Historical species definitions for many prokaryotes, including pathogens, have relied on phenotypic characteristics that are inconsistent with genome evolution. This scenario forces microbiologists and clinicians to face a tradeoff between taxonomic rigor and clinical interpretability. Using the Bacillus cereus group as a model, a conceptual framework for the taxonomic delineation of prokaryotes which reconciles genomic definitions of species with clinically and industrially relevant phenotypes is presented. The nomenclatural framework outlined here serves as a model for genomics-based bacterial taxonomy that moves beyond arbitrarily set genomospecies thresholds while maintaining congruence with phenotypes and historically important species names.
KEYWORDS: Bacillus anthracis, Bacillus cereus, Bacillus cereus group, Bacillus thuringiensis, bioterrorism, foodborne pathogens, phylogenetic analysis, taxonomy
ABSTRACT
The Bacillus cereus group comprises numerous closely related species, including bioterrorism agent B. anthracis, foodborne pathogen B. cereus, and biopesticide B. thuringiensis. Differentiating organisms capable of causing illness or death from those used in industry is essential for risk assessment and outbreak preparedness. However, current species definitions facilitate species-phenotype incongruences, particularly when horizontally acquired genes are responsible for a phenotype. Using all publicly available B. cereus group genomes (n = 2,231), we show that current species definitions lead to overlapping genomospecies clusters, in which 66.2% of genomes belong to multiple genomospecies at a conventional 95 average nucleotide identity (ANI) genomospecies threshold. A genomospecies threshold of ≈92.5 ANI is shown to reflect a natural gap in genome similarity for the B. cereus group, and medoid genomes identified at this threshold are shown to yield resolvable genomospecies clusters with minimal overlap (six of 2,231 genomes assigned to multiple genomospecies; 0.269%). We thus propose a nomenclatural framework for the B. cereus group which accounts for (i) genomospecies using resolvable genomospecies clusters obtained at ≈92.5 ANI, (ii) established lineages of medical importance using a formal collection of subspecies names, and (iii) heterogeneity of clinically and industrially important phenotypes using a formalized and extended collection of biovar terms. We anticipate that the proposed nomenclature will remain interpretable to clinicians, without sacrificing genomic species definitions, which can in turn aid in pathogen surveillance; early detection of emerging, high-risk genotypes; and outbreak preparedness.
INTRODUCTION
Historically, prokaryotic species have been defined using various methods (e.g., phenotypic characterization, 16S rRNA gene sequencing, and DNA-DNA hybridization) (1–3). However, contemporary species delineation practices have migrated to high-throughput, in silico average nucleotide identity (ANI)-based methods (4), for which two genomes belong to the same genomospecies if they share an ANI value above a set threshold (usually 95 ANI) (5). Paradoxically, evolutionary insights provided by ANI-based species delineation can lead to greater taxonomic ambiguity, as species names deeply ingrained in medicine and industry may be inconsistent with genome evolution (6–8). In these cases, microbiologists face a tradeoff: revise the taxonomy to reflect genomic differences, potentially sacrificing clinical interpretability, or continue to use established species names and ignore underlying genomic diversity.
The Bacillus cereus group, also known as B. cereus sensu lato, is one such species complex plagued by taxonomic inconsistencies. Notable members include B. anthracis, the etiological agent of anthrax and renowned bioterrorism agent (9–12); B. cereus sensu stricto, which is commonly regarded as a foodborne pathogen but has been associated with anthrax-like symptoms and other severe infections (13, 14); and B. thuringiensis, a popular industrial biopesticide control agent (15, 16). Phenotypic characteristics used for taxonomic assignment of B. cereus group species (e.g., motility and hemolysis) vary within and among species (1, 2, 17, 18). Furthermore, genomic determinants responsible for some phenotypes are plasmid mediated, such as synthesis of anthrax toxin/capsular proteins (19–22), bioinsecticidal crystal proteins (23–25), and emetic toxin (cereulide) synthetase proteins (26, 27). These traits can be lost, gained, heterogeneous in their presence within a species, or present across multiple species (28–31).
ANI-based genomospecies assignment, however, has done little to alleviate taxonomic ambiguity. An influx of novel B. cereus sensu lato species (three published between 2013 and 2016 [32–34] and nine in 2017 [35]) has relied on variable genomospecies thresholds ranging from 92 to 96 ANI (33–35). This can lead to overlapping genomospecies clusters where some genomes may belong to more than one genomospecies, depending on the threshold used. Further confusion arises when “novel” species encompass established lineages within their genomospecies thresholds. For example, B. paranthracis, a species published in 2017 (35), encompasses the established foodborne pathogen known as emetic “B. cereus” (13, 36–38) within its genomospecies boundaries at a conventional 95 ANI threshold (39).
Current species definitions do not account for species-phenotype incongruences, which can potentially lead to high-consequence misclassifications of an isolate’s virulence potential. For example, clinical diagnostics used to rule out the presence of B. anthracis (1, 2) may incorrectly exclude an anthrax-causing strain exhibiting phenotypic characteristics associated with “B. cereus” as the cause of illness (30, 40–42). Additionally, the ability to cause anthrax is attenuated in B. anthracis strains which lack genes required for anthrax toxin and capsule formation (43). The problem at hand requires the construction of an ontological framework which is accurate in terms of its adherence to widely accepted genomic and taxonomic definitions of bacterial genomospecies while still being informative, intuitive, and actionable to those in public health and industry. Here, we leverage all publicly available assembled B. cereus group genomes (n = 2,231) to construct a phylogenomically informed taxonomic framework with the flexibility to account for phenotypes of interest to those in public health and industry.
RESULTS
Current species definitions cannot reliably differentiate B. anthracis from neighboring lineages.
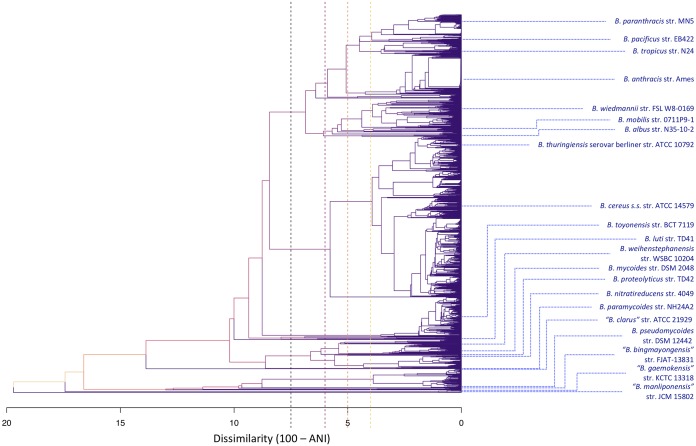
The practice of calculating ANI values between a genome of interest and the genomes of known B. cereus group species type strains (see Table S1 in the supplemental material) (33–35, 39) and using the widely accepted threshold of 95 ANI (5) as a hard genomospecies cutoff produced nonoverlapping genomospecies clusters for Bacillus albus, “B. bingmayongensis,” B. cytotoxicus, “B. gaemokensis,” B. luti, “B. manliponensis,” B. nitratireducens, B. paramycoides, B. proteolyticus, B. pseudomycoides, and B. toyonensis (Table S2). No genomes assigned to these genomospecies shared ≥95 ANI with any genomes assigned to a different genomospecies (Fig. 1 and 2A1). However, several type strain-centric genomospecies overlapped, including clusters formed by the type strains of (i) B. cereus sensu stricto and B. thuringiensis and (ii) B. mycoides and B. weihenstephanensis, as has been documented previously (Fig. 1 and 2A1; Table S2) (29, 34, 44). The type strains of B. mobilis and B. wiedmannii also produced overlapping genomospecies in which a genome could share ≥95 ANI with both species type strains (Fig. 1 and 2A1; Table S2). The largest source of ambiguity, however, stemmed from B. anthracis and neighboring lineages, as the genomospecies cluster formed by the B. anthracis reference genome overlapped with those of B. pacificus, B. paranthracis, and B. tropicus (Fig. 1 and 2A1; Table S2).
FIG 1.
Dendrogram constructed using symmetric pairwise average nucleotide identity (ANI) dissimilarities calculated between 2,218 B. cereus group genomes from NCBI’s RefSeq database with N50 of >20 kbp (i.e., in Materials and Methods) and the average linkage hierarchical clustering method implemented in the hclust function in R. Blue tip labels denote the location of species type strain/reference genomes in the dendrogram, while tree height corresponds to ANI dissimilarity. Branch colors correspond to branch height within the tree. Dashed vertical lines appear at dissimilarities of 7.5, 6, 5, and 4, which correspond to ANI thresholds of 92.5, 94, 95, and 96, respectively (from left to right in order of appearance along the x axis).
FIG 2.
Weighted undirected graphs constructed using symmetric pairwise average nucleotide identity (ANI) values calculated between 2,218 B. cereus group genomes from NCBI’s RefSeq database with N50 of >20 kbp (i.e., in Materials and Methods). Nodes represent individual genomes, while weighted edges connect each pair of genomes with a mean ANI value of ≥95 (A) and ≥92.5 (B), where edge weight corresponds to the mean ANI value of the pair. Nodes (i.e., genomes) are colored by (i) closest matching type strain genome or (ii) closest matching medoid genome of clusters formed at the respective ANI value. Graphs were constructed using the graphout layout algorithm implemented in R’s igraph package, using 1 million iterations and a charge of 0.02.
Species type strain/NCBI RefSeq reference genomes for 18 currently recognized B. cereus group species and three published putative species used in this study. Download Table S1, XLSX file, 0.01 MB (12KB, xlsx) .
Copyright © 2020 Carroll et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Proportion of genomes assigned to genomospecies X (rows) using the species type strain/reference genome at a threshold of 95 ANI which share ≥95 ANI with one or more genomes assigned to genomospecies Y (columns). Download Table S2, XLSX file, 0.01 MB (14.4KB, xlsx) .
Copyright © 2020 Carroll et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
The species overlap problem persisted at 95 ANI when medoid genomes were used to construct genomospecies clusters (Fig. 2A2; Table S3). All genomospecies which were nonoverlapping when type strains were used (e.g., B. pseudomycoides and B. toyonensis) remained nonoverlapping, except for B. proteolyticus (Fig. 2A2; Table S3). All overlapping genomospecies continued to produce multispecies classifications at 95 ANI, albeit at a lower rate than type strain-centric clusters: 405 (18.2%) and 1,478 (66.2%) genomes were assigned to 2 or more medoid- or type strain-centric genomospecies, respectively (Fig. 2A).
Proportion of genomes assigned to genomospecies X (rows) using the medoid genome at a threshold of 95 ANI which share ≥95 ANI with one or more genomes assigned to genomospecies Y (columns). Download Table S3, XLSX file, 0.02 MB (19.2KB, xlsx) .
Copyright © 2020 Carroll et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Genomic elements responsible for anthrax, emetic, and insecticidal toxin production exhibit heterogeneous presence in multiple species using current genomospecies definitions.
Additional nomenclatural discrepancies arise when a trait of interest is plasmid encoded, such as anthrax toxin genes cya (edema factor encoding), lef (lethal factor encoding), and pagA (protective antigen encoding) (45): 93 of 241 (38.6%) genomes most closely resembling the B. anthracis reference genome at ≥95 ANI did not possess anthrax toxin genes (Fig. 3A and B; Table S4). Notably, isolates which most closely resemble B. anthracis by current species definitions (i.e., ≥95 ANI), despite lacking anthrax toxin-encoding genes, do not appear to be uncommon. Such strains have been isolated from diverse environments (e.g., soil, animal feed, milk, spices, egg whites, and baby wipes) and from six continents, plus the International Space Station (Table S4). The classification of these isolates as B. anthracis could lead to incorrect assumptions of their anthrax-causing capabilities.
FIG 3.
Maximum likelihood phylogenies of 2,218 B. cereus group genomes with N50 of >20 kbp. Tip and branch labels are colored by genomospecies assignment using medoid genomes of genomospecies clusters formed at the widely used genomospecies threshold of 95 ANI (clusters are arbitrarily numbered) (A) and presence (colored) and absence (gray) of anthrax toxin genes cya, lef, and pagA (B); cereulide synthetase-encoding cesABCD (C); and one or more previously described Cry or Cyt insecticidal toxin-encoding genes (D). Phylogenies were constructed using core SNPs identified in 79 single-copy orthologous gene clusters present in 2,231 B. cereus group genomes. The type strain of “B. manliponensis” (i.e., the most distantly related member of the group) was treated as an outgroup on which each phylogeny was rooted. Virulence genes (cya, lef, and pagA and cesABCD) were detected using BTyper version 2.3.2 (default thresholds), while insecticidal toxin-encoding genes were detected using BtToxin_scanner version 1.0 (default settings; presence and absence of high-confidence, previously known Cry- and Cyt-encoding genes are shown, with predicted putative novel insecticidal toxin-encoding genes excluded).
Genomes most closely resembling the B. anthracis strain Ames species reference genome with ≥95 ANI in which anthrax toxin genes cya, lef, and pagA were not detected. Download Table S4, XLSX file, 0.02 MB (19KB, xlsx) .
Copyright © 2020 Carroll et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Importantly, isolates which display phenotypic characteristics associated with “B. cereus” (e.g., motility and gamma bacteriophage resistance) can cause anthrax (2, 30, 31, 40–42). Despite the assertion that it is a clonal species with low diversity (46–48), the B. anthracis genomospecies cluster formed at 95 ANI encompasses lineages which fall outside the one most commonly associated with anthrax illness (Fig. 3A and B). At a 95 ANI genomospecies threshold, three of seven genomes deposited in RefSeq as anthrax-causing “B. cereus” most closely resembled the B. anthracis reference genome (Fig. 3A and B; Table S5), while also sharing ≥95 ANI with the B. paranthracis type strain genome (Table S5). The remaining four anthrax-causing “B. cereus” genomes most closely resembled the B. tropicus type strain, shared ≥95 ANI with the B. paranthracis type strain, and shared between 94 and 95 ANI with the B. anthracis species reference genome (Table S5). The separation of anthrax-causing “B. cereus” genomes into two genomospecies at 95 ANI was maintained when medoid genomes were used (Fig. 2A2; Table S5). As such, several anthrax-causing “B. cereus” strains are technically still B. anthracis at 95 ANI (Fig. 3A and B) and despite having a mosaic of phenotypic characteristics attributed to “B. cereus” and B. anthracis.
Genomes submitted to NCBI’s RefSeq database as anthrax-causing “B. cereus.” Download Table S5, XLSX file, 0.01 MB (13.6KB, xlsx) .
Copyright © 2020 Carroll et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Similar issues plague emetic “B. cereus,” designated as such by its ability to produce cereulide, a toxin responsible for foodborne illness characterized by vomiting symptoms (13, 38, 49). At 95 ANI, all 30 emetic “B. cereus” genomes most closely resembled the B. paranthracis type strain, were confined to a single medoid-centric genomospecies, and were interspersed among genomes which lacked cereulide synthetase-encoding genes cesABCD (Fig. 3A and C; Table S6). cesABCD were detected in five genomes representing two additional medoid-based genomospecies at 95 ANI (Fig. 3A and C; Table S6). One contained the type strains of B. weihenstephanensis and B. mycoides, which is unsurprising considering that cereulide-producing B. weihenstephanensis has been isolated in rare cases (28, 50). However, two genomes categorized previously as emetic “B. weihenstephanensis” belonged to a completely separate genomospecies at 95 ANI (Fig. 3A and C; Table S6).
Genomes used in this study (n = 2,231). Download Table S6, XLSX file, 0.5 MB (508.7KB, xlsx) .
Copyright © 2020 Carroll et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
The Cry and Cyt insecticidal proteins associated with B. thuringiensis (i.e., Bt toxins), which can be plasmid mediated, face similar issues, as B. thuringiensis has historically been defined by its ability to produce insecticidal toxins (e.g., Cry and Cyt toxins) (51). However, genes encoding known insecticidal toxins were detected in nine of 21 B. cereus group type strain-centric genomospecies at 95 ANI (Fig. 3A and D). These results are consistent with previous findings, as Bt toxin production has been attributed to numerous lineages (29, 51, 52).
ANI-based comparisons to medoid genomes using a lowered genomospecies threshold of ≈92.5 eliminate the species overlap problem.
Numerous bacterial genomospecies have showcased a breakpoint in genome similarity which is close to 95 ANI (5); however, ANI values among a significant proportion of B. cereus group genomes, particularly B. anthracis and neighboring lineages, fall within the 93 to 95 ANI range, with a breakpoint occurring at ≈92.5 ANI (Fig. 4; Fig. S1). Using a hard 92.5 ANI threshold for B. cereus group genomospecies assignment, rather than 95, nearly eliminates the species overlap problem: only six of 2,231 genomes (0.269%) were assigned to 2 or more medoid-based genomospecies (Fig. 2B2; Table S7), compared to 18.2% and 66.2% of genomes assigned to multiple genomospecies at 95 ANI when medoid genomes and species type strain/reference genomes were used, respectively (Fig. 2; Tables S2 and S3). Eighteen genomospecies were present at a 92.5 ANI threshold, compared to 36 medoid-centric genomospecies at 95 ANI (Fig. 3A and Fig. 5; Tables S3 and S7). Notably, at 92.5 ANI, seven genomospecies did not possess type strains of any published species (Table S7), indicating that putative novel genomospecies may be present. While one of these genomospecies has recently been proposed as novel species “B. clarus” (53), the remaining six are uncharacterized (Table S8).
FIG 4.
Histogram of pairwise average nucleotide identity (ANI) values calculated between 2,231 B. cereus group genomes downloaded from NCBI’s RefSeq database. FastANI version 1.0 was used to calculate all pairwise ANI values. For histograms colored according to closest species type strain/reference genome at a conventional ≥95 ANI threshold, or histograms showing pairwise ANI values calculated between genomes meeting additional quality thresholds, see Fig. S1 and S2, respectively.
FIG 5.
Maximum likelihood phylogeny of 2,218 B. cereus group genomes with N50 of >20 kb. Tip and branch labels are colored by genomospecies assignment using medoid genomes of genomospecies clusters formed at the proposed genomospecies threshold of 92.5 ANI. Phylogeny was constructed using core SNPs identified in 79 single-copy orthologous gene clusters present in 2,231 B. cereus group genomes. The type strain of “B. manliponensis” (i.e., the most distantly related member of the group) was treated as an outgroup on which the phylogeny was rooted.
Histograms of pairwise ANI values between 2,218 B. cereus group genomes with N50 of >20 kbp. For each of 18 published species and 3 proposed putative species, ANI values calculated between two genomes for which one or more genome(s) shared ≥95 ANI with the species type strain or reference genome are colored in pink. ANI values calculated between two genomes for which both genomes shared <95 ANI with the species type strain/reference genome are colored in gray. See Table S1 for details regarding species type strain genomes. Download FIG S1, PDF file, 0.5 MB (541.8KB, pdf) .
Copyright © 2020 Carroll et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Proportion of genomes assigned to genomospecies X (rows) using the medoid genome at a threshold of 92.5 ANI which share ≥92.5 ANI with one or more genomes assigned to genomospecies Y (columns). Download Table S7, XLSX file, 0.01 MB (15.2KB, xlsx) .
Copyright © 2020 Carroll et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Genomes belonging to one of six previously unpublished genomospecies clusters formed at ANI of ≥92.5 (i.e., putative novel genomospecies). Download Table S8, XLSX file, 0.01 MB (15.1KB, xlsx) .
Copyright © 2020 Carroll et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Sample of histograms constructed using pairwise ANI values calculated between B. cereus group genomes meeting various quality thresholds. Because distribution breakpoints and shape were robust to the exclusion of genomes at all tested thresholds, the six histograms shown represent pairwise ANI values calculated between raw genomes downloaded directly from NCBI’s RefSeq database (i.e., the least stringent quality thresholds, with no filtering criteria employed; n = 2,231 genomes) (A), genomes with N50 of >20 kb (the set of genomes from which medoid genomes were selected; n = 2,218) (B), genomes with N50 of >0 and no contigs assigned to a domain other than Bacteria (n = 1,291) (C), genomes with N50 of >20 kb and no contigs assigned to a domain other than Bacteria (n = 1,289) (D), genomes with N50 of >0 and no contigs assigned to a genus outside Bacillus (n = 1,074) (E), and genomes with N50 of >100 kb and no contigs assigned to a genus outside Bacillus (i.e., the most stringent quality thresholds tested; n = 998) (F). Download FIG S2, PDF file, 0.1 MB (144.5KB, pdf) .
Copyright © 2020 Carroll et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
DISCUSSION
When applied to bacteria, the concept of “species” is notoriously ambiguous, particularly in cases where it is intertwined with a phenotype, and even more so when that phenotype is an established component of the medical or industrial lexicon. Taxonomic definitions based on phenotype lack nuance in the omics era, as they ignore underpinning genomic diversity which can be leveraged to improve assessment of an isolate’s pathogenic potential or industrial utility. Furthermore, taxonomy based on phenotype can be ambiguous—and even misleading—when a trait is lost, gained, or not widespread throughout a lineage. A notable example is provided by botulinum neurotoxin (BoNT)-producing species, to which the Clostridium botulinum label has historically been applied, despite multiple genomospecies exhibiting BoNT production capabilities (6). Adherence to a nomenclature just for the sake of taxonomic rigor, however, can be equally problematic when a lineage has deep roots in medicine or industry. Shigella spp. and Escherichia coli, for example, constitute a single genomospecies but are considered to be distinct entities, despite genomic inconsistencies reflected in their nomenclature (7, 54, 55).
An ideal taxonomy should be interpretable, without sacrificing the resolution provided by contemporary technologies. Several publications have appended the term “biovar” to species names to denote isolates which exhibit interesting phenotypes (e.g., anthrax-causing “B. cereus” as B. cereus biovar anthracis and Cry-producing B. wiedmannii as B. wiedmannii biovar thuringiensis) (41, 52). We therefore propose a taxonomic framework consisting of (i) an amended collection of genomospecies, corresponding to resolvable genomospecies obtained at ≈92.5 ANI; (ii) a formal collection of subspecies, which account for established lineages of medical importance; and (iii) a formalized and extended collection of biovars, which account for phenotypic heterogeneity (Fig. 6). Note that a recently proposed “genomovar” framework for B. cereus sensu stricto/B. thuringiensis (56) is not adopted here, due to the lack of genomospecies boundaries between their type strains (shown here and elsewhere [33–35], including the paper proposing the framework [56]), as well as the lack of a standardized species definition for B. thuringiensis (B. thuringiensis has been used to refer to any B. cereus group species capable of producing Bt toxins [29] or to the genomospecies formed by the B. thuringiensis type strain genome [56]).
FIG 6.
Taxonomic hierarchy for the proposed B. cereus group nomenclature. Taxonomic levels are listed in the left margin, with levels which are optional/not applicable to all organisms denoted as such. Rounded boxes shaded in light green correspond to possible taxonomic designations at their respective level, while blue boxes correspond to requirements that an isolate and/or its genome must meet to be assigned that designation. Possible forms which the final taxonomic assignment can take can be found in the gray box at the bottom of the chart.
Proposed taxonomic nomenclature. (A) Genomospecies.
The B. cereus group currently consists of eight published genomospecies (designated I to VIII), four previously proposed genomospecies (designated ix to xii), and six putative novel genomospecies (designated xiii to xviii) (Fig. 5). A genome belongs to a genomospecies if it shares ⪆92.5 ANI with the genomospecies medoid genome (see Table S7 in the supplemental material). Due to the resolvability of genomospecies at this threshold, it follows that (i) a genome does not belong to a genomospecies if it shares ⪅92.5 ANI with the genomospecies medoid genome, (ii) two genomes belong to the same genomospecies if they share ⪆92.5 ANI with each other, and (iii) two genomes belong to different genomospecies if they share ⪅92.5 ANI with each other (i.e., in practice, a genomospecies medoid genome does not need to be used for genomospecies assignment, but rather any genome of known genomospecies; see Tables S6 and S7 for a comprehensive list of genomospecies assignments). When written, genomospecies names immediately follow the genus name (Bacillus or B.) and are italicized and lowercase.
Published genomospecies.
(I) Bacillus pseudomycoides. The B. pseudomycoides genomospecies contained 111 genomes, including the genome of species type strain B. pseudomycoides strain DSM 12442. All genomes previously classified as B. pseudomycoides relative to the type strain at a 95 ANI threshold remain in this genomospecies, and no additional genomes belong to the genomospecies. As such, this genomospecies remains consistent with its previous classification, and its name remains unchanged.
(II) Bacillus paramycoides. The B. paramycoides genomospecies contained six genomes, including the genome of species type strain B. paramycoides strain NH24A2. All genomes previously classified as B. paramycoides relative to the type strain at a 95 ANI threshold remain in this genomospecies, and no additional genomes belong to the genomospecies. As such, this genomospecies remains consistent with its previous classification, and its name remains unchanged.
(III) Bacillus mosaicus. The B. mosaicus genomospecies contained 722 genomes, including type strains and reference genomes of species formerly known as B. albus (now B. mosaicus strain N35-10-2), B. anthracis (now B. mosaicus subsp. anthracis strain Ames; see “Subspecies” and “Biovars” below), B. mobilis (now B. mosaicus strain 0711P9-1), B. pacificus (now B. mosaicus strain EB422), B. paranthracis (now B. mosaicus strain MN5), B. tropicus (now B. mosaicus strain N24), and B. wiedmannii (now B. mosaicus strain FSL W8-0169). Additionally, all members of the lineage formerly known as emetic “B. cereus” belong to B. mosaicus (see “Subspecies” and “Biovars” below). While the species formerly known as B. anthracis is the oldest described former species in this group, it is not proposed as the genomospecies name, as doing so could lead to incorrect assumptions of an isolate’s anthrax-causing potential. As such, the proposed genomospecies name (mosaicus) is chosen to reflect the diversity of lineages and phenotypes present among members of this genomospecies. All genomes previously assigned to the abovementioned former species using their respective type strain or reference genomes at a 95 ANI threshold belong to B. mosaicus.
(IV) Bacillus cereus sensu stricto. The B. cereus sensu stricto genomospecies contained 949 genomes, including those of type strains B. cereus sensu stricto (B. cereus sensu stricto strain ATCC 14579) and former species B. thuringiensis (now B. cereus sensu stricto serovar Berliner biovar Thuringiensis strain ATCC 10792; see “Biovars” below). B. cereus sensu stricto was chosen as the genomospecies name, with Thuringiensis proposed as a biovar to account for phenotypic heterogeneity within B. cereus sensu stricto, as well as the presence of insecticidal toxins in other genomospecies (see “Biovars” below). All genomes previously assigned to the species B. cereus sensu stricto and former species B. thuringiensis at a 95 ANI threshold using these type strains belong to B. cereus sensu stricto.
(V) Bacillus toyonensis. The B. toyonensis genomospecies contained 230 genomes, including the type strain of B. toyonensis (B. toyonensis strain BCT-7112). All genomes previously classified as B. toyonensis relative to the type strain at a 95 ANI threshold remain in this genomospecies, and no additional genomes belong to the genomospecies. As such, this genomospecies remains consistent with its previous classification, and its name remains unchanged.
(VI) Bacillus mycoides. The B. mycoides genomospecies contained 164 genomes, including the type strain of B. mycoides (B. mycoides strain DSM 2048), and former species B. nitratireducens (now B. mycoides strain 4049), B. proteolyticus (now B. mycoides strain TD42), and B. weihenstephanensis (now B. mycoides strain WSBC 10204). Additionally, all members of the lineages formerly known as emetic B. weihenstephanensis belong to B. mycoides (see “Biovars” below). B. mycoides was selected as the genomospecies name, as it is the oldest of the published former species described in this cluster (and remains consistent with taxonomic changes recently proposed by others [44]). All genomes previously assigned to the abovementioned species using their respective type strain or reference genomes and a 95 ANI threshold belong to B. mycoides.
(VII) Bacillus cytotoxicus. The B. cytotoxicus genomospecies contained 14 genomes, including the type strain of B. cytotoxicus (B. cytotoxicus strain NVH 391-98). All genomes previously classified as B. cytotoxicus relative to the type strain at a 95 ANI threshold remain in this genomospecies, and no additional genomes belong to the genomospecies. As such, this genomospecies remains consistent with its previous classification, and its name remains unchanged.
(VIII) Bacillus luti. The B. luti genomospecies contained nine genomes, including the type strain of B. luti (B. luti strain TD41). All genomes previously classified as B. luti relative to the type strain at a 95 ANI threshold remain in this genomospecies, and no additional genomes belong to the genomospecies. As such, this genomospecies remains consistent with its previous classification, and its name remains unchanged.
Previously proposed putative genomospecies.
The following putative B. cereus group genomospecies which have been proposed previously remain unchanged: (IX) “B. bingmayongensis” (including type strain “B. bingmayongensis” strain FJAT-13831), (X) “B. gaemokensis” (including type strain “B. gaemokensis” strain KCTC 13318), (XI) “B. manliponensis” (including type strain “B. manliponensis” strain JCM 15802), and (XII) “B. clarus” (including type strain “B. clarus” strain ATCC 21929).
Putative novel genomospecies.
Six putative genomospecies (xiii to xviii [Table S8]) have not been proposed as novel genomospecies. Future novel B. cereus group genomospecies should (i) share <92.5 ANI with all B. cereus group genomes and (ii) share ≥97% 16S rRNA gene similarity with known B. cereus group species (a definition used in previous studies [35]).
(B) Subspecies.
The following subspecies are proposed to ensure that the medically important lineages formerly known as B. anthracis and emetic “B. cereus” remain interpretable. When written, subspecies names are italicized and lowercase and can optionally (i) be appended to the species name, after the nonitalicized delimiter “subspecies” or “subsp.,” prior to a serotype designation (if applicable); or (ii) follow the genus name (Bacillus or B.) directly, with the species name omitted, prior to a serotype designation (if applicable).
-
a.
Bacillus mosaicus subsp. anthracis (can be written as B. mosaicus subsp. anthracis; B. anthracis) refers to the comparatively clonal lineage of former species B. anthracis commonly associated with anthrax illness. Isolates which are assigned to this subspecies (i) exhibit distinguishing phenotypic characteristics (e.g., lack of motility and lack of hemolysis on sheep red blood cell [RBC] agar) associated with the classical definition of B. anthracis as outlined in the Bacteriological Analytical Manual (BAM) chapter on B. cereus (2) and/or (ii) share ⪆99.9 ANI with former species reference genome B. anthracis strain Ames (now B. mosaicus subsp. anthracis; RefSeq accession no. GCF_000007845.1), a threshold previously identified for this lineage (5) which was replicated here. The use of the term “subspecies anthracis” does not indicate whether an isolate produces anthrax toxin or possesses the machinery required for anthrax toxin synthesis (see “biovar Anthracis” below).
-
b.
Bacillus mosaicus subsp. cereus (can be written as B. mosaicus subsp. cereus; B. cereus) refers to the lineage formerly known as emetic “B. cereus.” All genomes possessing cereulide synthetase genes (cesABCD) which did not belong to the B. mycoides species cluster (see “Genomospecies” above) shared ≥97.5 ANI with the emetic reference strain formerly known as B. cereus strain AH187 (now B. mosaicus subsp. cereus biovar Emeticus; RefSeq accession no. GCF_000021225.1). As such, isolates assigned to this subspecies (i) produce cereulide and belong to the species B. mosaicus, (ii) possess cesABCD and belong to the species B. mosaicus, and/or (iii) share ⪆97.5 ANI with emetic reference genome B. cereus strain AH187 (now B. mosaicus subsp. cereus biovar Emeticus; RefSeq accession no. GCF_000021225.1). The use of the term “subspecies cereus” does not indicate whether an isolate produces cereulide or possesses the machinery required for cereulide synthesis (see “Biovar Emeticus” below).
(C) Biovars.
The following biovars are proposed to account for phenotypes of clinical and industrial importance which can be distributed across species and heterogeneous in their appearance in individual lineages. While phenotypic evidence of a trait is ideal, biovars can be predicted at the genomic level. When written, (i) the first letter of the biovar is capitalized; (ii) the biovar name is not italicized; (iii) the biovar is appended to the end of a species, subspecies (if applicable), or serotype name (if applicable), following the nonitalicized delimiter “biovar”; (iv) if applicable, multiple biovars follow the nonitalicized, plural delimiter “biovars,” are listed in alphabetical order, and are each separated by a comma and a single space; (v) biovar(s) may follow the genus name (Bacillus or B.) directly, with the species, subspecies (if applicable), and serotype (if applicable) names omitted
-
a.
Biovar Anthracis is applied to an isolate (i) known to produce anthrax toxin (preferred) and/or (ii) known to possess anthrax toxin-encoding genes cya, lef, and pagA. Capsular genes (e.g., cap, has, and bps) (21, 22, 57) are deliberately excluded from this definition as a conservative measure (i.e., to avoid cases in which an isolate might cause anthrax via a previously unknown capsule). Examples include B. mosaicus subsp. anthracis biovar Anthracis (i.e., anthrax-causing members of the “clonal” lineage often associated with anthrax disease; can be written as B. anthracis biovar Anthracis or B. Anthracis); B. mosaicus biovar Anthracis (i.e., anthrax-causing lineages formerly known as “anthrax-causing B. cereus”; can be written as B. Anthracis).
-
b.
Biovar Emeticus is applied to an isolate known to produce cereulide (preferred) and/or to possess cereulide synthetase-encoding genes (cesABCD). Examples include B. mosaicus subsp. cereus biovar Emeticus (i.e., cereulide-producing lineages formerly known as emetic “B. cereus”; can be written as B. cereus biovar Emeticus or B. Emeticus) and B. mycoides biovar Emeticus (i.e., cereulide-producing lineages formerly known as “emetic B. weihenstephanensis”; can also be written as B. Emeticus).
-
c.
Biovar Thuringiensis can be applied to an isolate known to produce one or more Bt toxins (e.g., Cry, Cyt, or Vip toxins; preferred) and/or to possess Bt toxin-encoding genes. Examples include B. mosaicus biovar Thuringiensis and B. cereus sensu stricto biovar Thuringiensis (both of which can be written as B. Thuringiensis).
The proposed taxonomy offers numerous advantages. Most importantly, it is consistent; it provides an explicit, standardized framework for taxonomic classification using genomic and/or phenotypic methods, and it resolves previous nomenclatural ambiguities. Second, the proposed taxonomy is backwards compatible with important medical and industrial taxonomic definitions. For example, any B. cereus group isolate capable of producing Bt toxins can be referred to as B. Thuringiensis, which is equivalent to the traditional species definition (29). All isolates capable of producing anthrax toxin can be referred to as B. Anthracis, while members of the “clonal” anthrax lineage remain B. anthracis (using subspecies notation). Finally, the proposed taxonomy is flexible and can be extended to account for additional lineages or phenotypes through the adoption of novel subspecies or biovars, respectively. For example, biovars can be proposed to describe B. cereus group members capable of causing diarrheal foodborne disease (i.e., biovar Cereus), as this disease involves multiple toxins and is not fully understood (58). The nomenclature proposed here not only provides a standardized framework for taxonomic classification which accounts for both phylogenomic diversity and phenotypic heterogeneity, but also serves as a model taxonomic framework which moves beyond arbitrary genomospecies thresholds while maintaining historical congruence.
MATERIALS AND METHODS
Acquisition and initial characterization of Bacillus cereus group genomes.
All genomes in the NCBI RefSeq Assembly database (59) which were submitted as one of 18 published B. cereus group species (35) were downloaded, along with the type strain genomes of three proposed effective B. cereus group species (60–62) (n = 2,231, accessed 19 November 2018) (see Tables S1 and S6 in the supplemental material). QUAST version 4.0 (63) was used to assess the quality of each genome, and BTyper version 2.3.2 (31) was used to detect B. cereus group virulence genes in each genome, using default minimum amino acid sequence identity and coverage thresholds (50% and 70%, respectively) (Table S6) (31, 64). Prokka version 1.12 (65) was used to annotate each genome, and the resulting coding sequences were used as input for the command-line implementation of BtToxin_scanner version 1.0 (BtToxin_scanner2.pl), which was used to identify Bt toxin genes in each genome using default settings (66).
Calculation of pairwise ANI values, hierarchical clustering, and medoid genome identification.
FastANI version 1.0 (5) was used to calculate ANI values between each of 2,231 genomes (4,977,361 comparisons). To ensure that the breakpoints and shape of the distribution of pairwise ANI calculations were robust, genomes which (i) fell below various N50 thresholds (i.e., ≤10 kbp, 20 kbp, 50 kbp, and 100 kbp) and/or (ii) contained any contigs classified in domains other than Bacteria, phyla other than Firmicutes, and/or genera other than Bacillus using Kraken version 2.0.8-beta (67, 68) and the complete standard Kraken database (accessed 6 August 2019) were removed (Fig. S2). For medoid genome identification (described below), all genomes with N50 of >20 kbp in the original set of 2,231 RefSeq genomes were used in subsequent steps (n = 2,218) (Table S6 and Fig. S2).
The resulting pairwise ANI values were used to construct a similarity matrix, SANI, using R version 3.6.0 (69) and the reshape2 package (70) as follows, where n = 2,218:
Let g1, g2, … gn be a set of n genomes, denoted by G (G = {g1, g2, … gn}). Similarity function ANI(gi, gj) denotes the ANI value shared by query and reference genomes gi and gj, respectively, where ANI: G × G→[0,100].
Similarity matrix SANI can be defined as SANI = (sij); sij = ANIij = ANI(gi, gj).
Similarity matrix SANI was converted to a dissimilarity matrix, DANI, as follows, where J denotes an n × n matrix where each element is equal to 1: DANI = 100J −SANI.
ANI as a similarity function is not symmetric [i.e., for all gi, gj, ANI(gi, gj) ≠ ANI(gj, gi)], as minor differences between corresponding values in the upper and lower triangles of DANI existed: max[d(gi, gj), d(gj, gi)] = 0.504; min[d(gi, gj), d(gj, gi)] = 0; mean[d(gi, gj), d(gj, gi)] = 0.056; median[d(gi, gj), d(gj, gi)] = 0.046.
As such, DANI is not a symmetric matrix (i.e., DANI ≠ DANIT). To coerce DANI to a symmetric matrix, , the following transformation was applied: .
The hclust function in R’s stats package was used to perform average linkage hierarchical clustering, using as the dissimilarity structure, and the resulting dendrogram was annotated using the ggplot2 (71), dendextend (72), and viridis (73) packages. Dendrogram clusters formed at various species thresholds (denoted here by Td, where Td = [5, 7.5], corresponding to ANI values of 95 and 92.5, respectively) were obtained by treating lineages which coalesced prior to Td as members of the same cluster (i.e., genomospecies) and those which did not as members of different clusters. Medoid genomes were identified within each cluster at each threshold, using the pam function in R’s cluster package (74) and as a dissimilarity structure, where medoid genome is defined as
where d(gi, gj) = 100 − ANI(gi, gj).
To construct a graph with each of 2,218 genomes represented as nodes and ANI values represented as weighted edges, was converted to a symmetric similarity matrix, , as follows:
The igraph (75) package in R was used to construct each graph, with treated as an adjacency matrix, and edges with weights (i.e., ANI values) less than a similarity threshold Ts (i.e., Ts = [92.5, 95]) removed.
Genomospecies assignment.
FastANI version 1.0 was used to assign each of 2,231 B. cereus group genomes to a genomospecies, using (i) species reference/type strain genomes (n = 21) (Table S1) and medoid genomes identified at (ii) 95 ANI (n = 36) (Table S3) and (iii) 92.5 ANI (n = 18) (Table S7) as reference genomes for each of three separate runs.
Phylogeny construction.
Amino acid sequences of protein-encoding features produced by Prokka were used as input for OrthoFinder version 2.3.3 (76). Single-copy orthologous clusters (i.e., genes) present in all 2,231 genomes were identified using an iterative approach, in which OrthoFinder was used to identify single-copy genes core to n of the 2,231 genomes, sampled randomly without replacement, where n = 30 or n = 11 for 74 and 1 (the remainder) iteration(s), respectively. The union of single-copy genes present in all n genomes in each random sample of genomes was then queried again using OrthoFinder, which identified a total of 79 single-copy genes core to all 2,231 genomes. Nucleotide sequences of each of the 79 single-copy core genes were aligned using PRANK v.170427 (77). The resulting alignments were concatenated, and SNP-sites version 2.4.0 (78) was used to produce an alignment of variant sites, excluding gaps and ambiguous characters. IQ-TREE version 1.6.10 (79) was used to construct a maximum likelihood phylogeny, using the alignment of core single nucleotide polymorphisms (SNPs) detected in all 2,231 genomes. The GTR+G+ASC nucleotide substitution model (i.e., general time reversible model [80] with a gamma parameter [81] to allow rate heterogeneity among sites and an ascertainment bias correction [82] to account for the use of solely variant sites) was used, along with 1,000 replicates of the ultrafast bootstrap approximation (83). The resulting phylogeny was annotated in R using the ggplot2 (71), ape (84), phytools (85), phylobase (86), ggtree (87), and phangorn (88) packages.
Data availability.
Accession numbers for all genomes queried in this study are available in Table S6. BTyper3, a command-line tool for characterizing B. cereus group genomes using the framework outlined here, is available at https://github.com/lmc297/BTyper3. An R package, bactaxR, is available for identifying medoid genomes and constructing plots using the methods described here at https://github.com/lmc297/bactaxR.
ACKNOWLEDGMENTS
This material is based on work supported by the National Science Foundation Graduate Research Fellowship Program under grant no. DGE-1650441 and USDA National Institute of Food and Agriculture Hatch Appropriations under project no. PEN04646 and accession no. 1015787.
Footnotes
This article is a direct contribution from Martin Wiedmann, a Fellow of the American Academy of Microbiology, who arranged for and secured reviews by Ben Tall, United States Food and Drug Administration, and Sandra Tallent, FDA.
Citation Carroll LM, Wiedmann M, Kovac J. 2020. Proposal of a taxonomic nomenclature for the Bacillus cereus group which reconciles genomic definitions of bacterial species with clinical and industrial phenotypes. mBio 11:e00034-20. https://doi.org/10.1128/mBio.00034-20.
REFERENCES
- 1.Tallent SM, Kotewicz KM, Strain EA, Bennett RW. 2012. Efficient isolation and identification of Bacillus cereus group. J AOAC Int 95:446–451. doi: 10.5740/jaoacint.11-251. [DOI] [PubMed] [Google Scholar]
- 2.Tallent SM, Rhodehamel EJ, Harmon SM, Bennett RW. 2012. Bacillus cereus. Bacteriological analytical manual. US Food and Drug Administration, Washington, DC. [Google Scholar]
- 3.Skerman VBD, McGowan V, Sneath PHA, Moore W. 1989. Approved lists of bacterial names (amended). American Society for Microbiology, Washington, DC. [PubMed] [Google Scholar]
- 4.Richter M, Rosselló-Móra R. 2009. Shifting the genomic gold standard for the prokaryotic species definition. Proc Natl Acad Sci U S A 106:19126–19131. doi: 10.1073/pnas.0906412106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jain C, Rodriguez RL, Phillippy AM, Konstantinidis KT, Aluru S. 2018. High throughput ANI analysis of 90K prokaryotic genomes reveals clear species boundaries. Nat Commun 9:5114. doi: 10.1038/s41467-018-07641-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith T, Williamson CHD, Hill K, Sahl J, Keim P. 2018. Botulinum neurotoxin-producing bacteria. Isn’t it time that we called a species a species? mBio 9:e01469-18. doi: 10.1128/mBio.01469-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pettengill EA, Pettengill JB, Binet R. 2015. Phylogenetic analyses of Shigella and enteroinvasive Escherichia coli for the identification of molecular epidemiological markers: whole-genome comparative analysis does not support distinct genera designation. Front Microbiol 6:1573. doi: 10.3389/fmicb.2015.01573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forbes BA. 2017. Mycobacterial taxonomy. J Clin Microbiol 55:380–383. doi: 10.1128/JCM.01287-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoffmaster AR, Fitzgerald CC, Ribot E, Mayer LW, Popovic T. 2002. Molecular subtyping of Bacillus anthracis and the 2001 bioterrorism-associated anthrax outbreak, United States. Emerg Infect Dis 8:1111–1116. doi: 10.3201/eid0810.020394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takahashi H, Keim P, Kaufmann AF, Keys C, Smith KL, Taniguchi K, Inouye S, Kurata T. 2004. Bacillus anthracis incident, Kameido, Tokyo, 1993. Emerg Infect Dis 10:117–120. doi: 10.3201/eid1001.030238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abbara A, Brooks T, Taylor GP, Nolan M, Donaldson H, Manikon M, Holmes A. 2014. Lessons for control of heroin-associated anthrax in Europe from 2009–2010 outbreak case studies, London, UK. Emerg Infect Dis 20:1115–1122. doi: 10.3201/eid2007.131764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanczaruk M, Reischl U, Holzmann T, Frangoulidis D, Wagner DM, Keim PS, Antwerpen MH, Meyer H, Grass G. 2014. Injectional anthrax in heroin users, Europe, 2000–2012. Emerg Infect Dis 20:322–323. doi: 10.3201/eid2002.120921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stenfors Arnesen LP, Fagerlund A, Granum PE. 2008. From soil to gut: Bacillus cereus and its food poisoning toxins. FEMS Microbiol Rev 32:579–606. doi: 10.1111/j.1574-6976.2008.00112.x. [DOI] [PubMed] [Google Scholar]
- 14.Bottone EJ. 2010. Bacillus cereus, a volatile human pathogen. Clin Microbiol Rev 23:382–398. doi: 10.1128/CMR.00073-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jouzani GS, Valijanian E, Sharafi R. 2017. Bacillus thuringiensis: a successful insecticide with new environmental features and tidings. Appl Microbiol Biotechnol 101:2691–2711. doi: 10.1007/s00253-017-8175-y. [DOI] [PubMed] [Google Scholar]
- 16.Chattopadhyay P, Banerjee G, Mukherjee S. 2017. Recent trends of modern bacterial insecticides for pest control practice in integrated crop management system. 3 Biotech 7:60. doi: 10.1007/s13205-017-0717-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamar R, Gohar M, Jehanno I, Rejasse A, Kallassy M, Lereclus D, Sanchis V, Ramarao N. 2013. Pathogenic potential of Bacillus cereus strains as revealed by phenotypic analysis. J Clin Microbiol 51:320–323. doi: 10.1128/JCM.02848-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller RA, Jian J, Beno SM, Wiedmann M, Kovac J. 2018. Intraclade variability in toxin production and cytotoxicity of Bacillus cereus group type strains and dairy-associated isolates. Appl Environ Microbiol 84:e02479-17. doi: 10.1128/AEM.02479-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okinaka RT, Cloud K, Hampton O, Hoffmaster AR, Hill KK, Keim P, Koehler TM, Lamke G, Kumano S, Mahillon J, Manter D, Martinez Y, Ricke D, Svensson R, Jackson PJ. 1999. Sequence and organization of pXO1, the large Bacillus anthracis plasmid harboring the anthrax toxin genes. J Bacteriol 181:6509–6515. doi: 10.1128/JB.181.20.6509-6515.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ezzell JW, Welkos SL. 1999. The capsule of Bacillus anthracis, a review. J Appl Microbiol 87:250. doi: 10.1046/j.1365-2672.1999.00881.x. [DOI] [PubMed] [Google Scholar]
- 21.Oh SY, Budzik JM, Garufi G, Schneewind O. 2011. Two capsular polysaccharides enable Bacillus cereus G9241 to cause anthrax-like disease. Mol Microbiol 80:455–470. doi: 10.1111/j.1365-2958.2011.07582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scarff JM, Seldina YI, Vergis JM, Ventura CL, O’Brien AD. 2018. Expression and contribution to virulence of each polysaccharide capsule of Bacillus cereus strain G9241. PLoS One 13:e0202701. doi: 10.1371/journal.pone.0202701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reyes-Ramírez A, Ibarra JE. 2008. Plasmid patterns of Bacillus thuringiensis type strains. Appl Environ Microbiol 74:125–129. doi: 10.1128/AEM.02133-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meric G, Mageiros L, Pascoe B, Woodcock DJ, Mourkas E, Lamble S, Bowden R, Jolley KA, Raymond B, Sheppard SK. 2018. Lineage-specific plasmid acquisition and the evolution of specialized pathogens in Bacillus thuringiensis and the Bacillus cereus group. Mol Ecol 27:1524–1540. doi: 10.1111/mec.14546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gonzalez JM Jr, Brown BJ, Carlton BC. 1982. Transfer of Bacillus thuringiensis plasmids coding for delta-endotoxin among strains of B. thuringiensis and B. cereus. Proc Natl Acad Sci U S A 79:6951–6955. doi: 10.1073/pnas.79.22.6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ehling-Schulz M, Fricker M, Grallert H, Rieck P, Wagner M, Scherer S. 2006. Cereulide synthetase gene cluster from emetic Bacillus cereus: structure and location on a mega virulence plasmid related to Bacillus anthracis toxin plasmid pXO1. BMC Microbiol 6:20. doi: 10.1186/1471-2180-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rasko DA, Rosovitz MJ, Okstad OA, Fouts DE, Jiang L, Cer RZ, Kolsto AB, Gill SR, Ravel J. 2007. Complete sequence analysis of novel plasmids from emetic and periodontal Bacillus cereus isolates reveals a common evolutionary history among the B. cereus-group plasmids, including Bacillus anthracis pXO1. J Bacteriol 189:52–64. doi: 10.1128/JB.01313-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thorsen L, Hansen BM, Nielsen KF, Hendriksen NB, Phipps RK, Budde BB. 2006. Characterization of emetic Bacillus weihenstephanensis, a new cereulide-producing bacterium. Appl Environ Microbiol 72:5118–5121. doi: 10.1128/AEM.00170-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng J, Gao Q, Liu L, Liu H, Wang Y, Peng D, Ruan L, Raymond B, Sun M. 2017. Comparative genomics of Bacillus thuringiensis reveals a path to specialized exploitation of multiple invertebrate hosts. mBio 8:e00822-17. doi: 10.1128/mBio.00822-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klee SR, Brzuszkiewicz EB, Nattermann H, Bruggemann H, Dupke S, Wollherr A, Franz T, Pauli G, Appel B, Liebl W, Couacy-Hymann E, Boesch C, Meyer FD, Leendertz FH, Ellerbrok H, Gottschalk G, Grunow R, Liesegang H. 2010. The genome of a Bacillus isolate causing anthrax in chimpanzees combines chromosomal properties of B. cereus with B. anthracis virulence plasmids. PLoS One 5:e10986. doi: 10.1371/journal.pone.0010986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carroll LM, Kovac J, Miller RA, Wiedmann M. 2017. Rapid, high-throughput identification of anthrax-causing and emetic Bacillus cereus group genome assemblies using BTyper, a computational tool for virulence-based classification of Bacillus cereus group isolates using nucleotide sequencing data. Appl Environ Microbiol 83:e01096-17. doi: 10.1128/AEM.01096-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guinebretiere MH, Auger S, Galleron N, Contzen M, De Sarrau B, De Buyser ML, Lamberet G, Fagerlund A, Granum PE, Lereclus D, De Vos P, Nguyen-The C, Sorokin A. 2013. Bacillus cytotoxicus sp. nov. is a novel thermotolerant species of the Bacillus cereus group occasionally associated with food poisoning. Int J Syst Evol Microbiol 63:31–40. doi: 10.1099/ijs.0.030627-0. [DOI] [PubMed] [Google Scholar]
- 33.Jiménez G, Urdiain M, Cifuentes A, López-López A, Blanch AR, Tamames J, Kämpfer P, Kolstø A-B, Ramón D, Martínez JF, Codoñer FM, Rosselló-Móra R. 2013. Description of Bacillus toyonensis sp. nov., a novel species of the Bacillus cereus group, and pairwise genome comparisons of the species of the group by means of ANI calculations. Syst Appl Microbiol 36:383–391. doi: 10.1016/j.syapm.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 34.Miller RA, Beno SM, Kent DJ, Carroll LM, Martin NH, Boor KJ, Kovac J. 2016. Bacillus wiedmannii sp. nov., a psychrotolerant and cytotoxic Bacillus cereus group species isolated from dairy foods and dairy environments. Int J Syst Evol Microbiol 66:4744–4753. doi: 10.1099/ijsem.0.001421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Y, Du J, Lai Q, Zeng R, Ye D, Xu J, Shao Z. 2017. Proposal of nine novel species of the Bacillus cereus group. Int J Syst Evol Microbiol 67:2499–2508. doi: 10.1099/ijsem.0.001821. [DOI] [PubMed] [Google Scholar]
- 36.Ehling-Schulz M, Frenzel E, Gohar M. 2015. Food-bacteria interplay: pathometabolism of emetic Bacillus cereus. Front Microbiol 6:704. doi: 10.3389/fmicb.2015.00704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ehling-Schulz M, Svensson B, Guinebretiere MH, Lindback T, Andersson M, Schulz A, Fricker M, Christiansson A, Granum PE, Martlbauer E, Nguyen-The C, Salkinoja-Salonen M, Scherer S. 2005. Emetic toxin formation of Bacillus cereus is restricted to a single evolutionary lineage of closely related strains. Microbiology 151:183–197. doi: 10.1099/mic.0.27607-0. [DOI] [PubMed] [Google Scholar]
- 38.Ehling-Schulz M, Fricker M, Scherer S. 2004. Bacillus cereus, the causative agent of an emetic type of food-borne illness. Mol Nutr Food Res 48:479–487. doi: 10.1002/mnfr.200400055. [DOI] [PubMed] [Google Scholar]
- 39.Carroll LM, Wiedmann M, Mukherjee M, Nicholas DC, Mingle LA, Dumas NB, Cole JA, Kovac J. 2019. Characterization of emetic and diarrheal Bacillus cereus strains from a 2016 foodborne outbreak using whole-genome sequencing: addressing the microbiological, epidemiological, and bioinformatic challenges. Front Microbiol 10:144. doi: 10.3389/fmicb.2019.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marston CK, Ibrahim H, Lee P, Churchwell G, Gumke M, Stanek D, Gee JE, Boyer AE, Gallegos-Candela M, Barr JR, Li H, Boulay D, Cronin L, Quinn CP, Hoffmaster AR. 2016. Anthrax toxin-expressing Bacillus cereus isolated from an anthrax-like eschar. PLoS One 11:e0156987. doi: 10.1371/journal.pone.0156987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Antonation KS, Grutzmacher K, Dupke S, Mabon P, Zimmermann F, Lankester F, Peller T, Feistner A, Todd A, Herbinger I, de Nys HM, Muyembe-Tamfun JJ, Karhemere S, Wittig RM, Couacy-Hymann E, Grunow R, Calvignac-Spencer S, Corbett CR, Klee SR, Leendertz FH. 2016. Bacillus cereus biovar anthracis causing anthrax in sub-Saharan Africa—chromosomal monophyly and broad geographic distribution. PLoS Negl Trop Dis 10:e0004923. doi: 10.1371/journal.pntd.0004923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilson MK, Vergis JM, Alem F, Palmer JR, Keane-Myers AM, Brahmbhatt TN, Ventura CL, O’Brien AD. 2011. Bacillus cereus G9241 makes anthrax toxin and capsule like highly virulent B. anthracis Ames but behaves like attenuated toxigenic nonencapsulated B. anthracis Sterne in rabbits and mice. Infect Immun 79:3012–3019. doi: 10.1128/IAI.00205-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mikesell P, Ivins BE, Ristroph JD, Dreier TM. 1983. Evidence for plasmid-mediated toxin production in Bacillus anthracis. Infect Immun 39:371–376. doi: 10.1128/IAI.39.1.371-376.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Y, Lai Q, Shao Z. 2018. Genome analysis-based reclassification of Bacillus weihenstephanensis as a later heterotypic synonym of Bacillus mycoides. Int J Syst Evol Microbiol 68:106–112. doi: 10.1099/ijsem.0.002466. [DOI] [PubMed] [Google Scholar]
- 45.Dai Z, Sirard JC, Mock M, Koehler TM. 1995. The atxA gene product activates transcription of the anthrax toxin genes and is essential for virulence. Mol Microbiol 16:1171–1181. doi: 10.1111/j.1365-2958.1995.tb02340.x. [DOI] [PubMed] [Google Scholar]
- 46.Rodriguez RL, Gunturu S, Harvey WT, Rossello-Mora R, Tiedje JM, Cole JR, Konstantinidis KT. 2018. The Microbial Genomes Atlas (MiGA) webserver: taxonomic and gene diversity analysis of Archaea and Bacteria at the whole genome level. Nucleic Acids Res 46:W282–W288. doi: 10.1093/nar/gky467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vergnaud G, Girault G, Thierry S, Pourcel C, Madani N, Blouin Y. 2016. Comparison of French and worldwide Bacillus anthracis strains favors a recent, post-Columbian origin of the predominant North-American clade. PLoS One 11:e0146216. doi: 10.1371/journal.pone.0146216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sahl JW, Pearson T, Okinaka R, Schupp JM, Gillece JD, Heaton H, Birdsell D, Hepp C, Fofanov V, Noseda R, Fasanella A, Hoffmaster A, Wagner DM, Keim P. 2016. A Bacillus anthracis genome sequence from the Sverdlovsk 1979 autopsy specimens. mBio 7:e01501-16. doi: 10.1128/mBio.01501-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Agata N, Mori M, Ohta M, Suwan S, Ohtani I, Isobe M. 1994. A novel dodecadepsipeptide, cereulide, isolated from Bacillus cereus causes vacuole formation in HEp-2 cells. FEMS Microbiol Lett 121:31–34. doi: 10.1111/j.1574-6968.1994.tb07071.x. [DOI] [PubMed] [Google Scholar]
- 50.Hoton FM, Fornelos N, N’guessan E, Hu X, Swiecicka I, Dierick K, Jääskeläinen E, Salkinoja-Salonen M, Mahillon J. 2009. Family portrait of Bacillus cereus and Bacillus weihenstephanensis cereulide-producing strains. Environ Microbiol Rep 1:177–183. doi: 10.1111/j.1758-2229.2009.00028.x. [DOI] [PubMed] [Google Scholar]
- 51.Johler S, Kalbhenn EM, Heini N, Brodmann P, Gautsch S, Bağcioğlu M, Contzen M, Stephan R, Ehling-Schulz M. 2018. Enterotoxin production of Bacillus thuringiensis isolates from biopesticides, foods, and outbreaks. Front Microbiol 9:1915. doi: 10.3389/fmicb.2018.01915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lazarte JN, Lopez RP, Ghiringhelli PD, Beron CM. 2018. Bacillus wiedmannii biovar thuringiensis: a specialized mosquitocidal pathogen with plasmids from diverse origins. Genome Biol Evol 10:2823–2833. doi: 10.1093/gbe/evy211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Acevedo MM, Carroll LM, Mukherjee M, Mills E, Xiaoli L, Dudley EG, Kovac J. 2019. Bacillus clarus sp. nov. is a new Bacillus cereus group species isolated from soil bioRxiv 508077. doi: 10.1101/508077. [DOI] [PMC free article] [PubMed]
- 54.Chattaway MA, Schaefer U, Tewolde R, Dallman TJ, Jenkins C. 2017. Identification of Escherichia coli and Shigella species from whole-genome sequences. J Clin Microbiol 55:616–623. doi: 10.1128/JCM.01790-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sahl JW, Morris CR, Emberger J, Fraser CM, Ochieng JB, Juma J, Fields B, Breiman RF, Gilmour M, Nataro JP, Rasko DA. 2015. Defining the phylogenomics of Shigella species: a pathway to diagnostics. J Clin Microbiol 53:951–960. doi: 10.1128/JCM.03527-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baek I, Lee K, Goodfellow M, Chun J. 2019. Comparative genomic and phylogenomic analyses clarify relationships within and between Bacillus cereus and Bacillus thuringiensis: Proposal for the recognition of two Bacillus thuringiensis genomovars. Front Microbiol 10:1978. doi: 10.3389/fmicb.2019.01978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Uchida I, Makino S, Sekizaki T, Terakado N. 1997. Cross-talk to the genes for Bacillus anthracis capsule synthesis by atxA, the gene encoding the trans-activator of anthrax toxin synthesis. Mol Microbiol 23:1229–1240. doi: 10.1046/j.1365-2958.1997.3041667.x. [DOI] [PubMed] [Google Scholar]
- 58.Doll VM, Ehling-Schulz M, Vogelmann R. 2013. Concerted action of sphingomyelinase and non-hemolytic enterotoxin in pathogenic Bacillus cereus. PLoS One 8:e61404. doi: 10.1371/journal.pone.0061404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pruitt KD, Tatusova T, Maglott DR. 2007. NCBI reference sequences (RefSeq): a curated non-redundant sequence database of genomes, transcripts and proteins. Nucleic Acids Res 35:D61–D65. doi: 10.1093/nar/gkl842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu B, Liu G-H, Hu G-P, Sengonca C, Cetin S, Lin N-Q, Tang J-Y, Tang W-Q, Lin Y-Z. 2014. Bacillus bingmayongensis sp. nov., isolated from the pit soil of Emperor Qin’s terra-cotta warriors in China. Antonie Van Leeuwenhoek 105:501–510. doi: 10.1007/s10482-014-0150-3. [DOI] [PubMed] [Google Scholar]
- 61.Jung MY, Paek WK, Park I-S, Han J-R, Sin Y, Paek J, Rhee M-S, Kim H, Song HS, Chang Y-H. 2010. Bacillus gaemokensis sp. nov., isolated from foreshore tidal flat sediment from the Yellow Sea. J Microbiol 48:867–871. doi: 10.1007/s12275-010-0148-0. [DOI] [PubMed] [Google Scholar]
- 62.Jung MY, Kim JS, Paek WK, Lim J, Lee H, Kim PI, Ma JY, Kim W, Chang YH. 2011. Bacillus manliponensis sp. nov., a new member of the Bacillus cereus group isolated from foreshore tidal flat sediment. J Microbiol 49:1027–1032. doi: 10.1007/s12275-011-1049-6. [DOI] [PubMed] [Google Scholar]
- 63.Gurevich A, Saveliev V, Vyahhi N, Tesler G. 2013. QUAST: quality assessment tool for genome assemblies. Bioinformatics 29:1072–1075. doi: 10.1093/bioinformatics/btt086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kovac J, Miller RA, Carroll LM, Kent DJ, Jian J, Beno SM, Wiedmann M. 2016. Production of hemolysin BL by Bacillus cereus group isolates of dairy origin is associated with whole-genome phylogenetic clade. BMC Genomics 17:581. doi: 10.1186/s12864-016-2883-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Seemann T. 2014. Prokka: rapid prokaryotic genome annotation. Bioinformatics 30:2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 66.Ye W, Zhu L, Liu Y, Crickmore N, Peng D, Ruan L, Sun M. 2012. Mining new crystal protein genes from Bacillus thuringiensis on the basis of mixed plasmid-enriched genome sequencing and a computational pipeline. Appl Environ Microbiol 78:4795–4801. doi: 10.1128/AEM.00340-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wood DE, Salzberg SL. 2014. Kraken: ultrafast metagenomic sequence classification using exact alignments. Genome Biol 15:R46. doi: 10.1186/gb-2014-15-3-r46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wood DE, Lu J, Langmead B. 2019. Improved metagenomic analysis with Kraken 2. bioRxiv 762302. doi: 10.1101/762302. [DOI] [PMC free article] [PubMed]
- 69.R Core Team. 2018. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: https://www.R-project.org/. [Google Scholar]
- 70.Wickham H. 2007. Reshaping data with the reshape package. J Stat Softw 21:1–20. [Google Scholar]
- 71.Wickham H. 2009. Ggplot2: elegant graphics for data analysis. Springer, New York, NY. [Google Scholar]
- 72.Galili T. 2015. dendextend: an R package for visualizing, adjusting and comparing trees of hierarchical clustering. Bioinformatics 31:3718–3720. doi: 10.1093/bioinformatics/btv428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Garnier S. 2018. viridis: default color maps from ‘matplotlib’, vR package version 0.5.1. https://CRAN.R-project.org/package=viridis.
- 74.Maechler M, Rousseeuw P, Struyf A, Hubert M, Hornik K. 2017. cluster: cluster analysis basics and extensions, v2.0.6.
- 75.Csardi G, Nepusz T. 2006. The igraph software package for complex network research. InterJournal Complex Systems:1695. [Google Scholar]
- 76.Emms DM, Kelly S. 2015. OrthoFinder: solving fundamental biases in whole genome comparisons dramatically improves orthogroup inference accuracy. Genome Biol 16:157. doi: 10.1186/s13059-015-0721-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Loytynoja A. 2014. Phylogeny-aware alignment with PRANK. Methods Mol Biol 1079:155–170. doi: 10.1007/978-1-62703-646-7_10. [DOI] [PubMed] [Google Scholar]
- 78.Page AJ, Taylor B, Delaney AJ, Soares J, Seemann T, Keane JA, Harris SR. 2016. SNP-sites: rapid efficient extraction of SNPs from multi-FASTA alignments. Microb Genom 2:e000056. doi: 10.1099/mgen.0.000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nguyen LT, Schmidt HA, von Haeseler A, Minh BQ. 2015. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol 32:268–274. doi: 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tavare S. 1986. Some probabilistic and statistical problems in the analysis of DNA sequences. Lect Math Life Sci 17:57–86. [Google Scholar]
- 81.Yang Z. 1994. Maximum likelihood phylogenetic estimation from DNA sequences with variable rates over sites: approximate methods. J Mol Evol 39:306–314. doi: 10.1007/bf00160154. [DOI] [PubMed] [Google Scholar]
- 82.Lewis PO. 2001. A likelihood approach to estimating phylogeny from discrete morphological character data. Syst Biol 50:913–925. doi: 10.1080/106351501753462876. [DOI] [PubMed] [Google Scholar]
- 83.Hoang DT, Chernomor O, von Haeseler A, Minh BQ, Vinh LS. 2018. UFBoot2: improving the ultrafast bootstrap approximation. Mol Biol Evol 35:518–522. doi: 10.1093/molbev/msx281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Paradis E, Schliep K. 2019. ape 5.0: an environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 35:526–528. doi: 10.1093/bioinformatics/bty633. [DOI] [PubMed] [Google Scholar]
- 85.Revell LJ. 2012. phytools: an R package for phylogenetic comparative biology (and other things). Methods Ecol Evol 3:217–223. doi: 10.1111/j.2041-210X.2011.00169.x. [DOI] [Google Scholar]
- 86.R Hackathon. 2017. phylobase: base package for phylogenetic structures and comparative data, v0.8.4. https://CRAN.R-project.org/package=phylobase.
- 87.Yu G, Smith DK, Zhu H, Guan Y, Lam T. 2017. ggtree: an R package for visualization and annotation of phylogenetic trees with their covariates and other associated data. Methods Ecol Evol 8:28–36. doi: 10.1111/2041-210X.12628. [DOI] [Google Scholar]
- 88.Schliep KP. 2011. phangorn: phylogenetic analysis in R. Bioinformatics 27:592–593. doi: 10.1093/bioinformatics/btq706. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Species type strain/NCBI RefSeq reference genomes for 18 currently recognized B. cereus group species and three published putative species used in this study. Download Table S1, XLSX file, 0.01 MB (12KB, xlsx) .
Copyright © 2020 Carroll et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Proportion of genomes assigned to genomospecies X (rows) using the species type strain/reference genome at a threshold of 95 ANI which share ≥95 ANI with one or more genomes assigned to genomospecies Y (columns). Download Table S2, XLSX file, 0.01 MB (14.4KB, xlsx) .
Copyright © 2020 Carroll et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Proportion of genomes assigned to genomospecies X (rows) using the medoid genome at a threshold of 95 ANI which share ≥95 ANI with one or more genomes assigned to genomospecies Y (columns). Download Table S3, XLSX file, 0.02 MB (19.2KB, xlsx) .
Copyright © 2020 Carroll et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Genomes most closely resembling the B. anthracis strain Ames species reference genome with ≥95 ANI in which anthrax toxin genes cya, lef, and pagA were not detected. Download Table S4, XLSX file, 0.02 MB (19KB, xlsx) .
Copyright © 2020 Carroll et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Genomes submitted to NCBI’s RefSeq database as anthrax-causing “B. cereus.” Download Table S5, XLSX file, 0.01 MB (13.6KB, xlsx) .
Copyright © 2020 Carroll et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Genomes used in this study (n = 2,231). Download Table S6, XLSX file, 0.5 MB (508.7KB, xlsx) .
Copyright © 2020 Carroll et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Histograms of pairwise ANI values between 2,218 B. cereus group genomes with N50 of >20 kbp. For each of 18 published species and 3 proposed putative species, ANI values calculated between two genomes for which one or more genome(s) shared ≥95 ANI with the species type strain or reference genome are colored in pink. ANI values calculated between two genomes for which both genomes shared <95 ANI with the species type strain/reference genome are colored in gray. See Table S1 for details regarding species type strain genomes. Download FIG S1, PDF file, 0.5 MB (541.8KB, pdf) .
Copyright © 2020 Carroll et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Proportion of genomes assigned to genomospecies X (rows) using the medoid genome at a threshold of 92.5 ANI which share ≥92.5 ANI with one or more genomes assigned to genomospecies Y (columns). Download Table S7, XLSX file, 0.01 MB (15.2KB, xlsx) .
Copyright © 2020 Carroll et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Genomes belonging to one of six previously unpublished genomospecies clusters formed at ANI of ≥92.5 (i.e., putative novel genomospecies). Download Table S8, XLSX file, 0.01 MB (15.1KB, xlsx) .
Copyright © 2020 Carroll et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Sample of histograms constructed using pairwise ANI values calculated between B. cereus group genomes meeting various quality thresholds. Because distribution breakpoints and shape were robust to the exclusion of genomes at all tested thresholds, the six histograms shown represent pairwise ANI values calculated between raw genomes downloaded directly from NCBI’s RefSeq database (i.e., the least stringent quality thresholds, with no filtering criteria employed; n = 2,231 genomes) (A), genomes with N50 of >20 kb (the set of genomes from which medoid genomes were selected; n = 2,218) (B), genomes with N50 of >0 and no contigs assigned to a domain other than Bacteria (n = 1,291) (C), genomes with N50 of >20 kb and no contigs assigned to a domain other than Bacteria (n = 1,289) (D), genomes with N50 of >0 and no contigs assigned to a genus outside Bacillus (n = 1,074) (E), and genomes with N50 of >100 kb and no contigs assigned to a genus outside Bacillus (i.e., the most stringent quality thresholds tested; n = 998) (F). Download FIG S2, PDF file, 0.1 MB (144.5KB, pdf) .
Copyright © 2020 Carroll et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Data Availability Statement
Accession numbers for all genomes queried in this study are available in Table S6. BTyper3, a command-line tool for characterizing B. cereus group genomes using the framework outlined here, is available at https://github.com/lmc297/BTyper3. An R package, bactaxR, is available for identifying medoid genomes and constructing plots using the methods described here at https://github.com/lmc297/bactaxR.