Key Points
Question
What are the clinical effects of zilucoplan, a subcutaneously self-administered macrocyclic peptide inhibitor of complement component 5, in a broad population of patients with moderate to severe acetylcholine receptor autoantibody–positive generalized myasthenia gravis?
Findings
In a randomized, double-blind, placebo-controlled, multicenter phase 2 trial, zilucoplan yielded rapid, clinically meaningful, statistically significant, and sustained improvements in the primary and key secondary end points. Near-complete complement inhibition was associated with a faster onset and greater magnitude of benefit than submaximal complement inhibition, and favorable safety and tolerability were observed.
Meaning
The findings support a potential therapeutic role for zilucoplan in generalized myasthenia gravis and further evaluation in a phase 3 study.
Abstract
Importance
Many patients with generalized myasthenia gravis (gMG) have substantial clinical disability, persistent disease burden, and adverse effects attributable to chronic immunosuppression. Therefore, there is a significant need for targeted, well-tolerated therapies with the potential to improve disease control and enhance quality of life.
Objective
To evaluate the clinical effects of zilucoplan, a subcutaneously (SC) self-administered macrocyclic peptide inhibitor of complement component 5, in a broad population of patients with moderate to severe gMG.
Design, Setting, and Participants
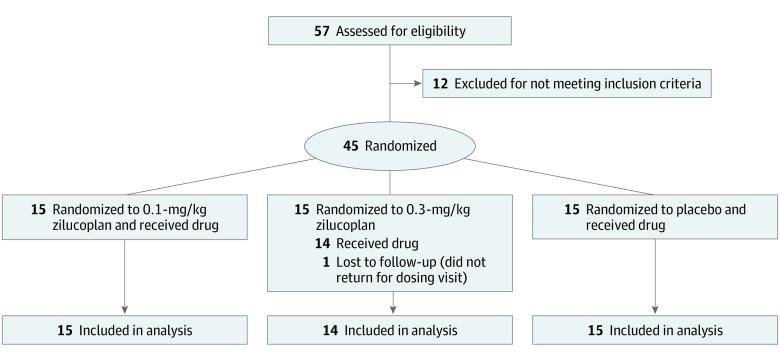
This randomized, double-blind, placebo-controlled phase 2 clinical trial at 25 study sites across North America recruited participants between December 2017 and August 2018. Fifty-seven patients were screened, of whom 12 did not meet inclusion criteria and 1 was lost to follow-up after randomization but before receiving study drug, resulting in a total of 44 acetylcholine receptor autoantibody (AChR-Ab)–positive patients with gMG with baseline Quantitative Myasthenia Gravis (QMG) scores of at least 12, regardless of treatment history.
Interventions
Patients were randomized 1:1:1 to a daily SC self-injection of placebo, 0.1-mg/kg zilucoplan, or 0.3-mg/kg zilucoplan for 12 weeks.
Main Outcomes and Measures
The primary and key secondary end points were the change from baseline to week 12 in QMG and MG Activities of Daily Living scores, respectively. Significance testing was prespecified at a 1-sided α of .10. Safety and tolerability were also assessed.
Results
The study of 44 patients was well balanced across the 3 treatment arms with respect to key demographic and disease-specific variables. The mean age of patients across all 3 treatment groups ranged from 45.5 to 54.6 years and most patients were white (average proportions across 3 treatment groups: 78.6%-86.7%). Clinically meaningful and statistically significant improvements in primary and key secondary efficacy end points were observed. Zilucoplan at a dose of 0.3 mg/kg SC daily resulted in a mean reduction from baseline of 6.0 points in the QMG score (placebo-corrected change, –2.8; P = .05) and 3.4 points in the MG Activities of Daily Living score (placebo-corrected change, –2.3; P = .04). Clinically meaningful and statistically significant improvements were also observed in other secondary end points, the MG Composite and MG Quality-of-Life scores. Outcomes for the 0.1-mg/kg SC daily dose were also statistically significant but slower in onset and less pronounced than with the 0.3-mg/kg dose. Rescue therapy (intravenous immunoglobulin or plasma exchange) was required in 3 of 15, 1 of 15, and 0 of 14 participants in the placebo, 0.1-mg/kg zilucoplan, and 0.3-mg/kg zilucoplan arms, respectively. Zilucoplan was observed to have a favorable safety and tolerability profile.
Conclusions and Relevance
Zilucoplan yielded rapid, meaningful, and sustained improvements over 12 weeks in a broad population of patients with moderate to severe AChR-Ab–positive gMG. Near-complete complement inhibition appeared superior to submaximal inhibition. The observed safety and tolerability profile of zilucoplan was favorable.
Trial Registration
ClinicalTrials.gov Identifier: NCT03315130.
This randomized clinical trial evaluates the clinical effects of zilucoplan, a subcutaneously self-administered macrocyclic peptide inhibitor of complement component 5, in a broad population of patients with moderate to severe generalized myasthenia gravis.
Introduction
Myasthenia gravis (MG) is a rare autoimmune disease characterized by the production of autoantibodies targeting proteins that are critical for normal neuromuscular synaptic transmission.1 The most common target of autoantibodies in MG is the nicotinic acetylcholine receptor (AChR), with approximately 80% to 88% of all patients with generalized MG (gMG) having detectable anti-AChR antibodies.2,3,4,5
There is substantial evidence supporting a role for the terminal complement cascade in the pathogenesis of all patients with AChR autoantibody (AChR-Ab)–positive gMG. Activation of the classical complement cascade is initiated by AChR-specific autoantibodies (typically complement-activating immunoglobulin [IgG1 and IgG3 isotypes]) but not by anti–muscle-specific kinase autoantibodies (typically IgG4 isotype, which does not bind complement).2,6,7 Complement-mediated structural disruption and simplification of the postsynaptic membrane and reduced AChR density at the postsynaptic membrane are the ultrastructural correlates of impaired neuromuscular signal transduction in gMG.8,9 A phase 3 clinical trial with eculizumab, a monoclonal antibody complement component 5 (C5) inhibitor, restricted to patients considered refractory to prior therapies, validated complement inhibition as a new therapeutic approach in AChR-Ab–positive gMG that led to its approval by the US Food and Drug Administration in 2017.10,11,12
Zilucoplan is a small (3.5 kDa), 15–amino acid macrocyclic peptide that binds to C5 with high affinity and specificity. This prevents the cleavage of C5 into complement components C5a and C5b. Zilucoplan binds to the domain of C5 that corresponds to C5b and thereby blocks binding of C5b to complement component C6.13 This dual mechanism of action prevents activation of the terminal complement pathway and prevents assembly of the terminal complement complex (previously known as membrane attack complex [C5b-9]), a large hydrophilic pore that can damage and destroy the postsynaptic membrane, disrupt ionic channel conductance, and impair neuromuscular transmission.2,14 Complement inhibition represents a targeted approach toward addressing the main mechanism of tissue damage in gMG. This contrasts with existing therapies, which focus on nonspecifically augmenting the AChR signal (eg, pyridostigmine) or nonspecifically suppressing the autoimmune response (eg, corticosteroids and other immunosuppressants).15 These treatments lack strong evidence from clinical trials to support their efficacy,15 are often poorly tolerated, and can be associated with considerable long-term toxicities.1 Nonspecific depletion of circulating antibodies by plasma exchange (PLEX) or intravenous immunoglobulin (IVIg), with the latter also considered to have broader immunomodulatory effects,16 carries a significant treatment burden and can only partially and transiently reduce autoantibody load, given that complete antibody elimination is not feasible and would incur significant safety risk. While these medications have had a meaningful effect on survival and quality of life of patients with gMG over the last 30 years, they are fraught with significant short-term and long-term toxicities and considerable treatment burden, and improved treatment options are needed for patients with gMG.1
We conducted a randomized, double-blind, placebo-controlled, multicenter phase 2 clinical trial to explore the clinical effect of self-administered subcutaneous (SC) zilucoplan, at 0.1 mg/kg or 0.3 mg/kg daily, in adult patients with AChR-Ab–positive gMG. In addition, this study was designed to determine whether near-complete complement inhibition is necessary to achieve maximal clinical benefit in patients with gMG, as well as whether complement inhibition can be effective across a broad spectrum of patients with AChR-Ab–positive gMG regardless of duration of disease, treatment history, or response to prior therapies (ie, including both refractory and nonrefractory patients).
Methods
Trial Design
This was a randomized, double-blind, placebo-controlled, multicenter phase 2 clinical trial designed to explore the clinical effects of zilucoplan in adult patients with AChR-Ab–positive gMG. Participants were randomly assigned 1:1:1 to zilucoplan, 0.1 mg/kg or 0.3 mg/kg, or placebo daily over 12 weeks. A study period of 12 weeks was considered of appropriate and sufficient duration based on the characteristics of response to complement inhibition observed in prior MG studies.10,17 Eligible participants had the opportunity to enter an open-label extension thereafter. Participants were evaluated at baseline and weeks 1, 2, 4, 8, and 12.
Participants were required to continue taking their existing standard-of-care treatments for MG, with no change in dose, during the 12-week placebo-controlled study period. In the event of worsening MG, administration of IVIg or PLEX was allowed as rescue therapy per investigator discretion.
The study was conducted in accordance with the International Conference on Harmonisation Guideline for Good Clinical Practice and applicable regulatory standards in the United States and Canada. Independent ethics committees or institutional review boards provided written approval for the study protocol and all amendments. The formal trial protocol can be found in Supplement 1.
Participants
Twenty-five study sites across North America recruited participants. All participants were required to provide written informed consent. Participants were enrolled between December 2017 and August 2018.
Key eligibility criteria included age 18 to 85 years; clinically confirmed diagnosis of MG (Myasthenia Gravis Foundation of America [MGFA] Class II-IVa)18; presence of AChR-Ab; Quantitative Myasthenia Gravis (QMG) score of at least 12 points with at least 4 items having a score of 2 or more; no change in standard-of-care therapy for MG, including pyridostigmine, corticosteroids, and immunosuppressive therapy, for 4 weeks before randomization; no thymectomy within the past 6 months; no IVIg or PLEX administration for 4 weeks before randomization; and no rituximab treatment for 6 months before randomization. A full list of inclusion and exclusion criteria can be found in the protocol.
Intervention
The study drug (0.1-mg/kg or 0.3-mg/kg zilucoplan or matching placebo) was provided in identical prefilled syringes (BD UltraSafe PLUS; BD Medical) for daily SC self-injection, using a 29-gauge autoretracting needle. Nominal doses of zilucoplan were provided in 3 weight-bracketed presentations. Dosing was based on each participant’s weight at screening. Each prefilled syringe contained a single dose of zilucoplan in a volume less than 1 mL. Participants were instructed and trained on how to self-inject the study drug at the baseline visit and subsequently continued to self-administer the study drug at home throughout the 12-week study period. Electronic device–based reminders were used to enhance compliance with daily self-administration.
Randomization and Blinding
Participants were randomized 1:1:1 to placebo, 0.1-mg/kg zilucoplan, or 0.3-mg/kg zilucoplan using a central computerized randomization algorithm at an independent vendor (Bioclinica). Randomization was stratified based on the screening QMG score (≤17 vs ≥18). All site investigators, participants, and study personnel, including the sponsor and their representatives, remained blinded to treatment assignment for the study duration and until after database lock.
Outcome Measures
Efficacy Assessments
The prespecified primary end point was the change in QMG score19 from baseline to week 12. The QMG score is a 13-item categorical scale assessing muscle weakness, with each item scored from 0 to 3 points. A total score of 0 represents no weakness, and a score of 39 represents severe weakness. Improvements in the QMG score of 2 to 3 points may be considered clinically meaningful depending on baseline disease severity.19,20 Participants were required to be not receiving pyridostigmine for at least 10 hours before performing each QMG assessment.
The key secondary end point was the change in MG Activities of Daily Living (MG-ADL) score21 from baseline to week 12. The MG-ADL is an 8-item categorical scale that assesses the effect of MG on daily functions that are typically affected by the disease, with each item scored from 0 to 3 points. A total score of 0 represents normal function, and a score of 24 represents a severe effect on activities of daily living from MG (total score of 0-24). The MG-ADL has been shown to correlate with other validated MG outcome measures including the QMG, the MG Composite (MGC), and the 15-item Myasthenia Gravis Quality-of-Life Revised Scale (MG-QoL15r).21,22,23,24 A 2-point improvement in MG-ADL score is considered clinically meaningful.24
Additional secondary end points included the change in MGC25 and MG-QoL15r23,26 scores from baseline to week 12; the proportion of patients with at least a 3-point improvement in the QMG score; and the proportion of participants requiring rescue therapy with PLEX or IVIg. In addition, the proportion of patients with minimal symptom expression (MSE; defined as MG-ADL of 0 or 1),27 a newly defined end point published after finalization of the protocol, was added as a predefined end point in the statistical analysis plan before database lock. All clinical evaluators underwent standardized training on the measurement of the primary and secondary end points, and patients were expected to be assessed by the same evaluator throughout the study.
Pharmacodynamic and Safety Assessments
Plasma samples were analyzed for the ability of zilucoplan to inhibit classical complement pathway-mediated hemolysis ex vivo in an antibody-sensitized sheep red blood cell lysis assay.28 Safety assessments included clinical evaluations, adverse event (AE) collection, standard laboratory assessments, evaluation of injection-site reactions (ISRs), and immunogenicity. Long-term pharmacologic inhibition of C5 with eculizumab, as well as inherited deficiencies of terminal complement pathway proteins, are both known to increase the susceptibility to infection with encapsulated bacteria, in particular Neisseria meningitidis.29,30 Therefore, all participants were required to receive meningococcal vaccination according to local standard of care at least 2 weeks before starting study treatment.
Statistical Methods and Sample Size Calculation
For the primary efficacy end point (change from baseline to week 12 in QMG score), group differences were assessed using an analysis of covariance model, with treatment as a factor and baseline QMG score as a covariate. For the continuous primary and secondary efficacy end points, least-squares means are presented. Observations occurring after rescue medication use were censored and imputed using the last observation carried forward method. The primary comparison was 0.3-mg/kg zilucoplan vs placebo at a 1-sided α of .10. Continuous secondary end points were analyzed similarly. Categorical end points were analyzed using Fisher exact test.
The planned enrollment of 36 participants provided approximately 81% power to detect a difference between groups on the primary end point (mean change from baseline to week 12 in QMG score), assuming a group mean difference of 4.5 in the QMG score and an SD of 5.0.
Results
Baseline Data
Forty-five of 57 screened patients were randomized (Figure 1). One patient was lost to follow-up shortly after randomization but before receiving the study drug. Owing to high demand for participation and a rapid surge in screenings over the last 2 months of the enrollment period, the study recruited more participants (n = 45) than originally planned (n = 36). A total of 44 patients received doses in the study, all of whom were included in the analyses (Figure 1). All participants completed the 12-week study period with no early withdrawals.
Figure 1. CONSORT Diagram of the Zilucoplan Phase 2 Study in Patients With Acetylcholine Receptor Autoantibody (AChR-Ab)–Positive Generalized Myasthenia Gravis.
Two patients discontinued the study drug before week 12: 1 placebo-treated patient discontinued owing to an adverse event of worsening myasthenia gravis and 1 patient receiving zilucoplan, 0.3 mg/kg, discontinued because of a prolonged admission at an outside hospital owing to exacerbation of preexisting diverticulitis with paracolic abscess.
The study population was representative of a broad range of patients with gMG and was well balanced across the 3 treatment arms with respect to key demographic and disease-specific variables (Table 1). Time since diagnosis of MG ranged widely, from 1 month to 26 years. Except for 1 patient who was naive to all standard-of-care therapies for MG (Table 1), patients had previously received pyridostigmine (93% to 100% for the 3 treatment arms), corticosteroids (87% to 100%), and/or immunosuppressive treatment (64% to 80%). More than one-half of patients had also been treated with IVIg (53% to 71%) and/or PLEX (47% to 60%). A small number of patients had previously received less frequently used immunosuppressive therapies, including cyclosporine (n = 4), rituximab (n = 3), methotrexate (n = 2), tacrolimus (n = 2), cyclophosphamide (n = 1), and eculizumab (n = 1; remote history of exposure 8 years before randomization during a clinical trial). One-third of patients had a history of thymoma (27% to 33%), and fewer than half had undergone thymectomy (33% to 53%).
Table 1. Demographics and Baseline Characteristics.
| Characteristic | No. (%) | ||
|---|---|---|---|
| Placebo (n = 15) | Zilucoplan | ||
| 0.1 mg/kg (n = 15) | 0.3 mg/kg (n = 14) | ||
| Age, mean (SD), y | 48.4 (15.7) | 45.5 (15.7) | 54.6 (15.5) |
| Male | 4 (26.7) | 7 (46.7) | 10 (71.4) |
| Weight, mean (SD), kg | 85.3 (21.44) | 93.7 (24.72) | 110.9 (30.79) |
| BMI, mean (SD) | 30.9 (7.39) | 32.8 (6.55) | 36.0 (8.24) |
| Race/ethnicity | |||
| White | 12 (80.0) | 13 (86.7) | 11 (78.6) |
| Asian | 1 (6.7) | 0 | 1 (7.1) |
| Black/African American | 2 (13.3) | 2 (13.3) | 2 (14.3) |
| MGFA class at screening | |||
| II | 7 (46.7) | 5 (33.3) | 5 (35.7) |
| III | 8 (53.3) | 10 (66.7) | 5 (35.7) |
| IV | 0 | 0 | 4 (28.6) |
| Age at disease onset, mean (SD), y | 40.3 (17.79) | 37.3 (16.04) | 46.9 (19.48) |
| Duration of disease, median (range), y | 6.3 (0.1-20.9) | 6.5 (1.6-24.1) | 5.3 (0.5-26.0) |
| Baseline score, mean (SD) | |||
| QMG | 18.7 (4.0) | 18.7 (4.0) | 19.1 (5.1) |
| MG-ADL | 8.8 (3.6) | 6.9 (3.3) | 7.6 (2.6) |
| MGC | 18.7 (5.7) | 14.5 (6.3) | 14.6 (6.3) |
| MG-QoL15r | 15.9 (7.4) | 19.1 (5.0) | 16.5 (7.3) |
| Prior MG therapies (standard of care) | |||
| Pyridostigmine | 14 (93.3) | 15 (100.0) | 14 (100.0) |
| Corticosteroids | 13 (86.7) | 13 (86.7) | 14 (100.0) |
| Immunosuppressants | 12 (80.0) | 12 (80.0) | 9 (64.3) |
| Prior IVIg | 9 (60.0) | 8 (53.3) | 10 (71.4) |
| Prior plasma exchange | 7 (46.7) | 9 (60.0) | 7 (50.0) |
| Diagnosis of thymoma | 4 (26.7) | 5 (33.3) | 4 (28.6) |
| Prior thymectomy | 5 (33.3) | 8 (53.3) | 7 (50.0) |
| Prior MG crisis requiring intubation | 3 (20.0) | 4 (26.7) | 2 (14.3) |
| MG therapy at baseline | |||
| Pyridostigmine | 14 (93.3) | 14 (93.3) | 13 (92.9) |
| Prednisone | 11 (73.3) | 9 (60.0) | 11 (78.6) |
| Azathioprine | 3 (20.0) | 3 (20.0) | 3 (21.4) |
| Mycophenolate mofetil | 5 (33.3) | 6 (40.0) | 1 (7.1) |
| Cyclosporine | 0 | 1 (6.7) | 0 |
| Tacrolimus | 1 (6.7) | 0 | 0 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); IVIg, intravenous immunoglobulin; MG, myasthenia gravis; MG-ADL, Myasthenia Gravis Activities of Daily Living; MGC, Myasthenia Gravis Composite; MGFA, Myasthenia Gravis Foundation of America; MG-QoL15r, Myasthenia Gravis Quality-of-Life Revised Scale; QMG, Quantitative Myasthenia Gravis.
Mean baseline QMG, MG-ADL, MGC, and MG-QoL15r scores confirmed that, as intended, moderately to severely affected patients were enrolled (mean [SD] baseline QMG score, 18.8 [4.3]), with approximately half of the population being MGFA class III or IV at baseline. All disease severity measures were well balanced across treatment arms except for the MG-QoL15r, which was approximately 3 points higher in the 0.1-mg/kg zilucoplan arm than in the other 2 arms at baseline.
Efficacy Analyses
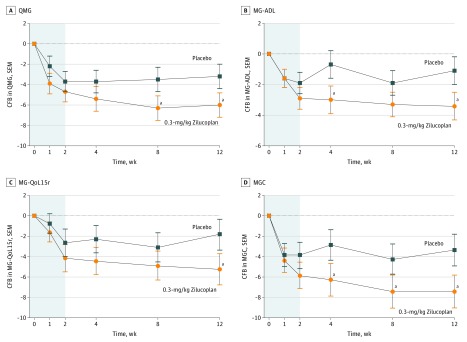
The study met its prespecified primary efficacy end point, showing a rapid, statistically significant and clinically meaningful difference in the QMG score between the 0.3-mg/kg zilucoplan and placebo arms, favoring zilucoplan (Figure 2A). The mean change from baseline to week 12 in the QMG score was –6.0 points in the 0.3-mg/kg zilucoplan arm compared with –3.2 points in the placebo arm, with a mean difference of –2.8 (P = .05; Table 2). The onset of action was rapid, with separation of the 0.3-mg/kg arm from the placebo arm beginning after 1 week (Figure 2A).
Figure 2. Change From Baseline Over 12 Weeks for 0.3-mg/kg Zilucoplan vs Placebo in Quantitative Myasthenia Gravis (QMG), Myasthenia Gravis Activities of Daily Living (MG-ADL), Myasthenia Gravis Quality-of-Life Revised Scale (MG-QoL15r), and Myasthenia Gravis Composite (MGC).
CFB indicates change from baseline.
aP < .10.
Table 2. Clinical Efficacy Outcomes at Week 12.
| Variable | Placebo, Mean (SEM) | 0.3-mg/kg Zilucoplan, Mean (SEM) | 0.3-mg/kg Zilucoplan vs Placebo | 0.1-mg/kg Zilucoplan, Mean (SEM) | 0.1-mg/kg Zilucoplan vs Placebo | ||
|---|---|---|---|---|---|---|---|
| Difference, Mean (SEM) | P Valuea | Difference, Mean (SEM) | P Valuea | ||||
| No. | 15 | 14 | NA | NA | 15 | NA | NA |
| QMG | –3.2 (1.2) | –6.0 (1.2) | –2.8 (1.7) | .05 | –5.5 (1.2) | –2.3 (1.7) | .09 |
| MG-ADL | –1.1 (0.9) | –3.4 (0.9) | –2.3 (1.3) | .04 | –3.3 (0.9) | –2.2 (1.3) | .05 |
| MG-QoL15r | –2.1 (1.7) | –5.9 (1.7) | –3.7 (2.4) | .06 | –7.4 (1.7) | –5.3 (2.4) | .02 |
| MGC | –3.3 (1.6) | –7.4 (1.6) | –4.1 (2.2) | .04 | –5.3 (1.5) | –2.0 (2.2) | .19 |
| QMG decrease ≥3, No. (%) | 8 (53.3) | 10 (71.4) | NA | .27 | 10 (66.7) | NA | .36 |
| Rescue received, No. (%) | 3 (20.0) | 0 | NA | .12 | 1 (6.7) | NA | .30 |
Abbreviations: MG-ADL, Myasthenia Gravis Activities of Daily Living; MGC, Myasthenia Gravis Composite; MG-QoL15r, Myasthenia Gravis Quality-of-Life Revised Scale; NA, not applicable; QMG, Quantitative Myasthenia Gravis.
One-sided P values.
The magnitude of the response with 0.1-mg/kg zilucoplan, although still clinically meaningful and statistically significant, was less pronounced and exhibited a slower onset of action, with separation from placebo beginning only after 4 weeks of therapy (eFigure 1A in Supplement 2).
Consistent with the primary end point, the MG-ADL showed a rapid, clinically meaningful, and statistically significant mean reduction from baseline of 3.4 points (placebo-corrected change, –2.3; P = .04), favoring 0.3-mg/kg zilucoplan at week 12 (Table 2 and Figure 2B). Similarly, the response with the 0.1-mg/kg zilucoplan dose was delayed and less pronounced, although still statistically significant when compared with placebo at week 12 (Table 2; eFigure 1B in Supplement 2).
The MG-QoL15r and MGC followed a similar pattern (Table 2 and Figure 2C and D; eFigure 1C and D in Supplement 2), although the MG-QoL15r showed a reversal of the dose response, perhaps a result of higher baseline scores in the 0.1-mg/kg zilucoplan arm (Table 1). An analysis of covariance sensitivity analysis evaluated the relevance of additional covariates on QMG and MG-ADL response and found that only the baseline value of each outcome variable proved significant (data not shown). Additional covariates, including age, sex, duration of disease, treatment history, prior thymectomy, and history of thymoma, were not significant in the analysis of covariance model (eTable 1 in Supplement 2).
A remarkable consistency in the response with zilucoplan was observed across all continuous clinical outcome measures tested (QMG, MG-ADL, MGC, and MG-QoL15r) with continued improvement through week 8 (eFigure 2A in Supplement 2). This contrasted with the placebo arm, where the effect was most pronounced during the first week, with some fluctuations thereafter (eFigure 2B in Supplement 2).
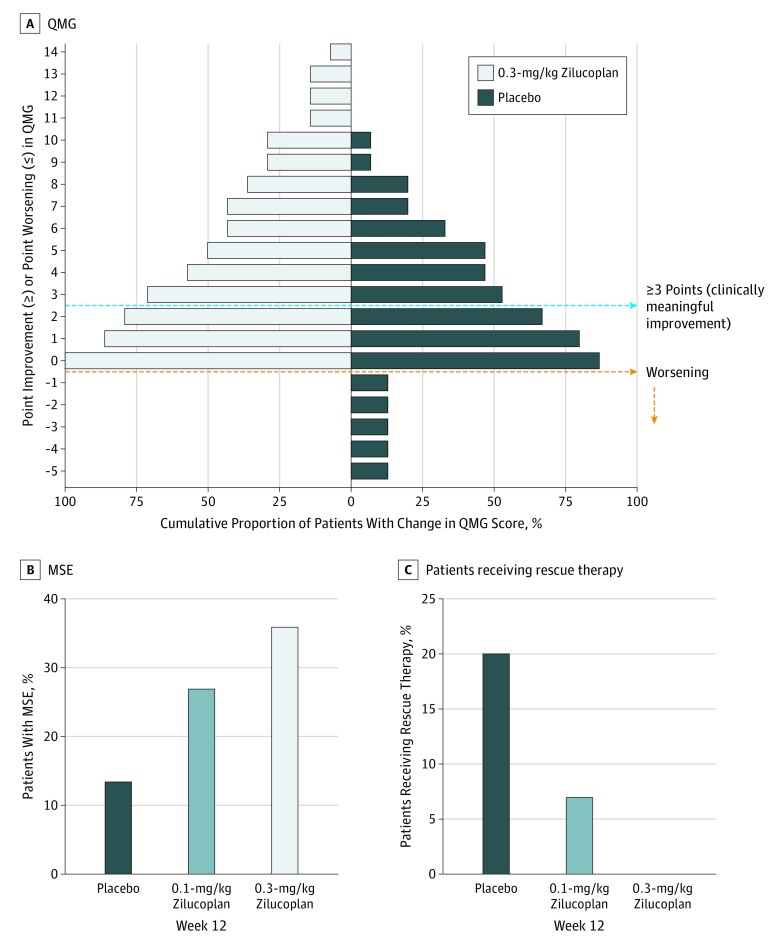
A higher proportion of patients achieved at least a 3-point difference in the QMG score with zilucoplan compared with placebo, although this difference did not reach statistical significance. More patients receiving zilucoplan improved by progressively higher scores, and, in contrast to placebo, none in the 0.3-mg/kg zilucoplan arm experienced worsening (Figure 3A). The proportion of participants achieving MSE (defined as MG-ADL of 0 or 1) was also higher with 0.3-mg/kg zilucoplan (n = 5 [35.7%]) than with 0.1-mg/kg zilucoplan (n = 4 [26.7%]) or placebo (n = 2 [13.3%]) (Figure 3B), although this did not reach statistical significance.
Figure 3. Categorical End Points.
A, Responder analysis for Quantitative Myasthenia Gravis (QMG) score with 0.3-mg/kg zilucoplan daily subcutaneously. B, Minimal symptom expression (MSE), defined as Myasthenia Gravis Activities of Daily Living score of 0 or 1 at 12 weeks. C, Patients who received treatment with rescue therapy with intravenous immunoglobulin or plasma exchange.
All participants remained receiving stable doses of their prior gMG therapy. Rescue therapy (IVIg or PLEX only) was administered per investigator’s discretion in 3 of 15 participants (20%) receiving placebo, 1 of 15 (7%) receiving 0.1-mg/kg zilucoplan, and none (0 of 14) receiving 0.3-mg/kg zilucoplan (Figure 3C). Although not statistically significant, the trend in these results is consistent with other efficacy analyses.
Pharmacodynamic Analyses
Daily self-administered SC 0.3-mg/kg zilucoplan achieved rapid, sustained, and near-complete complement inhibition as measured in the sheep red blood cell lysis assay, with greater than 97% inhibition at trough. In contrast, the 0.1-mg/kg zilucoplan dose achieved rapid, sustained but submaximal (approximately 88%) complement inhibition at trough (eFigure 3 in Supplement 2).
Safety Analyses
All 44 of 45 randomized patients who received the study drug completed the 12-week placebo-controlled study. The study drug was well tolerated, with a low incidence of local ISRs (2 of 15, 4 of 15, and 3 of 14 patients receiving placebo, 0.1-mg/kg zilucoplan, and 0.3-mg/kg zilucoplan, respectively). The only moderate ISR occurred in a placebo-treated patient. All other ISRs were mild, and there was no difference in patterns between the zilucoplan and placebo arms. Thirty-nine participants reported treatment-emergent AEs, with no apparent pattern across treatment arms (eTable 2 in Supplement 2). Most events were mild, considered unrelated to the study drug, and resolved spontaneously without modification of study drug administration. Eight serious AEs were reported, none of which were considered related to the study drug: 3 in the placebo arm, 0 in the 0.1-mg/kg zilucoplan arm, and 5 in the 0.3-mg/kg zilucoplan arm. Two patients discontinued the study drug before week 12: 1 placebo-treated patient owing to an AE of worsening MG and 1 patient receiving 0.3-mg/kg zilucoplan because of a prolonged admission at an outside hospital owing to exacerbation of preexisting diverticulitis with paracolic abscess. There were no meningococcal infections, life-threatening AEs, or deaths during the study. No antizilucoplan antibodies were detected.
Discussion
The SC peptide C5 inhibitor zilucoplan met the prespecified primary efficacy end point and demonstrated a favorable safety and tolerability profile in this multicenter, randomized, double-blind, placebo-controlled phase 2 clinical trial, assessing a broad spectrum of patients with moderate to severe gMG regardless of their treatment history.
Consistent with the primary efficacy analysis, all continuous secondary end points also demonstrated clinically meaningful and statistically significant improvements except for the MGC score between the 0.1-mg/kg zilucoplan and placebo arms. The proportion of participants requiring rescue therapy (a proxy for patients’ use of health care resources) and the proportion of participants achieving MSE also favored zilucoplan over placebo. The totality of the clinical efficacy data supports further investigation of the 0.3-mg/kg daily SC dose of zilucoplan, which showed a more rapid and profound improvement in clinical disease measures compared with the 0.1-mg/kg dose.
Although none of the participants receiving the 0.3-mg/kg dose deteriorated, and most improved significantly, it is interesting that 28% of participants did not improve by the minimal clinically important difference of 3 points on the QMG. We speculate that failure to respond to near-complete C5 inhibition in this subset of patients may be attributed to one or more of the following factors: insensitivity of the outcome measures to detect clinical improvement; previously unrecognized fixed weakness; or the complement-independent effects of blocking or modulating autoantibodies that sterically hinder binding of ACh to the receptor or decrease AChR density, respectively.2,31
Until this study, to our knowledge, it was not known whether complete inhibition of the terminal complement pathway is necessary to achieve optimal clinical response in patients with AChR-Ab–positive gMG. Our data suggest that maximal complement inhibition is necessary to provide rapid and pronounced disease suppression, although submaximal inhibition was still superior to placebo.
Although cross-study comparisons of clinical effects must be made with caution, the magnitude of the clinical effect of zilucoplan in this study appears similar to that observed in prior studies of the complement inhibitor eculizumab conducted in a restricted population of patients defined as having refractory gMG.10,17 Importantly, this study provides evidence for the efficacy of C5 inhibition in a broader population of patients with moderate to severe gMG than were enrolled in the eculizumab studies, including those early in their disease course, without regard to failure of prior therapies, and inclusive of patients with a history of thymoma. This observation is important because in gMG, disease severity frequently peaks within the first few years after diagnosis, before all treatment options have been exhausted32 and before patients may be formally declared treatment refractory. A 2019 analysis33 of the MGFA Patient Registry showed that many patients with MG, including those who have not yet been treated with multiple immunosuppressive therapies, experience considerable disease burden.33 Taken together, these findings indicate that there is no biologic relevance of refractory disease with respect to complement inhibition and no scientific rationale for C5 inhibitors to be reserved for use as a last-line option only. Indeed, C5 inhibition deployed earlier in the disease course may have the potential to reduce or eliminate the need for more invasive treatments, as reflected by abrogation of the need for rescue therapy in the high-dose zilucoplan arm.
Strengths and Limitations
As a self-administered C5 inhibitor, zilucoplan was well tolerated, and the safety profile was similar across the zilucoplan and placebo arms. All participants were vaccinated against N meningitidis, and no cases of Neisseria infection were observed during the study. However, the overall exposure was too limited to allow for a complete characterization of the risk profile given that the absolute incidence rate of Neisseria infection associated with C5 inhibition is extremely low.30 Furthermore, it is noteworthy that zilucoplan administration was not associated with any AEs suggestive of local or systemic inflammatory reactions.
Our phase 2 study was designed to provide an initial assessment of clinical activity and to support dose selection for phase 3. Given the similarity in the safety profile for both doses, as well as the more rapid and pronounced clinical effect seen with 0.3-mg/kg zilucoplan, this dose has been selected for further testing in a pivotal phase 3 study (ClinicalTrials.gov Identifier: NCT04115293).
Conclusions
In summary, our study suggests that complement inhibition appears to be effective across a broad spectrum of patients with moderate to severe AChR-Ab–positive gMG, regardless of prior therapies; that near-complete complement inhibition is superior to submaximal complement inhibition; and that the safety and tolerability profile of zilucoplan in gMG appears to be favorable. Confirmation of the clinical effect of zilucoplan in gMG will be further assessed through the open-label long-term extension of the phase 2 study and an upcoming registrational phase 3 clinical program.
Trial Protocol.
eTable 1. Efficacy of Zilucoplan Is Independent of Prior Therapies
eTable 2. Treatment-Emergent AEs and Injection-Site Reactions
eFigure 1. Change From Baseline Over 12 Weeks for 0.1 mg/kg Zilucoplan and Placebo in (A) QMG (B) MG-ADL, (C) MG-QoL15r, and (D) MGC Scores
eFigure 2. Radar Plots Suggest Consistency of Improvement Across All 4 End Points When Treated With (A) 0.3 mg/kg Zilucoplan Daily Subcutaneously vs (B) Placebo.
eFigure 3. Mean Complement Activity as Measured by Sheep Red Blood Cell Assay (% Hemolysis)
Data Sharing Statement.
References
- 1.Gilhus NE. Myasthenia gravis. N Engl J Med. 2016;375(26):2570-2581. doi: 10.1056/NEJMra1602678 [DOI] [PubMed] [Google Scholar]
- 2.Gilhus NE, Tzartos S, Evoli A, Palace J, Burns TM, Verschuuren JJGM. Myasthenia gravis. Nat Rev Dis Primers. 2019;5(1):30. doi: 10.1038/s41572-019-0079-y [DOI] [PubMed] [Google Scholar]
- 3.Lindstrom JM, Seybold ME, Lennon VA, Whittingham S, Duane DD. Antibody to acetylcholine receptor in myasthenia gravis: prevalence, clinical correlates, and diagnostic value. Neurology. 1976;26(11):1054-1059. doi: 10.1212/WNL.26.11.1054 [DOI] [PubMed] [Google Scholar]
- 4.Meriggioli MN, Sanders DB. Autoimmune myasthenia gravis: emerging clinical and biological heterogeneity. Lancet Neurol. 2009;8(5):475-490. doi: 10.1016/S1474-4422(09)70063-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vincent A, Newsom-Davis J. Acetylcholine receptor antibody as a diagnostic test for myasthenia gravis: results in 153 validated cases and 2967 diagnostic assays. J Neurol Neurosurg Psychiatry. 1985;48(12):1246-1252. doi: 10.1136/jnnp.48.12.1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McConville J, Farrugia ME, Beeson D, et al. Detection and characterization of MuSK antibodies in seronegative myasthenia gravis. Ann Neurol. 2004;55(4):580-584. doi: 10.1002/ana.20061 [DOI] [PubMed] [Google Scholar]
- 7.Rødgaard A, Nielsen FC, Djurup R, Somnier F, Gammeltoft S. Acetylcholine receptor antibody in myasthenia gravis: predominance of IgG subclasses 1 and 3. Clin Exp Immunol. 1987;67(1):82-88. https://www.ncbi.nlm.nih.gov/pubmed/3621677. [PMC free article] [PubMed] [Google Scholar]
- 8.Conti-Fine BM, Milani M, Kaminski HJ. Myasthenia gravis: past, present, and future. J Clin Invest. 2006;116(11):2843-2854. doi: 10.1172/JCI29894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engel AG, Lindstrom JM, Lambert EH, Lennon VA. Ultrastructural localization of the acetylcholine receptor in myasthenia gravis and in its experimental autoimmune model. Neurology. 1977;27(4):307-315. doi: 10.1212/WNL.27.4.307 [DOI] [PubMed] [Google Scholar]
- 10.Howard JF Jr, Utsugisawa K, Benatar M, et al. ; REGAIN Study Group . Safety and efficacy of eculizumab in anti-acetylcholine receptor antibody-positive refractory generalised myasthenia gravis (REGAIN): a phase 3, randomised, double-blind, placebo-controlled, multicentre study. Lancet Neurol. 2017;16(12):976-986. doi: 10.1016/S1474-4422(17)30369-1 [DOI] [PubMed] [Google Scholar]
- 11.SOLIRIS . (Eculizumab). Prescribing Information. Boston, MA: Alexion Pharmaceuticals, Inc; 2018. [Google Scholar]
- 12.Muppidi S, Utsugisawa K, Benatar M, et al. ; Regain Study Group . Long-term safety and efficacy of eculizumab in generalized myasthenia gravis. Muscle Nerve. 2019;60(1):14-24. doi: 10.1002/mus.26447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ricardo A, Arata M, DeMarco S, et al. Preclinical evaluation of RA101495, a potent cyclic peptide inhibitor of C5 for the treatment of paroxysmal nocturnal hemoglobinuria [abstract]. Blood. 2015;126:939. doi: 10.1182/blood.V126.23.939.93926065653 [DOI] [Google Scholar]
- 14.Jackson MB, Stephens CL, Lecar H. Single channel currents induced by complement in antibody-coated cell membranes. Proc Natl Acad Sci U S A. 1981;78(10):6421-6425. doi: 10.1073/pnas.78.10.6421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farmakidis C, Pasnoor M, Dimachkie MM, Barohn RJ. Treatment of myasthenia gravis. Neurol Clin. 2018;36(2):311-337. doi: 10.1016/j.ncl.2018.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hartung HP. Advances in the understanding of the mechanism of action of IVIg. J Neurol. 2008;255(suppl 3):3-6. doi: 10.1007/s00415-008-3002-0 [DOI] [PubMed] [Google Scholar]
- 17.Howard JF Jr, Barohn RJ, Cutter GR, et al. ; MG Study Group . A randomized, double-blind, placebo-controlled phase II study of eculizumab in patients with refractory generalized myasthenia gravis. Muscle Nerve. 2013;48(1):76-84. doi: 10.1002/mus.23839 [DOI] [PubMed] [Google Scholar]
- 18.Myasthenia Gravis Foundation of America . Myasthenia gravis: a manual for the health care provider. https://myasthenia.org/Portals/0/Provider%20Manual_ibook%20version.pdf. Accessed September 3, 2019.
- 19.Barohn RJ, McIntire D, Herbelin L, Wolfe GI, Nations S, Bryan WW. Reliability testing of the quantitative myasthenia gravis score. Ann N Y Acad Sci. 1998;841:769-772. doi: 10.1111/j.1749-6632.1998.tb11015.x [DOI] [PubMed] [Google Scholar]
- 20.Katzberg HD, Barnett C, Merkies IS, Bril V. Minimal clinically important difference in myasthenia gravis: outcomes from a randomized trial. Muscle Nerve. 2014;49(5):661-665. doi: 10.1002/mus.23988 [DOI] [PubMed] [Google Scholar]
- 21.Wolfe GI, Herbelin L, Nations SP, Foster B, Bryan WW, Barohn RJ. Myasthenia gravis activities of daily living profile. Neurology. 1999;52(7):1487-1489. doi: 10.1212/WNL.52.7.1487 [DOI] [PubMed] [Google Scholar]
- 22.Burns TM, Conaway MR, Cutter GR, Sanders DB; Muscle Study Group . Less is more, or almost as much: a 15-item quality-of-life instrument for myasthenia gravis. Muscle Nerve. 2008;38(2):957-963. doi: 10.1002/mus.21053 [DOI] [PubMed] [Google Scholar]
- 23.Burns TM, Grouse CK, Conaway MR, Sanders DB; MG Composite and MG-QoL15 study group . Construct and concurrent validation of the MG-QOL15 in the practice setting. Muscle Nerve. 2010;41(2):219-226. doi: 10.1002/mus.21609 [DOI] [PubMed] [Google Scholar]
- 24.Muppidi S, Wolfe GI, Conaway M, Burns TM; MG Composite And MG-QoL15 Study Group . MG-ADL: still a relevant outcome measure. Muscle Nerve. 2011;44(5):727-731. doi: 10.1002/mus.22140 [DOI] [PubMed] [Google Scholar]
- 25.Burns TM, Conaway M, Sanders DB; MG Composite and MG-QOL15 Study Group . The MG composite: a valid and reliable outcome measure for myasthenia gravis. Neurology. 2010;74(18):1434-1440. doi: 10.1212/WNL.0b013e3181dc1b1e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burns TM, Sadjadi R, Utsugisawa K, et al. International clinimetric evaluation of the MG-QOL15, resulting in slight revision and subsequent validation of the MG-QOL15r. Muscle Nerve. 2016;54(6):1015-1022. doi: 10.1002/mus.25198 [DOI] [PubMed] [Google Scholar]
- 27.Vissing J, Jacob S, Fujita K, O’Brien F, Howard JF Jr. Minimal symptom expression with eculizumab in myasthenia gravis. Muscle Nerve. 2018;58:S98. doi: 10.1002/mus.26353 [DOI] [Google Scholar]
- 28.Costabile M. Measuring the 50% haemolytic complement (CH50) activity of serum. J Vis Exp. 2010;e1923(37). doi: 10.3791/1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lewis LA, Ram S. Meningococcal disease and the complement system. Virulence. 2014;5(1):98-126. doi: 10.4161/viru.26515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Socié G, Caby-Tosi MP, Marantz JL, et al. Eculizumab in paroxysmal nocturnal haemoglobinuria and atypical haemolytic uraemic syndrome: 10-year pharmacovigilance analysis. Br J Haematol. 2019;185(2):297-310. doi: 10.1111/bjh.15790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruff RL, Lisak RP. Nature and action of antibodies in myasthenia gravis. Neurol Clin. 2018;36(2):275-291. doi: 10.1016/j.ncl.2018.01.001 [DOI] [PubMed] [Google Scholar]
- 32.Grob D, Brunner N, Namba T, Pagala M. Lifetime course of myasthenia gravis. Muscle Nerve. 2008;37(2):141-149. doi: 10.1002/mus.20950 [DOI] [PubMed] [Google Scholar]
- 33.Cutter G, Xin H, Aban I, et al. Cross-sectional analysis of the Myasthenia Gravis Patient Registry: disability and treatment. Muscle Nerve. 2019;60(6):707-715. doi: 10.1002/mus.26695 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol.
eTable 1. Efficacy of Zilucoplan Is Independent of Prior Therapies
eTable 2. Treatment-Emergent AEs and Injection-Site Reactions
eFigure 1. Change From Baseline Over 12 Weeks for 0.1 mg/kg Zilucoplan and Placebo in (A) QMG (B) MG-ADL, (C) MG-QoL15r, and (D) MGC Scores
eFigure 2. Radar Plots Suggest Consistency of Improvement Across All 4 End Points When Treated With (A) 0.3 mg/kg Zilucoplan Daily Subcutaneously vs (B) Placebo.
eFigure 3. Mean Complement Activity as Measured by Sheep Red Blood Cell Assay (% Hemolysis)
Data Sharing Statement.