Abstract
Maintenance of regulatory T (Treg) cells is crucial for the regulatory function of Treg cells in immune homeostasis and self-tolerance; however, the detailed underlying mechanisms remain elusive. In the current study, we found that the cytokine suppressor CIS is required for maintenance of Treg cell identity. Mice with Treg specific Cis-deficiency displayed aggravated experimental allergic asthma and in adulthood, developed splenomegaly, lymphadenopathy and spontaneous eosinophilic airway inflammation, accompanied by accumulation of effector memory helper T (TH) cells. Cis-deficiency led to loss of Foxp3 expression and decrease in suppressive function of Treg cells. Cis-deficient Treg cells expressed TH2 cell signature genes, Gata3, Irf4 and Il4, and excessive IL-4-STAT6 signals resulted in repressive chromatin modification in the Foxp3 locus and permissive modification in the Il4 loci. In vitro, blockade of IL-4 restored the expression of Foxp3 and the suppressive function of iTreg cells. Thus, we identified a novel feedback loop in stabilization of Treg cells and suppression of TH2 type inflammation in a Treg-intrinsic manner.
Keywords: Treg plasticity, TH2 cell, Cytokine induced SH-2 protein, Allergy
INTRODUCTION
CD4+CD25+Foxp3+ Treg cells play a crucial role in the maintenance of self-tolerance and immune homeostasis 1, 2. Treg cells are classified into two major subsets, thymus-derived Treg [tTreg, or natural Treg (nTreg)] cells and peripherally developed Treg (pTreg) cells. In vitro generated inducible Treg (iTreg) cells also possess suppressive function, but rapidly lose Foxp3 expression in vivo 3. Foxp3, the master and signature transcription factor of Treg cells, governs the development, self-maintenance and function of both tTreg and iTreg cells 4, 5. In humans, mutation or deletion of gene encoding Foxp3 leads to Treg cell dysfunction or deficiency, resulting in severe autoimmune diseases 6. In contrast, forced overexpression of Foxp3 in conventional T cells confers suppressive activity 7, 8, suggesting a pivotal role of Foxp3 in mediating Treg cell function. Manipulation of Treg cells is a promising immune therapy for autoimmune and inflammatory diseases, such as type 1 diabetes, graft versus host disease and inflammatory bowel disease 9-12. However, destabilization of Treg cells is associated with a variety of inflammatory diseases, including allergic disorders and presents a huge hurdle for their therapeutic potentials 13-17.
Signal transducer and activator of transcription (STAT) proteins are one of the most common cytokine signaling mediators, and cytokine-induced STAT signaling presents as a major factor affecting Treg cell stability. It has been shown that IL-6 activates STAT3 to drive the loss of Foxp3 expression in Treg cells, followed by the reprogramming of Treg towards TH17 cells 13, 16, 18. Additionally, IL-4-induced STAT6 signaling also destabilizes Treg cells 14, 15, 19, 20. Activated STAT3 and STAT6 undergo nuclear translocation, bind to the Foxp3 locus, and diminish Foxp3 expression 19, 21. Thus, the activation of STAT by inflammatory cytokines in milieu is one of the most important factors affecting Treg cell stability.
STAT signals are controlled by several negative regulatory strategies 22-24. The Suppressor of cytokine signaling (SOCS) family proteins are broadly involved in negative regulation of STAT signaling. In this study, we found that CIS (Cytokine induced SH-2 protein, also termed CIS1, SOCS and CISH) 25, a SOCS family member, plays an essential role in the maintenance of Treg cell identity. CIS has been shown to disrupt STAT5 signaling induced by cytokines and hormones 22-24, 26, 27 In addition, our previous study has showed that CIS also inhibits STAT3 and STAT6 signaling in CD4+ T cells 28. Both tTreg and iTreg cells express CIS 28. In the current study, we utilized Treg specific Cis-deficiency mouse model to investigate the role of CIS in Treg cell stability and function. We found that Treg-specific Cis-deficiency exacerbated experimental asthma and in adult mice, resulted in spontaneous eosinophilic airway inflammation. The exacerbated inflammatory responses were due to impaired suppressive function of Cis-deficient Treg cells, which was associated with activation of an endogenous TH2 cell programs. Excessive IL-4-STAT6 signals drove the loss of Foxp3 expression in Treg cells. Concluding, CIS stabilizes Treg cells through inhibition of a Treg-intrinsic TH2 program and negatively control allergic airway inflammation.
RESULTS
Treg-specific Cis-deficiency leads to spontaneous chronic inflammation with enhanced T effector programs
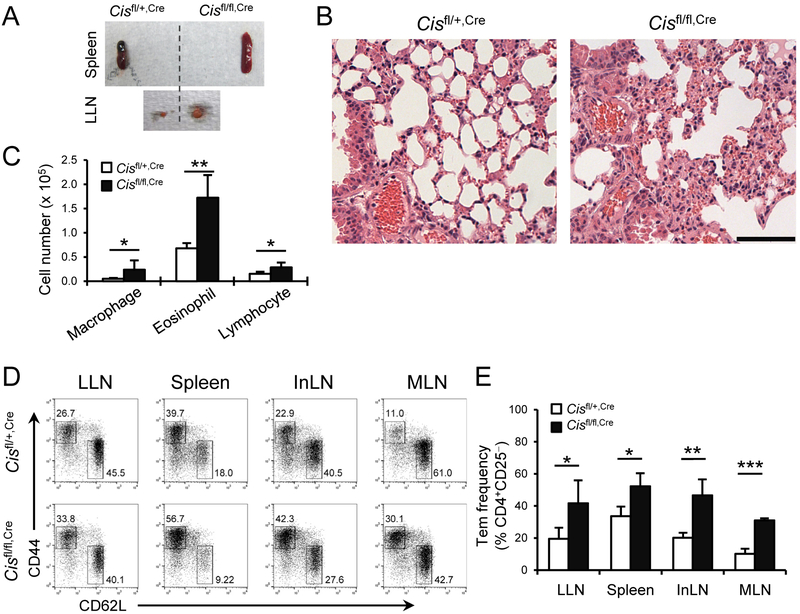
We have previously shown that Cis-deficiency on the B6 background leads to spontaneous airway disease with aging, associated with increased TH2 and TH9 cell responses 28. Genetic background is known to shape gene function. On a 129;B6 mixed background, Cis-deficiency caused severe splenomegaly and lymphadenopathy (Supplemental Fig. 1), resembling Treg-deficiency induced autoimmune diseases. Thus, we conceived that CIS may be required for the function of Treg cells. To validate this, we generated Treg specific Cis-deficient mice, Cisfl/fl;Foxp3-YfpCre (Cisfl/fl,Cre) mice on the 129;B6 F1 background (hereafter, the mice will be all on the 129;B6 F1 background) by breeding B6.Cisfl/fl,Cre with 129.Cisfl/+ (the latter was generated by crossing B6.Cisfl/fl with 129 mice for 7-8 generations). The deletion of Cis in Treg cells was confirmed by RT-quantitative (q) PCR (Supplemental Fig. 2). We first examined the inflammatory phenotypes and TH cell profiles in Cisfl/fl,Cre mice compared with their Cisfl/+,Cre littermates (Cisfl/+,Cre mice had a similar phenotype as Cisfl/fl and Cis+/+,Cre mice; data not shown). At the age of 6 months, Cisfl/fl,Cre mice exhibited apparent splenomegaly and lymphadenopathy (Fig. 1A), coupled with aggravated lung inflammation relative to the control mice (Fig. 1B). The lungs of Cisfl/fl,Cre mice displayed significantly more immune infiltrates including lymphocytes, eosinophils and macrophages (Fig. 1C). We also assessed the kidney and pancreas but did not observe appreciable immune infiltrates (Data not shown). Since Treg cells are essential in the maintenance of immune homeostasis, we asked whether Cis-deficiency in Treg cells affects T effector cell programs. We measured the frequencies of effector memory CD4+ T cells in 6-month old animals and found that Cisfl/fl,Cre mice displayed significantly increased CD4+CD25−CD44hiC62L− effector memory T cells in lung draining mediastinal (LLNs), inguinal and mesenteric lymph nodes and spleen compared with Cisfl/+,Cre mice (Fig. 1D, E). Furthermore, we analyzed the CD4+ T compartment by intracellular stain of respective signature cytokines, and found that Cisfl/fl,Cre mice had significantly higher frequencies and numbers of TH1, TH2 and TH17 cells in the lungs, LLNs and spleens than Cisfl/+,Cre mice (Fig. 2A, B). In Peryer’s patches, Treg-specific Cis-deficiency led to increased frequencies of TH2 cells but not TH1 and TH17 cells (Supplemental Fig. 3). Interestingly, the elevated inflammatory response in Cisfl/fl,Cre mice was associated with aging. Young (6-8-week old) Cisfl/fl,Cre and Cisfl/+,Cre mice both had very few TH1, TH2 and TH17 cells in the lung, and the numbers of these cells in the lung, LLN and spleen were comparable between the two groups of mice (Supplemental Figs. 4 and 5). At the age of 6-7 months, Cisfl/fl,Cre mice had increased serum levels of IL-5 and IL-13 but not IL-17 and IFNγ relative to Cisfl/+,Cre mice, whereas there were no significant differences in the serum cytokines of 6-8-week old Cisfl/fl,Cre and Cisfl/+,Cre mice (Supplemental Fig. 6). Collectively, these data indicate a pivotal role of CIS in maintaining the regulatory function of Treg cells during aging.
Fig. 1. Treg-specific Cis-deficient mice spontaneously develop splenomegaly, lymphadenopathy and chronic lung inflammation.
(A) Spleens and Lung associated mediastinal LNs (LLNs) of 6-month old Cisfl/+,Cre and Cisfl/fl,Cre mice. (B) H&E stain of left lung lopes from mice in (A). Scale bar, 100 μm. (C) Cellular profiles of lung infiltrates. (D) Cellular profiles of CD4+CD25− T cells in LLNs, spleens, inguinal lymph nodes (InLNs) and mesenteric lymph nodes (MLNs). (E) Statistical analysis of effector memory TH cells (Tem) in (D). (C, E) Data are a representative of 2 experiments (4-5 mice per group). Values are means and S.D. Student t test, * p< 0.05, ** p< 0.005, *** p< 0.0005.
Fig. 2. Treg-specific Cis-deficiency enhances T effector programs in secondary lymphoid tissues and lungs.
(A) Cytokine expression by CD4+ cells in the lung, LLN and spleen of 6-month old Cisfl/+,Cre and Cisfl/fl,Cre mice. (B, C) Statistical analysis of (A). Data are a representative of 2 experiments (4-5 mice per group). Values are means and S.D. Student t test, * p< 0.05, ** p< 0.005, *** p< 0.0005.
Treg-specific Cis-deficiency enhances experimental asthma
Germ-line Cis-deficiency on the B6 background causes spontaneous airway disease associated with elevated TH2 and TH9 responses 28. Our results above also demonstrate a critical role of Treg-specific Cis-deficiency in lung inflammation and immune homeostasis. We asked whether Treg-specific Cis-deficiency also plays a role in allergic airway inflammation. To answer this question, we employed an experimental allergic asthma model using a standard protocol as described before 29, 30. Young Cisfl/fl,Cre mice (6-8 weeks old) had only a few lung infiltrates but were otherwise health (Supplemental Fig. 4). After induction of allergic asthma, we assessed the cellular profiles of bronchoalveolar lavage fluids (BALFs) and found that Cisfl/fl,Cre BALFs contained more CD4+ T lymphocytes and eosinophils than Cisfl/+,Cre BALFs (Fig. 3A).
Fig. 3. Treg-specific Cis-deficiency enhances allergic asthma.
(A) Cellular profile of bronchoalveolar lavage fluids (BALFs) of Cisfl/+,Cre and Cisfl/fl,Cre mice after induction of asthma. (B) H&E stain of left lung lopes from mice in (A). Scale bar, 100 μm. (C) Intracellular stain of TH2 cytokines in the BALF, LLN and Spleen. (D) Statistical analysis of (C). (E) ELISA of cytokine expression by LLN cells and Splenocytes following recall with Ovalbumin (OVA) at indicated concentrations for 3 d. Data are a representative of 3 (A-D) and 2 (E) independent experiments (4-5 mice per group). Values are means and S.D. Student t test, * p< 0.05, ** p< 0.005.
Histological analyses further confirmed that Cisfl/fl,Cre lungs had more immune infiltrates than the Cisfl/+,Cre counterparts (Fig. 3B). The asthmatic Cisfl/fl,Cre mice had increased frequencies of TH2 cells in the BALF, LLN and spleen (Fig. 3C-D) relative to Cisfl/+,Cre mice. Moreover, upon ex vivo recall with chicken Ovalbumin (OVA), a model antigen used to elicit asthma, Cisfl/fl,Cre LLN cells and splenocytes secreted significantly higher amounts of TH2 cytokines, IL-4, IL-5 and IL-13, but not TH1 cytokine IFNγ, than Cisfl/+,Cre cells (Fig. 3E). In the Cisfl/fl,Cre lung, we also observed increased frequencies of TH2 cells with no change in TH1 cells (Supplemental Fig. 7).
Next, we assessed whether Cis-deficiency in Treg cells affects asthmatic responses by using an adoptive transfer system. This was achieved by transfer of Cis-deficient (or -sufficient) Treg cells with Treg-depleted Cis-sufficient CD4+ T cells to prevent egress of tTreg cells into the environment as well as de novo generation of Cis-deficient iTreg cells. In agreement with our above observation, we found that mice receiving Cisfl/fl,Cre Treg cells had increased TH2 responses and eosinophilia relative to those receiving Cisfl/+,Cre Treg cells (Supplemental Fig. 8). Together, these data suggest that CIS plays a crucial role in maintaining Treg suppressive function during allergic inflammation and the loss of CIS in Treg cells results in exacerbate allergic responses.
Cis-deficiency leads to decreased Foxp3 expression and suppressive function of Treg
Since our above results demonstrate a crucial role of CIS in Treg cells in maintaining immune homeostasis in vivo, we asked whether CIS directly affects Treg suppressive function. To explore this, we isolated CD4+Foxp3-GFP+NRP1hi tTreg cells by FACS-sorting from Cis−/−;Foxp3Gfp (Cis−/−,GFP) and Cis+/+,GFP mice to assess their suppressive function. Additionally, we polarized naïve CD4+ T cells from the above mice towards iTreg cells and purified Foxp3 expressing cells using a CD4+Foxp3-GFP+ gate. The suppressive function of tTreg and iTreg cells (both purity > 99%) were assessed by measuring 3H-thymidine incorporation of effector CD4+ T cells during proliferation. We found that Cis-deficiency resulted in impaired suppressive function of tTreg cells as well as iTreg cells, whereas the impairments were more pronounced in iTreg cells (Fig. 4A-B). Together, these data demonstrate that CIS is required for the maintenance of both iTreg and tTreg cell suppressive function.
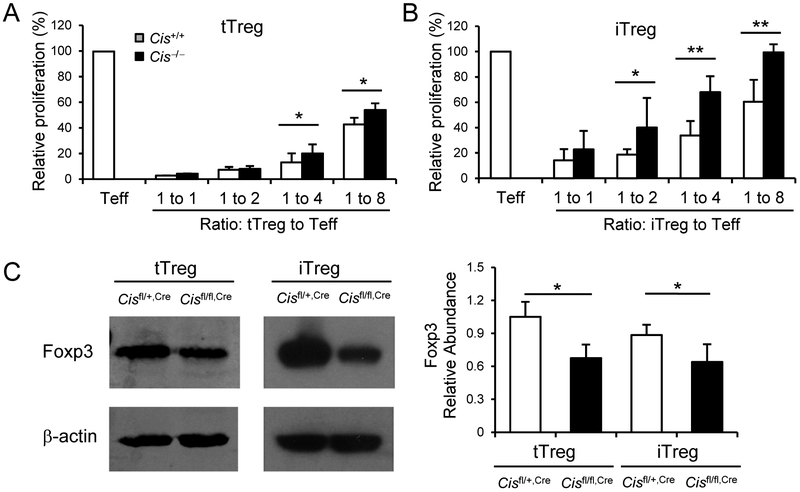
Fig. 4. Cis-deficiency leads to decreased Foxp3 expression in Treg cells and impaired their suppressive activity.
(A, B) 3H-thymidine incorporation of effector CD4+ T cells in the presence or absence of (A) tTreg (CD4+Foxp3-GFP+Nrp-1hi) cells isolated from Cis+/+,GFP and Cis−/−,GFP mice or (B) purified Foxp3-GFP+ WT and Cis−/− iTreg cells differentiated from naïve cells of above mice. Teff, effector T cells. (C) Immunoblot of Foxp3 expression in purified Cisfl/fl,Cre and Cisfl/+,Cre tTreg cells and iTreg cells. β-actin was used as a loading control. Right, statistical analysis of Foxp3 abundances relative to β-actin. Data are a combination of 2 (A, B, n = 4-6 biological replicates per group) or 4 (C) experiments. Values are means and S.D. Student t test, * p< 0.05, ** p< 0.005.
As we know, Foxp3, the master transcription factor, governs the development, maintenance and function of Treg cells 4, 5. Thus, we assessed Foxp3 expressing Treg cells in LLNs from 6-month old Cisfl/+,Cre and Cisfl/fl,Cre mice by flow cytometry following intracellular stain and found comparable percentages and numbers of Foxp3+ cells in CD4+ T populations (Supplemental Fig. 9A). Although Cis-deficiency in Treg cells did not affect the frequencies and numbers of the Treg cells population, it led to lower expression of Foxp3 in both tTreg and iTreg cells (Fig. 4C, Supplemental Fig. 10), indicating a pivotal role of CIS in sustaining Foxp3 expression in Treg cells in vivo and in vitro. Together, these data suggest CIS is required for maintaining Foxp3 expression in Treg cells.
Cis-deficiency drives loss of Treg cell identity and activates effector programs
Our above findings suggest that Cis-deficiency results in decreased expression of Foxp3 and impaired function of Treg cells. We then asked whether CIS is required for maintenance of Treg cell identity. To address this, we employed a Treg cell fate-mapping mice model, in which Foxp3-YFPCre excised a floxed stop code sequence and led to constitutive expression of enhanced YFP from the Rosa26stop-eYfp (R26Y) locus that marked Foxp3lo (that partially lost Foxp3 expression) and Foxp3hi Treg cells. We analyzed the Treg cell fate in splenocytes from 6-month old Cisfl/+,Cre R26Y and Cisfl/fl,Cre R26Y mice with a CD4+YFP+ gate. As expected, Cisfl/fl,Cre R26Y cells displayed a higher frequency of Foxp3lo Treg cells compared with control cells (Fig. 5A), suggesting a critical role of CIS in Treg cell stability. Interestingly, albeit Cis-deficiency led to decreased Foxp3 expression in Treg cells, we observed similar frequencies and numbers of Treg cells in LLNs of Cisfl/fl,Cre mice compared with Cisfl/+,Cre controls (Supplemental Fig. 9A and data not shown). This may be explained by higher proliferation rates of Cisfl/fl,Cre over Cisfl/+,Cre Treg cells as measured by the proliferation status marker Ki67 (Supplemental Fig. 9B). We next asked whether Foxp3lo Treg cells could maintain the expression of Treg-associated functional molecules. We found that Cis-deficient (and -sufficient) Foxp3lo Treg cells lost the expression of CD25, but largely remained the expression of NRP1 and had comparable levels of PD1 (Supplemental Fig. 11A). Interestingly, Cisfl/fl,Cre mice had more CD25loFoxp3lo, Nrp1−Foxp3lo and PD1loFoxp3lo cells in the CD4+YFP+ compartment than Cisfl/+,Cre mice. Furthermore, CIS did not affect the expression of CD39, CTLA-4 and ICOS in either Foxp3hi or Foxp3lo Treg cells (Supplemental Fig. 11B). Together, our data suggest Cis-deficiency diminishes the expression of Foxp3 and CD25, which at least in part, accounts for the impaired suppressive function of Cis-deficient Treg cells.
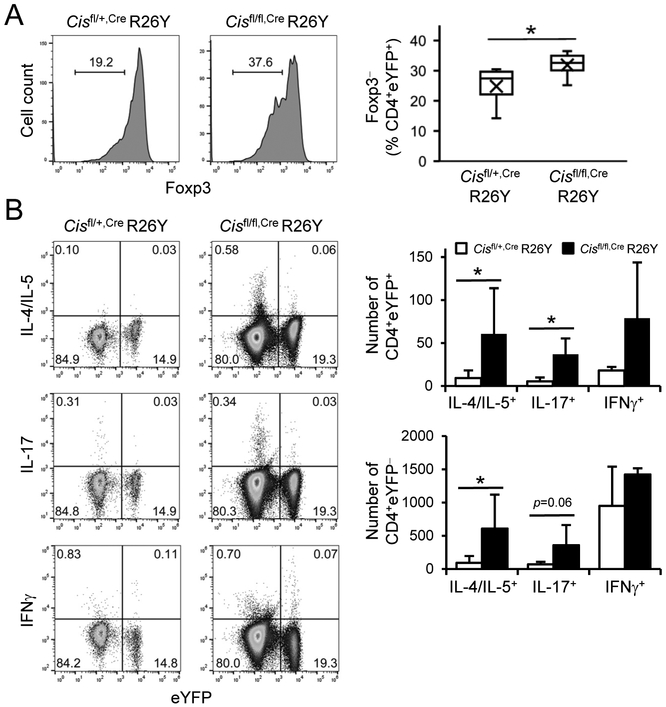
Fig. 5. Cis-deficiency results in loss of Treg cell identity and activation of effector programs.
(A) Intracellular stain of Foxp3 in LLN CD4+YFP+ Treg cells from 6-month old Cisfl/fl,Cre R26Y and Cisfl/+,Cre R26Y mice. Right, statistical analysis of Foxp3lo Treg (CD4+YFP+Foxp3lo) cells. Box indicates the 1st and 3rd quartiles and whiskers indicate the maximum and minimum. “X” indicates mean point. N = 8 mice per group. (B) Intracellular stain of cytokine expressing cells in LLNs from 6-month old Cisfl/fl,Cre R26Y and Cisfl/+,Cre R26Y mice on a CD4+ gate. Data represent 2 experiments (n = 4 mice per group). Values are means and S.D. Student t test, * p< 0.05.
Our above results indicate that Cis-deficiency impaired Treg cell program. Nevertheless, it remains unclear whether Cis-deficiency leads to adoption of effector programs in Treg cells. To test this, we assessed the expression of effector cytokines in Treg-originated cells from the fate-mapping mice and found that indeed, CD4+YFP+ LLN cells from 6-month old Cisfl/fl,Cre R26Y mice contained more IL-4/IL-5+ TH2 and IL-17+ TH17 cells but not IFNγ+ TH1 cells compared with those from Cisfl/+,Cre R26Y mice (Fig. 5B). Interestingly, conventional CD4+YFP− LLN cells from Cisfl/fl,Cre R26Y mice also contained more TH2 cells than the Cisfl/+,Cre R26Y counterparts (Fig. 5B), in agreement with the impaired Treg cell function. To further understand whether Foxp3lo or Foxp3hi Treg cells express effector cytokines, we co-stained Foxp3 with the effector cytokines and found that both Foxp3lo and Foxp3hi Treg cells from Cisfl/fl,Cre R26Y and Cisfl/+,Cre R26Y mice contained a small portion of cytokine expressing cells, but there were no differences between the two groups (Supplemental Fig. 12). Our data suggest CIS expression in Treg cells silences TH effector programs regardless of Foxp3 expression status.
CIS antagonizes IL-4 signals to maintain Treg cell function
To gain further insights into how CIS regulates the stability and function of Treg cells, we profiled the gene expression patterns in the presence or absence of CIS. CD4+YFP+ (Foxp3+) CD25+ Treg cells were FACS-sorted from Cisfl/fl,Cre and Cisfl/+,Cre mice and mRNA expressions of genes associated with various TH cell programs were assessed by RT-qPCR. In alignment with our above results, mRNA expression of transcription factor Foxp3 and IL-2 receptor CD25 (but not the common gamma chain) was downregulated in Cis-deficient Treg cells relative to Cis-sufficient counterparts (Fig. 6A, Supplemental Fig. 13). Surprisingly, mRNA expression of anti-inflammatory cytokine IL-10 was increased in Cis-deficient Treg cells compared with Cis-sufficient Treg cells, which might reflect an effector but not regulatory T cell program since effector T cells express various amounts of IL-10. Cis-deficiency did not alter the expression of CTLA-4, TGFβ and IL-35 (encoded by p35 and Ebi3; although Cis-deficiency increased the expression of Ebi3). Noteworthy, Cis-deficiency resulted in increased expression of TH2 signature genes, including those encoding cytokine IL-4, and transcription factors, GATA3 and IRF4 (and a trend of increase in IL-4Ra) (Fig. 6A). In line with this, Cis-deficiency promoted the expression of TH2 genes in either CD4+CD25loYFP+ (containing Foxp3lo cells) or CD4+CD25hiYFP+ (Foxp3hi) Treg cells sorted from the fate mapping mice, but only downregulated Foxp3 expression in CD4+CD25loYFP+ but not CD4+CD25hiYFP+ population, suggesting that the upregulation of the TH2 program might be an earlier event than the loss of Foxp3 in Cis-deficient Treg cells (Supplemental Fig. 14). In addition, Cis-deficiency also led to a trend of increases in the expression of other TH program genes, such as Tbx21 (encoding TH1 transcription factor T-bet), Rorgt (encoding TH17 transcription factor RORγt) and Il17 (Fig. 6A). By using ELISA, we found that Cis-deficient Treg cells expressed heightened amounts of TH2 cytokines, IL-4 an IL-5 but not TH17 cytokine IL-17 and TH1 cytokine IFNγ compared with Cis-sufficient counterparts (Fig. 6B). Similarly, we observed an increase in mRNA expression of Irf4 and Il4, and a decrease in expression of Foxp3 in purified Cis−/− relative to Cis+/+ iTreg cells (Supplemental Fig. 15). In summary, these findings confirmed that Cis-deficient Treg cells generated both in vivo and in vitro possess the propensity to lose Treg tolerance program and to gain T effector programs, especially the TH2 program that may serve as an early source of IL-4, promoting conventional TH2 cell differentiation and accumulation.
Fig. 6. Cis-deficiency impairs Treg cell function in an IL-4 signaling-dependent manner.
(A) RT-qPCR of gene expression in CD4+Foxp3-YFP+CD25+ Treg cells sorted from Cisfl/fl,Cre and Cisfl/+,Cre mice. Ebi3 and p35 encode IL-35. Tbx21 encodes T-bet. Actb was used as a loading control. (B) ELISA of effector cytokine expression in purified Treg cells from above mice following stimulation with plate-bound α-CD3 and α-CD28. (C) Intracellular stain of Foxp3 in Cisfl/fl,Cre and Cisfl/+,Cre iTreg cells cultured on α-CD3 and α-CD28 coated plate for 8 h in the presence of α-IL-4, α-IL-6 + IL-21R, α-IFNγ or a control antibody (Ctr). The numbers are the percentages of Foxp3lo cells (Cisfl/+,Cre/Cisfl/fl,Cre). (D) 3H-thymidine incorporation of effector CD4+ T cells in the presence or absence of purified Foxp3-GFP+ WT and Cis−/− iTreg cells differentiated from naïve cells isolated from Cis+/+,GFP and Cis−/−,GFP mice. Data shown are a representative of 2 experiments (3 biological replicates per group) (A, B), 2 (C) independent experiments, or a combination of 2 experiments (4-6 biological replicates per group) (D). Values (A, B, D) are means and S.D. (B, D). Student t test, * p< 0.05, ** p< 0.005.
We next examined whether Treg cell-specific Cis-deficiency inhibits iTreg cell polarization through the induction of a Treg-intrinsic TH2 cell program. Cis-deficient T cells displayed increased TH2 cell responses as well as decreased differentiation of iTreg cells which could be neutralized by blocking IL-4 in vitro 28. We further showed Treg cell-specific Cis-deficiency also resulted in a decrease in polarization of Foxp3+ cells and treatment with anti-IL-4 rescued Foxp3+ iTreg cell induction (Supplemental Fig. 16). This indicates a pivotal role of Treg-expression of CIS in inhibition of IL-4 signals during iTreg cell polarization. To understand how CIS antagonizes pro-inflammatory cytokine signals and stabilizes Treg cells, we cultured purified Cis-deficient and -sufficient iTreg cells on a plate coated with anti-CD3 and anti-CD28 in the presence of IL-2 with or without blockade of cytokine signals. We found that after treatment with a control antibody, Cis-deficiency resulted in dramatic loss of Foxp3, whereas anti-IL-4 treatment largely restored Foxp3 expression in Cis-deficient iTreg cells (Fig. 6C). Interestingly, treatment with anti-IL-6 plus IL-21R (blocking the TH17 pathway) or anti-IFNγ (blocking the TH1 pathway) only slightly prevents the loss of Foxp3 expression in Cis-deficient iTreg cells. Therefore, CIS plays a critical role in guarding Treg cell stability by inhibiting a TH2 dominant effector program. We then asked whether Cis-deficiency impairs Treg cell function through IL-4 signals. To answer this, we examined the regulatory function of purified Cis-deficient and -sufficient iTreg cells in the presence or absence of IL-4 neutralizing antibody. As expected, Cis−/− iTreg cells had impaired suppressive function compared with Cis+/+ iTreg, whereas the attenuated function of Cis−/− iTreg cells was restored by addition of anti-IL-4 but not a control antibody into the culture (Fig. 6D), suggesting a central role of CIS in governing IL-4 signals in regulating Treg suppressive function.
CIS inhibits STAT6 binding to and silencing the Foxp3 locus
As mentioned above, STAT6 phosphorylation is the major downstream signal event in response to IL-4 in CD4+ T helper cells. Thus, we examined the effects of CIS on STAT6 activation in Treg cells by intracellular stain. First, we measured in vivo expression of phosphor- (p) STAT6 in Treg cells and observed a slightly increase in the levels of pSTAT6 in Cisfl/fl,Cre Treg cells compared with Cisfl/+,Cre Treg cells (Fig. 7A). Second, we assessed pSTAT6 expression in iTreg cells polarized from Cisfl/fl,Cre and Cisfl/+,Cre naïve T cells and found that Cisfl/fl,Cre iTreg cells expressed higher amounts of pSTAT6 than the control cells (Fig. 7A). The absence of STAT6 abolished IL-4 expression in Cis-deficient Treg cells (Supplemental Fig. 17). Concluding, Cis-deficiency led to elevated STAT6 activation in Treg cells, which was likely triggered by autocrine IL-4 induced by activated STAT6.
Fig. 7. Cis-deficiency silences the Treg program and promotes a TH2 phenotype through the IL-4-STAT6 axis.
(A) Intracellular phosphor-stain of pSTAT6 in tTreg or iTreg cells. (B) ChIP assays of pSTAT6 and pSTAT5 binding onto the Foxp3 locus in Cisfl/fl,Cre and Cisfl/+,Cre iTreg cells. (C-D) ChIP assays of H3K4m3 (C) and H3K27m3 (D) modifications on the Foxp3, IL-4 and IL-17 loci in indicated iTreg cells. Data are a representative of 2-3 independent experiments (3 biological replicates per group). (A right, B-D) Values are means and S.D. Student t test, * p< 0.05, ** p< 0.005. *** p< 0.0005.
To understand how Cis-deficiency induced IL-4 signaling destabilizes iTreg cells, we performed chromatin immunoprecipitation (ChIP) assays to uncover the transcriptional accessibility of Treg and effector T cell signature gene loci. At the conserved non-coding sequence 2 (CNS2) and the promoter of Foxp3 gene, pSTAT5 binding is required for the maintenance of Treg cell identity, whereas pSTAT6 was thought to compete with pSTAT5 binding at this locus and may lead to decrease expression or even silence of Foxp3 gene 31-33. We measured the binding levels of pSTAT6 and pSTAT5 at the Foxp3 locus in iTreg cells in the absence of exogenous IL-4, and found higher pSTAT6 binding to both Foxp3 promoter and CNS2 regions in Cisfl/fl,Cre relative to Cisfl/+,Cre iTreg cells (Fig. 7B, left). There was a trend showing a decrease in pSTAT5 bindings to these two sites in Cisfl/fl,Cre iTreg cells compared with that in Cisfl/+,Cre iTreg cells; however, it did not reach significance (Fig. 7B, right). These data suggest CIS blocks IL-4-induced activation of STAT6 in iTreg cells and prevents pSTAT6-mediated silence of Foxp3 expression.
To gain further insight into the role of CIS in stabilization of Treg cells, we assessed epigenetic modifications of histone h3 lysine 4 trimethylation (H3K4m3), indicating permissive epigenetic modification, and H3K27m3, associated with gene inactivation, at the Foxp3, Il4 and Il17 loci, (Fig. 6A). We observed a dramatic decrease in H3K4m3 modifications on both Foxp3 promoter and CNS2 regions in Cis-deficient relative to Cis-sufficient iTreg cells (Fig. 7C, left), although no distinction has been found on H3K27m3 modifications on these sites (Fig. 7D, left). In addition, the H3K4m3 modification at the Il4 HS2 region but not the Il17 promoter was significantly enhanced (Fig. 7C, right), and meanwhile, the H3K27m3 modifications were consistently decreased at the Il4 HS2 and HS3 regions as well as the Il17 promoter in Cisfl/fl,Cre compared with that in Cisfl/+,Cre iTreg cells (Fig. 7D, right). Together, these results unravel that CIS stabilizes Treg cells via antagonizing IL-4-STAT6 signals that represses the Foxp3 locus and activates the Il4 gene.
Taken together, we have uncovered an unappreciated and pivotal role of CIS in maintaining Treg cell identity and function via antagonizing IL-4-STAT6 signaling and stabilizing Foxp3 expression (summarized in Supplemental Fig. 18).
DISCUSSION
Treg cells are crucial for maintaining immune tolerance and homeostasis in mice and humans. The stability of Foxp3 expression in Treg cells is considered as a determinant factor of immune homeostasis 4, 5. Many transcription factors, including STAT5 (activated through IL-2), SMADs (activated through TGFβ), NFAT and CREB, have been shown to stabilize Foxp3 expression in Treg cells through targeting CNS2 or other regulatory elements at the Foxp3 locus 3, 34. However, under certain pro-inflammatory circumstances, Foxp3+ Treg cells become unstable and lose Foxp3 expression and might further adopt a CD4+ T effector phenotype. Several studies concerning STATs regulation of Foxp3 locus showed that IL-4 induced pSTAT6 and IL-6 induced pSTAT3 are enabled to compete for the pSTAT5-binding sites at the Foxp3 locus and interact with DNA methyltransferases Dnmt1, which is believed to be responsible for de novo DNA methylation 32, leading to diminished Foxp3 expression 19, 21. In concert with aforementioned studies, our study provides evidence that dysregulation of pro-inflammatory cytokine signals in Cis-deficient Treg cells led to loss of Foxp3 expression and impairment of suppressive function of Treg cells. Mice carrying Cis-deficient Treg cells manifested a spontaneous phenotype of eosinophilic airway inflammation. Consistently, Treg cell-specific deletion of Cis led to exacerbated type 2 immune responses in an asthma model.
Pro-inflammatory cytokine signaling is largely dependent on STAT protein activation. As one of the classical negative regulatory strategies on STATs signaling, SOCS family proteins are broadly involved in the restriction of STAT activation and stabilization of Treg cells in pro-inflammatory conditions. As shown in previous studies, SOCS1 and SOCS2 are both essential for Treg cell stability. SOCS1 stabilizes Treg cells through inhibiting STAT1 and STAT3 activation 35, whereas SOCS2 maintains pTreg cells likely through inhibition of IL-4-STAT6 signal36. CIS, another member of the SOCS protein family, has been shown to disrupt STAT5 signaling induced by cytokines and hormones 22-24, 26, 27. In addition, our previous studies showed that CIS also inhibits STAT3 and STAT6 signaling in CD4+ T cells 28. In this study, we found that in the absence of CIS, Treg cells, generated either in vivo or in vitro, had impaired suppressive function and diminished Foxp3 expression, which was largely mediated by TH2 type cytokine IL-4 but not TH1 type cytokine IFNγ and TH17 type cytokines IL-21 and IL-6. Consistent with this, we detected increased STAT6 binding to the Foxp3 locus, accompanied with decreased epigenetic permissive marker H3K4me3 levels on the Foxp3 CNS and promoter in Cis-deficient relative to Cis-sufficient Treg cells. Together, these data indicate a critical role of CIS in antagonizing IL-4-mediated STAT6 activation and in maintenance of chromatin accessibility of the Foxp3 locus.
GATA3 is the major transcription factor of TH2 cells 37. Treg cells residing in barrier sites also express GATA3 that is required for Treg cell maintenance under inflammatory conditions but not the steady state 38, 39, predisposing a risk of instability in Treg cells. In our study, we found that Cis-deficiency not only caused loss of Foxp3 expression but also rendered an endogenous TH2 cell program in Treg cells generated in vivo with elevation of mRNAs encoding TH2 cell signature transcription factors, GATA3 and IRF4, and cytokine, IL-4. In iTreg cells, Cis-deficiency also resulted in increased mRNA expression of Irf4 and Il4, but did not alter Gata3 mRNA expression, indicating that increased expression of IL-4 by Cis-deficient iTreg cells did not require further upregulation of GATA3. The autocrine IL-4 triggered activation of STAT6 that bound to and occupied the Foxp3 promoter and CNS2. This overloaded IL-4-STAT6 signaling circuit drove the loss of Foxp3 expression in Treg cells, and meanwhile promoted TH2 cell effector cytokine expression, therefore enforcing a feedforward loop that impaired Treg function and led to spontaneous inflammation. Taken together, CIS plays a crucial role in stabilizing Treg cells through antagonizing an autocrine (and maybe also other sourced) IL-4 mediated STAT6 signaling, highlighting a cell-intrinsic circuit important for Treg cell self-maintenance.
As we observed that CIS was required to maintain Treg cell suppressive function via stabilizing the expression of Foxp3, we examined the relationship between CIS and Treg-associated functional molecules. By leverage a Treg-specific reporter system, we observed Foxp3lo Treg cells that were highly present in Cis-deficient animals lost expression of CD25 but remained other Treg-associated surface markers, such as CTLA4, CD39, PD1 and ICOS, suggesting that the Foxp3lo Treg cells may partially remain their suppressive function. As revealed by intracellular stain, Treg-expression of CIS suppressed the expression of effector cytokines, IL-4/IL-5, IL-17, or IFNγ by Treg cells. Interestingly, both Foxp3lo and Foxp3hi Treg cells expressed these effector cytokines even in Cis-deficient animals; however, only a few Foxp3lo Treg cells expressed effector cytokines. Therefore, Cis-deficiency destabilizes Treg cells through an autonomous TH2 pathway, which impairs Treg cell function, resulting in excessive conventional TH2 cell responses. In summary, our study suggests a previously unappreciated role of CIS in the regulation of Treg cell stability though a feedback control of the Treg-intrinsic TH2 program and in the inhibition of allergic airway inflammation. These findings provide novel insights into the stabilization of Treg cells and may suggest therapeutic intervention in human allergic airway diseases.
MATERIALS AND METHODS
Animals
Cisfl/fl;Foxp3-YfpCre (Cisfl/fl,Cre) mice on the C57BL/6 (B6);129 F1 background were generated by breeding B6.Cisfl/fl,Cre with 129.Cisfl/+ mice; Cisfl/+,Cre (or Cisfl/fl or Cisfl/+,Cre) littermates were used as control. B6.Cisfl/fl,Cre mice were obtained by breeding B6.Cisfl/fl mice with B6.Foxp3-YfpCre mice (purchased from the Jackson Laboratory) 40, whereas 129.Cisfl/+ mice were obtained by crossing B6.Cisfl/fl with 129 mice for 7-8 generations. Cis−/−,GFP mice on the B6;129 F1 background were generated by breeding B6.Cis+/−,GFP mice (obtained by crossing B6.Cis−/− mice with B6.Foxp3-Gfp 18, 41), with 129.Cis+/− mice; Cis+/+,GFP littermates were used as control. Similarly, Cisfl/fl,Cre R26Y and Cisfl/+,Cre R26Y fate mapping mice on the B6;129 F1 background were generated as described above. R26Y (Rosa26stop-eYfp) mice were purchased from the Jackson Laboratory 42. All mice were housed in the specific pathogen-free animal facility at the University of New Mexico Health Sciences Center. All experiments were performed with protocols approved by the Institutional Animal Care and Use Committee of the University of New Mexico.
Induction of experimental allergic asthma
For induction of allergic asthma, 6-8-week old sex-matched mice were immunized intranasally with 25 μg papain and 50 μg chicken Ovalbumin (OVA) for three times on d 0, d 1 and d 14. In some experiments, Treg cells (0.5 × 106 cells per recipient) isolated from indicated mice by using a Treg isolation kit (Miltenyi Biotec) were co-transferred intraperitoneally with Treg-depleted Cis-sufficient CD4+ T cells (5 × 106 cells per recipient) into Rag1−/− mice, and the recipient mice were subjected to induction of asthma. On d 15, BALFs were collected for analysis of airway infiltrates as described 29, 30. Additionally, BALFs, LLNs and spleens were collected for analysis of cellular profiles. LLN and spleen cells (4 × 106 cells ml−1) were recalled with various concentrations of Ova for 3 days and the supernatants were collected for measurement of cytokines expression by ELISA using a standard protocol.
In vitro Treg cell differentiation
CD4+CD25−CD44loC62L+ naïve T cells were isolated from indicated mice, and activated by plate-bound α-CD3/α-CD28 in a Treg-polarizing condition (100 unit ml−1 IL-2, 2 ng ml−1 TGFβ, 2 μg ml−1 anti-IFN-γ with or without 5 μg ml−1 anti-IL-4 as indicated).
In vitro Treg suppression assay
Wild-type (WT) naïve CD4+ T cells were co-cultured with purified Treg cells at indicated ratios of Treg to naïve CD4+ T cells in the presence of irradiated splenic antigen presenting cells (APCs) and anti-CD3 for 3 d. In some experiments, anti-IL-4 (11B11) neutralizing antibodies (10 μg ml−1) were added as indicated. Proliferation was assessed by 3H-thymidine incorporation for the final 8 h before harvest.
RT-qPCR
Gene mRNA expression levels were determined by RT-qPCR using primers in Supplemental Table 1. Data were normalized to an Actb reference gene.
Chromatin Immunoprecipitation (ChIP)
Cell lysates were prepared as described 28. The lysates were first pre-cleared with Protein G magnetic beads at 4 °C for 30 m. After removal of the Protein G beads, supernatants were collected and incubated with an antibody against the target protein or a control antibody overnight at 4 °C, followed by Protein G magnetic beads incubation for 2 h at 4 °C on d 2 morning. The bead-antibody-chromatin complexes were magnetically separated and washed 2 times with low salt TE buffer and additional 2 times with high salt TE buffer. Finally, the bead-antibody-chromatin complexes were collected and resuspended in TE buffer for reverse crosslink in the presence of 0.2 M NaCl for 4 h at 65 °C. The samples were then subjected for qPCR. The primers used were in Supplemental Table 2. The antibodies used were in Supplemental Table 3.
Flow cytometry
For intracellular cytokine stain, the cells were collected and stimulated with phorbol 12-myristate 13-acetate (PMA) and ionomycin in the presence of Golgi-stop for 4 hr. Phosphor-protein stain of pSTAT6 was performed using a previously described protocol with minor modifications 43. In brief, the cells were collected in vivo or in vitro and fixed by 1.6% paraformaldehyde for 20 m, re-suspended in 50% ethanol for 1 m, and permeabilized with methanol for 20 m. The resulting cells were stained with anti-pSTAT6 in cold 0.1% BSA-PBS for 30 min. The flow cytometry antibodies were in Supplemental Table 3.
Statistical analysis
The statistical significance of differences between groups was calculated with the unpaired Student’s t test. P values of 0.05 or less were considered significant.
Supplementary Material
Acknowledgements
This work was supported in part by NIH grants AI116772 and AI142200 (X.O.Y.), GM121176 (X.O.Y., M.L.; PD, V. Deretic), and DK110439 (M.L.). We acknowledge the University of New Mexico Flow Cytometry Facility supported by NIH CA118100 and the Central South University collaboration program supported by NSFC 81828004. H.Z. was a recipient of Careers in Immunology Fellowship, American Association of Immunologists and Ruby Fellowship.
Footnotes
Competing Interests
The authors declare that they have no competing interests.
REFERENCES
- 1.Rudensky AY. Regulatory T cells and Foxp3. Immunol Rev 2011; 241(1): 260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell 2008; 133(5): 775–787. [DOI] [PubMed] [Google Scholar]
- 3.Li X, Liang Y, LeBlanc M, Benner C, Zheng Y. Function of a Foxp3 cis-element in protecting regulatory T cell identity. Cell 2014; 158(4): 734–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fontenot JD, Rudensky AY. A well adapted regulatory contrivance: regulatory T cell development and the forkhead family transcription factor Foxp3. Nat Immunol 2005; 6(4): 331–337. [DOI] [PubMed] [Google Scholar]
- 5.Williams LM, Rudensky AY. Maintenance of the Foxp3-dependent developmental program in mature regulatory T cells requires continued expression of Foxp3. Nat Immunol 2007; 8(3): 277–284. [DOI] [PubMed] [Google Scholar]
- 6.Bennett CL, Ochs HD. IPEX is a unique X-linked syndrome characterized by immune dysfunction, polyendocrinopathy, enteropathy, and a variety of autoimmune phenomena. Curr Opin Pediatr 2001; 13(6): 533–538. [DOI] [PubMed] [Google Scholar]
- 7.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol 2003; 4(4): 330–336. [DOI] [PubMed] [Google Scholar]
- 8.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science 2003; 299(5609): 1057–1061. [DOI] [PubMed] [Google Scholar]
- 9.McMurchy AN, Bushell A, Levings MK, Wood KJ. Moving to tolerance: clinical application of T regulatory cells. Semin Immunol 2011; 23(4): 304–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Game DS, Cao X, Jiang S. Regulatory T cells: the cunning fox and its clinical application. Sci Signal 2008; 1(51): mr3. [DOI] [PubMed] [Google Scholar]
- 11.Brusko T, Bluestone J. Clinical application of regulatory T cells for treatment of type 1 diabetes and transplantation. Eur J Immunol 2008; 38(4): 931–934. [DOI] [PubMed] [Google Scholar]
- 12.Marek-Trzonkowska N, Mysliwec M, Siebert J, Trzonkowski P. Clinical application of regulatory T cells in type 1 diabetes. Pediatr Diabetes 2013; 14(5): 322–332. [DOI] [PubMed] [Google Scholar]
- 13.Bettelli E, Carrier YJ, Gao WD, Korn T, Strom TB, Oukka M et al. Reciprocal developmental pathways for the generation of pathogenic effector T(H)17 and regulatory T cells. Nature 2006; 441(7090): 235–238. [DOI] [PubMed] [Google Scholar]
- 14.Dardalhon V, Awasthi A, Kwon H, Galileos G, Gao W, Sobel RA et al. IL-4 inhibits TGF-beta-induced Foxp3(+) T cells and, together with TGF-beta, generates IL-9(+) IL-10(+) Foxp3(−) effector T cells. Nat Immunol 2008; 9(12): 1347–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kastner L, Dwyer D, Qin FXF. Synergistic Effect of IL-6 and IL-4 in Driving Fate Revision of Natural Foxp3(+) Regulatory T Cells. J Immunol 2010; 185(10): 5778–5786. [DOI] [PubMed] [Google Scholar]
- 16.Xu LL, Kitani A, Fuss I, Strober W. Cutting edge: Regulatory T cells induce CD4(+)CD25(−)Foxp3(−) T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-beta. J Immunol 2007; 178(11): 6725–6729. [DOI] [PubMed] [Google Scholar]
- 17.Jin HS, Park Y, Elly C, Liu YC. Itch expression by Treg cells controls Th2 inflammatory responses. J Clin Invest 2013; 123(11): 4923–4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang XO, Nurieva R, Martinez GJ, Kang HS, Chung Y, Pappu BP et al. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity 2008; 29(1): 44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takaki H, Ichiyama K, Koga K, Chinen T, Takaesu G, Sugiyama Y et al. STAT6 Inhibits TGF-beta1-mediated Foxp3 induction through direct binding to the Foxp3 promoter, which is reverted by retinoic acid receptor. J Biol Chem 2008; 283(22): 14955–14962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chapoval S, Dasgupta P, Dorsey NJ, Keegan AD. Regulation of the T helper cell type 2 (Th2)/T regulatory cell (Treg) balance by IL-4 and STAT6. J Leukoc Biol 2010; 87(6): 1011–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu L, Kitani A, Stuelten C, McGrady G, Fuss I, Strober W. Positive and negative transcriptional regulation of the Foxp3 gene is mediated by access and binding of the Smad3 protein to enhancer I. Immunity 2010; 33(3): 313–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Shea JJ, Gadina M, Schreiber RD. Cytokine signaling in 2002: new surprises in the Jak/Stat pathway. Cell 2002; 109 Suppl: S121–131. [DOI] [PubMed] [Google Scholar]
- 23.Palmer DC, Restifo NP. Suppressors of cytokine signaling (SOCS) in T cell differentiation, maturation, and function. Trends Immunol 2009; 30(12): 592–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoshimura A, Naka T, Kubo M. SOCS proteins, cytokine signalling and immune regulation. Nat Rev Immunol 2007; 7(6): 454–465. [DOI] [PubMed] [Google Scholar]
- 25.Yoshimura A, Ohkubo T, Kiguchi T, Jenkins NA, Gilbert DJ, Copeland NG et al. A novel cytokine-inducible gene CIS encodes an SH2-containing protein that binds to tyrosine-phosphorylated interleukin 3 and erythropoietin receptors. EMBO J 1995; 14(12): 2816–2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aman MJ, Migone TS, Sasaki A, Ascherman DP, Zhu M, Soldaini E et al. CIS associates with the interleukin-2 receptor beta chain and inhibits interleukin-2-dependent signaling. J Biol Chem 1999; 274(42): 30266–30272. [DOI] [PubMed] [Google Scholar]
- 27.Matsumoto A, Masuhara M, Mitsui K, Yokouchi M, Ohtsubo M, Misawa H et al. CIS, a cytokine inducible SH2 protein, is a target of the JAK-STAT5 pathway and modulates STAT5 activation. Blood 1997; 89(9): 3148–3154. [PubMed] [Google Scholar]
- 28.Yang XO, Zhang H, Kim BS, Niu X, Peng J, Chen Y et al. The signaling suppressor CIS controls proallergic T cell development and allergic airway inflammation. Nat Immunol 2013; 14(7): 732–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng H, Zhang X, Castillo EF, Luo Y, Liu M, Yang XO. Leptin Enhances TH2 and ILC2 Responses in Allergic Airway Disease. J Biol Chem 2016; 291(42): 22043–22052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng H, Wu D, Wu X, Zhang X, Zhou Q, Luo Y et al. Leptin promotes allergic airway inflammation through targeting the unfolded protein response pathway. Sci Rep 2018; (1): 8905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yao Z, Kanno Y, Kerenyi M, Stephens G, Durant L, Watford WT et al. Nonredundant roles for Stat5a/b in directly regulating Foxp3. Blood 2007; 109(10): 4368–4375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feng Y, Arvey A, Chinen T, van der Veeken J, Gasteiger G, Rudensky AY. Control of the inheritance of regulatory T cell identity by a cis element in the Foxp3 locus. Cell 2014; 158(4): 749–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng Y, Josefowicz S, Chaudhry A, Peng XP, Forbush K, Rudensky AY. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature 2010; 463(7282): 808–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol 2012; 30: 531–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takahashi R, Nishimoto S, Muto G, Sekiya T, Tamiya T, Kimura A et al. SOCS1 is essential for regulatory T cell functions by preventing loss of Foxp3 expression as well as IFN-{gamma} and IL-17A production. J Exp Med 2011; 208(10): 2055–2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knosp CA, Schiering C, Spence S, Carroll HP, Nel HJ, Osbourn M et al. Regulation of Foxp3+ inducible regulatory T cell stability by SOCS2. J Immunol 2013; 190(7): 3235–3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yagi R, Zhu J, Paul WE. An updated view on transcription factor GATA3-mediated regulation of Th1 and Th2 cell differentiation. Int Immunol 2011; 23(7): 415–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Y, Su MA, Wan YY. An essential role of the transcription factor GATA-3 for the function of regulatory T cells. Immunity 2011; 35(3): 337–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wohlfert EA, Grainger JR, Bouladoux N, Konkel JE, Oldenhove G, Ribeiro CH et al. GATA3 controls Foxp3(+) regulatory T cell fate during inflammation in mice. J Clin Invest 2011; 121(11): 4503–4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity 2008; 28(4): 546–558. [DOI] [PubMed] [Google Scholar]
- 41.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity 2005; 22(3): 329–341. [DOI] [PubMed] [Google Scholar]
- 42.Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol 2001; 1: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krutzik PO, Nolan GP. Intracellular phospho-protein staining techniques for flow cytometry: monitoring single cell signaling events. Cytometry A 2003; 55(2): 61–70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.