Abstract
Objective:
Restricting dietary methionine to 0.17% in male mice increases energy expenditure, reduces fat deposition, and improves metabolic health. The goal of this work was to compare each of these responses in post-weaning male and female mice and in physically mature male and female mice.
Methods:
Methionine-restricted (MR) diets were fed to age-matched cohorts of male and female mice for 8-10 weeks beginning at 8 weeks of age or beginning at 4 months of age. The physiological and transcriptional responses to MR were compared in the respective cohorts.
Results:
Dietary MR produced sexually dimorphic changes in body composition in young growing animals, with males preserving lean at the expense of fat and females preserving fat at the expense of lean. The effects of MR on energy balance were comparable between sexes when the diet was initiated after attainment of physical maturity (4 months), and metabolic and endocrine responses were also comparable between males and females after 8 weeks on the MR diet.
Conclusions:
The sexually dimorphic effects of MR are limited to nutrient partitioning between lean and fat tissue deposition in young, growing mice. Introduction of the diet after physical maturity produced comparable effects on growth and metabolic responses in male and female mice.
Keywords: essential amino acid, nutrient sensing, sex, obesity, lipid, FGF21
Introduction
Metabolic disease is a cluster of pathologies that includes obesity, insulin resistance, systemic inflammation, dysregulation of lipid metabolism, and disordered carbohydrate metabolism. Pharmaceuticals are available to treat specific components of metabolic disease (e.g., lower circulating lipids, enhance insulin action, etc.), but lifestyle modifications leading to weight reduction are the most effective approach for collective improvement of multiple biomarkers of metabolic syndrome (1,2). Chronic reductions in energy intake produce reproducible and predictable weight loss. However, the high rates of recidivism and weight regain after the active phase of calorie restriction (CR) ends have underscored the need for development of alternative weight loss strategies. Bariatric surgical approaches produce a combined restrictive and malabsorptive state that is highly effective in producing weight loss and its attendant benefits, but the risks of surgery and its associated complications are not insignificant in obese patients (3).
Dietary strategies that produce weight loss without food restriction represent an appealing approach to treat metabolic disease. Dietary methionine restriction (MR) is one such alternative based on original studies showing that rats chronically fed a MR diet were smaller, leaner, and lived longer despite higher weight-specific food consumption than controls (4-6). Subsequent preclinical studies have established that the MR-dependent hyperphagia is compensated for by a significant increase in energy expenditure (EE) that effectively limits fat deposition and reduces adiposity (7-10). The translational potential of dietary MR was recently tested in patients with metabolic syndrome using the medical food, Hominex-2® (11). Hominex-2® was developed by Abbott Labs (Abbott Park, IL) for patients with pyridoxine-unresponsive homocystinuria or hypermethionemia (12), and is effective because it lacks methionine but provides all other amino acids. Hominex-2® also contains cysteine and although the significance of this detail was not recognized when planning the original study (11), it was recently established that even small amounts of cysteine reverse key effects of the MR diet, including induction of hepatic FGF21 and increases in EE (13, 14). Thus, it is likely that cysteine in Hominex-2® spared methionine and limited the diet’s full efficacy to increase EE and impact energy balance.
With respect to translation, a significant remaining question has emerged from studies comparing the biological efficacy of protein dilution and/or dietary MR in male and female rodents (15-18). In these studies, the experimental diet (e.g., MR, protein dilution) was reported to be less effective in females than males. Given that a major goal of our work is to develop therapeutic diets that reduce methionine, it is important to establish the efficacy of dietary MR across sex. Using cohorts of young, growing mice and mature, weight-stable mice of both sexes, we establish that dietary MR produces sexually dichotomous effects on body composition in young, growing mice, but is fully effective in producing robust reductions of fat mass in both sexes of mature mice.
Materials and Methods
Animals and Diets.
All experiments were approved by Pennington Biomedical Research Center Institutional Animal Care and Use Committee based on guidelines approved by the National Research Council, Animal Welfare Act, and PHS Policy on humane care and use of animals in scientific research. Male and female C57BL/6J mice were generated from internal breeding colonies and weaned at 28 days of age. The first experiment used age-matched male and female mice beginning at 8 weeks of age and the second used age-matched male and female mice beginning at 4 months of age.
The mice were singly housed in shoebox cages with corncob bedding and mice were randomly assigned to one of two dietary treatments. Using the feeding paradigm described previously (19,20), mice assigned to the Control diet (Con) received a purified diet containing 0.86% methionine and no cysteine while mice in the methionine-restricted group (MR) received the same diet with methionine restricted to 0.17% and no cysteine. The diets were formulated as extruded pellets and provided ad libitum. The energy content of both diets (Dyets Inc., Bethlehem, PA) was 15.96 kJ/g, with 18.9% of energy from fat (corn oil), 64.9% from carbohydrate, and 14.8% from a custom mixture of L-amino acids. The amino acid content of the diet on a weight basis was 14.1%. Details of diet composition were provided previously (20). Housing temperature was maintained at 22-23° C and lights were on 12 h cycle from 7 AM-7 PM.
Experiment 1. Eight week-old male and female mice (n = 16 per sex) were adapted to the Con diet for a period of four days in a Promethion indirect calorimetry (IDC) system. On day 5, half the mice of each sex were randomized to receive the MR diet while the other half continued to receive the Con diet. Mice remained in the calorimeter for an additional ten days to measure their responses to the respective diets before being returned to home cages. Thereafter, animals were maintained on assigned diets for eight weeks. Body weight, composition, and food and water intake were assessed at weekly intervals during this period. Body composition was assessed using nuclear magnetic resonance (NMR; Brucker MiniSpec, Billerica, MA). Food intake was calculated as the difference between the weight of food provided less the food remaining in the hopper and wasted food. Food waste was collected by sifting the bedding on a screen and weighing the food. Water intake was calculated as the change in weight of the Hydropack at weekly intervals. Thereafter, mice were euthanized following a four-hour fast. Tissues were harvested and snap frozen in liquid nitrogen for subsequent analysis.
Experiment 2. Three month-old male and female mice (n = 16 per sex) were placed on the Con diet for a period of one month prior to randomization of half the mice of each sex to the MR diet while the remaining mice continued on the Con diet for the following eight weeks. Body weight, composition, and food and water intake were measured at weekly intervals as in Experiment 1. Thereafter, mice were acclimated to the Promethion indirect calorimetry system for one week, followed by measurement of VO2 consumption and VCO2 production for an additional 5 days. Thereafter, animals were returned to home cages for one week prior to euthanization. Animals were fasted for four hours before euthanasia, and tissues were harvested and snap frozen in liquid nitrogen.
Analysis of Energy Expenditure –
VO2 is expressed as liters (L) of O2 consumed per h, while Respiratory Exchange Ratio (RER) is the ratio of VCO2 produced to VO2 consumed. EE was calculated as (VO2 X (3.815 + (1.232 X RER)) X 0.96 kCal/h), expressed as kCal/h/mouse as described by manufacturer (Promethion, Sable Systems, N Las Vegas, NV). Group differences (e.g., Diet, Sex) in 24 h EE (kCal/h/mouse) were compared using Analysis of Covariance (ANCOVA) (JMP Software, Version 12; SAS Institute Inc., Cary, NC) to calculate least squares means that accounted for variation in EE attributable to differences in lean mass, fat mass, and activity among the mice. The significance of model effects and least squares means ± SEM for the two-way interaction were compared using residual variance as the error term (21).
RNA Isolation and Quantitative PCR.
Total RNA was isolated using RNeasy Mini Kit (QIAGEN, Inc.; Valencia, CA). One microgram of RNA was used to produce complementary DNA by reverse transcription. Gene expression was measured by qPCR (Applied Biosystems; Foster City, CA) using SYBR green. Expression of each target gene was normalized to the expression of cyclophilin in the same sample.
Western Blotting.
Whole cell lysates of BAT were prepared by homogenizing tissue in buffer (150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 10 mM Tris, 1% Triton x-100, 0.5% NP-40) and protein quantitated by Lowry assay. Three μg were loaded per lane, separated by SDS-PAGE, and transferred to polyvinylidene fluoride membranes (BioRad; Hercules, CA). Expression of UCP1 was detected as described previously and standardized to PDC-E2 (21). Blots were developed using enhanced chemiluminescence and band densities determined using Image J software.
Statistical Analysis.
Body weight and composition, food and water intake, gene expression data, and protein expression levels were analyzed using two-way ANOVA (GraphPad Prism; San Diego, CA) with diet and sex as main effects. Group differences in EE (kcal/hr/mouse) were compared using ANCOVA (15), calculating the least squares means that take into account the variation in EE due to differences in fat mass, lean mass, activity, sex, diet, and the sex by diet interaction (JMP; SAS Institute, Cary, NC). The least square means ± SEM for each sex x diet interaction were compared using two-way ANOVA and the significance of the model effects and interaction were tested using residual variance calculated by the ANCOVA. Protection against type I errors was set at 5% (α = 0.05).
Results
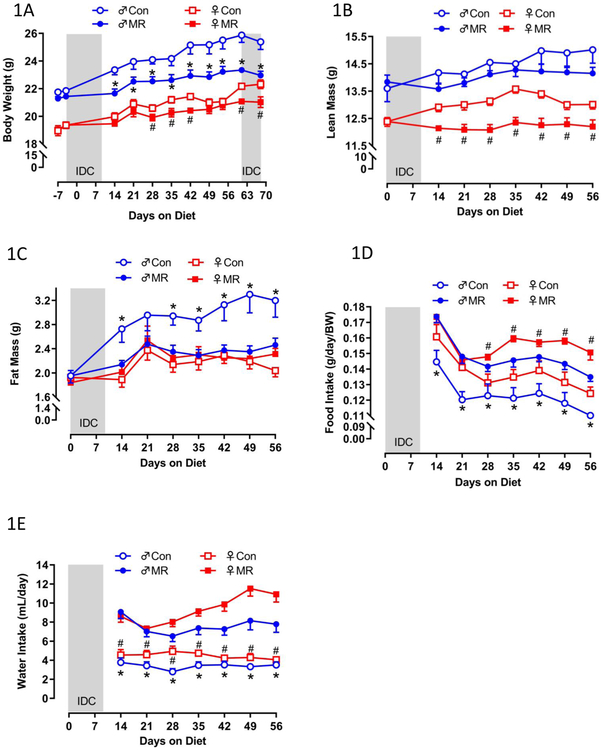
Experiment 1. Relative to Controls, MR produced a significant reduction in body weight accumulation (BW) of male mice within 14 d of its introduction, whereas in female mice an initial and smaller reduction of BW accumulation was not detected until day 28 (Fig. 1A). The overall reduction in BW accumulation by MR was 2-fold greater in males than females (Fig. 1A). In contrast, the effects of MR on body composition were completely different between the sexes, with MR producing a significant reduction in lean mass accumulation from day 14 through day 56 in females but no significant effect in males over this period (Fig. 1B). Conversely, MR produced a significant reduction in fat mass accumulation in male mice between day 14 and day 56 while MR was without effect on fat mass in females over this entire period (Fig. 1C). The effect on food consumption was comparable between the sexes, with weight-adjusted food consumption comparably increased by MR in males and females from day 28 through day 56 (Fig. 1D). The MR-induced increase in water consumption was also comparable between sexes over the entire study (Fig. 1E).
Figure 1 – Energy Balance Data for Experiment 1.
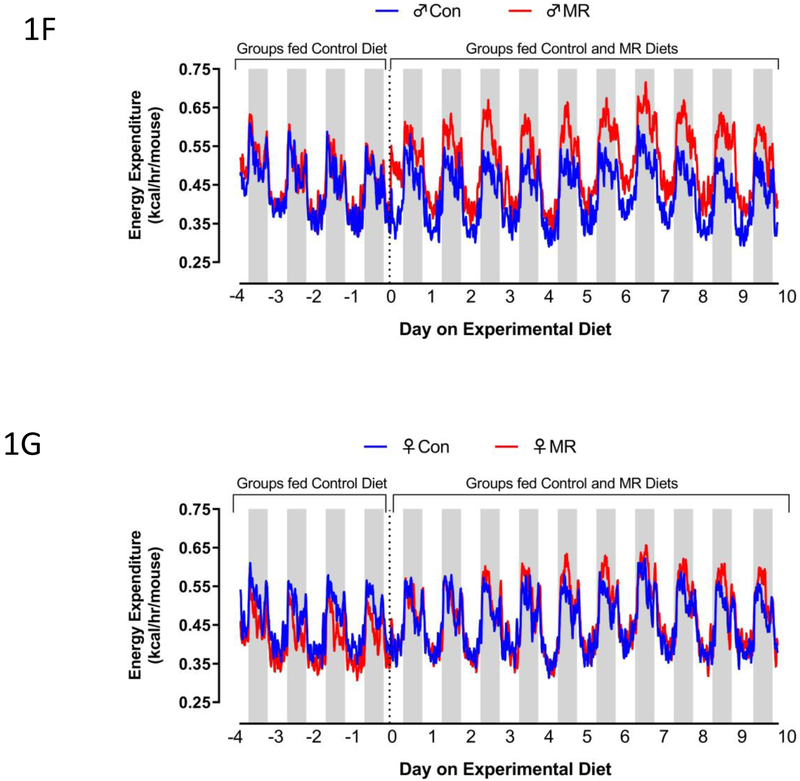
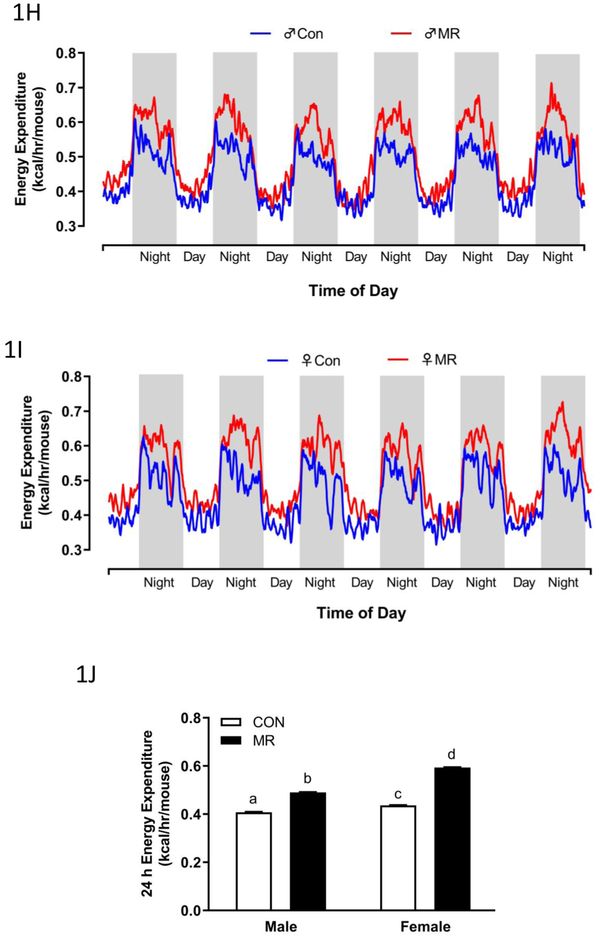
Body weight (A), lean mass (B) fat mass (C) food intake (D), and water intake (E) were measured at weekly intervals over the course of the 8 week study and analyzed using a repeated measures two way ANOVA with sex and diet as main effects. Means at specific time points that are annotated with an asterisk (*) differ between Con and MR for male mice at p < 0.05 or annotated with a pound symbol (#) differ between Con and MR for female mice at p < 0.05. Energy expenditure (EE) was measured via indirect calorimetry (IDC) during the first 10 days of the experiment (F-males,G-females) and for the last 7 days of the experiment (H-males, I-females). Error bars are omitted for clarity in Figs. 1F-1I. Prior to beginning the experiment, 16 animals of each sex were placed in the calorimeter chambers for 4 days with all animals receiving the control diet. Then on day 0, half the mice of each sex were switched to the MR diet while the other half of the mice continued on the control diet. EE was measured for the next 10 days. At the end of the study, all mice were returned to the calorimeter chambers for measurement of EE for 6 days. The time courses of EE means are summarized in Fig. 1H for males and Fig. 1I for females The time periods the mice were in the calorimeter chambers is designated with grey bars in Figs. 1A-1E. Mean 24 h EE was calculated from the measurements made during the last week of the study and compared by ANCOVA (J). All values are expressed as mean ± SEM for 8 mice of each diet x sex. In Fig. 1J, the least square means for 24 h EEnot sharing a common letter differ at p < 0.05.
The effects of MR on EE were examined at the beginning and end of Experiment 1. There were notable differences between sexes in the initial increase in EE after introduction of the MR diet, with both daytime and nighttime increases evident in males but not females within 2 d (Fig. 1F). The magnitude of the daytime and nighttime increases in EE produced by MR expanded in males between day 2 and 10 (Fig. 1F). In contrast, the magnitude of the initial MR-induced increase in EE at day 3 in females was significantly less than in males (Fig. 1G), and the significant increase in EE between day 3 and 10 in males (Fig. 1F) was present but more muted in females (Fig. 1G). The total 24 h EE was significantly increased by MR in females (Fig. 1G), but it is clear that the increase produced by MR in males during days 2-10 is significantly larger (Fig. 1F). In contrast, measurements made at the end of the experiment show that the MR-induced increase in EE was slightly larger in females than males (Figs. 1H, 1I, 1J). Together, these findings indicate that MR-induced increases in EE are slower to develop in young female mice, but are no less robust than the responses in males after chronic consumption of the diet.
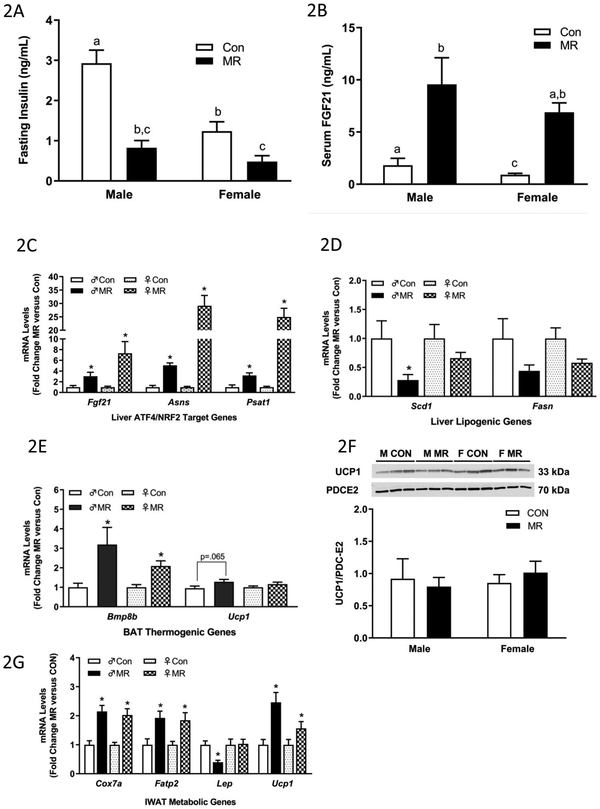
The physiological and transcriptional effects of the diets in liver and adipose tissue from Experiment 1 are illustrated in Figs. 2A-2G. Dietary MR produced a significant reduction in fasting insulin in both male and female mice (Fig. 2A), despite the lower fasting insulin in females on the Con diet. This is probably a reflection of the much lower fat mass in females relative to males at the ages being studied (Fig. 1C). MR also produced a comparable increase in serum FGF21 in male and female mice after 8 weeks on the diet (Fig. 2B). Given its essential role in mediating MR-induced increases in EE, this finding is consistent with the comparable increase in EE produced by 8 weeks of MR in both sexes (Fig. 1J). In liver, the MR diet faithfully reproduced its previously reported activation of nuclear factor erythroid 2-related factor 2 (NRF2) and activating transcription factor 4 (ATF4) target genes (14,22) (Fig. 2C). Hepatic Scd1 mRNA was reduced by the MR diet in males but not females, and the MR diet failed to decrease hepatic Fasn mRNA in either sex (Fig. 2D). In BAT, the MR diet increased the thermogenic markers, Bmp8b and Ucp1 mRNA in males, while Bmp8b but not Ucp1 mRNA was increased in females (Fig. 2E). Surprisingly, UCP1 protein was not increased in BAT of either sex (Fig. 2F). In IWAT, the transcriptional responses to MR were comparable in males and females, with the diet increasing Cox7a, Fatp2, and Ucp1 mRNA in both sexes (Fig. 2G). The only difference was with Leptin mRNA, where MR produced a decrease in male but not female IWAT (Fig. 2G). This finding is consistent with the failure of MR to reduce adiposity in females in Experiment 1. Overall, with only a couple of noted exceptions, the transcriptional responses to MR were comparable in tissues from male and female mice.
Figure 2 – Endocrine and Transcriptional Endpoints for Experiment 1.
Fasting insulin (A) and serum FGF21 levels (B) in blood samples collected at end of study. Hepatic gene expression (C,D), brown adipose tissue gene expression (E), and inguinal white adipose tissue (G) gene expression were measured via qPCR or by Western blot (F) for UCP1 expression in brown adipose tissue. The mitochondrial marker, PDC-E2 (pyruvate dehydrogenase dihydrolipoamide acetyltransferase) was used as a loading control for the UCP1 Western blot (E). Insulin and serum FGF21 were measured via ELISA. Con – control; MR – methionine restricted diets; IWAT – inguinal white adipose tissue; BAT – brown adipose tissue; Fgf21 – fibroblast growth factor 21; Asns – asparagine synthase; Psat1 - Phosphoserine Aminotransferase 1; Scd1 – stearoyl CoA desaturase 1; Fasn – fatty acid synthase; Bmp8b – bone morphogenetic protein 8b; Ucp1 – uncoupling protein 1; Cox7a – cytochrome C oxidase subunit 7A; Lep – Leptin; Fatp2 – fatty acid transport protein 2. Each variable was analyzed using a two way ANOVA with sex and diet as main effects and residual variance used as the error term. All values are expressed as mean ± SEM for 8 mice of each diet x sex. Means for fold-change of gene expression denoted with an asterisk (*) indicates significant difference from Con at p < 0.05. For fasting insulin and serum FGF21, group means not sharing a common letter differ at p < 0.05.
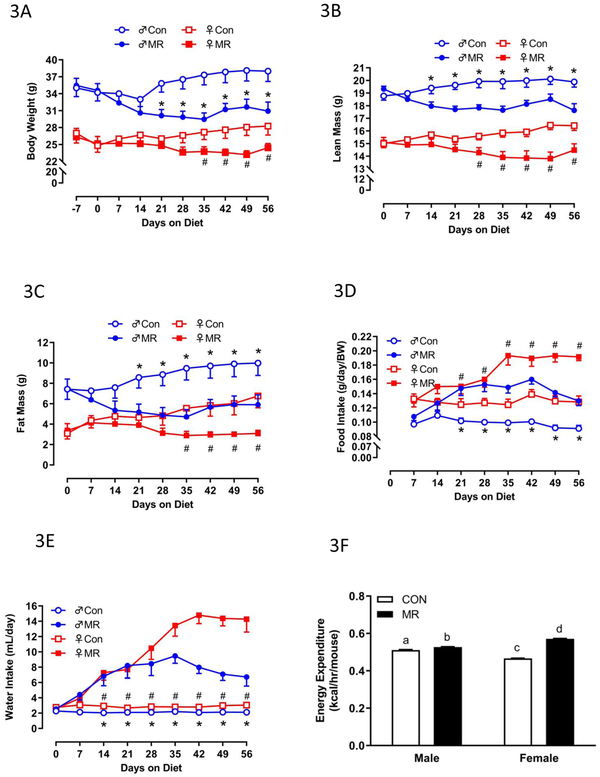
Experiment 2. To compare the responses between young, growing animals and physically mature animals, we tested the efficacy of dietary MR in 4 month-old male and female mice. Although the males were significantly heavier than females at this age, both sexes were fairly weight stable on the Con diet, only increasing their BW by 5-8% over the following 2 months (Fig. 3A). Over the same period, the MR diet decreased BW by 12% in males and 10% in females (Fig. 3A). Unsurprisingly, lean mass paralleled BW in male and female mice on the Con diet, with males increasing lean mass by 6% over the 8-week study and females increasing lean mass by 9.3% (Fig. 3B). Dietary MR produced comparable reductions in lean mass across sex, with males decreasing lean mass by 9% and females decreasing lean mass by 6% (Fig. 3B). The greatest percentage of tissue expansion over the 8-week study was in fat mass, with males on the Con diet increasing fat mass by 34% and females by 52% (Fig. 3C). MR produced a comparable reduction in fat mass across sex, with males decreasing fat mass accumulation by 22% and females decreasing fat mass accumulation by 25% (Fig. 3C). Dietary MR increased food intake per unit BW by 40-50% in both males and females over the last 5 weeks of the study (Fig. 3D). Interestingly, MR increased water intake by 4-fold in both sexes during the first month of the study, but for the last month, MR increased water intake to 7-fold higher than Con females (Fig. 3E). Lastly, after 8 weeks on each diet, measurement of VO2 and VCO2 by indirect calorimetry revealed that MR produced significant increases in 24 h EE in both sexes, with the effect in females significantly larger than the effect in males (Fig. 3F). The higher EE in females on the MR diet compared to males is reflective of their significantly higher weight-adjusted food consumption over the last month of the study (Fig. 3D). The underlying mechanism of this subtle sex difference is not clear.
Figure 3 – Energy Balance Data for Experiment 2.
Body weight (A), lean mass (B) fat mass (C) food intake (D), and water intake (E) were measured for 8 mice of each diet x sex combination at weekly intervals over the course of the 8 week study and analyzed using a repeated measures two way ANOVA with sex and diet as main effects. Means at specific time points denoted with an asterisk (*) differ between Con and MR for male mice at p < 0.05 and denoted with a pound symbol (#) differ between Con and MR for female mice at p < 0.05. . Energy expenditure (EE) was measured via indirect calorimetry (IDC) during the last 4 days of the experiment. Least squares means for 24 h EE were calculated from the measurements made during the last week of the study by ANCOVA (F). For 24 h EE, groups not sharing a common letter differ at p < 0.05.
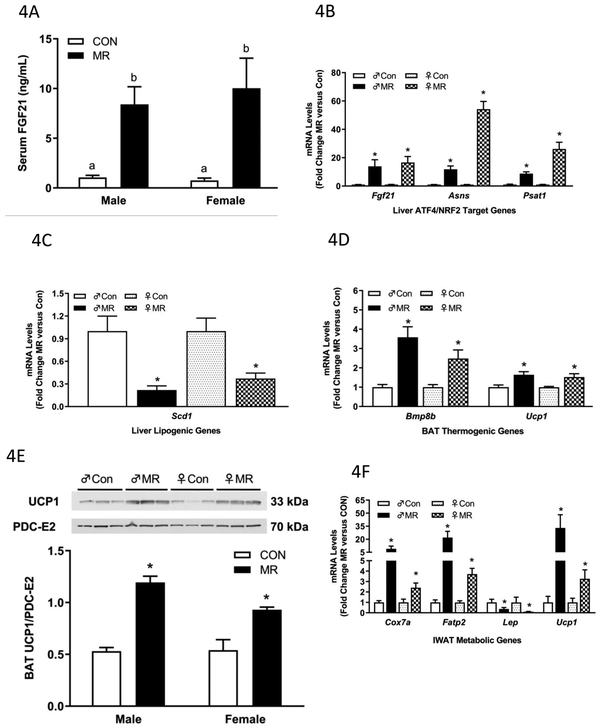
Without exception, the MR diet was fully effective in producing comparable physiological and transcriptional effects of dietary MR in serum and tissues from mature, weight-stable male and female mice (Figs. 4A-4F). For example, after 8 weeks of MR, the diet produced a comparable increase in serum FGF21 in male and female mice (Fig. 4A), and a comparable increase in hepatic Fgf21 mRNA (Fig. 4B). Dietary MR also produced a comparable increase in hepatic ATF4 target genes in male and female mice (Fig. 4B), and a comparable 4- to 5-fold decrease in hepatic Scd1 mRNA between the sexes (Fig. 4C). In these mature mice, dietary MR produced comparable increases in mRNA levels of BAT thermogenic genes, Bmp8b and Ucp1 (Fig. 4D), and UCP1 protein expression was comparably increased in BAT from male and female mice (Fig. 4E). In IWAT, Cox7a, Fatp2, and Ucp1 mRNA were all significantly increased by MR in male and female mice (Fig. 4F). However, the magnitude of the increase in each metabolic gene was significantly greater in males than females (Fig. 4F). The significance of this difference is unknown, although it is worth noting that the diet-induced decrease in Leptin mRNA was comparable in IWAT of male and female mice (Fig. 4F). Aside from this minor deviation, dietary MR produced comparable changes in target genes in liver, BAT, and IWAT of male and female mice.
Figure 4 – Endocrine and Transcriptional Endpoints for Experiment 1.
Serum FGF21 (A) in blood samples collected at end of study. Hepatic gene expression (B,C), brown adipose tissue gene expression (D), and inguinal white adipose tissue (F) gene expression was measured via qPCR or by Western blot (E) for UCP1 expression in brown adipose tissue. The mitochondrial marker, PDC-E2 (pyruvate dehydrogenase dihydrolipoamide acetyltransferase) was used as a loading control for the UCP1 Western blot. Serum FGF21 was measured via ELISA. Con – control; MR – methionine restricted diets; IWAT – inguinal white adipose tissue; BAT – brown adipose tissue; Fgf21 – fibroblast growth factor 21; Asns – asparagine synthase; Psat1 – Phosphoserine Aminotransferase 1; Scd1 – stearoyl CoA desaturase 1; Bmp8b – bone morphogenetic protein 8b; Ucp1 – uncoupling protein 1; Cox7a – cytochrome C oxidase subunit 7A; Lep – Leptin; Fatp2 – fatty acid transport protein 2. Each variable was analyzed using a two way ANOVA with sex and diet as main effects and residual variance used as the error term. All values are expressed as mean ± SEM for 8 mice of each diet x sex. Means for fold-change of gene expression denoted with an asterisk (*) indicates significant difference from Con at p < 0.05. For serum FGF21, means not sharing a common letter differ at p < 0.05.
Discussion
Dietary restriction is a well-documented nutritional approach that improves health and longevity of mammals. It involves either limiting overall food intake to produce CR or dilution of dietary macronutrients, such as protein or individual essential amino acids. CR increases lifespan in pre-clinical models and acutely improves metabolic health in both rodents and humans (23,24). However, long-term weight reduction is rarely maintained after CR because of high rates of recidivism and weight regain due to poor compliance (25,26). Dilution of dietary protein or restriction of individual essential amino acids also improves biomarkers of metabolic health in rodents. These approaches are attractive alternatives for treatment of obesity because they increase EE and limit fat deposition without food restriction (7,27-29).
For example, dietary MR produces a series of transcriptional, physiological, and behavioral responses that reduce fat deposition, enhance insulin sensitivity, reduce circulating lipids, and confer resistance to metabolic disease (7-9,30-36). A major ongoing goal of this work has been to understand how the MR diet produces these effects and translate the well-documented preclinical efficacy of dietary MR into a therapeutic approach for treatment of metabolic disease. A significant caveat of most preclinical work to date is that the MR diet has been studied primarily in male rodents. Given the need for a therapeutic diet that is effective in both males and females, a key objective of the present work was to conduct careful side by side comparisons of the responses of age-matched male and female mice to dietary MR. The rationale for this work stems from recent studies reporting fundamentally different responses to protein dilution (15), methionine deprivation (17), and CR (37) between males and females. Of the three experimental diets, protein dilution (e.g., protein restriction) is most relevant to dietary MR because both diets rely on FGF21 as an endocrine mediator of their weight-reducing and metabolic effects (27,28,38). Dietary MR and protein restriction also produce the same behavioral and physiological responses in male mice, as well as transcriptional effects in liver, WAT and BAT (7,27,28,33,36,39), so it was surprising to see the report that female mice were mostly unresponsive to protein dilution (15). Larson et al. (15) used 8-12 week old C57BL/6J mice and compared the responses of male and female mice to 2 to 4 weeks of protein restriction. In males, protein restriction produced fat loss in both studies while in females, the low protein diet produced no loss of body weight, lean mass, or fat mass (15). The sex-specific differences in response to the low protein diet were attributed in part to a blunting of the diet-induced increase in plasma FGF21 in females and a lack of induction of BAT and IWAT Ucp1 mRNA by the low protein diet (15). Chaumontet et al. (40) conducted a slightly longer protein restriction study with younger female BALB/c mice and found that fat mass was unchanged after 8 weeks of the low protein diet while lean mass was significantly decreased and accounted for essentially all of the body weight loss over this period. And although BAT Ucp1 mRNA was not increased by the low protein diet in this study, energy intake and EE were both increased by the low protein diet (40). The common feature of both studies is that female mice responded to the low protein diet by preserving their fat mass at the expense of lean tissue, and when the diet produced weight loss, lean tissue was the affected tissue component (15,40). In contrast, male mice preserved lean mass on the low protein diet and lost fat mass (15). This sexual dichotomy with respect to body composition is also observed with CR, where females responded to 5-20% reduction in food intake by increasing fat deposition while remaining weight stable (37,41). In contrast, males subjected to 20% CR reduced fat mass to maintain weight stability (37). Collectively, these findings suggest that preservation of fat mass is prioritized in female mice subjected to dietary restriction or CR while male mice prioritize retention of lean mass over fat mass.
In the studies conducted herein, we looked for sex-specific differences in the responses of young, growing mice and adult, weight stable mice to 8 weeks of dietary MR. We examined variables associated with energy balance, nutrient partitioning to lean and fat deposition, and the well-documented endocrine and transcriptional responses in liver, BAT, and IWAT. An initial objective was to establish whether the reported unresponsiveness of young, growing female mice to protein dilution (15,40) would be replicated with dietary MR. We observed two fundamental differences in the way young female mice responded to MR compared to age-matched males. First, female mice on the MR diet deposited less lean tissue than mice on the control diet, while fat deposition in the two groups of mice did not differ. In practical terms, this means that female mice on the MR diet prioritized deposition and retention of fat tissue over lean tissue. In contrast, lean deposition in age-matched male mice on the control and MR diets did not differ, while fat deposition was significantly decreased in the MR group. This finding illustrates that males on the MR diet prioritized deposition and retention of lean tissue at the expense of fat. The other notable difference is that the initial MR-induced during the first 10 days was less robust in females than males. However, when measured after 8 weeks on the diets, dietary MR produced a slightly larger increase in 24h EE in females than males. Most of the transcriptional responses in liver and adipose tissues were comparably affected by the MR diet in young male and female mice. One notable endocrine difference was fasting insulin, but this is probably explained by the significantly higher insulin levels in males compared to females on the control diet. Nevertheless, MR produced a significant decrease in fasting insulin in male and female mice, and a comparable increase in serum FGF21 across sex.
A long-term goal of this work is to develop therapeutic diets that produce clinically-significant weight loss and remediation of components of metabolic disease, so from a translational perspective, the more relevant application of dietary MR will be in adult, weight-stable patients with metabolic dysfunction. These conditions were modeled using 4 month-old mice in the second study. In mice on the control diet, little additional body weight was added in either male or females over the 8-week study. The MR diet produced comparable increases in food intake in each sex while also producing comparable decreases in body weight, lean mass, and fat mass accumulation in male and female mice. Interestingly, the MR-induced increase in EE was larger in female than male mice, but the diet-induced transcriptional responses were fairly comparable across tissues. Collectively, these findings provide convincing evidence that the response of female mice to dietary MR is not compromised compared to male mice and only differs in young growing animals because female mice prioritize fat deposition under nutritional challenges. It seems possible that this sex difference in nutrient partitioning in young mice stems from the greater demands on energy reserves to support reproduction in female mice. However, under our experimental conditions both male and female mice lose body weight when dietary MR is initiated after attainment of adult body size. The lost weight is primarily adipose tissue in both sexes and the transcriptional responses across target tissues provide little evidence for sex-specific molecular responses to the diet. We conclude that if the technical and palatability obstacles could be overcome, application of dietary MR as a therapeutic approach to treat metabolic syndrome would be efficacious in both male and female patients.
What is already known about this subject?
Restriction of dietary methionine to 0.17% reduces adiposity, enhances browning of white adipose tissue, increases energy expenditure, decreases hepatic lipogenic genes, and improves insulin sensitivity.
Methionine restriction and dietary protein dilution produce essentially the same effects on adiposity, browning of white adipose, increases in energy expenditure, decreases in hepatic lipogenic genes, and improvements in insulin sensitivity.
Protein dilution was reported to produce sexually dimorphic effects on the hormonal and metabolic responses to the diet.
Short term methionine deprivation was reported to produce sexually dimorphic metabolic improvements in mice.
What does your study add?
Initiation of dietary methionine restriction in young, growing animals produces sexually dimorphic changes in body composition, with males preserving lean tissue at the expense of fat and females preserving fat at the expense of lean.
The increase in energy expenditure after introduction of methionine restriction was slower to develop in young females than age-matched males, but the metabolic, endocrine, and transcriptional responses to methionine restriction were comparable between sexes after 8 weeks on the diet.
The effects of methionine restriction on energy balance were comparable between sexes when the diet was initiated after attainment of physical maturity (4 months), and metabolic and endocrine responses were also comparable between males and females.
Therefore, the sexually dimorphic effects of dietary methionine restriction are limited to the post-weaning period of growth and pertain only to nutrient partitioning between lean and fat tissue.
Potential of work to change clinical practice
Given that a major goal of our work is to develop therapeutic diets that reduce obesity by reducing dietary methionine, it is important to establish the efficacy of dietary MR in males and females in pre-clinical studies.
Using cohorts of young, growing mice, and mature, weight-stable mice of both sexes, we establish that dietary MR produces sexually dichotomous effects on body composition in young growing mice, but is fully effective in producing robust reductions of fat mass in both sexes of mature mice.
Acknowledgments
This work was supported BY NIH DK-096311 (TWG). This work also made use of the Genomics Core Facility supported by NIH P20-GM103528 (TWG) and NIH 2P30 DK072476. This research project used the Transgenic and Animal Phenotyping core facilities that are supported in part by the NORC (NIH 2P30 DK072476) and by an equipment grant (S10OD023703) from the NIH. LAF was supported by an ADA mentor-based postdoctoral fellowship award (ADA 7-13-MI-05).
Footnotes
Disclosure: The authors have no conflicts of interest to disclose.
Reference List
- 1.Grundy SM, Hansen B, Smith SC Jr., Cleeman JI, Kahn RA. Clinical management of metabolic syndrome: report of the American Heart Association/National Heart, Lung, and Blood Institute/American Diabetes Association conference on scientific issues related to management. Arterioscler Thromb Vasc Biol 2004;24(2):e19–e24. [DOI] [PubMed] [Google Scholar]
- 2.Knowler WC, Barrett-Connor E, Fowler SE et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346(6):393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lager CJ, Esfandiari NH, Subauste AR et al. Roux-En-Y Gastric Bypass Vs. Sleeve Gastrectomy: Balancing the Risks of Surgery with the Benefits of Weight Loss. Obes Surg 2017;27(1):154–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Orentreich N, Matias JR, DeFelice A, Zimmerman JA. Low methionine ingestion by rats extends life span. J Nutr 1993;123(2):269–74. [DOI] [PubMed] [Google Scholar]
- 5.Richie JP Jr., Leutzinger Y, Parthasarathy S, Malloy V, Orentreich N, Zimmerman JA. Methionine restriction increases blood glutathione and longevity in F344 rats. FASEB J 1994;8(15):1302–7. [DOI] [PubMed] [Google Scholar]
- 6.Zimmerman JA, Malloy V, Krajcik R, Orentreich N. Nutritional control of aging. Exp Gerontol 2003;38(1-2):47–52. [DOI] [PubMed] [Google Scholar]
- 7.Malloy VL, Krajcik RA, Bailey SJ, Hristopoulos G, Plummer JD, Orentreich N. Methionine restriction decreases visceral fat mass and preserves insulin action in aging male Fischer 344 rats independent of energy restriction. Aging Cell 2006;5(4):305–14. [DOI] [PubMed] [Google Scholar]
- 8.Perrone CE, Mattocks DA, Jarvis-Morar M, Plummer JD, Orentreich N. Methionine restriction effects on mitochondrial biogenesis and aerobic capacity in white adipose tissue, liver, and skeletal muscle of F344 rats. Metabolism 2009;59:1000–11. [DOI] [PubMed] [Google Scholar]
- 9.Hasek BE, Stewart LK., Henagan TM et al. Dietary methionine restriction enhances metabolic flexibility and increases uncoupled respiration in both fed and fasted states. Am J Physiol Regul Integr Comp Physiol 2010;299:R728–R739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Plaisance EP, Henagan TM, Echlin H et al. Role of ß-adrenergic receptors in the hyperphagic and hypermetabolic responses to dietary methionine restriction. Am J Physiol Regul Integr Comp Physiol 2010;299:R740–R750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Plaisance EP, Greenway FL, Boudreau A et al. Dietary methionine restriction increases fat oxidation in obese adults with metabolic syndrome. J Clin Endocrinol Metab 2011;96(5):E836–E840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morris AA, Kozich V, Santra S et al. Guidelines for the diagnosis and management of cystathionine beta-synthase deficiency. J Inherit Metab Dis 2017;40(1):49–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elshorbagy AK, Valdivia-Garcia M, Mattocks DA et al. Cysteine supplementation reverses methionine restriction effects on rat adiposity: significance of stearoyl-coenzyme A desaturase. J Lipid Res 2011,’52(1): 104–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wanders D, Stone KP, Forney LA et al. Role of GCN2-independent signaling through a non-canonical PERK/NRF2 pathway in the physiological responses to dietary methionine restriction. Diabetes 2016;65(6):1499–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larson KR, Russo KA, Fang Y, Mohajerani N, Goodson ML, Ryan KK. Sex Differences in the Hormonal and Metabolic Response to Dietary Protein Dilution. Endocrinology 2017;158(10):3477–87. [DOI] [PubMed] [Google Scholar]
- 16.Ables GP, Ouattara A, Hampton TG et al. Dietary methionine restriction in mice elicits an adaptive cardiovascular response to hyperhomocysteinemia. Sci Rep 2015;5:8886. doi: 10.1038/srep08886.:8886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu D, Yang SE, Miller BR et al. Short-term methionine deprivation improves metabolic health via sexually dimorphic, mTORC1-independent mechanisms. FASEB J 2018;32(6):3471–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kane AE, Sinclair DA, Mitchell JR, Mitchell SJ. Curr Opin Physiol 2018;6:28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forney LA, Wanders D, Stone KP, Pierse A, Gettys TW. Concentration-dependent linkage of dietary methionine restriction to the components of its metabolic phenotype. Obesity (Silver Spring) 2017;25:730–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wanders D, Forney LA, Stone KP, Hasek BE, johnson WD, Gettys TW. The Components of Age-Dependent Effects of Dietary Methionine Restriction on Energy Balance in Rats. Obesity (Silver Spring) 2018;26(4):740–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wanders D, Burk DH, Cortez CC et al. UCP1 is an essential mediator of the effects of methionine restrictin on energy balance but not insulin sensitivity. FASEB J 2015;29(June 2015):2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stone KP, Wanders D., Calderon LF, Spurgin SB, Scherer PE, Gettys TW. Compromised responses to dietary methionine restriction in adipose tissue but not liver of ob/ob mice. Obesity 2015;23(9):1836–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ravussin E, Redman LM, Rochon J et al. A 2-Year Randomized Controlled Trial of Human Caloric Restriction: Feasibility and Effects on Predictors of Health Span and Longevity. J Gerontol A Biol Sci Med Sci 2015;70(9):1097–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Redman LM, Heilbronn LK, Martin CK et al. Metabolic and behavioral compensations in response to caloric restriction: implications for the maintenance of weight loss. PLoS ONE 2009;4(2):e4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson JW, Konz EC, Frederich RC, Wood CL. Long-term weight-loss maintenance: a meta-analysis of US studies. Am J Clin Nutr 2001;74(5):579–84. [DOI] [PubMed] [Google Scholar]
- 26.Kraschnewski JL, Boan J, Esposito J et al. Long-term weight loss maintenance in the United States. Int J Obes (Lond) 2010;34(11):1644–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laeger T, Henagan TM, Albarado DC et al. FGF21 is an endocrine signal of protein restriction. J Clin Invest 2014;124:3913–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laeger T, Albarado DC, Burke SJ et al. Metabolic Responses to Dietary Protein Restriction Require an Increase in FGF21 that Is Delayed by the Absence of GCN2. Cell Rep 2016;16(3):707–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wanders D, Stone KP, Dille KN, Simon J, Pierse A, Gettys TW. Metabolic responses to dietary leucine restriction involve remodeling of adipose tissue and enhanced hepatic insulin signaling. Biofactors 2015;41:391–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stone KP, Wanders D, Orgeron M, Cortez CC, Gettys TW. Mechanisms of increased in vivo insulin sensitivity by dietary methionine restriction in mice. Diabetes 2014;63:3721–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hasek BE, Boudreau A, Shin J et al. Remodeling the integration of lipid metabolism between liver and adipose tissue by dietary methionine restriction in rats. Diabetes 2013;62:3362–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lees EK, Krol E, Grant L et al. Methionine restriction restores a younger metabolic phenotype in adult mice with alterations in fibroblast growth factor 21. Aging Cell 2014;13(5):817–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghosh S, Wanders D, Stone KP, Van NT, Cortez CC, Gettys TW. A systems biology analysis of the unique and overlapping transcriptional responses to caloric restriction and dietary methionine restriction in rats. FASEB J 2014;28:2577–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wanders D, Ghosh S, Stone K., Van NT., Gettys TW. Transcriptional impact of dietary methionine restriction on systemic inflammation: Relevance to biomarkers of metabolic disease during aging. Biofactors 2013;40:13–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ables GP, Perrone CE, Orentreich D, Orentreich N. Methionine-restricted C57BL/6J mice are resistant to diet-induced obesity and insulin resistance but have low bone density. PLoS ONE 2012;7(12):e51357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perrone CE, Mattocks DA, Plummer JD et al. Genomic and Metabolic Responses to Methionine-Restricted and Methionine-Restricted, Cysteine-Supplemented Diets in Fischer 344 Rat Inguinal Adipose Tissue, Liver and Quadriceps Muscle. J Nutrigenet Nutrigenomics 2012;5(3):132–57. [DOI] [PubMed] [Google Scholar]
- 37.Mitchell SJ, Madrigal-Matute J, Scheibye-Knudsen M et al. Effects of Sex, Strain, and Energy Intake on Hallmarks of Aging in Mice. Cell Metab 2016;23(6):1093–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wanders D, Forney LA, Stone KP, Burk DH, Pierse A, Gettys TW. FGF21 Mediates the Thermogenic and Insulin-Sensitizing Effects of Dietary Methionine Restriction but not its Effects on Hepatic Lipid Metabolism. Diabetes 2017;66:858–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ghosh S, Forney LA, Wanders D, Stone KP, Gettys TW. An integrative analysis of tissue-specific transcriptomic and metabolomic responses to short-term dietary methionine restriction in mice. PLoS ONE 2017;12(5):e0177513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chaumontet C, Azzout-Marniche D, Blais A et al. Low protein and methionine, high starch diets increase energy intake and expenditure, increase FGF21, decrease IGF-1, and has little effect on adiposity in mice. Am J Physiol Regul Integr Comp Physiol 2019;316(February 8):R486–R501. [DOI] [PubMed] [Google Scholar]
- 41.Li X, Cope MB, Johnson MS, Smith DL Jr., Nagy TR. Mild calorie restriction induces fat accumulation in female C57BL/6J mice. Obesity (Silver Spring) 2010;18(3):456–62. [DOI] [PMC free article] [PubMed] [Google Scholar]