Key Points
Question
What are the 20-year efficacy and safety outcomes of adjuvant high-dose chemotherapy with hematopoietic stem cell transplant compared with conventional-dose chemotherapy for patients with stage III breast cancer?
Findings
This 20-year follow-up of a multicenter randomized phase 3 trial of 885 patients with stage III breast cancer showed no overall improvement in long-term survival after high-dose chemotherapy compared with conventional chemotherapy but showed clinically important survival benefit for patients with 10 or more involved axillary lymph nodes.
Meaning
Adjuvant high-dose chemotherapy with stem cell support should not be used in unselected patients with stage III breast cancer, but the survival benefit in subgroups of patients suggests that further research is needed.
Abstract
Importance
Trials of adjuvant high-dose chemotherapy (HDCT) have failed to show a survival benefit in unselected patients with breast cancer, but long-term follow-up is lacking.
Objective
To determine 20-year efficacy and safety outcomes of a large trial of adjuvant HDCT vs conventional-dose chemotherapy (CDCT) for patients with stage III breast cancer.
Design, Setting, and Participants
This secondary analysis used data from a randomized phase 3 multicenter clinical trial of 885 women younger than 56 years with breast cancer and 4 or more involved axillary lymph nodes conducted from August 1, 1993, to July 31, 1999. Additional follow-up data were collected between June 1, 2016, and December 31, 2017, from medical records, general practitioners, the Dutch national statistical office, and nationwide cancer registries. Analysis was performed on an intention-to-treat basis. Statistical analysis was performed from February 1, 2018, to October 14, 2019.
Interventions
Participants were randomized 1:1 to receive 5 cycles of CDCT consisting of fluorouracil, 500 mg/m2, epirubicin, 90 mg/m2, and cyclophosphamide, 500 mg/m2, or HDCT in which the first 4 cycles were identical to CDCT and the fifth cycle was replaced by cyclophosphamide, 6000 mg/m2, thiotepa, 480 mg/m2, and carboplatin, 1600 mg/m2, followed by hematopoietic stem cell transplant.
Main Outcomes and Measures
Main end points were overall survival and safety and cumulative incidence risk of a second malignant neoplasm or cardiovascular events.
Results
Of the 885 women in the study (mean [SD] age, 44.5 [6.6] years), 442 were randomized to receive HDCT, and 443 were randomized to receive CDCT. With 20.4 years median follow-up (interquartile range, 19.2-22.0 years), the 20-year overall survival was 45.3% with HDCT and 41.5% with CDCT (hazard ratio, 0.89; 95% CI, 0.75-1.06). The absolute improvement in 20-year overall survival was 14.6% (hazard ratio, 0.72; 95% CI, 0.54-0.95) for patients with 10 or more invoved axillary lymph nodes and 15.4% (hazard ratio, 0.67; 95% CI, 0.42-1.05) for patients with triple-negative breast cancer. The cumulative incidence risk of a second malignant neoplasm at 20 years or major cardiovascular events was similar in both treatment groups (20-year cumulative incidence risk for second malignant neoplasm was 12.1% in the HDCT group vs 16.2% in the CDCT group, P = .10), although patients in the HDCT group more often had hypertension (21.7% vs 14.3%, P = .02), hypercholesterolemia (15.7% vs 10.6%, P = .04), and dysrhythmias (8.6% vs 4.6%, P = .005).
Conclusions and Relevance
High-dose chemotherapy provided no long-term survival benefit in unselected patients with stage III breast cancer but did provide improved overall survival in very high-risk patients (ie, with ≥10 involved axillary lymph nodes). High-dose chemotherapy did not affect long-term risk of a second malignant neoplasm or major cardiovascular events.
Trial Registration
ClinicalTrials.gov Identifier: NCT03087409
This secondary analysis of a randomized clinical trial examines 20-year efficacy and safety outcomes of a large trial of adjuvant high-dose chemotherapy and hematopoietic stem cell transplant (HSCT) vs conventional-dose chemotherapy for patients with stage III breast cancer.
Introduction
In the 1980s and 1990s, high-dose chemotherapy (HDCT) with autologous stem cell support was investigated as treatment for breast cancer (BC) until phase 3 trials showed no overall survival (OS) benefit compared with conventional-dose chemotherapy (CDCT).1 Since then, however, additional subgroup analyses suggested an OS benefit after HDCT in subgroups of patients with 10 or more involved axillary lymph nodes (ALNs), Erb-B2 receptor tyrosine kinase 2 (ERBB2)–negative BC, and triple-negative BC (TNBC).1,2,3
The potential benefit of HDCT is accompanied by toxic effects during and after treatment, such as severe mucositis, myeloablation-causing transfusion dependency, and prolonged neutropenia with corresponding infection risk. Some studies of high-dose alkylating agents showed an increased incidence of a second malignant neoplasm and cardiovascular events.4,5,6 However, this increase was not seen in other studies,7,8,9,10,11,12 and some studies even reported fewer patients with myeloid leukemia and/or myelodysplastic syndrome with HDCT compared with CDCT.13,14
The importance of long-term follow-up of BC studies for efficacy has been well established.15,16 Similarly, extended follow-up is important to establish long-term safety, especially after HDCT. However, to our knowledge, only 1 of the studies that compared HDCT with CDCT has reported follow-up at 12 years.7,17 The largest multicenter randomized clinical trial comparing HDCT with CDCT for women with invasive BC was conducted in the Netherlands.2 We report an efficacy and safety analysis of the Dutch trial with 20-year follow-up.
Methods
Study Design
This multicenter randomized clinical trial was conducted between August 1, 1993, and July 31, 1999, in 10 hospitals in the Netherlands. Full trial details were published in 2003.2 In brief, 885 women younger than 56 years with BC involving 4 or more ALNs who underwent breast surgery plus complete axillary clearance were included. Patients were randomized 1:1 to receive CDCT consisting of 5 cycles of fluorouracil, 500 mg/m2, epirubicin, 90 mg/m2, and cyclophosphamide, 500 mg/m2 (FEC) or to receive HDCT in which the first 4 cycles were identical to CDCT and the fifth cycle was replaced by cyclophosphamide, 6000 mg/m2, thiotepa, 480 mg/m2, and carboplatin, 1600 mg/m2, supported with autologous hematopoietic stem cell transplant. All patients received radiotherapy according to the local standard as well as 2 years of tamoxifen treatment. After the Early Breast Cancer Trialists’ Collaborative Group demonstrated a benefit to tamoxifen for patients with estrogen receptor (ER)–positive BC,18 patients with ER-positive tumors were offered 5 years of tamoxifen treatment. Informed consent was obtained from all patients in the trial, and the medical ethical review board at each of the participating centers approved the trial. The medical ethical review boards of the Netherlands Cancer Registry, the nationwide network and registry of histopathology and cytopathology in the Netherlands (PALGA), and Statistics Netherlands approved this follow-up analysis, which is registered with ClinicalTrials.gov (NCT03087409; trial protocol in Supplement 1).
Collection of Data
The characteristics of the patients, the date of diagnosis, the histologic features of the primary tumor, ALN involvement, treatment data, follow-up data, and adverse events were recorded during follow-up of the original trial. The frequency and duration of follow-up visits more than 3 years after the end of treatment were at the discretion of the treating physician.
Follow-up data beyond 3 years were collected between June 1, 2016, and December 31, 2017, from patients’ medical records using case record forms. Questionnaires were sent to the general practitioner of each patient and to medical specialists if the patients had received treatment for cancer or cardiovascular disease. To complete and verify follow-up data, we linked the data set with patient-level data from the population-based municipal Personal-Records Database, the Netherlands Cancer Registry, PALGA, and Statistics Netherlands (eMethods 1 in Supplement 2).
Statistical Analysis
Statistical analyses were performed from February 1, 2018, to October 14, 2019. The main end point of this long-term analysis was OS, defined as the time from randomization to death from any cause.19 For patients last known to be alive, OS data were censored at last follow-up visit, last date known to be alive according to the questionnaire(s), or at last linkage to the Personal-Records Database—whichever was the latest date. Secondary end points were BC-specific survival (BCSS) and safety. Breast cancer–specific survival was defined using STEEP (Standardized Definitions for Efficacy End Points) criteria.19 In the original trial, subgroup analyses were preplanned based on the number of involved ALNs (4-9 vs ≥10). For this update, we performed preplanned subgroup analyses based on the number of involved ALNs and BC subtype (ER-positive and ERBB2-negative, ERBB2-positive, or TNBC).
All randomized patients were included in the intention-to-treat population. Efficacy and safety analyses of HDCT compared with CDCT were performed for the intention-to-treat population. eMethods 2 in Supplement 2 contains details on statistical analyses. All P values were from 2-sided tests, and the results were deemed statistically significant at P < .05. Analyses were performed with R, version 3.5.3 (R Project for Statistical Computing).
Results
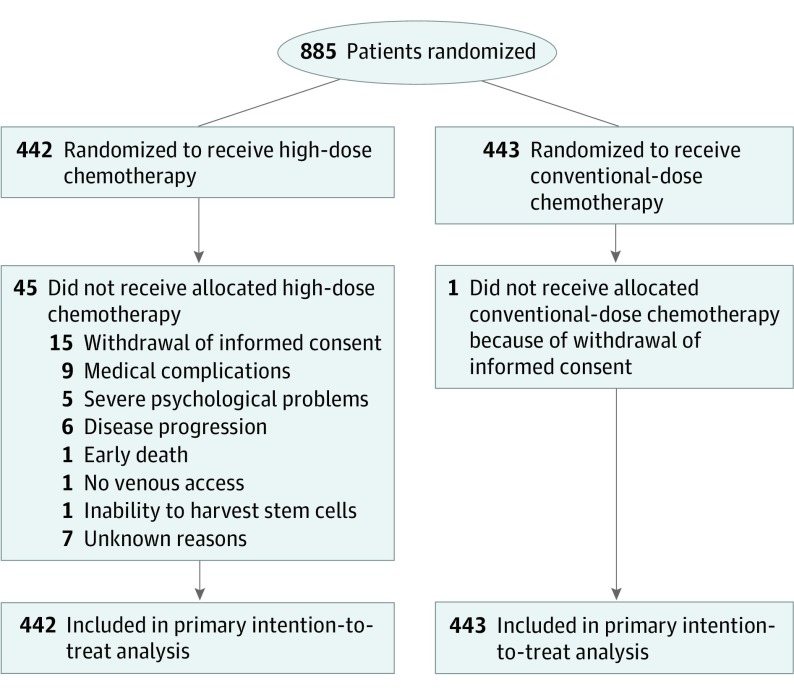
Of 885 patients included (mean [SD] age, 44.5 [6.6] years), 442 were randomized to receive HDCT and 443 to receive to CDCT (Figure 1). Baseline characteristics were well-balanced between groups (eTable 1 in Supplement 2), as were the subgroups based on the number of involved ALNs in BC subtypes (eTable 2 in Supplement 2). Treatment details are described in the original study.2 For this long-term update, follow-up data on vital status, cause of death, and occurrence of second malignant neoplasms were complete for 99% or more of patients. Data on incidence of cardiovascular events and risk factors were complete for 85.8% of patients (759 of 885).
Figure 1. CONSORT Flow Diagram.
CONSORT flow diagram showing the 885 patients who were included in the intention-to-treat analysis and safety population. Information on the number of patients screened for eligibility was not collected and is thus not reported.
Long-term Efficacy Analyses
Overall Survival
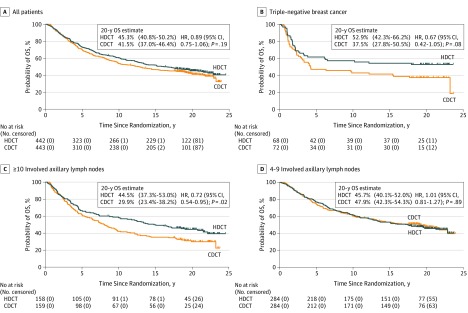
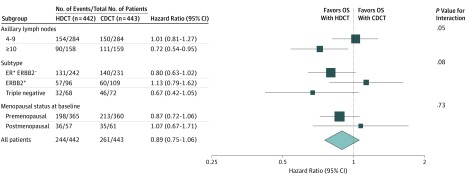
After a median follow-up of 20.4 years (interquartile range, 19.2-22.0 years), the 20-year OS estimate was 45.3% for those receiving HDCT and 41.5% for those receiving CDCT (hazard ratio, 0.89; 95% CI, 0.75-1.06) (Figure 2A). For patients with 10 or more involved ALNs, the absolute difference in 20-year OS was 14.6% (hazard ratio, 0.72; 95% CI, 0.54-0.95) in favor of HDCT compared with a 2.2% difference (hazard ratio, 1.01; 95% CI, 0.81-1.27) for patients with 4 to 9 involved ALNs (P = .05 for interaction; Figure 2C and D and Figure 3). For patients with TNBC, the absolute difference in 20-year OS was 15.4% (hazard ratio, 0.67; 95% CI, 0.42-1.05), whereas for patients with ER-positive and ERBB2-negative disease, the 20-year absolute difference was 7.0% (hazard ratio, 0.80; 95% CI, 0.63-1.02) (Figure 2B and Figure 3).
Figure 2. Overall Survival (OS) in All Patients and Subgroups.
A, All patients. B, Patients with triple-negative breast cancer. C, Patients with 10 or more involved axillary lymph nodes. D, Patients with 4 to 9 involved axillary lymph nodes. For the OS analysis for all patients and subgroups, 20-year estimates with corresponding 95% CIs and hazard ratios (HRs) with corresponding 95% CIs are reported. CDCT indicates conventional-dose chemotherapy; and HDCT, high-dose chemotherapy.
Figure 3. Stratified Overall Survival Analysis in Subgroups.
CDCT indicates conventional-dose chemotherapy; ER+, estrogen receptor–positive; ERBB2−, Erb-B2 receptor tyrosine kinase 2 negative; ERBB2+, Erb-B2 receptor tyrosine kinase 2 positive; HDCT, high-dose chemotherapy; and OS, overall survival.
Breast Cancer–Specific Survival
Breast cancer was the cause of death for most patients in both groups (HDCT group, 204 of 244 [83.6%]; CDCT group, 238 of 261 [91.2%]; Table). Mortality from a second malignant neoplasm and cardiovascular disease was comparable between groups. Five of 442 patients (1.1%) receiving HDCT died within 6 months after completion of treatment. Fifteen patients, of whom 14 were in the HDCT group, died of other causes. Twenty-year BCSS estimates were 52.3% among those who received HDCT vs 45.4% among those who received CDCT (eFigure 1 in Supplement 2). Subgroup analyses for BCSS showed comparable effects as seen for OS.
Table. Causes of Death Among Study Patients.
| Cause of Death | Patients, No. (%) | |
|---|---|---|
| HDCT Group (n = 244) | CDCT Group (n = 261) | |
| Breast cancer | 204 (83.6) | 238 (91.2) |
| Nonbreast cancer malignant neoplasm | 13 (5.3) | 18 (6.9) |
| Cardiovascular disease | 4 (1.6) | 2 (0.8) |
| Treatment-related (<6 mo after randomization) | 5 (2.1) | 0 |
| Othera | 14 (5.7) | 1 (0.4) |
| Unknown | 4 (1.6) | 2 (0.8) |
Abbreviations: CDCT, conventional-dose chemotherapy; HDCT, high-dose chemotherapy.
Other causes include infection (n = 5; 10-19 years after completion of chemotherapy), suicide (n = 2; 7 and 9 years after completion of chemotherapy), accidents (n = 2; 4 and 6 years after completion of chemotherapy), idiopathic pancreatitis (n = 1; 6 years after completion of chemotherapy), liver cirrhosis (n = 1; 16 years after completion of chemotherapy), dementia without signs of a central nervous system relapse (n = 1; 18 years after completion of chemotherapy), epilepsy without sign of central nervous system relapse (n = 1; 19 years after completion of chemotherapy), pneumothorax with bleeding (n = 1; 1 year after completion of chemotherapy), and acute vascular disorder of the intestines (n = 1; 9 years after completion of chemotherapy).
Long-term Safety Analyses
Thirty months after randomization, fewer patients in the HDCT group remained premenopausal compared with the CDCT group (26 of 442 [5.9%] vs 93 of 443 [21.0%]; eTable 1 in Supplement 2). During follow-up, 58 patients in the HDCT group (13.1%) developed 65 malignant neoplasms, while 74 patients in the CDCT group (16.7%) developed 81 malignant neoplasms. Breast cancer was the most common second malignant neoplasm (eTable 3 in Supplement 2). The cumulative incidence of a second malignant neoplasm at 20 years was 12.1% (95% CI, 11.8%-12.4%) after HDCT and 16.2% (95% CI, 15.9%-16.6%) after CDCT (P = .10; eFigure 2 in Supplement 2). The incidence of major cardiovascular events did not differ between treatment groups, but more patients in the HDCT group than in the CDCT group developed hypertension, hypercholesterolemia, and dysrhythmia (eTable 4 in Supplement 2).
Discussion
Although initial studies of HDCT for BC showed promising early results, later data from randomized studies failed to confirm large enough benefits of HDCT compared with CDCT to outweigh its toxic effects in unselected patients with BC.2,6,8,9,10,11,13,20,21,22 Interest in HDCT decreased, and long-term follow-up of the HDCT studies was not performed. Our analysis with 20-year follow-up data from, to our knowledge, the largest randomized clinical trial of HDCT for BC suggests that selected subgroups may benefit from this treatment.
Our analysis confirms earlier results that HDCT has no significant OS benefit compared with CDCT for unselected patients with stage III BC.1,2 However, we found a 14.6% improvement in 20-year OS estimates with HDCT in the predefined subgroup of patients with 10 or more involved ALNs. This result obtains the highest grade, namely an “A,” on the European Society of Medical Oncology Magnitude of Clinical Benefit Scale (ESMO-MCBS), which is considered a clinically meaningful benefit.23 In addition, our data suggest an OS benefit for patients with TNBC, with a 15.4% absolute difference in 20-year OS estimates between groups. However, this latter subgroup analysis was not planned when the trial was designed and therefore was not graded on the ESMO-MCBS.
Although HDCT did not improve OS in the overall population, it did improve BCSS. This discrepancy can be attributed to excess mortality in the HDCT group owing to noncancer and noncardiovascular deaths. Of 15 “other deaths,” which comprise many different causes, 14 occurred in the HDCT group. Most other deaths occurred 10 to 20 years after completion of treatment. Given the wide variety of causes and the different timing of these deaths, however, an association with prior HDCT is not immediately evident. Two patients who died owing to other causes did not receive the HDCT to which they were allocated. Nevertheless, given the number of non-BC deaths, BCSS results should be interpreted with caution, and we focus mainly on the OS results in this update analysis.
In our 20-year follow-up analysis, there was no increase in cumulative risk for a second malignant neoplasm or for incidence of major cardiovascular events after HDCT. Dysrhythmia, hypertension, and hypercholesterolemia occurred more frequently in the HDCT group. Part of the increased incidence of hypertension and hypercholesterolemia may be owing to a more frequent induction of menopause in women receiving HDCT.2 Furthermore, carboplatin may cause vascular endothelial dysfunction possibly related to long-term circulating platinum residuals, making hypertension a possible indirect late effect of HDCT.24 Because the type of dysrhythmia was often not specified, a potential causative role for HDCT is uncertain. Early data on cardiac toxic effects from a small trial did not show a difference in left ventricular ejection fraction change after HDCT or CDCT.12 Consequently, assessment of cardiovascular risk factors, monitoring, and, if necessary, treatment according to guidelines should be considered after HDCT.
Limitations
The nationwide registries of vital status, cancer incidence, pathologic findings, and cause-of-death data in the Netherlands enabled us to perform the present analysis on the efficacy and safety of treatment for all initially included patients in the trial after a unique long-term follow-up. However, this unplanned follow-up analysis does have limitations. First, current standard systemic therapy for BC differs from the regimen used in the control group: taxanes, platinum, and capecitabine were not used; ERBB2-blockade was not yet available for ERBB2-positive BC; and the type (eg, tamoxifen and aromatase inhibitor) and duration of endocrine therapy for ER-positive BC differed from current standards. Therefore, it is likely that the observed benefit of HDCT is smaller when compared with contemporary control treatment.
Second, an indirect effect of HDCT via induction of menopause after HDCT cannot be ruled out based on our data because fewer patients in the HDCT group compared with the CDCT group remained premenopausal 30 months after randomization (5.9% [26 of 442] vs 21.0% [93 of 443]; eTable 1 in Supplement 2). In a post hoc subgroup analysis based on baseline menopausal status, we found a stronger hazard ratio with HDCT for premenopausal vs postmenopausal women but no significant association between menopausal status and treatment (Figure 3). However, because of the inclusion criterion of age being younger than 56 years, the subgroup of postmenopausal patients was small. However, HDCT appeared to be particularly effective for patients with TNBC, which makes an endocrinal cause less likely to be solely responsible for the observed effects. The distribution of menopausal status was similar between BC subtypes (eTable 5 in Supplement 2).
A third limitation of our analysis is that some data were collected retrospectively (eg, incidence of cardiovascular disease), resulting in missing incidence dates and susceptibility to misclassification bias. No baseline data on cardiovascular risk factors were available. Fourth, apart from the number of involved ALNs, other subgroup analyses by BC subtypes were not planned when the original trial was designed in 1993, and these results must be interpreted with caution. In addition, the group of patients with TNBC is small, resulting in wide CIs.
To validate the current findings in light of these limitations, the SUBITO (Substantially Improving the Cure Rate of High-risk BRCA1-like Breast Cancer Patients With Personalized Therapy) trial (NCT02810743) will compare the effectiveness, toxic effects, and cost-effectiveness of HDCT with an optimal control group according to current standard treatment containing dose-dense doxorubicin-cyclophosphamide, paclitaxel-carboplatin, and a PARP (poly–adenosine diphosphate ribose polymerase) inhibitor for patients with stage III BC. Patients with residual disease at surgery will also receive adjuvant capecitabine. Because the observed absolute OS benefit with HDCT in the present analysis was 15.4% for patients with TNBC and 9% for patients with ERBB2-negative BC (both ER-positive and ERBB2-negative and TNBC; eFigure 3 in Supplement 2), the effect of HDCT may be associated with tumors that are deficient in homologous recombination. The SUBITO study, therefore, includes only tumors with a BRCA1-like signature.25,26,27
Conclusions
High-dose chemotherapy provides no long-term OS benefit for unselected patients with stage III BC. However, HDCT did improve long-term BCSS of unselected patients as well as the long-term OS in a subgroup of patients with 10 or more involved ALNs, resulting in 20-year benefit rate of 14.6%.
Trial Protocol
eMethods 1. Additional Details on Data Sources and Collection of Data
eMethods 2. Additional Details on Statistical Analysis
eReferences
eTable 1. Baseline Characteristics
eTable 2. Distribution Number of OS Events in Breast Cancer Subtypes Split for Number of Involved Axillary Lymph Nodes and Between Treatment Groups
eTable 3. Second Malignant Neoplasms
eTable 4. Cardiovascular Events and Cardiovascular Risk Factors
eTable 5. Distribution Number of OS Events in Breast Cancer Subtypes Split for Menopausal Status and Between Treatment Groups
eFigure 1. Breast-Cancer Specific Survival in All Patients
eFigure 2. Cumulative Incidence of a Second Malignancy Treating Death as Competing Risk in All Patients
eFigure 3. Overall Survival in Patients With HER2-Negative Breast Cancer
Data Sharing Statement
References
- 1.Berry DA, Ueno NT, Johnson MM, et al. . High-dose chemotherapy with autologous stem-cell support as adjuvant therapy in breast cancer: overview of 15 randomized trials. J Clin Oncol. 2011;29(24):3214-3223. doi: 10.1200/JCO.2010.32.5910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodenhuis S, Bontenbal M, Beex LVAM, et al. ; Netherlands Working Party on Autologous Transplantation in Solid Tumors . High-dose chemotherapy with hematopoietic stem-cell rescue for high-risk breast cancer. N Engl J Med. 2003;349(1):7-16. doi: 10.1056/NEJMoa022794 [DOI] [PubMed] [Google Scholar]
- 3.Rodenhuis S, Bontenbal M, van Hoesel QGCM, et al. ; Netherlands Working Party on Autologous Transplantation in Solid Tumours . Efficacy of high-dose alkylating chemotherapy in HER2/neu–negative breast cancer. Ann Oncol. 2006;17(4):588-596. doi: 10.1093/annonc/mdl001 [DOI] [PubMed] [Google Scholar]
- 4.Basser RL, Abraham R, To LB, Fox RM, Green MD. Cardiac effects of high-dose epirubicin and cyclophosphamide in women with poor prognosis breast cancer. Ann Oncol. 1999;10(1):53-58. doi: 10.1023/A:1008390203340 [DOI] [PubMed] [Google Scholar]
- 5.Cardinale D, Sandri MT, Martinoni A, et al. . Myocardial injury revealed by plasma troponin I in breast cancer treated with high-dose chemotherapy. Ann Oncol. 2002;13(5):710-715. doi: 10.1093/annonc/mdf170 [DOI] [PubMed] [Google Scholar]
- 6.Tallman MS, Gray R, Robert NJ, et al. . Conventional adjuvant chemotherapy with or without high-dose chemotherapy and autologous stem-cell transplantation in high-risk breast cancer. N Engl J Med. 2003;349(1):17-26. doi: 10.1056/NEJMoa030684 [DOI] [PubMed] [Google Scholar]
- 7.Hanrahan EO, Broglio K, Frye D, et al. . Randomized trial of high-dose chemotherapy and autologous hematopoietic stem cell support for high-risk primary breast carcinoma: follow-up at 12 years. Cancer. 2006;106(11):2327-2336. doi: 10.1002/cncr.21906 [DOI] [PubMed] [Google Scholar]
- 8.Leonard RCF, Lind M, Twelves C, et al. ; Anglo-Celtic Cooperative Oncology Group . Conventional adjuvant chemotherapy versus single-cycle, autograft-supported, high-dose, late-intensification chemotherapy in high-risk breast cancer patients: a randomized trial. J Natl Cancer Inst. 2004;96(14):1076-1083. doi: 10.1093/jnci/djh188 [DOI] [PubMed] [Google Scholar]
- 9.Nitz UA, Mohrmann S, Fischer J, et al. ; West German Study Group . Comparison of rapidly cycled tandem high-dose chemotherapy plus peripheral-blood stem-cell support versus dose-dense conventional chemotherapy for adjuvant treatment of high-risk breast cancer: results of a multicentre phase III trial. Lancet. 2005;366(9501):1935-1944. doi: 10.1016/S0140-6736(05)67784-7 [DOI] [PubMed] [Google Scholar]
- 10.Coombes RC, Howell A, Emson M, et al. . High dose chemotherapy and autologous stem cell transplantation as adjuvant therapy for primary breast cancer patients with four or more lymph nodes involved: long-term results of an international randomised trial. Ann Oncol. 2005;16(5):726-734. doi: 10.1093/annonc/mdi166 [DOI] [PubMed] [Google Scholar]
- 11.Peters WP, Rosner GL, Vredenburgh JJ, et al. . Prospective, randomized comparison of high-dose chemotherapy with stem-cell support versus intermediate-dose chemotherapy after surgery and adjuvant chemotherapy in women with high-risk primary breast cancer: a report of CALGB 9082, SWOG 9114, and NCIC MA-13. J Clin Oncol. 2005;23(10):2191-2200. doi: 10.1200/JCO.2005.10.202 [DOI] [PubMed] [Google Scholar]
- 12.Meinardi MT, van Veldhuisen DJ, Gietema JA, et al. . Prospective evaluation of early cardiac damage induced by epirubicin-containing adjuvant chemotherapy and locoregional radiotherapy in breast cancer patients. J Clin Oncol. 2001;19(10):2746-2753. doi: 10.1200/JCO.2001.19.10.2746 [DOI] [PubMed] [Google Scholar]
- 13.Bergh J, Wiklund T, Erikstein B, et al. . Tailored fluorouracil, epirubicin, and cyclophosphamide compared with marrow-supported high-dose chemotherapy as adjuvant treatment for high-risk breast cancer: a randomised trial: Scandinavian Breast Group 9401 study. Lancet. 2000;356(9239):1384-1391. doi: 10.1016/S0140-6736(00)02841-5 [DOI] [PubMed] [Google Scholar]
- 14.Wilking N, Lidbrink E, Wiklund T, et al. . Long-term follow-up of the SBG 9401 study comparing tailored FEC-based therapy versus marrow-supported high-dose therapy. Ann Oncol. 2007;18(4):694-700. doi: 10.1093/annonc/mdl488 [DOI] [PubMed] [Google Scholar]
- 15.Colleoni M, Sun Z, Price KN, et al. . Annual hazard rates of recurrence for breast cancer during 24 years of follow-up: results from the International Breast Cancer Study Group Trials I to V. J Clin Oncol. 2016;34(9):927-935. doi: 10.1200/JCO.2015.62.3504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pan H, Gray R, Braybrooke J, et al. ; EBCTCG . 20-Year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N Engl J Med. 2017;377(19):1836-1846. doi: 10.1056/NEJMoa1701830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hortobagyi GN, Buzdar AU, Theriault RL, et al. . Randomized trial of high-dose chemotherapy and blood cell autografts for high-risk primary breast carcinoma. J Natl Cancer Inst. 2000;92(3):225-233. doi: 10.1093/jnci/92.3.225 [DOI] [PubMed] [Google Scholar]
- 18.EBCTCG Tamoxifen for early breast cancer: an overview of the randomised trials: Early Breast Cancer Trialists’ Collaborative Group. Lancet. 1998;351(9114):1451-1467. doi: 10.1016/S0140-6736(97)11423-4 [DOI] [PubMed] [Google Scholar]
- 19.Hudis CA, Barlow WE, Costantino JP, et al. . Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. J Clin Oncol. 2007;25(15):2127-2132. doi: 10.1200/JCO.2006.10.3523 [DOI] [PubMed] [Google Scholar]
- 20.Colleoni M, Sun Z, Martinelli G, et al. ; International Breast Cancer Study Group . The effect of endocrine responsiveness on high-risk breast cancer treated with dose-intensive chemotherapy: results of International Breast Cancer Study Group Trial 15-95 after prolonged follow-up. Ann Oncol. 2009;20(8):1344-1351. doi: 10.1093/annonc/mdp024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roche H, Viens P, Biron P, Lotz J-P, Asselain B; PEGASE Group . High-dose chemotherapy for breast cancer: the French PEGASE experience. Cancer Control. 2003;10(1):42-47. doi: 10.1177/107327480301000105 [DOI] [PubMed] [Google Scholar]
- 22.Rettig RA, Jacobson P, Farquhar CM, Aubry WM. False Hope: Bone Marrow Transplantation for Breast Cancer. New York, NY: Oxford University Press; 2007. [Google Scholar]
- 23.Cherny NI, Dafni U, Bogaerts J, et al. . ESMO—magnitude of clinical benefit scale version 1.1. Ann Oncol. 2017;28(10):2340-2366. doi: 10.1093/annonc/mdx310 [DOI] [PubMed] [Google Scholar]
- 24.Cameron AC, Touyz RM, Lang NN. Vascular complications of cancer chemotherapy. Can J Cardiol. 2016;32(7):852-862. doi: 10.1016/j.cjca.2015.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vollebergh MA, Lips EH, Nederlof PM, et al. . Genomic patterns resembling BRCA1- and BRCA2-mutated breast cancers predict benefit of intensified carboplatin-based chemotherapy. Breast Cancer Res. 2014;16(3):R47. doi: 10.1186/bcr3655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vollebergh MA, Lips EH, Nederlof PM, et al. . An aCGH classifier derived from BRCA1-mutated breast cancer and benefit of high-dose platinum-based chemotherapy in HER2-negative breast cancer patients. Ann Oncol. 2011;22(7):1561-1570. doi: 10.1093/annonc/mdq624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schouten PC, Gluz O, Harbeck N, et al. . BRCA1-like profile predicts benefit of tandem high dose epirubicin-cyclophospamide-thiotepa in high risk breast cancer patients randomized in the WSG-AM01 trial. Int J Cancer. 2016;139(4):882-889. doi: 10.1002/ijc.30078 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eMethods 1. Additional Details on Data Sources and Collection of Data
eMethods 2. Additional Details on Statistical Analysis
eReferences
eTable 1. Baseline Characteristics
eTable 2. Distribution Number of OS Events in Breast Cancer Subtypes Split for Number of Involved Axillary Lymph Nodes and Between Treatment Groups
eTable 3. Second Malignant Neoplasms
eTable 4. Cardiovascular Events and Cardiovascular Risk Factors
eTable 5. Distribution Number of OS Events in Breast Cancer Subtypes Split for Menopausal Status and Between Treatment Groups
eFigure 1. Breast-Cancer Specific Survival in All Patients
eFigure 2. Cumulative Incidence of a Second Malignancy Treating Death as Competing Risk in All Patients
eFigure 3. Overall Survival in Patients With HER2-Negative Breast Cancer
Data Sharing Statement