Abstract
Objective
To assess the variation of effect estimates in the analysis of mortality and length of stay (LOS) in patients with infections caused by extended-spectrum beta-lactamase (ESBL)-producing Enterobacteriaceae.
Design
Systematic review and meta-analysis
Methods
Literature search for clinical studies from 1 January 1960 to 1 October 2018 was conducted in PubMed. Primary outcomes were risk ratios (RRs) of all-cause and attributable mortality and weighted mean differences (WMDs) in LOS in patients with bloodstream infections (BSIs) and non-invasive infections. Any change in the effect estimates was assessed by grouping studies according to design, setting, economy-based country classification, reporting period, microbiological aetiology, infection type and adjustment for appropriateness of empirical treatment. The impact of ESBL production was calculated using random-effect meta-analysis and heterogeneity was evaluated by I2 statistics and metaregression.
Results
Eighty-four studies including 22 030 patients and 149 outcome measures were included in the meta-analysis. Most studies were retrospective cohorts from high-income countries, providing unadjusted estimates. ESBL production in patients with BSIs (56 studies) increased the RR for all-cause mortality by a factor of 1.70 (95% CI 1.52 to 1.90; p<0.001), attributable mortality (16 studies) by 1.75 (95% CI 1.448 to 2.108; p<0.001) and WMD in the intensive care unit by 3.07 days (95% CI 1.61 to 4.54; p<0.001). WMD in hospital LOS was significantly higher in BSIs (4.41 days; 95% CI 3.37 to 5.46; p<0.001) and non-invasive (2.19 days; 95% CI 1.56 to 2.81; p<0.001). Subgroup analyses showed variation of estimates by study design, population, strain and assessment of appropriateness of empiric treatment. High heterogeneity was observed in all analyses.
Conclusions
Current evidence of the clinical burden of infections caused by ESBL-producing bacteria is highly heterogeneous and based mainly on unadjusted estimates derived from retrospective studies. Despite these limitations, ESBL production in strains causing BSIs seems associated with higher all-cause and attributable mortality and longer hospitalisation.
Keywords: extended-spectrum beta-lactamase, mortality, bloodstream infection, length of stay, meta-analysis, systematic review
Strengths and limitations of this study.
Evidence of the impact of extended-spectrum beta-lactamase production on mortality and length of stay in strains causing bacteraemic and non-bacteraemic infections was collected systematically.
Effect of multiple epidemiological and clinical variables was assessed in the calculation of estimates.
Heterogeneity among studies was assessed.
Only few studies had been performed in high-risk populations or low-income countries.
Introduction
Infections caused by extended-spectrum beta-lactamase (ESBL)-producing Enterobacteriaceae are responsible for high morbidity and mortality worldwide.1–3 The 2018 WHO list of antibiotic-resistant pathogens identified mortality as the most important criteria to prioritise bacteria for research and development of new, effective antibiotics.1 In this prioritisation exercise, ESBL-producing Enterobacteriaceae were designated a critical priority because of their high all-cause mortality and high prevalence globally in healthcare-associated and community-acquired infections. The incidence and attributable mortality of multidrug-resistant bacterial infections, including ESBL-producing Enterobacteriaceae, in European countries has been recently estimated using a modelling analysis.4 In 2015, ESBL-producing Escherichia coli was responsible for almost 300 000 infections in Europe and 9000 attributable deaths, and ESBL-producing Klebsiella pneumoniae caused around 70 000 infections and more than 3500 deaths. The major limitation of this analysis is the sparseness of evidence on mortality due to ESBL-producing bacteria, which was limited largely to studies conducted in high-income countries (HICs).
Two systematic reviews have been performed to define the impact of ESBL production on mortality due to Enterobacteriaceae.2 3 Both meta-analyses included studies targeting bloodstream infections (BSIs) and showed doubling all-cause mortality for ESBL-associated bacteraemia compared with non-ESBL Enterobacteriaceae bacteraemia. A major drawback of the analyses, highlighted by the authors, was the lack of control for confounding and limited adjustment for empiric therapy. No systematic review has been performed to assess attributable mortality and other indicators of clinical impact such as length of stay (LOS).
Because estimates of clinical burden drive policy design for antibiotic stewardship and infection control interventions, precise and current estimates are essential. The objective of this systematic review and meta-analysis was to assess the variation of effect estimates in the analysis of mortality and LOS in patients with infections due to ESBL-producing Enterobacteriaceae.
Methods
Literature search strategy
The search was performed by two researchers (BPG and PS) in PubMed on 5 October 2018 using search terms (online supplementary table S1) relevant to the following combinations: (ESBL AND Escherichia coli AND mortality) OR (ESBL AND Klebsiella pneumoniae AND mortality) OR (ESBL AND Escherichia coli AND length of stay OR length of hospitalisation) OR (ESBL AND Klebsiella pneumoniae AND length of stay OR length of hospitalisation). Reference lists of retrieved articles were also searched.
bmjopen-2019-030266supp001.pdf (43.8KB, pdf)
Eligibility criteria
We included all clinical studies with a comparison group assessing all-cause mortality, attributable mortality and overall LOS and intensive care unit stay (ICU) LOS in hospitalised patients with ESBL infections. Studies published from 1 January 1960 to 1 October 2018 irrespective of the clinical setting and study design were included. No language restriction has been applied. Diagnostic studies, reviews, case reports, non-clinical studies and abstracts of conference presentations were not included.
Data extraction
Two reviewers (PS and BPG) independently assessed the eligibility of trials and extracted data. In case of disagreement, a third reviewer (DL) was consulted. Extracted data were collected in an electronic worksheet, using EpiInfo 7.1: authors, journal, country, year of publication, year of study, time of data collection, study design, comparison group, study setting, population, aetiology, type and site of infection, and raw data related to mortality and LOS/ICU-LOS. Countries were classified as high-income, middle-income or low-income using the World Bank Atlas method.5 Adjusted effect estimates such as ORs or HRs and quality indicators such as reporting of antibiotic therapy, appropriateness of empirical treatment, resistance mechanisms and minimum inhibitory concentrations (MICs) were also extracted.
Mortality data were extracted as all-cause mortality or attributable mortality as defined in the studies. Where available, prespecified time periods for mortality assessment (ie, 14 days, 28 days, in hospital) were also extracted. LOS and ICU-LOS were extracted as days with mean and SD or median and IQR.
Data analysis
The primary outcomes for the clinical impact of ESBL infections were RRs of all-cause and attributable mortality and the weighted mean difference (WMD) in LOS and ICU-LOS in patients with ESBL infections compared with those in patients with non-ESBL infections and, where available, with uninfected patients. The impact of ESBL production on attributable and all-cause mortality was calculated with random-effect meta-analysis and expressed as RR with 95% CI. WMD in days with 95% CI was calculated to express the excess in LOS and ICU-LOS.
Variation of the effect estimate was assessed by grouping the studies according to the following study/outcome characteristics: mortality time assessment (7 vs 14 days), aetiology (E. coli vs K. pneumoniae), infection localisation, clinical setting (paediatric, oncology, ICU), economic country areas (HICs vs low-income and medium-income countries (LMICs)), study design, assessment of empiric therapy and year. Studies were classified according to the type of infections evaluated. Studies on BSIs were defined as those in which patients had positive blood cultures and were admitted to the hospitals with signs and symptoms of systemic inflammatory response and requiring therapy, similarly to the definition adopted by the most recent cohort studies on ESBL infections.6 Non-invasive infections included non-bacteraemic patients with only localised signs and symptoms of infection (such as urinary tract infections (UTIs) or superficial surgical site infections).
Subgroup analysis was computed only if more than two studies were available for each group. Heterogeneity was evaluated by using I2 statistics and metaregression. Overall significance testing was carried out using Wald tests adjusted using the Bonferroni correction. The unadjusted ORs were compared with the adjusted ORs to estimate the effect of adjustment. Reporting and publication bias was presented in funnel plots (online supplementary figures 1 and 2) and tested by Egger’s test. Statistical analyses were performed using Stata V.15. Risk of bias was assessed independently by two authors (PS, DL) using the Newcastle-Ottawa Quality Assessment Scale for cohort studies.7 Studies were classified as low, moderate or high quality according to Agency for Health Research and Quality (AHRQ) standards (online supplementary table S2). All meta-analyses were performed in accordance with the Cochrane Collaboration recommendations8 and reported according to the Preferred Reporting Items for Systematic Reviews andMeta-Analyses statement.9
bmjopen-2019-030266supp002.pdf (64KB, pdf)
bmjopen-2019-030266supp003.pdf (62.8KB, pdf)
bmjopen-2019-030266supp004.pdf (63.9KB, pdf)
The protocol is available online (https://im1-tuebingen.de/wp-content/uploads/2019/03/ClinicalImpactAMR_SR_studyprotocol_2018.pdf).
Patient and public involvement
There was no patient or public involvement in this systematic review of published literature.
Results
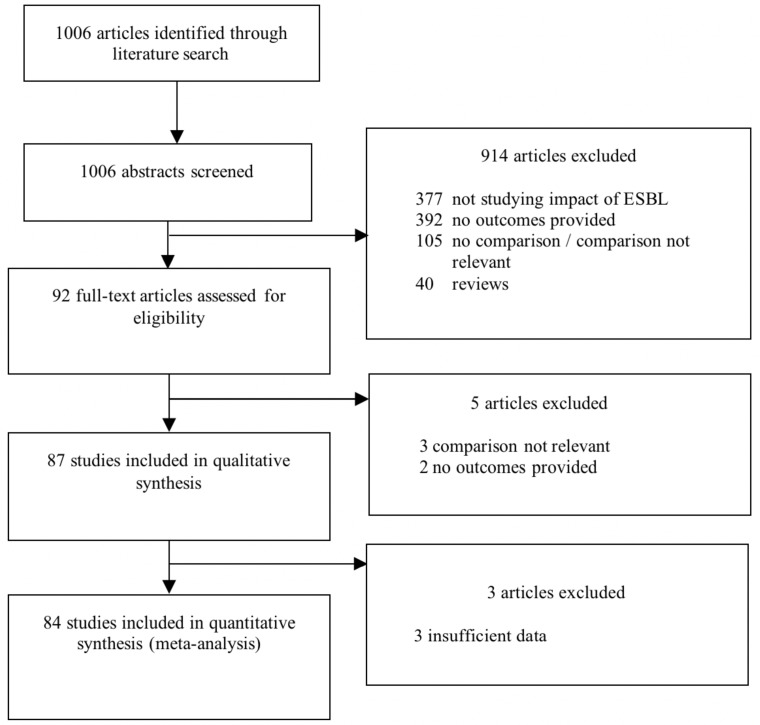
Our literature search identified 1006 studies, and 92 (9.2%) met the eligibility criteria on the basis of abstract screening. Full-text screening excluded an additional 5 articles, providing an evidence base of 87 studies (figure 1).10–96 The 87 studies included in the qualitative analysis were conducted between 1991 and 2017 in 25 countries, mainly in South Korea (14 studies), Thailand (7), USA (7), Taiwan (7) and Spain (7). Sixty (68.9%) studies were performed in HICs, 26 (29.9%) in LMICs and 1 included both HICs and LMICs.56 About half (44, 50.6%) were retrospective cohort studies, 24 (27.6%) case cohort studies and 18 (20.7%) prospective cohort studies; 1 study had an interventional design.57 The comparison group was patients with infections caused by gram-negative non-ESBL producers in 82 (94.3%) studies, non-infected patients in 2 (2.3%) and both control groups in 3 (3.5%). Most (57, 65.5%) studies included data from the entire hospital, while a few focused on specific settings, mainly ICUs (9, 10.3%) and paediatric wards (8, 9.2%). The most common ESBL-producing bacteria were E. coli (23, 26.4%) and K. pneumoniae (17, 19.5%). An overview of study characteristics is provided in online supplementary table 3.
Figure 1.
Literature search and study inclusion and exclusion. ESBL, extended-spectrum beta-lactamase.
bmjopen-2019-030266supp005.pdf (104.6KB, pdf)
Because data in 3 studies22 61 87 were insufficient for quantitative analysis, 84 (96.6%) studies were included in the meta-analysis analysing data from 22 030 patients and 149 outcome measures. Fifty-seven studies analysed BSIs and 10 non-invasive infections. Study characteristics for all studies are provided in online supplementary table 4. Forty-nine (58.3%) studies were of high quality, 23 (27.3%) were of moderate quality and 12 (14.3%) were of low quality (online supplementary table S5).
bmjopen-2019-030266supp006.pdf (158.8KB, pdf)
bmjopen-2019-030266supp007.pdf (69.4KB, pdf)
All-cause mortality
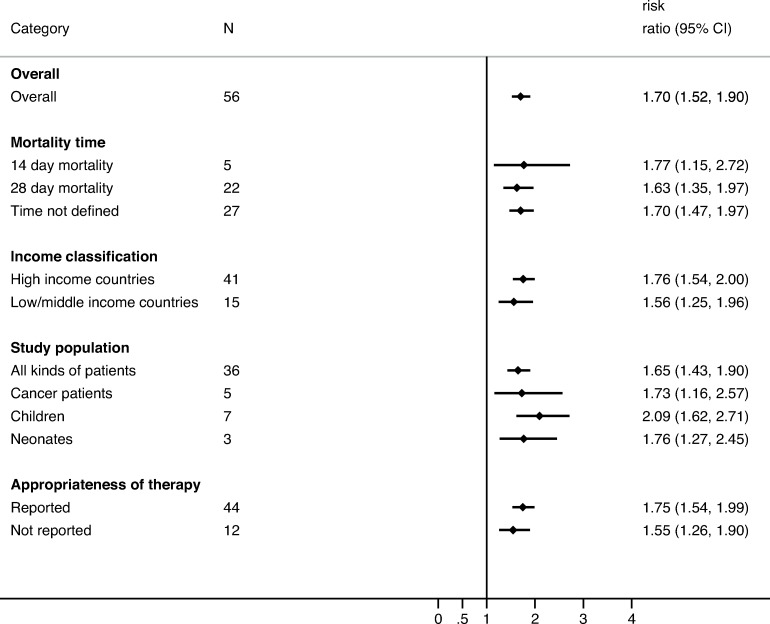
All-cause mortality was reported in 81 studies including 21 942 patients (56 on BSIs and 7 on non-invasive infections). ESBL production in patients with BSIs increased all-cause mortality by a factor of 1.70 (95% CI 1.52 to 1.90; p<0.001; I2=45.3%; p<0.001) while studies including non-invasive reported an RR of 1.58 (95% CI 1.23 to 2.02; p<0.001; online supplementary figure 3). Among the patients with BSI, the RR increased over time from 1.56 (95% CI 1.15 to 2.11; p=0.004) in 1991–1999 to 1.74 (95% CI 1.50 to 2.01; p<0.001) in 2000–2009, and it was stable in 2010–2018 (1.72, 95% CI 1.39 to 2.13; p<0.001). The RR was higher in studies assessing appropriateness of empiric therapy (RR=1.75; 95% CI 1.54 to 1.99; p<0.001) than in those that did not (RR=1.55; 95% CI 1.26 to 1.90; p<0.001). The subgroup analysis by pathogen showed that ESBL production increased the RR in BSIs due to E. coli (RR=1.82; 95% CI 1.50 to 2.21; p<0.001) compared with those due to K. pneumoniae (RR=1.48; 95% CI 1.17 to 1.87; p=0.001). Stratification by population age showed a higher RR in paediatric population (RR=2.09; 95% CI 1.62 to 2.71; p<0.001). Effect estimates did not vary significantly by study country, mortality time assessment (14 vs 28 days), ESBL molecular resistance mechanisms, or study design (figure 2 and online supplementary figure 4). Adjusted estimates for inappropriate empirical antibiotic therapy were provided for 14 studies. The pooled unadjusted OR for all-cause mortality was 2.91 (95% CI 2.23 to 3.81; p<0.001, I2=27.1%; p=0.164) and the pooled OR after adjusting for receipt of appropriate empirical treatment was 3.22 (95% CI 1.53 to 6.76; p=0.002; I2=87.5%; p<0.001). The impact of ESBL production on LOS and mortality varied according to the infection type, with higher effect in intra-abdominal, respiratory and BSIs (online supplementary figures 5 and 6).
Figure 2.
Pooled risk ratios for all-cause mortality in patients with extended-spectrum beta-lactamase (ESBL) bloodstream infections compared with patients with non-ESBL bloodstream infections—subgroups not included in attributable mortality.
bmjopen-2019-030266supp008.pdf (33.5KB, pdf)
bmjopen-2019-030266supp009.pdf (34.6KB, pdf)
bmjopen-2019-030266supp010.pdf (41KB, pdf)
bmjopen-2019-030266supp011.pdf (39KB, pdf)
Attributable mortality
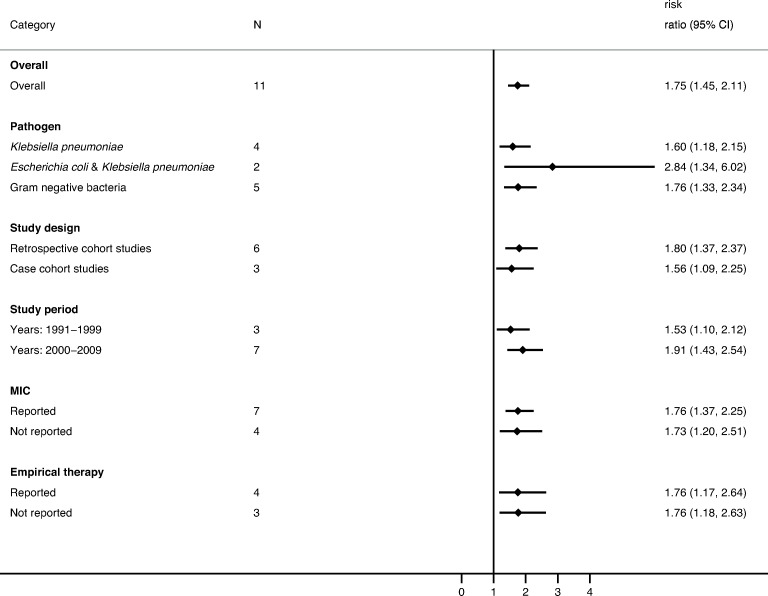
Attributable mortality was analysed in 16 studies including 2885 patients. All studies were performed in HICs. ESBL production in patients with BSIs increased the risk of attributable mortality by a factor of 1.75 (95% CI 1.45 to 2.11; p<0.001; I2=0%; p<0.001). The RR increased over time from 1.53 (95% CI 1.10 to 2.12; p=0.011) in 1991–1999 to 1.91 (95% CI 1.43 to 2.54; p<0.001) in 2000–2009 (figure 3). Pathogen-specific RR for attributable mortality was 1.60 (95% CI 1.18 to 2.15; p=0.002) for K. pneumoniae and 1.76 (95% CI 1.33 to 2.34; p<0.001) when the gram-negative organisms were analysed all together without species differentiation. The subgroup analysis showed the RR was lower in case cohort studies (1.56; 95% CI 1.09 to 2.25; p=0.016) than in cohort studies (1.80; 95% CI 1.37 to 2.37; p<0.001).
Figure 3.
Pooled risk ratios for attributable mortality in patients with extended-spectrum beta-lactamase (ESBL) bloodstream infections compared with patients with non-ESBL bloodstream infections. MIC, minimum inhibitory concentration.
Length of stay
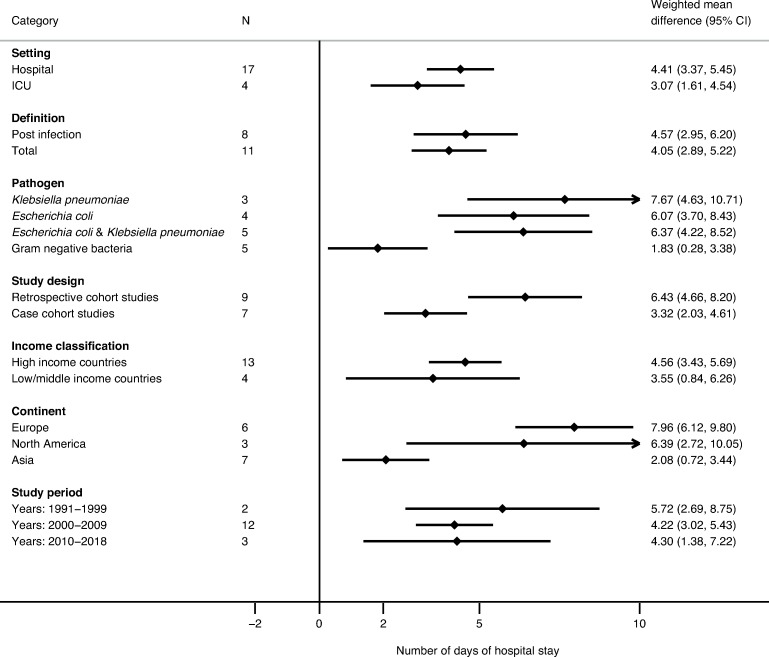
LOS data were provided in 37 studies (17 on BSIs and 8 on non-invasive) analysing 38 outcome measures. The WMD of LOS in patients with BSIs was 4.41 days (95% CI 3.37 to 5.46; p<0.001) and decreased from 5.72 days (95% CI 2.69 to 8.75; p<0.001) in 1991–1999 to 4.22 days (95% CI 3.02 to 5.43; p<0.001) in 2000–2009 and was stable up to 2018 (4.30 days; 95% CI 1.38 to 7.22; p=0004). Higher WMD (p<0.001) was observed for BSIs due to K. pneumoniae (7.67 days; 4.63–10.71) than for those due to E. coli (6.07 days; 95% CI 3.71 to 8.43). Retrospective cohort studies reported higher (p<0.001) WMD (6.43 days; 95% CI 4.66 to 8.21; p<0.001) than case cohort studies (3.32 days; 95% CI 2.03 to 4.61). Studies in HICs showed higher WMD (4.56 days; 95% CI 3.43 to 5.70; p<0.001) than studies in LMICs (3.55 days; 95% CI 0.84 to 6.26; p=0.01) (figure 4).
Figure 4.
Weighted mean difference in the length of stay for patients with extended-spectrum beta-lactamase (ESBL) bloodstream infections compared with patients with non-ESBL bloodstream infections. ICU, intensive care unit.
Studies with non-invasive infections reported a WMD of 2.19 days (95% CI 1.56 to 2.81; p<0.001), which decreased from 7.66 (95% CI 5.83 to 9.46; p<0.001) in 2000–2009 to 1.44 (95% CI 0.77 to 2.10; p<0.001) in 2010–2018 (online supplementary figure 7).
bmjopen-2019-030266supp012.pdf (32.2KB, pdf)
The data on ICU-LOS were provided in seven studies and showed that BSIs caused by ESBL producers had a WMD of LOS of 3.07 days (95% CI 1.61 to 4.54; p<0.001).
Heterogeneity of the studied effect modifiers did not reach statistical significance when assessed by metaregression (online supplementary table S6). Sensitivity analysis based on the quality of studies revealed no notable difference in the effect estimates after exclusion of low-quality studies (data not shown). Egger’s test and the funnel plots (online supplementary figures 1 and 2) showed evidence for small-study effects (p<0.001) and publication bias.
bmjopen-2019-030266supp013.pdf (37.2KB, pdf)
Discussion
This systematic review shows that ESBL production has a significant impact on the most relevant patient-related clinical outcomes. In the subgroup analyses, all-cause mortality, attributable mortality and LOS both in hospital and in ICU were higher for patients with BSIs due to ESBL-producing Enterobacteriaceae than for patients with BSIs due to non-ESBL-producing strains. Non-invasive infections caused by ESBL-producing strains were associated with higher all-cause mortality and prolonged LOS. Within the limitation of the low number of studies evaluating specific patient populations, paediatric patient and patients with cancer seemed to suffer a higher impact of ESBL invasive infections than the overall population. Stratifying by pathogen type, the impact of ESBL production was higher for E. coli BSIs than for K. pneumoniae BSIs. No relevant differences in mortality analysis emerged with stratification by study design or country income level. Impact of ESBL infections on mortality became more evident in more recent studies. Studies reporting on appropriateness of empirical therapy, ESBL resistance mechanisms and MICs showed a higher clinical impact of ESBL infections than studies not assessing these variables. In particular, pooled ORs adjusted for inappropriate empirical treatment showed a remarkably higher OR for mortality in patients with ESBL infections.
Our findings confirm the results of previous systematic reviews. Schwaber et al performed a systematic review comparing mortality in ESBL BSIs and non-ESBL BSIs in studies published through 2003.2 The authors show a pooled RR for all-cause mortality of 1.85 but, in contrast to our study, they combined E. coli, Klebsiella spp and Proteus spp in the analysis because of sample size limitations. Rottier et al analysed studies published through 2010 and adjusting results for inappropriate empirical treatment found an adjusted OR of 1.37.3 Our study, adding more than 50 studies in 17 years to the Rottier systematic review, confirmed the clinical importance of ESBL production to all-cause mortality and for the first time assessed the role of ESBL production on attributable mortality. We addressed relevant effect modifiers through subgroup analyses and found that population, pathogen and assessment of empirical therapy all had an impact on estimates. Because we believe that appropriate empirical treatment plays a relevant role in invasive infections, we performed a secondary analysis by pooling only adjusted ORs and confirming the significant impact of antibiotic resistance as already shown in a previously published systematic review.97 The lack of consideration of appropriateness of therapy in the studies evaluating mortality seems to underestimate the impact of ESBL production on mortality. However, studies assessing the impact of appropriate therapy did not provide homogeneous definition and could refer either to empirical or definite therapy or a single component irrespective of the dosage, making results difficult to interpret. Especially in infections with different sources and different clinical severity, the sole contribution of empirical therapy remains challenging to measure. For example, patients with UTI receiving inappropriate empirical antibiotic therapy can potentially show a favourable outcome, most probably due to the high concentration of antibiotic reached in the urinary tract.98
Community acquired ESBL infections emerged in the late 1990s and show an increasing trend.99 100 Recent study shows that community onset ESBL infections are associated with lower mortality compared with healthcare-associated and hospital-acquired infections.101 The place of acquisition could not be appropriately addressed in our meta-analysis due to the lack of data in included studies.
Our systematic review contributes to the discussion on the limitation of current evidence for the estimation of mortality due to antibiotic-resistant infections. The impact of ESBL production on LOS in our study has shown that both BSIs and non-invasive infections lead to prolongation of hospitalisation.
Our study has some limitations. Although results of the meta-analyses were significant in all the subgroups, we could analyse only a limited number of studies providing information for subgroups such as haematological patients and low-income countries, making generalisability of results less certain for these specific patient populations. Only a few studies reported MIC data or specific ESBL molecular resistant phenotype (ie, AmpC). Moreover, publication bias was detected in both the main analyses (all-cause mortality and LOS), thus implying the possibility that results from small studies with non-significant results might have been conducted and not published, resulting in a possible overestimation of our results. The non-homogeneous reporting of some relevant data in published literature (eg, infection type, presence of bacteraemia, disease severity, underlying comorbidities and resistance mechanism) may also have affected the precision of the estimate. A limited number of patients with non-bacteraemic infections was included in our systematic review, thus limiting the generalisability of results to this patients’ population. Moreover, patients with ESBL are intrinsically at higher risk of mortality and complications because they are often older, have more comorbidities or higher antibiotic exposure, and are at higher risk of receiving inappropriate empirical treatment.102 Finally, due to resource constraints, we had to limit our search to PubMed database with the chance of missing relevant studies.
In summary, our systematic review emphasises the importance of suspicion and confirmation of ESBL production as soon as possible for invasive infections and demonstrates that ESBL production increases the risk of attributable mortality and LOS in both hospital and ICU for invasive and non-invasive infections. Patients with ESBL infections remain at higher risk of mortality and prolonged LOS even after adjustment for empirical inappropriate treatment. Control for other relevant effect modifiers is hindered by the sparseness of published data. Individual patient data network meta-analyses are needed to define differences in outcomes between severe intravascular infections and bacteraemia. Future studies addressing the clinical burden of drug-resistant infections must include ESBL production and should assess both the impact of molecular mechanisms of resistance and effect on specific patient populations such as haematological patients and those in LMIC.
Supplementary Material
Acknowledgments
The authors thank Anne McDonough, a professional medical writer who provided medical writing support funded by the authors.
Footnotes
Contributors: ET contributed to the study concept. BPG and PS performed data analysis. PS and AC extracted data and wrote the first draft of the manuscript. DL contributed to the first draft of the manuscript. EC and ET wrote the final version of the manuscript. CB reviewed the paper. All authors read, edited and approved the final manuscript. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Funding: This work was supported by the German Center for Infection Research Clinical Research Unit for Health-Care Associated Infections grant number TTU 08.701 and DRIVE-AB (115618).
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request. Requests for data should be addressed to the corresponding author.
References
- 1. Tacconelli E, Carrara E, Savoldi A, et al. . Discovery, research, and development of new antibiotics: the who priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis 2018;18:318–27. 10.1016/S1473-3099(17)30753-3 [DOI] [PubMed] [Google Scholar]
- 2. Schwaber MJ, Carmeli Y. Mortality and delay in effective therapy associated with extended-spectrum beta-lactamase production in Enterobacteriaceae bacteraemia: a systematic review and meta-analysis. J Antimicrob Chemother 2007;60:913–20. 10.1093/jac/dkm318 [DOI] [PubMed] [Google Scholar]
- 3. Rottier WC, Ammerlaan HSM, Bonten MJM. Effects of confounders and intermediates on the association of bacteraemia caused by extended-spectrum β-lactamase-producing Enterobacteriaceae and patient outcome: a meta-analysis. J Antimicrob Chemother 2012;67:1311–20. 10.1093/jac/dks065 [DOI] [PubMed] [Google Scholar]
- 4. Cassini A, Högberg LD, Plachouras D, et al. . Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European economic area in 2015: a population-level modelling analysis. Lancet Infect Dis 2019;19:56–66. 10.1016/S1473-3099(18)30605-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. The World Bank What is the world bank atlas method? Available: https://datahelpdesk.worldbank.org/knowledgebase/articles/77933-what-is-the-world-bank-atlas-method [Accessed 14 Jan 2019].
- 6. Rodríguez-Baño J, Gutiérrez-Gutiérrez B, Machuca I, et al. . Treatment of Infections Caused by Extended-Spectrum-Beta-Lactamase-, AmpC-, and Carbapenemase-Producing Enterobacteriaceae. Clin Microbiol Rev 2018;31 10.1128/CMR.00079-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wells GA, Shea B, O'Connell D, et al. . The Newcastle–Ottawa scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Ottawa, Canada: The Ottawa Health Research Institute; http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm (Accessed 14 Apr 2015). [Google Scholar]
- 8. Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration (editors), 2011. Available: http://handbook.cochrane.org
- 9. Moher D, Liberati A, Tetzlaff J, et al. . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lautenbach E, Patel JB, Bilker WB, et al. . Extended-Spectrum -Lactamase-Producing Escherichia coli and Klebsiella pneumoniae: Risk Factors for Infection and Impact of Resistance on Outcomes. Clinical Infectious Diseases 2001;32:1162–71. 10.1086/319757 [DOI] [PubMed] [Google Scholar]
- 11. Leistner R, Sakellariou C, Gürntke S, et al. . Mortality and molecular epidemiology associated with extended-spectrum β-lactamase production in Escherichia coli from bloodstream infection. Infect Drug Resist 2014;7:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lin J-N, Chen Y-H, Chang L-L, et al. . Clinical characteristics and outcomes of patients with extended-spectrum β-lactamase-producing bacteremias in the emergency department. Intern Emerg Med 2011;6:547–55. 10.1007/s11739-011-0707-3 [DOI] [PubMed] [Google Scholar]
- 13. Kim HJ, Park JH, Park DI, et al. . Clinical impact of extended-spectrum β-lactamase-producing Enterobacteriaceae in patients with biliary tract infection. Dig Dis Sci 2013;58:841–9. 10.1007/s10620-012-2398-7 [DOI] [PubMed] [Google Scholar]
- 14. Kim S-H, Kwon J-C, Choi S-M, et al. . Escherichia coli and Klebsiella pneumoniae bacteremia in patients with neutropenic fever: factors associated with extended-spectrum β-lactamase production and its impact on outcome. Ann Hematol 2013;92:533–41. 10.1007/s00277-012-1631-y [DOI] [PubMed] [Google Scholar]
- 15. Pillay T, Pillay D, Adhikari M, et al. . Piperacillin/Tazobactam in the Treatment of Klebsiella Pneumoniae Infections in Neonates. Am J Perinatol 1998;15:47–51. 10.1055/s-2007-993898 [DOI] [PubMed] [Google Scholar]
- 16. Kim B-N, Woo J-H, Kim M-N, et al. . Clinical implications of extended-spectrum beta-lactamase-producing Klebsiella pneumoniae bacteraemia. J Hosp Infect 2002;52:99–106. 10.1053/jhin.2002.1288 [DOI] [PubMed] [Google Scholar]
- 17. Kim Y-K, Pai H, Lee H-J, et al. . Bloodstream infections by extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae in children: epidemiology and clinical outcome. Antimicrob Agents Chemother 2002;46:1481–91. 10.1128/AAC.46.5.1481-1491.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bhavnani SM, Ambrose PG, Craig WA, et al. . Outcomes evaluation of patients with ESBL- and non–ESBL-producing Escherichia coli and Klebsiella species as defined by CLSI reference methods: report from the SENTRY antimicrobial surveillance program. Diagn Microbiol Infect Dis 2006;54:231–6. 10.1016/j.diagmicrobio.2005.09.011 [DOI] [PubMed] [Google Scholar]
- 19. Blomberg B, Jureen R, Manji KP, et al. . High rate of fatal cases of pediatric septicemia caused by gram-negative bacteria with extended-spectrum beta-lactamases in Dar ES Salaam, Tanzania. J Clin Microbiol 2005;43:745–9. 10.1128/JCM.43.2.745-749.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Panhotra BR, Saxena AK, Al-Ghamdi AM. Extended-Spectrum beta-lactamase-producing Klebsiella pneumoniae Hospital acquired bacteremia. risk factors and clinical outcome. Saudi Med J 2004;25:1871–6. [PubMed] [Google Scholar]
- 21. Marra AR, Wey SB, Castelo A, et al. . Nosocomial bloodstream infections caused by Klebsiella pneumoniae: impact of extended-spectrum beta-lactamase (ESBL) production on clinical outcome in a hospital with high ESBL prevalence. BMC Infect Dis 2006;6:24 10.1186/1471-2334-6-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Skippen I, Shemko M, Turton J, et al. . Epidemiology of infections caused by extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella spp.: a nested case-control study from a tertiary hospital in London. J Hosp Infect 2006;64:115–23. 10.1016/j.jhin.2006.05.010 [DOI] [PubMed] [Google Scholar]
- 23. Schwaber MJ, Navon-Venezia S, Kaye KS, et al. . Clinical and economic impact of bacteremia with extended- spectrum-beta-lactamase-producing Enterobacteriaceae. Antimicrob Agents Chemother 2006;50:1257–62. 10.1128/AAC.50.4.1257-1262.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Apisarnthanarak A, Kiratisin P, Saifon P, et al. . Clinical and molecular epidemiology of community-onset, extended-spectrum β-lactamase-producing Escherichia coli infections in Thailand: A case-case-control study. Am J Infect Control 2007;35:606–12. 10.1016/j.ajic.2007.05.008 [DOI] [PubMed] [Google Scholar]
- 25. Tumbarello M, Spanu T, Sanguinetti M, et al. . Bloodstream infections caused by extended-spectrum-beta-lactamase-producing Klebsiella pneumoniae: risk factors, molecular epidemiology, and clinical outcome. Antimicrob Agents Chemother 2006;50:498–504. 10.1128/AAC.50.2.498-504.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Apisarnthanarak A, Kiratisin P, Saifon P, et al. . Risk factors for and outcomes of healthcare-associated infection due to extended-spectrum beta-lactamase-producing Escherichia coli or Klebsiella pneumoniae in Thailand. Infect Control Hosp Epidemiol 2007;28:873–6. 10.1086/518725 [DOI] [PubMed] [Google Scholar]
- 27. Kanafani Z, Mehio-Sibai A, Araj G, et al. . Epidemiology and risk factors for extended-spectrum β-lactamase-producing organisms: a case control study at a tertiary care center in Lebanon. Am J Infect Control 2005;33:326–32. 10.1016/j.ajic.2005.03.009 [DOI] [PubMed] [Google Scholar]
- 28. Zaoutis TE, Goyal M, Chu JH, et al. . Risk factors for and outcomes of bloodstream infection caused by extended-spectrum ss-Lactamase-Producing Escherichia coli and Klebsiella species in children. Pediatrics 2005;115:942–9. 10.1542/peds.2004-1289 [DOI] [PubMed] [Google Scholar]
- 29. Loh L-C, Nor Izran Hanim BtAS, Rosdara Masayuni BtMS, et al. . Hospital outcomes of adult respiratory tract infections with extended-spectrum B-Lactamase (ESBL) producing Klebsiella pneumoniae. Malays J Med Sci 2007;14:36–40. [PMC free article] [PubMed] [Google Scholar]
- 30. Melzer M, Petersen I. Mortality following bacteraemic infection caused by extended spectrum beta-lactamase (ESBL) producing E. coli compared to non-ESBL producing E. coli. J Infect 2007;55:254–9. 10.1016/j.jinf.2007.04.007 [DOI] [PubMed] [Google Scholar]
- 31. Song K-H, Jeon JH, Park WB, et al. . Clinical outcomes of spontaneous bacterial peritonitis due to extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiellaspecies: a retrospective matched case-control study. BMC Infect Dis 2009;9:41 10.1186/1471-2334-9-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bennett JW, Robertson JL, Hospenthal DR, et al. . Impact of extended spectrum beta-lactamase producing Klebsiella pneumoniae infections in severely burned patients. J Am Coll Surg 2010;211:391–9. 10.1016/j.jamcollsurg.2010.03.030 [DOI] [PubMed] [Google Scholar]
- 33. Trecarichi EM, Tumbarello M, Spanu T, et al. . Incidence and clinical impact of extended-spectrum-beta-lactamase (ESBL) production and fluoroquinolone resistance in bloodstream infections caused by Escherichia coli in patients with hematological malignancies. J Infect 2009;58:299–307. 10.1016/j.jinf.2009.02.002 [DOI] [PubMed] [Google Scholar]
- 34. Tuon FF, Kruger M, Terreri M, et al. . Klebsiella ESBL bacteremia-mortality and risk factors. Braz J Infect Dis 2011;15:594–8. 10.1590/S1413-86702011000600016 [DOI] [PubMed] [Google Scholar]
- 35. Kang C-I, Song J-H, Chung DR, et al. . Risk factors and treatment outcomes of community-onset bacteraemia caused by extended-spectrum β-lactamase-producing Escherichia coli. Int J Antimicrob Agents 2010;36:284–7. 10.1016/j.ijantimicag.2010.05.009 [DOI] [PubMed] [Google Scholar]
- 36. Peña C, Gudiol C, Calatayud L, et al. . Infections due to Escherichia coli producing extended-spectrum beta-lactamase among hospitalised patients: factors influencing mortality. J Hosp Infect 2008;68:116–22. 10.1016/j.jhin.2007.11.012 [DOI] [PubMed] [Google Scholar]
- 37. Tumbarello M, Spanu T, Di Bidino R, et al. . Costs of Bloodstream Infections Caused by Escherichia coli and Influence of Extended-Spectrum- -Lactamase Production and Inadequate Initial Antibiotic Therapy. Antimicrob Agents Chemother 2010;54:4085–91. 10.1128/AAC.00143-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kang C-I, Chung DR, Ko KS, et al. . Risk factors for infection and treatment outcome of extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae bacteremia in patients with hematologic malignancy. Ann Hematol 2012;91:115–21. 10.1007/s00277-011-1247-7 [DOI] [PubMed] [Google Scholar]
- 39. YH W, Chen PL, Hung YP, et al. . Risk factors and clinical impact of levofloxacin or cefazolin nonsusceptibility or ESBL production among uropathogens in adults with community-onset urinary tract infections. J Microbiol Immunol Infect 2014;47:197–203. [DOI] [PubMed] [Google Scholar]
- 40. Rodríguez‐Baño J, Picón E, Gijón P, et al. . Community‐Onset Bacteremia Due to Extended‐Spectrum β‐Lactamase–Producing Escherichia coli: Risk Factors and Prognosis. Clin Infect Dis 2010;50:40–8. 10.1086/649537 [DOI] [PubMed] [Google Scholar]
- 41. Gürntke S, Kohler C, Steinmetz I, et al. . Molecular epidemiology of extended-spectrum beta-lactamase (ESBL)-positive Klebsiella pneumoniae from bloodstream infections and risk factors for mortality. J Infect Chemother 2014;20:817–9. 10.1016/j.jiac.2014.08.012 [DOI] [PubMed] [Google Scholar]
- 42. MM O, Chae JY, Kim JW, et al. . Positive culture for extended-spectrum beta-lactamase during acute prostatitis after prostate biopsy is a risk factor for progression to chronic prostatitis. Urology 2013;81:1209–12. [DOI] [PubMed] [Google Scholar]
- 43. Leistner R, Gürntke S, Sakellariou C, et al. . Bloodstream infection due to extended-spectrum beta-lactamase (ESBL)-positive K. pneumoniae and E. coli: an analysis of the disease burden in a large cohort. Infection 2014;42:991–7. 10.1007/s15010-014-0670-9 [DOI] [PubMed] [Google Scholar]
- 44. NS K, Chung HS, Choi JY, et al. . Clinical usefulness of the 2010 clinical and laboratory Standards Institute revised breakpoints for cephalosporin use in the treatment of bacteremia caused by Escherichia coli or Klebsiella spp. Biomed Res Int 2015;2015:831074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Anunnatsiri S, Towiwat P, Chaimanee P. Risk factors and clinical outcomes of extended spectrum beta-lactamase (ESBL)-producing Escherichia coli septicemia at Srinagarind University Hospital, Thailand. Southeast Asian J Trop Med Public Health 2012;43:1169–77. [PubMed] [Google Scholar]
- 46. Kang C-I, Kim S-H, Kim DM, et al. . Risk factors for and clinical outcomes of bloodstream infections caused by extended-spectrum beta-lactamase-producing Klebsiella pneumoniae. Infect Control Hosp Epidemiol 2004;25:860–7. 10.1086/502310 [DOI] [PubMed] [Google Scholar]
- 47. Raviv Y, Shitrit D, Amital A, et al. . Multidrug-resistant Klebsiella pneumoniae acquisition in lung transplant recipients. Clin Transplant 2012;26:E388–94. 10.1111/j.1399-0012.2012.01671.x [DOI] [PubMed] [Google Scholar]
- 48. MacVane SH, Tuttle LO, Nicolau DP. Impact of extended-spectrum β-lactamase-producing organisms on clinical and economic outcomes in patients with urinary tract infection. J Hosp Med 2014;9:232–8. 10.1002/jhm.2157 [DOI] [PubMed] [Google Scholar]
- 49. Abhilash KP, Veeraraghavan B, Abraham OC. Epidemiology and outcome of bacteremia caused by extended spectrum beta-lactamase (ESBL)-producing Escherichia coli and Klebsiella spp. in a tertiary care teaching hospital in south India. J Assoc Physicians India 2010;58:13–17. [PubMed] [Google Scholar]
- 50. Shanthi M, Sekar U. Extended spectrum beta lactamase producing Escherichia coli and Klebsiella pneumoniae: risk factors for infection and impact of resistance on outcomes. J Assoc Physicians India 2010;58:41–4. [PubMed] [Google Scholar]
- 51. Han SB, Jung SW, Bae EY, et al. . Extended-Spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae bacteremia in febrile neutropenic children. Microb Drug Resist 2015;21:244–51. 10.1089/mdr.2014.0092 [DOI] [PubMed] [Google Scholar]
- 52. Lee S, Song DY, Cho SH, et al. . Impact of extended-spectrum beta-lactamase on acute pyelonephritis treated with empirical ceftriaxone. Microbial Drug Resistance 2014;20:39–44. 10.1089/mdr.2013.0075 [DOI] [PubMed] [Google Scholar]
- 53. Chayakulkeeree M, Junsriwong P, Keerasuntonpong A, et al. . Epidemiology of extended-spectrum beta-lactamase producing gram-negative bacilli at Siriraj Hospital, Thailand, 2003. Southeast Asian J Trop Med Public Health 2005;36:1503–9. [PubMed] [Google Scholar]
- 54. Apisarnthanarak A, Kiratisin P, Mundy LM. Predictors of Mortality From Community-Onset Bloodstream Infections Due to Extended-Spectrum β -Lactamase-Producing Escherichia coli and Klebsiella pneumoniae. Infect Control Hosp Epidemiol 2008;29:671–4. 10.1086/588082 [DOI] [PubMed] [Google Scholar]
- 55. Apisarnthanarak A, Kiratisin P, Saifon P, et al. . Predictors of mortality among patients with community-onset infection due to extended-spectrum beta-lactamase-producing Escherichia coli in Thailand. Infect Control Hosp Epidemiol 2008;29:80–2. 10.1086/524321 [DOI] [PubMed] [Google Scholar]
- 56. Jean S-S, Ko W-C, Xie Y, et al. . Clinical characteristics of patients with community-acquired complicated intra-abdominal infections: a prospective, multicentre, observational study. Int J Antimicrob Agents 2014;44:222–8. 10.1016/j.ijantimicag.2014.05.018 [DOI] [PubMed] [Google Scholar]
- 57. Lee J, Pai H, Kim YK, et al. . Control of extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae in a children's Hospital by changing antimicrobial agent usage policy. J Antimicrob Chemother 2007;60:629–37. 10.1093/jac/dkm225 [DOI] [PubMed] [Google Scholar]
- 58. Briongos-Figuero LS, Gómez-Traveso T, Bachiller-Luque P, et al. . Epidemiology, risk factors and comorbidity for urinary tract infections caused by extended-spectrum beta-lactamase (ESBL)-producing enterobacteria. Int J Clin Pract 2012;66:891–6. 10.1111/j.1742-1241.2012.02991.x [DOI] [PubMed] [Google Scholar]
- 59. YE H, Kang CI, Cha MK, et al. . Epidemiology and clinical outcomes of bloodstream infections caused by extended-spectrum beta-lactamase-producing Escherichia coli in patients with cancer. Int J Antimicrob Agents 2013;42:403–9. [DOI] [PubMed] [Google Scholar]
- 60. Du B, Long Y, Liu H, et al. . Extended-Spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae bloodstream infection: risk factors and clinical outcome. Intensive Care Med 2002;28:1718–23. 10.1007/s00134-002-1521-1 [DOI] [PubMed] [Google Scholar]
- 61. Stone PW, Gupta A, Loughrey M, et al. . Attributable Costs and Length of Stay of an Extended-Spectrum Beta-Lactamase-Producing Klebsiella pneumoniae Outbreak in a Neonatal Intensive Care Unit. Infect Control Hosp Epidemiol 2003;24:601–6. 10.1086/502253 [DOI] [PubMed] [Google Scholar]
- 62. Peña C, Pujol M, Ardanuy C, et al. . An outbreak of hospital-acquired Klebsiella pneumoniae bacteraemia, including strains producing extended-spectrum beta-lactamase. J Hosp Infect 2001;47:53–9. 10.1053/jhin.2000.0862 [DOI] [PubMed] [Google Scholar]
- 63. Kola A, Maciejewski O, Sohr D, et al. . Clinical impact of infections caused by ESBL-producing E. coli and K. pneumoniae. Scand J Infect Dis 2007;39:975–82. 10.1080/00365540701466140 [DOI] [PubMed] [Google Scholar]
- 64. Tsai M-H, Lee I-T, Chu S-M, et al. . Clinical and molecular characteristics of neonatal extended-spectrum β-lactamase-producing gram-negative bacteremia: a 12-year Case-Control-Control study of a referral center in Taiwan. PLoS One 2016;11:e0159744 10.1371/journal.pone.0159744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Maslikowska JA, Walker SAN, Elligsen M, et al. . Impact of infection with extended-spectrum β-lactamase-producing Escherichia coli or Klebsiella species on outcome and hospitalization costs. J Hosp Infect 2016;92:33–41. 10.1016/j.jhin.2015.10.001 [DOI] [PubMed] [Google Scholar]
- 66. Onken A, Said AK, Jørstad M, et al. . Prevalence and antimicrobial resistance of microbes causing bloodstream infections in Unguja, Zanzibar. PLoS One 2015;10:e0145632 10.1371/journal.pone.0145632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Nguyen M-L, Toye B, Kanji S, et al. . Risk factors for and outcomes of bacteremia caused by extended-spectrum ß-Lactamase– producing Escherichia coli and Klebsiella species at a Canadian tertiary care hospital. Can J Hosp Pharm 2015;68:136–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Denis B, Lafaurie M, Donay J-L, et al. . Prevalence, risk factors, and impact on clinical outcome of extended-spectrum beta-lactamase-producing Escherichia coli bacteraemia: a five-year study. Int J Infect Dis 2015;39:1–6. 10.1016/j.ijid.2015.07.010 [DOI] [PubMed] [Google Scholar]
- 69. Chopra T, Marchaim D, Johnson PC, et al. . Risk factors for bloodstream infection caused by extended-spectrum β-lactamase–producing Escherichia coli and Klebsiella pneumoniae: a focus on antimicrobials including cefepime. Am J Infect Control 2015;43:719–23. 10.1016/j.ajic.2015.02.030 [DOI] [PubMed] [Google Scholar]
- 70. Artero A, Esparcia A, Alberola J, et al. . Prospective cohort study of risk factors for extended-spectrum ß-lactamase-producing Escherichia coli urinary tract infections in elderly patients admitted to hospital. Int J Clin Pract 2017;71:e13001 10.1111/ijcp.13001 [DOI] [PubMed] [Google Scholar]
- 71. Chen I-L, Huang H-C, Wu C-T, et al. . Analysis of early-onset bloodstream infection due to Escherichia coli infection in premature babies. Medicine 2017;96:e7748 10.1097/MD.0000000000007748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Islas-Muñoz B, Volkow-Fernández P, Ibanes-Gutiérrez C, et al. . Bloodstream infections in cancer patients. risk factors associated with mortality. Int J Infect Dis 2018;71:59–64. 10.1016/j.ijid.2018.03.022 [DOI] [PubMed] [Google Scholar]
- 73. Ma J, Li N, Liu Y, et al. . Antimicrobial resistance patterns, clinical features, and risk factors for septic shock and death of nosocomial E coli bacteremia in adult patients with hematological disease. Medicine 2017;96:e6959 10.1097/MD.0000000000006959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Man MY, Shum HP, Chan YH, et al. . Clinical predictors and outcomes of Klebsiella pneumoniae bacteraemia in a regional hospital in Hong Kong. J Hosp Infect 2017;97:35–41. 10.1016/j.jhin.2017.06.007 [DOI] [PubMed] [Google Scholar]
- 75. Marando R, Seni J, Mirambo MM, et al. . Predictors of the extended-spectrum-beta lactamases producing Enterobacteriaceae neonatal sepsis at a tertiary Hospital, Tanzania. Int J Med Microbiol 2018;308:803–11. 10.1016/j.ijmm.2018.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Namikawa H, Yamada K, Fujimoto H, et al. . Clinical characteristics of bacteremia caused by extended-spectrum beta-lactamase-producing Escherichia coli at a tertiary hospital. Intern Med 2017;56:1807–15. 10.2169/internalmedicine.56.7702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Shi S-H, Feng X-N, Lai M-C, et al. . Biliary diseases as main causes of pyogenic liver abscess caused by extended-spectrum beta-lactamase-producing Enterobacteriaceae. Liver Int 2017;37:727–34. 10.1111/liv.13267 [DOI] [PubMed] [Google Scholar]
- 78. Tanır Basaranoglu S, Ozsurekci Y, Aykac K, et al. . A comparison of blood stream infections with extended spectrum beta-lactamase-producing and non-producing Klebsiella pneumoniae in pediatric patients. Ital J Pediatr 2017;43:79 10.1186/s13052-017-0398-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Razazi K, Mekontso Dessap A, Carteaux G, et al. . Frequency, associated factors and outcome of multi-drug-resistant intensive care unit-acquired pneumonia among patients colonized with extended-spectrum β-lactamase-producing Enterobacteriaceae. Ann Intensive Care 2017;7:61 10.1186/s13613-017-0283-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ray S, Anand D, Purwar S, et al. . Association of high mortality with extended–spectrum β-lactamase (ESBL) positive cultures in community acquired infections. J Crit Care 2018;44:255–60. 10.1016/j.jcrc.2017.10.036 [DOI] [PubMed] [Google Scholar]
- 81. Haruki Y, Hagiya H, Haruki M, et al. . Clinical characteristics and outcome of critically ill patients with bacteremia caused by extended-spectrum β-lactamase-producing and non-producing Escherichia coli. J Infect Chemother 2018;24:944–7. 10.1016/j.jiac.2018.04.016 [DOI] [PubMed] [Google Scholar]
- 82. Lin W-T, Tang H-J, Lai C-C, et al. . Clinical manifestations and bacteriological features of culture-proven gram-negative bacterial arthritis. J Microbiol Immunol Infect 2017;50:527–31. 10.1016/j.jmii.2015.08.026 [DOI] [PubMed] [Google Scholar]
- 83. Buys H, Muloiwa R, Bamford C, et al. . Klebsiella pneumoniae bloodstream infections at a South African children’s hospital 2006–2011, a cross-sectional study. BMC Infect Dis 2016;16:570 10.1186/s12879-016-1919-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Lee C-C, Lee C-H, Hong M-Y, et al. . Propensity-matched analysis of the impact of extended-spectrum β-lactamase production on adults with community-onset Escherichia coli, Klebsiella species, and Proteus mirabilis bacteremia. J Microbiol Immunol Infect 2018;51:519–26. 10.1016/j.jmii.2017.05.006 [DOI] [PubMed] [Google Scholar]
- 85. Huang YY, Alleyne A, Leung V, et al. . Urosepsis due to extended-ppectrum beta-lactamase-producing Escherichia coli: a retrospective, single-centre review of risk factors and clinical outcomes. Can J Hosp Pharm 2018;71:119–27. [PMC free article] [PubMed] [Google Scholar]
- 86. Komatsu Y, Kasahara K, Inoue T, et al. . Molecular epidemiology and clinical features of extended-spectrum beta-lactamase- or carbapenemase-producing Escherichia coli bacteremia in Japan. PLoS One 2018;13:e0202276 10.1371/journal.pone.0202276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Liu M, Li M, Wu L, et al. . Extended-Spectrum β-lactamase-producing E. coli septicemia among rectal carriers in the ICU. Medicine 2018;97:e12445 10.1097/MD.0000000000012445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Nivesvivat T, Piyaraj P, Thunyaharn S, et al. . Clinical epidemiology, risk factors and treatment outcomes of extended-spectrum beta-lactamase producing Enterobacteriaceae bacteremia among children in a tertiary care Hospital, Bangkok, Thailand. BMC Res Notes 2018;11:624 10.1186/s13104-018-3729-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Cordery RJ, Roberts CH, Cooper SJ, et al. . Evaluation of risk factors for the acquisition of bloodstream infections with extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella species in the intensive care unit; antibiotic management and clinical outcome. J Hosp Infect 2008;68:108–15. 10.1016/j.jhin.2007.10.011 [DOI] [PubMed] [Google Scholar]
- 90. Daikos GL, Kosmidis C, Tassios PT, et al. . Enterobacteriaceae bloodstream infections: presence of integrons, risk factors, and outcome. Antimicrob Agents Chemother 2007;51:2366–72. 10.1128/AAC.00044-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Gudiol C, Calatayud L, Garcia-Vidal C, et al. . Bacteraemia due to extended-spectrum beta-lactamase-producing Escherichia coli (ESBL-EC) in cancer patients: clinical features, risk factors, molecular epidemiology and outcome. J Antimicrob Chemother 2010;65:333–41. 10.1093/jac/dkp411 [DOI] [PubMed] [Google Scholar]
- 92. Marchaim D, Gottesman T, Schwartz O, et al. . National multicenter study of predictors and outcomes of bacteremia upon hospital admission caused by Enterobacteriaceae producing extended-spectrum beta-lactamases. Antimicrob Agents Chemother 2010;54:5099–104. 10.1128/AAC.00565-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Menashe G, Borer A, Yagupsky P, et al. . Clinical significance and impact on mortality of extended-spectrum beta lactamase-producing Enterobacteriaceae isolates in nosocomial bacteremia. Scand J Infect Dis 2001;33:188–93. [DOI] [PubMed] [Google Scholar]
- 94. Ortega M, Marco F, Soriano A, et al. . Analysis of 4758 Escherichia coli bacteraemia episodes: predictive factors for isolation of an antibiotic-resistant strain and their impact on the outcome. J Antimicrob Chemother 2009;63:568–74. 10.1093/jac/dkn514 [DOI] [PubMed] [Google Scholar]
- 95. Szilágyi E, Füzi M, Böröcz K, et al. . Risk factors and outcomes for bloodstream infections with extended-spectrum β -lactamase-producing Klebsiella pneumoniae; Findings of the nosocomial surveillance system in Hungary. Acta Microbiol Immunol Hung 2009;56:251–62. 10.1556/AMicr.56.2009.3.5 [DOI] [PubMed] [Google Scholar]
- 96. Tsai SS, Huang JC, Chen ST, et al. . Characteristics of Klebsiella pneumoniae bacteremia in community-acquired and nosocomial infections in diabetic patients. Chang Gung Med J 2010;33:532–9. [PubMed] [Google Scholar]
- 97. Carrara E, Pfeffer I, Zusman O, et al. . Determinants of inappropriate empirical antibiotic treatment: systematic review and meta-analysis. Int J Antimicrob Agents 2018;51:548–53. 10.1016/j.ijantimicag.2017.12.013 [DOI] [PubMed] [Google Scholar]
- 98. Horcajada JP, Shaw E, Padilla B, et al. . Healthcare-Associated, community-acquired and hospital-acquired bacteraemic urinary tract infections in hospitalized patients: a prospective multicentre cohort study in the era of antimicrobial resistance. Clin Microbiol Infect 2013;19:962–8. 10.1111/1469-0691.12089 [DOI] [PubMed] [Google Scholar]
- 99. Pitout JDD, Nordmann P, Laupland KB, et al. . Emergence of Enterobacteriaceae producing extended-spectrum β-lactamases (ESBLs) in the community. J Antimicrob Chemother 2005;56:52–9. 10.1093/jac/dki166 [DOI] [PubMed] [Google Scholar]
- 100. Pitout JDD, Laupland KB. Extended-Spectrum beta-lactamase-producing Enterobacteriaceae: an emerging public-health concern. Lancet Infect Dis 2008;8:159–66. 10.1016/S1473-3099(08)70041-0 [DOI] [PubMed] [Google Scholar]
- 101. Palacios-Baena ZR, Gutierrez-Gutierrez B, De Cueto M, et al. . Development and validation of the INCREMENT-ESBL predictive score for mortality in patients with bloodstream infections due to extended-spectrum-beta-lactamase-producing Enterobacteriaceae. J Antimicrob Chemother 2017;72:906–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Gandra S, Barter DM, Laxminarayan R. Economic burden of antibiotic resistance: how much do we really know? Clin Microbiol Infect 2014;20:973–80. 10.1111/1469-0691.12798 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2019-030266supp001.pdf (43.8KB, pdf)
bmjopen-2019-030266supp002.pdf (64KB, pdf)
bmjopen-2019-030266supp003.pdf (62.8KB, pdf)
bmjopen-2019-030266supp004.pdf (63.9KB, pdf)
bmjopen-2019-030266supp005.pdf (104.6KB, pdf)
bmjopen-2019-030266supp006.pdf (158.8KB, pdf)
bmjopen-2019-030266supp007.pdf (69.4KB, pdf)
bmjopen-2019-030266supp008.pdf (33.5KB, pdf)
bmjopen-2019-030266supp009.pdf (34.6KB, pdf)
bmjopen-2019-030266supp010.pdf (41KB, pdf)
bmjopen-2019-030266supp011.pdf (39KB, pdf)
bmjopen-2019-030266supp012.pdf (32.2KB, pdf)
bmjopen-2019-030266supp013.pdf (37.2KB, pdf)