Abstract
Objectives
We employed a comprehensive systematic review and meta-analysis to assess benefits and risks of a threshold of haemoglobin level below 7 g/dL versus liberal transfusion strategy among critically ill patients, and even patients with septic shock.
Design
Systematic review and meta-analysis.
Data sources
We performed systematical searches for relevant randomised controlled trials (RCTs) in the Cochrane Library, EMBASE and PubMed databases up to 1 September 2019.
Eligibility criteria
RCTs among adult intensive care unit (ICU) patients comparing 7 g/dL as restrictive strategy with liberal transfusion were incorporated.
Data extraction and synthesis
The clinical outcomes, including short-term mortality, length of hospital stay, length of ICU stay, myocardial infarction (MI) and ischaemic events, were screened and analysed after data collection. We applied odds ratios (ORs) to analyse dichotomous outcomes and standardised mean differences (SMDs) to analyse continuous outcomes with fixed or random effects models based on heterogeneity evaluation for each outcome.
Results
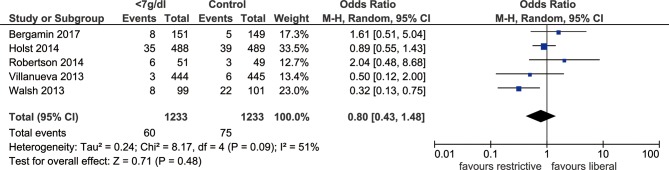
Eight RCTs with 3415 patients were included. Compared with a more liberal threshold, a red blood cell (RBC) transfusion threshold <7 g/dL haemoglobin showed no significant difference in short-term mortality (OR: 0.90, 95% CI: 0.67 to 1.21, p=0.48, I2=53%), length of hospital stay (SMD: −0.11, 95% CI: −0.30 to 0.07, p=0.24, I2=71%), length of ICU stay (SMD: −0.03, 95% CI: −0.14 to 0.08, p=0.54, I2=0%) or ischaemic events (OR: 0.80, 95% CI: 0.43 to 1.48, p=0.48, I2=51%). However, we found that the incidence of MI (OR: 0.54, 95% CI: 0.30 to 0.98, p=0.04, I2=0%) was lower in the group with the threshold <7 g/dL than that with the more liberal threshold.
Conclusions
An RBC transfusion threshold <7 g/dL haemoglobin is incapable of decreasing short-term mortality in ICU patients according to currently published evidences, while it might have potential role in reducing MI incidence.
Keywords: red blood cells, transfusion, intensive care units, septic shock
Strengths and limitations of this study.
This meta-analysis focused on the feasibility of a transfusion threshold of haemoglobin <7 g/dL with regard to short-term mortality in critically ill patients through only including randomised controlled trials that specified the restrictive red blood cell transfusion threshold as a pretransfusion haemoglobin concentration <7 g/dL.
In this meta-analysis, we performed an updated and comprehensive analysis that focused on intensive care unit patients with septic shock.
The number of studies we enrolled was not large enough due to the strict inclusion criteria of a restrictive transfusion threshold of haemoglobin <7 g/dL.
There was imperfect blinding of the study participants in the trials mainly owing to the nature of the interventions.
Introduction
Allogenic red blood cell (RBC) transfusion remains a commonly used and crucial treatment among patients admitted to the intensive care unit (ICU), as anaemia is commonly complicated and critically involved in poor outcomes.1 Every year, approximately 75 million units of blood are reportedly obtained worldwide, with higher levels of consumption in the UK, Canada and USA.2 3 In ICU settings, 40%~50% of critically ill patients receive at least one unit of RBC transfusion, and the average consumption reaches five units during their ICU stay.4 Undoubtedly, appropriate blood transfusion can benefit critically ill patients by increasing oxygen delivery and reducing oxygen debt, protecting against multiple organ dysfunction.5 While these data also urge the cautious use of RBCs because of the substantial cost and supply shortage. For example, Holst and colleagues have reported that the units of RBCs used for liberal transfusion trigger strategies are almost twice the amount of RBCs transfusion with restrictive strategies, but no significant difference is noted between restrictive and liberal triggers in assessment of primary outcomes.6 Additionally, the risk of complications, such as volume overload, infection, transfusion reactions and even increased mortality, also raises concerns about the threshold for RBC transfusion in ICU patients.7–9 However, the optimal thresholds for RBC transfusion in diverse critical care settings remain controversial. The results of the transfusion requirements in critical care (TRICC) study have confirmed the superiority of a restrictive transfusion strategy (RBC transfusions were given when haemoglobin concentration was below 7 g/dL) in controlling the 30-day mortality of critically ill patients with younger age and lower acute physiology and chronic health evaluation II score. Indeed, conservative blood transfusion could result in a marked decline in the use of RBCs, which further decreases the in-hospital cost of ICU patients.2 10 Recently, various studies have extensively discussed transfusion strategies to optimise the outcomes. For instance, no significant difference was shown between restrictive and liberal transfusion strategies in terms of adverse effects, as reported by some studies.11 12 In addition, other researchers found that blood transfusions triggered at a threshold of 7 g/dL were much safer in critically ill patients with cardiovascular diseases.10 13 However, Silva Junior et al have found that RBC transfusion was an independent risk factor for mortality of critically ill patients, followed with longer ICU and hospital stay, which was associated with different decisions regarding transfusion triggers.14 Other indices, such as oxygen delivery (DO2) and oxygen consumption (VO2), also show marked deviation among various studies. Study by Conrad and colleagues revealed significant improvement in DO2 but no influence in VO2 after blood transfusion on septic patients.15 While Steffesand colleagues have reported that blood transfusion is capable of elevating DO2 and VO2 in septic surgical patients.16 Therefore, the thresholds for blood transfusion should be different for patients with various diseases and need to be carefully evaluated.
Actually, the benefits and harms of blood transfusions in patients admitted to ICUs have been discussed by many systematic reviews and meta-analyses, but the results remain controversial due to the distinct inclusion criteria and outcome measurement across studies.9 11 12 17–19 Salpeter and colleagues found that restrictive blood transfusion trigger at 7 g/dL could significantly reduce mortality of disparate phase, as well as diverse transfusion-related complications compared with the liberal transfusion trigger. However, they did not distinguish paediatric and adult ICU settings, and merely enrolled three randomised controlled trials (RCTs).17 Systematic reviews conducted by Fominskiy et al revealed no statistical difference in 90-day mortality between two transfusion thresholds.18 Nevertheless, recently updated publication by Chong and colleagues incorporated almost same RCTs as Fominskiy et al did, while they identified a significant reduction of 30-day mortality in ICU patients with restrictive strategy in comparison with those with more liberal transfusion trigger.19 In addition, the specific thresholds of haemoglobin concentration are essential for decision of RBC transfusion regarding various clinical practice. However, no studies have reported the impact of the transfusion threshold of 7 g/dL on the short-term outcomes of critically ill patients or the financial value of a different transfusion strategy, even though it is considered as a common trigger to implement restrictive transfusion strategy. Furthermore, different types of clinical conditions also show remarkable deviation in RBCs administration. For example, septic shock is commonly recognised as a substantial threat to ICU, and it is related to high hospital costs and poor outcomes.20 Anaemia is also commonly complicated during the progression of sepsis, as it presents with insufficient tissue perfusion, like hypovolemic shock, and dysfunction of cellular metabolism, which cannot be reversed by prompt fluid resuscitation and administration of vasoactive drugs. Indeed, blood transfusion is frequently administered as an efficient remedy for patients with septic shock, but the protocol for transfusion is different in patients with septic shock from patients with other critical illnesses.1 21 22 In fact, there is still a lack of conclusive data regarding the rational transfusion threshold for patients with septic shock.22 23 The transfusion requirements in septic shock (TRISS) trial did provide strong evidences that no significant difference was noted between RBC transfusion with lower and higher haemoglobin thresholds in long-term mortality and adverse reactions.22 However, other researchers found that RBC transfusion was related to unfavourable outcomes of septic patients, such as sequential organ failure assessment score and length of stay in ICU. In addition, the association between RBC transfusion and short-term outcomes of septic patients has not been established yet. In the present study, we aim to perform a comprehensive systematic review and meta-analysis specifically determining whether haemoglobin level below 7 g/dL is an optimal trigger for blood transfusion among adult ICU patients when compared with more liberal transfusion thresholds by evaluating its impacts on short-term mortality and adverse reactions. Additionally, a subgroup analysis is further performed with patients with or without septic shock to seek the optimal transfusion strategy for this unique subset of critically ill patients.
Materials and methods
This systematic review and meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis statement.24
Patient and public involvement
There were no patients’ involvement in the development of the research question, outcome measurement, design of this study, or the recruitment to and conduct of the study. The results will not be disseminated to study participants.
Search strategy and information sources
Online databases, including Cochrane Library, EMBASE and PubMed, were systematically searched. We conceived strategy comprised of following combination of exploded Medical Subject Heading terms: ‘critical care’, ‘intensive care unit’, ‘blood transfusion’. Detailed search strategy was presented in online supplementary file 1. Relevant studies up to 1 September 2019 were searched without any language limitations. In addition, ongoing trials and conference abstracts were identified to obtain additional evidences. We also obtained references by searching the reference lists of reviews and trial registries.
bmjopen-2019-030854supp001.pdf (64.3KB, pdf)
Eligibility and exclusion criteria
This meta-analysis included RCTs among adult ICU patients (age >18 years) who underwent allogenic RBC transfusion. The recruited studies had to compare two distinct blood transfusion thresholds, a restrictive threshold and a liberal one. The definition of transfusion thresholds in this systematic review was based on haemoglobin or hematocrit levels. Blood transfusion initiated at haemoglobin thresholds below 7 g/dL were termed restrictive strategies, while the liberal transfusions were conducted at haemoglobin thresholds between 8.5 and 10 g/dL. Other types of studies, including observational, cohort and case–control, were excluded. Trials with pretransfusion haemoglobin concentrations higher than 7 g/dL were eliminated as well. Only ICU patients were considered, while participants in other hospital departments were not eligible.
Study selection
Two reviewers (RQY and CR) independently screened the titles and abstracts of the relevant trials. If the abstract of a potentially eligible article failed to provide adequate information, the full-text version was then screened to determine its eligibility. Differing opinions between the two authors were settled by discussion and consensus. If a consensus could not be reached, a consulting group including two experts (ZFX and YMY) resolved the disagreements.
Data collection
Two reviewers (RQY and CR) extracted the data from all eligible trials with a standardised and predesigned form. First author, year of publication, baseline characteristics, the total number of included patients and the clinical settings were recorded. The clinical outcomes (short-term mortality, length of hospital stay, length of ICU stay, myocardial infarction (MI) and ischaemic events) and study design were also obtained.
Risk of bias assessment
The Cochrane Collaboration tool was used to evaluate the risk of bias of the RCTs. The randomisation sequence, allocation concealment, blinding of personnel and participants, risk of incomplete outcome data, selective reporting bias and other sources of bias were assessed independently by two authors. Each clause was rated as ‘low’, ‘high’ or ‘unclear’ bias. The summarised risk of bias of each RCT was ranked as low, moderate or high.
Grading quality of evidence
The quality of evidence of each outcome was evaluated in accordance with the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) methods. This procedure was conducted with GRADE Pro software V.3.6.
Outcomes
The primary endpoint was all-cause short-term mortality, which was preferentially analysed by 28-day or 30-day mortality. In the case of unreported short-term mortality, we contacted the authors for the original data or considered the closest available mortality data. Secondary outcomes included the following indicators: length of hospital stay, length of ICU stay, MI and ischaemic events.
Data synthesis and analysis
The statistical analysis was conducted with ReviewManager (RevMan V.5.3, Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). We applied ORs to analyse dichotomous outcomes and standardised mean differences (SMDs) for continuous outcomes. The pooled results were calculated with 95% CIs. Heterogeneity among studies for each outcome was assessed by applying both χ2 test and I2 statistics. Either I2 greater than 50% or p value of χ2 test <0.10 was deemed as statistically significant heterogeneity. If remarkable heterogeneity existed in pooled results, random effect models combined with the Mantel-Haenszel (M-H) method were used, or else, fixed effect models was applied accordingly. For the small study bias, the funnel plot of the pooled short-term mortality data was scanned visually by reviewers. Besides, by using Stata software, V.12, we performed Begg’s and Egger’s tests to further assess the possible small study bias. A sensitivity analysis was also performed by means of excluding each study one at a time from the pooled effect. Additionally, we performed a subgroup analysis based on the M-H model to determine the difference between septic shock and non-sepsis groups.
Results
Search results and the characteristics of the included studies
This systematic review and meta-analysis identified 4641 relevant citations; we removed duplicates and then scanned the titles and abstracts of 4600 studies. Eventually, the full-text articles for 41 trials were reviewed, and eight RCTs met the inclusion criteria and were presented with full paper, with ICU patients older than 18 years who received RBC transfusions at haemoglobin thresholds below 7 g/dL (figure 1).
Figure 1.
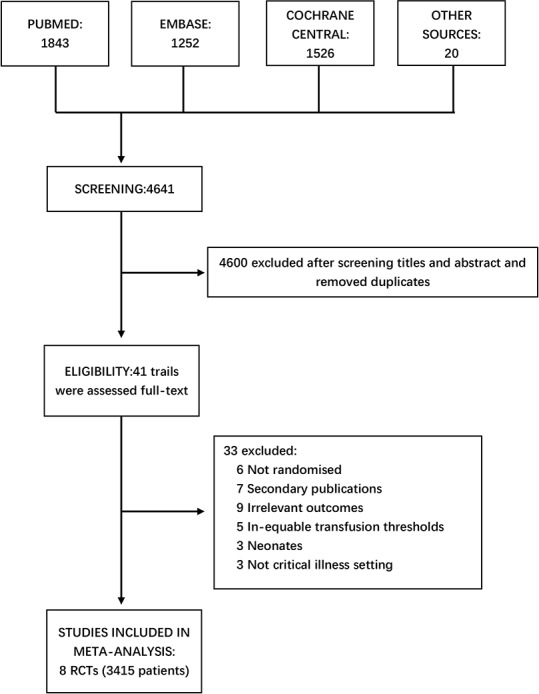
Flow chart for study selection. Online databases, including Cochrane Library, EMBASE and PubMed, were systematically searched. Finally, nine RCTs with 3415 patients were included in the meta-analysis. RCTs, randomised controlled trials.
The eight included RCTs ranged in publication year from 1999 to 2019 and contained a total of 3415 patients.10 22 23 25–29 The patient population sizes of the included trials were very diverse, ranging from 44 to 998. Three studies enrolled more than 800 patients, while three trials enrolled fewer than 200 eligible patients. Four studies enrolled 1480 patients with septic shock, including one study complicated by cancer diagnoses. In addition, four trials were multicentre studies (table 1)
Table 1.
Characteristics of the included studies
| Author | Year of publication | No of sites | Population | Transfusion triggers | Mortality data | References | |||
| Clinical settings | Details | Number of participants | Restrictive | Liberal | |||||
| Hebert et al | 1999 | 25 | Critical illness | Euvolemic critically ill patients | 838 | Hb 7 | Hb 10 | 30-day mortality | |
| 60-day mortality | 10 | ||||||||
| ICU mortality | |||||||||
| Hospital mortality | |||||||||
| Holst et al | 2014 | 32 | Critical illness | Patients with septic shock | 998 | Hb 7 | Hb 9 | 90-day mortality | 22 |
| Mazza et al | 2015 | Single | Critical illness | Patients with septic shock | 46 | Hb 7 | Hb 9 | Hospital mortality | 23 |
| Robertson et al | 2014 | 2 | Traumatic brain injury | Patients with closed head injuries | 200 | Hb 7 | Hb 10 | 6-month mortality | 25 |
| Villanueva et al | 2013 | Single | Upper gastrointestinal bleeding | Patients with hematemesis, melena or both | 889 | Hb 7 | Hb 9 | 45-day mortality | 26 |
| Walsh et al | 2013 | 6 | Critical illness | Older critically ill patients receiving mechanical ventilation | 100 | Hb 7 | Hb 9 | 30-day mortality | |
| 60-day mortality | |||||||||
| 180-day mortality | 27 | ||||||||
| ICU mortality | |||||||||
| Bergamin et al | 2017 | Single | Critical illness | Patients with cancer with septic shock | 300 | Hb 7 | Hb 9 | Hospital mortality 28-day mortality |
|
| 60-day mortality | 29 | ||||||||
| 90-day mortality | |||||||||
| Gobatto et al | 2019 | Single | Traumatic brain injury | Patients with moderate or severe traumatic brain injury | 44 | Hb 7 | Hb 9 | Hospital mortality ICU mortality |
28 |
ICU, intensive care unit.
Risk of bias
Most of the RCTs met the randomisation requirements and used rational distribution methods. In some of the included trials, however, it was challenging to blind the attending physicians and nurses to the outcome assessment based on the intervention, which resulted in high risk of performance bias (online supplementary figure 1).
bmjopen-2019-030854supp002.pdf (2.1MB, pdf)
Quality of evidence
The summary of findings for the outcomes of interest and the levels of evidence were provided (online supplementary table 1). The qualities of the primary outcome data and some secondary outcome data, including MI and ischaemic events, were all ranked as moderate. However, the length of stay both in hospital and ICU displayed low quality.
bmjopen-2019-030854supp003.pdf (92.5KB, pdf)
Primary outcome: short-term mortality
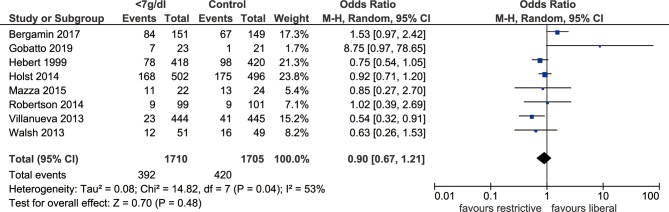
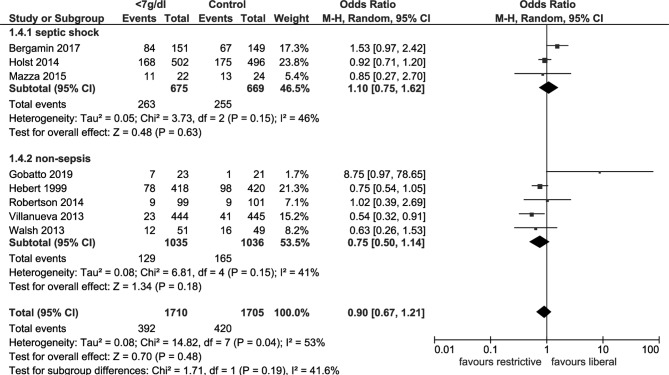
Within this meta-analysis, there were three RCTs that reported 28-day or 30-day mortality, and four reported in-hospital mortality only. The published study from Holst et al did provide solid conclusions about the impacts of blood transfusion with liberal and restrictive haemoglobin thresholds on long-term mortality and rates of ischaemic events, which presented with similar effects, while the information about short-term outcomes was missing.22 Therefore, we wrote a letter asking for the important evidence of short-term mortality rates, as its analysis was based on a large sample size and was essential for our conclusions. After generating the forest plot, we found no significant difference in short-term mortality between the transfusion threshold of haemoglobin <7 g/dL and the more liberal strategy (OR: 0.90, 95% CI: 0.67 to 1.21, p=0.48, I2=53%). Meanwhile, we noticed that the RCT reported by Bergamin et al 29 was the main resource of heterogeneity, and removing that study resulted in a marked reduction in heterogeneity (I2=29%, p=0.21) (figure 2).
Figure 2.
Forest plot of all-cause short-term mortality in ICU patients. The OR and 95% CI for short-term mortality between the restrictive and liberal transfusion thresholds are presented in the forest plot. The threshold of haemoglobin <7 g/dL showed no obvious improvement in short-term mortality when compared with the liberal threshold. ICU, intensive care unit; M-H, Mantel-Haenszel.
Secondary outcome: length of hospital stay, length of ICU stay, MI and ischaemic events
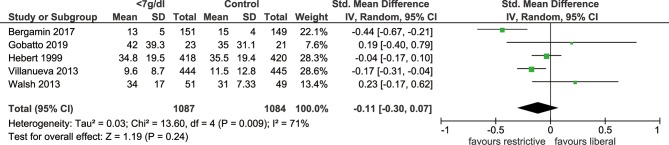
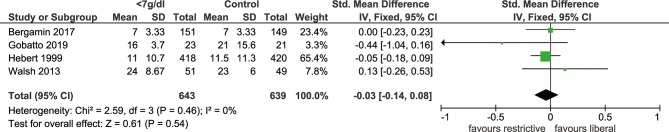
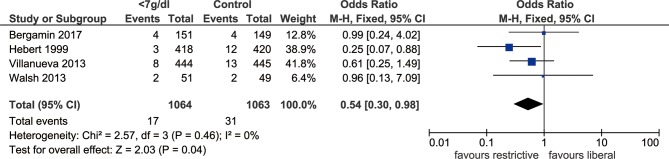
Five included studies documented the length of hospital stay, which revealed no significant difference in hospital stays when the threshold of haemoglobin <7 g/dL was used, comparing with the more liberal threshold (SMD: −0.11, 95% CI: −0.30 to 0.07, p=0.24, I2=71%, figure 3). Sensitivity analysis indicated that study by Bergamin et al was the main source of heterogeneity, exclusion of which could significantly reduce heterogeneity (I2=45%, p=0.51). The outcome of length of ICU stay was reported by four trials, and there was no significant difference between the two thresholds (SMD: −0.03, 95% CI: −0.14–0.08, p=0.54, I2=0%, figure 4). In addition, we identified that MI events was decreased among patients with transfusion trigger of haemoglobin <7 g/dL when compared with those with the liberal transfusion strategy (OR: 0.54, 95% CI: 0.30 to 0.98, p=0.04, I2=0%, figure 5). However, no significant differences were noted between the two transfusion thresholds for critically ill patients in ischaemic/thromboembolic events (OR, 0.80, 95% CI, 0.43 to 1.48, p=0.48, I2=51%, figure 6). After removing study conducted by Walsh et al, the heterogeneity of this outcome decreased significantly (I2=0%, p=0.21), which indicated the main source of heterogeneity.
Figure 3.
Forest plot of the length of hospital stay. The forest plot shows the mean difference and 95% CI for the length of hospital stay between the two groups. Blood transfusion at the restrictive threshold resulted in no significant difference of hospital stays compared with blood transfusion at the more liberal threshold.
Figure 4.
Forest plot of the length of ICU stay. The difference in the length of ICU stay in the groups with different transfusion thresholds is shown by the mean difference and 95% CI in the forest plot. No marked improvement was seen in the length of ICU stay with a transfusion threshold of haemoglobin <7 g/dL. ICU, intensive care unit.
Figure 5.
Forest plot of myocardial infarction in ICU patients after RBCs transfusion. The forest plot shows the ORs and 95% CI for myocardial infarction in the groups of ICU patients with different transfusion thresholds. Blood transfusion at a threshold of haemoglobin <7 g/dL significantly decrease in the rate of myocardial infarction compared with the more liberal threshold. ICU, intensive care unit; M-H, Mantel-Haenszel; RBC, red blood cell.
Figure 6.
Forest plot of ischaemic events/thromboembolic events in ICU patients after RBC transfusions. The ORs and 95% CI for ischaemic/thromboembolic events are presented in the forest plot. No significant difference was noted in ischaemic/thromboembolic events between the group with the threshold of 7 g/dL haemoglobin compared with the group with the more liberal threshold. ICU, intensive care unit; M-H, Mantel-Haenszel; RBC, red blood cell.
Small study bias
We constructed a funnel plot to assess the possible small study bias. After inspecting the funnel plot, we found no evidence of small study bias. Furthermore, we used Begg’s test (p=0.71) and Egger’s test (p=0.62) to evaluate the funnel plot asymmetry, which also showed no significant statistical evidence of small study bias (online supplementary figure 2).
bmjopen-2019-030854supp004.pdf (352.8KB, pdf)
Subgroup analysis
The subgroup analysis of the septic shock and non-sepsis groups investigated short-term mortality. From the forest plot, there were no significant differences in short-term mortality between two thresholds in either the septic shock group (OR: 1.10, 95% CI: 0.75 to 1.62, p=0.63, I2=46%) or the non-sepsis group (OR: 0.75, 95% CI: 0.50 to 1.14, p=0.15, I2=41%) (figure 7).
Figure 7.
Forest plot for short-term mortality following subgroup analysis. The forest plot shows the ORs and 95% CI for the all-cause short-term mortality of patients receiving RBC transfusions at various thresholds according to the subgroup analysis of the septic shock and non-sepsis groups. Restrictive transfusion was incapable of decreasing short-term mortality in septic ICU patients. ICU, intensive care unit; M-H, Mantel-Haenszel; RBC, red blood cell.
Discussion
Major findings
The current study demonstrated that restricting the transfusion threshold to a haemoglobin concentration <7 g/dL did not result in significant differences in short-term mortality, ICU/hospital length of stay or ischaemic events, when compared with more liberal thresholds. Of note, the length of stay of both ICU and hospital displayed low quality of evidence. Additionally, pooled data also revealed that the incidence of MI was decreased among patients applied 7 g/dL as transfusion threshold. Nevertheless, we should be cautious when interpreting this finding. After removing the study conducted by Villanueva and colleagues, as well as changing effects model from random effects models to fixed effects models could alter the consequence, indicating the instability of this outcome.
Within the primary outcome analysis, the heterogeneity of enrolled trials was moderate, with an I2 of 53% according to the heterogeneity test, while sensitivity analysis revealed that remove of the Transfusion Requirements in Critically Ill Oncological Patients (TRICOP) trial resulted in dramatically decreased heterogeneity (I2=29%, p=0.21). As this study enrolled patients diagnosed with both solid cancer and septic shock, the baseline characteristic of this unique subset might differ from other ordinary ICU patients, which could partially explain the source of heterogeneity. Also, this finding was assumed to be due to different clinical settings, especially for patients with septic shock. We further performed a subgroup analysis after classifying the studies into a septic shock group and a non-sepsis group, as septic shock was recognised as one of the major causes of death in critically ill patients. In septic shock group, patients with a transfusion threshold <7 g/dL showed no significant difference in short-term mortality compared with those with a more liberal transfusion threshold, while the heterogeneity was markedly decreased (I2=46%, p=0.15). In non-sepsis group, no significant difference in short-term mortality was noted between the two thresholds with only five trials included. Additionally, the highly disparate sample sizes of included studies could be another resource of heterogeneity. Given the fact that several studies came from conference abstracts, we were unable to evaluate their methodology and data quality in detail.
Relations to other meta-analysis
Carefully designed meta-analyses on RBC transfusions in critically ill patients have been published recently. In 2014, the first time Salpeter and colleagues reported the benefits of restrictive blood transfusion at haemoglobin trigger <7 g/dL in critically ill patients via conducting meta-analysis, which presented with significant reductions in total mortality (RR: 0.80, 95% CI: 0.65 to 0.98), in-hospital mortality (RR: 0.74, 95% CI: 0.60 to 0.92), 30-day mortality (RR: 0.77, 95% CI: 0.61 to 0.96), acute coronary syndrome (RR: 0.44, 95% CI: 0.22 to 0.89), pulmonary oedema (RR: 0.48, 95% CI: 0.33 to 0.72), rebleeding (RR: 0.64, 95% CI: 0.45 to 0.90) and bacterial infections (RR: 0.86, 95% CI: 0.73 to 1.00) when compared with the liberal transfusion threshold group.17 However, this meta-analysis did not provide a convincing conclusion with only three RCTs included, and also failed to separate adult and paediatric participants, as each population shared different transfusion protocols.
Recently, in a review by Fominskiy et al,18 the restrictive and liberal RBC transfusion thresholds in critically ill patients showed no significant difference in all-cause 90-day mortality (OR: 1.10, 95% CI: 0.99 to 1.23, p=0.07, I2=34%). In fact, this study was the first comprehensive meta-analysis to address different transfusion thresholds among critically ill and perioperative patients, but it lacked a valid analysis of secondary outcomes which were noteworthy factors for the effects of RBC transfusions. Furthermore, Chong and colleagues also conducted an updated analysis on the effects of RBC transfusion, which included two more RCTs other than the same 10 trials included in Fominskiy’s study.18 19 23 30 These results suggested that RBC transfusion with restrictive threshold significantly reduced the risk of overall 30-day mortality (OR: 0.82, 95% CI: 0.70 to 0.97, p=0.019) when compared with that with liberal threshold, accompanied with declining risk of stroke/transient ischaemic attack (OR: 0.63, 95% CI: 0.40 to 0.99, p=0.04), transfusion reactions (OR: 0.48, 95% CI: 0.29 to 0.80, p=0.005), allogenic blood exposure (OR: 0.04, 95% CI: 0.01 to 0.14, p=0.001) and length of hospital stay (95% CI: 0.42 to 1.64, p=0.001), hinting the safety of using restrictive transfusion protocol. Actually, above two studies focused on different primary outcomes, 30-day and 90-day mortality, respectively, and further drew different conclusions even though both included similar RCTs, indicating that the effects of RBC transfusion varied with the stage of critical settings. However, Hovaguimian and Myles31 performed a context-specific systematic review and meta-analysis comparing the restrictive and liberal transfusion thresholds and found no significant differences in early mortality (OR: 0.94, 95% CI: 0.73 to 1.20, p=0.09, I2=45%) between the two thresholds, indicating that the specific types and severity of critical illness might be in need of different strategies of RBC transfusion, especially for patients with major surgery.
In the present study, we specifically concentrated on the restrictive transfusion threshold of haemoglobin <7 g/dL in ICU patients. We included data from the newly published Transfusion Requirements after Head Trauma trial and the TRICOP trial, which presented with increased mortality rate in the group with restrictive transfusion thresholds in comparison with liberal transfusion threshold group.28 29 This study showed that RBC transfusion with restrictive threshold <7 g/dL did not result in significant improvement in short-term mortality when compared with those using liberal thresholds.
Subgroup analysis
The first review with regard to the impact of blood transfusion on the prognosis of septic shock patients was conducted by Dupuis and colleagues.32 They showed no association between RBC transfusion and mortality rate in patients with septic shock, and also failed to determine correlations between the two different transfusion thresholds or to infer the optimal transfusion threshold for septic shock patients because of a shortage of high-quality RCTs.32 In fact, a 10 g/dL haemoglobin threshold has been universally proposed for treatment of septic shock as the crucial role of RBC transfusions in early goal-directed therapy.33 Nonetheless, severe adverse events caused by extensive blood transfusion have been reported as a great threat for septic shock patients by several studies.34–36 The restrictive strategy, as reported previously, was beneficial for the improvement of microcirculation, while also saving blood products.10 37 The landmark TRISS trial that was conducted by Holst et al 22 revealed no significant differences in 90-day mortality between patients in the group with the transfusion thresholds of 7 g/dL and those with the more liberal thresholds. In addition, the number of patients experiencing ischaemic events and severe adverse reactions was also similar between the two groups. The TRISS trial demonstrated the safety and economic efficiency of the restrictive blood transfusion threshold, with a well-controlled risk of bias. Mazzaet al 23 performed a randomised physiological study of septic shock patients with the endpoint of abnormal lactate and ScvO2 under distinct pretransfusion haemoglobin concentrations. However, they failed to provide valid data on mortality with a relatively small sample size provided. Recently, Bergamin and colleagues focused on cancer patients who developed septic shock in the ICU through a single-centre RCT.29 Indeed, tumour patients who were complicated by septic shock were in urgent need of blood transfusion as high risk of anaemia.22 38 Ideally, the more restrictive threshold for transfusion might reduce the occurrence of multiple transfusion-related complications. In this study, we conducted a comprehensive meta-analysis after enrolling all recently published RCTs that covered septic shock cases. No marked difference in mortality was observed between the transfusion threshold of haemoglobin <7 g/dL and the more liberal transfusion threshold (OR: 1.08, 95% CI: 0.82 to 1.41, p=0.54, I2=20%). We assumed that these results might be, at least in part, due to the overwhelming weight that the TRISS trial carried and the relatively low quality of the other three studies. Moreover, the study by Mazza et al 23 enrolled participants with a diagnosis of malignant tumorous, which might generate heterogeneity. Taken together, we cannot determine that blood transfusion at thresholds of 7 g/dL is the optimal transfusion threshold for patients with septic shock based on current evidences, which urges more as well as large clinical trials.
Strengths and limitations
This study mainly focused on analysing the impact of the transfusion threshold of 7 g/dL on the short-term outcomes of critically ill patients, which remains an essential clinical practice but with controversy. Indeed, the blood transfusion is given with different triggers by different organisations, such as Surviving Sepsis Campaign, the American college of critical care medicine and the WHO.39–41 Further analysis shows that these guidelines are provided mainly based on long-term effects of blood transfusion on ICU patients. Our meta-analysis is the first report concerning the feasibility of a transfusion threshold of haemoglobin <7 g/dL with regard to short-term mortality in critically ill patients, which is an essential issue for survival of ICU patients based on specific clinical characters. In addition, unlike the previously published meta-analyses, which enrolled studies with different restrictive transfusion thresholds, we only included RCTs that specified the restrictive RBC transfusion threshold as a pretransfusion haemoglobin concentration <7 g/dL to get relative solid conclusions. Simultaneously, we performed an updated and comprehensive analysis that focused on ICU patients with septic shock. Meanwhile, this analysis revealed no evidence of significant small study bias according to visual inspection of the funnel plot, Begg’s test and Egger’s test.
Some limitations are also noted in the current systematic review and meta-analysis. First, the number of studies we enrolled was not large enough due to the strict inclusion criteria of a restrictive transfusion threshold of haemoglobin <7 g/dL. Five relevant studies that discussed the two different transfusion thresholds among critically ill patients were excluded because of their different definitions of restrictive RBC transfusion thresholds.30 42–45 Second, the heterogeneity in our meta-analysis was relatively high, which was caused by different outcome measurements and clinical settings. Some trials with low-quality evidence and insufficient participants might be another source of heterogeneity. Correspondingly, we tried to eliminate the heterogeneity by conducting a subgroup analysis and analysing the effects. Third, there was imperfect blinding of the study participants in the trials mainly owing to the nature of the interventions. Fourth, the sample sizes of all incorporated RCTs were varied. We applied the M-H method to address this diversity in sample sizes and to avoid our results from being dominated by the larger studies. Finally, we failed to testify if haemoglobin level <7 g/dL was the optimal threshold for the blood transfusions in critically ill patients and in those with septic shock basing on a lack of sufficient evidence.
Conclusions and clinical implications
The present meta-analysis of RCTs focused on the effect of RBC transfusions at the threshold of haemoglobin <7 g/dL on the survival and prognosis of ICU patients. RBC transfusions at the threshold of haemoglobin <7 g/dL did not result in significant difference in short-term mortality when compared with transfusions administered at a more liberal threshold. However, it might associate with decreased MI events, suggesting its potentially protecting role for critically ill patients. Besides, regarding ICU patients with septic shock, RBC transfusions at the restrictive threshold did not improve short-term mortality compared with transfusions at the more liberal threshold. Therefore, we recommend a haemoglobin trigger of 7 g/dL for critically ill patients with or without septic shock due to the cost and resource saving effects, as well as its latent value in reducing severe adverse effects. Still, further studies are required to validate our findings. This study indeed provides novel conclusions on the impact of blood transfusion on short-term outcomes of critically ill patients as well as patients with septic shock. Even though it was hard to determine that the haemoglobin trigger of 7 g/dL was the optimal strategy for RBC transfusion, but it did show advantages in managing the use of RBC units and urged prudent decision-making in blood transfusion for critically ill patients.
Supplementary Material
Acknowledgments
We greatly appreciated the excellent job and kind share of professor Holst in providing important evidence on the impact of blood transfusion with liberal and restrictive haemoglobin thresholds on short-term mortality.
Footnotes
R-qY and CR contributed equally.
Contributors: YMY and ZFX conceived the meta-analysis. RQY and CR extracted all data. YBZ and ZCZ undertook and refined the searches. RQY and CR co-wrote the paper. RQY undertook the statistical analyses. All authors contributed to and revised the final manuscript.
Funding: This work was supported by grants from the National Natural Science Foundation (Nos. 81730057, 81930057) and the National Key Research and Development Program of China (No. 2017YFC1103302).
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available in a public, open access repository. All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1. Rosland RG, Hagen MU, Haase N, et al. Red blood cell transfusion in septic shock - clinical characteristics and outcome of unselected patients in a prospective, multicentre cohort. Scand J Trauma Resusc Emerg Med 2014;22:14 10.1186/1757-7241-22-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Klein HG, Spahn DR, Carson JL. Red blood cell transfusion in clinical practice. Lancet 2007;370:415–26. 10.1016/S0140-6736(07)61197-0 [DOI] [PubMed] [Google Scholar]
- 3. Amin M, Fergusson D, Aziz A, et al. The cost of allogeneic red blood cells - a systematic review. Transfus Med 2003;13:275–86. 10.1046/j.1365-3148.2003.00454.x [DOI] [PubMed] [Google Scholar]
- 4. Corwin HL, Surgenor SD, Gettinger A. Transfusion practice in the critically ill. Crit Care Med 2003;31:S668–71. 10.1097/01.CCM.0000099348.99451.84 [DOI] [PubMed] [Google Scholar]
- 5. Kristof K, Büttner B, Grimm A, et al. Anaemia requiring red blood cell transfusion is associated with unfavourable 90-day survival in surgical patients with sepsis. BMC Res Notes 2018;11:879 10.1186/s13104-018-3988-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Holst LB. Benefits and harms of red blood cell transfusions in patients with septic shock in the intensive care unit. Dan Med J 2016;63:B5209. [PubMed] [Google Scholar]
- 7. Madjdpour C, Spahn DR. Allogeneic red blood cell transfusions: efficacy, risks, alternatives and indications. Br J Anaesth 2005;95:33–42. 10.1093/bja/aeh290 [DOI] [PubMed] [Google Scholar]
- 8. Marik PE, Corwin HL. Efficacy of red blood cell transfusion in the critically ill: a systematic review of the literature*. Crit Care Med 2008;36:2667–74. 10.1097/CCM.0b013e3181844677 [DOI] [PubMed] [Google Scholar]
- 9. Hopewell S, Omar O, Hyde C, et al. A systematic review of the effect of red blood cell transfusion on mortality: evidence from large-scale observational studies published between 2006 and 2010. BMJ Open 2013;3:e002154 10.1136/bmjopen-2012-002154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. transfusion requirements in critical care Investigators, Canadian critical care Trials Group. N Engl J Med 1999;340:409–17. 10.1056/NEJM199902113400601 [DOI] [PubMed] [Google Scholar]
- 11. Holst LB, Petersen MW, Haase N, et al. Restrictive versus liberal transfusion strategy for red blood cell transfusion: systematic review of randomised trials with meta-analysis and trial sequential analysis. BMJ 2015;350:h1354 10.1136/bmj.h1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Carson JL, Carless PA, Hebert PC. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. The Cochrane database of systematic reviews 2012;4:Cd002042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hébert PC, Yetisir E, Martin C, et al. Is a low transfusion threshold safe in critically ill patients with cardiovascular diseases? Crit Care Med 2001;29:227–34. 10.1097/00003246-200102000-00001 [DOI] [PubMed] [Google Scholar]
- 14. Silva Junior JMda, Rezende E, Amendola CP, et al. Red blood cell transfusions worsen the outcomes even in critically ill patients undergoing a restrictive transfusion strategy. Sao Paulo Med J 2012;130:77–83. 10.1590/S1516-31802012000200002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Conrad SA, Dietrich KA, Hebert CA, et al. Effect of red cell transfusion on oxygen consumption following fluid resuscitation in septic shock. Circ Shock 1990;31:419–29. [PubMed] [Google Scholar]
- 16. Steffes CP, Bender JS, Levison MA. Blood transfusion and oxygen consumption in surgical sepsis. Crit Care Med 1991;19:512–7. 10.1097/00003246-199104000-00010 [DOI] [PubMed] [Google Scholar]
- 17. Salpeter SR, Buckley JS, Chatterjee S. Impact of more restrictive blood transfusion strategies on clinical outcomes: a meta-analysis and systematic review. Am J Med 2014;127:124–31. 10.1016/j.amjmed.2013.09.017 [DOI] [PubMed] [Google Scholar]
- 18. Fominskiy E, Putzu A, Monaco F, et al. Liberal transfusion strategy improves survival in perioperative but not in critically ill patients. A meta-analysis of randomised trials. Br J Anaesth 2015;115:511–9. 10.1093/bja/aev317 [DOI] [PubMed] [Google Scholar]
- 19. Chong MA, Krishnan R, Cheng D, et al. Should transfusion trigger thresholds differ for critical care versus perioperative patients? A meta-analysis of randomized trials. Crit Care Med 2018;46:252–63. 10.1097/CCM.0000000000002873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Singer M, Deutschman CS, Seymour CW, et al. The third International consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016;315:801–10. 10.1001/jama.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zarychanski R, Doucette S, Fergusson D, et al. Early intravenous unfractionated heparin and mortality in septic shock*. Crit Care Med 2008;36:2973–9. 10.1097/CCM.0b013e31818b8c6b [DOI] [PubMed] [Google Scholar]
- 22. Holst LB, Haase N, Wetterslev J, et al. Lower versus higher hemoglobin threshold for transfusion in septic shock. N Engl J Med 2014;371:1381–91. 10.1056/NEJMoa1406617 [DOI] [PubMed] [Google Scholar]
- 23. Mazza BF, Freitas FGR, Barros MMO, et al. Blood transfusions in septic shock: is 7.0g/dL really the appropriate threshold? Revista Brasileira de Terapia Intensiva 2015;27:36–43. 10.5935/0103-507X.20150007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264–9. 10.7326/0003-4819-151-4-200908180-00135 [DOI] [PubMed] [Google Scholar]
- 25. Robertson CS, Hannay HJ, Yamal J-M, et al. Effect of erythropoietin and transfusion threshold on neurological recovery after traumatic brain injury. JAMA 2014;312:36–47. 10.1001/jama.2014.6490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med 2013;368:11–21. 10.1056/NEJMoa1211801 [DOI] [PubMed] [Google Scholar]
- 27. Walsh TS, Boyd JA, Watson D, et al. Restrictive versus liberal transfusion strategies for older mechanically ventilated critically ill patients. Crit Care Med 2013;41:2354–63. 10.1097/CCM.0b013e318291cce4 [DOI] [PubMed] [Google Scholar]
- 28. Gobatto ALN, Link MA, Solla DJ, et al. Transfusion requirements after head trauma: a randomized feasibility controlled trial.. Crit Care 2019;23:89 10.1186/s13054-018-2273-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bergamin FS, Almeida JP, Landoni G, et al. Liberal versus restrictive transfusion strategy in critically ill oncologic patients: the transfusion requirements in critically ill oncologic patients randomized controlled trial. Crit Care Med 2017;45:766–73. 10.1097/CCM.0000000000002283 [DOI] [PubMed] [Google Scholar]
- 30. Jairath V, Kahan BC, Gray A, et al. Restrictive versus liberal blood transfusion for acute upper gastrointestinal bleeding (trigger): a pragmatic, open-label, cluster randomised feasibility trial. Lancet 2015;386:137–44. 10.1016/S0140-6736(14)61999-1 [DOI] [PubMed] [Google Scholar]
- 31. Hovaguimian F, Myles PS. Restrictive versus liberal transfusion strategy in the perioperative and acute care settings: a context-specific systematic review and meta-analysis of randomized controlled trials. Anesthesiology 2016;125:46–61. 10.1097/ALN.0000000000001162 [DOI] [PubMed] [Google Scholar]
- 32. Dupuis C, Sonneville R, Adrie C, et al. Impact of transfusion on patients with sepsis admitted in intensive care unit: a systematic review and meta-analysis. Ann Intensive Care 2017;7:5 10.1186/s13613-016-0226-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 2001;345:1368–77. 10.1056/NEJMoa010307 [DOI] [PubMed] [Google Scholar]
- 34. Cata JP, Wang H, Gottumukkala V, et al. Inflammatory response, immunosuppression, and cancer recurrence after perioperative blood transfusions. Br J Anaesth 2013;110:690–701. 10.1093/bja/aet068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Amato A, Pescatori M, Cochrane Colorectal Cancer Group . Perioperative blood transfusions and recurrence of colorectal cancer. Cochrane Database Syst Rev 2006;32:Cd005033 10.1002/14651858.CD005033.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mynster T, Christensen IJ, Moesgaard F, et al. Effects of the combination of blood transfusion and postoperative infectious complications on prognosis after surgery for colorectal cancer. Danish RANX05 colorectal cancer Study Group. Br J Surg 2000;87:1553–62. 10.1046/j.1365-2168.2000.01570.x [DOI] [PubMed] [Google Scholar]
- 37. den Uil CA, Lagrand WK, Spronk PE, et al. Does red blood cell transfusion result in a variate microvascular response in sepsis? Crit Care Med 2007;35:2464–5. 10.1097/01.CCM.0000284756.78940.C1 [DOI] [PubMed] [Google Scholar]
- 38. Soares M, Caruso P, Silva E, et al. Characteristics and outcomes of patients with cancer requiring admission to intensive care units: a prospective multicenter study*. Crit Care Med 2010;38:9–15. 10.1097/CCM.0b013e3181c0349e [DOI] [PubMed] [Google Scholar]
- 39. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med 2017;43:304–77. 10.1007/s00134-017-4683-6 [DOI] [PubMed] [Google Scholar]
- 40. Davis AL, Carcillo JA, Aneja RK, et al. American College of critical care medicine clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock. Crit Care Med 2017;45:1061–93. 10.1097/CCM.0000000000002425 [DOI] [PubMed] [Google Scholar]
- 41. Obonyo NG, Byrne L, Tung J-P, et al. Pre-Clinical study protocol: blood transfusion in endotoxaemic shock. MethodsX 2019;6:1124–32. 10.1016/j.mex.2019.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Carson JL, Brooks MM, Abbott JD, et al. Liberal versus restrictive transfusion thresholds for patients with symptomatic coronary artery disease. Am Heart J 2013;165:964–71. 10.1016/j.ahj.2013.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cooper HA, Rao SV, Greenberg MD, et al. Conservative versus liberal red cell transfusion in acute myocardial infarction (the CRIT randomized pilot study). Am J Cardiol 2011;108:1108–11. 10.1016/j.amjcard.2011.06.014 [DOI] [PubMed] [Google Scholar]
- 44. Hebert PC, Wells G, Marshall J, et al. Transfusion requirements in critical care. A pilot study. Canadian critical care Trials Group. JAMA 1995;273:1439–44. [DOI] [PubMed] [Google Scholar]
- 45. Blair SD, Janvrin SB, McCollum CN, et al. Effect of early blood transfusion on gastrointestinal haemorrhage. Br J Surg 1986;73:783–5. 10.1002/bjs.1800731007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2019-030854supp001.pdf (64.3KB, pdf)
bmjopen-2019-030854supp002.pdf (2.1MB, pdf)
bmjopen-2019-030854supp003.pdf (92.5KB, pdf)
bmjopen-2019-030854supp004.pdf (352.8KB, pdf)