shortening in myocardium with heart weight gain and cause of death. Sci Rep. 2013;3:2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sharifi-Sanjani M, Oyster NM, Tichy ED, Bedi KC Jr., Harel O, Margulies KB, Mourkioti F. Cardiomyocyte-specific telomere shortening is a distinct signature of heart failure in humans. J Am Heart Assoc. 2017;6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Childs BG, Li H, van Deursen JM. Senescent cells: A therapeutic target for cardiovascular disease. J Clin Invest. 2018;128:1217–1228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764 [DOI] [PubMed] [Google Scholar]
- 119.Loffredo FS, Steinhauser ML, Jay SM, Gannon J, Pancoast JR, Yalamanchi P, Sinha M, Dall’Osso C, Khong D, Shadrach JL, Miller CM, Singer BS, Stewart A, Psychogios N, Gerszten RE, Hartigan AJ, Kim MJ, Serwold T, Wagers AJ, Lee RT. Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell. 2013;153:828–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sinha M, Jang YC, Oh J, Khong D, Wu EY, Manohar R, Miller C, Regalado SG, Loffredo FS, Pancoast JR, Hirshman MF, Lebowitz J, Shadrach JL, Cerletti M, Kim MJ, Serwold T, Goodyear LJ, Rosner B, Lee RT, Wagers AJ. Restoring systemic gdf11 levels reverses age-related dysfunction in mouse skeletal muscle. Science. 2014;344:649–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Katsimpardi L, Litterman NK, Schein PA, Miller CM, Loffredo FS, Wojtkiewicz GR, Chen JW, Lee RT, Wagers AJ, Rubin LL. Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science. 2014;344:630–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Schafer MJ, Atkinson EJ, Vanderboom PM, Kotajarvi B, White TA, Moore MM, Bruce CJ, Greason KL, Suri RM, Khosla S, Miller JD, Bergen HR 3rd, LeBrasseur NK. Quantification of gdf11 and myostatin in human aging and cardiovascular disease. Cell Metab. 2016;23:1207–1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Morissette MR, Stricker JC, Rosenberg MA, Buranasombati C, Levitan EB, Mittleman MA, Rosenzweig A. Effects of myostatin deletion in aging mice. Aging Cell. 2009;8:573–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Egerman MA, Cadena SM, Gilbert JA, Meyer A, Nelson HN, Swalley SE, Mallozzi C, Jacobi C, Jennings LL, Clay I, Laurent G, Ma S, Brachat S, Lach-Trifilieff E, Shavlakadze T, Trendelenburg AU, Brack AS, Glass DJ. Gdf11 increases with age and inhibits skeletal muscle regeneration. Cell Metab. 2015;22:164–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Smith SC, Zhang X, Zhang X, Gross P, Starosta T, Mohsin S, Franti M, Gupta P, Hayes D, Myzithras M, Kahn J, Tanner J, Weldon SM, Khalil A, Guo X, Sabri A, Chen X, MacDonnell S, Houser SR. Gdf11 does not rescue aging-related pathological hypertrophy. Circ Res. 2015;117:926–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tian Z, Miyata K, Kadomatsu T, Horiguchi H, Fukushima H, Tohyama S, Ujihara Y, Okumura T, Yamaguchi S, Zhao J, Endo M, Morinaga J, Sato M, Sugizaki T, Zhu S, Terada K, Sakaguchi H, Komohara Y, Takeya M, Takeda N, Araki K, Manabe I, Fukuda K, Otsu K, Wada J, Murohara T, Mohri S, Yamashita JK, Sano M, Oike Y. Angptl2 activity in cardiac pathologies accelerates heart failure by perturbing cardiac function and energy metabolism. Nature communications. 2016;7:13016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shimamoto A, Kagawa H, Zensho K, Sera Y, Kazuki Y, Osaki M, Oshimura M, Ishigaki Y, Hamasaki K, Kodama Y, Yuasa S, Fukuda K, Hirashima K, Seimiya H, Koyama H, Shimizu T, Takemoto M, Yokote K, Goto M, Tahara H. Reprogramming suppresses premature senescence phenotypes of werner syndrome cells and maintains chromosomal stability over long-term culture. PloS one. 2014;9:e112900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tian Z, Miyata K, Morinaga J, Horiguchi H, Kadomatsu T, Endo M, Zhao J, Zhu S, Sugizaki T, Sato M, Terada K, Okumura T, Murohara T, Oike Y. Circulating angptl2 levels increase in humans and mice exhibiting cardiac dysfunction. Circ J. 2018;82:437–447 [DOI] [PubMed] [Google Scholar]
- 129.Lenk K, Erbs S, Hollriegel R, Beck E, Linke A, Gielen S, Winkler SM, Sandri M, Hambrecht R, Schuler G, Adams V. Exercise training leads to a reduction of elevated myostatin levels in patients with chronic heart failure. Eur J Prev Cardiol. 2012;19:404–411 [DOI] [PubMed] [Google Scholar]
- 130.Lenk K, Schur R, Linke A, Erbs S, Matsumoto Y, Adams V, Schuler G. Impact of exercise training on myostatin expression in the myocardium and skeletal muscle in a chronic heart failure model. Eur J Heart Fail. 2009;11:342–348 [DOI] [PubMed] [Google Scholar]
- 131.McIntyre RL, Daniels EG, Molenaars M, Houtkooper RH, Janssens GE. From molecular promise to preclinical results: Hdac inhibitors in the race for healthy aging drugs. EMBO Mol Med. 2019:e9854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhang W, Song M, Qu J, Liu GH. Epigenetic modifications in cardiovascular aging and diseases. Circ Res. 2018;123:773–786 [DOI] [PubMed] [Google Scholar]
- 133.Okano M, Bell DW, Haber DA, Li E. DNA methyltransferases dnmt3a and dnmt3b are essential for de novo methylation and mammalian development. Cell. 1999;99:247–257 [DOI] [PubMed] [Google Scholar]
- 134.Heyn H, Moran S, Esteller M. Aberrant DNA methylation profiles in the premature aging disorders hutchinson-gilford progeria and werner syndrome. Epigenetics. 2013;8:28–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Issa JP. Aging and epigenetic drift: A vicious cycle. J Clin Invest. 2014;124:24–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang W, Zhang Y, Wang R, Shrestha Y, Xu Y, Peng L, Zhang J, Li J, Zhang L. Risk factors and epigenetic markers of left ventricular diastolic dysfunction with preserved ejection fraction in a community-based elderly chinese population. Clin Interv Aging. 2019;14:1719–1728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gilsbach R, Preissl S, Gruning BA, Schnick T, Burger L, Benes V, Wurch A, Bonisch U, Gunther S, Backofen R, Fleischmann BK, Schubeler D, Hein L. Dynamic DNA methylation orchestrates cardiomyocyte development, maturation and disease. Nature communications. 2014;5:5288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Movassagh M, Choy MK, Knowles DA, Cordeddu L, Haider S, Down T, Siggens L, Vujic A, Simeoni I, Penkett C, Goddard M, Lio P, Bennett MR, Foo RS. Distinct epigenomic features in end-stage failing human hearts. Circulation. 2011;124:2411–2422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Xiao D, Dasgupta C, Chen M, Zhang K, Buchholz J, Xu Z, Zhang L. Inhibition of DNA methylation reverses norepinephrine-induced cardiac hypertrophy in rats. Cardiovasc Res. 2014;101:373–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dorn LE, Lasman L, Chen J, Xu X, Hund TJ, Medvedovic M, Hanna JH, van Berlo JH, Accornero F. The n(6)-methyladenosine mrna methylase mettl3 controls cardiac homeostasis and hypertrophy. Circulation. 2019;139:533–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.O’Sullivan RJ, Kubicek S, Schreiber SL, Karlseder J. Reduced histone biosynthesis and chromatin changes arising from a damage signal at telomeres. Nat Struct Mol Biol. 2010;17:1218–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Piazzesi A, Papic D, Bertan F, Salomoni P, Nicotera P, Bano D. Replication-independent histone variant h3.3 controls animal lifespan through the regulation of pro-longevity transcriptional programs. Cell Rep. 2016;17:987–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Haberland M, Montgomery RL, Olson EN. The many roles of histone deacetylases in development and physiology: Implications for disease and therapy. Nat Rev Genet. 2009;10:32–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Montgomery RL, Potthoff MJ, Haberland M, Qi X, Matsuzaki S, Humphries KM, Richardson JA, Bassel-Duby R, Olson EN. Maintenance of cardiac energy metabolism by histone deacetylase 3 in mice. J Clin Invest. 2008;118:3588–3597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Montgomery RL, Davis CA, Potthoff MJ, Haberland M, Fielitz J, Qi X, Hill JA, Richardson JA, Olson EN. Histone deacetylases 1 and 2 redundantly regulate cardiac morphogenesis, growth, and contractility. Genes Dev. 2007;21:1790–1802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wang L, Quan N, Sun W, Chen X, Cates C, Rousselle T, Zhou X, Zhao X, Li J. Cardiomyocyte-specific deletion of sirt1 gene sensitizes myocardium to ischaemia and reperfusion injury. Cardiovasc Res. 2018;114:805–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lyu G, Guan Y, Zhang C, Zong L, Sun L, Huang X, Huang L, Zhang L, Tian XL, Zhou Z, Tao W. Tgf-beta signaling alters h4k20me3 status via mir-29 and contributes to cellular senescence and cardiac aging. Nature communications. 2018;9:2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Soci UPR, Fernandes T, Barauna VG, Hashimoto NY, de Fatima Alves Mota G, Rosa KT, Irigoyen MC, Philips MI, de Oliveira EM. Epigenetic control of exercise training-induced cardiac hypertrophy by mir-208. Clinical science (London, England : 1979). 2016;130:2005–2015 [DOI] [PubMed] [Google Scholar]
- 149.Zhao D, Sun Y, Tan Y, Zhang Z, Hou Z, Gao C, Feng P, Zhang X, Yi W, Gao F. Short-duration swimming exercise after myocardial infarction attenuates cardiac dysfunction and regulates mitochondrial quality control in aged mice. Oxidative medicine and cellular longevity. 2018;2018:4079041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Pillai VB, Sundaresan NR, Gupta MP. Regulation of akt signaling by sirtuins: Its implication in cardiac hypertrophy and aging. Circ Res. 2014;114:368–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Jiang YW, Veschambre P, Erdjument-Bromage H, Tempst P, Conaway JW, Conaway RC, Kornberg RD. Mammalian mediator of transcriptional regulation and its possible role as an end-point of signal transduction pathways. Proc Natl Acad Sci U S A. 1998;95:8538–8543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Anand P, Brown JD, Lin CY, Qi J, Zhang R, Artero PC, Alaiti MA, Bullard J, Alazem K, Margulies KB, Cappola TP, Lemieux M, Plutzky J, Bradner JE, Haldar SM. Bet bromodomains mediate transcriptional pause release in heart failure. Cell. 2013;154:569–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Duan Q, McMahon S, Anand P, Shah H, Thomas S, Salunga HT, Huang Y, Zhang R, Sahadevan A, Lemieux ME, Brown JD, Srivastava D, Bradner JE, McKinsey TA, Haldar SM. Bet bromodomain inhibition suppresses innate inflammatory and profibrotic transcriptional networks in heart failure. Sci Transl Med. 2017;9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zamore PD, Haley B. Ribo-gnome: The big world of small rnas. Science. 2005;309:1519–1524 [DOI] [PubMed] [Google Scholar]
- 155.Gupta SK, Foinquinos A, Thum S, Remke J, Zimmer K, Bauters C, de Groote P, Boon RA, de Windt LJ, Preissl S, Hein L, Batkai S, Pinet F, Thum T. Preclinical development of a microrna-based therapy for elderly patients with myocardial infarction. J Am Coll Cardiol. 2016;68:1557–1571 [DOI] [PubMed] [Google Scholar]
- 156.Boon RA, Iekushi K, Lechner S, Seeger T, Fischer A, Heydt S, Kaluza D, Treguer K, Carmona G, Bonauer A, Horrevoets AJ, Didier N, Girmatsion Z, Biliczki P, Ehrlich JR, Katus HA, Muller OJ, Potente M, Zeiher AM, Hermeking H, Dimmeler S. Microrna-34a regulates cardiac ageing and function. Nature. 2013;495:107–110 [DOI] [PubMed] [Google Scholar]
- 157.Huang W, Feng Y, Liang J, Yu H, Wang C, Wang B, Wang M, Jiang L, Meng W, Cai W, Medvedovic M, Chen J, Paul C, Davidson WS, Sadayappan S, Stambrook PJ, Yu XY, Wang Y. Loss of microrna-128 promotes cardiomyocyte proliferation and heart regeneration. Nat Commun. 2018;9:700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Elmén J, Lindow M, Schütz S, Lawrence M, Petri A, Obad S, Lindholm M, Hedtjärn M, Hansen HF, Berger U, Gullans S, Kearney P, Sarnow P, Straarup EM, Kauppinen S. Lna-mediated microrna silencing in non-human primates. Nature. 2008;452:896–899 [DOI] [PubMed] [Google Scholar]
- 159.Janssen HL, Reesink HW, Lawitz EJ, Zeuzem S, Rodriguez-Torres M, Patel K, van der Meer AJ, Patick AK, Chen A, Zhou Y, Persson R, King BD, Kauppinen S, Levin AA, Hodges MR. Treatment of hcv infection by targeting microrna. New England Journal Of Medicine. 2013;368:1685–1694 [DOI] [PubMed] [Google Scholar]
- 160.van Almen GC, Verhesen W, van Leeuwen RE, van de Vrie M, Eurlings C, Schellings MW, Swinnen M, Cleutjens JP, van Zandvoort MA, Heymans S, Schroen B. Microrna-18 and microrna-19 regulate ctgf and tsp-1 expression in age-related heart failure. Aging Cell. 2011;10:769–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Eulalio A, Mano M, Dal Ferro M, Zentilin L, Sinagra G, Zacchigna S, Giacca M. Functional screening identifies mirnas inducing cardiac regeneration. Nature. 2012;492:376–381 [DOI] [PubMed] [Google Scholar]
- 162.Hou Z, Qin X, Hu Y, Zhang X, Li G, Wu J, Li J, Sha J, Chen J, Xia J, Wang L, Gao F. Longterm exercise-derived exosomal mir-342-5p. Circ Res. 2019;124:1386–1400 [DOI] [PubMed] [Google Scholar]
- 163.Zhang X, Cheng L, Xu L, Zhang Y, Yang Y, Fu Q, Mi W, Li H. The lncrna, h19 mediates the protective effect of hypoxia postconditioning against hypoxia-reoxygenation injury to senescent cardiomyocytes by targeting microrna-29b-3p. Shock. 2019;52:249–256 [DOI] [PubMed] [Google Scholar]
- 164.Zhu B, Zhang L, Liang C, Liu B, Pan X, Wang Y, Zhang Y, Zhang Y, Xie W, Yan B, Liu F, Yip HK, Yu XY, Li Y. Stem cell-derived exosomes prevent aging-induced cardiac dysfunction through a novel exosome/lncrna malat1/nf-kappab/tnf-alpha signaling pathway. Oxidative medicine and cellular longevity. 2019;2019:9739258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Chun Yang X, Hui Zhao D, Bond Lau W, Qiang Liu K, Yu Tian J, Chao Cheng Z, Liang Ma X, Hua Liu J, Fan Q. Lncrna ensmust00000134285 increases mapk11 activity, regulating aging-related myocardial apoptosis. J Gerontol A Biol Sci Med Sci. 2018;73:1010–1017 [DOI] [PubMed] [Google Scholar]
- 166.Huang S, Li X, Zheng H, Si X, Li B, Wei G, Li C, Chen Y, Chen Y, Liao W, Liao Y, Bin J. Loss of super-enhancer-regulated circrna nfix induces cardiac regeneration after myocardial infarction in adult mice. Circulation. 2019;139:2857–2876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Garikipati VNS, Verma SK, Cheng Z, Liang D, Truongcao MM, Cimini M, Yue Y, Huang G, Wang C, Benedict C, Tang Y, Mallaredy V, Ibetti J, Grisanti L, Schumacher SM, Gao E, Rajan S, Wilusz JE, Goukassian D, Houser SR, Koch WJ, Kishore R. Circular rna circfndc3b modulates cardiac repair after myocardial infarction via fus/vegf-a axis. Nature communications. 2019;10:4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gruner H, Cortes-Lopez M, Cooper DA, Bauer M, Miura P. Circrna accumulation in the aging mouse brain. Sci Rep. 2016;6:38907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gude NA, Broughton KM, Firouzi F, Sussman MA. Cardiac ageing: Extrinsic and intrinsic factors in cellular renewal and senescence. Nat Rev Cardiol. 2018;15:523–542 [DOI] [PubMed] [Google Scholar]
- 170.Linnane AW, Marzuki S, Ozawa T, Tanaka M. Mitochondrial DNA mutations as an important contributor to ageing and degenerative diseases. Lancet. 1989;1:642–645 [DOI] [PubMed] [Google Scholar]
- 171.Vermulst M, Wanagat J, Kujoth GC, Bielas JH, Rabinovitch PS, Prolla TA, Loeb LA. DNA deletions and clonal mutations drive premature aging in mitochondrial mutator mice. Nat Genet. 2008;40:392–394 [DOI] [PubMed] [Google Scholar]
- 172.Baris OR, Ederer S, Neuhaus JF, von Kleist-Retzow JC, Wunderlich CM, Pal M, Wunderlich FT, Peeva V, Zsurka G, Kunz WS, Hickethier T, Bunck AC, Stockigt F, Schrickel JW, Wiesner RJ. Mosaic deficiency in mitochondrial oxidative metabolism promotes cardiac arrhythmia during aging. Cell Metab. 2015;21:667–677 [DOI] [PubMed] [Google Scholar]
- 173.Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink JN, Rovio AT, Bruder CE, Bohlooly YM, Gidlof S, Oldfors A, Wibom R, Tornell J, Jacobs HT, Larsson NG. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature. 2004;429:417–423 [DOI] [PubMed] [Google Scholar]
- 174.Dai DF, Chen T, Wanagat J, Laflamme M, Marcinek DJ, Emond MJ, Ngo CP, Prolla TA, Rabinovitch PS. Age-dependent cardiomyopathy in mitochondrial mutator mice is attenuated by overexpression of catalase targeted to mitochondria. Aging Cell. 2010;9:536–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, Hofer T, Seo AY, Sullivan R, Jobling WA, Morrow JD, Van Remmen H, Sedivy JM, Yamasoba T, Tanokura M, Weindruch R, Leeuwenburgh C, Prolla TA. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science. 2005;309:481–484 [DOI] [PubMed] [Google Scholar]
- 176.Trifunovic A, Hansson A, Wredenberg A, Rovio AT, Dufour E, Khvorostov I, Spelbrink JN, Wibom R, Jacobs HT, Larsson NG. Somatic mtdna mutations cause aging phenotypes without affecting reactive oxygen species production. Proc Natl Acad Sci U S A. 2005;102:17993–17998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Migliaccio E, Giorgio M, Mele S, Pelicci G, Reboldi P, Pandolfi PP, Lanfrancone L, Pelicci PG. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature. 1999;402:309–313 [DOI] [PubMed] [Google Scholar]
- 178.Rota M, LeCapitaine N, Hosoda T, Boni A, De Angelis A, Padin-Iruegas ME, Esposito G, Vitale S, Urbanek K, Casarsa C, Giorgio M, Luscher TF, Pelicci PG, Anversa P, Leri A, Kajstura J. Diabetes promotes cardiac stem cell aging and heart failure, which are prevented by deletion of the p66shc gene. Circ Res. 2006;99:42–52 [DOI] [PubMed] [Google Scholar]
- 179.Camici GG, Schiavoni M, Francia P, Bachschmid M, Martin-Padura I, Hersberger M, Tanner FC, Pelicci P, Volpe M, Anversa P, Luscher TF, Cosentino F. Genetic deletion of p66(shc) adaptor protein prevents hyperglycemia-induced endothelial dysfunction and oxidative stress. Proc Natl Acad Sci U S A. 2007;104:5217–5222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Francia P, Cosentino F, Schiavoni M, Huang Y, Perna E, Camici GG, Luscher TF, Volpe M. P66(shc) protein, oxidative stress, and cardiovascular complications of diabetes: The missing link. J Mol Med (Berl). 2009;87:885–891 [DOI] [PubMed] [Google Scholar]
- 181.Safdar A, Khrapko K, Flynn JM, Saleem A, De Lisio M, Johnston AP, Kratysberg Y, Samjoo IA, Kitaoka Y, Ogborn DI, Little JP, Raha S, Parise G, Akhtar M, Hettinga BP, Rowe GC, Arany Z, Prolla TA, Tarnopolsky MA. Exercise-induced mitochondrial p53 repairs mtdna mutations in mutator mice. Skelet Muscle. 2016;6:7. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 182.Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, Lindsley RC, Mermel CH, Burtt N, Chavez A, Higgins JM, Moltchanov V, Kuo FC, Kluk MJ, Henderson B, Kinnunen L, Koistinen HA, Ladenvall C, Getz G, Correa A, Banahan BF, Gabriel S, Kathiresan S, Stringham HM, McCarthy MI, Boehnke M, Tuomilehto J, Haiman C, Groop L, Atzmon G, Wilson JG, Neuberg D, Altshuler D, Ebert BL. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371:2488–2498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Jaiswal S, Natarajan P, Silver AJ, Gibson CJ, Bick AG, Shvartz E, McConkey M, Gupta N, Gabriel S, Ardissino D, Baber U, Mehran R, Fuster V, Danesh J, Frossard P, Saleheen D, Melander O, Sukhova GK, Neuberg D, Libby P, Kathiresan S, Ebert BL. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med. 2017;377:111–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Sano S, Oshima K, Wang Y, Katanasaka Y, Sano M, Walsh K. Crispr-mediated gene editing to assess the roles of tet2 and dnmt3a in clonal hematopoiesis and cardiovascular disease. Circ Res. 2018;123:335–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Sano S, Oshima K, Wang Y, MacLauchlan S, Katanasaka Y, Sano M, Zuriaga MA, Yoshiyama M, Goukassian D, Cooper MA, Fuster JJ, Walsh K. Tet2-mediated clonal hematopoiesis accelerates heart failure through a mechanism involving the il-1beta/nlrp3 inflammasome. J Am Coll Cardiol. 2018;71:875–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Xie M, Lu C, Wang J, McLellan MD, Johnson KJ, Wendl MC, McMichael JF, Schmidt HK, Yellapantula V, Miller CA, Ozenberger BA, Welch JS, Link DC, Walter MJ, Mardis ER, Dipersio JF, Chen F, Wilson RK, Ley TJ, Ding L. Age-related mutations associated with clonal hematopoietic expansion and malignancies. Nat Med. 2014;20:1472–1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ, Group CT. Antiinflammatory therapy with canakinumab for atherosclerotic disease. New England Journal Of Medicine. 2017;377:1119–1131 [DOI] [PubMed] [Google Scholar]
- 188.Cho S, Vashisth M, Abbas A, Majkut S, Vogel K, Xia Y, Ivanovska IL, Irianto J, Tewari M, Zhu K, Tichy ED, Mourkioti F, Tang HY, Greenberg RA, Prosser BL, Discher DE. Mechanosensing by the lamina protects against nuclear rupture, DNA damage, and cell-cycle arrest. Dev Cell. 2019;49:920–935 e925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Brayson D, Shanahan CM. Current insights into lmna cardiomyopathies: Existing models and missing lincs. Nucleus. 2017;8:17–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Lee J, Termglinchan V, Diecke S, Itzhaki I, Lam CK, Garg P, Lau E, Greenhaw M, Seeger T, Wu H, Zhang JZ, Chen X, Gil IP, Ameen M, Sallam K, Rhee JW, Churko JM, Chaudhary R, Chour T, Wang PJ, Snyder MP, Chang HY, Karakikes I, Wu JC. Activation of pdgf pathway links lmna mutation to dilated cardiomyopathy. Nature. 2019;572:335–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ragnauth CD, Warren DT, Liu Y, McNair R, Tajsic T, Figg N, Shroff R, Skepper J, Shanahan CM. Prelamin a acts to accelerate smooth muscle cell senescence and is a novel biomarker of human vascular aging. Circulation. 2010;121:2200–2210 [DOI] [PubMed] [Google Scholar]
- 192.Wei X, Liu X, Rosenzweig A. What do we know about the cardiac benefits of exercise? Trends Cardiovasc Med. 2014 [DOI] [PMC free article] [PubMed]
- 193.Calvert JW, Elston M, Pablo Aragón J, Nicholson CK, Moody BF, Hood RL, Sindler A, Gundewar S, Seals DR, Barouch LA, Lefer DJ. Exercise protects against myocardial ischemia-reperfusion injury via stimulation of {beta}3-adrenergic receptors and increased nitric oxide signaling: Role of nitrite and nitrosothiols. Circulation research. 2011 [DOI] [PMC free article] [PubMed]
- 194.Platt C, Houstis N, Rosenzweig A. Using exercise to measure and modify cardiac function. Cell Metab. 2015;21:227–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.