Abstract
Skeletal muscle plays a pivotal role in the maintenance of physical and metabolic health and, critically, mobility. Accordingly, strategies focused on increasing the quality and quantity of skeletal muscle are relevant, and resistance exercise is foundational to the process of functional hypertrophy. Much of our current understanding of skeletal muscle hypertrophy can be attributed to the development and utilization of stable isotopically labeled tracers. We know that resistance exercise and sufficient protein intake act synergistically and provide the most effective stimuli to enhance skeletal muscle mass; however, the molecular intricacies that underpin the tremendous response variability to resistance exercise-induced hypertrophy are complex. The purpose of this review is to discuss recent studies with the aim of shedding light on key regulatory mechanisms that dictate hypertrophic gains in skeletal muscle mass. We also aim to provide a brief up-to-date summary of the recent advances in our understanding of skeletal muscle hypertrophy in response to resistance training in humans.
Keywords: resistance exercise, muscle, protein, hypertrophy
Introduction
Skeletal muscle is the organ of locomotion but is also a large contributor to resting energy expenditure 1 and is the largest reservoir for post-prandial insulin-stimulated disposal of blood glucose 2. Thus, beyond skeletal muscle’s obvious role in locomotion and mobility, its maintenance is critical for metabolic health. Indeed, lower-than-predicted norms of skeletal muscle mass and function are associated with a variety of negative health outcomes such as cardiovascular disease, cancer, and increased risk for disability 3. Therefore, concerted efforts to maintain, increase, or regain lost skeletal muscle mass (for example, due to muscle disuse) are of relevance to human health 4.
Skeletal muscle exhibits an extraordinary range of phenotypic plasticity in response to changing contractile stimuli. Skeletal muscle hypertrophy can be defined as an increase in muscle axial cross-sectional area (CSA), assessed via magnetic resonance imaging (MRI), computed tomography, ultrasound, and/or biopsies examining muscle fiber CSA (fCSA). Presently, chronic resistance exercise (RE) training (RET) and sufficient dietary protein feeding provide the most effective non-pharmacological strategies to promote skeletal muscle hypertrophy 5. Significant attention has been directed towards deciphering the mechanistic underpinnings of what gives rise to skeletal muscle hypertrophy. The purpose of this review is to provide a brief up-to-date narrative on recent advances in our understanding of RET-induced skeletal muscle hypertrophy. It is notable that similar topical reviews have recently been published (see references 6– 8), and they should be consulted to obtain other viewpoints on this topic.
Exogenous versus endogenous variables in determining hypertrophy
Muscle hypertrophy is influenced by factors that can be broadly grouped into two categories: exogenous and endogenous variables. Exogenous factors include RE-related variables (load, reps, time under tension, volume, etc.), diet-related variables such as protein supplementation, energy intake, and consumption of anabolic supplements (i.e. creatine), and administration of anabolic hormones. The hypertrophic response to RET can be augmented marginally via greater-than-recommended protein ingestion, but the response is saturated around self-reported intakes of ~1.6 g protein/kg body mass/day 5; however, in resistance-trained individuals, protein intake may need to be greater (~2.0–2.2 g protein/kg body mass/day) to maximize whole-body anabolism 5, 9. Specifically, leucine has been repeatedly shown to be the most potent, and possibly exclusively in human skeletal muscle 10, amino acid agonist that induces muscle protein synthesis (MPS) 10– 12.
Endogenous variables, namely genomic, epigenetic, transcriptomic, and proteomic variables 13, are determinants of muscle hypertrophy. Importantly, each of these variables can ultimately be affected by exogenous variables, such as nutrition and RET paradigms, to which they may show differential responses. Extant literature demonstrates that manipulation of some RET variables has, at best, statistically significant but relatively small effects that are for the most part related to greater mechanical work (although this too would have a ceiling) and are most easily outwardly manifested by high(er) degrees of effort 14. What is abundantly clear is that transient post-exercise rises in systemic concentrations of various anabolic hormones (testosterone, growth hormone, and insulin-like growth factor 1 [IGF-1]) are unrelated to muscle hypertrophy 15, 16.
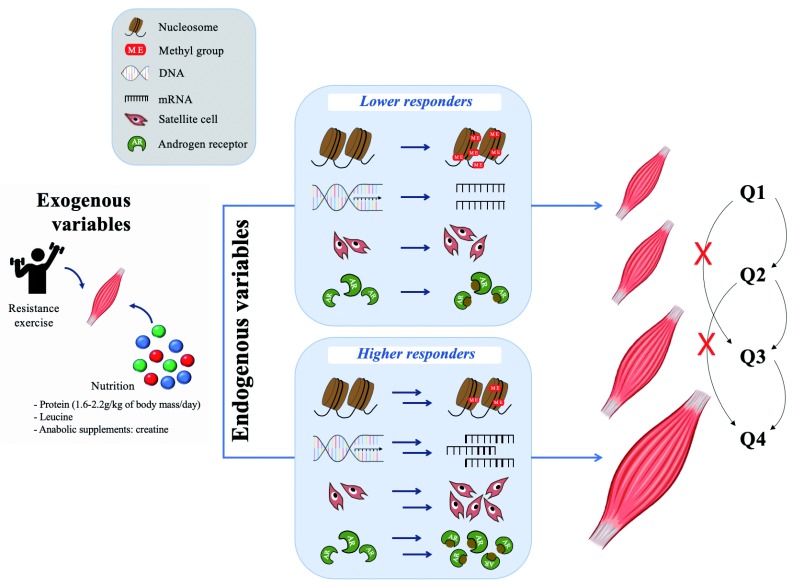
Although exogenous variables are important, it is becoming more widely appreciated that the endogenous molecular responses to RE are paramount in determining the hypertrophic response. Intramuscular mechanosensitive signaling pathways and extracellular supporting structures (i.e. extracellular matrix and capillaries) appear to play important roles in hypertrophy 17. While evidence is equivocal 18, 19, our laboratory has demonstrated that individuals exhibiting greater hypertrophy in response to RET appear to have greater androgen receptor content at rest 16, and the change in androgen receptor content is positively correlated with increased fCSA following RET 20. Moreover, an enhanced satellite cell (SC) proliferation in response to loading 21 differentiates higher from lower hypertrophic “responders” to RET. Furthermore, the aforementioned endogenous variables—higher androgen receptor content and augmented SC proliferation—have been reported to be greater in “high” compared to “low” responders to RET 22– 24. Stimulation of MPS can also occur owing to increased efficiency of translation, with more mRNA translated per ribosomal unit 25, or to increased translational capacity, which occurs by adding more ribosomes to translate existing mRNA. Therefore, ribosomal biogenesis has also been purported as an endogenous variable related to muscle hypertrophy 6, 26. This concept is discussed in more detail further in the review. A schematic of these relationships is summarized in Figure 1. A tenet illustrated in this figure is that in response to mechanical loading, there are degrees of hypertrophic response on which people can, but also cannot, improve. Thus, similar to variability in response to any external stimulus, there is a response variability in exercise-induced hypertrophy that is propelled by external variables but predominantly translated into muscle growth through endogenous variables. Clearly, we do not have a complete picture of the loading-induced hypertrophic process, and further research is needed to define the relationship between exogenous variables and their effect on endogenous variables that directly mediate pathways leading to muscle hypertrophy.
Figure 1. Current understanding of the relationship between exogenous and endogenous variables for skeletal muscle hypertrophy.
Appropriate exogenous stimuli are required to modulate endogenous variables related to muscle protein synthesis and induce skeletal muscle hypertrophy. Resistance exercise and nutrition variables such as dietary protein (especially leucine) as well as anabolic supplements are considered to be the most reliable exogenous variables for skeletal muscle hypertrophy. However, the red arrow-headed line and red dotted line illustrate that exogenous variables do not induce skeletal muscle hypertrophy independently of the endogenous variable modulation. Therefore, endogenous variables are affected by exogenous variables, such as modification to histones, transcription factors, satellite cells, and/or androgen receptor content, which are key determinants of skeletal muscle hypertrophy. The blue arrow-headed line describes the exogenous stimuli that must act through endogenous variables, as represented by thin blue lines, to induce skeletal muscle hypertrophy. Furthermore, depending on the extent of the endogenous variables’ response to exogenous stimuli, higher responders may have greater skeletal muscle hypertrophy compared to lower responders.
Protein turnover and its role in skeletal muscle hypertrophy
Skeletal muscle hypertrophy occurs as the result of recurrent periods of positive net protein balance (NPB), when the rate of MPS exceeds that of muscle protein breakdown (MPB). In the post-absorptive (i.e. fasted) state, rates of MPB exceed MPS, resulting in a negative NPB 27. Importantly, nutrition and contractile activity are potent modulators of MPS and, to a lesser extent, MPB in both trained 28– 30 and untrained individuals 31. Specifically, in the post-absorptive state, RE stimulates increases in both MPS and MPB, and while MPS is stimulated to a greater extent, NPB remains negative 30. Ingestion of dietary protein containing sufficient essential amino acids 30, in close temporal proximity to RE, augments MPS and attenuates the exercise-induced increase in MPB. Therefore, only when RE is coupled with protein feeding does NPB become positive, facilitating small periods of muscle protein accrual with RET that sum to yield eventual hypertrophy 27.
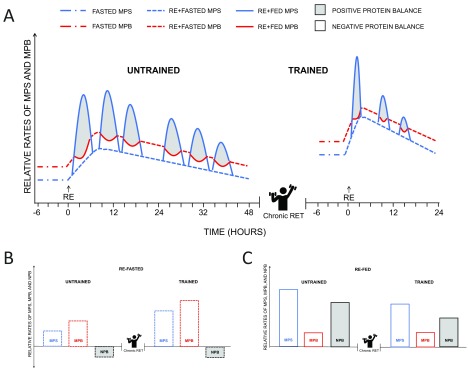
Changes in post-absorptive MPS are modified with RET (for review, see 32). Elevated post-absorptive MPS has been proposed as a primary contributor to muscle hypertrophy with RET (>6 weeks) 6. Indeed, early observations in humans show that post-absorptive MPS is elevated in the trained state 30, 33, 34. However, identical to what is seen in untrained individuals, NPB in the post-absorptive state is always negative because of a concomitant elevation of MPB in trained individuals 30, 32. Thus, the trained state is demarked by an enhanced overall rate of protein turnover—elevated rates of MPS and MPB—that favors only net protein accretion, as demonstrated multiple times 26, 32, 35, in the fed state. The elevation in MPB in the trained state is also supported by molecular evidence 36. Acute intermittent elevations in MPS in response to, and with persistent practice of, RE in combination with sufficient protein feeding are undeniably the major drivers of muscle protein accretion and skeletal muscle hypertrophy 37. We speculate that the overall increased protein turnover (as a result of cumulative greater acute periods of positive NPB) observed with chronic RET is advantageous and is reflective of a general increase in turnover of muscle proteins (i.e. upregulation of MPS and MPB) that favors efficient remodeling of protein that leads to a gradual muscle protein accrual manifested as hypertrophy 32; these concepts are depicted schematically in Figure 2.
Figure 2. Current understanding of changes in muscle protein turnover with chronic resistance exercise training.
Skeletal muscle hypertrophy can occur only under periods of positive protein balance: that is, when relative rates of muscle protein synthesis (MPS) (blue line) exceed that of muscle protein breakdown (MPB) (red line). In the fasted state, rates of MPB exceed those of MPS, resulting in a negative net protein balance (NPB). Compared to untrained individuals ( A), trained individuals ( B) display higher fasted rates of MPS; however, protein balance remains negative because of the concomitant elevation of MPB in the trained state. Regardless of training status, nutritional and contractile stimuli are potent regulators of MPS and, to a lesser extent, MPB. Resistance exercise (RE) stimulates increases in both MPS and MPB, and NPB remains negative. Ingestion of dietary protein—in particular, essential amino acids—in close temporal proximity to RE augments MPS and attenuates the exercise-induced increase in MPB, resulting in a temporary state of positive protein balance. Chronic RE training (RET) modulates the time course of the increase in MPS following a bout of RE. Specifically, the initial increase in MPS following a bout of RE is less pronounced in the untrained state than in the trained state; however, it is longer lived and peaks later in the untrained than the trained state. MPS, MPB, and NPB during periods of ( B) RE+Fasted and ( C) RE+Fed in the untrained and trained state.
At the molecular level, RE and protein feeding increase MPS through mechanistic target of rapamycin complex 1 (mTORC1)-dependent 38 and -independent 38, 39 mechanisms. Typically, mTORC1 phosphorylation activates several downstream kinases, augmenting translational efficiency (i.e. an increase in the rate of translation of mRNA by a constant pool of ribosomes) and, with RET, translational capacity (i.e. total number of available ribosomes) 26, 38. Recently, it has been suggested that increased translational capacity is central to changes in post-absorptive MPS with chronic RET 6. Several groups have demonstrated that chronic RET results in increased total RNA 19, 40– 42 and ribosomal RNA (rRNA) content 24, 40 in addition to increases in regulators of rRNA synthesis 24, 40– 42. In contrast, other groups reported a reduction in biomarkers of ribosomal biogenesis 43 or no change following 12 weeks of RET 19. Increases in RNA content—following 16 weeks 44, 45 and 6 weeks 18 of RET—were similar between individuals showing either no change (i.e. “non/low responders”) or an extreme increase (i.e. “extreme/high responders”) in vastus lateralis muscle fCSA. In contrast, Stec and colleagues 41 reported that only “extreme” responders to 4 weeks of RET had increases in total RNA and rRNA content. Conflicting results may be attributed to differences in participant characteristics, experimental design, and analytical techniques 26; however, current evidence does not demonstrate a clear connection between translational capacity and skeletal muscle hypertrophy in humans 37. We hypothesize that early on in a RET program, ribosomal capacity may be increased as a general response to a need for greater rates of global protein synthesis 46. However, with persistent practice of RET once protein synthetic responses and transcriptional programs become “refined” and more specific to the stimulus of RET 34—as well as being shorter in duration 32—further increasing ribosomal capacity is not required and would either stabilize 40, 42 or possibly decline 43, 47. This thesis would underpin why early during a RET bout a very short-term MPS response does not align well with eventual hypertrophy 48, but this is not the case with further RET where MPS shares common variance with hypertrophy 46. It should also be noted that the stabilization of ribosomal capacity following chronic RET 40, 42 does not indicate a loss of muscle ribosomes per se; instead, this likely reflects a dilution of the ribosomal capacity by larger, hypertrophied myofibers.
Understanding changes in translational capacity with RET is limited owing to a number of methodological constraints. Specifically, the study of ribosomal biogenesis relies heavily on static measures (i.e. immunoblotting and quantifying total RNA content and assuming rRNA content is responsible), and traditional stable isotope tracer investigations provide insight into only acute (i.e. hours) metabolic fluctuations 49. Recent advances in mass spectrometry techniques have led to the reintroduction of deuterium oxide (D 2O) 50, 51, which enables the assessment of metabolic flux in response to a variety of stimuli, such as skeletal muscle loading 11, 12, 42, 46, unloading 52– 54, and feeding 28, 42, 46 under longer-term, “free-living” conditions (i.e. integrated over days to weeks). Brook and colleagues 50 recently validated the use of D 2O in monitoring the synthesis of ribonucleotides, providing the first dynamic measure of RNA synthesis in human skeletal muscle in response to RET. Of particular note in this study, RNA synthesis was increased above basal rates over the 0–6-week period with continuous RET 50. Importantly, myofibrillar MPS in these individuals was not significantly increased above basal levels during this period 42, showing a discordance between translational capacity and MPS with long-term muscle adaptations. Future studies incorporating dynamic measures of RNA synthesis and integrated rates of MPS in concert with omic-level measurements should provide a platform to elucidate the relative contribution, and time-course, of translational efficiency and capacity to changes in MPS and hypertrophy in response to chronic RET.
Omic-based science and skeletal muscle hypertrophy
Our present mechanistic understanding of muscle hypertrophy has largely been informed by the use of “targeted” analytical approaches providing static snapshots (i.e. qPCR and immunoblotting). However, the increased usage of “omic” technologies can offer an unbiased and integrative understanding of the processes regulating muscle hypertrophy. Proteomic profiling has tremendous potential to advance our understanding of muscle growth; however, it is currently constrained by a relatively limited coverage of highly abundant proteins in the proteome versus a far larger coverage of RNA: <500 proteins reliably detected 55, 56 versus ~30,000 RNA species 55. This low protein:RNA ratio results in an incomplete understanding of downstream ontology/pathway analyses 57 but could also mask the important role of less-abundant regulatory proteins in muscle hypertrophy (i.e. signaling molecules 57 or integrin receptors 58). It is possible to circumvent these limitations by studying the expressed RNA complement of the cell (via transcriptomics) or translatome of the cell (via polysomal RNA and transcriptomics), given the close association between mRNA and protein abundance under most conditions 59, 60 and, in particular, the global translatome in skeletal muscle 61, 62.
Early applications of transcriptomics have shown that older adults, and lower hypertrophic responders in general 63, express a pro-inflammatory gene profile at rest and respond to an acute bout of RE with an exaggerated inflammatory response 64, linking inflammation with an attenuated muscle growth response to RET. Elderly adults also have an elevated expression of p21 65, a cell cycle inhibitor that affects SC proliferation 66 and may therefore impair muscle growth following RET 21. In contrast, higher hypertrophy responders to RET express higher levels of several well-known growth and remodeling genes prior to training compared to lower responders, which is suggestive of a “primed” basal state of protein turnover 63. Higher RET responders also express greater levels of oxidative, angiogenic, and extracellular matrix remodeling genes after RET 65, 67. Two noteworthy yet ill-characterized genes that are also upregulated in high responders in the basal state include NAP1L1 and DGKZ 63, which encode a nucleosome-associated protein and diacylglycerol kinase zeta (DGKζ), respectively. The protein encoded by NAP1L1 controls chromatin compaction but has also been shown to bind to and regulate the nuclear-cytoplasmic shuttling of DGKζ 68. Importantly, DGKζ was shown recently to play a pivotal role in mechanical overload-induced muscle hypertrophy in rodents, but only if the nuclear localization signal of DGKζ was intact 69. While the nature of this interaction in humans warrants further investigation, the example attests to the hypothesis-generating power of transcriptome profiling and its inherent potential for biological discovery.
An ongoing challenge in transcriptomics is the use of gene ontology (i.e. DAVID 70) and network analytical tools (ingenuity pathway analysis [IPA] 71), which are commonly used to uncover functional relationships from large lists of RET-regulated genes. These tools rely on the function(s) of a gene product being known 56. However, data-driven networks (DDNs) are networks constructed on the basis of experimentally derived gene co-expression similarities, without a priori knowledge of gene function. Clarke and colleagues 72 used a DDN approach to construct gene networks from pre- and post-muscle transcriptome samples obtained from the HERITAGE study 73 (endurance-based training) and identified EIF6 as an exercise-responsive highly interconnected “hub” gene. EIF6 was therefore predicted, on the basis of being highly connected to other regulated genes, to play an important role in the adaptation to endurance training. Indeed, subsequent development of a mutant EIF6 murine model was shown to affect many of the same signaling pathways predicted by the HERITAGE study 72, 73 that affect phenotype. Greater use of DDNs and network modeling could be applied to the study of muscle hypertrophy with RET with, we propose, great potential.
SCs and their role in RET-induced hypertrophy
In humans, increases in muscle fiber size are commonly reported with a concomitant increase in the number of myonuclei 74, an observation that lends credence to the myonuclear domain theory of muscle growth 75. This theory suggests that each myonucleus governs a set volume within the muscle fiber and, when the ceiling of the muscle fiber volume is reached, the transcriptional capacity of an existing myonucleus is reached and new myonuclei must be added to maintain (or re-establish) transcriptional control over a defined myonuclear domain. Skeletal muscle is a post-mitotic tissue; therefore, the addition of new myonuclei must come from a new source, which occurs via donation from skeletal muscle stem cells, i.e. SCs.
Activation of SCs occurs following various stimuli such as injury, damage, and exercise. Once activated, SCs progress from proliferation to terminal differentiation, eventually fusing and donating their nuclei to existing myofibers, a process termed the myogenic program. Although common dogma had long associated SCs with skeletal muscle hypertrophy 76, 77, this concept has recently been challenged. McCarthy and colleagues 78 were the first to use the Pax7-DTA mouse strain that results in conditional SC ablation to demonstrate that significant overload-induced hypertrophy, via synergist ablation, can occur in SC-depleted rodent skeletal muscle. The same group reinforced these findings using hind-limb suspension, to induce atrophy, followed by reloading and regrowth of muscle which was not affected by SC depletion, in the Pax7-DTA mouse 79. Importantly, while interesting, these results highlight that SCs are not necessary for hypertrophy in short-term extreme models of hypertrophy but do not address the question of whether SCs are involved in a more physiologically relevant hypertrophic situation (i.e. following RET). This notion was further challenged by a study from Egner and colleagues 80, in which they describe impaired hypertrophy with 2 weeks of overload, via synergist ablation, using the same Pax7-DTA mouse strain 78, 79. Further to this, work by Murach and colleagues 81 demonstrated that myonuclear accretion via the SC is necessary to support overload-induced hypertrophy in younger growing mice, highlighting that the requirement of SCs to support hypertrophy is affected by age. Notably, the extent of hypertrophy is attenuated following 8 (versus 2) weeks of overload-induced hypertrophy in Pax7-DTA mice 82, suggesting that SCs are involved in muscle growth. Importantly, the researchers described an accumulation of the extracellular matrix in SC-depleted mice following 8 weeks of overload, which resulted in the impaired hypertrophic response 82. These data suggest that SCs are able to support muscle growth not only by fusing to existing fibers resulting in myonuclear accretion but also by their interaction with other cell types to regulate the extracellular matrix deposition 83. Although work in rodent models has been essential in providing insight into the basic cellular and molecular mechanisms that result in muscle hypertrophy, these results cannot always easily be translated to humans. For example, cerebral palsy, a developmental motor disorder characterized by a reduction in muscle fiber size, is also associated with a reduction in SC content 84, 85, and it is postulated that the reduction in SC content may contribute to the impairment in muscle growth 86. For obvious reasons, it isn’t possible to study the effects of SC depletion in humans, and the observation of SCs in a human model with a reduced (although not ablated) SC content is often confounded by the presence of chronic disease, where factors other than SC content may contribute to the inability of muscle to hypertrophy.
Importantly, the majority of evidence stemming from human studies has implicated a role for SCs in contributing to increases in muscle fiber size. Several studies have described a positive relationship between muscle fiber size and number of myonuclei in human muscle 19, 21, 47, 87– 92. In addition, studies have also described an increase in myonuclear number with training-induced fiber hypertrophy concomitant with an increase in SC content 80, 87– 90. It is, however, important to note that several groups have reported an increase in fCSA without an increase in SC/myonuclear content 92– 94. This may be due to several factors, one of which is the ability of existing myonuclei to increase their transcriptional capacity to support the increase in muscle fiber size 95.
Interestingly, individuals classified as “extreme” (hypertrophy) responders to RET had greater basal SC content compared to “lower” and “moderate” responders, which translated to a greater expansion of the SC pool with training and was accompanied by an increase in myonuclear content; however, the myonuclear domain also increased 21. Thus, similar to transcriptional observations, the basal characteristics of skeletal muscle (i.e. SC content) may play a role in response plasticity to hypertrophic stimulus. Congruent with previous work 21, we demonstrated that the acute SC response to a bout of unaccustomed RE is related to the increase in quadriceps volume observed following training 87. Although SCs likely contribute to hypertrophic adaptation via myonuclear accretion, it is important to recognize the ability of resident myonuclei to respond to varying stimuli such as RET and their inherent ability to support growth. The concept of muscle “memory”, manifested through possible epigenetic changes, is also likely an important contributor to the ability of skeletal muscle to hypertrophy. Seaborne and colleagues 96 demonstrated that prior RE-induced hypertrophy enhanced the subsequent response to a bout of resistance training, following a period of detraining, which may be a consequence of the widespread hypomethylation incurred during the first adaptive response. Together, the evidence in humans reporting an increase in muscle fiber size with a concomitant increase in myonuclei 19, 21, 47, 87– 92 highlights that SCs likely play a role in mediating skeletal muscle hypertrophy. However, as shown by Kirby and colleagues 95, using a time-course experiment following synergist ablation in the Pax7-DTA mouse model, the ability of existing resident myonuclei to support periods of fiber growth cannot be disregarded.
Conclusion and future directions
Skeletal muscle plays an indispensable role in an array of mechanical and metabolic functions 97. Typically, as we age, the quantity and quality of skeletal muscle deteriorates owing to the infiltration of non-muscle tissue including adipose and connective tissue 98. Therefore, concerted efforts to increase and maintain skeletal muscle mass should be made by a range of individuals spanning from those striving to improve athletic performance to those focused on extending the healthspan. RE and dietary protein act synergistically and, at present, provide the most effective strategy to augment skeletal muscle mass 37. Skeletal muscle hypertrophy is a complex process with multiple regulatory gene/protein hubs that have recently received significant attention in helping to decipher the mechanistic underpinnings that dictate the skeletal muscle adaptive response. As a result, a number of exogenous factors that influence endogenous pathways have been identified to play an important role in skeletal muscle hypertrophy.
MPS is the principal locus of control that influences muscle protein accretion in response to anabolic stimuli, as opposed to MPB 28– 31. However, the relative contribution of increased translational efficiency and translational capacity in affecting hypertrophy remains unclear. Intermittent elevations in rates of MPS in response to exogenous stimuli (i.e. RE and protein nutrition) drive muscle hypertrophy 28– 31. Nevertheless, research focused on translational capacity is in its infancy, and the proposed importance 6 of ribosomal biogenesis has yet to be confirmed.
What is clearly evident is that muscle hypertrophy is a multi-faceted process. However, targeted approaches that probe specific genes and proteins will provide only an incomplete picture of muscle growth. Unbiased, global “omic” technologies have the potential to provide a more comprehensive understanding of the underlying prerequisites for muscle growth but have inherent limitations that need to be considered.
Myonuclear accretion, due to a loading stimulus, is a means by which the transcriptional capacity of the skeletal muscle may be increased. The addition of new myonuclei is due to the activation and subsequent fusion of SCs to muscle fibers, and substantial evidence shows a role for SCs in muscle hypertrophy in humans. Although this is speculative, we hypothesize that resident myonuclei likely possess the ability, possibly through epigenetic modification, to increase transcriptional capacity to a certain extent, ultimately supporting muscle growth.
Although significant progress has been made, considerable work remains to be done in order to deepen our understanding of the processes that govern RET-induced muscle hypertrophy. Future studies incorporating dynamic measures of RNA synthesis, integrated rates of MPS, and SC/myonuclei assessments in concert with “omic” technologies and DDNs will provide a platform to elucidate the relative contribution, and time-course, of translational efficiency and capacity to changes in MPS and hypertrophy in response to chronic RET.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
John J McCarthy, Department of Physiology, College of Medicine, University of Kentucky, Lexington, KY, USA
Michael Roberts, School of Kinesiology, Auburn University, Auburn, AL, USA
Funding Statement
SMP holds grants from the Canadian Institutes for Health Research and the National Science and Engineering Council of Canada and thanks the Canada Research Chairs Program for their support.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; peer review: 2 approved]
References
- 1. Zurlo F, Larson K, Bogardus C, et al. : Skeletal muscle metabolism is a major determinant of resting energy expenditure. J Clin Invest. 1990;86(5):1423–7. 10.1172/JCI114857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Thiebaud D, Jacot E, DeFronzo RA, et al. : The effect of graded doses of insulin on total glucose uptake, glucose oxidation, and glucose storage in man. Diabetes. 1982;31(11):957–63. 10.2337/diacare.31.11.957 [DOI] [PubMed] [Google Scholar]
- 3. Mcleod JC, Stokes T, Phillips SM: Resistance Exercise Training as a Primary Countermeasure to Age-Related Chronic Disease. Front Physiol. 2019;10:645. 10.3389/fphys.2019.00645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Phillips SM, McGlory C: CrossTalk proposal: The dominant mechanism causing disuse muscle atrophy is decreased protein synthesis. J Physiol. 2014;592(24):5341–3. 10.1113/jphysiol.2014.273615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Morton RW, Murphy KT, McKellar SR, et al. : A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br J Sports Med. 2018;52(6):376–84. 10.1136/bjsports-2017-097608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Figueiredo VC: Revisiting the roles of protein synthesis during skeletal muscle hypertrophy induced by exercise. Am J Physiol Regul Integr Comp Physiol. 2019;317(5):R709–R718. 10.1152/ajpregu.00162.2019 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 7. Lavin KM, Roberts BM, Fry CS, et al. : The Importance of Resistance Exercise Training to Combat Neuromuscular Aging. Physiology (Bethesda). 2019;34(2):112–22. 10.1152/physiol.00044.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 8. Roberts MD, Haun CT, Mobley CB, et al. : Physiological Differences Between Low Versus High Skeletal Muscle Hypertrophic Responders to Resistance Exercise Training: Current Perspectives and Future Research Directions. Front Physiol. 2018;9:183. 10.3389/fphys.2018.00834 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 9. Mazzulla M, Sawan SA, Williamson E, et al. : Protein Intake to Maximize Whole-Body Anabolism during Postexercise Recovery in Resistance-Trained Men with High Habitual Intakes is Severalfold Greater than the Current Recommended Dietary Allowance. J Nutr. 2019. pii: nxz249. 10.1093/jn/nxz249 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 10. Atherton PJ, Smith K, Etheridge T, et al. : Distinct anabolic signalling responses to amino acids in C2C12 skeletal muscle cells. Amino Acids. 2010;38(5):1533–9. 10.1007/s00726-009-0377-x [DOI] [PubMed] [Google Scholar]
- 11. Devries MC, McGlory C, Bolster DR, et al. : Protein leucine content is a determinant of shorter- and longer-term muscle protein synthetic responses at rest and following resistance exercise in healthy older women: A randomized, controlled trial. Am J Clin Nutr. 2018;107(2):217–26. 10.1093/ajcn/nqx028 [DOI] [PubMed] [Google Scholar]
- 12. Devries MC, McGlory C, Bolster DR, et al. : Leucine, Not Total Protein, Content of a Supplement Is the Primary Determinant of Muscle Protein Anabolic Responses in Healthy Older Women. J Nutr. 2018;148(7):1088–1095. 10.1093/jn/nxy091 [DOI] [PubMed] [Google Scholar]
- 13. Turner DC, Seaborne RA, Sharples AP: Comparative Transcriptome and Methylome Analysis in Human Skeletal Muscle Anabolism, Hypertrophy and Epigenetic Memory. Sci Rep. 2019;9(1):4251. 10.1038/s41598-019-40787-0 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 14. Morton RW, Colenso-Semple L, Phillips SM: Training for strength and hypertrophy: An evidence-based approach. Curr Opin Physiol. 2019;10:90–5. 10.1016/j.cophys.2019.04.006 [DOI] [Google Scholar]
- 15. Morton RW, Oikawa SY, Wavell CG, et al. : Neither load nor systemic hormones determine resistance training-mediated hypertrophy or strength gains in resistance-trained young men. J Appl Physiol. 2016;121(1):129–38. 10.1152/japplphysiol.00154.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Morton RW, Sato K, Gallaugher MPB, et al. : Muscle Androgen Receptor Content but Not Systemic Hormones Is Associated With Resistance Training-Induced Skeletal Muscle Hypertrophy in Healthy, Young Men. Front Physiol. 2018;9:1373. 10.3389/fphys.2018.01373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Goodman CA: Role of mTORC1 in mechanically induced increases in translation and skeletal muscle mass. J Appl Physiol (1985). 2019;127(2):581–90. 10.1152/japplphysiol.01011.2018 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 18. Haun CT, Vann CG, Mobley CB, et al. : Pre-training Skeletal Muscle Fiber Size and Predominant Fiber Type Best Predict Hypertrophic Responses to 6 Weeks of Resistance Training in Previously Trained Young Men. Front Physiol. 2019;10:297. 10.3389/fphys.2019.00297 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 19. Mobley CB, Haun CT, Roberson PA, et al. : Biomarkers associated with low, moderate, and high vastus lateralis muscle hypertrophy following 12 weeks of resistance training. PLoS One. 2018;13(4):e0195203. 10.1371/journal.pone.0195203 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 20. Mitchell CJ, Churchward-Venne TA, Bellamy L, et al. : Muscular and systemic correlates of resistance training-Induced muscle hypertrophy. PLoS One. 2013;8(10):e78636. 10.1371/journal.pone.0078636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Petrella JK, Kim JS, Mayhew DL, et al. : Potent myofiber hypertrophy during resistance training in humans is associated with satellite cell-mediated myonuclear addition: a cluster analysis. J Appl Physiol (1985). 2008;104(6):1736–42. 10.1152/japplphysiol.01215.2007 [DOI] [PubMed] [Google Scholar]
- 22. Ahtiainen JP, Hulmi JJ, Kraemer WJ, et al. : Heavy resistance exercise training and skeletal muscle androgen receptor expression in younger and older men. Steroids. 2011;76(1–2):183–92. 10.1016/j.steroids.2010.10.012 [DOI] [PubMed] [Google Scholar]
- 23. Damas F, Libardi CA, Ugrinowitsch C, et al. : Early- and later-phases satellite cell responses and myonuclear content with resistance training in young men. PLoS One. 2018;13(1):e0191039. 10.1371/journal.pone.0191039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Figueiredo VC, Caldow MK, Massie V, et al. : Ribosome biogenesis adaptation in resistance training-induced human skeletal muscle hypertrophy. Am J Physiol Endocrinol Metab. 2015;309(1):E72–83. 10.1152/ajpendo.00050.2015 [DOI] [PubMed] [Google Scholar]
- 25. Chaillou T, Kirby TJ, McCarthy JJ: Ribosome biogenesis: emerging evidence for a central role in the regulation of skeletal muscle mass. J Cell Physiol. 2014;229(11):1584–94. 10.1002/jcp.24604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McGlory C, Devries MC, Phillips SM: Skeletal Muscle and Resistance Exercise Training; The Role of Protein Synthesis in Recovery and Remodeling. J Appl Physiol. 2017;122(3):541–8. 10.1152/japplphysiol.00613.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stokes T, Hector AJ, Morton RW, et al. : Recent Perspectives Regarding the Role of Dietary Protein for the Promotion of Muscle Hypertrophy With Resistance Exercise Training. Nutrients. 2018;10(2): pii: E180. 10.3390/nu10020180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Davies RW, Bass JJ, Carson BP, et al. : Differential Stimulation of Post-Exercise Myofibrillar Protein Synthesis in Humans Following Isonitrogenous, Isocaloric Pre-Exercise Feeding. Nutrients. 2019;11(7): pii: E1657. 10.3390/nu11071657 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 29. McKendry J, Shad BJ, Smeuninx B, et al. : Comparable Rates of Integrated Myofibrillar Protein Synthesis Between Endurance-Trained Master Athletes and Untrained Older Individuals. Front Physiol. 2019;10:1084. 10.3389/fphys.2019.01084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Phillips SM, Parise G, Roy BD, et al. : Resistance-training-induced Adaptations in Skeletal Muscle Protein Turnover in the Fed State. Can J Physiol Pharmacol. 2002;80(11):1045–53. 10.1139/y02-134 [DOI] [PubMed] [Google Scholar]
- 31. Tang JE, Perco JG, Moore DR, et al. : Resistance Training Alters the Response of Fed State Mixed Muscle Protein Synthesis in Young Men. Am J Physiol Regul Integr Comp Physiol. 2008;294(1):R172–R178. 10.1152/ajpregu.00636.2007 [DOI] [PubMed] [Google Scholar]
- 32. Damas F, Phillips S, Vechin FC, et al. : A Review of Resistance Training-Induced Changes in Skeletal Muscle Protein Synthesis and Their Contribution to Hypertrophy. Sports Med. 2015;45(6):801–7. 10.1007/s40279-015-0320-0 [DOI] [PubMed] [Google Scholar]
- 33. Kim PL, Staron RS, Phillips SM: Fasted-state Skeletal Muscle Protein Synthesis After Resistance Exercise Is Altered With Training. J Physiol. 2005;568(Pt 1):283–90. 10.1113/jphysiol.2005.093708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wilkinson SB, Phillips SM, Atherton PJ, et al. : Differential Effects of Resistance and Endurance Exercise in the Fed State on Signalling Molecule Phosphorylation and Protein Synthesis in Human Muscle. J Physiol. 2008;586(15):3701–17. 10.1113/jphysiol.2008.153916 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 35. Kumar V, Atherton P, Smith K, et al. : Human Muscle Protein Synthesis and Breakdown During and After Exercise. J Appl Physiol (1985). 2009;106(6):2026–39. 10.1152/japplphysiol.91481.2008 [DOI] [PubMed] [Google Scholar]
- 36. Seaborne RA, Hughes DC, Turner DC, et al. : UBR5 is a novel E3 ubiquitin ligase involved in skeletal muscle hypertrophy and recovery from atrophy. J Physiol. 2019;597(14):3727–49. 10.1113/JP278073 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 37. Brook MS, Wilkinson DJ, Smith K, et al. : It's not just about protein turnover: the role of ribosomal biogenesis and satellite cells in the regulation of skeletal muscle hypertrophy. Eur J Sport Sci. 2019;19(7):952–63. 10.1080/17461391.2019.1569726 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 38. Hodson N, West DWD, Philp A, et al. : Molecular regulation of human skeletal muscle protein synthesis in response to exercise and nutrients: a compass for overcoming age-related anabolic resistance. Am J Physiol Cell Physiol. 2019;317(6):C1061–C1078. 10.1152/ajpcell.00209.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. You JS, McNally RM, Jacobs BL, et al. : The role of raptor in the mechanical load-induced regulation of mTOR signaling, protein synthesis, and skeletal muscle hypertrophy. FASEB J. 2019;33(3):4021–34. 10.1096/fj.201801653RR [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 40. Hammarström D, Øfsteng S, Koll L, et al. : Benefits of higher resistance-training volume are related to ribosome biogenesis. J Physiol. 2020;598(3):543–65. 10.1113/JP278455 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 41. Stec MJ, Kelly NA, Many GM, et al. : Ribosome biogenesis may augment resistance training-induced myofiber hypertrophy and is required for myotube growth in vitro. Am J Physiol Endocrinol Metab. 2016;310(8):E652–E661. 10.1152/ajpendo.00486.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 42. Brook MS, Wilkinson DJ, Mitchell WK, et al. : Synchronous deficits in cumulative muscle protein synthesis and ribosomal biogenesis underlie age-related anabolic resistance to exercise in humans. J Physiol. 2016;594(24):7399–417. 10.1113/JP272857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fyfe JJ, Bishop DJ, Bartlett JD, et al. : Enhanced skeletal muscle ribosome biogenesis, yet attenuated mTORC1 and ribosome biogenesis-related signalling, following short-term concurrent versus single-mode resistance training. Sci Rep. 2018;8(1):560. 10.1038/s41598-017-18887-6 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 44. Bamman MM, Petrella JK, Kim JS, et al. : Cluster analysis tests the importance of myogenic gene expression during myofiber hypertrophy in humans. J Appl Physiol. 2007;102(6):2232–9. 10.1152/japplphysiol.00024.2007 [DOI] [PubMed] [Google Scholar]
- 45. Kim JS, Petrella JK, Cross JM, et al. : Load-mediated downregulation of myostatin mRNA is not sufficient to promote myofiber hypertrophy in humans: a cluster analysis. J Appl Physiol (1985). 2007;103(5):1488–95. 10.1152/japplphysiol.01194.2006 [DOI] [PubMed] [Google Scholar]
- 46. Damas F, Phillips SM, Libardi CA, et al. : Resistance training-induced changes in integrated myofibrillar protein synthesis are related to hypertrophy only after attenuation of muscle damage. J Physiol. 2016;594(18):5209–22. 10.1113/JP272472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kadi F, Schjerling P, Andersen LL, et al. : The effects of heavy resistance training and detraining on satellite cells in human skeletal muscles. J Physiol. 2004;558(Pt 3):1005–12. 10.1113/jphysiol.2004.065904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mitchell CJ, Churchward-Venne TA, Parise G, et al. : Acute Post-Exercise Myofibrillar Protein Synthesis Is Not Correlated with Resistance Training-Induced Muscle Hypertrophy in Young Men. PLoS One. 2014;9(2):e89431. 10.1371/journal.pone.0089431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mitchell CJ, Churchward-Venne TA, Cameron-Smith D, et al. : What is the relationship between the acute muscle protein synthesis response and changes in muscle mass? J Appl Physiol (1985). 2015;118(4):495–7. 10.1152/japplphysiol.00609.2014 [DOI] [PubMed] [Google Scholar]
- 50. Brook MS, Wilkinson DJ, Mitchell WK, et al. : A novel D 2O tracer method to quantify RNA turnover as a biomarker of de novo ribosomal biogenesis, in vitro, in animal models, and in human skeletal muscle. Am J Physiol Endocrinol Metab. 2017;313(6):E681–E689. 10.1152/ajpendo.00157.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 51. Wilkinson DJ, Franchi MV, Brook MS, et al. : A validation of the application of D 2O stable isotope tracer techniques for monitoring day-to-day changes in muscle protein subfraction synthesis in humans. Am J Physiol Endocrinol Metab. 2014;306(5):E571–9. 10.1152/ajpendo.00650.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. McGlory C, Gorissen SHM, Kamal M, et al. : Omega-3 fatty acid supplementation attenuates skeletal muscle disuse atrophy during two weeks of unilateral leg immobilization in healthy young women. FASEB J. 2019;33(3):4586–97. 10.1096/fj.201801857RRR [DOI] [PubMed] [Google Scholar]
- 53. McGlory C, von Allmen MT, Stokes T, et al. : Failed Recovery of Glycemic Control and Myofibrillar Protein Synthesis With 2 wk of Physical Inactivity in Overweight, Prediabetic Older Adults. J Gerontol A Biol Sci Med Sci. 2018;73(8):1070–7. 10.1093/gerona/glx203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Oikawa SY, McGlory C, D'Souza LK, et al. : A randomized controlled trial of the impact of protein supplementation on leg lean mass and integrated muscle protein synthesis during inactivity and energy restriction in older persons. Am J Clin Nutr. 2018;108(5):1060–8. 10.1093/ajcn/nqy193 [DOI] [PubMed] [Google Scholar]
- 55. Timmons JA, Atherton PJ, Larsson O, et al. : A coding and non-coding transcriptomic perspective on the genomics of human metabolic disease. Nucleic Acids Res. 2018;46(15):7772–92. 10.1093/nar/gky570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Timmons JA, Szkop KJ, Gallagher IJ: Multiple sources of bias confound functional enrichment analysis of global -omics data. Genome Biol. 2015;16:186. 10.1186/s13059-015-0761-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Potts GK, McNally RM, Blanco R, et al. : A map of the phosphoproteomic alterations that occur after a bout of maximal-intensity contractions. J Physiol. 2017;595(15):5209–5226. 10.1113/JP273904 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 58. Boppart MD, Mahmassani ZS: Integrin signaling: linking mechanical stimulation to skeletal muscle hypertrophy. Am J Physiol Cell Physiol. 2019;317(4):C629–C641. 10.1152/ajpcell.00009.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Li JJ, Bickel PJ, Biggin MD: System wide analyses have underestimated protein abundances and the importance of transcription in mammals. PeerJ. 2014;2:e270. 10.7717/peerj.270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Li JJ, Biggin MD: Gene expression. Statistics requantitates the central dogma. Science. 2015;347(6226):1066–7. 10.1126/science.aaa8332 [DOI] [PubMed] [Google Scholar]
- 61. Chen YW, Nader GA, Baar KR, et al. : Response of rat muscle to acute resistance exercise defined by transcriptional and translational profiling. J Physiol. 2002;545(1):27–41. 10.1113/jphysiol.2002.021220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Roberts MD, Childs TE, Brown JD, et al. : Early depression of Ankrd2 and Csrp3 mRNAs in the polyribosomal and whole tissue fractions in skeletal muscle with decreased voluntary running. J Appl Physiol (1985). 2012;112(8):1291–9. 10.1152/japplphysiol.01419.2011 [DOI] [PubMed] [Google Scholar]
- 63. Thalacker-Mercer A, Stec M, Cui X, et al. : Cluster analysis reveals differential transcript profiles associated with resistance training-induced human skeletal muscle hypertrophy. Physiol Genomics. 2013;45(12):499–507. 10.1152/physiolgenomics.00167.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Thalacker-Mercer AE, Dell'Italia LJ, Cui X, et al. : Differential genomic responses in old vs. young humans despite similar levels of modest muscle damage after resistance loading. Physiol Genomics. 2010;40(3):141–9. 10.1152/physiolgenomics.00151.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Raue U, Trappe TA, Estrem ST, et al. : Transcriptome signature of resistance exercise adaptations: mixed muscle and fiber type specific profiles in young and old adults. J Appl Physiol (1985). 2012;112(10):1625–36. 10.1152/japplphysiol.00435.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Li J, Han S, Cousin W, et al. : Age-specific functional epigenetic changes in p21 and p16 in injury-activated satellite cells. Stem Cells. 2015;33(3):951–61. 10.1002/stem.1908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Damas F, Ugrinowitsch C, Libardi CA, et al. : Resistance training in young men induces muscle transcriptome-wide changes associated with muscle structure and metabolism refining the response to exercise-induced stress. Eur J Appl Physiol. 2018;118(12):2607–2616. 10.1007/s00421-018-3984-y [DOI] [PubMed] [Google Scholar]
- 68. Okada M, Hozumi Y, Ichimura T, et al. : Interaction of nucleosome assembly proteins abolishes nuclear localization of DGKζ by attenuating its association with importins. Exp Cell Res. 2011;317(20):2853–63. 10.1016/j.yexcr.2011.09.014 [DOI] [PubMed] [Google Scholar]
- 69. You JS, Dooley MS, Kim CR, et al. : A DGKζ-FoxO-ubiquitin proteolytic axis controls fiber size during skeletal muscle remodeling. Sci Signal. 2018;11(530): pii: eaao6847. 10.1126/scisignal.aao6847 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 70. Huang da W, Sherman BT, Zheng X, et al. : Extracting biological meaning from large gene lists with DAVID. Curr Protoc Bioinformatics. 2009;Chapter 13:Unit 13.11. 10.1002/0471250953.bi1311s27 [DOI] [PubMed] [Google Scholar]
- 71. Krämer A, Green J, Pollard J, Jr, et al. : Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics. 2014;30(4):523–30. 10.1093/bioinformatics/btt703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Clarke K, Ricciardi S, Pearson T, et al. : The Role of Eif6 in Skeletal Muscle Homeostasis Revealed by Endurance Training Co-expression Networks. Cell Rep. 2017;21(6):1507–1520. 10.1016/j.celrep.2017.10.040 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 73. Timmons JA, Knudsen S, Rankinen T, et al. : Using molecular classification to predict gains in maximal aerobic capacity following endurance exercise training in humans. J Appl Physiol (1985). 2010;108(6):1487–96. 10.1152/japplphysiol.01295.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Murach KA, Fry CS, Kirby TJ, et al. : Starring or Supporting Role? Satellite Cells and Skeletal Muscle Fiber Size Regulation. Physiology (Bethesda). 2018;33(1):26–38. 10.1152/physiol.00019.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 75. Allen DL, Roy RR, Edgerton VR: Myonuclear domains in muscle adaptation and disease. Muscle Nerve. 1999;22(10):1350–60. [DOI] [PubMed] [Google Scholar]
- 76. Adams GR, Caiozzo VJ, Haddad F, et al. : Cellular and molecular responses to increased skeletal muscle loading after irradiation. Am J Physiol Cell Physiol. 2002;283(4):C1182–95. 10.1152/ajpcell.00173.2002 [DOI] [PubMed] [Google Scholar]
- 77. Rosenblatt JD, Parry DJ: Gamma irradiation prevents compensatory hypertrophy of overloaded mouse extensor digitorum longus muscle. J Appl Physiol (1985). 1992;73(6):2538–43. 10.1152/jappl.1992.73.6.2538 [DOI] [PubMed] [Google Scholar]
- 78. McCarthy JJ, Mula J, Miyazaki M, et al. : Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development. 2011;138(17):3657–66. 10.1242/dev.068858 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 79. Jackson JR, Mula J, Kirby TJ, et al. : Satellite cell depletion does not inhibit adult skeletal muscle regrowth following unloading-induced atrophy. Am J Physiol Cell Physiol. 2012;303(8):C854–C861. 10.1152/ajpcell.00207.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Egner IM, Bruusgaard JC, Gundersen K: Satellite cell depletion prevents fiber hypertrophy in skeletal muscle. Development. 2016;143(16):2898–906. 10.1242/dev.134411 [DOI] [PubMed] [Google Scholar]
- 81. Murach KA, White SH, Wen Y, et al. : Differential requirement for satellite cells during overload-induced muscle hypertrophy in growing versus mature mice. Skelet Muscle. 2017;7(1):14. 10.1186/s13395-017-0132-z [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 82. Fry CS, Lee JD, Jackson JR, et al. : Regulation of the muscle fiber microenvironment by activated satellite cells during hypertrophy. FASEB J. 2014;28(4):1654–65. 10.1096/fj.13-239426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Fry CS, Kirby TJ, Kosmac K, et al. : Myogenic Progenitor Cells Control Extracellular Matrix Production by Fibroblasts during Skeletal Muscle Hypertrophy. Cell Stem Cell. 2017;20(1):56–69. 10.1016/j.stem.2016.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 84. Smith LR, Chambers HG, Lieber RL: Reduced satellite cell population may lead to contractures in children with cerebral palsy. Dev Med Child Neurol. 2013;55(3):264–70. 10.1111/dmcn.12027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Dayanidhi S, Dykstra PB, Lyubasyuk V, et al. : Reduced satellite cell number in situ in muscular contractures from children with cerebral palsy. J Orthop Res. 2015;33(7):1039–45. 10.1002/jor.22860 [DOI] [PubMed] [Google Scholar]
- 86. Dayanidhi S, Lieber RL: Skeletal muscle satellite cells: mediators of muscle growth during development and implications for developmental disorders. Muscle Nerve. 2014;50(5):723–32. 10.1002/mus.24441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Bellamy LM, Joanisse S, Grubb A, et al. : The acute satellite cell response and skeletal muscle hypertrophy following resistance training. PLoS One. 2014;9(10):e109739. 10.1371/journal.pone.0109739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Farup J, Rahbek SK, Riis S, et al. : Influence of exercise contraction mode and protein supplementation on human skeletal muscle satellite cell content and muscle fiber growth. J Appl Physiol (1985). 2014;117(8):898–909. 10.1152/japplphysiol.00261.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Kadi F, Thornell LE: Concomitant increases in myonuclear and satellite cell content in female trapezius muscle following strength training. Histochem Cell Biol. 2000;113(2):99–103. 10.1007/s004180050012 [DOI] [PubMed] [Google Scholar]
- 90. Leenders M, Verdijk LB, van der Hoeven L, et al. : Elderly men and women benefit equally from prolonged resistance-type exercise training. J Gerontol A Biol Sci Med Sci. 2013;68(7):769–79. 10.1093/gerona/gls241 [DOI] [PubMed] [Google Scholar]
- 91. Olsen S, Aagaard P, Kadi F, et al. : Creatine supplementation augments the increase in satellite cell and myonuclei number in human skeletal muscle induced by strength training. J Physiol. 2006;573(Pt 2):525–34. 10.1113/jphysiol.2006.107359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Petrella JK, Kim JS, Cross JM, et al. : Efficacy of myonuclear addition may explain differential myofiber growth among resistance-trained young and older men and women. Am J Physiol Endocrinol Metab. 2006;291(5):E937–46. 10.1152/ajpendo.00190.2006 [DOI] [PubMed] [Google Scholar]
- 93. Fry CS, Noehren B, Mula J, et al. : Fibre type-specific satellite cell response to aerobic training in sedentary adults. J Physiol. 2014;592(12):2625–35. 10.1113/jphysiol.2014.271288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Murach KA, Walton RG, Fry CS, et al. : Cycle training modulates satellite cell and transcriptional responses to a bout of resistance exercise. Physiol Rep. 2016;4(18): pii: e12973. 10.14814/phy2.12973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kirby TJ, Patel RM, McClintock TS, et al. : Myonuclear transcription is responsive to mechanical load and DNA content but uncoupled from cell size during hypertrophy. Mol Biol Cell. 2016;27(5):788–98. 10.1091/mbc.E15-08-0585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Seaborne RA, Strauss J, Cocks M, et al. : Human Skeletal Muscle Possesses an Epigenetic Memory of Hypertrophy. Sci Rep. 2018;8(1):1898. 10.1038/s41598-018-20287-3 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 97. Frontera WR, Ochala J: Skeletal muscle: a brief review of structure and function. Calcif Tissue Int. 2015;96(3):183–95. 10.1007/s00223-014-9915-y [DOI] [PubMed] [Google Scholar]
- 98. Nilwik R, Snijders T, Leenders M, et al. : The decline in skeletal muscle mass with aging is mainly attributed to a reduction in type II muscle fiber size. Exp Gerontol. 2013;48(5):492–8. 10.1016/j.exger.2013.02.012 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation