Key Points
Question
Among patients undergoing surgery for lower limb fractures related to major trauma, is there a difference in deep surgical infection rates when the wound is treated with incisional negative pressure wound therapy vs standard wound dressing?
Findings
In this randomized clinical trial that included 1548 adults, there was no statistically significant difference in the rate of deep surgical site infection at 30 days between incisional negative pressure wound therapy (5.8%) and standard wound dressing (6.7%).
Meaning
The findings do not support the use of incisional negative pressure wound therapy for surgical wounds associated with lower limb fractures from major trauma, although the event rate at 30 days was lower than expected.
Abstract
Importance
Following surgery to treat major trauma–related fractures, deep wound infection rates are high. It is not known if negative pressure wound therapy can reduce infection rates in this setting.
Objective
To assess outcomes in patients who have incisions resulting from surgery for lower limb fractures related to major trauma and were treated with either incisional negative pressure wound therapy or standard wound dressing.
Design, Setting, and Participants
A randomized clinical trial conducted at 24 trauma hospitals representing the UK Major Trauma Network that included 1548 patients aged 16 years or older who underwent surgery for a lower limb fracture caused by major trauma from July 7, 2016, through April 17, 2018, with follow-up to December 11, 2018.
Interventions
Incisional negative pressure wound therapy (n = 785), which involved a specialized dressing used to create negative pressure over the wound, vs standard wound dressing not involving negative pressure (n = 763).
Main Outcomes and Measures
The primary outcome measure was deep surgical site infection at 30 days diagnosed according to the criteria from the US Centers for Disease Control and Prevention. A preplanned secondary analysis of the primary outcome was performed at 90 days. The secondary outcomes were patient-reported disability (Disability Rating Index), health-related quality of life (EuroQol 5-level EQ-5D), surgical scar assessment (Patient and Observer Scar Assessment Scale), and chronic pain (Douleur Neuropathique Questionnaire) at 3 and 6 months, as well as other local wound healing complications at 30 days.
Results
Among 1548 participants who were randomized (mean [SD] age, 49.8 [20.3] years; 561 [36%] were aged ≤40 years; 583 [38%] women; and 881 [57%] had multiple injuries), 1519 (98%) had data available for the primary outcome. At 30 days, deep surgical site infection occurred in 5.84% (45 of 770 patients) of the incisional negative pressure wound therapy group and in 6.68% (50 of 749 patients) of the standard wound dressing group (odds ratio, 0.87 [95% CI, 0.57 to 1.33]; absolute risk difference, −0.77% [95% CI, −3.19% to 1.66%]; P = .52). There was no significant difference in the deep surgical site infection rate at 90 days (11.4% [72 of 629 patients] in the incisional negative pressure wound therapy group vs 13.2% [78 of 590 patients] in the standard wound dressing group; odds ratio, 0.84 [95% CI, 0.59 to 1.19]; absolute risk difference, −1.76% [95% CI, −5.41% to 1.90%]; P = .32). For the 5 prespecified secondary outcomes reported, there were no significant differences at any time point.
Conclusions and Relevance
Among patients who underwent surgery for major trauma–related lower limb fractures, use of incisional negative pressure wound therapy, compared with standard wound dressing, resulted in no significant difference in the rate of deep surgical site infection. The findings do not support the use of incisional negative pressure wound therapy in this setting, although the event rate at 30 days was lower than expected.
Trial Registration
isrctn.org Identifier: ISRCTN12702354
This randomized clinical trial compares the effects of incisional negative pressure wound therapy vs standard wound dressing on deep surgical site infection (SSI) at 30 days among patients undergoing surgery for major trauma–related lower limb fractures.
Introduction
In 2010, trauma was the leading cause of death worldwide among those younger than 45 years.1 Major trauma, defined as when more than 1 body system is injured or an isolated limb has been subjected to severe trauma, is a significant cause of short- and long-term morbidity and a key cost driver in both acute health care and subsequent social care.2 Eighty-five percent of patients with major trauma sustain serious limb injuries, most commonly fractured bones.3 Treating these fractures is complicated by the systematic inflammatory response to major trauma, as well as the extensive soft tissue injuries adjacent to the broken bone which, taken together, may cause high rates of wound infection following surgery for lower limb fractures after major trauma.4,5
One of the factors that may reduce the risk of infection in the surgical wounds of patients with major trauma is the type of dressing applied over the incision at the end of the operation. New techniques for wound management, such as incisional negative pressure wound therapy, have shown promising results following some types of surgery and these techniques have the potential for reducing wound infections; however, there is limited evidence among patients with wounds associated with surgery for lower limb fractures caused by major trauma.6,7
The aim of this randomized clinical trial was to determine if incisional negative pressure wound therapy was more effective than standard wound dressing in reducing the rate of deep surgical site infection in wounds associated with surgery for a fracture in the context of major trauma to the lower limb.
Methods
The National Research Ethics Service approved the study. The trial protocol appears in Supplement 1 and the statistical analysis plan appears in Supplement 2 and were both published8,9; modifications to the protocol appear in Supplement 3. The trial was overseen by independent steering and data and safety monitoring committees. Eligible patients were approached by a local researcher and were provided with verbal and written information about the trial before being asked to provide written informed consent.
For patients with acute confusional states or temporary impairment of consciousness, we approached a “consultee” to provide agreement on behalf of the patient as per the UK Mental Capacity Act 2005. All participants randomized under this provision were subsequently approached for written consent once capacity was restored, with the option to continue or discontinue involvement in the trial. For this reason, higher levels of postrandomization withdrawal were anticipated than might be expected in most clinical trials.
The trial took place at 24 major trauma hospitals representing the UK Major Trauma Network. In the United Kingdom, patients with major trauma are transported directly to a specialist trauma center. Eligible patients were aged 16 years or older with a lower extremity fracture caused by major trauma that required surgery and, postoperatively, had a wound that could be closed. Patients had to present to the trial hospital within 72 hours of their injury, including those who were transferred from other hospitals.
Patients were excluded if they were unable to adhere to the trial procedures or complete questionnaires (eg, had a preexisting diagnosis of dementia or an open fracture and the wound could not be closed after the first surgery). Patients with open fractures that cannot be closed at the first surgery are at the highest risk of surgical site infection. Incisional negative pressure wound therapy cannot be applied to these types of wounds.
Randomization and Masking
A computer-generated randomization algorithm was created and delivered by an accredited clinical trials unit to ensure the allocation sequence was concealed. Each patient was randomized to treatment on a 1:1 basis, stratified by trial recruitment center, Injury Severity Score (ISS) of 15 or less vs ISS of 16 or greater (ISS was used as a surrogate for the degree of systemic inflammatory response associated with the injuries) and open or closed fracture at presentation (only those open fractures for which the wound could be closed primarily after the first surgical wound debridement were eligible for inclusion because incisional negative pressure wound therapy cannot be applied to wounds that are left open). A probabilistic element was included in the minimization algorithm to ensure unpredictability of treatment assignment. When a patient entered the trial, nonidentifiable details were logged on the secure, encrypted, web-based system.
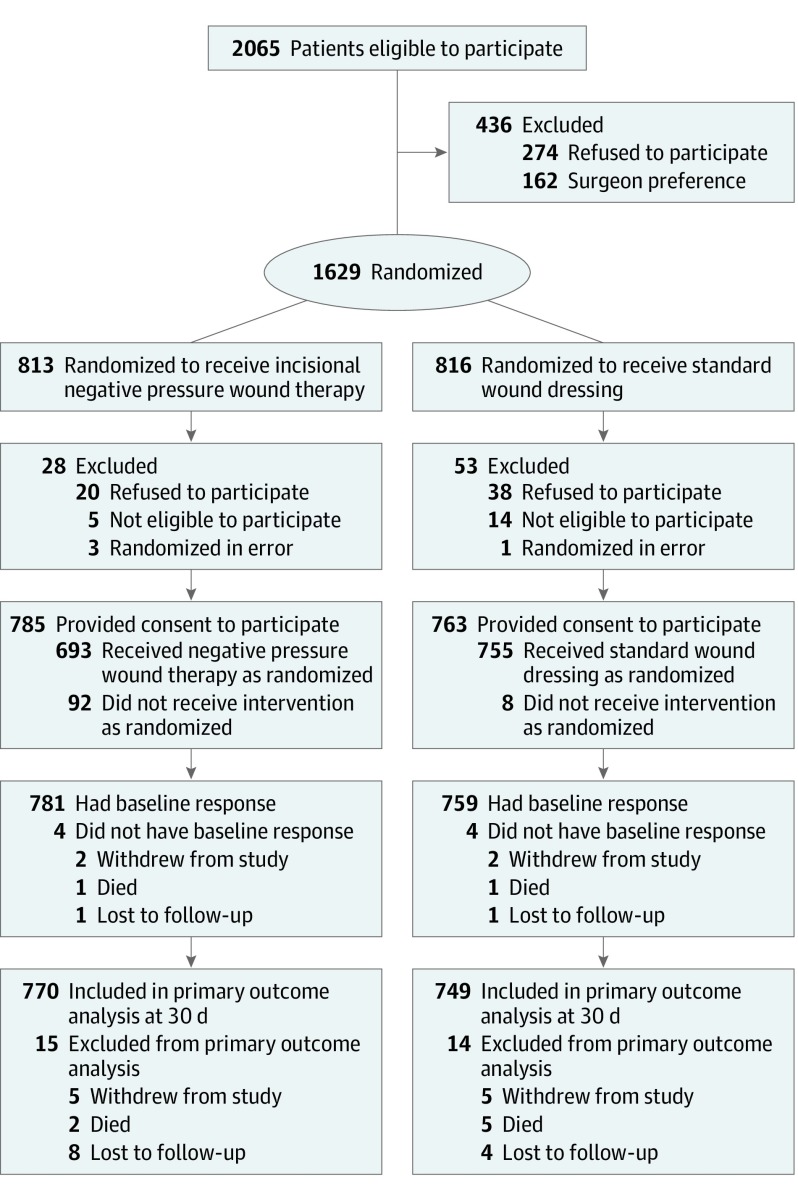
Participants were assigned treatment intraoperatively at the end of surgery once it was determined that the surgical wound could be closed but before any wound dressing was applied (Figure). It was not possible to blind trial participants to treatment because the wound dressing was clearly visible. In addition, the treating surgeons could not be blinded to the intervention; however, the surgical and health care teams were not involved in any trial assessments and the primary outcome data were collected by independent research associates.
Figure. Patient Recruitment, Randomization, and Follow-up in the WHIST Study.
Interventions
All patients received a general or regional anesthetic. The operative treatment followed standard clinical practice with relevant details recorded by the research team. At the end of the operation, a dressing was applied to the surgical wound. The patient was randomized to incisional negative pressure wound therapy or standard wound dressing. All other elements of postoperative care remained the same. The wound could be redressed again on the ward at the discretion of the clinical team; any further wound dressing was recorded and followed the randomized treatment unless otherwise clinically indicated.
Incisional Negative Pressure Wound Therapy Group
Incisional negative pressure wound therapy uses a silicone contact layer with a silicon-based adhesive, an airlock layer, a superabsorbent layer, and a polyurethane (semipermeable) layer on the top that makes the system waterproof while allowing water vapor to pass. A sealed tube connects the dressing to a built-in mini-pump that creates a partial vacuum (−80 mm Hg of negative pressure) over the wound. Incisional negative pressure wound therapy was applied to the wound at the end of the operation as per the treating surgeon’s normal practice and according to the dressing manufacturer’s instructions.
Standard Wound Dressing Group
All centers used a sterile dressing obtained from sealed packages that prevented external contamination. However, the precise details of the materials used were left to the discretion of the treating health care team as per routine care at their center. Details of each dressing applied in the trial were recorded and classified according to British National Formulary classification.
Data Collection and Outcome Measures
The primary outcome measure for this study was deep surgical site infection. The US Centers for Disease Control and Prevention (CDC) defines surgical site infection as a “deep infection” involving the tissues deep to the skin that occurs within 30 days of injury.10 The treating clinical team recorded any signs or symptoms of wound-related infection in the medical record per routine clinical practice. The treating clinicians were not part of the research team.
The participants were clinically assessed, and the medical records were reviewed by an independent research associate who recorded the primary outcome of deep surgical site infection against the CDC criteria for the diagnosis of deep infection. The first criterion was whether fluid was leaking from the wound and whether the fluid was pus. The second criterion included at least one description from each of the following: (1) the wound was gaping open (dehisced) or a surgeon had deliberately opened the wound and (2) the area around the wound was painful or tender or the participant had a fever higher than 38°C. The third criterion was whether there was any sign of abscess or infection on direct examination or imaging (eg, ultrasound).
Shortly after the start of this trial, the CDC updated its criteria for a deep surgical site infection in patients treated for fracture fixation. Specifically, the end point for wounds involving an implant was changed from 30 days to 90 days. Because infection at 90 days was captured under the outcome measure for wound complications, no changes were made to data collection. However, to facilitate future evidence synthesis, and after consultation with the trial steering committee, we included a secondary analysis of the primary outcome of deep surgical site infection at 90 days within the statistical analysis plan.
There were several secondary outcomes measured during the 6 months after randomization: (1) patient-reported Disability Rating Index (range, 0 to 100 points [0, normal function; 100, complete disability]; minimal clinically important difference of 8 points)11; (2) health-related quality of life using the EuroQol 5-level EQ-5D (range, −0.594 to 1 [0, death; a higher score relates to better quality of life])12; (3) self-reported quality of wound healing using the patient scale from the Patient and Observer Scar Assessment Scale (range, 1 to 10 [1, normal skin; 10, very different from normal skin])13; and (4) patient-reported Douleur Neuropathique Questionnaire for chronic pain (responses to 7 questions; neuropathic pain considered present if answered yes to ≥3 questions).14 Local wound complications other than deep surgical site infection were collected up to 30 days. Resource use data were collected but are not reported herein.
Statistical Analysis
In the only prior randomized clinical trial7 that compared incisional negative pressure wound therapy vs standard wound dressing for surgical incisions associated with major trauma to the lower limb, the rate of late (deep) infection was reduced by 6% in the incisional negative pressure wound therapy group. In the absence of a commonly accepted minimum clinically important difference for deep wound infection, surgeons in the UK Orthopaedic Trauma Society who perform surgery for major trauma were surveyed to determine what they believed was a clinically important reduction in deep surgical site infection rates. The survey (based on 120 respondents) showed that a 6% reduction in the rate of “deep infection” would be sufficient to change clinical practice with regard to the choice of wound dressing.
Assuming a reduction from 15% (based on a prior randomized clinical trial7) to 9% in the proportion of patients having a deep surgical site infection, 615 patients would be required in each group to provide 90% power at the 5% level. Therefore, we aimed to recruit 1540 participants to account for up to 20% of patients being lost to follow-up.
Per the prespecified analysis plan, which was approved by the independent data and safety monitoring committee, the rates of deep surgical site infection in the 2 groups were compared using a mixed-effects logistic regression model. The model included a random effect due to recruitment center, and fixed effects to adjust for open vs closed fractures at presentation, ISS level (≤15 vs ≥16), participant age, and participant sex. The odds ratio (OR), 95% CI, and associated P value were used to compare the 2 treatment groups; the absolute risk difference also was reported. This analysis was performed according to the randomization group (randomized population).
A secondary analysis was performed for the primary outcome measure according to the type of dressing actually applied (per-protocol population). Binary secondary outcome measures were analyzed using similar logistic regression models. Analogous multilevel, mixed-effects linear regression models were used for the continuous secondary outcome measures. The models used repeated measures nested within participants and were adjusted for fixed and random effects as in the primary outcome model. The models relied on assumptions of linearity, normality of residuals, homoscedasticity, and without correlation between level 1 and level 2 residuals; these assumptions were checked via appropriate plots. All secondary analyses were performed according to randomization group (randomized population).
The main analyses for the primary and secondary outcomes were performed using available cases only. In a sensitivity analysis, multiple imputation for the missing primary outcome data was performed under the missing at random assumption.
A 2-sided significance level of .05 was used throughout. Because of the potential for type I error due to multiple comparisons, the findings for the secondary end point analyses should be interpreted as exploratory. Stata version 15.0 (StataCorp) was used for all analyses.
Results
A total of 1629 patients were randomized between July 7, 2016, and April 17, 2018, with follow-up to December 11, 2018. Eighty-one patients did not have sufficient mental capacity to participate in the study prior to surgery or were unable or unwilling to provide informed consent after randomization. Among the remaining 1548 participants (mean [SD] age, 49.8 [20.3] years; 561 [36%] were aged ≤40 years; 583 [38%] women; 964 [62%] men; and 881 [57%] had multiple injuries), 1519 (98%) had data available for the primary outcome (Figure). Data sum to 1547 for men and women because we were unable to report sex for 1 person who withdrew immediately after randomization. The majority of participants had a closed fracture (81%) with an ISS of 15 or less (78%). The characteristics of the 2 groups were well balanced after randomization (Table 1).
Table 1. Baseline Characteristics of Participants.
| No./Total No. (%)a | ||
|---|---|---|
| Incisional Negative Pressure Wound Therapy |
Standard Wound Dressing |
|
| Sex | ||
| Male | 482/784 (61.5) | 482/763 (63.2) |
| Female | 302/784 (38.5) | 281/763 (36.8) |
| Age ≤40 y | 283/784 (36.1) | 278/763 (36.4) |
| Race/ethnicity | ||
| White | 701/773 (90.7) | 667/741 (90.0) |
| Black Caribbean | 9/773 (1.2) | 6/741 (0.8) |
| Black African | 15/773 (1.9) | 13/741 (1.8) |
| Other black | 2/773 (0.3) | 4/741 (0.5) |
| Indian | 7/773 (0.9) | 11/741 (1.5) |
| Pakistani | 7/773 (0.9) | 14/741 (1.9) |
| Bangladeshi | 1/773 (0.1) | 3/741 (0.4) |
| Chinese | 0/773 | 1/741 (0.1) |
| Otherb | 31/773 (4.0) | 22/741 (3.0) |
| Body mass index, mean (SD)c | 26.4 (5.9) | 26.7 (6.0) |
| Mechanism of injury | ||
| Road traffic collision | 298/780 (38.2) | 273/759 (36.0) |
| Low energy fall | 275/780 (35.3) | 252/759 (33.2) |
| High energy fall | 139/780 (17.8) | 145/759 (19.1) |
| Crush injury | 16/780 (2.1) | 16/759 (2.1) |
| Contact sports | 10/780 (1.3) | 12/759 (1.6) |
| Other | 42/780 (5.4) | 61/759 (8.0) |
| Preinjury diagnosis of diabetes | 63/775 (8.1) | 85/750 (11.3) |
| Regular smoker | 218/762 (28.6) | 216/740 (29.2) |
| Alcohol consumption ≤14 U/wkd | 627/756 (82.9) | 609/730 (83.4) |
| Injury Severity Score ≤15e | 609/784 (77.7) | 598/763 (78.4) |
| Wound closed at presentation | 637/784 (81.3) | 622/763 (81.5) |
| Wound above the knee | 474/781 (60.7) | 447/759 (58.9) |
| Type of closure | ||
| Subcuticular suture | 242/774 (31.3) | 220/756 (29.1) |
| Skin clips | 198/774 (25.6) | 216/756 (28.6) |
| Interrupted sutures | 203/774 (26.2) | 200/756 (26.5) |
| Other skin closure | 131/774 (16.9) | 120/756 (15.9) |
Unless otherwise indicated.
Includes unspecified race/ethnicity.
Calculated as weight in kilograms divided by height in meters squared.
A unit is equivalent to 10 mL of pure alcohol.
An anatomical score that measures the overall severity of injured patients in 6 body regions; range, 1 to 75. Injuries were assigned an Abbreviated Injury Scale code and an associated score from 1 (minor injury) to 6 (an injury that is thought to be incompatible with life). Patients with multiple injuries are scored by adding together the squares of the 3 highest scores; for example, 52 + 52 + 52 equals 75, which is the maximum survivable score. By convention, a patient with an Abbreviated Injury Scale code of 6 in 1 body region is given an Injury Severity Score of 75.
There was no significant difference in the primary outcome measure of deep surgical site infection at 30 days between the groups as randomized. The rate of deep surgical site infection at 30 days was 5.84% (45/770) in the incisional negative pressure wound therapy group vs 6.68% (50/749) in the standard wound dressing group (OR, 0.87 [95% CI, 0.57 to 1.33]; absolute risk difference, −0.77% (95% CI, −3.19% to 1.66%); P = .52) (Table 2).
Table 2. Comparison of Treatment Groups on Primary and Secondary Outcomes.
| Incisional Negative Pressure Wound Therapya |
Standard Wound Dressinga |
Adjusted Treatment Effect (95% CI) |
P Valueb | |
|---|---|---|---|---|
| Primary Outcome | ||||
| Deep surgical site infection at 30 d, No./Total No. (%) | ||||
| Randomized population | 45/770 (5.84) | 50/749 (6.68) | OR, 0.87 (0.57 to 1.33) | .52 |
| Per-protocol population | 41/668 (6.14) | 48/731 (6.57) | OR, 0.93 (0.60 to 1.44) | .76 |
| Primary analysis population | 46/784 (5.87) | 51/763 (6.68) | OR, 0.86 (0.57 to 1.31) | .49 |
| Secondary analysis of primary outcome at 90 d in randomized population, No./Total No. (%) | 72/629 (11.4) | 78/590 (13.2) | OR, 0.84 (0.59 to 1.19) | .32 |
| Self-Reported Secondary Outcomes | ||||
| Disability Rating Index, mean (95% CI)c | ||||
| At 3 mo | (n = 507) 51.6 (49.5 to 53.6) |
(n = 456) 51.1 (48.9 to 53.3) |
MD, −0.01 (−2.79 to 2.78) | >.99 |
| At 6 mo | (n = 469) 40.6 (38.3 to 42.8) |
(n = 432) 40.2 (37.7 to 42.8) |
MD, 0.03 (−2.82 to 2.88) | .98 |
| Health-related quality of life assessed by EuroQol 5-level EQ-5D, mean (95% CI)d | ||||
| At 3 mo | (n = 528) 0.50 (0.47 to 0.52) |
(n = 470) 0.49 (0.47 to 0.52) |
MD, 0 (−0.03 to 0.04) | .84 |
| At 6 mo | (n = 486) 0.58 (0.55 to 0.60) |
(n = 446) 0.58 (0.55 to 0.60) |
MD, 0 (−0.03 to 0.04) | .86 |
| Visual analog scale on EuroQol 5-level EQ-5D, mean (95% CI)e | ||||
| At 3 mo | (n = 531) 64.1 (62.2 to 66.0) |
(n = 478) 64.7 (62.7 to 66.8) |
MD, −0.73 (−3.30 to 1.84) | .58 |
| At 6 mo | (n = 489) 69.7 (67.8 to 71.5) |
(n = 449) 69.4 (67.4 to 71.5) |
MD, 0.08 (−2.57 to 2.74) | .95 |
| Scar assessment score, mean (95% CI)f | ||||
| At 30 d | (n = 657) 4.35 (4.15 to 4.56) |
(n = 616) 4.58 (4.37 to 4.79) |
MD, −0.18 (−0.46 to 0.10) | .22 |
| At 3 mo | (n = 523) 4.71 (4.47 to 4.95) |
(n = 470) 4.86 (4.61 to 5.11) |
MD, −0.11 (−0.41 to 0.20) | .51 |
| At 6 mo | (n = 483) 4.61 (4.36 to 4.86) |
(n = 437) 4.52 (4.27 to 4.77) |
MD, 0.11 (−0.21 to 0.42) | .52 |
| Chronic neuropathic pain assessed by Douleur Neuropathique Questionnaire, No./Total No. (%)g | ||||
| At 3 mo | 113/362 (31.2) | 109/339 (32.2) | OR, 0.94 (0.68 to 1.31) | .72 |
| At 6 mo | 117/414 (28.3) | 117/367 (31.9) | OR, 0.84 (0.61 to 1.15) | .27 |
Abbreviations: MD, mean difference; OR, odds ratio.
Adjusted for open vs closed wounds, Injury Severity Score, participant age, sex, and, when appropriate, baseline scores (Disability Rating Index; health-related quality of life and visual analog scale on EuroQol 5-level EQ-5D) as fixed effects, and for randomizing center as a random effect. As a sensitivity analysis, multiple imputation was used for missing primary outcome data under the missing at random assumption.
Calculated from mixed-effects logistic regression models for binary variables and from repeated-measures mixed-effects linear regression models for continuous variables.
Range, 0 to 100; higher scores indicate less disability. Scores were calculated as a mean across all 12 questions and were imputed for participants who answered at least 10 questions.
Range, −0.594 to 1; higher scores indicate better quality of life. Scores were converted to multiattribute utility values using the crosswalk and EQ-5D-3L value sets.
Range, 0 to 100; higher scores indicate better quality of life.
Range, 1 to 10; 1 indicates normal skin; 10, very different from normal skin.
Binary measure of neuropathic pain. If a participant responded yes to 3 or more of the 7 questions about pain characteristics, neuropathic pain was considered to be present. Data were available for the randomized population.
There was no significant difference in the secondary analysis of the per-protocol population or in the sensitivity analysis using multiple imputation. In the per-protocol population, the rate of deep surgical site infection at 30 days was 6.14% (41/668) in the incisional negative pressure wound therapy group vs 6.57% (48/731) in the standard wound dressing group (OR, 0.93 [95% CI, 0.60 to 1.44]; absolute risk difference, 0.33% [95% CI, −2.93% to 2.15%]; P = .76). In the randomized population, there was no significant difference in deep surgical site infection at 90 days; the rate was 11.4% (72/629) in the incisional negative pressure wound therapy group vs 13.2% (78/590) in the standard wound dressing group (OR, 0.84 [95% CI, 0.59 to 1.19]; absolute risk difference, −1.76% [95% CI, −5.41% to 1.90%]; P = .32).
Similarly, there was no significant difference between the groups for the secondary outcome measures. The mean score for participants’ self-reported Disability Rating Index at 6 months was 40.6 (95% CI, 38.3 to 42.8) in the incisional negative pressure wound therapy group vs 40.2 (95% CI, 37.7 to 42.8) in the standard wound dressing group (between-group mean difference, 0.03 [95% CI, −2.82 to 2.88]; P = .98). The mean score for health-related quality of life (EuroQol 5-level EQ-5D) at 6 months was 0.58 (95% CI, 0.55 to 0.60) in the incisional negative pressure wound therapy group vs 0.58 (95% CI, 0.55 to 0.60) in the standard wound dressing group (between-group mean difference, 0 [95% CI, −0.03 to 0.04]; P = .86).
The mean score for overall self-assessment of the surgical scar at 30 days was 4.35 (95% CI, 4.15 to 4.56) in the incisional negative pressure wound therapy group vs 4.58 (95% CI, 4.37 to 4.79) in the standard wound dressing group (mean between-group difference, −0.18 [95% CI, −0.46 to 0.10]; P = .22). The proportion of patients reporting chronic neuropathic pain (Douleur Neuropathique Questionnaire) at 6 months was 28.3% in the incisional negative pressure wound therapy group vs 31.9% in the standard wound dressing group (OR, 0.84 [95% CI, 0.61 to 1.15]; absolute risk difference, −3.5% [95% CI, −9.9% to 2.8%]; P = .27) (Table 2).
Other wound healing complications not meeting the CDC criteria for deep surgical site infection appear in Table 3. No significant differences between the 2 groups were identified. The most common complication was a painful or tender wound reported by 24.4% of participants in the incisional negative pressure wound therapy group vs 27.6% of participants in the standard wound dressing group (adjusted OR, 0.84 [95% CI, 0.65 to 1.08]; absolute risk difference, −3.0% [95% CI, −7.4% to 1.4%]; P = .18). Only a small number of participants received an antibiotic to treat a wound complication that was not a deep surgical site infection (3.5% in the incisional negative pressure wound therapy group vs 3.9% in the standard wound dressing group; adjusted OR, 0.87 [95% CI, 0.50 to 1.51]; absolute risk difference, −0.5% [95% CI, −2.5% to 1.5%]; P = .61).
Table 3. Other Wound Complications at or Prior to 30 Days.
| No./Total No. (%) | Absolute Risk Difference (95% CI), % |
Adjusted Odds Ratio (95% CI) |
P Value | ||
|---|---|---|---|---|---|
| Incisional Negative Pressure Wound Therapy (n = 739) |
Standard Wound Dressing (n = 713) |
||||
| Wound healing complicationa | |||||
| Red and inflamed | 76/715 (10.6) | 90/684 (13.2) | −2.7 (−6.0 to 6.5) | 0.76 (0.55 to 1.07) | .11 |
| Swollen | 147/715 (20.6) | 137/684 (20.0) | 0.5 (−3.5 to 4.6) | 1.03 (0.79 to 1.36) | .81 |
| Painful or tender | 173/710 (24.4) | 188/682 (27.6) | −3.0 (−7.4 to 1.4) | 0.84 (0.65 to 1.08) | .18 |
| Fever >38°C | 61/713 (8.6) | 67/681 (9.8) | −1.5 (−4.5 to 1.4) | 0.82 (0.56 to 1.19) | .30 |
| Fluid leaking (not pus) | 50/715 (7.0) | 60/687 (8.7) | −1.8 (−4.7 to 0.9) | 0.76 (0.51 to 1.14) | .18 |
| Treatment for complication | |||||
| Surgicallyb | 1/573 (0.2) | 2/575 (0.3) | −0.2 (−0.8 to 0.4) | 0.39 (0.03 to 5.06) | .47 |
| Antibiotic | 25/724 (3.5) | 27/689 (3.9) | −0.5 (−2.5 to 1.5) | 0.87 (0.50 to 1.51) | .61 |
Did not meet the definition of deep surgical site infection from the US Centers for Disease Control and Prevention. Some participants experienced more than 1 wound healing complication.
Data were not available for all participants.
Discussion
This multicenter trial of patients undergoing surgery for fractures of the lower limb associated with major trauma found no significant difference in the rate of deep surgical site infection at 30 days between patients whose surgical wound was treated with incisional negative pressure wound therapy and those treated with standard wound dressing. There was no significant difference in the rate of deep surgical site infection at 90 days. Furthermore, there was no evidence of any significant differences in patient self-reported disability, health-related quality of life, scar healing, chronic pain, or other wound healing complications.
Patients with open fractures of the lower limb that could not be closed primarily at the first surgical wound debridement were excluded from this trial. These patients are at the highest risk of surgical site infection and the use of negative pressure wound therapy has previously been reported in the Wound Management of Open Lower Limb Fractures (WOLLF) trial.15 The WOLLF trial found no evidence that surface negative pressure wound therapy reduced patient-reported disability for those with open wounds associated with fractures of the lower limb.
The trial reported herein addresses the use of a different type of negative pressure wound therapy placed over a closed surgical incision for wounds that have a high risk for deep wound infection. At the inception of this trial, there had been only 1 other trial7 comparing incisional negative pressure wound therapy vs standard wound dressing applied to surgical wounds in the context of major trauma. That trial7 indicated that incisional negative pressure wound therapy reduced the rate of deep surgical site infection vs standard wound dressing. However, the previous study was considerably smaller (n = 249) than the trial reported herein.
A systematic review of the literature conducted since the start of this trial shows 1 additional small randomized trial16 of incisional negative pressure wound therapy vs standard wound dressing for surgical wounds following trauma. In that trial, 66 patients undergoing surgery for fixation of an acetabular fracture were randomized to incisional negative pressure wound therapy vs standard gauze dressing. There was no evidence of a statistically significant difference in the rate of deep surgical site infection; however, the number of deep surgical site infections was small with only 2 patients (6.1%) in the standard wound dressing group and 5 (15.2%) in the incisional negative pressure wound therapy group. The only other trial17 reported since the current trial started, was a mechanistic study involving 20 patients in which ultrasonography was used to assess wound seroma formation following the use of incisional negative pressure wound therapy in patients receiving surgery for spinal fractures.
Limitations
This study has several limitations. First, because of the emergency nature of the surgery, it was anticipated that some patients who were randomized would subsequently be unable or unwilling to participate. However, the majority of patients (85%) agreed to participate.
Second, 100 patients did not receive the randomized intervention. As expected, when testing a relatively new intervention such as incisional negative pressure wound therapy, the majority of the crossovers were from the incisional negative pressure wound therapy group to the standard wound dressing group (n = 92). The analysis by the treatment received confirmed the result of the primary analysis (ie, there was no significant difference between the groups of participants).
Third, in terms of assessing the primary outcome of infection, the event rate at 30 days was lower than reported in the literature and lower than anticipated during the trial development. This may reflect recent improvements in the care pathways for patients with major trauma.18 Or it may simply reflect changes in the way that data relating to deep surgical site infection are reported. The secondary analysis of the primary outcome of deep surgical site infection at 90 days, as per the change in the CDC criteria after the trial started, found an event rate much closer to that previously reported in the literature and used in the sample size calculation for this trial. There was also no significant difference between treatment groups at this time point.
However, this estimate at 90 days is less precise than that at 30 days due to higher levels of missing data. In mitigation of the lower than expected rate of infection at the primary end point, the rate of those lost to follow-up at 30 days was less than 2%, which was considerably lower than anticipated.
Conclusions
Among patients who underwent surgery for major trauma–related lower limb fractures, use of incisional negative pressure wound therapy, compared with standard wound dressing, resulted in no significant difference in the rate of deep surgical site infection. The findings do not support the use of incisional negative pressure wound therapy in this setting, although the event rate at 30 days was lower than expected.
Statistical analysis plan
Trial protocol
Trial protocol changes
Data sharing statement
References
- 1.Lozano R, Naghavi M, Foreman K, et al. . Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010 [published correction appears in Lancet. 2013;381(9867):628]. Lancet. 2012;380(9859):2095-2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Audit Office Major trauma: assessment and initial management. https://www.nice.org.uk/guidance/ng39. Accessed January 10, 2020.
- 3.Herron J, Hutchinson R, Lecky F, et al. . The impact of age on major orthopaedic trauma. Bone Joint J. 2017;99-B(12):1677-1680. [DOI] [PubMed] [Google Scholar]
- 4.McFerran MA, Smith SW, Boulas HJ, Schwartz HS. Complications encountered in the treatment of pilon fractures. J Orthop Trauma. 1992;6(2):195-200. [DOI] [PubMed] [Google Scholar]
- 5.Young MJ, Barrack RL. Complications of internal fixation of tibial plateau fractures. Orthop Rev. 1994;23(2):149-154. [PubMed] [Google Scholar]
- 6.Glass GE, Murphy GF, Esmaeili A, et al. . Systematic review of molecular mechanism of action of negative-pressure wound therapy. Br J Surg. 2014;101(13):1627-1636. [DOI] [PubMed] [Google Scholar]
- 7.Stannard JP, Volgas DA, McGwin G III, et al. . Incisional negative pressure wound therapy after high-risk lower extremity fractures. J Orthop Trauma. 2012;26(1):37-42. [DOI] [PubMed] [Google Scholar]
- 8.Achten J, Vadher K, Bruce J, et al. . Standard wound management versus negative-pressure wound therapy in the treatment of adult patients having surgical incisions for major trauma to the lower limb-a two-arm parallel group superiority randomised controlled trial: protocol for Wound Healing in Surgery for Trauma (WHIST). BMJ Open. 2018;8(6):e022115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knight R, Spoors LM, Costa ML, Dutton SJ. Wound Healing In Surgery for Trauma (WHIST): statistical analysis plan for a randomised controlled trial comparing standard wound management with negative pressure wound therapy. Trials. 2019;20(1):186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36(5):309-332. [DOI] [PubMed] [Google Scholar]
- 11.Salén BA, Spangfort EV, Nygren AL, Nordemar R. The Disability Rating Index. J Clin Epidemiol. 1994;47(12):1423-1435. [DOI] [PubMed] [Google Scholar]
- 12.Brooks R. EuroQol: the current state of play. Health Policy. 1996;37(1):53-72. [DOI] [PubMed] [Google Scholar]
- 13.Draaijers LJ, Tempelman FR, Botman YA, et al. . The Patient and Observer Scar Assessment Scale. Plast Reconstr Surg. 2004;113(7):1960-1965. [DOI] [PubMed] [Google Scholar]
- 14.Bouhassira D, Lantéri-Minet M, Attal N, et al. . Prevalence of chronic pain with neuropathic characteristics in the general population. Pain. 2008;136(3):380-387. [DOI] [PubMed] [Google Scholar]
- 15.Costa ML, Achten J, Bruce J, et al. . Effect of negative pressure wound therapy vs standard wound management on 12-month disability among adults with severe open fracture of the lower limb. JAMA. 2018;319(22):2280-2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Webster J, Scuffham P, Sherriff KL, et al. . Negative pressure wound therapy for skin grafts and surgical wounds healing by primary intention. Cochrane Database Syst Rev. 2012;(4):CD009261. [DOI] [PubMed] [Google Scholar]
- 17.Crist BD, Oladeji LO, Khazzam M, et al. . Role of acute negative pressure wound therapy over primarily closed surgical incisions in acetabular fracture ORIF. Injury. 2017;48(7):1518-1521. [DOI] [PubMed] [Google Scholar]
- 18.Moran CG, Lecky F, Bouamra O, et al. . Changing the system—major trauma patients and their outcomes in the NHS (England) 2008-17. EClinicalMedicine. 2018;2-3:13-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Statistical analysis plan
Trial protocol
Trial protocol changes
Data sharing statement