Abstract
Objective:
Symptom heterogeneity in major depressive disorder (MDD) obscures diagnostic and treatment-responsive biomarker identification. Whether symptom constellations are differentially changed by electroconvulsive therapy (ECT) remains unknown. We investigate the clustering of depressive symptoms over ECT index and whether ECT differentially influences symptom clusters.
Methods:
The 17-item Hamilton Depression Rating Scale (HDRS-17) was collected from 111 patients with current depressive episode before and after ECT from four independent participating sites of the Global ECT-MRI Research Collaboration (GEMRIC). Exploratory factor analysis of HDRS-17 items pre- and post-ECT treatment identified depressive symptom dimensions before and after ECT. A two-way ANCOVA was used to determine whether baseline symptom clusters were differentially changed by ECT between treatment remitters (defined as patients with post-treatment HDRS-17 total score ≤8) and non-remitters, while controlling for pulse width, titration method, concurrent antidepressant treatment, use of benzodiazepine, and demographic variables.
Results:
A three-factor solution grouped pretreatment HDRS-17 items into core mood/anhedonia, somatic, and insomnia dimensions. A two-factor solution best described the symptoms at post-treatment despite poorer separation of items. Among remitters, core mood/anhedonia symptoms were significantly more reduced than somatic and insomnia dimensions. No differences in symptom dimension trajectories were observed among non-remitting patients.
Conclusions:
ECT targets the underlying source of depressive symptomatology and may confer differential degrees of improvement in certain core depressive symptoms. Our findings of differential trajectories of symptom clusters over ECT index might help related predictive biomarker studies to refine their approaches by identifying predictors of change along each latent symptom dimension.
Keywords: Major depressive disorder, Symptom heterogeneity, Electroconvulsive therapy, Hamilton Depression Rating Scale, Factor analysis
Introduction
Major depressive disorder (MDD) is among the most common and debilitating psychiatric disorders [1] and is a leading cause of disability worldwide [2]. Antidepressants and psychotherapy are typical first-line interventions; however, up to a third of patients remain unresponsive to pharmacotherapeutic treatments [3]. Contributing to this failure rate is depressive symptom heterogeneity. The Diagnostic and Statistical Manual of Mental Disorders-5th edition (DSM-5) diagnosis of MDD requires the presence of one of two core symptoms and an additional three or four of seven other symptoms (or a minimum of 5 of 9 symptoms), which amounts to 227 potential depressive symptom constellations [4]. Consequently, patients with the same diagnosis can experience different symptomatic burdens. Additionally, antidepressant treatments (e.g., monoamine oxidase inhibitors, N-methyl-D-aspartate receptor antagonists, atypical antipsychotic augmentation, different modes of neurostimulation [5]) available to unresponsive patients have different mechanisms and targets. MDD heterogeneity and the manifold mechanisms by which different treatments act on the central nervous system calls into question whether certain antidepressant interventions are more effective at targeting particular MDD features and can be tailored for patients with particular symptom profiles.
Electroconvulsive therapy (ECT), considered the most effective treatment for severe or treatment resistant MDD, is typically prescribed when patients have failed to respond to multiple interventions or need a rapid response [6]. Compared to most interventions, ECT elicits fast-acting (patients typically respond in 2-4 weeks) and prominent antidepressant effects [7]. However, while the general efficacy of ECT is well established, it remains unknown whether particular symptoms, or sets of symptoms, are most effectively targeted.
While several validated depression rating scales are available [8], the Hamilton Depression Rating Scale (HDRS [9]) remains amongst the most frequently used for determining treatment efficacy. The psychometric validity and factor structure of the HDRS has been addressed previously [10]. The HDRS is reportedly more sensitive to treatment-related symptom changes than the Beck Depression Inventory [11] and the Zung Self-Rating Depression Scale [12]), and has been validated in cross-cultural cohorts [13, 14]. However, the HDRS is criticized for its multidimensional factorial structure and inability to define unidimensional depressive symptoms [15], and the relatively low interrater and internal reliability for some items [10]. Recognition of these limitations has spurred efforts to modify the HDRS for better classifying depression symptom severity. Bech et al. [16] used Rasch analysis [17] and identified a unidimensional subset of six HDRS items (HDRS-6) [18-20]. Using factor analysis and item response theory techniques, others identified unidimensional HDRS sub-scales comprised of partially overlapping core items [21, 22]. Notably, not all HDRS-items are equally sensitive for detecting antidepressant treatment response [21-24]. Meta-analyses have shown that unidimensional subscales, including the HDRS-6 [16], are more sensitive to treatment effects of selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs) and tricyclic antidepressants compared to placebo than the total HDRS score [21, 25, 26].
Given the varied MDD symptoms, different modes of antidepressant treatment (i.e., pharmacological, behavioral, neurostimulation) may distinctly influence different symptom dimensions tied to overlapping and unique neurobiological mechanisms of response. Further, specific symptomatic profiles or depression biotypes [27] might benefit from select antidepressant interventions. Addressing how change in HDRS items covary over distinct treatments could inform the mechanisms of therapeutic response, and inform more effective personalized treatments. Previously, Okazaki and colleagues [28] evaluated ECT-related changes along the three factors of the Montgomery-Asberg Depression Rating Scale (MADRS), dysphoria, retardation, and vegetative symptoms, in a cohort of 24 Japanese patients with MDD. Okazaki reported that pretreatment dysphoric symptoms were significantly more elevated among patients who subsequently responded to ECT compared to those who did not. As with most ECT studies, however, this previous study is limited by its modest sample size.
Using the 17-item HDRS (HDRS-17) [9], we investigated how symptomatic dimensions of depression vary with ECT in a multi-site cohort of 111 patients participating in the Global ECT-MRI Research Collaboration (GEMRIC) [29]. Specifically, we evaluated the effects of ECT on multidimensional features of depression symptom severity. Exploratory factor analysis (EFA) and hierarchical clustering were used to identify covarying HDRS-17 items before and after the ECT index series (ECT-IS). Given the prevalence of somatic symptoms in treatment resistant MDD [30], expected overlap with antidepressant drug therapies that show predictive effects with regard to the presence and severity of psychic and somatic anxiety symptom subscales [31], and previous HDRS factor analyses, we hypothesized that HDRS-17 items would segregate into psychic and somatic clusters. We further hypothesized that HDRS-17 factor(s) identified with EFA with prominent weightings from more unidimensional constructs found in the HDRS-6 (Depressed Mood, Feelings of Guilt, Work and Interests, Retardation, Psychic Anxiety, and Somatic Symptoms) would be most reduced by ECT.
Materials and Methods
Patients
Amongst GEMRIC participants [29], 111 patients with a current major depressive episode (N=68 female; age=52.16 ± 14.67) from four independent sites had complete item-level HDRS-17 scores available before and following ECT treatment for analysis. Data for 28 participants were acquired from Site 1, 39 from Site 2, 16 from Site 3, and 28 from Site 4. Also included were nine patients with bipolar disorder, 19 patients with psychotic features, and six patients with a single major depressive episode. Ninety-six patients were diagnosed with recurrent MDD. Patient clinical and demographic information is summarized in Table 1.
Table 1.
Demographic and clinical features
| Overall | Site 1 | Site 2 | Site 3 | Site 4 | |
|---|---|---|---|---|---|
| N | 111 | 28 | 39 | 16 | 28 |
| Age, mean (SD) years | 52.16 (14.67) | 41.07 (14.45) | 64.43 (9.08) | 52.12 (10.82) | 46.17 (10.22) |
| Male/Female | 43/68 | 14/14 | 13/26 | 5/11 | 11/17 |
| Clinical Info | |||||
| RUL | 91 | 26 | 37 | 0 | 28 |
| BT | 21 | 1 | 2 | 15 | 3 |
| Others | 1 LART | 1 LART | |||
| RUL number, mean (SD) | 9.22 (5.26) | 8.0 (3.63) | 10.32 (3.06) | -- | 13.46 (4.46) |
| BT number, mean (SD) | 6.69 (6.50) | -- | 7.28 (3.54) | 14.20 (4.98) | 6.33 (1.52) |
| ECT Device | -- | MECTA | Thymatron | Thymatron | Thymatron |
| Titration Method | -- | Seizure threshold (n=28) | Seizure threshold (n=39) | Seizure threshold (n=2) ½ age (n=14) |
½ age (n=28) |
| ECT pulse width, milliseconds | -- | 0.3 (n=26) 0.5 (n=2) |
0.25 (n=36) 1 (n=3) |
0.5 (n=15) NA (n=1) |
0.5 (n=28) |
| ECT pulse amplitude, milliamps | -- | 800 (n=28) | 900 (n=39) | 900 (n=15) NA (n=1) |
900 (n=28) |
| Antidepressant medication | -- | None (n=28) | None (n=2) SSRI (n=17) SNRI (n=16) TCA (n=4) |
None (n=3) SSRI (n=4) SNRI (n=3) TCA (n=6) |
None (n=5) SSRI (n=4) SNRI (n=15) TCA (n=4) |
| Antipsychotic medication, Yes/No/NA | -- | 0/28/0 | 20/19/0 | 8/7/1 | 19/0/9 |
| Benzodiazepines, Yes/No/NA | -- | 0/28/0 | 16/22/1 | 8/8/0 | 0/28/0 |
| Lithium, Yes/No/NA | -- | 0/28/0 | 0/39/0 | 1/15/0 | 4/0/24 |
| Bipolar | 9 | 4 | 0 | 5 | 0 |
| Psychotic features | 19 | 0 | 16 | 3 | 0 |
| Single Episode | 6 | 0 | 2 | 2 | 2 |
| Treatment resistant, Yes/No, count | 105/3 | 28/0 | 36/3 | 13/0 | 28/0 |
| HDRS 17 Baseline Score, mean (SD) | 24.42 (6.31) | 23.89 (6.82) | 25.28 (6.70) | 28.0 (4.87) | 21.71 (4.80) |
| HDRS 17 Follow-up Score, mean (SD) | 12.0 (9.11) | 20.0 (9.41) | 6.64 (7.16) | 13.00 (6.71) | 10.92 (6.57) |
Abbreviations: RUL: Right unilateral stimulation; BT: Bi-temporal stimulation; LART: Left anterior right temporal stimulation; SSRI: Selective serotonin reuptake inhibitors; SNRI: Serotonin–norepinephrine reuptake inhibitor; TCA: Tricyclic antidepressants.
All ECT protocols were naturalistic (stimulus parameters were not manipulated for research purposes). Individual need for ECT was clinically determined at each site. Site 1 used a MECTA Spectrum 5000Q device (Oswego, Oregon) while all other sites used Thymatron IV devices (Somatics Inc., USA). Barbiturate (Methohexitol or Thiopental) induction was used at all sites. Treatment resistance was defined as failure to respond to at least two prior adequate medication trials for Sites 1 and 2; At least one unsuccessful psychotherapy for Site 4; and there were no strict criteria for treatment resistance for Site 3. Patients completed clinical assessments before and <2 weeks following ECT-IS, where ECT was administered 2-3 times weekly for Site 4 and 3 times weekly for Sites 1, 2, and 3. ECT-IS duration was clinically determined based on response speed and magnitude, ending when maximal response was achieved or lack of appreciable and sustained benefit. Within and across sites, modes of ECT administration included bitemporal, left anterior right temporal, and right unilateral electrode placement. Suicidal ideation was not exclusionary at any site. Patients at Site 1 were tapered off antidepressant medication before treatment, but at the other study sites participant’s antidepressant medications continued.
All participants provided written informed consent as approved by their local ethical committees or Institutional Review Boards (IRBs), and centralized analysis of pooled data was approved by the Regional Ethic Committee South-East in Norway (2013/1032 ECT and Neuroradiology, June 1st 2015).
Depression Symptom Severity Measure
Trained psychiatrists administered the HDRS-17 at each site immediately before and within two weeks following ECT-IS. As previously outlined, the HDRS-17 is among the most commonly used depression rating scales and has been validated in cross-cultural cohorts [13, 14]. However, its psychometric properties are criticized as it is a multidimensional scale and items have variety of ranges; some range between 0-4 while others range from 0-2.
Statistical methods
We conducted exploratory factor analyses (EFAs) of HDRS-17 items independently at each time point, with oblique, oblimin rotations [32] to allow for between-factor correlations, to identify latent symptom dimensions. A range of factor numbers was considered. For each solution, we assessed item cross loadings and average weighting onto each factor as goodness of fit criteria, favoring solutions with minimal cross loadings and higher average item weightings. Only items with loadings ≥0.3 were included for each factor and we required a minimum of 3 items loaded onto each factor. Subject-level factor loadings were then computed by a unit-weighting approach; that is, each factor was an unweighted sum of all HDRS items meeting the loading ≥0.3 criterion. Hierarchical clustering of HDRS-17 items was used to corroborate factor structures. This proceeded by defining the pairwise Euclidean distances between each item across all subject’s scores. All items were scaled to a range of ± 1. We applied Ward’s hierarchical agglomerative clustering method [33] to the Euclidean distance matrices to identify separable clusters.
We used an ANCOVA model to determine whether ECT conferred differential degrees of change on baseline symptom dimensions as a function of remission status. Here, remission was defined as a post-treatment HDRS-17 total score of ≤8. Because unit-weighted symptom dimensions had differing scales due to loadings from variable numbers of HDRS items with differing ranges, we z-transformed the baseline factor scores and z-transformed the factor scores at follow-up by scaling them relative to the baseline scores. Thus, the degree of change along each symptom dimension over ECT-IS was calculated as the difference between the z-transformed follow-up and baseline scores (follow-up - baseline). Symptom dimension change was then predicted from main effects of dimension ID (categorical), remission status (categorical), and an interaction of dimension ID and remission status. The model further included covariates for ECT parameters (pulse width, titration method (seizure threshold or ½ age) and number of RUL treatments), concurrent antidepressant treatment and antidepressant class, use of benzodiazepine, patient age, sex, and site. We adjusted for multiple comparisons in post-hoc tests using Tukey’s Range Test. All analyses were conducted in R 3.4.0 (https://www.r-project.org/).
Results
Demographic and clinical effects
Age differed significantly between the majority sites. Only sites 1 and 4 as well as 3 and 4 did not significantly differ in age, with Site 2 having the oldest patients (age=64.43 ± 9.08) on average. The proportion of female patients was consistent across the sites (χ2=2.35, df=3, p=0.50). Forty-eight patients achieved remission following ECT-IS. Remitting patients were significantly older (t=−3.75, df=108.54, p < 0.05) with the mean age of remitting and non-remitting patients being 57.6 and 47.9 years, respectively. Proportions of males and females did not differ significantly between remitting and non-remitting patients (χ2=0.67, df=1, p=0.41).
Pre-treatment HDRS factor structure
Baseline items exhibited a Kaiser-Meyer-Olkin (KMO) score [34] of 0.62 suggesting that they are only moderately amenable to EFA. Individual item-level measures of sampling adequacy (iMSA) were mostly moderate. However, several items’ iMSAs were low including middle insomnia (0.58), psychomotor retardation (0.58), psychomotor agitation (0.55), weight loss (0.55), and insight (0.49), suggesting they have poor psychometric properties or are unrelated to more dominant symptom constellations in this sample. The internal validity of baseline HDRS-17 items was acceptable with a Cronbach’s α (an estimate of reliability [35]) of 0.73, which was unaffected by dropping items with low iMSA.
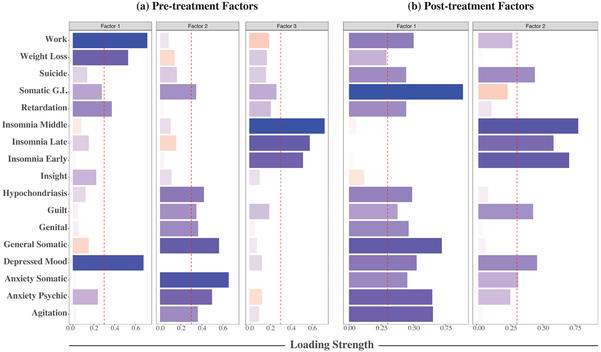
A three-factor solution was favored for baseline HDRS-17 items (see Figure 1(a)). All items cross-loaded, but the following items were predominantly loaded on Factor 1: work and interests, weight loss, psychomotor retardation, and depressed mood. Factor 2: consisted predominantly of somatic gastrointestinal (G.I.) symptoms, hypochondriasis, feelings of guilt, genital symptoms, general somatic symptoms, somatic anxiety, anxiety psychic, and psychomotor agitation. Factor 3: early insomnia, middle insomnia, and late insomnia. Suicide was spread across all three factors with loadings less than 0.2. Insight failed to adequately load onto a specific factor. Cronnbach’s α scores for each factor were moderate: Factor 1 (0.66), Factor 2 (0.69), and Factor 3 (0.63). In Supplemental Figure 1 we illustrate the clustering of HDRS-17 items by time point using hierarchical clustering. Hierarchical clustering of items largely mirrored the factor structure. No significant difference was observed between the percent change in the three factors over ECT-IS.
Figure 1.
(a) Pre-treatment HDRS factor structure and item loadings. Vertical dashed red lines indicate loading cutoff of 0.3, below which items are not considered adequately loaded onto a factor. Blue bars indicate positive factor loadings while red bars indicate negative loadings. (b) Post-treatment HDRS factor structure and item loadings.
Post-treatment HDRS factor structure
The KMO score for items at follow-up was 0.87. Individual MSA scores were high (above 0.8) except for late insomnia (0.73), weight loss (0.63), and insight (0.59). Cronbach’s α was also high (0.88). Two- and three-factor solutions were comparably viable; however, the average correlation between latent factors was lower in the two-factor solution (r=0.63, versus 0.73 in the three-factor solution). Here, Factor 1 consisted of depressed mood, guilt, suicide, work and interests, psychomotor retardation, psychomotor agitation, anxiety psychic, somatic anxiety, somatic G.I. symptoms, general somatic symptoms, genital symptoms, and hypochondriasis. Factor 2 comprised depressed mood, feelings of guilt, suicide, early insomnia, middle insomnia, late insomnia, and somatic anxiety. All solutions resulted in high degrees of cross-loading. Cronbach’s α scores for the factors were high: Factor 1 (0.90) and Factor 2 (0.86). The post-treatment factor structure is illustrated in Figure 1 (b).
Treatment-related changes in baseline HDRS factor structure
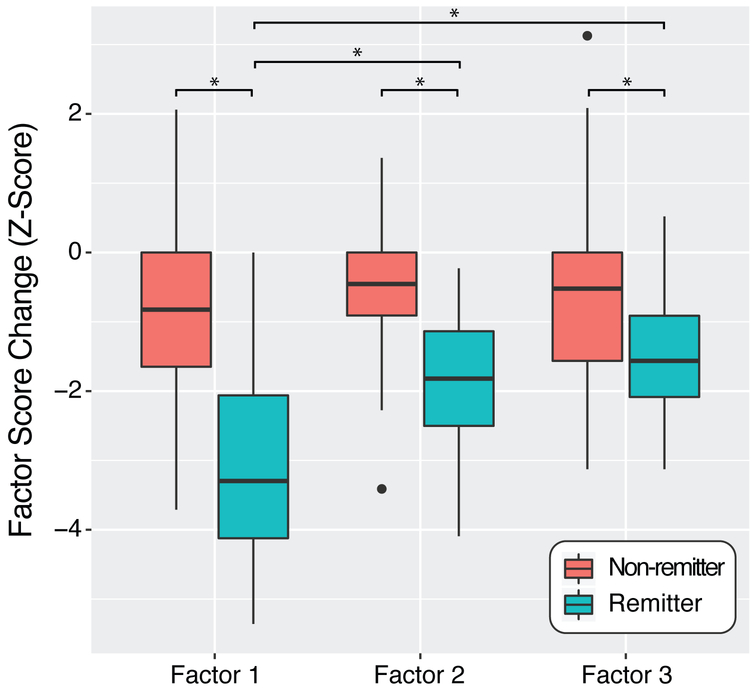
We observed a significant symptom dimension-by-remission status interaction (F=10.50, df=2, p<0.05) indicating that factors change differentially as a function of remission status. Among remitting patients, Factor 1 (core mood/anhedonia) was reduced significantly more than both Factor 2 (mean difference Z=1.13, p<0.05) and Factor 3 (mean difference Z=1.51, p<0.05). No difference was observed between Factors 2 and 3 among remitting patients. There was no evidence of significant differences in the change of symptom dimensions among non-remitting patients. All symptom dimensions were reduced significantly more among remitting patients compared to non-remitting patients (all p<0.05; see Figure 2).
Figure 2.
Symptom dimension changes over treatment by remission group.
Discussion
To determine whether ECT outcomes may benefit from patient selection based on features of a major depressive episode, we evaluated how ECT affects symptom dimensions of depression. EFA examined the factor structure of symptom dimensions as captured by HDRS-17 at pre-treatment and post-treatment. This revealed a three-factor representation of HDRS-17 items at baseline and a more uniform two-factor representation at follow-up. We further found a significant symptom dimension-by-remission status interaction wherein particular symptom dimensions changed differentially between remitting and non-remitting patients. Notably, the symptom dimension capturing core mood and anhedonia symptoms was relatively more improved than the other two symptom dimensions among remitting patients; however, no discernable differences in the change of symptom clusters was observed among non-remitting patients. Further, the severity of symptom dimensions did not significantly differ at pretreatment between subsequent remitting and non-remitting patients.
Our overarching premise is that depressive symptoms are heterogeneous and identifying sets of symptoms or symptom dimensions most responsive to ECT would improve clinical outcomes following treatment. As a number of previous studies have endeavored to predict response following ECT [36-39] or identify biomarkers associated with ECT outcome [40], a more precise measure of response defined along more homogenous symptom dimensions could improve the statistical power of related studies. Studies of antidepressant pharmacotherapuetic treatments show improved sensitivity for determining treatment efficacy by focusing only on unidimensional depression scales [21, 25]. To date, ECT studies have relied predominantly on depression symptom severity total scores, which have mainly been multidimensional in nature, to define response. Unfortunately, this approach has limited the ability to determine if ECT is more effective at treating specific depressive symptoms.
Factor analysis of the HDRS
Since depressive symptomatology is multidimensional and the HDRS-17 captures unidimensional depressive features, we conducted EFAs to identify latent symptom dimensions that might provide homogenous bases for measuring and investigating depressive symptomatology.
Most studies [41-43] used principal component analysis with a varimax rotation to identify factors, requiring the eigenvalues of each factor to be ≥1, and item loadings to be ≥0.4. However, this enforces that the recovered factors are orthogonal, which implies the absence of correlations and cross loadings across factors [44]. This restriction is unrealistic for the exploration of depressive symptom sets which are interrelated. Consequently, we instead used an approach that allowed for cross loading of HDRS-17 items and correlation between factors.
At baseline, HDRS-17 items mapped onto a three-factor solution. The first factor captured core aspects of MDD with contributions from depressed mood and work and interests (e.g., decreased pleasure, anhedonia). The second factor was largely comprised of items reflecting somatic symptoms (e.g., headache, gastrointestinal) and anxiety. The third factor was more clearly defined as it was comprised exclusively of the insomnia items.
These factor structures are somewhat consistent with previous findings. A meta-analysis by Bagby [10] reviewed 15 studies that factor analyzed the HDRS-17 and reported that the number of factors ranged between two to eight. The Bagby report showed that with 13 factorizations, the insomnia items loaded on a single factor, consistent with our third factor. Further, a ‘general depression’ factor that consisted of depressed mood, feelings of guilt, and suicide was observed in six studies. Moreover, six samples identified a common factorization of psychomotor agitation, psychic anxiety, somatic anxiety, and somatic G.I. symptoms, which support the existence of an anxiety and somatization factor. Indeed, multiple studies have supported that the HDRS-17 has an anxiety and somatization factor score [45].
Similar to Bagby’s meta-analysis, our baseline factorization observed a unidimensional sleep disturbance factor, and evidence for an overlapping anxiety/somatization factor. However, the ‘general depression’ factor was not replicated here as depressed mood, work and interests, and weight loss loaded onto the same factor. Interestingly, the suicide item was not significantly loaded onto any baseline factor, but exhibited sub-threshold cross-loadings onto each factor. As suicide was not exclusionary for any site this is unlikely to be an artifact of inclusion criteria.
The HDRS-17 items at follow-up appeared more homogeneous as our analysis found a two-factor solution. The second factor was composed predominantly of sleep-disturbance items alongside suicide, feelings of guilt, and depressed mood, which were heavily cross-loaded with the first factor. The first factor had significant contributions from all items with the exception of the insight and three insomnia items. Since most HDRS-17 items were more similar at post-treatment due to the robust antidepressant effects of ECT, the data may have been more spherical and expressed fewer discernible dimensions.
Differential Changes in Symptom Dimensions
Although all three pretreatment symptom dimensions were significantly reduced among remitting patients, the core mood/anhedonia symptom dimension was improved to a differentially higher degree compared to the somatic disturbances and insomnia symptom dimensions. This, however, was not observed among non-remitting patients. This finding supports that ECT disproportionately affects core symptoms of depression.
Taken together, our findings suggest that depressive symptoms cluster into discernable constellations, but these constellations become less distinct after ECT-IS. Although certain core symptoms of depression are disproportionately improved by ECT, each symptom dimension was significantly reduced among remitting patients. Among non-remitting patients, however, no discernible difference in the change of symptoms was observed. Further, remitters and non-remitters were not distinguished on the basis of pre-treatment symptom severities. These findings beg the question of whether unique neural mechanisms underlie these pre-treatment symptom constellations and if they uniquely or uniformly are affected by ECT, which warrants additional research. Further work is also warranted to identify pretreatment biomarkers predictive of the degree of response along each of these symptom dimensions.
Limitations
Several limitations must be considered. As a multi-site study, treatment protocols were naturalistic and uncontrolled across site. However, this is also a strength as it allowed us to capture diverse and realistic implementations of clinically administered ECT protocols. Although ECT stimulation parameters were uncontrolled across sites, we did control for such variables in the analyses. Prior studies have shown that ECT stimulus parameters and other treatment manipulations can affect the extent of response and magnitude of cognitive adverse effects [47-49]. These parameters might also influence the trajectory of symptom changes. We evaluated the effect of treatment parameters, however, and observed no significant associations with the change along each of the three latent symptom dimensions identified at baseline. Associations with ECT parameters were confounded with the study site, which is unavoidable in a naturalistic multi-site study. There were also confounds between patient age and site; however, no significant associations were found between symptom changes and patient age. Inclusion and exclusion criteria varied though the data was collected at sites that required that patients had failed at least one previous treatment or be in immediate need of ECT. Further, a minority of patients with bipolar disorder, psychotic features, and single-episode depression were included in this analysis. Although symptom constellations in these groups might differ from those with recurrent unipolar depression, they reflect the naturalistic design of this study and help account for the expected variability in patients eligible for ECT. Notably, patients from Site 1 exhibited the least symptom improvement (Table 1); perhaps due to the higher proportion of males or absence of concurrent antidepressant medication.
Conclusions and future directions
To our knowledge, this is the first study to evaluate the trajectory of HDRS-17 symptom dimensions over ECT-IS, and identify latent factors for HDRS-17 items pre and post ECT. We demonstrated that there were latent depressive symptom dimensions at baseline consistent with prior research, and there were discernable changes in latent depressive symptom trajectories over ECT-IS. Our results suggested that ECT may disproportionately improve particular symptom constellations. Additional studies are needed to evaluate related antidepressant treatments to determine whether other treatment modalities differentially affect specific depressive symptom dimensions. Future studies seeking to minimize the heterogeneity of depressive symptoms and the multidimensionality inherent in the HDRS-17 scale could leverage these dimensions. Our ongoing work will investigate relationships between these depressive symptom dimensions and neuroimaging data available in the GEMRIC data to determine their underlying neurobiology to optimize antidepressant neuromodulation treatment delivery and precision medicine approaches.
Supplementary Material
Supplementary Figure 1. Dendograms illustrating hierarchical clustering of HDRS items by time point.
Acknowledgments
Conflicts of Interest and Source of Funding: This work is supported in part by the National Institute of Mental Health (MH092301, MH110008 and MH102743 to UCLA investigators). This study is supported by Western Norway Regional Health Authority, Haukeland University Hospital and the University of Bergen, Norway. Additionally, individual sites acknowledge support from: The Muriel Harris Chair in Geriatric Psychiatry (RE); The Lundbeck Foundation (MJB); the Münster Cohort was funded by the German Research Foundation (DFG, grant FOR2107, 1151/5-1 to UD) and Innovative Medizinische Forschung (IMF, RE111604 to RR); Centers of Biomedical Research Excellence (2P20GM103472-01 to CA); BD is supported by the Swiss National Science Foundation (NCCR Synapsy, project grant Nr 32003B 159780), Foundation Parkinson Switzerland and Foundation Synapsis. LREN is very grateful to the Roger de Spoelberch and Partridge Foundations for their generous financial support; for UCLA, funding was obtained through Award Numbers R01MH092301 and K24MH102743 from the National Institute of Mental Health. BW is supported by a NARSAD Young Investigator Grant (#27786).
Financial support
This work is supported in part by a NARSAD Young Investigator Grant (grant number 27786 to BW) and the National Institute of Mental Health (MH092301, MH110008 and MH102743 to UCLA investigators). This study is supported by Western Norway Regional Health Authority, Haukeland University Hospital and the University of Bergen, Norway. Additionally, individual sites acknowledge support from: The Muriel Harris Chair in Geriatric Psychiatry (RE); The Lundbeck Foundation (MJB); the Münster Cohort was funded by the German Research Foundation (DFG, grant FOR2107, 1151/5-1 to UD) and Innovative Medizinische Forschung (IMF, RE111604 to RR); Centers of Biomedical Research Excellence (2P20GM103472-01 to CA); BD is supported by the Swiss National Science Foundation (NCCR Synapsy, project grant Nr 32003B 159780), Foundation Parkinson Switzerland and Foundation Synapsis. LREN is very grateful to the Roger de Spoelberch and Partridge Foundations for their generous financial support; for UCLA, funding was obtained through Award Numbers R01MH092301 and K24MH102743 from the National Institute of Mental Health.
Footnotes
Conflict of interest
The authors have no conflicts of interest related to this work.
References
- [1].Kessler RC and Bromet EJ, “The epidemiology of depression across cultures,” Annu Rev Public Health, vol. 34, pp. 119–38, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].World Health Organization. (2018, 22 August). Depression. Available: http://www.who.int/news-room/fact-sheets/detail/depression
- [3].Trivedi MH et al. , “Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice,” Am J Psychiatry, vol. 163, no. 1, pp. 28–40, January 2006. [DOI] [PubMed] [Google Scholar]
- [4].van Loo HM, de Jonge P, Romeijn JW, Kessler RC, and Schoevers RA, “Data-driven subtypes of major depressive disorder: a systematic review,” (in eng), BMC Med, vol. 10, p. 156, December 4 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Holtzheimer PE and Nemeroff CB, “Novel targets for antidepressant therapies,” Curr Psychiatry Rep, vol. 10, no. 6, pp. 465–73, December 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].American Psychiatric Association, The Practice of Electroconvulsive Therapy, 2 ed. 2001, p. 368. [Google Scholar]
- [7].Pagnin D, de Queiroz V, Pini S, and Cassano GB, “Efficacy of ECT in depression: a meta-analytic review,” J ECT, vol. 20, no. 1, pp. 13–20, March 2004. [DOI] [PubMed] [Google Scholar]
- [8].McClintock SM, Haley C, and Bernstein IH, “Psychometric considerations of depression symptom rating scales,” (in English), Neuropsychiatry, vol. 1, no. 6, pp. 611–623, December 2011. [Google Scholar]
- [9].Hamilton M, “A rating scale for depression,” J Neurol Neurosurg Psychiatry, vol. 23, pp. 56–62, February 1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bagby RM, Ryder AG, Schuller DR, and Marshall MB, “The Hamilton Depression Rating Scale: has the gold standard become a lead weight?,” Am J Psychiatry, vol. 161, no. 12, pp. 2163–77, December 2004. [DOI] [PubMed] [Google Scholar]
- [11].Edwards BC, Lambert MJ, Moran PW, McCully T, Smith KC, and Ellingson AG, “A meta-analytic comparison of the Beck Depression Inventory and the Hamilton Rating Scale for Depression as measures of treatment outcome,” Br J Clin Psychol, vol. 23 (Pt 2), pp. 93–9, May 1984. [DOI] [PubMed] [Google Scholar]
- [12].Lambert MJ, Hatch DR, Kingston MD, and Edwards BC, “Zung, Beck, and Hamilton Rating Scales as measures of treatment outcome: a meta-analytic comparison,” J Consult Clin Psychol, vol. 54, no. 1, pp. 54–9, February 1986. [DOI] [PubMed] [Google Scholar]
- [13].Hamdi E, Amin Y, and Abou-Saleh MT, “Performance of the Hamilton Depression Rating Scale in depressed patients in the United Arab Emirates,” Acta Psychiatr Scand, vol. 96, no. 6, pp. 416–23, December 1997. [DOI] [PubMed] [Google Scholar]
- [14].Tylee A and Gandhi P, “The importance of somatic symptoms in depression in primary care,” Prim Care Companion J Clin Psychiatry, vol. 7, no. 4, pp. 167–76, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gibbons RD, Clark DC, and Kupfer DJ, “Exactly what does the Hamilton Depression Rating Scale measure?,” (in eng), J Psychiatr Res, vol. 27, no. 3, pp. 259–73, Jul-Sep 1993. [DOI] [PubMed] [Google Scholar]
- [16].Bech P et al. , “The Hamilton depression scale. Evaluation of objectivity using logistic models,” Acta Psychiatr Scand, vol. 63, no. 3, pp. 290–9, March 1981. [DOI] [PubMed] [Google Scholar]
- [17].Boone WJ, “Rasch Analysis for Instrument Development: Why, When, and How?,” CBE Life Sciences Education, vol. 15, no. 4, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bech P, Allerup P, Maier W, Albus M, Lavori P, and Ayuso JL, “The Hamilton scales and the Hopkins Symptom Checklist (SCL-90). A cross-national validity study in patients with panic disorders,” Br J Psychiatry, vol. 160, pp. 206–11, February 1992. [DOI] [PubMed] [Google Scholar]
- [19].Bech P, Tanghoj P, Andersen HF, and Overo K, “Citalopram dose-response revisited using an alternative psychometric approach to evaluate clinical effects of four fixed citalopram doses compared to placebo in patients with major depression,” Psychopharmacology (Berl), vol. 163, no. 1, pp. 20–5, August 2002. [DOI] [PubMed] [Google Scholar]
- [20].Ostergaard SD, Bech P, and Miskowiak KW, “Fewer study participants needed to demonstrate superior antidepressant efficacy when using the Hamilton melancholia subscale (HAM-D(6)) as outcome measure,” J Affect Disord, vol. 190, pp. 842–845, January 15 2016. [DOI] [PubMed] [Google Scholar]
- [21].Faries D, Herrera J, Rayamajhi J, DeBrota D, Demitrack M, and Potter WZ, “The responsiveness of the Hamilton Depression Rating Scale,” J Psychiatr Res, vol. 34, no. 1, pp. 3–10, Jan-Feb 2000. [DOI] [PubMed] [Google Scholar]
- [22].Williams JB, “Standardizing the Hamilton Depression Rating Scale: past, present, and future,” Eur Arch Psychiatry Clin Neurosci, vol. 251 Suppl 2, pp. II6-12, 2001. [DOI] [PubMed] [Google Scholar]
- [23].Santen G, Gomeni R, Danhof M, and Della Pasqua O, “Sensitivity of the individual items of the Hamilton depression rating scale to response and its consequences for the assessment of efficacy,” J Psychiatr Res, vol. 42, no. 12, pp. 1000–9, October 2008. [DOI] [PubMed] [Google Scholar]
- [24].Bech P, “The responsiveness of the different versions of the Hamilton Depression Scale,” World Psychiatry, vol. 14, no. 3, pp. 309–10, October 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Entsuah R, Shaffer M, and Zhang J, “A critical examination of the sensitivity of unidimensional subscales derived from the Hamilton Depression Rating Scale to antidepressant drug effects,” J Psychiatr Res, vol. 36, no. 6, pp. 437–48, Nov-Dec 2002. [DOI] [PubMed] [Google Scholar]
- [26].Webb CA et al. , “Personalized prediction of antidepressant v. placebo response: evidence from the EMBARC study,” (in eng), Psychol Med, pp. 1–10, July 2 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Drysdale AT et al. , “Resting-state connectivity biomarkers define neurophysiological subtypes of depression,” Nat Med, vol. 23, no. 1, pp. 28–38, January 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Okazaki M et al. , “Predictors of response to electroconvulsive therapy obtained using the three-factor structure of the Montgomery and Asberg Depression Rating Scale for treatment-resistant depressed patients,” J ECT, vol. 26, no. 2, pp. 87–90, June 2010. [DOI] [PubMed] [Google Scholar]
- [29].Oltedal L et al. , “The Global ECT-MRI Research Collaboration (GEMRIC): Establishing a multi-site investigation of the neural mechanisms underlying response to electroconvulsive therapy,” Neuroimage Clin, vol. 14, pp. 422–432, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Papakostas GI et al. , “Somatic symptoms in treatment-resistant depression,” Psychiatry Res, vol. 118, no. 1, pp. 39–45, May 1 2003. [DOI] [PubMed] [Google Scholar]
- [31].Papakostas GI et al. , “Psychic and somatic anxiety symptoms as predictors of response to fluoxetine in major depressive disorder,” Psychiatry Res, vol. 161, no. 1, pp. 116–20, October 30 2008. [DOI] [PubMed] [Google Scholar]
- [32].Brown JD, “Choosing the Right Type of Rotation in PCA and EFA,” Shiken: JALT Testing & Evaluation SIG Newsletter, vol. 13, no. 3, pp. 20–25, 2009. [Google Scholar]
- [33].Murtagh F and Legendre P, “Ward’s Hierarchical Agglomerative Clustering Method: Which Algorithms Implement Ward’s Criterion?,” (in English), Journal of Classification, vol. 31, no. 3, pp. 274–295, October 2014. [Google Scholar]
- [34].Kaiser HF, “An index of factorial simplicity,” Psychometrika, vol. 39, no. 1, pp. 31–36, 1974/March/01 1974. [Google Scholar]
- [35].Cronbach LJ, “Coefficient alpha and the internal structure of tests,” Psychometrika, vol. 16, no. 3, pp. 297–334, 1951/September/01 1951. [Google Scholar]
- [36].van Waarde JA, Scholte HS, van Oudheusden LJ, Verwey B, Denys D, and van Wingen GA, “A functional MRI marker may predict the outcome of electroconvulsive therapy in severe and treatment-resistant depression,” Mol Psychiatry, vol. 20, no. 5, pp. 609–14, May 2015. [DOI] [PubMed] [Google Scholar]
- [37].Wade BS et al. , “Effect of Electroconvulsive Therapy on Striatal Morphometry in Major Depressive Disorder,” (in eng), Neuropsychopharmacology, vol. 41, no. 10, pp. 2481–91, September 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Wade BSC et al. , “Data-driven cluster selection for subcortical shape and cortical thickness predicts recovery from depressive symptoms,” in 2017 IEEE 14th International Symposium on Biomedical Imaging (ISBI 2017), 2017, pp. 502–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Jiang R et al. , “SMRI Biomarkers Predict Electroconvulsive Treatment Outcomes: Accuracy with Independent Data Sets,” Neuropsychopharmacology, July 31 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Leaver AM, Espinoza R, Pirnia T, Joshi SH, Woods RP, and Narr KL, “Modulation of intrinsic brain activity by electroconvulsive therapy in major depression,” Biol Psychiatry Cogn Neurosci Neuroimaging, vol. 1, no. 1, pp. 77–86, January 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Addington D, Addington J, and Atkinson M, “A psychometric comparison of the Calgary Depression Scale for Schizophrenia and the Hamilton Depression Rating Scale,” Schizophr Res, vol. 19, no. 2-3, pp. 205–12, May 1996. [DOI] [PubMed] [Google Scholar]
- [42].Akdemir A, Turkcapar MH, Orsel SD, Demirergi N, Dag I, and Ozbay MH, “Reliability and validity of the Turkish version of the Hamilton Depression Rating Scale,” Compr Psychiatry, vol. 42, no. 2, pp. 161–5, Mar-Apr 2001. [DOI] [PubMed] [Google Scholar]
- [43].Fleck MP, Poirier-Littre MF, Guelfi JD, Bourdel MC, and Loo H, “Factorial structure of the 17-item Hamilton Depression Rating Scale,” (in eng), Acta Psychiatr Scand, vol. 92, no. 3, pp. 168–72, September 1995. [DOI] [PubMed] [Google Scholar]
- [44].Costello AB and Osborne JW, “best practices in exploratory factor analysis: Four Recommendations for Getting the Most From Your Analysis,” Practical Assessment, Research & Evaluation, vol. 10, 2005. [Google Scholar]
- [45].McClintock SM et al. , “Assessing anxious features in depressed outpatients,” Int J Methods Psychiatr Res, vol. 20, no. 4, pp. e69–82, December 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Suzuki A et al. , “A three-factor model of the MADRS in major depressive disorder,” Depress Anxiety, vol. 21, no. 2, pp. 95–7, 2005. [DOI] [PubMed] [Google Scholar]
- [47].Spellman T, Peterchev AV, and Lisanby SH, “Focal electrically administered seizure therapy: a novel form of ECT illustrates the roles of current directionality, polarity, and electrode configuration in seizure induction,” (in eng), Neuropsychopharmacology, vol. 34, no. 8, pp. 2002–10, July 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Kolshus E, Jelovac A, and McLoughlin DM, “Bitemporal v. high-dose right unilateral electroconvulsive therapy for depression: a systematic review and meta-analysis of randomized controlled trials,” (in eng), Psychol Med, vol. 47, no. 3, pp. 518–530, February 2017. [DOI] [PubMed] [Google Scholar]
- [49].Sackeim HA et al. , “A prospective, randomized, double-blind comparison of bilateral and right unilateral electroconvulsive therapy at different stimulus intensities,” Arch Gen Psychiatry, vol. 57, no. 5, pp. 425–34, May 2000. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Dendograms illustrating hierarchical clustering of HDRS items by time point.