This cohort study assesses whether consuming processed meat, unprocessed red meat, poultry, or fish is associated with incident cardiovascular disease and all-cause mortality among US adults participating in 6 long-term cohort studies.
Key Points
Question
Is consuming processed meat, unprocessed red meat, poultry, or fish associated with incident cardiovascular disease and all-cause mortality?
Findings
In this cohort study of 29 682 US adults pooled from 6 prospective cohort studies, intake of processed meat, unprocessed red meat, or poultry was significantly associated with incident cardiovascular disease, but fish intake was not. Intake of processed meat or unprocessed red meat was significantly associated with all-cause mortality, but intake of poultry or fish was not.
Meaning
The findings of this study appear to have critical public health implications given that dietary behaviors are modifiable and most people consume these 4 food types on a daily or weekly basis.
Abstract
Importance
Although the associations between processed meat intake and cardiovascular disease (CVD) and all-cause mortality have been established, the associations of unprocessed red meat, poultry, or fish consumption with CVD and all-cause mortality are still uncertain.
Objective
To identify the associations of processed meat, unprocessed red meat, poultry, or fish intake with incident CVD and all-cause mortality.
Design, Setting, and Participants
This cohort study analyzed individual-level data of adult participants in 6 prospective cohort studies in the United States. Baseline diet data from 1985 to 2002 were collected. Participants were followed up until August 31, 2016. Data analyses were performed from March 25, 2019, to November 17, 2019.
Exposures
Processed meat, unprocessed red meat, poultry, or fish intake as continuous variables.
Main Outcomes and Measures
Hazard ratio (HR) and 30-year absolute risk difference (ARD) for incident CVD (composite end point of coronary heart disease, stroke, heart failure, and CVD deaths) and all-cause mortality, based on each additional intake of 2 servings per week for monotonic associations or 2 vs 0 servings per week for nonmonotonic associations.
Results
Among the 29 682 participants (mean [SD] age at baseline, 53.7 [15.7] years; 13 168 [44.4%] men; and 9101 [30.7%] self-identified as non-white), 6963 incident CVD events and 8875 all-cause deaths were adjudicated during a median (interquartile range) follow-up of 19.0 (14.1-23.7) years. The associations of processed meat, unprocessed red meat, poultry, or fish intake with incident CVD and all-cause mortality were monotonic (P for nonlinearity ≥ .25), except for the nonmonotonic association between processed meat intake and incident CVD (P for nonlinearity = .006). Intake of processed meat (adjusted HR, 1.07 [95% CI, 1.04-1.11]; adjusted ARD, 1.74% [95% CI, 0.85%-2.63%]), unprocessed red meat (adjusted HR, 1.03 [95% CI, 1.01-1.06]; adjusted ARD, 0.62% [95% CI, 0.07%-1.16%]), or poultry (adjusted HR, 1.04 [95% CI, 1.01-1.06]; adjusted ARD, 1.03% [95% CI, 0.36%-1.70%]) was significantly associated with incident CVD. Fish intake was not significantly associated with incident CVD (adjusted HR, 1.00 [95% CI, 0.98-1.02]; adjusted ARD, 0.12% [95% CI, −0.40% to 0.65%]). Intake of processed meat (adjusted HR, 1.03 [95% CI, 1.02-1.05]; adjusted ARD, 0.90% [95% CI, 0.43%-1.38%]) or unprocessed red meat (adjusted HR, 1.03 [95% CI, 1.01-1.05]; adjusted ARD, 0.76% [95% CI, 0.19%-1.33%]) was significantly associated with all-cause mortality. Intake of poultry (adjusted HR, 0.99 [95% CI, 0.97-1.02]; adjusted ARD, −0.28% [95% CI, −1.00% to 0.44%]) or fish (adjusted HR, 0.99 [95% CI, 0.97-1.01]; adjusted ARD, −0.34% [95% CI, −0.88% to 0.20%]) was not significantly associated with all-cause mortality.
Conclusions and Relevance
These findings suggest that, among US adults, higher intake of processed meat, unprocessed red meat, or poultry, but not fish, was significantly associated with a small increased risk of incident CVD, whereas higher intake of processed meat or unprocessed red meat, but not poultry or fish, was significantly associated with a small increased risk of all-cause mortality. These findings have important public health implications and should warrant further investigations.
Introduction
Processed meat, unprocessed red meat, poultry, and fish are major components of the US diet, representing more than 40% of protein intake, 42% of dietary cholesterol intake, and 26% of total energy intake in adults.1,2 From 1999 to 2016, the mean consumption of processed meat and fish did not change, whereas the mean consumption of unprocessed red meat decreased and poultry increased among US adults.3 The positive associations between processed meat intake and cardiovascular disease (CVD) and mortality have been established, but the associations of unprocessed red meat, poultry, or fish intake with CVD and mortality remain uncertain, partly owing to heterogeneity across studies, methodological limitations, and limited data from long-term prospective cohort studies.4
To address the foregoing research gaps, we pooled individual-level data from 6 prospective cohort studies of US adults. The primary objective of this study was to establish the associations of processed meat, unprocessed red meat, poultry, or fish intake with incident CVD and all-cause mortality.
Methods
This cohort study, which consisted of secondary data analysis of deidentified data, was conducted from March 25, 2019, to November 17, 2019. The study was approved by the Northwestern University Institutional Review Board, which determined that specific consent from participants was not required. Each of the 6 original cohort studies obtained written informed consent from all of their respective participants.
Study Sample
The included 6 cohorts were part of the Lifetime Risk Pooling Project,5 comprising the ARIC (Atherosclerosis Risk in Communities) study, CARDIA (Coronary Artery Risk Development in Young Adults) study, CHS (Cardiovascular Health Study), FHS (Framingham Heart Study), FOS (Framingham Offspring Study), and MESA (Multi-Ethnic Study of Atherosclerosis). Baseline diet data from 1985 to 2002 were collected. The baseline visit for FHS (1986-1990) was examination 20 and for FOS (1991-1995) was examination 5, owing to the availability of validated diet data. The original baseline visit was used for the other 4 cohorts: 1986-1989 for ARIC, 1985-1986 for CARDIA, 1989-1990 for CHS, and 2000-2002 for MESA. Eligible participants were free of CVD at baseline, had self-reported total caloric intake between 500 and 6000 kcal per day, and had no missing data for the study variables.
Diet Data Assessment
Dietary intake was collected using a validated food frequency questionnaire or diet history.6,7,8,9,10 Diet data were harmonized using a standardized protocol. The same definitions for food groups were applied, and serving sizes were unified. Primary dietary exposures were processed meat, unprocessed red meat, poultry, or fish (including shellfish) intake. Foods in mixed dishes were considered. One serving was defined primarily according to the widely used Willett Food Frequency Questionnaire.11 One serving was equivalent to 4 oz of unprocessed red meat or poultry or 3 oz of fish. For processed meat, 1 serving consisted of 2 slices of bacon, 2 small links of sausage, or 1 hot dog. Details about the diet data harmonization methods have been described.12 Only baseline diet data were analyzed.
Outcome Ascertainment
All events were adjudicated by each original cohort using similar criteria.5 Two primary outcomes were incident CVD and all-cause mortality. Incident CVD was a composite end point that included fatal and nonfatal coronary heart disease, fatal and nonfatal stroke, fatal and nonfatal heart failure, and other CVD deaths. Vital status was ascertained for 98% of the participants. The last event was ascertained on the last follow-up date of August 31, 2016.
Covariate Assessment
The following variables were self-reported: age, sex, race/ethnicity, educational level, smoking status and pack-years, alcohol intake, physical activity, medication use, and medical conditions. Race/ethnicity was self-reported through answering questions with fixed categories. The inclusion of racially/ethnically diverse samples increased generalizability and allowed the exploration of racial/ethnic differences in the study findings. The following variables were measured according to standard protocols: body mass index (calculated as weight in kilograms divided by height in meters squared), blood pressure, and serum lipid levels.
Statistical Analysis
Cause-specific hazard models were used to identify the associations of processed meat, unprocessed red meat, poultry, or fish intake with incident CVD. Standard proportional hazards models were used for all-cause mortality. Cause-specific hazard models are recommended for examining the origin of an association in the presence of competing risks (eg, non-CVD death as a competing risk for incident CVD).13 For incident CVD, only the first event was considered. Intake of the 4 food types was winsorized at the 0.5 and 99.5 percentiles. The supremum test was used to assess proportional hazards assumption.14 Violation of the assumption was corrected through stratifying 1 or more of the following variables: age groups, sex, and race/ethnicity. To evaluate for a nonmonotonic association, polynomial terms were included if the model fit was significantly improved. The unit of interpretation was based on each additional intake of 2 servings per week (approximately 0.29 serving per day), close to the median intake in the study sample, for monotonic associations or a comparison of 2 with 0 servings per week for nonmonotonic associations.
Cohort-stratified models were adjusted for age, sex, race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, Chinese, or other), and educational level (less than high school, high school, or some college or higher) (model 1); plus total energy, cohort-specific physical activity z score, smoking status (never, former, or current), smoking pack-years (0, 0.1-4.9, 5.0-9.9, 10-19.9, 20-29.9, 30-39.9, or ≥40), alcohol intake (grams), and hormone therapy (yes or no) (model 2); plus 3 of the 4 exposure variables, eggs, fruits, vegetables (excluding potatoes and legumes), potatoes, legumes, whole grains, refined grains, nuts and seeds, low-fat dairy products, high-fat dairy products, and sugar-sweetened beverages (model 3).
For each hazard ratio (HR) from models 1 to 3, an absolute risk difference (ARD) was computed using R packages riskRegression,15 pec,16 and survival.17 The mean value of the covariates, a prespecified length of follow-up (10, 20, or 30 years) and an intake difference of 2 servings per week for the exposures were used. The 95% CI was derived from 500 bootstrap samples.
A secondary objective of this study was to examine the associations of processed meat, unprocessed red meat, poultry, or fish intake with incident CVD and all-cause mortality by the following subgroups: age (<45, 45-64, or ≥65 years), sex (male or female), race/ethnicity (non-Hispanic white, non-Hispanic black, or other), educational level (<high school, high school, or ≥ some college), smoking status (never, former, or current), weight status (normal/underweight, overweight, or obese), diabetes (yes or no), hypertension (yes or no), hyperlipidemia (yes or no; defined as use of lipid-lowering drugs or total cholesterol level of ≥240 mg/dL [to convert to millimoles per liter, multiply by 0.0259]), low lipids level (yes or no; defined as nonuse of lipid-lowering drugs and low-density lipoprotein cholesterol level of <70 mg/dL [to convert to millimoles per liter, multiply by 0.0259] or non-high-density lipoprotein cholesterol level of <100 mg/dL), higher-quality diet (yes or no; defined as an Alternate Healthy Eating Index 201018 score in the highest quartile), high-saturated-fat diet (yes or no; defined as percentage of energy from saturated fat in the highest quartile), low-saturated-fat diet (yes or no; defined as percentage of energy from saturated fat <7%), high-fat diet (yes or no; defined as percentage of energy from fat in the highest quartile), high-protein diet (yes or no; defined as percentage of energy from protein in the highest quartile), and high-carbohydrate diet (yes or no; defined as percentage of energy from carbohydrates in the highest quartile). P value for interaction was obtained with the joint test19 for monotonic associations and likelihood ratio test20 for nonmonotonic associations.
Eight sensitivity analyses were conducted as follows: (1) missing data were imputed using multiple imputation by chained equations21; (2) events that occurred within 2 years or within 5 years of follow-up were excluded; (3) follow-up was arbitrarily truncated at 10 and 20 years; (4) any 1 of the 6 cohorts was removed; (5) cohort-specific quintiles of the intake were used; (6) energy density models were used and the 4 food types were in energy-adjusted form (ie, servings per week per 1000 kcal); (7) subdistribution hazard models were used that may be more appropriate for prediction rather than cause investigation13; and (8) fish was divided into fatty fish and nonfatty fish.
A 2-tailed P < .01 was set a priori to account for multiple comparisons for primary analyses, including 4 exposures and 2 outcomes. Bonferroni correction (0.05/8 = .006) was inappropriately conservative because of the correlation within primary exposures and outcomes. Results from subgroup analyses were undertaken as exploratory analyses. Statistical analyses were conducted using R, version 3.6.1 (R Project for Statistical Computing) and SAS, version 9.4 (SAS Institute Inc).
Results
Among the 29 682 total participants, the mean (SD) age was 53.7 (15.7) years at baseline, 13 168 (44.4%) were male, and 9101 (30.7%) self-identified as non-white (Table 1). The median (interquartile range [IQR]) intake in servings per week was 1.5 (0.5-3.8) for processed meat, 3.0 (1.5-5.0) for unprocessed red meat, 2.0 (1.0-3.0) for poultry, and 1.6 (0.9-3.4) for fish. Compared with participants with lower total intake of these 4 food types, participants with higher total intake (1) were younger and more likely to be male, non-Hispanic black, and current smokers and to have diabetes, higher body mass index, higher non-high-density lipoprotein cholesterol levels, higher energy intake, and higher alcohol intake; (2) had lower high-density lipoprotein cholesterol levels, had lower diet quality, and were less likely to use lipid-lowering drugs and hormone therapy; and (3) had higher incidence of CVD and all-cause mortality. Partial correlation coefficients between the 4 food types and a range of dietary and other lifestyle factors are shown in eTable 1 in the Supplement.
Table 1. Baseline Characteristics of the Study Sample, Overall and by Quartiles of the Total Intake of Meat, Poultry, and Fish.
| Variable | No. (%) | ||||
|---|---|---|---|---|---|
| Total (N = 29 682) | Quartile 1 (n = 7423) | Quartile 2 (n = 7387) | Quartile 3 (n = 7451) | Quartile 4 (n = 7421) | |
| Age, mean (SD), y | 53.7 (15.7) | 55.3 (16.6) | 53.7 (15.1) | 53.6 (14.7) | 52.3 (16.3) |
| Sex | |||||
| Female | 16 514 (55.6) | 4840 (65.2) | 4389 (59.4) | 3945 (52.9) | 3340 (45.0) |
| Male | 13 168 (44.4) | 2583 (34.8) | 2998 (40.6) | 3506 (47.1) | 4081 (55.0) |
| Race/ethnicity | |||||
| Non-Hispanic white | 20 581 (69.3) | 5082 (68.5) | 5415 (73.3) | 5377 (72.2) | 4707 (63.4) |
| Non-Hispanic black | 7004 (23.6) | 1413 (19.0) | 1482 (20.1) | 1705 (22.9) | 2404 (32.4) |
| Hispanic | 1348 (4.5) | 618 (8.3) | 311 (4.2) | 218 (2.9) | 201 (2.7) |
| Chinese | 731 (2.5) | 305 (4.1) | 178 (2.4) | 147 (2.0) | 101 (1.4) |
| Othera | 18 (0.1) | 5 (0.1) | 1 (0) | 4 (0.1) | 8 (0.1) |
| Educational level | |||||
| <High school | 5541 (18.7) | 1417 (19.1) | 1194 (16.2) | 1298 (17.4) | 1632 (22.0) |
| High school | 8461 (28.5) | 2078 (28.0) | 2142 (29.0) | 2132 (28.6) | 2109 (28.4) |
| ≥Some college | 15 680 (52.8) | 3928 (52.9) | 4051 (54.8) | 4021 (54.0) | 3680 (49.6) |
| Smoking status | |||||
| Never | 14 772 (49.8) | 4021 (54.2) | 3749 (50.8) | 3613 (48.5) | 3389 (45.7) |
| Former | 8853 (29.8) | 2144 (28.9) | 2254 (30.5) | 2215 (29.7) | 2240 (30.2) |
| Current | 6057 (20.4) | 1258 (16.9) | 1384 (18.7) | 1623 (21.8) | 1792 (24.1) |
| Physical activity z score, median (IQR) | −0.2 (−0.7 to 0.4) | −0.2 (−0.7 to 0.4) | −0.2 (−0.7 to 0.4) | −0.2 (−0.7 to 0.5) | −0.2 (−0.7 to 0.5) |
| BMI, median (IQR) | 26.2 (23.3 to 29.7) | 25.5 (22.7 to 29.0) | 26.1 (23.3 to 29.4) | 26.5 (23.6 to 30.0) | 26.8 (23.8 to 30.4) |
| SBP, mean (SD), mm Hg | 123.0 (20.1) | 122.9 (21.1) | 122.1 (19.7) | 123.2 (19.8) | 123.7 (19.7) |
| HDL-C, mean (SD), mg/dL | 52.2 (15.7) | 53.5 (15.6) | 52.7 (15.9) | 51.7 (15.7) | 50.8 (15.3) |
| Non-HDL-C, mean (SD), mg/dL | 151.2 (41.8) | 147.4 (41.2) | 151.7 (42.1) | 153.5(41.9) | 152.4 (41.7) |
| With diabetes | 2570 (8.7) | 567 (7.6) | 566 (7.7) | 648 (8.7) | 789 (10.6) |
| Using antihypertension drug | 7613 (25.6) | 2001 (27.0) | 1823 (24.7) | 1948 (26.1) | 1841 (24.8) |
| Using lipid-lowering drug | 1659 (5.6) | 579 (7.8) | 432 (5.8) | 378 (5.1) | 270 (3.6) |
| Using hormone therapy | 2598 (8.8) | 838 (11.3) | 738 (10.0) | 622 (8.3) | 400 (5.4) |
| Total energy, median (IQR), kcal/d | 1678 (1260 to 2227) | 1225 (943 to 1586) | 1525 (1207 to 1907) | 1781 (1431 to 2227) | 2319 (1847 to 2985) |
| Meat, poultry, and fish intake, median (IQR), servings/wkb | 9.9 (6.4 to 14.4) | 4.6 (3.4 to 5.6) | 8.2 (7.4 to 9.0) | 11.9 (10.9 to 13.0) | 18.4 (16.0 to 22.4) |
| Processed meat intake, median (IQR), servings/wk | 1.5 (0.5 to 3.8) | 0.5 (0 to 1.0) | 1.1 (0.5 to 2.0) | 2.0 (0.9 to 4.0) | 4.6 (2.1 to 7.9) |
| Unprocessed red meat intake, median (IQR), servings/wk | 3.0 (1.5 to 5.0) | 1.3 (0.7 to 2.0) | 2.5 (1.5 to 3.9) | 3.9 (2.3 to 5.5) | 5.9 (3.9 to 8.2) |
| Poultry intake, median (IQR), servings/wk | 2.0 (1.0 to 3.0) | 1.0 (0.5 to 1.5) | 1.9 (1.0 to 3.0) | 2.6 (1.0 to 3.3) | 3.0 (2.0 to 5.0) |
| Fish intake, median (IQR), servings/wkb | 1.6 (0.9 to 3.4) | 0.9 (0.4 to 1.5) | 1.5 (0.9 to 2.6) | 2.0 (1.0 to 3.7) | 3.3 (1.5 to 5.9) |
| Alcohol intake, median (IQR), g/d | 0.6 (0 to 7.7) | 0.1 (0 to 5.0) | 0.9 (0 to 7.5) | 0.9 (0 to 8.7) | 1.3 (0 to 10.8) |
| AHEI-2010 score, mean (SD)c | 44.6 (10.1) | 45.2 (9.9) | 45.1 (10.0) | 44.3 (10.0) | 43.6 (10.5) |
| Incident CVDd | |||||
| No. of events | 6963 | 1482 | 1557 | 1857 | 2067 |
| Follow-up years | 528 218 | 129 120 | 133 166 | 132 693 | 133 239 |
| Rate per 1000 person-years | 13.2 | 11.5 | 11.7 | 14.0 | 15.5 |
| All-cause mortality | |||||
| No. of events | 8875 | 2024 | 1991 | 2265 | 2595 |
| Follow-up years | 562 624 | 136 294 | 140 770 | 141 996 | 143 563 |
| Rate per 1000 person-years | 15.8 | 14.9 | 14.1 | 16.0 | 18.1 |
Abbreviations: AHEI, Alternate Healthy Eating Index; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CVD, cardiovascular disease; HDL-C, high-density lipoprotein cholesterol; IQR, interquartile range; SBP, systolic blood pressure.
SI conversion factor: To convert HDL-C level to millimoles per liter, multiply by 0.0259.
For participants who reported mixed race/ethnicity or checked the Other option for the race/ethnicity questions.
Included shellfish intake.
Unprocessed red meat and processed meat were excluded from the calculation. The original version of the AHEI-2010 has a score range of 0 to 110 points. In the present study, the AHEI-2010 score range was 0 to 100 points because the meat item was removed. The higher the AHEI-2010 score, the higher the diet quality. Currently, no cutoff score has been established for defining high or low diet quality based on the AHEI-2010 score. A score around 40 to 50 was considered as poor diet quality, according to a study using data from the National Health and Nutrition Examination Survey.22
Included fatal and nonfatal coronary heart disease, fatal and nonfatal stroke, fatal and nonfatal heart failure, and other CVD deaths.
Primary Outcomes
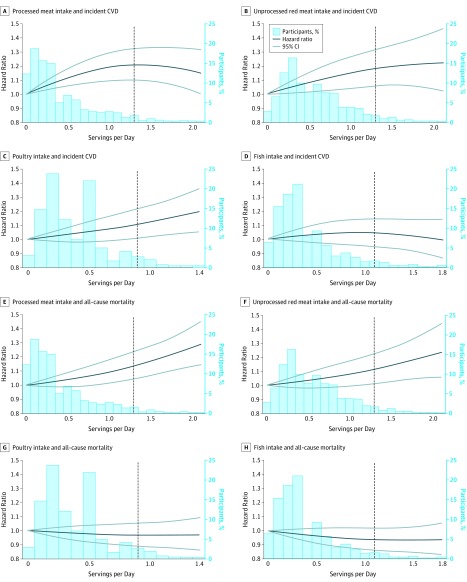
During a total of 562 624 follow-up years, 6963 incident CVD events (2687 coronary heart disease events [38.6%], 1740 stroke events [25.0%], 2366 heart failure events [34.0%], and 170 other CVD deaths [2.4%]) and 8875 all-cause deaths occurred. The median (IQR) follow-up duration was 19.0 (14.1-23.7) years. The associations of processed meat, unprocessed red meat, poultry, or fish intake with incident CVD and all-cause mortality were monotonic (P for nonlinearity ≥.25), except for the association between processed meat intake and incident CVD (P for nonlinearity = .006) (Figure 1).
Figure 1. Associations of Meat, Poultry, or Fish Intake With Incident Cardiovascular Disease (CVD) and All-Cause Mortality .
Cohort-stratified cause-specific hazard models for incident CVD and cohort-stratified standard proportional hazards models for all-cause mortality were applied. The models were further stratified by age groups, sex, and race/ethnicity for panel E and by sex for panel F, to satisfy proportional hazards assumption. The dotted vertical line indicates the 95th percentile cutoff. The distribution is shown up to the 99th percentile. All panels were created with the fully adjusted models specified in the Methods. P values for the quadratic term of the food intake were P = .006 for A, P = .35 for B, P = .62 for C, P = .25 for D, P = .36 for E, P = .57 for F, P = .63 for G, and P = .48 for H.
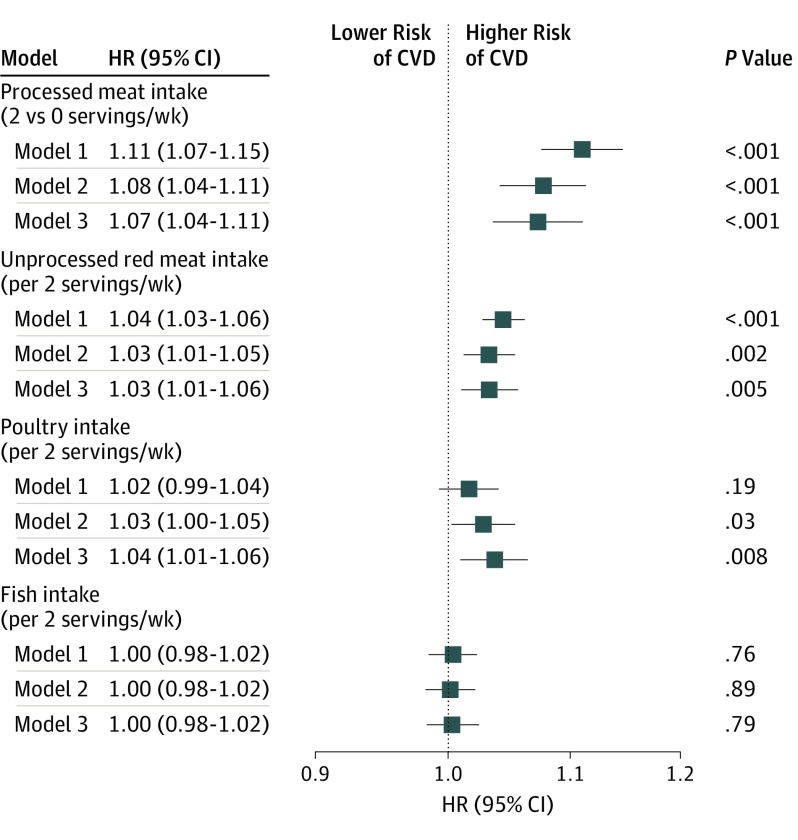
Based on the most fully adjusted model 3, processed meat intake was statistically significantly associated with incident CVD (comparing 2 vs 0 servings per week; HR, 1.07 [95% CI, 1.04-1.11]; 30-year ARD, 1.74% [95% CI, 0.85%-2.63%]) (Figure 2 and Table 2). Each additional 2 servings of unprocessed red meat consumed per week was significantly associated with incident CVD (HR, 1.03 [95% CI, 1.01-1.06]; 30-year ARD, 0.62% [95% CI, 0.07%-1.16%]). Each additional 2 servings of poultry consumed per week was significantly associated with incident CVD (HR, 1.04 [95% CI, 1.01-1.06]; 30-year ARD, 1.03% [95% CI, 0.36%-1.70%]). Each additional 2 servings of fish consumed per week was not significantly associated with incident CVD (HR, 1.00 [95% CI, 0.98-1.02]; 30-year ARD, 0.12% [95% CI, −0.40% to 0.65%]).
Figure 2. Associations of Meat, Poultry, or Fish Intake With Incident Cardiovascular Disease (CVD).
All models were stratified by cohort. Model 1 was adjusted for age, sex, race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, Chinese, or other), and educational level (less than high school, high school, or some college or higher). Model 2 was adjusted for model 1 variables plus total energy, smoking status (current, former, or never), smoking pack-years (0, 0.1-4.9, 5.0-9.9, 10-19.9, 20-29.9, 30-39.9, or ≥40), cohort-specific physical activity z score, alcohol intake (grams), and hormone therapy (yes or no). Model 3 was adjusted for model 2 variables plus fruits, legumes, potatoes, other vegetables excluding legumes and potatoes, nuts and seeds, whole grains, refined grains, low-fat dairy products, high-fat dairy products, sugar-sweetened beverages, eggs, and 3 of the 4 food types (processed meat, unprocessed red meat, poultry, and fish); a term of processed meat squared was also included. HR indicates hazard ratio.
Table 2. Absolute Risk Difference for the Associations of Meat, Poultry, or Fish Intake With Incident Cardiovascular Disease and All-Cause Mortalitya.
| Variable | Absolute Risk Difference (95% CI) | |||
|---|---|---|---|---|
| Processed Meat Intakeb | Unprocessed Red Meat Intake | Poultry Intake | Fish Intake | |
| 10-y Risk Difference, % | ||||
| Incident CVDc | ||||
| Model 1d | 0.63 (0.44 to 0.82) | 0.25 (0.15 to 0.34) | 0.10 (−0.05 to 0.25) | 0.02 (−0.10 to 0.14) |
| Model 2e | 0.44 (0.24 to 0.63) | 0.18 (0.06 to 0.30) | 0.16 (0.02 to 0.31) | 0.01 (−0.10 to 0.13) |
| Model 3f | 0.40 (0.19 to 0.62) | 0.17 (0.05 to 0.29) | 0.20 (0.05 to 0.35) | 0.01 (−0.11 to 0.14) |
| All-cause mortality | ||||
| Model 1d | 0.28 (0.22 to 0.34) | 0.21 (0.15 to 0.27) | −0.11 (−0.21 to −0.01) | −0.04 (−0.12 to 0.04) |
| Model 2e | 0.16 (0.09 to 0.22) | 0.10 (0.03 to 0.17) | −0.08 (−0.18 to 0.03) | −0.07 (−0.14 to −0.001) |
| Model 3f | 0.13 (0.06 to 0.19) | 0.11 (0.03 to 0.19) | −0.04 (−0.15 to 0.07) | −0.05 (−0.13 to 0.03) |
| 20-y Risk Difference, % | ||||
| Incident CVDc | ||||
| Model 1d | 1.50 (1.03 to 1.97) | 0.57 (0.33 to 0.82) | 0.28 (−0.07 to 0.63) | 0.07 (−0.22 to 0.36) |
| Model 2e | 1.09 (0.56 to 1.62) | 0.43 (0.13 to 0.73) | 0.45 (0.07 to 0.82) | 0.05 (−0.24 to 0.35) |
| Model 3f | 1.02 (0.50 to 1.55) | 0.41 (0.10 to 0.72) | 0.54 (0.14 to 0.93) | 0.05 (−0.28 to 0.37) |
| All-cause mortality | ||||
| Model 1d | 1.00 (0.81 to 1.20) | 0.75 (0.53 to 0.98) | −0.38 (−0.76 to −0.01) | −0.14 (−0.42 to 0.14) |
| Model 2e | 0.58 (0.35 to 0.82) | 0.38 (0.11 to 0.64) | −0.28 (−0.65 to 0.09) | −0.26 (−0.54 to 0.02) |
| Model 3f | 0.48 (0.25 to 0.71) | 0.40 (0.09 to 0.71) | −0.15 (−0.54 to 0.24) | −0.18 (−0.49 to 0.12) |
| 30-y Risk Difference, % | ||||
| Incident CVDc | ||||
| Model 1d | 2.32 (1.48 to 3.15) | 0.82 (0.41 to 1.23) | 0.61 (−0.002 to 1.22) | 0.15 (−0.36 to 0.66) |
| Model 2e | 1.81 (0.95 to 2.67) | 0.67 (0.13 to 1.21) | 0.90 (0.27 to 1.52) | 0.17 (−0.33 to 0.67) |
| Model 3f | 1.74 (0.85 to 2.63) | 0.62 (0.07 to 1.16) | 1.03 (0.36 to 1.70) | 0.12 (−0.40 to 0.65) |
| All-cause mortality | ||||
| Model 1d | 1.86 (1.46 to 2.26) | 1.40 (0.97 to 1.84) | −0.70 (−1.36 to −0.04) | −0.26 (−0.76 to 0.25) |
| Model 2e | 1.10 (0.66 to 1.53) | 0.71 (0.19 to 1.22) | −0.52 (−1.23 to 0.18) | −0.48 (−1.03 to 0.07) |
| Model 3f | 0.90 (0.43 to 1.38) | 0.76 (0.19 to 1.33) | −0.28 (−1.00 to 0.44) | −0.34 (−0.88 to 0.20) |
Abbreviation: CVD, cardiovascular disease.
Absolute risk difference was estimated using 3 R packages: riskRegression,15 pec,16 and survival.17 The 95% CI was derived from 500 bootstrap samples. A follow-up time of 10 years, 20 years, or 30 years; the mean value of the included covariates; and a difference of 2 servings per week in consumption of these 4 food types were used.
For estimating the absolute risk difference of the association between processed meat intake and incident CVD, a quadratic term for processed meat intake was used in addition to its original linear term. The associations were monotonic for the other 3 food types. For processed meat, the comparison was 2 servings per week vs 0 serving per week. For the other 3 food types, the comparison was an intake difference of 2 servings per week (eg, 2 vs 0 servings per week, 3 vs 1 servings per week, etc.).
A composite end point of fatal and nonfatal coronary heart disease, fatal and nonfatal stroke, fatal and nonfatal heart failure, and other cardiovascular deaths.
Model 1 was adjusted for cohort, age, sex, race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, Chinese, or other), and educational level (less than high school, high school, or some college or higher).
Model 2 was adjusted for model 1 variables plus total energy, smoking status (current, former, or never), smoking pack-years (0, 0.1-4.9, 5.0-9.9, 10-19.9, 20-29.9, 30-39.9, or ≥40), cohort-specific physical activity z score, alcohol intake (grams), and hormone therapy (yes or no).
Model 3 was adjusted for model 2 variables plus fruits, legumes, potatoes, other vegetables excluding legumes and potatoes, nuts and seeds, whole grains, refined grains, low-fat dairy products, high-fat dairy products, sugar-sweetened beverages, eggs, and 3 of the 4 food types (processed meat, unprocessed red meat, poultry, and fish); a term of processed meat squared was also included when analyzing the incident CVD outcome.
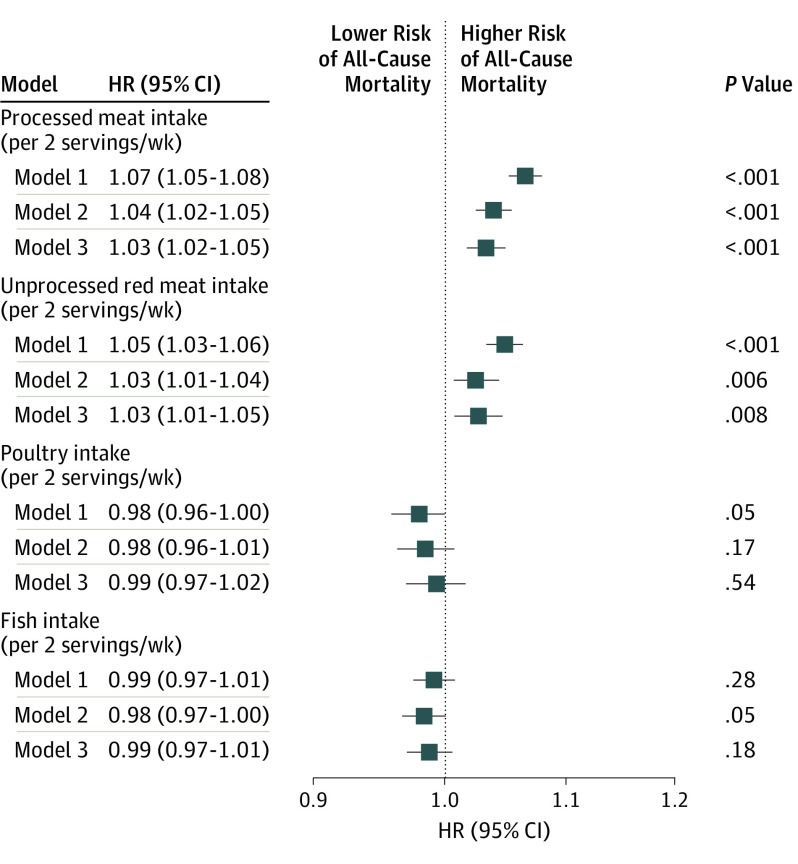
Based on the most fully adjusted model 3, each additional 2 servings of processed meat consumed per week was statistically significantly associated with all-cause mortality (HR, 1.03 [95% CI, 1.02-1.05]; 30-year ARD, 0.90% [95% CI, 0.43%-1.38%]) (Figure 3 and Table 2). Each additional 2 servings of unprocessed red meat consumed per week was significantly associated with all-cause mortality (HR, 1.03 [95% CI, 1.01-1.05]; 30-year ARD, 0.76% [95% CI, 0.19%-1.33%]). Each additional 2 servings of poultry consumed per week was not significantly associated with all-cause mortality (HR, 0.99 [95% CI, 0.97-1.02]; 30-year ARD, −0.28% [95% CI, −1.00% to 0.44%]). Each additional 2 servings of fish consumed per week was not significantly associated with all-cause mortality (HR, 0.99 [95% CI, 0.97-1.01]; 30-year ARD, −0.34% [95% CI, −0.88% to 0.20%]).
Figure 3. Associations of Meat, Poultry, or Fish Intake With All-Cause Mortality.
All models were stratified by cohort. Model 1 was adjusted for age, sex, race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, Chinese, or other), and educational level (less than high school, high school, or some college or higher). Model 2 was adjusted for model 1 variables plus total energy, smoking status (current, former, or never), smoking pack-years (0, 0.1-4.9, 5.0-9.9, 10-19.9, 20-29.9, 30-39.9, or ≥40), cohort-specific physical activity z score, alcohol intake (grams), and hormone therapy (yes or no). Model 3 was adjusted for model 2 variables plus fruits, legumes, potatoes, other vegetables excluding legumes and potatoes, nuts and seeds, whole grains, refined grains, low-fat dairy products, high-fat dairy products, sugar-sweetened beverages, eggs, and 3 of the 4 food types (processed meat, unprocessed red meat, poultry, and fish). HR indicates hazard ratio.
For these associations, the increased relative risks ranged from approximately 3% to 7%. The increased absolute risks were less than 2% over the 30 years of follow-up.
Subgroup Analyses
The strength of the association between processed meat intake and incident CVD decreased with age (HR, 1.17 [95% CI, 1.01-1.36] for adults aged <45 years; 1.12 [95% CI, 1.07-1.17] for those aged 45-64 years; 1.00 [95% CI, 0.95-1.05] for those aged ≥65 years; P for interaction = .008) (eFigure 1 in the Supplement). The association between unprocessed red meat intake and incident CVD was stronger in participants who consumed a higher-quality diet compared with those who did not (HR, 1.09 [95% CI, 1.05-1.14] vs 1.02 [95% CI, 0.99-1.04]; P for interaction < .001) (eFigure 2 in the Supplement) and was stronger in participants who consumed a non-high-fat diet compared with those who did not (HR, 1.06 [95% CI, 1.03-1.09] vs 0.99 [95% CI, 0.96-1.03]; P for interaction < .001). The association between poultry intake and incident CVD was stronger in participants with a non-high-fat diet compared with those without such a diet (HR, 1.06 [95% CI, 1.03-1.09] vs 0.98 [95% CI, 0.93-1.03]; P for interaction = .004) (eFigure 3 in the Supplement). The association between fish intake and incident CVD was stronger in participants who consumed a high-protein diet than those who did not (HR, 0.96 [95% CI, 0.93-0.99] vs 1.02 [95% CI, 0.99-1.05]; P for interaction = .002) (eFigure 4 in the Supplement). The associations of processed meat, unprocessed red meat, poultry, or fish intake with all-cause mortality were similar across all subgroups (P for interaction ≥ .01) (eFigures 5-8 in the Supplement).
Sensitivity Analyses
The included participants were different from their excluded (primarily because of missing data) counterparts (eg, mean [SD] age, 53.7 [15.7] years vs 59.3 [15.4] years; non-Hispanic black, 7004 [23.6%] vs 1698 [35.2%]) (eTable 2 in the Supplement). Results from analyzing imputed data were similar to those from the primary analyses of complete data (eTable 3 in the Supplement). Results remained qualitatively similar after excluding events ascertained within 2 or 5 years or after truncating follow-up at 10 or 20 years, with 1 exception (HR, 0.95; 95% CI, 0.92-0.98) (eTable 4 in the Supplement). The inverse association between fish intake and all-cause mortality became statistically significant (HR, 0.95; 95% CI, 0.92-0.98) when follow-up was truncated at 10 years, after excluding any 1 of the 6 cohorts (eTable 5 in the Supplement), using cohort-specific quintiles of the intake (eTable 6 in the Supplement), and using energy density models (eTable 7 in the Supplement). When subdistribution hazard models were applied, the association between unprocessed red meat intake and incident CVD was no longer significant (HR, 1.02; 95% CI, 0.99-1.04) (eTable 8 in the Supplement). No significant difference was found between fatty fish and nonfatty fish intake in relation to incident CVD and all-cause mortality (eTable 9 in the Supplement).
Discussion
Among the 29 682 US adults followed up for a median of 19 years and up to 3 decades, higher intake of processed meat, unprocessed red meat, or poultry, but not fish, was significantly associated with a higher risk of incident CVD. Higher intake of processed meat or unprocessed red meat, but not poultry or fish, was significantly associated with a higher risk of all-cause mortality. The effect sizes of these association estimates were small.
Evidence from meta-analyses consistently revealed a significant positive association between processed meat intake and a range of CVD and mortality outcomes.23,24,25,26,27,28,29 However, the association with unprocessed red meat intake varied within and between health outcomes. Overall, the studies included in these meta-analyses were heterogeneous, with differences in consumption between the highest and lowest intake category, covariate adjustments, outcome definitions, and dietary assessment approaches. Nevertheless, the robust significant positive association between processed meat intake and health outcomes was retained despite the challenges introduced by these heterogeneities. This study harmonized diet and other data across 6 cohorts, which largely attenuated these heterogeneities. The significant positive associations of processed meat or unprocessed red meat intake with incident CVD and all-cause mortality remained after adjusting for a comprehensive list of covariates.
Poultry intake was significantly inversely associated with stroke in 1 meta-analysis29 but was not associated with all-cause and CVD mortality in another meta-analysis.24 The present study identified a significant positive association between poultry intake and incident CVD, which also remained in multiple sensitivity analyses. This association may be related to the poultry intake including fried chicken. Fried food consumption has been significantly positively associated with adverse outcomes.30 Food preparation methods were not consistently and universally assessed across the cohorts in this study. Therefore, separating fried chicken from poultry intake was not possible. Poultry intake was not significantly associated with all-cause mortality in this study. The reasons underlying the differential associations between poultry intake and different outcomes require further investigation.
This study did not reveal significant associations between fish intake and incident CVD and all-cause mortality. A major limitation was the lack of detail on food preparation methods (eg, fried vs nonfried). After the arbitrary truncation of follow-up time at 10 years in a sensitivity analysis, fish intake became significantly inversely associated with all-cause mortality. This finding may be by chance because the association between fish intake and incident CVD remained similar and not significant with truncation. Otherwise, the misclassification of fish intake using one-time measurement in this long-term prospective study may have biased the association with all-cause mortality toward null. However, fish consumption among US adults did not change from 1999 to 2016.3 Meta-analyses generally reported inverse associations between fish intake and CVD or mortality-related outcomes, but most of these associations were modest with P values close to .05.31,32 Uncertainty remains regarding the associations between fish intake and incident CVD and all-cause mortality.
The effect size estimates of association in this study were small but comparable with those reported in the literature.33 This study revealed approximately 3% to 7% higher relative risks and less than 2% higher absolute risks of incident CVD and all-cause mortality over the 30 years of follow-up. This finding is partly the result of using 2 servings per week as the unit of interpretation. People who consume more servings per week would have greater risks. Furthermore, risks of CVD and mortality are determined by a range of factors, including but not limited to genetic predisposition, demographic factors, socioeconomic status, weight, lifestyle factors (eg, smoking, sleep, physical activity, and diet), and the built environment. Greater intake of a single type of food is not likely independently associated with a substantially higher risk of incident CVD and all-cause mortality. In spite of the small effect sizes, findings of this study have critical public health implications because dietary behaviors are modifiable and most people consume these 4 food types on a daily or weekly basis.
Several subgroup differences were found, including the seemingly counterintuitive finding that unprocessed red meat intake was significantly positively associated with incident CVD only among participants who consumed a higher-quality diet. This subgroup finding does not conflict with evidence showing that people with a healthier diet have a lower CVD risk, thereby favoring adoption of healthy dietary behaviors.34 Rather, this subgroup finding suggests that participants also benefited from reducing their unprocessed red meat intake even if the overall quality of their diet was high. This result may further reflect the difficulty of identifying a small association in the context of lower-quality diet and higher-background CVD incidence. The age difference in the association between processed meat intake and incident CVD was not found in the NIH-AARP Diet and Health Study,35 but that study did not include younger adults (<50 years) for whom the association was the strongest in the present study. Reasons were unclear for the differences in the associations between unprocessed red meat or poultry intake and incident CVD according to various fat intake levels and for the differences in the associations between fish intake and incident CVD according to various protein intake levels. These subgroup findings warrant future investigations.
Limitations
This study has several limitations. First, measurement error was unavoidable for self-reported diet and other data. Measurement error may result in an overestimation or underestimation of an association. Second, more detailed diet data were unavailable on food preparation methods (eg, fried vs nonfried). Third, only 1 dietary measurement was used, but participants’ dietary behaviors may have changed over time. Robust results were seen when follow-up was truncated at different times, except for the association of fish intake with all-cause mortality. Fourth, a comprehensive set of confounders was considered, but residual confounding was still likely. Fifth, the data pertained to only US adults; thus, caution should be taken when generalizing the findings to other countries and to children. Sixth, this study could not establish causality.
Conclusions
This study’s findings suggest that among US adults, higher intake of processed meat, unprocessed red meat, or poultry, but not fish, was significantly associated with a small increased risk of incident CVD. Higher intake of processed meat or unprocessed red meat, but not poultry or fish, was significantly associated with a small increased risk of all-cause mortality.
eTable 1. Partial Correlation Coefficients Between the 4 Foods and Dietary and Other Lifestyle Factors
eTable 2. Key Characteristics Between the Included and Excluded Participants
eTable 3. Imputation Analysis for the Associations Between the 4 Foods and Incident CVD and All-Cause Mortality (n = 34504)
eTable 4. Associations Between the 4 Foods and Incident CVD and All-Cause Mortality After Excluding Early Events and Arbitrary Truncations
eTable 5. Associations Between the 4 Foods and Incident CVD and All-Cause Mortality After Excluding One Cohort
eTable 6. Associations Between Quintiles of the Intake for Each of the 4 Foods and Incident CVD and All-Cause Mortality
eTable 7. Energy Density Models for the Associations Between the 4 Foods and Incident CVD and All-Cause Mortality
eTable 8. Associations Between the 4 Foods and Incident CVD Using Subdistribution Hazard Models
eTable 9. Associations of Fatty Fish or Nonfatty Fish Intake With Incident CVD and All-Cause Mortality
eFigure 1. Association Between Processed Meat Intake (2 vs 0 Servings/Week) and Incident CVD Among Different Subgroups
eFigure 2. Association Between Each Additional 2 Servings of Unprocessed Red Meat Consumed per Week and Incident CVD Among Different Subgroups
eFigure 3. Association Between Each Additional 2 Servings of Poultry Consumed per Week and Incident CVD Among Different Subgroups
eFigure 4. Association Between Each Additional 2 Servings of Fish Consumed per Week and Incident CVD Among Different Subgroups
eFigure 5. Association Between Each Additional 2 Servings of Processed Meat Consumed per Week and All-Cause Mortality Among Different Subgroups
eFigure 6. Association Between Each Additional 2 Servings of Unprocessed Red Meat Consumed per Week and All-Cause Mortality Among Different Subgroups
eFigure 7. Association Between Each Additional 2 Servings of Poultry Consumed per Week and All-Cause Mortality Among Different Subgroups
eFigure 8. Association Between Each Additional 2 Servings of Fish Consumed per Week and All-Cause Mortality Among Different Subgroups
References
- 1.Pasiakos SM, Agarwal S, Lieberman HR, Fulgoni VL III. Sources and amounts of animal, dairy, and plant protein intake of US adults in 2007-2010. Nutrients. 2015;7(8):7058-7069. doi: 10.3390/nu7085322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu Z, McClure ST, Appel LJ. Dietary cholesterol intake and sources among U.S. adults: results from National Health and Nutrition Examination Surveys (NHANES), 2001-2014. Nutrients. 2018;10(6):E771. doi: 10.3390/nu10060771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zeng L, Ruan M, Liu J, et al. . Trends in processed meat, unprocessed red meat, poultry, and fish consumption in the United States, 1999-2016. J Acad Nutr Diet. 2019;119(7):1085-1098.e12. doi: 10.1016/j.jand.2019.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mozaffarian D. Dietary and policy priorities for cardiovascular disease, diabetes, and obesity: a comprehensive review. Circulation. 2016;133(2):187-225. doi: 10.1161/CIRCULATIONAHA.115.018585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilkins JT, Karmali KN, Huffman MD, et al. . Data resource profile: the Cardiovascular Disease Lifetime Risk Pooling Project. Int J Epidemiol. 2015;44(5):1557-1564. doi: 10.1093/ije/dyv150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stevens J, Metcalf PA, Dennis BH, Tell GS, Shimakawa T, Folsom AR. Reliability of a food frequency questionnaire by ethnicity, gender, age and education. Nutr Res. 1996;16(5):735-745. doi: 10.1016/0271-5317(96)00064-4 [DOI] [Google Scholar]
- 7.Liu K, Slattery M, Jacobs D Jr, et al. . A study of the reliability and comparative validity of the cardia dietary history. Ethn Dis. 1994;4(1):15-27. [PubMed] [Google Scholar]
- 8.Posner BM, Martin-Munley SS, Smigelski C, et al. . Comparison of techniques for estimating nutrient intake: the Framingham Study. Epidemiology. 1992;3(2):171-177. doi: 10.1097/00001648-199203000-00016 [DOI] [PubMed] [Google Scholar]
- 9.Mayer-Davis EJ, Vitolins MZ, Carmichael SL, et al. . Validity and reproducibility of a food frequency interview in a multi-cultural epidemiology study. Ann Epidemiol. 1999;9(5):314-324. doi: 10.1016/S1047-2797(98)00070-2 [DOI] [PubMed] [Google Scholar]
- 10.Kumanyika S, Tell GS, Fried L, Martel JK, Chinchilli VM. Picture-sort method for administering a food frequency questionnaire to older adults. J Am Diet Assoc. 1996;96(2):137-144. doi: 10.1016/S0002-8223(96)00042-9 [DOI] [PubMed] [Google Scholar]
- 11.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135(10):1114-1126. doi: 10.1093/oxfordjournals.aje.a116211 [DOI] [PubMed] [Google Scholar]
- 12.Zhong VW, Van Horn L, Cornelis MC, et al. . Associations of dietary cholesterol or egg consumption with incident cardiovascular disease and mortality. JAMA. 2019;321(11):1081-1095. doi: 10.1001/jama.2019.1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lau B, Cole SR, Gange SJ. Competing risk regression models for epidemiologic data. Am J Epidemiol. 2009;170(2):244-256. doi: 10.1093/aje/kwp107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin DY, Wei LJ, Ying Z. Checking the Cox model with cumulative sums of Martingale-based residuals. Biometrika. 1993;80(3):557-572. doi: 10.1093/biomet/80.3.557 [DOI] [Google Scholar]
- 15.Package riskRegression [software]. Version 2019.11.03. https://cran.r-project.org/web/packages/riskRegression/riskRegression.pdf. Accessed November 17, 2019.
- 16.Package pec [software]. Version 2019.11.03. https://cran.r-project.org/web/packages/pec/pec.pdf. Accessed November 17, 2019.
- 17.Package survival [software]. Version 3.1-8. https://cran.r-project.org/web/packages/survival/survival.pdf. Accessed November 17, 2019.
- 18.Chiuve SE, Fung TT, Rimm EB, et al. . Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142(6):1009-1018. doi: 10.3945/jn.111.157222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.SAS. The PHREG procedure. Type 3 tests and joint tests. http://support.sas.com/documentation/cdl/en/statug/67523/HTML/default/viewer.htm#statug_phreg_details32.htm. Accessed November 17, 2019.
- 20.Buse A. The likelihood ratio, Wald, and Lagrange multiplier tests: an expository note. Am Stat. 1982;36(3, pt 1):153-157. doi: 10.2307/2683166 [DOI] [Google Scholar]
- 21.White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30(4):377-399. doi: 10.1002/sim.4067 [DOI] [PubMed] [Google Scholar]
- 22.Wang DD, Leung CW, Li Y, et al. . Trends in dietary quality among adults in the United States, 1999 through 2010. JAMA Intern Med. 2014;174(10):1587-1595. doi: 10.1001/jamainternmed.2014.3422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larsson SC, Orsini N. Red meat and processed meat consumption and all-cause mortality: a meta-analysis. Am J Epidemiol. 2014;179(3):282-289. doi: 10.1093/aje/kwt261 [DOI] [PubMed] [Google Scholar]
- 24.Abete I, Romaguera D, Vieira AR, Lopez de Munain A, Norat T. Association between total, processed, red and white meat consumption and all-cause, CVD and IHD mortality: a meta-analysis of cohort studies. Br J Nutr. 2014;112(5):762-775. doi: 10.1017/S000711451400124X [DOI] [PubMed] [Google Scholar]
- 25.Yang C, Pan L, Sun C, Xi Y, Wang L, Li D. Red meat consumption and the risk of stroke: a dose-response meta-analysis of prospective cohort studies. J Stroke Cerebrovasc Dis. 2016;25(5):1177-1186. doi: 10.1016/j.jstrokecerebrovasdis.2016.01.040 [DOI] [PubMed] [Google Scholar]
- 26.Wang X, Lin X, Ouyang YY, et al. . Red and processed meat consumption and mortality: dose-response meta-analysis of prospective cohort studies. Public Health Nutr. 2016;19(5):893-905. doi: 10.1017/S1368980015002062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Micha R, Michas G, Mozaffarian D. Unprocessed red and processed meats and risk of coronary artery disease and type 2 diabetes—an updated review of the evidence. Curr Atheroscler Rep. 2012;14(6):515-524. doi: 10.1007/s11883-012-0282-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cui K, Liu Y, Zhu L, Mei X, Jin P, Luo Y. Association between intake of red and processed meat and the risk of heart failure: a meta-analysis. BMC Public Health. 2019;19(1):354. doi: 10.1186/s12889-019-6653-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim K, Hyeon J, Lee SA, et al. . Role of total, red, processed, and white meat consumption in stroke incidence and mortality: a systematic review and meta-analysis of prospective cohort studies. J Am Heart Assoc. 2017;6(9):e005983. doi: 10.1161/JAHA.117.005983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun Y, Liu B, Snetselaar LG, et al. . Association of fried food consumption with all cause, cardiovascular, and cancer mortality: prospective cohort study. BMJ. 2019;364:k5420. doi: 10.1136/bmj.k5420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Djoussé L, Akinkuolie AO, Wu JH, Ding EL, Gaziano JM. Fish consumption, omega-3 fatty acids and risk of heart failure: a meta-analysis. Clin Nutr. 2012;31(6):846-853. doi: 10.1016/j.clnu.2012.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jayedi A, Shab-Bidar S, Eimeri S, Djafarian K. Fish consumption and risk of all-cause and cardiovascular mortality: a dose-response meta-analysis of prospective observational studies. Public Health Nutr. 2018;21(7):1297-1306. doi: 10.1017/S1368980017003834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeraatkar D, Han MA, Guyatt GH, et al. . Red and processed meat consumption and risk for all-cause mortality and cardiometabolic outcomes: a systematic review and meta-analysis of cohort studies [published online October 1, 2019]. Ann Intern Med. [DOI] [PubMed] [Google Scholar]
- 34.Schwingshackl L, Bogensberger B, Hoffmann G. Diet quality as assessed by the Healthy Eating Index, Alternate Healthy Eating Index, Dietary Approaches to Stop Hypertension Score, and health outcomes: an updated systematic review and meta-analysis of cohort studies. J Acad Nutr Diet. 2018;118(1):74-100.e11. doi: 10.1016/j.jand.2017.08.024 [DOI] [PubMed] [Google Scholar]
- 35.Etemadi A, Sinha R, Ward MH, et al. . Mortality from different causes associated with meat, heme iron, nitrates, and nitrites in the NIH-AARP Diet and Health Study: population based cohort study. BMJ. 2017;357:j1957. doi: 10.1136/bmj.j1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Partial Correlation Coefficients Between the 4 Foods and Dietary and Other Lifestyle Factors
eTable 2. Key Characteristics Between the Included and Excluded Participants
eTable 3. Imputation Analysis for the Associations Between the 4 Foods and Incident CVD and All-Cause Mortality (n = 34504)
eTable 4. Associations Between the 4 Foods and Incident CVD and All-Cause Mortality After Excluding Early Events and Arbitrary Truncations
eTable 5. Associations Between the 4 Foods and Incident CVD and All-Cause Mortality After Excluding One Cohort
eTable 6. Associations Between Quintiles of the Intake for Each of the 4 Foods and Incident CVD and All-Cause Mortality
eTable 7. Energy Density Models for the Associations Between the 4 Foods and Incident CVD and All-Cause Mortality
eTable 8. Associations Between the 4 Foods and Incident CVD Using Subdistribution Hazard Models
eTable 9. Associations of Fatty Fish or Nonfatty Fish Intake With Incident CVD and All-Cause Mortality
eFigure 1. Association Between Processed Meat Intake (2 vs 0 Servings/Week) and Incident CVD Among Different Subgroups
eFigure 2. Association Between Each Additional 2 Servings of Unprocessed Red Meat Consumed per Week and Incident CVD Among Different Subgroups
eFigure 3. Association Between Each Additional 2 Servings of Poultry Consumed per Week and Incident CVD Among Different Subgroups
eFigure 4. Association Between Each Additional 2 Servings of Fish Consumed per Week and Incident CVD Among Different Subgroups
eFigure 5. Association Between Each Additional 2 Servings of Processed Meat Consumed per Week and All-Cause Mortality Among Different Subgroups
eFigure 6. Association Between Each Additional 2 Servings of Unprocessed Red Meat Consumed per Week and All-Cause Mortality Among Different Subgroups
eFigure 7. Association Between Each Additional 2 Servings of Poultry Consumed per Week and All-Cause Mortality Among Different Subgroups
eFigure 8. Association Between Each Additional 2 Servings of Fish Consumed per Week and All-Cause Mortality Among Different Subgroups