This cross-sectional study analyzes the distributional, convergent, and predictive validity of National Institutes of Health Stroke Scale (NIHSS) values in the National Inpatient Sample.
Key Points
Question
Do the National Institutes of Health Stroke Scale (NIHSS) deficit severity scores reported by US hospitals nationally show expected distribution patterns and outcome-predictive power?
Findings
In this cross-sectional study of 154 165 ischemic stroke admissions in the US National Inpatient Sample, optionally reported NIHSS scores were recorded in nearly 1 in 7 patients. The NIHSS score distribution nationally was similar to that in clinical registries and population-based studies, with associations with functional outcome and early mortality.
Meaning
These data support the validity and robust prognostic power of national, administratively reported NIHSS scores, highlighting their potential to aid care quality assessment in the United States.
Abstract
Importance
Comparative assessment of acute ischemic stroke care quality provided by hospitals in the United States has been hampered by the unavailability of the National Institutes of Health Stroke Scale (NIHSS) in administrative data sets, preventing adequate adjustment for variations in patient case-mix risk. In response to stakeholder concerns, the US Centers for Medicare & Medicaid Services in 2016 implemented optional reporting of NIHSS scores.
Objective
To analyze the distributional, convergent, and predictive validity of nationally submitted NIHSS values in the National Inpatient Sample.
Design, Setting, and Participants
This population-based retrospective cross-sectional study took place from October 1 to December 31, 2016. The nationally representative sample included US adults who had ischemic stroke hospitalizations during the first calendar quarter in which optional NIHSS reporting was implemented. Analysis began September 2019.
Main Outcomes and Measures
Distribution of NIHSS scores, functional independence at discharge, inpatient mortality, and administrative reporting of NIHSS.
Results
Among 154 165 ischemic stroke hospitalizations during the first 3 months of the reporting policy, NIHSS scores were reported in 21 685 patients (14%) (10 925 women [50.4%]; median [interquartile range] age, 72 [61-82] years). Median (interquartile range) NIHSS score was 4 (2-11), and frequency of severity categories included absent (NIHSS score, 0) in 2080 patients (9.6%), minor (NIHSS score, 1-4) in 8760 patients (40.4%), and severe (NIHSS score, 21-42) in 1930 patients (8.9%). National Institutes of Health Stroke Scale score of 10 or more, an indicator of possible large vessel occlusions, was present in 6290 patients (29%). Presenting NIHSS score was higher in very elderly patients (age ≥80 y) and women and also in patients receiving endovascular thrombectomy vs intravenous thrombolysis alone vs no reperfusion therapy (median [interquartile range], 17 [12-22] vs 6 [4-12] vs 4 [2-9], respectively) (P < .001). National Institutes of Health Stroke Scale scores were similarly higher for discharge outcomes of mortality vs discharge to skilled nursing facility vs discharge home (median [interquartile range], 19 [12-25] vs 7 [3-15] vs 2 [1-5], respectively) (P < .001). Likelihood of NIHSS scores being reported independently increased with interfacility transfer, receipt of acute reperfusion therapies, larger hospital size, academic centers, and region other than the West.
Conclusions and Relevance
In the initial national optional reporting period in the United States, NIHSS scores were reported in nearly 1 in 7 ischemic stroke hospitalizations. The distribution of NIHSS scores was similar to that from narrow population-based studies and registries, and NIHSS scores were powerfully associated with discharge outcome, supporting the validity and potential to aid care quality assessment.
Introduction
The National Institutes of Health Stroke Scale (NIHSS) is the most widely used measure of presenting stroke deficit severity and a dominant predictor of patient functional outcome.1,2 Until recently, attempts to measure the quality of stroke care provided by hospitals in the United States and the world were hampered by the unavailability of the NIHSS in administrative data sets, preventing adequate adjustment for variations in patient case-mix risk when comparing functional outcomes and mortality after stroke across facilities. The lack of regularly documented and recorded NIHSS score also constrained understanding of the generalizability of clinical trial findings to patients encountered in routine clinical care and of secular trends over time and variation by region in presenting stroke severity. Clinical registries have provided important insights regarding presenting stroke severity, but NIHSS values have had high missingness rates in registries, and registry hospitals and patients are not fully representative of all facilities and individuals affected by stroke.3
Recognizing the urgent societal need to improve documentation of presenting stroke severity, major stroke organizations, including the American Heart Association/American Stroke Association, the European Stroke Organisation, and the World Stroke Organization, advocated for the addition of the NIHSS score as a data element to administrative data sets overseen by regulatory authorities, including the World Health Organization and the US Centers for Medicare & Medicaid Services (CMS).4 In response, fields for NIHSS strokes were included in the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) and became available to be completed for stroke admission claims by hospital coders in October 2016.5 The Centers for Medicare & Medicaid Services has indicated that, beginning in 2023, the NIHSS will be incorporated into the risk-adjustment formulas for the 30-day Stroke Mortality Measure and 30-day Stroke Readmission Measure and that failure to report the NIHSS codes will affect hospital performance on these indices.6
To our knowledge, no data have been reported on the NIHSS documentation under the CMS program during the current initial optional reporting period, including the range and patterns of NIHSS scores observed nationally, their convergent validity with patterns seen in prior large data set, the initial frequency of reporting of NIHSS, and characteristics of hospitals with high and low reporting. With the publication of the 2016 National Inpatient Sample (NIS), data regarding NIHSS reporting in claims from the first quarter (Q4 of 2016) of implementation of optional reporting became available for analysis in a nationally representative sample of US hospitals and patients. Therefore, we examined in the NIS data set: (1) the distribution of administratively reported NIHSS scores in all ischemic stroke hospitalizations; (2) the association of administratively reported NIHSS scores with outcomes; (3) the NIHSS reporting rate and its predictors across US hospitals; and (4) the patient-level and hospital-level determinants of administrative NIHSS reporting.
Methods
Study Design and Population
We performed a retrospective cross-sectional study using data from the largest US all-payer inpatient claims-based database, the NIS. Beginning in 2012, the NIS approximates a 20% stratified sample of all discharges from US hospitals, including data on all patients. At present, 46 states plus the District of Columbia are included in the NIS.7 Hospitalizations for ischemic stroke from the last quarter of 2016 were identified using validated ICD-10–Clinical Modification (ICD-10-CM) diagnosis and procedural codes8 and methodology.9 Hospitalizations involving reperfusion therapies (ie, intravenous thrombolysis and mechanical thrombectomy) were identified using 03CG3ZZ and 3E03317 procedural codes, respectively.9 For the purpose of this analysis, patients younger than 18 years were excluded. Data from the Healthcare Cost and Utilization Project are encrypted and anonymized so that individual patient identifications cannot be determined. This study met US Department of Health and Human Services criteria for exemption from institutional review board approval and from individual patient informed consent as all NIS data are publicly available and deidentified.
Measurements
As of October 1, 2016, reporting of the NIHSS score was made available with the collection of ICD-10-CM codes. According to the ICD-10-CM Official Guidelines for Coding and Reporting, the NIHSS codes (R29.7xx) can be used in conjunction with acute stroke codes (I63) to characterize the patient’s neurological status and the severity of the stroke. The code assignment should reflect the initial NIHSS at presentation and may be acquired based on the documentation from clinicians who were part of the treating team.
In-hospital mortality was identified in the database. The discharge destination (ie, disposition), a predictor of functional status, was derived from the Healthcare Cost and Utilization Project meta-label DISPUNIFORM. Total number of NIHSS reporting performance were tabulated for each hospital in the data set. A hospital was considered “NIHSS-reporting” if it performed 1 or more NIHSS report for ischemic stroke hospitalizations within the study period.
Statistical Analysis
The distribution of documented NIHSS scores was delineated with median and interquartile range (IQR) and histogram analyses. National Institutes of Health Stroke Scale score frequencies were also characterized in the 5 severity groupings identified by CMS: 0, no measured stroke symptoms; 1 to 4, minor stroke; 5 to 15, moderate stroke; 16 to 20, moderate to severe stroke; and 21 to 42, severe stroke. Patient-level variations in NIHSS scores were evaluated by calculating median and IQR in demographic, clinical, and financial subgroups of age (<50, 50-79, ≥80 years), sex, race/ethnicity (white, African American, Hispanic, Asian or Pacific Islander, and other [including Native American]), extent of comorbidities (quantified with the Charlson Comorbidity Index), type of acute stroke treatment (no reperfusion therapy, intravenous thrombolysis only, intra-arterial reperfusion therapy with or without intravenous thrombolysis), and primary payer. The association of NIHSS score with outcome was evaluated for 2 outcomes at discharge: (1) discharge destination (home vs other) and (2) inpatient mortality.
All analyses were performed using data weighted to produce national estimates. Concerning median NIHSS scores by hospital reporting quintiles, differences between groups were analyzed using a nonparametric Kruskal-Wallis test. Multivariable logistic regression modeling was performed to identify the independent predictors of NIHSS reporting performance following stroke admission. Statistically significant baseline clinical covariates with 2-sided P value of less than .05 were used in modeling logistic regression. All analyses for NIHSS reporting performance were adjusted for index hospital stroke volume in the study period. Results of regression models were reported using odds ratios and 95% confidence intervals. Statistical analysis was performed using SAS, version 9.4 (SAS Institute Inc). Analysis began September 2019.
Missing Data
Missing data were identified as more than 5% for race. Accordingly, missing race/ethnicity was handled using multiple imputation according to the Healthcare Cost and Utilization Project’s recommendations for dealing with missing data.
Results
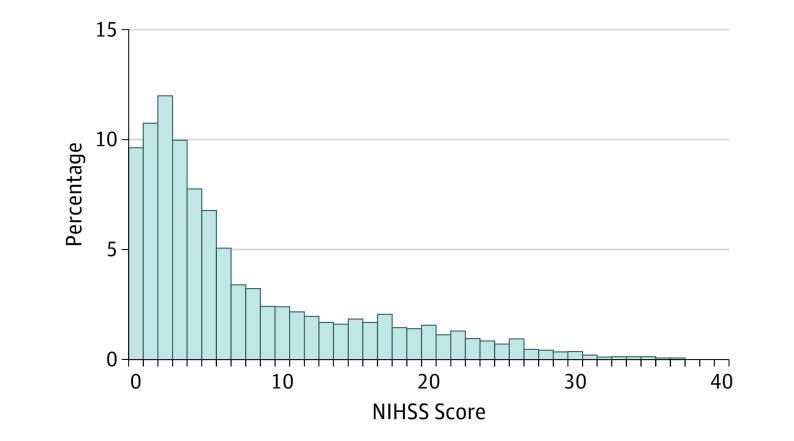
A total of 154 165 ischemic stroke admissions were identified during the 3-month period. Overall, NIHSS score was reported for 21 685 acute ischemic stroke hospitalizations (14%) (10 925 women [50.4%]; median [IQR] age, 72 [61-82] years). The median (IQR) NIHSS score was 4 (2-11). The overall distribution of the NIHSS scores is shown in the Figure 1. Considered in broad categories, presenting deficit severity was absent (NIHSS score, 0) in 2080 individuals (9.6%), minor (NIHSS score, 1-4) in 8760 individuals (40.4%), moderate (NIHSS score, 5-15) in 7115 individuals (32.8%), moderate to severe (NIHSS score, 16-20) in 1800 individuals (8.3%), and severe (NIHSS score, 21-42) in 1930 individuals (8.9%). National Institutes of Health Stroke Scale scores of 10 or higher, an indicator of patients likely having large vessel occlusions, were present in 6290 individuals (29%).
Figure 1. Distribution of Administratively Recorded National Institutes of Health Stroke Scale (NIHSS) Scores in Ischemic Stroke Hospitalizations in US National Inpatient Sample.
The distribution of NIHSS scores according to various patient and hospital characteristics are shown in Table 1. Presenting deficit severity was higher in very elderly patients (age, ≥80 years) and in women. There were no significant differences in the distribution of NIHSS scores by patients’ race/ethnicity or by the urban/rural or regional geographic location of the hospitals, or by hospital teaching status. National Institutes of Health Stroke Scale scores differed by reperfusion treatments applied, being highest in patients receiving endovascular thrombectomy with or without intravenous thrombolysis, intermediate in patients receiving intravenous thrombolysis only, and lowest in patients not receiving any reperfusion therapy (Table 1).
Table 1. Distribution of NIHSS Scores by Patient Subgroups in the United States.
| Subgroup | No. | Median (IQR) | P Value |
|---|---|---|---|
| Age, y | |||
| <55 | 3015 | 3 (1-8) | <.001 |
| 55-79 | 12 110 | 4 (2-10) | |
| ≥80 | 6560 | 6 (2-16) | |
| Sex | |||
| Female | 10 925 | 5 (2-13) | <.001 |
| Male | 10 745 | 4 (2-10) | |
| Race/ethnicity | |||
| White | 15 205 | 4 (2-11) | .59 |
| African American | 3745 | 5 (2-11) | |
| Hispanic | 1495 | 5 (2-12) | |
| Asian/Pacific Islander | 650 | 5 (2-11) | |
| Othera | 590 | 5 (2-13) | |
| Reperfusion therapy | |||
| No reperfusion | 17 570 | 4 (2-9) | <.001 |
| Any IV thrombolysis | 3240 | 7 (4-14) | |
| IV thrombolysis without EVT | 2960 | 6 (4-12) | |
| IV thrombolysis + EVT | 280 | 17 (13-22) | |
| Any EVT | 1155 | 17 (12-22) | |
| EVT without IV thrombolysis | 875 | 17 (11-22) | |
| Region | |||
| Northeast | 4960 | 4 (2-10) | .12 |
| Midwest | 5215 | 5 (2-12) | |
| South | 8240 | 4.5 (2-11) | |
| West | 3270 | 5 (2-13) | |
| Hospital status | |||
| Rural | 860 | 4 (2-10) | .21 |
| Urban nonteaching | 4065 | 4 (2-10) | |
| Urban teaching | 16760 | 5 (2-12) | |
| Discharge status | |||
| Home | 7190 | 2 (1-5) | <.001 |
| Skilled nursing | 9625 | 7 (3-15) | |
| Died | 1230 | 19 (12-25) |
Abbreviations: EVT, endovascular thrombectomy; IQR, interquartile range; IV, intravenous; NIHSS, National Institutes of Health Stroke Scale.
Includes Native American.
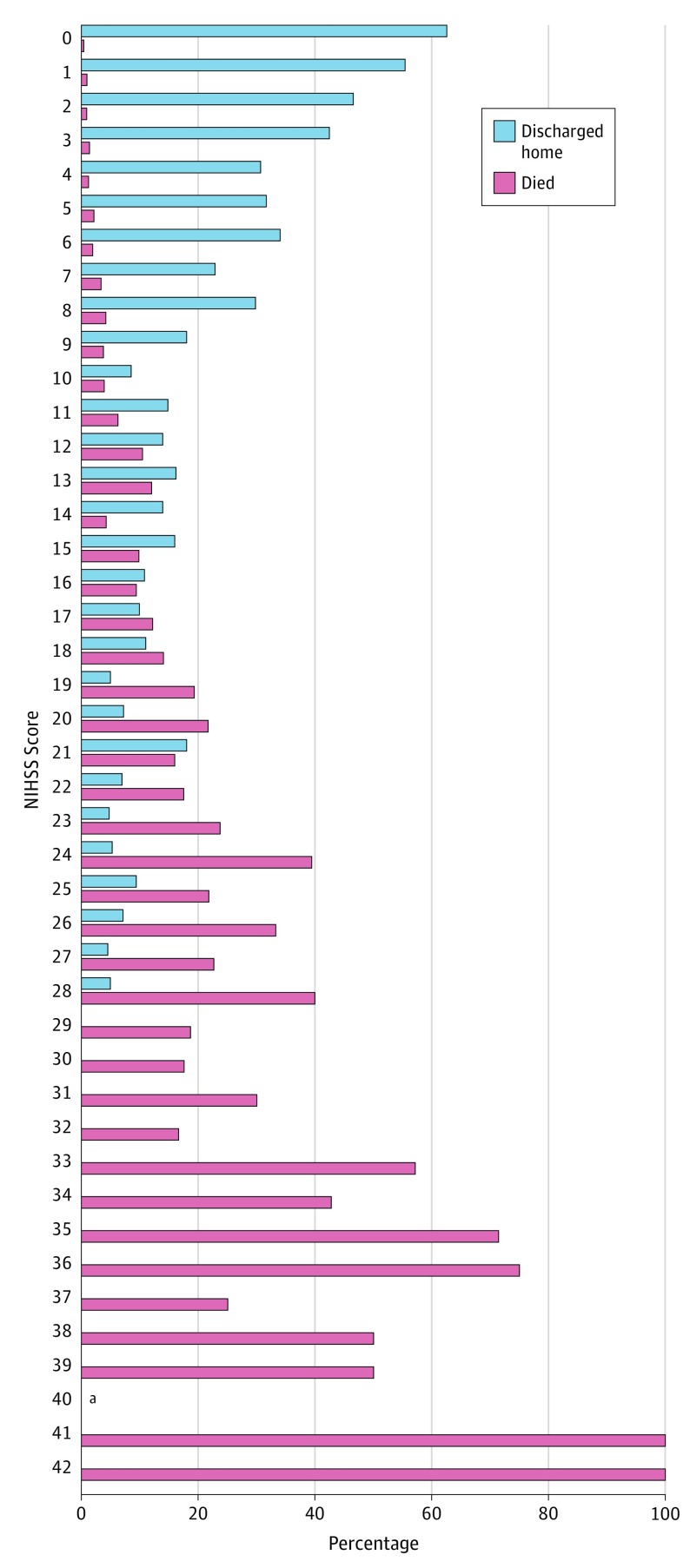
Patient outcomes also varied substantially by initial NIHSS scores, with initial deficit severity scores highest among those with inpatient mortality, intermediate among those discharged alive to another care facility, and lowest among those discharged to home (Table 1). Figure 2 shows the differential distribution of NIHSS scores among hospitalizations with in-hospital mortality compared with those discharged to home (eFigures 1 and 2 in the Supplement).
Figure 2. Distributions of Administratively Recorded NIHSS Scores in Patients With Excellent (Discharged Home) and Fatal Outcomes at Completion of Acute Stroke Hospitalization in US National Inpatient Sample.
aNo patient had a National Institutes of Health Stroke Scale (NIHSS) score of 40.
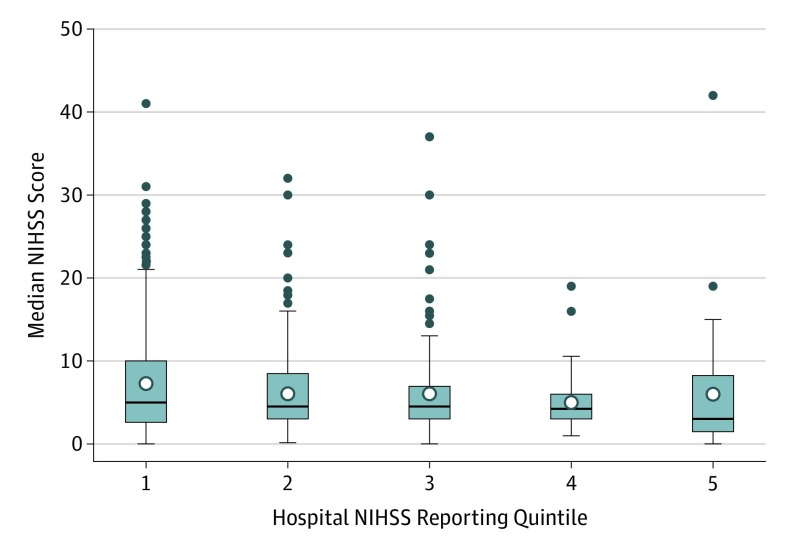
Among 2957 hospitals with at least 1 ischemic stroke hospitalization during the study period, 1108 (37.5%) reported at least 1 NIHSS score. The distribution of NIHSS score reporting rates across hospitals with at least 1 NIHSS score report performance is shown in eFigure 3 in the Supplement. The median (IQR) Charlson Comorbidity Index score was similar in discharges with or without NIHSS score report (3 [2-4]). The proportion of patients among whom NIHSS score was reported did not vary substantially by month across the 3 months of the initial CMS reporting period. The variation of median NIHSS scores among quintiles of hospital NIHSS reporting performance did not reach statistical significance (Figure 3). Comparing hospitals with the highest vs lowest quintiles of discharges with NIHSS score reported, NIHSS scores in the highest reporting quintile were a median (IQR) of 3 (2-10) and in the lowest reporting quintile median (IQR) of 5.0 (1.5-8.5).
Figure 3. Distribution of Median NIHSS Scores Among Quintiles of Hospital NIHSS Reporting Performance.
From lowest to highest quintile of reporting frequency, median National Institutes of Health Stroke Scale (NIHSS) values were 5, 4.5, 4.5, 4.25, and 3, respectively (P = .06).
In multivariable analysis including patient- and hospital-level characteristics, independent factors associated with a patient’s NIHSS value being reported were white (vs other) race/ethnicity, receipt of intravenous or intra-arterial reperfusion therapy, patient arrival by interfacility transfer, larger hospital size, teaching hospital status, hospital region in the Northeast, Midwest, or South, and Medicare (vs Medicaid) payer (Table 2).
Table 2. Multivariate Logistic Regression Analysis for Patient- and Hospital-Level Factors Predicting NIHSS Reporting Performance.
| Characteristic | Odds Ratio (95% CI) |
|---|---|
| Age | 1.00 (0.99-1.01) |
| Female | 0.97 (0.90-1.03) |
| Race/ethnicity | |
| Black | 0.98 (0.89-1.07) |
| Hispanic | 0.89 (0.77-1.01) |
| Asian/Pacific Islander | 1.06 (0.87-1.3) |
| Othera | 0.70 (0.55-0.89) |
| White | 1 [Reference] |
| Transfer | |
| In | 1.16 (1.09-1.25) |
| Out | 1.00 (0.96-1.03) |
| Reperfusion therapy | |
| IV thrombolysis only | 2.84 (2.56-3.16) |
| EVT + IV thrombolysis | 2.82 (2.38-3.34) |
| No reperfusion therapy | 1 [Reference] |
| Admission weekend | 1.05 (0.97-1.13) |
| Hospital bed size | |
| Small | 0.76 (0.67-0.86) |
| Medium | 0.77 (0.70-0.85) |
| Large | 1 [Reference] |
| Hospital status | |
| Rural | 0.53 (0.44-0.63) |
| Urban | |
| Nonteaching | 0.74 (0.67-0.82) |
| Teaching | 1 [Reference] |
| Hospital region | |
| Northeast | 1.60 (1.42-1.79) |
| Midwest | 1.51 (1.35-1.68) |
| South | 1.11 (1-1.22) |
| West | 1 [Reference] |
| Payer | |
| Medicaid | 0.82 (0.71-0.94) |
| Other | 1.08 (0.98-1.19) |
| Medicare | 1 [Reference] |
| Charlson Comorbidity Index score | 1.01 (0.99-1.03) |
Abbreviations: EVT, endovascular thrombectomy; IV, intravenous; NIHSS, National Institutes of Health Stroke Scale.
Includes Native American.
Discussion
In this study of the initial implementation of NIHSS score deficit severity documentation in nationally representative claims data for US patients with acute ischemic stroke, findings were encouraging regarding the feasibility and effect of this enhanced stroke characterization process. During the initial optional reporting period, NIHSS scores were already being reported in about 1 in 7 acute ischemic stroke hospitalizations. The distribution of NIHSS scores closely resembled that available from the most comprehensive US clinical registry, Get with the Guidelines–Stroke,2 and from formal, population-based study of acute ischemic stroke presentations in New York10 and in the Cincinnati/Northern Kentucky Stroke Study,11 providing external validity. National Institutes of Health Stroke Scale score severities varied in expected ways with stroke-presenting features, treatment, and outcome, being higher in the very elderly and women, higher in those treated with intravenous and endovascular reperfusion therapies, and higher in those with acute hospitalization outcome characterized by discharge to another care facility or death rather than discharge to home.
The findings from this study are consonant with, and extend, prior investigations. The study confirms, in a nationally representative data set, that the distribution of deficit severity in acute ischemic stroke is skewed toward less severe deficits. The median presenting NIHSS score of 4 falls far to the left of the portion of the entire NIHSS scale range from 0 to 42. More than half of patients with acute ischemic stroke had minor deficits (NIHSS score, 0-4) at presentation. These findings accord with large clinical registries and population-based studies.10,11 Nonetheless, a substantial proportion of hospitalizations were characterized with moderate (1 in 3), moderate to severe (1 in 12), and severe (1 in 11) deficits. The national median NIHSS score of 17 of stroke hospitalizations undergoing endovascular reperfusion therapy accords with the deficit severity of patients with large vessel occlusions in the pivotal trials of endovascular thrombectomy.12 The national median NIHSS score of 7 of patients undergoing intravenous thrombolysis in 2016 is lower than that of patients enrolled in the initial intravenous thrombolytic trials (median, 14) and that reported in the national US registry in 2010 to 2011 (median, 11).13,14 This finding is consistent with subsequent regional US studies that have indicated increasing treatment of patients with mild deficit in clinical practice.15,16 However, it may also reflect undercoding of the use of intravenous thrombolysis when coadministered to patients receiving endovascular thrombectomy.
This study’s findings additionally provide some insight into the national prevalence of acute ischemic strokes due to large vessel occlusions, which may be treatable by endovascular approaches. Prior studies have found NIHSS values of 10 or more at presentation to identify patients with large vessel occlusions with high positive predictive values.17,18 In the NIS, 29% of the acute ischemic stroke admissions had an NIHSS score of 10 or higher. This indication that more than a quarter of arterial ischemic stroke hospitalizations have a large vessel occlusion accords with prior, less nationally representative studies. Nationwide administrative documentation and analysis of presenting NIHSS scores should provide a powerful tool for monitoring trends in large vessel occlusion frequency, sites of initial presentation and transfer, receipt of endovascular therapy, and outcomes in different regions and hospital settings across the United States. This study additionally confirmed that the NIHSS is a potent prognostic variable for stroke hospitalization outcome, including excellent functional outcome (discharge to home) and acute mortality.
These findings indicate that administratively recorded NIHSS values maintain the strength of association with outcome seen with NIHSS values recorded in clinical trials and clinical registries and that adding the NIHSS to risk-adjustment models is likely to substantially improve CMS’ and other organizations’ assessments of hospital care quality. However, it continues to be important to note that stroke is a disease that is associated with disability rather than death or recurrence. As a result, quality measures such as risk-adjusted mortality and risk-adjusted readmission rates, even when improved risk-adjustment formulas are used, are poor indices of care quality for ischemic stroke, sampling tails of outcome distributions and sensitive to care aspects that are less central to the trajectory of most patients. The major treatments of proven benefit for acute ischemic stroke, intravenous thrombolysis, and endovascular thrombectomy have no or minimal effects on early mortality and readmission but have profound effects on attainment of nondisabled and functionally independent outcomes. Construction of an administratively functional outcome measure that can be monitored, such as risk-adjusted home time during the first 30 or 90 days after stroke, remains highly desirable.
While reporting and incorporating NIHSS scores into the risk-adjustment model could improve the discrimination of the stroke mortality measure and substantially enhance nationwide stroke surveillance measures, failure to report NIHSS scores would constrain this potential. In the current analysis, smaller hospital size, rural and nonacademic centers, and Western geographic region were hospital factors associated with nonreporting of NIHSS scores. Hospitals should assess whether NIHSS score documentation improvement opportunities exist at their institutions, and efforts should be made by the stroke community to encourage NIHSS score reporting by hospitals, such as the incorporation of NIHSS score reporting as a performance measure by the Joint Commission and the American Heart Association/American Stroke Association in accrediting Comprehensive Stroke Centers.19
Limitations
Our study has limitations. Hospital reporting of NIHSS scores was voluntary, and the reported NIHSS score distribution may be more representative of the larger academic hospitals. The identification of diagnoses and procedures were largely dependent on ICD-10-CM codes collected for billing purposes and thus may be susceptible to measurement error. The timing of the NIHSS scores after arrival was not documented, and some may be more reflective of postadmission than initial presentation status. However, CMS instructions to hospitals do direct that sites should “report the initial score documented.”5 National Institutes of Health Stroke Scale values in recurrent strokes may represent residual deficits of prior stroke hospitalizations. There was not a requirement that NIHSS raters have completed formal training and certification in scale administration, but the similar distribution of scores to studies using certified raters suggests generally reliable performance of NIHSS assessments.
Conclusions
In conclusion, in the initial national optional reporting period in the United States, NIHSS scores were reported in administrative claims data in nearly 1 in 7 acute ischemic stroke hospitalizations. The distribution of NIHSS scores was similar to that from narrow population-based studies and clinical registries. Presenting NIHSS scores were higher in very elderly patients, women, and in those who received treatment with reperfusion therapies. Documented NIHSS scores were powerfully associated with functional outcome and early mortality. These findings support the validity and the potential to aid care quality assessment of discharge claims-reported NIHSS values.
eFigure 1. In-hospital mortality rates associated with different initial administratively-reported NIHSS scores in United States National Inpatient Sample
eFigure 2. Rates of excellent outcome (discharge home) associated with different initial administratively-reported NIHSS scores in United States National Inpatient Sample
eFigure 3. The distribution of NIHSS score reporting rates across hospitals with at least one NIHSS report performance
References
- 1.Adams HP Jr, Davis PH, Leira EC, et al. . Baseline NIH Stroke Scale score strongly predicts outcome after stroke: a report of the Trial of Org 10172 in Acute Stroke Treatment (TOAST). Neurology. 1999;53(1):126-131. doi: 10.1212/WNL.53.1.126 [DOI] [PubMed] [Google Scholar]
- 2.Fonarow GC, Saver JL, Smith EE, et al. . Relationship of national institutes of health stroke scale to 30-day mortality in Medicare beneficiaries with acute ischemic stroke. J Am Heart Assoc. 2012;1(1):42-50. doi: 10.1161/xJAHA.111.000034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reeves MJ, Smith EE, Fonarow GC, et al. . Variation and trends in the documentation of National Institutes of Health Stroke Scale in GWTG-stroke hospitals. Circ Cardiovasc Qual Outcomes. 2015;8(6)(suppl 3):S90-S98. doi: 10.1161/CIRCOUTCOMES.115.001775 [DOI] [PubMed] [Google Scholar]
- 4.Yale New Haven health Services Corporation/Center for Outcomes Research & Evaluation Claims-Based and Hybrid Measures of 30-Day Mortality Following Acute Ischemic Stroke Hospitalization Incorporating Risk Adjustment for Stroke Severity. July 2015. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/MMS/Downloads/Claims-Based-and-Hybrid-Measures-of-30-Day-Mortality-Following-Acute-Ischemic-Stroke-Hospitalization-Incorporating-Risk-Adjustment-for-Stroke-Severity-Technical-Report-.pdf. Accessed December 27, 2019. [Google Scholar]
- 5.ICD-10-CM Official Guidelines for Coding and Reporting. https://www.cms.gov/Medicare/Coding/ICD10/Downloads/2019-ICD10-Coding-Guidelines-.pdf. Accessed October 15, 2019. [Google Scholar]
- 6.Centers for Medicare & Medicaid Services Measure methodology. 2019. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/Measure-Methodology. Accessed September 29, 2019.
- 7.Healthcare Cost and Utilization Project NIS overview. https://www.hcup-us.ahrq.gov/nisoverview.jsp. Accessed October 16, 2018.
- 8.Tirschwell DL, Longstreth WT Jr. Validating administrative data in stroke research. Stroke. 2002;33(10):2465-2470. doi: 10.1161/01.STR.0000032240.28636.BD [DOI] [PubMed] [Google Scholar]
- 9.Saber H, Navi BB, Grotta JC, et al. . Real-world treatment trends in endovascular stroke therapy. Stroke. 2019;50(3):683-689. doi: 10.1161/STROKEAHA.118.023967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sacco RL, Shi T, Zamanillo MC, Kargman DE. Predictors of mortality and recurrence after hospitalized cerebral infarction in an urban community: the Northern Manhattan Stroke Study. Neurology. 1994;44(4):626-634. doi: 10.1212/WNL.44.4.626 [DOI] [PubMed] [Google Scholar]
- 11.Reeves M, Khoury J, Alwell K, et al. . Distribution of National Institutes of Health stroke scale in the Cincinnati/Northern Kentucky Stroke Study. Stroke. 2013;44(11):3211-3213. doi: 10.1161/STROKEAHA.113.002881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goyal M, Menon BK, van Zwam WH, et al. ; HERMES collaborators . Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723-1731. doi: 10.1016/S0140-6736(16)00163-X [DOI] [PubMed] [Google Scholar]
- 13.National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333(24):1581-1587. doi: 10.1056/NEJM199512143332401 [DOI] [PubMed] [Google Scholar]
- 14.Schwamm LH, Ali SF, Reeves MJ, et al. . Temporal trends in patient characteristics and treatment with intravenous thrombolysis among acute ischemic stroke patients at Get With The Guidelines-Stroke hospitals. Circ Cardiovasc Qual Outcomes. 2013;6(5):543-549. doi: 10.1161/CIRCOUTCOMES.111.000095 [DOI] [PubMed] [Google Scholar]
- 15.Asdaghi N, Wang K, Ciliberti-Vargas MA, et al. ; FL-PR CReSD Investigators and Collaborators . Predictors of thrombolysis administration in mild stroke: Florida-Puerto Rico collaboration to reduce stroke disparities. Stroke. 2018;49(3):638-645. doi: 10.1161/STROKEAHA.117.019341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Domino JS, Baek J, Meurer WJ, et al. . Emerging temporal trends in tissue plasminogen activator use: results from the BASIC project. Neurology. 2016;87(21):2184-2191. doi: 10.1212/WNL.0000000000003349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hastrup S, Damgaard D, Johnsen SP, Andersen G. Prehospital acute stroke severity scale to predict large artery occlusion: design and comparison with other scales. Stroke. 2016;47(7):1772-1776. doi: 10.1161/STROKEAHA.115.012482 [DOI] [PubMed] [Google Scholar]
- 18.Noorian AR, Sanossian N, Shkirkova K, et al. ; FAST-MAG Trial Investigators and Coordinators . Los Angeles motor scale to identify large vessel occlusion: prehospital validation and comparison with other screens. Stroke. 2018;49(3):565-572. doi: 10.1161/STROKEAHA.117.019228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joint Commission. Specifications manual for joint commission national quality measures. https://manual.jointcommission.org/releases/TJC2019A/TableOfContentsTJC.html. Accessed October 15, 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. In-hospital mortality rates associated with different initial administratively-reported NIHSS scores in United States National Inpatient Sample
eFigure 2. Rates of excellent outcome (discharge home) associated with different initial administratively-reported NIHSS scores in United States National Inpatient Sample
eFigure 3. The distribution of NIHSS score reporting rates across hospitals with at least one NIHSS report performance