Abstract
Introduction
One in four diabetes patients will develop a foot ulcer over their lifetime. The role of glycaemic control in the healing of foot ulcers in diabetes patients is not supported by randomised controlled trial (RCT) data.
Objectives
To determine the feasibility of an RCT of glycaemic control with intensive insulin therapy in diabetic foot ulcer, by assessing: entry criteria, fasting capillary blood glucose (FCBG) medication satisfaction and sensitivity of different ulcer-healing endpoints to glycaemic control.
Design
Two substudies: one cross-sectional and one single-arm prospective.
Setting
Single-centre secondary care diabetic foot clinic in New Zealand.
Participants
Substudy 1: 78 participants consisting of all people ≥18 years with a diabetic foot ulcer presenting to the clinic over 35 weeks in 2015.
Substudy 2: 15 participants from Substudy 1 consenting to intensive insulin therapy.
Intervention
Substudy 1: None.
Substudy 2: Intensive insulin therapy with standard podiatry care over 24 weeks.
Outcome
Substudy 1: Proportion of participants satisfying potential RCT entry criteria; medication satisfaction (Diabetes Medication Satisfaction).
Substudy 2: FCBG, index ulcer healing time, index ulcer size, health-related quality of life (HRQoL; EuroQol 5 Dimensions 5 Levels and Diabetic Foot Ulcer Scale-Short Form).
Results
Proportion in Substudy 1 satisfying all entry criteria was 31% (95% CI 21 to 42). FCBG values decreased between baseline and study end (difference −3.7 mmol/L, 95% CI −6.5 to −0.8); 83% (95% CI 44 to 95) of ulcers healed by 24 weeks. FCBG correlated negatively with medication satisfaction. Ulcer area logarithm was most sensitive to FCBG changes, displaying significant negative correlation with HRQoL outcomes. Detecting a 30% between-group difference in this outcome (80% power, α=5%) requires 220 participants per arm, achievable within 1 year with 15 centres similar to study setting.
Conclusions
An adequately powered RCT requires cooperation between a large number of centres. Ulcer area logarithm should be primary endpoint.
Trial registration number
ANZCTR ACTRN12617001414303
Keywords: diabetic foot, wound management, statistics & research methods
Strengths and limitations of this study.
First study in 15 years to examine the feasibility of a definitive randomised controlled trial (RCT) of intensive insulin therapy for diabetic foot ulcers.
Use of imaging techniques allowed assessment of various ulcer-size-related outcomes as potential primary endpoints for an RCT.
The target sample size of 20 for Substudy 2, examining the relationship between ulcer healing and glycaemic control, was not achieved during the study period.
The study was conducted in a single centre in New Zealand, limiting generalisability to other populations and settings with different pathways for diabetes and diabetic complications.
Introduction
One in four diabetes patients will develop a foot ulcer in their lifetime.1 Diabetic foot ulcer is one of the most significant complications of diabetes1–4 and often responds poorly to treatment, with only one-third of those managed in secondary care healing by 3 months and one-half at 6 months.5 Non-healing ulcers are an important cause of lower extremity amputation. Most notable causes of foot ulceration are peripheral neuropathy, peripheral vascular disease and structural foot disease.6 7 These factors are linked to hyperglycaemia8–10 and pathological states associated with diabetes.
A meta-analysis of nine randomised controlled trials in nearly 11 000 participants showed that intensive glycaemic control potentially improved the incidence of diabetic foot ulcer, decreased the risk of amputation and improved sensory nerve function compared with less intensive control.11 As a result, clinical practice guidelines from the Society for Vascular Surgery, American Podiatric Medical Association and Society for Vascular Medicine now recommend adequate glycaemic control (glycosylated haemoglobin (HbA1c) <53 mmol/mol) to reduce the incidence of diabetic foot ulcers.1
The evidence that improved glycaemic control can accelerate the healing of foot ulcers and reduce the incidence of ulceration and amputation remains observational.12–18 There is no randomised trial evidence that tight glycaemic control improves ulcer wound healing.11 A previous feasibility study in this area concluded 15 years ago that a definitive randomised trial in this area19 was not feasible, possibly due to exacting entry criteria. To investigate further whether a randomised controlled trial (RCT) evaluating the efficacy of intensive insulin therapy on diabetic foot ulcer healing is possible, this two-part feasibility study sought appropriate but less stringent trial entry criteria to improve accrual rates (Substudy 1) and sought appropriate endpoints for such a trial (Substudy 2). In Substudy 2, presence or absence of peripheral neuropathy, peripheral vascular disease and foot deformities were noted as they were identified factors in the genesis of diabetic foot ulcers. Data on microcirculation in the feet were also collected using novel laser imaging technology. This report focuses on data from Substudy 1 and Substudy 2; the laser imaging data analysis will be reported separately.
The primary objective of Substudy 1 was to estimate the proportion of participants satisfying the entry criteria for the planned RCT. Entry criteria used in the previous feasibility study19 were revised as follows: removing the requirement for chronic ulcers (>4 weeks), removing the ulcer size criterion (25 to 2500 mm2) and including participants with a higher HbA1c (≥60 mmol/mol), renal disease and/or a history of hypoglycaemia. Secondary objectives were to estimate the length of the recruitment period for the intended RCT, and determine participant satisfaction with their diabetes medication.
In Substudy 2, the main objective was to determine a primary endpoint for the RCT by analysing sensitivity of ulcer healing-related outcomes (ulcer area, change in ulcer area and time to complete healing) to glycaemic control accounting for standard podiatry care. The ulcer healing outcome measure with the best association with glycaemic control was to be assessed for convergent validity with an established foot ulcer scale. Secondary objectives included examining the relationship between improved glycaemic control as well as attendance, and satisfaction with diabetes medication, and evaluating health-related quality of life (HRQoL) measures in this population.
Materials and methods
Setting and study design
The study was conducted at the Counties Manukau Health (CMH) Diabetes Foot Clinic in Auckland, New Zealand, between 2 February 2015 and 28 September 2015, with Substudy 2 follow-up to 20 February 2016. Substudy 1 was a cross-sectional study enrolling all people aged ≥18 years with diabetes and a current foot ulcer presenting at the CMH Diabetes Foot Clinic over a period of 24 weeks. Substudy 2 was a single-arm interventional study enrolling Substudy 1 participants meeting all entry criteria (table 1) and treating them with intensive insulin therapy for 24 weeks.
Table 1.
Entry criteria assessed in substudy 1
| Criteria | Notation | Description |
| Inclusion | IC1 | Male or female aged ≥18 years |
| IC2 | Type 1 or type 2 diabetes mellitus for more than 1 year with an HbA1c ≥60 mmol/mol | |
| IC3 | Incident foot ulcer(s) located below the level of the malleoli | |
| IC4 | Able and willing to undertake home blood glucose monitoring and administer insulin up to four times daily under the supervision of the diabetes nurse specialist | |
| IC5 | Able and willing to provide informed consent to participate in the study | |
| Exclusion | EC1 | Ulcers with radiological features of osteomyelitis |
| EC2 | Significant peripheral vascular disease under consideration for re-vascularisation | |
| EC3 | Significant bone deformity as determined by the investigator which may delay wound healing | |
| EC4 | Non-adherence to standard care | |
| EC5 | Any other disease or condition in the opinion of the investigator could make them unsuitable for entry | |
| Full | FC | All inclusion criterion satisfied (5-Yes) and all exclusion criterion satisfied (5-No) |
EC, exclusion criteria;FC, full criteria; HbA1c, glycosylated haemoglobin; IC, inclusion criteria.
All participants provided informed written consent.
Accrual periods and sample size
The accrual period for Substudy 1 (35 weeks) was selected as long as possible while respecting contractual obligations with the study funder. To distinguish between recruitment of existing patients and new patients, we prospectively defined two recruitment periods. During the first period of recruitment (Period A) all patients attending the diabetic foot clinic were to be recruited. Period A was to finish from the moment patients already attending the clinic were recruited, at which point only newly enrolled clinic patients started to be recruited in the study, giving way to Period B (figure 1).
Figure 1.
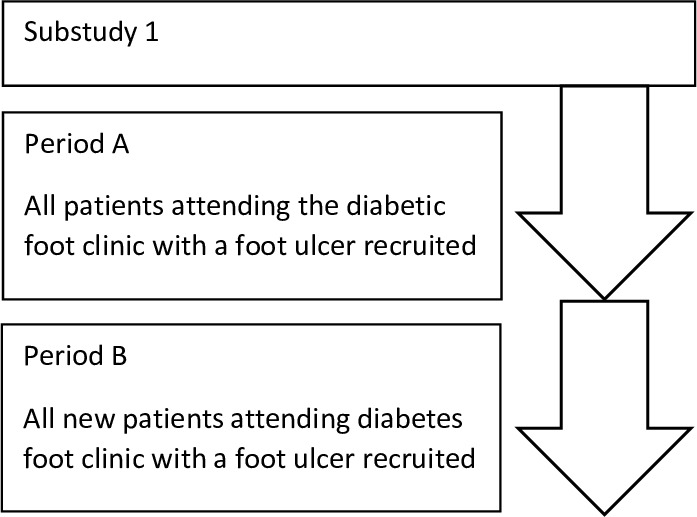
Substudy 1 recruitment flow diagram.
Substudy 2 aimed to recruit 20 participants. Assuming 10% attrition, this number is sufficient to estimate the SD of a continuous variable (eg, ulcer area) with a coefficient of variation inferior to 0.2. This criterion is consistent with the goal of identifying a sensitive primary endpoint endowed with good precision.
Patient and public involvement
Patients’ priorities, experience and preferences were taken into account through the clinical experience of the study team, who devised the research question associated with the intended full study. The participants were not involved in the design of this study; however, one of the objectives of the feasibility study was to obtain feedback from participants that would inform the design of a potentially larger study. Patients were not involved in the recruitment or conduct of the study. The participants will be provided access to the research paper. The results of the study will be displayed in the podiatry clinic where the participants attend.
Study procedures and outcomes
Substudy 1 participants completed a Diabetes Medication Satisfaction (DiabMedSat) questionnaire.20 21 Their foot wound(s) (index ulcers) were inspected, and entry criteria status (table 1) and demographic data recorded. In Substudy 2, visits occurred weekly between weeks 1 (baseline) and 4, then at 6, 8, 12, 16, 20 and 24 weeks, or until the index ulcers healed. The following were undertaken at each visit: ulcer examination: digital photographic planimetry of ulcer area (using the SilhouetteStar camera, ARANZ Medical, New Zealand), fasting capillary blood glucose (FCBG) measurement (in mmol/L), medication review and adverse events assessment. FCBG was used in the analyses as a measure of glycaemic control22 HbA1c was assessed at baseline and at the end of trial, providing an assessment of chronic hyperglycaemia. By contrast, fasting capillary glucose or mean daily capillary glucose may provide evidence of acute improvement of glycaemia in a short-term clinical trial.22 In addition, participants completed three questionnaires at each visit: DiabMedSat,20 21 EuroQol 5 Dimensions 5 Levels (EQ-5D-5L)23 24 and Diabetic Foot Ulcer Scale-Short Form (DFS-SF).25
Intervention
On entry to Substudy 2, intermediate-acting or long-acting insulin was initiated or adjusted, and given in addition to usual oral hypoglycaemic tablet therapy (first line metformin, second line sulphonylurea). In addition participants received cholesterol-lowering medication, anti-hypertensives and aspirin to prevent cardiovascular disease consistent with international guidelines.26 Short-acting mealtime insulin was provided as appropriate. The goal was to maintain FCBG at 4 to 7 mmol/L, with ≤2 episodes of mild hypoglycaemia per week. If >2 episodes of mild hypoglycaemia occurred the target FCBG was raised. Within these parameters the choice of regimen was determined by the Diabetes Nurse Specialist.
Substudy 2 participants received usual podiatry care at each visit, including ulcer debridement, orthotics prescription and adjustments, antibiotics if indicated and education.
Statistical analyses
Substudy 1
Descriptive statistics were produced for participant demographic characteristics, recruitment rate, entry criteria fulfilment and DiabMedSat subscores. Multivariate analysis of variance (MANOVA) was used to detect differences in subscores based on demographic or participant characteristics. A Poisson exact test was used to compare recruitment rates over two recruitment periods and mixed logistic regression used to compare differences in entry criteria fulfilment between the periods. Indicators of criterion satisfaction were fitted jointly by themselves in a first model, and interacting with period in a second model. Models were compared using deviance tests.
Substudy 2
The analysis set for this feasibility study consisted of all participants having initiated treatment. All analyses were carried out on the index ulcers, present at the first visit. All participants had a single index ulcer.
Linear mixed models were used to determine the relationship between four different ulcer area-related outcomes at each visit (ulcer area in cm2, log(ulcer area +0.01), absolute and relative rates of change in ulcer area) and glycaemic control. (The value of 0.01 in the logarithmic endpoint has been previously validated as a surrogate marker of ulcer healing27 was chosen based on the data to improve the normality of the outcome.) FCBG was the fixed effect and participant of the random effect. Time-to-healing was considered as a fifth possible outcome. A Kaplan-Meier estimate of ulcer persistence probability was produced and Cox regression used to estimate the HR of ulcer healing under changes in FCBG, taken as a time-dependent covariate. Time-adjusted and/or baseline ulcer area-adjusted estimates, as well as unadjusted estimates, were produced to assess potential confounding, as tight management in a foot clinic may promote ulcer healing in several ways. The most sensitive outcome was selected by consideration of its adjusted observed significance level and its equivalent Cohen’s effect size where appropriate. To estimate correlations and their 95% CIs using all available longitudinal data, we fitted outcomes jointly using a heterogeneous compound symmetry covariance model, and using only intercepts as fixed effects. We thus obtained correlations between DFS-SF subscores and selected ulcer healing outcome; between DiabMedSat subscores and FCBG; and between DFS-SF subscores and EQ-5D-5L.
Descriptive statistics were produced on completed follow-ups (defined as healing before 24 weeks or attending the 24 week visit) and attended visits. Attendance probability was regressed on the most recently available FCBG, measure of ulcer area and DiabMedSat subscores using mixed logistic regression. The final attendance model was selected using Akaike’s information criterion (AIC).
No data were missing for time-to-healing analyses. Other analyses all involved mixed models on longitudinal data, known to alleviate selection bias due to missingness.28
Results
Participants
Seventy-eight participants (all unique clinic visitors during the recruitment period) were enrolled in Substudy 1 (table 2). Mean age was 57 years (SD 14). The majority were men and most were of Pacific or European ethnicity. No data were missing for Substudy 1. All participants were identified as having some measure of foot deformity, judged unlikely to affect ulcer healing by the treating podiatrists. However, no objective measure of foot deformity, to our knowledge, has been assessed for association with ulcer healing.
Table 2.
Characteristics of participants included in both substudies
| Substudy 1 participants (n=78) |
Substudy 2 participants (n=15) |
|
| Median age (IQR), years | 58.5 (18.2) | 51 (16.5) |
| Women, n (%) | 30 (38.5) | 6 (40) |
| Mean baseline HbA1c (SD), mmol/mol | Not collected | 93 (29) |
| Mean baseline FCBG (SD), mmol/L | Not collected | 11.3 (5.7) |
| Ethnicity, n (%) | ||
| Asian | 5 (6.4) | 1 (6.7) |
| European | 27 (34.6) | 4 (26.7) |
| Māori | 13 (16.7) | 2 (13.3) |
| Pacific | 33 (42.3) | 7 (46.7) |
| Type 1 diabetes mellitus, n (%) | 12 (15.4) | 3 (20.0) |
| Type 2 diabetes mellitus, n (%) | 66 (84.6) | 12 (80.0) |
| Duration of diabetes, n (%) | ||
| 0–10 years | 15 (19.2) | 3 (20.0) |
| 10–20 years | 31 (39.7) | 6 (40.0) |
| 20–30 years | 22 (28.2) | 4 (26.7) |
| 30–40 years | 7 (9.0) | 2 (13.3) |
| 40–50 years | 3 (3.9) | 0 (0.0) |
| Peripheral neuropathy, n (%) | Not collected | 15 (100.0) |
| Peripheral vascular disease, n (%) | Not collected | 1 (6.7) |
FCBG, fasting capillary blood glucose; HbA1c, glycosylated haemoglobin.
Seventeen participants entered Substudy 2. One withdrew consent after Visit 1 and another was healed on Visit 1, leaving 15 participants in the analysis set, assessed on a total of 90 occasions.
Completeness
Two participants with unhealed ulcers did not attend the 24 week visit (13%, 95% CI 2 to 40). Of the 102 scheduled visits, 12 were missed, yielding an attendance proportion of 91% (95% CI 79 to 96), accounting for clustering by participant. Average follow-up time to healing or last visit was 17.6 weeks (SD 7.8). Clinical constraints or patient choice prevented some measurements from being taken, yielding completeness proportions for specific outcomes between 78% (DiabMedSat and FCBG) and 85% (ulcer area).
Recruitment rate and entry criteria
Period A (as defined earlier) finished after 6 weeks, and was the most active with 42 participants recruited compared with 36 participants over the remaining 29 weeks, forming Period B.
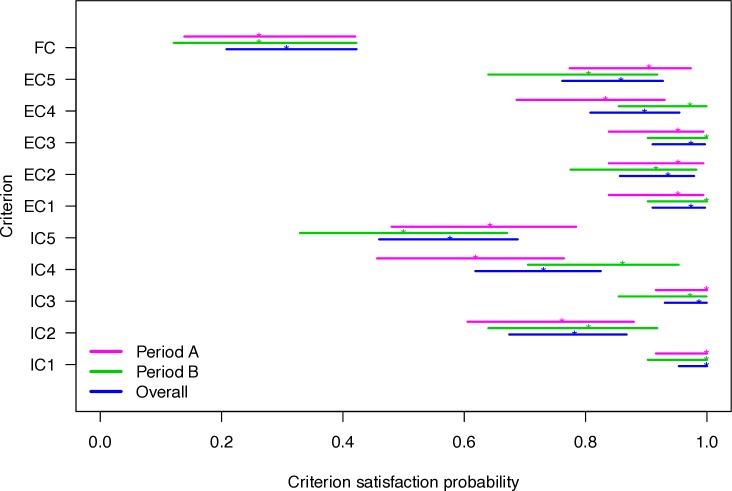
All participants satisfied criterion inclusion criteria 1 (IC1), 78.2% met IC2, 98.7% met IC3, 73.1% met IC4 and 57.7% met IC5. Twenty-four participants (30.8%, 95% CI 20.8 to 42.2) met all criteria. Removal of any single criteria increased eligibility proportion appreciably only in the case of IC2 (to 37.2%, 95% CI 26.5 to 48.9) and IC5 (to 38.5%, 95% CI 27.7 to 50.2). The probabilities of participants meeting entry criteria differed between periods A and B (figure 2); the criterion-period interaction term was significant (p=0.009). The A-to-B rate ratio of eligibility to a full study was 4.1 (95% CI 1.8 to 9.2). The eligibility rate in period B was 0.45 participant per week (95% CI 0.24 to 0.77).
Figure 2.
Point estimate and CI of probability of each criterion being met by recruitment period. Period A was 0 to 5 weeks and period B was 6 to 35 weeks.EC, exclusion criteria; FC, full criteria; IC, inclusion criteria
Diabetes medication satisfaction
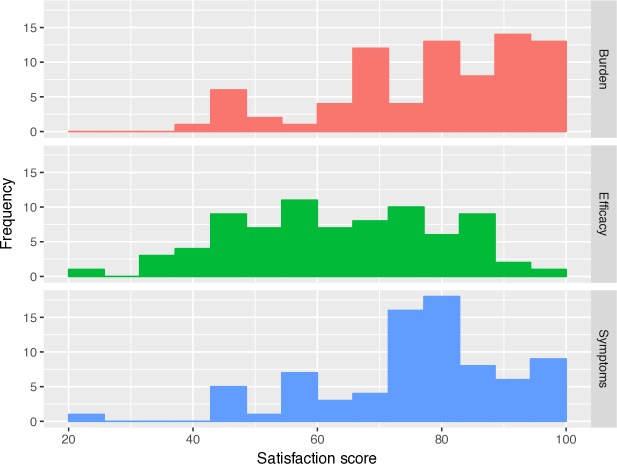
Higher scores on the DiabMedSat subscales indicate increased satisfaction for all three subscales. The subscale histograms are displayed in figure 3. Median score for the Efficacy subscale was 63.3 (IQR 26.7), and was numerically lower than those of the Burden (81.8 (IQR 22.8)) and Symptom subscales (80.0 (IQR 16.0)). MANOVA demonstrated no significant variation in subscale scores based on ethnicity, sex or diabetes duration, but there was significant variation in the Burden (p=0.005) and Symptom (p=0.026) scores based on age (generally higher in older participants).
Figure 3.
Histograms of participant scores on the three subscales of the Diabetes Medication Satisfaction questionnaire: (a) burden, (b) symptoms and (c) efficacy subscales. Subscales are scored from 0 to 100, higher scores indicating greater satisfaction.
Glycaemic control
FCBG values during the study indicated that participants were generally adhering to their glycaemic control regimen. Between study end and baseline, the mean difference in FCBG was −3.7 mmol/L (95% CI −6.5 to −0.8) and in HbA1c was −9.4 mmol/mol (95% CI −19.0 to 0.3). The fasting blood glucose of participants of Substudy 2 over time are shown in figure 4.
Figure 4.
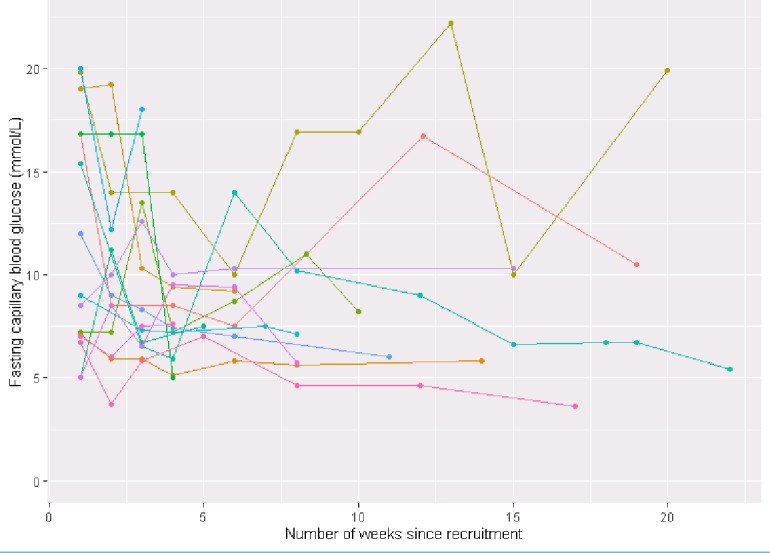
Fasting capillary blood glucose against time from study entry during intensive insulin therapy in the 15 participants of substudy 2.
Selection of primary endpoint for RCT
Twelve Substudy 2 participants experienced complete healing between weeks 3 and 24; the remaining three had unhealed ulcers at the time of their last visit on or before week 24. Median ulcer-healing time was 7 weeks. The Kaplan-Meier estimate of the proportion of ulcers not healing by week 24 was 16.7% (95% CI 5.0 to 56.1). The Cox model showed no significant association between time to ulcer healing and FCBG (table 3).
Table 3.
Unadjusted and adjusted regression results for five ulcer-healing endpoints against fasting capillary blood glucose
| Ulcer outcome | Adjustment status | Change in outcome per 1 mmol/L increase in FCBG |
Equivalent effect size | P value | |
| Estimate | 95% CI | ||||
| Ulcer area (cm2) * | Unadjusted | 0.010 | –0.041 to 0.062 | 0.004 | 0.69 |
| Adjusted† | 0.004 | −0.024 to 0.032 | 0.001 | 0.77 | |
| Log(ulcer area +0.01) * | Unadjusted | 0.134 | 0.049 to 0.214 | 0.10 | 0.002** |
| Adjusted† | 0.114 | 0.020 to 0.194 | 0.08 | 0.0145* | |
| Rate of absolute change (cm2/week) * | Unadjusted | 0.011 | −0.018 to 0.041 | 0.01 | 0.45 |
| Adjusted‡ | 0.009 | −0.014,0.032 | 0.01 | 0.70 | |
| Rate of relative change (%/week-1) * | Unadjusted | 5.7 | 1.1 to 10.3 | 0.06 | 0.015* |
| Adjusted‡ | 5.7 | 1.1 to 10.3 | 0.06 | 0.015* | |
| HR§ | Unadjusted | 0.90 | 0.74 to 1.10 | N/A | 0.31 |
| Adjusted ¶ | 0.88 | 0.71 to 1.08 | N/A | 0.21 | |
*Mixed linear regression, additive change reported.
†Adjusted for baseline value and time since Visit 1.
‡Adjusted for time since Visit 1 only.
§Cox regression, multiplicative change reported.
¶Adjusted for baseline ulcer area.
FCBG, fasting capillary blood glucose.
Log(ulcer area in cm2 +0.01) (hereafter ‘log of ulcer area’) and ulcer area relative rate of change were the only ulcer healing outcomes sensitive to change in FCBG (table 3). The former was selected as most sensitive to changes in FCBG, with effect size of 0.08 per mmol/L increase in FCBG, adjusted for both time and baseline value. This corresponds to a 30% reduction in ulcer area with a 3 mmol/L improvement in FCBG. Time adjustment was intended to account for non-glycaemia-related improvements and interventions such as podiatric care. Between-participant and within-participant outcome variances were estimated at 1.3 and 0.4, respectively.
Validation of selected outcome with QoL measures
DFS-SF scores tended to increase over time (ie, improved HRQoL); increases reached statistical significance for four of the six subscales (Leisure (p=0.05), Dependence (p=0.01), Negative emotion (p=0.001) and Worried about ulcers (p=0.04)). All six subscales showed statistically significant, moderate-to-strong negative correlation with log of ulcer area (table 4).
Table 4.
Estimated Pearson correlation coefficients between diabetic foot ulcer scale-short form subscale scores and both log(ulcer area +0.01) and EuroQol 5 dimension 5 level
| Correlates | log(ulcer area +0.01)* | EQ-5D-5L VAS | ||||
| DFS-SF subscale | Est. | 95% CI | P value | Est. | 95% CI | P value |
| Leisure | −0.48 | –0.66 to 0.25 | <0.0001*** | 0.50 | 0.23 to 0.77 | 0.0002*** |
| Physical health | −0.48 | –0.66 to 0.26 | <0.0001*** | 0.64 | 0.44 to 0.84 | <0.0001*** |
| Dependence | −0.54 | –0.71 to 0.33 | <0.0001*** | 0.58 | 0.36 to 0.81 | <0.0001*** |
| Negative emotion | −0.64 | –0.80 to 0.42 | <0.0001*** | 0.38 | 0.04 to 0.72 | 0.03* |
| Worried about ulcers | −0.54 | –0.71 to 0.32 | <0.0001*** | 0.62 | 0.38 to 0.86 | <0.0001*** |
| Bothered by ulcer care | −0.46 | –0.63 to 0.24 | 0.0001*** | 0.36 | 0.04 to 0.69 | 0.03* |
*Area in cm2.
DFS-SF, Diabetic Foot Ulcer Scale-Short Form; EQ-5D-5L, EuroQol 5 Dimension 5 Level; VAS, visual analogue scale.
Participant satisfaction and improvement of fasting capillary blood glucose
Glycaemia levels displayed weak-to-moderate negative correlation with the DiabMedSat scores. The correlation of FCBG with the Burden subscale was −0.35 (95% CI −0.59 to −0.09; p=0.01), with the Efficacy subscale −0.42 (95% CI −0.61 to −0.18; p=0.0009) and with the Symptoms subscale −0.21 (95% CI −0.47 to 0.08, p=0.15).
Health-related QoL
The EQ-5D-5L visual analogue scale (VAS) displayed moderate-to-strong positive correlation with all six DFS-SF subscale scores (table 4).
Modelling of attendance
The model explaining attendance with smallest AIC involved the DiabMedSat Burden score only, with an attendance OR of 1.78 (95% CI 1.26 to 2.51; p=0.001) per 10-point score increase.
Discussion
Key findings
This feasibility study showed that by using less stringent entry criteria, more people with diabetic foot ulcers attending our foot clinic (30%) may be eligible for a proposed RCT investigating the efficacy of an intensive insulin regimen on diabetic foot ulcer healing than reported in a previous feasibility study (0.5% (1/200)).19 The study also showed that the primary endpoint of log of ulcer area, with ulcer area measured using digital photographic planimetry, may be sensitive enough to glycaemic control to base an RCT on in this population.
The mean HbA1c of Substudy group 2 prior to the intervention was 93 mmol/mol and the mean FCBG was 11.3 mmol/L. Depending on the model used to estimate HbA1c from FCBG there may appear to be a discrepancy. However, this is explained by the small number of participants, and the variability of contribution between FCBG and postprandial capillary blood glucose between individuals especially at higher HbA1c concentrations.22
In terms of design and conduct of an RCT of intensive glycaemic control versus standard care in people with diabetic foot ulcers, analysis showed that the largest gains in eligibility from removal of a single criterion would occur by waiving IC2 (type 1 or type 2 diabetes for greater than 1 year with an HbA1c of >60 mmol/mol) or IC5. IC5 cannot of course be waived, since participant consent is compulsory in any ethical clinical trial. However, if glycaemic control is efficacious in individuals with recently developed diabetes, IC2 could perhaps advantageously be relaxed. Those with recent onset diabetes maybe different to those with long-standing diabetes in ways that impact on ulcer healing. On the other hand, those with recently diagnosed diabetes will likely have years of exposure to risk factors they share with those with long-standing diabetes that predispose them to ulcer formation also.29
Further findings are indications that acting on the medication burden may improve attendance and that acting on medication satisfaction in general, and satisfaction with efficacy in particular, may improve adherence to the control regimen. In both cases, however, the evidence obtained is only correlational and not causal.
Relationship to other studies
Ulcer healing data suggested an early beneficial effect of intensive insulin therapy; median healing time was 7 weeks after the initial visit, and by week 21 an estimated 17% of ulcers had not healed. Over a similar timeframe, much lower healing rates have been reported with standard care (eg, 31% at 20 weeks in a meta-analysis30). Even when standard care included insulin, a retrospective cohort study found that only 30% of ulcers had healed after 1.1 month.31 The baseline mean HbA1c was lower in the participants of that study (7.9% or 63 mmol/mol) compared with our own (10.8% or 94 mmol/mol). While this finding is promising, a randomised controlled trial is needed to confirm that this is the effect of intensive blood glucose control. There are other factors that may account for more rapid ulcer healing in our study such as the high (weekly) frequency of the first four visits in Substudy 2, allowing more opportunities for treatment such as wound debridement, and orthotic or medication adjustments.
Implications for a full study
The log of the ulcer area outcome proved sensitive to glycaemic control even controlling for time since study entry, and was correlated with DFS-SF subscores, supporting the use of this measure. This is consistent with prior validation of log ulcer area as a surrogate endpoint for ulcer healing.27 Using this measure as primary endpoint, a target reduction in ulcer size of 30% would correspond to a 3 mmol/L average difference in FCBG (corresponding to the difference between the lower bounds of normal glycaemia and a diagnosis of diabetes), with intensive glycaemic control versus standard care. In an RCT, 220 participants per arm would be required to detect a between-group decrease of 30% with 80% power at a 5% significance level. At the differential eligibility rates observed in both periods and assuming a loss to follow-up of 10%, such a number could be achieved within about 1 year if 15 centres similar to ours were recruiting participants.
Good reductions in FCBG over the first 4 weeks of intensive insulin therapy were seen, but more variable levels observed afterwards. To achieve optimal improvements in glycaemic control over the course of a longer-term RCT more regular visits may be necessary after the first 4 to 6 weeks of therapy than were used in our feasibility study and more daytime FCBG recordings done to optimise therapy.
The EQ-5D VAS appeared to have good convergent validity with the specialised DFS-SF, indicating its appropriateness as a generic QoL measure in our study population, opening the door to valid economic analyses. We also realise the importance of objective quantification of neuropathy, peripheral vascular disease and foot deformities enabling stratification at randomisation in the larger trial as they are the most notable causative factors of diabetic foot ulceration.11
Tight glycaemic control relies on long-term patient adherence.1 32 Satisfied patients are more likely to adhere to recommendations regarding not only medication use and follow-up visits but also dietary habits and physical activity.33 Our findings showed that our participants’ perception of diabetes medication burden was strongly associated with fasting capillary blood glucose and attendance, suggests, although does not prove, that intervening on burden may promote attendance.
Limitations
Some limitations temper the interpretation of our results. The most important limitation was that Substudy 2 did not reach the target sample size of 20. This shortfall was largely due to availability of personnel and funder timelines. These issues, particularly around staffing, have the potential to affect any future RCT. Moreover, the study was conducted at a single centre in New Zealand, limiting generalisability to other populations and settings with different care pathways for diabetes and diabetic complications.
Another limitation of this study is that FCBG was the only surrogate for medication adherence. The addition of other surrogates such as a record of whether prescriptions had been filled would be preferable. Furthermore, in a study aiming to evaluate the four times a day blood glucose testing is preferable to FCBG and HbA1c.
Non-adherence to standard care was an exclusion criterion of this study. This was included so that the impact of glycaemic control would be the focus of this study. However, this criterion limits the application of the study to the real world as non-adherence is a major issue in most real-life clinical settings.
Conclusion
The study has produced evidence of moderate quality that tight glycaemic control may be beneficial for ulcer healing, and that an outcome derived from ulcer area could be sensitive to glycaemic control as expressed by FCBG.
This feasibility study is the first since 2005 to investigate issues relevant to the initiation of a definitive RCT evaluating the impact of intensive insulin therapy on ulcer healing in people with diabetes. The results of such a trial would be useful to inform evidence-based clinical practice guidelines. However, we have also demonstrated that a reasonably powered trial would require the involvement of a large number of centres, increasing the complexity of such an undertaking.
Supplementary Material
Acknowledgments
Medical writing assistance was provided by Nicola Ryan, independent medical writer. The authors thank Benjamin Elliott (independent data manager), Lawrence Kingi (podiatrist, Counties Manukau Health) and Dr John Baker (Middlemore Clinical Trials) for their assistance with this study.
Footnotes
Contributors: ACV and AD designed the study and co-wrote the manuscript with VB. AD oversaw the intervention and contributed to the discussion. ACV contributed to data management, data monitoring and analysis planning and conduct. DP contributed to statistical analyses. BM coordinated the research. RG and AN provided podiatric care and contributed to discussion. AD and ACV take responsibility for the contents of the article, study design, access to data and the decision to submit and publish the results.
Funding: This work was supported by the Health Research Council of New Zealand’s Feasibility Study grant number 14/605.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: Ethics approval was given for the study by the New Zealand Northern A Health and Disability Ethics Committee, ethics approval number 14/NTA/195.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request.
References
- 1. Hingorani A, LaMuraglia GM, Henke P, et al. The management of diabetic foot: a clinical practice guideline by the Society for vascular surgery in collaboration with the American podiatric Medical association and the Society for vascular medicine. J Vasc Surg 2016;63:3S–21. 10.1016/j.jvs.2015.10.003 [DOI] [PubMed] [Google Scholar]
- 2. Australian Institute of health and welfare, 2008. Available: http://wwwaihwgovau/WorkArea/DownloadAssetaspx?id=6442453674
- 3. Ragnarson Tennvall G, Apelqvist J. Health-economic consequences of diabetic foot lesions. Clin Infect Dis 2004;39:S132–9. 10.1086/383275 [DOI] [PubMed] [Google Scholar]
- 4. Stockl K, Vanderplas A, Tafesse E, et al. Costs of lower-extremity ulcers among patients with diabetes. Diabetes Care 2004;27:2129–34. 10.2337/diacare.27.9.2129 [DOI] [PubMed] [Google Scholar]
- 5. Treece KA, Macfarlane RM, Pound N, et al. Validation of a system of foot ulcer classification in diabetes mellitus. Diabetic Medicine 2004;21:987–91. 10.1111/j.1464-5491.2004.01275.x [DOI] [PubMed] [Google Scholar]
- 6. Clayton W, Elasy TA. A review of the pathophysiology, classification, and treatment of foot ulcers in diabetic patients. Clinical Diabetes 2009;27:52–8. 10.2337/diaclin.27.2.52 [DOI] [Google Scholar]
- 7. Schaper NC. Lessons from Eurodiale. Diabetes Metab Res Rev 2012;28:21–6. 10.1002/dmrr.2266 [DOI] [PubMed] [Google Scholar]
- 8. Ikem R, Ikem I, Adebayo O, et al. An assessment of peripheral vascular disease in patients with diabetic foot ulcer. Foot 2010;20:114–7. 10.1016/j.foot.2010.09.002 [DOI] [PubMed] [Google Scholar]
- 9. Ogbera OA, Osa E, Edo A, et al. Common clinical features of diabetic foot ulcers: perspectives from a developing nation. Int J Low Extrem Wounds 2008;7:93–8. 10.1177/1534734608318236 [DOI] [PubMed] [Google Scholar]
- 10. Tesfaye S, Selvarajah D. Advances in the epidemiology, pathogenesis and management of diabetic peripheral neuropathy. Diabetes Metab Res Rev 2012;28:8–14. 10.1002/dmrr.2239 [DOI] [PubMed] [Google Scholar]
- 11. Fernando ME, Seneviratne RM, Tan YM, et al. Intensive versus conventional glycaemic control for treating diabetic foot ulcers. Cochrane Database Syst Rev 2016;22 10.1002/14651858.CD010764.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hasan R, Firwana B, Elraiyah T, et al. A systematic review and meta-analysis of glycemic control for the prevention of diabetic foot syndrome. J Vasc Surg 2016;63:22S–8. 10.1016/j.jvs.2015.10.005 [DOI] [PubMed] [Google Scholar]
- 13. Brem H, Sheehan P, Rosenberg HJ, et al. Evidence-Based protocol for diabetic foot ulcers. Plast Reconstr Surg 2006;117:193S–209. 10.1097/01.prs.0000225459.93750.29 [DOI] [PubMed] [Google Scholar]
- 14. Christman AL, Selvin E, Margolis DJ, et al. Hemoglobin A1c predicts healing rate in diabetic wounds. J Invest Dermatol 2011;131:2121–7. 10.1038/jid.2011.176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lan C-CE, Liu I-H, Fang A-H, et al. Hyperglycaemic conditions decrease cultured keratinocyte mobility: implications for impaired wound healing in patients with diabetes. Br J Dermatol 2008;159:1103–15. 10.1111/j.1365-2133.2008.08789.x [DOI] [PubMed] [Google Scholar]
- 16. Marston WA, Dermagraft Diabetic Foot Ulcer Study Group . Risk factors associated with healing chronic diabetic foot ulcers: the importance of hyperglycemia. Ostomy Wound Manage 2006;52:26–8. [PubMed] [Google Scholar]
- 17. Miner A, Kirsner RS. Diabetic control affects healing rates in neuropathic and vasculopathic patients. J Invest Dermatol 2011;131:1962 10.1038/jid.2011.269 [DOI] [PubMed] [Google Scholar]
- 18. Rathsman B, Jensen-Urstad K, Nyström T. Intensified insulin treatment is associated with improvement in skin microcirculation and ischaemic foot ulcer in patients with type 1 diabetes mellitus: a long-term follow-up study. Diabetologia 2014;57:1703–10. 10.1007/s00125-014-3248-2 [DOI] [PubMed] [Google Scholar]
- 19. Idris I, Game F, Jeffcoate W. Does close glycaemic control promote healing in diabetic foot ulcers? report of a feasibility study. Diabet Med 2005;22:1060–3. 10.1111/j.1464-5491.2005.01606.x [DOI] [PubMed] [Google Scholar]
- 20. Brod M, Christensen T, Kongsø JH, et al. Examining and interpreting responsiveness of the diabetes medication satisfaction measure. J Med Econ 2009;12:309–16. 10.3111/13696990903337017 [DOI] [PubMed] [Google Scholar]
- 21. Brod M, Skovlund SE, Wittrup-Jensen KU. Measuring the impact of diabetes through patient report of treatment satisfaction, productivity and symptom experience. Qual Life Res 2006;15:481–91. 10.1007/s11136-005-1624-6 [DOI] [PubMed] [Google Scholar]
- 22. Monnier L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA1c. Diabetes Care 2003;26:881–5. 10.2337/diacare.26.3.881 [DOI] [PubMed] [Google Scholar]
- 23. Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727–36. 10.1007/s11136-011-9903-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Janssen MF, Pickard AS, Golicki D, et al. Measurement properties of the EQ-5D-5L compared to the EQ-5D-3L across eight patient groups: a multi-country study. Qual Life Res 2013;22:1717–27. 10.1007/s11136-012-0322-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bann CM, Fehnel SE, Gagnon DD. Development and Validation of the Diabetic Foot Ulcer Scale???Short Form (DFS-SF). Pharmacoeconomics 2003;21:1277–90. 10.2165/00019053-200321170-00004 [DOI] [PubMed] [Google Scholar]
- 26. International Diabetes Federation Global guideline for type 2 diabetes, 2012. [Google Scholar]
- 27. Margolis DJ, Gelfand JM, Hoffstad O, et al. Surrogate end points for the treatment of diabetic neuropathic foot ulcers. Diabetes Care 2003;26:1696–700. 10.2337/diacare.26.6.1696 [DOI] [PubMed] [Google Scholar]
- 28. Fielding S, Fayers P, Ramsay CR. Analysing randomised controlled trials with missing data: choice of approach affects conclusions. Contemp Clin Trials 2012;33:461–9. 10.1016/j.cct.2011.12.002 [DOI] [PubMed] [Google Scholar]
- 29. Lee CC, Perkins BA, Kayaniyil S, et al. Peripheral neuropathy and nerve dysfunction in individuals at high risk for type 2 diabetes: the promise cohort. Diabetes Care 2015;38:793–800. 10.2337/dc14-2585 [DOI] [PubMed] [Google Scholar]
- 30. Margolis DJ, Kantor J, Berlin JA. Healing of diabetic neuropathic foot ulcers receiving standard treatment. A meta-analysis. Diabetes Care 1999;22:692–5. 10.2337/diacare.22.5.692 [DOI] [PubMed] [Google Scholar]
- 31. Vatankhah N, Jahangiri Y, Landry GJ, et al. Effect of systemic insulin treatment on diabetic wound healing. Wound Rep and Reg 2017;25:288–91. 10.1111/wrr.12514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pascal I, Ofoedu J, Uchenna N, et al. Blood glucose control and medication adherence among adult type 2 diabetic Nigerians attending a primary care clinic in under-resourced environment of Eastern Nigeria. N Am J Med Sci 2012;4:310–5. 10.4103/1947-2714.98590 10.4103/1947-2714.98590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Al-Aujan S, Al-Aqeel S, Al-Harbi A, et al. Patients' satisfaction with diabetes medications in one Hospital, Saudi Arabia. Patient Prefer Adherence 2012;6:735–40. 10.2147/PPA.S32859 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.