This systematic review and meta-analysis compares the rates of complications associated with autologous vs homologous vs Tutoplast cartilage grafts in patients undergoing dorsal augmentation rhinoplasty.
Key Points
Question
Is there a difference in the rate of complications associated with autologous vs homologous cartilage grafts in patients undergoing dorsal augmentation rhinoplasty?
Findings
In this systematic review and meta-analysis of 28 retrospective cohort studies comprising 1041 patients, no difference in outcomes was found between autologous and homologous costal cartilage grafts, including rates of warping, resorption, infection, contour irregularity, or revision surgery.
Meaning
Autologous and homologous cartilage grafts may be equivalent options for dorsal augmentation rhinoplasty with respect to multiple outcomes.
Abstract
Importance
Augmentation rhinoplasty requires adding cartilage to provide enhanced support to the structure of the nose. Autologous costal cartilage and irradiated homologous costal cartilage (IHCC) are well-accepted rhinoplasty options. Tutoplast is another alternative cartilage source. No studies, to our knowledge, have definitively demonstrated a higher rate of complications with IHCC grafts compared with autologous costal cartilage grafts.
Objective
To compare rates of outcomes in the published literature for patients undergoing septorhinoplasty with autologous costal cartilage vs IHCC grafts vs Tutoplast grafts.
Data Sources
For this systematic review and meta-analysis, the MEDLINE, Embase, Scopus, Cochrane Central Register of Controlled Trials, and ClinicalTrials.gov databases were searched for articles published from database inception to February 2019 using the following keywords: septorhinoplasty, rhinoplasty, autologous costal cartilage graft, cadaveric cartilage graft, and rib graft.
Study Selection
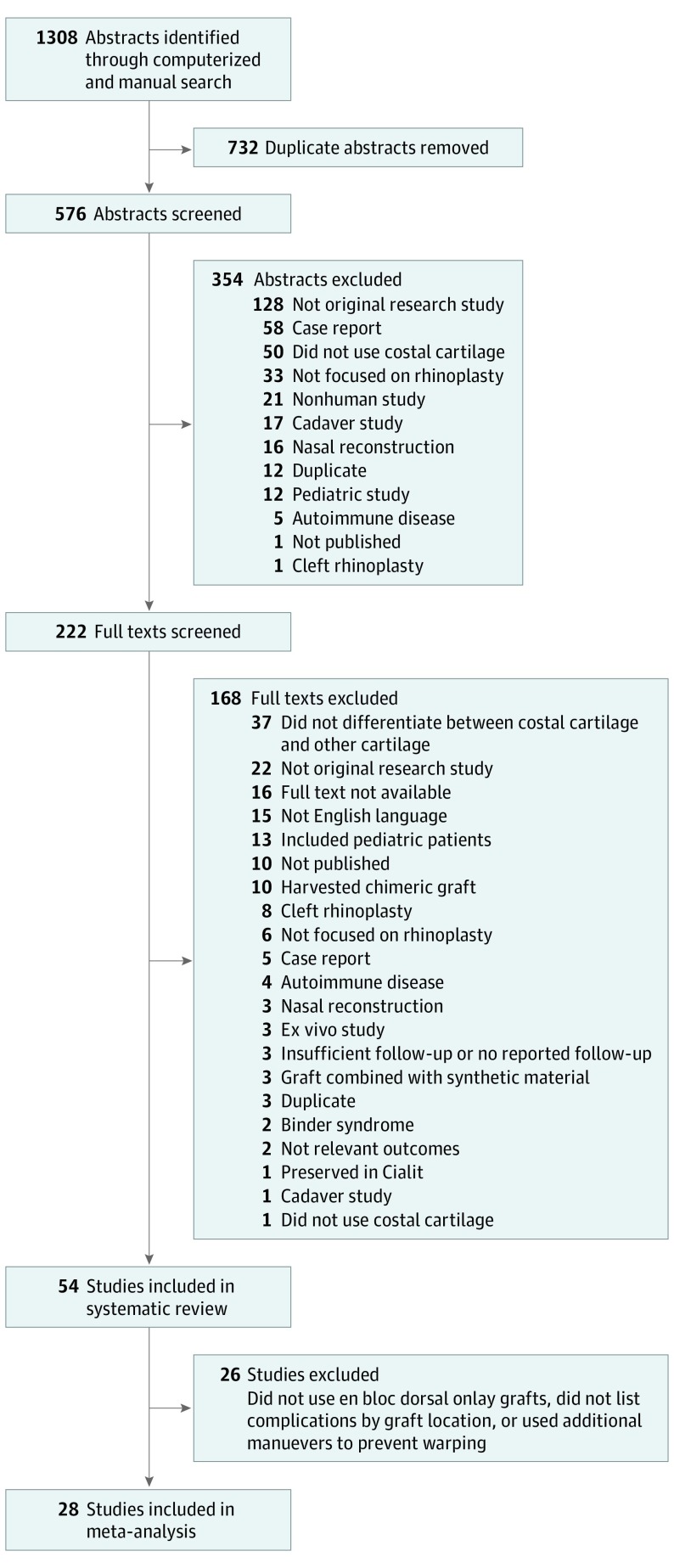
Abstracts and full texts were reviewed in duplicate, and disagreements were resolved by consensus. Only patients who underwent an en bloc dorsal onlay graft were included for comparison to ensure a homogenous study sample. A total of 1308 results were found. After duplicate records were removed, 576 unique citations remained. Studies were published worldwide between January 1, 1990, and December 31, 2017.
Data Extraction and Synthesis
Independent extraction by 2 authors was performed. Data were pooled using a random-effects model.
Main Outcomes and Measures
All reported outcomes after septorhinoplasty and rates of graft warping, resorption, infection, contour irregularity, and revision surgery among patients receiving autologous grafts vs IHCC vs Tutoplast cartilage grafts.
Results
Of 576 unique citations, 54 studies were included in our systematic review; 28 studies were included after applying inclusion and exclusion criteria. Our search captured 1041 patients of whom 741 received autologous grafts and 293 received IHCC grafts (regardless of type). When autologous cartilage (n = 748) vs IHCC (n = 153) vs Tutoplast cartilage (n = 140) grafts were compared, no difference in warping (5%; 95% CI, 3%-9%), resorption (2%; 95% CI, 0%-2%), contour irregularity (1%; 95% CI, 0%-3%), infection (2%; 95% CI, 0%-4%), or revision surgery (5%; 95% CI, 2%-9%) was found.
Conclusions and Relevance
No difference was found in outcomes between autologous and homologous costal cartilage grafts, including rates of warping, resorption, infection, contour irregularity, or revisions, in patients undergoing dorsal augmentation rhinoplasty. En bloc dorsal onlay grafts are commonly used in augmentation rhinoplasty to provide contour and structure to the nasal dorsum.
Introduction
Augmentation rhinoplasty requires the addition of cartilage to provide enhanced support to the structure of the nose. Although septal cartilage is an excellent source if available, additional material is often required for revision. Costal and auricular cartilages are well-accepted sources and thought to be superior to alloplastic implants because of the lower risk of infection and extrusion.1,2 Because of the larger amount of cartilage available with costal cartilage compared with auricular cartilage, costal cartilage is often the graft of choice in augmentation rhinoplasty.
However, the use of costal cartilage has risks. The risk of warping is often discussed,3,4,5,6,7,8 which has spurred various maneuvers to mitigate this risk, including carving techniques, suture techniques, microplate fixation, and Kirschner wires. In addition, the added morbidity associated with harvesting costal cartilage, including potential pneumothorax, scarring, and postoperative pain, as well as the added operative time raise the question of whether homologous costal cartilage can provide a similar result without harvesting autologous cartilage.2,9,10
More than 30 years ago, a landmark article by Welling et al11 reported a higher rate of infection and resorption with irradiated homologous costal cartilage (IHCC) grafts. However, this study11 was not focused on rhinoplasty and used grafts in various locations around the head and neck, including the maxilla, infraorbital rims, and auricles. Various studies2,9,12,13 later reported the opposite results, showing a low rate of infection and resorption in rhinoplasty with IHCC, which led to a resurgence in its use. Another alternative cartilage source that has been described is Tutoplast (RTI Surgical Holdings Inc). In contrast to IHCC, which is irradiated to 30 000 to 50 000 Gy and maintained in normal saline, Tutoplast is dehydrated with peroxide and acetone before irradiation up to 25 000 Gy. Song et al14 concluded in a study of 35 patients that Tutoplast cartilage is inadequate for dorsal augmentation, with an overall complication rate of 31%, including resorption, warping, and fracture of dorsal onlay grafts. Menger and Nolst Trenité15 also noted an increased rate of complications with Tutoplast cartilage, especially when used as shield grafts, with 1 patient having complete resorption of a columellar strut graft and one-third of 27 patients having some amount of resorption postoperatively.
The location of the grafts may be another factor to consider in augmentation rhinoplasty. Although a landmark study by Kridel et al2 found no difference in warping or resorption rates between IHCC and autologous grafts, Suh et al16 found that use of IHCC for septal extension grafts may be associated with a higher rate of resorption, perhaps because of the high tensile force to support tip projection and rotation. In addition, various experienced rhinoplasty surgeons17,18 have reported that there is no difference in warping between IHCC and autologous cartilage grafts but that IHCC should not be used for structural grafts, such as columellar struts, septal extension grafts, lateral crural strut grafts, or alar rim grafts, because of the risk of resorption. Others state that warping rates in autologous cartilage grafts are higher than IHCC because the IHCC has already been given time to warp after harvest.19
Given the varying results on outcomes with IHCC in rhinoplasty, no studies, to our knowledge, have definitively demonstrated that patients experience higher complications, including warping or resorption, with IHCC grafts compared with autologous costal cartilage grafts. Our goal was to compare rates of complications associated with autologous vs IHCC grafts in patients undergoing augmentation rhinoplasty in the published literature, including graft resorption, infection, warping, contour irregularity, and revision rates.
Methods
This systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline.20
Search Strategy and Study Selection
A review protocol was published for this study in PROSPERO.21 Using the PICOS (population, intervention, comparator, outcome, and study design) framework for this systematic review, the population of interest was patients older than 15 years who underwent septorhinoplasty; the intervention was septorhinoplasty with autologous costal cartilage graft; the comparator was septorhinoplasty with cadaveric cartilage graft; the outcomes were donor site morbidity, perioperative complications, aesthetic outcomes, patient-reported outcome measures, warping or resorption of grafts, and revision rates; and the study design was all study types except case reports.
A medical librarian (L.H.Y.) searched the literature for records that included the concepts of septorhinoplasty, rhinoplasty, autologous costal cartilage graft, and cadaveric cartilage graft. The librarian created search strategies using a combination of the following keywords and controlled vocabulary in MEDLINE, Embase, Scopus, Cochrane Central Register of Controlled Trials, and ClinicalTrials.gov: septorhinoplasty, rhinoplasty, autologous costal cartilage graft, cadaveric cartilage graft, and rib graft. All searches were performed for articles published from database inception to February 2019, and a total of 1308 results were found. With use of EndNote, 692 duplicate records were identified by the automatic duplicate finder and another 40 duplicates were removed by hand, resulting in a total of 576 unique citations included in the project library. Fully reproducible search strategies for each database can be found in eFigure 1 and eFigure 2 in the Supplement.
Abstracts and full texts were reviewed independently in duplicate (P.M.V., L.M.J.), and disagreements were resolved by consensus. At the abstract review stage, we excluded studies for the following reasons: (1) not an original research study, (2) case report, (3) did not use costal cartilage, (4) not focused on rhinoplasty, (5) nonhuman study, (6) cadaver study, (7) nasal reconstruction, (8) duplicate study, (9) pediatric study, (10) autoimmune disease, (11) not a published manuscript and thus study details could not be assessed, and (12) cleft rhinoplasty. At the full text review stage, we excluded studies for the following reasons: (1) did not differentiate between costal and other sources of cartilage, (2) full text was not available, (3) not an original research study, (4) not English language, (5) included pediatric patients, (6) not a published manuscript and thus study details could not be assessed, (7) cleft rhinoplasty, (8) not focused on rhinoplasty, (9) case report, (10) nasal reconstruction, (11) ex vivo study, (12) autoimmune disease, (13) harvested chimeric graft (rib bone in addition to cartilage), (14) Binder syndrome, (15) not relevant outcomes, (16) no reported follow-up, (17) duplicate publication, (18) did not use costal cartilage, (19) graft combined with synthetic material, (20) homologous cartilage preserved in Cialit, a process no longer used in current practice, and (21) cadaver study (Figure 1).
Figure 1. PRISMA Flow Diagram.
Data Extraction
Outcome measures used in each study were extracted and recorded in a database. The required data for completing a modified version of the Cochrane Collaboration’s Risk of Bias Tool were also extracted, including information on blinding and selective outcome reporting.22 Because none of the included studies were randomized clinical trials, the domains specific to randomized clinical trials (sequence generation and allocation concealment) were marked as not applicable.
Statistical Analysis
Random-effects meta-analysis of proportions was performed. We used the Freeman-Tukey Double Arcsine method to calculate the pooled estimate or rate. All analyses were performed in Stata software, version 14.2 (StataCorp LLC) and Microsoft Excel, version 15.2 (Microsoft Corp). The primary meta-analyses performed were reported rates of warping (defined as the patient or surgeon noting a change in cartilage shape postoperatively from the intraoperative appearance), resorption (defined as cartilage loss postoperatively), contour irregularity (defined as an unnatural appearance or palpable graft problem not noted intraoperatively), infection (defined as erythema and swelling requiring antibiotic therapy), and revision rates (defined as in-office or return to operating room for revision of the index surgery). Rates were calculated and reported as the number of events among the number of patients in the study. The groups that were compared were patients who received autologous costal cartilage grafts, IHCC grafts, and Tutoplast cartilage grafts. Because of the limitations in the way that outcomes are reported in the literature, we were unable to study complications by each subsite where cartilage was used. However, because of the larger number of studies focused on dorsal onlay grafts and the ease of detecting complications, such as warping at that site, we chose to focus on that subset of studies. We examined studies that used an en bloc dorsal onlay graft and excluded studies that used additional maneuvers, such as diced cartilage, additional suture techniques, K-wires, or screws, to make the study population as homogenous as possible and focused on the differences in the cartilage grafts. If studies did not separate complications by graft location, we excluded them from the analysis. To ensure that studies that failed to report length of follow-up did not bias our conclusions, we performed a sensitivity analysis after excluding those studies to compare the results.
Results
The search strategy resulted in 576 unique citations. After inclusion and exclusion criteria were applied, 54 studies were included in our systematic review (Figure 1). Twenty-eight studies were included for meta-analysis, all of which were retrospective cohort studies. These studies comprised 1041 patients of whom 741 received autologous grafts and 293 received IHCC grafts. Studies were published between January 1, 1990, and December 31, 2017; the mean sample size was 36 patients (range, 9-157 patients); and studies were from countries around the world, including Turkey (n = 9),3,23,24,25,26,27,28,29,30 the United States (n = 5),9,13,31,32,33 South Korea (n = 4),14,18,19,34 Italy (n = 2),35,36 India (n = 2),37,38 the United Kingdom (n = 1),39 Germany (n = 1),40 the Netherlands (n = 1),15 Egypt (n = 1),41 South Africa (n = 1),42 and Saudi Arabia (n = 1).43 Mean follow-up time ranged from 6 to 48 months in the studies that reported follow-up, although 8 studies did not. No difference was found in follow-up between groups, with mean follow-up periods of 23.2 months (95% CI, 13.8-32.7 months) for autologous costal cartilage studies, 31.2 months (95% CI, 5.4-57.0) for IHCC studies, and 18.7 months (95% CI, 0.0-79.1 months) for Tutoplast studies. A sensitivity analysis was performed to assess whether excluding studies that did not report follow-up time would influence the results, and no differences were found.
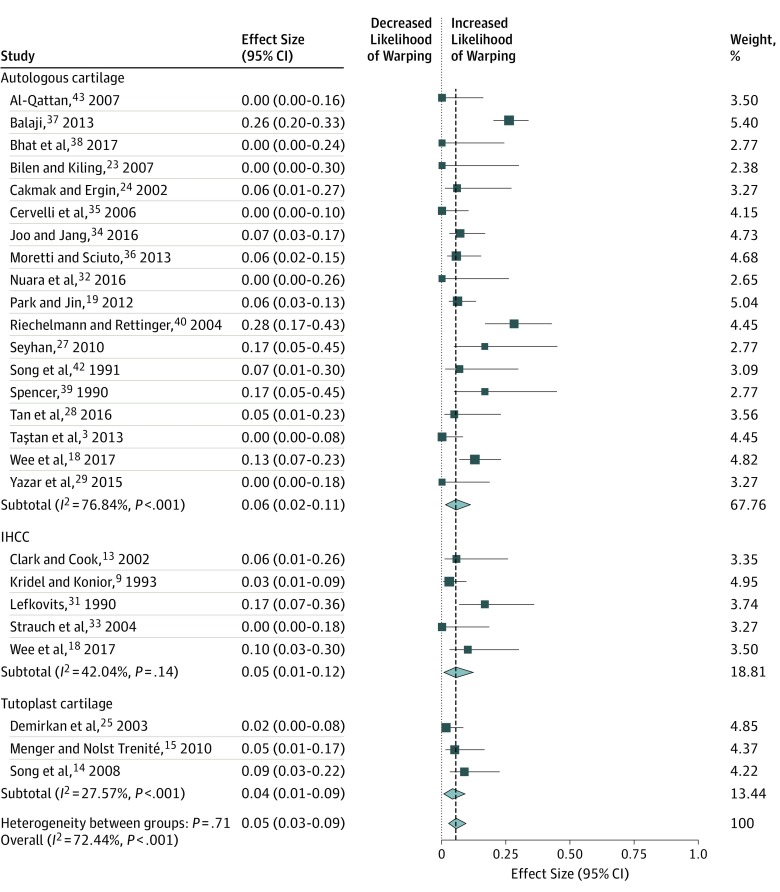
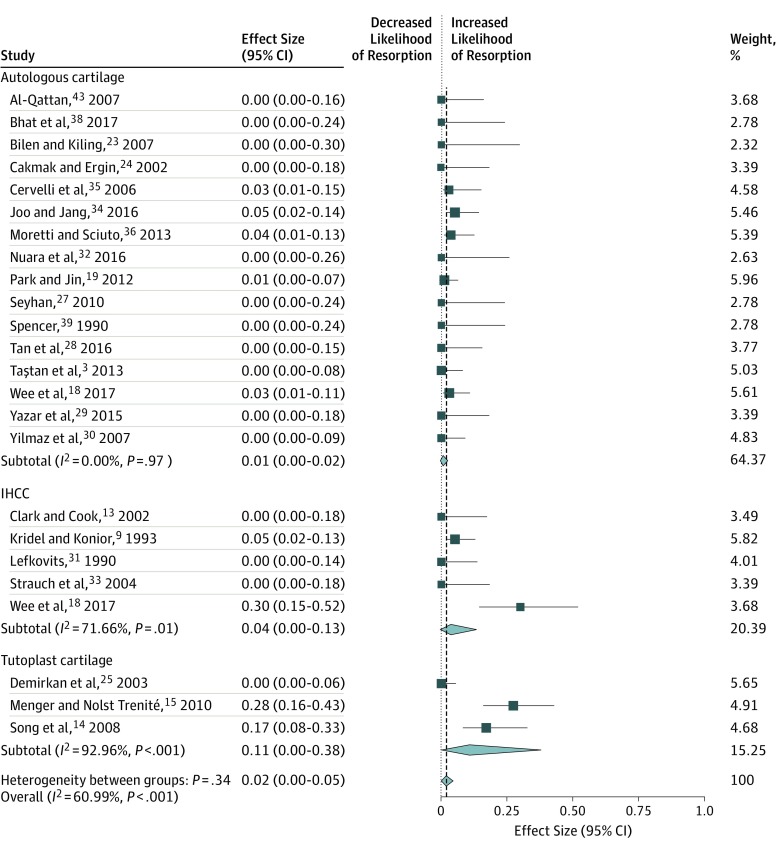
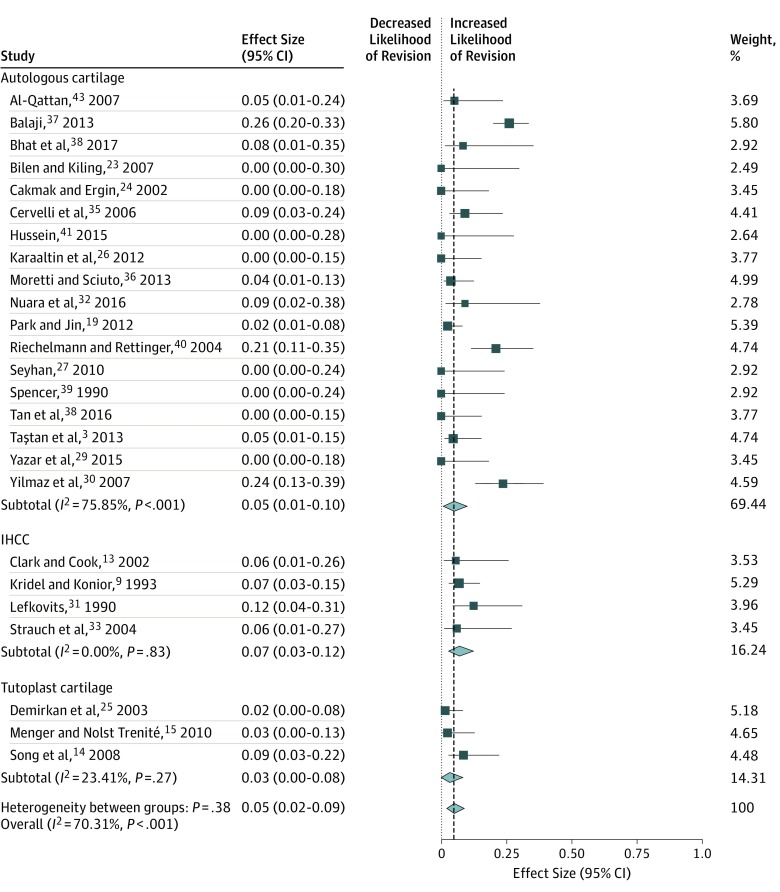
When comparing autologous cartilage (n = 748) vs IHCC (n = 153) vs Tutoplast cartilage (n = 140) used for en bloc dorsal onlay grafts, we found no difference in the rate of warping (5%; 95% CI, 3%-9%), resorption (2%; 95% CI, 0%-2%), contour irregularity (1%; 95% CI, 0%-3%), infection (2%; 95% CI, 0%-4%), or revision surgery (5%; 95% CI, 2%-9%). The Table gives a summary of the meta-analyses that were performed. Figure 2, Figure 3, and Figure 4 display the forest plots for each meta-analysis. The risk of bias was rated as high for 23 studies and unclear for 5 studies. The primary reason for the high risk of bias rating in those studies was lack of blinding, with the primary surgeon being responsible for the patient aesthetic evaluation (eg, warping and contour irregularity). Three studies18,19,34 included a blinded assessment of patient photographs, which satisfied criteria for a low risk of bias in that domain. A more detailed listing of the risk of bias assessment can be found in the eTable in the Supplement.
Table. Overall Summary of Meta-analyses.
| Outcome, Graft Type | Total No. | Pooled Event Rate, % (95% CI) | Heterogeneity, I2 | |
|---|---|---|---|---|
| Studiesa | Patients | |||
| Warping | ||||
| Autologous cartilage | 18 | 679 | 6 (2-11) | 76.8 |
| Irradiated homologous | 5 | 153 | 5 (1-12) | 42.0 |
| Tutoplast homologous | 3 | 140 | 4 (1-9) | 27.6 |
| Overall | 26 | 972 | 5 (3-9) | 72.4 |
| Resorption | ||||
| Autologous cartilage | 16 | 502 | 1 (0-2) | 0 |
| Irradiated homologous | 5 | 153 | 4 (0-13) | 71.7 |
| Tutoplast homologous | 3 | 140 | 11 (0-48) | 93.0 |
| Overall | 24 | 795 | 2 (0-5) | 61.0 |
| Contour Irregularity | ||||
| Autologous cartilage | 9 | 215 | 0 (0-3) | 0 |
| Irradiated homologous | 3 | 109 | 3 (0-7) | 0 |
| Tutoplast homologous | 2 | 75 | 4 (0-10) | 9.7 |
| Overall | 14 | 399 | 1 (0-3) | 0 |
| Infection | ||||
| Autologous cartilage | 15 | 493 | 2 (0-5) | 45.5 |
| Irradiated homologous | 5 | 153 | 3 (1-8) | 0 |
| Tutoplast homologous | 2 | 100 | 0 (0-2) | 64.6 |
| Overall | 22 | 746 | 2 (0-4) | 41.7 |
| Revisions | ||||
| Autologous cartilage | 18 | 613 | 5 (1-10) | 75.9 |
| Irradiated homologous | 4 | 133 | 7 (3-12) | 0 |
| Tutoplast homologous | 3 | 140 | 3 (0-8) | 23.4 |
| Overall | 25 | 886 | 5 (2-9) | 70.3 |
Study totals do not equal 28 because not all studies reported all listed outcomes.
Figure 2. Meta-analysis of Studies on Warping Rates by Cartilage Graft Types.
Squares indicate mean values, with lines indicating 95% CIs. Diamonds indicate the pooled effect estimates, with the tips of the diamonds indicating the 95% CIs. IHCC indicates irradiated homologous costal cartilage.
Figure 3. Meta-analysis of Studies on Resorption Rates by Cartilage Graft Types.
Squares indicate mean values, with lines indicating 95% CIs. Diamonds indicate the pooled effect estimates, with the tips of the diamonds indicating the 95% CIs. IHCC indicates irradiated homologous costal cartilage.
Figure 4. Meta-analysis of Studies on Revision Rates by Cartilage Graft Types.
Squares indicate mean values, with lines indicating 95% CIs. Diamonds indicate the pooled effect estimates, with the tips of the diamonds indicating the 95% CIs. IHCC indicates irradiated homologous costal cartilage.
Discussion
This systematic review and meta-analysis of more than 1000 en bloc dorsal onlay grafts in dorsal augmentation rhinoplasty found no difference in outcomes between autologous costal cartilage, IHCC, and Tutoplast cartilage grafts. When examining heterogeneity among studies, there was low heterogeneity in the rates of contour irregularity and infection. This finding demonstrates that the rate of these 2 outcomes are relatively robust and do not vary greatly among published studies, meaning that contour irregularity and infection are not sensitive to technique, not sensitive to graft type, or both. However, significant heterogeneity was found among studies in the rates of warping, resorption, and revision. Of note, the studies with the highest reported warping rates were also the studies with the highest revision rates. Whether the technique used in those studies led to higher warping rates and thus required higher revision rates is unknown. However, when pooling all included studies, no difference was found in warping or revision rates between autologous and homologous grafts. The possibility that warping and/or resorption may be subtle in certain cases and thus not reported equally among all studies may explain the variability that we observed. Revision rates are likely influenced by individual surgeons, and surgeons’ willingness to revise their procedures substantially contributes to the variation noted among studies. Our results help to address a controversial debate among rhinoplasty surgeons regarding the equivalency of homologous compared with autologous rib cartilage in dorsal onlay grafts. The question of whether homologous grafts can be used as structural grafts (eg, columellar strut grafts and lateral crural grafts) is worthy of future study,
Limitations
We were unable to determine whether homologous grafts can be used as structural grafts from our data because of the limitations in the way results are reported in the literature. For example, if a study reported using multiple grafts per procedure and that patient subsequently had warping of the cartilage, it is impossible to determine where warping occurred and which graft was responsible. Thus, by limiting our analysis to dorsal onlay grafts, the analysis was much more specific in that warping could only happen in the dorsal onlay graft if detected.
Another domain that we were unable to include in the meta-analysis because of paucity of reporting is that of patient-reported outcome measures. Although outcomes such as warping and resorption are important to tracking outcomes after surgery, patient-reported outcome measures, such as the Nasal Obstruction Symptom Evaluation scale44 or the more recent and specific Standardized Cosmesis and Health Nasal Outcomes Survey,45 provide additional information that may help us better hone techniques and protocols in the future. The authors believe that rhinoplasty surgeons should be using patient-reported outcome measures to even further refine their results.
When studies discuss revision rates, it is impossible to know whether patients went to another surgeon after being lost to follow-up, but this is a universal problem with all published studies. Thus, there may be an overall underestimation of the revision rates reported. In addition, some surgeons may be quicker to perform revision surgery than others. Similarly, there may be a surgeon bias for selecting autologous or homologous cartilage grafts for dorsal augmentation rhinoplasty. It is theoretically possible that patients self-selected surgeons who preferred using one type of graft, introducing a selection bias to the results. However, by including various studies from different institutions and including studies that used either type of graft exclusively, we believe that this possibility is minimized and does not affect our results.
Conclusions
We found no difference in outcomes between autologous cartilage and IHCC grafts, including rates of warping, resorption, infection, contour irregularity, or revisions. Whether autologous cartilage and IHCC cartilage are equivalent in structural grafts in rhinoplasty, such as columellar strut or lateral crural strut grafts, remains a question for future research.
eFigure 1. Meta-analysis of Studies Examining Rates of Contour Irregularity Between Cartilage Graft Types
eFigure 2. Meta-analysis of Studies Examining Infection Rates Between Cartilage Graft Types
eTable. Risk of Bias Assessment Among Included Studies
References
- 1.Parker Porter J. Grafts in rhinoplasty: alloplastic vs. autogenous. Arch Otolaryngol Head Neck Surg. 2000;126(4):558-561. doi: 10.1001/archotol.126.4.558 [DOI] [PubMed] [Google Scholar]
- 2.Kridel RW, Ashoori F, Liu ES, Hart CG. Long-term use and follow-up of irradiated homologous costal cartilage grafts in the nose. Arch Facial Plast Surg. 2009;11(6):378-394. doi: 10.1001/archfacial.2009.91 [DOI] [PubMed] [Google Scholar]
- 3.Taştan E, Yücel OT, Aydin E, Aydoğan F, Beriat K, Ulusoy MG. The oblique split method: a novel technique for carving costal cartilage grafts. JAMA Facial Plast Surg. 2013;15(3):198-203. doi: 10.1001/jamafacial.2013.671 [DOI] [PubMed] [Google Scholar]
- 4.Swanepoel PF, Fysh R. Laminated dorsal beam graft to eliminate postoperative twisting complications. Arch Facial Plast Surg. 2007;9(4):285-289. doi: 10.1001/archfaci.9.4.285 [DOI] [PubMed] [Google Scholar]
- 5.Kim DW, Shah AR, Toriumi DM. Concentric and eccentric carved costal cartilage: a comparison of warping. Arch Facial Plast Surg. 2006;8(1):42-46. doi: 10.1001/archfaci.8.1.42 [DOI] [PubMed] [Google Scholar]
- 6.Guyuron B, Wang DZ, Kurlander DE. The cartilage warp prevention suture. Aesthetic Plast Surg. 2018;42(3):854-858. doi: 10.1007/s00266-017-1052-3 [DOI] [PubMed] [Google Scholar]
- 7.Gunter JP, Clark CP, Friedman RM. Internal stabilization of autogenous rib cartilage grafts in rhinoplasty: a barrier to cartilage warping. Plast Reconstr Surg. 1997;100(1):161-169. doi: 10.1097/00006534-199707000-00026 [DOI] [PubMed] [Google Scholar]
- 8.Eren F, Öksüz S, Melikoğlu C, Karagöz H, Ülkür E. Saddle-nose deformity repair with microplate-adapted costal cartilage. Aesthetic Plast Surg. 2014;38(4):733-741. doi: 10.1007/s00266-014-0344-0 [DOI] [PubMed] [Google Scholar]
- 9.Kridel RW, Konior RJ. Irradiated cartilage grafts in the nose: a preliminary report. Arch Otolaryngol Head Neck Surg. 1993;119(1):24-30. doi: 10.1001/archotol.1993.01880130026003 [DOI] [PubMed] [Google Scholar]
- 10.Wee JH, Park MH, Oh S, Jin HR. Complications associated with autologous rib cartilage use in rhinoplasty: a meta-analysis. JAMA Facial Plast Surg. 2015;17(1):49-55. doi: 10.1001/jamafacial.2014.914 [DOI] [PubMed] [Google Scholar]
- 11.Welling DB, Maves MD, Schuller DE, Bardach J. Irradiated homologous cartilage grafts: long-term results. Arch Otolaryngol Head Neck Surg. 1988;114(3):291-295. doi: 10.1001/archotol.1988.01860150073018 [DOI] [PubMed] [Google Scholar]
- 12.Murakami CS, Cook TA, Guida RA. Nasal reconstruction with articulated irradiated rib cartilage. Arch Otolaryngol Head Neck Surg. 1991;117(3):327-330. doi: 10.1001/archotol.1991.01870150095013 [DOI] [PubMed] [Google Scholar]
- 13.Clark JMC, Cook TA. Immediate reconstruction of extruded alloplastic nasal implants with irradiated homograft costal cartilage. Laryngoscope. 2002;112(6):968-974. doi: 10.1097/00005537-200206000-00006 [DOI] [PubMed] [Google Scholar]
- 14.Song HM, Lee BJ, Jang YJ. Processed costal cartilage homograft in rhinoplasty: the Asan Medical Center experience. Arch Otolaryngol Head Neck Surg. 2008;134(5):485-489. doi: 10.1001/archotol.134.5.485 [DOI] [PubMed] [Google Scholar]
- 15.Menger DJ, Nolst Trenité GJ. Irradiated homologous rib grafts in nasal reconstruction. Arch Facial Plast Surg. 2010;12(2):114-118. doi: 10.1001/archfacial.2010.6 [DOI] [PubMed] [Google Scholar]
- 16.Suh MK, Lee SJ, Kim YJ. Use of irradiated homologous costal cartilage in rhinoplasty: complications in relation to graft location. J Craniofac Surg. 2018;29(5):1220-1223. doi: 10.1097/SCS.0000000000004440 [DOI] [PubMed] [Google Scholar]
- 17.Toriumi DM. Choosing autologous vs irradiated homograft rib costal cartilage for grafting in rhinoplasty. JAMA Facial Plast Surg. 2017;19(3):188-189. doi: 10.1001/jamafacial.2017.0036 [DOI] [PubMed] [Google Scholar]
- 18.Wee JH, Mun SJ, Na WS, et al. . Autologous vs irradiated homologous costal cartilage as graft material in rhinoplasty. JAMA Facial Plast Surg. 2017;19(3):183-188. doi: 10.1001/jamafacial.2016.1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park JHJ, Jin HR. Use of autologous costal cartilage in Asian rhinoplasty. Plast Reconstr Surg. 2012;130(6):1338-1348. doi: 10.1097/PRS.0b013e31826d9f03 [DOI] [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264-269, w264. [DOI] [PubMed] [Google Scholar]
- 21.Jeanpierre LM, Vila PM, Rizzi CJ, Yaeger LH, Chi JJ Systematic review and meta-analysis of outcomes and complications in rhinoplasty with autologous versus homologous costal cartilage grafts. https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=134581. Accessed June 25, 2019. [DOI] [PMC free article] [PubMed]
- 22.Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration, 2011. https://www.handbook.cochrane.org. Accessed June 25, 2019. [Google Scholar]
- 23.Bilen BTK, Kilinç H. Reconstruction of saddle nose deformity with three-dimensional costal cartilage graft. J Craniofac Surg. 2007;18(3):511-515. doi: 10.1097/scs.0b013e318052fecd [DOI] [PubMed] [Google Scholar]
- 24.Cakmak O, Ergin T. The versatile autogenous costal cartilage graft in septorhinoplasty. Arch Facial Plast Surg. 2002;4(3):172-176. doi: 10.1001/archfaci.4.3.172 [DOI] [PubMed] [Google Scholar]
- 25.Demirkan F, Arslan E, Unal S, Aksoy A. Irradiated homologous costal cartilage: versatile grafting material for rhinoplasty. Aesthetic Plast Surg. 2003;27(3):213-220. doi: 10.1007/s00266-003-0118-6 [DOI] [PubMed] [Google Scholar]
- 26.Karaaltin MV, Batioglu-Karaaltin A, Orhan KS, Demirel T, Guldiken Y. Autologous fascia lata graft for contour restoration and camouflage in tertiary rhinoplasty. J Craniofac Surg. 2012;23(3):719-723. doi: 10.1097/SCS.0b013e31824dbb92 [DOI] [PubMed] [Google Scholar]
- 27.Seyhan T. Correction of major saddle nose deformities with nasomaxillary depression using an intraoral and external open rhinoplasty approach. Aesthetic Plast Surg. 2010;34(5):587-595. doi: 10.1007/s00266-010-9512-z [DOI] [PubMed] [Google Scholar]
- 28.Tan O, Algan S, Cinal H, Barin EZ, Kara M, Inaloz A. Management of saddle nose deformity using dermal fat and costal cartilage “sandwich” graft: a problem-oriented approach and anthropometric evaluation. J Oral Maxillofac Surg. 2016;74(9):1848, e1841-e1848. [DOI] [PubMed] [Google Scholar]
- 29.Yazar M, Yazar SK, Sevim KZ, et al. . Key and keyhole model for dorsal onlay cartilage grafts in correcting nasal deformities. Ann Plast Surg. 2015;75(4):418-423. doi: 10.1097/SAP.0000000000000151 [DOI] [PubMed] [Google Scholar]
- 30.Yilmaz M, Vayvada H, Menderes A, Mola F, Atabey A. Dorsal nasal augmentation with rib cartilage graft: long-term results and patient satisfaction. J Craniofac Surg. 2007;18(6):1457-1462. doi: 10.1097/scs.0b013e31814e07b4 [DOI] [PubMed] [Google Scholar]
- 31.Lefkovits G. Irradiated homologous costal cartilage for augmentation rhinoplasty. Ann Plast Surg. 1990;25(4):317-327. doi: 10.1097/00000637-199010000-00014 [DOI] [PubMed] [Google Scholar]
- 32.Nuara MJ, Loch RB, Saxon SA. Reconstructive rhinoplasty using multiplanar carved costal cartilage. JAMA Facial Plast Surg. 2016;18(3):207-211. doi: 10.1001/jamafacial.2015.2251 [DOI] [PubMed] [Google Scholar]
- 33.Strauch B, Erhard HA, Baum T. Use of irradiated cartilage in rhinoplasty of the non-Caucasian nose. Aesthet Surg J. 2004;24(4):324-330. doi: 10.1016/j.asj.2004.04.008 [DOI] [PubMed] [Google Scholar]
- 34.Joo YH, Jang YJ. Comparison of the surgical outcomes of dorsal augmentation using expanded polytetrafluoroethylene or autologous costal cartilage. JAMA Facial Plast Surg. 2016;18(5):327-332. doi: 10.1001/jamafacial.2016.0316 [DOI] [PubMed] [Google Scholar]
- 35.Cervelli V, Bottini DJ, Gentile P, et al. . Reconstruction of the nasal dorsum with autologous rib cartilage. Ann Plast Surg. 2006;56(3):256-262. doi: 10.1097/01.sap.0000199153.26947.5e [DOI] [PubMed] [Google Scholar]
- 36.Moretti A, Sciuto S. Rib grafts in septorhinoplasty. Acta Otorhinolaryngol Ital. 2013;33(3):190-195. [PMC free article] [PubMed] [Google Scholar]
- 37.Balaji SM. Costal cartilage nasal augmentation rhinoplasty: study on warping. Ann Maxillofac Surg. 2013;3(1):20-24. doi: 10.4103/2231-0746.110070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bhat U, Gupta T, Nair M, Mantri M, Pawar M, Baliarsing A. Three component cartilage framework reconstruction for correction of post-traumatic nasal septal collapse. Indian J Plast Surg. 2017;50(3):236-243. doi: 10.4103/ijps.IJPS_74_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spencer MG. Chondroplastic graft augmentation rhinoplasty. J Laryngol Otol. 1990;104(7):539-543. doi: 10.1017/S0022215100113118 [DOI] [PubMed] [Google Scholar]
- 40.Riechelmann H, Rettinger G. Three-step reconstruction of complex saddle nose deformities. Arch Otolaryngol Head Neck Surg. 2004;130(3):334-338. doi: 10.1001/archotol.130.3.334 [DOI] [PubMed] [Google Scholar]
- 41.Hussein WKAH. Saddle nose: autologous augmentation techniques and their relevant patient satisfaction. Egypt J Ear Nose Throat Allied Sci. 2015;16(2):113-122. doi: 10.1016/j.ejenta.2015.04.003 [DOI] [Google Scholar]
- 42.Song C, Mackay DR, Chait LA, Manders EK, Kelly MA. Use of costal cartilage cantilever grafts in negroid rhinoplasties. Ann Plast Surg. 1991;27(3):201-209. doi: 10.1097/00000637-199109000-00003 [DOI] [PubMed] [Google Scholar]
- 43.Al-Qattan MM. Augmentation of the nasal dorsum with autogenous costal cartilage using the “edge-on” technique. Ann Plast Surg. 2007;59(6):642-644. doi: 10.1097/01.sap.0000258952.61173.12 [DOI] [PubMed] [Google Scholar]
- 44.Stewart MG, Witsell DL, Smith TL, Weaver EM, Yueh B, Hannley MT. Development and validation of the Nasal Obstruction Symptom Evaluation (NOSE) scale. Otolaryngol Head Neck Surg. 2004;130(2):157-163. doi: 10.1016/j.otohns.2003.09.016 [DOI] [PubMed] [Google Scholar]
- 45.Moubayed SP, Ioannidis JPA, Saltychev M, Most SP. The 10-item Standardized Cosmesis and Health Nasal Outcomes Survey (SCHNOS) for functional and cosmetic rhinoplasty. JAMA Facial Plast Surg. 2018;20(1):37-42. doi: 10.1001/jamafacial.2017.1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Meta-analysis of Studies Examining Rates of Contour Irregularity Between Cartilage Graft Types
eFigure 2. Meta-analysis of Studies Examining Infection Rates Between Cartilage Graft Types
eTable. Risk of Bias Assessment Among Included Studies