Abstract
Numerous human diseases involve abnormal metabolism, and proton exchange is an effective source of magnetic resonance imaging (MRI) contrast for assessing metabolism. One MRI technique that capitalizes on proton exchange is R1 relaxation in the rotating frame (R1ρ). Here we investigated the sensitivity of R1ρ to various proton-exchange mechanisms at spin-lock pulses within FDA safety guidelines for radiofrequency-induced heating. We systematically varied pH known to change the rate of proton exchange as well as the glucose and lysine concentrations, thus changing the number of amide, hydroxyl, and amine exchangeable sites in a series of egg-white albumin phantoms. The resulting effects on quantitative relaxation time measurements of R1ρ, R1, and R2 were observed at 3T. Using spin-lock amplitudes available for human imaging (less than 23.5μT) at near physiologic temperatures, we found R1ρ was more sensitive to physiologic changes in pH than to changes in glucose and lysine concentrations. In addition, R1ρ was more sensitive to pH changes than R1 and R2. Models of proton exchange fit to the relaxation measurements suggest that amide groups were the primary source of pH sensitivity. Together, these experiments suggest an optimal spin-lock amplitude for measuring pH changes while not exceeding FDA subject heating limitations.
Keywords: Multi-parametric imaging, R1ρ dispersion, physical phantom, temperature, glucose, lysine, pH
INTRODUCTION
Proton exchange is being increasingly utilized as a source of intrinsic contrast in magnetic resonance imaging (MRI). In tissue, water protons are continuously exchanged with protons on amide (O=C-NH-), hydroxyl (-OH) and amine (-NH2) groups abundant in macromolecules such as proteins. The magnetization of protons in water and macromolecules is sensitive to this exchange. The rate of exchange (kex) depends on the forward (k1) and reverse (k-1) rate constants and can range from 10–500s−1 for amide protons to 500–10,000s−1 for hydroxyl and amine protons1.
| (1) |
The two most commonly used MRI techniques sensitive to proton exchange are R1 relaxation in the rotating frame (R1ρ) and chemical exchange saturation transfer (CEST). These techniques have been used to study several psychiatric and neurological disorders including Alzheimer’s Disease2–4, Parkinson’s Disease 5, Huntington’s Disease 6, panic disorder 7, and bipolar disorder 8,9. Both static8,9 and functional7 changes in R1ρ relaxation rates have been observed, which have been interpreted to be driven at least in part by abnormalities in brain pH. However, solvent concentrations or other macromolecules could also contribute to the observed changes. Relatively little work has been done to characterize R1ρ relaxation rates using commonly employed clinical MRI field strengths (e.g. 3T) and spin-lock amplitudes that are within the Food and Drug Administration (FDA) radiofrequency heating limits.
MRI pulse sequences for acquiring R1ρ typically involve a spin-lock block comprised of the following: 1) a 90° radiofrequency (RF) pulse tipping the magnetization into the transverse plane; 2) a spin-lock RF pulse of constant amplitude; and 3) a second 90° RF pulse restoring the magnetization to the longitudinal direction10. To help minimize B1 and B0 sensitivity of the spin-lock block, adiabatic 90° RF pulses can be used11, and the phase of the spin-lock pulse can be alternated for each half of its duration12. R1ρ relaxation rate can be calculated from two or more images with different spin-lock pulse durations (TSL) using Equation 2:
| (2) |
where STSL is the signal collected after applying the spin-lock block and S0 is the signal without applying the spin-lock pulse. Previous work has attributed the sensitivity at lower spin-lock amplitudes to amide exchange and other low frequency processes13,14 while work at ultra-high magnetic fields and large spin-lock amplitudes (greater than 23.5μT) have found that spin-lock pulses are sensitive to hydroxyl and amine protons15,16.
While a number of studies have aimed to discern factors affecting R1ρ relaxation rates, these studies have almost exclusively been conducted with high spin-lock amplitudes greater than 23.5μT (equivalent to a frequency of more than 1000 Hz) and/or using ultrahigh-field (7T or higher) small bore scanners where specimen heating was not a concern or was not monitored17–19. Such studies have identified a number of important factors20 contributing to R1ρ relaxation rates including solute concentration (glucose21 and glutamate17), pH22, temperature23 and molecular rotational rate24. Other contributions to R1ρ relaxation rates have been identified including interactions between large macromolecules and water via dipolar and diffusion relaxation pathways25,26. Moreover, outcomes from R1ρ relaxation rate studies depended on both field strength27 and the amplitude of the applied spin-lock pulse28. To better understand the contributions from several sources including proton exchange, diffusion, temperature, and pH Spear and Gore recently undertook an extensive study to explore their effect on R1ρ relaxation rates29. They showed that temperature and pH were two key factors that influenced the rate of proton exchange. In addition, they showed that the multiple pools of exchanging protons behave independently and that multiple R1ρ measurements can be used to derive an exchange rate constant.
The goal of this work was to better understand the role of proton exchange with compounds abundant in brain tissue and thought to potentially contribute to R1ρ changes by building upon the fundamental framework of Spear and Gore. These studies may be helpful in understanding the findings previously observed in prior human studies at 3T that have identified differences in R1ρ relaxation times between individuals with psychiatric and neurological disorders and controls. Using well-controlled phantoms, three experiments were performed to investigate the effects of changing the chemical environment in the presence of proteins on R1ρ relaxation rates. This was done by adjusting pH (known to alter rate of amide proton transfer) within the phantom as well as changing the number of amide, hydroxyl, and amine exchangeable sites by adding glucose and lysine in the physiological range of concentration. These studies were performed in a 3T MRI scanner across a range of temperatures (9–34°C) while maintaining the specific absorption rate (SAR) levels within allowed FDA levels. Based on our previous R1ρ imaging work in phantoms and animals30, we hypothesized that at 3T R1ρ would exhibit a greater sensitivity to pH as compared to the other metabolites studied.
MATERIALS & METHODS
Phantom Preparation
Phantoms were created that allowed for the systematic variation of pH, glucose, and lysine concentrations while controlling temperature. The spherical shell of a Magphan® EMR051 phantom (The Phantom Laboratory; Greenwich, NY) was used for this study. The interior of each phantom housed three fiberglass plates that were printed to hold seven 50mL Falcon tubes inside the sphere. The plugs at either end of the phantom were replaced with nylon barbs used to connect tygon tubing to the phantom providing a constant temperature water bath to the tubes inside the phantom. The other end of the hoses was connected to a water recirculating system (Endocal RTE-110, NESLAB) housed outside of the scan room (Supplementary Materials, Figure S1).
Experiments were performed using four separate phantoms, which contained various concentrations of pH, glucose, lysine, and water (control) added to 45 mL liquid egg-white albumin (HyVee generic brand, 0.124mM of proteins). The first phantom tested the effects of pH on R1ρ. Varying amounts of hydrochloric acid (HCl, 1N) were added to different Falcon tubes to produce pH values across a physiologically relevant range (pH 6.01, 6.51, 7.02, 7.25, 7.51, 7.79 and 8.0). pH was confirmed using an Accumet AB15 pH meter (Fisher Scientific; Pittsburg, PA). The second phantom tested the effects of changing the concentration of hydroxyl exchange sites by changing glucose concentration. Glucose concentration was varied from 0, 5, 10, 15, 20, 30, and 40 mM (pH 9.03 for all vials). The third phantom tested the effects of changing the number of amine exchange sites. Lysine concentration was varied from 0, 0.5, 1.0, 1.5, 2.0, 2.5, and 3.0 mM (pH 9.03 for all vials). The pH, glucose, and lysine concentrations were selected to encompass the physiological range expected in the in vivo brain. The first three phantoms allowed for the exploration of proton exchange mediated by changing the rate of exchange versus changes in the number of exchangeable sites. The fourth phantom was a water control phantom that contained two sets of three vials diluted with either water or HCl (1N) (1, 2, and 3mL). The seventh vial contained only liquid egg whites. pH was then measured in the tubes, and the pH for the water-containing vials was 9.03, 9.04, and 9.05 while the HCl-containing vials had pH values of 7.42, 6.46 and 5.93, respectively. The tube containing only egg whites had a pH of 9.00.
MR Imaging
All MR imaging was performed on a Siemens 3T TIM Trio MRI scanner (Erlangen, Germany). The spherical phantom was placed in a circularly polarized head coil. The thermostatic bath was then set to the appropriate temperature (9, 22, 34°C) for the imaging experiment and allowed to circulate for 30 minutes before initiating imaging. Temperature within the phantom was measured at the start and end of each imaging experiment. Imaging included a three-plane localizer, B0 mapping, B1 mapping, and R1ρ, R2, and R1 relaxation rate mapping. R1ρ mapping was collected in the coronal plane using a continuous-wave spin-lock preparation pulse and adiabatic excitation in a turbo spin-echo sequence with the following parameters: TR = 5000 ms, TE = 12 ms, slice thickness = 5.0 mm, FOV = 22.0×22.0 cm, matrix = 256×256, bandwidth = 130 Hz/pixel, time of spin-lock (TSL) = 0 and 100 ms, turbo factor = 4. Johnson et al. have shown that optimal SNR can be achieved with two spin-lock times31, thus the spin-lock times here were chosen while maintaining SAR within FDA limits. At room temperature, the egg-white medium had an R1ρ of 3.92. R2 mapping was acquired using Malcom-Levitt (MLEV) preparation with four refocusing hard pulses, which provided effective echo times of 0 and 100 ms. All other imaging parameters were the same as the R1ρ based turbo spin-echo sequence. R1 mapping was collected using a coronal inversion recovery (IR) sequence with the following parameters: TR = 5000 ms, TE = 12 ms, slice thickness = 5.0 mm, FOV = 22.0×22.0 cm, matrix = 256×256, Bandwidth = 130 Hz/pixel. Sequential acquisitions were performed with TI = 50, 150, 250, 450, 750, and 1150 ms. Three separate measurements were obtained for R1ρ, R2 and R1 in each phantom preparation and temperature with different vial placements for each measurement.
For the pH, glucose, and lysine phantoms, R1ρ imaging was performed at four spin-lock field strengths (4.7, 9.4, 14.1, and 18.8μT) corresponding to frequencies of 200, 400, 600 and 800 Hz to assess sensitivity across a variety of spin-lock amplitudes that may be applied in vivo. These phantoms were imaged at 9, 22, and 34°C. The water control phantom was assessed using only the 9.4μT spin-lock pulse at 22°C.
MR Image Analysis
To generate the relaxation maps, voxel-by-voxel calculation of the relaxation rates was performed using MATLAB (MathWorks; Natick, MA). R1ρ and R2 were calculated by fitting the respective TSL or TE to a single exponential decay model (Eq. 2), and R1 was calculated using a single exponential recovery model as specified in Equation 3.
| (3) |
Once the relaxation maps were generated, regions of interest (ROI) were manually defined for each tube in MATLAB (Fig. 3). The resulting ROIs were placed in regions where the B0 field map was between 0.5–1.2Hz and the B1 map was between ±10°. The median value and standard deviation were obtained from each ROI. This was done independently for each of the repeated measurements, relaxation property, and phantom preparations. This allowed us to evaluate both the sensitivity as well as the reliability of the measurements. To visualize the results, the plots were normalized to one of the metabolite concentrations. The relaxation parameters were normalized to the results obtained at 7.02 (pH), 15mM (glucose), and 1.5mM (lysine), respectively. The change in the relaxation rates relative to these reference concentrations were plotted as percent changes. Error propagation was used to translate the standard deviation at each vial onto the percentage change plots.
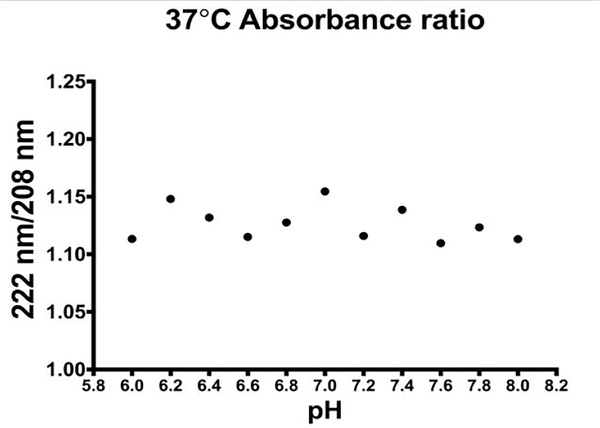
Figure 3.
Results from the circular dichroism experiment showing the ratio of absorbance between 222 and 208 nm wavelengths. The results show no change in the protein folding across the pH range studied.
Circular Dichroism Experiment
To determine if the proteins in the pH phantom were denatured as result of adding HCl to the egg white albumin, circular dichroism measurements were obtained. Eleven vials (50mL) were produced with egg white albumin, phosphate buffered saline solutions at pH ranging from 6 to 8 with steps of 0.2 by doping the solutions with HCl and sodium hydroxide (NaOH). At temperatures similar to those used in the imaging experiments (4, 22, 37°C), circular dichroism spectra were obtained in the samples at wavelengths 190–260. These measurements were repeated 5 times. A ratio of absorbance at 222 and 208nm was used to assess if changes in protein folding were observed32.
Two-Pool Model Fit to Relaxation Measurements
To estimate the type of solute protons responsible for the pH sensitivity of the R1ρ measurements, a two-pool proton exchange model, based on the work of Chopra et al.27 and adapted by Cobb et al.33,34, was fit to the R1ρ relaxation measurements. This model describes R1ρ for protons undergoing two-site exchange as follows 29,35:
| (4) |
In Equation 4, R2 is the transverse relaxation rate for the system and R1ρ∞ is the value of R1ρ when then spin lock amplitude approaches infinity. R1b (=1/T1b) and R2b (=1/T2b) are the longitudinal and transverse relaxation rates of bound protons in the laboratory frame. The proton exchange rate is rb and Δωb is the chemical-shift of the solute protons (from the water frequency) while ω1 is the applied spin-lock amplitude.
For each pH value, Equation 4 was fit to the experimental R1ρ measurements as a function of the applied spin-lock amplitude, with Δωb and rb set to values appropriate for exchange with amide protons (Δωb =3.35ppm, rb =20s−1)36, hydroxyl groups (Δωb =1.25ppm, rb =1500s−1)36, and amines (Δωb =3ppm, rb =4000s−1)36. The relaxation rates of amide protons (R1b = 0.51 s−1 and R2b = 4.19 s−1), glucose (R1b = 0.20 s−1 and R2b= 0.50 s−1), and lysine (R1b = 0.50 s−1 and R2b = 1.00 s−1) as measured by Sykes et al. for ovalbumin at B0=100MHz (2.35T)37 were used. The fitnlm function in MATLAB was then used to generate a non-linear fit of Equation 4 to the R1ρ dispersion measurements at each pH, solving for , R2, and rb. The values specified above for rb was used as an initial condition for the fitting procedure while initial values were assigned to R2 and based on the R2 and R1 relaxation rates observed in the MR imaging experiments. These values were just initial conditions and the parameters were then allowed to float during model fitting. The resulting estimates for the relaxation rates were compared to those obtained experimentally.
RESULTS
Example R1ρ relaxation rate maps obtained in the pH, glucose, and lysine phantoms are shown in Figure S2 of the Supplementary Materials. ROI based measurements obtained from these maps for R1ρ, R2, and R1 relaxation rates are summarized below. Examples of the B0 maps are shown in Figure S3 of the Supplementary Materials.
pH Phantom
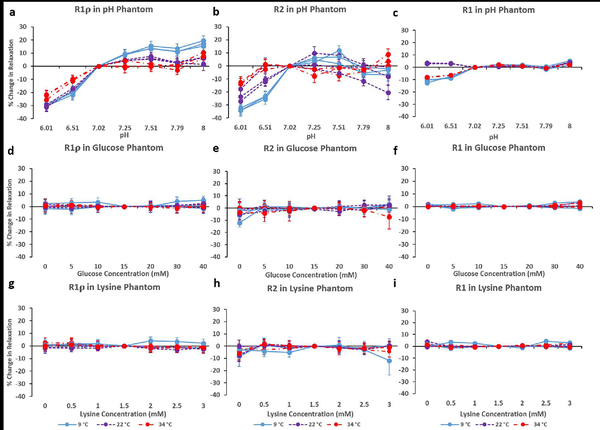
R1ρ mapping of the pH phantom showed the expected sensitivity to pH. Relaxation rate percent change plots for R1ρ with 400Hz spin-lock amplitude are shown in Figure 1a. The sensitivity to pH was observed across the entire experimental temperature range and at 34°C R1ρ showed the greatest sensitivity to pH changes as compared to R2 and R1 relaxation. Changes in R1ρ relaxation rates did show a subtle dependence on temperature with a larger decrease observed at 9°C (approx. −30%) as compared to 34°C (approx. −25%) across the pH range studied (6.0–8.0). The R1ρ relaxation rates ranged from 2.18 to 3.7 s−1 at 9°C and from 2.41 to 3.4 s−1 at 34°C. Little difference was observed between the R1ρ relaxation rates at 34°C and 22°C. In the water control phantom, small changes in the water concentration did not change the R1ρ relaxation rates (Supplementary Materials Figure S4).
Figure 1.
Along the columns, change in R1ρ and R2 and R1 values are shown for the pH (a,b,c), glucose (d,e,f), and lysine (g,h,i) phantoms respectively, Measurements were obtained at three temperatures (9, 22, and 34 °C) and three independent measurements were obtained for each condition and relaxation parameter. Data for 9 °C is shown as a solid line (light blue), 22 °C is a dashed line (purple), and 34 °C is a dot/dashed line (red).
R2 relaxation rates were sensitive to pH in the phantom similar to those observed for R1ρ relaxation rates (Figure 1b). However, the R2 experiments showed a greater temperature dependence. A 35% decrease in R2 relaxation rates at pH 6.0 relative to pH 7.02 was observed at 9°C, which as reduced to 12% at 34°C. Only small variations (approx. 5%) in the R2 relaxation rates were observed above pH 7.0. Figure S5b in the Supplementary Materials shows the temperature sensitivity for the R2 measurements.
The R1 relaxation rates showed very little sensitivity to pH with decreases of less than 10% between pH 6.0 and 7.0 (Figure 1c). In addition, the pH sensitivity did not change with temperature.
Glucose Phantom
By adding varying concentrations of glucose to liquid egg-white solutions, the number of hydroxyl exchangeable sites was increased. Figure 1 shows the relative change plots of the relaxation rates acquired with respect to temperature. R1ρ relaxation rates showed very little change in response to glucose concentration at three temperatures studied (Figure 1d). Across the glucose concentration range studied in this experiment, R1ρ relaxation rates showed changes of 6%, 4%, and 5% at 9, 22, and 34°C respectively. Similar small changes were observed for R2 (Figure 1e) and R1 (Figure 1f) relaxation rates in the phantom at 34°C. At lower temperatures, variation in the R2 relaxation rates were observed between the tubes containing 0 and 5mM concentrations of glucose, but little change was observed between 5 and 40 mM.
Lysine Phantom
Fast exchanging amine protons were added to liquid egg-white solutions by adding lysine at varying concentrations. All three of the quantitative relaxation measures (R1ρ, R2, and R1) showed little sensitivity to the lysine concentrations added to the various tubes in the phantom (Figure 1, g–i). R1ρ showed had 4% change across the full concentration range used in this study at 34°C.
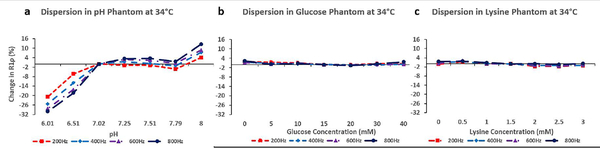
Dispersion Data
The impact of the R1ρ spin-lock pulse amplitude was also explored in this study. The overall RF energy deposited in the body is limited by the FDA and practically, this limits the maximum amplitude of the spin-lock pulse to be 1000Hz or less to keep the RF levels below the FDA limit in a clinically acceptable acquisition time. The sensitivity of R1ρ to changes in pH, glucose, and lysine concentrations was evaluated for spin-lock amplitudes ranging from 4.7 to 18.8 μT (200–800Hz) to determine if the sequence was sensitive to different exchange rates based on the amplitude of the spin-lock pulse. In Figure 2 the relative changes in R1ρ relaxation rates are shown for each phantom at 34°C. For R1ρ, the 18.8 μT (800Hz) spin-lock produced the greatest change (approx. 43%) in R1ρ from pH 7.01 to 6.01. The other spin-lock amplitudes also showed pH sensitivity but with a smaller change of 40% (14.2 μT), 35% (9.4 μT) and 20% (4.7 μT). R1ρ relaxation rates showed no sensitivity to glucose and lysine in the phantoms across all the spin-lock amplitudes used in this study.
Figure 2.
Plots of relative change in R1ρ at 34 °C with varying spin-lock frequency (dispersion). R1ρ dispersion is shown for the pH (a), glucose (b) and lysine (c) phantoms.
Circular Dichroism Measurements
To discern the mechanism underlying the sensitivity of R1ρ imaging to pH, circular dichroism measurements were obtained. Figure 3 shows the absorbance ratio 222nm/208nm as a function of pH at 37°C. Across the pH range, there was only a slight variance in the absorption at 222nm when compared with the 208 nm reference wavelength. This means the absorption by the helical structures remained constant throughout the experiments. Thus, there was little or no denaturation of the proteins, suggesting the pH sensitivity of R1ρ is not likely due to changes in protein conformation.
Two-Pool Model Fit to Relaxation Measurements
To evaluate the type of exchange processes contributing to the observed R1ρ changes, the two-pool model described in Equation 4 was fit using parameters for amide protons, hydroxyl groups, and amines. The model behavior was only reasonable for the parameters associated with amide exchange. The model for amide exchange generated similar trends for the R2 measurements as observed in the phantom (Table 1). Table 1 also includes the R2 and R1 relaxation rates measured from the imaging experiments for comparison. An error of approximately 8% was observed for the R2 values generated from the model as compared to the experimental results. Measured R1ρ values were consistently between measured R2 and calculated for the same conditions. The model fit for the measurements were approximately a factor of 4 shorter than the observed R1 values in the phantom. The model behavior for parameters suitable for amines and hydroxyl groups generated unrealistic values for the fitted parameters (i.e. < 0, or extremely large rb) and thus the corresponding models were considered to be invalid.
Table 1:
Two-Pool model estimates for exchange rate (rb), transverse relaxation rate (R2), and . R1ρ, R1, and R2 relaxation rates measured from the imaging experiments at 34°C are shown as well.
| pH | Phantom R1ρ (s−1)* 800 Hz SL | Phantom R1 (s−1)* | Model Fit (s−1)* | Phantom R2 (s−1)* | Model Fit R2(s−1)* | Exchange Rate rb (s−1)* |
|---|---|---|---|---|---|---|
| 6.01 | 1.8±0.03 | 0.45±0.01 | 1.4±0.17 | 3.5±0.03 | 3.1±0.12 | 225±67 |
| 6.51 | 2.1 ±0.09 | 0.46±0.01 | 1.6±0.24 | 4.1±0.06 | 3.7±0.17 | 220±76 |
| 7.02 | 2.6±0.06 | 0.5±0.01 | 2.1±0.31 | 4.1±0.03 | 3.8±0.16 | 281±62 |
| 7.25 | 2.7±0.06 | 0.51±0.01 | 2.2±0.34 | 3.9±0.14 | 3.7±0.14 | 331±148 |
| 7.51 | 2.7±0.09 | 0.51±0.02 | 2.3±0.23 | 3.9±0.11 | 3.8±0.13 | 261±105 |
| 7.79 | 2.6±0.05 | 0.49±0.01 | 2.2±0.37 | 3.9±0.005 | 3.6±0.16 | 310±83 |
| 8 | 2.9±0.16 | 0.52±0.02 | 2.5±0.65 | 4.2±0.20 | 3.9±0.33 | 276±304 |
Mean ± Standard Deviation
DISCUSSION
We have previously observed R1ρ relaxation rate changes in studies of psychiatric7–9 and neurological disorders2,5,6, which were interpreted as likely due to changes in brain pH and metabolism. This study was undertaken to better understand factors that may influence R1ρ relaxation rates including pH, other solutes (glucose and lysine), as well as temperature. Since the manipulation of a single compound in vivo is challenging, this study used a physical phantom to systematically vary compound concentrations. Imaging was performed on a 3T MRI scanner while using spin-lock amplitudes within FDA SAR limits. The key findings from the experiments showed that R1ρ relaxation was only sensitive to changes in pH while showing little to no sensitivity to glucose and lysine concentrations within the physiological range. Furthermore, the two-pool proton exchange modeling showed that this pH sensitivity was likely the result of amide proton exchange rates and not due to amine and hydroxyl exchange. R1ρ relaxation showed the greatest sensitivity to pH near body temperature as compared to R2 and R1 relaxation rates. Finally, the results of this work were in good agreement with the work by Spear and Gore, which also found that pH and temperature were two of the primary contributing factors to changes in proton exchange29. This work did extend the findings of Spear and Gore by using a more heterogenous protein media for the phantoms, a lower MR field strength, and a broader temperature range.
In the present study, a direct relationship between R1ρ relaxation rates and pH was observed between 6.0–7.0 at all spin-lock amplitudes (4.7 to 18.8 μT) used. This relationship was stronger as the spin-lock amplitude increased and appeared to be reaching a plateau at 18.8μT. The higher spin-lock amplitudes (14.1 and 18.8μT) showed sensitivity to pH up to 7.5, which were not evident at the lower spin-lock amplitudes. We restricted the maximum spin-lock amplitude to value consistent with clinical SAR limitations while maintaining maximum spin-lock duration of 100ms. These findings are in good agreement with previous studies reported in the literature (Table 2)17,22,30,38–40. A similar relationship between R1ρ relaxation rates and pH has been observed with spin-lock amplitudes greater than 25μT (1065Hz)39, but the greatest pH sensitivity were observed when the spin-lock amplitude was less than 23.5μT (1000Hz)40 by Kettunen et al. as well as Spear and Gore29. Magnotta et al. previously reported a similar relationship between R1ρ and pH in agar phantoms, which contained potassium phosphate buffer (KPB) and bovine serum albumin (BSA) at 3T30. The only difference between the results presented here and by Magnotta et al.30 was the wider range of the R1ρ sensitivity to pH likely due to differences in the proteins used for the phantom construction (BSA versus egg white ovalbumin). Finally, Willumsen observed exchange rate changes in the pH range 6.5–7.5 in aqueous ovalbumin media using deuterium experiments41.
Table 2:
Relationship between amine, hydroxyl, and amine exchange and relaxation times from the literature spin-lock amplitudes (B1) and static (B0) field used in this study are included. The correlations are summarized in the table as positive (+), negative (−), or no correlation (↔). n/r signifies values not reported while empty cells were not measured in the study
| Reference | Relaxation Trend with pH | |||||
|---|---|---|---|---|---|---|
| R1ρ | B1 (μT) | R2 | R1 | pH Range | B0 (T) | |
| Kettunen et al.22 | + | 56.4 | + | 7.27–6.81 a | 4.7 | |
| Magnotta et al.30 | + | 23.5 | 8.0–6.0b | 4.7 | ||
| Jokivarsi et al.38 | + | 40 | + | 7.31-n/r a | 4.7 | |
| Mäkelä et al.39 | + | 25.1–27.7 | + | - | 8.0–5.5 | 4.7 |
| Jin et al.17 | + | 2.9–94 | 7.5–6.0 | 9.4 | ||
| Koenig et al.45 | - | 5.2–4.0 | 0.1 | |||
| - | 7.4–6.5 | 0.1 | ||||
| + | 10–8.4 | 0.1 | ||||
| Meiboom et al.43 | + | ↔ | 8–6 | 0.7 | ||
| - | ↔ | 10–8 | 0.7 | |||
| Schilling et al.44 | + | ↔ | 7–6 | 2.4 | ||
| - | ↔ | 7–8 | 2.4 | |||
| Owusu et al. | + | 4.7–18.8 | + | + | 8.0–6.0 | 3.0 |
| Relaxation Trend with Hydroxyl Protons | Concentration | |||||
| Schuenke et al.42 | + | 5.0 | 5–20 mM | 7.0 | ||
| Jin et al.17 | + | 2.9–93.9 | 0–30 mM | 9.4 | ||
| Spear et al.29 | + | 1.2–235 | 0–200 mM | 4.7,7.0 | ||
| Owusu et al. | ↔ | 4.7–18.8 | ↔ | ↔ | 0–40 mM | 3.0 |
| Relaxation Trend with Amine Protons | ||||||
| Jin et al.17 | - | 2.9–93.9 | 0–20 mM b | 9.4 | ||
| Owusu et al. | ↔ | 4.7–18.8 | ↔ | ↔ | 0–3.0 mM | 3.0 |
in vivo data
agarose phantom.
R1ρ showed little sensitivity to glucose and lysine in the presence of protein in the current study. In addition, no significant changes were observed in R2 and R1 relaxation rates. These findings contrast with previous work by Schuenke et al.42 and Jin and Kim17 conducted at ultra-high field. Both studies found a relationship between R1ρ relaxation rates and hydroxyl concentrations by adjusting glucose levels while the work by Jin and Kim also found a relationship with amine group concentrations by adjusting glutamate levels. A possible explanation for the insensitivity to hydroxyl concentration changes in the current study may be due to the different field strengths employed across the studies. The strength of the main magnetic field affects the chemical shift of the solute protons. The chemical shift for the hydroxyl group hydrogen is close to water (approximately 100Hz at 3T) and higher field strengths will increase the chemical shift and thus the ability to differentiate these two pools. Thus, the sensitivity to proton exchange is dependent on the strength of the static field; the current study employed a 3T scanner, which is more than 2.5 times lower than the previous field strengths used. The effect of spin-lock amplitude on sensitivity to hydroxyl concentration changes was studied by Spear and Gore29. Between spin-lock amplitudes of 200 to 800Hz, the authors showed a 40% increase in R1ρ when the concentration of glucose was increased from 0 to 50mM. We were unable to detect this much change in R1ρ signal. The decrease in SNR from spin-lock amplitude may have contributed to the lack of detection. Furthermore, a likely explanation for the R1ρ insensitivity to changes in amine group concentrations in the current study is the low amplitude spin-lock pulses. Amine protons exchange at rates faster than that of amide protons and require higher spin-lock amplitudes for sensitivity than were achievable at 3T while adhering to FDA limits for subject heating.
The Two-Pool model based on Cobb et al.33 for proton exchange suggests that the sensitivity of R1ρ in these experiments to pH at spin-lock amplitudes available when performing human imaging studies (less than 23.5μT) is likely due to changes in amide exchange. The resulting model fit to the dispersion data was statistically significant and the resulting estimated parameters for R2 exhibited similar trends as observed in our imaging experiments. Furthermore, the model fit using hydroxyl, and amine exchange parameters did not generate reasonable estimates for the relaxation measurements. It should be noted that the resulting model fit did not change significantly based on the initial values assigned to rb, R2, and . The calculated values for were significantly shorter than the observed R1 values. This indicates that additional factors may need to be added to the two-pool model to fully characterize the relaxation behavior. Finally, no changes in protein structure were observed based on the circular dichroism experiments.
In addition to R1ρ sensitivity to pH, R2 and R1 relaxation also showed sensitivity to pH as summarized in Table 2. The R2 relaxation measurements conducted in this study showed sensitivity to pH. This work agrees with prior studies, which have shown similar R2 sensitivity to pH35,43,44. Meiboom et al. attributed the pH sensitivity of R2 relaxation rates to the breaking and forming of hydrogen bonds43. In addition, we observed the greatest R2 sensitivity to pH at 9 °C which agrees with work by Chopra et al.35 R1 relaxation rates also exhibited a dependence on pH at 9°C and 34°C with little change at 22°C in the range of pH 6.0–7.0. The literature has reported mixed results related to R1 sensitivity to pH with some prior studies reporting pH sensitivity39,45 while others finding no pH sensitivity43,45. These discrepancies may be related to the protein used, the ionic strength of the solutions in these experiments, the temperature, or pH range over which the measurements were made.
The results of this study suggest that changes in R1ρ relaxation rates that have been previously observed in various psychiatric and neurological disorders using spin-lock amplitudes of less than 23.5μT (1000Hz) may be due to changes in pH. Furthermore, we have previously shown a 500s−1 increase in the R1ρ relaxation rate in bipolar disorder as compared to controls8. Based on the results obtained at 34°C in this study, this magnitude of a R1ρ relaxation time change cannot be explained by hydroxyl and amine proton exchange based on the phantoms and modeling used in this study.
The pH, glucose and lysine concentration ranges tested here are broader than those occurring in brain tissue. However, there are limitations that need to be considered when extrapolating results from this phantom study to imaging of the brain in vivo. In egg white, there are two other proteins (ovotransferrin and ovomucoid) in solution apart from ovalbumin that exist in relatively large concentrations. The average acid dissociation constants (i.e. isoelectric point) of these proteins do not vary greatly (between 4–6). However, the brain expresses on the order of 15,000 proteins46, with a range of isoelectric points. Different isoelectric points are likely to alter the relative pH sensitivity R1ρ relaxation rate. Differences in temperature before and after scanning were minimized due to the re-circulator; however, temperature did drift slightly during the experiment. While this temperature change was limited to approximately 2° C, it may have added to some of the variations in the relaxation measurements observed in the phantoms. In biological tissue, however, proton exchange is not the only contributor to R1ρ change. Diffusion of protons through regions where magnetic susceptibility change have also been shown to affect R1ρ considerably independent of proton exchange47,48. Though this work was conducted at 7T, the effects of magnetic susceptibility are relevant at all static field strengths. Recently, Ali et al. have concluded that the sensitivity of R1ρ to macromolecular change is significant and thus must be considered 49. Due to pathophysiology, cellular density and metabolism will lead to changes in macromolecular concentration, which may also influence R1ρ relaxation times.
In conclusion, R1ρ is more sensitive to physiological changes in pH than glucose or lysine concentration in a protein at 3T. Near physiologic temperature, R1ρ is more sensitive to pH changes in the physiologic range than R2 and R1, although R2 and R1 also show some sensitivity to pH changes. R1ρ results also reflected the pH dependence of exchange in proteins. This suggests that changes in acidity in the presence of proteins may be an underlying physiologic response driving the changes in R1ρ observed in applications to neurologic or psychiatric disorders at 3T. Additionally, we show that spin-lock pulses at low amplitudes produce adequate contrast to see pH related changes in tissue, well below SAR limits.
Supplementary Material
Figure S1 a) Diagram of experimental setup for imaging with temperature control. The water circulation system is placed in the scanner control room and the phantom in the transmit-receive coil with tubing attached. Both phantom and coil are moved into the scanner bore before imaging. The directional arrows indicate the flow of water to and from the phantom. b) A cross-sectional view of the phantom with Falcon tubes inserted.
Figure S4 Relative change plot of R1ρ relaxation rate in separate egg-white phantom containing HCl and water. The relative change data were at 22 °C.
Figure S3 Example B0 maps of the (a) pH, (b) glucose and (c) lysine phantoms at 22 °C. The color scale represents the homogeneity of spins as the result of a 90-degree hard pulse. 0° represents a rightly tipped spin and ±40° represents over or under tipped spins according to the sign.
Figure S5 R1ρ (a) and R2 (b) relaxation rates in the pH phantom showing temperature dependence. Differences based on temperature are much more pronounced in the R2 relaxation rates as compared to R1ρ. The values plotted are averages of three repetitions at each temperature. Error bars here are based on standard deviations of the relaxation rates from the three repetitions at each pH.
Figure S2 Example of image quality obtained in the phantom for R1ρ imaging in the a) pH, b) glucose and c) lysine phantoms. An example ROI used for the measurements is shown in the vial with the pH measured as 7.25. The median value within the ROI was used for the relaxation measurements.
ACKNOWLEDGEMENTS
This work was supported by the National Institute of Mental Health (5R01MH11157802) and that of Biomedical Imaging and Bioengineering (5R01EB02201902).
ABBREVIATIONS
- R1
Longitudinal relaxation rate in the laboratory frame
- R2
Transverse relaxation rate in the laboratory frame
- R1ρ(ω)
Longitudinal relaxation rate in the rotating frame
- T1ρ
1/R1ρ
- TSL
Duration of the spin-lock pulse block
- CEST
Chemical exchange saturation transfer
- B0
Static magnetic field
- B1
Magnetic component of the radiofrequency field
- SAR
Specific absorption
- Glc
Glucose
- Glu
Glutamate
- HCl
Hydrochloric acid
- NaOH
Sodium hydroxide
- BSA
Bovine serum albumin
- kex
Proton exchange rate
Footnotes
ADDITIONAL INFORMATION
The authors declare that they have no competing interests.
R1ρ sensitivity to different exchange rates at clinically accessible (B0 ≤ 3T) field strengths is not well characterized and this work is to address that question for 3T. Multiparametric mapping of three phantoms with gradations of metabolite concentrations and pH values shows R1ρ sensitivity to physiologic pH range in protein solutions at 3T. Modeling of proton exchange with relaxation measures suggests amide proton exchange is the likely source of pH sensitivity.
REFERENCE
- 1.Bai Y, Milne JS, Mayne L & Englander SW Primary structure effects on peptide group hydrogen exchange. Proteins 17, 75–86 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haris M, McArdle E, Fenty M, Singh A, Davatzikos C, Trojanowski JQ, Melhem ER, Clark CM & Borthakur A Early marker for Alzheimer’s disease: hippocampus T1rho (T(1rho)) estimation. J Magn Reson Imaging 29, 1008–1012 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haris M, Singh A, Cai K, Nath K, Crescenzi R, Kogan F, Hariharan H & Reddy R MICEST: a potential tool for non-invasive detection of molecular changes in Alzheimer’s disease. J. Neurosci. Methods 212, 87–93 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haris M, Nath K, Cai K, Singh A, Crescenzi R, Kogan F, Verma G, Reddy S, Hariharan H, Melhem ER & Reddy R Imaging of Glutamate Neurotransmitter Alterations in Alzheimer’s Disease. NMR in biomedicine 26, 386 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haris M, Singh A, Cai K, Davatzikos C, Trojanowski JQ, Melhem ER, Clark CM & Borthakur A T1rho (T1ρ) MR imaging in Alzheimer’ disease and Parkinson’s disease with and without dementia. J Neurol 258, 380–385 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wassef SN, Wemmie J, Johnson CP, Johnson H, Paulsen JS, Long JD & Magnotta VA T1ρ imaging in premanifest Huntington disease reveals changes associated with disease progression. Mov. Disord (2015). doi: 10.1002/mds.26203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Magnotta VA, Johnson CP, Follmer R & Wemmie JA Functional t1ρ imaging in panic disorder. Biol. Psychiatry 75, 884–891 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson CP, Follmer RL, Oguz I, Warren LA, Fiedorowicz JG, Magnotta VA & Wemmie JA Brain abnormalities in bipolar disorder detected by quantitative T1ρ mapping. Mol. Psychiatry 20, 201–206 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson CP, Christensen GE, Fiedorowicz JG, Mani M, Shaffer JJ, Magnotta VA & Wemmie JA Alterations of the cerebellum and basal ganglia in bipolar disorder mood states detected by quantitative T1ρ mapping. Bipolar Disorders 20, 381–390 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sepponen RE, Pohjonen JA, Sipponen JT & Tanttu JI A method for T1 rho imaging. J Comput Assist Tomogr 9, 1007–1011 (1985). [DOI] [PubMed] [Google Scholar]
- 11.Sepponen RE Imaging method. (1996).
- 12.Yuan J, Li Y, Zhao F, Chan Q, Ahuja AT & Wang Y-XJ Quantification of T 1ρ relaxation by using rotary echo spin-lock pulses in the presence of B 0 inhomogeneity. Phys. Med. Biol 57, 5003 (2012). [DOI] [PubMed] [Google Scholar]
- 13.Zu Z, Afzal A, Li H, Xie J & Gore JC Spin-lock imaging of early tissue pH changes in ischemic rat brain. NMR in Biomedicine 31, e3893 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown RD & Koenig SH 1 /T1p and Low-Field 1 / T1 of Tissue Water Protons Arise from Magnetization Transfer to Macromolecular Solid-State Broadened Lines. Magn. Reson. Med 28, 145–152 (1992). [DOI] [PubMed] [Google Scholar]
- 15.Zong X, Wang P, Kim S-G & Jin T Sensitivity and Source of Amine-Proton Exchange and Amide-Proton Transfer Magnetic Resonance Imaging in Cerebral Ischemia. Magn. Reson. Med 71, 118–132 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin T, Wang P, Zong X & Kim S-G Magnetic resonance imaging of the Amine–Proton EXchange (APEX) dependent contrast. NeuroImage 59, 1218–1227 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin T & Kim S-G Characterization of non-hemodynamic functional signal measured by spin-lock fMRI. Neuroimage 78, 385–395 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duvvuri U, Poptani H, Feldman M, Nadal-Desbarats L, Gee MS, Lee WM, Reddy R, Leigh JS & Glickson JD Quantitative T1rho magnetic resonance imaging of RIF-1 tumors in vivo: detection of early response to cyclophosphamide therapy. Cancer Res. 61, 7747–7753 (2001). [PubMed] [Google Scholar]
- 19.Gröhn OHJ, Kettunen MI, Mäkelä HI, Penttonen M, Pitkänen A, Lukkarinen JA & Kauppinen RA Early detection of irreversible cerebral ischemia in the rat using dispersion of the magnetic resonance imaging relaxation time, T1rho. J. Cereb. Blood Flow Metab 20, 1457–1466 (2000). [DOI] [PubMed] [Google Scholar]
- 20.Gilani IA & Sepponen R Quantitative rotating frame relaxometry methods in MRI. NMR Biomed. 29, 841–861 (2016). [DOI] [PubMed] [Google Scholar]
- 21.Jin T, Mehrens H, Hendrich KS & Kim S-G Mapping brain glucose uptake with chemical exchange-sensitive spin-lock magnetic resonance imaging. J. Cereb. Blood Flow Metab 34, 1402–1410 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kettunen MI, Gröhn OHJ, Silvennoinen MJ, Penttonen M & Kauppinen RA Effects of intracellular pH, blood, and tissue oxygen tension on T1rho relaxation in rat brain. Magn Reson Med 48, 470–477 (2002). [DOI] [PubMed] [Google Scholar]
- 23.Peng ZY, Simplaceanu V, Lowe IJ & Ho C Rotating-frame relaxation studies of slow motions in fluorinated phospholipid model membranes. Biophysical Journal 54, 81–95 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Menon RS & Allen PS Solvent proton relaxation of aqueous solutions of the serum proteins alpha 2-macroglobulin, fibrinogen, and albumin. Biophysical Journal 57, 389–396 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.III, A. G. P., Fairbrother WJ, Cavanagh J, Skelton NJ & Rance M Protein NMR Spectroscopy, Second Edition: Principles and Practice. (Academic Press, 2006). [Google Scholar]
- 26.Knispel RR, Thompson RT & Pintar MM Dispersion of proton spin-lattice relaxation in tissues. Journal of Magnetic Resonance (1969) 14, 44–51 (1974). [Google Scholar]
- 27.Mäkelä HI, Vita ED, Gröhn OHJ, Kettunen MI, Kavec M, Lythgoe M, Garwood M, Ordidge R & Kauppinen RA B0 dependence of the on-resonance longitudinal relaxation time in the rotating frame (T1ρ) in protein phantoms and rat brain in vivo. Magnetic Resonance in Medicine 51, 4–8 (2004). [DOI] [PubMed] [Google Scholar]
- 28.Virta A, Komu M & Kormano M T1ρ of protein solutions at very low fields: Dependence on molecular weight, concentration, and structure. Magn. Reson. Med 37, 53–57 (1997). [DOI] [PubMed] [Google Scholar]
- 29.Spear JT & Gore JC New insights into rotating frame relaxation at high field. NMR Biomed. 29, 1258–1273 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Magnotta VA, Heo H-Y, Dlouhy BJ, Dahdaleh NS, Follmer RL, Thedens DR, Welsh MJ & Wemmie JA Detecting activity-evoked pH changes in human brain. Proc. Natl. Acad. Sci. U.S.A 109, 8270–8273 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson CP, Thedens DR & Magnotta VA Precision-guided sampling schedules for efficient T1ρ mapping. J Magn Reson Imaging 41, 242–250 (2015). [DOI] [PubMed] [Google Scholar]
- 32.Greenfield NJ Using circular dichroism spectra to estimate protein secondary structure. Nature Protocols 1, 2876–2890 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cobb JG Quantitave Proton Relaxation in the Rotating Frame With Magnetic Resonance Imaging. (Vanderbilt University, 2011). [Google Scholar]
- 34.Cobb JG, Xie J & Gore JC Contributions of chemical and diffusive exchange to T1ρ dispersion. Magnetic Resonance in Medicine 69, 1357–1366 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chopra S, McClung RED & Jordan RB Rotating-frame relaxation rates of solvent molecules in solutions of paramagnetic ions undergoing solvent exchange. Journal of Magnetic Resonance (1969) 59, 361–372 (1984). [Google Scholar]
- 36.van Zijl PCM & Yadav NN Chemical exchange saturation transfer (CEST): what is in a name and what isn’t? Magn Reson Med 65, 927–948 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sykes BD, Hull WE & Snyder GH Experimental evidence for the role of cross-relaxation in proton nuclear magnetic resonance spin lattice relaxation time measurements in proteins. Biophys J 21, 137–146 (1978). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jokivarsi KT, Hiltunen Y, Gröhn H, Tuunanen P, Gröhn OHJ & Kauppinen RA Estimation of the onset time of cerebral ischemia using T1rho and T2 MRI in rats. Stroke 41, 2335–2340 (2010). [DOI] [PubMed] [Google Scholar]
- 39.Mäkelä HI, Gröhn OHJ, Kettunen MI & Kauppinen RA Proton Exchange as a Relaxation Mechanism for T1 in the Rotating Frame in Native and Immobilized Protein Solutions. Biochemical and Biophysical Research Communications 289, 813–818 (2001). [DOI] [PubMed] [Google Scholar]
- 40.Kettunen MI, Sierra A, Närväinen MJ, Valonen PK, Ylä-Herttuala S, Kauppinen RA & Gröhn OHJ Low Spin-Lock Field T1 Relaxation in the Rotating Frame as a Sensitive MR Imaging Marker for Gene Therapy Treatment Response in Rat Glioma. Radiology 243, 796–803 (2007). [DOI] [PubMed] [Google Scholar]
- 41.Willumsen L Hydrogen-deuterium exchange in ovalbumin. C R Trav Lab Carlsberg 36, 247–263 (1967). [PubMed] [Google Scholar]
- 42.Schuenke P, Koehler C, Korzowski A, Windschuh J, Bachert P, Ladd ME, Mundiyanapurath S, Paech D, Bickelhaupt S, Bonekamp D, Schlemmer H-P, Radbruch A & Zaiss M Adiabatically prepared spin-lock approach for T1ρ-based dynamic glucose enhanced MRI at ultrahigh fields. Magn. Reson. Med 78, 215–225 (2016). [DOI] [PubMed] [Google Scholar]
- 43.Meiboom S, Luz Z & Gill D Proton Relaxation in Water. The Journal of Chemical Physics 27, 1411–1412 (1957). [Google Scholar]
- 44.Schilling A, Blankenburg F, Bernarding J, Heidenreich J & Wolf K Intracerebral pH affects the T2 relaxation time of brain tissue. Neuroradiology 44, 968–972 (2002). [DOI] [PubMed] [Google Scholar]
- 45.Koenig SH & Schillinger WE Nuclear Magnetic Relaxation Dispersion in Protein Solutions I. APOTRANSFERRIN. J. Biol. Chem 244, 3283–3289 (1969). [PubMed] [Google Scholar]
- 46.The human proteome in brain - The Human Protein Atlas. Available at: https://www.proteinatlas.org/humanproteome/brain (Accessed: 18th May 2018)
- 47.Spear JT, Zu Z & Gore JC Dispersion of relaxation rates in the rotating frame under the action of spin-locking pulses and diffusion in inhomogeneous magnetic fields: Dispersion of Relaxation Rates in the Rotating Frame. Magnetic Resonance in Medicine 71, 1906–1911 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spear JT & Gore JC Effects of diffusion in magnetically inhomogeneous media on rotating frame spin–lattice relaxation. Journal of Magnetic Resonance 249, 80–87 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ali SO, Fessas P, Kaggie JD, Zaccagna F, Houston G, Reid S, Graves MJ & Gallagher FA Evaluation of the sensitivity of R1ρ MRI to pH and macromolecular density. Magn Reson Imaging 58, 156–161 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 a) Diagram of experimental setup for imaging with temperature control. The water circulation system is placed in the scanner control room and the phantom in the transmit-receive coil with tubing attached. Both phantom and coil are moved into the scanner bore before imaging. The directional arrows indicate the flow of water to and from the phantom. b) A cross-sectional view of the phantom with Falcon tubes inserted.
Figure S4 Relative change plot of R1ρ relaxation rate in separate egg-white phantom containing HCl and water. The relative change data were at 22 °C.
Figure S3 Example B0 maps of the (a) pH, (b) glucose and (c) lysine phantoms at 22 °C. The color scale represents the homogeneity of spins as the result of a 90-degree hard pulse. 0° represents a rightly tipped spin and ±40° represents over or under tipped spins according to the sign.
Figure S5 R1ρ (a) and R2 (b) relaxation rates in the pH phantom showing temperature dependence. Differences based on temperature are much more pronounced in the R2 relaxation rates as compared to R1ρ. The values plotted are averages of three repetitions at each temperature. Error bars here are based on standard deviations of the relaxation rates from the three repetitions at each pH.
Figure S2 Example of image quality obtained in the phantom for R1ρ imaging in the a) pH, b) glucose and c) lysine phantoms. An example ROI used for the measurements is shown in the vial with the pH measured as 7.25. The median value within the ROI was used for the relaxation measurements.