Abstract
The human liver is an organ with a diverse array of immunologic functions. Its unique anatomic position that leads to it receiving all the mesenteric venous blood, combined with its unique micro anatomy, allows it to serve as a sentinel for the body’s immune system. Hepatocytes, biliary epithelial cells, Kupffer cells, stellate cells, and liver sinusoidal endothelial cells express key molecules that recruit and activate innate and adaptive immunity. Additionally, a diverse array of lymphoid and myeloid immune cells resides within and traffic to the liver in specific circumstances. Derangement of these trafficking mechanisms underlie the pathophysiology of autoimmune liver diseases, NASH, and liver transplantation. Here, we review these pathways and interactions along with potential targets that have been identified to be exploited for therapeutic purposes.
Keywords: Liver immunology, chemotaxis, immunobiology
INTRODUCTION
The liver is responsible for numerous important tasks that support and impact all organ systems. It is essential for the metabolism of carbohydrates, lipids, amino acids, and vitamins as well as the storage of nutrients. The liver also plays a key role in digestion, producing bile that allows for absorption of lipids. Additionally, it is responsible for the breakdown and clearance of numerous toxic substances and drugs. Early in fetal development, the liver is also responsible for hematopoiesis. Although not often thought of as such, the liver is a unique and complex immunologic organ as well. The liver houses a diverse population of immune cells despite not being considered a lymphoid organ and is responsible for the production of acute phase proteins important for immune responses.1,2 Here, we will review the unique aspects of the liver and its array of resident immune cells and functions, as well as the specialized mechanisms that have developed in order to direct immune cells to the liver in both normal and pathologic conditions.
ANATOMY OF LIVER AS AN IMMUNOLOGIC ORGAN
Gross Blood Supply
The liver receives 80% of its blood supply from mesenteric venous circulation and 20% from the systemic arterial circulation (Figure 1). This not only supplies the liver with nutrients from the gastrointestinal (GI) tract, but also signaling molecules, intact cells and microorganisms from both intestinal and systemic circulation that facilitate its metabolic, detoxification, and immunologic functions. Potentially pathogenic and malignant cells are carried to the liver via mesenteric circulation, while systemic antigens are brought to the liver via arterial circulation. With this constant inundation of pro-inflammatory antigens, the liver has developed mechanisms to remain in a homeostatic state and to allow for pro-inflammatory response only when appropriate.3,4 For example, animal models have shown that antigens are better tolerated when introduced via the portal vein versus systemic circulation, proving the liver’s protective role from over-inflammation.5
Figure 1:
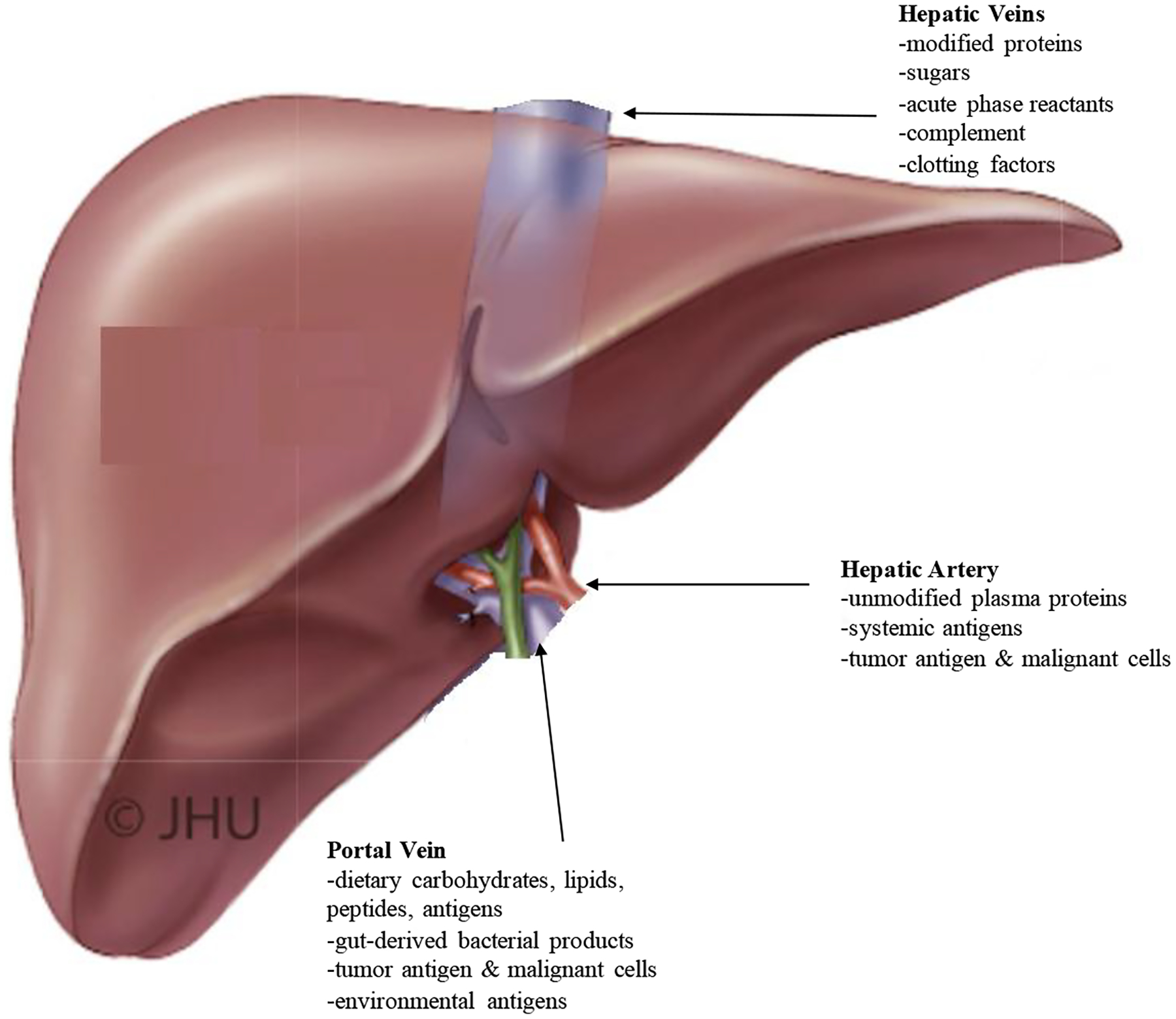
Blood Circulation to the Liver: The possesses a unique dual circulation, receiving blood both from the systemic arterial system via the hepatic artery and from the mesenteric system via the portal vein. This arrangement allows the liver to monitor and process substrates from both areas of the body and to release appropriate products systemically. Adapted with permission from Current Surgical Therapy, 12th Edition p. 393.
Microcirculation
Both the arterial and portal circulation terminate into the same thin, porous network of specialized capillaries made up of liver sinusoidal endothelial cells (LSECs).6 The liver sinusoids lack a basement membrane and instead have a subendothelial compartment, called the space of Disse, where lymph collects into lymphatics.7 Blood drains through the fenestrations within the sinusoids, passing through the space of Disse and to hepatic parenchymal cells (Figure 2). Blood flow is very slow within the sinusoids allowing for longer exposure of antigens within the sinusoids.8 This network of slow-flowing capillaries facilitates the recognition and processing of antigens by the many immune as well as non-immune cells within the liver.
Figure 2:
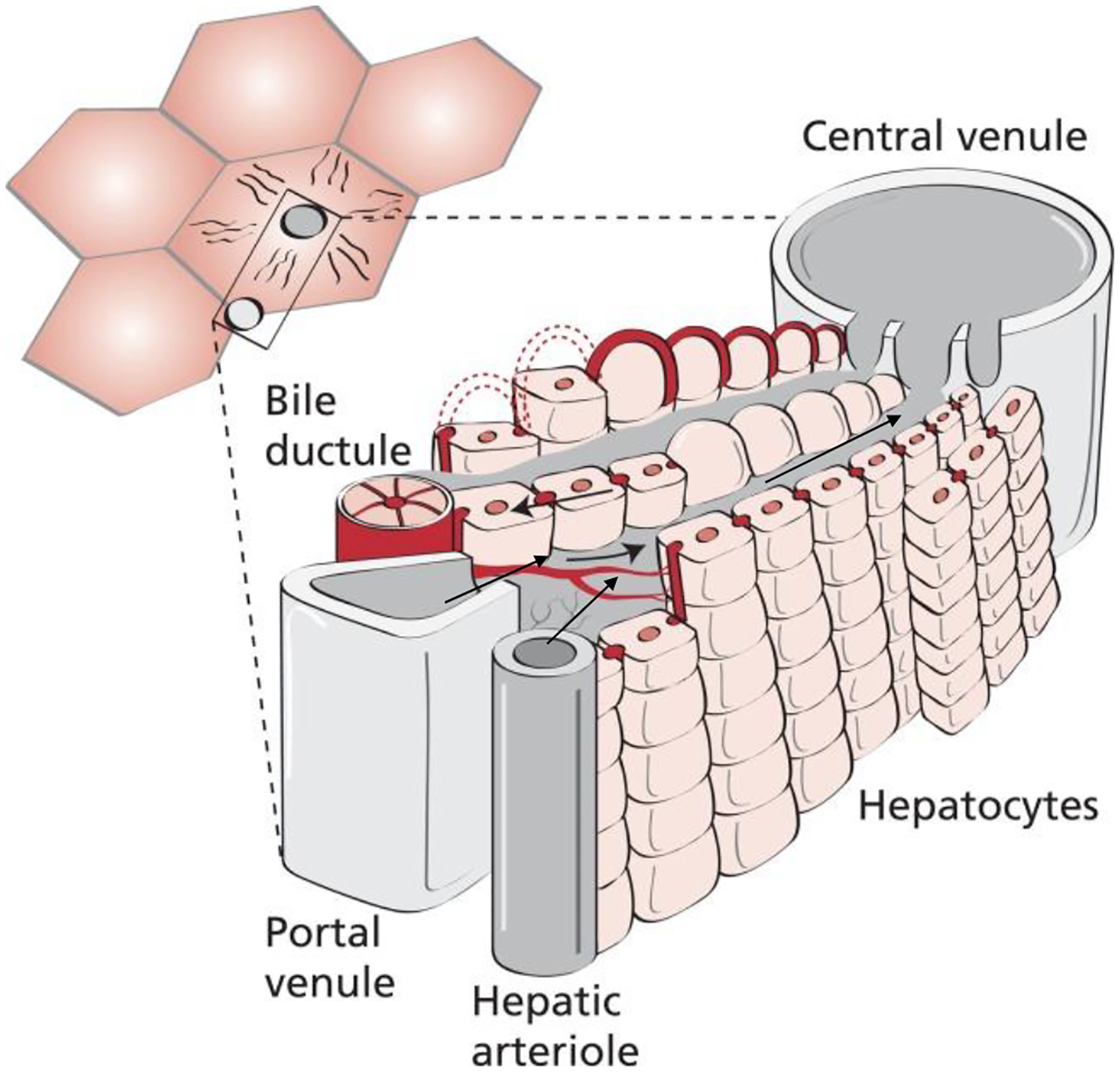
Microcirculation of hepatic lobule. Terminal branches of the hepatic artery and portal vein both drain into liver sinusoids where blood is then carried to the central vein, a branch of the hepatic vein. Multiple sets of hepatic artery and portal vein branches drain into a single central vein. Adapted with permission from Juza et al. Clin Anat 2014;27(5):764–769.256
IMMUNE FUNCTION OF LIVER CELLS
Hepatocytes comprise approximately 80% of the cells within the liver (Figure 3). They are the main drivers of the liver’s metabolic functions and are responsible for protein synthesis, carbohydrate storage and transformation, synthesis of bile and lipids, detoxification and processing of drugs. Although they are not immune cells, hepatocytes express innate immune receptors and can serve as antigen-presenting cells (APCs).9 They constantly express intercellular adhesion molecules and can be induced to express moderate levels of human leukocyte antigen (HLA) class I molecules.10 With high doses of interferon gamma (IFNγ), such as in an inflammatory state, hepatocytes express HLA class II molecules in vitro.11 They can prime naïve CD3+CD8+ T cells and numerous in vivo experiments have confirmed hepatocytes’ ability to serve as APCs.11–13
Figure 3.
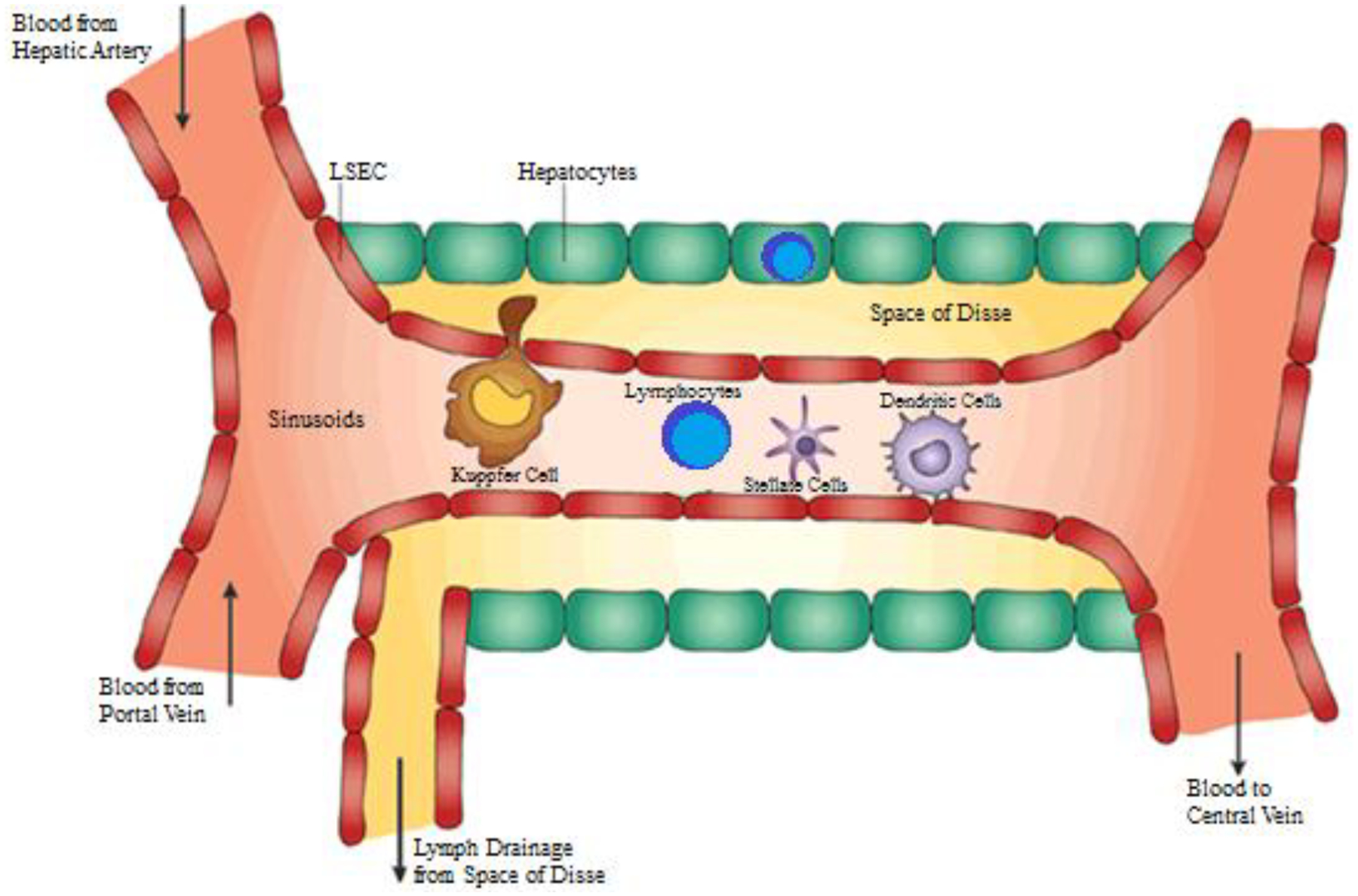
Resident Immune Cells within Liver. The liver is home to cells with a diversity of immunologic functions. Antigens from systemic and portal circulation are carried into the sinusoids where they are met by resident KCs, lymphocytes, dendritic cells, and HSCs. LSECs line the sinusoids and can also present antigens to activate the immune system. Within the sinusoids are fenestrations where antigens can extrude into the Space of Disse and also through which hepatocytes can sample antigens within sinusoidal lumen. Lymphocytes also reside within the parenchyma amongst hepatocytes. Adapted from with permission from Crispe, Nat Rev Immunol, 2003;3(1):51–62.
LSECs also play a unique role in physiological tolerance and hepatic immune reactivity. At rest, human LSECs express intercellular adhesion molecule-1 (ICAM-1) at a detectable level; however stimulation by tumor necrosis factor alpha (TNFα) and IFNγ induces increased expression of ICAM-1 as well high levels of major histocompatibility complex (MHC) Class II, CD40, and vascular cell adhesion molecule-1 (VCAM-1) that are previously unexpressed at rest, priming LSECs to interact with immune cells.14 Additionally, they have the ability to express other molecules necessary for antigen presentation including CD11b, CD11c, CD40, CD80, and CD86 in mice.15–24 Antigen presentation by LSECs normally leads to an anti-inflammatory, homeostatic environment. In mice, primed LSECs induce tolerant antigen-specific CD8+ T cells that is conserved following adoptive transfer from ovalbumin-fed to unfed mice.25,26 Endotoxin in liver downregulates expression of MHC class II, CD80 and CD86, but induces IL-10 secretion which suppresses murine LSECs’ antigen-presenting ability. LSECs also fail to induce activation of naïve CD4+ T cells in the presence of lipopolysaccharide (LPS).27–29 Data also suggest that Fas/Fas ligand and programmed death (PD)-1 ligation pathways are important and that LSECs can suppress dendritic cell (DC) activation of naïve CD8+ T cells through direct contact, but the underlying mechanism remains unclear.16,30,31 These interactions leads a relative resistance of the normal inflammatory activation by LPS seen in other tissues and a net regulatory effect by LSECs. However, in the setting of chronic liver diseases, LSECs become pro-inflammatory and no longer promote homeostatic conditions. In mice, after fibrotic injury caused by hepatotoxins, antigen presentation by LSECs induces IFNγ, IL-6, and TNFα secretion and an immunogenic T cell phenotype.28 Additionally in a murine model of hepatitis, infection of mouse hepatitis virus 3 and attenuated variants led to LSECs to release more pro-inflammatory factors and less IL-10 through TLR2 dependent pathways.32 Although the precise role LSECs play in infection remains unclear, they certainly are important mediators of continuing liver injury in settings of chronic liver disease.
The liver is also an important reservoir of macrophages, with 80–90% of the body’s total macrophages consisting of Kupffer cells (KCs) that reside within the hepatic sinusoids.33 Unlike other macrophages, KCs express a unique complement receptor that binds C3b, allowing them to catch bacteria under flow and shear conditions.34–36 However, these pathogens are only captured and held in place to be killed by neutrophils and other immune cells.37 Although they express the necessary markers to activate T cells, continuous exposure to LPS reduces KCs ability to activate lymphocytes.15,28,38 They can become potent activators of T cells in the presence of other pathogen-associated molecules or inflammatory cytokines.39 Additionally, they capture and clear activated neutrophils and depending on which receptor complex activates them, KCs can produce either pro- or anti-inflammatory cytokines in order to regulate inflammation and protect from collateral damage.40,41
DCs are also localized throughout the parenchyma but are mostly concentrated around the central vein where they lie in wait rather than patrol within tissues.42 Unlike their counterparts in other tissues, and consistent with the high-LPS environment in which they reside, hepatic DCs require much higher levels of LPS in order to activate T cells. Under basal conditions, they have an immature phenotype lacking costimulatory molecules necessary for T cell activation.43 The cytokine milieu within the liver, where IL-10 is high and IL-12 low, contributes to relative tolerance by promoting a shift from helper T cell (Th)1 to Th2 responses and the development of regulatory T cells (Tregs). However, DCs also have a greater capacity for phagocytosis and production of cytokines.44,45 The potential for robust activation of T cells resides within all hepatic DCs and is realized with blockage of IL-10 or activation by pathogen-associated molecules that leads to increased expression of co-stimulatory molecules.46 In fact, such stress is required for DCs to stimulate liver regeneration, as this is impaired in mice that are germ-free and resistant to LPS.47
Stellate cells (HSCs), also known as Ito cells, also play an immunologic role. Residing within space of Disse, under normal conditions they have a central role in vitamin A and lipid storage. Like other resident cells in the liver, HSCs express the prerequisite molecules for antigen presentation, but at insignificant levels under basal conditions.48–50 Although they have the ability to endocytose exogenous antigens, the mechanism for this remains unknown. Under inflammatory conditions, stellate cells differentiate into myofibroblasts that lead to liver fibrosis in chronic liver disease. There is some evidence that their ability to present antigens and directly activate T, natural killer (NK), and natural killer T (NKT) cells also is enhanced in such disease states.51
Cholangiocytes – the epithelial cells of the bile ducts – are primarily involved with secretion of bile from the liver. These cells are targeted in cholangiopathies, such as primary sclerosing cholangitis (PSC) and primary biliary cirrhosis (PBC). These cells also have secondary roles in liver immunity. Being contiguous with the intestinal epithelium, they share similar mucosal immune functions, such as secretion of IgA.52 In vitro and in vivo studies have revealed that human cholangiocytes express ICAM-1, VCAM-1, lymphocyte function associated antigen (LFA)-3, HLA-I and HLA-II.53–56 They also possess the necessary co-stimulation molecules necessary for antigen presentation, albeit at very low levels.57 Additionally, cholangiocytes participate in recruitment of immune cells via cytokine and endotoxin induced expression of CXCL8, CX3CL1, CXCL12, and CXCL16.58–61
The liver is rich in lymphocytes with about 1010 cells in an average liver. They reside within the portal tracts, sinusoids, as well as throughout the parenchyma.62–65 The vast majority of lymphocytes are CD3+CD56− T cells, CD3−CD56+ NK cells, and CD3+CD56+ NKT cells. Only approximately 5% of lymphocytes are B cells. Although the same populations are present in the peripheral circulation, the liver-resident lymphocytes vastly differ in the proportions of the different sub-types. Conventional αβ T cells comprise about 80% of CD3+ lymphocytes, with γδ cells comprising the remainder. This is in contrast to the periphery, where the proportion of γδ cells is 5-times lower.66,67 The role of γδ cells in liver immune homeostasis remains unclear, however there is evidence that it is mediated via IL-17A pathways.68 The population of conventional T cells is also enriched in CD8 cells, with a reversal of the normal 2:1 CD4:CD8 ratio seen in the periphery. Most CD8 T cells have an activated phenotype, expressing CD25 and CD69.69 NK and NKT cells in the liver make up a much larger proportion of lymphocytes when compared to the periphery. NK cells comprise one-third to one-half of hepatic lymphoid cells, three times greater than in periphery.70 They release cytotoxic granules as well as large amounts of cytokines, especially IFNγ, to direct immune responses.71 NKT cells produce cytokines to promote either inflammatory or anti-inflammatory responses and they are also the only immune cells that actively patrol the sinusoids, seeking out antigen.72,73
TRAFFICKING OF IMMUNE CELLS TO THE LIVER
Pattern Recognition Receptors
Trafficking to the liver begins with the recognition of antigens by one of the many types of immune cells described above. Hepatocytes, LSECs, HSCs, KCs, and lymphocytes express pattern recognition receptors (PRR) that recognize and bind microbial-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) that are abundant on the immunogenic molecules the liver is exposed to.74–76 It is the recognition of PAMPs and DAMPs that is the basis for targeted responses of the immune system. A specialized group of PRRs called the Toll-like receptors (TLRs) are the best characterized group. This family of PRRs recognize many different pathogenic molecules including LPS, bacterial flagella, and both RNA and DNA derived from bacteria and viruses. Depending on the type of TLR involved, binding can lead to activation, cytokine production and release, and modulation of many other cellular functions.9,77 Unlike elsewhere in the body, binding of TLRs in the liver usually promotes immunosuppressive effects to prevent over-inflammation in response to bacterial and dietary antigens the liver is exposed to regularly, especially for LPS via the TLR4 pathway. However, TLR-mediated immune regulation can be overcome by stimulation via other TLR-subtypes by non-LPS molecules such as flagella (via TLR5), viral double-stranded RNA (via TLR3), and single-stranded RNA (via TLR7 and TLR8).39,78–80
LSECs have abundant expression of both scavenger and carbohydrate receptors. Scavenger receptors recognize targets via glycosylation patterns, peptide motifs, and lipid moieties. These ligands can be internalized via endocytosis and processed to be presented to immune cells.81,82 These receptors are involved in the recognition of Mycobacterium tuberculosis and serve as an entry point for hepatitis C virus (HCV).83–85 LSECs also highly express carbohydrate receptors that recognize specific sugar moieties such as N-acetylglucosamine and mannose which are present on both self and non-self molecules.86 These receptors mediate internalization of Candida, M. tuberculosis, and other pathogens.87,88
Hepatic immune cells are capable of humoral immune system interactions through the expression of receptors for immunoglobulin (FcRs) and for complement. Both KCs and LSECs express FcRs that, upon binding of its ligand, facilitate phagocytosis of the target and modulation of cellular function depending on the type of FcR bound. KCs preferentially bind larger immune complexes while LSECs bind smaller complexes via FcRs.89–92 Many different complement receptors are expressed by KCs, including a special class of receptor localized only to the liver (and possibly splenic macrophages) called complement receptor of the immunoglobulin superfamily (CRIg).35 This receptor mediates the capture and clearance of C3b coated bacterial and viral targets allowing KCs to bind them under shear conditions and contributing to the sentinel role they play in the immune system.36,93
Adhesion Molecules
For leukocytes to act on invasive pathogens, migration must first occur from blood vessels into target tissues. Initial interactions occur between leukocytes and endothelial cells via a rolling interaction. In most organs, this takes place in post-capillary venules and is mediated by selectins, a family of adhesion proteins found on leukocytes (L-selectin), endothelial (E-selectin) cells, as well as platelets (P-selectin).94 These adhesion molecules are constitutively expressed and bind glycans on leukocytes to mediate tethering and rolling in organs.95 These are relatively weak interactions that are followed by tighter interactions mediated by integrins on immune cells and their ligands on endothelial cells.96 Unlike selectins, integrin expression requires activation and their affinity for their ligands can be modified by chemokine stimuli.96 Once leukocyte integrins bind to their respective endothelial cells, they then transmigrate from blood vessels and into tissue in most organs.
However, this model of leukocyte extravasation does not apply in the liver. Adhesion occurs within the sinusoids and actually at a much higher proportion than in post-capillary venules. These interactions occur without any notable selectin-mediated rolling.95,97 Additionally, leukocytes do not require transmigration to interact with liver immune cells. Extensions of hepatocytes and KCs protrude through sinusoid fenestrations and directly interact with leukocytes within the lumen.98 Therefore, the interactions between integrins on immune cells and their ligands on different liver cells are the most important mediators of immune interactions and are mediated chiefly by two families of integrins: α4 and β2.
The α4 integrin group includes α4β1 and α4β7, molecules that are expressed on lymphocytes and monocytes (Figure 4)99. Their ligands on endothelial cells are VCAM-1 and mucosal addressin cell adhesion molecule 1 (MadCAM-1), respectively.99 VCAM-1 is inducible in all tissues; however the level of expression in liver under homeostatic conditions is comparable to that seen in other tissues during inflammatory conditions.100 MadCAM-1 expression is normally expressed on intestinal endothelium, but can also be expressed by LSECs mediating liver inflammation in PSC.101 β2 integrins are a family of adhesion molecules expressed on all types of leukocytes and mediates their firm adhesion in many tissues.95,102,103 The most important ligand for β2 integrins is ICAM-1. In tissues outside the liver, ICAM-1 is only highly expressed in post-capillary venules; basally LSECS express ICAM-1 at similar levels to hepatic post-capillary venule, i.e. central vein.104 Interactions between VCAM-1 on LSECS and α4β1 integrin on activated CD8 T cells mediate non-specific adhesion which in turn adhere via interactions between β2 and ICAM-1 if recognition of antigens presented by hepatocytes or LSECs occurs.100
Figure 4.
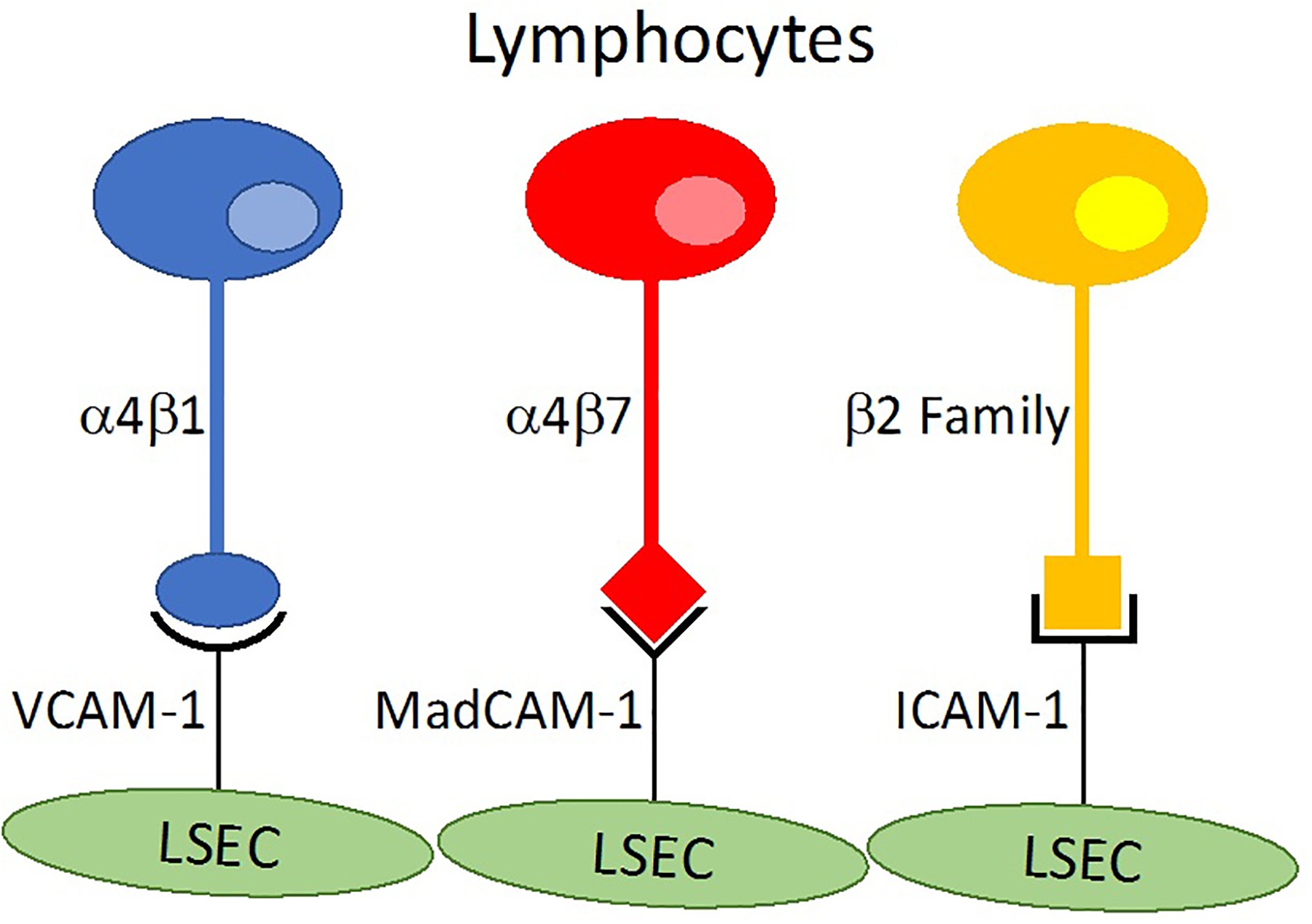
A schematic representation of adhesion molecules within liver sinusoids. LSECs express a number of adhesion molecules, including VCAM-1, MadCAM-1, and ICAM-1 that bind to the integrins α4β1, α4β7, and the β2 family. VCAM-1 is expressed at levels within sinusoids that other tissues only express under inflammatory conditions. ICAM-1 is also expressed by LSECs at levels that are normally seen in post-capillary venules. MadCAM-1 is normally only expressed in the gut, directing immune cells to the intestine; however, this becomes an important mediator in PSC when aberrantly expressed by LSECs. ICAM-1 is expressed on the basal membrane, whereas VCAM-1 and MadCAM-1 are expressed on the luminal membrane.
Other adhesion molecules also play a role in liver immune cell trafficking. Vascular adhesion protein-1 (VAP-1) is a glycoprotein expressed by LSECs that is particularly important in the liver. It promotes shear-dependent adhesion and transmigration across hepatic sinusoids and mediates activation of LSECs to upregulate other molecules that promote immune cell recruitment.105–109 Under homeostatic conditions, little VAP-1 is expressed but expression increases significantly in the setting of inflammation.110 However, the adhesion molecule on leukocytes that serves as its ligand remains to be identified. CD44 is another glycoprotein expressed by leukocytes that plays an important role in trafficking immune cells to the liver. It interacts with hyaluronan that is expressed on many different cell types, including LSECs. More recently, common lymphatic endothelial and vascular endothelial receptor (CLEVER-1) has been described and found to support adhesion and migration of human lymphocytes on lymphatic vessels and endothelial venules.111 Inhibition of CLEVER-1 reduces Treg migration into hepatic sinusoids by 40% and by >80% when ICAM-1 and VAP-1 were also inhibited.112
Chemokine Signals and Receptors
Chemokines are polypeptides that are secreted in response to both pro- and anti-inflammatory stimuli and bind cell-surface receptors that direct chemotaxis. They include four conserved cysteine residues that form two disulfide bonds pairing the first with the third, and the second with the fourth cysteine residues. They are grouped into four categories based on the arrangement of the N-terminal 2 cysteine residue. They are CXC, where one amino acid separates the 1st two cysteines; CC, where the two are adjacent to each other; CX3C, where 3 amino acids are between the two residues; and (X)C where the first and third cysteines are missing.113 Lymphocytes in the periphery express many of these receptors in homeostatic conditions.114–120 In normal and pathologic conditions of the liver, there are many chemokine-chemokine receptor interactions that mediate pro- and anti-inflammatory responses. The chemokine receptors CXCR3, CXCR6, CCR5, CCR2 and CCR1 on liver infiltrating effector T cells are the main mediators of recruitment (Table 1).121–124
Table 1.
Chemokine Receptors and Ligands in Immune Cell Trafficking
| Receptor | Chemokine | Distribution of Interactions | Trafficking in Liver |
|---|---|---|---|
| CC subgroup | |||
| CCR1 | CCL3, CCL5, CCL7, CCL8, CCL13–16 | monocyte, effector & memory T cell recruitment | |
| CCR2 | CCL2, CCL7, CCL8, CCL13 | monocyte, effector & memory T cells, Th1 recruitment | |
| CCR3 | CCL5, CCL7, CCL11, CCL15–16, CCL24, CCL26 | Th2 recruitment | |
| CCR4 | CCL17, CCL22 | Th17, Th2, Treg recruitment and retention | |
| CCR5 | CCL3, CCL4, CCL5, CCL8 | Th1, monocyte recruitment | portal veins and venules |
| CCR6 | CCL20 | all subtypes of T cells, B cell recruitment | malignancy |
| CCR7 | CCL19, CCL21 | recruitment to secondary lymphoid tissue | periportal lymph nodes |
| CCR8 | CCL1 | Th2, monocyte recruitment | |
| CCR9 | CCL25 | recruitment to GI tract | sinusoids in PSC |
| CCR10 | CCL25, CCL28 | recruitment to GI tract | bile ducts |
| CXC subgroup | |||
| CXCR1 | CXCL6, CXCL7, CXCL8 | neutrophil, monocyte recruitment | |
| CXCR2 | CXCL1–3, CXCL5–8 | neutrophil, monocyte recruitment | |
| CXCR3 | CXCL9–11 | Th1, Th17, Treg recruitment | sinusoids in non-specific liver inflammation |
| CXCR4 | CXCL12 | B cell recruitment | sinusoids and bile ducts; HCC |
| CXCR5 | CXCL13 | B cell recruitment | |
| CXCR6 | CXCL16 | NK and T cell recruitment | sinusoids and biliary epithelium |
| CX3CR1 | CX3CL1 | monocyte and NK cell recruitment | biliary epithelium in PBC |
CXCR3 and its ligands CXCL9, CXCL10, and CXCL11 are closely linked to pro-inflammatory Th1 responses. Effector CD4+ and CD8+ T cells within liver express high levels of CXCR3.125–127 Under inflammatory conditions, KCs and infiltrating innate immune cells release TNFα and IFNγ, which promote expression of ligands for CXCR3 by hepatocytes, HSCs, LSECs, and damaged or inflamed bile ducts.128 CD154+ immune cells that infiltrate the liver in states of inflammation trigger secretion of CXCR3 ligands by interaction with CD40 on liver cells.129,130 Blockade of CXCL9 and CXCL10 in mice reduces recruitment of host-derived mononuclear cells, especially those expressing CXCR3.131 Although these molecules are important mediators for adhesion and transmigration of lymphocytes across LSECs, blockade of individual molecules only partially reduced migration of lymphocytes, indicating the presence of redundant mechanisms.132,133 Tregs also use CXCR3-mediated processes to migrate into human liver tissue, indicating that its overall immune impact involves fine-tuning the balance of effector and regulatory cells.134
CXCR6 is expressed on Th1 and effector T cells in peripheral blood in homeostatic conditions, but much higher expression is found on CD4 and CD8 T cells that have infiltrated the liver. It interacts with CXCL16, regulating recruitment of activated T cells to inflamed liver in humans and mice.61 Inflamed bile ducts, hepatocytes, and LSECs in mice highly express CXCL16 allowing them to interact with CXCR6+ inflammatory cells as well as promoting β1 integrin dependent adhesion.135 In particular, CXCR6 is required for NK and NKT cell homing to the liver.73 In HCV, there is a specific subset of CXCR6+CD8+ T cells that express CD161, a C-type lectin involved in NK cell function and production of IFNγ and IL-17.136 In a mouse model of Graft vs Host Disease (GVHD), knock out of CXCR6 significantly reduces the accumulation of activated donor-derived CD8 T cells in recipient liver without changing the frequencies of CD8 T cells in peripheral circulation.137
CCR5, CCR2 and CCR1 have all been shown to interact with common ligands and promote recruitment of leukocytes to the liver. CCR5 interacts with CCL3, CCL4, CCL5, and CCL8; CCR2 interacts with CCL2, CC7, CCL8, and CCL13; while CCR1 interacts with CCL3, CCL5, CCL7, CCL14–16, and CCL23. Positivity for all three receptors is characteristic of memory T cells.138,139 CD8 T cells in inflamed human livers are enriched with CCR2 and CCR5, while CCR1 has been shown to regulate hepatic inflammation in mouse models.121,123 In portal endothelium, CCR5 is highly expressed and in mouse models of GvHD, leads to recruitment of effector cells to the portal tracts.140–142 However, in CCR5 knockout mice, liver inflammation is much more extensive and mediated by CCR1+ effectors, indicating that CCR5 may also recruit anti-inflammatory cells.143 However, in humans, blockade of CCR5 reduces liver inflammation and injury in GvHD.144
CCR6 is also an important signal responder in leukocyte recruitment to the liver. Although deficiency in this receptor leads to an increase in the recruitment of CD4+ T cells, it leads to a reduction of IL-17+ cells in liver injury in mice.145 Interactions with its ligand CCL20 on small intestine and inflamed bile ducts redirects Th17 cells from the periphery to these injured areas.146 In addition to mediating recruitment of inflammatory cells, CCR6 is also responsible for inducing migration of γδ T cells in chronic injury that inhibit HSC-mediated fibrosis and dampen excessive inflammation.147
The CX3CR1-CXC3L1 chemokine axis is another important signaling pathway that is essential for recruitment of immune cells to the liver. CX3CL1, also known as fractalkine, is expressed by hepatocytes, HSCs, BECs and epithelial cells of the liver.59,148 It can act as a free ligand in serum and promotes migration of immune cells, in particular monocytes.149,150 In chronic hepatitis C, liver injury, and injured BECs, CX3CL1 expression is increased in intrahepatic cells.60,151–155 This chemokine axis is responsible for the recruitment and accumulation of NK cells, which express CX3CR1, around injured bile ducts. In addition to pro-inflammatory effects, CX3CL1-CX3CR1 interactions mediate effects that protect the liver by preventing hepatocyte apoptosis, fibrosis, and activation of HSCs.150,156,157
Treg recruitment to the liver is mediated in large part by chemokines, many of which overlap with their effector counterparts allowing co-localization to sites of inflammation.158 The explanted chronically inflamed livers in human transplantation contain Tregs that express CXCR3 at levels that are higher than those found in peripheral blood. Through CXCR3 and α4β1, these Tregs are able to bind and transmigrate through sinusoids under flow conditions. Additionally, Tregs that are derived from the liver express a tissue infiltrating phenotype with high levels of CXCR3, but low levels of CCR7. DCs in chronically inflamed livers also recruit Tregs through expression CCL17 and CCL22, two ligands of CCR4.119 Once through the sinusoids, Tregs localize to bile ducts via interactions between CCR10 and CCL28 expressed on cholangiocytes. In HCV, expression of CCL28 is increased leading to increased infiltration of all subtypes of CCR10+ T cells, but with a predominance of Tregs.134
Other chemokine receptors expressed by naïve lymphocytes signal them to leave the liver, thus the loss of expression can lead to localization in infiltrated tissues. In particular, CCR7 expression on T cells allows for circulation out of liver through peripheral lymph nodes and secondary lymphoid tissue. CCR7 mediates this signaling by interacting with CCL19 and CCL21 as well as through L-selectin.159 However, CCR7 expression in non-naïve lymphocytes has been observed in certain disease states. Many T cells in autoimmune and HCV hepatitis are CCR7+ and have the ability to migrate through the periphery, but are also CD62L-low and LFA-1 high, a phenotype that is characteristic of memory T cells.159,160 These central memory cells are in contrast to effector memory T cells that do not express CCR7 and are therefore only localized to tissue.
Chemokine interactions also play an important role in recruiting non-lymphoid immune cells to the liver. CXCR2 expression directs neutrophils to sites of inflammation. When induced by LPS, neutrophils will migrate to the sinusoids and into tissue via CD44-hyalarunon interactions.161 Diapedesis does not occur in post-sinusoidal epithelium due to the absence of the hyalarunon ligand.162 Monocytes are recruited to inflamed livers by CX3CR1 and VAP-1.149 CCR2 is an inducible chemokine receptor found on monocytes not expressed under homeostatic conditions that direct these cells to sites of inflammation.163,164 Once directed to the liver, CCR2 expression keeps monocytes in hepatic tissue.165 There is evidence that these monocytes differentiate into DCs that express CXCR1 and have the ability to produce TNFα, inducible nitric oxide synthase (iNOS), and IL-10 to mediate both pro- and anti-inflammatory processes166,167 CCR8 has been implicated in liver inflammation and fibrosis; inhibition of it or its ligand CCL1 blocks differentiation of hepatic DCs and T cells protecting against injury.168
Gut-Liver Immune Axis
The liver and is bombarded with environmental, dietary, and bacterial antigens in the portal circulation. Intestinal and liver immunity are thus closely intertwined. First-line defense against pathogenic antigens is the gut mucosa that is coated with IgA and other anti-microbial substances.169 Intestinal mucosa is also rich in lymphoid tissue from Peyer’s patches and mucosal associated lymphatic tissue, which are rich in T cells, innate lymphoid cells, and gut associated dendritic cells.52 LSECs, in turn, can imprint naïve lymphocytes with a gut-homing phenotype.170 Mucosal memory T cells also preferentially recirculate through the liver and are not dependent on the expression of gut homing receptors, contributing to the liver’s function as the main sentinel of the GI tract for the immune system.171 The microbiome also contributes to gut immunity; alterations in microbiome homeostasis can lead to gut inflammation and in certain circumstances turn commensal organisms pathogenic.172
Intestinal immunity also uses chemokine signaling and adhesion molecules that are unique to the gut. CCL25 and α4β7 integrin secreted by small bowel epithelium interacts with CCR9 and MadCAM-1 to activate leukocytes, a mechanism that is confined to the intestine under normal conditions.173,174 Lymphocytes are imprinted with a gut-homing phenotype by CD103+ DCs within lymphoid tissue and IL-7 by retinoic acid-dependent mechanisms. Down-regulation of CCR7 and L-selectin combined with upregulation of CCR9 and α4β7 leads to the loss of the ability to re-enter peripheral lymphoid tissue.174 These DCs can also direct Tregs to the gut in the presence of TGF-β and IgA-producing B cells in the presence of IL-5 and IL-6. Tregs and B cells also interact with intestinal epithelium via CCR10-CCL28 interactions.175–177
Biliary epithelium is contiguous with intestinal mucosa and performs many of the same immune functions by similar mechanisms. Cholangiocytes normally express HLA class I molecules but not HLA class II and do not participate in antigen presentation. They are also an important source of IFNγ and TNFα.129,178 Bile ducts secrete IgA antibody and express similar sets of TLRs to intestinal epithelium.9 In response to cytokines and endotoxin, biliary epithelial cells (BECs) actively participate in leukocyte recruitment by upregulating CXCL8, CX3CL1, CXCL12, and CXCL16.58–61 These signals lead to upregulation of α4β1 to VCAM1 interactions. BECs can also upregulate CCL28 to recruit Tregs expressing CCR10 to the gut and liver.134 Increase in bile duct expression of CXCL12 and CXC3L1 recruits Th17 cells and upregulates secretion of pro-inflammatory cytokines.179–181
IMMUNE CELL TRAFFICKING IN LIVER DISEASE
As described earlier, in homeostatic conditions, the liver is in a relatively anti-inflammatory state. The various liver APCs (LSECs, Kuppfer cells, DCs) are resistant to activation by the various antigens, including LPS, that bombard the liver constantly and in fact promote a regulatory environment.182 This balance however, can be shifted to produce a physiologic immune response against pathogens. One such proposed mechanism is through the production of type 1 interferons (IFN-α/β). Viral infections of the liver promote synthesis of IFN-α/β by hepatocytes which in turn leads to recruitment of naïve T cells, increases production of IL-15, and promotes survival of CD8+ T cells.183–186 Moreover, innate lymphocytes (e.g. NK & NKT cells) are relatively abundant in the liver compared to other tissues of the body. They have the ability to both recognize many other non-protein antigens produced by microorganisms, infected cells, and tumors and activate physiological immunity within the liver.187
The interactions of the gut and liver immune systems plays an important role in the pathophysiology of PSC. PSC is characterized by massive T-cell mediated inflammation of the portal tracts and bile ducts, leading to biliary strictures and eventually liver failure152 There is a high incidence of PSC in patients with inflammatory bowel disease. Much of the T-cell recruitment is mediated by interactions between CCR9 on T cells and CCL25. Normally, expression of CCR9 is restricted to mucosal T cells in the intestine, while that of CCL25 is restricted to intestinal epithelium; this localization regulates recruitment of immune cells to the bowel.174,188,189 CCR9-CCL25 interactions upregulate expression of MadCAM-1 in gut vessels and in turn increase adhesion of leukocytes expressing α4β7 integrins. However, in PSC, there is aberrant expression of CCL25 and MadCAM-1 on LSECs. A large proportion of liver effector T cells in PSC express CCR9 and α4β7 leading to inflammatory interactions within the liver that are normally localized to the intestine.190,191
Aberrant immune cell trafficking also underlies the pathology of PBC and autoimmune hepatitis. Antimitochondrial antibodies are present in almost all patients with PBC, with the E2 component of pyruvate dehydrogenase complex being the main autoantigen.192–194 The large numbers of CD4+ T cells (both Tregs and Th17) that infiltrate portal tracts suggest cellular mechanisms play a large role in this disease’s pathophysiology.195 Studies of explanted livers from transplanted patients has shed light on the trafficking mechanisms at play. Chemokine receptors CXCR3 on LSECs and CCR4 ligands secreted by dendritic cells are involved recruiting T cells in autoimmune liver disease.119 CX3CL1 is also upregulated by injured bile ducts, recruiting CX3CR1+ CD4 and CD8 T cells to portal tracts.59 In advanced stages of autoimmune liver diseases, systemic levels of CXCL9 and CXCL10 are elevated but return to normal after successful treatment, suggesting that these signaling pathways are important mediators of pathologic inflmmation.196
Nonalcoholic fatty liver disease (NAFLD) is characterized by an aberrant increase in the accumulation of fat in the liver. Visceral fat, and particularly that in the liver, can produce an inflammatory response that can progress to non-alcoholic steatohepatitis (NASH).197 This is an increasing cause of cirrhosis and liver failure in the world. In individuals who develop NASH, excessive free-fatty acids induce expression of Cyr61/CTGF/NOV (CCN1), a member of a family of extracellular matrix-associated signaling proteins. Its overexpression in the liver through TLR4 pathways leads to recruitment of myeloid-derived macrophages and subsequent severe inflammation.198 Mouse models of NAFLD have revealed that both CCR2 and CD44 are also important mediators of leukocyte recruitment. Lack of CCR2 completely and CD44 partially reduces leukocyte recruitment, but this did not prevent the development of steatosis and inflammation, indicating there are redundant pathways of leukocyte recruitment produced by hepatic lipid accumulation.199 On the other hand, blockade of CCL2, the ligand for CCR2 and CCR4 in another mouse model of steatohepatitis leads to a reduction of macrophage infiltration and inflammation in chronic hepatic injury.200
Infection with HCV exploits lymphocyte recruitment mechanisms to cause chronic inflammation and injury. HCV infection promotes differentiation of lymphocytes into a Th1 profile.201,202 Increased CXCR3 and CCR5 levels are detectable in the periphery a few weeks after infection with a delayed infiltration of antigen-specific intrahepatic T cells detected 2–3 months later.203,204 This recruitment is in part mediated by the upregulation of CXCL11 in infected hepatocytes.204HCV also induces increased expression of CXCL16 on BECs and portal endothelium, attracting CXCR6+ lymphocytes that contribute to chronic infection and inflammation.61,120,205 The combination of serum CCL2 correlating with the severity of inflammation in HCV hepatitis and the enrichment of CCR2+ CD8 T cells in the inflamed liver suggests a role for the CCR2-CCL2 chemokine axis.206 Modulation of host responses occurs via manipulation of promoter genes, such as that for CXCL8.207 The impact HCV has on all these chemokine axes also leads to reduced effectiveness of the liver APCs to present antigen, allowing for viral survival.
Several chemokine axes have also been implicated in malignant conditions of the liver. In early studies of hepatocellular carcinoma (HCC), the CXCL12-CXCR4 axis had been identified as critical in the growth and progression of HCC.208 In biopsies from HCC patients, there is higher expression in tumor tissue than in surrounding, non-cancerous liver.209 There is also some evidence that the level of expression correlates to invasiveness, metastasis, and survival.210,211 In contrast, other studies have shown that expression of CXCL12 and CXCR4 in HCC tissue lacks an association with survival.212 CCL20-CCR6 is another pair of chemokines that are significantly upregulated in human HCC tumors.213 This axis promotes growth of the hepatoma cell line Huh7 in in vitro experiments, suggesting CCL20-CCR6 interactions importance in tumor growth.214 Additionally, CCL20 has been found to be overexpressed in colorectal liver metastatic lesions in humans, suggesting a role in spread of tumor.215 Finally, CX3CL1-CX3CR1 interactions have also been implicated as an important part of immune responses against HCC.216 Specifically CX3CL1 enhances anti-tumor effects of HCC in mice and high expression in serum of human patients is associated with a lower occurrence of metastasis.217 Expression of the CCR5, CCR6, and CXCR3 in peripheral lymphocytes was reduced but higher (particularly CXCR3) in tumor infiltrating cells of HCC patient, suggesting that these receptors are important in directing lymphocytes to malignancy in the liver.218 Although these interactions described play some role in growth progression of liver tumors, the specific mechanisms by which these interactions mediate their effect remains unclear.
In liver transplantation, changes in immune cell trafficking are important in both ischemia/reperfusion (I/R) injury as well as in rejection. I/R injury results in oxidative liver damage and systemic inflammation. The initial inciting event seems to be damage to LSECs during cold preservation.219 Once warm reperfusion takes place, KCs mediate early recruitment of leukocytes with later phases of injury mediated by neutrophil accumulation and CXC chemokine production.220–222 These chemokines also mediate systemic injury; for example, CXCL2 has been observed to be a key mediator in I/R injury to the lungs in a rodent model of liver transplant.223 DAMPS also serve as important mediators for immune cell trafficking in I/R injury. Formyl-peptide receptor 1, promotes neutrophil chemotaxis to transplanted liver grafts.224 Matrix metalloproteinase 9 is also important for leukocyte migration by degrading ECM to allow for movement; this also produces ECM fragments that are highly chemotactic for other immune cells.225,226 There are therefore multiple processes specific to reperfusion that mediate leukocyte trafficking and I/R injury in liver transplantation.
In the setting of graft dysfunction and liver rejection, immune cell trafficking has been well described. In the first week post-transplant higher serum levels of chemokines CCL2, CXCL8, CCL5, CXCL8, CXCL10 and IL-2R are associated with early allograft dysfunction. Pre-transplant, lower pre-operative IL-6 and higher IL-2R levels correlated with increased incidence and risk of early allograft dysfunction.227 Graft dysfunction due specifically to acute cellular rejection also correlates with high CXCL9 and in particular to low CD44 levels.228 On vascular and sinusoidal endothelium, CXCL9, CXCL10, and CXCL11 are highly upregulated in rejection, increasing interactions and recruitment of CXCR3+ B, NK, and CD4 T cells.229–231 Rodent models of liver rejection have revealed that interactions between VCAM-1 on LSECs and α4β1 integrin on effector T cells are critical to adhesion and transmigration across PV endothelial cells.232 CCL3 increases infiltration of recipient-derived NK and T cells.229,233 CCL20-CCR6 have been detected at much higher levels in portal fields with significant increases in B cells and plasma cells, suggesting that axis’ role in recruitment and promotion of humoral rejection processes.230 CCR2 and CCR5 are involved with recruitment of infiltrating lymphocytes in acute and chronic rejection.230 Other trafficking molecules that have been identified as important mediators in rejection include CCL2, CCL3L1, and CCL5.234 Blockade of each of these pathways in multiple experimental models have ameliorated rejection, but no single one has completely eliminated alloimmune responses in liver transplantation.
POTENTIAL TARGETS OF THERAPY
As reviewed earlier, there are multiple redundant pathways and mechanisms that traffic immune cells to the liver in both homeostatic and pathologic conditions. Despite differences between the sinusoidal and non-hepatic vascular endothelium, no single receptor has been identified that directs immune cells only to the liver.235 Instead, patterns of chemokine and adhesion molecule expression induce both pro- and anti-inflammatory responses. Multiple animal models have been developed to explore modulation of trafficking for potential therapeutic applications. Transcriptional regulators, such as rosiglitazone, an agonist of PPAR-γ, reduces CXCL10 production as well as CCL2 and CCL20 in a mouse model of Crohn’s Disease and PSC.236–240 Antibodies have also been developed that target and neutralize specific chemokine receptors and ligands. Those against CXCL16 improve survival of mice with immune-mediated liver injury; against CXCL10 reduced hepatic fibrosis in chronic toxic liver injury; and those against CCL20 improved liver function tests, reduced expression of inflammatory and pro-fibrotic genes in a model of acute and chronic toxic liver injury.241–243 Peptides that block chemokine receptors have also been developed; a recombinant analogue of CCL5 antagonizes CCR5 and CCR1, inhibiting HSC proliferation and reducing chemokine production and collagen deposition.244 Inhibition of immune cell trafficking at multiple points has shown potential for therapeutic application in rodent models. Dual inhibition of CCR2 and CCR5 reduces recruitment of monocytes and macrophages, as well as HSC activation in rodent liver fibrosis, significantly reducing fibrosis and inflammation.245 CXCR1 and CXCR2 antagonism also dramatically inhibits myeloid recruitment in a I/R rat model of transplantation, leading to decreased necrosis.246
A handful of pre-clinical models of trafficking blockade have been translated to clinical use. Blockade of CCR5 using the agent maraviroc has been applied to management of GvHD in hematopoietic stem-cell transplantation. A noncompetitive antagonist, maraviroc prevents CCL3, CCL4, and CCL5 binding and activation of signaling pathways.247 It was initially developed for use in subtypes of human immunodeficiency virus (HIV) that used only CCR5 to enter cells.248 In GvHD, this agent prevented of internalization of CCL5-blocked T-cell chemotaxis without impairing overall T-cell function or engraftment. This lead to a clinically significant reduction in both liver and gut GvHD.
Inflammatory bowel disease is another area where targeting leukocyte trafficking pathways has been applied. Vedolizumab and natalizumab are two monoclonal antibodies that have been used in refractory, severe forms of both Crohn’s disease and ulcerative colitis.249 Vedolizumab is specifically an antagonist for alpha4beta7, but not alpha4beta1. This prevents binding to MAdCAM-1 but not VCAM-1, thus exerting its anti-inflammatory effects in the gut without affecting leukocyte adhesion in other tissues.250,251 Natalizumab is an alternative immune-modulating agent that targets the same pathway. It is a humanized IgG4 monoclonal antibody that binds to the alpha4 chains of integrins to inhibit translocation of leukocytes.252 It antagonizes alpha4beta1 in addition to alpha4beta7 interactions; this non-specific binding has led to natalizumab’s use as an agent for multiple sclerosis as well as inflammatory bowel disease.253 Although these agents are effective in treating intestinal manifestations of Crohn’s disease and ulcerative colitis, they fail to have any therapeutic effect in concomitant PSC.254,255 Currently, there is an open label single arm study investigating anti-CXCL10 monoclonal antibodies’ potential application in PBC patients.
Conclusions
As a main sentinel for the human immune system, multiple mechanisms have developed to signal immune cells to travel to the liver in both homeostatic and pathological conditions. However, no one dominant signal exists that direct leukocytes to the liver, rather patterns of expression of multiple different signaling pathways are responsible. Although much has been revealed about these pathways, there remains much about the trafficking of pro-inflammatory and regulatory cells to be described. As with pathological conditions of other organ systems, there remains great potential to exploit these pathways for treating liver disease.
Funding:
SC received support from the NIH 5T32HL007854-19 grant. AG was supported by the Louis J. Gerstner, Jr. Foundation Award and the American Association for the Study of Liver Diseases Career Development Award in the Memory of the University of Michigan Transplant Team, NIH-NIAID R56 AI122332 and by the National Center for Advancing Translational Sciences, National Institutes of Health through Grant Number UL1TR001873. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosure: AG receives research support from United Therapeutics. SC and JE have no conflicts of interest to report.
Abbreviations:
- APC
Antigen Presenting Cell
- BEC
Biliary Epithelial Cell
- CLEVER
Common Lymphatic Endothelial and Vascular Endothelial Receptor
- DAMP
Damage Associated Molecular Patterns
- DC
Dendritic Cells
- GI
gastrointestinal
- GvHD
Graft vs Host Disease
- HCC
Hepatocellular Carcinoma
- HCV
Hepatitis C Virus
- HIV
Human Immunodeficiency Virus
- HLA
Human Leukocyte Antige
- HSC
Hepatic Stellate Cell
- ICAM
Intercellular Adhesion Molecule
- I/R
Ischemia/Reperfusion
- iNOS
inducible nitric oxide synthase
- KC
Kupffer Cells
- LFA
Lymphocyte Function-Associated Antigen
- LPS
lipopolysaccharide
- LSEC
Liver Sinusoidal Endothelial Cell
- MadCAM
Mucosal Addressin Cell Adhesion Molecule
- MHC
Major Histocompatibility Complex
- NAFLD
Nonalcoholic Fatty Liver Disease
- NASH
Nonalcoholic Steatohepatitis
- NK
Natural Killer
- NKT
Natural Killer T
- PAMP
Pathogen Associated Molecular Patterns
- PBC
Primary Biliary Cirrhosis
- PD
Programmed Death
- PRR
Pattern Recognition Receptors
- PSC
Primary Sclerosing Cholangitis
- Th
Helpter T cell
- Treg
Regulatory T cell
- TLR
Toll-like Receptors
- VAP
Vascular Adhesion Protein
- VCAM
Vascular Cell Adhesion Molecule
References:
- 1.Nemeth E, Baird AW, O’Farrelly C. Microanatomy of the liver immune system. Semin Immunopathol. 2009;31(3):333–343. [DOI] [PubMed] [Google Scholar]
- 2.O’Farrelly C, Crispe IN. Prometheus through the looking glass: reflections on the hepatic immune system. Immunology today. 1999;20(9):394–398. [DOI] [PubMed] [Google Scholar]
- 3.Sheth K, Bankey P. The liver as an immune organ. Curr Opin Crit Care. 2001;7(2):99–104. [DOI] [PubMed] [Google Scholar]
- 4.Kita H, Mackay IR, Van De Water J, Gershwin ME. The lymphoid liver: considerations on pathways to autoimmune injury. Gastroenterology. 2001;120(6):1485–1501. [DOI] [PubMed] [Google Scholar]
- 5.Cantor HM, Dumont AE. Hepatic suppression of sensitization to antigen absorbed into the portal system. Nature. 1967;215(5102):744–745. [DOI] [PubMed] [Google Scholar]
- 6.Elvevold K, Smedsrod B, Martinez I. The liver sinusoidal endothelial cell: a cell type of controversial and confusing identity. American journal of physiology Gastrointestinal and liver physiology. 2008;294(2):G391–400. [DOI] [PubMed] [Google Scholar]
- 7.Wisse E, Braet F, Luo D, et al. Structure and function of sinusoidal lining cells in the liver. Toxicol Pathol. 1996;24(1):100–111. [DOI] [PubMed] [Google Scholar]
- 8.Oda M, Yokomori H, Han JY. Regulatory mechanisms of hepatic microcirculation. Clin Hemorheol Microcirc. 2003;29(3–4):167–182. [PubMed] [Google Scholar]
- 9.Seki E, Brenner DA. Toll-like receptors and adaptor molecules in liver disease: update. Hepatology. 2008;48(1):322–335. [DOI] [PubMed] [Google Scholar]
- 10.Franco A, Barnaba V, Natali P, Balsano C, Musca A, Balsano F. Expression of class I and class II major histocompatibility complex antigens on human hepatocytes. Hepatology. 1988;8(3):449–454. [DOI] [PubMed] [Google Scholar]
- 11.Schroder AJ, Blaheta RA, Scholz M, Kronenberger B, Encke A, Markus BH. Effects of proinflammatory cytokines on cultivated primary human hepatocytes. Fluorometric measurement of intercellular adhesion molecule-1 and human leukocyte antigen-A, -B, -C, and DR expression. Transplantation. 1995;59(7):1023–1028. [DOI] [PubMed] [Google Scholar]
- 12.Bertolino P, McCaughan GW, Bowen DG. Role of primary intrahepatic T-cell activation in the ‘liver tolerance effect ’. Immunology and cell biology. 2002;80(1):84–92. [DOI] [PubMed] [Google Scholar]
- 13.Bertolino P, Trescol-Biemont MC, Rabourdin-Combe C. Hepatocytes induce functional activation of naive CD8+ T lymphocytes but fail to promote survival. European journal of immunology. 1998;28(1):221–236. [DOI] [PubMed] [Google Scholar]
- 14.Xu B, Broome U, Uzunel M, et al. Capillarization of hepatic sinusoid by liver endothelial cell-reactive autoantibodies in patients with cirrhosis and chronic hepatitis. Am J Pathol. 2003;163(4):1275–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knolle PA, Germann T, Treichel U, et al. Endotoxin down-regulates T cell activation by antigen-presenting liver sinusoidal endothelial cells. Journal of immunology. 1999;162(3):1401–1407. [PubMed] [Google Scholar]
- 16.Onoe T, Ohdan H, Tokita D, et al. Liver sinusoidal endothelial cells tolerize T cells across MHC barriers in mice. Journal of immunology. 2005;175(1):139–146. [DOI] [PubMed] [Google Scholar]
- 17.Scoazec JY, Feldmann G. In situ immunophenotyping study of endothelial cells of the human hepatic sinusoid: results and functional implications. Hepatology. 1991;14(5):789–797. [DOI] [PubMed] [Google Scholar]
- 18.Scoazec JY, Feldmann G. The cell adhesion molecules of hepatic sinusoidal endothelial cells. Journal of hepatology. 1994;20(2):296–300. [DOI] [PubMed] [Google Scholar]
- 19.Pusztaszeri MP, Seelentag W, Bosman FT. Immunohistochemical expression of endothelial markers CD31, CD34, von Willebrand factor, and Fli-1 in normal human tissues. J Histochem Cytochem. 2006;54(4):385–395. [DOI] [PubMed] [Google Scholar]
- 20.Mousa SA. Expression of adhesion molecules during cadmium hepatotoxicity. Life Sci. 2004;75(1):93–105. [DOI] [PubMed] [Google Scholar]
- 21.Daneker GW, Lund SA, Caughman SW, et al. Culture and characterization of sinusoidal endothelial cells isolated from human liver. In Vitro Cell Dev Biol Anim. 1998;34(5):370–377. [DOI] [PubMed] [Google Scholar]
- 22.Matsumura T, Takesue M, Westerman KA, et al. Establishment of an immortalized human-liver endothelial cell line with SV40T and hTERT. Transplantation. 2004;77(9):1357–1365. [DOI] [PubMed] [Google Scholar]
- 23.Karrar A, Broome U, Uzunel M, Qureshi AR, Sumitran-Holgersson S. Human liver sinusoidal endothelial cells induce apoptosis in activated T cells: a role in tolerance induction. Gut. 2007;56(2):243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knolle PA, Limmer A. Neighborhood politics: the immunoregulatory function of organ-resident liver endothelial cells. Trends in immunology. 2001;22(8):432–437. [DOI] [PubMed] [Google Scholar]
- 25.Limmer A, Ohl J, Kurts C, et al. Efficient presentation of exogenous antigen by liver endothelial cells to CD8+ T cells results in antigen-specific T-cell tolerance. Nature medicine. 2000;6(12):1348–1354. [DOI] [PubMed] [Google Scholar]
- 26.Limmer A, Ohl J, Wingender G, et al. Cross-presentation of oral antigens by liver sinusoidal endothelial cells leads to CD8 T cell tolerance. European journal of immunology. 2005;35(10):2970–2981. [DOI] [PubMed] [Google Scholar]
- 27.Knolle PA, Schmitt E, Jin S, et al. Induction of cytokine production in naive CD4(+) T cells by antigen-presenting murine liver sinusoidal endothelial cells but failure to induce differentiation toward Th1 cells. Gastroenterology. 1999;116(6):1428–1440. [DOI] [PubMed] [Google Scholar]
- 28.Knolle P, Schlaak J, Uhrig A, Kempf P, Meyer zum Buschenfelde KH, Gerken G. Human Kupffer cells secrete IL-10 in response to lipopolysaccharide (LPS) challenge. Journal of hepatology. 1995;22(2):226–229. [DOI] [PubMed] [Google Scholar]
- 29.Lohse AW, Knolle PA, Bilo K, et al. Antigen-presenting function and B7 expression of murine sinusoidal endothelial cells and Kupffer cells. Gastroenterology. 1996;110(4):1175–1181. [DOI] [PubMed] [Google Scholar]
- 30.Diehl L, Schurich A, Grochtmann R, Hegenbarth S, Chen L, Knolle PA. Tolerogenic maturation of liver sinusoidal endothelial cells promotes B7-homolog 1-dependent CD8+ T cell tolerance. Hepatology. 2008;47(1):296–305. [DOI] [PubMed] [Google Scholar]
- 31.Schildberg FA, Hegenbarth SI, Schumak B, Scholz K, Limmer A, Knolle PA. Liver sinusoidal endothelial cells veto CD8 T cell activation by antigen-presenting dendritic cells. European journal of immunology. 2008;38(4):957–967. [DOI] [PubMed] [Google Scholar]
- 32.Bleau C, Filliol A, Samson M, Lamontagne L. Mouse Hepatitis Virus Infection Induces a Toll-Like Receptor 2-Dependent Activation of Inflammatory Functions in Liver Sinusoidal Endothelial Cells during Acute Hepatitis. Journal of virology. 2016;90(20):9096–9113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bilzer M, Roggel F, Gerbes AL. Role of Kupffer cells in host defense and liver disease. Liver Int. 2006;26(10):1175–1186. [DOI] [PubMed] [Google Scholar]
- 34.Lee WY, Moriarty TJ, Wong CH, et al. An intravascular immune response to Borrelia burgdorferi involves Kupffer cells and iNKT cells. Nature immunology. 2010;11(4):295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Helmy KY, Katschke KJ Jr., Gorgani NN, et al. CRIg: a macrophage complement receptor required for phagocytosis of circulating pathogens. Cell. 2006;124(5):915–927. [DOI] [PubMed] [Google Scholar]
- 36.Gorgani NN, He JQ, Katschke KJ Jr., et al. Complement receptor of the Ig superfamily enhances complement-mediated phagocytosis in a subpopulation of tissue resident macrophages. Journal of immunology. 2008;181(11):7902–7908. [DOI] [PubMed] [Google Scholar]
- 37.Gregory SH, Sagnimeni AJ, Wing EJ. Bacteria in the bloodstream are trapped in the liver and killed by immigrating neutrophils. Journal of immunology. 1996;157(6):2514–2520. [PubMed] [Google Scholar]
- 38.You Q, Cheng L, Kedl RM, Ju C. Mechanism of T cell tolerance induction by murine hepatic Kupffer cells. Hepatology. 2008;48(3):978–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang LR, Wohlleber D, Reisinger F, et al. Intrahepatic myeloid-cell aggregates enable local proliferation of CD8(+) T cells and successful immunotherapy against chronic viral liver infection. Nature immunology. 2013;14(6):574–583. [DOI] [PubMed] [Google Scholar]
- 40.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. The Journal of clinical investigation. 1998;101(4):890–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shi J, Gilbert GE, Kokubo Y, Ohashi T. Role of the liver in regulating numbers of circulating neutrophils. Blood. 2001;98(4):1226–1230. [DOI] [PubMed] [Google Scholar]
- 42.Prickett TC, McKenzie JL, Hart DN. Characterization of interstitial dendritic cells in human liver. Transplantation. 1988;46(5):754–761. [DOI] [PubMed] [Google Scholar]
- 43.Pillarisetty VG, Shah AB, Miller G, Bleier JI, DeMatteo RP. Liver dendritic cells are less immunogenic than spleen dendritic cells because of differences in subtype composition. Journal of immunology. 2004;172(2):1009–1017. [DOI] [PubMed] [Google Scholar]
- 44.Jomantaite I, Dikopoulos N, Kroger A, et al. Hepatic dendritic cell subsets in the mouse. European journal of immunology. 2004;34(2):355–365. [DOI] [PubMed] [Google Scholar]
- 45.Hsu W, Shu SA, Gershwin E, Lian ZX. The current immune function of hepatic dendritic cells. Cell Mol Immunol. 2007;4(5):321–328. [PubMed] [Google Scholar]
- 46.Kingham TP, Chaudhry UI, Plitas G, Katz SC, Raab J, DeMatteo RP. Murine liver plasmacytoid dendritic cells become potent immunostimulatory cells after Flt-3 ligand expansion. Hepatology. 2007;45(2):445–454. [DOI] [PubMed] [Google Scholar]
- 47.Cornell RP, Liljequist BL, Bartizal KF. Depressed liver regeneration after partial hepatectomy of germ-free, athymic and lipopolysaccharide-resistant mice. Hepatology. 1990;11(6):916–922. [DOI] [PubMed] [Google Scholar]
- 48.Bomble M, Tacke F, Rink L, Kovalenko E, Weiskirchen R. Analysis of antigen-presenting functionality of cultured rat hepatic stellate cells and transdifferentiated myofibroblasts. Biochemical and biophysical research communications. 2010;396(2):342–347. [DOI] [PubMed] [Google Scholar]
- 49.Winau F, Hegasy G, Weiskirchen R, et al. Ito cells are liver-resident antigen-presenting cells for activating T cell responses. Immunity. 2007;26(1):117–129. [DOI] [PubMed] [Google Scholar]
- 50.Vinas O, Bataller R, Sancho-Bru P, et al. Human hepatic stellate cells show features of antigen-presenting cells and stimulate lymphocyte proliferation. Hepatology. 2003;38(4):919–929. [DOI] [PubMed] [Google Scholar]
- 51.Chang J, Hisamatsu T, Shimamura K, et al. Activated hepatic stellate cells mediate the differentiation of macrophages. Hepatology research : the official journal of the Japan Society of Hepatology. 2013;43(6):658–669. [DOI] [PubMed] [Google Scholar]
- 52.Trivedi PJ, Adams DH. Mucosal immunity in liver autoimmunity: a comprehensive review. J Autoimmun. 2013;46:97–111. [DOI] [PubMed] [Google Scholar]
- 53.Scholz M, Cinatl J, Blaheta RA, Kornhuber B, Markus BH, Doerr HW. Expression of human leukocyte antigens class I and class II on cultured biliary epithelial cells after cytomegalovirus infection. Tissue antigens. 1997;49(6):640–643. [DOI] [PubMed] [Google Scholar]
- 54.Dillon PW, Belchis D, Minnick K, Tracy T. Differential expression of the major histocompatibility antigens and ICAM-1 on bile duct epithelial cells in biliary atresia. Tohoku J Exp Med. 1997;181(1):33–40. [DOI] [PubMed] [Google Scholar]
- 55.Morita M, Watanabe Y, Akaike T. Inflammatory cytokines up-regulate intercellular adhesion molecule-1 expression on primary cultured mouse hepatocytes and T-lymphocyte adhesion. Hepatology. 1994;19(2):426–431. [PubMed] [Google Scholar]
- 56.Bloom S, Fleming K, Chapman R. Adhesion molecule expression in primary sclerosing cholangitis and primary biliary cirrhosis. Gut. 1995;36(4):604–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen XM, O’Hara SP, LaRusso NF. The immunobiology of cholangiocytes. Immunology and cell biology. 2008;86(6):497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Terada R, Yamamoto K, Hakoda T, et al. Stromal cell-derived factor-1 from biliary epithelial cells recruits CXCR4-positive cells: implications for inflammatory liver diseases. Laboratory investigation; a journal of technical methods and pathology. 2003;83(5):665–672. [DOI] [PubMed] [Google Scholar]
- 59.Shimoda S, Harada K, Niiro H, et al. CX3CL1 (fractalkine): a signpost for biliary inflammation in primary biliary cirrhosis. Hepatology. 2010;51(2):567–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Isse K, Harada K, Zen Y, et al. Fractalkine and CX3CR1 are involved in the recruitment of intraepithelial lymphocytes of intrahepatic bile ducts. Hepatology. 2005;41(3):506–516. [DOI] [PubMed] [Google Scholar]
- 61.Heydtmann M, Lalor PF, Eksteen JA, Hubscher SG, Briskin M, Adams DH. CXC chemokine ligand 16 promotes integrin-mediated adhesion of liver-infiltrating lymphocytes to cholangiocytes and hepatocytes within the inflamed human liver. Journal of immunology. 2005;174(2):1055–1062. [DOI] [PubMed] [Google Scholar]
- 62.Norris S, Collins C, Doherty DG, et al. Resident human hepatic lymphocytes are phenotypically different from circulating lymphocytes. Journal of hepatology. 1998;28(1):84–90. [DOI] [PubMed] [Google Scholar]
- 63.Norris S, Doherty DG, Collins C, et al. Natural T cells in the human liver: cytotoxic lymphocytes with dual T cell and natural killer cell phenotype and function are phenotypically heterogenous and include Valpha24-JalphaQ and gammadelta T cell receptor bearing cells. Human immunology. 1999;60(1):20–31. [DOI] [PubMed] [Google Scholar]
- 64.Doherty DG, Norris S, Madrigal-Estebas L, et al. The human liver contains multiple populations of NK cells, T cells, and CD3+CD56+ natural T cells with distinct cytotoxic activities and Th1, Th2, and Th0 cytokine secretion patterns. Journal of immunology. 1999;163(4):2314–2321. [PubMed] [Google Scholar]
- 65.Hata K, Zhang XR, Iwatsuki S, Van Thiel DH, Herberman RB, Whiteside TL. Isolation, phenotyping, and functional analysis of lymphocytes from human liver. Clin Immunol Immunopathol. 1990;56(3):401–419. [DOI] [PubMed] [Google Scholar]
- 66.Brandes M, Willimann K, Moser B. Professional antigen-presentation function by human gammadelta T Cells. Science. 2005;309(5732):264–268. [DOI] [PubMed] [Google Scholar]
- 67.Carding SR, Egan PJ. Gammadelta T cells: functional plasticity and heterogeneity. Nature reviews Immunology. 2002;2(5):336–345. [DOI] [PubMed] [Google Scholar]
- 68.Zhao N, Hao J, Ni Y, et al. Vgamma4 gammadelta T cell-derived IL-17A negatively regulates NKT cell function in Con A-induced fulminant hepatitis. Journal of immunology. 2011;187(10):5007–5014. [DOI] [PubMed] [Google Scholar]
- 69.Pruvot FR, Navarro F, Janin A, et al. Characterization, quantification, and localization of passenger T lymphocytes and NK cells in human liver before transplantation. Transplant international : official journal of the European Society for Organ Transplantation. 1995;8(4):273–279. [DOI] [PubMed] [Google Scholar]
- 70.Doherty DG, O’Farrelly C. Innate and adaptive lymphoid cells in the human liver. Immunological reviews. 2000;174:5–20. [DOI] [PubMed] [Google Scholar]
- 71.Crispe IN. The liver as a lymphoid organ. Annual review of immunology. 2009;27:147–163. [DOI] [PubMed] [Google Scholar]
- 72.Matsuda JL, Mallevaey T, Scott-Browne J, Gapin L. CD1d-restricted iNKT cells, the ‘Swiss-Army knife’ of the immune system. Current opinion in immunology. 2008;20(3):358–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Geissmann F, Cameron TO, Sidobre S, et al. Intravascular immune surveillance by CXCR6+ NKT cells patrolling liver sinusoids. PLoS Biol. 2005;3(4):e113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kubes P, Mehal WZ. Sterile inflammation in the liver. Gastroenterology. 2012;143(5):1158–1172. [DOI] [PubMed] [Google Scholar]
- 75.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140(6):805–820. [DOI] [PubMed] [Google Scholar]
- 76.Janeway CA, Jr. The immune system evolved to discriminate infectious nonself from noninfectious self. Immunology today. 1992;13(1):11–16. [DOI] [PubMed] [Google Scholar]
- 77.Wu J, Meng Z, Jiang M, et al. Toll-like receptor-induced innate immune responses in non-parenchymal liver cells are cell type-specific. Immunology. 2010;129(3):363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McDonald B, McAvoy EF, Lam F, et al. Interaction of CD44 and hyaluronan is the dominant mechanism for neutrophil sequestration in inflamed liver sinusoids. The Journal of experimental medicine. 2008;205(4):915–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jenne CN, Wong CH, Petri B, Kubes P. The use of spinning-disk confocal microscopy for the intravital analysis of platelet dynamics in response to systemic and local inflammation. PLoS One. 2011;6(9):e25109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jenne CN, Wong CH, Zemp FJ, et al. Neutrophils recruited to sites of infection protect from virus challenge by releasing neutrophil extracellular traps. Cell Host Microbe. 2013;13(2):169–180. [DOI] [PubMed] [Google Scholar]
- 81.Knolle PA, Limmer A. Control of immune responses by savenger liver endothelial cells. Swiss Med Wkly. 2003;133(37–38):501–506. [DOI] [PubMed] [Google Scholar]
- 82.Steffan AM, Gendrault JL, McCuskey RS, McCuskey PA, Kirn A. Phagocytosis, an unrecognized property of murine endothelial liver cells. Hepatology. 1986;6(5):830–836. [DOI] [PubMed] [Google Scholar]
- 83.Schafer G, Guler R, Murray G, Brombacher F, Brown GD. The role of scavenger receptor B1 in infection with Mycobacterium tuberculosis in a murine model. PLoS One. 2009;4(12):e8448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Eyre NS, Drummer HE, Beard MR. The SR-BI partner PDZK1 facilitates hepatitis C virus entry. PLoS pathogens. 2010;6(10):e1001130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dreux M, Dao Thi VL, Fresquet J, et al. Receptor complementation and mutagenesis reveal SR-BI as an essential HCV entry factor and functionally imply its intra- and extra-cellular domains. PLoS pathogens. 2009;5(2):e1000310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Magnusson S, Berg T. Extremely rapid endocytosis mediated by the mannose receptor of sinusoidal endothelial rat liver cells. Biochem J. 1989;257(3):651–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stahl PD, Ezekowitz RA. The mannose receptor is a pattern recognition receptor involved in host defense. Current opinion in immunology. 1998;10(1):50–55. [DOI] [PubMed] [Google Scholar]
- 88.Kerrigan AM, Brown GD. C-type lectins and phagocytosis. Immunobiology. 2009;214(7):562–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Monteiro RC, Van De Winkel JG. IgA Fc receptors. Annual review of immunology. 2003;21:177–204. [DOI] [PubMed] [Google Scholar]
- 90.Ganesan LP, Kim J, Wu Y, et al. FcgammaRIIb on liver sinusoidal endothelium clears small immune complexes. Journal of immunology. 2012;189(10):4981–4988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ravetch JV, Bolland S. IgG Fc receptors. Annual review of immunology. 2001;19:275–290. [DOI] [PubMed] [Google Scholar]
- 92.Skogh T, Blomhoff R, Eskild W, Berg T. Hepatic uptake of circulating IgG immune complexes. Immunology. 1985;55(4):585–594. [PMC free article] [PubMed] [Google Scholar]
- 93.He JQ, Katschke KJ Jr., Gribling P, et al. CRIg mediates early Kupffer cell responses to adenovirus. J Leukoc Biol. 2013;93(2):301–306. [DOI] [PubMed] [Google Scholar]
- 94.Kansas GS. Selectins and their ligands: current concepts and controversies. Blood. 1996;88(9):3259–3287. [PubMed] [Google Scholar]
- 95.Lee WY, Kubes P. Leukocyte adhesion in the liver: distinct adhesion paradigm from other organs. Journal of hepatology. 2008;48(3):504–512. [DOI] [PubMed] [Google Scholar]
- 96.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110(6):673–687. [DOI] [PubMed] [Google Scholar]
- 97.Wong J, Johnston B, Lee SS, et al. A minimal role for selectins in the recruitment of leukocytes into the inflamed liver microvasculature. The Journal of clinical investigation. 1997;99(11):2782–2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wisse E, De Zanger RB, Charels K, Van Der Smissen P, McCuskey RS. The liver sieve: considerations concerning the structure and function of endothelial fenestrae, the sinusoidal wall and the space of Disse. Hepatology. 1985;5(4):683–692. [DOI] [PubMed] [Google Scholar]
- 99.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76(2):301–314. [DOI] [PubMed] [Google Scholar]
- 100.John B, Crispe IN. Passive and active mechanisms trap activated CD8+ T cells in the liver. Journal of immunology. 2004;172(9):5222–5229. [DOI] [PubMed] [Google Scholar]
- 101.Crispe IN. Migration of lymphocytes into hepatic sinusoids. Journal of hepatology. 2012;57(1):218–220. [DOI] [PubMed] [Google Scholar]
- 102.Luo BH, Carman CV, Springer TA. Structural basis of integrin regulation and signaling. Annual review of immunology. 2007;25:619–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tan SM. The leucocyte beta2 (CD18) integrins: the structure, functional regulation and signalling properties. Biosci Rep. 2012;32(3):241–269. [DOI] [PubMed] [Google Scholar]
- 104.Iigo Y, Suematsu M, Higashida T, et al. Constitutive expression of ICAM-1 in rat microvascular systems analyzed by laser confocal microscopy. The American journal of physiology. 1997;273(1 Pt 2):H138–147. [DOI] [PubMed] [Google Scholar]
- 105.Lalor PF, Edwards S, McNab G, Salmi M, Jalkanen S, Adams DH. Vascular adhesion protein-1 mediates adhesion and transmigration of lymphocytes on human hepatic endothelial cells. Journal of immunology. 2002;169(2):983–992. [DOI] [PubMed] [Google Scholar]
- 106.McNab G, Reeves JL, Salmi M, Hubscher S, Jalkanen S, Adams DH. Vascular adhesion protein 1 mediates binding of T cells to human hepatic endothelium. Gastroenterology. 1996;110(2):522–528. [DOI] [PubMed] [Google Scholar]
- 107.Yoong KF, McNab G, Hubscher SG, Adams DH. Vascular adhesion protein-1 and ICAM-1 support the adhesion of tumor-infiltrating lymphocytes to tumor endothelium in human hepatocellular carcinoma. Journal of immunology. 1998;160(8):3978–3988. [PubMed] [Google Scholar]
- 108.Jalkanen S, Karikoski M, Mercier N, et al. The oxidase activity of vascular adhesion protein-1 (VAP-1) induces endothelial E- and P-selectins and leukocyte binding. Blood. 2007;110(6):1864–1870. [DOI] [PubMed] [Google Scholar]
- 109.Lalor PF, Sun PJ, Weston CJ, Martin-Santos A, Wakelam MJ, Adams DH. Activation of vascular adhesion protein-1 on liver endothelium results in an NF-kappaB-dependent increase in lymphocyte adhesion. Hepatology. 2007;45(2):465–474. [DOI] [PubMed] [Google Scholar]
- 110.Bonder CS, Norman MU, Swain MG, et al. Rules of recruitment for Th1 and Th2 lymphocytes in inflamed liver: a role for alpha-4 integrin and vascular adhesion protein-1. Immunity. 2005;23(2):153–163. [DOI] [PubMed] [Google Scholar]
- 111.Salmi M, Koskinen K, Henttinen T, Elima K, Jalkanen S. CLEVER-1 mediates lymphocyte transmigration through vascular and lymphatic endothelium. Blood. 2004;104(13):3849–3857. [DOI] [PubMed] [Google Scholar]
- 112.Shetty S, Weston CJ, Oo YH, et al. Common lymphatic endothelial and vascular endothelial receptor-1 mediates the transmigration of regulatory T cells across human hepatic sinusoidal endothelium. Journal of immunology. 2011;186(7):4147–4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zlotnik A, Yoshie O. The chemokine superfamily revisited. Immunity. 2012;36(5):705–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Annunziato F, Cosmi L, Santarlasci V, et al. Phenotypic and functional features of human Th17 cells. The Journal of experimental medicine. 2007;204(8):1849–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Annunziato F, Cosmi L, Liotta F, Maggi E, Romagnani S. The phenotype of human Th17 cells and their precursors, the cytokines that mediate their differentiation and the role of Th17 cells in inflammation. International immunology. 2008;20(11):1361–1368. [DOI] [PubMed] [Google Scholar]
- 116.Hirota K, Yoshitomi H, Hashimoto M, et al. Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. The Journal of experimental medicine. 2007;204(12):2803–2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Singh SP, Zhang HH, Foley JF, Hedrick MN, Farber JM. Human T cells that are able to produce IL-17 express the chemokine receptor CCR6. Journal of immunology. 2008;180(1):214–221. [DOI] [PubMed] [Google Scholar]
- 118.Nistala K, Moncrieffe H, Newton KR, Varsani H, Hunter P, Wedderburn LR. Interleukin-17-producing T cells are enriched in the joints of children with arthritis, but have a reciprocal relationship to regulatory T cell numbers. Arthritis and rheumatism. 2008;58(3):875–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Oo YH, Weston CJ, Lalor PF, et al. Distinct roles for CCR4 and CXCR3 in the recruitment and positioning of regulatory T cells in the inflamed human liver. Journal of immunology. 2010;184(6):2886–2898. [DOI] [PubMed] [Google Scholar]
- 120.Guidotti LG, Iannacone M. Effector CD8 T cell trafficking within the liver. Mol Immunol. 2013;55(1):94–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shields PL, Morland CM, Salmon M, Qin S, Hubscher SG, Adams DH. Chemokine and chemokine receptor interactions provide a mechanism for selective T cell recruitment to specific liver compartments within hepatitis C-infected liver. Journal of immunology. 1999;163(11):6236–6243. [PubMed] [Google Scholar]
- 122.Kunkel EJ, Boisvert J, Murphy K, et al. Expression of the chemokine receptors CCR4, CCR5, and CXCR3 by human tissue-infiltrating lymphocytes. Am J Pathol. 2002;160(1):347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Boisvert J, Kunkel EJ, Campbell JJ, Keeffe EB, Butcher EC, Greenberg HB. Liver-infiltrating lymphocytes in end-stage hepatitis C virus: subsets, activation status, and chemokine receptor phenotypes. Journal of hepatology. 2003;38(1):67–75. [DOI] [PubMed] [Google Scholar]
- 124.Leroy V, Vigan I, Mosnier JF, et al. Phenotypic and functional characterization of intrahepatic T lymphocytes during chronic hepatitis C. Hepatology. 2003;38(4):829–841. [DOI] [PubMed] [Google Scholar]
- 125.Dal-Secco D, Wang J, Zeng Z, et al. A dynamic spectrum of monocytes arising from the in situ reprogramming of CCR2+ monocytes at a site of sterile injury. The Journal of experimental medicine. 2015;212(4):447–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Apolinario A, Majano PL, Alvarez-Perez E, et al. Increased expression of T cell chemokines and their receptors in chronic hepatitis C: relationship with the histological activity of liver disease. The American journal of gastroenterology. 2002;97(11):2861–2870. [DOI] [PubMed] [Google Scholar]
- 127.Larrubia JR, Calvino M, Benito S, et al. The role of CCR5/CXCR3 expressing CD8+ cells in liver damage and viral control during persistent hepatitis C virus infection. Journal of hepatology. 2007;47(5):632–641. [DOI] [PubMed] [Google Scholar]
- 128.Manousou P, Kolios G, Drygiannakis I, et al. CXCR3 axis in patients with primary biliary cirrhosis: a possible novel mechanism of the effect of ursodeoxycholic acid. Clinical and experimental immunology. 2013;172(1):9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Alabraba EB, Lai V, Boon L, Wigmore SJ, Adams DH, Afford SC. Coculture of human liver macrophages and cholangiocytes leads to CD40-dependent apoptosis and cytokine secretion. Hepatology. 2008;47(2):552–562. [DOI] [PubMed] [Google Scholar]
- 130.Ahmed-Choudhury J, Russell CL, Randhawa S, Young LS, Adams DH, Afford SC. Differential induction of nuclear factor-kappaB and activator protein-1 activity after CD40 ligation is associated with primary human hepatocyte apoptosis or intrahepatic endothelial cell proliferation. Mol Biol Cell. 2003;14(4):1334–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kakimi K, Lane TE, Wieland S, et al. Blocking chemokine responsive to gamma-2/interferon (IFN)-gamma inducible protein and monokine induced by IFN-gamma activity in vivo reduces the pathogenetic but not the antiviral potential of hepatitis B virus-specific cytotoxic T lymphocytes. The Journal of experimental medicine. 2001;194(12):1755–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Harvey CE, Post JJ, Palladinetti P, et al. Expression of the chemokine IP-10 (CXCL10) by hepatocytes in chronic hepatitis C virus infection correlates with histological severity and lobular inflammation. J Leukoc Biol. 2003;74(3):360–369. [DOI] [PubMed] [Google Scholar]
- 133.Curbishley SM, Eksteen B, Gladue RP, Lalor P, Adams DH. CXCR 3 activation promotes lymphocyte transendothelial migration across human hepatic endothelium under fluid flow. Am J Pathol. 2005;167(3):887–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Eksteen B, Miles A, Curbishley SM, et al. Epithelial inflammation is associated with CCL28 production and the recruitment of regulatory T cells expressing CCR10. Journal of immunology. 2006;177(1):593–603. [DOI] [PubMed] [Google Scholar]
- 135.Germanov E, Veinotte L, Cullen R, Chamberlain E, Butcher EC, Johnston B. Critical role for the chemokine receptor CXCR6 in homeostasis and activation of CD1d-restricted NKT cells. Journal of immunology. 2008;181(1):81–91. [DOI] [PubMed] [Google Scholar]
- 136.Northfield JW, Kasprowicz V, Lucas M, et al. CD161 expression on hepatitis C virus-specific CD8+ T cells suggests a distinct pathway of T cell differentiation. Hepatology. 2008;47(2):396–406. [DOI] [PubMed] [Google Scholar]
- 137.Sato T, Thorlacius H, Johnston B, et al. Role for CXCR6 in recruitment of activated CD8+ lymphocytes to inflamed liver. Journal of immunology. 2005;174(1):277–283. [DOI] [PubMed] [Google Scholar]
- 138.Rot A, von Andrian UH. Chemokines in innate and adaptive host defense: basic chemokinese grammar for immune cells. Annual review of immunology. 2004;22:891–928. [DOI] [PubMed] [Google Scholar]
- 139.Viola A, Luster AD. Chemokines and their receptors: drug targets in immunity and inflammation. Annu Rev Pharmacol Toxicol. 2008;48:171–197. [DOI] [PubMed] [Google Scholar]
- 140.Murai M, Yoneyama H, Harada A, et al. Active participation of CCR5(+)CD8(+) T lymphocytes in the pathogenesis of liver injury in graft-versus-host disease. The Journal of clinical investigation. 1999;104(1):49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Goddard S, Williams A, Morland C, et al. Differential expression of chemokines and chemokine receptors shapes the inflammatory response in rejecting human liver transplants. Transplantation. 2001;72(12):1957–1967. [DOI] [PubMed] [Google Scholar]
- 142.Murai M, Yoneyama H, Ezaki T, et al. Peyer’s patch is the essential site in initiating murine acute and lethal graft-versus-host reaction. Nature immunology. 2003;4(2):154–160. [DOI] [PubMed] [Google Scholar]
- 143.Ajuebor MN, Aspinall AI, Zhou F, et al. Lack of chemokine receptor CCR5 promotes murine fulminant liver failure by preventing the apoptosis of activated CD1d-restricted NKT cells. Journal of immunology. 2005;174(12):8027–8037. [DOI] [PubMed] [Google Scholar]
- 144.Reshef R, Luger SM, Hexner EO, et al. Blockade of lymphocyte chemotaxis in visceral graft-versus-host disease. N Engl J Med. 2012;367(2):135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Affo S, Rodrigo-Torres D, Blaya D, et al. Chemokine Receptor Ccr6 Deficiency Alters Hepatic Inflammatory Cell Recruitment and Promotes Liver Inflammation and Fibrosis. PLoS One. 2015;10(12):e0145147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Klonowski KD, Williams KJ, Marzo AL, Blair DA, Lingenheld EG, Lefrancois L. Dynamics of blood-borne CD8 memory T cell migration in vivo. Immunity. 2004;20(5):551–562. [DOI] [PubMed] [Google Scholar]
- 147.Hammerich L, Bangen JM, Govaere O, et al. Chemokine receptor CCR6-dependent accumulation of gammadelta T cells in injured liver restricts hepatic inflammation and fibrosis. Hepatology. 2014;59(2):630–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bourd-Boittin K, Basset L, Bonnier D, L’Helgoualc’h A, Samson M, Theret N. CX3CL1/fractalkine shedding by human hepatic stellate cells: contribution to chronic inflammation in the liver. J Cell Mol Med. 2009;13(8A):1526–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Aspinall AI, Curbishley SM, Lalor PF, et al. CX(3)CR1 and vascular adhesion protein-1-dependent recruitment of CD16(+) monocytes across human liver sinusoidal endothelium. Hepatology. 2010;51(6):2030–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Karlmark KR, Zimmermann HW, Roderburg C, et al. The fractalkine receptor CX(3)CR1 protects against liver fibrosis by controlling differentiation and survival of infiltrating hepatic monocytes. Hepatology. 2010;52(5):1769–1782. [DOI] [PubMed] [Google Scholar]
- 151.Efsen E, Grappone C, DeFranco RM, et al. Up-regulated expression of fractalkine and its receptor CX3CR1 during liver injury in humans. Journal of hepatology. 2002;37(1):39–47. [DOI] [PubMed] [Google Scholar]
- 152.Borchers AT, Shimoda S, Bowlus C, Keen CL, Gershwin ME. Lymphocyte recruitment and homing to the liver in primary biliary cirrhosis and primary sclerosing cholangitis. Semin Immunopathol. 2009;31(3):309–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sasaki M, Miyakoshi M, Sato Y, Nakanuma Y. Modulation of the microenvironment by senescent biliary epithelial cells may be involved in the pathogenesis of primary biliary cirrhosis. Journal of hepatology. 2010;53(2):318–325. [DOI] [PubMed] [Google Scholar]
- 154.Sasaki M, Miyakoshi M, Sato Y, Nakanuma Y. Chemokine-chemokine receptor CCL2-CCR2 and CX3CL1-CX3CR1 axis may play a role in the aggravated inflammation in primary biliary cirrhosis. Digestive diseases and sciences. 2014;59(2):358–364. [DOI] [PubMed] [Google Scholar]
- 155.Zhang W, Ono Y, Miyamura Y, Bowlus CL, Gershwin ME, Maverakis E. T cell clonal expansions detected in patients with primary biliary cirrhosis express CX3CR1. J Autoimmun. 2011;37(2):7178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.White GE, Greaves DR. Fractalkine: a survivor’s guide: chemokines as antiapoptotic mediators. Arterioscler Thromb Vasc Biol. 2012;32(3):589–594. [DOI] [PubMed] [Google Scholar]
- 157.Aoyama T, Inokuchi S, Brenner DA, Seki E. CX3CL1-CX3CR1 interaction prevents carbon tetrachloride-induced liver inflammation and fibrosis in mice. Hepatology. 2010;52(4):1390–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lee JH, Kang SG, Kim CH. FoxP3+ T cells undergo conventional first switch to lymphoid tissue homing receptors in thymus but accelerated second switch to nonlymphoid tissue homing receptors in secondary lymphoid tissues. Journal of immunology. 2007;178(1):301–311. [DOI] [PubMed] [Google Scholar]
- 159.Mora JR, Cheng G, Picarella D, Briskin M, Buchanan N, von Andrian UH. Reciprocal and dynamic control of CD8 T cell homing by dendritic cells from skin- and gut-associated lymphoid tissues. The Journal of experimental medicine. 2005;201(2):303–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Faint JM, Annels NE, Curnow SJ, et al. Memory T cells constitute a subset of the human CD8+CD45RA+ pool with distinct phenotypic and migratory characteristics. Journal of immunology. 2001;167(1):212–220. [DOI] [PubMed] [Google Scholar]
- 161.Heit B, Robbins SM, Downey CM, et al. PTEN functions to ‘prioritize’ chemotactic cues and prevent ‘distraction’ in migrating neutrophils. Nature immunology. 2008;9(7):743–752. [DOI] [PubMed] [Google Scholar]
- 162.Lesley J, Hascall VC, Tammi M, Hyman R. Hyaluronan binding by cell surface CD44. J Biol Chem. 2000;275(35):26967–26975. [DOI] [PubMed] [Google Scholar]
- 163.Serbina NV, Jia T, Hohl TM, Pamer EG. Monocyte-mediated defense against microbial pathogens. Annual review of immunology. 2008;26:421–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Varol C, Landsman L, Fogg DK, et al. Monocytes give rise to mucosal, but not splenic, conventional dendritic cells. The Journal of experimental medicine. 2007;204(1):171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Karlmark KR, Weiskirchen R, Zimmermann HW, et al. Hepatic recruitment of the inflammatory Gr1+ monocyte subset upon liver injury promotes hepatic fibrosis. Hepatology. 2009;50(1):261–274. [DOI] [PubMed] [Google Scholar]
- 166.Arnold L, Henry A, Poron F, et al. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. The Journal of experimental medicine. 2007;204(5):1057–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Shi C, Velazquez P, Hohl TM, Leiner I, Dustin ML, Pamer EG. Monocyte trafficking to hepatic sites of bacterial infection is chemokine independent and directed by focal intercellular adhesion molecule-1 expression. Journal of immunology. 2010;184(11):6266–6274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Heymann F, Hammerich L, Storch D, et al. Hepatic macrophage migration and differentiation critical for liver fibrosis is mediated by the chemokine receptor C-C motif chemokine receptor 8 in mice. Hepatology. 2012;55(3):898–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Robinson CJ, Bohannan BJ, Young VB. From structure to function: the ecology of host-associated microbial communities. Microbiol Mol Biol Rev. 2010;74(3):453–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wilbanks A, Zondlo SC, Murphy K, et al. Expression cloning of the STRL33/BONZO/TYMSTRligand reveals elements of CC, CXC, and CX3C chemokines. Journal of immunology. 2001;166(8):5145–5154. [DOI] [PubMed] [Google Scholar]
- 171.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends in immunology. 2004;25(12):677–686. [DOI] [PubMed] [Google Scholar]
- 172.Lodes MJ, Cong Y, Elson CO, et al. Bacterial flagellin is a dominant antigen in Crohn disease. The Journal of clinical investigation. 2004;113(9):1296–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Papadakis KA, Prehn J, Nelson V, et al. The role of thymus-expressed chemokine and its receptor CCR9 on lymphocytes in the regional specialization of the mucosal immune system. Journal of immunology. 2000;165(9):5069–5076. [DOI] [PubMed] [Google Scholar]
- 174.Briskin M, Winsor-Hines D, Shyjan A, et al. Human mucosal addressin cell adhesion molecule-1 is preferentially expressed in intestinal tract and associated lymphoid tissue. Am J Pathol. 1997;151(1):97–110. [PMC free article] [PubMed] [Google Scholar]
- 175.Annacker O, Coombes JL, Malmstrom V, et al. Essential role for CD103 in the T cell-mediated regulation of experimental colitis. The Journal of experimental medicine. 2005;202(8):1051–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Mora JR, Iwata M, Eksteen B, et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314(5802):1157–1160. [DOI] [PubMed] [Google Scholar]
- 177.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. The Journal of experimental medicine. 2007;204(8):1757–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lleo A, Liao J, Invernizzi P, et al. Immunoglobulin M levels inversely correlate with CD40 ligand promoter methylation in patients with primary biliary cirrhosis. Hepatology. 2012;55(1):153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lemmers A, Moreno C, Gustot T, et al. The interleukin-17 pathway is involved in human alcoholic liver disease. Hepatology. 2009;49(2):646–657. [DOI] [PubMed] [Google Scholar]
- 180.Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Karow M, Flavell RA. Interleukin-22 but not interleukin-17 provides protection to hepatocytes during acute liver inflammation. Immunity. 2007;27(4):647–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Zhang JY, Zhang Z, Lin F, et al. Interleukin-17-producing CD4(+) T cells increase with severity of liver damage in patients with chronic hepatitis B. Hepatology. 2010;51(1):81–91. [DOI] [PubMed] [Google Scholar]
- 182.Crispe IN. Hepatic T cells and liver tolerance. Nature reviews Immunology. 2003;3(1):51–62. [DOI] [PubMed] [Google Scholar]
- 183.Liu ZX, Govindarajan S, Okamoto S, Dennert G. NK cells cause liver injury and facilitate the induction of T cell-mediated immunity to a viral liver infection. Journal of immunology. 2000;164(12):6480–6486. [DOI] [PubMed] [Google Scholar]
- 184.Salazar-Mather TP, Orange JS, Biron CA. Early murine cytomegalovirus (MCMV) infection induces liver natural killer (NK) cell inflammation and protection through macrophage inflammatory protein 1alpha (MIP-1alpha)-dependent pathways. The Journal of experimental medicine. 1998;187(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zhang X, Sun S, Hwang I, Tough DF, Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8(5):591–599. [DOI] [PubMed] [Google Scholar]
- 186.Mattei F, Schiavoni G, Belardelli F, Tough DF. IL-15 is expressed by dendritic cells in response to type I IFN, double-stranded RNA, or lipopolysaccharide and promotes dendritic cell activation. Journal of immunology. 2001;167(3):1179–1187. [DOI] [PubMed] [Google Scholar]
- 187.Doherty DG. Immunity, tolerance and autoimmunity in the liver: A comprehensive review. J Autoimmun. 2016;66:60–75. [DOI] [PubMed] [Google Scholar]
- 188.Adams DH, Eksteen B. Aberrant homing of mucosal T cells and extra-intestinal manifestations of inflammatory bowel disease. Nature reviews Immunology. 2006;6(3):244–251. [DOI] [PubMed] [Google Scholar]
- 189.Hillan KJ, Hagler KE, MacSween RN, et al. Expression of the mucosal vascular addressin, MAdCAM-1, in inflammatory liver disease. Liver. 1999;19(6):509–518. [DOI] [PubMed] [Google Scholar]
- 190.Grant AJ, Lalor PF, Hubscher SG, Briskin M, Adams DH. MAdCAM-1 expressed in chronic inflammatory liver disease supports mucosal lymphocyte adhesion to hepatic endothelium (MAdCAM-1 in chronic inflammatory liver disease). Hepatology. 2001;33(5):1065–1072. [DOI] [PubMed] [Google Scholar]
- 191.Eksteen B, Grant AJ, Miles A, et al. Hepatic endothelial CCL25 mediates the recruitment of CCR9+ gut-homing lymphocytes to the liver in primary sclerosing cholangitis. The Journal of experimental medicine. 2004;200(11):1511–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Mackay IR, Degail P. Thymic “Germinal Centres” and Plasma Cells in Systemic Lupus Erythematosus. Lancet. 1963;2(7309):667. [DOI] [PubMed] [Google Scholar]
- 193.Walker JG, Doniach D, Roitt IM, Sherlock S. Serological Tests in Diagnosis of Primary Biliary Cirrhosis. Lancet. 1965;1(7390):827–831. [DOI] [PubMed] [Google Scholar]
- 194.Gershwin ME, Mackay IR, Sturgess A, Coppel RL. Identification and specificity of a cDNA encoding the 70 kd mitochondrial antigen recognized in primary biliary cirrhosis. Journal of immunology. 1987;138(10):3525–3531. [PubMed] [Google Scholar]
- 195.Shimoda S, Miyakawa H, Nakamura M, et al. CD4 T-cell autoreactivity to the mitochondrial autoantigen PDC-E2 in AMA-negative primary biliary cirrhosis. J Autoimmun. 2008;31(2):110–115. [DOI] [PubMed] [Google Scholar]
- 196.Nishioji K, Okanoue T, Itoh Y, et al. Increase of chemokine interferon-inducible protein-10 (IP-10) in the serum of patients with autoimmune liver diseases and increase of its mRNA expression in hepatocytes. Clinical and experimental immunology. 2001;123(2):271–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science. 2011;332(6037):1519–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Bian Z, Peng Y, You Z, et al. CCN1 expression in hepatocytes contributes to macrophage infiltration in nonalcoholic fatty liver disease in mice. J Lipid Res. 2013;54(1):44–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Egan CE, Daugherity EK, Rogers AB, Abi Abdallah DS, Denkers EY, Maurer KJ. CCR2 and CD44 promote inflammatory cell recruitment during fatty liver formation in a lithogenic diet fed mouse model. PLoS One. 2013;8(6):e65247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Baeck C, Wehr A, Karlmark KR, et al. Pharmacological inhibition of the chemokine CCL2 (MCP-1) diminishes liver macrophage infiltration and steatohepatitis in chronic hepatic injury. Gut. 2012;61(3):416–426. [DOI] [PubMed] [Google Scholar]
- 201.Napoli J, Bishop GA, McGuinness PH, Painter DM, McCaughan GW. Progressive liver injury in chronic hepatitis C infection correlates with increased intrahepatic expression of Th1-associated cytokines. Hepatology. 1996;24(4):759–765. [DOI] [PubMed] [Google Scholar]
- 202.Bromley SK, Mempel TR, Luster AD. Orchestrating the orchestrators: chemokines in control of T cell traffic. Nature immunology. 2008;9(9):970–980. [DOI] [PubMed] [Google Scholar]
- 203.Thimme R, Binder M, Bartenschlager R. Failure of innate and adaptive immune responses in controlling hepatitis C virus infection. FEMS Microbiol Rev. 2012;36(3):663–683. [DOI] [PubMed] [Google Scholar]
- 204.Kang W, Shin EC. Clinical implications of chemokines in acute and chronic hepatitis C virus infection. Yonsei Med J. 2011;52(6):871–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Kim CH, Kunkel EJ, Boisvert J, et al. Bonzo/CXCR6 expression defines type 1-polarized T-cell subsets with extralymphoid tissue homing potential. The Journal of clinical investigation. 2001;107(5):595–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Neuman MG, Benhamou JP, Marcellin P, et al. Cytokine--chemokine and apoptotic signatures in patients with hepatitis C. Transl Res. 2007;149(3):126–136. [DOI] [PubMed] [Google Scholar]
- 207.Fahey S, Dempsey E, Long A. The role of chemokines in acute and chronic hepatitis C infection. Cell Mol Immunol. 2014;11(1):25–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Yoong KF, Afford SC, Jones R, et al. Expression and function of CXC and CC chemokines in human malignant liver tumors: a role for human monokine induced by gamma-interferon in lymphocyte recruitment to hepatocellular carcinoma. Hepatology. 1999;30(1):100–111. [DOI] [PubMed] [Google Scholar]
- 209.Li W, Gomez E, Zhang Z. Immunohistochemical expression of stromal cell-derived factor-1 (SDF-1) and CXCR4 ligand receptor system in hepatocellular carcinoma. J Exp Clin Cancer Res. 2007;26(4):527–533. [PubMed] [Google Scholar]
- 210.Sutton A, Friand V, Brule-Donneger S, et al. Stromal cell-derived factor-1/chemokine (C-X-C motif) ligand 12 stimulates human hepatoma cell growth, migration, and invasion. Mol Cancer Res. 2007;5(1):21–33. [DOI] [PubMed] [Google Scholar]
- 211.Liu H, Pan Z, Li A, et al. Roles of chemokine receptor 4 (CXCR4) and chemokine ligand 12 (CXCL12) in metastasis of hepatocellular carcinoma cells. Cell Mol Immunol. 2008;5(5):373–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Nahon P, Sutton A, Rufat P, et al. Chemokine system polymorphisms, survival and hepatocellular carcinoma occurrence in patients with hepatitis C virus-related cirrhosis. World journal of gastroenterology : WJG. 2008;14(5):713–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Rubie C, Frick VO, Wagner M, et al. Enhanced expression and clinical significance of CC-chemokine MIP-3 alpha in hepatocellular carcinoma. Scandinavian journal of immunology. 2006;63(6):468–477. [DOI] [PubMed] [Google Scholar]
- 214.Fujii H, Itoh Y, Yamaguchi K, et al. Chemokine CCL20 enhances the growth of HuH7 cells via phosphorylation of p44/42 MAPK in vitro. Biochemical and biophysical research communications. 2004;322(3):1052–1058. [DOI] [PubMed] [Google Scholar]
- 215.Rubie C, Frick VO, Wagner M, et al. Chemokine expression in hepatocellular carcinoma versus colorectal liver metastases. World journal of gastroenterology : WJG. 2006;12(41):6627–6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Matsubara T, Ono T, Yamanoi A, Tachibana M, Nagasue N. Fractalkine-CX3CR1 axis regulates tumor cell cycle and deteriorates prognosis after radical resection for hepatocellular carcinoma. J Surg Oncol. 2007;95(3):241–249. [DOI] [PubMed] [Google Scholar]
- 217.Tang L, Hu HD, Hu P, et al. Gene therapy with CX3CL1/Fractalkine induces antitumor immunity to regress effectively mouse hepatocellular carcinoma. Gene Ther. 2007;14(16):1226–1234. [DOI] [PubMed] [Google Scholar]
- 218.Liu Y, Poon RT, Feng X, Yu WC, Luk JM, Fan ST. Reduced expression of chemokine receptors on peripheral blood lymphocytes in patients with hepatocellular carcinoma. The American journal of gastroenterology. 2004;99(6):1111–1121. [DOI] [PubMed] [Google Scholar]
- 219.Peralta C, Jimenez-Castro MB, Gracia-Sancho J. Hepatic ischemia and reperfusion injury: effects on the liver sinusoidal milieu. Journal of hepatology. 2013;59(5):1094–1106. [DOI] [PubMed] [Google Scholar]
- 220.Gujral JS, Bucci TJ, Farhood A, Jaeschke H. Mechanism of cell death during warm hepatic ischemia-reperfusion in rats: apoptosis or necrosis? Hepatology. 2001;33(2):397–405. [DOI] [PubMed] [Google Scholar]
- 221.Teoh NC, Farrell GC. Hepatic ischemia reperfusion injury: pathogenic mechanisms and basis for hepatoprotection. J Gastroenterol Hepatol. 2003;18(8):891–902. [DOI] [PubMed] [Google Scholar]
- 222.Jaeschke H Mechanisms of Liver Injury. II. Mechanisms of neutrophil-induced liver cell injury during hepatic ischemia-reperfusion and other acute inflammatory conditions. American journal of physiology Gastrointestinal and liver physiology. 2006;290(6):G1083–1088. [DOI] [PubMed] [Google Scholar]
- 223.Shimizu H, Kataoka M, Ohtsuka M, et al. Extended cold preservation of the graft liver enhances neutrophil-mediated pulmonary injury after liver transplantation. Hepatogastroenterology. 2005;52(64):1172–1175. [PubMed] [Google Scholar]
- 224.Honda M, Takeichi T, Hashimoto S, et al. Intravital Imaging of Neutrophil Recruitment Reveals the Efficacy of FPR1 Blockade in Hepatic Ischemia-Reperfusion Injury. Journal of immunology. 2017;198(4):1718–1728. [DOI] [PubMed] [Google Scholar]
- 225.Hamada T, Fondevila C, Busuttil RW, Coito AJ. Metalloproteinase-9 deficiency protects against hepatic ischemia/reperfusion injury. Hepatology. 2008;47(1):186–198. [DOI] [PubMed] [Google Scholar]
- 226.Lohr KM, Kurth CA, Xie DL, Seyer JM, Homandberg GA. The amino-terminal 29- and 72-Kd fragments of fibronectin mediate selective monocyte recruitment. Blood. 1990;76(10):2117–2124. [PubMed] [Google Scholar]
- 227.Friedman BH, Wolf JH, Wang L, et al. Serum cytokine profiles associated with early allograft dysfunction in patients undergoing liver transplantation. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2012;18(2):166–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Raschzok N, Reutzel-Selke A, Schmuck RB, et al. CD44 and CXCL9 serum protein levels predict the risk of clinically significant allograft rejection after liver transplantation. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2015;21(9):1195–1207. [DOI] [PubMed] [Google Scholar]
- 229.Obara H, Nagasaki K, Hsieh CL, et al. IFN-gamma, produced by NK cells that infiltrate liver allografts early after transplantation, links the innate and adaptive immune responses. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2005;5(9):2094–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Krukemeyer MG, Moeller J, Morawietz L, et al. Description of B lymphocytes and plasma cells, complement, and chemokines/receptors in acute liver allograft rejection. Transplantation. 2004;78(1):65–70. [DOI] [PubMed] [Google Scholar]
- 231.Mitsuhashi N, Wu GD, Zhu H, et al. Rat chemokine CXCL11: structure, tissue distribution, function and expression in cardiac transplantation models. Mol Cell Biochem. 2007;296(1–2):1–9. [DOI] [PubMed] [Google Scholar]
- 232.Kariya T, Ueta H, Xu XD, et al. Direct evidence for activated CD8+ T cell transmigration across portal vein endothelial cells in liver graft rejection. J Gastroenterol. 2016;51(10):985–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Baldwin WM, 3rd, Larsen CP, Fairchild RL. Innate immune responses to transplants: a significant variable with cadaver donors. Immunity. 2001;14(4):369–376. [DOI] [PubMed] [Google Scholar]
- 234.Liu B, Li J, Yan LN. Chemokines in chronic liver allograft dysfunction pathogenesis and potential therapeutic targets. Clinical & developmental immunology. 2013;2013:325318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Peiseler M, Sebode M, Franke B, et al. FOXP3+ regulatory T cells in autoimmune hepatitis are fully functional and not reduced in frequency. Journal of hepatology. 2012;57(1):125–132. [DOI] [PubMed] [Google Scholar]
- 236.Pascual G, Fong AL, Ogawa S, et al. A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-gamma. Nature. 2005;437(7059):759–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Antonelli A, Rotondi M, Ferrari SM, et al. Interferon-gamma-inducible alpha-chemokine CXCL10 involvement in Graves’ ophthalmopathy: modulation by peroxisome proliferator-activated receptor-gamma agonists. J Clin Endocrinol Metab. 2006;91(2):614–620. [DOI] [PubMed] [Google Scholar]
- 238.Antonelli A, Ferrari SM, Fallahi P, et al. Monokine induced by interferon gamma (IFNgamma) (CXCL9) and IFNgamma inducible T-cell alpha-chemoattractant (CXCL11) involvement in Graves’ disease and ophthalmopathy: modulation by peroxisome proliferator-activated receptor-gamma agonists. J Clin Endocrinol Metab. 2009;94(5):1803–1809. [DOI] [PubMed] [Google Scholar]
- 239.Antonelli A, Ferrari SM, Frascerra S, et al. Peroxisome proliferator-activated receptor alpha agonists modulate Th1 and Th2 chemokine secretion in normal thyrocytes and Graves’ disease. Exp Cell Res. 2011;317(11):1527–1533. [DOI] [PubMed] [Google Scholar]
- 240.Lee JW, Bajwa PJ, Carson MJ, et al. Fenofibrate represses interleukin-17 and interferon-gamma expression and improves colitis in interleukin-10-deficient mice. Gastroenterology. 2007;133(1):108–123. [DOI] [PubMed] [Google Scholar]
- 241.Xu HB, Gong YP, Cheng J, Chu YW, Xiong SD. CXCL16 participates in pathogenesis of immunological liver injury by regulating T lymphocyte infiltration in liver tissue. World journal of gastroenterology : WJG. 2005;11(32):4979–4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Hintermann E, Bayer M, Pfeilschifter JM, Luster AD, Christen U. CXCL10 promotes liver fibrosis by prevention of NK cell mediated hepatic stellate cell inactivation. J Autoimmun. 2010;35(4):424–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Affo S, Morales-Ibanez O, Rodrigo-Torres D, et al. CCL20 mediates lipopolysaccharide induced liver injury and is a potential driver of inflammation and fibrosis in alcoholic hepatitis. Gut. 2014;63(11):1782–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Berres ML, Koenen RR, Rueland A, et al. Antagonism of the chemokine Ccl5 ameliorates experimental liver fibrosis in mice. The Journal of clinical investigation. 2010;120(11):4129–4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Lefebvre E, Moyle G, Reshef R, et al. Antifibrotic Effects of the Dual CCR2/CCR5 Antagonist Cenicriviroc in Animal Models of Liver and Kidney Fibrosis. PLoS One. 2016;11(6):e0158156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Berahovich RD, Zabel BA, Lewen S, et al. Endothelial expression of CXCR7 and the regulation of systemic CXCL12 levels. Immunology. 2014;141(1):111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Dorr P, Westby M, Dobbs S, et al. Maraviroc (UK-427,857), a potent, orally bioavailable, and selective small-molecule inhibitor of chemokine receptor CCR5 with broad-spectrum anti-human immunodeficiency virus type 1 activity. Antimicrob Agents Chemother. 2005;49(11):4721–4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.van Lelyveld SF, Wensing AM, Hoepelman AI. The MOTIVATE trials: maraviroc therapy in antiretroviral treatment-experienced HIV-1-infected patients. Expert Rev Anti Infect Ther. 2012;10(11):1241–1247. [DOI] [PubMed] [Google Scholar]
- 249.Rawla P, Sunkara T, Raj JP. Role of biologics and biosimilars in inflammatory bowel disease: current trends and future perspectives. J Inflamm Res. 2018;11:215–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Hesterberg PE, Winsor-Hines D, Briskin MJ, et al. Rapid resolution of chronic colitis in the cotton-top tamarin with an antibody to a gut-homing integrin alpha 4 beta 7. Gastroenterology. 1996;111(5):1373–1380. [DOI] [PubMed] [Google Scholar]
- 251.Soler D, Chapman T, Yang LL, Wyant T, Egan R, Fedyk ER. The binding specificity and selective antagonism of vedolizumab, an anti-alpha4beta7 integrin therapeutic antibody in development for inflammatory bowel diseases. The Journal of pharmacology and experimental therapeutics. 2009;330(3):864–875. [DOI] [PubMed] [Google Scholar]
- 252.Gensicke H, Leppert D, Yaldizli O, et al. Monoclonal antibodies and recombinant immunoglobulins for the treatment of multiple sclerosis. CNS Drugs. 2012;26(1):11–37. [DOI] [PubMed] [Google Scholar]
- 253.Zhang Y, Wang H. Integrin signalling and function in immune cells. Immunology. 2012;135(4):268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Tse CS, Loftus EV Jr., Raffals LE, Gossard AA, Lightner AL. Effects of vedolizumab, adalimumab and infliximab on biliary inflammation in individuals with primary sclerosing cholangitis and inflammatory bowel disease. Aliment Pharmacol Ther. 2018;48(2):190–195. [DOI] [PubMed] [Google Scholar]
- 255.Christensen B, Micic D, Gibson PR, et al. Vedolizumab in patients with concurrent primary sclerosing cholangitis and inflammatory bowel disease does not improve liver biochemistry but is safe and effective for the bowel disease. Aliment Pharmacol Ther. 2018;47(6):753–762. [DOI] [PMC free article] [PubMed] [Google Scholar]


