Background
The exponential rise in obesity and prevalence of alcohol misuse has resulted in liver disease becoming the third most common cause of death in the working-age population.1 A key aspect of managing patients with liver disease, from the stages of compensated cirrhosis through to liver failure, is early recognition and treatment of malnutrition. Malnutrition is defined as a deficiency or excess (or imbalance) of energy, protein and other nutrients and is most often associated with undernutrition.2 3 In cirrhosis, this is closely associated with a reduction in muscle mass, function and strength, known as sarcopenia.4 Collectively, malnutrition and sarcopenia negatively impact on a patients’ ability to complete daily tasks, quality of life, liver-related morbidity and mortality.1 5–10 Given the increasing prevalence of obesity, diabetes and their association with non-alcoholic fatty liver disease (NAFLD), an imbalance of nutritional intake is becoming increasingly observed in patients with cirrhosis.11 12 The resultant combination of adiposity and sarcopenia, termed ‘sarcopenic obesity’, poses unique nutritional challenges, with regard to optimising metabolic risk factors (ie, weight loss, glycaemic/lipid control) and muscle function.
Recently published guidelines by European Association for Studies of Liver (EASL)3 and European Society for Clinical Nutrition and Metabolism (ESPEN)13 provide comprehensive overviews of nutrition in cirrhosis. Herein, we provide a case-based practical guide of the causes of malnutrition, assessments tools, daily requirements and dietary interventions in both compensated and decompensated cirrhosis.
Case Part 1: compensated liver cirrhosis
A 49-year-old man, with NAFLD cirrhosis and portal hypertension, presented with fatigue, tiredness and unintentional weight loss (3 kg over 6 months). He had a past medical history of type 2 diabetes mellitus, hypertension and hyperlipidaemia. Current medications included Metformin 1 g two times per day, Amlodipine 10 mg one time per day and Atorvastatin 20 mg one time day. He never smoked and drank 0–2 units of alcohol per week. He was fully independent with his activities of daily living, however admitted to no additional exercise.
Clinical examination revealed no signs of liver failure. With the exception of mild splenomegaly, examination was unremarkable. His bloods revealed well-compensated disease (table 1), with Child-Pugh A cirrhosis and a model for end-stage liver disease score of 11. Abdominal ultrasound scan confirmed splenomegaly (15 cm) and a patent portal vein. His recent upper gastrointestinal (GI) endoscopy showed mild portal hypertensive gastropathy with small oesophageal varices.
Table 1.
Test results
| First outpatient review (compensated cirrhosis) | Inpatient (decompensated cirrhosis) |
Discharge follow-up (pre-transplant assessment) |
| Blood tests | ||
| Na+ 135 (mmol/L) | Na+ 126 (mmol/L) | Na+ 135 (mmol/L) |
| K+ 4.3 (mmol/L) | K+ 4.9 (mmol/L) | K+ 4.3 (mmol/L) |
| Urea 3 (mmol/L) | Urea 2.1 (mmol/L) | Urea 6.0 (mmol/L) |
| Creatinine (70 mmol/L) | Creatinine 110 (mmol/L) | Creatinine 80 (mmol/L) |
| HbA1c 58 (mmol/L) | HbA1c 65 (mmol/L) | HbA1c 55 (mmol/L) |
| Albumin 38 (g/L) | Albumin 28 (g/L) | Albumin 32 (g/L) |
| ALT 50 (IU/L) | ALT 50 (IU/L) | ALT 49 (IU/L) |
| ALP 112 (IU/L) | ALP 127 (IU/L) | ALP 120 (IU/L) |
| Bilirubin 21 (μmol/L) | Bilirubin 60 (μmol/L) | Bilirubin 50 (μmol/L) |
| WCC 5.1 (109/L) | WCC 5.1 (109/L) | WCC 6.0 (109/L) |
| HB 135 (g/dL) | HB 85 (g/dL) | HB 110 (g/dL) |
| Platelets 90 | Platelets 70 | Platelets 87 |
| INR 1.2 | INR 1.6 | INR 1.5 |
| Child-Pugh A | Child-Pugh C | Child-Pugh C |
| Anthropometry | ||
| MAMC 28.3 cm (50th centile) | MAMC 26.7 cm (25-50th centile) | MAMC 29.1 cm (50-75th centile) |
| HGS 30 (75% normal) | HGS 23 (58% normal) | HGS 30 (75% normal) |
ALT, alkaline phosphatase; HB, haemoglobin; HbA1c, haemoglobin A1c; HGS, hand grip strength; INR, international normalised ratio; MAMC, mid arm muscle circumference; WCC, white cell count.
Dietetic intake consisted of high fat content foods (crisps, chips, fried meats, fizzy drinks), but infrequent breakfasts. His current body weight was 108 kg, with a body mass index (BMI) 36 kg/m2. His hand grip strength in his non-dominant hand was 30 kg (75% normal) and his mid arm muscle circumference (MAMC) was 28.3 cm (50 centile).
Review of case (Part 1)
What are the mechanisms of malnutrition in liver cirrhosis
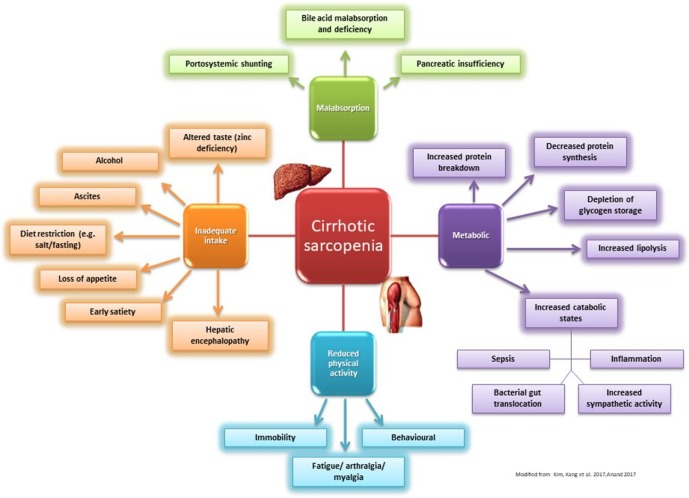
Malnutrition in liver cirrhosis is multi-factorial and is summarised in figure 1. Energy supply must balance total energy expenditure (TEE) to maintain nutritional equilibrium. TEE includes a combination of resting energy expenditure, physical activity expenditure and food-related thermogenesis.3 14 Even though physical activity is reduced in many patients with liver cirrhosis (hospitalised, fatigue and sarcopenia), the TEE ranges from 28 to 37.5 kcal/kg BW/day.15 16 This is largely due to hypermetabolism and a state of ‘accelerated starvation’, in which the main fuel source switches from glucose to fatty acids.15 17 In the fasted state, healthy individuals maintain their hepatic glycogen reserves by gluconeogenesis from amino acids derived from muscle protein. However, as patients with cirrhosis have poor glycogen reserves, an overnight fast is the equivalent of a 3-day fast in healthy individuals. Therefore, patients with cirrhosis have excessive muscle protein breakdown (proteolysis) to mobilise amino acids to maintain gluconeogenesis, thereby resulting in sarcopenia. This process is further enhanced by poor dietary intake (impaired gut motility, loss of appetite, altered taste, salt restriction, iatrogenic fasting for clinical investigations, alcohol dependence, encephalopathy); malabsorption (portal hypertension induced enteropathy, bile acid deficiency, bacterial overgrowth and variceal haemorrhage) and ascitic protein loss.3 13 15 18
Figure 1.
Causes and risk factors of sarcopenia in liver cirrhosis.
Which tools are available for nutritional screening
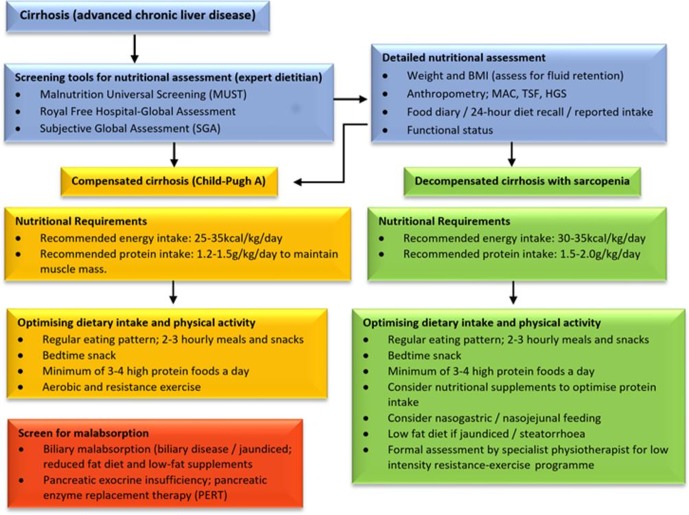
All patients with liver cirrhosis, irrespective of BMI, should be screened and assessed for malnutrition. This can be performed using validated screening tools, anthropometry, functional assessment; and detailed food and diet history (figure 2). 24-hour diet intake recall is quick and can be specifically adapted to the individual, however is reliant on the patient’s ability to do so. Alternatively, a 3-day to 7-day food diary provides a more detailed overview of typical intake.
Figure 2.
Assessment and management of malnutrition in liver cirrhosis.
Nutritional screening tools available to identify those at risk of malnutrition, which include the standard Malnutrition Universal Screening Tool (MUST) and the Nutritional Screening Risk-2002. They require further validation for use in patients with liver cirrhosis. The Royal Free Hospital nutritional prioritising tool score uses a short algorithm similar to the MUST but adapted to cirrhosis.19 20 The latter is quick (3 min), predicts clinical deterioration and transplant‐free survival. However, it is limited by subjectivity and has a low negative predictive value.3 13
BMI alone can be used in initial screening for malnutrition; however, careful adjustments are needed in patients with fluid retention. A dry body weight and BMI should be recorded, using weight following paracentesis or via estimation by subtracting a percentage of body weight based on ascites (mild 5%: moderate 10%; and severe 15%) and peripheral oedema (5% if bilateral).3 21 22 Due to the increasing prevalence of sarcopenic obesity in patients with cirrhosis,3 12 23 24 undernutrition (especially protein intake) should be formally assessed in all, irrespective of BMI.
For example, in a 80 kg patient with mild ascites, estimated dry weight would be 80 kg −(5% of 80=4 kg)=76 kg.
Anthropometry can be carried out to obtain measures of muscle mass (MAMC) and contractile function (hand grip strength), both of which predict mortality.25 26 Hand grip strength should be performed three times in the non-dominant hand and compared with historical ‘normal’ values for women (29 kg) and men (40 kg). MAMC is obtained by measuring the mid arm circumference (MAC) which incorporates muscle and adipose and tricep skin fold (TSF), an estimate of adipose thickness2 13 :
MAMC=MAC – (3.14 × TSF). MAMC and MAC in cm, TSF in mm.
Functional status can be assessed immediately as the patient arrives to a consultation: Do they walk unaided? What is the strength of patients hand shake? Are they able rise from a chair? Assess how well a person performs their activities of daily living and their perceived exercise tolerance.
Direct, validated measures of functional ability in cirrhosis include short physical performance battery testing, incremental shuttle walk tests and most recently the liver frailty index, which incorporates hand grip strength, timed chair stands and balance. The liver frailty index, which has only been validated in American cohorts to date, is a predictor of complications of cirrhosis, liver-related death and post-transplant outcomes.27 28
Energy and protein requirements
Understanding a patient’s energy expenditure is key o accurately calculate their nutritional energy requirements to protect and optimise muscle function. Indirect calorimetry (uses oxygen uptake, carbon dioxide production ± nitrogen excretion to determine this) is the most accurate method of doing so, but is impractical in clinic. Therefore, the following international targets are used3 13:
Compensated cirrhosis (Child-Pugh A)
Recommended energy intake: 25–35 kcal/kg/day.
Recommended protein intake: 1.2–1.5 g/kg/day to maintain muscle mass.
Decompensated cirrhosis with sarcopenia
Recommended energy intake: 30–35 kcal/kg/day.
Recommended protein intake: 1.5–2.0 g/kg/day to prevent further loss and reverse sarcopenia.
Optimising dietary intake
A regular eating pattern of 2–3 hourly meals and snacks, including a bedtime snack, should be encouraged with the aim of reducing starvation time, and minimising the breakdown of muscle and fat stores for use as a metabolic fuel. In the current case, the patient frequently missed breakfast, thereby prolonging his starvation time and accelerating the progression of sarcopenia. Optimising protein intake is also essential due to increased total body protein breakdown and reduced muscle protein synthesis.13 A minimum of 3–4 sources of high protein foods a day should be recommended including eggs and lean meat.
Fat content of food should also be addressed. In patients with obesity ± sarcopenia, several studies have shown that controlled weight loss improves outcomes with compensated cirrhosis, although with close supervision.3 29 30
It is also important to review glycaemic control, particularly in the presence of diabetes and subsequent anti-diabetic medications. Of note, haemoglobin (HbA1c) as a direct marker of glycaemic control should be used in clinical context, as it can be falsely reassuring in the context of anaemia. In this case, his HbA1c was elevated (table 1). A review of his intake identified a high sugar intake. He was advised to reduce his intake of high sugar foods/fluids (ie, fizzy drinks) and alternative snacks and fluid choices were discussed for example, protein yoghurt, cold meats.
Nutritional supplementation: oral supplementation
An adequate calorie and protein intake can be difficult to achieve, particularly for patients who have sarcopenia and nutritional supplements are often required. This typically involves the use of low volume, high protein sip feeds, tailored to each patient’s individual needs (ie, protein deficit, satiety and taste). Some examples of the commonly used nutritional supplements are summarised in table 2.
Table 2.
Nutritional Supplements profile
| Fortisip compact protein | Fortijuice | Ensure compact | Ensure plus advance | Ensure plus juice | Meritene shake | Renapro powder | Renapro Shot | ProSource plus | |
| Volume (mL) | 12 | 200 | 12 | 220 | 220 | 30 (sachet) | 20 g (sachet) | 60 | 30 |
| Calories (kcal) | 300 | 300 | 300 | 330 | 330 | 200* | 74 | 80 | 100 |
| Carbohydrate (g) | 30 | 67 | 36 | 37 | 44 | 27 g* | 0.7 g | 1.2 | 11 |
| Fat (g) | 12 (high) |
0 (low) |
12 (high) |
11 (high) |
0 (low) |
4* (low) |
0.2 (low) |
0.1 (low) |
0 (low) |
| Protein (g) | 18 (high) |
8 (low) |
13 (mod) |
20 (high) |
11 (low) |
16* (high) |
20 (high) |
20 (high) |
15 (high) |
| Protein source | Skimmed milk | Skimmed milk | Skimmed milk | Skimmed milk | Skimmed milk | Skimmed milk | Whey | Bovine | Collagen |
*Made with 200 mL semi-skimmed milk.
Websites for further details
https://www.nutriciahcp.com/adult/products/
https://ensure.com/nutrition-products
https://www.nestlehealthscience.co.uk/brands/meritene
https://www.stanningleypharma.co.uk
https://www.nestlehealthscience.co.uk/brands/meritene
Deficiencies in fat-soluble vitamins (ADEK) are common as they require bile salt transporters and undergo hepatic metabolism which is impaired in cirrhosis. It is usually more prevalent in those with biliary disease rather than parenchymal disease. This can lead to steatorrhoea and a significant increase in faecal fat excretion. Some patients will have additional malabsorption secondary to pancreatic insufficiency. In both, it is important not to advise significantly reduced fat content in a patient’s diet and to ensure their diet is adequate in all nutrients and fatty acids. Patients with pancreatic insufficiency will benefit from exogenous pancreatic enzyme replacement (eg, Creon).
Bone diseases including osteoporosis and osteomalacia are common in cirrhosis, with a higher prevalence in those with cholestasis. Standard recommendations of a lumbar spine and hip bone densitometry scan (DEXA) should be performed, in addition to laboratory calcium and vitamin D levels.3 31
Physical activity
Physical activity and exercise should be encouraged to support weight management (in individuals who are overweight or obese) and to improve muscle mass and strength; it should be individually tailored. A structured exercise programme including a warm up, a mixture of both aerobic (walking, swimming, cycling) and resistance exercise (chair stands, squats, etc), followed by a cool down for balance and flexibility training is beneficial at a moderate intensity, in which the patient can still speak a two-word to three-word sentence.4 32 Patients should also be educated on the positive effects of physical activity on other disease types, including mental health status, cardiovascular risk, diabetes and osteoarthritis.
Case Part 2: decompensated cirrhosis
After 3 months, the patient was admitted with decompensation secondary to an oesophageal variceal haemorrhage. On review, he had grade 2 hepatic encephalopathy (HE) and moderate ascites. He had progressed to Child-Pugh C cirrhosis (table 1). He underwent fluid resuscitation with intravenous albumin and blood transfusion. He was commenced on intravenous terlipressin and broad-spectrum antibiotics. There was no evidence of spontaneous bacterial peritonitis on ascitic tap. Upper GI endoscopy showed grade three oesophageal varices with active haemorrhage, subsequently managed with band ligation. His further management included rifaximin and lactulose for HE and abdominal paracentesis.
His anthropometry measures following his paracentesis showed a dry weight of 90 kg (18 kg weight loss) and a BMI of 29 kg/m2. His hand grip strength had reduced to 23 (58% normal) and MAMC of 26.7 cm (25-50th centile). He was unable to stand from a chair unaided and was mobilising with a frame.
Review of case Part 2
The importance of nutritional care at this stage involved addressing his change in requirements given his decompensation and variceal bleeding, and the need for enteral feeding.
What are the new factors affecting nutritional status?
Gross ascites can reduce dietary intake and increased protein loss. We would recommend small, frequent meals with snacks and optimising protein intake. A low sodium diet (<60 mmol sodium per day13) is recommended; this needs to be balanced with serum sodium levels, which are usually low and the degree of urinary sodium excretion, in the context of their fluid status. Patients are often on diuretics which enhance urinary loss and other drugs which contain sodium. Patients with ascites should avoid added salt (reduced amount in cooking, avoid high salt foods and avoid salt at the table).
HE does not indicate the need for a reduced protein intake, as the recommended increased protein intake has not been found to worsen HE. Enteral feeding via nasogastric (NG) tube should be considered in those with Grade III-IV HE who are unable to eat.3 33 34 BCAA can be considered in such patients with decompensated cirrhosis and HE; however, the practicalities and implementation in practice are variable.3 It is important to treat suspected vitamin B deficiency in malnourished patients or those with a history of alcohol excess, as this can enhance or cause HE in the form of Wernicke’s or Korsokoff’s syndrome.
Refeeding syndrome can occur in malnourished patients and it is important to monitor electrolytes and replace in these patients in the early stages of feeding.
Nutritional optimisation
The patient’s nutritional requirements were reviewed (35–40 kcal/kg a day for energy and 1.5 g/kg for protein)12 and due to reduced mobility, encephalopathy and ascitic fluid, his dietary intake had reduced. He was still managing three meals a day; however, his portion sizes had reduced. Low muscle mass is thought to be an independent risk factor for HE.12 To avoid long periods of fasting and minimise muscle breakdown overnight, NG enteral feeding was established to improve his energy and protein intake while awaiting his appetite to improve. Nutritional supplements (whey protein), which are low in calories and high in protein, were recommended inbetween meals. Collectively, these met his minimum daily protein requirements (130 g a day; 1.5 g/kg) and 50% of his energy requirements (3100 kcal; 35 kcal/kg). He was subsequently discharged on oral whey protein, after achieving nutritional stability.
Nutritional supplementation: enteral
Recent ESPEN and EASL guidelines support the use of enteral feeding in those who are unable to reach their requirements orally with the use of NG feeding. The evidence is limited but we recommend that varcieal banding should not delay placement of an NG tube for more than 24 hours, unless there is uncontrolled bleeding. In light of the need for high protein content, the most commonly used preparations had 7.5 g of protein/100 mL and for those requiring reduced fat content when clinically jaundiced, aim for <5 g of fat/100 mL. Percutaneous endoscopic gastrostomy should be avoided due to higher rates of complications.3 13
Parenteral nutrition should be considered when both oral feeding and enteral feeding have failed. It should involve a specialist nutrition team.
Rehabilitation
He had significant functional decline as he was previously able to ambulate independently. He was formally assessed by a physiotherapist and supported with a low-intensity resistance-exercise (eg, lunges and frog squats) programme to improve his mobility, muscle strength and psychological confidence.35
Case Part 3: decompensated cirrhosis with improved sarcopenia
Following his discharge, he was reviewed in outpatient clinic 3 months later and was able to walk into clinic unsupported (table 1). His dry weight had increased to 92 kg with a BMI 30 kg/m2. His hand grip strength had improved to 30 kg (75% of normal) with a MAMC 29.1 cm (50–75th centile).
His ascites was controlled with diuretics. He was on an endoscopic variceal banding programme. He had a degree of ongoing HE despite rifaximin and was referred for liver transplantation assessment.
Review of case Part 3
Although he had improved since his decompensation, his muscle mass and function had not yet corrected to his previous baseline. Regular monitoring through a multidisciplinary team approach is key to ensure that this patient maintains their physical fitness during assessment and while on a liver transplantation waiting list.
Summary
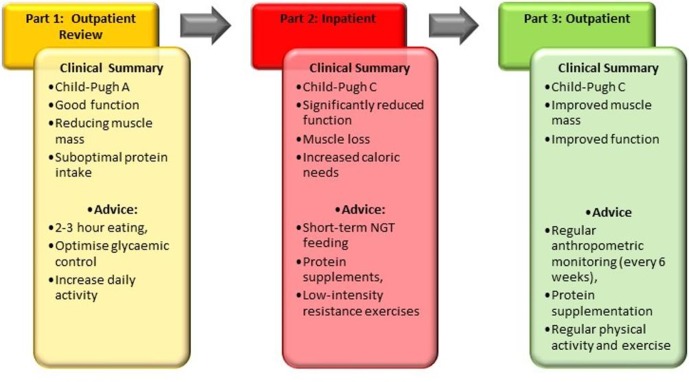
This case highlights several important and frequently encountered challenges in managing patients with liver cirrhosis. An overview is provided in figure 3. The key points are summarised later.
Figure 3.
Clinical summary and overview of advice at each stage.
Key messages
Accurate assessment of nutritional status in cirrhosis is key, including specialist dietitian input.
Protection of muscle is paramount in compensated cirrhosis
Minimise fasting time to combat prolonged starvation time and avoid muscle breakdown (3–5 frequent meals with a late evening snack)
Protein targets (1.5–2.0/kg/day) are higher in decompensated cirrhosis
Functional assessment/intervention is crucial—‘use it or lose it’
Consider symptoms of HE, ascites and malabsorption
The case used in this article is fictious and based on clinical experience.
Footnotes
Contributors: AD and MJA conceived the original article. AD drafted the original manuscript. JT contributed to the original manuscript. JT and MJA contributed to editing of the manuscript, figures and tables. MJA, AME and JML provided critical review and enhanced the quality of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Williams R, Alexander G, Aspinall R, et al. . Gathering momentum for the way ahead: fifth report of the Lancet standing Commission on liver disease in the UK. The Lancet 2018;392:2398–412. 10.1016/S0140-6736(18)32561-3 [DOI] [PubMed] [Google Scholar]
- 2. Cederholm T, Barazzoni R, Austin P, et al. . ESPEN guidelines on definitions and terminology of clinical nutrition. Clin Nutr 2017;36:49–64. 10.1016/j.clnu.2016.09.004 [DOI] [PubMed] [Google Scholar]
- 3. European association for the study of the liver. electronic address eee, European association for the study of the L. EASL clinical practice guidelines on nutrition in chronic liver disease. J Hepatol 2019;70:172–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Locquet M, Beaudart C, Petermans J, et al. . EWGSOP2 versus EWGSOP1: impact on the prevalence of sarcopenia and its major health consequences. J Am Med Dir Assoc 2019. [DOI] [PubMed] [Google Scholar]
- 5. Carey EJ, Lai JC, Wang CW, et al. . A multicenter study to define sarcopenia in patients with end-stage liver disease. Liver Transpl 2017;23:625–33. 10.1002/lt.24750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim G, Kang SH, Kim MY, et al. . Prognostic value of sarcopenia in patients with liver cirrhosis: a systematic review and meta-analysis. PLoS One 2017;12:e0186990 10.1371/journal.pone.0186990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van Vugt JLA, Alferink LJM, Buettner S, et al. . A model including sarcopenia surpasses the MELD score in predicting waiting list mortality in cirrhotic liver transplant candidates: a competing risk analysis in a national cohort. J Hepatol 2018;68:707–14. 10.1016/j.jhep.2017.11.030 [DOI] [PubMed] [Google Scholar]
- 8. Montano-Loza AJ, Meza-Junco J, Prado CMM, et al. . Muscle wasting is associated with mortality in patients with cirrhosis. Clin Gastroenterol Hepatol 2012;10:166–73. 10.1016/j.cgh.2011.08.028 [DOI] [PubMed] [Google Scholar]
- 9. Nutritional status in cirrhosis. Italian multicentre cooperative project on nutrition in liver cirrhosis. J Hepatol 1994;21:317–25. [PubMed] [Google Scholar]
- 10. Lautz HU, Selberg O, Körber J, et al. . Protein-Calorie malnutrition in liver cirrhosis. Clin Investig 1992;70:478–86. 10.1007/BF00210228 [DOI] [PubMed] [Google Scholar]
- 11. Berzigotti A, Garcia-Tsao G, Bosch J, et al. . Obesity is an independent risk factor for clinical decompensation in patients with cirrhosis. Hepatology 2011;54:555–61. 10.1002/hep.24418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Montano-Loza AJ, Angulo P, Meza-Junco J, et al. . Sarcopenic obesity and myosteatosis are associated with higher mortality in patients with cirrhosis. J Cachexia Sarcopenia Muscle 2016;7:126–35. 10.1002/jcsm.12039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Plauth M, Bernal W, Dasarathy S, et al. . ESPEN guideline on clinical nutrition in liver disease. Clin Nutr 2019;38:485–521. 10.1016/j.clnu.2018.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Greco AV, Mingrone G, Benedetti G, et al. . Daily energy and substrate metabolism in patients with cirrhosis. Hepatology 1998;27:346–50. 10.1002/hep.510270205 [DOI] [PubMed] [Google Scholar]
- 15. Dasarathy S, Merli M. Sarcopenia from mechanism to diagnosis and treatment in liver disease. J Hepatol 2016;65:1232–44. 10.1016/j.jhep.2016.07.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dasarathy S. Cause and management of muscle wasting in chronic liver disease. Curr Opin Gastroenterol 2016;32:1–65. 10.1097/MOG.0000000000000261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Glass C, Hipskind P, Tsien C, et al. . Sarcopenia and a physiologically low respiratory quotient in patients with cirrhosis: a prospective controlled study. J Appl Physiol 2013;114:559–65. 10.1152/japplphysiol.01042.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Anand AC. Nutrition and muscle in cirrhosis. J Clin Exp Hepatol 2017;7:340–57. 10.1016/j.jceh.2017.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tandon P, Raman M, Mourtzakis M, et al. . A practical approach to nutritional screening and assessment in cirrhosis. Hepatology 2017;65:1044–57. 10.1002/hep.29003 [DOI] [PubMed] [Google Scholar]
- 20. Borhofen SM, Gerner C, Lehmann J, et al. . The Royal free Hospital-Nutritional prioritizing tool is an independent predictor of deterioration of liver function and survival in cirrhosis. Dig Dis Sci 2016;61:1735–43. 10.1007/s10620-015-4015-z [DOI] [PubMed] [Google Scholar]
- 21. Tandon P, Ney M, Irwin I, et al. . Severe muscle depletion in patients on the liver transplant wait list: its prevalence and independent prognostic value. Liver Transpl 2012;18:1209–16. 10.1002/lt.23495 [DOI] [PubMed] [Google Scholar]
- 22. Tandon P, Low G, Mourtzakis M, et al. . A model to identify sarcopenia in patients with cirrhosis. Clin Gastroenterol Hepatol 2016;14:1473–80. 10.1016/j.cgh.2016.04.040 [DOI] [PubMed] [Google Scholar]
- 23. Carias S, Castellanos AL, Vilchez V, et al. . Nonalcoholic steatohepatitis is strongly associated with sarcopenic obesity in patients with cirrhosis undergoing liver transplant evaluation. J Gastroenterol Hepatol 2016;31:628–33. 10.1111/jgh.13166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Choudhary NS, Saigal S, Saraf N, et al. . Sarcopenic obesity with metabolic syndrome: a newly recognized entity following living donor liver transplantation. Clin Transplant 2015;29:211–5. 10.1111/ctr.12505 [DOI] [PubMed] [Google Scholar]
- 25. Giusto M, Lattanzi B, Albanese C, et al. . Sarcopenia in liver cirrhosis: the role of computed tomography scan for the assessment of muscle mass compared with dual-energy X-ray absorptiometry and anthropometry. Eur J Gastroenterol Hepatol 2015;27:328–34. 10.1097/MEG.0000000000000274 [DOI] [PubMed] [Google Scholar]
- 26. Tandon P, Tangri N, Thomas L, et al. . A rapid bedside screen to predict unplanned hospitalization and death in outpatients with cirrhosis: a prospective evaluation of the clinical frailty scale. Am J Gastroenterol 2016;111:1759–67. 10.1038/ajg.2016.303 [DOI] [PubMed] [Google Scholar]
- 27. Kobashigawa J, Dadhania D, Bhorade S, et al. . Report from the American Society of transplantation on frailty in solid organ transplantation. Am J Transplant 2019;19:984–94. 10.1111/ajt.15198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lai JC, Covinsky KE, McCulloch CE, et al. . The liver frailty index improves mortality prediction of the subjective clinician assessment in patients with cirrhosis. Am J Gastroenterol 2018;113:235–42. 10.1038/ajg.2017.443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zenith L, Meena N, Ramadi A, et al. . Eight weeks of exercise training increases aerobic capacity and muscle mass and reduces fatigue in patients with cirrhosis. Clin Gastroenterol Hepatol 2014;12:1920–6. 10.1016/j.cgh.2014.04.016 [DOI] [PubMed] [Google Scholar]
- 30. Macías-Rodríguez RU, Ilarraza-Lomelí H, Ruiz-Margáin A, et al. . Changes in hepatic venous pressure gradient induced by physical exercise in cirrhosis: results of a pilot randomized open clinical trial. Clin Transl Gastroenterol 2016;7:e180 10.1038/ctg.2016.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Guañabens N, Parés A. Liver and bone. Arch Biochem Biophys 2010;503:84–94. 10.1016/j.abb.2010.05.030 [DOI] [PubMed] [Google Scholar]
- 32. Tandon P, Ismond KP, Riess K, et al. . Exercise in cirrhosis: translating evidence and experience to practice. J Hepatol 2018;69:1164–77. 10.1016/j.jhep.2018.06.017 [DOI] [PubMed] [Google Scholar]
- 33. Gheorghe L, Iacob R, Vădan R, et al. . Improvement of hepatic encephalopathy using a modified high-calorie high-protein diet. Rom J Gastroenterol 2005;14:231–8. [PubMed] [Google Scholar]
- 34. Córdoba J, López-Hellín J, Planas M, et al. . Normal protein diet for episodic hepatic encephalopathy: results of a randomized study. J Hepatol 2004;41:38–43. 10.1016/j.jhep.2004.03.023 [DOI] [PubMed] [Google Scholar]
- 35. Williams FR, Vallance A, Faulkner T, et al. . Home‐Based exercise in patients awaiting liver transplantation: a feasibility study. Liver Transpl 2019;384 10.1002/lt.25442 [DOI] [PubMed] [Google Scholar]