Abstract
Currently, testing for immunoglobulin E (IgE) sensitization is the cornerstone of diagnostic evaluation in suspected allergic conditions. This review provides a thorough and updated critical appraisal of the most frequently used diagnostic tests, both in vivo and in vitro. It discusses skin tests, challenges, and serological and cellular in vitro tests, and provides an overview of indications, advantages and disadvantages of each in conditions such as respiratory, food, venom, drug, and occupational allergy. Skin prick testing remains the first line approach in most instances; the added value of serum specific IgE to whole allergen extracts or components, as well as the role of basophil activation tests, is evaluated. Unproven, non-validated, diagnostic tests are also discussed. Throughout the review, the reader must bear in mind the relevance of differentiating between sensitization and allergy; the latter entails not only allergic sensitization, but also clinically relevant symptoms triggered by the culprit allergen.
Keywords: IgE, Allergy, In vitro tests, Skin tests, Diagnostic strategies
Abbreviations: AAAAI, American Academy of Allergy Asthma and Immunology; ABA, Allergen Bead Array; ACAAI, American College of Allergy Asthma and Immunology; AIT, allergen immunotherapy; AEC, Allergen Exposure Chambers; Anti-IgE, Antibody against IgE; AP, Alkaline Phosphatase; AU/mL, Allergenic Units milliLiter; BAT, Basophil Activation Test; BAU/mL, Biologic Allergenic Units milliLiter; CaFE, Calibrated Fluorescence Enhancement; CBA, Cytometric Bead Array; CCD, Cross-reactive Carbohydrate Determinants; CDER, Center for Drug Evaluation and Research (USA); CL, Chemiluminescence; DBPCFC, Double-Blind Placebo-Controlled Food Challenge; EAACI, European Academy of Allergy and Immunology; EIA, Enzyme Immune Assay; ELISA, Enzyme Linked Immuno Sorbent Analysis; EMEA, European MEdicine Agencies; ENPP-3, EctoNucleotide Pyrophosphatase/Phosphodiesterase 3; FACS, Fluorescence-Activated Cell Sorting; FDA, Food and Drug Administration (U.S. Department of Health and Human Services); FEIA, Fluorescent Enzyme Immunoassays; FcεRI, High affinity IgE receptor; H1, Histamine 1 receptor; H2, Histamine 2 receptor; HPO, Horseradish Peroxidase; IDT, Intradermal Test; IgE, immunoglobulin E; ISAC, Immuno-Solid phase Allergen Chip; IUIS, International Union of Immunological Societies; IVD, in vitro diagnostic tool; kUA/L, kilo Units of Allergen/Liter for allergen-specific IgE antibody assays; LAMP-3, Lysosomal-Associated Membrane Protein; mAb, Monoclonal Antibody; MRGPRX2, Mas-related G protein receptor 2; MBAD, Molecule Based Allergy Diagnostics; NIH, National Institutes of Health (USA); NMBAs, NeuroMuscular Blocking Agents; NPA, Negative Percent Agreement; NSAIDs, Non-Steroidal Anti-Inflammatory Drugs; pNPP, p-Nitrophenylphosphate; PPA, Positive Percent Agreement; PPT, Prick-Prick Test; sIgE, specific IgE; RAST, Radio Allergo Sorbent Test; SCAR, severe cutaneous adverse drug reactions; SPT, Skin prick test; w/v, weight /volume
Key statements
-
•
Clinical suspicion of allergic sensitization is confirmed by demonstrating the presence of allergen-specific IgE antibodies in vivo (skin tests) or in vitro.
-
•
Confirmation of allergen sensitization and the identification of causal allergens are essential for optimizing the management of allergic conditions.
-
•
Skin prick testing (SPT) is the most frequently used method for the detection of IgE antibodies, due to its rapidity, simplicity and low cost. Skin prick tests and other skin test results must be interpreted by a clinician with adequate knowledge of medical history, clinical findings, and relevant type I allergens (including environmental, food, animal, insect, fungal, and drug allergens). Skin tests should include the relevant allergens in the given geographical area and ideally carried out only using standardized allergenic extracts.
-
•
In vitro tests, including molecular based allergy diagnostics, using either in single-plex and in multi-plexed strategies and other more functional tests, such as Basophil Activation Tests allow to better define the IgE profile of the patient. This approach is in line with the Precision Medicine statements.
Introduction
Allergic diseases are amongst the most prevalent diseases worldwide and the burden of these diseases continues to increase. An accurate diagnosis coupled with optimal therapy requires the use of appropriate tests to confirm the allergen sensitization and detailed information about exposure to the putative allergen. Skin tests, especially SPT, represent the most reliable and cost-effective tool for the diagnosis and management of IgE-mediated diseases,. They demonstrate a good correlation with outcomes of nasal, conjunctival, dermal, oral and bronchial challenges.
Once the diagnosis has been established, and the relevant allergens have been identified, specific treatments, including medications, environmental control measures and/or allergen immunotherapy (AIT) are required to achieve optimal, long-term outcomes. Allergy diagnosis, hence, may be categorized as precision medicine.
Several types of skin tests are used in allergy diagnostics:
-
1)
Skin Prick Test (SPT): This represents the first level of approach for the diagnosis of type I, immediate, IgE-mediated allergy. It is safe, has high sensitivity and good specificity when performed and interpreted correctly; a specific variant of type I skin tests is prick-to-prick testing (PPT) with native allergens.
-
2)
Intradermal Test (IDT): This can be used to evaluate both immediate IgE-mediated allergy and delayed-type hypersensitivity, according to the time of read-out. It has increased sensitivity and decreased specificity compared to SPT.
-
3)
Patch test: This is used for delayed type, cell-mediated, hypersensitivity reactions. It has no relevance for IgE-mediated allergy and thus will not be further examined in the present document.
The in vitro diagnosis of IgE-mediated allergic diseases is useful in the identification of the causative allergen(s) and usually involves different laboratory procedures. In particular:
-
1)
The total IgE assay which is nonspecific and provides only gross information.
-
2)
Serum specific IgE assays against allergen sources/molecules are the most commonly used in vitro diagnostic approach. They can be performed by a singleplexed or multiplexed strategy.
-
3)
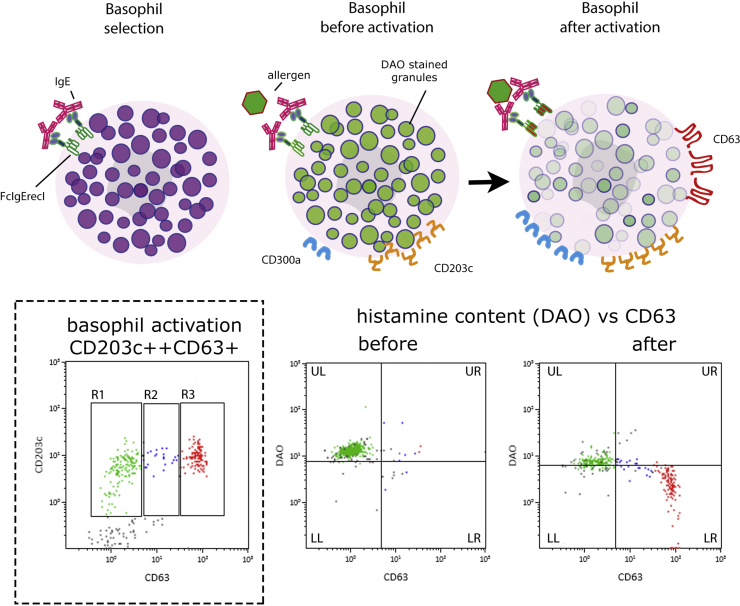
The Basophil Activation Test (BAT) which is quite specific, but complex to perform, and therefore limited to selected situations.
The first part of the present manuscript focuses on skin testing in the diagnosis of IgE-mediated allergy and is intended for all practitioners. There have been criticisms that the procedure is often left to technicians and nurses with limited expertise and poor attention towards quality control and methodological standardization.1 Surveys have highlighted the variability of the technical methodology2, 3, 4 and also the interpretation and communication of results5,6 by different practitioners. In the present document, recommendations for its clinical use, technical aspects, reporting, and interpretation of the results have been revised and updated. The second part deals with the in vitro techniques (serum IgE assays and cell-based assays), discussing characteristics, performance and indications for the various approaches. The third part includes allergen provocation testing. A final part is dedicated to special situations, where the confirmation or exclusion of an IgE-mediated disease mechanism is required (allergies to foods, drugs, insect venoms or occupational agents).
In vivo diagnosis: skin tests
Skin prick test
General information
The credit for the first skin testing devices goes to Charles H. Blackley, who in 1865 abraded a quarter-inch area of skin with a lancet, producing a dermographic reaction. In 1924, Lewis and Grant first described the skin prick test (SPT) method.
SPT is the simplest in vivo method to assess the presence of IgE sensitization in humans. When a specific allergen is introduced through a lancet into the skin of allergic individuals, dermal mast cells begin to degranulate mainly due to the cross-linking of allergen-specific IgE bound to their membrane receptors. Degranulation leads to the immediate release of histamine and other mediators, inducing a cutaneous response, clinically characterized by a wheal (sometimes with pseudopods) and surrounding erythema (flare) that can be measured in order to assess the degree of cutaneous sensitivity. Thus SPT represents a surrogate indicator of systemic allergic sensitization (i.e., nose, lungs, eyes, gut) through the presence of cutaneous reactivity to specific allergens.
When should skin prick tests be performed?
The diagnosis of allergy requires an appropriate medical history and physical examination. If the clinical information suggests type I (immediate-type) allergy, SPTs are indicated to detect the presence of specific IgE to relevant causative allergens: inhalant, food, hymenoptera venoms, drugs and, in some cases, occupational allergens. Type I hypersensitivity (immediate) is suspected clinically when reactions occur within 30–120 minutes of exposure.
In general, clinical conditions where SPT is indicated are the following:
-
•
Asthma;
-
•
Rhinitis/rhinosinusitis/rhino-conjunctivitis/conjunctivitis;
-
•
Eczema/atopic dermatitis (in the setting of selectively high clinical suspicion for underlying presence of IgE hypersensitivity to specific allergens);
-
•
Suspected food allergy (oral allergy syndrome, anaphylaxis/acute onset or exacerbation of urticaria or eczema that is temporally correlated with food ingestion);
-
•
Suspected drug allergy;
-
•
Hymenoptera venom allergy (systemic reactions immediately following insect sting);
-
•
Suspected occupational disease or exposure to selected potential allergens;
-
•
Chronic urticaria in rare selected cases which strongly suggest an allergen as potential trigger/aggravating factor;
-
•
Less common disorders, such as eosinophilic esophagitis, eosinophilic gastroenteritis or allergic bronchopulmonary aspergillosis, where IgE sensitization is one of the characteristics of its pathogenesis. However, there is controversy regarding the utility of SPT for these illnesses.
On the contrary, SPT is not routinely indicated in the following instances in the absence of other existing features of allergic disease:
-
•
Suspected food intolerance (e.g., irritable bowel syndrome, etc.);
-
•
Chronic urticaria in the absence of allergic features in the history;
-
•
Desire to lose weight (according to non-conventional approaches, obesity may be due to food intolerance, but no supporting scientific data have been reported in the literature);
-
•
Non-specific food-associated symptoms to food additives/preservatives/colorants;
-
•
Evaluation of the effectiveness of allergen immunotherapy (but may be supportive in Hymenoptera venom immunotherapy);
-
•
Non-specific respiratory symptoms to irritants (i.e., smoke, perfumes, detergents, chemicals and other strong odors);
-
•
Screening for allergic sensitization patterns in the absence of clinical symptoms (i.e., family history of allergy);
-
•
Non-specific cutaneous rashes in the absence of atopic features or other allergic symptoms; migraine, except for the indication of specific hypersensitivity to hormones. However, strong scientific data are still missing.
-
•
Chronic fatigue syndrome.
SPTs with great fidelity provide an objective and reliable confirmation of allergic sensitization. However, the clinical relevance of IgE-mediated sensitizations should always be carefully considered since, sometimes, positive SPTs do not directly imply allergic manifestations. A correct diagnosis of type I allergic conditions is quite important in order to choose proper avoidance measures and to prescribe allergen immunotherapy, when needed. When indicated, SPTs are convenient, simple, biologically relevant, reproducible, time- and cost-effective, and highly sensitive. They can be performed in parallel to serum specific IgE (sIgE) detection, and in specific cases, accompanied by other allergen challenge tests to evaluate the clinical relevance of the allergic sensitizations. SPTs assess the presence of allergen specific IgE bound to mast cells in the dermis. These mast cells can bind individual allergen specific IgE molecules for over one year. Allergen specific IgE blood tests measure of the presence of this antibody. These tests may be viewed as complementary.
The clinician who performs/interprets the SPT and the setting
A clinician with adequate knowledge of the important, relevant suspected allergens, based upon the patient's history and the geographic area, should decide which specific allergens are tested and interpret the clinical significance of the test results. SPT must be performed under medical supervision, with emergency equipment available for the treatment of anaphylaxis. The risk of systemic reactions in clinically stable patients is extremely low when using standardized respiratory allergen extracts.
Skin tests reporting form
As for any medical procedure, it is essential that proper documentation be recorded. An ideal skin testing form should list the following information:
-
•
name and date of birth of the patient;
-
•
date of the skin test;
-
•
name, address, and telephone number of the responsible physician;
-
•
region tested (e.g., back, forearm);
-
•
name of technician/nurse/doctor/health professional trainee who performed the test;
-
•
type of device used;
-
•
negative and positive controls (type and concentrations), with the respective results;
-
•
name of each allergen tested as reported on the commercial bottle (with genus and species identifier), followed by local/common names, concentration, and manufacturer;
-
•
if the allergen extract is diluted, both diluent and dilution should be recorded;
-
•
size of the resulting wheal and flare for each allergen after pricking;
-
•
time point of reading the result, usually 15–20 minutes;
-
•
optional: check box for pre-medications potentially interfering with the result before starting.
Records of extract source, lot number as well as expiration date may be kept separately.
Drugs possibly interfering with skin prick tests
Before testing, the clinician should verify that the patient has not been taking medications that might interfere with testing. According to the guideline recommendations of the Joint Task Force on Practice Parameters of the American Academy of Allergy Asthma and Immunology (AAAAI), the American College of Allergy Asthma and Immunology (ACAAI) and the European Academy of Allergy and Immunology (EAACI), certain medications should be discontinued to avoid suppressive effects on the immediate wheal and flare skin test response (Table 1, Table 2).
-
•
This effect is attributed to a combination of a decrease in mast cell recruitment and an increase of mast cell apoptosis.
Table 1.
Recommendations on medications that possibly interfere with SPT
|
|
|
|
|
Table 2.
Suppressant effects of drugs on immediate skin testing
| Drugs | Generic drug | Day(s) suppressed Despite the intervals indicated, the higher limit of the interval is recommended |
|---|---|---|
| H1-antihistamines | ||
| First generation | Chlorpheniramine | 2–6 |
| Clemastine | 5–10 | |
| Cyproheptadine | 9–11 | |
| Dexchlorpheniramine | 4 | |
| Diphenhydramine | 2–5 | |
| Hydroxyzine | 5–8 | |
| Promethazine | 3–5 | |
| Tripelennamine | 3–7 | |
| Second generation | Azelastine nasal | 3–10 |
| Ebastine | 3–10 | |
| Cetirizine | 3–10 | |
| Fexofenadine | 2 | |
| Loratadine | 7–10 | |
| Desloratadine | 3–10 | |
| Levocetirizine | 3–10 | |
| Bilastine | 4–5 | |
| Levocabastine nasal | Do not suppress skin tests | |
| Levocabastine ophthalmic | Do not suppress skin tests | |
| Rupatadine | 3–7 | |
| Tricyclic antidepressants and tranquilizers | Desipramine | 2 |
| Imipramine | >10 | |
| Doxepin | 6–11 | |
| Doxepin topical | 11 | |
| H2-antagonists | Ranitidine | 1 |
| Anti-IgE monoclonal antibody | Omalizumab | Prick tests can be performed after 6 weeks but false negative results can occur up to one year226 |
| Cysteinyl leukotriene receptor antagonists | Montelukast | Does not suppress skin tests |
| Zafirlukast | Does not suppress skin tests | |
| Short term oral corticosteroids | 30 mg of prednisone daily for 1 week | Do not suppress skin tests |
| Long term and relatively high dose corticosteroids | >20 mg/d | Possible suppression of immediate skin test reactions |
| Potent topical corticosteroids** | >3 weeks | Suppress immediate skin test over areas where they have been applied |
| Local anesthetic | EMLA (Eutectic Mixture of Local Anesthetic) cream | 1 h before test suppression (only suppresses erythema) |
* NB: Where this article reports in fractions of days, the total has been rounded up. Maximum days would apply to most patients, but there may be exceptions where suppression could last longer. Adapted from Bernstein IL, Li JT, Bernstein DI et al. Allergy diagnostic testing: an updated practice parameter. Ann Allergy Asthma Immunol 2008; 100 (Suppl 3):S1–S148.227
Allergen extracts for SPT
The number of skin tests and the selection of allergens for skin testing should be determined based on specific clinical history, allergen exposure pattern (seasonal versus perennial, or sporadic), distribution of allergenic sources in the local environment as well as living conditions, occupation, hobbies, or recreational activities.7
Patient populations tend to be highly mobile, hence exposure to different allergens at different places may occur.
Allergenic extracts consist of mixtures of allergenic (proteins, glycoproteins, polysaccharides) and non-allergenic components (lipids, salts, pigments, metabolites) derived from the allergenic source. Crude extracts thus usually contain both genuine sensitizers and cross-reactive proteins. Allergens in general are mainly proteins or glycoproteins, but carbohydrates or other low-molecular weight haptenic chemicals, when transformed to complete antigens, can also induce allergic sensitization.
Allergen extracts are usually obtained from natural sources by aqueous extraction. Their composition and biologic properties may be influenced by the quality and purity of the source material, the methods of extraction and processing, as well as the storage conditions. Extracts should not contain more than one allergenic source (mixes can be used if containing sources from a homogeneous taxonomic family) and should not contain interfering preservatives such as thiomersal.
Diagnostic extracts made of recombinant or highly purified allergenic proteins are available in some countries where they are approved for allergy diagnostics. Recombinant and natural allergen preparations have been evaluated and compared. In general, skin testing with synthesized allergens is highly specific and avoids the creation of false positives by the elimination of cross-reactive allergens. However, the precise role of recombinant allergens as an in vivo diagnostic tool remains to be fully determined.
Allergenic extracts used for diagnostics should ideally be standardized, both in vivo and in vitro (meaning that manufacturers should quantify the presence of major allergens in their allergenic extracts). Standardization facilitates the comparison of extracts from different manufacturers, lot to lot variability, and the reliability and reproducibility of test results.8
Current standardization is crucial particularly in European countries, where strict regulatory rules have led sometimes to a problematic and costly registration of some products.9 Allergenic extracts should also be cost effective.
Stability and potency of the allergen test extracts are also important issues. Since allergen extract potency deteriorates with time, accelerated by dilution and higher temperatures, allergen skin test extracts are usually preserved with 50% glycerin. All extracts should be stored in a refrigeration unit at 2–8 °C to improve stability.
For diagnostic use, both standardized and non-standardized products are commercially available with labeling in a variety of potency units (some examples are provided in Table 3).
Table 3.
Examples of skin test concentrations of standardized and non-standardized allergens.
| Allergen product | Skin prick test concentration | Intradermal test concentration |
|---|---|---|
| Standardized short ragweed | 1:20 w/v | 1:1000 w/v |
| Standardized cat hair | 10,000 BAU/mL | 200 BAU/mL |
| Standardized grass pollens | 10,000–100,000 BAU/mL | 200 BAU/mL |
| Standardized Hymenoptera venoms | 100 μg protein/mL | 0.1–1 μg protein/mL |
| Standardized mites | 10,000 AU/mL | 200 AU/mL |
| Non standardized allergens | 1:40–1:20 w/v | 1:1000 w/v |
Adapted from Dolen WK, MD. Immunology and Allergy Clinics of North America. Volume 21, number 2 May 2001. Saunders. Selection of allergen products for skin testing by Robert E. Esch, PhD.228
Number of skin tests
A pan-European study of skin tests, supported by the Global Allergy and Asthma European Network (GA2LEN), showed that, for respiratory/conjunctival allergies, it is not necessary to include a large number of allergen extracts for skin tests, at least in Europe. The total number of allergens tested depends, as mentioned above, on the local exposure framework. The suggested panel for respiratory allergy includes 18 allergens, as follows: alder (Alnus incana), birch (Betula alba/verrucosa), cypress (Cupressus sempervirens/arizonica), hazel (Corylus avellana), plane (Platanus vulgaris), grass mix (including Poa pratensis, Dactilis glomerata, Lolium perenne, Phleum pratense, Festuca pratensis, Helictotrichon pratense), olive (Olea europea), mugwort (Artemisia vulgaris), ragweed (Ambrosia artemisiifolia), Alternaria alternata (tenuis), Cladosporium herbarum, Aspergillus fumigatus, Parietaria, cat (Felis domesticus), dog (Canis familiaris), dust mite (Dermatophagoides pteronyssinus/farinae), and cockroach (Blatella germanica).10 In tropical countries, testing with Blomia tropicalis is recommended.11 The number of tests performed should be much lower when testing infants.
Information regarding cross-reactivity among allergens is important when interpreting results. Cross-reactivity describes the phenomenon whereby an immediate type skin reaction by a particular allergen (genuine sensitizer) can also be elicited by other similar allergens and is explained by IgE cross-reactivity to homologous (cross-reactive) allergens. Cross-reactivity of pollens is frequent among taxonomically related plants or in the case of highly conserved proteins across different species (Table 4).
Table 4.
Cross-reacting pollen allergen groups.
| Cross-reacting groups | Representative generaa | |
|---|---|---|
| Grass Pollens | Pooideae | Poa (bluegrass), Bromus (brome), Dactylis (orchard), Festuca (fescue), Lolium (perennial rye), Agrostis (redtop), Anthoxanthum (sweet vernal), Avena (cultivated oat), Holcus (velvet), Phalaris (reed canary), Phleum (timothy), Agropyron (quack), Elymus (wild rye), Secal e (cultivated rye), Triticum (cultivated wheat) |
| Chloridoideae | Cynodon (Bermuda), Bouteloua (blue grama, mosquito Grass), Distichlis (salt) | |
| Panicoideae | Paspalum (Bahia), Sorghum (Johnson), Panicum (Para grass), Zea (corn) | |
| Tree Pollens | Aceraceae | Acer (maples and box elder) |
| Betulaceae | Alnus (alder), Betula (birches), Corylus (hazelnut) | |
| Cupressaceae | Cupressus (cypress), Juniperus (junipers and cedars), Taxodium (bald-cypress), Cryptomeria (Japanese cedar) | |
| Fabaceae | Acacia (mimosa), Robinia (locust), Prosopis (mesquite tree) | |
| Fagaceae | Quercus (oaks), Fagus (beech) | |
| Juglandaceae | Carya (hickory and pecan), Juglans (walnut) | |
| Moraceae | Morus (mulberry), Broussonetia (paper mulberry) | |
| Oleaceae | Olea (olive), Fraxinus (ash), Ligustrum (privet) | |
| Pinaceae | Pinus (pines) | |
| Platanaceae | Platanus (sycamore) | |
| Salicaceae | Populus (cottonwood and poplars), Salix (willows) | |
| Ulmaceae | Ulmus (elms) | |
| Weed Pollens | Chenopodiaceae | Atriplex (scales and saltbush), Chenopodium (lamb's quarter), Salsola (Russian thistle), Kochia (firebush), Allenrolfea (iodine bush) |
| Asteraceae: Artemisia | Artemisia (mugworts, wormwood, sages) | |
| Asteraceae: Ambrosia | Ambrosia (ragweeds), Xanthium (cocklebur), Iva (poverty weed hemp) | |
| Amaranthaceae | Amaranthus (careless weed, pigweeds), Acnida (Western water hemp) | |
| Plantaginaceae | Plantago (plantain) | |
| Polygonaceae | Rumex (dock and sorrel) | |
| Urticaceae | Parietaria |
Representative genera are members of the same botanical family or subfamily. Manufacturers currently offer allergen products derived from one or more species of each listed genus
Generally, fewer tests to suspected allergens are required in infants and very young children (<2 years of age) because children are not likely to be sensitized to as many allergens as older children and adults. In toddlers, allergic sensitization reflects intense and/or prolonged exposure to allergens encountered early in life, such as foods, house dust mites, indoor molds, and animal dander rather than pollens.12
Relatively few foods account for most IgE-mediated allergic reactions in both children and adults. The more common food allergens in infants and young children are: cow's milk, hen's egg, peanuts, tree nuts, soybeans, and wheat, whereas the adult counterparts are peanuts, tree nuts, fish, crustaceans, mollusks, fruits, and vegetables. However, this generalization does not preclude the possibility that larger numbers of tests may be required, if multiple or hidden food allergies are suspected; this must be weighed against a high false positive response rate. PPT (Prick-Prick testing) whereby a fresh food sample is pricked followed by immediate pricking of the skin in suspected vegetable and fruit IgE-mediated reactions can provide greater sensitivity. Occupationally related allergy (e.g., latex, rodents, flour, food inhalants, etc.) is a special clinical condition for which a limited number of reliable skin test reagents are available.
The general recommendations for SPT are summarized in Table 5.
Table 5.
Recommendations for SPT
|
|
|
|
|
|
Available devices for skin prick testing
There are a variety of devices for performing skin tests throughout the world, developed in an attempt to improve reproducibility of the skin prick test method. Devices used generally are designed with a sharp point (0.9 or 1 mm) and a shoulder to prevent excessive penetration into the dermis. The most popular instruments are the Morrow Brown standardized needle, the “Greer Pick®” (DKL), the Stallerpoint®, and the Phazet®. Puncture tests can also be performed with a bifurcated smallpox vaccination needle, a 23G intravenous needle or with other devices. Several plastic devices with multiple heads (“multi-headed” skin test devices) have also been developed to apply several skin tests at the same time, which may limit technician time, and increase efficiency.
The inter-device wheal size variability is highly significant (Table 6). Potential causes of this variability include depth of penetration into the skin, amount of antigen entering the skin, angle of penetration, and skill of the investigator. It is imperative that the allergist/immunologist understands the characteristics of the device chosen (Table 7).
Table 6.
Recommendations on SPT devices
|
|
|
Table 7.
Wheal size indicating a positive response to skin tests using various devicesa.
| Devices for which a 3-mm wheal would be significant |
Devices for which a wheal >3 mm should be used as significant |
||
|---|---|---|---|
| Device | 99th Percentile of reactions at the negative control sites | Device | 99th Percentile of reactions at the negative control sites |
| Quintest (HS) puncture | 0 mm | DuoTip (Lincoln) twist | 3.5 mm |
| Smallpox needle (HS) prick | 0 mm | Bifurcated needle(ALO) prick | 4.0 mm |
| DuoTip (Lincoln) prick | 1.5 mm | MultiTest (Lincoln) Puncture - | 4.0 mm |
| Lancet (HS) puncture | 2.0 mm | Bifurcated needle (ALO) puncture | 4.5 mm |
| Lancet (ALK) Puncture | 3.0 mm | Quick Test (Pantrex)- | 4.0 mm |
| DermaPICK II (Biomedixs) Prick or puncture | 0 mm | Greer Track(Greer) | 3.5 mm |
Abbreviations: HS, Hollister Steir; Greer, Greer Laboratories; ALO, Allergy Labs of Ohio; Lincoln, Lincoln Diagnostics; ALK, ALK America. Positive response is defined as a wheal greater than 99% of wheals generated by the administration of saline to the subject's back by the same operator.
Adapted from Bernstein IL, Li JT, Bernstein DI et al. Allergy diagnostic testing: an updated practice parameter. Ann Allergy Asthma Immunol 2008; 100(Suppl 3):S1–S148.12
Skin prick test technique
SPT must be performed on normal skin. SPT are usually applied, for practical reasons on the volar surface of the forearm; but other sites are equally effective. The antecubital fossa is the most reactive portion of the arm, whereas the wrist is the less reactive. The ulnar side of the arm is more reactive than the radial area. In infants, the back is the preferred site for SPT. It is recommended that tests should not be placed within 5 cm of the wrist and 3 cm of the antecubital fossa. Skin tests should not be performed on skin sites with active dermatitis, severe dermographism and tattoos. Ageing of the skin, and sun damage will affect the skin's reactivity. The location of each allergenic drop can be marked with a pen or a test grid. The allergenic solution placed on the skin should be immediately pricked.
The selected test site is cleansed and disinfected with alcohol and allowed to dry. After marking the skin sites to place the extract drops with numbers, codes, or using a template, the allergen extracts are applied to the skin, depositing a drop of allergen extract on the skin of the forearm of the patient. The puncture device is passed through the drop at a 45°–60° angle to the skin, achieving penetration of small amounts of allergen extract just below the epidermis. This is called the skin prick test (alternatively, the skin device may be passed through the drop at a 90° angle to the skin with gentle pressure for 1 second, this is called a puncture test). The drops must be placed 2 cm or more apart each other to avoid mixing or overlapping and therefore false-positive reactions. If mast cells are sensitized with specific IgE in the patient's tissue, the penetration of the allergen causes the release of histamine, resulting in a wheal and flare response.
Because of inter-patient variability in cutaneous reactivity, it is necessary to include negative and positive controls at the same time as allergen tests in every skin test evaluation. Positive control solutions (histamine phosphate, used at a concentration of 5.43 mmol/L or 2.7 mg/mL, equivalent to 1 mg/mL of histamine base) are used to detect suppression by medications or disease, detect the exceptional patients who are poorly reactive to histamine, and determine variations in technician performance and/or the potency of the testing reagent. Histamine dihydrochloride is commercially available and has been approved for the use for in vivo testing in Japan.
The negative control (saline or 50% glycerinated human serum albumin–saline) will also detect traumatic reactivity induced by the skin test device (with a wheal which may approach a diameter of 3 mm with some devices) and/or the technique of the tester or the presence of dermographism.
Some of the most common errors in skin prick testing are listed in Table 8.
Table 8.
Common errors in skin prick testing.
|
|
|
|
Adapted from Mansmann HC, Jr, Bierman CW, Pearlmann DS (eds). Allergic diseases in infancy, childhood and adolesence.1980: p.289, with permission. Copyright, Elsevier; Bousquet J et al. Practical guide to skin prick test in allergy to aeroallergens. Allergy 2011.
Measurement and interpretation of skin prick test
Skin tests should be read at the peak of their reaction and in a standardized manner. Whatever the method, the immediate skin test induces a response that reaches a peak in 8–10 minutes for histamine and 15–20 minutes for allergens. The reading and evaluation of skin tests using an arbitrary scale of 0–4 + is not recommended unless the specific criteria for the scale are defined on the skin test form. The least variable method, which is both objective and reproducible, occurs when the wheal's size is measured in millimeters (mm) with a ruler. The size of the reaction may be recorded as a mean wheal diameter, D + d/2 (with D indicating the largest diameter of the wheal and d indicating the largest diameter orthogonal to D). Other Authors suggests (D + d)/2. Pseudopods are not included in the measurement, but can be marked separately. A prick/puncture test with a response of at least 3-mm diameter in wheal more than simultaneously performed diluent control is required as proof of the presence of cutaneous hypersensitivity, indicative of the presence of specific IgE.
The presence of allergic sensitization (a positive SPT with no correlative allergic disease) is a common finding, occurring in 8–30% of the population when using a local standard panel of aeroallergens. Interestingly, a prospective study13 reported that 60% of skin prick test positive (wheal > 4 mm) asymptomatic subjects developed clinical allergy, thus, the presence of positive SPT in asymptomatic subjects may predict the subsequent development of allergic symptoms.
False-positive tests may be provoked by impurities, contaminants, and non-specific mast cell secretagogues in the extract, as well as dermographism. Devices used and techniques applied should also be considered when comparing measurements to the negative control.
The most common cause of false-negative tests is the ingestion of a drug that inhibits the effect of histamine. In addition, skin reactivity is decreased in infants and the elderly and in skin that has suffered chronic solar injury. Technical factors that result in false negative results include improper technique, too short or too long interval from application to measurement and extracts of reduced potency due either to aging of the extract or poor original quality of the extract.
Since the interpretation of skin tests can have significant impact on daily life, in terms of avoidance measures and therapies, the specialist must pay attention to different clinical aspects:
-
•
positive tests may occur in absence of clinical relevant symptoms (sensitization);
-
•
negative skin prick test results can miss the presence of IgE-mediated sensitization (e.g., due to lack of major allergens in commercial extracts);
-
•
negative SPT results in children do not exclude the possibility of development of allergic diseases in the future.
When the SPT result is not clear or does not correlate with clinical history, a serum specific IgE assay or, more rarely a challenge with the culprit allergen, may be needed (Table 9).
Table 9.
Recommendations in SPT interpretation
|
|
Since the interpretation of SPT results is crucial for a proper treatment approach, in order to find out “reading keys” for interpretation of test results, a GA2LEN (Global Allergy and Asthma European Network) survey investigated the correlation between SPT wheal size and self-reported clinical relevance (i.e., symptoms related to asthma, allergic rhinitis, atopic dermatitis, and food allergy) for 18 allergens tested in 3068 patients in 17 European centers. With the exception of Aspergillus fumigatus, with larger wheal sizes the prevalence of allergic symptoms increased significantly. This correlation was variable among the different allergens, ranging from 40% (Blatella) to 87–89% (grass, Dermatophagoides pteronyssinus) of the positive SPT wheals associated with patient-reported clinical symptoms. In general, children with positive SPT were less symptomatic than adults in relation to hazel tree (P < 0.001) and dog (P < 0.001); no difference was found for house dust mites. The frequency of symptoms was slightly higher among skin test positive women than skin test positive men for hazel tree (P = 0.012), dog (P = 0.031), and Dermatophagoides pteronyssinus (P = 0.064). Furthermore, when geographical influence was assessed, a lower frequency of symptoms in the Mediterranean vs. Nordic and Central European regions was noted in relation to positive SPTs to hazel tree and dog; on the contrary, no differences were found for grass or house dust mites. Regarding the correlation between positive SPTs and physician-reported diagnosis, positive SPT for grass (OR 2.96, 95% CI 2.4–3.7), cat (OR 2.0, CI 1.6–2.6), Dermatophagoides pteronyssinus (OR 1.7, CI 1.4–2.1) and hazel tree (OR 1.7, CI 1.1–2.5) statistically increased the risk of allergic rhinitis. Positive SPT to Dermatophagoides pteronyssinus (OR 2.2, 95% CI 1.8–2.6), cat (OR 1.4, CI 1.1–1.8), and grass (OR 1.2, CI 1.0–1.5) significantly increased the risk of developing asthma, particularly in children (OR 4.2, CI 3.4–5.2). Positive SPT reaction to cat (OR 1.3, 95% CI 1.0–1.7) and grass (OR 1.3, CI 1.0–1.6) were slightly correlated with a major risk for atopic dermatitis, especially in children (OR 1.5, CI 1.2–1.9) and in females (OR 1.5, CI 1.2–1.9). Cutaneous positivity to birch (OR 1.7, CI 1.1–2.6) augmented the risk of food allergy, particularly in females (OR 1.4, CI 1.1–1.8). For each allergen, the wheal size in mm (ranging from 3 to 10 mm) with an 80% positive predictive value (PPV) for clinical relevance was calculated and reported in a GA2LEN “reading key” form that can be a useful tool for interpreting SPT results.14
Intradermal test
General aspects
In a patient with a strong clinical suspicion of an IgE-mediated disease with negative skin prick tests, the intradermal test (IDT) can be considered. IDTs are frequently used for inhalant allergen sensitization in the United States. Challenge studies have not confirmed the predictability of the test, however, it could be applied in specific situations (i.e., IDTs to foods are sometimes utilized for delayed food anaphylaxis from alpha-gal allergy).15 IDTs are applied when assessing hypersensitivity to drugs or hymenoptera venoms (see below).
It is important to consider the relative advantages of prick and intradermal testing (see Table 10).
Table 10.
Relative advantages/disadvantages of prick and intradermal allergy skin testing.
| Prick test | Intradermal test | |
|---|---|---|
| Simplicity | ++++ | ++ |
| Speed | ++++ | ++ |
| Interpretation of positive and negative reactions | ++++ | ++ |
| Discomfort | + | +++ |
| False-positive reactions | Possible | Likely |
| False-negative reactions | Possible | Rare |
| Reproducibility | +++ | ++++ |
| Sensitivity | +++ | ++++ |
| Specificity | ++++ | +++ |
| Indicative of IgE antibodies | Yes | Yes |
| Safety | ++++ | ++ |
| Testing of infants | Yes | Difficult |
Adapted from Adkinson: Middleton's Allergy: Principles and Practice, 7th ed. 2008. Chapter: 71 – In Vivo Methods for the Study of Allergy. Pascal Demoly, Jean Bousquet, and Antonino Romano.229
Intradermal testing (IDT) is important to reveal both immediate IgE-mediated allergy and delayed-type hypersensitivity. When used for type 1 allergy diagnosis, it is characterized by an increased risk for adverse reactions, thus requiring high levels of technical and interpretative expertise: for this reason, it is generally restricted to a clinical setting where emergency equipment and treatment are readily available.16,17 Delayed IDT readings are performed for delayed reactions, but will not be discussed in this manuscript.
Indications and contraindications
IDT is usually not required for the diagnosis of respiratory allergy. It is mainly indicated in case of suspected respiratory allergies with negative SPT, venom allergy and drug allergy. It has an established place in testing β-lactam (in particular, penicillin and cephalosporin) allergy, but may also be used for testing a number of other drugs such as insulin, opiates, anesthetics, neuromuscular relaxants, proton-pump inhibitors, enzymes, and chemotherapeutic agents.18, 19, 20
Contraindications for IDT include:
-
•
Diffuse dermatological conditions, such as eczema, urticaria, and dermographism;
-
•
Poor subject cooperation;
-
•
Patients being unable to stop antihistamines/other interfering drug treatments;
- •
-
•
*May be utilized in specific situations (i.e., IDT for foods are sometimes utilized for delayed food anaphylaxis from alpha-gal)15
Relative contraindications/precautions:
-
•
Persistent/unstable asthma;
-
•
Pregnancy (due to risk of anaphylaxis with hypotension and uterine contractions);
-
•
Infants or younger children.
Technique
In IDT, allergens (usually, 0.02 mL) are injected intradermally with small needles to produce a small bleb, and the outcome measure is an increase in the size of the wheal with flare reaction at 20 minutes. Allergenic extract must be diluted (10–1000 fold or more) from the concentrations used for SPT. IDT should always be preceded by SPT including negative and positive controls.
The skin end point titration can be defined as the intradermal injection of allergens at increasing concentrations to measure their allergic response. This is typically done for venom and drug allergy assessments but not for inhalant allergens. To avoid severe allergic reactions, testing starts with highly diluted extracts. After 15–20 minutes, the injection site is measured in terms of the size of the wheal and flare reaction. The end point, typically, is the concentration of antigen that causes an increase in the size of the wheal. For other Authors,23 the endpoint is the first dose of antigen provoking minimal erythema.
This method allows one to grossly quantify the individual's skin response and, subsequently, their degree of allergic sensitivity. Titration methods for IDTs have not been validated.24
Precautions and contraindications of all type I skin testing
Adverse reactions
The prick/puncture test is safe, with systemic reactions occasionally observed with commercial extracts. Foods were identified as the most relevant trigger (75%), with nuts having the highest risk. In general, history of severe allergic symptoms and large skin test reactions were recognized as predictors of possible severe adverse reactions to allergy skin testing (Table 11).
Table 11.
Type I skin testing summary precautions.
|
Adapted from Adkinson: Middleton's Allergy: Principles and Practice, 7th ed. 2008. Chapter: 71 – In Vivo Methods for the Study of Allergy. Pascal Demoly, Jean Bousquet, and Antonino Romano.229
Frequency of skin testing
Skin tests may be repeated for a variety of reasons including: changes in clinical manifestations or exposures; lack of clinical correlation with sensitization patterns; or the resolution of venom immunotherapy. However, routine repeated skin testing is not recommended.
Age for performing skin testing
Prick/puncture tests may be performed at any age if indicated, recognizing that positive reactions tend to be smaller in infants and younger children (<2 years), and in the elderly.
In vitro diagnosis
General concepts
Identification of the causative allergen (usually an allergenic protein) responsible for the causation of allergic disease is the main purpose of the allergy diagnostic evaluation. The identification of biological sources having allergenic properties dates back more than 100 years, and it is still in progress. Raw extracts obtained from allergenic sources have been used for decades to help demonstrate clinically relevant sensitization by means of skin testing, as well as provocation testing, such as oral or mucosal (nasal, conjunctival or bronchial) challenges. Since the purification of IgE in 1967, serological testing has become a commonly used test in the evaluation of allergic diseases.25
Using the first in vitro specific-IgE tests, some drawbacks of in vitro allergy testing were reported,26 but the progressive introduction of high-performance laboratory-based IgE methods, particularly after the development of second-generation in vitro systems,27 has greatly increased their diagnostic accuracy. Currently, automated workstations reduce the required labor, costs and errors, thus improving the consistency of in vitro tests. To date, the results achieved with in vitro specific IgE measurement and skin testing are nearly comparable with some well-known advantages and disadvantages for each diagnostic approach.28,29 The main problem with in vitro tests concerns the intrinsic nature of the crude extracts used, which are often are an unpredictable mixture of allergenic and non-allergenic substances. In addition, different producers have different extracts and the same producer may have different extract from material collected in different years.30 Despite these intrinsic differences which were an obstacle to full standardization,31,32 these “raw” extracts were used as allergen source for in vivo and in vitro tests for several decades. However, during the first years of the 1990s, molecular allergists began the production of recombinant allergens (termed components) that were thus used as reagents for in vitro diagnostics. It was immediately evident that molecular components did not completely correlate with the results of SPT and of sIgE tests for a number of reasons that will be discussed. But, it was also evident that the specific characteristics of each molecular component, when used in diagnostics, provided a real added value. Indeed, we are now able to classify components according to different strategies, represented by well-defined molecular characteristics (Table 12).
Table 12.
Different classification of allergens and molecular components are based on specific functional, clinical or biochemical characteristics (exemplary allergens in parentheses)
| Classification of allergen extract | Classification of molecular components | Cross reactivity of molecular components | Risk of molecular components | Frequency of molecular components | Evolution of molecular components | Susceptibility of molecular components |
|---|---|---|---|---|---|---|
| Inhalants | Inhalants (Phl p 1) | Genuine (Phl p 1) | Potentially dangerous (Ara h 1) | Frequent (major component) Phl p 1 | Age related (Bos d 1) | Heat Sensitive: Mal d 1 Resistant: (Pru p 3) |
| Food | Food (Mal d 1) | Pan allergen Phl p 12 | Virtually innocuous (Ara h 8) | Rare (minor components) Phl p 2 | Related to allergy march (Gal d 1) | Low pH Sensitive: Mal d 1 Resistant: (Pru p 3) |
| Contact (latex) | Contact (latex) (Hev b 6) | Cross-reactive Bet v 1 | Related to allergy march (Phl p 12) | Gut peptidase Sensitive: Mal d 1 Resistant: (Pru p 3) |
||
| Hymenoptera | Hymenoptera (Ves v 5) |
The full assessment of molecular components is quite complicated: at the moment (September 2018), in fact, >4700 distinct molecules have been described, including >3200 isoforms (such as Ara h 2.0101, Ara h 2.0102, Ara h 2.0201 and Ara h 2.0202 or Ara h 2) (http://www.allergome.org/script/statistic.php). This very large number of potential reagents offers unexpected possibilities in allergy diagnostics. So, in this chapter, the characteristics of in vitro IgE tests will be discussed in light of the most recent advances in basic and clinical research.
Diagnostic strategy
Before describing the different methods available and their extended technical possibilities, it is necessary to analyze the medical and diagnostic contexts in which these in vitro methods are used. The comparison of the results obtained by using SPT in vivo and sIgE in vitro started virtually immediately after the development of laboratory methods for the detection of specific IgE. Two groups of spirited partisans arose. SPT followers maintained that SPT is in general simple and painless; but sIgE supporters considered that SPT, in small children, can be really difficult, requiring a certain amount of co-operation from the patient. SPT followers maintain that in vivo assessment offers quick results (tens of minutes) but sIgE followers showed that modern sIgE requires just a couple of hours. SPT followers indicated that SPT can be performed with virtually any allergen (the prick-to-prick procedure) but sIgE countered by maintaining that the number of allergens to be tested in vitro is just limited by the costs. Moreover, sIgE followers noted that sIgE assays can be performed by any laboratory technician, are not affected by anti-histamine drugs, the results – despite certain differences between reagents and producers – are not dependent on the operator's experience, and potentially dangerous allergens are not administered to the patient. Nowadays, the two parties (SPT followers and sIgE followers) are still debating.33, 34, 35 As a general concept, SPTs are more sensitive than in vitro tests, whereas serum specific IgE detection is more quantitative than SPT. This discussion could have certain significance only if in vitro assays should replace SPT in the future (for example, if a reagent for SPT cannot be produced like for a drug). But at present, SPT are still available and the top-down strategy of allergy diagnosis36 remains the most frequent approach followed in clinical allergy diagnostics. However, in this context, it should be noted that SPT is considered a functional test by which the skin reactivity to an allergen (no matter whether mediated by IgE or not) is measured by the diameter of the wheal. On the contrary, different sIgE tests are assays used to detect the presence of IgE antibodies able to bind an extract allergen or a molecular component. So, specific IgEs are suitable to identify the presence of serum IgE to one or more allergens provided that IgE are present and detectable (Table 13). Sensitivity and specificity can be evaluated, for every new laboratory assay, by comparing the results of the novel test to that of a gold standard. For specific IgE, a gold standard does not seem to exist. Indeed, it is a common notion that every subject recognizes antigens or allergens on the basis of her/his genetic substrate: so, certain epitopes are recognized by certain haplotypes, while others are not. In addition, in vitro assays are in general based on an excess of allergen bound to the solid phase: for this reason, a large low-affinity immune response cannot be easily distinguished from a high affinity one.
Table 13.
Interpretation of non-concordance between allergen extract and allergen molecular IgE assay results (modified from Matricardi, Kleine-Tebbe 2016 144)
Allergen extract positive but its molecules negative
|
To this it should be added that, like any other serological method, every specific IgE test has certain characteristics, related to the structure of the solid phase (if any), the amount of allergen in the tube, the time and conditions for the incubation, the characteristics of the anti-IgE, the behavior of the labeled antigen the detection method used (like Enzyme Immune Assay [EIA] or Chemiluminescence [CL]), and the dynamic range of the reading etc. For example, in vitro assays based on chemiluminescence are more positive (in particular in the presence of low levels of specific IgE) than assays based on ELISA or similar techniques. So, in the presence of a positive result with CL and a negative result with ELISA, the question that must be asked is: is this a false positive result or is this result the demonstration of the presence of a small (but specific) IgE response to the allergen? In other words, is ELISA underestimating the presence of sIgE? Along this line, even the solid phase structure may have an unexpected effect on the serological result. For example, it has been shown that certain solid phases express CCD, thus causing false positive results in patients with sIgE to CCD.37 What is known from basic immunology is that a positive result can be observed in any situation just by changing the experimental conditions or increasing the sensitivity of the assay itself. Antibodies are characterized by a spectrum of specificities for the antigens: they are extremely specific under certain experimental conditions but, in other conditions, antibodies can efficiently recognize similar epitopes. So, the answer to this question lies squarely in the hands of the allergist who must decide whether what is observed is just a sensitization or a real allergy. Keeping in mind all these relevant points, it is evident that the comparison between methods may be sometimes an oversimplification of the problem, causing more confusion rather than shedding light on the argument. Along this line, from a practical point of view, an allergist should build her/his experience on a well-defined in vitro test, in order to have a clear strategy for the interpretation of the in vitro results. All these considerations are even more relevant when the tools of molecular allergy diagnostics are used. Indeed, at present, if an accurate diagnosis of allergic sensitization is required, specific IgE to allergen extracts are not always as precise as wished for, and a more specific description of the IgE profile may be mandated. This is particularly true when allergen immunotherapy (AIT) is provided to the patient. Even though large discussions on this topic are still ongoing, suggestive data seems to indicate that AIT is probably more effective in patients sensitized to genuine allergens,38, 39, 40, 41, 42, 43,42,43 while its activity is less impressive in patients with a sensitization to cross-reacting components or pan-allergens. In this context, the in vitro evidence of specific IgE involvement in the patient's symptoms associated with the detection of IgE specific for genuine components seems to be the entry level diagnostic for patients that could have a real modification of their allergy by AIT.
These considerations should be always be taken into account. One cannot directly compare the two methods (SPT and sIgE tests) as they are profoundly different in many aspects. Specific IgE (to the allergen extract or to molecular components) provide a real added value in those cases where the allergist requires a precise diagnosis before starting AIT and to identify accurate therapeutic or prophylactic strategies in food and hymenoptera allergy. Along this line, it should be clearly noted that specific IgE tests are poor or not significant at all for the very large majority of drug allergies. Although a few drug reagents are available (in particular, antigens derived from penicillin and some peptide hormones) they do not encompass the requirements of allergists in this field.
Total serum IgE
In 1967, the first solid-phase sandwich immunoassay for the measurement of total and specific IgE was described.44 The amount of total IgE was considered in the early studies as the simplest way to identify allergic subjects,45,46 but it became evident soon that total IgE levels could not be considered a reliable marker of allergy status.47,48 IgE levels significantly higher than the normal threshold, are usually associated with atopic disorders, but also with other conditions (see below). On the contrary, low or normal values do not exclude the presence of IgE-mediated diseases. As a consequence, total levels of IgE should be carefully interpreted and not considered as an indication for the presence of allergic diseases.
Serum IgE concentration is largely age-dependent. Very low levels of IgE are found in cord serum (<4.8 ng/mL) with a progressive increase observed up to the age of 15 years, similar to serum IgA. Total serum IgE then declines from the 2nd through the 8th decades of life.
Very high IgE levels are observed in parasitic infestations, rarely in multiple myeloma patients producing IgE, and in some primary immunodeficiencies (e.g., Immune dysregulation polyendocrinopathy enteropathy X-linked syndrome (IPEX), Omenn syndrome, Wiskott-Aldrich syndrome, Comel-Netherton syndrome, hyper-IgE syndrome and atypical complete Di George syndrome).49 Allergic broncho-pulmonary aspergillosis (ABPA) is the only clinical condition described to date, where the presence of high levels of IgE is strictly related to disease severity.50 Increased serum IgE levels can be seen also in a proportion of smokers.
In the past, total IgE levels were calculated using a number of immunoassays that utilize specific antibodies for human IgE as both capture and/or detection reagents. These antibodies, in the vast majority of cases, are conjugated on a solid phase (capture antibody) and/or directly labeled with radio-nuclide, enzyme, or fluorophore. Automated platforms significantly improved accuracy and reproducibility, increasing both specificity and sensitivity as well. Commercially available assays have been cross-standardized to a common primary human IgE standard (WHO 11/234).51 Total IgE values are currently reported in International Units of IgE per volume (IU/mL); a conversion factor (1 IU = 2.42 ng) is sometimes applied. Nowadays it is very common to be reported in its equivalence of kU/L.
Different scenarios can be considered. Table 14 shows these different conditions.
Table 14.
Relationships between Total IgE and specific IgE results.
| Normal Total IgE level | High total IgE level | |
|---|---|---|
| Absent sIgE | Non-allergic patient | See non-allergic high IgE conditionsa |
| Present sIgE | Patients with sensitization(s) | Patients with sensitization(s) |
This condition may also occur when specific IgE to the relevant allergen are not tested in the in vitro assay, or the specific allergen is missing in the allergen panel used.
Allergen-specific IgE assays
General concepts
The laboratory methods
The measurement of specific IgE recognizing allergenic epitopes can be achieved both through the usage of single reagents (singleplex) or with a pre-defined panel of a number of molecules to be tested simultaneously (multiplex).52
In general, there are some positive and negative aspects for both in vitro allergy testing methods that distinguish between the two different techniques.
The structure of the assay
Since the time of the original assays,25 the assay for the detection of specific IgE has been based on the classic sandwich technique with minor variation. The main elements are represented by the solid phase, the allergen conjugated with the solid phase, the patient's serum, the anti-IgE antibody, the labelling of the anti-IgE, and the substrate (if any) for the production of the signal. Washing and stop reagents are also present. However, the specific characteristics of the former are fundamental for the results while the latter (washings and stop solution) are less relevant.
The main reagents used in the assay
-
•
The reaction site (carrying the allergen) can be: a polyethylene cap with an internal sponge matrix, a plastic (polyethylene) or glass tube, a plastic microtiter plate well, a plastic stick, or a carbohydrate filament-coated silicone chip. To ameliorate the antibody-binding capacity, a variety of carbohydrate-based allergo-sorbents (other than Sephadex and paper), such as agarose and microcrystalline cellulose, can be used. The most important advance was the development of an encapsulated hydrophilic carrier polymer to which the allergen is covalently coupled.53
-
•
The allergen-containing reagent can be represented by a solid-phase allergo-sorbent or liquid-phase conjugated allergen. This is the most complex and highly variable component in terms of preparation from raw material, quality control and validation,54 conferring specificity on the IgE antibody assay. For example, RAST represented a non-competitive, immuno-radiometric assay that used allergen coupled on allergo-sorbent paper discs. Subsequently several other variants of the same assay were developed.55,56 In other systems, a liquid-phase is used with coupled anti-IgE that captures free serum IgE. These systems seemed not to recognize low affinity IgE, claimed to have less relevance from a clinical point of view, and have been rarely used. They are described as research tools.27 Usually, each allergen represents a distinct reagent, but a multi-allergen assay can be achieved by mixing a group of allergens into one reagent. In addition, solid-phase reagents are sometimes supplemented with recombinant molecules in order to improve the extract's performance (e.g., latex extract supplemented with rHev b 5).57
-
•
The nature of allergens used in the in vitro test for specific IgE.
As previously mentioned, allergens can be both raw extract allergens or single molecules. These molecules can be obtained by recombinant DNA technology or by biochemical purification from natural extracts (Table 16). Of note, raw extracts and highly purified extracts have certain post-translational modifications (such as glycosylation) that are absent in molecules produced in E.coli. There are two distinct types of molecules used in assays for specific IgE. The first one is represented by the so-called “genuine” markers of exposure, such as Phl p 1 from timothy grass pollen or Par j 2 from pellitory. These allergenic molecules belong to a specific biological source and are able to not only identify IgE sensitization, but also point towards the presence of the related allergenic sources in the environment.58,59 On that basis, epidemiological studies became possible. The first large scale surveys based on the routine use of ISAC biochips,60,61 allowed for the understanding of allergic sensitization in different geographical regions, and provided prevalence data for the development of molecule-based immunotherapy.62 The second group of molecules is represented by the so-called “cross-reactive molecules” or “pan-allergens”.63 They are families of strictly related proteins that are widely distributed among different species because they are involved in crucial cellular processes. Several panels of pan-allergens families are now identified (e.g. Bet v 1–like molecules, lipid transfer proteins, profilins, tropomyosins, parvalbumins, lipocalins, serum albumins, 2S albumins, vicilins, and 11S albumins). These panels of homologous molecules facilitate the diagnosis of sensitization in individual patients and also enhance the accuracy of epidemiologic research. Furthermore, despite the high sequence identity among components of every single group of pan-allergens, IgE co-recognition of homologous molecules64 does not always reflect what is predicted on the basis of amino acid sequence, but rather on the molecular 3D-structure.65 The use of several representative homologous molecules within every single pan-allergen group would provide more information on IgE epitope recognition, allergen structures, and, possibly, the identification of clinical phenotypes.66 The clinical picture, in fact, depends on which exposures determine the type of sensitization pattern (as previously mentioned) and may range from total absence of symptoms, (despite the presence of IgE reactivity) to severe, life threatening generalized reactions.67, 68, 69
-
•
The human sample. Both human sera and plasma have been used in diagnostics. In the original assays, undiluted serum samples were used. Following the introduction of novel and automated assays, the sample volume was reduced. It is evident that the concentration of the human sample in the test tube determines the results. Every laboratory method has its specific serum volume and concentration calibrated on the other reagents, such as the allergen amount and the anti-IgE “detection” antiserum (or monoclonal antibody)
-
•
The anti-human IgE Fc detection reagents (ε heavy-chain specific) usually are polyclonal rabbit, goat, sheep, horse, or murine anti-IgE monoclonal antibodies. Combinations of polyclonal and monoclonal (mAb) anti-human IgE and labeled human α-FcεR170 have also been used to detect human (but also horse, dog, and cat) IgE.
-
•
Antibody labelling and detection methods. Anti-human anti-sera or mAb were originally labeled with 125I (the original Radio Allergo-Sorbent Test – RAST). Nowadays, other labelling techniques using enzymes such as b-Galactosidase (b-Gal), Alkaline phosphatase (AP), and Horseradish Peroxidase (HPO) are used. A strictly related reagent is represented by the enzyme substrates: from pNPP for AP to “perox” for HPO. The sensitivity of the assay is improved when specific substrates (named chemiluminescent) are used. In this second case, a different reading method is necessary: indeed, for substrates emitting in the optical range, a photometer is needed, while for chemiluminescence, a fluorometer is required.
-
•
The calibration system (e.g., reference serum containing a known amount of IgE) defines the level of IgE antibody measured by the assay creating a calibration curve. The reference curve, as stated above, can be obtained by means of a “heterologous” or “homologous” interpolation approach. In the first approach, quantitative allergen-specific IgE antibodies are expressed in IUA/mL, where the “A” means “allergen-specific”, differentiating this measurement from the IU/mL utilized for the total IgE assay. In the second, the measurement is indicated by arbitrary units using a homologous calibration curve.27
-
•
The reaction buffer medium (salts, proteins) normalizes pH and gives a protein matrix for the analyses of interest to warrant the nonspecific binding.
-
•
The control samples, containing positive serum controls and not containing (negative serum controls) allergen-specific IgE antibody.
-
•
The data-processing software, for managing of results and data processing.
Table 16.
Allergen extracts and molecular components available from different commercial sources in single-plex allergy diagnostics
| Producer | Total number of available allergens | Number of extract allergens | Number of molecular component | Laboratory method |
|---|---|---|---|---|
| Thermofisher | 566 | 460 | 106 (of which 28 N and 78 R) | Enzyme immunoassay |
| Siemens | 439 | 413 | 26 (of which 20 N and 6R) | Chemiluminescence |
| Hycor | 79 | 69 | 10 | Chemiluminescence |
| Euroimmun (in 92 strips) | 316 | 285 | 31 of which 20 R and 11 N) | Dot blot |
Singleplex assay
The presence of allergen-specific IgE antibody in serum identifies sensitized individuals, and a large fraction of them can be considered allergic if the symptoms they observe are in agreement with the allergen(s) detected.
A number of articles have been published to try to define the sensitivity and the specificity of in vitro IgE tests. This may prove useful for more epidemiological reasons, if, in the future, in vivo SPT are abandoned. From a scientific point of view, it is a common notion that not all allergic reactions are mediated by IgE. Thus, defining the sensitivity of an in vitro assay that is well known to be able to detect only allergen specific IgE, by comparing it with clinical diagnosis of allergy, could lead to partially wrong conclusions. The diagnosis of allergy is a clinical diagnosis. The presence of specific IgE that could better correlate with the clinical picture, could be useful to better define the allergic profile of the patient before starting AIT. This is particularly true for inhalant allergy, while for food allergy, the picture is much more complex. For example, the dynamic range of specific IgE to inhalant allergens is wide, while the same range for food IgE is significantly smaller. Thus, the use of a single interval of reference values in these two different situations should not be accepted in a laboratory medicine setting. Indeed, not all hormones have the same range interval of reference values either. Although discrepancies remain, the original reference intervals used for allergens never changed. In the future, a more accurate approach will be required to define reference intervals, particularly in the presence of low concentrations of IgE. In this context, if should be noted that a certain variability between different methods (and laboratories) is expected, as was recently observed.71,72
There are a large number of singleplexed diagnostics systems in the world, and four major ones can be identified (Table 15). Each has specific characteristics and performances. Probably, this is not the right place to discuss differences between different laboratory solutions. Each has pros and cons. But in general, an IgE profile derived from one of these four producers is acceptable. As stated before, the specific characteristics of each method should be well known to the allergist. So, at least in follow up (in particular in the pediatric age), the use of different methods could sometimes generate results that could be difficult to interpret and/or manage.
Table 15.
The most commonly used systems for specific IgE detection include the following distinct components
| Producer | Solid phase | Allergens | Patient's serum | Anti-IgE | Anti-IgE Labelling | Enzyme substrate | Stop solution | Reading system |
|---|---|---|---|---|---|---|---|---|
| RAST | Sephadex or paper | Extract | 0.05 mL/sample | Polyclonal | 125 I | none | NN | Gamma-counter |
| Phadia | Polymer of hydrophilic, highly branched cellulose derivative enclosed in a capsule. | Extract or recombinant bound covalently to the solid phase | 0.04 mL | Mouse monoclonal anti-human IgE | ß-Galattosidase | 4-metilumbelliferil-ß-D-galattoside | Na Carbonate | Photometer |
| Siemens | Streptavidin-covered polystyrene ball conjugated with streptavidin- | Extract or recombinant allergens covalently to soluble biotinylated polylysine polymers. | anti-IgE antibody (mAb ? pAb?) | Alkaline phosphatase | 4-methoxy-4-(3-phosphatephenyl)-spiro-(1,2-dioxetane-3,2′-adamantane) | N.S. | Light emission detector (chemiluminescence) | |
| Hycor | Magnetic, streptavidin-coated microparticles incubated with a biotinylated allergen | Extract or recombinant | 0.04 mL | A mixture of two mouse monoclonal Anti-IgE | Horseradish Peroxidase | acridin based chemiluminescent substrate | N.S. | Light emission detector (chemiluminescence) |
| Euroimmun | Paper | Extract or recombinant | 1000 mL | Alkaline phospatase | Water | Scanner |
From a practical point of view, allergists use the singleplexed diagnostics in two different manners. The first is related to the results of SPT performed in the patient, in order to verify whether a positive or a negative result is confirmed by the presence of IgE to that allergen. Using this approach, the allergist focuses her/his attention to a very select (and small) number of allergens. In recent years, the availability of molecular components (identifying both genuine and cross-reacting molecules) has substantially improved the singleplexed strategy by allowing mixing whole allergen extracts and selected components, in order to have a clear description of the IgE profile of the patient. Nevertheless, it is evident that the allergist, by a singleplexed and censored choice of the allergen to be tested, can only detect IgE for the allergens required. Along this line, in some complex situations, for example a polysensitized patient with a large number of positive results by SPT, the list of allergens and components to be tested could be long.
Another approach (more focused on primary care) is related to the use of allergen panels. It is evident that panels for adults are different from panels for children, panels for a respiratory allergy are different from panels for a food allergy, and panels for northern countries are different from panels of southern countries, etc. So, the best approach, should be that every allergist develops her/his list of different panels suitable for her/his patients. This could be more difficult in primary care: for this reason, panels were developed in the late 1970s but at present their use is limited. A producer developed many different panels for different ages, climates, and symptoms. However, the choice of each allergist still remains fundamental. Panels with a mixture of whole extract allergens and molecular components could be a very interesting method, provided that the significance of a positive component is known in primary care.
The sensitivity of specific serum IgE antibody measurements could be considered as comparable to that obtained with skin prick testing for respiratory and food allergy, but only complementary to the intra-dermal skin test for drug and venom allergy diagnosis. The accuracy and reproducibility of the modern fully automated systems, providing reliable quantitative measures, has prompted investigators to assess the possible relationship between allergen-specific IgE antibody levels in serum and the clinical risk of an adverse reaction (probability-based risk assessment), especially in the field of food allergy.73, 74, 75, 76, 77, 78, 79 Higher risk of adverse reactions to food, as assessed by the reference standard for the diagnosis of food allergy, the double-blind placebo-controlled food challenge, was associated with defined diagnostic cut-off levels of food-specific IgE antibody.74 Unfortunately conflicting results were obtained in similar studies performed by other investigators attempting to replicate these findings,75 probably because predictive values should be carefully defined for each specific population separately.76
In the last several years, with the continued development of molecular diagnostics, it has become possible to perform singleplex assays with the use of recombinant or purified allergens (i.e., not only with allergenic extracts), thus increasing sensitivity, specificity, and diagnostic accuracy of the tests.8 The choice of using diagnostic recombinants in singleplex instead of in multiplex, is made on a case-by-case basis (considering previous history and clinical profile) and in an allergen-dependent manner (i.e., allergen source and availability of single recombinants).
The use of whole allergens and molecular components has pros and cons as it does in SPT. It is evident that the sensitivity of molecular components, when compared with that of whole allergens, is lower. This is due to many reasons. First, only in rare situations, a single component is so frequently positive in sensitized patients that the molecular component is representative of the sensitization. For example, Par j 2 is a major allergen of Parietaria judaica, and in clinical practice, the correlation between these two reagents is complete. But, for example, Phl p 1, one of the major components of Phleum pratense is positive in 70–80% of patients characterized by a positive reaction to the extract. To reach 90%, Phl p 1 should be associated with the other major component, Phl p 5. Second, not every molecular allergen is available in diagnostics. For this reason, at present, it is virtually impossible to describe a positive IgE reaction only by molecular allergen. For example, the IgE profile of dust mites, such as Dermatophagoides pteronyssinus, can be well detected by Der p 1, Der p 2 and Der p 10. But only with Der p 23, a fraction of originally extract-positive/component-negative sera becoming available, could the discrepancies between extracts and molecules be reduced.
As stated before, the singleplexed strategy of allergy diagnostics is strictly related to the classic “top-down” approach: the patient is seen by the allergist, SPT are performed, then if necessary, specific IgE for a selected panel of allergens are tested and, in a minority of situations, the study is deepened by using allergen components, in order to have a very accurate picture of the IgE profile.
More recently, a totally different approach, the so called “bottom-up” approach, was introduced in allergy diagnostics. Patients are originally screened by the use of a very large panel of reagents and, based on those results, the allergist then decides how many and which other components to test to improve the diagnosis and suggest a treatment. This approach was considered iconoclastic by classic allergists, but it may have certain advantages, at least in a precision medicine setting, where the best description of the patient's situation is required for any further decision.
In certain situations the use of recombinants is more useful and convenient, as compared with whole allergen extracts, such as in cases of multiple IgE sensitivities, particularly when assessing the following:
-
-
potential clinical risk and severity of allergic manifestations;
-
-
presence of cross-reactivities;
-
-
primary IgE sensitization;
-
-
polysensitization that is difficult to interpret.
A representative example is insuring that a complete screening includes specific IgE for Bet v 1, Bet v 2, and Bet v 4 recombinants to assess the type of sensitivity to birch (primary sensitization, possibility of cross-reactivities, and risk assessment for potential allergic reactions to cross-reactive foods). Another is the detection of sIgE for Der p 10 which is associated with IgE sensitivity to the tropomyosin protein family, increasingly the likelihood of reactions to ingestion of crustaceans and mollusks due to dust mite sensitivity, even if tropomyosin sensitization is primarily driven by seafood and not mite.80
Qualitative, semi-quantitative, and/or quantitative IgE antibody immuno-assays are currently available.
A qualitative assay generates only negative or positive results. It does not provide any measurement of IgE concentration, but it is defined by the presence of the analyte above a given positive threshold level of the assay. If levels are close to the cut-off point of the system, they can be considered as “borderline”.
A semi-quantitative assay produces a series of increasing classes (e.g., from I to VI), thus defining the amount of the response. These assays are not characterized by linearity, dilution recovery, or parallelism of quantitative assays.
A quantitative assay provides the measurement of IgE antibody concentration, on the basis of the interpolation from a multipoint calibration curve,77,78 obtained using both heterologous and/or homologous methods. A reliable quantitative assay should report results in units traceable to an internationally recognized standard (e.g., WHO 11/234).51 Unfortunately, it is not possible to use individual homologous calibrated IgE antibodies, for each of the hundreds of biologic sources tested. Therefore, the calibration method generally adopted is a heterologous interpolation of specific IgE antibody from a single total serum IgE reference curve. Quantitative systems results are reported in gravimetric (μg/L [total serum IgE assays]) or international units (kU/L [total serum IgE assays] or kUA/L [allergen-specific IgE antibody assays]).
Most specific IgE blood tests are immunoassays that include enzyme-linked immunosorbent assays (ELISAs), fluorescent enzyme immunoassays (FEIAs), chemiluminescent assays, or radioallergosorbent assays (RASTs). Since 2010, the National Institute of Allergy and Infectious Diseases (NIAID) / National Institutes of Health (NIH) have recommended discontinuation of the RAST as a diagnostic tool for allergy in favor of more sensitive fluorescence enzyme-labeled assays, in which a fluorescent antibody binds to the patient's sIgE, and the amount of IgE present is calculated from the amount of fluorescence. With over 4000 scientific articles showing its clinical value, ImmunoCAP at the moment is perceived as “reference standard” for in vitro IgE testing.81 However, the presence of this antibody only proves sensitization, not allergy, the latter being sensitization in the context of clinical symptoms upon exposure to the allergen.
The “CAP inhibition” technique is a variation of the direct CAP: allergic serum (containing sIgE) is first mixed with the soluble unknown allergen; then a standard amount of the solid-phase (immobilized) allergen is added. The CAP inhibition is important to evaluate the total allergenic activity of a diagnostic or therapeutic extract. Furthermore, it is still performed to distinguish multiple sensitizations from cross-reactive sensitivities, in particular as a diagnostic tool in Hymenoptera venom allergy; however, the development of molecular-based diagnostics in allergy has significantly reduced the need of CAP inhibition performance for certain Authors,82 while, for others, the CAP inhibition still remains fundamental.83, 84, 85
ELISA assays can represent a valid alternative to FEIAs, being relatively simple and inexpensive for the assessment of serum total and specific IgE for various common allergens. Their usefulness is more greatly appreciated where ImmunoCAP is not available. Academic medical centers may be able to provide in vitro allergy assays in areas without ImmunoCAP.86 Various systems currently employed in assessing allergen specific IgE often have substantial allergen by allergen discordance especially in the lower end of the allergen specific IgE assay range. This is likely due to variance in the allergens employed in these systems.
Multiplex assay
Genomic microarrays were developed and introduced about fifteen years ago in biomedicine to monitor the expression of many genes in parallel and to investigate differences in the level of mRNA expression from thousands of genes at the same time, in order to gain information about transcriptional changes involved in specific pathways.87 Genomic microarrays have provided insight into transcriptional gene expression of specific biologic pathways in the normal state and in disease.88 The proof of concept of the possible application of a proteomic microarray approach in the diagnosis of allergic sensitization appeared in the literature in 2002,89 followed by several other reports90, 91, 92 verifying the same array, widely known as the ISAC (Immuno Solid-phase Allergen Chip) system. A number of other studies have been published on the development of microarray prototypes,.93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106
Currently, IgE detection by means of microarray systems is classified as an in vitro diagnostic (IVD) tool.27 A few years ago the first version of the ISAC microarray became commercially available. It had 74 different allergenic proteins spotted on the microarray (ISAC74). The number of allergens immobilized on this microarray is growing and a version with 112 allergens (ISAC112) is currently available. The small amount of material necessary for the process of identification (from 0.1 to 1 ng)107 allows the use of both recombinant and highly purified natural molecules.
A study compared ImmunoCAP sIgE singleplex tests and the ISAC 112 IgE multiplex assay in 101 patients sensitized to grass pollens. The ISAC multiplex test correlated well with ImmunoCAP singleplex results, with a positive percent agreement (PPA) and negative percent agreement (NPA) of corresponding allergens varying between 60 and 100% for PPA and 78–97% for NPA.108
Interestingly, a deeper analysis of the ISAC 112's characteristics also demonstrated that it is a highly reproducible and accurate method which may be considered as a single analyte assay in the view of the EN ISO 15,189 accreditation procedure.109 This was further demonstrated in another work.110 Other authors108 observed that results obtained by allergen microarray correlated well with ImmunoCAP singleplexed results. In addition, technical improvement seems to ameliorate some of the less reproducible properties of the allergen microarray. Monroe et al.111 showed that their use of a calibrated fluorescence enhancement (CaFE) technique seems to more accurately measure probe density and bound target for a variety of antibody-antigen pairs.
At the same time, some at least partially unexpected cross-reactions between components of ISAC were identified.112 Not only can nPhl p4, a highly glycosylated protein cross-react with IgE specific for CCD, but also nCry j 1, nCup a 1, nCyn d 1, nPla a 1, and nJug r 2 can be non-specifically positive in patients sensitized to CCD. For this reason, the real clinical significance of a positive nJug r 2 must be carefully evaluated in the context of other component results and the patient's clinical picture.
Allergen microarrays were also used to evaluate the presence of specific IgE in fluids different from serum or plasma. Valenta and co-workers analyzed the presence of IgE in samples of breast milk.113 Leonardi et al.114 recently showed that in vernal keratoconjunctivitis (VKC), ISAC is able to detect the presence of specific IgE to grass, trees, mites, and animals but also food allergen-specific IgE in tears. What was particularly interesting was that in some patients, specific IgE were absent in serum but detectable in tears. The presence of specific IgE only in tears of VKC patients reinforces the concept of possible local sensitization. These two examples of the use of microarray technology for specimens different from plasma or serum opens new fields not only for research but also in clinics.
Another interesting and innovative use of allergen microarray is the monitoring of Allergen Immunotherapy. Indeed, it has been recently observed115 that allergen microarrays are useful to monitor the development of allergen-specific IgG responses during SIT, both against the allergen present in the SIT vaccine as well as against cross-reactive allergens. This application of the technique may finally offer a general-purpose tool for monitoring the immunological effects of AIT, resulting in better control of the treatment and, even more, a better understanding of therapeutically positive and negative results. This data was further supported by the article of Schmid JM et al.116 that demonstrated that pre-treatment allergen component-specific IgE appears to determine the induction of IgG4 during the updosing phase. Induced IgG4 seems to suppress IgE levels on ISAC, resulting in a marked decrease in ISAC-measured specific IgE levels after updosing of SCIT. They conclude that the decrease in ISAC IgE levels can be used to monitor the blocking effect of allergen-specific immunotherapy-induced non-IgE antibodies.
A number of studies have been performed in order to define the conditions where allergen microarray can or cannot be useful in different clinical pictures. For example, D'Amelio et al.117 argued whether the performance of ImmunoCAP ISAC 112 is sufficient to diagnose peach and apple allergies. They concluded that although the sensitivity of the peach components in ISAC is improvable, it can be sufficient in their region.115 The same authors118 concluded that the diagnostic performance of ISAC was adequate for hazelnut and walnut allergy but not for peanut allergy. Finally, in another different situation,119 even if the standard ImmunoCAP has, for apple and peach, a wider number of available components (in particular Mad d 3, a lipid transfer protein [LTP]), the evaluation of Pru p 3 (largely homologous to Mal d 3) may support the identification of an apple sensitivity even if “the presence of sIgE against Pru p 3 in LTP sensitized patients can be due to cross-reactivity and should therefore not be used to predict clinical symptoms”.
On the contrary, in idiopathic anaphylaxis120,121 the ISAC array contributed to the diagnosis in 20% of patients and may offer additional information where a careful allergy history and follow-on testing have not revealed the cause of the anaphylaxis.
Microarray technology is progressively improving, as shown by the MeDALL-chip which was developed within the MeDALL European project (Mechanisms of the Development of ALLergy) and is based on 170 relevant allergens. The MeDALL chip provided new information for some allergens and seemed to be more sensitive in detecting allergic sensitization than ImmunoCAP sIgE or SPT.119,122
In the period when ISAC 103 and ISAC 112 were used in clinics, other different attempts to modify the strategy of multiplexed molecular diagnostics were developed. The main attempt was related to the works of the MeDALL group that, in strict cooperation with industry, developed a novel microarray by adding more than 70 new components to the standard panel of ISAC 112.123 These new components were comprised of: Peanut allergens, Almond, cashew and pistachio allergens, Cow's milk allergens, Wheat allergens, Olive pollen allergens, Mite allergens, Dog allergens, Insect venom allergens, Staphylococcus aureus toxins, and Maltose binding protein (MBP). The clinical features of the MeDALL microarray were further evaluated during the so-called “allergen march”, from childhood to adolescence.124,125 The prevalence of allergic sensitization increased in all three diagnostic tests from age 10–16 years. It was similar by SPT and ImmunoCAP but significantly higher with the MeDALL-chip at 10 years. All three tests were comparable for identification of allergic sensitization among children with current rhinitis or asthma.125
A different approach has been developed by an English/Swedish company that designed and further implemented a microarray where not only single allergen components but also extractive whole components were spotted.126 This combination of extractive allergens and recombinant components was tested with other three allergy test methods (SPT, ImmunoCAP, and ISAC 112) and a total of 3485 pairwise test results were analyzed and compared. The four methods showed comparable results with a positive/negative agreement of 81–88% for any pair of test methods compared, which is in line with data in the literature. The most prevalent allergens (cat, dog, mite, timothy, birch and peanut) and their individual allergen components showed agreement between methods with correlation coefficients between 0.73 and 0.95. All four methods revealed deviating individual patient results for a minority of patients. These results indicate that microarray platforms are efficient and useful tools to characterize the specific IgE profile of allergic patients using a small volume of serum sample. The results produced by the Microtest system were in agreement with diagnostic tests in current use.
More recently, a different tool, termed ALEX (as in ALlergen EXplorer) was developed by MacroArrayDX in Vienna (Austria). The ALEX test is performed using an array of allergens spotted on a solid phase by way of nano-particles. ALEX contains 282 reagents (157 allergen extracts and 125 recombinant or highly purified molecules). So, in this chip, second level diagnostics (represented by extract allergens) and third level diagnostics (represented by single molecules) are all available. ALEX has been evaluated and the quality of ALEX results has been described.127 In consideration of the very large number of allergens and components and the significant complexity of the interpretation of the results (at least for non-professional molecular allergists), ALEX has been linked to a new version of the expert system, Allergenius, originally developed for the interpretation of ISAC results.128 In its present form, ALEX seems to be a good reagent for the “bottom-up” strategy of allergy diagnostics.
Another group of tests are represented by the multiple allergen simultaneous tests (MAST)-immunoblot, such as EUROLINE, which is a commercially available assay for component resolved allergy diagnostics based on the immunoblot technique. One “line blot” consists of several membrane chips containing allergen components coated in single lines. The membrane chips principle allows for optimized coating conditions of the respective components. Due to the simultaneous determination of sIgE to different allergen components, a sensitization profile can be generated in a single test run using low amounts of serum. This technique allows the detection of specific IgE quickly and efficiently with the use of immunoblot strips containing optimized combinations of relevant allergen components. Each profile is tailored to a specific indication: several indication-specific profiles for molecular allergy diagnostics are already available for inhalant allergens, insect venoms, and food allergens. When compared to ImmunoCAP, substantial agreement between MAST and ImmunoCAP was found for inhalant, food, and venom allergens thus representing a valid diagnostic alternative.129
A flow cytometric bead array (CBA) has been developed to detect soluble factors.130,131 Naked fluorescent micro-beads (Flex Set), can also be customized to detect specific antibodies,132,133 but not many reports on this specific application are found in the literature.134 CBA Flex Set, unlike other micro-bead-based systems,135 is designed for flow cytometry, an instrument widely used in almost all routine or research laboratories. The feasibility of the CBA Flex Set for specific IgE detection in human sera using allergenic molecules coupled on the fluorescent micro-beads, thus obtaining a “flexible” microarray for testing IgE, has been demonstrated.136 The system was named Allergen Bead Array (ABA), and the simultaneous measurement of up to thirty different micro-beads can be carried out just using the Fluorescence-Activated Cell Sorting (FACS) facilities. The opportunity of multiple fluorescence parameters evaluation could also permit the measurement of distinct antibody isotypes involved in antigen recognition in the same sample.136
Some criticisms have arisen against the multiplex approach, including poor flexibility in the list of allergens and the possible lack of relevant allergens. The allergist is forced to test the entire set of molecules in the commercial product, and it is not possible to test a “patient-tailored” set of molecules. Despite this, the allergen array based approach is in line with the modern concept of “precision medicine”. However, this approach may sometimes generate more questions than answers and complicate the management of, and the recommendations for, a specific patient.137 A recent paper compared the classic top-down approach (represented by the visit of the patient, then SPT, then — if considered useful — by specific IgE to whole extract allergens and/or single components and, finally, in a very select number of cases, the use of multiplexed IgE detection assays) and the so-called “bottom-up” strategy, represented by the immediate analysis of the patient's IgE profile (the patient's phenotype) followed by in vitro and in vivo tests useful to better describe the patient's endotype and how they can relate to therapeutic management.138
Informatics
The introduction of Expert System technologies to support Molecule Based Allergy Diagnostics (MBAD) has also led to the introduction of new concepts to the diagnostic approach.128 Indeed, ISAC seems to be redundant to some extent: for example, the number of profilins and LTPs measured seem to be higher than needed. Nevertheless, it has been recently observed that a hierarchy of cross-reacting components can be identified using large-scale MBAD assays.139 It was shown that IgE reactivity of PR-10 proteins is characterized by a hierarchical interrelationship: Bet v 1 > Mal d 1 > Cor a 1.04 > Ara h 8 > Pru p 1 > Aln g 1 > Api g 1 > Act d 8 > Gly m 4. For this reason, it is evident that having many cross-reacting components indicates a lot more than having only a few. Along this line, the rule is that if the number of positive components is >40% of the total number of components of a given family, the patient can be considered sensitized to the whole family of cross-reacting molecules.128 In addition, the first sensitizer in the member of the family of cross-reacting components can be identified as the one with the highest IgE score. Other added values can be derived from these rules: for example, if a discrepancy is identified between the results of SPT (or sIgE) of a certain extract of allergen and the ISAC results (namely, a positive SPT result with negative specific components derived from that allergen), an expert system can evaluate whether other cross-reacting components (belonging to other allergen sources but cross-reacting with components well known to be detectable in the whole allergen) are also positive. So, for example, if Ambrosia is positive in SPT but Amb a 1 is negative, other cross reacting components need evaluation. Profilins, PR-10, and CBP are all well represented in Ambrosia but are not present in ISAC. If at least one of these cross-reacting components is positive, then a discrepancy is not considered. On the contrary, if all the possible cross-reacting components are negative, a clear discrepancy is evident. By using this approach, the number of apparent discrepancies is reduced significanly. Another added value is related to the capacity of an expert system to evaluate the sum of the score of genuine inhalants and the sum of the score of inhalant components belonging to cross-reacting families. It has been demostrated that patients sensitized to genuine components are able to better respond to AIT than people sensitized to cross-reacting components.43,140 In addition, by the use of applying cluster analysis techniques to data from thousands of ISAC patients,141 it has been clearly shown that there are five different clusters of patients: Groups I and II (characterized by a sensitization to a large fraction of genuine components) are the only optimal targets for AIT, while Groups III and IV have a worse expectation of success. Group V relates to food allergies. Starting from this evidence, a new era characterized by the integration between artificial intelligence tools and MBAD seems ready to begin. Of note, many of these problems (in particular discrepancies between the result of the allergen extract and those of relevant components) can be easily overcome by the strategy used in ALEX, where both extracts and single components are assayed in the same allergen array.
A different approach that also works was used by Prosperi et al. By using the techniques of machine learning, they studied how microarray results can be applied to allergic diseases. A reasonable discrimination ability for asthma, rhino-conjunctivitis, wheeze and airway hyper-reactivity, but not for eczema, starting from ISAC phenotyping, was observed142
Interpretation of multiplexed in vitro allergy assays.
The interpretation of the results of a 112-allergen assay, and even more a 282 allergen assay, may be challenging, even for the experienced and trained allergen-array user. Some points should be carefully considered.
-
•
Detection of sIgE is indicative of a sensitization, and not of an allergy. Thus, a positive sIgE response in the absence of a history of allergic symptoms or a negative provocation should be referred to as clinically irrelevant.143
-
•
Clear differences have been described144 between allergen extracts and molecular components (revised in Table 13). Sometimes these discrepancies are quantitative, and sIgE levels to the allergen extract are lower than for the individual allergens when components are in low abundance in the extract. In cases when sIgE to allergen extract are positive but its genuine components are negative, sensitization to minor allergenic molecules or CCD determinants responsible for cross-reactivity should be ruled out. Of note, the recent suggestion to introduce an inhibitor of CCD in the sample diluent37 in order to reduce the signal related to reactivity to CCD is routinely used in the novel allergen array ALEX.127 This seems to be particularly relevant when highly purified components derived from natural extracts — and, for this reason, characterized by the presence of a post translational glycosylation — are used. For example, in ImmunoCAP ISAC, Walnut nJug r 2, Bermuda grass nCyn d 1, Timothy grass nPhl p 4, Japanese cedar nCry j 1, Arizona cypress nCup a 1, and Plane nPla a 2 may be positive for their content of glycidic chains This point was also noted in a AIT algorithm40 that suggested a specific strategy to follow before administering AIT for pollens in Mediterranean regions.
-
•
This introduces another relevant point, represented by geographical differences in sensitization. For example, Ole e 1 would be a marker of genuine sensitization to olive pollen in the South Spain but a marker of genuine sensitization to ash tree in Northern France. An example of a geographic distribution of sensitization has been recently published145
-
•
Differences in high or low risk markers and component combinations create differences in the interpretation of risk phenotypes. Generally, allergens resistant to heat and digestion, like seed-storage proteins or lipid transfer proteins, often trigger more severe allergic reactions and have been proposed as markers for severe reactions. Again, the specific relevance of each of these markers of severity will vary according to local molecular profiles (e.g. in USA and Northern Europe Ara h 2 is the best predictive marker for severe reactions to peanut, while in the Mediterranean area it is Ara h 9). On the other hand, Bet v-1 homologues and profilins are labile allergens, which typically induce local symptoms such as oral allergy syndrome (OAS) and have been proposed as markers of mild reactions.
-
•
However, the clinician needs to be aware that there may be exceptions to this rule in situations when large quantities of allergens are consumed, cofactors are associated, or in regions with large quantities of pollen exposure. Examples of this are severe anaphylactic reactions reported in patients mono-sensitized to Bet v1 homologues when drinking apple juice after performing exercise,146 or severe reactions in patients mono-sensitized to profilin in areas with over-exposure to grass pollen.147 In addition to considering the individual markers of severe allergic reactions, component combinations can define phenotypes with different clinical expression. It has been recently reported that, in an Italian cohort, sensitization to more than 5 nsLTPs out of the 8 present in ImmunoCAP ISAC ® is related to a higher incidence of food-induced systemic reactions, while co-sensitization to PR-10 or profilin pan-allergens is associated with milder symptoms.148 According to this, the assessment of IgE sensitization to the three key allergens — Bet v 1 homologues, LTPs and profilins — is of paramount importance for the interpretation of molecular diagnosis to fruits and vegetables, especially in the Mediterranean area.149
-
•
An advantage of multiplex analysis is also one of its main pitfalls: the generation of an extensive IgE sensitization profile, detecting IgE to unexpected allergens, which may sometimes induce confusion in the clinician if there is no suggestive pre-test clinical history. That is often the case in insect venom allergy. Due to the high prevalence of insect venom sensitization in approximately 25% of the population, nonspecific screening would generate an abundance of clinically irrelevant results and mainly serve to unsettle patients and their physicians.150 No recommendations are currently available on how to effectively manage these cases,137 but it seems reasonable to act in the same way as with other clinically irrelevant sensitizations to food or respiratory allergens that don't need any intervention other than to follow the patient to detect possible future reactions. On the other hand, the detection of silent sensitivities may give the allergist the chance to investigate other hypersensitivities and to alert the patient towards possible risks.151 In the case of sensitization to allergens responsible for food-pollen cross-reactive syndromes, the clinician should re-interrogate the patient for symptoms upon consumption of foods containing those allergens. However, sensitization itself should not drive avoidance measures.152 It is important to state that according to current guidelines the indication of an elimination diet should be recommended only if food allergy due to cross-reactions is based on a clear history or on a clinical observation after oral provocation tests.153
-
•
A further approach can be based on some simple rules from proteomics. It has been defined that a cross-reaction may occur if the homology between two or more molecular allergens is >70%. This is a very simple rule that can be easily verified by comparing the sequences of two components which are suspected to be related. It is evident that components belonging to the same family (such as PR-10) are highly homogeneous, such as Act c 8 (Green Kiwi), Ara h 8 (Peanut), Aspa o 17kD (Asparagus officinalis), Cas s 1 (Chestnut), Fag s 1 (Fagus sylvatica), Fic c 1 (Ficus carica), Jug r 5 (Walnut), Mor a 1 (White Mulberry), Ost c 1 (Ostrya carpinifolia), Rub i 1 (Rubus idaeus), Sol a l 4 (Tomato), Tri fg 4 (Trigonella foenum-graecum), Vig r 1 (Vigna radiata), etc. In these cases, the presence of cross reactions always should be considered. Other situations can be different. For example, in the presence of a weak positivity to Ambrosia a., the presence of a strong IgE reaction to Artemisia v. should be verified, because of the presence of a Amb a 1 like protein in Artemsia. Finally, a well known exception is represented by LTP from Par j 2, that when compared with Pru p 3 (a prototypic nsLTP) shares a 26% identity and a 52% positivity, while Pru p 3 compared with Mal d 3 (another nsLTP) has an identity = 77% and positivity = 83%, clearly justifying the presence of a cross reaction.
Cellular assays: basophil activation test
Cellular assays assess selected and defined functions of effector cells within the allergic cascade, and therefore play an increasing role within in vitro allergy diagnostic tests. This is particularly true in case of equivocal and/or negative results obtained with other in vitro and in vivo tests, and in case of discrepant results. In this context, the basophil activation test (BAT) has gained increasing interest within the scientific community and supplanted traditional histamine release assays.154
General aspects
Basophils, like mast cells, are recognized as important effector cells in immediate hypersensitivity responses. Basophils express the high-affinity IgE receptor (FcεRI), and thus they carry specific IgE (sIgE) antibodies on their surface and degranulate when the allergen cross-links these sIgE/FcεRI complexes. This degranulation of basophils can be detected and quantified by flow cytometry techniques.155 Since mast cells, a tissue-resident cell also expressing FcεRI, are not accessible for in vitro diagnostic tests, the basophil represents a unique alternative to study sIgE/FcεRI-dependent degranulation. Moreover, comparative analyses between mast cells and basophils might benefit studies of sIgE/FcεRI-independent effector cell activation, such as by off-target occupancy of the MRGPRX2 receptors,156 as basophils, unlike cutaneous mast cells, barely express this receptor.157
Over the last two decades several important advances have been achieved, announcing the BAT as an increasingly attractive in vitro diagnostic tool, to be applied in selected contexts. However, some methodological aspects need to be taken into consideration in order to interpret the data correctly:
-
o
The BAT is a flow-cytometric based assay: therefore, the investigator must be trained in flow-cytometric techniques, and proper equipment is required. The principles of the BAT and HistaFlow technique, that studies intracellular histamine content, are summarized elsewhere.155,158,159
-
o
There are several surface-marker combinations, which allow the correct identification of basophils. They include the combinations CCR3+/CD3-, or CD123+/HLA-DR-, or IgE+/CD203c+. The only lineage-specific basophil marker is CD203c. The precise identification of the population of basophils is a prerequisite for a valid interpretation of test results.
-
o
In a second step, the appearance and/or up-regulation of the desired activation/degranulation marker is investigated. CD63 or (lysosomal-associated membrane protein [LAMP-3]), is a degranulation marker that appears during compounded degranulation of the cell. CD203c, or ectonucleotide pyrophosphatase/phosphodiesterase 3 (ENPP-3), is not only a lineage specific marker of basophils, but it also serves as an activation marker. In the resting basophil, the expression of CD203c is low, and activation results in a rapid and marked increase in CD203c. Other activation/degranulation markers can also be used, amongst them analysis of the intracellular histamine content using an enzyme affinity method (HistaFlow).159 Fig. 1 illustrates the principle of the BAT/HistaFlow.
-
o
Although commercial basophil activation assays are available, these are rarely thoroughly validated and require additional investigation before they can enter mainstream application.
-
o
Standardization and harmonization of the BAT techniques and interpretation of outcome values and results are still lacking. This is particularly important in the definition of decision cut-off values. Until international agreement and standardization has been achieved, each laboratory should establish its own cut-off values. The best current available definition of a positive test result is based on the frequency of activated basophils following stimulation. For proteinaceous allergens, a test result of at least 15% activated basophils is generally considered as a reliable cut-off value, eventually together with a stimulation index (compared to the negative control) of at least 2. For drugs and small chemical substances, which are known to be less potent stimulators, lower decision thresholds frequently apply. Anyhow, it is recommended to abandon arbitrarily chosen decision thresholds. As a matter of fact, broad dose-finding experiments, spanning different stimulation concentrations and receiver operating characteristic (ROC) analysis are required to calculate allergen-specific cut-offs. Obviously, viability and cytotoxicity studies are mandatory to exclude false-negative results.
-
o
Pre-analytical considerations are of utmost importance, in order to obtain valid test results. In this regard, the BAT is best performed not later than 6–12 months after clinical reaction. However, BAT can stay positive over years, even for drugs.
-
o
It has been demonstrated that antihistamines do NOT influence the outcome in BAT.160 Therefore, unlike for skin and provocation tests, these drugs should not be discontinued.
-
o
A key problem still remains, the so-called “anergic”, that is, non-responders in the BAT. About 10% of donors present “anergic” basophils. A paper published in 2017 showed how inactivation of basophils (“basophil anergy”) seems to be associated with a down regulation of basophil Syk and an apparent reduction in the incidence of allergic rhinitis.161 For this, anti-IgE can be used as a positive control in BAT to identify non-responders.
-
o
Of great advantage, particularly in comparison to in vitro IgE antibody diagnostic tests, is the fact that the BAT assesses IgE-dependent as well as IgE-independent mechanisms. Activation of the basophils is not only obtained through IgE-dependent signal transduction pathways, but it can also be the consequence of a non-IgE mediated reaction. In addition, as already exemplified, comparisons between mast cell and basophil activation can benefit identification and investigation of alternative activation pathways, such as occupancy of the MRGPRX-receptor.
Fig. 1.
The principle of the BAT/HistaFlow
BAT in food allergy
The application of BAT in food allergy has to be seen in the overall context of molecular diagnostics.162 A major issue that is not addressed here is the allergen source. BAT might significantly differ according to the variety employed (e.g. it has been proven for peanut).163 Nevertheless, BAT can benefit diagnosis in difficult cases despite not very reliable in plant-derived food allergy.
Cow's milk allergy is one of the most common food allergies in early childhood. In a study by Rubio et al.164 several diagnostic methods were compared in order to assess the diagnostic value for each. The comparison of skin test, anti-cow's milk IgE antibody measurement, and BAT revealed that BAT yielded the highest specificity (90%) and sensitivity (91%), the highest positive predictive value with 81%, and the highest negative predictive value with 96%.
In another study165 food allergy was assessed in a cohort of 120 patients with suspected irritable bowel disease. The presence of food allergy was established by double-blind, placebo-controlled food challenges. The comparison of specific IgE measurements and BAT revealed a significantly higher diagnostic accuracy for the BAT (sensitivity 86%, specificity 88%).
These data have been confirmed in a study, where basophil activation was statistically correlated with double-blind placebo-controlled food challenge (DBPCFC) outcomes and severity scores, and compared to SPT and serum sIgE.166
Furthermore, the BAT assay has been investigated and correlated with the degree of cow's milk tolerance in 132 subjects divided into 3 groups: baked-milk-reactive, baked-milk-tolerant or “outgrown milk allergy”, based on the oral food challenge outcomes. BAT showed a significantly higher diagnostic accuracy compared to specific-IgE measurements (sensitivity 86%, specificity 88%). The basophil reactivity was significantly higher in baked-milk-reactive patients rather than in baked-milk-tolerant subjects (P < 0.01), and statistically higher in baked-milk-tolerant individuals than in those who have overcome their allergy (P < 0.05).167
Similar results were obtained with other foods, such as egg and tree nut allergens. A study group compared the basophil allergen threshold sensitivity (CD-sens) to peanut allergen-specific IgE antibody levels in relation to the DBPCFC outcome. BATs were performed with both peanut extract and purified rAra h 2: they showed positive results in 92% of the samples, in agreement with a positive DBPC food challenge. Negative outcome in CD-sens was associated with negative results in DBPCFC.168, 169, 170
Although these data must be confirmed by additional studies, this initial set of results indicates that at least for certain food allergens and in certain populations, the BAT plays a significant role in establishing the diagnosis.
BAT in drug allergy
Needless to say, one of the major applications of BAT is immediate drug hypersensitivity, as (in contrast to food allergy) there is no need for difficult extract preparation, and other in vitro tests are frequently lacking and skin tests still associated with considerable uncertainty. Moreover, it has been shown that drug challenges based on skin testing are not absolutely predictive to give a green light for safe subsequent re-exposure.171
To date, as reviewed in Mangodt et al and Ebo et al,172,173 BAT has mainly been studied in immediate hypersensitivity drug reactions due to neuromuscular blocking agents (NMBAs), antibiotics (β-lactams and fluoroquinolones), and iodinated contrast media (ICM). BAT sensitivity generally ranges between 50% and 60%, and specificity is 80%, except for fluoroquinolones where lower sensitivity of the BAT probably reflects an alternative mechanistic endotype.157 Presently, BAT appears to be the only reliable technique to diagnose IgE-mediated opiate hypersensitivity.174,175
BAT in venom allergy
The potential and limitations of BAT in the diagnosis of venom allergy should be viewed in the context of recent developments in molecular diagnostic tests that have greatly improved diagnosis in difficult cases,176,177 mainly patients demonstrating double positive sIgE results and at risk for inappropriate venom immunotherapy. In addition, BAT can benefit diagnosis in patients with negative results. BAT can be performed using native extracts but also with recombinant venom proteins that are increasingly characterized and cloned. Another application of BAT in venom allergy is monitoring of treatment. From these follow-up studies it emerges that it is important to distinguish between basophil sensitivity and reactivity, as the effect of venom immunotherapy on basophils is mainly seen when the cells are stimulated with suboptimal concentrations.178
Allergen provocation test
Allergen extracts can be applied to the conjunctival, bronchial or nasal mucosa in order to provoke symptoms and to clinically demonstrate relevance of IgE-mediated sensitization (as identified by skin-testing or by in vitro parameters) in patients with allergic rhinoconjunctivitis and/or allergic asthma.179,180 Allergen provocation tests are useful in confirming the diagnosis of underlying allergic disease, if by clinical history, skin tests and specific IgE determinations are not conclusive. There is, however, the potential for a systemic reaction, especially with inhalation challenges.
As such, these challenges should be standardized to some extent, in order to control environmental conditions (e.g., temperature, humidity), and can be performed repeatedly in selected individual patients. However, an international standardization of protocols for these provocation tests has been identified as an important unmet need,180 which has been followed by the European Academy of Allergy and Clinical Immunology (EAACI) in a Position Paper on “standardization of nasal allergen challenges“181 and “conjunctival allergen provocation test: guidelines for daily practice“.182 In the “Guideline on the clinical development of products for specific immunotherapy for the treatment of allergic diseases” the European Medicines Agency (EMA) has stated that in phase II “dose finding trials on allergen immunotherapy (AIT), provocation tests may be used as primary endpoints”. Besides that, they can be used in pharmacodynamic trials or in Phase III (pivotal) trials to support proof of efficacy.183
Allergen Exposure Chambers (AECs) have the advantage of mimicking real-life allergen exposures by challenging not only single target organs but the entire patient. In particular the conjunctival mucosa as well as upper and lower airways are exposed simultaneously. The number of technically validated AEC facilities has increased during the past two decades, and they are frequently used in clinical trials.183,184 Through the 2016 revision of the “Allergic Rhinitis: Developing Drug Products for Treatment Guidance for Industry“ document, the Center for Drug Evaluation and Research (CDER) of the U.S. Department of Health and Human Services Food and Drug Administration (FDA) underlines the role of AECs as useful tools in clinically assessing allergic rhinitis.185 In line with this, the EMA acknowledges AEC facilities as a promising tool for the evaluation of efficacy in AIT but calls for further clinical validation.183 Therefore, a published EAACI Position paper overviewed current concepts and unmet needs in AECs aimed to enhance progress towards a broader use of these facilities in the future.186
Taken together, allergen challenge tests can be ideally used to demonstrate the clinical relevance of inhalant allergens and play a central role in the diagnostic methods for allergenic diseases. However, a thorough standardization of these procedures as well as further validation is needed. Even though the number of AECs world-wide is still limited, these facilities may play an important role as diagnostic and therapy-monitoring tools in the future.185
Special cases in allergy diagnosis
IgE testing in drug allergy
IgE-mediated drug allergy accounts for only a limited portion of immune-mediated adverse drug reactions. Even when the clinical picture is consistent with an immediate drug reaction suggesting the involvement of IgE, the sensitivity of skin prick/intradermal testing and serum specific IgE assays remains low for many drug groups. The reasons include the following:187
-
•
Drugs seldom are complete antigens, but frequently act as haptens which require protein binding to become a complete antigen, in this case an “allergen” with IgE-binding capability.
-
•
Some drugs trigger allergic reactions through their metabolites.
-
•
Some immediate reactions may not be IgE-mediated.
All these factors contribute to challenges in consistently reproducing the pathogenic mechanism(s) involved in allergic drug reactions.188
Taking into account these limitations, skin testing with suspected drugs is still a widely used first-line procedure to assess the possibility of a drug allergy. One of the major problems is that concentration ranges allowing for an acceptable sensitivity but lacking an irritant effect have only been reported for a limited number of drugs, such as β-lactams, neuromuscular blocking agents (NMBAs), platinum salts, iodinated contrast media, and heparins (see Table 8). Others have been published only as isolated case reports or small case series.
The positive and negative predictive value of skin testing with drugs or specific-IgE determination varies, according to each specific drug, to the selection criteria of the patients to be tested (likelihood of allergic reaction) and to the time elapsed between the last reaction and testing, since the sensitivity of the latter decreases progressively with time.189, 190, 191
Skin testing for drug allergy
A variety of factors need consideration for performing and interpreting drug allergy skin testing. Positive and negative control tests should be performed simultaneously, usually with histamine and normal saline respectively. Skin testing with drugs should be performed in a setting with sufficient resources to facilitate management of systemic reactions. Patients may sign an informed consent document, which might be mandatory in some countries. Tests should be started by SPTs, and if these are negative, followed by IDT starting with the lowest concentrations, until a positive reaction is obtained or until the maximum concentration is reached. Whenever the test is performed with a drug for which the appropriate concentrations have not been validated, it may be necessary to test control subjects, such as the physician and clinical staff, to rule out non-specific responses.
In β-lactam skin testing, it is recommended to use the major and minor determinants of benzylpenicillin, as well as the culprit drug and possibly other β-lactams, to ascertain any cross-reactivity.189 Benzylpenicilloyl-poly-L-lysine (PPL, Pre-pen™, AllerQuest LLC, Plainville, CT, USA) and benzylpenicilloyl-octa-L-lysine (BP-OL, DAP™, Diater, Leganes, Spain) are the major determinants in use today, while the minor determinants are benzylpenicillin and sodium benzylpenilloate (DAP™). However, they are not commercially available in all countries. The sensitivity of skin testing with β-lactams differs among studies; it can reach 80% in immediate reactions (i.e., occurring within 1 hour of the last drug administration).187 For most antibiotics other than β-lactams, the value of skin tests appears to be less clear or not yet validated. SPT and IDT with undiluted intravenous quinolones are irritative. Higher dilutions have to be used in order to avoid these irritating results. Recommended highest non-irritative dilutions in the literature vary greatly. In any case, the sensitivity of skin testing with quinolones appears to be low and recommendations on concentrations are currently not possible. In immediate hypersensitivity reactions to macrolides, positive skin tests appear to be rare. In some studies, undiluted concentrations of i.v. macrolides have been reported to be irritating in the SPT. Table 17 summarizes the concentrations recommended for the drugs most frequently involved in IgE-mediated reactions.
Table 17.
Highest drug concentrations recommended for skin testing.
| Drug | SPT | IDT | Reference |
|---|---|---|---|
| β-lactam antibiotics | 189,190 | ||
| Amoxicillin | 20 mg/ml | 20 mg/ml | |
| Ampicillin | 20 mg/ml | 20 mg/ml | 190 |
| Penicillin G | 10,000 UI/ml | 10,000 UI/ml | |
| Cephalosporins | 2–20 mg/ml | 20 mg/ml230 | |
| Aztreonam | 2 mg/ml | 2 mg/ml | |
| Imipenem/cilastin | 0.5 mg/ml each | 0.5 mg/ml each | 190 |
| Meropenem | 1 mg/ml | 1 mg/ml | |
| Neuromuscular blocking agents (NMBAs) | 190 | ||
| Atracurium | 1 mg/ml | 0.01 mg/ml | |
| Cisacurium | 2 mg/ml | 0.02 mg/ml | |
| Mivacurium | 0.2 mg/ml | 0.002 mg/ml | |
| Rocuronium | 10 mg/ml | 0.05 mg/ml | |
| Vecuronium | 4 mg/ml | 0.4 mg/ml | |
| Pancuronium | 2 mg/ml | 0.2 mg/ml | |
| Suxamethonium | 10 mg/ml | 0.1 mg/ml | |
| Platinum salts | 190 | ||
| Carboplatin | 10 mg/ml | 1 mg/ml | |
| Oxaliplatin | 1 mg/ml | 0.1 mg/ml | |
| Cisplatin | 1 mg/ml | 0.1 mg/ml | |
| Iodinated contrast media | Undiluted | 1/10 in normal saline | 190 |
| Heparins | Undiluted | 1/10 in normal saline | |
| Pyrazolones (Metamizol) and other NSAIDs | Undiluted | 0.1 mg/ml | |
*Adapted from K. Brockow et al.: Skin test concentrations for systemically administered drugs - an ENDA/EAACI Drug Allergy Interest Group position paper.231
In pediatric patients, the definitive diagnosis of β-lactam and non β-lactam antibiotics (NBLAs) hypersensitivity frequently depends on drug provocation tests. Studies including children showed that only 7.8–36% of suspected hypersensitivity (both immediate and delayed) to NBLAs could be confirmed by skin and/or provocation tests, hence the need of a common guideline on a standardized diagnostic approach in the pediatric population.192
For neuromuscular blocking agents (NMBAs), skin tests are generally considered to have a high sensitivity. Cross-reactivity is reported with NMBAs in up to 60–70% of cases,193 even if it is possible that different results are observed.
If a skin test with the NMBA to which the patient was exposed is positive, the remaining available NMBAs should be tested, in order to rule out cross-sensitizations and identify safe alternatives. NMBAs can induce a non-specific direct histamine release in the skin, increasing the risk of false positive tests, especially in intradermal testing.194 There remains the possibility of low specificity based upon exposure and sensitization to common products (cosmetics and cough medicine) which lead to false positive results.195
Hagau and colleagues demonstrated that subjects with a history of antibiotic hypersensitivity reactions seem to have an increased incidence of positive skin tests for NMBAs, in particular atracurium (P = 0.02). These data suggest that there is a subset of patients that might be at higher risk for developing intraoperative anaphylaxis after undergoing general anesthesia, compared to the general population.196 With regard to platinum salts (carboplatin, oxaliplatin, and cisplatin), there is sufficient information to recommend the use of undiluted drugs for SPT and a tenfold dilution for IDT.197
Skin testing with iodinated contrast media (ICM) is suggested in patients who have previously experienced contrast-related reactions. On the contrary, there are no indications in non-ICM-exposed and/or non-previous-reactors for testing as the predictability of these skin tests has not been confirmed. In those with positive reactions, testing a panel of different compounds is recommended to detect any cross-reactivity. SPT should be performed using undiluted solutions. For IDT, preparations should be used in a 1:10 dilution, as undiluted contrast media may be irritating.198 Sese et al. published their experience with a low-dose intravenous provocation test with ICM that, added to skin tests, seem to have a NPV of 80% in a cohort of 37 patients with suspected hypersensitivity reactions to ICM. Only one subject experienced a mild adverse reaction. Further studies however are needed to confirm the safety and predictive value of this procedure.198
Heparin preparations should be used in a 1:10 dilution for intradermal tests. Immediate-type intradermal-test positivity may be observed in up to 10% using this dilution. Lower concentrations decrease the sensitivity of these tests.199
For non-steroidal anti-inflammatory drugs (NSAIDs), the sensitivity of skin testing is low except for pyrazolones.200 In fact, the vast majority of immediate hypersensitivity reactions (e.g., urticaria, bronchospasm, and anaphylaxis) to NSAIDs, excluding pyrazolones, are not due to an IgE-mediated mechanism, but are related to an aberrant arachidonic acid metabolism. In immediate hypersensitivity reactions to pyrazolones, positive skin tests may occur in up to 40% of patients. Nevertheless, some cases have been published for NSAIDs other than pyrazolones, in which skin tests have rendered positive results (Table 17). Although immediate corticosteroid allergy is infrequent, skin tests may be useful for diagnosis. However, skin testing or sIgE is not generally recognized of value for ICM or NSAIDs allergies in the USA.
In vitro testing for drug-specific IgE
The possibility of proving the existence of serum specific IgE to the suspected drug in an allergic reaction would make it possible to avoid performing in vivo tests, reducing the possibility of unwanted adverse reactions. Nevertheless, only a few IgE assays are commercially available for drug allergy diagnosis, with variable predictive values. In some cases, individual laboratory assays, such as ELISA or FEIA, are used. Table 18 summarizes the value of IgE testing for drug allergy for the most relevant studied drug groups. However, there is an honest difference of opinion regarding the interpretation of these results between US and European allergists.
Table 18.
Summary of IgE testing for drug allergy in different drug groups
| Drug | Techniques | Sensitivity | Specificity |
|---|---|---|---|
| NMBA | RIA/RAST/CAP FEIA | 47–97% | 91–100% |
| β-lactams | RAST/CAP FEIA/Sepharose-RIA | 0–87% | 67–97% |
| Quinolones | Sepharose-RIA | 32–55% | 100%, ND |
| Propyphenazone | ELISA | 58% | ND |
ELISA, enzyme-linked immunosorbent assay; FEIA, fluorometric enzyme immunoassay; RAST radioallergosorbent test; RIA, radioimmunoassay; ND, not done. Adapted from.232
BAT for drug allergy
This topic is discussed in the BAT chapter of this article.
IgE assays for occupational allergens
Occupational allergen sources may be materials that a large part of the general population is exposed to, but only occupational settings provide sufficiently high, prolonged and relevant exposure to cause sensitization and, eventually, allergic diseases. Two examples are flour dust and industrial detergent enzymes where the majority of reported cases come from industries and bakeries where large amounts of these source materials are handled.
IgE-testing plays a particularly important role in occupational allergy because of the relatively rare occurrence of the individual allergenic sources, and thus a correspondingly low number of standardized extracts for skin testing.201 Even locally or ad hoc produced skin test extracts may suffer from problems of toxicity that may limit the use of skin test as a diagnostic procedure. The lack of pre-produced extracts, however, may also be a problem for IgE-testing in settings where it is not possible to develop new assays based on an extract of the occupational material that is suspected of causing allergy. For this reason, the basophil histamine release assay or BAT may be a helpful technique, since it is possible to make an extract of the suspected material and use it for challenging the basophils. It is pertinent though, that the proper controls (no activation of basophils from healthy persons, no interference with the assay in general) are carried out.
The range of occupations in which IgE-mediated diseases have been reported is vast and varied, and it is important to realize that Occupational Allergy is a field with not only protein allergens but also small molecules, examples of which are given below. In many cases the specific allergen(s) from an occupational exposure have been identified, but there are also cases where a whole extract of the allergen source is the only available diagnostic tool. An interesting example in this respect is latex, which has been reported as an important allergen for health care workers,202,203 in which an outbreak of an allergy "epidemic" was observed in the years following the increased utilization of rubber gloves in the 1980s. Latex, the raw product of natural rubber, contains more than 200 polypeptides, and as of today 14 proteins, Hev b 1 to Hev b 14 have been given an IUIS nomenclature designation, with many isoforms and variants within these. Additional latex proteins have been isolated but have not received a designated allergen name. Health care workers seem to be particularly frequently sensitized to Hev b 5 and b 6.02, but many other sensitizing allergens have been reported.203 The ideal diagnostic work-up, if the reagents are available, is for a two-step process, where the sensitization to the whole extract is first demonstrated, after which the sensitization to individual allergens can be analyzed. While the sensitization to the whole extract is of relevance for the occupational exposure (since the patient is of course exposed to the whole product), the elucidation of the sensitization to individual allergenic molecules may be of relevance in advising the patient about cross-reactivities that may be experienced outside of work. Latex sensitization to Hev b 6.02 may confer cross-reactivity to endochitinases contained in avocado, chestnut, banana, and sweet pepper, whereas sensitization to Hev b 5 may cause cross-reactivity to kiwi fruit. Another aspect of cross-reactivity that should be considered is the possibility that the patient may be sensitized to pollens which may cause recognition of cross-reacting carbohydrate determinants (CCD)204 or profilins (Hev b 8 in latex, Phl p 12 in Timothy grass).205
A very important area of occupational allergy occurs with exposure to proteins in the handling of food whether it is in the industrial, workshop-based (bakeries, butchers) or catering sector. The most important exposure route is likely to be via inhalation of dust or aerosols created during the manufacturing process, but also absorption via the skin should be considered. A list of the foods that have been reported to act as occupational allergens in causing asthma include food from both the plant kingdom (cereals, seeds, whole plants, vegetables, fruits, spices, teas) and the animal kingdom (seafood and fish, meats from mammals and birds, milk and eggs).206,207 This list was updated in 2014.207
Probably the best-described occupational allergy to food is baker's asthma where the patient may be sensitized to the flour but also to additives in the form of enzymes (fungal alpha-amylase added to improve the leavening of the dough) or insect pests in the form of cockroaches that may be contaminants. IgE-reactivity to wheat flour may serve as an interesting lesson regarding occupational versus non-occupational reactivity. Wheat IgE-mediated allergy manifests itself as food allergy and as occupational inhalant allergy (baker's asthma),208 and identical allergens seem to be responsible in both allergies, although their relative importance differs.209,210 Nevertheless, healthy patients may also display positive skin test or specific IgE towards wheat, due to the cross-reactivity between grass pollen and cereals which may have an impact on the specificity of the diagnostic tests.74,211 This was confirmed in a study, showing up to 35% positivity against wheat for both skin prick tests and sIgE tests, when testing grass pollen allergic subjects that tolerated eating wheat and other cereals and without a history of baker's asthma.212 Thus also for occupational allergens, specificity remains an important issue.
In addition to the raw products involved, food additives and contaminants including insects, fungi and parasites should also be considered. To compensate for a low content of natural amylases, bioindustrially produced α-amylases are added to wheat flour to improve the leavening of the dough. One such α-amylase is derived from Aspergillus oryzae and formulated as the product Fungamyl®, which has been used as an additive to flour for more than 40 years. This preparation of α-amylase complies with the FAO/WHO JECFA recommended specifications for food grade enzymes,213 and it is generally considered as safe for human ingestion. Occupational exposure to enzyme dust, however, may cause type I allergic sensitization and allergic symptoms like asthma, rhinitis, and urticaria may be elicited on subsequent exposure.214 Preparations of α-amylase derived from Aspergillus oryzae, including Fungamyl®, in several cases, have been reported to cause sensitization of workers in enzyme production plants,215 the pharmaceutical industry,216 and bakeries.217
Other enzymes such as those used in the detergent industry have also been known to cause problems. The initial proteases used in detergents were produced by fermentation of Bacillus subtilis, and thus called subtilisins. Soon after the introduction of a subtilisin in detergent products, severe IgE-mediated asthma reactions appeared among workers in detergent factories.218 Strict exposure control programs reduced the exposures to low levels (15 ng/m3 range), which reduced sensitization and prevented the onset of allergic symptoms.219 Large scale studies of sensitization and allergic symptoms suggest that sensitization comes first, and only in some cases leads to development of symptoms. It has also been suggested that not only enzymes, but also whole microorganisms used in the food and feed industries may cause an allergy risk. Cleaning workers having asthma and/or rhinitis should also be evaluated, even though only a minority could actually be sensitized.
Other exposures to high molecular weight allergens may be seen in occupations with live animals such as farms or experimental animal facilities (where rodent urinary proteins seem to be a strong sensitizing allergen) and with plants from the farming and the gardening industry.220
Finally, occupational allergies may develop to small organic molecules, that are either complete allergens with a symmetric structure such as chlorhexidine221 or incomplete allergens that act as haptens by binding to carrier molecules, which are generally human proteins.222 Examples are ethylene oxide, formaldehyde, isocyanates, and phthallic anhydride that are used for the production of polyurethane.223 The isocyanate group compromise different chemical species, and there seems to be a certain cross-reactivity.224 Notably, in these cases, skin test or specific IgE in serum are not useful tools for diagnosis.225
In conclusion, IgE-based diagnostic methods for occupational allergies are not, in principle, different from other types of allergies. The large number of different allergenic sources for which occupational sensitizations have been reported, however, has made the research area more extensive, and for many occupations the specific allergens are still not complete. In the past different allergy "epidemics" such as latex, industrial enzymes, and proteins from experimental animals have gradually been contained even though new sensitizations still occur. On the other hand new processes, and particularly those that involve handling of, and exposure to, proteins, may also create new and hitherto unknown exposures that may cause new IgE-mediated allergies.
Unproven diagnostic approaches
Food allergy is a frequent allergic disorder, as 6–8% of children and 2–3% of adults are affected. However, the public perception of food allergy/intolerance is higher, as one out of three people believes they are allergic or intolerant to one or more foods. This perception is at least in part based on the results of unproven diagnostic approaches (Table 19). Therefore these unreliable diagnostic approaches may be costly for patients, delaying appropriate diagnosis and therapy.
Table 19.
Unproven diagnostic approaches
| Test | Description | Scientific evidence | References |
|---|---|---|---|
| Specific IgG antibodies | Food specific IgG or IgG4 panels are available as diagnostic tool for food allergy (NOTE: healthy subjects can produce specific IgG and IgG4 to commonly eaten foods without being allergic). | The titer of specific IgG-antibodies does not correlate with oral food challenges. In children with proven milk allergy (positive oral challenge) no increase in specific IgG was detected. There is no evidence that IgG subclasses are a reliable diagnostic tool. Seventy-three patients were challenged in a double-blind manner to the IgG4 positive food, with no adverse reactions reported. | 233, 234, 235 |
| Cytotoxic test | This is an in vitro test in which food allergens (up to 180) are put into contact with whole blood. Any change in leukocytes' shape indicates a positive reaction. | In controlled conditions the reproducibility and diagnostic efficiency were largely insufficient. | 236,237 |
| Hair analysis | Nutritional deficiencies, detectable in hair (e.g., zinc, magnesium) are due to food allergy/intolerance. | Nine allergic patients to fish (positive challenge) and 9 healthy controls underwent the test. The test did not recognize allergic patients, and additional allergies were found without clinical significance. Hair samples of two teenagers were tested in 13 different laboratories, which reported different results. | 238,239 |
| Iridology | Anatomical/morphological changes in the iris may suggest systemic diseases. | A systematic review on iridology concluded that this test as a diagnostic tool is not supported by scientific evidence. | 240 |
| Kinesiology | The patient holds a vial with a specific food in one hand, while the examiner tests the muscular strength of the opposite arm by applying a light pressure. Food allergy/intolerance is indicated by a decreased muscular contraction when the offending substance is held. | According to two controlled studies the scientific evidence suggests that this diagnostic modality is not validated, and kinesiology as a diagnostic tool is no better than random guessing. | 241,242 |
| Electrodermal testing | The patient is placed in a circuit where a galvanometer measures skin conductance. Vials with food extract are sequentially inserted into the circuit. A positive response consists of a drop in conductance. | According to two double-blind placebo-controlled studies this method failed to distinguish between allergic and non-allergic patients, and between positive and negative tests. Poor reproducibility was always reported. | 243,244 |
Conclusion
For type I IgE-mediated allergic disease, skin tests are still considered the first-line approach for indicating the presence of allergen-specific IgE antibodies on the surface of mast cells in the skin of a sensitized patient. Skin testing is a simple and generally safe method, reliable in skilled hands; the results are reproducible when standardized extracts are employed. As complementary or alternative diagnostics tools, in vitro serum IgE detection with the use of highly purified allergen or recombinants, in singleplex or multiplex manner, is an alternative diagnostic procedure. Serum IgE testing entails no risk to the patient other than a blood draw and is preferable if the patient has an unstable or uncontrolled medical condition, is at high risk of anaphylaxis, is taking essential medication that interferes with testing, is very young such that the procedure would be unduly stressful, or has a skin condition that limits available skin for testing. The development of screening tests with multiple allergens or multiplex tests that identify multiple specific-IgEs with a small blood volume makes this testing more appealing to very young children. However, food allergy panels need to be adapted and improved in order to be cost-effective and more specific.
Furthermore, the development of cellular assays such as the BAT may improve the diagnostic accuracy of testing, particularly for food, venoms, and drugs. However, BAT, standardized extracts, component testing, multiplex testing, and microarrays are only available for specific allergens, thus, having limited clinical availability.
Great effort is now focused on the standardization process, both for testing and production of immunotherapy extracts. Standardization will result in optimal, reproducible, accurate testing that will improve patient outcomes and general health care. Additional population studies are necessary to verify if more specific testing, such as component assays, are relevant before these assays can be recommended. The ultimate value of allergy testing depends upon the pretest probability derived from history and physical examination that precedes the testing, as the clinical judgment of the healthcare provider influences greatly the predictive value of allergy testing. No test can substitute for the importance of an adequate assessment, and remote testing should be discouraged to limit misdiagnosis.
Position paper review and endorsement
This position paper was reviewed and endorsed by the following national member societies of the World Allergy Organization.
American College of Allergy, Asthma and Immunology
Australasian Society of Clinical Immunology and Allergy
Belarus Association of Allergology & Clinical Immunology
Belgian Society of Allergology and Immunology
Canadian Society of Allergy and Clinical Immunology
Croatian Society of Allergology and Clinical Immunology
Cuban Society of Allergy, Asthma and Clinical Immunology
Czech Society of Allergology and Clinical Immunology
Egyptian Society of Pediatric Allergy and Immunology
Hungarian Society of Allergology and Clinical Immunology
Indian College of Allergy, Asthma and Applied Immunology
Japanese Society of Allergology
Korean Academy of Allergy, Asthma and Clinical Immunology
Kuwait Society of Allergy and Clinical Immunology
Paraguayan Society of Immunology and Allergy
Polish Society of Allergology
Portuguese Society of Allergology and Clinical Immunology
Romanian Society of Allergology and Clinical Immunology
Spanish Society of Allergology and Clinical Immunology
Swiss Society of Allergology and Immunology
Uruguayan Society of Allergology
The Jordanian Society for Allergy and Clinical Immunology reviewed and approved of publication of the position paper. The Austrian Society of Allergology and Immunology also reviewed the paper.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
Not applicable.
Funding
Not applicable.
Competing interest
E Jensen-Jarolim discloses she is a shareholder, and inventor on EP 2894478, with Biomedical International R+D GmbH Vienna; has received a grant and lecture honoraria from Bencard GmbH Germany; has received lecture honoraria from Sanofi GmbH, Roxall, Meda, and Novartis; and is a joint Editor-in-Chief of this journal.
R Valenta has received research grants from Viravaxx, Vienna, Austria, and serves as a consultant for this company.
All other authors have declared they have no competing interests related to this work.
Authors’ contributions
IJA, GM, GWC, LC, EV, ME, GP, ES, DE, RMG, OLS, JO, EJJ, and DF equally contributed to this manuscript, coordinating the different sections and reviewing all the content. All other authors critically reviewed the manuscript.
Acknowledgements
The authors wish to thank the allergy societies from the membership of the World Allergy Organization who provided critical review of the draft and the WAO Board of Directors for supporting this work.
Footnotes
Full list of author information is available at the end of the article
References
- 1.Dreborg S. Diagnosis of food allergy: tests in vivo and in vitro. Pediatr Allergy Immunol. 2001;12(Suppl 14):24–30. doi: 10.1034/j.1399-3038.2001.121406.x. [DOI] [PubMed] [Google Scholar]
- 2.Portnoy J. Diagnostic testing for allergies. Ann Allergy Asthma Immunol. 2006;96(1):3–4. doi: 10.1016/S1081-1206(10)61031-9. [DOI] [PubMed] [Google Scholar]
- 3.Oppenheimer J., Nelson H.S. Skin testing: a survey of allergists. Ann Allergy Asthma Immunol. 2006;96(1):19–23. doi: 10.1016/S1081-1206(10)61034-4. [DOI] [PubMed] [Google Scholar]
- 4.Malling H.J., Allesen-Holm P., Karved L.S., Poulsen L.K. Proficiency testing of skin prick testers as part of a quality assurance system. Clin Transl Allergy. 2016;6:36. doi: 10.1186/s13601-016-0126-7. eCollection 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Portnoy J.M. What do allergy skin tests really mean? Ann Allergy Asthma Immunol. 2002;89(4):335–336. doi: 10.1016/S1081-1206(10)62028-5. [DOI] [PubMed] [Google Scholar]
- 6.McCann W.A., Ownby D.R. The reproducibility of the allergy skin test scoring and interpretation by board-certified/board-eligible allergists. Ann Allergy Asthma Immunol. 2002;89(4):368–371. doi: 10.1016/S1081-1206(10)62037-6. [DOI] [PubMed] [Google Scholar]
- 7.Pawankar R., Holgate S.T., Canonica G.W., Lockey R.F., Blaiss M., editors. WAO White Book on Allergy 2013 Update, World Allergy Organization. 2013. https://www.worldallergy.org/wao-white-book-on-allergy [Google Scholar]
- 8.Canonica G.W., Ansotegui I.J., Pawankar R. A WAO - ARIA - GA(2)LEN consensus document on molecular-based allergy, diagnostics. World Allergy Organ J. 2013;6(1):17. doi: 10.1186/1939-4551-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klimek L., Hoffmann H.J., Renz H. Diagnostic test allergens used for in vivo diagnosis of allergic diseases are at risk: a European Perspective. Allergy. 2015;70(10):1329–1331. doi: 10.1111/all.12676. Epub 2015 Aug 19. [DOI] [PubMed] [Google Scholar]
- 10.Heinzerling L., Mari A., Bergmann K.C. The skin prick test - European standards. Clin Transl Allergy. 2013;3(1):3. doi: 10.1186/2045-7022-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matricardi P.M., Kleine-Tebbe J., Hoffmann H.J. EAACI molecular allergology user's guide. Pediatr Allergy Immunol. 2016;27(Suppl 23):1–250. doi: 10.1111/pai.12563. [DOI] [PubMed] [Google Scholar]
- 12.Oppenheimer J., Nelson H.S. Skin testing. Ann Allergy Asthma Immunol. 2006;96(2 Suppl 1) doi: 10.1016/s1081-1206(10)60895-2. S6-12. [DOI] [PubMed] [Google Scholar]
- 13.Bodtger U., Poulsen L.K., Malling H.J. Asymptomatic skin sensitization to birch predicts later development of birch pollen allergy in adults: a 3-year follow-up study. J Allergy Clin Immunol. 2003;111(1):149–154. doi: 10.1067/mai.2003.37. [DOI] [PubMed] [Google Scholar]
- 14.Haahtela T., Burbach G.J., Bachert C. Clinical relevance is associated with allergen-specific wheal size in skin prick testing. Clin Exp Allergy. 2014;44(3):407–416. doi: 10.1111/cea.12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Commins S.P., Satinover S.M., Hosen J. Delayed anaphylaxis, angioedema, or urticaria after consumption of red meat in patients with IgE antibodies specific for galactose-alpha-1,3-galactose. J Allergy Clin Immunol. 2009;123(2):426–433. doi: 10.1016/j.jaci.2008.10.052. Epub Dec 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bernstein D.I., Wanner M., Borish L., Liss G.M. Twelve-year survey of fatal reactions to allergen injections and skin testing: 1990-2001. J Allergy Clin Immunol. 2004;113(6):1129–1136. doi: 10.1016/j.jaci.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 17.Amin H.S., Liss G.M., Bernstein D.I. Evaluation of near-fatal reactions to allergen immunotherapy injections. J Allergy Clin Immunol. 2006;117(1):169–175. doi: 10.1016/j.jaci.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 18.Rueff F., Przybilla B., Bilo M.B. Clinical effectiveness of hymenoptera venom immunotherapy: a prospective observational multicenter study of the European academy of allergology and clinical immunology interest group on insect venom hypersensitivity. PLoS One. 2013;8(5) doi: 10.1371/journal.pone.0063233. e63233. Print 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwindt C.D., Hutcheson P.S., Leu S.Y., Dykewicz M.S. Role of intradermal skin tests in the evaluation of clinically relevant respiratory allergy assessed using patient history and nasal challenges. Ann Allergy Asthma Immunol. 2005;94(6):627–633. doi: 10.1016/S1081-1206(10)61319-1. [DOI] [PubMed] [Google Scholar]
- 20.Romano A., Warrington R. Antibiotic allergy. Immunol Allergy Clin N AM. 2014;34(3):489–506. doi: 10.1016/j.iac.2014.03.003. 2014 Aug. [DOI] [PubMed] [Google Scholar]
- 21.James J.M.B.A. Food allergy: current diagnostic methods and interpretation of results. In: Kemp S.F.L.R., editor. Diagnostic Testing of Allergic Disease. Marcel Dekker Inc.; New York: 2000. pp. 199–215. [Google Scholar]
- 22.Sampson H.A. Food allergy. Part 2: diagnosis and management. J Allergy Clin Immunol. 1999;103(6):981–989. doi: 10.1016/s0091-6749(99)70167-3. [DOI] [PubMed] [Google Scholar]
- 23.Turkeltaub P.C. Precutaneous and intracutaneous diagnostic tests of IgE-mediated diseases (immediate hypersensitivity. In: Kemp S.F.L.R., editor. Diagnostic Testing of Allergic Disease. Marcel Dekker Inc; New York: 2000. p. 66. [PubMed] [Google Scholar]
- 24.Gungor A., Houser S.M., Aquino B.F. A comparison of skin endpoint titration and skin-prick testing in the diagnosis of allergic rhinitis. Ear Nose Throat J. 2004;83(1):54–60. [PubMed] [Google Scholar]
- 25.Wide L., Bennich H., Johansson S.G. Diagnosis of allergy by an in-vitro test for allergen antibodies. Lancet. 1967;2(7526):1105–1107. doi: 10.1016/s0140-6736(67)90615-0. [DOI] [PubMed] [Google Scholar]
- 26.Cox L., Williams B., Sicherer S. Pearls and pitfalls of allergy diagnostic testing: report from the American College of allergy, asthma and immunology/American academy of allergy, asthma and immunology specific IgE test Task Force. Ann Allergy Asthma Immunol. 2008;101(6):580–592. [PubMed] [Google Scholar]
- 27.Hamilton R.G.M.P., Hovanec-Burns D., Mark Van Cleve M. Analytical performance characteristics, quality assurance and clinical utility of immunological assays for human IgE antibodies of defined allergen specificities. (CLSI-ILA20-A3) J Allergy Clin Immunol February. 2015;135(2 - Suppl) AB8. [Google Scholar]
- 28.Nolte H., DuBuske L.M. Performance characteristics of a new automated enzyme immunoassay for the measurement of allergen-specific IgE. Summary of the probability outcomes comparing results of allergen skin testing to results obtained with the HYTEC system and CAP system. Ann Allergy Asthma Immunol. 1997;79(1):27–34. doi: 10.1016/S1081-1206(10)63080-3. [DOI] [PubMed] [Google Scholar]
- 29.Kam K.L., Hsieh K.H. Comparison of three in vitro assays for serum IgE with skin testing in asthmatic children. Ann Allergy. 1994;73(4):329–336. [PubMed] [Google Scholar]
- 30.Asero R., Scala E., Villalta D. Shrimp allergy: analysis of commercially available extracts for in vivo diagnosis. J Investig Allergol Clin Immunol. 2017;27(3):175–182. doi: 10.18176/jiaci.0127. [DOI] [PubMed] [Google Scholar]
- 31.Ciardiello M.A., Giangrieco I., Tuppo L. Influence of the natural ripening stage, cold storage, and ethylene treatment on the protein and IgE-binding profiles of green and gold kiwi fruit extracts. J Agric Food Chem. 2009;57(4):1565–1571. doi: 10.1021/jf802966n. [DOI] [PubMed] [Google Scholar]
- 32.Hildebrandt S., Steinhart H., Paschke A. Comparison of different extraction solutions for the analysis of allergens in hen's egg. Food Chem. 2008;108(3):1088–1093. doi: 10.1016/j.foodchem.2007.11.051. Epub Nov 29. [DOI] [PubMed] [Google Scholar]
- 33.Jensen-Jarolim E., Jensen A.N., Canonica G.W. Debates in allergy medicine: molecular allergy diagnosis with ISAC will replace screenings by skin prick test in the future. World Allergy Organ J. 2017;10(1):33. doi: 10.1186/s40413-017-0162-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Larenas-Linnemann D., Luna-Pech J.A., Mosges R. Debates in Allergy Medicine: allergy skin testing cannot be replaced by molecular diagnosis in the near future. World Allergy Organ J. 2017;10(1):32. doi: 10.1186/s40413-017-0164-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mothes-Luksch N., Jordakieva G., Hinterholzl L. Allergy diagnosis from symptoms to molecules, or from molecules to symptoms: a comparative clinical study. World Allergy Organ J. 2018;11(1):22. doi: 10.1186/s40413-018-0199-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kowalski M.L., Ansotegui I., Aberer W. Risk and safety requirements for diagnostic and therapeutic procedures in allergology: world Allergy Organization Statement. World Allergy Organ J. 2016;9(1):33. doi: 10.1186/s40413-016-0122-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hemmer W., Altmann F., Holzweber F., Gruber C., Wantke F., Wohrl S. ImmunoCAP cellulose displays cross-reactive carbohydrate determinant (CCD) epitopes and can cause false-positive test results in patients with high anti-CCD IgE antibody levels. J Allergy Clin Immunol. 2018;141(1):372–381 e3. doi: 10.1016/j.jaci.2017.04.028. [DOI] [PubMed] [Google Scholar]
- 38.Valenta R., Niederberger V. Recombinant allergens for immunotherapy. J Allergy Clin Immunol. 2007;119(4):826–830. doi: 10.1016/j.jaci.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 39.Passalacqua G., Melioli G., Bonifazi F. The additional values of microarray allergen assay in the management of polysensitized patients with respiratory allergy. Allergy. 2013;68(8):1029–1033. doi: 10.1111/all.12194. [DOI] [PubMed] [Google Scholar]
- 40.Douladiris N., Savvatianos S., Roumpedaki I., Skevaki C., Mitsias D., Papadopoulos N.G. A molecular diagnostic algorithm to guide pollen immunotherapy in southern Europe: towards component-resolved management of allergic diseases. Int Arch Allergy Immunol. 2013;162(2):163–172. doi: 10.1159/000353113. [DOI] [PubMed] [Google Scholar]
- 41.Camacho G.D., Arjona A.M.M., Padial J.F., Jesus R.C. How molecular diagnosis may modify immunotherapy prescription in multi-sensitized pollen-allergic children. J Allergol Immunopathol (Madr). 2018 Nov-Dec;46(6):552–556. doi: 10.1016/j.aller.2018.03.002. Epub 2018 Jul 13. [DOI] [PubMed] [Google Scholar]
- 42.Sastre J., Landivar M.E., Ruiz-Garcia M., Andregnette-Rosigno M.V., Mahillo I. How molecular diagnosis can change allergen-specific immunotherapy prescription in a complex pollen area. Allergy. 2012;67(5):709–711. doi: 10.1111/j.1398-9995.2012.02808.x. [DOI] [PubMed] [Google Scholar]
- 43.Schmid-Grendelmeier P. [Recombinant allergens. For routine use or still only science?] Hautarzt. 2010;61(11):946–953. doi: 10.1007/s00105-010-1967-y. [DOI] [PubMed] [Google Scholar]
- 44.Wide L., Bennich H., Johansson S.G. Diagnosis of allergy by an in-vitro test for allergen antibodies. Lancet. 1967;2(7526):1105–1107. doi: 10.1016/s0140-6736(67)90615-0. [DOI] [PubMed] [Google Scholar]
- 45.Kjellman N.M., Johansson S.G., Roth A. Serum IgE levels in healthy children quantified by a sandwich technique (PRIST) Clin Allergy. 1976;6(1):51–59. doi: 10.1111/j.1365-2222.1976.tb01411.x. [DOI] [PubMed] [Google Scholar]
- 46.Wittig H.J., Belloit J., De Fillippi I., Royal G. Age-related serum immunoglobulin E levels in healthy subjects and in patients with allergic disease. J Allergy Clin Immunol. 1980;66(4):305–313. doi: 10.1016/0091-6749(80)90026-3. [DOI] [PubMed] [Google Scholar]
- 47.Meyers D.A., Postma D.S., Panhuysen C.I. Evidence for a locus regulating total serum IgE levels mapping to chromosome 5. Genomics. 1994;23(2):464–470. doi: 10.1006/geno.1994.1524. [DOI] [PubMed] [Google Scholar]
- 48.Cookson W.O., Young R.P., Sandford A.J. Maternal inheritance of atopic IgE responsiveness on chromosome 11q. Lancet. 1992;340(8816):381–384. doi: 10.1016/0140-6736(92)91468-n. [DOI] [PubMed] [Google Scholar]
- 49.Ozcan E., Notarangelo L.D., Geha R.S. Primary immune deficiencies with aberrant IgE production. J Allergy Clin Immunol. 2008;122(6):1054–1062. doi: 10.1016/j.jaci.2008.10.023. quiz 63-4. [DOI] [PubMed] [Google Scholar]
- 50.Greenberger P.A. Allergic bronchopulmonary aspergillosis. J Allergy Clin Immunol. 2002;110(5):685–692. doi: 10.1067/mai.2002.130179. [DOI] [PubMed] [Google Scholar]
- 51.Thorpe S.J., Heath A., Fox B., Patel D., Egner W. The 3rd International Standard for serum IgE: international collaborative study to evaluate a candidate preparation. Clin Chem Lab Med. 2014;52(9):1283–1289. doi: 10.1515/cclm-2014-0243. [DOI] [PubMed] [Google Scholar]
- 52.Lee S., Lim H.S., Park J., Kim H.S. A new automated multiple allergen simultaneous test-chemiluminescent assay (MAST-CLA) using an AP720S analyzer. Clin Chim Acta. 2009;402(1-2):182–188. doi: 10.1016/j.cca.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 53.Ewan P.W., Coote D. Evaluation of a capsulated hydrophilic carrier polymer (the ImmunoCAP) for measurement of specific IgE antibodies. Allergy. 1990;45(1):22–29. doi: 10.1111/j.1398-9995.1990.tb01080.x. [DOI] [PubMed] [Google Scholar]
- 54.PNJ M., RG H., RE E. 2009. Analytical Performance Characteristics and Clinical Utility of Immunological Assays for Human Immunoglobulin E (IgE) Antibodies and Defined Allergen Specificities. [Google Scholar]
- 55.Dolen W.K. Skin testing and immunoassays for allergen-specific IgE. Clin Rev Allergy Immunol. 2001;21(2-3):229–239. doi: 10.1385/CRIAI:21:2-3:229. [DOI] [PubMed] [Google Scholar]
- 56.Hamilton R.G., Franklin Adkinson N., Jr. In vitro assays for the diagnosis of IgE-mediated disorders. J Allergy Clin Immunol. 2004;114(2):213–225. doi: 10.1016/j.jaci.2004.06.046. quiz 26. [DOI] [PubMed] [Google Scholar]
- 57.Lundberg M., Chen Z., Rihs H.P., Wrangsjo K. Recombinant spiked allergen extract. Allergy. 2001;56(8):794–795. doi: 10.1034/j.1398-9995.2001.056008794.x. [DOI] [PubMed] [Google Scholar]
- 58.Fernandez-Rivas M., Bolhaar S., Gonzalez-Mancebo E. Apple allergy across Europe: how allergen sensitization profiles determine the clinical expression of allergies to plant foods. J Allergy Clin Immunol. 2006;118(2):481–488. doi: 10.1016/j.jaci.2006.05.012. Epub 2006 Jun 27. [DOI] [PubMed] [Google Scholar]
- 59.Barber D., de la Torre F., Feo F. Understanding patient sensitization profiles in complex pollen areas: a molecular epidemiological study. Allergy. 2008;63(11):1550–1558. doi: 10.1111/j.1398-9995.2008.01807.x. [DOI] [PubMed] [Google Scholar]
- 60.Scala E., Alessandri C., Bernardi M.L. Cross-sectional survey on immunoglobulin E reactivity in 23,077 subjects using an allergenic molecule-based microarray detection system. Clin Exp Allergy. 2010;40(6):911–921. doi: 10.1111/j.1365-2222.2010.03470.x. Epub 2010 Mar 1. [DOI] [PubMed] [Google Scholar]
- 61.Melioli G., Marcomini L., Agazzi A. The IgE repertoire in children and adolescents resolved at component level: a cross-sectional study. Pediatr Allergy Immunol. 2012;23(5):433–440. doi: 10.1111/j.1399-3038.2011.01228.x. [DOI] [PubMed] [Google Scholar]
- 62.Valenta R., Niederberger V. Recombinant allergens for immunotherapy. J Allergy Clin Immunol. 2007;119(4):826–830. doi: 10.1016/j.jaci.2007.01.025. Epub 2007 Mar 1. [DOI] [PubMed] [Google Scholar]
- 63.Sastre J. Molecular diagnosis in allergy. Clin Exp Allergy. 2010;40(10):1442–1460. doi: 10.1111/j.1365-2222.2010.03585.x. [DOI] [PubMed] [Google Scholar]
- 64.Ferreira F., Hawranek T., Gruber P., Wopfner N., Mari A. Allergic cross-reactivity: from gene to the clinic. Allergy. 2004;59(3):243–267. doi: 10.1046/j.1398-9995.2003.00407.x. [DOI] [PubMed] [Google Scholar]
- 65.Scala E., Alessandri C., Palazzo P. IgE recognition patterns of profilin, PR-10, and tropomyosin panallergens tested in 3,113 allergic patients by allergen microarray-based technology. PLoS One. 2011;6(9) doi: 10.1371/journal.pone.0024912. e24912. Epub 2011 Sep. 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Asero R., Monsalve R., Barber D. Profilin sensitization detected in the office by skin prick test: a study of prevalence and clinical relevance of profilin as a plant food allergen. Clin Exp Allergy. 2008;38(6):1033–1037. doi: 10.1111/j.1365-2222.2008.02980.x. Epub 2008 Apr 13. [DOI] [PubMed] [Google Scholar]
- 67.Kleine-Tebbe J., Vogel L., Crowell D.N., Haustein U.F., Vieths S. Severe oral allergy syndrome and anaphylactic reactions caused by a Bet v 1- related PR-10 protein in soybean, SAM22. J Allergy Clin Immunol. 2002;110(5):797–804. doi: 10.1067/mai.2002.128946. [DOI] [PubMed] [Google Scholar]
- 68.Ballmer-Weber B.K., Holzhauser T., Scibilia J. Clinical characteristics of soybean allergy in Europe: a double-blind, placebo-controlled food challenge study. J Allergy Clin Immunol. 2007;119(6):1489–1496. doi: 10.1016/j.jaci.2007.01.049. Epub 2007 Mar 26. [DOI] [PubMed] [Google Scholar]
- 69.Zuidmeer L., van Ree R. Lipid transfer protein allergy: primary food allergy or pollen/food syndrome in some cases. Curr Opin Allergy Clin Immunol. 2007;7(3):269–273. doi: 10.1097/ACI.0b013e32814a5401. [DOI] [PubMed] [Google Scholar]
- 70.Stedman K., Lee K., Hunter S., Rivoire B., McCall C., Wassom D. Measurement of canine IgE using the alpha chain of the human high affinity IgE receptor. Vet Immunol Immunopathol. 2001;78(3-4):349–355. doi: 10.1016/s0165-2427(01)00242-2. [DOI] [PubMed] [Google Scholar]
- 71.Wojtalewicz N., Kabrodt K., Goseberg S., Schellenberg I. Evaluation of the manufacturer-dependent differences in sIgE results for indoor allergens. Ann Allergy Asthma Immunol. 2018 Oct;121(4):490–495. doi: 10.1016/j.anai.2018.07.016. Epub 2018 Jul 17. [DOI] [PubMed] [Google Scholar]
- 72.Goikoetxea M.J., Sanz M.L., Garcia B.E. Recommendations for the use of in vitro methods to detect specific immunoglobulin E: are they comparable? J Investig Allergol Clin Immunol. 2013;23(7):448–454. quiz 2 pp. preceding 55. [PubMed] [Google Scholar]
- 73.Jahn-Schmid B., Harwanegg C., Hiller R. Allergen microarray: comparison of microarray using recombinant allergens with conventional diagnostic methods to detect allergen-specific serum immunoglobulin E. Clin Exp Allergy. 2003;33(10):1443–1449. doi: 10.1046/j.1365-2222.2003.01784.x. [DOI] [PubMed] [Google Scholar]
- 74.Sampson H.A. Utility of food-specific IgE concentrations in predicting symptomatic food allergy. J Allergy Clin Immunol. 2001;107(5):891–896. doi: 10.1067/mai.2001.114708. [DOI] [PubMed] [Google Scholar]
- 75.Celik-Bilgili S., Mehl A., Verstege A. The predictive value of specific immunoglobulin E levels in serum for the outcome of oral food challenges. Clin Exp Allergy. 2005;35(3):268–273. doi: 10.1111/j.1365-2222.2005.02150.x. [DOI] [PubMed] [Google Scholar]
- 76.Eigenmann P.A. Are specific immunoglobulin E titres reliable for prediction of food allergy? Clin Exp Allergy. 2005;35(3):247–249. doi: 10.1111/j.1365-2222.2005.02183.x. [DOI] [PubMed] [Google Scholar]
- 77.Yunginger J.W., Ahlstedt S., Eggleston P.A. Quantitative IgE antibody assays in allergic diseases. J Allergy Clin Immunol. 2000;105(6 Pt 1):1077–1084. doi: 10.1067/mai.2000.107041. [DOI] [PubMed] [Google Scholar]
- 78.Wickman M., Ahlstedt S., Lilja G., van Hage Hamsten M. Quantification of IgE antibodies simplifies the classification of allergic diseases in 4-year-old children. A report from the prospective birth cohort study--BAMSE. Pediatr Allergy Immunol. 2003;14(6):441–447. doi: 10.1046/j.0905-6157.2003.00079.x. [DOI] [PubMed] [Google Scholar]
- 79.Park K.H., Lee J., Sim D.W., Lee S.C. Comparison of singleplex specific IgE detection immunoassays: ImmunoCAP phadia 250 and immulite 2000 3gAllergy. Ann Lab Med. 2018;38(1):23–31. doi: 10.3343/alm.2018.38.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Barber D., Arias J., Boquete M. Analysis of mite allergic patients in a diverse territory by improved diagnostic tools. Clin Exp Allergy. 2012;42(7):1129–1138. doi: 10.1111/j.1365-2222.2012.03993.x. [DOI] [PubMed] [Google Scholar]
- 81.Boyce J.A., Assa'ad A., Burks A.W. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010;126(6 Suppl):S1–S58. doi: 10.1016/j.jaci.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hamilton R.G., Wisenauer J.A., Golden D.B., Valentine M.D., Adkinson N.F., Jr. Selection of Hymenoptera venoms for immunotherapy on the basis of patient's IgE antibody cross-reactivity. J Allergy Clin Immunol. 1993;92(5):651–659. doi: 10.1016/0091-6749(93)90007-3. [DOI] [PubMed] [Google Scholar]
- 83.Savi E., Peveri S., Makri E., Pravettoni V., Incorvaia C. Comparing the ability of molecular diagnosis and CAP-inhibition in identifying the really causative venom in patients with positive tests to Vespula and Polistes species. Clin Mol Allergy. 2016;14:3. doi: 10.1186/s12948-016-0040-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Savi E., Incorvaia C., Boni E. Which immunotherapy product is better for patients allergic to Polistes venom? A laboratory and clinical study. PLoS One. 2017;12(7) doi: 10.1371/journal.pone.0180270. e0180270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Quercia O., Cova V., Martini M. CAP-inhibition, molecular diagnostics, and total IgE in the evaluation of Polistes and vespula double sensitization. Int Arch Allergy Immunol. 2018;177(4):365–369. doi: 10.1159/000491939. [DOI] [PubMed] [Google Scholar]
- 86.Negrini A.C., Troise C., Voltolini S. ELISA in diagnosis of respiratory allergy. A comparison with RAST and skin tests. Allergy. 1985;40(4):238–241. doi: 10.1111/j.1398-9995.1985.tb00227.x. [DOI] [PubMed] [Google Scholar]
- 87.Schulze A., Downward J. Analysis of gene expression by microarrays: cell biologist's gold mine or minefield? J Cell Sci. 2000;113(Pt 23):4151–4156. doi: 10.1242/jcs.113.23.4151. [DOI] [PubMed] [Google Scholar]
- 88.MacBeath G., Schreiber S.L. Printing proteins as microarrays for high-throughput function determination. Science. 2000;289(5485):1760–1763. doi: 10.1126/science.289.5485.1760. [DOI] [PubMed] [Google Scholar]
- 89.Hiller R., Laffer S., Harwanegg C. Microarrayed allergen molecules: diagnostic gatekeepers for allergy treatment. FASEB J. 2002;16(3):414–416. doi: 10.1096/fj.01-0711fje. Epub 2002 Jan 14. [DOI] [PubMed] [Google Scholar]
- 90.Untersmayr E., Lukschal A., Hemmer W. Exercise with latex sport bands represents a risk for latex allergic patients. Immunol Lett. 2008;115(2):98–104. doi: 10.1016/j.imlet.2007.10.008. Epub 2007 Nov 6. [DOI] [PubMed] [Google Scholar]
- 91.Wohrl S., Vigl K., Zehetmayer S. The performance of a component-based allergen-microarray in clinical practice. Allergy. 2006;61(5):633–639. doi: 10.1111/j.1398-9995.2006.01078.x. [DOI] [PubMed] [Google Scholar]
- 92.Ott H., Schroder C.M., Stanzel S., Merk H.F., Baron J.M. Microarray-based IgE detection in capillary blood samples of patients with atopy. Allergy. 2006;61(9):1146–1147. doi: 10.1111/j.1398-9995.2006.01074.x. [DOI] [PubMed] [Google Scholar]
- 93.Alcocer M.J., Murtagh G.J., Wilson P.B., Progias P., Lin J., Archer D.B. The major human structural IgE epitope of the Brazil nut allergen Ber e 1: a chimaeric and protein microarray approach. J Mol Biol. 2004;343(3):759–769. doi: 10.1016/j.jmb.2004.08.065. [DOI] [PubMed] [Google Scholar]
- 94.Cerecedo I., Zamora J., Shreffler W.G. Mapping of the IgE and IgG4 sequential epitopes of milk allergens with a peptide microarray-based immunoassay. J Allergy Clin Immunol. 2008;122(3):589–594. doi: 10.1016/j.jaci.2008.06.040. [DOI] [PubMed] [Google Scholar]
- 95.Constantin C., Quirce S., Poorafshar M. Micro-arrayed wheat seed and grass pollen allergens for component-resolved diagnosis. Allergy. 2009;64(7):1030–1037. doi: 10.1111/j.1398-9995.2009.01955.x. Epub 2009 Feb 11. [DOI] [PubMed] [Google Scholar]
- 96.Cretich M., di Carlo G., Longhi R. High sensitivity protein assays on microarray silicon slides. Anal Chem. 2009;81(13):5197–5203. doi: 10.1021/ac900658c. [DOI] [PubMed] [Google Scholar]
- 97.Fall B.I., Eberlein-Konig B., Behrendt H., Niessner R., Ring J., Weller M.G. Microarrays for the screening of allergen-specific IgE in human serum. Anal Chem. 2003;75(3):556–562. doi: 10.1021/ac026016k. [DOI] [PubMed] [Google Scholar]
- 98.Gadermaier G., Wopfner N., Wallner M. Array-based profiling of ragweed and mugwort pollen allergens. Allergy. 2008;63(11):1543–1549. doi: 10.1111/j.1398-9995.2008.01780.x. [DOI] [PubMed] [Google Scholar]
- 99.Gaudin J.C., Rabesona H., Choiset Y. Assessment of the immunoglobulin E-mediated immune response to milk-specific proteins in allergic patients using microarrays. Clin Exp Allergy. 2008;38(4):686–693. doi: 10.1111/j.1365-2222.2008.02952.x. Epub 2008 Feb 26. [DOI] [PubMed] [Google Scholar]
- 100.Heyries K.A., Loughran M.G., Hoffmann D., Homsy A., Blum L.J., Marquette C.A. Microfluidic biochip for chemiluminescent detection of allergen-specific antibodies. Biosens Bioelectron. 2008;23(12):1812–1818. doi: 10.1016/j.bios.2008.02.025. Epub Mar 7. [DOI] [PubMed] [Google Scholar]
- 101.Lebrun S.J., Petchpud W.N., Hui A., McLaughlin C.S. Development of a sensitive, colorometric microarray assay for allergen-responsive human IgE. J Immunol Methods. 2005;300(1-2):24–31. doi: 10.1016/j.jim.2005.01.019. Epub 2005 Apr 13. [DOI] [PubMed] [Google Scholar]
- 102.Ott H., Baron J.M., Heise R. Clinical usefulness of microarray-based IgE detection in children with suspected food allergy. Allergy. 2008;63(11):1521–1528. doi: 10.1111/j.1398-9995.2008.01748.x. [DOI] [PubMed] [Google Scholar]
- 103.Ott H., Folster-Holst R., Merk H.F., Baron J.M. Allergen microarrays: a novel tool for high-resolution IgE profiling in adults with atopic dermatitis. Eur J Dermatol. 2010;20(1):54–61. doi: 10.1684/ejd.2010.0810. Epub 2009 Oct 2. [DOI] [PubMed] [Google Scholar]
- 104.Ott H., Schroder C., Raulf-Heimsoth M. Microarrays of recombinant Hevea brasiliensis proteins: a novel tool for the component-resolved diagnosis of natural rubber latex allergy. J Investig Allergol Clin Immunol. 2010;20(2):129–138. [PubMed] [Google Scholar]
- 105.Shreffler W.G., Lencer D.A., Bardina L., Sampson H.A. IgE and IgG4 epitope mapping by microarray immunoassay reveals the diversity of immune response to the peanut allergen, Ara h 2. J Allergy Clin Immunol. 2005;116(4):893–899. doi: 10.1016/j.jaci.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 106.Melioli G., Bonifazi F., Bonini S. The ImmunoCAP ISAC molecular allergology approach in adult multi-sensitized Italian patients with respiratory symptoms. Clin Biochem. 2011;44(12):1005–1011. doi: 10.1016/j.clinbiochem.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 107.Deinhofer K., Sevcik H., Balic N. Microarrayed allergens for IgE profiling. Methods. 2004;32(3):249–254. doi: 10.1016/j.ymeth.2003.08.018. [DOI] [PubMed] [Google Scholar]
- 108.Huss-Marp J., Gutermuth J., Schaffner I. Comparison of molecular and extract-based allergy diagnostics with multiplex and singleplex analysis. Allergo J Int. 2015;24:46–53. doi: 10.1007/s40629-015-0046-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lambert C., Sarrat A., Bienvenu F. The importance of EN ISO 15189 accreditation of allergen-specific IgE determination for reliable in vitro allergy diagnosis. Allergy. 2015;70(2):180–186. doi: 10.1111/all.12546. [DOI] [PubMed] [Google Scholar]
- 110.Martinez-Aranguren R., Lizaso M.T., Goikoetxea M.J. Is the determination of specific IgE against components using ISAC 112 a reproducible technique? PLoS One. 2014;9(2) doi: 10.1371/journal.pone.0088394. e88394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Monroe M.R., Reddington A.P., Collins A.D. Multiplexed method to calibrate and quantitate fluorescence signal for allergen-specific IgE. Anal Chem. 2011;83(24):9485–9491. doi: 10.1021/ac202212k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Villalta D., Conte M., Asero R., Da Re M., Stella S., Martelli P. Isolated IgE reactivity to native walnut vicilin-like protein (nJug r 2) on ISAC microarray is due to cross-reactive carbohydrate epitopes. Clin Chem Lab Med. 2013;51(10):1991–1995. doi: 10.1515/cclm-2013-0027. [DOI] [PubMed] [Google Scholar]
- 113.Hochwallner H., Alm J., Lupinek C. Transmission of allergen-specific IgG and IgE from maternal blood into breast milk visualized with microarray technology. J Allergy Clin Immunol. 2014;134(5):1213–1215. doi: 10.1016/j.jaci.2014.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Leonardi A., Borghesan F., Faggian D., Plebani M. Microarray-based IgE detection in tears of patients with vernal keratoconjunctivitis. Pediatr Allergy Immunol. 2015;26(7):641–645. doi: 10.1111/pai.12450. [DOI] [PubMed] [Google Scholar]
- 115.Wollmann E., Lupinek C., Kundi M., Selb R., Niederberger V., Valenta R. Reduction in allergen-specific IgE binding as measured by microarray: a possible surrogate marker for effects of specific immunotherapy. J Allergy Clin Immunol. 2015;136(3):806–809 e7. doi: 10.1016/j.jaci.2015.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schmid J.M., Wurtzen P.A., Dahl R., Hoffmann H.J. Pretreatment IgE sensitization patterns determine the molecular profile of the IgG4 response during updosing of subcutaneous immunotherapy with timothy grass pollen extract. J Allergy Clin Immunol. 2016;137(2):562–570. doi: 10.1016/j.jaci.2015.05.023. [DOI] [PubMed] [Google Scholar]
- 117.D'Amelio C.M., Goikoetxea M.J., Martinez-Aranguren R. Is the performance of ImmunoCAP ISAC 112 sufficient to diagnose peach and apple allergies? Ann Allergy Asthma Immunol. 2016;116(2):162–163. doi: 10.1016/j.anai.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 118.Goikoetxea M.J., D'Amelio C.M., Martinez-Aranguren R. Is microarray analysis really useful and sufficient to diagnose nut allergy in the mediterranean area? J Investig Allergol Clin Immunol. 2016;26(1):31–39. [PubMed] [Google Scholar]
- 119.Javaloyes G., Goikoetxea M.J., Garcia Nunez I. Pru p 3 acts as a strong sensitizer for peanut allergy in Spain. J Allergy Clin Immunol. 2012;130(6):1432–4 e3. doi: 10.1016/j.jaci.2012.08.038. [DOI] [PubMed] [Google Scholar]
- 120.Heaps A., Carter S., Selwood C. The utility of the ISAC allergen array in the investigation of idiopathic anaphylaxis. Clin Exp Immunol. 2014;177(2):483–490. doi: 10.1111/cei.12334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cardona V., Ansotegui I.J. Component-resolved diagnosis in anaphylaxis. Curr Opin Allergy Clin Immunol. 2016;16(3):244–249. doi: 10.1097/ACI.0000000000000261. [DOI] [PubMed] [Google Scholar]
- 122.Skrindo I., Lupinek C., Valenta R. The use of the MeDALL-chip to assess IgE sensitization: a new diagnostic tool for allergic disease? Pediatr Allergy Immunol. 2015;26(3):239–246. doi: 10.1111/pai.12366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lupinek C., Wollmann E., Baar A. Advances in allergen-microarray technology for diagnosis and monitoring of allergy: the MeDALL allergen-chip. Methods. 2014;66(1):106–119. doi: 10.1016/j.ymeth.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Skrindo I., Lupinek C., Valenta R. The use of the MeDALL-chip to assess IgE sensitization: a new diagnostic tool for allergic disease? Pediatr Allergy Immunol. 2015;26(3):239–246. doi: 10.1111/pai.12366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wickman M., Lupinek C., Andersson N. Detection of IgE reactivity to a handful of allergen molecules in early childhood predicts respiratory allergy in adolescence. EBioMedicine. 2017;26:91–99. doi: 10.1016/j.ebiom.2017.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Williams P., Onell A., Baldracchini F., Hui V., Jolles S., El-Shanawany T. Evaluation of a novel automated allergy microarray platform compared with three other allergy test methods. Clin Exp Immunol. 2016 Apr;184(1):1–10. doi: 10.1111/cei.12721. Epub 2016 Jan 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Heffler E., Puggioni F., Peveri S., Montagni M., Canonica G.W., Melioli G. Extended IgE profile based on an allergen macroarray: a novel tool for precision medicine in allergy diagnosis. World Allergy Organ J. 2018;11(1):7. doi: 10.1186/s40413-018-0186-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Melioli G., Spenser C., Reggiardo G. Allergenius, an expert system for the interpretation of allergen microarray results. World Allergy Organ J. 2014;7(1):15. doi: 10.1186/1939-4551-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Konopka E., Ceregra A., Maciorkowska E. Specific IgE antibodies in young children with atopic dermatitis--correlation of multiple allergen simultaneous immunoblot test and ImmunoCap system. Clin Lab. 2016;62(5):815–821. doi: 10.7754/clin.lab.2015.150816. [DOI] [PubMed] [Google Scholar]
- 130.Morgan E., Varro R., Sepulveda H. Cytometric bead array: a multiplexed assay platform with applications in various areas of biology. Clin Immunol. 2004;110(3):252–266. doi: 10.1016/j.clim.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 131.Varro R., Chen R., Sepulveda H., Apgar J. Bead-based multianalyte flow immunoassays: the cytometric bead array system. Methods Mol Biol. 2007;378:125–152. doi: 10.1007/978-1-59745-323-3_9. [DOI] [PubMed] [Google Scholar]
- 132.Ferbas J., Thomas J., Hodgson J., Gaur A., Casadevall N., Swanson S.J. Feasibility of a multiplex flow cytometric bead immunoassay for detection of anti-epoetin alfa antibodies. Clin Vaccine Immunol. 2007;14(9):1165–1172. doi: 10.1128/CVI.00157-07. Epub 2007 Jul 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Fulton R.J., McDade R.L., Smith P.L., Kienker L.J., Kettman J.R., Jr. Advanced multiplexed analysis with the FlowMetrix system. Clin Chem. 1997;43(9):1749–1756. [PubMed] [Google Scholar]
- 134.Kettman J.R., Davies T., Chandler D., Oliver K.G., Fulton R.J. Classification and properties of 64 multiplexed microsphere sets. Cytometry. 1998;33(2):234–243. [PubMed] [Google Scholar]
- 135.King E.M., Vailes L.D., Tsay A., Satinover S.M., Chapman M.D. Simultaneous detection of total and allergen-specific IgE by using purified allergens in a fluorescent multiplex array. J Allergy Clin Immunol. 2007;120(5):1126–1131. doi: 10.1016/j.jaci.2007.06.043. Epub 2007 Sep. 7. [DOI] [PubMed] [Google Scholar]
- 136.Pomponi D., Bernardi M.L., Liso M. Allergen micro-bead array for IgE detection: a feasibility study using allergenic molecules tested on a flexible multiplex flow cytometric immunoassay. PLoS One. 2012;7(4) doi: 10.1371/journal.pone.0035697. e35697. Epub 2012 Apr 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Incorvaia C., Mauro M., Ridolo E., Makri E., Montagni M., Ciprandi G. A pitfall to avoid when using an allergen microarray: the incidental detection of IgE to unexpected allergens. J Allergy Clin Immunol Pract. 2015;3(6):879–882. doi: 10.1016/j.jaip.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 138.Canonica G.W., Ferrando M., Baiardini I. Asthma: personalized and precision medicine. Curr Opin Allergy Clin Immunol. 2018;18(1):51–58. doi: 10.1097/ACI.0000000000000416. [DOI] [PubMed] [Google Scholar]
- 139.Westman M., Lupinek C., Bousquet J. Early childhood IgE reactivity to pathogenesis-related class 10 proteins predicts allergic rhinitis in adolescence. J Allergy Clin Immunol. 2015;135(5) doi: 10.1016/j.jaci.2014.10.042. 1199-11206 e1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Schmid-Grendelmeier P. [Pollen allergy and immunotherapy] Ther Umsch. 2012;69(4):239–248. doi: 10.1024/0040-5930/a000280. [DOI] [PubMed] [Google Scholar]
- 141.Melioli G., Passalacqua G., Canonica G.W., Baena-Cagnani C.E., Matricardi P. Component-resolved diagnosis in pediatric allergic rhinoconjunctivitis and asthma. Curr Opin Allergy Clin Immunol. 2013;13(4):446–451. doi: 10.1097/ACI.0b013e32836274d8. [DOI] [PubMed] [Google Scholar]
- 142.Prosperi M.C., Belgrave D., Buchan I., Simpson A., Custovic A. Challenges in interpreting allergen microarrays in relation to clinical symptoms: a machine learning approach. Pediatr Allergy Immunol. 2014;25(1):71–79. doi: 10.1111/pai.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hamilton R.G., Kleine-Tebbe J. Molecular allergy diagnostics: analytical features that support clinical decisions. Curr Allergy Asthma Rep. 2015;15(9):57. doi: 10.1007/s11882-015-0556-7. [DOI] [PubMed] [Google Scholar]
- 144.Matricardi P.M., Kleine-Tebbe J. Molecular allergology between precision medicine and the choosing wisely initiative. Clin Exp Allergy. 2016;46(5):664–667. doi: 10.1111/cea.12679. [DOI] [PubMed] [Google Scholar]
- 145.Scala E., Villalta D., Uasuf C.G. An atlas of IgE sensitization patterns in different Italian areas. A multicenter, cross-sectional study. Eur Ann Allergy Clin Immunol. 2018 Sep;50(5):217–225. doi: 10.23822/EurAnnACI.1764-1489.67. Epub 2018 Jul 24. [DOI] [PubMed] [Google Scholar]
- 146.Roseler S., Balakirski G., Plange J. [Anaphylaxis to PR-10 proteins (Bet v1 homologues)] Hautarzt. 2013;64(12):890–892. doi: 10.1007/s00105-013-2683-1. [DOI] [PubMed] [Google Scholar]
- 147.Alvarado M.I., Jimeno L., De La Torre F. Profilin as a severe food allergen in allergic patients overexposed to grass pollen. Allergy. 2014;69(12):1610–1616. doi: 10.1111/all.12509. [DOI] [PubMed] [Google Scholar]
- 148.Scala E., Till S.J., Asero R. Lipid transfer protein sensitization: reactivity profiles and clinical risk assessment in an Italian cohort. Allergy. 2015;70(8):933–943. doi: 10.1111/all.12635. [DOI] [PubMed] [Google Scholar]
- 149.Fernandez-Rivas M. Fruit and vegetable allergy. Chem Immunol Allergy. 2015;101:162–170. doi: 10.1159/000375469. [DOI] [PubMed] [Google Scholar]
- 150.Jakob T., Forstenlechner P., Matricardi P., Kleine-Tebbe J. Molecular allergy diagnostics using multiplex assays: methodological and practical considerations for use in research and clinical routine: Part 21 of the Series Molecular Allergology. Allergo J Int. 2015;24:320–332. doi: 10.1007/s40629-015-0087-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Macchia D., Melioli G., Pravettoni V. Guidelines for the use and interpretation of diagnostic methods in adult food allergy. Clin Mol Allergy. 2015;13:27. doi: 10.1186/s12948-015-0033-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rosario N.L.A.L. Does sensitization to food allergens in patients with rhinitis mean food allergy? J Allergy Ther. 2014;2(3):167. [Google Scholar]
- 153.Werfel T., Asero R., Ballmer-Weber B.K. Position paper of the EAACI: food allergy due to immunological cross-reactions with common inhalant allergens. Allergy. 2015;70(9):1079–1090. doi: 10.1111/all.12666. [DOI] [PubMed] [Google Scholar]
- 154.Demoly P., Lebel B., Arnoux B. Allergen-induced mediator release tests. Allergy. 2003;58(7):553–558. doi: 10.1034/j.1398-9995.2003.00253.x. [DOI] [PubMed] [Google Scholar]
- 155.Bridts C.H., Sabato V., Mertens C., Hagendorens M.M., De Clerck L.S., Ebo D.G. Flow cytometric allergy diagnosis: basophil activation techniques. Methods Mol Biol. 2014;1192:147–159. doi: 10.1007/978-1-4939-1173-8_11. [DOI] [PubMed] [Google Scholar]
- 156.McNeil B.D., Pundir P., Meeker S. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature. 2015;519(7542):237–241. doi: 10.1038/nature14022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Van Gasse A.L., Sabato V., Uyttebroek A.P. Immediate moxifloxacin hypersensitivity: is there more than currently meets the eye? Allergy. 2017;72(12):2039–2043. doi: 10.1111/all.13236. [DOI] [PubMed] [Google Scholar]
- 158.Ebo D.G., Sainte-Laudy J., Bridts C.H. Flow-assisted allergy diagnosis: current applications and future perspectives. Allergy. 2006;61(9):1028–1039. doi: 10.1111/j.1398-9995.2006.01039.x. [DOI] [PubMed] [Google Scholar]
- 159.Ebo D.G., Bridts C.H., Mertens C.H., Hagendorens M.M., Stevens W.J., De Clerck L.S. Analyzing histamine release by flow cytometry (HistaFlow): a novel instrument to study the degranulation patterns of basophils. J Immunol Methods. 2012;375(1-2):30–38. doi: 10.1016/j.jim.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 160.Sturm G.J., Kranzelbinder B., Sturm E.M., Heinemann A., Groselj-Strele A., Aberer W. The basophil activation test in the diagnosis of allergy: technical issues and critical factors. Allergy. 2009;64(9):1319–1326. doi: 10.1111/j.1398-9995.2009.02004.x. [DOI] [PubMed] [Google Scholar]
- 161.Puan K.J., Andiappan A.K., Lee B. Systematic characterization of basophil anergy. Allergy. 2017;72(3):373–384. doi: 10.1111/all.12952. Epub 2016 Aug 8. [DOI] [PubMed] [Google Scholar]
- 162.Faber M., Sabato V., De Witte L. State of the art and perspectives in food allergy (part I): diagnosis. Curr Pharmaceut Des. 2014;20(6):954–963. doi: 10.2174/13816128113199990046. [DOI] [PubMed] [Google Scholar]
- 163.Sabato V., van Hengel A.J., De Knop K.J. Basophil activation reveals divergent patient-specific responses to thermally processed peanuts. J Investig Allergol Clin Immunol. 2011;21(7):527–531. [PubMed] [Google Scholar]
- 164.Rubio A., Vivinus-Nebot M., Bourrier T., Saggio B., Albertini M., Bernard A. Benefit of the basophil activation test in deciding when to reintroduce cow's milk in allergic children. Allergy. 2011;66(1):92–100. doi: 10.1111/j.1398-9995.2010.02432.x. [DOI] [PubMed] [Google Scholar]
- 165.Carroccio A., Brusca I., Mansueto P. A cytologic assay for diagnosis of food hypersensitivity in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2010;8(3):254–260. doi: 10.1016/j.cgh.2009.11.010. Epub Nov 22. [DOI] [PubMed] [Google Scholar]
- 166.Song Y., Wang J., Leung N. Correlations between basophil activation, allergen-specific IgE with outcome and severity of oral food challenges. Ann Allergy Asthma Immunol. 2015;114(4):319–326. doi: 10.1016/j.anai.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 167.Ford L.S., Bloom K.A., Nowak-Wegrzyn A.H., Shreffler W.G., Masilamani M., Sampson H.A. Basophil reactivity, wheal size, and immunoglobulin levels distinguish degrees of cow's milk tolerance. J Allergy Clin Immunol. 2013;131(1):180–186. doi: 10.1016/j.jaci.2012.06.003. e1-3. Epub Jul 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sato S., Tachimoto H., Shukuya A. Utility of the peripheral blood basophil histamine release test in the diagnosis of hen's egg, cow's milk, and wheat allergy in children. Int Arch Allergy Immunol. 2011;155(Suppl 1):96–103. doi: 10.1159/000327490. Epub 2011 Jun 1. [DOI] [PubMed] [Google Scholar]
- 169.Glaumann S., Nopp A., Johansson S.G., Rudengren M., Borres M.P., Nilsson C. Basophil allergen threshold sensitivity, CD-sens, IgE-sensitization and DBPCFC in peanut-sensitized children. Allergy. 2012;67(2):242–247. doi: 10.1111/j.1398-9995.2011.02754.x. Epub 2011 Nov 30. [DOI] [PubMed] [Google Scholar]
- 170.Hoffmann-Sommergruber K., Pfeifer S., Bublin M. Applications of molecular diagnostic testing in food allergy. Curr Allergy Asthma Rep. 2015;15(9):56. doi: 10.1007/s11882-015-0557-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sabato V., Ebo D.G. Hypersensitivity to neuromuscular blocking agents: can skin tests give the green light for Re-exposure? J Allergy Clin Immunol Pract. 2018;6(5):1690–1691. doi: 10.1016/j.jaip.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 172.Mangodt E.A., Van Gasse A.L., Decuyper I. In vitro diagnosis of immediate drug hypersensitivity: should we go with the flow. Int Arch Allergy Immunol. 2015;168(1):3–12. doi: 10.1159/000440663. [DOI] [PubMed] [Google Scholar]
- 173.Ebo D.G., Faber M., Elst J. In vitro diagnosis of immediate drug hypersensitivity during anesthesia: a review of the literature. J Allergy Clin Immunol Pract. 2018;6(4):1176–1184. doi: 10.1016/j.jaip.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 174.Leysen J., De Witte L., Sabato V. IgE-mediated allergy to pholcodine and cross-reactivity to neuromuscular blocking agents: lessons from flow cytometry. Cytometry B Clin Cytom. 2013;84(2):65–70. doi: 10.1002/cyto.b.21074. [DOI] [PubMed] [Google Scholar]
- 175.Van Gasse A.L., Hagendorens M.M., Sabato V., Bridts C.H., De Clerck L.S., Ebo D.G. IgE to poppy seed and morphine are not useful tools to diagnose opiate allergy. J Allergy Clin Immunol Pract. 2015;3(3):396–399. doi: 10.1016/j.jaip.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 176.Ebo D.G., Van Vaerenbergh M., de Graaf D.C., Bridts C.H., De Clerck L.S., Sabato V. In vitro diagnosis of Hymenoptera venom allergy and further development of component resolved diagnostics. Expert Rev Clin Immunol. 2014;10(3):375–384. doi: 10.1586/1744666X.2014.881252. [DOI] [PubMed] [Google Scholar]
- 177.Blank S., Bilo M.B., Ollert M. Component-resolved diagnostics to direct in venom immunotherapy: important steps towards precision medicine. Clin Exp Allergy. 2018;48(4):354–364. doi: 10.1111/cea.13090. [DOI] [PubMed] [Google Scholar]
- 178.Nullens S., Sabato V., Faber M. Basophilic histamine content and release during venom immunotherapy: insights by flow cytometry. Cytometry B Clin Cytom. 2013;84(3):173–178. doi: 10.1002/cyto.b.21084. [DOI] [PubMed] [Google Scholar]
- 179.Agache I., Bilo M., Braunstahl G.J. In vivo diagnosis of allergic diseases--allergen provocation tests. Allergy. 2015;70(4):355–365. doi: 10.1111/all.12586. Epub 2015 Feb 12. [DOI] [PubMed] [Google Scholar]
- 180.Pfaar O., Demoly P., Gerth van Wijk R. Recommendations for the standardization of clinical outcomes used in allergen immunotherapy trials for allergic rhinoconjunctivitis: an EAACI Position Paper. Allergy. 2014;69(7):854–867. doi: 10.1111/all.12383. Epub 2014 Apr 25. [DOI] [PubMed] [Google Scholar]
- 181.Auge J., Vent J., Agache I. EAACI Position paper on the standardization of nasal allergen challenges. Allergy. 2018;73(8):1597–1608. doi: 10.1111/all.13416. [DOI] [PubMed] [Google Scholar]
- 182.Fauquert J.L., Jedrzejczak-Czechowicz M., Rondon C. Conjunctival allergen provocation test : guidelines for daily practice. Allergy. 2017;72(1):43–54. doi: 10.1111/all.12986. [DOI] [PubMed] [Google Scholar]
- 183.European Medicines Agency (EMA), Committee for Medicinal Products for Human Use (CHMP) 2009. Guideline on the Clinical Development of Products for Specific Immunotherapy for the Treatment of Allergic Diseases; pp. 3–13.https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-clinical-development-products-specific-immunotherapy-treatment-allergic-diseases_en.pdf Ref. CHMP/EWP/18504/20. [Google Scholar]
- 184.Rosner-Friese K., Kaul S., Vieths S., Pfaar O. Environmental exposure chambers in allergen immunotherapy trials: current status and clinical validation needs. J Allergy Clin Immunol. 2015;135(3):636–643. doi: 10.1016/j.jaci.2014.10.045. [DOI] [PubMed] [Google Scholar]
- 185.U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Allergic Rhinitis: Developing Drug Products for Treatment Guidance for Industry. 2016. http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm071293.pdf [Google Scholar]
- 186.Pfaar O., Calderon M.A., Andrews C.P. Allergen exposure chambers: harmonizing current concepts and projecting the needs for the future - an EAACI Position Paper. Allergy. 2017;25(10):13133. doi: 10.1111/all.13133. [DOI] [PubMed] [Google Scholar]
- 187.Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol. 2010;105(4):259–273. doi: 10.1016/j.anai.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 188.Ebo D.G., Clarke R.C., Mertes P.M., Platt P.R., Sabato V., Sadleir P.H.M. Molecular mechanisms and pathophysiology of perioperative hypersensitivity and anaphylaxis: a narrative review. Br J Anaesth. 2019 Jul;123(1):e38–e49. doi: 10.1016/j.bja.2019.01.031. Epub 2019 Mar 8. [DOI] [PubMed] [Google Scholar]
- 189.Blanca M., Romano A., Torres M.J. Update on the evaluation of hypersensitivity reactions to betalactams. Allergy. 2009;64(2):183–193. doi: 10.1111/j.1398-9995.2008.01916.x. [DOI] [PubMed] [Google Scholar]
- 190.Brockow K., Garvey L.H., Aberer W. Skin test concentrations for systemically administered drugs -- an ENDA/EAACI Drug Allergy Interest Group position paper. Allergy. 2013;68(6):702–712. doi: 10.1111/all.12142. Epub 2013 Apr 25. [DOI] [PubMed] [Google Scholar]
- 191.Torres M.J., Blanca M., Fernandez J. Diagnosis of immediate allergic reactions to beta-lactam antibiotics. Allergy. 2003;58(10):961–972. doi: 10.1034/j.1398-9995.2003.00280.x. [DOI] [PubMed] [Google Scholar]
- 192.Kuyucu S., Mori F., Atanaskovic-Markovic M. Hypersensitivity reactions to non-betalactam antibiotics in children: an extensive review. Pediatr Allergy Immunol. 2014;25(6):534–543. doi: 10.1111/pai.12273. [DOI] [PubMed] [Google Scholar]
- 193.Laxenaire M.C., Gastin I., Moneret-Vautrin D.A., Widmer S., Gueant J.L. Cross-reactivity of rocuronium with other neuromuscular blocking agents. Eur J Anaesthesiol Suppl. 1995;11:55–64. [PubMed] [Google Scholar]
- 194.Moneret-Vautrin D.A., Mertes P.M. Anaphylaxis to general anesthetics. Chem Immunol Allergy. 2010;95:180–189. doi: 10.1159/000315951. Epub 2010 Jun 1. [DOI] [PubMed] [Google Scholar]
- 195.Johansson S.G., Florvaag E., Oman H. National pholcodine consumption and prevalence of IgE-sensitization: a multicentre study. Allergy. 2010;65(4):498–502. doi: 10.1111/j.1398-9995.2009.02193.x. Epub 2009 Oct 1. [DOI] [PubMed] [Google Scholar]
- 196.Hagau N., Gherman N., Cocis M., Petrisor C. Antibiotic-induced immediate type hypersensitivity is a risk factor for positive allergy skin tests for neuromuscular blocking agents. Allergol Int. 2016;65(1):52–55. doi: 10.1016/j.alit.2015.07.007. Epub Aug 29. [DOI] [PubMed] [Google Scholar]
- 197.Castells M.C., Tennant N.M., Sloane D.E. Hypersensitivity reactions to chemotherapy: outcomes and safety of rapid desensitization in 413 cases. J Allergy Clin Immunol. 2008;122(3):574–580. doi: 10.1016/j.jaci.2008.02.044. Epub May 27. [DOI] [PubMed] [Google Scholar]
- 198.Sese L., Gaouar H., Autegarden J.E. Immediate hypersensitivity to iodinated contrast media: diagnostic accuracy of skin tests and intravenous provocation test with low dose. Clin Exp Allergy. 2016;46(3):472–478. doi: 10.1111/cea.12703. [DOI] [PubMed] [Google Scholar]
- 199.Bircher A.J., Harr T., Hohenstein L., Tsakiris D.A. Hypersensitivity reactions to anticoagulant drugs: diagnosis and management options. Allergy. 2006;61(12):1432–1440. doi: 10.1111/j.1398-9995.2006.01227.x. [DOI] [PubMed] [Google Scholar]
- 200.Gomez E., Blanca-Lopez N., Torres M.J. Immunoglobulin E-mediated immediate allergic reactions to dipyrone: value of basophil activation test in the identification of patients. Clin Exp Allergy. 2009;39(8):1217–1224. doi: 10.1111/j.1365-2222.2009.03237.x. Epub 2009 Apr 7. [DOI] [PubMed] [Google Scholar]
- 201.van Kampen V., de Blay F., Folletti I. EAACI position paper: skin prick testing in the diagnosis of occupational type I allergies. Allergy. 2013;68(5):580–584. doi: 10.1111/all.12120. Epub 2013 Feb 15. [DOI] [PubMed] [Google Scholar]
- 202.Turjanmaa K., Alenius H., Reunala T., Palosuo T. Recent developments in latex allergy. Curr Opin Allergy Clin Immunol. 2002;2(5):407–412. doi: 10.1097/00130832-200210000-00007. [DOI] [PubMed] [Google Scholar]
- 203.Cullinan P., Brown R., Field A. Latex allergy. A position paper of the British society of allergy and clinical immunology. Clin Exp Allergy. 2003;33(11):1484–1499. doi: 10.1046/j.1365-2222.2003.01818.x. [DOI] [PubMed] [Google Scholar]
- 204.Aalberse R.C. Food allergens. Environ Toxicol Pharmacol. 1997;4(1-2):55–60. doi: 10.1016/s1382-6689(97)10042-4. [DOI] [PubMed] [Google Scholar]
- 205.Santos A., Van Ree R. Profilins: mimickers of allergy or relevant allergens? Int Arch Allergy Immunol. 2011;155(3):191–204. doi: 10.1159/000321178. Epub 2011 Feb 2. [DOI] [PubMed] [Google Scholar]
- 206.Cartier A. The role of inhalant food allergens in occupational asthma. Curr Allergy Asthma Rep. 2010;10(5):349–356. doi: 10.1007/s11882-010-0130-2. [DOI] [PubMed] [Google Scholar]
- 207.Raulf M., Buters J., Chapman M. Monitoring of occupational and environmental aeroallergens-- EAACI position paper. Concerted action of the EAACI IG occupational allergy and aerobiology & air pollution. Allergy. 2014;69(10):1280–1299. doi: 10.1111/all.12456. [DOI] [PubMed] [Google Scholar]
- 208.Baur X. Baker's asthma: causes and prevention. Int Arch Occup Environ Health. 1999;72(5):292–296. doi: 10.1007/s004200050377. [DOI] [PubMed] [Google Scholar]
- 209.Palosuo K. Update on wheat hypersensitivity. Curr Opin Allergy Clin Immunol. 2003;3(3):205–209. doi: 10.1097/00130832-200306000-00009. [DOI] [PubMed] [Google Scholar]
- 210.Tatham A.S., Shewry P.R. Allergens to wheat and related cereals. Clin Exp Allergy. 2008;38(11):1712–1726. doi: 10.1111/j.1365-2222.2008.03101.x. Epub 2008 Sep 24. [DOI] [PubMed] [Google Scholar]
- 211.Scibilia J., Pastorello E.A., Zisa G. Wheat allergy: a double-blind, placebo-controlled study in adults. J Allergy Clin Immunol. 2006;117(2):433–439. doi: 10.1016/j.jaci.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 212.Martens M., Schnoor H.J., Malling H.J., Poulsen L.K. Sensitization to cereals and peanut evidenced by skin prick test and specific IgE in food-tolerant, grass pollen allergic patients. Clin Transl Allergy. 2011;1(1):15. doi: 10.1186/2045-7022-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Kanda N., Hau C.S., Tada Y., Sato S., Watanabe S. Decreased serum LL-37 and vitamin D3 levels in atopic dermatitis: relationship between IL-31 and oncostatin M. Allergy. 2012;67(6):804–812. doi: 10.1111/j.1398-9995.2012.02824.x. Epub 2012 Apr 10. [DOI] [PubMed] [Google Scholar]
- 214.Johnsen C.R., Sorensen T.B., Ingemann Larsen A. Allergy risk in an enzyme producing plant: a retrospective follow up study. Occup Environ Med. 1997;54(9):671–675. doi: 10.1136/oem.54.9.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Quirce S., Cuevas M., Diez-Gomez M. Respiratory allergy to Aspergillus-derived enzymes in bakers' asthma. J Allergy Clin Immunol. 1992;90(6 Pt 1):970–978. doi: 10.1016/0091-6749(92)90470-m. [DOI] [PubMed] [Google Scholar]
- 216.Baur X., Czuppon A.B. Allergic reaction after eating alpha-amylase (Asp o 2)-containing bread. A case report. Allergy. 1995;50(1):85–87. doi: 10.1111/j.1398-9995.1995.tb02487.x. [DOI] [PubMed] [Google Scholar]
- 217.Pepys J., Longbottom J.L., Hargreave F.E., Faux J. Allergic reactions of the lungs to enzymes of Bacillus subtilis. Lancet. 1969;1(7607):1181–1184. doi: 10.1016/s0140-6736(69)92166-7. [DOI] [PubMed] [Google Scholar]
- 218.Schweigert M.K., Mackenzie D.P., Sarlo K. Occupational asthma and allergy associated with the use of enzymes in the detergent industry--a review of the epidemiology, toxicology and methods of prevention. Clin Exp Allergy. 2000;30(11):1511–1518. doi: 10.1046/j.1365-2222.2000.00893.x. [DOI] [PubMed] [Google Scholar]
- 219.Larsen A.I., Johnsen C.R., Frickmann J., Mikkelsen S. Incidence of respiratory sensitisation and allergy to enzymes among employees in an enzyme producing plant and the relation to exposure and host factors. Occup Environ Med. 2007;64(11):763–768. doi: 10.1136/oem.2005.025304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Paulsen E., Skov P.S., Bindslev-Jensen C., Voitenko V., Poulsen L.K. Occupational type I allergy to christmas cactus (schlumbergera) Allergy. 1997;52(6):656–660. doi: 10.1111/j.1398-9995.1997.tb01045.x. [DOI] [PubMed] [Google Scholar]
- 221.Nagendran V., Wicking J., Ekbote A., Onyekwe T., Garvey L.H. IgE-mediated chlorhexidine allergy: a new occupational hazard? Occup Med (Lond) 2009;59(4):270–272. doi: 10.1093/occmed/kqp042. Epub 2009 Mar 26. [DOI] [PubMed] [Google Scholar]
- 222.Wisnewski A.V., Liu J., Redlich C.A. Antigenic changes in human albumin caused by reactivity with the occupational allergen diphenylmethane diisocyanate. Anal Biochem. 2010;400(2):251–258. doi: 10.1016/j.ab.2010.01.037. Epub Feb 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Wisnewski A.V., Jones M. Pro/Con debate: is occupational asthma induced by isocyanates an immunoglobulin E-mediated disease? Clin Exp Allergy. 2010;40(8):1155–1162. doi: 10.1111/j.1365-2222.2010.03550.x. Epub 2010 Jun 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Wisnewski A.V. Developments in laboratory diagnostics for isocyanate asthma. Curr Opin Allergy Clin Immunol. 2007;7(2):138–145. doi: 10.1097/ACI.0b013e3280895d22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Jares E.J., Baena-Cagnani C.E., Gomez R.M. Diagnosis of occupational asthma: an update. Curr Allergy Asthma Rep. 2012;12(3):221–231. doi: 10.1007/s11882-012-0259-2. [DOI] [PubMed] [Google Scholar]
- 226.Corren J., Shapiro G., Reimann J. Allergen skin tests and free IgE levels during reduction and cessation of omalizumab therapy. J Allergy Clin Immunol. 2008;121(2):506–511. doi: 10.1016/j.jaci.2007.11.026. [DOI] [PubMed] [Google Scholar]
- 227.Bernstein I.L., Li J.T., Bernstein D.I. Allergy diagnostic testing: an updated practice parameter. Ann Allergy Asthma Immunol. 2008;100(3 Suppl 3):S1–S148. doi: 10.1016/s1081-1206(10)60305-5. [DOI] [PubMed] [Google Scholar]
- 228.WK D. Saunders Company; Philadelphia, Pensylvania: 2001. Immunology and Allergy Clinics of North America. [Google Scholar]
- 229.Demoly P.P.V., Bousquet J. sixth ed. Mosby; New York: 2003. In Vivo Methods for Study of Allergy: Skin Tests, Techniques and Interpretation. [Google Scholar]
- 230.Torres M.J., Romano A., Celik G. Approach to the diagnosis of drug hypersensitivity reactions: similarities and differences between Europe and North America. Clin Transl Allergy. 2017;7:7. doi: 10.1186/s13601-017-0144-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Brockow K., Romano A. Skin tests in the diagnosis of drug hypersensitivity reactions. Curr Pharmaceut Des. 2008;14(27):2778–2791. doi: 10.2174/138161208786369821. [DOI] [PubMed] [Google Scholar]
- 232.Ebo D.G., Leysen J., Mayorga C., Rozieres A., Knol E.F., Terreehorst I. The in vitro diagnosis of drug allergy: status and perspectives. Allergy. 2011;66(10):1275–1286. doi: 10.1111/j.1398-9995.2011.02661.x. Epub 2011 Jun 7. [DOI] [PubMed] [Google Scholar]
- 233.Burks A.W., Williams L.W., Casteel H.B., Fiedorek S.C., Connaughton C.A. Antibody response to milk proteins in patients with milk-protein intolerance documented by challenge. J Allergy Clin Immunol. 1990;85(5):921–927. doi: 10.1016/0091-6749(90)90078-i. [DOI] [PubMed] [Google Scholar]
- 234.Firer M.A., Hoskings C.S., Hill D.J. Humoral immune response to cow's milk in children with cow's milk allergy. Relationship to the time of clinical response to cow's milk challenge. Int Arch Allergy Appl Immunol. 1987;84(2):173–177. doi: 10.1159/000234419. [DOI] [PubMed] [Google Scholar]
- 235.Antico A., Pagani M., Vescovi P.P., Bonadonna P., Senna G. Food-specific IgG4 lack diagnostic value in adult patients with chronic urticaria and other suspected allergy skin symptoms. Int Arch Allergy Immunol. 2011;155(1):52–56. doi: 10.1159/000318736. [DOI] [PubMed] [Google Scholar]
- 236.Chambers V.V., Glaser J. The incidence of subsequent ragweed pollinosis in symptom-free persons having positive reactions to ragweed pollen extract. J Allergy. 1958;29(3):249–257. doi: 10.1016/0021-8707(58)90009-1. [DOI] [PubMed] [Google Scholar]
- 237.Lehman C.W. The leukocytic food allergy test: a study of its reliability and reproducibility. Effect of diet and sublingual food drops on this test. Ann Allergy. 1980;45(3):150–158. [PubMed] [Google Scholar]
- 238.Sethi T.J., Lessof M.H., Kemeny D.M., Lambourn E., Tobin S., Bradley A. How reliable are commercial allergy tests? Lancet. 1987;1(8524):92–94. doi: 10.1016/s0140-6736(87)91922-2. [DOI] [PubMed] [Google Scholar]
- 239.Barrett S. Commercial hair analysis. Science or scam? J Am Med Assoc. 1985;254(8):1041–1045. [PubMed] [Google Scholar]
- 240.Ernst E. Iridology: a systematic review. Forschende Komplementärmed. 1999;6(1):7–9. doi: 10.1159/000021201. [DOI] [PubMed] [Google Scholar]
- 241.Garrow J.S. Kinesiology and food allergy. Br Med J. 1988;296(6636):1573–1574. doi: 10.1136/bmj.296.6636.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Ludtke R., Kunz B., Seeber N., Ring J. Test-retest-reliability and validity of the Kinesiology muscle test. Complement Ther Med. 2001;9(3):141–145. doi: 10.1054/ctim.2001.0455. [DOI] [PubMed] [Google Scholar]
- 243.Lewith G.T., Kenyon J.N., Broomfield J., Prescott P., Goddard J., Holgate S.T. Is electrodermal testing as effective as skin prick tests for diagnosing allergies? A double blind, randomised block design study. BMJ. 2001;322(7279):131–134. doi: 10.1136/bmj.322.7279.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Semizzi M., Senna G., Crivellaro M. A double-blind, placebo-controlled study on the diagnostic accuracy of an electrodermal test in allergic subjects. Clin Exp Allergy. 2002;32(6):928–932. doi: 10.1046/j.1365-2222.2002.01398.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.