Key Points
Question
What are the risks of hypophosphatemia following iron replacement with iron isomaltoside 1000 (now called ferric derisomaltose) vs ferric carboxymaltose?
Findings
In 2 randomized trials of 245 total patients (trial A: n = 123; trial B: n = 122) with iron-deficiency anemia, who were intolerant to or unresponsive to oral iron, the incidence of hypophosphatemia with use of iron isomaltoside, compared with ferric carboxymaltose, was 7.9% vs 75.0% in trial A and 8.1% vs 73.7% in trial B over 35 days; both differences were statistically significant.
Meaning
Iron isomaltoside, compared with ferric carboxymaltose, resulted in lower incidence of hypophosphatemia, but further research is needed to determine the clinical importance of these findings.
Abstract
Importance
Intravenous iron enables rapid correction of iron-deficiency anemia, but certain formulations induce fibroblast growth factor 23–mediated hypophosphatemia.
Objective
To compare risks of hypophosphatemia and effects on biomarkers of mineral and bone homeostasis of intravenous iron isomaltoside (now known as ferric derisomaltose) vs ferric carboxymaltose.
Design, Setting, and Participants
Between October 2017 and June 2018, 245 patients aged 18 years and older with iron-deficiency anemia (hemoglobin level ≤11 g/dL; serum ferritin level ≤100 ng/mL) and intolerance or unresponsiveness to 1 month or more of oral iron were recruited from 30 outpatient clinic sites in the United States into 2 identically designed, open-label, randomized clinical trials. Patients with reduced kidney function were excluded. Serum phosphate and 12 additional biomarkers of mineral and bone homeostasis were measured on days 0, 1, 7, 8, 14, 21, and 35. The date of final follow-up was June 19, 2018, for trial A and May 29, 2018, for trial B.
Interventions
Intravenous administration of iron isomaltoside, 1000 mg, on day 0 or ferric carboxymaltose, 750 mg, infused on days 0 and 7.
Main Outcomes and Measures
The primary end point was the incidence of hypophosphatemia (serum phosphate level <2.0 mg/dL) between baseline and day 35.
Results
In trial A, 123 patients were randomized (mean [SD] age, 45.1 [11.0] years; 95.9% women), including 62 to iron isomaltoside and 61 to ferric carboxymaltose; 95.1% completed the trial. In trial B, 122 patients were randomized (mean [SD] age, 42.6 [12.2] years; 94.1% women), including 61 to iron isomaltoside and 61 to ferric carboxymaltose; 93.4% completed the trial. The incidence of hypophosphatemia was significantly lower following iron isomaltoside vs ferric carboxymaltose (trial A: 7.9% vs 75.0% [adjusted rate difference, –67.0% {95% CI, –77.4% to –51.5%}], P < .001; trial B: 8.1% vs 73.7% [adjusted rate difference, –65.8% {95% CI, –76.6% to –49.8%}], P < .001). Beyond hypophosphatemia and increased parathyroid hormone, the most common adverse drug reactions (No./total No.) were nausea (iron isomaltoside: 1/125; ferric carboxymaltose: 8/117) and headache (iron isomaltoside: 4/125; ferric carboxymaltose: 5/117).
Conclusions and Relevance
In 2 randomized trials of patients with iron-deficiency anemia who were intolerant of or unresponsive to oral iron, iron isomaltoside (now called ferric derisomaltose), compared with ferric carboxymaltose, resulted in lower incidence of hypophosphatemia over 35 days. However, further research is needed to determine the clinical importance of this difference.
Trial Registration
ClinicalTrials.gov Identifiers: NCT03238911 and NCT03237065
Because intravenous (IV) iron can induce hypophosphatemia, the 2 clinical trials in this report directly compare the effects of 2 IV iron formulations, iron isomaltoside (now known as ferric derisomaltose) and ferric carboxymaltose, on phosphate levels in patients with iron-deficiency anemia unresponsive to oral iron.
Introduction
Iron-deficiency anemia is a global health problem.1,2 Iron isomaltoside 1000 (now known as ferric derisomaltose) and ferric carboxymaltose are intravenous iron formulations that were developed to rapidly correct iron-deficiency anemia, especially in patients who do not tolerate or fail to respond to oral iron.3,4 Both iron isomaltoside and ferric carboxymaltose effectively correct iron-deficiency anemia, but their safety profiles differ.5,6,7,8 Several studies have reported that ferric carboxymaltose causes high rates of hypophosphatemia by acutely increasing circulating concentrations of full-length, biologically active fibroblast growth factor 23, which causes hypophosphatemia by stimulating urinary phosphate excretion and reducing serum 1,25-dihydroxyvitamin D levels.9,10,11 Severe hypophosphatemia can cause serious complications, including rhabdomyolysis, heart failure, and respiratory failure, and chronic hypophosphatemia can be complicated by osteomalacia and fractures.12,13
Previous clinical trials suggested that the risk of hypophosphatemia may be lower with iron isomaltoside than with ferric carboxymaltose,5,7,8,14,15 but data from randomized trials that directly compared the 2 formulations are limited. Furthermore, no controlled studies have systematically investigated the effects of any intravenous iron on biomarkers of bone metabolism to link intravenous iron-associated changes in mineral metabolism to the skeletal complications described in case reports.13 Two randomized clinical trials were conducted to compare the incidence, severity and mechanisms of hypophosphatemia, and effects on biochemical biomarkers of mineral and bone homeostasis of treatment with iron isomaltoside (called ferric derisomaltose by the US Food and Drug Administration as of June 2019) or ferric carboxymaltose in patients with iron-deficiency anemia.
Methods
Trial Design
Two identically designed, open-label, randomized clinical trials were conducted at 30 sites across the United States between October 2017 and June 2018 (trial A) and October 2017 and May 2018 (trial B). The date of final follow-up was June 19, 2018, for trial A and May 29, 2018, for trial B. Trial protocols are available in Supplement 1 and Supplement 2, with revisions documented in eTable 1 in Supplement 3. These trials were conducted to support the US Food and Drug Administration submission package and the intended label of iron isomaltoside. Iron isomaltoside 1000 is also known as ferric derisomaltose. Iron isomaltoside 1000 is the generic name initially approved in the European Union, whereas ferric derisomaltose is the international nonproprietary name and United States Adopted Name. Two individually powered studies were performed in line with general regulatory recommendations to better demonstrate the robustness of results while decreasing the risk of findings occurring by chance. In both trials, a screening period was followed by a baseline randomization visit on day 0 and follow-up visits on days 1, 7, 8, 14, 21, and 35. Nonfasting blood and spot urine samples were collected at each visit. The day 1 and day 8 assessments were included to capture physiological responses 24 hours after iron administrations.
The trials were approved by a single institutional review board (Western Institutional Review Board, Puyallup, Washington; 98374-2115) and all patients provided written informed consent.
Patients
Both trials recruited adults aged 18 years and older with iron-deficiency anemia, defined as hemoglobin level of 11 g/dL or less and serum ferritin level of 100 ng/mL or less (to convert to pmol/L, multiply by 2.247), with a history of intolerance or unresponsiveness to 1 month or more of oral iron. Exclusion criteria included body weight less than 50 kg, estimated glomerular filtration rate less than 65 mL/min/1.73 m2, serum phosphate level less than 2.5 mg/dL, acute bleeding greater than 500 mL within 72 hours before study inclusion, hemochromatosis or other iron-storage disorder, or intravenous iron use within 30 days prior to screening. Additional inclusion and exclusion criteria are presented in eTable 1 in Supplement 3. Race/ethnicity data were collected as part of a comprehensive approach to describing the trials’ study populations and because of known differences in bone and mineral metabolism across racial groups. Race and ethnicity were ascertained by patient self-report based on fixed categories (white, black or African American, Asian, Hispanic or Latino, not Hispanic or not Latino, other).
Randomization
Patients were centrally randomized (1:1) using an interactive web response system (IBM Clinical Development eCRF system randomization module) that blinded investigators and patients to assignment to iron isomaltoside or ferric carboxymaltose. Randomization was stratified in blocks of 4 to try to ensure balance across the 2 groups in underlying gynecological cause of iron-deficiency anemia (yes or no) and screening serum phosphate level (<3.5 or ≥3.5 mg/dL).
Interventions
Iron isomaltoside was administered as a single dose of 1000 mg infused over 20 minutes on day 0, according to its anticipated US label. Ferric carboxymaltose was administered at 750 mg on day 0 and 750 mg on day 7, according to its Food and Drug Administration–approved label.16 The trials were open-label without blinding of the investigational products. During the trials, other forms of iron supplementation, blood transfusion, erythropoiesis-stimulating agents, radiotherapy, and chemotherapy were prohibited.
End Points
The primary end point was the incidence of hypophosphatemia, defined as serum phosphate level less than 2.0 mg/dL, at any time from baseline to day 35. There were multiple secondary safety and efficacy end points (eTable 2 in Supplement 3). Secondary safety end points reported in this article include prevalence of persistent hypophosphatemia at day 35; changes from baseline to each postrandomization visit in biomarkers of mineral and bone homeostasis: serum phosphate, urinary fractional excretion of phosphate, intact fibroblast growth factor 23 (measures only full-length peptide), C-terminal fibroblast growth factor 23 (measures full-length peptide and its C-terminal fragments), 25-hydroxyvitamin D, 1,25-dihydroxyvitamin D, 24,25-dihydroxyvitamin D, ionized calcium, and parathyroid hormone (PTH); and number of patients who experienced any adverse drug reactions.
Secondary efficacy end points reported in this article include changes in hemoglobin per gram of iron infused, ferritin, and transferrin saturation from baseline to each postrandomization visit. Post hoc analyses included study site–adjusted analyses of the primary end point, the incidence of severe hypophosphatemia (serum phosphate level ≤1.0 mg/dL) at any time from baseline to day 35, and the prevalence of hypophosphatemia at each postrandomization visit.
Exploratory end points reported in this article include changes in serum biomarkers of bone turnover, including total and bone-specific alkaline phosphatase, N-terminal propeptide of type 1 collagen, carboxy-terminal collagen crosslinks, and changes in hemoglobin level from baseline to each postrandomization visit. A central laboratory that was blinded to randomized treatment performed all laboratory assays, details of which are presented in eTable 3 in Supplement 3.
Sample Size
At the time of protocol development, there was no known minimal clinically important difference in rates of hypophosphatemia between different intravenous iron formulations. Conservatively assuming an incidence of hypophosphatemia of 15% for iron isomaltoside and 40% for ferric carboxymaltose based on prior studies,9,17,18,19,20,21 each trial required 49 patients in each treatment group to detect a significant difference between groups with 80% power and α of 5%. To account for potential loss to follow-up, 60 patients per treatment group were planned to be randomized in each trial.
Statistical Analysis
The statistical analysis plan is available in Supplement 4. The primary end point and all secondary safety end points were analyzed using the safety data sets, which included all patients who received at least 1 dose of study drug (trial A: iron isomaltoside, n = 63, ferric carboxymaltose, n = 60; trial B: iron isomaltoside, n = 62, ferric carboxymaltose, n = 57). For the secondary efficacy end points, patients were analyzed according to their randomization group (trial A: iron isomaltoside, n = 62, ferric carboxymaltose, n = 61, including 1 patient who erroneously received iron isomaltoside; trial B: iron isomaltoside, n = 61, ferric carboxymaltose, n = 61, including 1 patient who erroneously received iron isomaltoside).
For the primary end point, the difference between the incidence of hypophosphatemia in the iron isomaltoside group vs the ferric carboxymaltose group was calculated using the Cochran-Mantel-Haenszel method with 95% Newcombe CIs,22 adjusting for randomized strata (and trial, in the pooled analyses of both trials). In a post hoc analysis, the primary end point was analyzed using the Cochran-Mantel-Haenszel method with 95% Newcombe CIs, adjusting for individual study sites. For the patients with no postbaseline measurements (n = 3 across both trials), serum phosphate level was imputed as less than 2.0 mg/dL for the primary analysis. Prevalence of hypophosphatemia at individual time points was analyzed using the same methodology.
Longitudinal changes in biomarkers of bone and mineral homeostasis and in anemia and iron parameters were analyzed using mixed models for repeated measurements with a restricted maximum likelihood–based approach. The models included iron isomaltoside vs ferric carboxymaltose treatment, randomization strata, trial (in the pooled analyses), study day, and treatment-by-day interaction as fixed categorical effects. An unstructured covariance matrix was used to model within-patient error, with baseline values of the continuous dependent variables and baseline value-by-day interaction as fixed covariates. In the mixed-model analyses, patients without postbaseline values had their change from baseline set to zero at the first postbaseline visit. Otherwise, no imputation of missing values was applied.
The numbers of patients who experienced any adverse drug reactions were compared between treatment groups using Fisher exact tests.
Because of the potential for type I error due to multiple comparisons, findings for analyses of secondary end points should be interpreted as exploratory.
All statistical analyses were performed using SAS release 9.4 (SAS Institute) and 2-tailed P values less than .05 were considered statistically significant.
Results
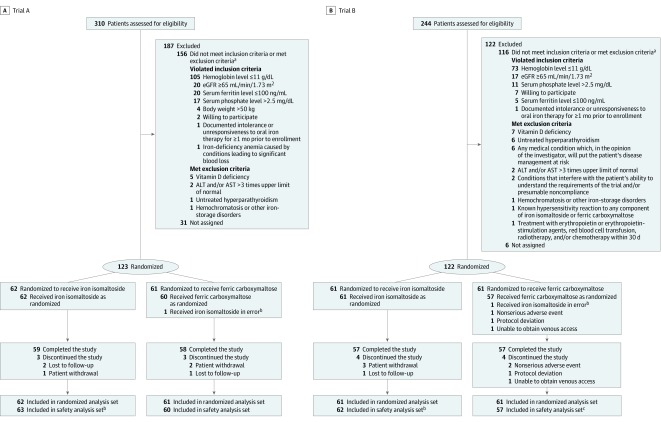
Of the 554 patients screened across the 2 trials, 123 were randomized to iron isomaltoside and 122 to ferric carboxymaltose; 231 of 245 enrollees completed the trials (Figure 1). Demographic and clinical characteristics were well balanced across the treatment groups in both trials (Table 1). The 2 trials enrolled mostly women with iron-deficiency anemia due to gynecological bleeding, which is among the most common causes of iron-deficiency anemia.1 Consistent with the known effects of untreated iron deficiency to stimulate FGF23 gene transcription and fibroblast growth factor 23 protein cleavage,11 C-terminal fibroblast growth factor 23 levels were markedly elevated at baseline.
Figure 1. Participant Flow in Trial A and Trial B Assessing the Effect of Iron Isomaltoside vs Ferric Carboxymaltose on Hypophosphatemia in Patients With Iron-Deficiency Anemia.
aSome potential study participants had more than 1 reason for exclusion.
bOne patient randomized to ferric carboxymaltose was erroneously treated with iron isomaltoside and included in the iron isomaltoside safety analysis set.
cThree patients randomized to ferric carboxymaltose were not treated and not included in the safety analysis set.
Table 1. Baseline Demographics and Laboratory Parameters.
| Trial A | Trial B | Pooled | ||||
|---|---|---|---|---|---|---|
| Iron Isomaltoside (n = 63) | Ferric Carboxymaltose (n = 60) | Iron Isomaltoside (n = 62) | Ferric Carboxymaltose (n = 57) | Iron Isomaltoside (n = 125) | Ferric Carboxymaltose (n = 117) | |
| Patient Demographics | ||||||
| Age, mean (SD), y | 43.9 (10.4) | 46.3 (11.6) | 42.2 (12.9) | 43.1 (11.5) | 43.0 (11.7) | 44.7 (11.6) |
| Sex, No. (%) | ||||||
| Female | 61 (96.8) | 57 (95.0) | 58 (93.5) | 54 (94.7) | 119 (95.2) | 111 (94.9) |
| Male | 2 (3.2) | 3 (5.0) | 4 (6.5) | 3 (5.3) | 6 (4.8) | 6 (5.1) |
| Race, No. (%) | ||||||
| White | 38 (60.3) | 38 (63.3) | 28 (45.2) | 29 (50.9) | 66 (52.8) | 67 (57.3) |
| African American | 22 (34.9) | 19 (31.7) | 32 (51.6) | 27 (47.4) | 54 (43.2) | 46 (39.3) |
| Asian | 2 (3.2) | 1 (1.7) | 0 | 0 | 2 (1.6) | 1 (0.9) |
| Other | 1 (1.6) | 2 (3.3) | 2 (3.2) | 1 (1.8) | 3 (2.4) | 3 (2.6) |
| Hispanic ethnicity | 37 (58.7) | 36 (60.0) | 23 (37.1) | 23 (40.4) | 60 (48.0) | 59 (50.4) |
| Weight, mean (SD), kg | 80.6 (16.6) | 77.4 (20.2) | 90.1 (29.2) | 84.2 (20.1) | 85.3 (24.0) | 80.7 (20.3) |
| BMI, mean (SD) | 30.6 (6.1) | 29.6 (7.0) | 32.3 (8.6) | 31.7 (7.9) | 31.5 (7.5) | 30.7 (7.5) |
| Gynecological cause of IDA, No. (%) | 41 (65.1) | 42 (70.0) | 44 (71.0) | 39 (68.4) | 85 (68.0) | 81 (69.2) |
| Laboratory Parameters | ||||||
| Hemoglobin, mean (SD), g/dLa,b | 9.8 (1.3) | 9.6 (1.3) | 9.6 (1.2) | 9.3 (1.4) | 9.7 (1.3) | 9.5 (1.4) |
| Ferritin, median (IQR), ng/mLa,c | 6.1 (2.9-12.9) | 4.8 (3.1-7.5) | 4.8 (2.8-8.7) | 5.1 (2.7-8.8) | 5.2 (2.8-11.2) | 4.8 (3.0-7.7) |
| Transferrin saturation, median (IQR), %a,d | 5.6 (3.5-9.7) | 4.7 (3.6-7.7) | 5.2 (3.5-8.8) | 4.8 (3.2-9.2) | 5.3 (3.5-9.7) | 4.8 (3.4-8.1) |
| Serum phosphate, mean (SD), mg/dLe | 3.3 (0.6) | 3.3 (0.5) | 3.4 (0.5) | 3.3 (0.5) | 3.4 (0.5) | 3.3 (0.5) |
| Urinary fractional excretion of phosphate, mean (SD), %f | 11.1 (6.7) | 10.3 (4.7) | 9.4 (4.9) | 10.2 (4.5) | 10.3 (5.9) | 10.3 (4.6) |
| C-terminal FGF23, median (IQR), RU/mLg | 507 (225-1256) | 351 (186-857) | 579 (162-1317) | 454 (89-1344) | 539 (196-1257) | 398 (142-1192) |
| Intact FGF23, mean (SD), pg/mLg | 59.0 (39.8) | 46.2 (20.5) | 60.9 (50.3) | 53.6 (35.3) | 59.9 (45.2) | 49.9 (29.0) |
| Ionized calcium, mean (SD), mg/dLh | 5.1 (0.2) | 5.1 (0.2) | 5.1 (0.2) | 5.1 (0.2) | 5.1 (0.2) | 5.1 (0.2) |
| Intact parathyroid hormone, mean (SD), pg/mLi | 55.1 (26.4) | 51.6 (26.4) | 55.4 (26.5) | 59.9 (33.9) | 55.3 (26.3) | 55.7 (30.5) |
| 25-Hydroxyvitamin D, mean (SD), ng/mLj | 23.2 (7.6) | 25.9 (7.8) | 23.2 (11.0) | 23.8 (10.0) | 23.2 (9.4) | 25.0 (8.9) |
| 1,25-Dihydroxyvitamin D, mean (SD), pg/mLk | 58.9 (18.2) | 63.9 (19.4) | 55.6 (16.4) | 59.6 (19.6) | 57.3 (17.3) | 61.8 (19.5) |
| 24,25-Dihydroxyvitamin D, mean (SD), ng/mLl | 2.1 (1.1) | 2.4 (1.2) | 2.0 (1.6) | 1.9 (1.1) | 2.0 (1.4) | 2.2 (1.2) |
| Alkaline phosphatase, mean (SD), IU/Lm | 70.0 (26.9) | 72.4 (27.5) | 71.8 (18.5) | 76.9 (26.8) | 70.9 (23.1) | 74.6 (27.1) |
| Bone-specific alkaline phosphatase, mean (SD), μg/Ln | 11.6 (4.1) | 12.5 (6.6) | 12.0 (3.5) | 12.8 (5.9) | 11.8 (3.8) | 12.7 (6.3) |
| N-terminal propeptide of type 1 collagen, mean (SD), ng/mL | 56.5 (26.3) | 57.3 (28.9) | 58.4 (25.4) | 65.6 (39.4) | 57.4 (25.7) | 61.4 (34.5) |
| Carboxy-terminal collagen crosslinks, mean (SD), ng/mL | 0.33 (0.16) | 0.29 (0.15) | 0.33 (0.15) | 0.38 (0.22) | 0.33 (0.16) | 0.34 (0.20) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); FGF23, fibroblast growth factor; IDA, iron-deficiency anemia; IQR, interquartile range.
SI conversion factors: To convert alkaline phosphatase to μkat/L, multiply by 0.0167; ferritin to pmol/L, multiply by 2.247; and ionized calcium to mmol/L, multiply by 0.25.
Data are presented for the as-randomized analysis set; all other data in the table are for the safety analysis set.
Reference range: women 18-59 y, 11.6-16.4 g/dL; men 18-59 y, 12.7-18.1 g/dL.
Reference range: women, 11.0-306.8 ng/mL; men, 23.9-336.2 ng/mL.
Calculcated as: (Total serum iron [μmol/L] × 5.586) / (transferrin [g/L] × 100) × 70.9.
Reference range: 2.2-5.1 mg/dL.
Calculcated as: (Urinary phosphate × serum creatinine) / (serum phosphate × urinary creatinine) × 100.
No reference range.
Reference range: 4.6-5.3 mg/dL.
Reference range: 14.0-72.0 pg/mL.
Reference range: 25.0-80.0 ng/mL.
Reference range: 20.8-105.4 pg/mL.
Reference range: 1.6-9.1 ng/mL.
Reference range: women 18-50 y, 31-106 IU/L; women 50-60 y, 35-123 IU/L; men 18-50 y, 31-129 IU/L; and men 50-60 y, 35-131 IU/L.
Reference range: premenopausal women, 2.9-14.5 μg/L; postmenopausal women, 3.8-22.6 μg/L; and men, 3.7-20.9 μg/L.
Primary End Point: Incidence of Hypophosphatemia
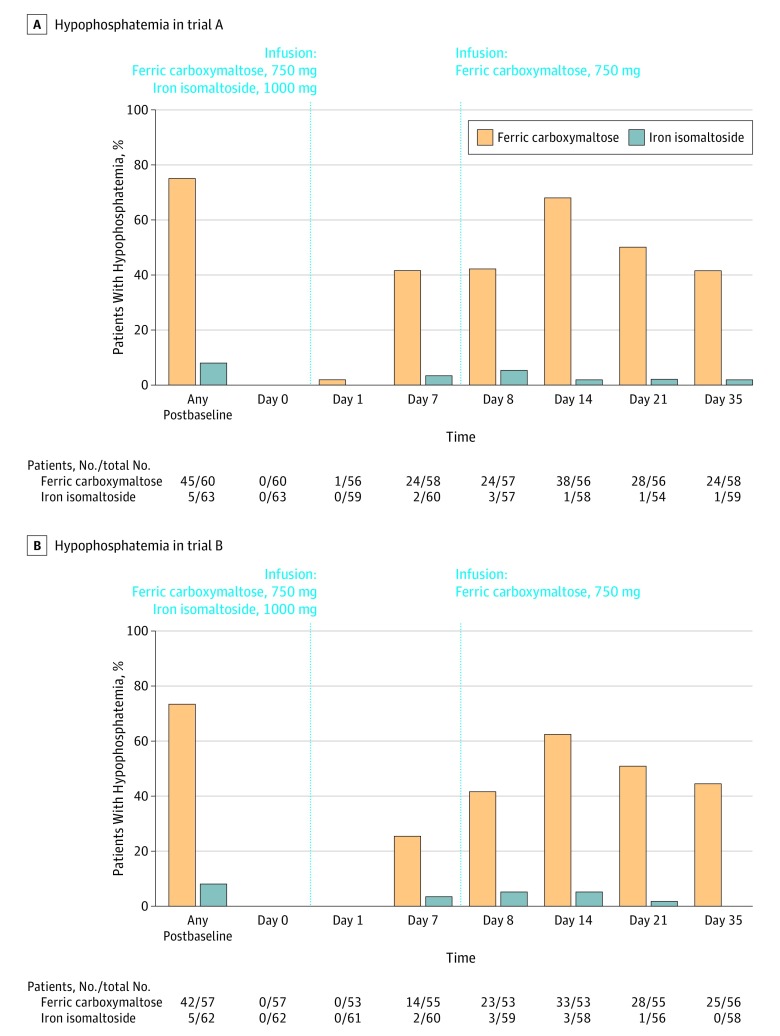
The incidence of hypophosphatemia at any time from baseline to day 35 was significantly lower among patients treated with iron isomaltoside than with ferric carboxymaltose (trial A: 7.9% vs 75.0% [adjusted rate difference, –67.0% {95% CI, –77.4% to –51.5%}], P < .001; trial B: 8.1% vs 73.7% [adjusted rate difference, –65.8% {95% CI, –76.6% to –49.8%}], P < .001; Figure 2; eTable 4 and eFigure 1 in Supplement 3).
Figure 2. Hypophosphatemia in Trial A and Trial B.
The leftmost columns correspond to the primary outcome of incident hypophosphatemia at any time during the trial. The remaining columns correspond to the proportions of patients with serum phosphate level less than 2.0 mg/dL at each individual time point in the safety analysis set.
Secondary End Points
Subsequent results of the biomarkers of mineral and bone homeostasis are derived from pooled analyses of trial A and trial B; trial-specific and pooled data for unadjusted and least squares mean changes from baseline are presented in eTable 5 and eTable 6 in Supplement 3.
Serum Phosphate and Urinary Excretion of Phosphate
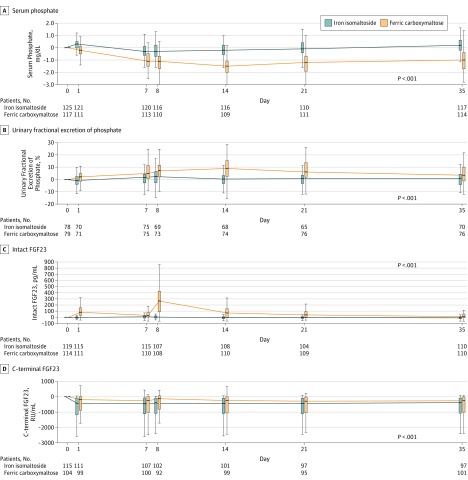
Beginning at day 1 and through all postbaseline visits, ferric carboxymaltose induced significantly larger magnitude reductions in serum phosphate than iron isomaltoside (Figure 3 and Figure 4; eTable 5 and eFigure 2 in Supplement 3). Urinary phosphate excretion was significantly higher in the ferric carboxymaltose group vs the iron isomaltoside group throughout the study period, with a peak at day 14, which coincided with the ferric carboxymaltose group’s nadir of serum phosphate (Figure 3 and Figure 4; eTable 5 and eFigure 2 in Supplement 3).
Figure 3. Changes From Baseline in Biomarkers of Mineral and Bone Homeostasis According to Iron Treatment: Pooled Data for Trial A and Trial B.
Tukey box plots indicate the interquartile range (25th, 75th percentiles) as vertical boxes, medians as horizontal lines within the boxes, and observations within 1.5 times above and below the interquartile range as vertical whiskers. Outliers are not shown. P values correspond to the treatment group-by-time interaction terms from the mixed models for repeated measures analyses of change from baseline in biomarkers, as described in the Methods section. FCM indicates ferric carboxymaltose; FGF23, fibroblast growth factor 23; and IIM, iron isomaltoside 1000 (now called ferric derisomaltose).
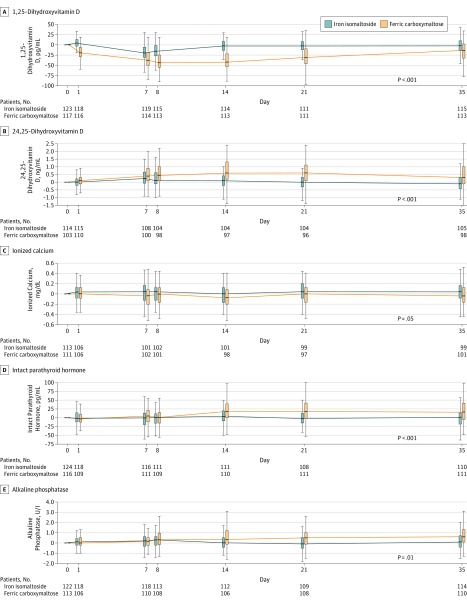
Figure 4. Changes From Baseline in Biomarkers of Mineral and Bone Homeostasis According to Iron Treatment: Pooled Data for Trial A and Trial B.
See the Figure 3 legend for descriptions of the data markers and analysis. FCM indicates ferric carboxymaltose; FGF23, fibroblast growth factor 23; and IIM, iron isomaltoside 1000 (now called ferric derisomaltose).
Fibroblast Growth Factor 23
Within 24 hours after the first dose of ferric carboxymaltose on day 0, mean biologically active intact fibroblast growth factor 23 increased from 46.2 pg/mL to 151.2 pg/mL and reached a peak of 343.6 pg/mL on day 8, which was 24 hours after the second dose of ferric carboxymaltose (Figure 3 and Figure 4; eTable 5 and eFigure 2 in Supplement 3). Thereafter, intact fibroblast growth factor 23 gradually decreased through day 35 in the ferric carboxymaltose group, but remained significantly higher than in the iron isomaltoside group at all postbaseline visits (Figure 3 and Figure 4; eTable 5 and eFigure 2 in Supplement 3). Concentrations of C-terminal fibroblast growth factor 23 declined within 24 hours of either iron isomaltoside or ferric carboxymaltose administration, but increased again in the ferric carboxymaltose group vs the iron isomaltoside group between days 8 and 21, coincident with that group’s peak in full-length fibroblast growth factor 23, which is also detected by the C-terminal assay (Figure 3 and Figure 4; eTable 5 and eFigure 2 in Supplement 3).
Vitamin D
Serum concentrations of the storage form of vitamin D, 25-hydroxyvitamin D, remained similar throughout the study in the iron isomaltoside and ferric carboxymaltose groups (eTable 5 in Supplement 3). In contrast, both treatment groups experienced decreases in the biologically active form, 1,25-dihydroxyvitamin D, but the decrease was significantly more pronounced in the ferric carboxymaltose group and persisted throughout the remainder of the study period (Figure 3 and Figure 4; eTable 5 and eFigure 2 in Supplement 3). Serum concentrations of the inactive vitamin D metabolite, 24,25-dihydroxyvitamin D, increased significantly in the ferric carboxymaltose vs the iron isomaltoside group from day 7 onward, and the ferric carboxymaltose group’s peak serum 24,25-dihydroxyvitamin D on day 14 coincided with its nadir in 1,25-dihydroxyvitamin D on days 8 to 14 (Figure 3 and Figure 4; eTable 5 and eFigure 2 in Supplement 3).
Calcium and PTH
Compared with iron isomaltoside, levels of ionized calcium decreased significantly on days 7, 8, and 21 in the ferric carboxymaltose group, whereas PTH increased significantly beginning on day 7. From day 14 throughout the duration of the trial, PTH remained significantly higher in the ferric carboxymaltose group (Figure 3 and Figure 4; eTable 5 and eFigure 2 in Supplement 3).
Iron and Anemia Parameters
In trial A and trial B and in the pooled analyses of both trials, iron isomaltoside and ferric carboxymaltose each increased hemoglobin levels, hemoglobin per gram of iron infused, and ferritin and transferrin saturation (eTable 7 and eFigure 3 in Supplement 3).
Exploratory End Points: Bone Turnover Markers
Compared with iron isomaltoside, ferric carboxymaltose induced significant increases in total and bone-specific alkaline phosphatase at multiple postbaseline visits (Figure 3 and Figure 4; eTable 5, eFigure 2, and eFigure 4 in Supplement 3). Compared with iron isomaltoside, ferric carboxymaltose induced significant decreases in N-terminal propeptide of type 1 collagen and carboxy-terminal collagen crosslinks at multiple postbaseline visits (eTable 5 and eFigure 4 in Supplement 3).
Post Hoc End Points and Analyses
The results of post hoc analyses of the primary end point that adjusted for study site were similar to the primary analyses (eTable 4 in Supplement 3).
By day 7 of both trials, the prevalence of hypophosphatemia was significantly lower in patients treated with iron isomaltoside vs ferric carboxymaltose, despite the ferric carboxymaltose group having received only 750 mg of iron by that time vs 1000 mg in the iron isomaltoside group (Figure 2; eTable 4 in Supplement 3). In both trials, the prevalence of hypophosphatemia peaked on day 14 in the ferric carboxymaltose group (1 week after the second 750-mg dose), and remained significantly higher than in the iron isomaltoside group at study end on day 35 (Figure 2; eTable 4 and eFigure 1 in Supplement 3). Severe hypophosphatemia (serum phosphate ≤1.0 mg/dL) was not observed in iron isomaltoside–treated patients, but developed in 11.3% of ferric carboxymaltose–treated patients in the pooled analysis (P < .001).
Adverse Events
Overall, site investigators reported more frequent adverse drug reactions in the ferric carboxymaltose group vs the iron isomaltoside group (trial A: 27/60 [45.0%] vs 7/63 [11.1%]; trial B: 28/57 [49.1%] vs 14/62 [22.6%]; Table 2). In the ferric carboxymaltose group, hypophosphatemia and blood phosphorus decreased were reported as adverse drug reactions in 38.5% of patients (Table 2). After excluding these, rates of adverse drug reactions remained higher in the ferric carboxymaltose group vs the iron isomaltoside group (Table 2). Overall, serious or severe hypersensitivity reactions occurred in 1 patient (0.8%) in the iron isomaltoside group (swollen eyelid unilaterally) and in 2 patients (1.7%) in the ferric carboxymaltose group (dyspnea and swelling).
Table 2. Adverse Drug Reactions Occurring at a Frequency of 5% or Greater in Either Treatment Group in the Safety Analysis Set.
| Adverse Drug Reactionsa | No. (%) | |||||
|---|---|---|---|---|---|---|
| Trial A | Trial B | Pooled | ||||
| Iron Isomaltoside (n = 63) | Ferric Carboxymaltose (n = 60) | Iron Isomaltoside (n = 62) | Ferric Carboxymaltose (n = 57) | Iron Isomaltoside (n = 125) | Ferric Carboxymaltose (n = 117) | |
| Any adverse drug reaction | 7 (11.1) | 27 (45.0) | 14 (22.6) | 28 (49.1) | 21 (16.8) | 55 (47.0) |
| Specific adverse drug reactions | ||||||
| Hypophosphatemia | 0 | 12 (20.0) | 2 (3.2) | 14 (24.6) | 2 (1.6) | 26 (22.2) |
| Blood | ||||||
| Phosphorus decreased | 0 | 12 (20.0) | 0 | 7 (12.3) | 0 | 19 (16.2) |
| Parathyroid hormone increased | 0 | 1 (1.7) | 4 (6.5) | 5 (8.8) | 4 (3.2) | 6 (5.1) |
| Headache | 1 (1.6) | 1 (1.7) | 3 (4.8) | 4 (7.0) | 4 (3.2) | 5 (4.3) |
| Nausea | 0 | 4 (6.7) | 1 (1.6) | 4 (7.0) | 1 (0.8) | 8 (6.8) |
| Serum ferritin increased | 0 | 0 | 0 | 3 (5.3) | 0 | 3 (2.6) |
The reporting of adverse drug reactions uses standard methodology (MedDRA terms). The listings for adverse drug reactions reflect adverse events that were judged by the local site investigator to be related or possibly related to the study drugs. For laboratory assessments, local site investigators saw the values and judged whether the decreased or increased levels necessitated reporting as an adverse drug reaction.
Discussion
In 2 randomized trials conducted in patients with iron-deficiency anemia who were intolerant of or unresponsive to oral iron, iron isomaltoside, compared with ferric carboxymaltose, resulted in lower incidence of hypophosphatemia over 35 days. These trials provide data about the incidence of an adverse effect that may have clinical consequences and mechanistic information about the role of fibroblast growth factor 23 in vitamin D metabolism in humans.
Detailed investigation of rare hereditary and acquired states of primary fibroblast growth factor 23 excess demonstrate that elevation of full-length, biologically active, intact fibroblast growth factor 23 causes hypophosphatemia by reducing proximal tubular reabsorption of filtered phosphate, and by suppressing circulating concentrations of 1,25-dihydroxyvitamin D, which is the active form of vitamin D.23,24,25 Reduced 1,25-dihydroxyvitamin D limits compensatory increases in dietary phosphate absorption that would otherwise occur in response to hypophosphatemia and limits dietary calcium absorption, which can decrease serum calcium.23,25 Secondary hyperparathyroidism in response to decreased serum calcium helps to maintain serum calcium within the normal range, but can further exacerbate hypophosphatemia by promoting renal phosphate losses via the known phosphaturic effects of elevated PTH.23,26
The findings of these 2 trials suggest that ferric carboxymaltose activated this entire pathophysiological cascade by acutely increasing intact fibroblast growth factor 23 within 1 day. This was followed by increased urinary phosphate excretion and decreased 1,25-dihydroxyvitamin D and ionized calcium, which precipitated secondary hyperparathyroidism that likely maintained renal phosphate wasting and hypophosphatemia even after intact fibroblast growth factor 23 returned toward normal. Although the mechanism by which ferric carboxymaltose acutely elevates intact fibroblast growth factor 23 remains unknown, it has been proposed that the carbohydrate carrier of iron in ferric carboxymaltose somehow inhibits cleavage of full-length fibroblast growth factor 23 that is normally upregulated in parallel with increased FGF23 gene transcription in iron deficiency.9,11,27
Animal studies have demonstrated that fibroblast growth factor 23 lowers 1,25-dihydroxyvitamin D concentrations by reducing its production via inhibition of Cyp27b1 (1α-hydroxylase) and by accelerating its degradation via stimulation of Cyp24a1 (24-hydroxylase).28 However, physiological evidence of the importance of fibroblast growth factor 23–mediated stimulation of the vitamin D degradation pathway in humans has been limited. The finding that ferric carboxymaltose significantly increased 24,25-dihydroxyvitamin D levels, a marker of increased 24-hydroxylase activity, in association with increased intact fibroblast growth factor 23, supports fibroblast growth factor 23–mediated activation of 24-hydroxylase as an important contributor to reduced 1,25-dihydroxyvitamin D in states of fibroblast growth factor 23 excess. Previous human studies may have failed to isolate the effects of fibroblast growth factor 23 on 24-hydroxylase because of competing effects of 1,25-dihydroxyvitamin D on the enzyme. For example, in states of chronically elevated fibroblast growth factor 23 in which 1,25-dihydroxyvitamin D levels are suppressed, the known effects of low 1,25-dihydroxyvitamin D to reduce levels of 24,25-dihydroxyvitamin D24 likely obscured the effects of fibroblast growth factor 23 excess to elevate 24,25-dihydroxyvitamin D. In contrast, the acute effects of ferric carboxymaltose enabled confirmation that abrupt elevation of fibroblast growth factor 23 significantly activates 24-hydroxylase activity.
Although there are numerous case reports of skeletal complications of ferric carboxymaltose,13,29,30,31,32 to our knowledge, no previous controlled studies investigated the effects of intravenous iron on biomarkers of bone turnover. Thus, an important finding of these trials is that ferric carboxymaltose induced increases in intact fibroblast growth factor 23 and its downstream metabolic consequences may have significant effects on bone, as evidenced by increased total and bone-specific alkaline phosphatase and decreases in N-terminal propeptide of type 1 collagen, and carboxy-terminal collagen crosslinks. The change in alkaline phosphatase, which is consistent with the pattern observed in patients with osteomalacia,33,34 provides new evidence that even a single course of ferric carboxymaltose may adversely affect the skeleton and may help explain why repeated dosing of ferric carboxymaltose has been associated with osteomalacia and fractures.13,29,30,31,32
Limitations
These trials have several limitations. First, the preponderance of patients with gynecological causes of iron-deficiency anemia, who tend to have higher rates of hypophosphatemia,10 likely explains the higher than anticipated incidence of hypophosphatemia following ferric carboxymaltose treatment; this may limit generalizability to other causes of iron-deficiency anemia.
Second, the dosing for ferric carboxymaltose and iron isomaltoside differed, which could have affected the results. However, a recent observational study that was conducted in Europe, where the dosing of both ferric carboxymaltose and iron isomaltoside were identical, demonstrated similarly higher rates of hypophosphatemia following ferric carboxymaltose vs iron isomaltoside,35 suggesting that the dosing is not the main driver of the current results.
Third, the end of follow-up at day 35 precluded a complete assessment of the duration until serum phosphate, 1,25-dihydroxyvitamin D, PTH, and alkaline phosphatase levels normalized after a single course of ferric carboxymaltose.
Fourth, the trials did not measure clinical outcomes.
Fifth, while the second dose within a single course of ferric carboxymaltose induced larger magnitude effects on intact fibroblast growth factor 23 and mineral metabolism than the first, the trials did not study whether the effects are further magnified by repeated courses of ferric carboxymaltose. Testing for such dose-stacking effects—whereby a second course of ferric carboxymaltose given during or shortly after an episode of hypophosphatemia from a prior course precipitates more severe and more protracted hypophosphatemia—is needed to further investigate the pathogenesis of ferric carboxymaltose–associated osteomalacia. However, this may be impossible in a controlled study because it would be ethically unacceptable to administer another course of ferric carboxymaltose to a patient who remains hypophosphatemic from a previous course.
Conclusions
In 2 randomized trials of patients with iron-deficiency anemia who were intolerant of or unresponsive to oral iron, iron isomaltoside, compared with ferric carboxymaltose, resulted in lower incidence of hypophosphatemia over 35 days. However, further research is needed to determine the clinical importance of this difference.
Trial Protocol
Trial Protocol
eTable 1. Inclusion and Exclusion Criteria, and Main Revisions to the Trial Protocols
eTable 2. Listing of All Secondary Safety and Efficacy End Points
eTable 3. Biochemical Assays
eTable 4. Prevalence of Hypophosphatemia at Each Time Point – Trial A, Trial B and Pooled Data for Trials A and B
eTable 5. Secondary and Additional Safety End Points – Trial A, Trial B, and Pooled Data for Trials A and B
eTable 6. Least Squares Mean Changes From Baseline in Biochemical and Bone Markers – Trial A, Trial B, and Pooled Data for Trials A and B
eTable 7. Secondary Efficacy End Points – Trial A, Trial B, and Pooled Data for Trial A and Trial B
eFigure 1. Incidence of Hypophosphatemia (Serum Phosphate <2.0 mg/dL) Overall and Prevalence of Hypophosphatemia at Each Time Point – Pooled Data for Trial A and Trial B
eFigure 2. Least Squares Mean Changes From Baseline in Biomarkers of Mineral and Bone Homeostasis According to Iron Treatment – Pooled Data for Trial A and Trial B
eFigure 3. Least Squares Mean Changes From Baseline in Iron Parameters – Pooled Data for Trial A and Trial B
eFigure 4. Changes in Bone Turnover Markers – Pooled Data for Trial A and Trial B
Statistical Analysis Plan
Data Sharing Statement
References
- 1.Camaschella C. Iron-deficiency anemia. N Engl J Med. 2015;372(19):1832-1843. doi: 10.1056/NEJMra1401038 [DOI] [PubMed] [Google Scholar]
- 2.Lopez A, Cacoub P, Macdougall IC, Peyrin-Biroulet L. Iron deficiency anaemia. Lancet. 2016;387(10021):907-916. doi: 10.1016/S0140-6736(15)60865-0 [DOI] [PubMed] [Google Scholar]
- 3.Girelli D, Ugolini S, Busti F, Marchi G, Castagna A. Modern iron replacement therapy: clinical and pathophysiological insights. Int J Hematol. 2018;107(1):16-30. doi: 10.1007/s12185-017-2373-3 [DOI] [PubMed] [Google Scholar]
- 4.Bhandari S, Pereira DIA, Chappell HF, Drakesmith H. Intravenous irons: from basic science to clinical practice. Pharmaceuticals (Basel). 2018;11(3):82. doi: 10.3390/ph11030082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Derman R, Roman E, Modiano MR, Achebe MM, Thomsen LL, Auerbach M. A randomized trial of iron isomaltoside versus iron sucrose in patients with iron deficiency anemia. Am J Hematol. 2017;92(3):286-291. doi: 10.1002/ajh.24633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holm C, Thomsen LL, Norgaard A, Langhoff-Roos J. Single-dose intravenous iron infusion or oral iron for treatment of fatigue after postpartum haemorrhage: a randomized controlled trial. Vox Sang. 2017;112(3):219-228. doi: 10.1111/vox.12477 [DOI] [PubMed] [Google Scholar]
- 7.Onken JE, Bregman DB, Harrington RA, et al. . A multicenter, randomized, active-controlled study to investigate the efficacy and safety of intravenous ferric carboxymaltose in patients with iron deficiency anemia. Transfusion. 2014;54(2):306-315. [DOI] [PubMed] [Google Scholar]
- 8.Onken JE, Bregman DB, Harrington RA, et al. . Ferric carboxymaltose in patients with iron-deficiency anemia and impaired renal function: the REPAIR-IDA trial. Nephrol Dial Transplant. 2014;29(4):833-842. doi: 10.1093/ndt/gft251 [DOI] [PubMed] [Google Scholar]
- 9.Wolf M, Koch TA, Bregman DB. Effects of iron deficiency anemia and its treatment on fibroblast growth factor 23 and phosphate homeostasis in women. J Bone Miner Res. 2013;28(8):1793-1803. doi: 10.1002/jbmr.1923 [DOI] [PubMed] [Google Scholar]
- 10.Wolf M, Chertow GM, Macdougall IC, Kaper R, Krop J, Strauss W. Randomized trial of intravenous iron-induced hypophosphatemia. JCI Insight. 2018;3(23):124486. doi: 10.1172/jci.insight.124486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edmonston D, Wolf M. FGF23 at the crossroads of phosphate, iron economy and erythropoiesis. Nat Rev Nephrol. 2020;16(1):7-19. doi: 10.1038/s41581-019-0189-5 [DOI] [PubMed] [Google Scholar]
- 12.Subramanian R, Khardori R. Severe hypophosphatemia: pathophysiologic implications, clinical presentations, and treatment. Medicine (Baltimore). 2000;79(1):1-8. doi: 10.1097/00005792-200001000-00001 [DOI] [PubMed] [Google Scholar]
- 13.Zoller H, Schaefer B, Glodny B. Iron-induced hypophosphatemia: an emerging complication. Curr Opin Nephrol Hypertens. 2017;26(4):266-275. doi: 10.1097/MNH.0000000000000329 [DOI] [PubMed] [Google Scholar]
- 14.Auerbach M, Lykke LL. A single infusion of iron isomaltoside 1000 allows a more rapid hemoglobin increment than multiple doses of iron sucrose with a similar safety profile in patients with iron deficiency anemia. Blood. 2018;132(suppl 1):2334. doi: 10.1182/blood-2018-99-110199 [DOI] [Google Scholar]
- 15.Emrich IE, Lizzi F, Seiler-Mußler S, et al. . Hypophosphatemia after high dosage iron substitution with ferric carboxymaltose (FCM) and iron isomaltoside (IM): the randomised controlled Home Afers 1 trial. Blood. 2018;132(suppl 1):3627. doi: 10.1182/blood-2018-99-114386 [DOI] [Google Scholar]
- 16.US Food and Drug Administration Highlights of prescribing information: Injectafer (ferric carboxymaltose injection). Vifor (International) Inc, Switzerland. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/203565s005lbl.pdf. Revised January 2018. Accessed October 22, 2019.
- 17.Birgegård G, Henry D, Glaspy J, Chopra R, Thomsen LL, Auerbach M. A randomized noninferiority trial of intravenous iron isomaltoside versus oral iron sulfate in patients with nonmyeloid malignancies and anemia receiving chemotherapy: the PROFOUND trial. Pharmacotherapy. 2016;36(4):402-414. doi: 10.1002/phar.1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dahlerup JF, Jacobsen BA, van der Woude J, Bark LÅ, Thomsen LL, Lindgren S. High-dose fast infusion of parenteral iron isomaltoside is efficacious in inflammatory bowel disease patients with iron-deficiency anaemia without profound changes in phosphate or fibroblast growth factor 23. Scand J Gastroenterol. 2016;51(11):1332-1338. doi: 10.1080/00365521.2016.1196496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reinisch W, Staun M, Tandon RK, et al. . A randomized, open-label, non-inferiority study of intravenous iron isomaltoside 1,000 (Monofer) compared with oral iron for treatment of anemia in IBD (PROCEED). Am J Gastroenterol. 2013;108(12):1877-1888. doi: 10.1038/ajg.2013.335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Wyck DB, Mangione A, Morrison J, Hadley PE, Jehle JA, Goodnough LT. Large-dose intravenous ferric carboxymaltose injection for iron deficiency anemia in heavy uterine bleeding: a randomized, controlled trial. Transfusion. 2009;49(12):2719-2728. doi: 10.1111/j.1537-2995.2009.02327.x [DOI] [PubMed] [Google Scholar]
- 21.Favrat B, Balck K, Breymann C, et al. . Evaluation of a single dose of ferric carboxymaltose in fatigued, iron-deficient women: PREFER a randomized, placebo-controlled study. PLoS One. 2014;9(4):e94217. doi: 10.1371/journal.pone.0094217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yan X, Su XG. Stratified Wilson and Newcombe confidence intervals for multiple binomial proportions. Stat Biopharm Res. 2010;2(3):329-335. doi: 10.1198/sbr.2009.0049 [DOI] [Google Scholar]
- 23.Quarles LD. Endocrine functions of bone in mineral metabolism regulation. J Clin Invest. 2008;118(12):3820-3828. doi: 10.1172/JCI36479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reichel H, Koeffler HP, Norman AW. The role of the vitamin D endocrine system in health and disease. N Engl J Med. 1989;320(15):980-991. doi: 10.1056/NEJM198904133201506 [DOI] [PubMed] [Google Scholar]
- 25.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266-281. doi: 10.1056/NEJMra070553 [DOI] [PubMed] [Google Scholar]
- 26.Potts JT. Parathyroid hormone: past and present. J Endocrinol. 2005;187(3):311-325. doi: 10.1677/joe.1.06057 [DOI] [PubMed] [Google Scholar]
- 27.Farrow EG, Yu X, Summers LJ, et al. . Iron deficiency drives an autosomal dominant hypophosphatemic rickets (ADHR) phenotype in fibroblast growth factor-23 (Fgf23) knock-in mice. Proc Natl Acad Sci U S A. 2011;108(46):E1146-E1155. doi: 10.1073/pnas.1110905108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimada T, Hasegawa H, Yamazaki Y, et al. . FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res. 2004;19(3):429-435. doi: 10.1359/JBMR.0301264 [DOI] [PubMed] [Google Scholar]
- 29.Klein K, Asaad S, Econs M, Rubin JE. Severe FGF23-based hypophosphataemic osteomalacia due to ferric carboxymaltose administration. BMJ Case Rep. 2018;2018:bcr-2017-222851. doi: 10.1136/bcr-2017-222851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schaefer B, Glodny B, Zoller H. Blood and bone loser. Gastroenterology. 2017;152(6):e5-e6. doi: 10.1053/j.gastro.2016.09.050 [DOI] [PubMed] [Google Scholar]
- 31.Urbina T, Belkhir R, Rossi G, et al. . Iron supplementation-induced phosphaturic osteomalacia: FGF23 is the culprit. J Bone Miner Res. 2018;33(3):540-542. doi: 10.1002/jbmr.3369 [DOI] [PubMed] [Google Scholar]
- 32.Burckhardt P. Iron-induced osteomalacia. Osteologie. 2018;27(1):20-23. [Google Scholar]
- 33.Nagata Y, Imanishi Y, Ishii A, et al. . Evaluation of bone markers in hypophosphatemic rickets/osteomalacia. Endocrine. 2011;40(2):315-317. doi: 10.1007/s12020-011-9512-z [DOI] [PubMed] [Google Scholar]
- 34.Peach H, Compston JE, Vedi S, Horton LWL. Value of plasma calcium, phosphate, and alkaline phosphatase measurements in the diagnosis of histological osteomalacia. J Clin Pathol. 1982;35(6):625-630. doi: 10.1136/jcp.35.6.625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Detlie TE, Lindstrøm JC, Jahnsen ME, et al. . Incidence of hypophosphatemia in patients with inflammatory bowel disease treated with ferric carboxymaltose or iron isomaltoside. Aliment Pharmacol Ther. 2019;50(4):397-406. doi: 10.1111/apt.15386 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Trial Protocol
eTable 1. Inclusion and Exclusion Criteria, and Main Revisions to the Trial Protocols
eTable 2. Listing of All Secondary Safety and Efficacy End Points
eTable 3. Biochemical Assays
eTable 4. Prevalence of Hypophosphatemia at Each Time Point – Trial A, Trial B and Pooled Data for Trials A and B
eTable 5. Secondary and Additional Safety End Points – Trial A, Trial B, and Pooled Data for Trials A and B
eTable 6. Least Squares Mean Changes From Baseline in Biochemical and Bone Markers – Trial A, Trial B, and Pooled Data for Trials A and B
eTable 7. Secondary Efficacy End Points – Trial A, Trial B, and Pooled Data for Trial A and Trial B
eFigure 1. Incidence of Hypophosphatemia (Serum Phosphate <2.0 mg/dL) Overall and Prevalence of Hypophosphatemia at Each Time Point – Pooled Data for Trial A and Trial B
eFigure 2. Least Squares Mean Changes From Baseline in Biomarkers of Mineral and Bone Homeostasis According to Iron Treatment – Pooled Data for Trial A and Trial B
eFigure 3. Least Squares Mean Changes From Baseline in Iron Parameters – Pooled Data for Trial A and Trial B
eFigure 4. Changes in Bone Turnover Markers – Pooled Data for Trial A and Trial B
Statistical Analysis Plan
Data Sharing Statement