Abstract
MBW protein complexes containing MYB, bHLH and WD40 repeat factors are known transcriptional regulators of secondary metabolites production such as proanthocyanidins and anthocyanins, and developmental processes such as trichome formation in many plant species. DkMYB2 and DkMYB4 (MYB-type), DkMYC1 (bHLH-type) and DkWDR1 (WD40-type) factors have been proposed by different authors to take part of persimmon MBW complexes for proanthocyanidin accumulation in immature fruit, leading to its characteristic astringent flavour with important agronomical and ecological effects. We have confirmed the nuclear localization of these proteins and their mutual physical interaction by bimolecular fluorescence complementation analysis. In addition, transient expression of DkMYB2, DkMYB4 and DkMYC1 cooperatively increase the expression of a persimmon anthocyanidin reductase gene (ANR), involved in the biosynthesis of cis-flavan-3-ols, the structural units of proanthocyanidin compounds. Collectively, these data support the presence of MBW complexes in persimmon fruit and suggest their coordinated participation in ANR regulation for proanthocyanidin production.
Subject terms: Plant development, Fruiting, Secondary metabolism, Agricultural genetics, Plant breeding
Introduction
Proanthocyanidins (PAs), or condensed tannins, are flavonoid polymers that accumulate in fruits, leaves, seeds and other tissues of many plants, providing protection against pathogens and herbivores. PAs also contribute to fruit flavour and colour and are considered beneficial for human health in virtue of their antioxidant properties, among other salutary attributes1. In persimmon (Diospyros kaki), the microstructure and accumulation of soluble PAs of the so called tannin cells has been found related to fruit astringent taste2,3, potentially affecting fruit palatability for frugivorous animals4. Interestingly, soluble tannins are reduced throughout persimmon fruit development and maturation3,5, providing a way to channel the action of frugivores when seeds are fully viable and ready for dispersal. In addition to a poorly known mechanism involving the transcriptional repression of PA biosynthetic enzymes6, soluble PA content is reduced during fruit ripening by the production of acetaldehyde by seeds, leading to PA insolubilization and the subsequent astringency loss7,8. Although the content of soluble tannins becomes undetectable from a sensory point of view at overripening stages, the concomitant loss of fruit firmness importantly limits fruit postharvest life and therefore the commercialization opportunities. To overcome this limitation, the fruit is harvested before overripening and subjected to deastringency postharvest treatments to remove astringency while maintaining high firmness9. Most of deastringency methods in persimmon are based on maintaining the fruit under anaerobic conditions or exposing them to products that induce anaerobic respiration. Under these conditions, soluble tannins are polymerized by acetaldehyde accumulated in the flesh10,11. Furthermore, natural non-astringent mutants exist into persimmon germplasm collections. Several of these non-astringent cultivars are hypothesized to carry a recessive mutation in a single gene known as AST12,13, but the molecular function and identity of this gene remain unknown. Postharvest treatments of astringent varieties improve the postharvest life and the organoleptic quality of treated fruit, however these treatments represent an important production cost and are often a challenge for new varieties or stressed orchards14. Thus, studying PAs biosynthesis and metabolism in persimmon fruit may help to better understand the different mechanisms employed by plants to drive frugivore-dependent dispersal of seeds, and to improve deastringency treatments and crop management with the aim to reduce costs and increase the sustainability of persimmon production.
The pathway of PAs biosynthesis has been genetically dissected by the analysis of different seed mutants in Arabidopsis thaliana, barley and maize among other species15. PAs are formed by condensation of trans- and cis-flavan-3-ols units, synthesized respectively by stereospecific leucoanthocyanidin reductase (LAR) and anthocyanidin reductase (ANR) enzymes16,17. These PA biosynthetic activities and genes are essentially conserved in persimmon, with some regulatory particularities18. In persimmon fruit, ANR gene is much more expressed than its counterpart LAR, consistent with the higher content of cis-flavan-3-ols stereoisomers in PA composition19. In addition, ANR is strongly repressed in advanced steps of fruit maturation, concomitantly with tannin decrease, which points to a role of ANR as a major integrative target of PA regulatory pathways in persimmon.
In Arabidopsis, regulation of the ANR orthologous gene BANYULS (BAN) and PA accumulation in seed coat requires the concerted action of TRANSPARENT TESTA2 (TT2), TRANSPARENT TESTA8 (TT8) and TRANSPARENT TESTA GLABRA1 (TTG1), encoding respectively a R2R3-MYB transcription factor, a basic helix-loop-helix (bHLH) transcription factor and a WD40-repeat (WDR) protein20. These regulatory factors form a ternary complex named MYB-bHLH-WD40 (MBW) that may invoke the participation of alternative MYB and bHLH components for the regulation of particular steps of PA and anthocyanin biosynthetic pathways21,22. MBW complexes also contribute to PAs production in edible fruits of crop plants such as grapevine23, strawberry24, apple25 and persimmon26.
In persimmon, DkMYB2 and DkMYB4 genes cause an altered pattern of PAs accumulation and expression of biosynthetic enzymes when misexpressed, and increase ANR promoter transcriptional activity in transient reporter assays when combined with a heterologous bHLH factor from Arabidopsis27,28. Interestingly, DkMYB2 and DkMYB4 specifically recognize different MYB-binding cis-elements by electrophoretic mobility shift assays, arguing for certain degree of subfunctionalization28. On the other hand, a persimmon bHLH gene named DkMYC1 is underexpressed in non-astringent cultivars, following an expression pattern in fruit similar to DkMYB429. DkMYC1 protein interacts with DkMYB2 and DkMYB4, and these, in turn, interact with the WD40-repeat protein DkWDR1 by two-hybrid analysis, suggesting the conserved participation of MBW complexes in PA synthesis regulation in persimmon fruit26. In this study, we go further on the cytological and molecular characterization of MBW complex in persimmon by approaching the subcellular localization, protein interaction and transcriptional regulation effects of these MYB (DkMYB2 and DkMYB4), bHLH (DkMYC1) and WD40 (DkWDR1) components.
Results
Subcellular localization of MBW complex components
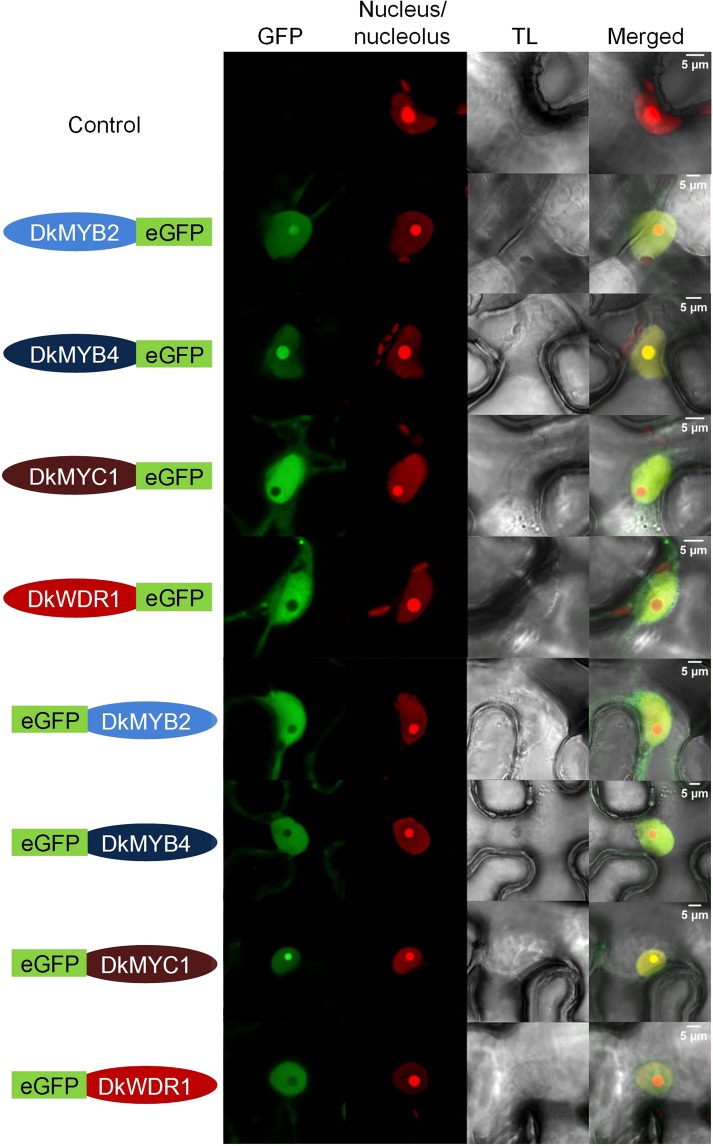
As transcription factors belonging to a hypothetical regulatory protein complex, DkMYB2, DkMYB4, DkMYC1 and DkWDR1 are expected to co-localize temporarily in the cell nucleus where they interact and perform their regulatory role within the framework of a developmental programme. The subcellular localization of these four putative MBW components in persimmon has been elucidated by transient expression in Nicotiana benthamiana leaves of the four corresponding genes fused to enhanced green fluorescent protein (eGFP) gene. According to the localization of specific nucleus and nucleolus markers, DkMYB2, DkMYB4 and DkMYC1 show differential nucleus/nucleolus partitioning when eGFP fusion is either at the N-terminal (Nt) or C-terminal (Ct), suggesting that eGFP position affects protein targeting (Fig. 1). Protein fusions with higher abundance in the nucleolus are DkMYB2-eGFP, DkMYB4-eGFP and eGFP-DkMYC1, whereas DkWDR1-eGFP shows appreciable presence in the cytoplasm in spite of its predominant localization in the nucleus. Overall, these four transcription factors show preferential localization in the nuclear compartment, and consequently physical interactions at the protein level occur most plausibly in the nucleus.
Figure 1.
Nuclear localization of MBW factors. Nicotiana benthamiana leaves agroinfiltrated with DkMYB2, DkMYB4, DkMYC1 and DkWDR1 constructs containing Ct (right green label) or Nt (left green label) eGFP tags, were co-expressed with nucleus/nucleolus markers. The green (GFP), red (nucleus/nucleolus marker), transmitted light (TL) channels and merged images are shown in the figure. The fluorescent signals were visualized at 72 hours post-infiltration. Scale bars are shown in merged images.
In vivo interaction of DkMYB2, DkMYB4, DkMYC1 and DkWDR1
Physical protein interactions among members of MBW complex involved in PAs accumulation in persimmon fruit has been only previously tested by the two-hybrid system in the yeast model, however no additional in planta evidences have been obtained on the formation of this complex. To asses this issue, we have assayed pair-wise interactions between DkMYB2, DkMYB4, DkMYC1 and DkWDR1 factors by bimolecular fluorescence complementation (BiFC) and transient expression in N. benthamiana. The Nt and Ct fragments of the yellow fluorescent protein (YFP), required for reassembling of the fluorescent reporter, have been tested on both Nt and Ct sides of each transcription factor. Positive protein interactions by BiFC are shown in Fig. 2a. According to these results, DkMYB4 and DkMYC1 are able to interact with the rest of the factors and with themselves, and DkWDR1 does not reconstitute YFP fluorescence when paired with DkMYB2 and itself. This reproduces previous yeast two-hybrid results with few exceptions (Fig. 2b). Particularly, DkMYC1 homodimerization was not observed, and DkMYB2 self-interaction was not assayed due to autoactivation issues in former yeast-two hybrid experiments. Moreover, DkMYB2-DkWDR1 and DkMYC1-DkWDR1 interactions have been exclusively detected by yeast-two hybrid and BiFC analysis, respectively. A western analysis of transiently transformed leaves confirms that DkWDR1 and DkMYB2 were successfully co-expressed in different construct combinations and hence, the absent interaction of DkWDR1 with itself and DkMYB2 are not due to deficient protein synthesis or accumulation (Supplementary Fig. S1). Overall, these data support the biochemical ability of these factors to associate in a putative MBW complex in planta.
Figure 2.
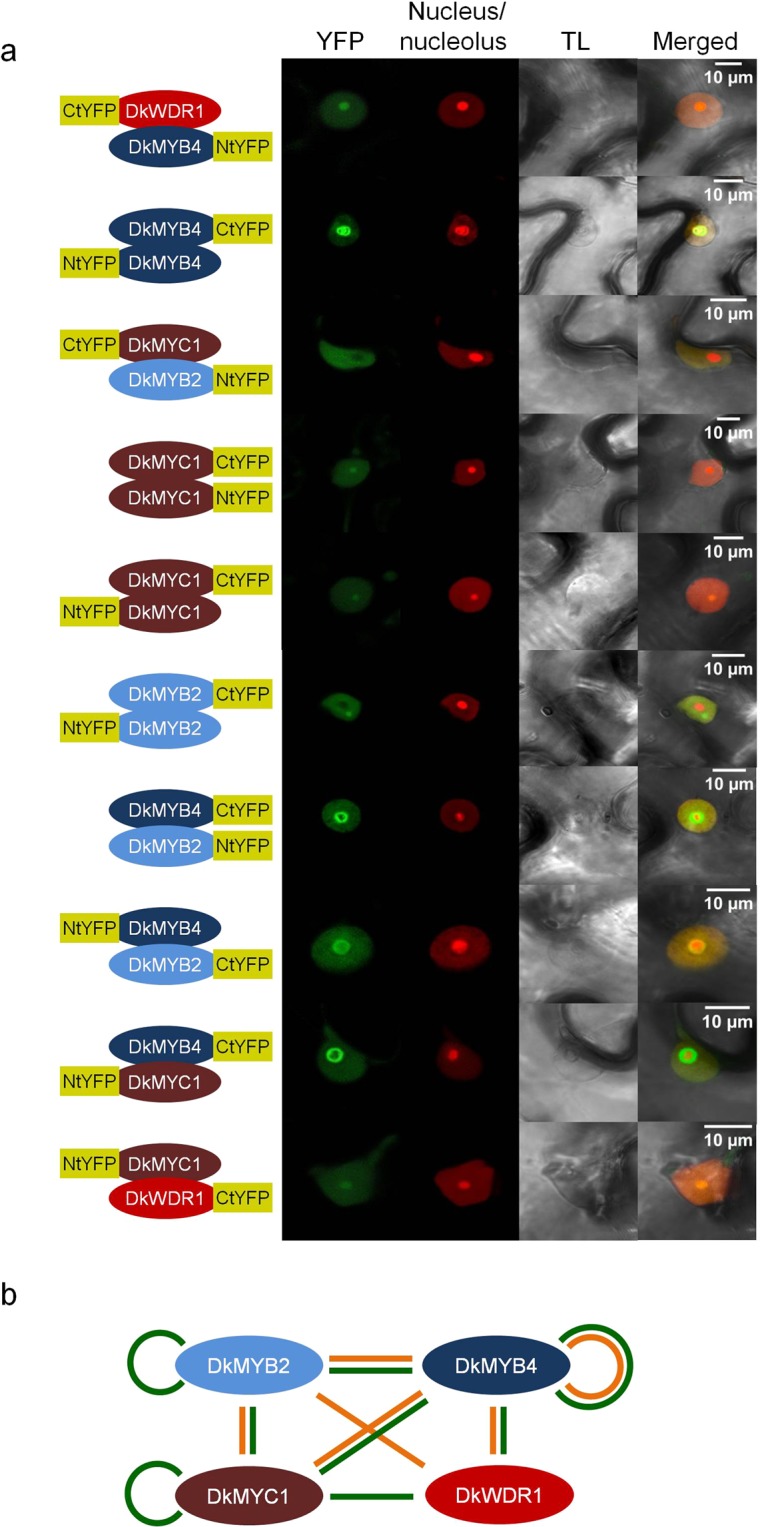
Protein interactions of MBW factors by bimolecular fluorescence complementation assays (BiFC). N. benthamiana leaves agroinfiltrated with different combinations of MBW factors fused to NtYFP or CtYFP peptides in Ct (right yellow label) or Nt (left yellow label) positions together with nucleus/nucleolus markers (a). The green reconstituted fluorescence (YFP), red (nucleus/nucleolus marker), transmitted light (TL) channels and merged images are shown in the figure. A diagram of previous yeast two-hybrid (orange lines) and positive BiFC interactions obtained in this study (green lines) (b). The fluorescent signals were visualized at 72 hours post-infiltration. Scale bars are shown in merged images.
DkANR expression correlates well with PA accumulation and astringency
PA content has been measured at different points of fruit development in ‘Hachiya’ cultivar, starting in July and finishing in September, after external colour change has been initiated and before ripening leads to fruit softening and natural deastringency (Fig. 3a). During that period fruit average weight increases three-fold and the percentage of PA decreases concomitantly (Fig. 3a). Fruit soluble PA content is a balance between PA biosynthesis and insolubilization. PA insolubilization is mediated by acetaldehyde accumulation as a result of ripening and deastringency treatments10,11. Acetaldehyde content in these ‘Hachiya’ fruit samples reaches values around 0.1 mg per 100 ml of juice (Fig. 3b), which is by far lower than acetaldehyde produced in stored fruit and fruit treated for deastringency3,30. Thus, PA insolubilization due to acetaldehyde accumulation is not expected to contribute significantly to reduce soluble PA level in our samples, and PA content is mostly dependent on its biosynthesis rate. When representing the total estimated amount of PAs per fruit instead of its relative percentage, PA amount remains almost unchanged during the whole interval, with the exception of an initial increase in July samples (Fig. 3b). As we consider that PA reduction by acetaldehyde-dependent insolubilization is relatively low, the observed decrease in relative PA content in Fig. 3a must be mostly due to a growth dilution effect, and the rate of PA biosynthesis is expected to be also low in this period.
Figure 3.
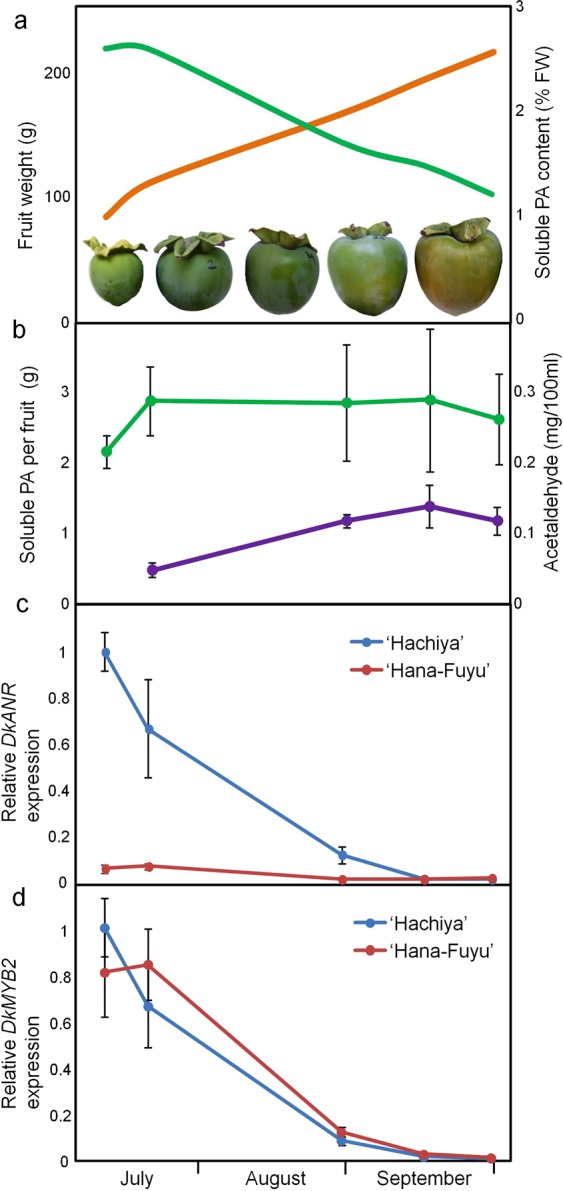
Proanthocyanidin (PA) content and gene expression during fruit development in persimmon. Fruit weight (orange line) and PA relative content (green line) in ‘Hachiya’ cultivar at different fruit development stages (a). Acetaldehyde accumulation (violet label) and total estimated PA content per fruit unit (green label) in ‘Hachiya’ (b). Relative gene expression of DkANR (c) and DkMYB2 (d) by qRT-PCR in the fruit samples shown in (a,b). The astringent cultivar ‘Hachiya’ (blue label) and the non-astringent cultivar ‘Hana Fuyu’ with the ast mutation (red label) have been analyzed. Data are means from four different fruits for anatomical and chemical analysis, and two biological replicates for gene expression. Error bars represent standard deviations.
As anthocyanidin reductase encoded by ANR gene has been postulated to perform a key role in PA biosynthesis, we have measured DkANR expression in ‘Hachiya’ fruit samples by qRT-PCR. DkANR transcript sharply decreases until a 0.01-fold change during the whole period (Fig. 3c), which cannot be explained by just growth dilution effects. On the contrary, it indicates a strong transcriptional repression in advanced developmental stages. The higher expression of DkANR in initial samples is in close agreement with the concurrent increase in total PA content per fruit (Fig. 3b). Then PA content remains steady in concordance with DkANR down-regulation (Fig. 3b,c). Such a positive correlation between DkANR expression and astringent-responsible PAs is confirmed in the low-PA non-astringent cultivar ‘Hana Fuyu’, which shows a constantly low DkANR expression during fruit development stages (Fig. 3c).
Other PA biosynthetic regulators, such as DkMYB4 and DkMYC1, reproduce well this low expression profile in ‘Hana Fuyu’ and other non-astringent cultivars, as shown in previous studies26,27,29. However, DkMYB2 relative expression decays in a similar way in both astringent and non-astringent cultivars (Fig. 3d), suggesting a common mechanism involving AST locus-dependent regulation of DkMYB4 and DkMYC1, with no impact on DkMYB2 expression.
Effect of MBW factors on the activity of ANR promoter
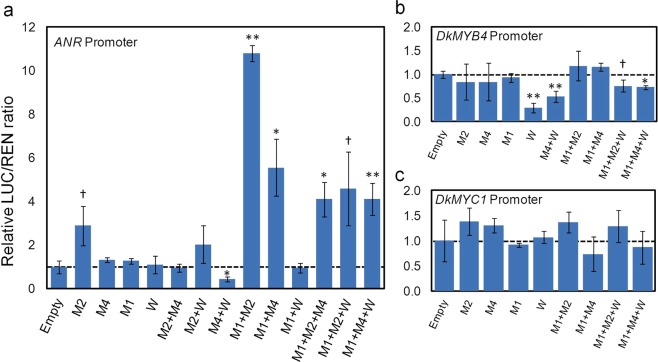
Based on PA-linked expression of these genes, we have cloned three DNA fragments (1.2–1.4 kb) of the promoter and 5′ UTR of DkMYB4, DkMYC1 and the D. lotus ANR, in the pGreenII-0800-LUC vector. These vectors synthesize luciferase reporter (LUC) under the action of our selected promoters, in order to test the regulatory effect of individual and combined MBW factors on the activity of these promoters by a dual luciferase assay, using expression of the REN reporter gene as internal reference.
DkMYB2 transient expression in N. benthamiana leaves increases ANR promoter transcriptional activity three-fold, whereas DkMYB4, DkMYC1 and DkWDR1 do not modify it significantly (Fig. 4a). Interestingly, any combination of two or three elements containing both DkMYC1 and a MYB gene (DkMYB2 or DkMYB4), strongly increases LUC/REN ratio, being highest when DkMYC1 and DkMYB2 are co-expressed. On the contrary, DkWDR1 does not improve ANR promoter expression under any gene combination.
Figure 4.
Analysis of the transcriptional activity of relevant PA biosynthesis regulatory promoters by dual luciferase assay. The LUC/REN ratio of N. benthamiana cells agroinfiltrated with ANR (a), DkMYB4 (b) and DkMYC1 (c) promoters driving the LUC gene reporter, and regulatory genes DkMYB2 (M2), DkMYB4 (M4), DkMYC1 (M1) and DkWDR1 (W) under different combinations, was made relative to mock agroinfiltrations with pGreenII-62-SK (value of 1 labelled with a discontinuous line). Data are means from three replicates with error bars represent standard deviations. Symbols indicate statistical differences with respect to empty sample determined by Student’s t test (†P < 0.1, *P < 0.05, **P < 0.01).
As DkMYB4 and DkMYC1 genes are similarly down-regulated during fruit development concomitantly with PA reduction, and are differentially expressed in a non-astringent cultivar26,27,29, their promoters have been also cloned and tested by dual luciferase assays in N. benthamiana. MBW factors assayed in the experiment do not increase the activity of DkMYB4 nor DkMYC1 promoters, however DkWDR1 transient expression associates with a significant decrease in LUC expression driven by DkMYB4 promoter (Fig. 4b,c).
Discussion
DkMYB2, DkMYB4, DkMYC1 and DkWDR1 have been postulated to co-regulate the expression of PA biosynthesis genes in persimmon as a complex26, and hence a coordinated nuclear co-localization of them is expected. Related components of MBW complexes in other species have been found located in the nucleus (TT2)31, or partitioned in nucleus and cytoplasm (TTG1 and VvMYC1)23,32. In this study, DkMYB2, DkMYB4, DkMYC1 and DkWDR1 have been mostly localized in the cell nucleus, but DkMYB2, DkMYB4, DkMYC1 show a differential nucleus-nucleolus partitioning depending on the Nt or Ct position of the eGFP fusion. To our knowledge, this has not been previously observed in other MBW factors, although in most of these cases, only one Nt or Ct fusion is assayed. Indeed, a systematic approach in yeast has shown that a high percentage of proteins display different subcellular localization when GFP is tagged at either the Nt or Ct33.
Transient expression in N. benthamiana and BiFC analysis support the ability of DkMYB2, DkMYB4, DkMYC1 and DkWDR1 proteins to interact with each other in vivo, which in fact reinforces previous two-hybrid data in the yeast Saccharomyces cerevisiae26. The only combinatorial interactions not confirmed by BiFC are DkMYB2-DkWDR1 and DkWDR1 with itself. DkMYB2-DkWDR1 interaction was observed in a previous two-hybrid study, thus only homodimerization of DkWDR1 is not sustained on experimental evidences. These BiFC and yeast two-hybrid interaction data are compatible with a multitude of possible combinations and sizes of the complex, which presumably enable a high degree of functional and regulatory versatility. Physical interactions among MBW factors involved in PA production have been also verified in Arabidopsis20, grapevine23, strawberry24, and tea plant34 among other PA and flavonoid biosynthesis complexes.
These BiFC and subcellular localization results strongly support the formation in vivo of protein complexes comprising at least several of these factors, but conclusive functional evidences about their coordinated recruitment to modify the expression of PA biosynthetic genes in persimmon fruit are scarce. Anthocyanidin reductase is the main enzyme specifically involved in PA production in persimmon, and its coding ANR gene is considered a major target of transcriptional regulation19, being consequently a proper candidate gene for studying PA biosynthesis regulation. Previous dual luciferase assays in N. benthamiana have shown that DkMYB2 and DkMYB4 increase the activity of ANR promoter when co-expressed with Arabidopsis AtEGL3 gene coding for a bHLH protein involved in regulation of the flavonoid pathway, but not when expressed individually28. On the contrary, in our hands, DkMYB2 is able to increase ANR promoter activity three-fold in the absence of other factors, which suggests that DkMYB2 does not require a complete MBW complex to enhance, at some level, ANR expression and consequently improve PA production (Fig. 5). Interestingly, the ectopic expression of DkMYB2 in kiwifruit calluses induces PA accumulation without additional MBW components28. DkMYB2 expression is regulated by fruit maturation factors in persimmon that markedly reduce it in advanced stages of development, similarly to ANR, DkMYB4 and DkMYC1, but in contrast to these genes it seems not to be impaired in ast non-astringent mutants. Unexpectedly, DkMYB2 has only a minor contribution to PA accumulation in these mutants, which is most likely due to its low expression level in fruit in comparison with DkMYB427.
Figure 5.
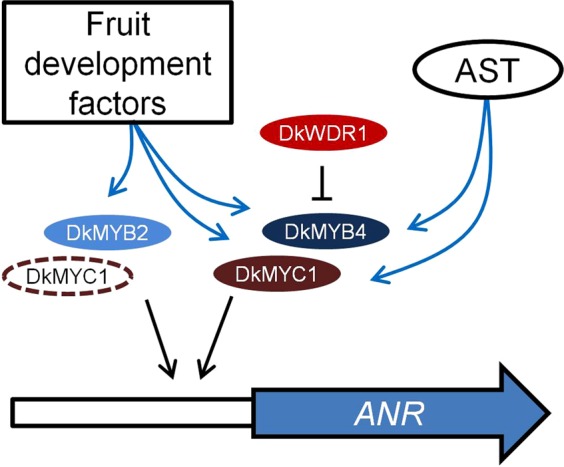
Diagram of regulatory factors affecting ANR expression. ANR coding gene is represented as a wide blue arrow, and its promoter as a contiguous white rectangle. Regulatory proteins are elliptic forms. The discontinuous ellipse of DkMYC1 indicates it is dispensable for the positive effect of DkMYB2. The transcriptional effect is labelled as a black arrow (inductive) or a black line ended in a perpendicular bar (repressive). Fruit development-dependent factors and AST locus modify the expression of MYB and bHLH factors by a yet unknown mechanism (blue arrows).
We have employed for the first time the persimmon bHLH (DkMYC1) and WD40 (DkWDR1) components in transient expression assays in combination with MYB factors, for the elucidation of the function of MBW complexes in the expression of PA responsive genes in persimmon fruit. DkMYC1 does not affect significantly ANR expression on its own, but consistently intensifies the effect of DkMYB2 and DkMYB4. In Arabidopsis, there is a similar synergistic effect of TT2 (MYB) and TT8 (bHLH) on the expression of BAN that responds to a stronger cooperative binding of the pair TT2-TT8 to BAN promoter20. Thus, the low expression of ANR gene from early stages of fruit development in the ast non-astringent cultivar ‘Hana Fuyu’ (Fig. 3c) seems to be caused by the concomitant defective expression of DkMYB4 and DkMYC1 genes in this mutant26 (Fig. 5). Contrarily to the positive effect of TTG1 (WD40) gene overexpression, and the negative effect of TTG1 silencing on BAN expression in Arabidopsis20, DkWDR1 expression does not affect ANR promoter activity in our transient expression experiments. The strawberry ortholog of TTG1 also increases Arabidopsis BAN expression in combination with its bHLH and MYB co-interactors24, which suggests the presence of certain functional particularities in persimmon DkWDR1 or perhaps regulatory differences between ANR and BAN promoters. On the other side, WD40 proteins act as structural platforms for facilitating protein-protein interaction, and consequently its effect on the positive transcriptional activity of the complex could be shaded by the ectopic overexpression of components of the complex and the presence of endogenous similar factors in N. benthamiana cells.
As DkMYB4 and DkMYC1 show a development and cultivar dependent expression profile highly similar to ANR26,27,29 (Fig. 3c), we considered plausible a self-regulatory loop in the expression of these genes, similar to the positive feedback mechanism operating in TT8 from Arabidopsis35. However, DkMYB4 and DkMYC1 promoters are not activated by any MBW element utilized in this study. On the contrary, DkWDR1 reduces the expression of DkMYB4 by itself and in combination with MYB genes. This repressive effect of DkWDR1 resembles the activity of MBW complexes containing MYB proteins showing at the C-terminal end an ethylene-responsive element binding factor-associated amphiphilic repression (EAR) motif36. Thus, binding of specific repressive MYB proteins has potentially the ability to turn MBW complex into a repressive factor. In light of these data we cannot discard a repressive role of DkWDR1 on the expression of DkMYB4 and other genes, which could depend on the binding of distinct MYB of bHLH elements with specific activating or repressive domains.
Methods
Plant material
Diospyros kaki Thunb. cvs ‘Hachiya’ (astringent fruit) and ‘Hana Fuyu’ (non-astringent fruit) were grown in an orchard located in Museros (Spain; 39°34′40″N, 0°21′46″W) under standard agricultural practices37. Four fruit samples per cultivar were collected at different maturation stages on the following dates in 2011: July 12, July 21, August 30, September 16 and September 3026. Soluble tannins were evaluated using the Folin-Denis method38, as described previously39, and results were expressed as percentage of fresh weight (FW).
Gene isolation
The Diospyros kaki (cv. ‘Hachiya’) genes DkMYB2 (AB503699.1), DkMYB4 (KR057233.1), DkMYC1 (KR057234.1) and DkWDR1 (KR057229.1) were obtained from pGADT7 plasmids described in a previous study26. In order to identify DkMYB4 and DkMYC1 promoter sequences, a manual assembly of D. lotus cv. Kunsenshi genome reads stored in the Sequence Read Archive (SRA) database (ID: SRP045872) was performed40. For amplifying ANR promoter, we designed primers (Supplementary Table S1) from the previously published sequence of D. lotus gene (AB504523.1). ANR promoter amplification was not possible in D. kaki, and therefore the D. lotus promoter was used in the analysis. DkMYB4 and DkMYC1 promoters were amplified from D. kaki cv. ‘Hachiya’ genomic DNA (Supplementary Table S1). The DNA of both D. kaki and D. lotus was extracted from fresh leaves following a standard CTAB DNA extraction protocol41.
Plasmid construction
For the construction of subcellular localization and BiFC vectors, each of the four genes were amplified (Supplementary Table S1) with a 15 bp target vector residue at the 5′ end needed for recombination with the In-Fusion HD Cloning kit (TAKARA BIO, Otsu, Japan). Fragments were purified and cloned into ampicillin resistant pSK + 35S-eGFP-PoPit vectors42 and two cassettes for each protein were made for subcellular localization. Four cassettes for each protein were made with ampicillin resistant pSK + 35S-(N-YFP or C-YFP)-PoPit vector43 for BiFC analysis. The fragments containing the expression cassette from pSK vectors were digested with HindIII and subcloned into the kanamycin resistant pMOG800 vector44.
In order to construct the vectors for transient expression, the four genes contained in pGADT7 plasmids26 were digested with SacI and XhoI to release the insert. The fragments were purified and inserted in a pGreenII-62-SK kanamycin resistant vector45, previously linearized with SacI/XhoI. The promoter sequences were amplified from vectors using specific primers with restriction enzyme sequences tails at 5′ (Supplementary Table S1). The purified PCR products were digested (HindIII/PstI for ANR and DkMYC1 promoters and HindIII/NcoI for DkMYB4 promoter), purified and cloned into linearized pGreenII-0800-LUC kanamycin resistant vector45.
All the described vectors were provided by Dr. J. A. Sánchez-Navarro (Instituto de Biología Molecular y Celular de Plantas “Primo Yúfera”, Valencia, Spain). Plasmids containing gene and promoter constructs were finally transferred to Agrobacterium tumefaciens strain C58 by electroporation. For pMOG800 vectors, transformation was carried out in bacteria containing the virulence helper plasmid pCH3246. All DNA constructions were verified by plasmid DNA sequencing.
Subcellular localization of MBW complex proteins in vivo
To characterize the subcellular localization of the MBW complex components, each protein was fused at either the Nt or the Ct of eGFP and transiently expressed in planta. For a better visualization of the fluorescence signal, all proteins were co-expressed with the silencing suppressor HC-Pro protein from the Tobacco Etch Virus43. A. tumefaciens C58 strains were grown overnight in LB media supplemented with kanamycin and rifampicin, at 28 °C. Cultures were centrifuged 5 min at 4,000 × g, and pellets were resuspended in infiltration media (MgCl2 10 mM + MES 10 mM pH 5.6) to an OD600 of 0.5 for each construct and an OD600 of 0.1 for the HC-Pro. N. benthamiana young plants (2 pairs of leaves) were agroinfiltrated as previously described47. Plants remained in a greenhouse at 24 °C (day) and 18 °C (night) with a 16 h light photoperiod. Three days after infiltration, leaf samples were collected and mounted in a microscope slide with a drop of water. Observation of the fluorescence in the underside epidermis was performed with a LEICA TCS SL confocal microscope (λexc = 488 nm; λem = 492–533 nm for eGFP). For the nucleus and nucleolus subcellular colocalization, the proteins were coinfiltrated with cultures (OD600 0.1) expressing the NLS of SV40 large T antigen fused to the red fluorescent protein and the fibrillarin fused to the cherry fluorescent protein, respectively (λexc = 561 nm; λem = 588–634 nm).
Bimolecular fluorescence complementation assays (BiFC)
In the BiFC assay48, addressed to characterize the interaction between the components of the hypothetical MBW complex, all the possible two-by-two combinations of homodimers and heterodimers were assayed in planta. Chimeric proteins were transiently co-expressed in N. benthamiana using A. tumefaciens (strain C58) cultures (OD600 = 0.4) transformed with the corresponding binary plasmids pMOG800, as previously described47. To increase the expression of the different proteins, we included an A. tumefaciens culture (OD600 = 0.1) expressing the HC-Pro. At 3 days post-infiltration, the fluorescence reconstitution was monitored in the confocal LEICA TCS SL (λexc = 488 nm; λem = 492–533 nm).
Western blot assay
Samples from the BiFC assay were immediately frozen in liquid nitrogen. Frozen leaves (50 mg) were ground and proteins were extracted with 200 µl of Laemmli buffer49, boiled for 5 min and centrifuged for 1 min at 15,800 × g to pellet cellular debris. For protein separation, 25 µl of the mixture was subjected to 12% sodium dodecylsulphate‐polyacrylamide gel electrophoresis (SDS‐PAGE). Gels were electrotransferred to nitrocellulose membranes following the manufacturer’s recommendations. Proteins were detected on western blots using an anti‐GFP N‐terminal antibody (SIGMA, St. Louis, MO, USA; cat. no. G1544) for N-terminal yellow fluorescent protein (NtYFP) fusions, and anti‐GFP antibody (ROCHE, Basel, Switzerland; cat. no. 11814460001) for C-terminal YFP (CtYFP) fusions, followed by a secondary peroxidase-labelled antibody and incubation with a chemiluminescence substrate (AMERSHAM, ECLTM Prime Western Blotting Detection Reagent). The chemiluminescence was detected exposing photographic film to the membranes.
Dual luciferase assay
To determine the effects of the hypothetical MBW protein complex on the promoters of ANR, DkMYB4 and DkMYC1 genes, a dual luciferase assay was performed. The effect of the homo and heterodimers formed by two and three proteins was assayed by the co-expression of the promoter and protein vectors as previously described in N. benthamiana with A. tumefaciens strain C58. HC-Pro was also used for enhancing the transient expression of the different proteins. After 3 days, 30 mg of agroinfiltrated leaves from 3 biological replicates, were sampled for each combination. Samples were immediately frozen in liquid nitrogen. For measuring promoter activity, samples were ground to powder and then Firefly (LUC) and Renilla (REN) luciferase activity was measured following the Dual-Luciferase Reporter Assay System (PROMEGA, Madison, WI, USA) with the aid of a PROMEGA GloMax Multi Microplate Reader luminometer. Promoter activity was measured as the quotient between the LUC/REN ratio of promoter plus transcription factors samples and the LUC/REN ratio of promoter without additional factors.
Isolation of RNA and quantitative real-time RT-PCR (qRT-PCR)
Total RNA was isolated from 150 mg of fruit flesh using a cetyltrimethylammonium bromide (CTAB)-based procedure50. Genomic DNA was removed with the RNase-Free DNase Set (QIAGEN, Hilden, Germany) according to manufacturer’s instructions. Purified RNA was reverse transcribed with PrimeScript RT Reagent Kit (TAKARA BIO). qRT-PCR was performed in a StepOnePlus Real-Time PCR System (LIFE TECHNOLOGIES, Carlsbad, CA, USA), using 1–2 µl of 10X diluted cDNA, SYBR premix Ex Taq (Tli RNaseH plus) (TAKARA BIO) and primers shown in Supplementary Table S1, in a total volume of 20 µl. The PCR protocol consisted of 10 min at 95 °C, followed by 40 cycles of 15 s at 95 °C, and 1 min at 60 °C. PCR specificity was confirmed by the presence of a single peak in the dissociation curve and by agarose electrophoresis. We used DkActin as reference gene26,27. A relative standard curve procedure was employed for measuring relative expression. Results were the average of two independent biological replicates with 2–3 technical replicates each.
Acknowledgements
This work was funded by the Instituto Nacional de Investigación y Tecnología Agraria y Alimentaria (INIA)-FEDER (grant no. RF2013-00043-C02-02 and RTA2017-00011-C03-01). FG-M was funded by a fellowship co-financed by the Generalitat Valenciana and European Social Fund (2014–2020) (grant no. ACIF/2016/115). We are grateful to Sara Selma for providing scientific and technical advice.
Supporting Information
Author contributions
J.A.S.N., M.L.B., M.M.N. and G.R. contributed to the experimental design. F.G.M. and M.M.N. made gene isolation and cloning. F.G.M. performed protein localization, BiFC, qRT-PCR, dual luciferase and western blot experiments. C.B. and A.S. performed fruit measurements. F.G.M., J.A.S.N., M.M.N. and G.R. analyzed the data. G.R. wrote the paper. All authors reviewed and approved the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-60635-w.
References
- 1.Dixon RA, Xie D-Y, Sharma SB. Proanthocyanidins–a final frontier in flavonoid research? New Phytol. 2005;165:9–28. doi: 10.1111/j.1469-8137.2004.01217.x. [DOI] [PubMed] [Google Scholar]
- 2.Yonemori K, Matsushima J. Property of development of the tannin cells in non-astringent type fruits of Japanese persimmon (Diospyros kaki) and its relationship to natural deastringency. J. Jpn. Soc. Hortic. Sci. 1985;54:201–208. doi: 10.2503/jjshs.54.201. [DOI] [Google Scholar]
- 3.Salvador A, et al. Physiological and structural changes during ripening and deastringency treatment of persimmon fruit cv. ‘Rojo Brillante’. Postharvest Biol. Tec. 2007;46:181–188. doi: 10.1016/j.postharvbio.2007.05.003. [DOI] [Google Scholar]
- 4.Bernays, E. A., Driver, G. C. & Bilgener, M. Herbivores and plant tannins. In Advances in Ecological Research (eds. Begon, M., Fitter, A. H., Ford, E. D. & MacFadyen, A.) 19, 263–302 (Academic Press, 1989).
- 5.Tessmer MA, et al. Microstructural changes while persimmon fruits mature and ripen. Comparison between astringent and non-astringent cultivars. Postharvest Biol. Tec. 2016;120:52–60. doi: 10.1016/j.postharvbio.2016.05.014. [DOI] [Google Scholar]
- 6.Nishiyama S, et al. Characterization of a gene regulatory network underlying astringency loss in persimmon fruit. Planta. 2018;247:733–743. doi: 10.1007/s00425-017-2819-0. [DOI] [PubMed] [Google Scholar]
- 7.Sugiura A, Yonemori K, Harada H, Tomama T. Changes of ethanol and acetaldehyde contents in Japanese persimmon fruits and their relation to natural deastringency. Studies from Inst. Hort. Kyoto Univ. 1979;9:41–47. [Google Scholar]
- 8.Sugiura A, Tomana T. Relationships of ethanol production by seeds of different types of Japanese persimmons and their tannin content. HortSci. 1983;18:319–321. [Google Scholar]
- 9.Ben-Arie R, Sonego L. Temperature affects astringency removal and recurrence in persimmon. J. Food Sci. 1993;58:1397–1400. doi: 10.1111/j.1365-2621.1993.tb06191.x. [DOI] [Google Scholar]
- 10.Matsuo T, Itoo S. A model experiment for de-astringency of persimmon fruit with high carbon dioxide treatment: in vitro gelation of kaki-tannin by reacting with acetaldehyde. Agr. Biol. Chem. Tokyo. 1982;46:683–689. [Google Scholar]
- 11.Pesis E, Ben-Arie R. Involvement of acetaldehyde and ethanol accumulation during induced deastringency of persimmon fruits. J. Food Sci. 1984;49:896–899. doi: 10.1111/j.1365-2621.1984.tb13236.x. [DOI] [Google Scholar]
- 12.Kanzaki S, Yonemori K, Sugiura A, Sato A, Yamada M. Identification of molecular markers linked to the trait of natural astringency loss of Japanese persimmon (Diospyros kaki) fruit. J. Am. Soc. Hortic. Sci. 2001;126:51–55. doi: 10.21273/JASHS.126.1.51. [DOI] [Google Scholar]
- 13.Yamada M, Sato A. Segregation for fruit astringency type in progenies derived from crosses of ‘Nishimurawase’×pollination constant non-astringent genotypes in oriental persimmon (Diospyros kaki Thunb.) Sci. Hortic.-Amsterdam. 2002;92:107–111. doi: 10.1016/S0304-4238(01)00285-0. [DOI] [Google Scholar]
- 14.Besada C, et al. Chloride stress triggers maturation and negatively affects the postharvest quality of persimmon fruit. Involvement of calyx ethylene production. Plant Physiol. Biochem. 2016;100:105–112. doi: 10.1016/j.plaphy.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 15.Lepiniec L, et al. Genetics and biochemistry of seed flavonoids. Annu. Rev. Plant Biol. 2006;57:405–430. doi: 10.1146/annurev.arplant.57.032905.105252. [DOI] [PubMed] [Google Scholar]
- 16.Tanner GJ, et al. Proanthocyanidin biosynthesis in plants. Purification of legume leucoanthocyanidin reductase and molecular cloning of its cDNA. J. Biol. Chem. 2003;278:31647–31656. doi: 10.1074/jbc.M302783200. [DOI] [PubMed] [Google Scholar]
- 17.Xie D-Y, Sharma SB, Paiva NL, Ferreira D, Dixon RA. Role of anthocyanidin reductase, encoded by BANYULS in plant flavonoid biosynthesis. Science. 2003;299:396–399. doi: 10.1126/science.1078540. [DOI] [PubMed] [Google Scholar]
- 18.Ikegami A, Eguchi S, Kitajima A, Inoue K, Yonemori K. Identification of genes involved in proanthocyanidin biosynthesis of persimmon (Diospyros kaki) fruit. Plant Science. 2007;172:1037–1047. doi: 10.1016/j.plantsci.2007.02.010. [DOI] [Google Scholar]
- 19.Akagi T, et al. Expression balances of structural genes in shikimate and flavonoid biosynthesis cause a difference in proanthocyanidin accumulation in persimmon (Diospyros kaki Thunb.) fruit. Planta. 2009;230:899–915. doi: 10.1007/s00425-009-0991-6. [DOI] [PubMed] [Google Scholar]
- 20.Baudry A, et al. TT2, TT8, and TTG1 synergistically specify the expression of BANYULS and proanthocyanidin biosynthesis in Arabidopsis thaliana. Plant J. 2004;39:366–380. doi: 10.1111/j.1365-313X.2004.02138.x. [DOI] [PubMed] [Google Scholar]
- 21.Xu W, et al. Complexity and robustness of the flavonoid transcriptional regulatory network revealed by comprehensive analyses of MYB-bHLH-WDR complexes and their targets in Arabidopsis seed. New Phytol. 2014;202:132–144. doi: 10.1111/nph.12620. [DOI] [PubMed] [Google Scholar]
- 22.Xu W, Dubos C, Lepiniec L. Transcriptional control of flavonoid biosynthesis by MYB-bHLH-WDR complexes. Trends Plant Sci. 2015;20:176–185. doi: 10.1016/j.tplants.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 23.Hichri I, et al. The basic helix-loop-helix transcription factor MYC1 is involved in the regulation of the flavonoid biosynthesis pathway in grapevine. Mol. Plant. 2010;3:509–523. doi: 10.1093/mp/ssp118. [DOI] [PubMed] [Google Scholar]
- 24.Schaart JG, et al. Identification and characterization of MYB-bHLH-WD40 regulatory complexes controlling proanthocyanidin biosynthesis in strawberry (Fragaria × ananassa) fruits. New Phytol. 2013;197:454–467. doi: 10.1111/nph.12017. [DOI] [PubMed] [Google Scholar]
- 25.Gesell A, Yoshida K, Tran LT, Constabel CP. Characterization of an apple TT2-type R2R3 MYB transcription factor functionally similar to the poplar proanthocyanidin regulator PtMYB134. Planta. 2014;240:497–511. doi: 10.1007/s00425-014-2098-y. [DOI] [PubMed] [Google Scholar]
- 26.Naval M, et al. A WD40-repeat protein from persimmon interacts with the regulators of proanthocyanidin biosynthesis DkMYB2 and DkMYB4. Tree Genet. Genomes. 2016;12:13. doi: 10.1007/s11295-016-0969-z. [DOI] [Google Scholar]
- 27.Akagi T, et al. DkMyb4 is a Myb transcription factor involved in proanthocyanidin biosynthesis in persimmon fruit. Plant Physiol. 2009;151:2028–2045. doi: 10.1104/pp.109.146985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akagi T, Ikegami A, Yonemori K. DkMyb2 wound-induced transcription factor of persimmon (Diospyros kaki Thunb.), contributes to proanthocyanidin regulation. Planta. 2010;232:1045–1059. doi: 10.1007/s00425-010-1241-7. [DOI] [PubMed] [Google Scholar]
- 29.Su F, Hu J, Zhang Q, Luo Z. Isolation and characterization of a basic Helix–Loop–Helix transcription factor gene potentially involved in proanthocyanidin biosynthesis regulation in persimmon (Diospyros kaki Thunb.) Sci. Hortic.-Amsterdam. 2012;136:115–121. doi: 10.1016/j.scienta.2012.01.013. [DOI] [Google Scholar]
- 30.Hribal J, Zavrtanik M, Simćić M, Vidrih R. Changes during storing and astringency removal of persimmon fruit Diospyros kaki L. Acta Aliment. Hung. 2000;29:123–136. [Google Scholar]
- 31.Nesi N, Jond C, Debeaujon I, Caboche M, Lepiniec L. The Arabidopsis TT2 gene encodes an R2R3 MYB domain protein that acts as a key determinant for proanthocyanidin accumulation in developing seed. Plant Cell. 2001;13:2099–2114. doi: 10.1105/TPC.010098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao M, Morohashi K, Hatlestad G, Grotewold E, Lloyd A. The TTG1-bHLH-MYB complex controls trichome cell fate and patterning through direct targeting of regulatory loci. Development. 2008;135:1991–1999. doi: 10.1242/dev.016873. [DOI] [PubMed] [Google Scholar]
- 33.Weill U, et al. Assessment of GFP tag position on protein localization and growth Fitness in yeast. J. Mol. Biol. 2019;431:636–641. doi: 10.1016/j.jmb.2018.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang P, et al. A sucrose-induced MYB (SIMYB) transcription factor promoting proanthocyanidin accumulation in the tea plant (Camellia sinensis) J. Agric. Food Chem. 2019;67:1418–1428. doi: 10.1021/acs.jafc.8b06207. [DOI] [PubMed] [Google Scholar]
- 35.Baudry A, Caboche M, Lepiniec L. TT8 controls its own expression in a feedback regulation involving TTG1 and homologous MYB and bHLH factors, allowing a strong and cell-specific accumulation of flavonoids in Arabidopsis thaliana. Plant J. 2006;46:768–779. doi: 10.1111/j.1365-313X.2006.02733.x. [DOI] [PubMed] [Google Scholar]
- 36.Albert NW, et al. A conserved network of transcriptional activators and repressors regulates anthocyanin pigmentation in eudicots. Plant Cell. 2014;26:962–980. doi: 10.1105/tpc.113.122069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bellini, E. Cultural practices for persimmon production. In Options Méditerranéennes. Série A: Séminaires Méditerranéens (CIHEAM) (eds. Bellini, E. & Giordani, E.) 51. 39–52 (CIHEAM-IAMZ, 2002).
- 38.Taira, S. Astringency in Persimmon. In Fruit Analysis (eds. Linskens, H. F. & Jackson, J. F.) 97–110 (Springer Berlin Heidelberg, 1995).
- 39.Arnal L, Rio MAD. Quality of persimmon fruit cv. Rojo brillante during storage at different temperatures. Span. J. Agric. Res. 2004;2:243–247. [Google Scholar]
- 40.Akagi T, Henry IM, Tao R, Comai L. A Y-chromosome–encoded small RNA acts as a sex determinant in persimmons. Science. 2014;346:646–650. doi: 10.1126/science.1257225. [DOI] [PubMed] [Google Scholar]
- 41.Doyle JJ, Doyle JL. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochemical Bulletin. 1987;19:11–15. [Google Scholar]
- 42.Herranz MC, Sanchez-Navarro JA, Aparicio F, Pallás V. Simultaneous detection of six stone fruit viruses by non-isotopic molecular hybridization using a unique riboprobe or ‘polyprobe’. J. Virol. Methods. 2005;124:49–55. doi: 10.1016/j.jviromet.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 43.Leastro MO, Pallás V, Resende RO, Sánchez-Navarro JA. The movement proteins (NSm) of distinct tospoviruses peripherally associate with cellular membranes and interact with homologous and heterologous NSm and nucleocapsid proteins. Virology. 2015;478:39–49. doi: 10.1016/j.virol.2015.01.031. [DOI] [PubMed] [Google Scholar]
- 44.Knoester M, et al. Ethylene-insensitive tobacco lacks nonhost resistance against soil-borne fungi. Proc. Natl. Acad. Sci. USA. 1998;95:1933–1937. doi: 10.1073/pnas.95.4.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hellens RP, Edwards EA, Leyland NR, Bean S, Mullineaux PM. pGreen: a versatile and flexible binary Ti vector for Agrobacterium-mediated plant transformation. Plant Mol. Biol. 2000;42:819–832. doi: 10.1023/A:1006496308160. [DOI] [PubMed] [Google Scholar]
- 46.Hamilton CM, Frary A, Lewis C, Tanksley SD. Stable transfer of intact high molecular weight DNA into plant chromosomes. Proc. Natl. Acad. Sci. USA. 1996;93:9975–9979. doi: 10.1073/pnas.93.18.9975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Genovés A, Pallás V, Navarro JA. Contribution of topology determinants of a viral movement protein to its membrane association, intracellular traffic, and viral cell-to-cell movement. J. Virol. 2011;85:7797–7809. doi: 10.1128/JVI.02465-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aparicio F, Sánchez-Navarro JA, Pallás V. In vitro and in vivo mapping of the Prunus necrotic ringspot virus coat protein C-terminal dimerization domain by bimolecular fluorescence complementation. J. Gen. Virol. 2006;87:1745–1750. doi: 10.1099/vir.0.81696-0. [DOI] [PubMed] [Google Scholar]
- 49.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 50.Gambino G, Perrone I, Gribaudo I. A Rapid and effective method for RNA extraction from different tissues of grapevine and other woody plants. Phytochem. Anal. 2008;19:520–525. doi: 10.1002/pca.1078. [DOI] [PubMed] [Google Scholar]