Abstract
Background
Inflammation may be a hidden process in the relationship between dietary intake and depression, but no study has evaluated the role of diet and inflammation jointly in explaining depression risk in early life. The current study aims to investigate the relationship between inflammatory dietary pattern (IDP) in childhood and depression in early adulthood.
Methods
This study used data prospectively collected over 10 years from the Avon Longitudinal Study of Parents and Children (ALSPAC) cohort (n = 6939) free from depression at baseline (age 8.5 years). An IDP score was empirically derived via reduced rank regression and stepwise linear regression based on dietary intake data from the food frequency questionnaire at 8.5 years and levels of inflammatory biomarkers, C-reactive protein and interleukin-6, at 9.5 years. At age 18 years, depression cases were identified via the International Statistical Classification of Diseases, 10th Revision (ICD-10) diagnosis and the Clinical Interview Schedule-Revised (CIS-R) depression score. Logistic regression models were constructed to examine the relationship between the IDP score and risk of depression adjusted for potential confounders. Analyses stratified by weight status were also conducted. Multiple imputations were utilized to minimize bias due to loss-to-follow-up.
Results
Participants in the highest tertile of IDP score had 1.34 times odds to develop depression compared to those in the lowest tertile (95% CI, 1.08–1.66; P-trend<0.01), after dietary misreporting status and energy intake were adjusted. After all covariates were adjusted, the relationship between IDP tertiles and depression was attenuated (highest tertile vs. lowest tertile: OR = 1.21; 95% CI, 0.96–1.51); in addition, the relationship was marginally significant among participants who were not overweight or obese (p < 0.10) but not significant among participants who were overweight or obese.
Conclusions
Higher IDP in childhood seems to be associated with higher depression risk in early adulthood. The study provides preliminary evidence that chronic inflammation may underlie the relationship between diet and depression even for children, especially those who are not overweight or obese.
Keywords: ALSPAC, Cohort, Children, Depression, Inflammatory dietary pattern, Inflammatory biomarkers, C-reactive protein, Interleukin-6, Reduced rank regression
Highlights
-
•
Inflammatory diet in childhood increases depression risk in early adulthood.
-
•
The relationship is more robust among children who are not overweight or obese.
-
•
Inflammation may underlie the link between diet and depression even for children.
-
•
Anti-inflammatory diet could be recommended for children to prevent depression.
1. Introduction
Depression influences about 16% of Americans over their lifetime, with a quarter of these cases starting before age 20 years (Kessler et al., 2003; Kessler et al., 2005; Murray and Lopez, 2013). Depression occurring in adolescence and early adulthood tends to persist into later life stages and can profoundly affect academic performance, social connections, and physical and mental health later in life, causing large costs to both individuals and society (Fergusson and Woodward, 2002; Fombonne et al., 2001). Childhood is a sensitive period of human development when adverse exposures can influence mental and physical disease risk for a lifetime (Ben-Shlomo and Kuh, 2002). Therefore, identifying early life etiologic factors that are modifiable may be particularly useful in reducing the burden of depression in the population. Where much depression research focuses on the psychosocial determinants of the disease (Bowes et al., 2015; Fletcher, 2009), researchers are increasingly considering whether dietary factors also play a role, though few have considered diet in early life as contributing to later life depression risk.
Recent prospective studies among adults have indicated that healthy dietary patterns (featuring vegetables, fruits, whole grains, fish, and mushrooms), such as the Mediterranean style diet (Rienks et al., 2013) and the Prudent diet (Jacka et al., 2014; Ruusunen et al., 2014), decrease depression risk, while unhealthy dietary patterns (featuring red meat, fried foods, refined grains, highly processed foods, and sugar sweetened beverages), such as the Western diet (Jacka et al., 2014; Le Port et al., 2012), increase depression risk (Jacka et al., 2014; Le Port et al., 2012; Rienks et al., 2013; Ruusunen et al., 2014). For example, one study of 6060 women aged 50–55 years found that higher adherence to the Mediterranean style diet at baseline was associated with lower incidence of depressive symptoms 3 years later (Rienks et al., 2013). Moreover, while it has been shown that dietary habits formed in childhood tend to be maintained into adulthood (Mikkila et al., 2005), knowledge of the effects of diet early in life on mental health is limited (Khalid et al., 2016; O’Neil et al., 2014). One cross-sectional study among 5003 Chinese adolescents aged 11–16 years derived three dietary patterns via factor analysis and indicated that the “snack” and “animal food” patterns were associated with higher levels of depression, while the “traditional” pattern (fresh fruits and vegetables, soy products, gruel, whole grains, etc.) was associated with lower levels of depression (Weng et al., 2012). Another cross-sectional study of 1799 adolescents found higher adherence to the “Western” dietary pattern and less adherence to the “Healthy” dietary pattern were significantly associated with attention deficit hyperactivity disorder (ADHD) diagnosis (Howard et al., 2011). While these studies and similar work are suggestive of an association between childhood dietary pattern and risk of mental health and behavioral problems later in life, studies in this area are subject to some limitations, including an overreliance on cross-sectional designs which raise concerns about reverse causality (Oddy et al., 2009; Oellingrath et al., 2013; Weng et al., 2012), short follow up period between exposure and outcome assessments (Jacka et al., 2013; McMartin et al., 2012; Trapp et al., 2016), and emphasis on childhood externalizing behavior problems, such as ADHD (Azadbakht and Esmaillzadeh, 2012; Howard et al., 2011; Khalid et al., 2016; O’Neil et al., 2014; van Egmond-Froehlich et al., 2012). Therefore, research is needed to better characterize the long-term prospective association between childhood dietary practices and depression risk later in life.
A plausible biologic mechanism linking early life dietary practices with depression risk is systemic inflammation. As part of the adaptive reaction to infection from the immune system, inflammation measured by levels of serum inflammatory markers, such as interleukin 6 (IL-6) and C-reactive protein (CRP), can trigger a set of metabolic syndromes and behavioral regulations (Yaffe et al., 2004). A large body of work has shown the bidirectional relationship between inflammation and depression. In particular, while some studies show that individuals with depression have a higher risk of developing inflammation (Copeland et al., 2012; Deverts et al., 2010; Duivis et al., 2011; Stewart et al., 2009), others show that inflammation can contribute to depression (Gimeno et al., 2009; Khandaker et al., 2014; Valkanova et al., 2013). For example, one prospective study among 4415 youths (the dataset used for the current analysis) has indicated that higher IL-6 levels at age 9 years were associated with an increased risk of depression at age 18 years (Khandaker et al., 2014). Other research suggests that an unhealthy diet that is high in saturated fats, trans fatty acids, sugar, and refined starches, but low in omega-3 (n-3) unsaturated fats (e.g. from fish oil), whole grains, fibers (from fruits and vegetables), and natural antioxidants may stimulate inflammation (Kaplan et al., 2015; Loprinzi et al., 2015). Taken together, these studies suggest that chronic inflammation may be a process through which dietary intake influences depression (Kiecolt-Glaser, 2010; Kiecolt-Glaser et al., 2015).
Compared to individual food items, dietary patterns, measured a priori via dietary indices (e.g. Healthy Eating Index) or derived a posteriori via statistical methods (e.g. factor analysis), can incorporate complex interactions among foods/nutrients and can better predict disease risk (Hu, 2002). A few studies among adults have integrated information on diet and inflammation to study the joint contribution of these factors on depression risk. Four studies utilized an a priori Dietary Inflammatory Index (DII) (Shivappa et al., 2014) and found that higher DII scores were associated with higher depression risk (Adjibade et al., 2017; Akbaraly et al., 2016; Sanchez-Villegas et al., 2015; Shivappa et al., 2016). However, like other a priori dietary indices, the DII focused only on selected aspects of diet and did not use all of the available dietary intake information from the study samples (Hoffmann et al., 2004; Hu, 2002). To overcome limitations of the a priori dietary index, one study empirically derived an Inflammatory Dietary Pattern (IDP), which incorporated biomarkers of inflammation and self-reported information on dietary intake via reduced rank regression (RRR) (Lucas et al., 2014). Through RRR, dietary patterns that maximally explain variation in inflammatory markers are empirically derived and may better predict disease, since both empiric information from study data and theorized information about disease-related variables are utilized (Hoffmann et al., 2004; Lucas et al., 2014). Using this approach, the study examined 43,685 Nurses’ Health Study participants aged 50–77 years and found those in the top IDP quintile exhibited 40% higher odds of developing depression compared with women in the lowest quintile, after confounders were controlled, over 12 years of follow up (Lucas et al., 2014). Taken together, these studies indicate that an inflammatory diet may contribute to depression risk and that empirically derived biomarker-based IDP measures may be more valid and precise than a priori measures. However, this approach has not been applied to a younger population, and thus it is not clear if an inflammatory dietary pattern in childhood could likewise be derived to predict depression risk. Furthermore, compared with traditional mediation analysis that investigates the mediating effect from inflammation on the relationship between diet and depression, RRR can identify the components of the diet that are most relevant to inflammation.
In addition, adipose tissue is a rich source of inflammatory factors and the relationship between adiposity and depression is bidirectional (Shelton and Miller, 2011). Adipose tissue produces inflammatory factors, including cytokines such as IL-6, TNF-α, and chemokines, which in turn may activate widespread immune reaction, potentially leading to or exacerbating inflammation-related diseases, including depression (Shelton and Miller, 2011). Previous studies that investigated the association between inflammatory diet and depression among adults indicate there may be a modifying effect of weight status. For example, a prospective study among 15,093 adults found a stronger association between DII score and depression among obese vs. non-obese participants (Sanchez-Villegas et al., 2015). It is not known whether such weight-status patterning in the inflammatory diet and depression association would be similarly evident earlier in the life course.
In the current study, we integrated biomarkers of inflammation with a widely-used dietary assessment to examine the association between inflammatory dietary patterns in childhood and incident depression in young adulthood. We leveraged nearly 10 years of prospectively collected life course information from the Avon Longitudinal Study of Parents and Children (ALSPAC), a large population-based birth cohort study from the United Kingdom. We hypothesized that children with higher IDP scores would have higher risk of depression in young adulthood compared to children with lower IDP scores, controlling for relevant social, behavioral, and demographic confounders. We also considered whether the association was modified by weight status. To our knowledge, this study is among the first to consider an inflammatory dietary pattern in childhood in association with depression risk later in life.
2. Material and methods
2.1. Study sample
The study sample originates from ALSPAC, an ongoing population-based trans-generational prospective study. The core aim of ALSPAC is to examine the effects of a wide range of social, biological, and environmental factors on women’s pregnancies and their children’s physical and mental health. Pregnant women who lived in Avon County in Southwest England and had an estimated delivery date between April 1st, 1991 and December 31st, 1992 were eligible to participate. About 85% of all eligible women enrolled, forming a cohort of 14,541 pregnant women who gave birth to 13,988 children alive at 1 year of age (phase I recruitment). 713 children were additionally recruited when they were around 7 years old (phase II and phase III recruitment) (Boyd et al., 2013; Fraser et al., 2013; Golding et al., 2001). The study website contains details of all the data that are available through a fully searchable data dictionary and variable search tool (http://www.bristol.ac.uk/alspac/researchers/our-data/). Questionnaires were mailed to mothers four times during pregnancy. After the children were born, child-related questionnaires were sent to mothers twice a year and mother/partner-related questionnaires were sent once a year. The survey collected data ranging from socio-demographic characteristics to life style factors, physical health, and mental health for study children, mothers, and partners. Researchers also collected biological and genetic samples (e.g. blood, urine, hair, nail, etc.) from both mothers and children during their clinic visits at certain time points (Boyd et al., 2013; Fraser et al., 2013; Golding et al., 2001; Ness, 2004).
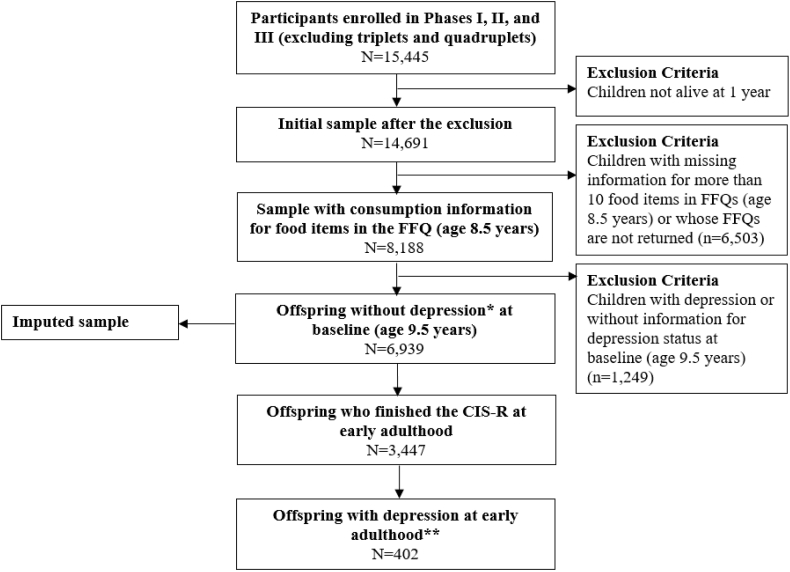
The present study uses data from a subset of ALSPAC participants, specifically children who had available dietary intake information at age 8.5 years. To ensure incident cases of depression were identified at follow up, we excluded children with depression at age 9.5 years (based on a score of ≥12 on the Short Moods and Feelings Questionnaire) (Angold et al., 1995). Thus, the base analytic sample included 6939 participants (Fig. 1). The ALSPAC Law and Ethics Committee, the ALSPAC Local Research Ethics Committee, and the Institutional Review Board of the University at Albany, State University of New York authorized the study protocol.
Fig. 1.
Flowchart of the study sample in the ALSPAC. ALSPAC, Avon Longitudinal Study of Parents and Children; FFQ, Food Frequency Questionnaire; CIS-R, Computerized Interview Schedule-Revised; SMFQ, Short Moods and Feelings Questionnaire; ICD-10, the International Statistical Classification of Diseases, 10th Revision. * Baseline depression (at age 9.5 years) was assessed via the SMFQ reported by mothers/main caregivers. ** Participants were defined as depression cases if they had depression score ≥ 9 (out of 21) measured via the CIS-R or had ICD-10 diagnosis of depression at early adulthood (i.e., at age 18 years).
2.2. Depression in early adulthood
During the clinic visit when participants were about 18 years old, 3447 (49.7% of the total sample of 6939) completed the computerized version of the Clinical Interview Schedule-Revised (CIS-R), a standardized and validated tool widely utilized to measure depression and anxiety (Lewis et al., 1992). The CIS-R incorporates core symptoms of depression from International Statistical Classification of Diseases, 10th Revision (ICD-10) to produce a composite depression score (ranging from 0 to 21) that includes sub-scores from five sources---depression, fatigue, concentration, sleep issues, and depressive thoughts---to indicate severity of depressive symptoms in the previous week (Bowes et al., 2015; Khandaker et al., 2014). We combined the criteria (score 9 as cut-point of the CIS-R depression score) in a previous study (Khandaker et al., 2014) with the ICD-10 diagnosis of probable depression to more comprehensively identify probable depression cases in early adulthood. Among the 3447 participants, 402 (11.7%) were classified as depression cases (Fig. 1).
2.3. Inflammatory dietary pattern score in childhood
The IDP score at age 9 years was derived by synthesizing parent-reported information on diet from a Food Frequency Questionnaire (FFQ) and child biomarkers of CRP and IL-6, and by conducting the RRR analysis. These variables and derivations are described in turn below.
Diet. When children were 8.5 years old, dietary intake was evaluated via a FFQ completed by mothers, or main caregivers (University of Bristol, 2000). The FFQ contains a series of questions inquiring about the “current” consumption frequency of a wide range of food and drinks, including 90 items (87 detailed items and 3 general items [“milk”, “bread”, and “spread”]). Participants were excluded if they failed to return the FFQ or had more than 10 food items missing over the 90 items in the FFQ. If ten or fewer items were unanswered, FFQ data were utilized and the missing items were assigned a value of 0 (Emmett et al., 2015; Northstone, Joinson, Emmett, Ness and Paus, 2012).
Most items had standardized options reflecting the consumption frequency of each food item: (1) “never or rarely”; (2) “once in 2 weeks”; (3) “1–3 times per week”; (4) “4–7 times per week”; or (5) “more than once per day”. To facilitate quantitative analysis, data were converted into a weekly basis (i.e. 0; 0.5; 2; 5.5; and 10 times per week corresponding to the five frequency options) (Emmett et al., 2015; Northstone and Emmett, 2005; Northstone et al., 2012). Some detailed food items have non-standardized consumption frequency options in the FFQ and we modified them to also represent weekly consumption. For example, items “wine”, “beer”, “spirits”, and “other alcohol” have options (“> once a week”, “once a week”, “< once a week”, and “no”) that were converted to weekly frequency 2, 1,0.5, and 0 times respectively (Northstone and Emmett, 2008). Detailed algorithm descriptions can be provided upon request.
Similar to previous studies (Hu et al., 1999; Northstone and Emmett, 2005; Northstone et al., 2012; Vermeulen et al., 2016), we combined some original detailed items in the FFQ before analysis since they belong to the same food type. Thus, 87 detailed food items in the FFQ were condensed into 38 food groups (e.g. “shellfish”, “white fish without coating”, “tuna”, and “other fish” were combined into the food group “fish & other seafood”) (Supplemental Table 1). Weekly consumption frequencies for each of the 38 food groups were then used as the 38 predictors/independent variables in the RRR model.
Inflammation. When children were about 9.5 years old, they were invited to a clinic visit for biological assessments. Children gave blood samples after their parents provided informed consent. The blood samples were promptly spun and refrigerated at −80 °C. Inflammatory markers were assessed in 2008 following a storage period (median 7.5 years) without any freeze-thaw cycle (Khandaker et al., 2014). Two biomarkers of inflammation were considered in this analysis: C-reactive protein (CRP) and Interleukin 6 (IL-6). Concentration of CRP (mg/L) was assessed via the automated particle-enhanced immunoturbidimetric assay (Roche, UK) and concentration of high sensitivity IL-6 (pg/mL) was assessed via enzyme-linked immunosorbent assay (R&D systems, Abingdon, UK). For both biomarkers, inter-assay coefficients of variation were less than 5% (Khandaker et al., 2014). Among the study sample, 3814 children had valid measurements of the two inflammatory biomarkers (CRP and IL-6) at age 9.5. To improve normality, both CRP and IL-6 levels were log-transformed and then were used as the dependent variables in the RRR model (Tabung et al., 2016).
Deriving the inflammatory dietary pattern score. An inflammatory dietary pattern (IDP) score was derived using data on dietary intake and inflammatory biomarkers via RRR (Tabung et al., 2016). The RRR model started from the covariance matrix of the two response variables (two biomarkers) and extracted linear functions of predictors (38 food groups) that explain the shared variation in the two response variables as much as possible (Hoffmann et al., 2004). The maximum number of factors derived equals the number of response variables (Hoffmann et al., 2004) and thus two factors (or dietary patterns) were derived. The first and second factor explained 1.40% and 0.31% respectively of the shared variation in both biomarkers. In alignment with previous studies (Ambrosini et al., 2016; Hoffmann et al., 2004; Lucas et al., 2014; Tabung et al., 2016), the first factor was kept for subsequent analyses since it explains the most shared variation. Then, the food groups that are more essential to IDP were identified via stepwise linear regression with the response score of the first factor as the dependent variable (p < 0.05). Finally, intakes of the several essential food groups identified via the final stepwise linear regression model were weighted by the corresponding regression coefficients in the stepwise linear regression. These weighted intakes were summed up to construct the IDP score (Lucas et al., 2014; Schulze et al., 2003; Tabung et al., 2016). The IDP score reflects the inflammatory potential of the diet, with a lower (more negative) score indicating an anti-inflammatory diet and higher (more positive) score indicating a pro-inflammatory diet. Spearman correlation coefficients were utilized to assess associations among factor score, response score, the IDP score, component food groups identified, and levels of the inflammatory biomarkers (Table 1). Among the study sample (n = 6939), 3814 participants who had biomarker measures were included to derive the IDP and then an IDP score was calculated for each participant in the study sample based on his/her dietary intake. Since the range of the IDP score was narrow (−0.78, 0.34) and to also facilitate interpretation, IDP scores were converted to z-scores (mean 0, standard deviation 1) and treated as continuous predictors in regression models. The IDP score was also divided into tertiles to assess potential non-linear associations between inflammatory diet and depression risk (Miki et al., 2015).
Table 1.
Components of IDP score and their correlations with inflammatory biomarkers among 3814 ALSPAC participantsa.
| RRR dietary pattern response scoreb | RRR dietary pattern factor scoreb | IDP score | CRP | IL-6 | Weights | |
|---|---|---|---|---|---|---|
| RRR dietary pattern response scoreb | 1.00 | 0.13*** | 0.10*** | 0.86*** | 0.82*** | NA |
| RRR dietary pattern factor scoreb | 0.13*** | 1.00 | 0.75*** | 0.13*** | 0.10*** | NA |
| IDP score | 0.10*** | 0.75*** | 1.00 | 0.09*** | 0.08*** | NA |
| IDP score component food groupsc | ||||||
| Positive associations | ||||||
| Sweetened beverages | 0.04* | 0.23*** | 0.31*** | 0.04* | 0.03 | 8.22 |
| Salad | 0.02 | 0.17*** | 0.21*** | 0.02 | 0.02 | 31.01 |
| Negative associations | ||||||
| Root vegetables | −0.04** | −0.25*** | −0.34*** | −0.05** | −0.03# | −20.26 |
| Nuts | −0.04** | −0.24*** | −0.33*** | −0.04** | −0.03* | −28.86 |
| Whole grains | −0.05** | −0.36*** | −0.48*** | −0.04* | −0.05** | −9.24 |
| Digestive biscuits | −0.05** | −0.38*** | −0.48*** | −0.05** | −0.03* | −29.87 |
IDP, Inflammatory Dietary Pattern; ALSPAC, Avon Longitudinal Study of Parents and Children; RRR, Reduced Rank Regression; CRP, C-reactive protein; IL-6, Interleukin-6; NA, Not Applicable.
#p < 0.10; *p < 0.05; **p < 0.01; ***p < 0.001.
Participants with food intake information at age 8.5 years and with CRP and IL-6 measured at age 9 years were included (n = 3814). Values except those in the last column are Spearman correlation coefficients. Values in the last column are 1000 times the regression coefficients for each IDP score component obtained from the last step of the stepwise linear regression analysis.
The RRR dietary pattern was the first factor obtained from RRR with all 38 food groups. The response score is a linear function of responses (biomarkers) and the factor score is a linear function of predictors (food groups). The response score was then utilized as the dependent variable in the stepwise linear regression analyses to identify the most important food groups contributing to the IDP score.
Six component food groups of the IDP score were identified through the stepwise linear regression with p < 0.05 for inclusion and exclusion. Sweetened beverages include sweetened fruit juice, squash, cola, other fizzy drinks (e.g. lemonade, fizzy water), and flavored milk; salad includes lettuce, cucumber, peppers, other raw vegetables, etc.; root vegetables include carrots and other root vegetables (e.g. turnip, swede, parsnip); nuts include peanuts/peanut butter and other nuts (e.g. cashew, nut roast); whole grains include crispbreads, oat cereals, bran cereals, brown/granary bread, wholemeal bread, chappatis/pitta, and naan; digestive biscuits include Rich tea, shortcake, digestive and chocolate digestive, Hob Nobs, etc.
2.4. Covariates
Children’s race (white vs. non-white), parental highest education level based on the UK classification system (below O-level, O-level, A-level, or above A-level), and parental highest social class (professional, managerial/technical, skilled non-manual, skilled manual, or partly skilled/unskilled) were collected from maternal questionnaires issued at about 32 weeks gestation. Children’s sex (male vs. female), maternal age at delivery (<25 years, 25–29 years, 30–34 years, or ≥ 35 years; range: 15–44 years) (Earls and Jung, 1987), and children’s birth weight (<2500 g (low birthweight), 2,500 g-<4,000 g (normal birthweight), or ≥ 4,000 g (high birthweight); range: 645–5640 g) (World Health Organization, 2016) were collected from questionnaires and hospital records. The following confounders were assessed via maternal questionnaires (with assessment time indicated by age of children in years shown in square brackets): children’s physical activity levels (frequency the child goes to the swimming pool or other sporting area: < once a month, once a month, once a week, or at least twice a week) [5 years], maternal smoking status (smokers vs. smokers) [5 years], maternal mental health (anxiety (yes vs. no) and depression (yes vs. no)) [6 years], and maternal marital status (married vs. other) [8 years]. At 8.5 years, energy intake (EI) (kJ/day) was calculated by the ALSPAC research team from University of Bristol using the fifth edition of “McCance and Widdowson’s The composition of Food” (The Royal Society of Chemistry and Ministry of Agriculture, Fisheries and Food, 1991) based on standard age-specific portion size (Ministry of Agriculture, Fisheries and Food, 1993) and consumption of each food item collected in the FFQ (Emmett et al., 2015; Northstone et al., 2012). Among the study sample, EI ranged from 2282.82 kJ/day to 18866.99 kJ/day with mean 7945.58 kJ/day and standard deviation 1934.48 kJ/day. The total coefficient of variation (CVt) equaled 24% (Black and Cole, 2000). To better reflect biological plausibility, one CVt was utilized to specify the range of misreporting of EI based on the individualized estimated energy requirement (EER) (Ambrosini et al., 2016; Huang et al., 2005; Johnson et al., 2008). EER was calculated according to gender and age group-specific formulae with the body weight that coincided with the EI estimation (Torun, 2005). The body weight at age 8.5 years was estimated by that measured at age 9.5 years. Thus, for each child, the estimated/reported EI within 76%–124% of his/her individualized EER can be considered as plausibly reported. Participants are then divided into three groups as underreporters (EI/EER<0.76), plausible reporters (0.76 ≤ EI/EER ≤ 1.24), or overreporters (EI/EER>1.24). At the clinic visit of age 9.5 years, height and weight were measured. BMI was calculated by dividing height in meters by square of weight in kilograms. Body weight status (underweight, normal weight, overweight, or obesity) was defined based on the age- and gender-specific BMI cut points from the International Obesity Task Force (Cole and Lobstein, 2012).
2.5. Statistical analysis
We conducted multiple imputation (MI) to decrease potential attrition bias while increasing statistical power. The ALSPAC includes many measures that are very useful to predict missingness of other critical variables. Thus, the assumption of “missing at random” (MAR), on which MI is based, is plausible (Bowes et al., 2015; Hammerton et al., 2015; Yuan, 2011). MI was conducted via fully conditional specification (FCS) within the statistical procedure SAS Proc MI (SAS Institute Inc.) with 10 cycles of burn-in iterations before each imputation (Bartlett et al., 2015). Suggested by previous studies (Bowes et al., 2015; White et al., 2011), the imputation model included both variables in the analysis model (exposure, outcome, and confounders) and additional variables, such as other demographic and SES factors, child mental health, child BMI, and maternal mental health indicators. The number of imputed data sets should be at least 100 times the maximum value of the fraction of missing information (FMI) that can be estimated by the maximum missing rate among the variables in the analysis model (White et al., 2011). Among the study sample, ICD-10 diagnosis of depression at early adulthood has the highest missing rate (0.50). Thus, we imputed the data set 50 times and the relative efficiency for each variable in the analysis model achieved at least 0.99.
SAS 9.4 (SAS Institute Inc., Cary, NC, USA) was utilized to conduct all statistical analyses based on the multiply imputed data sets. Associations between exposure (the IDP score in childhood) and outcome (depression in adulthood) and associations between each covariate considered in the analysis model and exposure or outcome were evaluated via bivariate analyses where the IDP score was divided into tertiles. For each categorical variable, chi-square tests were conducted over the exposure or outcome across multiply imputed data sets and the p-value was calculated based on pooled statistics from these tests (Ratitch, Lipkovich, & O’Kelly, 2013). For each continuous variable, type 3 analyses (over levels of exposure or outcome) were conducted across multiply imputed data sets and the p-value was calculated based on pooled statistics from these tests (Gantz, 2006; Wang et al., 2014). The significance level was set to be 0.10. If a covariate was significantly related to either exposure or outcome, then it was included in the adjusted statistical models.
Logistic regression models evaluated the association between the IDP score in childhood and depression in early adulthood. The IDP score was treated as a continuous z-score and also as tertiles. Trend tests were also conducted. For each form of the IDP score, four logistic models were separately constructed. Similar to previous studies (Ambrosini et al., 2016; Johnson et al., 2008; McMartin et al., 2012), model 1 was the crude model; model 2 adjusted for EI and EI misreporting status; model 3 adjusted for other covariates (sociodemographic, birth-related, maternal, child behavioral factors, and weight status); and model 4 was the fully adjusted model. Finally, like previous studies (Lucas et al., 2014; Sanchez-Villegas et al., 2015; Tabung et al., 2016), we conducted the analysis stratified by weight status (underweight and normal weight vs. overweight and obese) to investigate potential effect modification.
3. Results
3.1. IDP score
We identified six component food groups that significantly contributed to the IDP score. Spearman correlation coefficients between the IDP score and levels of inflammatory biomarkers were 0.09 (p < 0.01) and 0.08 (p < 0.01) for CRP and IL-6 respectively. The IDP was associated with high intakes of sweetened beverages and salad but low intakes of root vegetables, nuts, whole grains, and digestive biscuits (all p < 0.01). Except for salad, all the other food groups were significantly associated with at least one inflammatory biomarker (Table 1).
3.2. Confounders chosen for the statistical models
As shown in Table 2, compared to participants with the lowest tertile IDP score, participants with higher IDP scores had a higher probability of developing depression in early adulthood (p = 0.02; from the lowest to the highest IDP tertile, prevalence of depression was 11.7%, 12.6%, and 15.2% in each group respectively). In addition, higher proportions of participants in the higher IDP score groups were female, non-white race, less physically active, overweight or obese, and born to mothers who smoked and who had younger age at delivery (all p < 0.05). Higher IDP score was also associated with lower EI derived from FFQs which tended to be underreported (p < 0.05). Lower parental education level (less likely to have above A-level), lower parental social class (less likely to be professional), and non-married parents were also associated with higher IDP scores (all p < 0.05) (Table 2). Depression incidence by early adulthood was higher among female participants, those with higher IDP score and BMI, those who were less physically active, and those whose mothers were smokers, had anxiety and depression and were not married (all p < 0.05) (Table 3). Thus, EI misreporting status, EI, gender, race, parental education, parental social class, parental marital status, maternal age at delivery, birthweight, maternal smoking status, maternal anxiety, maternal depression, physical activity level, and weight status were included in the analysis model.
Table 2.
Characteristics by tertiles of the IDP score among 6939 ALSPAC participantsa.
| Characteristics | Total |
IDP score |
P-valueb | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1st Tertile |
2nd Tertile |
3rd Tertile |
|||||||
| n | (%) | n | (%) | n | (%) | n | (%) | ||
| Depression at age 18 yearsc | |||||||||
| Yes | 911 | (13.1) | 270 | (11.7) | 290 | (12.6) | 351 | (15.2) | 0.020* |
| No | 6028 | (86.9) | 2043 | (88.3) | 2023 | (87.4) | 1962 | (84.8) | |
| Socio-demographic factors | |||||||||
| Sex | |||||||||
| Male | 3488 | (50.3) | 1301 | (56.2) | 1138 | (49.2) | 1049 | (45.4) | <.001** |
| Female | 3451 | (49.7) | 1012 | (43.8) | 1175 | (50.8) | 1264 | (54.6) | |
| Race | |||||||||
| White | 6684 | (96.3) | 2246 | (97.1) | 2226 | (96.3) | 2211 | (95.6) | 0.039* |
| Non-White | 255 | (3.7) | 67 | (2.9) | 87 | (3.7) | 102 | (4.4) | |
| Parental education | |||||||||
| Below O-level | 881 | (12.7) | 230 | (9.9) | 309 | (13.4) | 342 | (14.8) | <.001** |
| O-level | 1775 | (25.6) | 514 | (22.2) | 615 | (26.6) | 646 | (27.9) | |
| A-level | 2370 | (34.2) | 777 | (33.6) | 791 | (34.2) | 803 | (34.7) | |
| Above A-level | 1912 | (27.6) | 792 | (34.2) | 598 | (25.8) | 523 | (22.6) | |
| Parental social class | |||||||||
| Professional | 1174 | (16.9) | 476 | (20.6) | 373 | (16.1) | 326 | (14.1) | <.001** |
| Managerial or technical | 3163 | (45.6) | 1091 | (47.2) | 1020 | (44.1) | 1053 | (45.5) | |
| Skilled non-manual | 1848 | (26.6) | 534 | (23.1) | 671 | (29.0) | 643 | (27.8) | |
| Skilled manual | 556 | (8.0) | 160 | (6.9) | 178 | (7.7) | 219 | (9.5) | |
| Partly skilled/unskilled | 197 | (2.8) | 52 | (2.3) | 72 | (3.1) | 73 | (3.2) | |
| Parental marital status | |||||||||
| Married | 5613 | (80.9) | 1946 | (84.1) | 1848 | (79.9) | 1820 | (78.7) | <.001** |
| Not married | 1326 | (19.1) | 367 | (15.9) | 465 | (20.1) | 493 | (21.3) | |
| Birth-related factors | |||||||||
| Maternal age at delivery | |||||||||
| <25 years | 992 | (14.3) | 277 | (12.0) | 331 | (14.3) | 383 | (16.6) | <.001** |
| 25–29 years | 2731 | (39.4) | 890 | (38.5) | 913 | (39.5) | 928 | (40.1) | |
| 30–34 years | 2365 | (34.1) | 850 | (36.8) | 777 | (33.6) | 738 | (31.9) | |
| 35- years | 851 | (12.3) | 296 | (12.8) | 292 | (12.6) | 263 | (11.4) | |
| Birthweight | |||||||||
| Low birthweight (<2500 g) | 298 | (4.3) | 96 | (4.2) | 110 | (4.7) | 92 | (4.0) | 0.083# |
| Normal birthweight (2500 g - < 4000 g) | 5699 | (82.1) | 1875 | (81.1) | 1924 | (83.2) | 1899 | (82.1) | |
| High birthweight (≥ 4000 g) | 942 | (13.6) | 341 | (14.8) | 279 | (12.1) | 321 | (13.9) | |
| Maternal factors | |||||||||
| Smoking status | |||||||||
| Smoker | 1407 | (20.3) | 406 | (17.6) | 494 | (21.4) | 506 | (21.9) | <.001** |
| Non-smoker | 5532 | (79.7) | 1907 | (82.4) | 1819 | (78.6) | 1807 | (78.1) | |
| Anxiety | |||||||||
| Yes | 1437 | (20.7) | 456 | (19.7) | 472 | (20.4) | 509 | (22.0) | 0.164 |
| No | 5502 | (79.3) | 1857 | (80.3) | 1841 | (79.6) | 1804 | (78.0) | |
| Depression | |||||||||
| Yes | 1497 | (21.6) | 482 | (20.8) | 502 | (21.7) | 513 | (22.2) | 0.543 |
| No | 5442 | (78.4) | 1831 | (79.2) | 1811 | (78.3) | 1800 | (77.8) | |
| Child behavioral factors | |||||||||
| Physical activities | |||||||||
| Less than once a month | 2109 | (30.4) | 655 | (28.3) | 695 | (30.0) | 759 | (32.8) | 0.019* |
| Once a month | 1934 | (27.9) | 643 | (27.8) | 667 | (28.8) | 624 | (27.0) | |
| Once a week | 2448 | (35.3) | 858 | (37.1) | 817 | (35.3) | 772 | (33.4) | |
| At least twice a week | 449 | (6.5) | 157 | (6.8) | 134 | (5.8) | 158 | (6.8) | |
| Energy intake | |||||||||
| Underreport | 1248 | (18.0) | 250 | (10.8) | 435 | (18.8) | 562 | (24.3) | <.001** |
| Plausible report | 4479 | (64.5) | 1521 | (65.8) | 1515 | (65.5) | 1442 | (62.4) | |
| Overreport | 1214 | (17.5) | 542 | (23.4) | 362 | (15.7) | 309 | (13.3) | |
| Energy intake (kJ/day) | |||||||||
| Mean (SE) | 7946 | (23.22) | 8511 | (39.54) | 7803 | (39.11) | 7523 | (39.34) | <.001** |
| Child weight status | |||||||||
| BMI | |||||||||
| Underweight | 603 | (8.7) | 202 | (8.7) | 213 | (9.2) | 187 | (8.1) | <.001** |
| Normal weight | 4895 | (70.5) | 1715 | (74.1) | 1609 | (69.5) | 1571 | (67.9) | |
| Overweight | 1189 | (17.1) | 331 | (14.3) | 407 | (17.6) | 452 | (19.5) | |
| Obesity | 252 | (3.6) | 65 | (2.8) | 84 | (3.6) | 103 | (4.4) | |
IDP, Inflammatory Dietary Pattern; ALSPAC, Avon Longitudinal Study of Parents and Children.
#p < 0.10; *p < 0.05; **p < 0.01.
Imputed sample, n = 6939 (number of imputations: 50). Participants without depressive disorders and with IDP score at baseline were included.
For categorical characteristics, p-values were obtained based on chi-squared tests pooled across multiply imputed samples. For continuous characteristics (e.g. birthweight), p-values were obtained based on type 3 analyses pooled across multiply imputed samples.
Depression cases were identified if participants had ICD-10 diagnosis or had depression score ≥ 9 (out of 21) in the Computerized Interview Schedule-Revised (CIS-R) at early adulthood.
Table 3.
Characteristics by depression status among 6939 ALSPAC participantsa.
| Characteristics | Depression at age 18 yearsb |
P-valuec | |
|---|---|---|---|
| n | (%) | ||
| IDP score | |||
| 1st Tertile | 270 | (11.7) | 0.020* |
| 2nd Tertile | 290 | (12.6) | |
| 3rd Tertile | 351 | (15.2) | |
| Socio-demographic factors | |||
| Sex | |||
| Male | 348 | (10.0) | <.001** |
| Female | 563 | (16.3) | |
| Race | |||
| White | 875 | (13.1) | 0.527 |
| Non-White | 36 | (14.2) | |
| Parental education | |||
| Below O-level | 116 | (13.2) | 0.553 |
| O-level | 234 | (13.2) | |
| A-level | 328 | (13.9) | |
| Above A-level | 232 | (12.1) | |
| Parental social class | |||
| Professional | 129 | (11.0) | 0.227 |
| Managerial or technical | 428 | (13.5) | |
| Skilled non-manual | 242 | (13.1) | |
| Skilled manual | 86 | (15.5) | |
| Partly skilled/unskilled | 26 | (13.1) | |
| Parental marital status | |||
| Married | 682 | (12.1) | 0.001** |
| Not married | 229 | (17.3) | |
| Birth-related factors | |||
| Maternal age at delivery | |||
| <25 years | 151 | (15.2) | 0.417 |
| 25–29 years | 356 | (13.0) | |
| 30–34 years | 294 | (12.4) | |
| 35- years | 110 | (13.0) | |
| Birthweight | |||
| Low birthweight (<2500 g) | 32 | (10.8) | 0.493 |
| Normal birthweight (2500 g - < 4000 g) | 759 | (13.3) | |
| High birthweight (≥ 4000 g) | 120 | (12.7) | |
| Maternal factors | |||
| Smoking status | |||
| Smoker | 236 | (16.8) | 0.003** |
| Non-smoker | 675 | (12.2) | |
| Anxiety | |||
| Yes | 246 | (17.1) | <.001** |
| No | 665 | (12.1) | |
| Depression | |||
| Yes | 240 | (16.0) | 0.009** |
| No | 671 | (12.3) | |
| Child behavioral factors | |||
| Physical activities | |||
| Less than once a month | 317 | (15.0) | 0.044* |
| Once a month | 265 | (13.7) | |
| Once a week | 282 | (11.5) | |
| At least twice a week | 48 | (10.6) | |
| Energy intake | |||
| Underreport | 175 | (14.0) | 0.616 |
| Plausible report | 582 | (13.0) | |
| Overreport | 155 | (12.7) | |
| Energy intake (kJ/day) | |||
| Mean (SE) | 7872 | (88.3) | 0.103 |
| Child weight status | |||
| BMI | |||
| Underweight | 75 | (12.5) | 0.265 |
| Normal weight | 615 | (12.6) | |
| Overweight | 178 | (14.9) | |
| Obesity | 43 | (17.1) | |
ALSPAC, Avon Longitudinal Study of Parents and Children; IDP, Inflammatory Dietary Pattern.
#p < 0.10; *p < 0.05; **p < 0.01.
Imputed sample, n = 6939 (number of imputations: 50). Participants without depressive disorders and with IDP score at baseline were included.
Depression cases were identified if participants had ICD-10 diagnosis or had depression score ≥ 9 (out of 21) in the Computerised Interview Schedule-Revised (CIS-R) at early adulthood.
For categorical characteristics, p-values were obtained based on chi-squared tests pooled across multiply imputed samples. For continuous characteristics (e.g. birthweight), p-values were obtained based on type 3 analyses pooled across multiply imputed samples.
3.3. Associations between IDP score in childhood and depression at early adulthood
Among 6939 children who were free from depression at baseline, 911 (13.1%) incident depression cases were identified in their early adulthood after a 10-year follow-up. One-unit increase in the IDP z-score corresponded to a 10% increase in the odds of developing depression (OR = 1.10; 95% CI: 1.01–1.21). After EI misreporting status and EI was adjusted, the association was nearly unchanged (OR = 1.10; 95% CI: 1.00–1.21). The relationship was attenuated after socio-demographics, birth-related factors, maternal factors, child behavioral factors, and child weight status were adjusted. When children were classified based on tertiles of the IDP score, participants in the highest tertile had 1.35 times the odds of developing depression (95% CI: 1.10–1.67) compared to those in the lowest tertile. The association remained relatively unchanged after dietary misreporting status and EI were adjusted (OR = 1.34; 95% CI: 1.08–1.66). A dose-response relation between IDP tertiles and depression risk was also observed (p-trend = 0.008). However, the association between IDP tertiles and depression became marginally significant as covariates were added to the model (OR = 1.22; 95% CI: 0.98–1.52 [highest tertile vs. lowest tertile]; p-trend = 0.088) and only the test for trend was marginally significant in the fully adjusted model (p-trend = 0.097) (Table 4).
Table 4.
Associations between IDP score in childhood and depression in early adulthood among 6939 ALSPAC participantsa.
| Model 1b |
Model 2c |
Model 3d |
Model 4e |
|||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| IDP z-score (continuous) | 1.10 | (1.01, 1.21) | 1.10 | (1.00, 1.21) | 1.05 | (0.95, 1.16) | 1.04 | (0.95, 1.15) |
| IDP score tertiles | ||||||||
| 1st Tertile | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| 2nd Tertile | 1.09 | (0.88, 1.35) | 1.08 | (0.87, 1.34) | 1.02 | (0.82, 1.27) | 1.01 | (0.81, 1.26) |
| 3rd Tertile | 1.35 | (1.10, 1.67) | 1.34 | (1.08, 1.66) | 1.22 | (0.98, 1.52) | 1.21 | (0.96, 1.51) |
| P for trend | 0.005 | 0.008 | 0.088 | 0.097 | ||||
IDP, Inflammatory Dietary Pattern; ALSPAC, Avon Longitudinal Study of Parents and Children; OR, Odds Ratio; CI, Confidence Interval.
Imputed sample, n = 6939 (number of imputations: 50). Participants without depressive disorders and with IDP score at baseline were included. Depression cases were identified if participants had ICD-10 diagnosis or had depression score ≥ 9 (out of 21) in the Computerized Interview Schedule-Revised (CIS-R) at early adulthood.
Crude model.
Adjusted for energy intake misreporting (underreporter, plausible reporter, or overreporter) and energy intake (range: 2282.82–18866.99 kJ/d).
Adjusted for sex (female vs. male), race (non-white vs white), parental education (below O-level, O-level, A-level, or above A-level), parental social class (professional, managerial/technical, skilled non-manual, skilled manual, or partly skilled/unskilled), parental marital status (married vs. other), maternal age at delivery (range: 15–44 years), birthweight (range: 645–5640 g), maternal smoking status (yes vs. no), maternal anxiety (yes vs. no), maternal depression (yes vs. no), physical activities level (frequency the child goes to the swimming pool or other sporting area: < once a month, once a month, once a week, or at least twice a week), and weight status (underweight, normal weight, overweight, or obesity).
Fully adjusted model (i.e. Model 3 plus energy intake misreporting (underreporter, plausible reporter, or overreporter) and energy intake (range: 2282.82–18866.99 kJ/d)).
The relationships between IDP score (the continuous IDP z-score and the IDP tertiles) and incident depression were stronger among participants with under or normal weight compared with those associations among participants who were overweight or obese. For example, among participants with under or normal weight, those in the highest tertile had 1.27 times the odds of developing depression in early adulthood (95% CI: 0.98–1.65) compared to those in the lowest tertile after all covariates were adjusted, while the corresponding OR was 0.99 (95% CI: 0.61–1.60) among participants who were overweight or obese. In addition, no significant associations between IDP score and incident depression were observed among participants with overweight and obesity (Table 5).
Table 5.
Associations between IDP score in childhood and depression in early adulthood among 6939 ALSPAC participants stratified by weight statusa.
| Weight status | Model 1b |
Model 2c |
Model 3d |
Model 4e |
||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Underweight or normal weight (n = 5498) | ||||||||
| IDP z-score (continuous) | 1.13 | (1.02, 1.25) | 1.12 | (1.01, 1.24) | 1.08 | (0.97, 1.20) | 1.07 | (0.96, 1.20) |
| IDP score tertiles | ||||||||
| 1st Tertile | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| 2nd Tertile | 1.20 | (0.93, 1.55) | 1.18 | (0.92, 1.53) | 1.14 | (0.87, 1.47) | 1.13 | (0.87, 1.46) |
| 3rd Tertile | 1.40 | (1.10, 1.80) | 1.38 | (1.08, 1.78) | 1.29 | (0.99, 1.67) | 1.27 | (0.98, 1.65) |
| P for trend | 0.008 | 0.011 | 0.056 | 0.070 | ||||
| Overweight or obese (n = 1441) | ||||||||
| IDP z-score (continuous) | 0.99 | (0.82, 1.20) | 0.98 | (0.80, 1.21) | 0.95 | (0.77, 1.16) | 0.94 | (0.76, 1.16) |
| IDP score tertiles | ||||||||
| 1st Tertile | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| 2nd Tertile | 0.74 | (0.47, 1.17) | 0.73 | (0.45, 1.16) | 0.69 | (0.43, 1.10) | 0.68 | (0.42, 1.10) |
| 3rd Tertile | 1.10 | (0.71, 1.70) | 1.08 | (0.68, 1.71) | 1.00 | (0.63, 1.60) | 0.99 | (0.61, 1.60) |
| P for trend | 0.549 | 0.595 | 0.836 | 0.872 | ||||
IDP, Inflammatory Dietary Pattern; ALSPAC, Avon Longitudinal Study of Parents and Children; OR, Odds Ratio; CI, Confidence Interval.
Imputed sample, n = 6939 (number of imputations: 50). Participants without depressive disorders and with IDP score at baseline were included. Depression cases were identified if participants had ICD-10 diagnosis or had depression score ≥ 9 (out of 21) in the Computerized Interview Schedule-Revised (CIS-R) at early adulthood.
Crude model.
Adjusted for energy intake misreporting (underreporter, plausible reporter, or overreporter) and energy intake (range: 2282.82–18866.99 kJ/d).
Adjusted for sex (female vs. male), race (non-white vs white), parental education (below O-level, O-level, A-level, or above A-level), parental social class (professional, managerial/technical, skilled non-manual, skilled manual, or partly skilled/unskilled), parental marital status (married vs. other), maternal age at delivery (range: 15–44 years), birthweight (range: 645–5640 g), maternal smoking status (yes vs. no), maternal anxiety (yes vs. no), maternal depression (yes vs. no), and physical activities level (frequency the child goes to the swimming pool or other sporting area: < once a month, once a month, once a week, or at least twice a week).
Fully adjusted model (i.e. Model 3 plus energy intake misreporting (underreporter, plausible reporter, or overreporter) and energy intake (range: 2282.82–18866.99 kJ/d)).
4. Discussion
In this large prospective cohort study, we synthesized biomarkers of inflammation with dietary behavioral information measured in childhood to empirically derive an inflammatory dietary pattern score for children who were free from depression at baseline. We found that after a 10-year follow-up period, depression risk increased as the inflammatory diet score increased, indicating that children with diets characterized by higher intake of pro-inflammatory foods may be at heightened risk of developing depression later in life. However, it is important to note that some of the associations were attenuated with full covariate adjustment. Thus, this study provides some preliminary evidence that adulthood depression risk may have some origins in inflammatory dietary patterns established in childhood. We encourage future work to build on this emerging evidence and continue to consider associations between early life inflammatory diets and the emergence of depression across the life course.
Based on previous studies (Ambrosini et al., 2016; Lucas et al., 2014; Northstone and Emmett, 2008), we expected that unhealthy food groups, such as fizzy drinks, white bread, cakes, and red meat, would be positively related to IDP score while healthy food groups, such as vegetables, nuts, and wholegrain bread, would be negatively related to IDP score. The six food groups identified as critical components of the IDP generally aligned with the expectation, except for salad which was positively related to IDP score. This counterintuitive finding could be due to the consumption of salad dressings, which often contain oils high in omega-6 fatty acids (i.e., soybean, corn, and safflower oils) and could be pro-inflammatory (DiNicolantonio & O’Keefe, 2018; Innes and Calder, 2018; Marchix et al., 2015; Patterson et al., 2012). Previous studies have identified other vegetables (e.g., corn, celery, mushrooms, green pepper, eggplant, summer squash, and mixed vegetables) as positively contributing to the IDP score (Lucas et al., 2014; Tabung et al., 2016). Further investigation is warranted to unravel the exact contents within the “salad” grouping. Method of food preparation should also be considered. In addition, based on previous studies that suggest food items be combined into food groups (Lucas et al., 2014; Northstone and Emmett, 2005; Northstone and Emmett, 2008; Tabung et al., 2016), we classified 90 food items into 38 food groups. Although the classification was subjective and can be done in multiple ways, it was verified by a registered dietitian co-investigator. Different methods for food group classification can be conducted in future research to further detect sensitivity of the current statistical method to derive IDP.
We found that only a small proportion of variation among levels of inflammatory biomarkers (2%) was explained by the dietary patterns extracted via RRR. Though low, this level is similar to what has been observed in prior work. For example, a previous study indicated that among middle aged and older women enrolled in NHS, only 4% of variation among inflammatory biomarkers was explained by empirically derived dietary patterns (Tabung et al., 2016). Compared with middle aged and older adults, children tend to be healthier and have lower levels of inflammation, which can help explain why a smaller amount of variation among inflammatory biomarkers was explained by dietary intake in this sample. Additionally, IDP in the current study was constructed based on the only two inflammatory biomarkers (CRP and IL-6) available in the ALSPAC data set. In the NHS-based study, researchers also included tumor necrosis factor α receptor 2 (TNFαR2) in addition to CRP and IL-6 as inflammatory biomarkers and thus constructed the RRR model based on three response variables to derive IDP (Tabung et al., 2016), which may contribute to the difference between the two studies in the proportion of variation among biomarkers explained by IDP. In future studies, we encourage researchers to incorporate a broad panel of inflammatory markers in deriving IDP scores in order to enhance the sensitivity of the measure.
Contrary to our expectations of finding a stronger effect of inflammatory diet on depression among those who were overweight or obese, our findings suggest the opposite. One possible explanation for this counterintuitive finding could be that compared to children with overweight or obesity, children with under or normal weight may have more variability in inflammation in response to their dietary intake and thus the effect of IDP on depression risk may be more easily detected. Adipose tissue produces inflammatory factors, including cytokines such as IL-6, TNF-α, and chemokines, which in turn may activate widespread immune reaction, potentially leading to or exacerbating inflammation-related diseases including depression (Shelton and Miller, 2011). Obesity is thus a pro-inflammatory condition and can cause a chronic low-grade inflammation state (Hotamisligil, 2006; Ouchi et al., 2011), which could potentially obscure any relationship between diet-induced inflammation and depression risk. An alternative possible explanation for the null findings among the overweight/obese group could be the statistical power. The 95% CIs among the overweight/obese group were much wider than those in the under/normal weight group since the sample size for overweight or obese children is relatively small (about 20% of the study sample) compared to that for children with under or normal weight, which may lead to lower power to detect the effect. Thus, the different associations observed across weight strata in this study could be attributable to existing levels of inflammation and/or power considerations. We encourage future research to consider these alternatives explicitly.
The IDP score allows for an integration of behavioral and physiologic factors that can jointly explain the incidence of depression. Intake of inflammation-inducing foods may lead to higher serum levels of inflammatory cytokines, such as IL-6, which have been shown to boost activation of the hypothalamic-pituitary-adrenal (HPA) axis and to increase oxidative stress in the brain. These effects could be responsible for the impaired mood and feelings observed in depression cases (Dantzer, O’Connor, Freund, Johnson and Kelley, 2008; Miller et al., 2009). Future work with measured markers of HPA-related activity should explicitly test the possibility that the IDP may trigger dysregulation of the HPA axis.
The current study has some significant advantages. First, it is a population-based study with large sample size. Second, it prospectively investigates and establishes the temporal relationship between IDP in childhood and depression risk in early adulthood. Third, participants were followed from age 9 to age 18, with covariate information measured from birth. Due to this lengthy follow-up time, long-term effects from IDP in childhood on mental health in young adulthood can be examined over the life course. Fourth, various familial and socio-demographic factors are adjusted for and the independent influence of childhood IDP on depression in youth can be examined. In addition, using RRR took advantage of objectively measured biomarkers to derive an inflammation prone dietary pattern, demonstrating the role of chronic inflammation in the etiology of depression through diet in early life.
However, the study also has several limitations. First, loss-to-follow-up is a concern for prospective studies. Young adults who came from families with higher SES and had better childhood health were more likely to attend clinic visits to have measurements for IL-6 or CRP and depressive symptoms, which raises concerns for selection effects. Second, only food items provided in home were included in the analysis and foods provided by others (e.g. at school) were not included, which may affect the precision of the dietary measure used in this study. Third, methods to prepare food were not assessed in the FFQ; it is unknown whether healthy food items were prepared in an unhealthy way. In addition, food intake was based on maternal report, and measurement errors cannot be ruled out. Dietary intake measured at a single time point was used, which may not represent dietary practices across childhood. However, prior work in the cohort indicates a high correlation of dietary scores across childhood which somewhat mitigates this concern (Northstone and Emmett, 2008). Finally, generalizability of the study is limited because about 95% of participants are White.
The physical health benefits of healthy eating are well known and are the focus of much public health research, practice, and intervention (Rodríguez-Monforte et al., 2015; Rosato et al., 2019; USDA & HHS, 2016). The results of this study suggest that healthy dietary practices, particularly when established early in life, may provide a mental health benefit as well. The current study illustrates the potential developmental origins of depression from the joint perspective of inflammation and diet. Study findings provide preliminary evidence that pro-inflammatory diets in early life may increase depression risk, which in turn suggests that interventions designed to promote anti-inflammatory diets could provide mental health benefits to children that persist into adulthood. Future research should build on these results and work to provide new insights into the role of diet in relation to depression prevention and mental health promotion across the life course.
Funding/Support
The UK Medical Research Council and the Wellcome Trust (Grant ref: 102215/2/13/2) and the University of Bristol provide core support for ALSPAC. The Wellcome Trust (Grant ref: 08426812/Z/07/Z) funds CIS-R assessment. This research was partially supported by a grant to the Center for Social and Demographic Analysis (CSDA) at the University at Albany from the National Institute of Child Health and Human Development (R24-HD044943).
Role of the sponsors
The funding sources were not involved in the data analysis, manuscript writing, and publication. This publication is the work of the authors, who will serve as guarantors for the contents of this paper.
Declaration of competing interest
None reported.
Acknowledgements
We are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists and nurses. The UK Medical Research Council and the Wellcome Trust (Grant ref: 102215/2/13/2) and the University of Bristol provide core support for ALSPAC. The Wellcome Trust (Grant ref: 08426812/Z/07/Z) funds CIS-R assessment. This publication is the work of the authors, who will serve as guarantors for the contents of this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbih.2019.100017.
Supplementary data
The following is the Supplementary data to this article:
References
- Adjibade M., Andreeva V.A., Lemogne C., Touvier M., Shivappa N., Hébert J.R. The inflammatory potential of the diet is associated with depressive symptoms in different subgroups of the general population. J. Nutr. 2017:jn245167. doi: 10.3945/jn.116.245167. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbaraly T.N., Kerleau C., Wyart M., Chevallier N., Ndiaye L., Shivappa N. Dietary inflammatory index and recurrence of depressive symptoms: results from the whitehall II study. Clinical Psychological Science. 2016;4(6):1125–1134. doi: 10.1177/2167702616645777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosini G.L., Johns D.J., Northstone K., Emmett P.M., Jebb S.A. Free sugars and total fat are important characteristics of a dietary pattern associated with adiposity across childhood and adolescence. J. Nutr. 2016;146(4):778–784. doi: 10.3945/jn.115.224659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angold A., Costello E.J., Messer S.C., Pickles A., Winder F., Silver D. Development of a short questionnaire for use in epidemiological studies of depression in children and adolescents. Int. J. Methods Psychiatr. Res. 1995;5(4):237–249. [Google Scholar]
- Azadbakht L., Esmaillzadeh A. Dietary patterns and attention deficit hyperactivity disorder among Iranian children. Nutrition. 2012;28(3):242–249. doi: 10.1016/j.nut.2011.05.018. [DOI] [PubMed] [Google Scholar]
- Bartlett J.W., Seaman S.R., White I.R., Carpenter J.R. Multiple imputation of covariates by fully conditional specification: accommodating the substantive model. Stat. Methods Med. Res. 2015;24(4):462–487. doi: 10.1177/0962280214521348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shlomo Y., Kuh D. A life course approach to chronic disease epidemiology: conceptual models, empirical challenges and interdisciplinary perspectives. Int. J. Epidemiol. 2002;31(2):285–293. doi: 10.1093/ije/31.2.285. [DOI] [PubMed] [Google Scholar]
- Black A.E., Cole T.J. Within- and between-subject variation in energy expenditure measured by the doubly-labelled water technique: implications for validating reported dietary energy intake. Eur. J. Clin. Nutr. 2000;54(5):386–394. doi: 10.1038/sj.ejcn.1600970. [DOI] [PubMed] [Google Scholar]
- Bowes L., Joinson C., Wolke D., Lewis G. Peer victimisation during adolescence and its impact on depression in early adulthood: prospective cohort study in the United Kingdom. BMJ Br. Med. J. (Clin. Res. Ed.) 2015;350:h2469. doi: 10.1136/bmj.h2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd A., Golding J., Macleod J., Lawlor D.A., Fraser A., Henderson J., Smith G.D. Cohort profile: the ’children of the 90s’-the index offspring of the Avon longitudinal study of parents and children. Int. J. Epidemiol. 2013;42(1):111–127. doi: 10.1093/ije/dys064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole T.J., Lobstein T. Extended international (IOTF) body mass index cut-offs for thinness, overweight and obesity. Pediatric Obesity. 2012;7(4):284–294. doi: 10.1111/j.2047-6310.2012.000064.x. [DOI] [PubMed] [Google Scholar]
- Copeland W.E., Shanahan L., Worthman C., Angold A., Costello E.J. Cumulative depression episodes predict later C-reactive protein levels: a prospective analysis. Biol. Psychiatry. 2012;71(1):15–21. doi: 10.1016/j.biopsych.2011.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R., O’Connor J.C., Freund G.G., Johnson R.W., Kelley K.W. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 2008;9(1):46–57. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deverts D.J., Cohen S., DiLillo V.G., Lewis C.E., Kiefe C., Whooley M., Matthews K.A. Depressive symptoms, race, and circulating C-reactive protein: the coronary artery risk development in young adults (CARDIA) study. Psychosom. Med. 2010;72(8):734–741. doi: 10.1097/PSY.0b013e3181ec4b98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNicolantonio J.J., O’Keefe J.H. Importance of maintaining a low omega–6/omega–3 ratio for reducing inflammation. Open Heart. 2018;5(2):e000946. doi: 10.1136/openhrt-2018-000946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duivis H.E., de Jonge P., Penninx B.W., Na B.Y., Cohen B.E., Whooley M.A. Depressive symptoms, health behaviors, and subsequent inflammation in patients with coronary heart disease: prospective findings from the heart and soul study. Am. J. Psychiatry. 2011;168(9):913–920. doi: 10.1176/appi.ajp.2011.10081163. [DOI] [PubMed] [Google Scholar]
- Earls F., Jung K. Temperament and home environment characteristics as causal factors in the early development of childhood psychopathology. Martha’s Vineyard Child Health Survey. 1987;26:491–498. doi: 10.1097/00004583-198707000-00005. [DOI] [PubMed] [Google Scholar]
- Emmett P.M., Jones L.R., Northstone K. Dietary patterns in the Avon longitudinal study of parents and children. Nutr. Rev. 2015;73:207–230. doi: 10.1093/nutrit/nuv055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergusson D.M., Woodward L.J. Mental health, educational, and social role outcomes of adolescents with depression. Arch. Gen. Psychiatr. 2002;59(3):225–231. doi: 10.1001/archpsyc.59.3.225. [DOI] [PubMed] [Google Scholar]
- Fletcher J.M. Childhood mistreatment and adolescent and young adult depression. Soc. Sci. Med. 2009;68(5):799–806. doi: 10.1016/j.socscimed.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Fombonne E., Wostear G., Cooper V., Harrington R., Rutter M. The Maudsley long-term follow-up of child and adolescent depression 1. Psychiatric outcomes in adulthood. Br. J. Psychiatry. 2001;179:210–217. doi: 10.1192/bjp.179.3.210. [DOI] [PubMed] [Google Scholar]
- Fraser A., Macdonald-Wallis C., Tilling K., Boyd A., Golding J., Davey Smith G. Cohort profile: the Avon longitudinal study of parents and children: ALSPAC mothers cohort. Int. J. Epidemiol. 2013;42(1):97–110. doi: 10.1093/ije/dys066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantz M. Creating RTF tables with univariate analyses of multiply imputed data. 2006. https://analytics.ncsu.edu/sesug/2006/PO16_06.PDF Retrieved from.
- Gimeno D., Kivimaki M., Brunner E.J., Elovainio M., De Vogli R., Steptoe A. Associations of C-reactive protein and interleukin-6 with cognitive symptoms of depression: 12-year follow-up of the Whitehall II study. Psychol. Med. 2009;39(3):413–423. doi: 10.1017/S0033291708003723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding J., Pembrey M., Jones R. ALSPAC-the Avon longitudinal study of parents and children—I. Study methodology. Paediatr. Perinat. Epidemiol. 2001;15(1):74–87. doi: 10.1046/j.1365-3016.2001.00325.x. [DOI] [PubMed] [Google Scholar]
- Hammerton G., Mahedy L., Mars B., Harold G.T., Thapar A., Zammit S., Collishaw S. Association between maternal depression symptoms across the first eleven years of their child’s life and subsequent offspring suicidal ideation. PLoS One. 2015;10(7):e0131885. doi: 10.1371/journal.pone.0131885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann K., Schulze M.B., Schienkiewitz A., Nothlings U., Boeing H. Application of a new statistical method to derive dietary patterns in nutritional epidemiology. Am. J. Epidemiol. 2004;159(10):935–944. doi: 10.1093/aje/kwh134. [DOI] [PubMed] [Google Scholar]
- Hotamisligil G.S. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- Howard A.L., Robinson M., Smith G.J., Ambrosini G.L., Piek J.P., Oddy W.H. ADHD is associated with a “western” dietary pattern in adolescents. J. Atten. Disord. 2011;15(5):403–411. doi: 10.1177/1087054710365990. [DOI] [PubMed] [Google Scholar]
- Hu F.B. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr. Opin. Lipidol. 2002;13(1):3–9. doi: 10.1097/00041433-200202000-00002. [DOI] [PubMed] [Google Scholar]
- Hu F.B., Rimm E., Smith-Warner S.A., Feskanich D., Stampfer M.J., Ascherio A. Reproducibility and validity of dietary patterns assessed with a food-frequency questionnaire. Am. J. Clin. Nutr. 1999;69(2):243–249. doi: 10.1093/ajcn/69.2.243. [DOI] [PubMed] [Google Scholar]
- Huang T.T.K., Roberts S.B., Howarth N.C., McCrory M.A. Effect of screening out implausible energy intake reports on relationships between diet and BMI. Obes. Res. 2005;13(7):1205–1217. doi: 10.1038/oby.2005.143. [DOI] [PubMed] [Google Scholar]
- Innes J.K., Calder P.C. Omega-6 fatty acids and inflammation. Prostagl. Leukot. Essent. Fat. Acids. 2018;132:41–48. doi: 10.1016/j.plefa.2018.03.004. [DOI] [PubMed] [Google Scholar]
- Jacka F.N., Cherbuin N., Anstey K.J., Butterworth P. Dietary patterns and depressive symptoms over time: examining the relationships with socioeconomic position, health behaviours and cardiovascular risk. PLoS One. 2014;9(1):e87657. doi: 10.1371/journal.pone.0087657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacka F.N., Rothon C., Taylor S., Berk M., Stansfeld S.A. Diet quality and mental health problems in adolescents from East London: a prospective study. Soc. Psychiatry Psychiatr. Epidemiol. 2013;48(8):1297–1306. doi: 10.1007/s00127-012-0623-5. [DOI] [PubMed] [Google Scholar]
- Johnson L., Mander A.P., Jones L.R., Emmett P.M., Jebb S.A. Energy-dense, low-fiber, high-fat dietary pattern is associated with increased fatness in childhood. Am. J. Clin. Nutr. 2008;87(4):846–854. doi: 10.1093/ajcn/87.4.846. [DOI] [PubMed] [Google Scholar]
- Kaplan B.J., Rucklidge J.J., Romijn A., McLeod K. The emerging field of nutritional mental health: inflammation, the microbiome, oxidative stress, and mitochondrial function. Clinical Psychological Science. 2015;3(6):964–980. doi: 10.1177/2167702614555413. [DOI] [Google Scholar]
- Kessler R.C., Berglund P., Demler O., Jin R., Koretz D., Merikangas K.R. The epidemiology of major depressive disorder—results from the national comorbidity survey replication (NCS-R) Jama-Journal of the American Medical Association. 2003;289(23):3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Kessler R.C., Berglund P., Demler O., Jin R., Walters E.E. Lifetime prevalence and age-of-onset distributions’ of DSM-IV disorders in the national comorbidity survey replication. Arch. Gen. Psychiatr. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Khalid S., Williams C.M., Reynolds S.A. Is there an association between diet and depression in children and adolescents? A systematic review. Br. J. Nutr. 2016;116(12):2097–2108. doi: 10.1017/S0007114516004359. [DOI] [PubMed] [Google Scholar]
- Khandaker G.M., Pearson R.M., Zammit S., Lewis G., Jones P.B. Association of serum interleukin 6 and C-reactive protein in childhood with depression and psychosis in young adult life A population-based longitudinal study. Jama Psychiatry. 2014;71(10):1121–1128. doi: 10.1001/jamapsychiatry.2014.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser J.K. Stress, food, and inflammation: psychoneuroimmunology and nutrition at the cutting edge. Psychosom. Med. 2010;72(4):365–369. doi: 10.1097/PSY.0b013e3181dbf489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser J.K., Derry H.M., Fagundes C.P. Inflammation: depression fans the flames and feasts on the heat. Am. J. Psychiatry. 2015;172(11):1075–1091. doi: 10.1176/appi.ajp.2015.15020152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Port A., Gueguen A., Kesse-Guyot E., Melchior M., Lemogne C., Nabi H. Association between dietary patterns and depressive symptoms over time: a 10-year follow-up study of the GAZEL cohort. PLoS One. 2012;7(12):e51593. doi: 10.1371/journal.pone.0051593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis G., Pelosi A.j., Araya R., Dunn G. Measuring psychiatric disorder in the community: a standardized assessment for use by lay interviewers. Psychol. Med. 1992;22(2):465–486. doi: 10.1017/S0033291700030415. [DOI] [PubMed] [Google Scholar]
- Loprinzi P.D., Lee I.-M., Andersen R.E., Crespo C.I., Smit E. Association of concurrent healthy eating and regular physical activity with cardiovascular disease risk factors in US youth. Am. J. Health Promot. 2015;30(1):2–8. doi: 10.4278/ajhp.140213-QUAN-71. [DOI] [PubMed] [Google Scholar]
- Lucas M., Chocano-Bedoya P., Shulze M.B., Mirzaei F., O’Reilly E.J., Okereke O.I. Inflammatory dietary pattern and risk of depression among women. Brain Behav. Immun. 2014;36:46–53. doi: 10.1016/j.bbi.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchix J., Choque B., Kouba M., Fautrel A., Catheline D., Legrand P. Excessive dietary linoleic acid induces proinflammatory markers in rats. J. Nutr. Biochem. 2015;26(12):1434–1441. doi: 10.1016/j.jnutbio.2015.07.010. [DOI] [PubMed] [Google Scholar]
- McMartin S.E., Kuhle S., Colman I., Kirk S.F.L., Veugelers P.J. Diet quality and mental health in subsequent years among Canadian youth. Public Health Nutr. 2012;15(12):2253–2258. doi: 10.1017/S1368980012000535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki T., Kochi T., Kuwahara K., Eguchi M., Kurotani K., Tsuruoka H. Dietary patterns derived by reduced rank regression (RRR) and depressive symptoms in Japanese employees: the Furukawa nutrition and health study. Psychiatry Res. 2015;229(1–2):214–219. doi: 10.1016/j.psychres.2015.07.033. [DOI] [PubMed] [Google Scholar]
- Mikkila V., Rasanen L., Raitakari O.T., Pietinen P., Viikari J. Consistent dietary patterns identified from childhood to adulthood: the cardiovascular risk in young finns study. Br. J. Nutr. 2005;93(6):923–931. doi: 10.1079/BJN20051418. [DOI] [PubMed] [Google Scholar]
- Miller A.H., Maletic V., Raison C.L. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol. Psychiatry. 2009;65(9):732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of Agriculture, Fisheries and Food . second ed. Her Majesty’s Stationery Office; London: 1993. Food Portion Sizes. [Google Scholar]
- Murray C.J.L., Lopez A.D. Measuring the global burden of disease. N. Engl. J. Med. 2013;369(5):448–457. doi: 10.1056/NEJMra1201534. [DOI] [PubMed] [Google Scholar]
- Ness A.R. The Avon Longitudinal Study of Parents and Children (ALSPAC)—a resource for the study of the environmental determinants of childhood. Eur. J. Endocrinol. 2004;151:U141–U149. doi: 10.1530/eje.0.151U141. [DOI] [PubMed] [Google Scholar]
- Northstone K., Emmett P. Multivariate analysis of diet in children at four and seven years of age and associations with socio-demographic characteristics. Eur. J. Clin. Nutr. 2005;59(6):751–760. doi: 10.1038/sj.ejcn.1602136. [DOI] [PubMed] [Google Scholar]
- Northstone Kate, Emmett P.M. Are dietary patterns stable throughout early and mid-childhood? A birth cohort study. Br. J. Nutr. 2008;100(5):1069–1076. doi: 10.1017/S0007114508968264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northstone Kate, Joinson C., Emmett P., Ness A., Paus T. Are dietary patterns in childhood associated with IQ at 8 years of age? A population-based cohort study. J. Epidemiol. Community Health. 2012;66(7):624–628. doi: 10.1136/jech.2010.111955. [DOI] [PubMed] [Google Scholar]
- Oddy W.H., Robinson M., Ambrosini G.L., O’Sullivan T.A., de Klerk N.H., Beilin L.J., Stanley F.J. The association between dietary patterns and mental health in early adolescence. Prev. Med. 2009;49(1):39–44. doi: 10.1016/j.ypmed.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Oellingrath I.M., Hersleth M., Svendsen M.V. Association between parental motives for food choice and eating patterns of 12-to 13-year-old Norwegian children. Public Health Nutr. 2013;16(11):2023–2031. doi: 10.1017/S1368980012004430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neil A., Quirk S.E., Housden S., Brennan S.L., Williams L.J., Pasco J.A., Jacka F.N. Relationship between diet and mental health in children and adolescents: a systematic review. Am. J. Public Health. 2014;104(10):E31–E42. doi: 10.2105/AJPH.2014.302110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouchi N., Parker J.L., Lugus J.J., Walsh K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011;11(2):85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson E., Wall R., Fitzgerald G.F., Ross R.P., Stanton C. Health implications of high dietary omega-6 polyunsaturated fatty acids. J. Nutr. Metabol. 2012 doi: 10.1155/2012/539426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratitch B., Lipkovich I., O’Kelly M. 2013. Combining Analysis Results from Multiply Imputed Categorical Data.https://www.pharmasug.org/proceedings/2013/SP/PharmaSUG-2013-SP03.pdf Retrieved from. [Google Scholar]
- Rienks J., Dobson A.J., Mishra G.D. Mediterranean dietary pattern and prevalence and incidence of depressive symptoms in mid-aged women: results from a large community-based prospective study. Eur. J. Clin. Nutr. 2013;67(1):75–82. doi: 10.1038/ejcn.2012.193. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Monforte M., Flores-Mateo G., Sánchez E. Dietary patterns and CVD: a systematic review and meta-analysis of observational studies. Br. J. Nutr. 2015;114(9):1341–1359. doi: 10.1017/S0007114515003177. [DOI] [PubMed] [Google Scholar]
- Rosato V., Temple N.J., La Vecchia C., Castellan G., Tavani A., Guercio V. Mediterranean diet and cardiovascular disease: a systematic review and meta-analysis of observational studies. Eur. J. Nutr. 2019;58(1):173–191. doi: 10.1007/s00394-017-1582-0. [DOI] [PubMed] [Google Scholar]
- Ruusunen A., Lehto S.M., Mursu J., Tolmunen T., Tuomainen T.-P., Kauhanen J., Voutilainen S. Dietary patterns are associated with the prevalence of elevated depressive symptoms and the risk of getting a hospital discharge diagnosis of depression in middle-aged or older Finnish men. J. Affect. Disord. 2014;159:1–6. doi: 10.1016/j.jad.2014.01.020. [DOI] [PubMed] [Google Scholar]
- Sanchez-Villegas A., Ruiz-Canela M., de la Fuente-Arrillaga C., Gea A., Shivappa N., Hebert J.R., Martinez-Gonzalez M.A. Dietary inflammatory index, cardiometabolic conditions and depression in the Seguimiento Universidad de Navarra cohort study. Br. J. Nutr. 2015;114(9):1471–1479. doi: 10.1017/S0007114515003074. [DOI] [PubMed] [Google Scholar]
- Schulze M.B., Hoffmann K., Kroke A., Boeing H. An approach to construct simplified measures of dietary patterns from exploratory factor analysis. Br. J. Nutr. 2003;89(3):409–418. doi: 10.1079/BJN2002778. [DOI] [PubMed] [Google Scholar]
- Shelton R.C., Miller A.H. Inflammation in depression: is adiposity a cause? Dialogues Clin. Neurosci. 2011;13(1):41–53. doi: 10.31887/DCNS.2011.13.1/rshelton. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivappa N., Schoenaker D.A.J.M., Hebert J.R., Mishra G.D. Association between inflammatory potential of diet and risk of depression in middle-aged women: the Australian Longitudinal Study on Women’s Health. Br. J. Nutr. 2016;116(6):1077–1086. doi: 10.1017/S0007114516002853. [DOI] [PubMed] [Google Scholar]
- Shivappa N., Steck S.E., Hurley T.G., Hussey J.R., Hebert J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014;17(8):1689–1696. doi: 10.1017/S1368980013002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J.C., Rand K.L., Muldoon M.F., Kamarck T.W. A prospective evaluation of the directionality of the depression-inflammation relationship. Brain Behav. Immun. 2009;23(7):936–944. doi: 10.1016/j.bbi.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabung F.K., Smith-Warner S.A., Chavarro J.E., Wu K., Fuchs C.S., Hu F.B., Giovannucci E.L. Development and validation of an empirical dietary inflammatory index. J. Nutr. 2016;146(8):1560–1570. doi: 10.3945/jn.115.228718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Royal Society of Chemistry and Ministry of Agriculture, Fisheries and Food . fifth ed. Her Majesty’s Stationery Office; London: 1991. McCance and Widdowson’s the Composition of Foods. [Google Scholar]
- Torun B. Energy requirements of children and adolescents. Public Health Nutr. 2005;8(7A):968–993. doi: 10.1079/PHN2005791. [DOI] [PubMed] [Google Scholar]
- Trapp G.S.A., Allen K.L., Black L.J., Ambrosini G.L., Jacoby P., Byrne S. A prospective investigation of dietary patterns and internalizing and externalizing mental health problems in adolescents. Food Sci. Nutr. 2016;4(6):888–896. doi: 10.1002/fsn3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- University of Bristol Quentionnaire KT: my daughter at home and at school (103 month) 2000. http://www.bristol.ac.uk/media-library/sites/alspac/migrated/documents/ques-cb18-my-daughter-at-home-and-at-school.pdf Retrieved from.
- USDA, HHS 2015-2020 dietary guidelines—health.gov. 2016. https://health.gov/dietaryguidelines/2015/guidelines/ Retrieved June 28, 2019, from.
- Valkanova V., Ebmeier K.P., Allan C.L. CRP, IL-6 and depression: a systematic review and meta-analysis of longitudinal studies. J. Affect. Disord. 2013;150(3):736–744. doi: 10.1016/j.jad.2013.06.004. [DOI] [PubMed] [Google Scholar]
- van Egmond-Froehlich A.W.A., Weghuber D., de Zwaan M. Association of symptoms of attention-deficit/hyperactivity disorder with physical activity, media time, and food intake in children and adolescents. PLoS One. 2012;7(11) doi: 10.1371/journal.pone.0049781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen E., Stronks K., Visser M., Brouwer I.A., Schene A.H., Mocking R.J.T. The association between dietary patterns derived by reduced rank regression and depressive symptoms over time: the Invecchiare in Chianti (InCHIANTI) study. Br. J. Nutr. 2016;115(12):2145–2153. doi: 10.1017/S0007114516001318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Fang Y., Jin M. 2014. Combining Type-III Analyses from Multiple Imputations.http://support.sas.com/resources/papers/proceedings14/1543-2014.pdf Retrieved from. [Google Scholar]
- Weng T.-T., Hao J.-H., Qian Q.-W., Cao H., Fu J.-L., Sun Y. Is there any relationship between dietary patterns and depression and anxiety in Chinese adolescents? Public Health Nutr. 2012;15(4):673–682. doi: 10.1017/S1368980011003077. [DOI] [PubMed] [Google Scholar]
- White I.R., Royston P., Wood A.M. Multiple imputation using chained equations: issues and guidance for practice. Stat. Med. 2011;30(4):377–399. doi: 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- World Health Organization . World Health Organization; 2016. Tenth Revision of the International Classification of Diseases. [Google Scholar]
- Yaffe K., Kanaya A., Lindquist K., Simonsick E.M., Harris T., Shorr R.I. The metabolic syndrome, inflammation, and risk of cognitive decline. Jama-Journal of the American Medical Association. 2004;292(18):2237–2242. doi: 10.1001/jama.292.18.2237. [DOI] [PubMed] [Google Scholar]
- Yuan Y. Multiple imputation using SAS software. J. Stat. Softw. 2011;45(6):1–25. doi: 10.18637/jss.v045.i01. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.