Key Points
Question
What can DSM-IV–based and Research Domain Criteria–based analytic approaches contribute to the understanding of threat and extinction learning in individuals with anxiety disorders?
Findings
In this cross-sectional study of 114 adults with and without anxiety disorders, the categorical DSM-IV–based approach indicated that all groups of individuals with an anxiety disorder, irrespective of DSM-IV diagnosis, exhibited hypoactivation in the ventromedial prefrontal cortex during extinction recall. The dimensional research domain criteria–based approach revealed that higher arousal to the unconditioned stimulus during threat learning was associated with higher threat responses during extinction recall.
Meaning
Personalized medicine may be better served with the inclusion of both DSM-IV–based and research domain criteria–based approaches, as they provide distinct yet complementary information.
Abstract
Importance
The Research Domain Criteria project of the National Institute of Mental Health aims to guide neuropsychiatry toward precision medicine. Its inception was partly in response to the overlap of clinical manifestations between different DSM-IV diagnoses within a category. For example, anxiety disorders comprise a DSM-IV category that includes diagnoses that differ from each other but are all characterized by dysregulated fear levels. Whether DSM-IV–based and Research Domain Criteria–based analytic approaches provide distinct or similar information with regard to the fear circuitry of individuals with anxiety disorders has not been directly tested.
Objective
To use a threat conditioning and extinction protocol to conduct categorical (DSM-IV–based) and dimensional (Research Domain Criteria–based) assessments of psychophysiological, neural, and psychometric responses in individuals with and without anxiety disorders.
Design, Setting, and Participants
This cross-sectional study was conducted at the Athinoula A. Martinos Center for Biomedical Imaging at Massachusetts General Hospital in Boston between March 2013 and May 2015. Functional magnetic resonance imaging was used to assess psychophysiological, neural, and psychometric responses among adults aged 18 to 65 years with specific phobia, generalized anxiety disorder, social anxiety disorder, and panic disorder as well as a control group of adults without anxiety disorders. Data were analyzed between May 2018 and April 2019.
Exposures
A 2-day threat conditioning and extinction protocol.
Main Outcomes and Measures
Skin conductance responses and blood oxygenated level–dependent responses were measured during the threat and extinction protocol. The categorical analysis was performed by grouping participants based on their primary DSM-IV diagnosis. The dimensional analysis was performed by regrouping participants, irrespective of their diagnoses, based on their skin conductance responses to shock delivery during threat conditioning.
Results
This cross-sectional study of 114 adults aged 18 to 65 years included 93 participants (34 men and 59 women; mean [SD] age, 29.7 [11.1] years) with at least 1 anxiety disorder (specific phobia, generalized anxiety disorder, social anxiety disorder, or panic disorder) and 21 participants (11 men and 10 women) without an anxiety disorder. The categorical DSM-IV–based approach indicated that all anxiety disorder groups exhibited hypoactivation in the ventromedial prefrontal cortex during extinction recall (ηp2 = 0.15; P = .004). The Research Domain Criteria–based approach revealed that higher arousal to the unconditioned stimulus was associated with higher threat responses during extinction recall (for skin conductance responses, ηp2 = 0.21; P = .01 and in functional magnetic resonance imaging results, ηp2 = 0.12; P = .02). The direct comparison of DSM-IV–based vs Research Domain Criteria–based results did not yield significant findings (ηp2 values ranged from 0.02 to 0.078; P values ranged from .09 to .98), suggesting no overlap between the approaches.
Conclusions and Relevance
The data obtained from both approaches indicated complementary yet distinct findings. The findings highlight the validity and importance of using both categorical and dimensional approaches to optimize understanding of the etiology and treatment of anxiety symptoms.
This cross-sectional study uses a threat conditioning and extinction protocol to conduct categorical (DSM-IV–based) and transdiagnostic dimensional (research domain criteria–based) assessments of psychophysiological, neural, and psychometric responses in adults with and without anxiety disorders.
Introduction
Anxiety disorders, such as specific phobia, social anxiety disorder, generalized anxiety disorder, and panic disorder, differ from each other based on various DSM-IV criteria ranging from the focus or trigger of the anxiety to the context and durability of the anxious response and its associated symptoms.1 The threat conditioning and extinction paradigm has been used to study anxiety and fear-based disorders because of its relevance to the etiology, the sustainability of symptoms, and the exposure-based cognitive behavioral therapies used to treat these disorders.2,3,4,5,6 Threat conditioning and extinction are modulated by a network of brain regions, including the insular cortex, amygdala, hippocampus, dorsal anterior cingulate cortex (dACC), and ventromedial prefrontal cortex (vmPFC).2,6,7 The functional activation of these brain regions has been reported to be impaired within the context of threat extinction learning and retention across a number of disorders, including posttraumatic stress disorder (PTSD),8,9,10,11,12 obsessive-compulsive disorder,13 and anxiety disorders.14,15
Despite the knowledge gained thus far, psychiatry is lagging behind other medical fields, and there is an increasing need for precision medicine. To overcome this gap, the National Institute of Mental Health has launched the Research Domain Criteria (RDoC) project,16 which emphasizes a multimodal approach to delineate links between neurobiological measures, clinical behaviors, and trait factors that are present across conventional diagnostic categories. The RDoC project therefore aims to identify biological markers underlying disease processes, irrespective of the diagnosis. Specific constructs identified by the RDoC project, such as acute threat (fear), are relevant to the study of anxiety. Studies have reported associations between brain activations in response to acute threat and transdiagnostic anxious and depressive symptomatology.17,18 Moreover, measuring characteristics that promote excessive anxiety and general negative emotionality could help the understanding of the factors modulating key regions that are implicated in the neurobiology of anxiety disorders. For example, neuroticism, which has been associated with anxious and depressed behaviors,19 has also been negatively associated with extinction memory and medial orbitofrontal cortex thickness.20 Thus, transdiagnostic and dimensional approaches have yielded informative data regarding the psychobehavioral mechanisms of anxiety.
These data, however, do not offer a clear view of whether the RDoC-based approach offers advantages or new insights that exceed the knowledge gained using the DSM-IV–based categories. Direct comparison of the 2 approaches has not been widely used in the study of anxiety disorders. Moreover, several RDoC pillars (eg, using biological measures to predict dysfunction or grouping anew using an investigator-determined variable, irrespective of the disorder) have not been tested. This exploratory study aims to shed light on the use of the RDoC pillars and to compare categorical and transdiagnostic dimensional approaches in individuals with and without anxiety disorders.
Methods
This cross-sectional study was conducted at the Athinoula A. Martinos Center for Biomedical Imaging at Massachusetts General Hospital in Boston between March 2013 and May 2015. We recruited 114 adults aged 18 to 65 years from the general community and through referrals from specialized anxiety clinics at Massachusetts General Hospital (the Center for Anxiety and Traumatic Stress Disorders) and McLean Hospital.
Of those, 93 participants were diagnosed with at least 1 of the following: specific phobia (n = 20), social anxiety disorder (n = 28), generalized anxiety disorder (n = 27), or panic disorder (n = 18). The remaining 21 participants without an anxiety disorder were assigned to the control group. Exclusion criteria included a history of seizures or severe head trauma, current substance abuse or dependency, metal implants, pregnancy, and breastfeeding (additional exclusion criteria available in the eMethods in the Supplement). Data were analyzed between May 2018 and April 2019. The study was approved by the institutional review board of Partners HealthCare. Written informed consent was obtained from all participants.
We used a 2-day threat conditioning and extinction protocol (eFigure 1 in the Supplement). On day 1, participants were exposed to threat conditioning, during which 2 cues were paired with a shock (CS+) and 1 cue was not paired with a shock (CS-). Participants then underwent extinction learning, during which 1 CS+ was presented without reinforcement (CS+E) along with the CS-. The next day, extinction memory recall was tested by presenting participants with all 3 cues (CS+, CS-, and CS+E). Skin conductance responses and blood oxygenated level–dependent signals in the fear network (the amygdala, hippocampus, insular cortex, dACC, and vmPFC) were recorded during the protocol (eMethods and eFigure 1 in the Supplement).
Participants completed the following 5 questionnaires: the Anxiety Sensitivity Index, a 36-item survey that measures the basic dimensions and hierarchic structure of anxiety sensitivity (scale range, 1-5, with 1 indicating strong disagreement and 5 indicating strong agreement);21 the Beck Anxiety Inventory, a 21-item multiple-choice self-report questionnaire that measures the severity of anxiety (score range, 0-63, with 0 indicating minimal anxiety and 63 indicating severe anxiety);22 the Beck Depression Inventory, a 21-item multiple-choice self-report questionnaire that measures the severity of depression (score range, 0-63, with 0 indicating minimal depression and 63 indicating severe depression);23 the State-Trait Anxiety Inventory–Trait Anxiety Scale, a 20-item self-report survey that uses a 4-point Likert scale to measure trait anxiety (ie, anxiety as a personal characteristic; score range, 20-80, with 20 indicating low anxiety and 80 indicating high anxiety);24 and the NEO Five-Factor Inventory, a 60-item self-report inventory that uses a 5-point Likert scale to measure the 5 primary dimensions (neuroticism, extraversion, openness, agreeableness, and conscientiousness) of personality (5 separate domain scores ranging from 12-60, with 12 indicating the lowest endorsement of the trait and 60 indicating the highest endorsement of the trait).25
Skin conductance responses and imaging data were computed as previously described.8,10,26,27,28,29 For the composite of anxiety, mood, and personality traits, a composite measure was derived from the scores obtained on the 5 questionnaires (eMethods and eFigure 1 in the Supplement).
Analytic Approach
For the DSM-IV–based approach, 5 groups were created based on the individual’s primary diagnosis (specific phobia, social anxiety disorder, generalized anxiety disorder, or panic disorder) or absence of an anxiety disorder diagnosis (control group; eFigure 1 in the Supplement). For all analyses pertaining to this DSM-IV–based approach, age and years of education were used as covariates.
For the RDoC-based approach, we grouped anew all of the individuals with an anxiety disorder based on their skin conductance responses to shock delivery (eFigure 1 in the Supplement). Irrespective of the DSM-IV diagnosis, we artificially created 4 groups based on the level of arousal in response to shock, with group 1 exhibiting the lowest level of arousal, group 2 exhibiting a low to moderate level of arousal, group 3 exhibiting a moderate to high level of arousal, and group 4 exhibiting the highest level of arousal. This analysis excluded individuals in the control group, who were included in the DSM-IV–based approach as a reference point. For all of the RDoC-based analyses, age was used as a covariate (eMethods in the Supplement).
To compare the results obtained from the 2 approaches, we selected each cluster within the regions of interest for which a significant result was obtained and computed an analysis of variance for the alternative approach (eMethods in the Supplement).
Results
This cross-sectional study of 114 adults aged 18 to 65 years included 93 participants (34 men and 59 women; mean [SD] age, 29.7 [11.1] years) with at least 1 anxiety disorder (specific phobia, generalized anxiety disorder, social anxiety disorder, and panic disorder) and 21 participants (11 men and 10 women) without an anxiety disorder. Of those with at least 1 anxiety disorder, 20 participants were diagnosed with specific phobia, 28 with social anxiety disorder, 27 with generalized anxiety disorder, and 18 with panic disorder.
DSM-IV–Based Categorical Approach
Post hoc tests indicated that the specific phobia group was substantially older than all other groups (mean [SD] age, 38.7 [15.0] years vs 27.4 [10.9] years in the social anxiety group, 30.3 [9.9] years in the generalized anxiety group, 27.2 [8.7] years in the panic disorder group, and 25.8 [4.8] years in the control group; P ≤ .05 for all comparisons). The control group had substantially more years of education than the social anxiety disorder group (mean [SD] 17.4 [1.7] years vs 15.2 [2.4] years, respectively; P = .01; eResults and eFigure 2 in the Supplement).
Skin Conductance Response
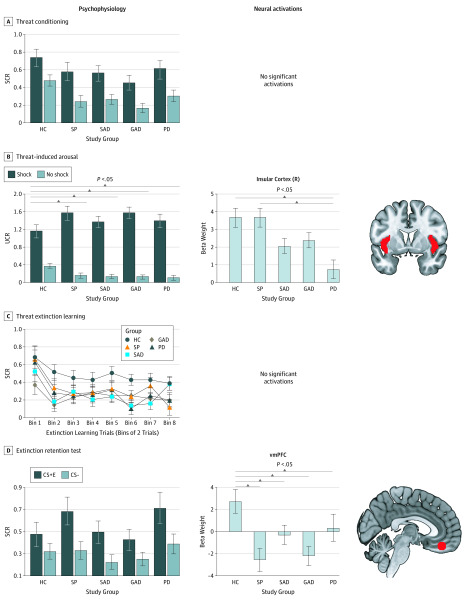
In the conditioned threat acquisition test, the stimulus (CS+ and CS-) × group analysis of covariance (ANCOVA) revealed a main effect of stimulus (F1,105 = 5.62; P = .02), indicating higher skin conductance response to the CS+ compared with the CS- stimulus (Figure 1A).
Figure 1. Psychophysiological and Neural Responses Based on Categorical (DSM-IV–Based) Comparison .
A-D, Psychophysiological and neural responses. The masks used for the different regions of interest are indicated in red. Error bars represent the SEM. CS- indicates cue with no shock; CS+E, cue with shock + extinction learning; GAD, generalized anxiety disorder; HC, healthy controls; PD, panic disorder; R, right; SAD, social anxiety disorder; SCR, skin conductance response; SP, specific phobia; UCR, unconditioned response; and vmPFC, ventromedial prefrontal cortex.
In the threat-induced arousal test, the stimulus (shock and no-shock) × group ANCOVA yielded a main effect of stimulus (F1,101 = 23.89; P = .001) and a significant interaction (F4,101 = 2.79; P = .03), which was in response to a group effect for the no-shock presentations (F4,101 = 4.32; P = .003). All anxiety disorder groups exhibited lower unconditioned responses to the shocks compared with the control group (P ≤ .008 for all comparisons; Figure 1B).
In the threat extinction learning test, the stimulus (CS+E and CS-) × time (early and late) × group ANCOVA revealed a marginal effect of time (F4.97,466.81 = 2.08; P = .07) and a significant effect of group (F4,94 = 3.59; P = .009), which was in response to the higher skin conductance responses of the control group throughout extinction compared with the other groups (P ≤ .05 for all comparisons; Figure 1C).
In the extinction memory retention test, the stimulus (CS+E and CS-) × group ANCOVA revealed no significant results (P ≥ .17 for all comparisons; Figure 1).
Functional Magnetic Resonance Imaging
In the conditioned threat acquisition analysis, no significant results were found in the CS+ vs CS- functional magnetic resonance imaging (fMRI) contrast (Figure 1A).
In the threat-induced arousal analysis during the shock vs no-shock fMRI contrast, a main effect of group was found in the right insular cortex (Montreal Neurological Institute [MNI] coordinates x, y, z = 36, 26, 6; cluster size = 157; F4104 = 8.80; probability of familywise error [pFWE] = 0.002; and search volume = 1770 voxels), with the panic disorder group having lower activation than the control and specific phobia groups (Figure 1B).
In the threat extinction learning analysis, no significant effects were detected in the CS+E vs CS- contrast during early or late extinction learning (Figure 1C).
In the extinction memory retention test, a main effect of group was found in the vmPFC of the early CS+E vs early CS- contrast, with all anxiety disorder groups having lower vmPFC activations compared with the control group (MNI coordinates x, y, z = 4, 36, −14; cluster size = 6; F4,96 = 5.26; pFWE = 0.024; search volume = 248 voxels; Figure 1D). When running the overall ANOVA using the extracted beta weights to evaluate the between-group differences, the overall ANOVA was ηp2 = 0.15 and P = .004.
RDoC-Based Dimensional Approach
With regard to the demographic characterization of the new grouping, which was based on the level of threat-induced arousal (with group 1 exhibiting the lowest level and group 4 exhibiting the highest level of arousal), participants in groups 1 and 2 (mean [SD] age, 36.6 [13.4] years and 31.9 [12.7] years, respectively) were older than those in group 4 (mean [SD] age, 22.9 [3.6] years; P < .04; eResults and eFigure 3 in the Supplement).
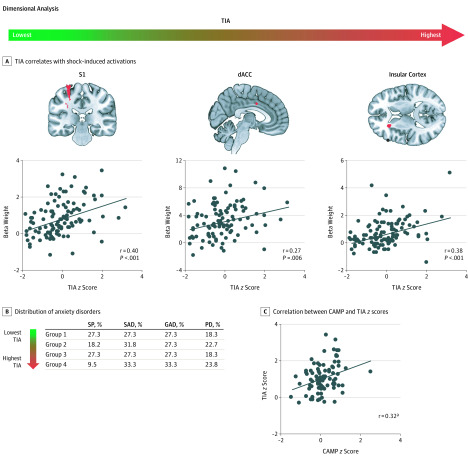
To validate the RDoC-based grouping variable, a whole-brain exploratory analysis was performed during the shock vs no-shock fMRI contrast using threat-induced arousal as the regressor. This analysis revealed a positive association with the primary somatosensory cortex, dACC, and insular cortex activations (for somatosensory cortex activation, MNI coordinates x, y, z = −28, −30, 66; cluster size = 53; t102 = 3.11; pFWE = 0.03; for dACC activation, MNI coordinates x, y, z = 2, 4, 36; cluster size = 13; t102 = 2.87; pFWE = 0.05; and for insular cortex activation, MNI coordinates x, y, z = −26, 28, 14; cluster size = 94; t102 = 3.91; pFWE = 0.003; Figure 2A). The newly formed groups did not differ in their distribution of anxiety disorders (Figure 2B). Across all participants with anxiety disorders, a significant association was found between the z scores of threat-induced arousal and the composite of anxiety, mood, and personality traits (r85 = 0.32; P = .002; Figure 2C). A split-half reliability analysis performed on odd and even shock presentations yielded a Spearman-Brown coefficient of 0.98.
Figure 2. Characterization of Groups Based on Transdiagnostic Dimensional (RDoC-Based) Approach.
A, Threat-induced arousal (TIA). Shown are voxelwise analyses of TIA (measured with skin conductance responses to shock delivery) z scores indicating brain activations during shock delivery. B, Shown is the distribution of anxiety disorders. Distribution across the 4 groups based on the level of threat-induced arousal (expressed as percentage). C, Composite of anxiety, mood, and personality (CAMP) traits and TIA z scores. The red areas indicate significant activations. Error bars represent the SEM. dACC indicates dorsal anterior cingulate cortex; GAD, generalized anxiety disorder; PD, panic disorder; S1, somatosensory cortex; SAD, social anxiety disorder; and SP, specific phobia.
aP < .05.
Skin Conductance Response
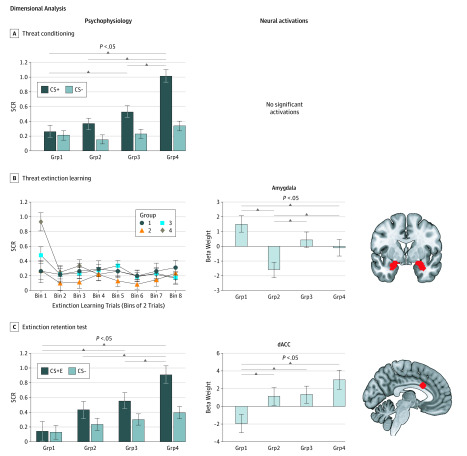
In the conditioned threat acquisition test, the stimulus (CS+ and CS-) × group ANCOVA revealed a stimulus effect (F1,81 = 11.59; P = .001), a group effect (F3,81 = 8.06; P < .001), and a significant interaction (F3,81 = 18.6; P < .001), which was in response to a group effect to the CS+ (F3,81 = 14.94; P = .001), with group 4 having higher skin conductance responses compared with all other groups and group 3 having higher skin conductance responses compared with group 1 (Figure 3A).
Figure 3. Psychophysiological and Neural Responses for Dimensional (RDoC-Based) Comparison.
A-C, Shown are skin conductance responses (SCRs) for threat conditioning, the threat extinction learning test, and the extinction memory retention test. Shown are extracted beta weights for significant differences in blood oxygenated level–dependent signal. Data for shock delivery are not displayed because groups 1 to 4 (Grp1-Grp4) were formed based on that measurement standard, and no significant findings were obtained for the no-shock replacements (SCR) or for the shock vs no-shock functional magnetic resonance imaging contrast. Grp1 to Grp4 are based on the skin conductance levels obtained in response to shock (threat-induced arousal). The masks used for the different regions of interest are indicated in red. The 16 extinction trials binned by 2 resulted in 8 points. Error bars indicate the SEM. CS+ indicates cue with shock; CS-, cue with no shock; CS+E, cue with shock + extinction learning; and dACC, dorsal anterior cingulate cortex.
In the threat-induced arousal test, the stimulus (shock and no-shock) × group ANCOVA revealed a main effect of stimulus (F1,82 = 101.89; P < .001), a group effect (F3,82 = 63.82; P < .001), and a significant interaction (F3,82 = 99.06; P < .001), which was in response to a group effect to the shocks (which was irrelevant because the new groups were formed based on skin conductance responses to the shocks).
In the threat extinction learning test, the stimulus (CS+E and CS-) × time (early and late) × group ANCOVA revealed a time by group interaction (F15.69,366.09 = 1.76; P = .04), which was in response to a significant effect of time for group 4 (F4.58,91.54 = 4.81; P = .001; Figure 3B).
In the extinction memory retention test, the stimulus (CS+E and CS-) × group ANCOVA yielded a main effect of group (F3,67 = 4.54; P = .006) and a stimulus × group interaction (F3,67 = 3.98; P = .01). The interaction was in response to a group effect for the CS+E stimulus (F3,67 = 5.92; P = .001; ηp2 = 0.21), with group 4 exhibiting higher skin conductance responses compared with all other groups (P ≤ .03 for all comparisons) and group 3 having higher skin conductance responses than group 1 (P = .02; Figure 3C).
Functional Magnetic Resonance Imaging
In both the conditioned threat acquisition (CS+ vs CS-) analysis and the threat-induced arousal (shock vs no-shock) analysis, no significant group effect was found in the shock vs no-shock fMRI contrast (Figure 3A).
In the threat extinction learning analysis, a main effect of group was found in the amygdala (MNI coordinates x, y, z = 26, 2, −20; cluster size = 24; F3,80 = 6.63; pFWE = 0.02; search volume = 248 voxels) during early extinction in the CS+E vs CS- contrast, with group 2 having lower activation than the other groups (P ≤ .05 for all comparisons) and group 4 having lower activation than group 1 (P = .05). No group differences were found during late extinction in the CS+E vs CS- contrast (Figure 3B).
In the extinction memory retention test, a main effect of group was found in the dACC (MNI coordinates x, y, z = 0, 18, 28; cluster size = 1; F3,75 = 4.78; pFWE = 0.048; search volume = 81 voxels) of the early CS+E vs early CS- contrast, with group 1 having lower dACC activation compared with the other 3 groups (P ≤ .03 for all comparisons; Figure 3C). Extracted beta weights from the dACC were associated with the composite of anxiety, mood, and personality traits (r = 0.29; P = .008; data not shown). When using the extracted beta weights to evaluate the between-group differences, the overall ANOVA was ηp2 = 0.12 and P = .02.
Categorical Analysis vs Dimensional Grouping
With regard to significant insular cortex activation in the shock vs no-shock fMRI contrast, the ANCOVA using the RDoC-based grouping did not reveal a main effect of group (F3,81 = 0.55; P = .65; eFigure 4 in the Supplement). The significant difference observed in the amygdala during extinction learning did not translate into a significant group effect when using the DSM-IV–based grouping (F3,79 = 2.23; ηp2 = 0.078; P = .09; eFigure 4 in the Supplement). During extinction recall, the significant vmPFC activation did not yield a main effect when using the RDoC-based grouping (F3,74 = 0.06; ηp2 = 0.02; P = .98; eFigure 4 in the Supplement). The dACC activation revealed by the threat-induced arousal grouping during extinction recall was not significant when using the DSM-IV–based approach (F3,74 = 0.9, P = .45; eFigure 4 in the Supplement).
Both higher threat-induced arousal and higher composite of anxiety, mood, and personality scores were associated with lower vmPFC activations during the various phases of the protocol. For detailed results of the whole-brain exploratory analyses using threat-induced arousal and the composite of anxiety, mood, and personality traits as regressors, see eResults and eFigure 5 in the Supplement.
Discussion
We examined the psychophysiological and neural mechanisms of threat conditioning, threat extinction, and extinction memory retention in individuals with and without anxiety disorders. We used 2 integrative analytic approaches: DSM-IV–based categories and RDoC-based dimensions (with skin conductance response to shock delivery as our measurement standard). The analytic approaches revealed different yet complementary results associated with activations within the threat extinction network. Yet, the results from the direct comparison of both approaches revealed no overlap. These results highlight the usefulness of both the RDoC-based and DSM-IV–based approaches, at least as they relate to anxiety disorders and the negative valence and arousal systems.
The categorical approach provides novel data given that, to our knowledge, no single study has examined these 4 anxiety disorders using the same experimental task. Most studies have either compared 1 particular anxiety disorder with a cohort of individuals without anxiety disorders or have compared individuals with anxiety disorders (regardless of the disorder) with individuals without anxiety disorders.30,31 One major finding of our DSM-IV–based approach is the hypoactivation of the vmPFC during threat extinction memory retention as a common dysfunction in each of the 4 anxiety disorders tested. Our study and others have previously reported vmPFC hypoactivations during threat extinction retention in individuals with anxiety disorders14 and other psychopathologies, notably PTSD, obsessive-compulsive disorder, and schizophrenia.8,10,12,13,32 Activation of the vmPFC has been associated with emotional regulation across multiple tasks in humans33,34,35,36,37 and with a reduction in conditioned responses in rodents.38 Individuals with a dysfunctional vmPFC are therefore likely to experience emotional rigidity or the inability to switch or regulate emotional responses.
Another key finding revealed by the DSM-IV–based analytic approach is that individuals in the control group had higher skin conductance responses to the no-shock presentations and during extinction learning, suggesting a blunted physiology in individuals with an anxiety disorder under some circumstances. In the same manner, we found that insular cortex activation was dampened across individuals with social anxiety disorder, generalized anxiety disorder, and panic disorder during the shock vs no-shock fMRI contrast, with the most prominent impairment in the panic disorder group. Moreover, threat-induced arousal was positively associated with insular cortex activations in the shock vs no-shock fMRI contrast.
The insular cortex has connections with multiple regions of the fear network, including the amygdala, the anterior cingulate, and the prefrontal cortices,39,40 and it is involved in interoception and in the linking of sensory experiences with emotions.39,40 It is likely that individuals with generalized anxiety disorder, social anxiety disorder, and panic disorder feel the shock delivery to a similar degree as those with a specific phobia and those without an anxiety disorder, but they may not attribute a negative valence to the shock to the same extent as the other groups. This sensory-emotion disconnection could likely play a role in dissociative states or numbness-related symptoms that could be observed, especially in individuals with panic disorder. It is also possible that this disconnection could be associated with an individual’s cognitive flexibility, which could reflect itself during extinction recall. In support of this possibility, studies have reported that the insular cortex is involved in both fear-related and extinction-related memories given its role in prediction error.41,42,43,44 This finding suggests that networks promoting fear and safety likely coexist within the insular cortex and that dysregulated activations of the insular cortex could promote either anxiogenic or anxiolytic patterns.
The transdiagnostic dimensional approach indicated that the groups exhibiting greater levels of threat-induced arousal also had higher physiological reactivity to the CS+E stimulus and greater activation in the dACC, a fear-promoting region. It is important to note that this higher arousal was not present during all phases of the protocol. Therefore, arousal in response to shock appears to specifically be associated with extinction memory retention, both in terms of psychophysiological and neural measurement standards. Extinction memory retention deficits are of high relevance to clinical outcomes. Our results suggest that threat-induced arousal could be used to identify individuals who may be at greater risk of relapse after therapy. Our results also highlight the importance of testing the association of arousal levels with clinical outcomes after exposure-based therapies. In support of this idea, a recent study has reported that skin conductance response measured in the aftermath of trauma was associated with the subsequent development of PTSD.45
One interesting finding that emerged from the RDoC-based approach is that the group with the lowest threat-induced arousal score exhibited higher amygdala activation during threat extinction learning. Although this may appear surprising, it is important to point out that this difference was observed during early threat extinction learning when the extinction memory had not yet been formed. Moreover, the role of the amygdala in extinction learning has been reported to be primordial.2 These results suggest that individuals with the lowest threat-induced arousal levels engage their amygdala early during extinction learning, which may promote a more adaptive learning of the safety memory, as was observed the next day during extinction memory testing.
Notably, the threat-induced arousal measurement standard that we propose is based on unconditioned responses to shock delivery, as measured by skin conductance response. As discussed in a previous study, it is necessary to have measurement standards with good reliability in the context of an RDoC-based framework.46 Our research group has previously demonstrated good reliability for such a measurement standard,47 further highlighting the relevance of using threat-induced arousal as a measure. The current study’s split-half reliability was high, adding weight to the reliability of the threat-induced arousal index. We considered creating RDoC-based groups using other biological measurement standards (ie, brain activations), but the data were not robust or consistent, which may be owing to the lower reliability of such a measurement standard relative to threat-induced arousal.
Another important and novel observation from our data is that our results do not favor 1 method over another (categorical vs dimensional). The results obtained with 1 method did not translate into significant differences when the second method was used. This finding highlights the importance of integrating multiple layers of information, such as neural, psychophysiological, clinical, and self-reported measures, to make a convergent use of these data to better understand individual differences and nuances that are present across disorders.
Based on our results, we propose an integrative working model in an attempt to begin conceptualizing the importance and functionality of each of the brain nodes within a clinical setting. The model first highlights the positive association between physiological reactivity to aversive cues (threat-induced arousal) and the composite of anxiety, mood, and personality traits (our self-reported measurement standards). Individuals who exhibit high levels of threat-induced arousal and/or have high composite scores of anxiety, mood, and personality traits are also likely to exhibit increased activation of regions involved in feeling, monitoring, and expressing fear, such as the dACC, insular cortex, and somatosensory cortex. Concurrently, the increased threat expression appears to be associated with reduced vmPFC and dACC activations. These later neurobiological impairments may be associated with emotional inflexibility, which is commonly observed in individuals with anxiety. When learning about threat regulation, these individuals tend to engage the amygdala and, to a lesser degree, the vmPFC, which are 2 key structures that are well known for their role in extinction learning. During threat extinction memory retention, this emotional inflexibility would be reflected by less engagement of safety-promoting regions, such as the vmPFC, but more engagement of the posterior hippocampus, which has been associated with more fear expression.
Limitations
This study had several limitations. One limitation was that groups were not matched for age and years of education. Because these factors have been associated with conditionability,48 we decided to include them as covariates in all analyses. The current results therefore necessitate replication in matched samples. Future studies should more closely monitor data pertaining to psychotropic medications and evaluate whether the use of these medications changes the results. Notably, results obtained from the DSM-IV–based analytic approach were based on the participants' primary diagnoses irrespective of comorbidities. Moreover, our sample size allowed us to detect medium to large effect sizes. It is possible that smaller effects may have been undetected. Another important point is that the current analyses were mostly exploratory, which warrants replication of the findings.
Conclusions
To our knowledge, this is the first study to use both categorical and dimensional analytic approaches in a sample of individuals with anxiety disorders. Our results suggest that personalized medicine could be better served with the inclusion of both approaches, as they provide distinct and complementary information.
eMethods. Participants, Paradigm, Data Processing, Analytic Approach, DSM-IV vs RDoC
eResults. Sample Characterization for DSM-IV Approach, Sample Characterization for RDoC Approach, and Whole-Brain Analysis
eFigure 1. Schematic Representation of the Experimental Protocol, the Measures, and the 2 Analytic Approaches
eFigure 2. Characterization of the Groups Based on the Categorical Approach
eFigure 3. Characterization of the Groups Based on the Dimensional Approach
eFigure 4. Comparison of Categorial vs Dimensional Analytic Approach
eFigure 5. Voxelwise Analyses of TIA and CAMP Scores Across the Different Phases of the Threat and Extinction Protocol
References
- 1.American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders. 4th ed Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 2.Milad MR, Quirk GJ. Fear extinction as a model for translational neuroscience: ten years of progress. Annu Rev Psychol. 2012;63:129-151. doi: 10.1146/annurev.psych.121208.131631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vervliet B, Craske MG, Hermans D. Fear extinction and relapse: state of the art. Annu Rev Clin Psychol. 2013;9:215-248. doi: 10.1146/annurev-clinpsy-050212-185542 [DOI] [PubMed] [Google Scholar]
- 4.Gillihan SJ, Foa EB. Fear extinction and emotional processing theory In: Schachtman TR, Reilly SS, eds. Associative Learning and Conditioning Theory. Oxford, UK: Oxford University Press; 2011:27-43. doi: 10.1093/acprof:oso/9780199735969.003.0017 [DOI] [Google Scholar]
- 5.Pitman RK, Rasmusson AM, Koenen KC, et al. Biological studies of post-traumatic stress disorder. Nat Rev Neurosci. 2012;13(11):769-787. doi: 10.1038/nrn3339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35(1):169-191. doi: 10.1038/npp.2009.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.VanElzakker MB, Dahlgren MK, Davis FC, Dubois S, Shin LM. From Pavlov to PTSD: the extinction of conditioned fear in rodents, humans, and anxiety disorders. Neurobiol Learn Mem. 2014;113:3-18. doi: 10.1016/j.nlm.2013.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Milad MR, Pitman RK, Ellis CB, et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry. 2009;66(12):1075-1082. doi: 10.1016/j.biopsych.2009.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Helpman L, Marin MF, Papini S, et al. Neural changes in extinction recall following prolonged exposure treatment for PTSD: a longitudinal fMRI study. Neuroimage Clin. 2016;12:715-723. doi: 10.1016/j.nicl.2016.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marin MF, Song H, VanElzakker MB, et al. Association of resting metabolism in the fear neural network with extinction recall activations and clinical measures in trauma-exposed individuals. Am J Psychiatry. 2016;173(9):930-938. doi: 10.1176/appi.ajp.2015.14111460 [DOI] [PubMed] [Google Scholar]
- 11.Garfinkel SN, Abelson JL, King AP, et al. Impaired contextual modulation of memories in PTSD: an fMRI and psychophysiological study of extinction retention and fear renewal. J Neurosci. 2014;34(40):13435-13443. doi: 10.1523/JNEUROSCI.4287-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rougemont-Bucking A, Linnman C, Zeffiro TA, et al. Altered processing of contextual information during fear extinction in PTSD: an fMRI study. CNS Neurosci Ther. 2011;17(4):227-236. doi: 10.1111/j.1755-5949.2010.00152.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Milad MR, Furtak SC, Greenberg JL, et al. Deficits in conditioned fear extinction in obsessive-compulsive disorder and neurobiological changes in the fear circuit. JAMA Psychiatry. 2013;70(6):608-618. doi: 10.1001/jamapsychiatry.2013.914 [DOI] [PubMed] [Google Scholar]
- 14.Marin MF, Zsido RG, Song H, et al. Skin conductance responses and neural activations during fear conditioning and extinction recall across anxiety disorders. JAMA Psychiatry. 2017;74(6):622-631. doi: 10.1001/jamapsychiatry.2017.0329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Britton JC, Grillon C, Lissek S, et al. Response to learned threat: an fMRI study in adolescent and adult anxiety. Am J Psychiatry. 2013;170(10):1195-1204. doi: 10.1176/appi.ajp.2013.12050651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Insel T, Cuthbert B, Garvey M, et al. Research Domain Criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167(7):748-751. doi: 10.1176/appi.ajp.2010.09091379 [DOI] [PubMed] [Google Scholar]
- 17.MacNamara A, Klumpp H, Kennedy AE, Langenecker SA, Phan KL. Transdiagnostic neural correlates of affective face processing in anxiety and depression. Depress Anxiety. 2017;34(7):621-631. doi: 10.1002/da.22631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo L, Becker B, Zheng X, et al. A dimensional approach to determine common and specific neurofunctional markers for depression and social anxiety during emotional face processing. Hum Brain Mapp. 2018;39(2):758-771. doi: 10.1002/hbm.23880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jylha P, Isometsa E. The relationship of neuroticism and extraversion to symptoms of anxiety and depression in the general population. Depress Anxiety. 2006;23(5):281-289. doi: 10.1002/da.20167 [DOI] [PubMed] [Google Scholar]
- 20.Rauch SL, Milad MR, Orr SP, Quinn BT, Fischl B, Pitman RK. Orbitofrontal thickness, retention of fear extinction, and extraversion. Neuroreport. 2005;16(17):1909-1912. doi: 10.1097/01.wnr.0000186599.66243.50 [DOI] [PubMed] [Google Scholar]
- 21.Taylor S, Cox BJ. An expanded anxiety sensitivity index: evidence for a hierarchic structure in a clinical sample. J Anxiety Disord. 1998;12(5):463-483. doi: 10.1016/S0887-6185(98)00028-0 [DOI] [PubMed] [Google Scholar]
- 22.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56(6):893-897. doi: 10.1037/0022-006X.56.6.893 [DOI] [PubMed] [Google Scholar]
- 23.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561-571. doi: 10.1001/archpsyc.1961.01710120031004 [DOI] [PubMed] [Google Scholar]
- 24.Spielberger CD, Gorsuch RL, Lushene RE, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory (Form Y1-Y2). Mountain View, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 25.Costa PT, McCrae RR. NEO PI-R Professional Manual. Lutz, FL: Psychological Assessment Resources; 1992. [Google Scholar]
- 26.Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry. 2007;62(5):446-454. doi: 10.1016/j.biopsych.2006.10.011 [DOI] [PubMed] [Google Scholar]
- 27.Milad MR, Quirk GJ, Pitman RK, Orr SP, Fischl B, Rauch SL. A role for the human dorsal anterior cingulate cortex in fear expression. Biol Psychiatry. 2007;62(10):1191-1194. doi: 10.1016/j.biopsych.2007.04.032 [DOI] [PubMed] [Google Scholar]
- 28.Milad MR, Orr SP, Lasko NB, Chang Y, Rauch SL, Pitman RK. Presence and acquired origin of reduced recall for fear extinction in PTSD: results of a twin study. J Psychiatr Res. 2008;42(7):515-520. doi: 10.1016/j.jpsychires.2008.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Linnman C, Zeidan MA, Furtak SC, Pitman RK, Quirk GJ, Milad MR. Resting amygdala and medial prefrontal metabolism predicts functional activation of the fear extinction circuit. Am J Psychiatry. 2012;169(4):415-423. doi: 10.1176/appi.ajp.2011.10121780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duits P, Cath DC, Lissek S, et al. Updated meta-analysis of classical fear conditioning in the anxiety disorders. Depress Anxiety. 2015;32(4):239-253. doi: 10.1002/da.22353 [DOI] [PubMed] [Google Scholar]
- 31.Lissek S, Powers AS, McClure EB, et al. Classical fear conditioning in the anxiety disorders: a meta-analysis. Behav Res Ther. 2005;43(11):1391-1424. doi: 10.1016/j.brat.2004.10.007 [DOI] [PubMed] [Google Scholar]
- 32.Holt DJ, Coombs G, Zeidan MA, Goff DC, Milad MR. Failure of neural responses to safety cues in schizophrenia. Arch Gen Psychiatry. 2012;69(9):893-903. doi: 10.1001/archgenpsychiatry.2011.2310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Etkin A, Buchel C, Gross JJ. The neural bases of emotion regulation. Nat Rev Neurosci. 2015;16(11):693-700. doi: 10.1038/nrn4044 [DOI] [PubMed] [Google Scholar]
- 34.Andrewes DG, Jenkins LM. The role of the amygdala and the ventromedial prefrontal cortex in emotional regulation: implications for post-traumatic stress disorder. Neuropsychol Rev. 2019;29(2):220-243. doi: 10.1007/s11065-019-09398-4 [DOI] [PubMed] [Google Scholar]
- 35.Delgado MR, Nearing KI, Ledoux JE, Phelps EA. Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron. 2008;59(5):829-838. doi: 10.1016/j.neuron.2008.06.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J Neurosci. 2007;27(33):8877-8884. doi: 10.1523/JNEUROSCI.2063-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Urry HL, van Reekum CM, Johnstone T, et al. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. J Neurosci. 2006;26(16):4415-4425. doi: 10.1523/JNEUROSCI.3215-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vidal-Gonzalez I, Vidal-Gonzalez B, Rauch SL, Quirk GJ. Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learn Mem. 2006;13(6):728-733. doi: 10.1101/lm.306106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Uddin LQ, Nomi JS, Hebert-Seropian B, Ghaziri J, Boucher O. Structure and function of the human insula. J Clin Neurophysiol. 2017;34(4):300-306. doi: 10.1097/WNP.0000000000000377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Namkung H, Kim SH, Sawa A. The insula: an underestimated brain area in clinical neuroscience, psychiatry, and neurology. Trends Neurosci. 2017;40(4):200-207. doi: 10.1016/j.tins.2017.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gogolla N. The insular cortex. Curr Biol. 2017;27(12):R580-R586. doi: 10.1016/j.cub.2017.05.010 [DOI] [PubMed] [Google Scholar]
- 42.Casanova JP, Madrid C, Contreras M, Rodriguez M, Vasquez M, Torrealba F. A role for the interoceptive insular cortex in the consolidation of learned fear. Behav Brain Res. 2016;296:70-77. doi: 10.1016/j.bbr.2015.08.032 [DOI] [PubMed] [Google Scholar]
- 43.Berret E, Kintscher M, Palchaudhuri S, et al. Insular cortex processes aversive somatosensory information and is crucial for threat learning. Science. 2019;364(6443):eaaw0474. doi: 10.1126/science.aaw0474 [DOI] [PubMed] [Google Scholar]
- 44.Greco JA, Liberzon I. Neuroimaging of fear-associated learning. Neuropsychopharmacology. 2016;41(1):320-334. doi: 10.1038/npp.2015.255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hinrichs R, van Rooij SJ, Michopoulos V, et al. Increased skin conductance response in the immediate aftermath of trauma predicts PTSD risk. Chronic Stress (Thousand Oaks). 2019;3:247054701984444. doi: 10.1177/2470547019844441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rodebaugh TL, Scullin RB, Langer JK, et al. Unreliability as a threat to understanding psychopathology: the cautionary tale of attentional bias. J Abnorm Psychol. 2016;125(6):840-851. doi: 10.1037/abn0000184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zeidan MA, Lebron-Milad K, Thompson-Hollands J, et al. Test-retest reliability during fear acquisition and fear extinction in humans. CNS Neurosci Ther. 2012;18(4):313-317. doi: 10.1111/j.1755-5949.2011.00238.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rosenbaum BL, Bui E, Marin MF, et al. Demographic factors predict magnitude of conditioned fear. Int J Psychophysiol. 2015;98(1):59-64. doi: 10.1016/j.ijpsycho.2015.06.010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Participants, Paradigm, Data Processing, Analytic Approach, DSM-IV vs RDoC
eResults. Sample Characterization for DSM-IV Approach, Sample Characterization for RDoC Approach, and Whole-Brain Analysis
eFigure 1. Schematic Representation of the Experimental Protocol, the Measures, and the 2 Analytic Approaches
eFigure 2. Characterization of the Groups Based on the Categorical Approach
eFigure 3. Characterization of the Groups Based on the Dimensional Approach
eFigure 4. Comparison of Categorial vs Dimensional Analytic Approach
eFigure 5. Voxelwise Analyses of TIA and CAMP Scores Across the Different Phases of the Threat and Extinction Protocol