Key Points
Question
Is serum C-reactive protein level sufficiently accurate to aid the diagnosis of late-onset infection in newborn infants?
Findings
In this systematic review and meta-analysis of 22 cohort studies (2255 infants) comparing the diagnostic test accuracy of serum C-reactive protein with microbiological culture, median specificity was 0.74 and pooled sensitivity was 0.62. Assuming a orwould miss 152 cases of infection and wrongly diagnose 156 cases.
Meaning
The findings suggest that serum C-reactive protein level is not sufficiently accurate to aid diagnosis or to inform treatment decisions in infants with suspected late-onset infection.
Abstract
Importance
Rapid and accurate diagnosis of late-onset infection in newborn infants could inform treatment decisions and avoid unnecessary administration of antibiotics.
Objective
To compare the accuracy of serum C-reactive protein (CRP) with that of microbiological blood culture for diagnosing late-onset infection in newborns.
Data Sources
MEDLINE (1946-2019), Embase (1946-2019), and Science Citation Index (1900-2019) databases were searched for references (any language). The MeSH search terms included were “exp infant, newborn/” or “premature birth/” plus free text synonyms; and “C-reactive protein/” plus free text synonyms; and “exp sepsis/” or “exp bacterial infections/” plus free text synonyms. The proceedings from relevant conferences and references of identified papers were scrutinized. Authors were contacted to request missing data.
Study Selection
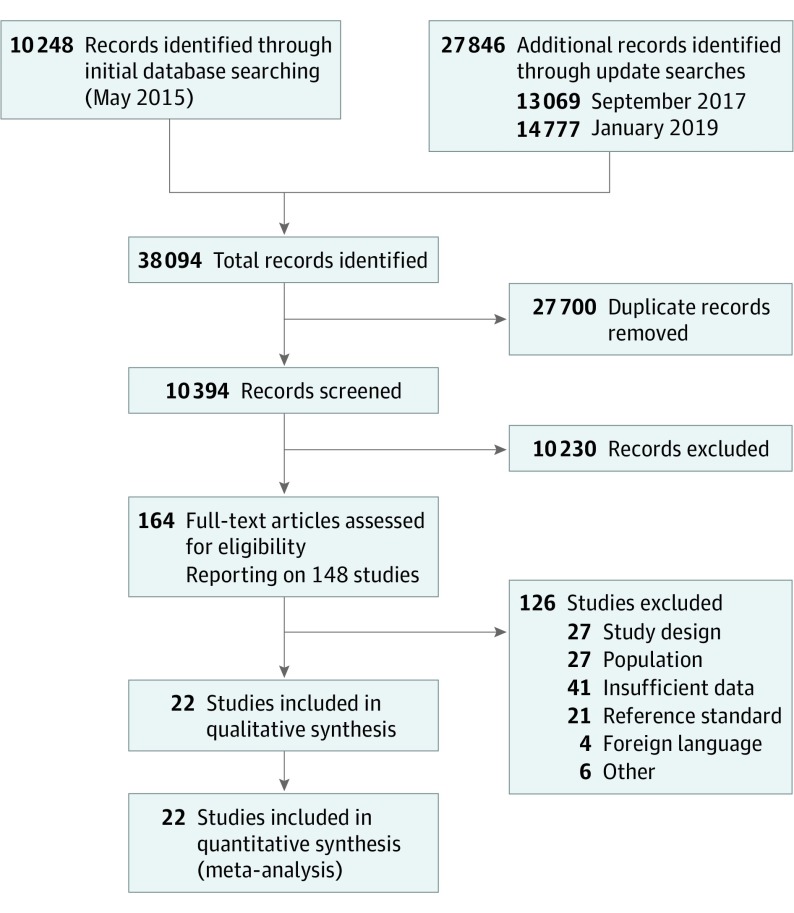
Cohort and cross-sectional studies were included that compared the accuracy of serum CRP levels with microbiological culture results to diagnose late-onset (>72 hours after birth) infection in newborns (any gestational age) hospitalized after birth. Two reviewers assessed study eligibility. Among 10 394 records, 148 studies were assessed as full texts.
Data Extraction and Synthesis
The Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guideline extension for Diagnostic Test Accuracy (DTA) reviews was followed. Two reviewers assessed the method quality of each study using guidance from the Cochrane Screening and Diagnostic Test Methods Group (adapted from the Quality Assessment of Diagnostic Accuracy Studies 2).
Main Outcomes and Measures
The primary meta-analysis outcome was diagnostic test accuracy of serum CRP level taken at initial investigation of an infant with suspected late-onset infection. The median specificity (proportion of true-negative results) and calculated pooled sensitivity (proportion of true-positive results) were determined by generating hierarchical summary receiver characteristic operating curves.
Results
In total, 22 studies with 2255 infants were included (sample size range, 11-590 infants). Participants in most studies were preterm (<37 weeks) or very low-birth weight (<1500 g) infants. Two studies additionally enrolled infants born at term. Most studies (14 of 16) used a prespecified CRP level cutoff for a “positive” index test (5-10 mg/L) and the culture of a pathogenic microorganism from blood as the reference standard. Risk of bias was low with independent assessment of index and reference tests. At median specificity (0.74), pooled sensitivity was 0.62 (95% CI, 0.50-0.72). Adding serum CRP level to the assessment of an infant with a 40% pretest probability of late-onset infection (the median for the included studies) generated posttest probabilities of 26% for a negative test result and 61% for a positive test result.
Conclusions and Relevance
The findings suggest that determination of serum CRP level at initial evaluation of an infant with suspected late-onset infection is unlikely to aid early diagnosis or to select infants to undergo further investigation or treatment with antimicrobial therapy or other interventions.
This systematic review and meta-analysis of cohort and cross-sectional studies compares the diagnostic test accuracies of serum C-reactive protein level vs microbiological culture for diagnosing late-onset infection among newborn infants.
Introduction
Late-onset infection (occurring >72 hours after birth) is one of the most common serious complications associated with intensive care for newborn infants.1 Preterm infants, particularly very preterm infants, with late-onset infection have a higher risk of mortality, morbidity, and need for intensive care and prolonged hospitalization than newborn infants without infection.2 Late-onset infection is associated with adverse neurodevelopmental outcomes, including cerebral palsy and visual, hearing, and cognitive impairments.3,4
Clinical signs of infection in neonates can be nonspecific, and the diagnosis of late-onset infection in newborn infants can be delayed if these signs are missed. Microbiological culture of a potentially pathogenic organism from a blood sample takes 24 to 48 hours to complete. Delayed treatment of late-onset infection may increase the risk of morbidity and mortality in newborn infants. However, empirical treatment of all infants with suspected infection will result in the administration of unnecessary courses of antibiotics.5 Such widespread use, particularly of broad-spectrum antibiotics, is associated with accelerated selective pressure and the emergence of drug resistance through mechanisms such as extended-spectrum β-lactamase production.6,7,8 In addition, the exposure to antibiotics in early life carries a risk of adversely altering the developing microbiome,9 which could be detrimental, especially to unwell or preterm infants in whom concerns regarding gut health already exist.10,11
To avoid the unnecessary stress on the already compromised organs, such as in the gastrointestinal tract of preterm infants, and the contribution to the wider problem of antimicrobial resistance, several biomarkers have been proposed as tests to support the diagnosis of late-onset infection in newborn infants. Used in conjunction with blood culture, biomarkers have the potential to indicate whether infection is more or less likely in infants in whom it is suspected.12,13 The most commonly used biomarker is the serum level of C-reactive protein (CRP), an acute-phase reactant synthesized by hepatocytes in response to inflammatory cytokines generated by white blood cells reacting to microbial pyrogens.14 Serum CRP might be a useful biomarker for late-onset infection in newborn infants if it can be shown to have acceptable levels of accuracy. Currently, in the absence of robust evidence to inform guideline or protocol development, the role of serum CRP in diagnostic algorithms for late-onset infection varies greatly.5,15 Most studies examining the accuracy of CRP and other biomarkers of late-onset infection have been conducted in single centers and, therefore, are limited by the small sample size. We conducted a systematic diagnostic test accuracy review and meta-analysis to identify, quality appraise, and synthesize the available evidence to inform policy and practice as well as future research.
Methods
We searched MEDLINE (1946-2019), Embase (1946-2019), and Science Citation Index (1900-2019) databases for references published in any language. The MeSH search terms included were “exp infant, newborn/” or “premature birth/” plus free text synonyms; and “C-reactive protein/” plus free text synonyms; and “exp sepsis/” or “exp bacterial infections/” plus free text synonyms. We examined the reference lists of all studies identified as potentially relevant and searched the abstracts from the annual meetings of the Pediatric Academic Societies (1993-2018), the European Society for Pediatric Research (1995-2018), the UK Royal College of Paediatrics and Child Health (2000-2018), and the Perinatal Society of Australia and New Zealand (2000-2018). Studies reported only as abstracts were eligible if sufficient information was available from the report or from contact with the authors to fulfill the inclusion criteria. The detailed search strategy can be found in eAppendix 1 in the Supplement. This systematic review and meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline extension for Diagnostic Test Accuracy (DTA) reviews. This study was excluded from internal review board review and the requirement for informed patient consent based on institutional policies for systematic reviews and meta-analyses.
Three reviewers (J.V.E.B., N.M., and J.C.) independently screened titles and abstracts and retrieved full-text publications for potentially relevant references. All records were screened by 2 of those 3. Disagreements were resolved by a third author (W.M.) if necessary. We included cohort and cross-sectional studies of hospitalized newborn infants of any gestational age with clinically suspected late-onset infection (including bacteremia, fungemia, meningitis, osteomyelitis, septic arthritis, and peritonitis) for whom diagnostic test accuracy data for serum CRP levels were reported. Studies that included infants younger than 72 hours after birth (with suspected early-onset infection) were only eligible for inclusion if data for late-onset infection could be extracted separately. We emailed the authors of studies published after 2004 to request unpublished data and clarification of the study method as needed. We excluded case-control studies because that design does not allow for valid assessment of diagnostic test accuracy in this clinical context. We excluded studies in which the reference standard incorporated the index test, that is, infection was defined as a positive microbiological culture test result and an increased serum CRP level. We did not include studies in which the participants were infants cared for at home or in another community setting and who then presented to a health care facility with possible infection.
Statistical Analysis
Two of 3 reviewers (J.V.E.B., N.M., or J.C.) assessed the method quality of each included study following guidance from the Cochrane Screening and Diagnostic Test Methods Group, which is adapted from the Quality Assessment of Diagnostic Accuracy Studies 2 tool.16 One author (J.V.E.B., N.M., or J.C.) extracted study characteristics, participant details, and diagnostic data to enable derivation of the number of true-positive results, false-positive results, false-negative results, and true-negative results from the included reports. A second author (J.V.E.B., N.M., or J.C.) checked data extraction. Disagreements were discussed and resolved with input from a third reviewer (W.M.) as needed. Only the index test conducted at the same time as the reference standard was used. We created forest plots with 95% CIs for sensitivity and specificity for each study using RevMan 5 (Review Manager 5) software, version 5.3.17
We calculated estimates of sensitivity at fixed values of specificity (median and lower and upper quartiles reported in the included studies) on the summary receiver operating characteristic (ROC) curves. Because the reported cutoff level for a positive CRP test differed among the studies, we fitted a hierarchical summary ROC model that assumed accuracy and thresholds varied among studies.18 Analyses were conducted using the NLMIXED procedure with the SAS system for Windows, version 9.4 (SAS Institute Inc). We also conducted bivariate meta-analyses for studies with a CRP level threshold of 5 to 10 mg/L to provide summary estimates of sensitivity and specificity at that threshold (to convert CRP levels to nanomoles per liter, multiply by 9.524).
To facilitate interpretation of diagnostic accuracy estimates, we presented the number of cases missed and the number of cases wrongly diagnosed in a hypothetical cohort of 1000 neonates suspected of late-onset sepsis based on sensitivity and median specificity estimates. We calculated these values when the expected prevalence rate was 20%, 40%, or 60% for late-onset sepsis.
We assessed heterogeneity by examining forest plots of sensitivity and specificity across studies for variability of study estimates and overlap of 95% CIs. We conducted metaregression analyses to explore the association of serum CRP cutoff levels (categorical covariate: standard threshold 5-10 mg/L vs any other threshold) and reporting a predefined threshold on heterogeneity (categorical covariate: reporting predefined threshold vs not reporting predefined threshold).
We planned to assess gestational age at birth, type of pathogens, and subtype of late-onset infection in metaregression analyses to evaluate the association of these participant level characteristics with the diagnostic accuracy of serum CRP. If sufficient data were available, we planned to explore whether study method quality was associated with the results in sensitivity analyses by removing studies considered at higher risk of bias across key domains (selection, verification).
We assessed publication bias using a funnel plot and the Deeks test.19 This systematic review of diagnostic test accuracy was registered prospectively (PROSPERO CRD42016045585). Protocol changes are given in eAppendix 2 in the Supplement.
Results
Among 10 394 records, 148 studies were assessed as full texts. Of those, 22 studies reported in 22 separate publications were included in this systematic review. Sample sizes ranged from 11 to 590 infants (total 2255 infants). See Figure 1, PRISMA flow diagram, for details of the study selection process. Most studies were carried out in high-income countries in Europe,20,21,22,23,24,25,26,27 Asia,28,29,30 North America,31,32,33 South America,34 or Australasia.35,36 Five studies were conducted in low- and middle-income countries.37,38,39,40,41 All but one31 of the studies were single-center investigations. Studies were published between 1990 and 2018, with most studies (17 of 22) published since 2000. Three studies were cohorts assembled retrospectively.22,23,33 The remaining 19 studies included prospectively observed cohorts.
Figure 1. Study Selection Flowchart.
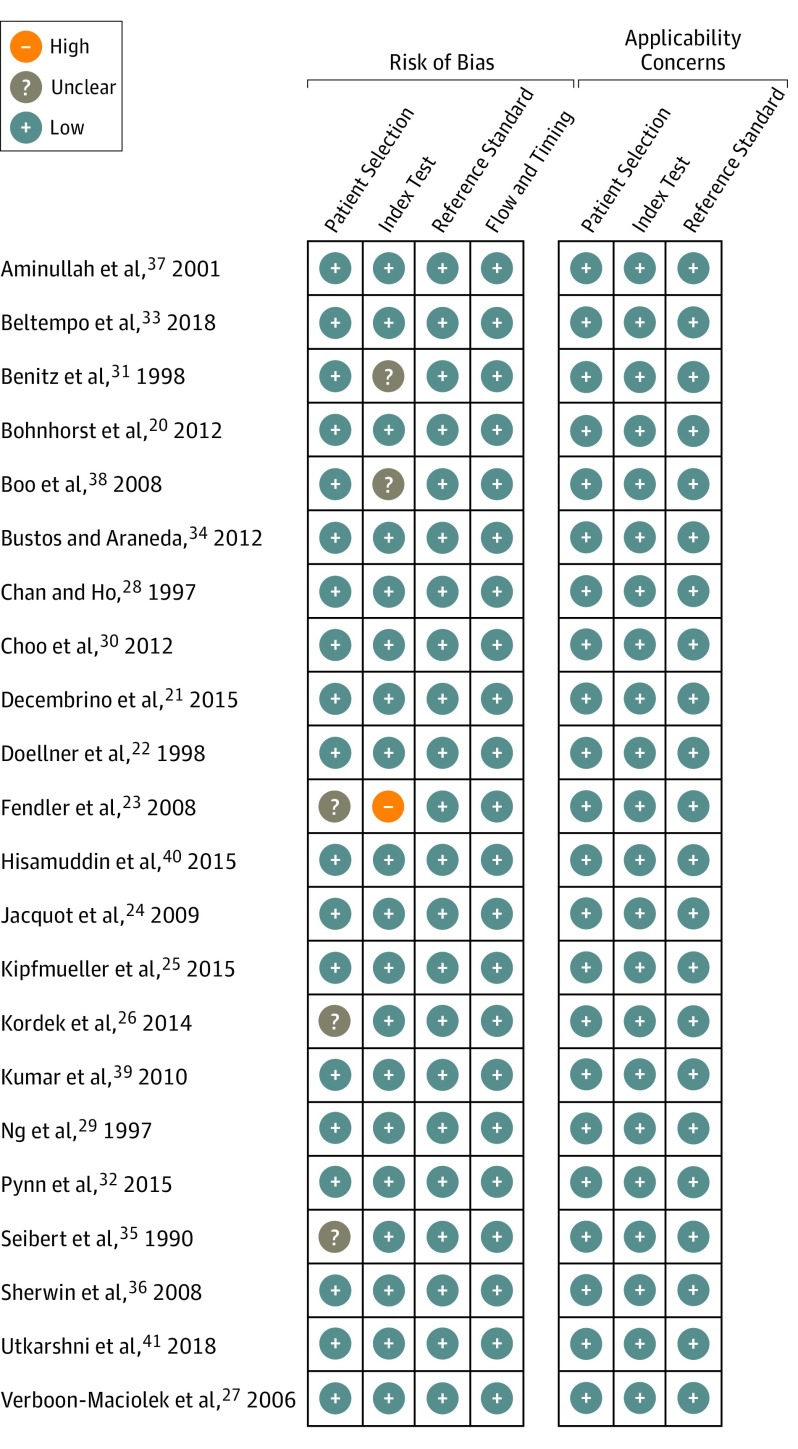
The characteristics of the included studies and the study level diagnostic data are summarized in the Table. The method quality of the included studies was good, and the risk of bias was low (Figure 2). In 17 studies, participants were predominantly preterm (or very low-birth-weight) infants. Two studies also included terms infants.22,36 Three studies did not report the gestational age of the included infants, but it is likely that most participants were preterm or low-birth-weight infants.21,39,41 Of those studies that reported the gestational age at birth of participants, the mean or median gestational age was younger than 30 weeks in 10 studies, 30 to 32 weeks in 6 studies, and older than 32 weeks in 3 studies (not reported in 3 studies). Birth weight data were not reliably reported in the included studies. Age of the infants at the time of enrollment was also reported poorly and appeared to occur either within 48 to 72 hours after birth or at a later time within the neonatal period (28 days).
Table. Characteristics of Included Studies.
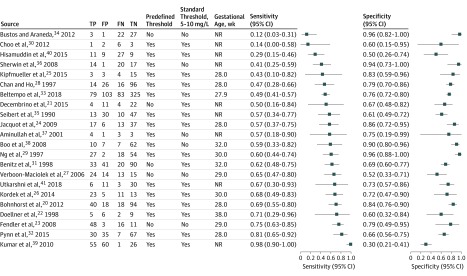
| Source | Country (No. of Study Sites) | Recruitment | Participants | Threshold Serum CRP Level, mg/L | Threshold Predefined | Total No. | True Positive No. | False Positive No. | False Negative No. | True Negative No. | Sensitivity (95% CI) |
Specificity (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aminullah et al,37 2001 | Indonesia (1) | Prospective | BW, >1 kg; GA, NR | 12 | Yes | 11 | 4 | 1 | 3 | 3 | 0.57 (0.18-0.90) | 0.75 (0.19-0.99) |
| Beltempo et al,33 2018 | Canada (1) | Retrospective | BW, NR; mean GA, 27.9 wk | 10 | Yes | 590 | 79 | 103 | 83 | 325 | 0.49 (0.41-0.57) | 0.76 (0.72-0.80) |
| Benitz et al,31 1998 | United States (3) | Prospective | BW, NR; mean GA, 32 wk | 10 | No | 184 | 33 | 41 | 20 | 90 | 0.62 (0.48-0.75) | 0.69 (0.60-0.77) |
| Bohnhorst et al,20 2012 | Germany (1) | Prospective | BW, NR; mean GA, 28 wk | 10 | Yes | 170 | 40 | 18 | 18 | 94 | 0.69 (0.55-0.80) | 0.84 (0.76-0.90) |
| Boo et al,38 | Malaysia (1) | Prospective | BW, NR; mean GA, 32 wk | 1 | Yes | 86 | 10 | 7 | 7 | 62 | 0.59 (0.33-0.82) | 0.90 (0.80-0.96) |
| Bustos and Araneda,34 2012 | Chile (1) | Prospective | BW, NR; GA, 23-35 wk | 111 | No | 53 | 3 | 1 | 22 | 27 | 0.12 (0.03-0.31) | 0.96 (0.82-1.00) |
| Chan and Ho,28 1997 | Singapore (1) | Prospective | BW, NR; mean GA, 28 wk | 10 | Yes | 152 | 14 | 26 | 16 | 96 | 0.47 (0.28-0.66) | 0.79 (0.70-0.86) |
| Choo et al,30 2012 | Korea (1) | Prospective | BW, NR; GA, >29 wk | 10 | Yes | 12 | 1 | 2 | 6 | 3 | 0.14 (0.00-0.58) | 0.60 (0.15-0.95) |
| Decembrino et al,21 2015 | Italy (1) | Prospective | BW, NR; GA, NR | 6 | No | 41 | 4 | 11 | 4 | 22 | 0.50 (0.16-0.84) | 0.67 (0.48-0.82) |
| Doellner et al,22 1998 | Norway (1) | Retrospective | BW, NR; mean GA, 38 wk | 10 | Yes | 22 | 5 | 6 | 2 | 9 | 0.71 (0.29-0.96) | 0.60 (0.32-0.84) |
| Fendler et al,23 2008 | Poland (1) | Retrospective | BW, NR; mean GA, 29 wk | 2.2 | No | 78 | 48 | 3 | 16 | 11 | 0.75 (0.63-0.85) | 0.79 (0.49-0.95) |
| Hisamuddin et al,40 2015 | Pakistan (1) | Prospective | BW, >1500 g; GA, >32 wk | 5 | Yes | 56 | 11 | 9 | 27 | 9 | 0.29 (0.15-0.46) | 0.50 (0.26-0.74) |
| Jacquot et al,24 2009 | France (1) | Prospective | BW NR; median GA, 28 wk | 10 | Yes | 73 | 17 | 6 | 13 | 37 | 0.57 (0.37-0.75) | 0.86 (0.72-0.95) |
| Kipfmueller et al,25 2015 | Germany (1) | Prospective | BW, NR; mean GA, 28 wk | 10 | Yes | 25 | 3 | 3 | 4 | 15 | 0.43 (0.10-0.82) | 0.83 (0.59-0.96) |
| Kordek et al,26 2014 | Poland (1) | Prospective | BW, NR; median GA, 30 wk | 5 | Yes | 52 | 23 | 5 | 11 | 13 | 0.68 (0.49-0.83) | 0.72 (0.47-0.90) |
| Kumar et al,39 2010 | Kenya (1) | Prospective | BW, NR; GA, NR | 5 | Yes | 142 | 55 | 60 | 1 | 26 | 0.98 (0.90-1.00) | 0.30 (0.21-0.41) |
| Ng et al,29 1997 | Hong Kong (1) | Prospective | BW, NR; mean GA 30 wk | 10 | Yes | 101 | 27 | 2 | 18 | 54 | 0.60 (0.44-0.74) | 0.96 (0.88-1.00) |
| Pynn et al,32 2015 | United States (1) | Prospective | BW, NR; median GA, 28 wk | 10 | Yes | 139 | 30 | 35 | 7 | 67 | 0.81 (0.65-0.92) | 0.66 (0.56-0.75) |
| Seibert et al,35 1990 | Australia (1) | Prospective | BW, NR, GA, 23-31 wk | 10 | Yes | 100 | 13 | 30 | 10 | 47 | 0.57 (0.34-0.77) | 0.61 (0.49-0.72) |
| Sherwin et al,36 2008 | New Zealand (1) | Prospective | BW, NR; GA, 23-42 wk | 18 | No | 52 | 14 | 1 | 20 | 17 | 0.41 (0.25-0.59) | 0.94 (0.73-1.00) |
| Utkarshni et al,41 2018 | India (1) | Prospective | BW, NR; GA, NR | 6 | Yes | 50 | 6 | 11 | 3 | 30 | 0.67 (0.30-0.93) | 0.73 (0.57-0.86) |
| Verboon-Maciolek et al,27 2006 | Netherlands (1) | Prospective | BW, NR; median GA, 29 wk | 14 | No | 66 | 24 | 14 | 13 | 15 | 0.65 (0.47-0.80) | 0.52 (0.33-0.71) |
Abbreviations: BW, birth weight; CRP, C-reactive protein; GA, gestational age; NR, not reported.
SI conversion factor: To convert CRP levels to nanomoles per liter, multiply by 9.524.
Figure 2. Summary of Risk of Bias in Included Studies.
Sixteen studies used a prespecified serum CRP level to determine the threshold level (cutoff) for a positive test. Those thresholds ranged from 1 to 12 mg/L, with most studies (14 of 16) using a cutoff CRP level between 5 and 10 mg/L. None of the studies reported sensitivity and specificity at multiple thresholds. Six studies determined the CRP threshold level retrospectively by modeling the area under the ROC curve.21,23,27,31,34,36 These studies determined thresholds ranging from 2.2 to 111 mg/L.
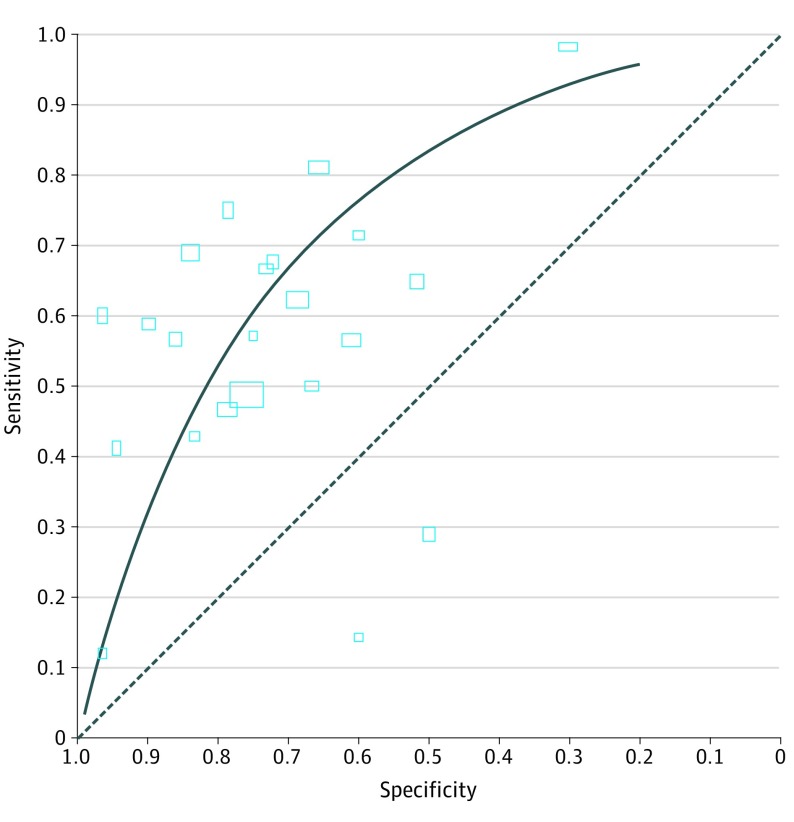
At the reported median specificity (0.74), sensitivity was 0.62 (95% CI, 0.50-0.72); at the reported lower quartile specificity (0.61), sensitivity was 0.76 (95% CI, 0.66-0.83); at the reported upper quartile specificity (0.84), sensitivity was 0.45 (95% CI, 0.34-0.57). See Figure 3 for the summary ROC curve and Figure 4 for forest plots. As is common with meta-analyses of diagnostic accuracy studies, there was substantial heterogeneity among the studies, with sensitivity and specificity estimates varying widely among them.
Figure 3. Summary Receiver Operating Characteristic (ROC) Plot of C-Reactive Protein for Neonatal Infection.
Study estimates of sensitivity and specificity are shown with the summary ROC curve. The dotted line indicates sensitivity equal to 1 minus specificity. Rectangle size is scaled to the inverse standard error.
Figure 4. Coupled Forest Plot Showing Sensitivity and Specificity of C-Reactive Protein (CRP) for Diagnosing Late-Onset Infection.
FN represents false-negative results; FP, false-positive results; NR, mean or median gestational age not reported; TP, true-positive results; TN, true-negative results. Squares represent mean values, with error bars representing 95% CIs. To convert CRP levels to nanomoles per liter, multiply by 9.524.
We used these data for sensitivity (0.62) and reported median specificity (0.74) to estimate posttest probabilities after a “positive” or “negative” CRP test result for a range of pretest probabilities in infants being evaluated for possible late-onset infection and receiving a CRP test (eTable in the Supplement).
The prevalence rates of late-onset infection in the included studies ranged from 20% to 82% (median, 40%; interquartile range, 27%-61%). We applied the diagnostic test accuracy estimates for sensitivity (0.62) and median specificity (0.74) from our meta-analysis to a hypothetical cohort of 1000 neonates with a prevalence rate of infection of 20% (resulting in a median of 76 cases of infection being missed and 208 cases being wrongly diagnosed as having infection), 40% (152 cases of infection would be missed, and 156 would be wrongly diagnosed as having infection), or 60% (228 cases would be missed, and 104 would be wrongly diagnosed as having infection). Adding serum CRP level to the assessment of an infant with a 40% pretest probability of late onset infection generated posttest probabilities of 26% for a negative test result and 61% for a positive test result.
Bivariate meta-analysis of studies using a threshold of 5 to 10 mg/L found similar estimates of sensitivity (0.61; 95% CI, 0.49-0.72) and specificity (0.73; 95% CI, 0.64-0.80) as the main analyses, including all thresholds. See eFigure 1 in the Supplement for the summary ROC curve.
When covariates with thresholds above or below 5 to 10 mg/L were added, likelihood ratio tests found no statistically significant difference in goodness of fit for any of these models compared with those without covariates.
Six studies did not report using a predefined threshold. There were no statistically significant differences in goodness of fit between any of these models, including a covariate for predefined threshold compared with models without covariates.
One study was an outlier and reported using a cutoff CRP level of 111 mg/L.34 Removing the study did not change effect estimates; at median specificity (0.73), sensitivity was 0.63 (95% CI, 0.52-0.73). We conducted a sensitivity analysis to explore the diagnostic performance of CRP in studies that included only preterm infants. That analysis is presented in eFigure 2 in the Supplement. Published reports of the included studies did not provide sufficient detail to enable us to conduct any of the planned metaregression analyses.
Visual assessment of the funnel plot did not identify important asymmetry and the Deeks test was not statistically significant (eFigure 3 in the Supplement). However, this finding does not rule out publication bias because the Deeks test lacks power, particularly in the presence of high heterogeneity.
Discussion
This systematic review and meta-analysis suggests that the serum CRP level at initial evaluation of an infant with suspected late-onset infection is unlikely to aid diagnosis or help triage infants for further investigation or treatment. Using the pooled estimate of sensitivity (0.62) at median specificity (0.74) and assuming a 40% prevalence rate of true infection (the median for the included studies) in a hypothetical cohort of 1000 newborn infants, assessing serum CRP level alone would miss 152 cases of infection (false-negative results) and wrongly diagnose 156 cases (false-positive results). For an individual infant with a 40% pretest probability of late-onset infection, adding the serum CRP level to the assessment would generate a posttest probability of 26% for a negative test (does not “rule out” infection) and a posttest probability of 61% for a positive test (does not “rule in” infection).
These findings are similar to those in a systematic review and meta-analysis examining the accuracy of serum CRP levels for diagnosing serious infection in children (aged 1 month to 18 years) with febrile illness.42 The possible explanations for the suboptimal diagnostic accuracy include the potential for false-positive results if CRP levels are increased by triggers, such as inflammation due to extravasation, cholestasis, or gastrointestinal pathology.43 Conversely, serum CRP levels may not increase, or increase only slowly, in some infants with infection, particularly very preterm infants with coagulase-negative staphylococcal bacteremia.13,44
Strengths and Limitations
We used methods to reduce reviewer error and bias, including independent and duplicate study selection as well as double-checking data extraction and risk of bias assessments. We assessed the included studies to be at low risk of bias but some use of the “unclear” category was unavoidable owing to missing detail in study reports. To ensure our analyses drew on the most complete evidence base possible, we complemented our comprehensive literature searches with a pragmatic but thorough approach to contacting study authors. This enabled us to use unpublished data and clarify important method questions for several of the included studies. We excluded case-control studies because this design is unlikely to allow for valid assessment of diagnostic test accuracy in this clinical context. We also excluded studies at high risk of incorporation bias (those in which the serum CRP level was part of the reference standard) because these studies overestimate test performance. The only “high” risk of bias was identified in a retrospective study in which the result of the reference standard was known before the index test was performed (although the laboratory test result was not likely to have been affected by this knowledge).
Heterogeneity in the estimates of sensitivity and specificity was evident on forest plot inspection, a well-recognized feature in reviews of diagnostic test accuracy.45,46 Although variations in case definition may have contributed to differences in the rates of confirmed infection in studied cohorts, it was likely that between-study differences in thresholds for investigating suspected infection were also important factors. Other factors might have been associated with the observed heterogeneity in findings, such as gestational age, blood sampling techniques, and methods used to determine the serum CRP level. However, the included studies provided insufficient details to explore the potential association of these factors.
The serum CRP cutoff level for a “positive” test used in the included studies was typically between 5 and 10 mg/L, consistent with current use in clinical practice. In most studies, the threshold was predefined, and the estimates of test performance were based on this cutoff for a positive test. Six studies did not predefine a cutoff for positivity. Five of these studies calculated levels between 2.2 and 18 mg/L (with 2 studies finding optimal cutoffs between 5 and 10 mg/L). One study was an outlier with an optimal cutoff of 111 mg/L.34 Neither the published study nor any unpublished data we received from the authors explained this unexpectedly high threshold. However, in a sensitivity analysis conducted without this study, there was no change in the estimates of sensitivity at the median, upper, or lower quartiles of specificity reported in the included studies.
The reference standard was microbiologically confirmed late-onset infection (more than 72 hours after birth) including bacteremia, fungemia, meningitis, osteomyelitis, septic arthritis, and peritonitis. There are concerns about how fully this reference standard defines all infants who truly have late-onset infection. Microbiological cultures may not detect cases of bacteremia or fungemia if an insufficient volume of the infant's blood is incubated. Conversely, microbiological cultures may also generate false-positive results if blood sampling techniques allow entry of contaminating microorganisms (typically coagulase-negative staphylococci from the infant’s skin).47 Insufficient data were available to undertake a subgroup analysis of infection with coagulase-negative staphylococci vs other bacteria to explore whether test accuracy was associated with the likelihood of identified microorganisms representing true bloodstream infections. Any such analysis, however, may be confounded by between-species differences in the capacity of microorganisms to trigger inflammatory cascades and in the generation of CRP.
We accepted the primary study authors’ definitions of late-onset infection based on the infant’s age at assessment. In the studies that provided this information (13 of 22), definitions ranged from 48 hours (3 studies) to 6 days after birth. The 3 studies that used the earlier onset definition contributed 158 of 2255 infants to our meta-analyses. Although using this earlier onset deviated from our proposed definition of more than 72 hours after birth, we adopted this broader definition to maximize available data and to reflect the variation in definitions of late onset that exists in clinical practice.48
Most included studies have assessed the accuracy of elevated serum CRP levels for diagnosing late-onset infection in preterm infants in neonatal units of high- or middle-income countries. Although these data are likely, therefore, to be applicable to preterm infants cared for in modern neonatal units in high- and (some) middle-income countries, the present review findings are less likely to be generalized to resource-limited settings in low- or middle-income countries in which the epidemiology, microbiology, pathogenesis, treatment options, and outcomes for late-onset infection in newborn infants differ.49,50
Our findings are specific to the accuracy of the serum CRP level in determining whether infection is less or more likely among infants in whom there is a suspicion based on clinical signs (ie, for diagnosing infection). The present review does not address whether serial monitoring of the serum CRP level may be useful in screening well neonates for infection before it is suspected clinically or in assessing the response to treatment, including helping to decide whether to stop antibiotics or rule out infection when CRP levels fail to increase.51 C-reactive protein level is frequently used in these contexts in current clinical practice; but as detection of an increased serum CRP level (index test) would be used to trigger application of the reference test, it is not possible to measure diagnostic accuracy. Rather, intervention studies should address this separate question and evaluate the diagnostic utility of using the serum CRP level to inform clinical decisions in these contexts.
Similarly, in clinical practice, CRP may not be the only biomarker that is measured in newborn infants with suspected sepsis. Our review investigated only the diagnostic test accuracy of CRP alone, not of CRP when used in conjunction with other tests.
Conclusions
The timely diagnosis of late-onset infection on the basis of clinical features and signs in newborn infants, particularly very preterm infants, remains challenging.52 Given the poor performance of serum CRP levels in this context, research efforts might focus on other serum biomarkers, such as procalcitonin, that are increased more quickly in response to infection or inflammation.13 Newer methods using molecular markers to identify pathogenic microorganisms (such as real-time polymerase chain reaction or microarray techniques) are worthy of further research. Those new techniques can provide results more quickly than standard microbiological culture (6-8 hours vs 24-36 hours), and evidence of their diagnostic accuracy is accumulating.15
Quick and accurate diagnosis of late-onset infection in newborn infants remains an important goal in clinical practice worldwide. Results from our systematic review and meta-analysis that included data from 2255 infants (22 studies) do not support the use of serum CRP level in this context. Although quick, the use of serum CRP level does not appear to be sufficiently accurate to aid in a diagnosis that is based on clinical features or to support treatment decisions with the aim of avoiding unnecessary administration of antibiotics.
eAppendix 1. Search Strategy
eAppendix 2. Changes Between Protocol and Review
eTable. Post-Test Probabilities for Late-Onset Infection for a Sample of Population Prevalence
eFigure 1. SROC Plot for Studies With CRP Threshold 5-10 mg/L (Bivariate Meta-analysis)
eFigure 2. SROC Plot of CRP for Preterm Infants Only
eFigure 3. Deeks’ Funnel Plot (Asymmetry Test)
References
- 1.McGuire W, Clerihew L, Fowlie PW. Infection in the preterm infant. BMJ. 2004;329(7477):1277-1280. doi: 10.1136/bmj.329.7477.1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shah J, Jefferies AL, Yoon EW, Lee SK, Shah PS; Canadian Neonatal Network . Risk factors and outcomes of late-onset bacterial sepsis in preterm neonates born at <32 weeks’ gestation. Am J Perinatol. 2015;32(7):675-682. [DOI] [PubMed] [Google Scholar]
- 3.Bassler D, Stoll BJ, Schmidt B, et al. ; Trial of Indomethacin Prophylaxis in Preterms Investigators . Using a count of neonatal morbidities to predict poor outcome in extremely low birth weight infants: added role of neonatal infection. Pediatrics. 2009;123(1):313-318. doi: 10.1542/peds.2008-0377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stoll BJ, Hansen NI, Adams-Chapman I, et al. ; National Institute of Child Health and Human Development Neonatal Research Network . Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA. 2004;292(19):2357-2365. doi: 10.1001/jama.292.19.2357 [DOI] [PubMed] [Google Scholar]
- 5.Dong Y, Speer CP. Late-onset neonatal sepsis: recent developments. Arch Dis Child Fetal Neonatal Ed. 2015;100(3):F257-F263. doi: 10.1136/archdischild-2014-306213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Man P, Verhoeven BA, Verbrugh HA, Vos MC, van den Anker JN. An antibiotic policy to prevent emergence of resistant bacilli. Lancet. 2000;355(9208):973-978. doi: 10.1016/S0140-6736(00)90015-1 [DOI] [PubMed] [Google Scholar]
- 7.Muller-Pebody B, Johnson AP, Heath PT, Gilbert RE, Henderson KL, Sharland M; iCAP Group (Improving Antibiotic Prescribing in Primary Care) . Empirical treatment of neonatal sepsis: are the current guidelines adequate? Arch Dis Child Fetal Neonatal Ed. 2011;96(1):F4-F8. doi: 10.1136/adc.2009.178483 [DOI] [PubMed] [Google Scholar]
- 8.Tsai MH, Chu SM, Hsu JF, et al. Risk factors and outcomes for multidrug-resistant Gram-negative bacteremia in the NICU. Pediatrics. 2014;133(2):e322-e329. doi: 10.1542/peds.2013-1248 [DOI] [PubMed] [Google Scholar]
- 9.Schulfer A, Blaser MJ. Risks of antibiotic exposures early in life on the developing microbiome. PLoS Pathog. 2015;11(7):e1004903. doi: 10.1371/journal.ppat.1004903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tapiainen T, Koivusaari P, Brinkac L, et al. Impact of intrapartum and postnatal antibiotics on the gut microbiome and emergence of antimicrobial resistance in infants. Sci Rep. 2019;9(1):10635. doi: 10.1038/s41598-019-46964-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gasparrini AJ, Crofts TS, Gibson MK, Tarr PI, Warner BB, Dantas G. Antibiotic perturbation of the preterm infant gut microbiome and resistome. Gut Microbes. 2016;7(5):443-449. doi: 10.1080/19490976.2016.1218584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shane AL, Stoll BJ. Recent developments and current issues in the epidemiology, diagnosis, and management of bacterial and fungal neonatal sepsis. Am J Perinatol. 2013;30(2):131-141. doi: 10.1055/s-0032-1333413 [DOI] [PubMed] [Google Scholar]
- 13.Gilfillan M, Bhandari V. Biomarkers for the diagnosis of neonatal sepsis and necrotizing enterocolitis: clinical practice guidelines. Early Hum Dev. 2017;105:25-33. doi: 10.1016/j.earlhumdev.2016.12.002 [DOI] [PubMed] [Google Scholar]
- 14.Steel DMWA, Whitehead AS. The major acute phase reactants: C-reactive protein, serum amyloid P component and serum amyloid A protein. Immunol Today. 1994;15(2):81-88. doi: 10.1016/0167-5699(94)90138-4 [DOI] [PubMed] [Google Scholar]
- 15.Pammi M, Flores A, Versalovic J, Leeflang MM. Molecular assays for the diagnosis of sepsis in neonates. Cochrane Database Syst Rev. 2017;2:CD011926. doi: 10.1002/14651858.CD011926.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whiting PF, Rutjes AW, Westwood ME, et al. ; QUADAS-2 Group . QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529-536. doi: 10.7326/0003-4819-155-8-201110180-00009 [DOI] [PubMed] [Google Scholar]
- 17.Review Manager (RevMan) [computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration; 2014.
- 18.Rutter CM, Gatsonis CA. A hierarchical regression approach to meta-analysis of diagnostic test accuracy evaluations. Stat Med. 2001;20(19):2865-2884. doi: 10.1002/sim.942 [DOI] [PubMed] [Google Scholar]
- 19.Deeks JJMP, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol. 2005;58(9):882-893. doi: 10.1016/j.jclinepi.2005.01.016 [DOI] [PubMed] [Google Scholar]
- 20.Bohnhorst B, Lange M, Bartels DB, Bejo L, Hoy L, Peter C. Procalcitonin and valuable clinical symptoms in the early detection of neonatal late-onset bacterial infection. Acta Paediatr. 2012;101(1):19-25. doi: 10.1111/j.1651-2227.2011.02438.x [DOI] [PubMed] [Google Scholar]
- 21.Decembrino L, De Amici M, Pozzi M, De Silvestri A, Stronati M. Serum calprotectin: a potential biomarker for neonatal sepsis. J Immunol Res. 2015;2015:147973. doi: 10.1155/2015/147973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doellner H, Arntzen KJ, Haereid PE, Aag S, Austgulen R. Interleukin-6 concentrations in neonates evaluated for sepsis. J Pediatr. 1998;132(2):295-299. doi: 10.1016/S0022-3476(98)70448-2 [DOI] [PubMed] [Google Scholar]
- 23.Fendler WM, Piotrowski AJ. Procalcitonin in the early diagnosis of nosocomial sepsis in preterm neonates. J Paediatr Child Health. 2008;44(3):114-118. doi: 10.1111/j.1440-1754.2007.01230.x [DOI] [PubMed] [Google Scholar]
- 24.Jacquot A, Labaune JM, Baum TP, Putet G, Picaud JC. Rapid quantitative procalcitonin measurement to diagnose nosocomial infections in newborn infants. Arch Dis Child Fetal Neonatal Ed. 2009;94(5):F345-F348. doi: 10.1136/adc.2008.155754 [DOI] [PubMed] [Google Scholar]
- 25.Kipfmueller F, Schneider J, Prusseit J, et al. Role of neutrophil CD64 index as a screening marker for late-onset sepsis in very low birth weight infants. PLoS One. 2015;10(4):e0124634. doi: 10.1371/journal.pone.0124634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kordek A, Łoniewska B, Podraza W, Nikodemski T, Rudnicki J. Usefulness of estimation of blood procalcitonin concentration versus C-reactive protein concentration and white blood cell count for therapeutic monitoring of sepsis in neonates. Postepy Hig Med Dosw (Online). 2014;68:1516-1523. doi: 10.5604/17322693.1133101 [DOI] [PubMed] [Google Scholar]
- 27.Verboon-Maciolek MA, Thijsen SF, Hemels MA, et al. Inflammatory mediators for the diagnosis and treatment of sepsis in early infancy. Pediatr Res. 2006;59(3):457-461. doi: 10.1203/01.pdr.0000200808.35368.57 [DOI] [PubMed] [Google Scholar]
- 28.Chan DK, Ho LY. Usefulness of C-reactive protein in the diagnosis of neonatal sepsis. Singapore Med J. 1997;38(6):252-255. [PubMed] [Google Scholar]
- 29.Ng PC, Cheng SH, Chui KM, et al. Diagnosis of late onset neonatal sepsis with cytokines, adhesion molecule, and C-reactive protein in preterm very low birthweight infants. Arch Dis Child Fetal Neonatal Ed. 1997;77(3):F221-F227. doi: 10.1136/fn.77.3.F221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choo YK, Cho HS, Seo IB, Lee HS. Comparison of the accuracy of neutrophil CD64 and C-reactive protein as a single test for the early detection of neonatal sepsis. Korean J Pediatr. 2012;55(1):11-17. doi: 10.3345/kjp.2012.55.1.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benitz WE, Han MY, Madan A, Ramachandra P. Serial serum C-reactive protein levels in the diagnosis of neonatal infection. Pediatrics. 1998;102(4):E41. doi: 10.1542/peds.102.4.e41 [DOI] [PubMed] [Google Scholar]
- 32.Pynn JM, Parravicini E, Saiman L, Bateman DA, Barasch JM, Lorenz JM. Urinary neutrophil gelatinase-associated lipocalin: potential biomarker for late-onset sepsis. Pediatr Res. 2015;78(1):76-81. doi: 10.1038/pr.2015.62 [DOI] [PubMed] [Google Scholar]
- 33.Beltempo M, Viel-Thériault I, Thibeault R, Julien AS, Piedboeuf B. C-reactive protein for late-onset sepsis diagnosis in very low birth weight infants. BMC Pediatr. 2018;18(1):16. doi: 10.1186/s12887-018-1002-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bustos BR, Araneda CH. Procalcitonin for the diagnosis of late onset sepsis in newborns of very low birth weight [in Spanish]. Rev Chilena Infectol. 2012;29(5):511-516. doi: 10.4067/S0716-10182012000600005 [DOI] [PubMed] [Google Scholar]
- 35.Seibert K, Yu VY, Doery JC, Embury D. The value of C-reactive protein measurement in the diagnosis of neonatal infection. J Paediatr Child Health. 1990;26(5):267-270. doi: 10.1111/j.1440-1754.1990.tb01069.x [DOI] [PubMed] [Google Scholar]
- 36.Sherwin C, Broadbent R, Young S, et al. Utility of interleukin-12 and interleukin-10 in comparison with other cytokines and acute-phase reactants in the diagnosis of neonatal sepsis. Am J Perinatol. 2008;25(10):629-636. doi: 10.1055/s-0028-1090585 [DOI] [PubMed] [Google Scholar]
- 37.Aminullah A, Sjachroel DN, Hadinegoro SR, Madiyono B. The role of plasma C-reactive protein in the evaluation of antibiotic treatment in suspected neonatal sepsis. Med J Indones. 2001;10(1):16-21. doi: 10.13181/mji.v10i1.3 [DOI] [Google Scholar]
- 38.Boo NY, Nor Azlina AA, Rohana J. Usefulness of a semi-quantitative procalcitonin test kit for early diagnosis of neonatal sepsis. Singapore Med J. 2008;49(3):204-208. [PubMed] [Google Scholar]
- 39.Kumar R, Musoke R, Macharia WM, Revathi G. Validation of C-reactive protein in the early diagnosis of neonatal sepsis in a tertiary care hospital in Kenya. East Afr Med J. 2010;87(6):255-261. [DOI] [PubMed] [Google Scholar]
- 40.Hisamuddin E, Hisam A, Wahid S, Raza G. Validity of C-reactive protein (CRP) for diagnosis of neonatal sepsis. Pak J Med Sci. 2015;31(3):527-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Utkarshni SJ, Paul S, Singh K, Neki NS. Role of procalcitonin as diagnostic marker in neonatal sepsis and its correlation with clinical, biochemical and haematological profile. Int J Curr Res Med Sci. 2018;4:27-39. [Google Scholar]
- 42.Van den Bruel A, Thompson MJ, Haj-Hassan T, et al. Diagnostic value of laboratory tests in identifying serious infections in febrile children: systematic review. BMJ. 2011;342:d3082. doi: 10.1136/bmj.d3082 [DOI] [PubMed] [Google Scholar]
- 43.Hofer N. Müller W, Resch B. Chapter 4: the role of C-reactive protein in the diagnosis of neonatal sepsis In: Resch B, et al. Neonatal Bacterial Infection. London, UK: InTech; 2013. doi: 10.5772/54255 [DOI] [Google Scholar]
- 44.Lai MYTM, Tsai MH, Lee CW, et al. Characteristics of neonates with culture-proven bloodstream infection who have low levels of C-reactive protein (≤10 mg/L). BMC Infect Dis. 2015;15:320. doi: 10.1186/s12879-015-1069-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reitsma JBGA, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol. 2005;58(10):982-990. doi: 10.1016/j.jclinepi.2005.02.022 [DOI] [PubMed] [Google Scholar]
- 46.Naaktgeboren CAOE, Ochodo EA, Van Enst WA, et al. Assessing variability in results in systematic reviews of diagnostic studies. BMC Med Res Methodol. 2016;16:6. doi: 10.1186/s12874-016-0108-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oeser C, Lutsar I, Metsvaht T, Turner MA, Heath PT, Sharland M. Clinical trials in neonatal sepsis. J Antimicrob Chemother. 2013;68(12):2733-2745. doi: 10.1093/jac/dkt297 [DOI] [PubMed] [Google Scholar]
- 48.Haque KN. Definitions of bloodstream infection in the newborn. Pediatr Crit Care Med. 2005;6(3)(suppl):S45-S49. doi: 10.1097/01.PCC.0000161946.73305.0A [DOI] [PubMed] [Google Scholar]
- 49.Zea-Vera A, Ochoa TJ. Challenges in the diagnosis and management of neonatal sepsis. J Trop Pediatr. 2015;61(1):1-13. doi: 10.1093/tropej/fmu079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vergnano S, Sharland M, Kazembe P, Mwansambo C, Heath PT. Neonatal sepsis: an international perspective. Arch Dis Child Fetal Neonatal Ed. 2005;90(3):F220-F224. doi: 10.1136/adc.2002.022863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ehl S, Gering B, Bartmann P, Högel J, Pohlandt F. C-reactive protein is a useful marker for guiding duration of antibiotic therapy in suspected neonatal bacterial infection. Pediatrics. 1997;99(2):216-221. doi: 10.1542/peds.99.2.216 [DOI] [PubMed] [Google Scholar]
- 52.Verstraete EHBK, Blot K, Mahieu L, Vogelaers D, Blot S. Prediction models for neonatal health care-associated sepsis: a meta-analysis. Pediatrics. 2015;135(4):e1002-e1014. doi: 10.1542/peds.2014-3226 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Search Strategy
eAppendix 2. Changes Between Protocol and Review
eTable. Post-Test Probabilities for Late-Onset Infection for a Sample of Population Prevalence
eFigure 1. SROC Plot for Studies With CRP Threshold 5-10 mg/L (Bivariate Meta-analysis)
eFigure 2. SROC Plot of CRP for Preterm Infants Only
eFigure 3. Deeks’ Funnel Plot (Asymmetry Test)