This economic analysis estimates the long-term association of banning indoor tanning devices or prohibiting their use by minors only with health and economic outcomes in North America and Europe.
Key Points
Question
Is there an association between regulatory actions for indoor tanning (banning all commercial indoor tanning and prohibiting use by minors only) and health and economic outcomes?
Findings
In this economic analysis of persons aged 12 to 35 years, despite decreasing indoor tanning rates across North America and Europe, banning indoor tanning for the next generation of individuals aged 12 to 35 years could avert 448 000 melanomas and 9.7 million keratinocyte carcinomas and generate health care cost savings of US $5.7 billion, whereas prohibiting only minors from indoor tanning could produce one-third of these benefits.
Meaning
Banning all commercial indoor tanning may be associated with substantially greater health and economic benefit than prohibiting use by minors only.
Abstract
Importance
UV radiation emissions from indoor tanning devices are carcinogenic. Regulatory actions may be associated with reduced exposure of UV radiation at a population level.
Objective
To estimate the long-term health and economic consequences of banning indoor tanning devices or prohibiting their use by minors only in North America and Europe compared with ongoing current levels of use.
Design, Setting, and Participants
This economic analysis modeled data for individuals 12 to 35 years old in North America and Europe, who commonly engage in indoor tanning. A Markov cohort model was used with outcomes projected during the cohort’s remaining life-years. Models were populated by extracting data from high-quality systematic reviews and meta-analyses, epidemiologic reports, and cancer registrations.
Main Outcomes and Measures
Main outcomes were numbers of melanomas and deaths from melanoma, numbers of keratinocyte carcinomas, life-years, and health care and productivity costs. Extensive sensitivity analyses were performed to assess the stability of results.
Results
In an estimated population of 110 932 523 in the United States and Canada and 141 970 492 in Europe, for the next generation of youths and young adults during their remaining lifespans, regulatory actions that ban indoor tanning devices could be expected to gain 423 000 life-years, avert 240 000 melanomas (−8.2%), and avert 7.3 million keratinocyte carcinomas (−7.8%) in North America and gain 460 000 life-years, avert 204 000 melanomas (−4.9%), and avert 2.4 million keratinocyte carcinomas (−4.4%) in Europe compared with ongoing current levels of use. Economic cost savings of US $31.1 billion in North America and €21.1 billion (US $15.9 billion) in Europe could occur. Skin cancers averted and cost savings after prohibiting indoor tanning by minors may be associated with one-third of the corresponding benefits of a total ban.
Conclusions and Relevance
Banning indoor tanning may be associated with reduced skin cancer burden and health care costs. Corresponding gains from prohibiting indoor tanning by minors only may be smaller.
Introduction
The incidence of skin cancer in fair-skinned populations is high and increasing globally1,2,3 such that total health care spending on its treatment is among the highest of all cancers in countries such as the United States, Denmark, and Australia.4,5,6 The most common skin cancers are basal cell carcinomas (BCCs) and squamous cell carcinomas (SCCs) (collectively known as keratinocyte carcinomas). Melanoma is less common overall but is the most frequently diagnosed cancer in young adults7 and is more serious, with 59 782 deaths from melanoma annually worldwide.8 Exposure to UV radiation is the predominant cause of keratinocyte carcinomas and melanoma,9 thus, these cancers are largely preventable by reducing personal exposure to UV radiation by sun protection10 or avoidance of indoor tanning.11
Indoor tanning devices (eg, sunbeds, tanning beds, and sunlamps) can emit high to extreme levels of UV radiation,12 and in 2009. the International Agency for Research on Cancer (IARC) classified indoor tanning devices as carcinogenic to humans.9 More than 20 countries have now legislated against indoor tanning for persons younger than 18 years (or even younger), whereas Australia has banned all commercial indoor tanning and Brazil has banned private and commercial indoor tanning.13 Other countries have introduced various restrictions, such as preventing indoor tanning by UV-sensitive people, banning unsupervised access, licensing indoor tanning establishments, mandating operator training, and taxing indoor tanning sessions. More broadly, educational campaigns promote awareness of the dangers of indoor tanning, including through warning notices and provision of consumer information.14 Public health concerns are greatest for youths, who are particularly vulnerable to skin cancers associated with high UV exposure.14
Although several studies15,16,17 have quantified the expected long-term health and economic outcomes after introduction of sunbed regulations, to our knowledge, none have predicted outcomes based on updated indoor tanning rates since regulatory actions have increased.9 The purpose of this study was to estimate the expected health and economic consequences in the next generation of youths and young adults if indoor tanning were regulated across North America and Europe. We assessed 2 regulatory actions, banning all commercial indoor tanning and prohibiting indoor tanning for minors only, and compared their long-term outcomes with those expected if current indoor tanning rates continued.
Methods
Population and Setting
This economic evaluation included fair-skinned populations from North America (United States and Canada) and Europe, where no country or province has banned indoor tanning (although some regions restrict use in minors). The models included individuals aged 12 to 35 years because they are the main users of indoor tanning devices. The models tracked the cohorts through their lifetime until death from melanoma or other causes. The study was exempt from ethics approval by the QIMR Berghofer Medical Research Institute Human Research Ethics Committee.
Strategies
Three strategies were compared: (1) continuing current levels of indoor tanning, (2) prohibiting minors (aged <18 years) from commercial indoor tanning, and (3) banning all commercial indoor tanning.
Model Structure
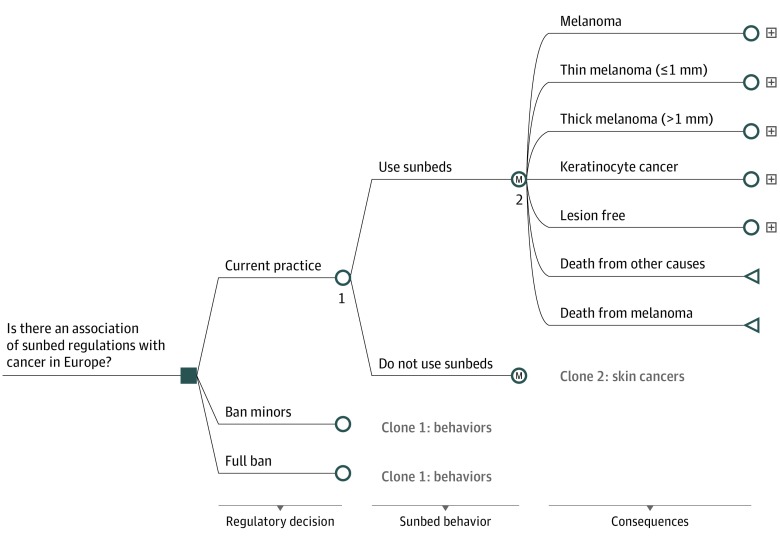
Two similar health economic models were constructed, one for each continent, and region-specific data inputs were applied. Europe broadly refers to the 28 nations in the European Union. We designed a decision tree with Markov chains in TreeAge Pro for Healthcare 2018 (TreeAge Software Inc) (Figure 1). The models started with an equal choice between the strategies followed by a proportion of the populations engaging or not engaging in indoor tanning depending on strategy and age. Markov health states were used to estimate the health consequences for the young cohorts for their remaining life. The models had annual cycles, and individuals could move among the health states according to transition probabilities (eFigure 1 in the Supplement). As the cohorts aged, variables associated with indoor tanning, such as skin cancers, health care costs, survival, and mortality, were aggregated. Competing risk of death by age was embedded in the model so that, annually, persons could die from other causes based on background mortality statistics.
Figure 1. Model Structure.
Model Probabilities and Skin Cancer Risk Ratios
Data to populate the models were obtained from published epidemiologic studies, cost reports, and official cancer registry reports1,4,15,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37 (Table 1). Studies on indoor tanning prevalence were selected if indoor tanning prevalence data were collected from 2009 onward, after the IARC carcinogenicity statement.41 A 2019 systematic review and meta-analysis41 provided the pooled mean prevalence of indoor tanning for both continents. Indoor tanning in the past year by adolescents 15 populations42,43,44,45,46,47,48,49) and for ever use by adults (26 populations49,50,51,52,53,54,55,56,57,58,59,60) were extracted and pooled.41 For European countries with multiple prevalence reports, we selected only the latest study (eFigure 2 in the Supplement) and included all estimates in a sensitivity analysis.41 The estimates for past-year use in adolescents in that analysis were 8% (95% CI, 5%-11%) for North America and 5% (95% CI, 0%-16%) for Europe. The estimates for adult ever use were 36% (95% CI, 23%-49%) for North America and 22% (95% CI, 12%-34%) for Europe (eTable 1 and eFigure 2 in the Supplement).
Table 1. Values, Description, and Sources of Model Inputs.
| Input | Best Estimate (Range) | Distribution and Variables | Source |
|---|---|---|---|
| Both Models | |||
| Starting age of the cohorts, y | Distribution (12-35) | Uniform:12-35 | Allocated by authors |
| Background all-cause mortality | By age | NA | US Life Tables, 2014,38 Eurostat mortality for European Union, 201639 |
| Discount rate, % | 3 (0-5) | NA | Guy et al15 and Pil et al,27 applied to costs and life-years globally in models |
| Time from sunbed exposure to cancer, y | 9 y | NA | Cust et al28 reported lag for melanoma and assumed same for BCCs and SCCs. |
| Rate ratio | |||
| Melanoma with no sunbed use | 1.00 | NA | [Reference] |
| Melanoma with sunbed use | 1.59 (1.36-1.85) | Log normal: μ = 0.40, σ = 0.36 | Boniol et al,18 figures for first use in those aged <35 y |
| Probability of melanoma in population | By age | NA | Bray et al,21 IARC Canada and US incidence by age and sex |
| Probability of subsequent melanomas | 0.0075 (0.0064-0.0086) | β: α = 44.1, β = 6060 | Youlden et al,22 15% variation in high and low estimates |
| Probability of multiple SCCS and BCCS | 0.1364 (0.1091-0.1637) | β: α = 38.2, β = 242.1 | Marcil et al,29 –3-y cumulative risk of 44% for another BCC and 18% for SCC |
| North American Model | |||
| Probabilities | |||
| Sunbed use | |||
| Current situation | Y = 0.2691ln(age) 0.6380 | NA | Meta-analysis findings and imputation (8% for persons aged 12-17 y, ≤36% for persons aged 57 y) |
| Ban for persons aged <18 y | Y = 0.2636ln(age) 0.6597 | NA | Assumed 1% for those aged 12-17 y, imputed approximately 4-5 percentage points lower use than current |
| Ban for all ages | By age | NA | Assumed 1% for persons aged 12-17 y and 2% in adults because of private use |
| Rate ratio | |||
| KCs with no sunbed use | 1.00 | NA | [Reference] |
| KCs with sunbed use | 1.48 (1.21-2.08) | Log normal: μ = 0.35, σ = 0.29 | Wehner et al,19 risk ratio for young at first use, weighted mean of BCC to SCC of 2.5:11 for North America and 6.8:1 for Europe. |
| SCCs and BCCs in general population | By age group | Rogers et al,1 by age adjusted for procedures by person (1.64) and 1.5% (assumed) increase annually to 2018 | |
| Thick melanoma | 0.27 (0.25-0.29) | β: n = 97 114, r = 26 221 | Aitken et al,30 general background data, 26 221/97 114 |
| Death from melanoma | |||
| >1-mm thick | By year from diagnosis | NA | Table using survival data from stage II, III, and IV cancer, latter from therapy trials31 |
| ≤1-mm thin | By age | NA | Green et al23 |
| Costs, $ | |||
| Age at death from melanoma, y | |||
| <65 | 111 573 (83 680-152 292) | γ: α = 44.4, λ = 0.0003 | Guy et al,15 adjusted for inflation |
| ≥65 | 74 383 (55 787-92 979) | γ: α = 44.4, λ = 0.001 | |
| Care for person in last year of life | 498 (374-622) | γ: α = 44.4, λ = 0.089 | |
| Treating SCC or BCC | 1312 (984-1477) | γ: α = 171, λ = 0.139 | Guy et al,24 adjusted for inflation |
| Thick melanoma in first year | 24 369 (17 059-31 680) | γ: α = 44.4, λ = 0.002 | Elliott et al,32 adjust for currency and inflation |
| Thick melanoma after year 1 | By year | ||
| Thin melanoma in first year | 5789 (6947-4631) | γ: α = 44.4, λ = 0.008 | Guy et al,15 adjusted for inflation |
| Thin melanoma after year 1 | By year | NA | Elliott et al,32 adjust for currency and inflation |
| Lost productivity from premature melanoma death | 521 140 | NA | Bristow et al,33 adjusted for inflation, applied to deaths from melanoma before life-expected retirement age (<70 y) |
| European Model | |||
| Probabilities | |||
| Sunbed use | |||
| Current situation | Y = 0.1331ln(Age) 0.29 (maximum, 22%) | NA | Rodriguez-Acevedo et al,34 5% for persons age 12-17 y, imputed up to 22% for persons aged 57 y) |
| Ban for persons aged <18 y | Y = 0.1287ln(Age) 0.315 | NA | Assumed 1% for persons aged 12-17 y, imputed 4-5 percentage points lower use than current |
| Ban for all ages | 1% < 18 y, 2% adults | NA | Assumed 1% for persons aged 12-17 y and 2% in adults because of private use |
| Rate ratio | |||
| KCs with no sunbed use | 1.00 | [Reference] | |
| KCs with sunbed use | 1.58 (1.12-2.76) | Log normal: μ = 0.35, σ = 0.29 | Wehner et al,19 risk ratio for young at first use, weighted mean of BCC to SCC of 2.5:11 for North America. |
| BCCs and SCCs in general population | By age group | NA | Birch-Johansen et al,20 Danish figures as a proxy for Europe, middle latitude adjusted by 4% annual increase to 2018 |
| Thick melanoma | 0.354 (0.283-0.425) | β: α = 28.3, β = 51.7 | Calculated from Sacchetto et al40 (18 cancer registries of Europe) |
| Death from melanoma | |||
| >1-mm thick | By years from diagnosis | NA | SEER survival data from stage II, III, and IV melanoma, latter from trials31 |
| ≤1-mm thin | By age | NA | Green et al,23 population-based 20-y survival data, Australia |
| Costs, € | |||
| Care for person in last months of life | 4150 (3528-4773) | γ: α = 44.4, λ = 0.011 | Round et al,36 adjusted cancer figure by −27% based on Reeve et al35 |
| Death from melanoma | 5271 (4480-6061) | γ: α = 44.4, λ = 0.008 | Round et al,36 UK study |
| Treating SCC or BCC | 2716 (2309-3124) | γ: α = 44.4, λ = 0.016 | Bentzen et al,4 Danish study |
| Thick melanoma in first year | 11 994 (10 195-13 793) | γ: α = 44.4, λ = 0.004 | |
| Thick melanoma after year 1 | By years from diagnosis | NA | Lyth et al,25 Swedish study |
| Thin melanoma in first year | 2834 (2409-3259) | γ: α = 44.4, λ = 0.016 | |
| Thin melanoma after year 1 | By years from diagnosis | NA | |
| Lost productivity owing to premature death from melanoma | 332 917 | NA | Hanly et al,26 applied to melanoma deaths before life expected retirement age (age <70 y) |
Abbreviations: BCC, basal cell carcinoma; IARC, International Agency for Research on Cancer; KC, keratinocyte carcinoma; NA, not applicable; SCC, squamous cell carcinoma; SEER, Surveillance, Epidemiology, and End Results.
We fitted logarithmic equations to represent prevalence of indoor tanning by age during the lifespan, reflecting a steep increase in prevalence during youth and leveling as individuals aged (eFigure 3 in the Supplement). We applied the pooled past-year exposure for adolescents as the starting point and imputed the rates throughout later life up to a maximum rate, which was the pooled estimate of ever exposure in adults.15,16 For the regulation strategies, we assumed 1% prevalence among minors and 2% prevalence among adults, allowing for private indoor tanning use. We assumed that when prohibition of use by minors occurred, prevalence would spike at 18 years of age but would be lower than current rates by approximately 4 percentage points and would remain proportionally lower thereafter.
In a meta-analysis,18 the estimated relative risk of developing melanoma from indoor tanning was 1.59 (95% CI, 1.36-1.85) when first exposure occurred at younger than 35 years.18 This estimate was applied to both continents, and the 95% CIs were tested in sensitivity analyses. The relative risk of developing keratinocyte carcinoma19 among indoor tanners was determined using the combined risks of BCC and SCC weighted by the ratios of BCC to SCC prevalence in the United States (BCC to SCC ratio, 2.5:11) and Europe (BCC to SCC ratio, 6.8:120). Incidence of keratinocyte carcinomas was estimated from epidemiologic reports1,20 and inflated to 2018 estimates based on annual growth rates (Table 1).
Incidence rates of melanoma by age group and region were provided in cancer registry reports.21,61 However, these reports cited invasive melanomas, and rates were inflated to account for in situ melanomas by 15% for North America62 and 25% for Europe40 because in situ melanomas are treated in the health care system and incur costs, although they do not metastasize. The models accounted for some individuals having multiple melanomas22 or multiple keratinocyte carcinomas1,20,63 during their lifetime. Mortality rates for melanoma were based on survival data23 and applied as mortality probabilities for thin and thick melanomas (approximately clinical stage I and stages IIa to IIId, respectively, with appropriate mortality weights). All rates were converted to annual probabilities.
Cost Inputs
The study used a societal cost perspective. Health care costs for the diagnosis and treatment of melanomas (separated into in situ and thin or thick) and keratinocyte carcinomas were derived from cost-of-illness reports in the United States15,24 and Europe.4,25 Results from a Danish study on skin cancer costs by Bentzen et al4 and a Swedish study by Lyth et al25 were considered to be reasonable proxies for mean health care spending on skin cancer in Europe because both countries are ranked in the center of health care spending across the European Union.64 Economic losses to society for premature deaths from melanoma were applied when a person died before expected retirement age and were US $511 003 and €332 817 per melanoma death in the United States and European Union, respectively.26,65 Health care costs were inflated to 2018 currencies when necessary by using medical price indexes and were converted to US dollars using purchasing power parities. Modeled future costs and life-years were discounted at 3% to reflect current values.
Statistical Analysis
Outcomes of interest were the number of melanomas, number of keratinocyte carcinomas, life-years, number of deaths from melanoma, health care costs, and productivity costs. These outcomes support a perspective beyond health care and present a full picture of the various burdens. However, we retained the classic approach to cost-effectiveness analysis and chose life-years gained as the key measure of benefit for determining cost-effectiveness. This approach was used because melanoma, although deadly at advanced stages, is mostly curable, and other causes of death become prominent with aging. Life-years gained is a generic outcome and allows comparisons across other interventions and health conditions. The model aggregated the probabilities, and values assigned to the different health states using 5000 Monte Carlo simulations or random draws of the different pathways were possible, randomly selecting individuals between 12 and 35 years of age. Mean costs, life-years, and skin cancer outcomes were calculated from the 5000 iterations and scaled up to the relevant estimated population size.66,67,68 For each regulation strategy against continued current levels of indoor tanning, the incremental cost per life-year was calculated (the difference in mean costs for 2 strategies divided by the difference in mean life-years).
Sensitivity Analysis
One-way sensitivity analyses were performed by rerunning the simulations to determine how variation in the model variables (one at a time) changed the key findings. Each model input was varied between the 95% confidence limits when available or other plausible high and low limits from the literature (Table 1). Probabilistic sensitivity analyses, changing multiple variables concurrently, were undertaken with Monte Carlo simulations and resampling 5000 times at random from probability distributions for each estimate (Table 1). The γ, β, and log normal distributions were assigned for cost, probability, and relative risk inputs, respectively. Cost-effectiveness was determined by using the traditional thresholds of the incremental cost-effectiveness ratios: US $50 000 per life-year saved for North America and €30 000 for Europe.17 Two modelers (L.G.G. and A.C.G.) undertook internal checking for model coherence, errors, and inconsistencies, and we validated the model by assessing the predicted survival with external reports.
Results
North America
On the basis of our modeling with an estimated population of 110 932 523 in the United States and Canada and 141 970 492 in Europe, in the lifetimes of individuals currently aged 12 to 35 years, we estimated that banning indoor tanning in North America could be expected to produce 89 193 fewer deaths from melanoma (−6.9%), 244 347 fewer melanomas (−8.2%), and 7.3 million fewer keratinocyte carcinomas (−7.8%) and save 428 781 life-years (−0.01%). Health care costs could be US$3.5 billion lower, and productivity gains could be worth US $27.5 billion (Table 2). The estimated benefits of banning indoor tanning were 3.7-fold higher than for an age prohibition on minors (Table 2 and Figure 2).
Table 2. Association of Sunbed Regulation With Health and Economic Outcomes in Young Cohorts (Aged 12-35 Years).
| Variable | United States and Canada | Europe | Total | ||
|---|---|---|---|---|---|
| No. | Change, % | No. | Change, % | ||
| Estimated population | 110 932 523 | NA | 141 970 492 | NA | 252 903 015 |
| Full Ban vs Current | |||||
| Life-years | 428 781 | 0.01 | 459 669 | 0.01 | 888 450 |
| Melanomas | 244 347 | −8.2 | 203 736 | −4.9 | 448 083 |
| Deaths from melanoma | 89 193 | −6.9 | 98 288 | −4.4 | 187 481 |
| Keratinocyte cancers | 7 323 570 | −7.8 | 2 425 705 | −4.4 | 9 749 276 |
| Health care costs | |||||
| Cost, € | NA | NA | 2 847 096 672 | NA | NA |
| PPP, $ | 3 540 031 348 | −7.2 | 2 135 322 504 | −2.3 | 5 675 353 852 |
| Productivity losses | |||||
| Cost, € | NA | NA | 18 301 449 960 | NA | NA |
| PPP, $ | 27 526 845 366 | −7.7 | 13 726 087 470 | −4.3 | 41 252 932 836 |
| Minors Ban vs Current | |||||
| Life-years | 114 552 | 0.00 | 178 766 | 0.00 | 293 318 |
| Melanomas | 67 068 | −2.3 | 79 948 | −1.9 | 147 017 |
| Deaths from melanoma | 25 095 | −1.9 | 39 088 | −1.8 | 64 183 |
| Keratinocyte cancers | 1 972 618 | −2.1 | 936 737 | −1.7 | 2 909 355 |
| Health care costs | |||||
| Costs, € | NA | NA | 1 044 434 862 | NA | NA |
| PPP, $ | 872 552 196 | −1.8 | 783 326 146 | −0.9 | 1 655 878 342 |
| Productivity losses | |||||
| Costs, € | NA | NA | 7 464 663 059 | NA | NA |
| PPP, $ | 7 988 990 677 | −2.2 | 5 598 497 294 | −1.8 | 13 587 487 971 |
Abbreviations: NA, not applicable; PPP, purchasing power parity.
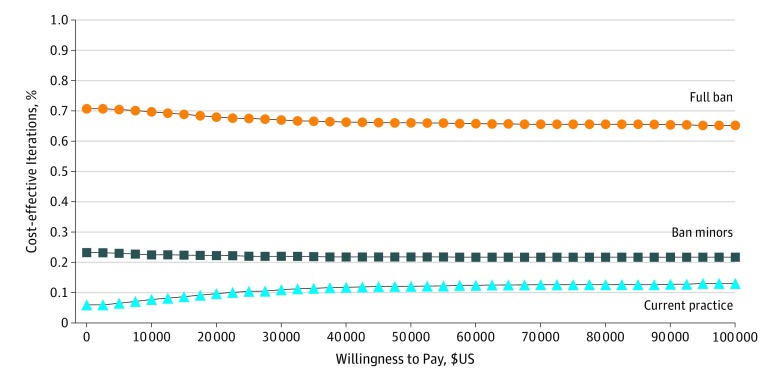
Figure 2. Cost-effectiveness Acceptability Curve for North American Model.
One-way sensitivity analyses indicated that for each regulation strategy, the findings were most sensitive to the increased risk of keratinocyte carcinomas and melanoma from indoor tanning, probability of thick melanoma, and first-year costs of thick melanoma (eTable 2 in the Supplement). Increased relative risk of skin cancers reflected greater cost savings associated with regulation, but life-years gained remained volatile at approximately 0, affecting the incremental cost per life-year ratios. The probability that prohibiting minors or banning indoor tanning was cost-effective was 83.4%. In most simulations, regulation would be associated with cost savings and with increased life-years (eFigure 4 in the Supplement).
Europe
With banning indoor tanning during the cohort’s remaining life, the long-term estimated numbers of skin cancers avoided in Europe were 203 736 melanomas (−4.9%) and 2.4 million keratinocyte carcinomas (−4.4%) (Table 2). This ban could also avert 98 288 premature deaths from melanoma (−4.4%) and produce a gain in 459 669 life-years (0.01%). Health care cost savings were €2.8 billion (US $2.1 billion), whereas society could gain €18.3 billion (US $13.7 billion) from additional productivity (Table 2). These benefits were a mean of 2.6-fold higher than when minors only were prohibited from indoor tanning (Table 2).
When the pooled estimate for adult ever exposure was more inclusive of all reported studies (with Denmark having greater use of indoor tanning than other parts of Europe), the pooled estimate increased from 22% to 31% and resulted in an estimated 1–percentage point increase across outcomes for the full ban. For each regulation strategy, the findings were most sensitive to the increased risk of keratinocyte carcinomas associated with indoor tanning, the probability of developing thick melanomas, and the increased risk of melanoma associated with indoor tanning (eTable 2 in the Supplement). For life-years, the likelihood that banning indoor tanning was cost-effective was 83.8% (eFigure 5 in the Supplement).
Discussion
Our primary findings suggest that banning indoor tanning could produce 888 000 additional life-years and cost savings for society for the next generation of youths and young adults across North America and Europe for their remaining lives. Banning indoor tanning could avert 448 000 melanomas, avert 9.7 million keratinocyte carcinomas, and generate health care cost savings of US $5.7 billion and US$41.3 billion in productivity gains. If countries prohibited only minors instead, the benefits would be approximately one-third those of a full ban, assuming perfect compliance to the age-based legislation.69 The expected outcomes were stable when the model inputs were varied around plausible values.
Since the earlier review of indoor tanning prevalence by Wehner et al70 in 2014, our findings show that indoor tanning in North America has been stable and has remained high in adults (ever-exposure of 35%-36%) and adolescents (past-year exposure of 8%-10%).41 However, in European youths and adults, indoor tanning has decreased notably since the 2009 IARC statement70 from 42% to 22%. The potential population health and economic gains associated with banning indoor tanning have been substantial in both continents. In the United States, 42 states and districts regulate the use of tanning facilities, but 20 states and 1 territory prohibit minors from the use of tanning facilities.71,72
Four studies15,16,17,73 have estimated the expected long-term health and economic outcomes associated with indoor tanning regulations and show that regulations would be significantly associated with reduced skin cancer incidence and health care costs. The most recent study, to our knowledge, in the United States with a cohort of individuals aged 14 years estimated that 4.7% of melanomas would be averted and 4.9% fewer deaths from melanoma would occur in association with regulations compared with no regulation.15 Our results are more favorable, estimating 8.2% fewer melanomas and 6.9% fewer deaths from melanoma, likely because of the larger population segment in our study and recent estimates of skin cancer incidence.15
Indoor tanning bans have been implemented in only 3 countries: Brazil, Iran, and Australia. Despite no scientific reports, to our knowledge, on the recent prevalence of indoor tanning from these countries, other available sources report that private or clandestine indoor tanning in Australia during the summer of 2016-2017 was less than 1%,74 whereas in Brazil, it was approximately 4%.75 Australia banned commercial indoor tanning establishments incrementally through each state authority during a 9-year period (2008-2016) after initially prohibiting use by minors only,74 whereas Brazil legislated a total national ban in 2009. In Australia and Brazil, buy-back schemes were implemented in which commercial operators received compensatory payments from governments that enabled safe environmental disposal and minimized device transfer into private homes. Selling obsolete commercial sunbeds privately was marketed in Australia through websites, but commercial sunbeds require industrial-grade electrical power, which is generally infeasible in domestic homes. However, sunbeds designed for private home use are legally marketed in Australia, and the desire to tan remains strong in some population segments.76 Spray tanning is popular, and manufacturers of self-tanning products are continually improving their look and feel to meet consumer demand.77 In Australia, there has been strong market growth (10.6% mean per year in 2011-2016) in sales of self-tanning products.77
The World Health Organization does not recommend the use of UV tanning devices for cosmetic purposes and publishes guides intended for government health authorities to assist them in developing public health policy to manage the risks associated with indoor tanning devices.14,78 UV devices specifically designed for medical phototherapy remain important for treating psoriasis, atopic dermatitis, and other conditions according to clinical guidelines. However, the scientific community acknowledges and supports the benefits of initiatives that protect citizens from UV-induced harm from cosmetic solaria.78 On the basis of the evidence of harms associated with sunbed use and supported by health economic evidence,16 Australian state and territory governments were motivated to ultimately ban commercial indoor tanning.79
Strengths and Limitations
Our model was based on the best available evidence and synthesized findings from high-quality data. We used melanoma incidence and mortality data from established cancer registries. Extensive sensitivity analyses were undertaken to assess the robustness of results and determine the effects of uncertainty in model estimates. These analyses showed that our key findings were stable.
This study has limitations. Some assumptions were necessary regarding the model structure and inputs. Pooling prevalence data across studies and countries loses the precision in inputs and the behavioral and other epidemiologic differences within regions and countries. For example, the reported prevalence estimates across Europe for ever use by adults ranged from 10% to 56%.60,80 The incidence of keratinocyte carcinomas is uncertain and may be underestimated because these cancers are rarely registered in provincial cancer registries and researchers have indirectly estimated their incidence through treatment (excision) rates.1 Our analyses focused on overall survival, which may have included death from causes unrelated to the health consequences of indoor tanning. Other causes of death cannot be ruled out, especially considering that the target sample comprised young persons aged 12 to 35 years, who may live another 45 to 68 years assuming a statistical life expectancy of 80 years. We have also excluded the direct costs incurred for implementing regulation, ongoing audits or necessary compliance activities, and tanning business closures or loss of income. Although banning indoor tanning might incur more implementation costs, such as device buy-back schemes, prohibiting minors could incur even higher ongoing costs for signage, materials, and auditing or policing. Of importance, the current evidence of poor compliance with prohibiting minors only69 exacerbates these costs and reduces effectiveness.
Conclusions
On the basis of recent international estimates of indoor tanning prevalence and burden of skin cancers, our findings suggest that regulating indoor tanning may be associated with decreases in public harm and the burden on health care systems. Banning all commercial indoor tanning may be associated with substantially greater health and economic benefit than prohibiting use by minors only.
eFigure 1. Illustration of Markov Model Health States and Possible Transitions
eFigure 2. Pooled Estimates of Ever Exposure to Indoor Tanning Among Adults After 2009
eFigure 3. Estimated Prevalence of Indoor Tanning by Age
eFigure 4. Incremental Cost per Life-year Scatterplot for Full Ban vs Current Use in North America
eFigure 5. Incremental Cost per Life-year Scatterplot for Full Ban vs Current Use in Europe
eTable 1. Comparison of Pooled Estimates on the Prevalence of Indoor Tanning
eTable 2. One-Way Sensitivity Analyze: Full Ban vs Current Sunbed Use
eReferences
References
- 1.Rogers HW, Weinstock MA, Feldman SR, Coldiron BM. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol. 2015;151(10):1081-1086. doi: 10.1001/jamadermatol.2015.1187 [DOI] [PubMed] [Google Scholar]
- 2.Robsahm TE, Helsing P, Veierod MB Cutaneous squamous cell carcinoma in Norway 1963-2011: increasing incidence and stable mortality. Cancer Med 2015;4(3):472-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whiteman DC, Green AC, Olsen CM. The growing burden of invasive melanoma: projections of incidence rates and numbers of new cases in six susceptible populations through 2031. J Invest Dermatol. 2016;136(6):1161-1171. doi: 10.1016/j.jid.2016.01.035 [DOI] [PubMed] [Google Scholar]
- 4.Bentzen J, Kjellberg J, Thorgaard C, Engholm G, Phillip A, Storm HH. Costs of illness for melanoma and nonmelanoma skin cancer in Denmark. Eur J Cancer Prev. 2013;22(6):569-576. doi: 10.1097/CEJ.0b013e328360150c [DOI] [PubMed] [Google Scholar]
- 5.Fransen M, Karahalios A, Sharma N, English DR, Giles GG, Sinclair RD. Non-melanoma skin cancer in Australia. Med J Aust. 2012;197(10):565-568. doi: 10.5694/mja12.10654 [DOI] [PubMed] [Google Scholar]
- 6.Housman TS, Feldman SR, Williford PM, et al. . Skin cancer is among the most costly of all cancers to treat for the Medicare population. J Am Acad Dermatol. 2003;48(3):425-429. doi: 10.1067/mjd.2003.186 [DOI] [PubMed] [Google Scholar]
- 7.Iannacone MR, Youlden DR, Baade PD, Aitken JF, Green AC. Melanoma incidence trends and survival in adolescents and young adults in Queensland, Australia. Int J Cancer. 2015;136(3):603-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karimkhani C, Green AC, Nijsten T, et al. . The global burden of melanoma: results from the Global Burden of Disease Study 2015. Br J Dermatol. 2017;177(1):134-140. doi: 10.1111/bjd.15510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization International Agency for Research in Cancer Radiation. IARC monographs on the evaluation of carcinogenic risks to humans, No. 100D. Lyon, France: International Agency for Research in Cancer; 2009. [Google Scholar]
- 10.Green AC, Williams GM, Logan V, Strutton GM. Reduced melanoma after regular sunscreen use: randomized trial follow-up. J Clin Oncol. 2011;29(3):257-263. doi: 10.1200/JCO.2010.28.7078 [DOI] [PubMed] [Google Scholar]
- 11.Janda M, Green A. Primary prevention of skin cancer In: Williams H, Bigby M, Herxheimer A, et al. , eds. Evidence-Based Dermatology. London, England: BMJ Books; 2014:223-230. [Google Scholar]
- 12.Nilsen LT, Hannevik M, Veierød MB. Ultraviolet exposure from indoor tanning devices: a systematic review. Br J Dermatol. 2016;174(4):730-740. doi: 10.1111/bjd.14388 [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization UV Radiation Programme. Global Health Observatory data. https://www.who.int/gho/phe/ultraviolet_radiation/sunbeds_legislation/en/ Published 2018. Accessed February 13, 2018.
- 14.World Health Organization Artificial Tanning Devices: Public Health Interventions to Manage Sunbeds Geneva, Switzerland: World Health Organization; 2017. [Google Scholar]
- 15.Guy GP Jr, Zhang Y, Ekwueme DU, Rim SH, Watson M. The potential impact of reducing indoor tanning on melanoma prevention and treatment costs in the United States: An economic analysis. J Am Acad Dermatol. 2017;76(2):226-233. doi: 10.1016/j.jaad.2016.09.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirst N, Gordon L, Gies P, Green AC. Estimation of avoidable skin cancers and cost-savings to government associated with regulation of the solarium industry in Australia. Health Policy. 2009;89(3):303-311. doi: 10.1016/j.healthpol.2008.07.003 [DOI] [PubMed] [Google Scholar]
- 17.Pil L, Hoorens I, Vossaert K, et al. . Burden of skin cancer in Belgium and cost-effectiveness of primary prevention by reducing ultraviolet exposure. Prev Med. 2016;93:177-182. doi: 10.1016/j.ypmed.2016.10.005 [DOI] [PubMed] [Google Scholar]
- 18.Boniol M, Autier P, Boyle P, Gandini S. Cutaneous melanoma attributable to sunbed use: systematic review and meta-analysis. BMJ. 2012;345:e4757. doi: 10.1136/bmj.e4757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wehner MR, Shive ML, Chren MM, Han J, Qureshi AA, Linos E. Indoor tanning and non-melanoma skin cancer: systematic review and meta-analysis. BMJ. 2012;345:e5909. doi: 10.1136/bmj.e5909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Birch-Johansen F, Jensen A, Mortensen L, Olesen AB, Kjær SK. Trends in the incidence of nonmelanoma skin cancer in Denmark 1978-2007: Rapid incidence increase among young Danish women. Int J Cancer. 2010;127(9):2190-2198. doi: 10.1002/ijc.25411 [DOI] [PubMed] [Google Scholar]
- 21.Bray F, Colombet M, Mery L, et al. . Cancer Incidence in Five Continents. Vol XI Lyon, France: International Agency for Research on Cancer; 2017. [Google Scholar]
- 22.Youlden DR, Baade PD, Soyer HP, et al. . Ten-year survival after multiple invasive melanomas is worse than after a single melanoma: a population-based study. J Invest Dermatol. 2016;136(11):2270-2276. doi: 10.1016/j.jid.2016.03.014 [DOI] [PubMed] [Google Scholar]
- 23.Green AC, Baade P, Coory M, Aitken JF, Smithers M. Population-based 20-year survival among people diagnosed with thin melanomas in Queensland, Australia. J Clin Oncol. 2012;30(13):1462-1467. doi: 10.1200/JCO.2011.38.8561 [DOI] [PubMed] [Google Scholar]
- 24.Guy GP Jr, Machlin SR, Ekwueme DU, Yabroff KR. Prevalence and costs of skin cancer treatment in the U.S., 2002-2006 and 2007-2011. Am J Prev Med. 2015;48(2):183-187. doi: 10.1016/j.amepre.2014.08.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lyth J, Carstensen J, Synnerstad I, Lindholm C. Stage-specific direct health care costs in patients with cutaneous malignant melanoma. J Eur Acad Dermatol Venereol. 2016;30(5):789-793. doi: 10.1111/jdv.13110 [DOI] [PubMed] [Google Scholar]
- 26.Hanly P, Soerjomataram I, Sharp L. Measuring the societal burden of cancer: the cost of lost productivity due to premature cancer-related mortality in Europe. Int J Cancer. 2015;136(4):E136-E145. doi: 10.1002/ijc.29105 [DOI] [PubMed] [Google Scholar]
- 27.Pil L, Hoorens I, Vossaert K, et al. . Cost-effectiveness and budget effect analysis of a population-based skin cancer screening. JAMA Dermatol. 2017;153(2):147-153. doi: 10.1001/jamadermatol.2016.4518 [DOI] [PubMed] [Google Scholar]
- 28.Cust AE, Armstrong BK, Goumas C, et al. . Sunbed use during adolescence and early adulthood is associated with increased risk of early-onset melanoma. Int J Cancer. 2011;128(10):2425-2435. doi: 10.1002/ijc.25576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marcil I, Stern RS. Risk of developing a subsequent nonmelanoma skin cancer in patients with a history of nonmelanoma skin cancer: a critical review of the literature and meta-analysis. Arch Dermatol. 2000;136(12):1524-1530. doi: 10.1001/archderm.136.12.1524 [DOI] [PubMed] [Google Scholar]
- 30.Aitken JF, Elwood M, Baade PD, Youl P, English D. Clinical whole-body skin examination reduces the incidence of thick melanomas. Int J Cancer. 2010;126(2):450-458. doi: 10.1002/ijc.24747 [DOI] [PubMed] [Google Scholar]
- 31.Wolchok JD, Rollin L, Larkin J. Nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2017;377(25):2503-2504. doi: 10.1056/NEJMc1714339 [DOI] [PubMed] [Google Scholar]
- 32.Elliott TM, Whiteman DC, Olsen CM, Gordon LG. Estimated healthcare costs of melanoma in Australia over 3 years post-diagnosis. Appl Health Econ Health Policy. 2017;15(6):805-816. doi: 10.1007/s40258-017-0341-y [DOI] [PubMed] [Google Scholar]
- 33.Bristow BN, Casil J, Sorvillo F, Basurto-Dávila R, Kuo T. Melanoma-related mortality and productivity losses in the USA, 1990-2008. Melanoma Res. 2013;23(4):331-335. doi: 10.1097/CMR.0b013e328361926c [DOI] [PubMed] [Google Scholar]
- 34.Rodriguez-Acevedo AJ, Green AC, Sinclair C, van Deventer E, Gordon LG. Indoor tanning prevalence after the International Agency for Research on Cancer statement on carcinogenicity of artificial tanning devices: systematic review and meta-analysis. [published online August 5, 2019]. Br J Dermatol. 2019. doi: 10.1111/bjd.18412 [DOI] [PubMed] [Google Scholar]
- 35.Reeve R, Srasuebkul P, Langton JM, Haas M, Viney R, Pearson SA; EOL-CC study authors . Health care use and costs at the end of life: a comparison of elderly Australian decedents with and without a cancer history. BMC Palliat Care. 2017;17(1):1. doi: 10.1186/s12904-017-0213-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Round J, Jones L, Morris S. Estimating the cost of caring for people with cancer at the end of life: A modelling study. Palliat Med. 2015;29(10):899-907. doi: 10.1177/0269216315595203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lomas A, Leonardi-Bee J, Bath-Hextall F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br J Dermatol. 2012;166(5):1069-1080. doi: 10.1111/j.1365-2133.2012.10830.x [DOI] [PubMed] [Google Scholar]
- 38.Arias E, Heron M, Xu J. United States life tables, 2014. Natl Vital Stat Rep. 2017;66(4):1-64. [PubMed] [Google Scholar]
- 39.Eurostat. 2019. Life Table. Eur Union. 2016;28. http://aoossi.eurostat.ec.europa.eu/nui/show.do?dataset=demo_mlifetable&lang=en. Accessed January 19, 2020. [Google Scholar]
- 40.Sacchetto L, Zanetti R, Comber H, et al. . Trends in incidence of thick, thin and in situ melanoma in Europe. Eur J Cancer. 2018;92:108-118. doi: 10.1016/j.ejca.2017.12.024 [DOI] [PubMed] [Google Scholar]
- 41.Rodriguez-Acevedo AJ, Green AC, Sinclair C, van Deventer E, Gordon LG Indoor tanning prevalence after the International Agency for Research on Cancer's statement on carcinogenicity of artificial tanning devices: systematic review and meta-analysis [published online August 5, 2019]. Br J Dermatol. [DOI] [PubMed] [Google Scholar]
- 42.Coups EJ, Stapleton JL, Delnevo CD. Indoor tanning among New Jersey high school students before and after the enactment of youth access restrictions. J Am Acad Dermatol. 2016;75(2):440-442. doi: 10.1016/j.jaad.2016.03.040 [DOI] [PubMed] [Google Scholar]
- 43.Guy GP Jr, Berkowitz Z, Tai E, Holman DM, Everett Jones S, Richardson LC. Indoor tanning among high school students in the United States, 2009 and 2011. JAMA Dermatol. 2014;150(5):501-511. doi: 10.1001/jamadermatol.2013.7124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guy GP Jr, Watson M, Seidenberg AB, Hartman AM, Holman DM, Perna F. Trends in indoor tanning and its association with sunburn among US adults. J Am Acad Dermatol. 2017;76(6):1191-1193. doi: 10.1016/j.jaad.2017.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meyer MKH, Køster B, Juul L, et al. . Sunbed use among 64,000 Danish students and the associations with demographic factors, health-related behaviours, and appearance-related factors. Prev Med. 2017;100:17-24. doi: 10.1016/j.ypmed.2017.03.012 [DOI] [PubMed] [Google Scholar]
- 46.Nadalin V, Marrett L, Atkinson J, Tenkate T, Rosen CF. Tanning among Ontario adolescents pre-legislation: prevalence and beliefs. Prev Med. 2016;91:244-249. doi: 10.1016/j.ypmed.2016.08.045 [DOI] [PubMed] [Google Scholar]
- 47.Nadalin V, Marrett LD, Cawley C, Minaker LM, Manske S. Intentional tanning among adolescents in seven Canadian provinces: provincial comparisons (CRAYS 2015). Prev Med. 2018;111:225-230. doi: 10.1016/j.ypmed.2018.03.004 [DOI] [PubMed] [Google Scholar]
- 48.Say M, Beauchet A, Vouldoukis I, et al. . Decrease in artificial tanning by French teenagers: 2011-2016. Photodermatol Photoimmunol Photomed. 2018;34(4):257-261. doi: 10.1111/phpp.12380 [DOI] [PubMed] [Google Scholar]
- 49.Schneider S, Görig T, Breitbart EW, Greinert R, Diehl K. Prevalence, risk groups, and reasons for sunbed use in Germany [in German]. Hautarzt. 2016;67(3):226-233. doi: 10.1007/s00105-015-3753-3 [DOI] [PubMed] [Google Scholar]
- 50.Andrulonis R, Secrest AM, Patton TJ, Grandinetti LM, Ferris LK. A cross-sectional study of indoor tanning use among patients seeking skin cancer screening. J Am Acad Dermatol. 2017;76(1):164-165. doi: 10.1016/j.jaad.2016.07.055 [DOI] [PubMed] [Google Scholar]
- 51.Daniel CL, Gassman NR, Fernandez AM, Bae S, Tan MCB. Intentional tanning behaviors among undergraduates on the United States’ Gulf Coast. BMC Public Health. 2018;18(1):441. doi: 10.1186/s12889-018-5345-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Day AK, Wilson CJ, Hutchinson AD, Roberts RM. Acculturation, skin tone preferences, and tanning behaviours among young adult Asian Australians. J Prim Prev. 2016;37(5):421-432. doi: 10.1007/s10935-016-0442-7 [DOI] [PubMed] [Google Scholar]
- 53.Diehl K, Görig T, Breitbart EW, et al. . First evaluation of the Behavioral Addiction Indoor Tanning Screener (BAITS) in a nationwide representative sample. Br J Dermatol. 2018;178(1):176-182. doi: 10.1111/bjd.15888 [DOI] [PubMed] [Google Scholar]
- 54.Gajda M, Kamińska-Winciorek G, Wydmański J, Tukiendorf A, Kowalska M. Behaviors of active sunbeds users and their knowledge on the potential health risks: results of cross-sectional study in Poland. J Cosmet Dermatol. 2018;17(3):538-544. doi: 10.1111/jocd.12548 [DOI] [PubMed] [Google Scholar]
- 55.Grange F, Mortier L, Crine A, et al. . Prevalence of sunbed use, and characteristics and knowledge of sunbed users: results from the French population-based Edifice Melanoma survey. J Eur Acad Dermatol Venereol. 2015;29(suppl 2):23-30. doi: 10.1111/jdv.12899 [DOI] [PubMed] [Google Scholar]
- 56.Heckman CJ, Handorf E, Auerbach MV. Prevalence and correlates of skin cancer screening among indoor tanners and nontanners. JAMA Dermatol. 2018;154(5):554-560. doi: 10.1001/jamadermatol.2018.0163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hillhouse J, Stapleton JL, Florence LC, Pagoto S. Prevalence and correlates of indoor tanning in nonsalon locations among a national sample of young women. JAMA Dermatol. 2015;151(10):1134-1136. doi: 10.1001/jamadermatol.2015.1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Parsons BG, Gren LH, Simonsen SE, Harding G, Grossman D, Wu YP. Opportunities for skin cancer prevention education among individuals attending a community skin cancer screening in a high-risk catchment area. J Community Health. 2018;43(2):212-219. doi: 10.1007/s10900-017-0406-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rodríguez VM, Daniel CL, Welles BF, Geller AC, Hay JL. Friendly tanning: young adults’ engagement with friends around indoor tanning. J Behav Med. 2017;40(4):631-640. doi: 10.1007/s10865-017-9832-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Savoye I, Cervenka I, Mahamat-Saleh Y, Boutron-Ruault MC, Kvaskoff M. Factors associated with sunbed use in women: the E3N-SunExp Study. Am J Health Behav. 2018;42(1):85-98. doi: 10.5993/AJHB.42.1.9 [DOI] [PubMed] [Google Scholar]
- 61.Australian Institute of Health and Welfare. Melanoma of the Skin. Australian Cancer Incidence and Mortality (ACIM) books. Canberra: Australian Institute of Health and Welfare; 2018. [Google Scholar]
- 62.Wei EX, Qureshi AA, Han J, et al. . Trends in the diagnosis and clinical features of melanoma in situ (MIS) in US men and women: A prospective, observational study. J Am Acad Dermatol. 2016;75(4):698-705. doi: 10.1016/j.jaad.2016.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pandeya N, Olsen CM, Whiteman DC. The incidence and multiplicity rates of keratinocyte cancers in Australia. Med J Aust. 2017;207(8):339-343. doi: 10.5694/mja17.00284 [DOI] [PubMed] [Google Scholar]
- 64.Luengo-Fernandez R, Leal J, Gray A, Sullivan R. Economic burden of cancer across the European Union: a population-based cost analysis. Lancet Oncol. 2013;14(12):1165-1174. doi: 10.1016/S1470-2045(13)70442-X [DOI] [PubMed] [Google Scholar]
- 65.Guy GP, Ekwueme DU. Years of potential life lost and indirect costs of melanoma and non-melanoma skin cancer: a systematic review of the literature. Pharmacoeconomics. 2011;29(10):863-874. doi: 10.2165/11589300-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 66.European Commission European Core Health Indicators. ECHI data tool. EU28 Current composition 2016 data by age group. European Union. https://ec.europa.eu/health/indicators_data/echi_en. Published 2018. Accessed August 16, 2018.
- 67.Statistics Canada Table 17-10-0005-01: population estimates on July 1st, by age and sex. https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1710000501 Published 2018. Accessed August 16, 2018.
- 68.US Census Bureau Estimates of U.S. population by age and sex: April 1, 2010 to July 1, 2017. https://www.census.gov/newsroom/press-releases/2018/pop-characteristics.html. Published 2018. Accessed August 16, 2018.
- 69.Reimann J, McWhirter JE, Papadopoulos A, Dewey C. A systematic review of compliance with indoor tanning legislation. BMC Public Health. 2018;18(1):1096. doi: 10.1186/s12889-018-5994-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wehner MR, Chren MM, Nameth D, et al. . International prevalence of indoor tanning: a systematic review and meta-analysis. JAMA Dermatol. 2014;150(4):390-400. doi: 10.1001/jamadermatol.2013.6896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Qin J, Holman DM, Jones SE, Berkowitz Z, Guy GP Jr. State indoor tanning laws and prevalence of indoor tanning among US high school students, 2009-2015. Am J Public Health. 2018;108(7):951-956. doi: 10.2105/AJPH.2018.304414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Centers for Disease Control and Prevention. Skin cancer prevention progress report. https://www.cdc.gov/cancer/skin/what_cdc_is_doing/progress_report.htm. Published 2018. Accessed November 12, 2018.
- 73.Gordon LG, Hirst NG, Gies PH, Green AC. What impact would effective solarium regulation have in Australia? Med J Aust. 2008;189(7):375-378. doi: 10.5694/j.1326-5377.2008.tb02082.x [DOI] [PubMed] [Google Scholar]
- 74.Cancer Council Victoria Peter MacCallum Cancer Centre. Clare Oliver's legacy 10 years on: solarium use low but internet sites still providing platform for illegal practice. https://www.sunsmart.com.au/about/media-campaigns/media-releases/2017-media-releases/clare-olivers-legacy-10-years-on.html. Published 2017. Accessed December 6, 2018.
- 75.Purim KSM, Wroblevski FC. Sun exposure and protection among medical students in Curitiba (PR) [in Spanish]. Rev Bras Educ Med. 2014;38(4):477-485. doi: 10.1590/S0100-55022014000400009 [DOI] [Google Scholar]
- 76.Gordon LG, Hirst NG, Green AC, Neale RE. Tanning behaviors and determinants of solarium use among indoor office workers in Queensland, Australia. J Health Psychol. 2012;17(6):856-865. doi: 10.1177/1359105311427476 [DOI] [PubMed] [Google Scholar]
- 77.Euromonitor International Sun Care in Australia. London, England: Euromonitor International; May 2017.
- 78.World Health Organization WHO guidance brochures: artificial tannng sunbeds—risks and guidance. Geneva, Switzerland: World Health Organization; 2003. [Google Scholar]
- 79.Sinclair CA, Makin JK, Tang A, Brozek I, Rock V. The role of public health advocacy in achieving an outright ban on commercial tanning beds in Australia. Am J Public Health. 2014;104(2):e7-e9. doi: 10.2105/AJPH.2013.301703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Koster B, Meyer MKH, Andersson TM, Engholm G, Dalum P. Skin cancer projections and cost savings 2014-2045 of improvements to the Danish sunbed legislation of 2014. Photodermatol Photoimmunol Photomed. 2018;10(10):12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Illustration of Markov Model Health States and Possible Transitions
eFigure 2. Pooled Estimates of Ever Exposure to Indoor Tanning Among Adults After 2009
eFigure 3. Estimated Prevalence of Indoor Tanning by Age
eFigure 4. Incremental Cost per Life-year Scatterplot for Full Ban vs Current Use in North America
eFigure 5. Incremental Cost per Life-year Scatterplot for Full Ban vs Current Use in Europe
eTable 1. Comparison of Pooled Estimates on the Prevalence of Indoor Tanning
eTable 2. One-Way Sensitivity Analyze: Full Ban vs Current Sunbed Use
eReferences