Abstract
Enzymatic noncovalent synthesis (ENS), a process that integrates enzymatic reactions and supramolecular (i.e., noncovalent) interactions for spatial organization of higher-order molecular assemblies, represents an emerging research area at the interface of physical and biological sciences. This review provides a few representative examples of ENS in the context of supramolecular soft matter. After a brief comparison of enzymatic covalent and noncovalent synthesis, we discuss ENS of man-made molecules for generating supramolecular nanostructures (e.g., supramolecular hydrogels) in cell-free conditions. Then, we introduce ENS in a cellular environment. To illustrate the unique merits for applications, we discuss intercellular, peri- or intracellular, and subcellular ENS for cell morphogenesis, molecular imaging, cancer therapy, and targeted delivery. Finally, we provide an outlook on the potential of ENS. We hope that this review offers a new perspective for scientists who develop supramolecular soft matter to address societal needs at various frontiers.
Introduction
Enzymatic synthesis refers to chemical transformations catalyzed by enzymes. For example, enzymes catalyze the synthesis of glucose from carbon dioxide and water under the irradiation of light, the synthesis of adenosine triphosphate (ATP) from the oxidation of glucose, and the synthesis of proteins from amino acids according to genetic codes by ribosomes. Besides serving as the essential process of life, enzymatic synthesis has found applications in industrial transformation, such as the synthesis of antibiotics and intermediates for drugs. Enzymatic synthesis falls in the category of enzymatic covalent synthesis because it uses enzymes to catalyze the formation of covalent bonds to generate individual molecules as the desired products.
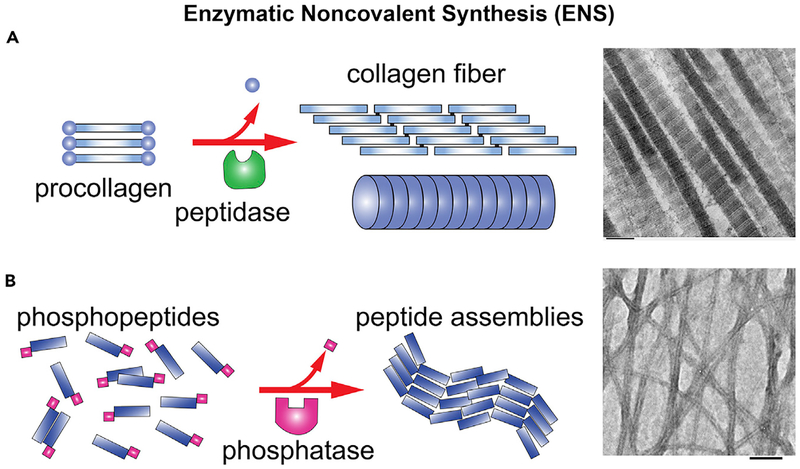
In addition to enzymatic reactions, supramolecular (i.e., noncovalent) interactions are also an inherent feature of life.1 Thus, nature has evolved processes that integrate enzymatic reactions and supramolecular (i.e., noncovalent) interactions for spatial organization of higher-order molecular assemblies. For example, noncovalent interactions between tubulins and enzymatic hydrolysis of guanine triphosphate (GTP) drive the formation of microtubules,2 enzymatic phosphorylation and dephosphorylation regulate the dynamics of inflammasome filaments,3 and a protease cleaves certain segments from procollagen to form collagens, which assemble via noncovalent interaction to form collagen fibers4 (Figure 1A). We refer to such processes as enzymatic noncovalent synthesis (ENS) because the key features of these processes are (1) enzymatic reaction(s) and (2) the formation of supramolecular (or noncovalent) assemblies of molecules.
Figure 1. Preliminary Examples of ENS.
(A) An example of endogenous ENS, forming collagen fibers.5
(B) An example of biomimetic ENS: enzymatic formation of the nanofibers of tetrapeptides via dephosphorylation.6 Scale bar, 100 nm.
Although enzymatic covalent synthesis is a well-established dogma in biology and has already found applications in industrial transformation,7 the exploration of ENS is just beginning. Interestingly, ENS has advanced most rapidly in the context of supramolecular chemistry, especially supramolecular soft matter, such as hydrogels,8 resulting from supramolecular nanofibers that are made by an enzymatic reaction (Figure 1B). Thus, the aim of this review is to present progress and the potential of ENS. Here, we provide a few representative examples of ENS in the context of supramolecular soft matter. Because enzymatic reactions are most useful for living organisms and enzymatic reactions of proteins are well documented, we emphasize ENS of small molecules in a cellular environment with a long-term goal in biomedicine. By discussing intercellular, peri- or intracellular, and subcellular ENS for cell morphogenesis, molecular imaging, cancer therapy, and organelle-targeted delivery, we illustrate the potential applications of ENS for biomedical applications. We also provide a perspective on the potential of ENS and hope this review is useful for scientists who develop supramolecular soft matter to address societal needs at various frontiers. We would like to apologize to researchers whose work9–25,26–54 is not discussed in more detail due to space limitations.
ENS for Making Soft Matter: Supramolecular Hydrogels
Enzymes catalyze many kinds of reactions in life. Over the years, the use of enzymes in modulating the formation of various nanostructures for possible biomedical applications has attracted increasing research efforts. In this section, we show the use of various enzymes to facilitate bond breaking and forming noncovalent interactions to yield supramolecular hydrogels. We focus on the cases of enzyme-catalyzed bond breaking because noncovalent interactions are indispensable for the formation of hydrogels, which are easily distinguishable from the conventional hydrogels made of covalent polymeric networks,55 including the covalent polymeric networks made by enzymes.56 Moreover, the last few decades have witnessed rapid progress in supramolecular hydrogels made of small molecules,8 especially peptides.57 We start the discussion with the use of ENS to form supramolecular hydrogels that consist of small molecules.
Alkaline Phosphatase-Catalyzed Self-Assembly
The First Abiological ENS of Small Molecules.
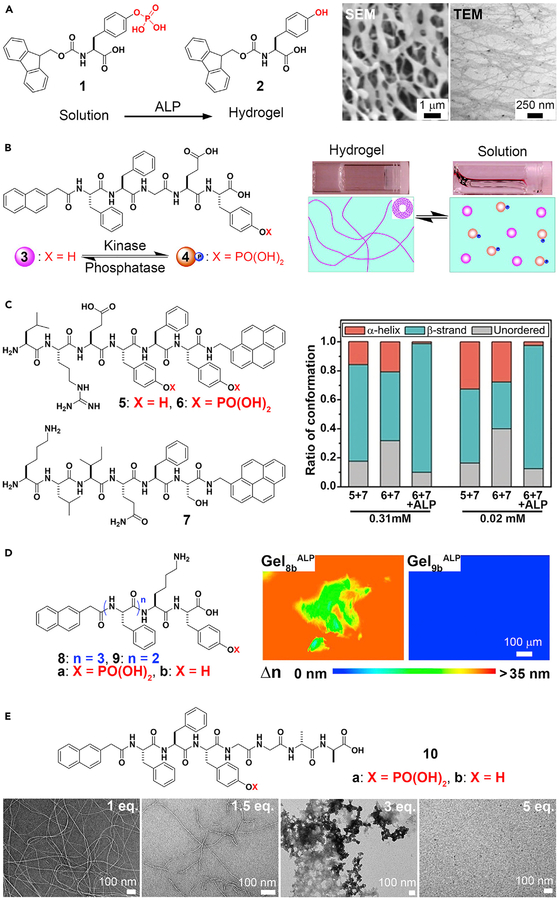
Although ENS is ubiquitous in biology, the application of this concept to small organic molecules occurred only 15 years ago. The first example of integrating an enzymatic reaction with self-assembly of small molecules involved alkaline phosphatase (ALP) and self-assembly of a derivative of an aromatic amino acid.58 In this case, ALP converts an ionic and water-soluble precursor, Fmoc-tyrosine phosphate (Fmoc-Yp, 1), into a hydrogelator (Fmoc-tyrosine, 2) by dephosphorylation (Figure 2A). After enzymatic cleavage of the phosphate group from the tyrosine residue, the aromatic interactions between fluorene-fluorene, fluorene-O-hydroxyl-phenyl, or O-hydroxyl-phenyl-O-hydroxyl-phenyl groups enhance intermolecular hydrogen bonding of the Fmoc-tyrosine in water. Thus, these hydrogelators self-assemble to form a nanofibrillar network that immobilizes water. This work illustrates the feasibility of using ALP for ENS to form supramolecular hydrogels. This concept is applicable for other small molecules,59 even when the small-molecule precursors aggregate, in a concentration-dependent manner, before enzymatic dephosphorylation60 or in the presence of other self-assembling molecules (co-assembly).37 Because hydrogelation is a simple assay, ALP has received the most extensive exploration for ENS, such as regulating reversible gel-sol transitions, controlling peptide secondary structures, kinetics, and a host of other functions.
Figure 2. Alkaline Phosphatase (ALP)-Catalyzed Noncovalent Synthesis.
(A) ALP-catalyzed supramolecular hydrogelation.
(B) Enzymatic-switch-regulated supramolecular hydrogelation.
(C) Circular dichroism spectra analysis of the peptide solutions at 0.31 and 0.02 mM, indicating enzymatic control of secondary peptide structures.
(D) Anisotropic supramolecular hydrogelation by combining an enzymatic reaction and aromatic-aromatic interaction. Gel8b or Gel9bALP, supramolecular hydrogel of 8b or 9b, induced by ALP.
(E) Structure and transmission electron microscopy (TEM) images of ENS of Nap-FFYpGGaa, which is controlled by the equivalents of vancomycin.
(A) Adapted with permission from Yang et al.58 Copyright 2004 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. (B) Adapted with permission from Yang et al.6 Copyright 2006 American Chemical Society. (C) Adapted with permission from Li et al.61 Copyright 2018 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. (D) Adapted with permission from Zhou et al.62 Copyright 2014 American Chemical Society. (E) Adapted with permission from Haburcak et al.63 Copyright 2016 American Chemical Society.
Reversible Gel-Sol Phase Transition.
Reversibility of hydrogelation is beneficial because it allows hydrogels to respond to enzymes in different organs and tissues, which ultimately may provide a way for control and adaptability of materials in situ. A facile strategy for controlling the states of a supramolecular hydrogel uses an enzymatic switch made of kinase and phosphatase.6 Nap-FFGEY (3) forms a hydrogel in less than 1 wt %. After adding tyrosine kinase to the gel in the presence of ATP, Nap-FFGEYp (4) is formed. Phosphorylation disrupts the self-assembled network of 3, thereby inducing a gel-sol transition. Treating the newly formed solution with ALP turns the solution to the gel again (Figure 2B). Given that many diseases overexpress kinases and phosphatases, using these enzymes as controllable switches is important, because it uses natural biological responses to control hydrogelation instead of external stimuli that may be conducive to the biological environment.
A Photo-Switchable Gel.
Further use of ALP in gel-sol transitions is to form photo-switchable hydrogels.64 Displaying cis-trans isomerization upon light irradiation, an azobenzene derivative has low solubility in water. Based on that many biological organisms use enzymatic reactions to regulate the solubility of proteins, a facile approach is to attach an azobenzene moiety to a known hydrogelator, Nap-FFKYp,65 to form the precursor. Upon enzymatic cleavage of phosphate from the precursor, the hydrogel forms. UV irradiation results in gel-sol transitions. The use of ENS should be applicable for making other light-responsive gels if the low solubility of the hydrogelator prevents hydrogelation. In fact, ENS is a general approach for making hydrogels that are difficult to make due to the low aqueous solubility of the hydrogelators.66,67
Modulating Secondary Structures of Peptides.
Post-translational modification is a strategy used by nature for controlling the secondary structures of proteins. Similarly, ALP-based ENS is able to select the secondary structures of homotypic and heterotypic assemblies of peptides.61 Phosphorylation of LREYFY-pyn (5) yields 6, which exhibits mainly unordered conformation from an α-helix dominant conformation. KLIQFS-pyn (7) exists in a β-strand dominant conformation. The direct assembly of 5 and 7 yields assemblies with both α-helix and β-strand conformations, and treating a mixture of 6 and 7 with ALP generates assemblies of 5 and 7 with predominantly β-strand conformation (Figure 2C). The relatively strong binding (Kd = 30.9 μM) and the selection of a single conformation, formed via ALP-catalyzed dephosphorylation, demonstrate the feasibility of using ENS to direct the final secondary structure of conformationally flexible peptides in supramolecular assemblies. Shi et al. demonstrated the use of a protein phosphatase to control the conformational landscape of self-assembling peptides.18
Spontaneous Alignment of Supramolecular Nanofibrils.
Whereas soft matter made by nature is usually anisotropic, supramolecular hydrogels made by synthetic molecules are largely isotropic. Surprisingly, ALP is able to direct the assembly of certain small peptides to form a spontaneously aligned supramolecular hydrogel, as revealed by the birefringence of the hydrogels.62 Birefringence is a property found in anisotropic materials that results in the refraction of light along different directions. The polarization of light changes due to a differential phase shift in two orthogonal polarized components. The retardance (i.e., the differential phase shift between two wave fronts of the orthogonal components of a birefringent material) and transmission electron microscopy (TEM) images of the hydrogels confirm the spontaneous alignment of supramolecular nanofibrils resulting from ENS. As shown in Figure 2D, the hydrogel of 8b formed by ALP-catalyzed dephosphorylation exhibits more directional order than the hydrogel of 8b formed by changing the pH. This result demonstrates the uniqueness of ENS and underscores the importance of aromatic-aromatic interactions in water, because the hydrogels of 9b (having one less phenylalanine than 8b) remain isotropic regardless of whether they are produced via ENS or by changing the pH. This work implies that ENS should be a useful way to develop anisotropic soft matter, which is much less explored.68
Ligand-Receptor Interactions Regulating ENS.
Ligand-receptor interactions are important for cell signaling, drug targeting, and a host of other biological and non-biological functions, including modulating soft matter. For example, noncovalent interactions between vancomycin (Van) and d-Ala-d-Ala are able to weaken or reinforce supramolecular hydrogels.69,70 Such ligand-receptor interactions are also able to regulate the supramolecular assemblies formed by ALP-catalyzed self-assembly.63 As shown in Figure 2E, a small peptide, Nap-FFYGGaa (“a” represents d-alanine), self-assembles to form nanofibrils after adding ALP to a solution of Nap-FFYpGGaa. The co-addition of vancomycin and ALP results in supramolecular assemblies that depend on the concentration of vancomycin. Although 1 equiv of vancomycin hardly disrupts the nanofibrils formed by the dephosphorylation of Nap-FFYpGGaa, higher concentrations of vancomycin eventually prevent the formation of the nanofibrils. This relatively simple experiment indicates the complexity and versatility of combining specific ligand-receptor interactions with ENS, which may help us understand the emergent behavior of sophisticated supramolecular systems that have multiple and parallel processes.
Other Examples of Various Substrates for ALP.
Conjugating various informational biomolecules to small peptides may provide insight on different biological interactions and exert control over cellular processes in vitro and in vivo. The conjugation of nucleobases (e.g., adenine, thymine, guanine, and cytosine) to the ALP-responsive self-assembling motif, FFKYp, creates a hydrogelator that self-assembles upon enzymatic cleavage of ALP.71 The use of nucleosides to create assemblies introduces additional versatility for interacting with biological molecules, because nucleobases are essential building blocks of life. An example is attaching Nap-FF to adenosine monophosphate (AMP).72 Conjugating non-informational molecules, such as cholesterol, to a tyrosine phosphate generates a substrate of ALP. These phosphor-tyrosine conjugates selectively kill cisplatin-resistant ovarian cancer cells.73,74 In another study reported by Pires et al.,75 adding a phosphate group to a self-assembling carbohydrate results in a substrate of ALP that inhibits osteosarcoma (Saos-2) cancer cells. These works and other related studies on ALP-based ENS indicate that ALP may be a ubiquitous enzyme in various cancers so that ALP can be exploited to catalyze the formation of supramolecular assemblies for developing therapeutics for cancer (see later).
Other Useful Enzymes for ENS
Enterokinase-Catalyzed Self-Assembly.
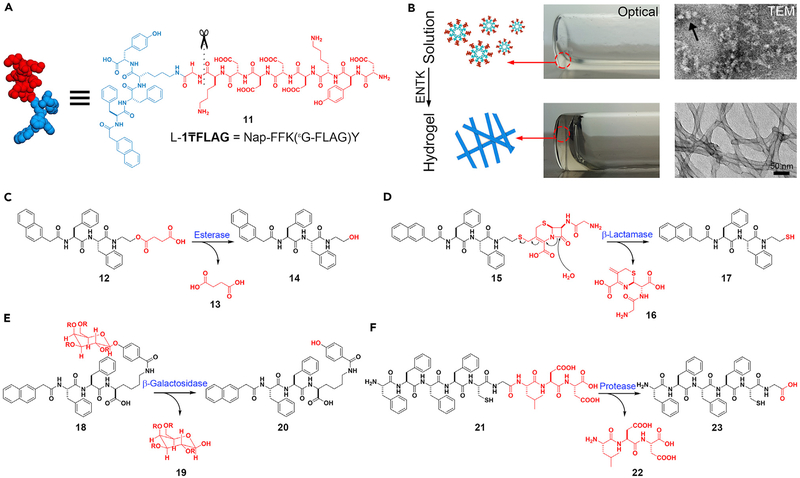
The cleavage of phosphate groups is only a small fraction of the many reactions that enzymes catalyze. It is worthwhile exploring ENS by enzymes other than phosphatases. Enterokinase (ENTK) is an enzyme involved in digestion, discovered by Pavlov.76 ENTK catalyzes cleavage of the sequence DDDDK off protrypsin, leading to the development of FLAG tag (DYKDDDDK)77 for protein purification and sometimes for identifying the proteins in cells. Using ENTK to cleave FLAG tag leads to the formation of supramolecular hydrogels by ENS. Figure 3A shows the structure of the molecule, (11), which is a substrate of ENTK. Before the addition of ENTK, forms a solution containing micelles. After the addition of ENTK at physiological pH in PBS, nanofibers form because ENTK cleaves the FLAG segment (Figure 3B).78 Using ENTK to catalyze the transition from the micelles to the nanofibers may lead to an application in drug delivery.79
Figure 3. Multiple Enzymes Are Useful in ENS.
(A and B) Enterokinase (ENTK)-catalyzed noncovalent synthesis. (A) The molecular structure of . (B) Optical and TEM images of solution (without ENTK) and hydrogel (activated by ENTK).
(C–F) The formation of the self-assembling peptides via the reactions catalyzed by (C) esterase, (D) β-lactamase, (E) β-galactosidase, and (F) protease.
(B) Adapted with permission from He et al.78 Copyright 2018 The Royal Society of Chemistry.
ENS can also utilize other enzymes that facilitate bond breaking to generate self-assembled nanostructures. As shown in Figure 3C, carboxylesterases (CESs) cleave the ester bond of 12 to form butyric diacid (13) and a hydrogelator (14) to form hydrogels.80 β-Lactamase catalyzes the opening of β-lactam rings to result in hydrogelation, which acts as an assay for screening the inhibitors of β-lactamases or reporting β-lactam antibiotic-resistant pathogens (Figure 3D).81 β-Galactosidase hydrolyzes the glycosidic bond between galactose and phenol to form aglycone (20), which further self-assembles to form a hydrogel.82 Figure 3F shows the use of matrix metal-loproteinase-9 (MMP-9), an enzyme overexpressed in various malignancies, to catalyze the synthesis of the hydrogel made of 23.83 Such a metalloprotease-catalyzed ENS is able to inhibit cancer cells selectively, as demonstrated by work from the Maruyama lab in 2015,29 and Ulijn lab.84,85 Recently, Ulijn and coworkers reported a useful guide for the systematic design of substrates of MMP-9 to generate various nanostructures by ENS.86
Applications of ENS in a Cellular Environment
ENS is an essential feature of cells. The integration of enzymatic reactions and molecular self-assembly allows functions to arise from the transition between different molecular or structural forms. That is, in addition to a molecular structure-determining function, the molecular process also dictates the functions, especially in the context of cells. Thus, exploring ENS in a cellular environment may lead to many exciting results because it is a largely unexplored research area. The following representative examples illustrate ENS in intercellular, pericellular, intracellular, and subcellular spaces.
Intercellular ENS
Cell Morphogenesis.
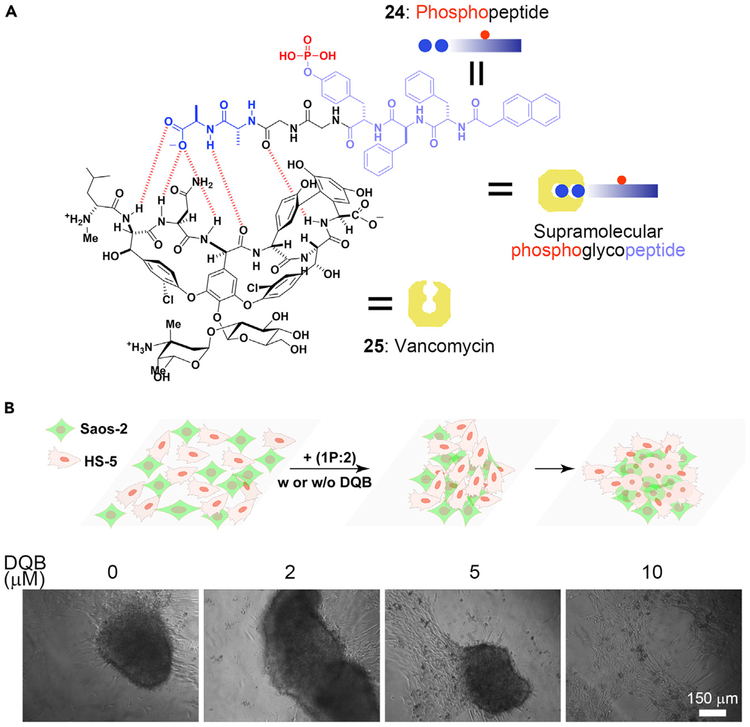
The extracellular matrix (ECM) houses many important proteins that dynamically undergo remodeling, serving as a model for the creation of new biomaterials in the intercellular space. Such a feature of ECM has led to the development of an ENS process to mimic the ECM in vitro to form 3D spheroids.87 Specifically, the ligand-receptor interaction between vancomycin and d-alanine-d-alanine (d-Ala-d-Ala)63,70 results in a supramolecular phosphoglycopeptide (sPGP), which self-assembles to form nanoparticles. Upon dephosphorylation, the nanoparticles of the sPGP become nanofibers. When incubated with sPGP, HS-5 cells transform from a 2D sheet to a 3D cell spheroid after 24 h. The dynamic assembly was further confirmed by attaching 4-nitrobenzo[c][1, 2, 5]oxadiazole (NBD) to the sPGP. Over a 24-h incubation period, fluorescence gradually increases as sPGP is dephosphorylated, indicating that the dynamic continuum of the assemblies of the peptides results in cell morphogenesis (i.e., from a 2D cell layer to 3D spheroids).
The sPGP (Figure 4A) also acts as a biomaterial to control cell morphogenesis in a context-dependent manner.88 In the co-culture of HS-5 with Saos-2 cells, ENS of the sPGP leads to different phenotypes according to the rate of dephosphorylation of the sPGP. Saos-2 cells overexpress ALP, which dephosphorylates sPGP rapidly to form the assemblies that kill the Saos-2 cells. Altering the ratio of HS-5 and Saos-2 cells or adding ALP inhibitor can slow down the assembling rate so that the cells form spheroids (Figure 4B). Severely inhibiting ALP prevents the assembly of the sPGP, and the cells remain as the 2D layer. Besides using sPGP, which forms heterotypic assemblies to control intercellular interactions, it is feasible to use homotypic assemblies for intercellular ENS to mimic protein dynamics and control cell morphogenesis.89 These results illustrate the use of ENS to manipulate the signal transduction of cells, which offers a new way to create intercellular biomaterials for modulating the cell microenvironment.
Figure 4. ENS in Intercellular Space.
(A) Interaction between vancomycin and the phosphopeptide containing d-Ala-d-Ala.
(B) Formation of a 3D cell spheroid activated by 1P:2, and the effect of (2,5-dimethoxy-N-(quinolin-3-yl) benzenesulfonamide (DQB, an ALP inhibitor).
(B) Adapted with permission from Wang et al.88 Copyright 2019 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Pericellular and Intracellular ENS
Imaging Pericellular Molecular Self-Assembly by ENS.
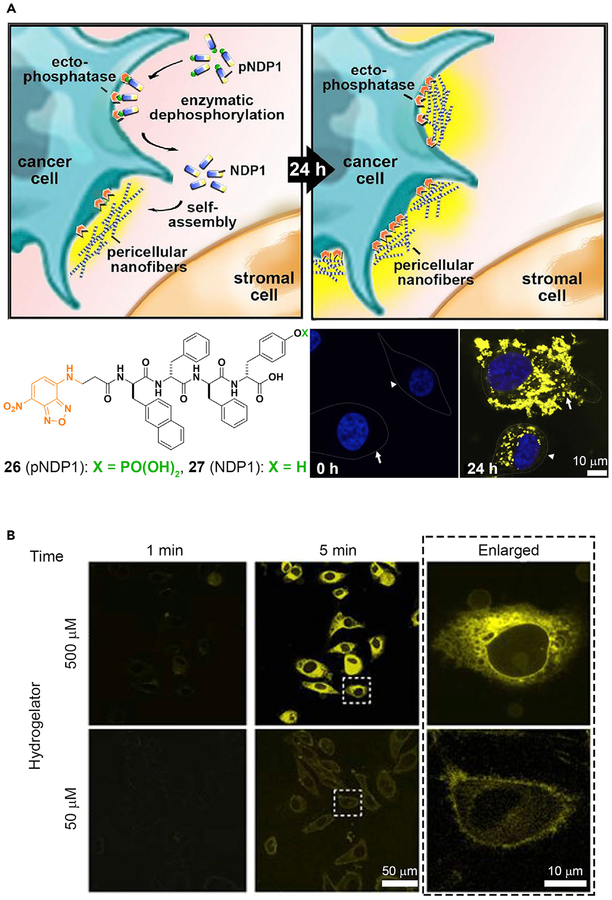
Molecular imaging on the micro- or nanoscale provides important information for understanding the underlying dynamics of living systems, thus contributing to elucidation of the biology and diagnosis of diseases.90 One interesting cellular dynamic structure is the lipid raft, a glycosphingolipid microdomain that floats freely in the membrane bilayer. Although lipid rafts have been hypothesized for more than two decades, the structures and functions of lipid rafts remain controversial. New advances in molecular imaging may be able to support this hypothesis, as shown by an active fluorescent probe that images membrane dynamics with high spatiotemporal quality.91 In this case, a tyrosine phosphate residue and an NBD fluorophore are conjugated with cholesterol. ALP residing in the lipid rafts cleaves the phosphate and allows the NBD-conjugated cholesterol to self-assemble within the lipid rafts and to fluoresce. In turn, the membrane heterogeneity is revealed. Just as imaging the constant changes of cell membranes is important, imaging the activities of an enzyme on or in the cells also deserves attention. For example, the conventional imaging probes of phosphatases have been unsuccessful for live-cell imaging due to inherent cytotoxicity. A fluorescent molecule (pNPD1, 26), which forms nondiffusive nanofibers of NPD1 (27) by ENS,92 is able to minimize this problem. Figure 5A shows the conversion of 26 into 27 by ectophosphatases on the cell surface. The bottom right images in Figure 5A show HS-5 (stromal cells) and HeLa (ovarian cancer cells) before and after dephosphorylation and shows an obvious contrast between 0 h and 24 h. The intensity and distribution of the fluorescent signals in HS-5 differ markedly from those of the HeLa cells, further confirming the utility of ENS for understanding cell biology. In addition, several other labs have reported the use of ENS for imaging the activities of ALP on cells.15,93,94
Figure 5. ENS in Pericellular or Intercellular Space.
(A) Peri- or intercellular ENS for spatiotemporal profiling of the ALP activity on cancer cells. White arrows, HeLa cells; white arrowheads, HS-5 cells.
(B) Intracellular ENS occurs on the endoplasmic reticulum of living cells.
(A) Adapted with permission from Zhou et al.92 Copyright 2016 Published by Elsevier Inc. (B) Adapted with permission from Gao et al.95 Copyright 2012 Springer Nature.
Imaging Intracellular Molecular Self-Assembly by ENS.
Although intracellular molecular self-assembly was reported in 2007,96,97 imaging ENS inside the cell is challenging. The first molecule to exhibit fluorescence for imaging ENS inside living cells was reported in 2012.95 Figure 5B shows that the fluorescent nanofibers of an NBD-conjugated tetrapeptide form mainly on the endoplasmic reticulum (ER) after dephosphorylation by PTP1B. In a related study, a D-peptide quinazolinone derivative, which minimizes the cytotoxicity of quinazolinone-based molecule probes, was shown to be promising for intracellular imaging of phosphatases.98
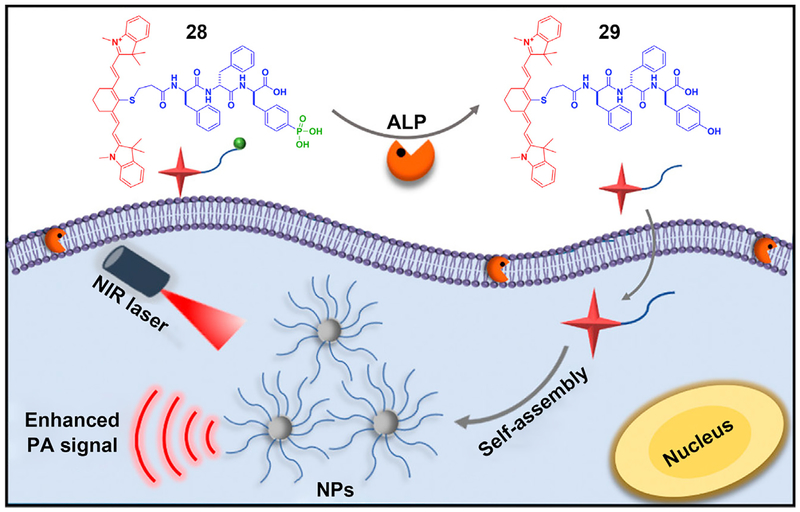
Imaging molecular self-assembly inside cells provides a new way to explore the activity of therapeutics to confirm the target location of a drug or to validate a diagnosis. Particularly, the ability to image cancerous tumors with high spatial resolution is important, because cancer remains a major challenge in health care. A promising modality for imaging tumors is photoacoustic (PA) imaging, in which ultrasound waves are excited by irradiation of the tissue with electromagnetic radiation. Tissue chromophores such as hemoglobin or water absorb the electromagnetic output, subsequently emitting acoustic waves that can be transduced by an ultrasound receiver.99 Based on this idea, Liang and coworkers developed an ALP-activatable probe for enhancing photoacoustic imaging of enzyme activity in vitro and in tumors (Figure 6).100 They designed and synthesized a phosphotripeptide (28), which turned into 29 after enzymatic cleavage of the phosphate from the tyrosine. Self-assembling to form nanoparticles, 29 exhibits a 6.4-fold increase in the 795 nm PA signal of 28. This innovative demonstration may improve the sensitivity for imaging deep tissues. Hopefully, by replacing the core peptides, more probes can be designed for imaging other cancers in the future. Since the demonstration of self-assembly for molecular imaging in animal models by Rao et al,90 applications of ENS for molecular imaging are receiving more research attention. These works90,101 are evidence of the potential of intracellular ENS.
Figure 6. The Use of ENS in Intracellular Imaging.
Schematic of ENS signal enhancement of photoacoustic (PA) imaging. NIR, near infrared; NP, nanoparticle. Reprinted with permission from Wu et al.100 Copyright 2018 American Chemical Society.
Subcellular ENS
Mitochondrial Targeting.
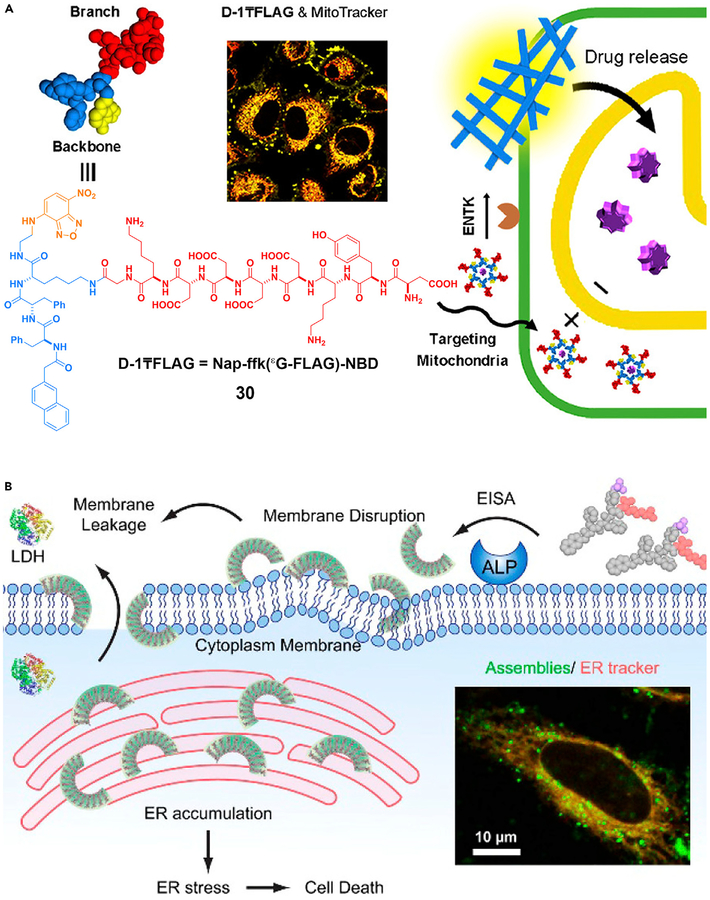
ENS offers a unique approach to target the subcellular organelles of cancer cells, as shown by the development of a triphenylphosphinium (TPP) conjugated phosphotetrapeptide to kill the cancer cells by targeting the mitochondria.102 After ALP, overexpressed on cancer cells, catalytically dephosphorylates the conjugates, the nanoscale assemblies formed on the cell surface are internalized by endocytosis. After escaping the lysosomes, the assemblies target the mitochondria to induce the release of cytochrome c, resulting in cell death. The use of ENS of 30 catalyzed by ENTK is able to target mitochondria (Figure 7A) as well.79 Targeting the subcellular organelles has an advantage over current cancer treatments, because it not only minimizes acquired drug resistance but also promises to enhance the efficacy of therapeutics without increasing toxicity.
Figure 7. ENS in Organelle Targeting.
ENS to target (A) mitochondria and (B) endoplasmic reticulum. LDH, lactate dehydrogenase.
(A) Reprinted with permission from He et al.79 Copyright 2018 American Chemical Society. (B) Reprinted with permission from Feng et al.103 Copyright 2018 American Chemical Society.
Endoplasmic Reticulum Targeting.
The ER is emerging as a target for cancer therapy, but it is difficult to target the ER of the cancer cells selectively. ENS offers a solution for this common challenge in translational medicine. As shown in Figure 7B, ENS generates crescent-shaped supramolecular assemblies of short peptides, which disrupt the cell membranes, target the ER, and result in cancer cell death.103 In a related study, ENS forms molecular condensates that sequester protein-tyrosine phosphatase 1B (PTP1B) and cyclooxygenase-2 (COX-2) on the ER.104 These ENS processes, similar to the formation of higher-order assemblies of proteins105 or protein phase transition,106 promise a new way to use assemblies of small molecules to modulate protein-protein interactions for biomedicine.
ENS for Cancer Therapy
Cancer remains a major burden to public health. Anticancer chemotherapy continues to be the most important adjuvant therapy to surgery, but multiple underlying cellular mechanisms complicate the treatment. Even when the treatment is effective initially, genomic instability causes the emergence of drug resistance, which is the most significant challenge in chemotherapy. Despite significant advances in cancer immunotherapy based on checkpoint blockade, multiple immunosuppressive mechanisms in the tumor microenvironment still result in the patients’ unresponsiveness to the treatment. One major challenge is that developing new inhibitors to further block immunosuppression may not be an option, because the targets are “undruggable.” Under these circumstance, ENS may be an out-of-the-box approach for cancer therapy, because it inhibits cancer cells based on enzymatic reactions rather than on enzyme inhibition.107 Since the observation that intracellular gelation by ENS selectively inhibits cancer cells,96 the development of ENS for cancer therapy has advanced rapidly;29,75 thus, it is worthwhile discussing this in a separate section.
ENS Selectively Gelling and Killing Cancer Cells.
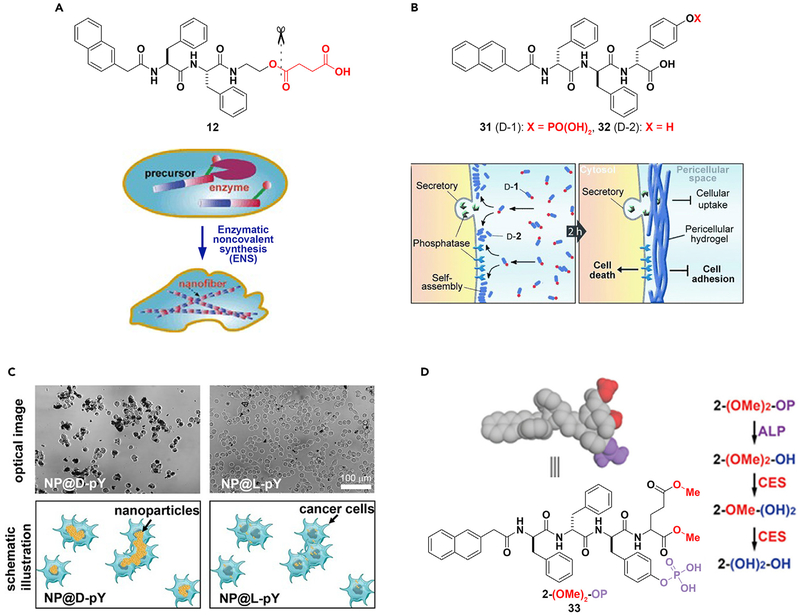
New advances in cancer therapy are necessary to eradicate such a malignant disease. ENS may be a promising method in combating this problem. The formation of nanofibers by ENS inside cells would likely gel the cytosol and change the microviscosity inside cells, and thus may lead to cell death. This hypothesis was first confirmed in the case of bacteria,97 which was followed by a test of the concept on mammalian cells.96 Specifically, the precursor (12) is a substrate of CES. Hydrolysis of 12 produces a hydrogelator that self-assembles to form nanofibers resulting in a supramolecular hydrogel. Although the high expression level of CES in HeLa cells catalyzes the formation of the hydrogelator that results in the formation of nanofibers as well as hydrogelation inside the cells and causes cell death (Figure 8A),96 the low level of expression of esterase in a fibroblast cell (e.g., NIH3T3) is unable to generate enough hydrogelator molecules to promote gelation, and thus the precursor is innocuous to the NIH3T3cells. As the first case of ENS creating cellular artificial nanostructures and thus controlling the fate of cells, this work illustrates the concept of molecular processes acting as novel therapeutics.
Figure 8. ENS in Cancer Therapy.
(A and B) (A) Intracellular and (B) peri- or intercellular ENS for cancer therapy.
(C) The incubation of HeLa cells with d- or l-phosphotyrosine-decorated magnetic nanoparticles (NP@D-pY or NP@L-pY).
(D) Modulation of self-assembly using ALP and CES.
(A) Adapted with permission from Yang et al.96 Copyright 2007 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. (B) Adapted with permission from Kuang et al.108 Copyright 2014 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. (C) Reprinted with permission from Du et al.109 Copyright 2017 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. (D) Adapted with permission from Feng et al.110 Copyright 2017 American Chemical Society.
The further development of ENS based on CES and D-peptides has greatly improved the efficacy for inhibiting cancer cells,111–113 as well as boosted the efficacy of cisplatin against drug-resistant cancer cells.113 In a related study, incorporating taurine in the D-peptide precursor greatly increases the retention of the D-peptide nanofibers inside the cells after ENS.114 A recent kinetic study on the CES-catalyzed ENS of D-peptide nanofibers has elucidated how stereochemistry affects ENS in the complex extra- and/or intracellular environment and provided insights for understanding the kinetics and cytotoxicity of peptide assemblies for killing cancer cells.111
Pericellular ENS for Selectively Inhibiting Cancer Cells.
Although intracellular gelation inhibiting cancer cells was demonstrated over a decade ago, pericellular gelation for inhibiting cancer cells selectively is a recent surprise.108 As shown in Figure 8B, ALP catalytically dephosphorylates 31 to form the hydrogelator (32). The incubation of HeLa cells with 31 resulted in the gel of 32 on the cell surface, which was a consequence of pericellular ENS. The accumulation of the hydrogelators results in nanonets surrounding the cancer cell (Figure 8B), thereby inhibiting cell adhesion, nutrient uptake, migration, all culminating in cell death. Further development of this ALP-catalyzed ENS shows that the nanofibers of 32 inhibit the growth of cancer cells in a co-culture by selectively inducing apoptosis.115 The relevant mechanistic study reveals that the nanofibers of 32 pleiotropically activate extrinsic death signaling for selectively killing cancer cells. This result illustrates the potential of ENS as a supramolecular cellular biochemical process (consisting of reaction, assembly, and binding) for multi-targeting that may lead to a new way for combating cancer drug resistance.74,102
The concept of ENS selectively inhibiting cancer cells is applicable to other molecules containing phosphates73,75 or ENS catalyzed by other enzymes.29 As expected, structural modification of the substrates, such as modifying the peptide C-terminal,116 altering the number of phosphates to be cleaved,117 conjugating to anticancer drugs65 or nonsteroidal anti-inflammatory drugs,118 and changing the structure of the core peptides,119 can improve the anticancer activity and selectivity of ENS. If L-peptides are the substrates for ENS, inhibiting proteolysis of the peptides is able to enhance the efficacy of ENS against cancer cells.120 Moreover, it is feasible to use nanoparticles to replace peptides for pericellular ENS that selectively targets cancer cells. As shown in Figure 8C, d-phosphotyrosine-decorated magnetic nanoparticles selectively adhere and inhibit HeLa cells, whereas l-phosphotyrosine-decorated magnetic nanoparticles barely interact with the HeLa cells.109,121 Because ENS of D-peptides forms nanonets around cancer cells, the nanonets are able entrap the secretome from cancer cells.122
ENS for Targeting Downregulation in Cancer Cells.
Cancer cells differ from normal cells mainly in two aspects: gain of functions (i.e., upregulation) and loss of functions (i.e., downregulation). Although it is straightforward to develop therapeutics for inhibiting upregulation, it is difficult to target downregulation in cancer cells. Because it is independent from inhibition, ENS may provide a novel approach for targeting downregulation in cancer cells, as reported in the case of targeting the cells that express ALP but downregulate CES. As shown in Figure 8D, the precursor (2-(OMe)2-OP) or 33 contains both the CES cleavage site (i.e., carboxylmethylester) and the ALP cleavage site (i.e., phosphotyrosine). Upon the action of ALP, 2-(OMe)2-OP turns into 2-(OMe)2-OH, which self-assembles to form nanotubes. Upon the action of CES, 2-(OMe)2-OH becomes 2-(OH)2-OH, which exhibits lower self-assembling ability than that of 2-(OMe)2-OH. When incubated with OVSAHO cells (which express ALP but downregulate CES) and HepG2 cells (which express ALP and upregulate CES), 33 exhibits half maximal inhibitory concentrations of 5 μM against OVSAHO cells and >200 μM toward HepG2 cells, confirming that 33 selectively inhibits OVSAHO over HepG2 cells.110 This design provides a way to reduce the liver toxicity of ENS against cancer cells and further confirms the concept of regulating the rate of ENS for targeting cancer cells.117
ENS Minimizes Acquired Drug Resistance.
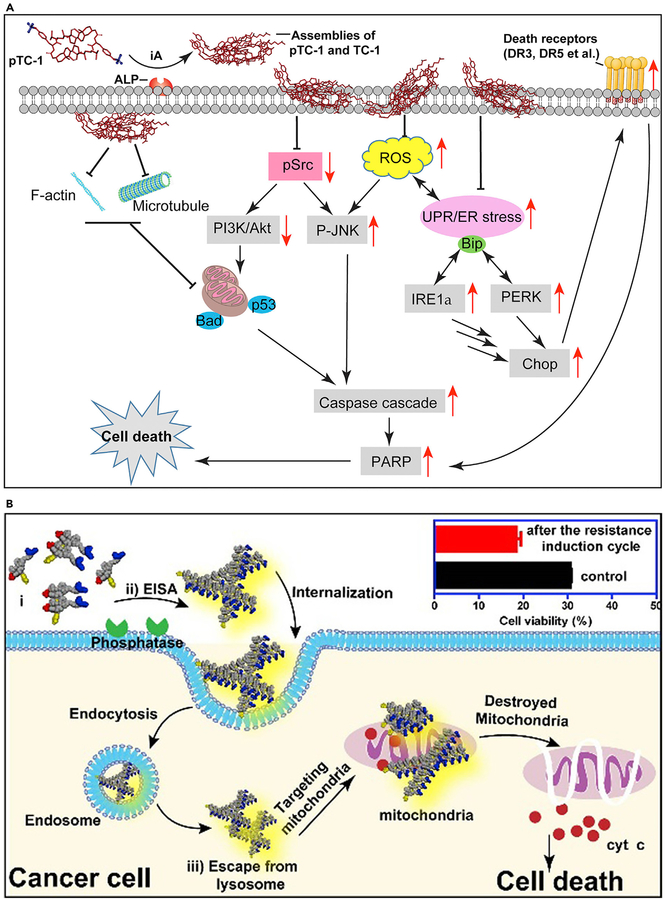
Acquired drug resistance remains a challenge in cancer therapy. A recent mechanistic study demonstrated in situ ENS of cholesterol derivatives to act as poly pharmaceuticals for selectively inducing cancer cell death via multiple pathways (Figure 9A).74 The tyrosine-cholesterol conjugates, after ENS, self-assemble in various parts of cancer cells. They induce stress-related processes such as aggregation of cell death receptors, interfering with the expression of glycoproteins, disruption of cytoskeleton dynamics, and a host of others that effectively kill cancer cells altogether.
Figure 9. ENS Combats Acquired Drug Resistance.
(A) ENS pathways to cell death. iA, instructed assembly.
(B) Schematic of endocytosis of nanofibers inducing cancer cell death. EISA, enzyme-instructed self-assembly
(A) Adapted with permission from Wang et al.74 Copyright 2018 American Association for Cancer Research. (B) Reprinted with permission from Wang et al.102 Copyright 2016 American Chemical Society.
Modulating the redox potential of mitochondria is a promising approach to destroy cancer cells that have acquired drug resistance. However, in using mitochondria to solve this problem, selectivity is an issue given that mitochondria are ubiquitous in all cells. Figure 9B shows a strategy for using TPP conjugated to a pair of enantiomeric phosphorylated peptides to selectively modulate the mitochondria of Saos2 cells and induce cell death.102 In this approach, ENS results in assemblies of the TPP-conjugated peptides on the surface of the cancer cells selectively. After endocytosis of the TPP-conjugated assemblies, a high concentration of TPP on the mitochondria of the cancer cells induces the release of cytochrome c from the mitochondria to trigger an intrinsic apoptosis process. Because the mitochondria are the metabolic center and signaling hub of cells, it is difficult for the cancer cells to evolve resistance to the insult of mitochondria.
ENS for Immunological Applications
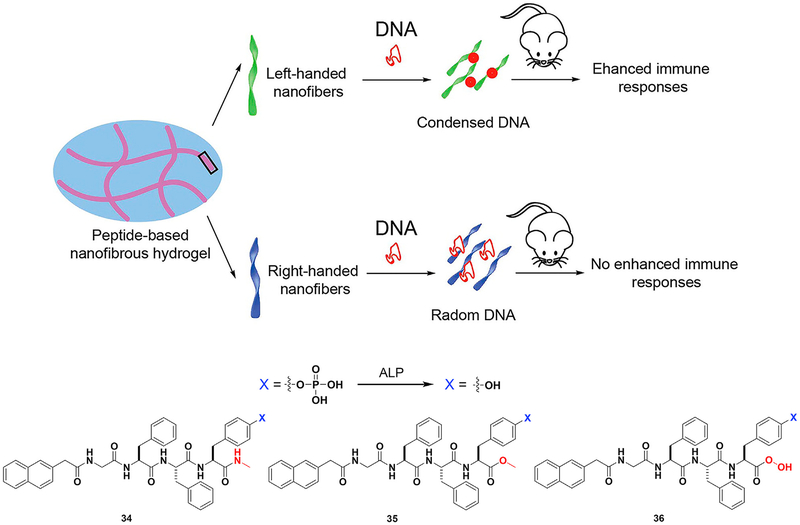
Generally, ENS generates nanostructures (e.g., nanoparticles or nanofibers), which may be first internalized by cells from immune systems. But the application of ENS in immunology is new. In addition to the demonstration of ENS of an immunoreceptor tyrosine-based inhibitory (ITIM) motif,123 the most impressive result has been the development of HIV vaccines based on ENS of a tetrapeptide, reported by Yang and coworkers.124 In that study, upon enzymatic dephosphorylation of a phosphotetrapeptide, the tetrapeptide self-assembles to form a hydrogel that encapsulates DNA. This mixture triggers humoral and cellular immune responses, which are significant signals for HIV prevention and therapy. The structure of the hydrogel is important for its function (Figure 10). For instance, the G-NMe moiety in the peptide enhances the immune response. Left-handed helical structures generated from 34 protect DNA from degradation, thus allowing its entry into mammalian cells. Previous works have focused on the immune response from structures 35 and 36. This study on the 34 nanovector seems to be a more effective approach for developing an HIV vaccine.
Figure 10. ENS in Immunology.
Peptide-based hydrogel formed by ENS mixed with DNA to yield nanovectors for HIV treatment. Adapted with permission from Tian et al.124 Copyright 2014 American Chemical Society.
Outlook
Noncovalent synthesis is a well-established concept, as demonstrated by the continuing development of supramolecular chemistry125,126 and the recent intensive development of metal-organic frameworks.127 The idea of noncovalent synthesis of organic fibers was presented by Menger et al. over two decades ago.128 ENS is simply a logical and natural extension of the concept of noncovalent synthesis. Enzymes make the exploration of functions (especially biological functions) of noncovalent synthesis relatively easy. ENS also frees researchers from the structure-function dogma, allowing the focus of the study to be on the dynamic-function or transition-function paradigm. One such transition-function example in the context of noncovalent synthesis of organic molecules is the liquid crystal in a liquid crystal display. Such transition-function examples of proteins in biology are simply too numerous to be mention here. Therefore, ENS for supramolecular soft matter is only at its exciting beginning.
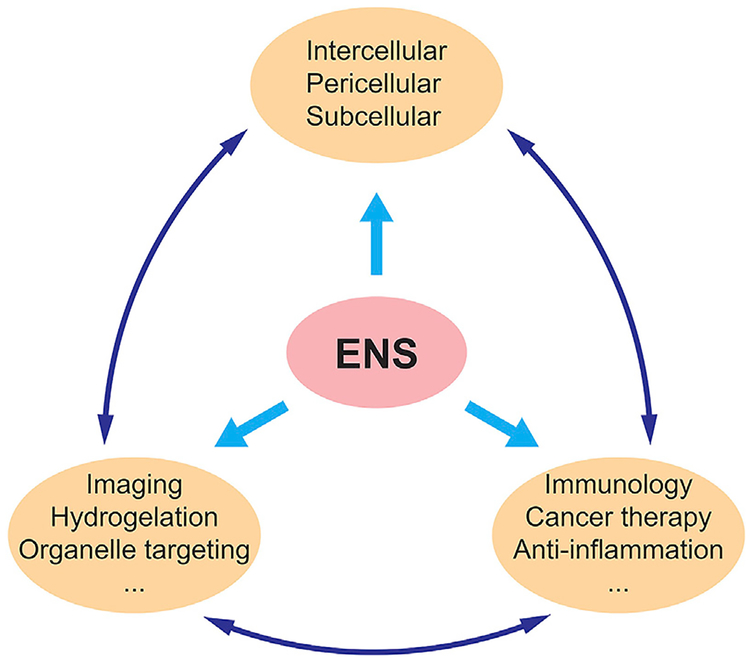
ENS can take many forms. It facilitates not only bond breaking but also bond forming.30,129–133 This review has shown the many modes of ENS, from the plethora of enzymes used to construct nanoscale assemblies to its use in different subspaces of the cells and to its wide range of applications. As the study of ENS continues to progress, new avenues may be explored. Recently, ENS has been used to decrease NF-κB activation, inducing synthetic lethality.134 Also, given the advantage of proteolytic resistance of D-peptides over their L-enantiomers, hopefully in the future they find applications to treat diseases other than cancer.120 A current example is to use ENS to release dexamethasone for treating fibrosis.135 The substrates of ENS can be diverse. Although current studies focus more on peptides, other organic or inorganic molecules should also be able to undergo ENS. A recent example even shows the ENS of nucleic acids, which recapitulates an essential cellular process in cell-free conditions.136 The prevalence of ENS in biology has already shown how nature combines enzymatic reactions and noncovalent interactions for controlling dynamics, emergent properties, and functions. This fact suggests the broad applicability of ENS (Scheme 1) for developing new promising soft matter in the field of biomedicine.
Scheme 1.
Potential Applications of ENS
Progress and Potential.
Enzymatic covalent synthesis is well established in industry, e.g., the use of bacteria or yeast in food. However, using enzymatic noncovalent synthesis (ENS) to make materials that are strong and effective yet biocompatible is still in its infancy. Biomaterials has become an important field in medicine and engineering because these synthetic molecules may be able to complete tasks that we humans cannot. This review discusses various studies of ENS of small biomolecules that can be used in different facets of medicine. Peptidic nanomaterials and their applications are highlighted along with the enzymes used to catalyze their reactions. In the future, more focus should be placed on the application of ENS with other organic and inorganic molecules such as DNA, hydrocarbon chains, or fatty acids.
ACKNOWLEDGMENTS
This work is partially supported by NIH (CA142746).
REFERENCES
- 1.Lehn J-M (1995). Supramolecular Chemistry (VCH; ). [Google Scholar]
- 2.Desai A, and Mitchison TJ (1997). Microtubule polymerization dynamics. Annu. Rev. Cell Dev. Biol 13, 83–117. [DOI] [PubMed] [Google Scholar]
- 3.Yang Y, Wang H, Kouadir M, Song H, and Shi F (2019). Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis. 10, 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Orgel JP, Irving TC, Miller A, and Wess TJ (2006). Microfibrillar structure of type I collagen in situ. Proc. Natl. Acad. Sci. U S A 103, 9001–9005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Howard L Lung TEM. http://remf.dartmouth.edu/images/mammalianLungTEM/source/9.html.
- 6.Yang Z, Liang G, Wang L, and Xu B (2006). Using a kinase/phosphatase switch to regulate a supramolecular hydrogel and forming the supramolecular hydrogel in vivo. J. Am. Chem. Soc 128, 3038–3043. [DOI] [PubMed] [Google Scholar]
- 7.Seelbach KLA, and Wadrey C (2006). Industrial Biotransformations (Wiley-VCH; ). [Google Scholar]
- 8.Estroff LA, and Hamilton AD (2004). Water gelation by small organic molecules. Chem. Rev 104, 1201–1218. [DOI] [PubMed] [Google Scholar]
- 9.Qi J, Yan Y, Cheng B, Deng L, Shao Z, Sun Z, and Li X (2018). Enzymatic formation of an injectable hydrogel from a glycopeptide as a biomimetic scaffold for vascularization. ACS Appl. Mater Interfaces 10, 6180–6189. [DOI] [PubMed] [Google Scholar]
- 10.Zhan J, Cai Y, Ji S, He S, Cao Y, Ding D, Wang L, and Yang Z (2017). Spatiotemporal control of supramolecular self-assembly and function. ACS Appl. Mater Interfaces 9, 10012–10018. [DOI] [PubMed] [Google Scholar]
- 11.Alarcon-Correa M, Gunther J-P, Troll J, Kadiri VM, Bill J, Fischer P, and Rothenstein D (2019). Self-assembled phage-based colloids for high localized enzymatic activity. ACS Nano 13, 5810–5815. [DOI] [PubMed] [Google Scholar]
- 12.Wang H, Luo Z, Wang Y, He T, Yang C, Ren C, Ma L, Gong C, Li X, and Yang Z (2016). Enzyme-catalyzed formation of supramolecular hydrogels as promising vaccine adjuvants. Adv. Funct. Mater 26, 1822–1829. [Google Scholar]
- 13.Magro M, Baratella D, Miotto G, Frommel J, Sebela M, Kopecna M, Agostinelli E, and Vianello F (2019). Enzyme self-assembly on naked iron oxide nanoparticles for aminoaldehyde biosensing. Amino Acids 51, 679–690. [DOI] [PubMed] [Google Scholar]
- 14.Vallooran JJ, Assenza S, and Mezzenga R (2019). Spatiotemporal control of enzyme induced crystallization under lyotropic liquid crystal nanoconfinement. Angew. Chem. Int. Ed 58, 7289–7293. [DOI] [PubMed] [Google Scholar]
- 15.Liu H-W, Li K, Hu X-X, Zhu L, Rong Q, Liu Y, Zhang X-B, Hasserodt J, Qu F-L, and Tan W (2017). In situ localization of enzyme activity in live cells by a molecular probe releasing a precipitating fluorochrome. Angew. Chem. Int. Ed 56, 11788–11792. [DOI] [PubMed] [Google Scholar]
- 16.Fores JR, Mendez MLM, Mao X, Wagner D, Schmutz M, Rabineau M, Lavalle P, Schaaf P, Boulmedais F, and Jierry L (2017). Localized supramolecular peptide self-assembly directed by enzyme-induced proton gradients. Angew. Chem. Int. Ed 56, 15984–15988. [DOI] [PubMed] [Google Scholar]
- 17.Sahoo JK, Pappas CG, Sasselli IR, Abul-Haija YM, and Ulijn RV (2017). Biocatalytic self-assembly cascades. Angew. Chem. Int. Ed 56, 6828–6832. [DOI] [PubMed] [Google Scholar]
- 18.Shi J, Fichman G, and Schneider JP (2018). Enzymatic control of the conformational landscape of self-assembling peptides. Angew. Chem. Int. Ed 57, 11188–11192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang C, Shafi R, Lampel A, MacPherson D, Pappas CG, Narang V, Wang T, Maldarelli C, and Ulijn RV (2017). Switchable hydrolase based on reversible formation of supramolecular catalytic site using a self-assembling peptide. Angew. Chem. Int. Ed 56, 14511–14515. [DOI] [PubMed] [Google Scholar]
- 20.Moldenhauer D, Werner JPF, Strassert CA, and Groehn F (2019). Light-responsive size of self-assembled spiropyran-lysozyme nanoparticles with enzymatic function. Biomacromolecules 20, 979–991. [DOI] [PubMed] [Google Scholar]
- 21.Nandi N, Gayen K, Ghosh S, Bhunia D, Kirkham S, Sen SK, Ghosh S, Hamley IW, and Banerjee A (2017). Amphiphilic peptide-based supramolecular, noncytotoxic, stimuli responsive hydrogels with antibacterial activity. Biomacromolecules 18, 3621–3629. [DOI] [PubMed] [Google Scholar]
- 22.West HT, Csizmar CM, and Wagner CR (2018). Tunable supramolecular assemblies from amphiphilic nucleoside phosphoramidate nanofibers by enzyme activation. Biomacromolecules 19, 2650–2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song J, He W, Shen H, Zhou Z, Li M, Su P, and Yang Y (2019). Self-assembly of a magnetic DNA hydrogel as a new biomaterial for enzyme encapsulation with enhanced activity and stability. Chem. Commun. (Camb.) 55, 2449–2452. [DOI] [PubMed] [Google Scholar]
- 24.Wang Z, Liang C, Shang Y, He S, Wang L, and Yang Z (2018). Narrowing the diversification of supramolecular assemblies by preorganization. Chem. Commun. (Camb.) 54, 2751–2754. [DOI] [PubMed] [Google Scholar]
- 25.Shang Y, Wang Z, Zhang R, Li X, Zhang S, Gao J, Li X, and Yang Z (2019). A novel thermogel system of self-assembling peptides manipulated by enzymatic dephosphorylation. Chem. Commun. (Camb.) 55, 5123–5126. [DOI] [PubMed] [Google Scholar]
- 26.Luo K, Park K-H, Lee D-H, Hong C-E, Song Y-W, Yoo S-H, and Kim Y-R (2019). Self-assembly kinetics of debranched short-chain glucans from waxy maize starch to form spherical microparticles and its applications. Colloids Surf. B Biointerfaces 176, 352–359. [DOI] [PubMed] [Google Scholar]
- 27.Cho H-J, Lee S, Park S-J, Lee Y-D, Jeong K, Park JH, Lee Y-S, Kim B, Jeong H-S, and Kim S (2019). Tumor microenvironment-responsive fluorogenic nanoprobe for ratiometric dual-channel imaging of lymph node metastasis. Colloids Surf. B Biointerfaces 179, 9–16. [DOI] [PubMed] [Google Scholar]
- 28.Cai Y, Shen H, Zhan J, Lin M, Dai L, Ren C, Shi Y, Liu J, Gao J, and Yang Z (2017). Supramolecular “Trojan Horse” for nuclear delivery of dual anticancer drugs. J. Am. Chem. Soc 139, 2876–2879. [DOI] [PubMed] [Google Scholar]
- 29.Tanaka A, Fukuoka Y, Morimoto Y, Honjo T, Koda D, Goto M, and Maruyama T (2015). Cancer cell death induced by the intracellular self-assembly of an enzyme-responsive supramolecular gelator. J. Am. Chem. Soc 137, 770–775. [DOI] [PubMed] [Google Scholar]
- 30.Toledano S, Williams RJ, Jayawarna V, and Ulijn RV (2006). Enzyme-triggered self-assembly of peptide hydrogels via reversed hydrolysis. J. Am. Chem. Soc 128, 1070–1071. [DOI] [PubMed] [Google Scholar]
- 31.Lancaster L, Bulutoglu B, Banta S, and Wheeldon I (2019). Enzyme colocalization in protein-based hydrogels. Methods Enzymol. 617, 265–285. [DOI] [PubMed] [Google Scholar]
- 32.Jiang T, Shen S, Wang T, Li M, He B, and Mo R (2017). A substrate-selective enzyme catalysis assembly strategy for oligopeptide hydrogel-assisted combinatorial protein delivery. Nano Lett. 17, 7447–7454. [DOI] [PubMed] [Google Scholar]
- 33.Yang C, Ren X, Ding D, Wang L, and Yang Z (2016). Enzymatic induction of supramolecular order and bioactivity. Nanoscale. 8, 10768–10773. [DOI] [PubMed] [Google Scholar]
- 34.Zhou Z, Xie X, Yi Q, Yin W, Kadi AA, Li J, and Zhang Y (2017). Enzyme-instructed self-assembly with photo-responses for the photo-regulation of cancer cells. Org. Biomol. Chem 15, 6892–6895. [DOI] [PubMed] [Google Scholar]
- 35.Bai J, Gong Z, Wang J, and Wang C (2017). Enzymatic hydrogelation of self-assembling peptide I4K2 and its antibacterial and drug sustained-release activities. RSC Adv. 7, 48631–48638. [Google Scholar]
- 36.Huang A, Ou C, Cai Y, Wang Z, Li H, Yang Z, and Chen M (2016). In situ enzymatic formation of supramolecular nanofibers for efficiently killing cancer cells. RSC Adv. 6, 32519–32522. [Google Scholar]
- 37.Abul-Haija YM, Roy S, Frederix PW, Javid N, Jayawarna V, and Ulijn RV (2014). Biocatalytically triggered co-assembly of two-component core/shell nanofibers. Small 10, 973–979. [DOI] [PubMed] [Google Scholar]
- 38.Zheng Z, Chen P, Xie M, Wu C, Luo Y, Wang W, Jiang J, and Liang G (2016). Cell environment-differentiated self-assembly of nanofibers. J. Am. Chem. Soc 138, 11128–11131. [DOI] [PubMed] [Google Scholar]
- 39.Hai Z, Li J, Wu J, Xu J, and Liang G (2017). Alkaline phosphatase-triggered simultaneous hydrogelation and chemiluminescence. J. Am. Chem. Soc 139, 1041–1044. [DOI] [PubMed] [Google Scholar]
- 40.Zheng W, Gao J, Song L, Chen C, Guan D, Wang Z, Li Z, Kong D, and Yang Z (2013). Surface-induced hydrogelation inhibits platelet aggregation. J. Am. Chem. Soc 135, 266–271. [DOI] [PubMed] [Google Scholar]
- 41.Zhan J, Cai Y, He S, Wang L, and Yang Z (2018). Tandem molecular self-assembly in liver cancer cells. Angew. Chem. Int. Ed 57, 1813–1816. [DOI] [PubMed] [Google Scholar]
- 42.Liang G, Ronald J, Chen Y, Ye D, Pandit P, Ma ML, Rutt B, and Rao J (2011). Controlled self-assembling of gadolinium nanoparticles as smart molecular magnetic resonance imaging contrast agents. Angew. Chem. Int. Ed 50, 6283–6286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang X, Chu X, Wang L, Wang H, Liang G, Zhang J, Long J, and Yang Z (2012). Rational design of a tetrameric protein to enhance interactions between self-assembled fibers gives molecular hydrogels. Angew. Chem. Int. Ed 51, 4388–4392. [DOI] [PubMed] [Google Scholar]
- 44.Liu S, Tang A, Xie M, Zhao Y, Jiang J, and Liang G (2015). Oligomeric hydrogels self-assembled from reduction-controlled condensation. Angew. Chem. Int. Ed 54, 3639–3642. [DOI] [PubMed] [Google Scholar]
- 45.Yuan Y, Wang L, Du W, Ding Z, Zhang J, Han T, An L, Zhang H, and Liang G (2015). Intracellular self-assembly of taxol nanoparticles for overcoming multidrug resistance. Angew. Chem. Int. Ed 54, 9700–9704. [DOI] [PubMed] [Google Scholar]
- 46.Wei Q, Xu M, Liao C, Wu Q, Liu M, Zhang Y, Wu C, Cheng L, and Wang Q (2016). Printable hybrid hydrogel by dual enzymatic polymerization with superactivity. Chem. Sci 7, 2748–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu C, Hu W, Wei Q, Qiao L, Gao Y, Lv Y, Liu M, Li C, Wang X, and Wang Q (2018). Controllable growth of core-shell nanogels via esterase-induced self-assembly of peptides for drug delivery. J. Biomed. Nanotechnol 14, 354–361. [DOI] [PubMed] [Google Scholar]
- 48.Wei Q, Xu W, Liu M, Wu Q, Cheng L, and Wang Q (2016). Viscosity-controlled printing of supramolecular-polymeric hydrogels via dual-enzyme catalysis. J. Mater. Chem. B 4, 6302–6306. [DOI] [PubMed] [Google Scholar]
- 49.Wu Q, He ZG, Wang X, Zhang Q, Wei QC, Ma SQ, Ma C, Li J, and Wang QG (2019). Cascade enzymes within self-assembled hybrid nanogel mimicked neutrophil lysosomes for singlet oxygen elevated cancer therapy. Nat. Commun 10, 240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ji W, Zhang SJ, Yukawa S, Onomura S, Sasaki T, Miyazawa K, and Zhang Y (2018). Regulating higher-order organization through the synergy of two self-sorted assemblies. Angew. Chem. Int. Ed 57, 3636–3640. [DOI] [PubMed] [Google Scholar]
- 51.Mang D, Zhang S, Wu X, Hu X, Mochizuki T, Li G, and Zhang Y (2019). Enzyme-mediated dual-targeted-assembly realizes a synergistic anticancer effect. Chem. Commun. (Camb.) 55, 6126–6129. [DOI] [PubMed] [Google Scholar]
- 52.Yao D, Yang S, Wang Y, Bian K, Yang W, Wang D, and Zhang B (2019). An ALP activatable and mitochondria-targeted probe for prostate cancer-specific bimodal imaging and aggregation-enhanced photothermal therapy. Nanoscale 11, 6307–6314. [DOI] [PubMed] [Google Scholar]
- 53.Wang Z, An HW, Hou D, Wang M, Zeng X, Zheng R, Wang L, Wang K, Wang H, and Xu W (2019). Addressable peptide self-assembly on the cancer cell membrane for sensitizing chemotherapy of renal cell carcinoma. Adv. Mater 31, e1807175. [DOI] [PubMed] [Google Scholar]
- 54.Liu H, Li Y, Lyu Z, Wan Y, Li X, Chen H, Chen H, and Li X (2014). Enzyme triggered supramolecular self-assembly of platinum prodrug with enhanced tumor-selective accumulation and reduced systemic toxicity.J. Mater. Chem. B 2, 8303–8309. [DOI] [PubMed] [Google Scholar]
- 55.Osada Y, and Khokhlov A (2001). Polymer Gels and Networks, First Edition (Taylor & Francis; ). [Google Scholar]
- 56.Hu B-H, and Messersmith PB (2003). Rational design of transglutaminase substrate peptides for rapid enzymatic formation of hydrogels. J. Am. Chem. Soc 125, 14298–14299. [DOI] [PubMed] [Google Scholar]
- 57.Du X, Zhou J, Shi J, and Xu B (2015). Supramolecular hydrogelators and hydrogels: from soft matter to molecular biomaterials. Chem. Rev 115, 13165–13307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang Z, Gu H, Fu D, Gao P, Lam JK, and Xu B (2004). Enzymatic formation of supramolecular hydrogels. Adv. Mater 16, 1440–1444. [Google Scholar]
- 59.Yang Z, Liang G, and Xu B (2008). Enzymatic hydrogelation of small molecules. Acc. Chem. Res 41, 315–326. [DOI] [PubMed] [Google Scholar]
- 60.Thornton K, Abul-Haija YM, Hodson N, and Ulijn RV (2013). Mechanistic insights into phosphatase triggered self-assembly including enhancement of biocatalytic conversion rate. Soft Matter 9, 9430–9439. [Google Scholar]
- 61.Li J, Zhan Z, Du X, Wang J, Hong B, and Xu B (2018). Selection of secondary structures of heterotypic supramolecular peptide assemblies by an enzymatic reaction. Angew. Chem 57, 11716–11721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou J, Du X, Gao Y, Shi J, and Xu B (2014). Aromatic-aromatic interactions enhance interfiber contacts for enzymatic formation of a spontaneously aligned supramolecular hydrogel. J. Am. Chem. Soc 136, 2970–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Haburcak R, Shi J, Du X, Yuan D, and Xu B (2016). Ligand-receptor interaction modulates the energy landscape of enzyme-instructed self-assembly of small molecules.J. Am. Chem. Soc 138, 15397–15404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li X, Gao Y, Kuang Y, and Xu B (2010). Enzymatic formation of a photoresponsive supramolecular hydrogel. Chem. Commun. (Camb.) 46, 5364–5366. [DOI] [PubMed] [Google Scholar]
- 65.Gao Y, Kuang Y, Guo Z-F, Guo Z, Krauss IJ, and Xu B (2009). Enzyme-instructed molecular self-assembly confers nanofibers and a supramolecular hydrogel of taxol derivative. J. Am. Chem. Soc 131, 13576–13577. [DOI] [PubMed] [Google Scholar]
- 66.Gao J, Wang H, Wang L, Wang J, Kong D, and Yang Z (2009). Enzyme promotes the hydrogelation from a hydrophobic small molecule. J. Am. Chem. Soc 131, 11286–11287. [DOI] [PubMed] [Google Scholar]
- 67.Zhao F, Gao Y, Shi J, Browdy HM, and Xu B (2011). Novel anisotropic supramolecular hydrogel with high stability over a wide pH range. Langmuir 27, 1510–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang S, Greenfield MA, Mata A, Palmer LC, Bitton R, Mantei JR, Aparicio C, de la Cruz MO, and Stupp SI (2010). A self-assembly pathway to aligned monodomain gels. Nat. Mater 9, 594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang Y, Yang Z, Yuan F, Gu H, Gao P, and Xu B (2004). Molecular recognition remolds the self-assembly of hydrogelators and increases the elasticity of the hydrogel by 106 fold. J. Am. Chem. Soc 126, 15028–15029. [DOI] [PubMed] [Google Scholar]
- 70.Zhang Y, Gu H, Yang Z, and Xu B (2003). Supramolecular hydrogels respond to ligand receptor interaction. J. Am. Chem. Soc 125, 13680–13681. [DOI] [PubMed] [Google Scholar]
- 71.Li X, Kuang Y, Lin H-C, Gao Y, Shi J, and Xu B (2011). Supramolecular nanofibers and hydrogels of nucleopeptides. Angew. Chem. Int. Ed 50, 9365–9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Du X, Li J, Gao Y, Kuang Y, and Xu B (2012). Catalytic dephosphorylation of adenosine monophosphate (AMP) to form supramolecular nanofibers/hydrogels. Chem. Commun. (Camb.) 48, 2098–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang H, Feng Z, Wu D, Fritzsching KJ, Rigney M, Zhou J, Jiang Y, Schmidt Rohr K, and Xu B (2016). Enzyme-regulated supramolecular assemblies of cholesterol conjugates against drug-resistant ovarian cancer cells. J. Am. Chem. Soc 138, 10758–10761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang H, Feng Z, Yang C, Liu J, Medina JE, Aghvami SA, Dinulescu DM, Liu J, Fraden S, and Xu B (2019). Unraveling the cellular mechanism of assembling cholesterols for selective cancer cell death. Mol. Cancer Res 17, 907–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pires RA, Abul-Haija YM, Costa DS, Novoa-Carballal R, Reis RL, Ulijn RV, and Pashkuleva I (2015). Controlling cancer cell fate using localized biocatalytic self-assembly of an aromatic carbohydrate amphiphile.J. Am. Chem. Soc 137, 576–579. [DOI] [PubMed] [Google Scholar]
- 76.Pavlov IP, and Thompson WH (1902). The Work of the Digestive Glands (Charles Griffin & Company; ). [Google Scholar]
- 77.Hopp TP, Prickett KS, Price VL, Libby RT, March CJ, Pat Cerretti D, Urdal DL, and Conlon PJ (1988). A short polypeptide marker sequence useful for recombinant protein identification and purification. Nat. Biotechnol 6, 1204–1210. [Google Scholar]
- 78.He H, Wang H, Zhou N, Yang D, and Xu B (2018). Branched peptides for enzymatic supramolecular hydrogelation. Chem. Commun. (Camb.) 54, 86–89. [DOI] [PubMed] [Google Scholar]
- 79.He H, Wang J, Wang H, Zhou N, Yang D, Green DR, and Xu B (2018). Enzymatic cleavage of branched peptides for targeting mitochondria. J. Am. Chem. Soc 140, 1215–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yang ZM, Xu KM, Guo ZF, Guo ZH, and Xu B (2007). Intracellular enzymatic formation of nanofibers results in hydrogelation and regulated cell death. Adv. Mater 19, 3152–3156. [Google Scholar]
- 81.Yang Z, Ho P-L, Liang G, Chow KH, Wang Q, Cao Y, Guo Z, and Xu B (2007). Using β-lactamase to trigger supramolecular hydrogelation. J. Am. Chem. Soc 129, 266–267. [DOI] [PubMed] [Google Scholar]
- 82.Zhao F, Weitzel CS, Gao Y, Browdy HM, Shi J, Lin H-C, Lovett ST, and Xu B (2011). β-Galactosidase-instructed formation of molecular nanofibers and a hydrogel. Nanoscale. 3, 2859–2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang Z, Ma M, and Xu B (2009). Using matrix metalloprotease-9 (MMP-9) to trigger supramolecular hydrogelation. Soft Matter 5, 2546–2548. [Google Scholar]
- 84.Kalafatovic D, Nobis M, Javid N, Frederix PWJM, Anderson KI, Saunders BR, and Ulijn RV (2015). MMP-9 triggered micelle-to-fibre transitions for slow release of doxorubicin. Biomater. Sci 3, 246–249. [DOI] [PubMed] [Google Scholar]
- 85.Kalafatovic D, Nobis M, Son J, Anderson KI, and Ulijn RV (2016). MMP-9 triggered self-assembly of doxorubicin nanofiber depots halts tumor growth. Biomaterials 98, 192–202. [DOI] [PubMed] [Google Scholar]
- 86.Son J, Kalafatovic D, Kumar M, Yoo B, Cornejo MA, Contel M, and Ulijn RV (2019). Customizing morphology, size, and response kinetics of matrix metalloproteinase responsive nanostructures by systematic peptide design. ACS Nano 13, 1555–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang H, Shi J, Feng Z, Zhou R, Wang S, Rodal AA, and Xu B (2017). An in situ dynamic continuum of supramolecular phosphoglycopeptides enables formation of 3D cell spheroids. Angew. Chem. Int. Ed 56, 16297–16301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang H, Feng Z, and Xu B (2019). Instructed-assembly as context-dependent nanoscale signals for death and morphogenesis of cells. Angew. Chem. Int. Ed 58, 5567–5571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang H, Feng Z, and Xu B (2019). Intercellular instructed-assembly mimics protein dynamics to induce cell spheroids.J. Am. Chem. Soc 141, 7271–7274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rao J, Dragulescu-Andrasi A, and Yao H (2007). Fluorescence imaging in vivo: recent advances. Curr. Opin. Biotechnol 18, 17–25. [DOI] [PubMed] [Google Scholar]
- 91.Wang H, Feng Z, Del Signore SJ, Rodal AA, and Xu B (2018). Active probes for imaging membrane dynamics of live cells with high spatial and temporal resolution over extended time scales and areas. J. Am. Chem. Soc 140, 3505–3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhou J, Du X, Berciu C, He H, Shi J, Nicastro D, and Xu B (2016). Enzyme-instructed self-assembly for spatiotemporal profiling of the activities of alkaline phosphatases on live cells. Chem 1, 246–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu H-W, Hu X-X, Zhu L, Li K, Rong Q, Yuan L, Zhang X-B, and Tan W (2017). In vivo imaging of alkaline phosphatase in tumor-bearing mouse model by a promising near infrared fluorescent probe. Talanta 175, 421–426. [DOI] [PubMed] [Google Scholar]
- 94.Liu H-W, Chen L, Xu C, Li Z, Zhang H, Zhang X-B, and Tan W (2018). Recent progresses in small-molecule enzymatic fluorescent probes for cancer imaging. Chem. Soc. Rev 47, 7140–7180. [DOI] [PubMed] [Google Scholar]
- 95.Gao Y, Shi J, Yuan D, and Xu B (2012). Imaging enzyme-triggered self-assembly of small molecules inside live cells. Nat. Commun 3, 1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yang Z, Xu K, Guo Z, Guo Z, and Xu B (2007). Intracellular enzymatic formation of nanofibers results in hydrogelation and regulated cell death. Adv. Mater 19, 3152–3156. [Google Scholar]
- 97.Yang Z, Liang G, Guo Z, Guo Z, and Xu B (2007). Intracellular hydrogelation of small molecules inhibits bacterial growth. Angew. Chem. Int. Ed 46, 8216–8219. [DOI] [PubMed] [Google Scholar]
- 98.Wang J, Zhou J, He H, Wu D, Du X, and Xu B (2019). Cell-compatible nanoprobes for imaging intracellular phosphatase activities. ChemBioChem. 20, 526–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Beard P (2011). Biomedical photoacoustic imaging. Interface Focus 1, 602–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wu C, Zhang R, Du W, Cheng L, and Liang G (2018). Alkaline phosphatase-triggered self-assembly of near-infrared nanoparticles for the enhanced photoacoustic imaging of tumors. Nano Lett. 18, 7749–7754. [DOI] [PubMed] [Google Scholar]
- 101.Lin Y-X, Qiao S-L, Wang Y, Zhang R-X, An H-W, Ma Y, Rajapaksha RPYJ, Qiao Z-Y, Wang L, and Wang H (2017). An in situ intracellular self-assembly strategy for quantitatively and temporally monitoring autophagy. ACS Nano 11, 1826–1839. [DOI] [PubMed] [Google Scholar]
- 102.Wang H, Feng Z, Wang Y, Zhou R, Yang Z, and Xu B (2016). Integrating enzymatic self-assembly and mitochondria targeting for selectively killing cancer cells without acquired drug resistance. J. Am. Chem. Soc 138, 16046–16055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Feng Z, Wang H, Wang S, Zhang Q, Zhang X, Rodal AA, and Xu B (2018). Enzymatic assemblies disrupt the membrane and target endoplasmic reticulum for selective cancer cell death. J. Am. Chem. Soc 140, 9566–9573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Feng Z, Wang H, and Xu B (2018).Instructed assembly of peptides for intracellular enzyme sequestration. J. Am. Chem. Soc 140, 16433–16437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wu H, and Fuxreiter M (2016). The structure and dynamics of higher-order assemblies: amyloids, signalosomes, and granules. Cell 165, 1055–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Boeynaems S, Alberti S, Fawzi NL, Mittag T, Polymenidou M, Rousseau F, Schymkowitz J, Shorter J, Wolozin B, Van den Bosch L, et al. (2018). Protein phase separation: a new phase in cell biology. Trends Cell Biol. 28, 420–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhou J, and Xu B (2015). Enzyme-instructed self-assembly: a multistep process for potential cancer therapy. Bioconjug. Chem 26, 987–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kuang Y, Shi J, Li J, Yuan D, Alberti KA,Xu Q, and Xu B (2014). Pericellular hydrogel/nanonets inhibit cancer cells. Angew. Chem. Int. Ed 53, 8104–8107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Du X, Zhou J, Wang J, Zhou R, and Xu B (2017). Chirality controls reaction-diffusion of nanoparticles for inhibiting cancer cells. ChemNanoMat 3, 17–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Feng Z, Wang H, Zhou R, Li J, and Xu B (2017). Enzyme-instructed assembly and disassembly processes for targeting downregulation in cancer Cells. J. Am. Chem. Soc 139, 3950–3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li J, Bullara D, Du X, He H, Sofou S,Kevrekidis IG, Epstein IR, and Xu B (2018). Kinetic analysis of nanostructures formed by enzyme-instructed intracellular assemblies against cancer cells. ACS Nano 12, 3804–3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li J, Shi J, Medina JE, Zhou J, Du X, Wang H, Yang C, Liu J, Yang Z, Dinulescu DM, and Xu B (2017). Selectively inducing cancer cell death by intracellular enzyme-instructed self-assembly (EISA) of dipeptide derivatives. Adv. Healthc. Mater 6, 1601400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li J, Kuang Y, Shi J, Zhou J, Medina JE, Zhou R, Yuan D, Yang C, Wang H, Yang Z, et al. (2015). Enzyme-instructed intracellular molecular self-assembly to boost activity of cisplatin against drug-resistant ovarian cancer cells. Angew. Chem. Int. Ed 54, 13307–13311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhou J, Du X, Li J, Yamagata N, and Xu B (2015). Taurine boosts cellular uptake of small D-peptides for enzyme-instructed intracellular molecular self-assembly. J. Am. Chem. Soc 137, 10040–10043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Du X, Zhou J, Wang H, Shi J, Kuang Y, Zeng W, Yang Z, and Xu B (2017). In situ generated D-peptidic nanofibrils as multifaceted apoptotic inducers to target cancer cells. Cell Death Dis 8, e2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Feng Z, Wang H, Du X, Shi J, Li J, and Xu B (2016). Minimal C-terminal modification boosts peptide self-assembling ability for necroptosis of cancer cells. Chem. Commun. (Camb.) 52, 6332–6335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhou J, Du X, and Xu B (2016). Regulating the rate of molecular self-assembly for targeting cancer cells. Angew. Chem. Int. Ed 55, 5770–5775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Li J, Li X, Kuang Y, Gao Y, Du X, Shi J, and Xu B (2013). Self-delivery multifunctional anti-HIV hydrogels for sustained release. Adv. Healthc. Mater 2, 1586–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhou J, Du X, Yamagata N, and Xu B (2016). Enzyme-instructed self-assembly of small D-peptides as a multiple-step process for selectively killing cancer cells. J. Am. Chem. Soc 138, 3813–3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li J, Du X, Powell DJ, Zhou R, Shi J, He H, Feng Z, and Xu B (2018). Down regulating proteolysis to enhance anticancer activity of peptide nanofibers. Chem. Asian J 13, 3464–3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Du X, Zhou J, Wu L, Sun S, and Xu B (2014). Enzymatic transformation of phosphate decorated magnetic nanoparticles for selectively sorting and inhibiting cancer cells. Bioconjug. Chem 25, 2129–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhou R, Kuang Y, Zhou J, Du X, Li J, Shi J, Haburcak R, and Xu B (2016). Nanonets collect cancer secretome from pericellular space. PLoS One 11, e0154126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yamagata N, Chen X, Zhou J, Li J, Du X, and Xu B (2017). Enzymatic self-assembly of an immunoreceptor tyrosine-based inhibitory motif (ITIM). Org. Biomol. Chem 15, 5689–5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tian Y, Wang H, Liu Y, Mao L, Chen W, Zhu Z, Liu W, Zheng W, Zhao Y, Kong D, et al. (2014). A peptide-based nanofibrous hydrogel as a promising DNA nanovector for optimizing the efficacy of HIV vaccine. Nano Lett 14, 1439–1445. [DOI] [PubMed] [Google Scholar]
- 125.Liu Y, Lehn J-M, and Hirsch AKH (2017). Molecular biodynamers: dynamic covalent analogues of biopolymers. Acc. Chem. Res 50, 376–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Aida T, Meijer EW, and Stupp IW (2012). Functional supramolecular polymers. Science 335, 813–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhou H-C, Long JR, and Yaghi OM (2012). Introduction to metal–organic frameworks. Chem. Rev 112, 673–674. [DOI] [PubMed] [Google Scholar]
- 128.Menger FM, Lee SS, and Tao X (1995). Noncovalent synthesis of organic fibers. Adv. Mater 7, 669–671. [Google Scholar]
- 129.Mart RJ, Osborne RD, Stevens MM, and Ulijn RV (2006). Peptide-based stimuli responsive biomaterials. Soft Matter 2, 822–835. [DOI] [PubMed] [Google Scholar]
- 130.Hirst AR, Roy S, Arora M, Das AK, Hodson N, Murray P, Marshall S, Javid N, Sefcik J, Boekhoven J, et al. (2010). Biocatalytic induction of supramolecular order. Nat. Chem 2, 1089–1094. [DOI] [PubMed] [Google Scholar]
- 131.Kumar M, Ing NL, Narang V, Wijerathne NK, Hochbaum AI, and Ulijn RV (2018). Amino-acid-encoded biocatalytic self-assembly enables the formation of transient conducting nanostructures. Nat. Chem 10, 696–703. [DOI] [PubMed] [Google Scholar]
- 132.Pappas CG, Shafi R, Sasselli IR, Siccardi H, Wang T, Narang V, Abzalimov R, Wijerathne N, and Ulijn RV (2016). Dynamic peptide libraries for the discovery of supramolecular nanomaterials. Nat. Nanotechnol 11, 960–967. [DOI] [PubMed] [Google Scholar]
- 133.Williams RJ, Smith AM, Collins R, Hodson N, Das AK, and Ulijn RV (2009). Enzyme-assisted self-assembly under thermodynamic control. Nat. Nanotechnol 4, 19–24. [DOI] [PubMed] [Google Scholar]
- 134.Zhou J, Du X, Chen X, Wang J, Zhou N, Wu D, and Xu B (2018). Enzymatic self-assembly confers exceptionally strong synergism with NF-kappa B targeting for selective necroptosis of cancer cells. J. Am. Chem. Soc 140, 2301–2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tang W, Zhao Z, Chong Y, Wu C, Liu Q, Yang J, Zhou R, Lian Z-X, and Liang G (2018). Tandem enzymatic self-assembly and slow release of dexamethasone enhances its antihepatic fibrosis effect. ACS Nano 12, 9966–9973. [DOI] [PubMed] [Google Scholar]
- 136.Agarwal S, and Franco E (2019). Enzyme-driven assembly and disassembly of hybrid DNA–RNA nanotubes. J. Am. Chem. Soc 141, 7831–7841. [DOI] [PubMed] [Google Scholar]