Abstract
Introduction
Percutaneous coronary intervention (PCI) aims to provide instant relief of symptoms, and improve functional capacity and prognosis in patients with coronary artery disease. Although patients may experience a quick recovery, continuity of care from hospital to home can be challenging. Within a short time span, patients must adjust their lifestyle, incorporate medications and acquire new support. Thus, CONCARDPCI will identify bottlenecks in the patient journey from a patient perspective to lay the groundwork for integrated, coherent pathways with innovative modes of healthcare delivery. The main objective of the CONCARDPCI is to investigate (1) continuity of care, (2) health literacy and self-management, (3) adherence to treatment, and (4) healthcare utilisation and costs, and to determine associations with future short and long-term health outcomes in patients after PCI.
Methods and analysis
This prospective multicentre cohort study organised in four thematic projects plans to include 3000 patients. All patients undergoing PCI at seven large PCI centres based in two Nordic countries are prospectively screened for eligibility and included in a cohort with a 1-year follow-up period including data collection of patient-reported outcomes (PRO) and a further 10-year follow-up for adverse events. In addition to PROs, data are collected from patient medical records and national compulsory registries.
Ethics and dissemination
Approval has been granted by the Norwegian Regional Committee for Ethics in Medical Research in Western Norway (REK 2015/57), and the Data Protection Agency in the Zealand region (REG-145-2017). Findings will be disseminated widely through peer-reviewed publications and to patients through patient organisations.
Trial registration number
Keywords: continuity of care, adherence to treatment, health literacy, healthcare utilisation, percutaneous coronary intervention, rehabilitation
Strengths and limitations of this study.
The CONCARDPCI is an interdisciplinary, multicentre effort with the unique combination of data from hospital medical records, patient self-report and national registries providing opportunities to identify novel pathways for continuity of care that contribute to outcomes.
Although the linkage to national registers will ensure complete follow-up of the study population, potential challenges include response rate of patient self-report at follow-up.
Non-participants will be compared with participants on a limited number of registry variables to account for potential selection bias.
Introduction
The widespread commitment to involve patients in planning and service development has become a key element of current healthcare policy. Health literacy, as the ability to access, process and comprehend health information and services, can be used to complement both individual patient care and community-level development. Understanding the varying health literacy of patients, particularly in those who experience poor access and outcomes, is thereby pivotal.1 The American Heart Association (AHA) recently published a scientific statement2 addressing health literacy in cardiovascular disease as of fundamental relevance to primary and secondary prevention. European leaders in secondary prevention have called for action in the postacute aftercare of patients with coronary artery disease (CAD).3 Although CAD is the single most common cause of death in Europe, there has been an encouraging decrease in mortality ascribed to improvements in risk factor management, pharmacological treatment and revascularisation techniques.4 Since more people need to manage life with CAD as a chronic disease, modern developments in primary healthcare provision have led to increased interest in continuity of care as an essential element.5–7 Patients’ transition from hospital to home is particularly challenging because patients need to adjust their lifestyle, incorporate new medications and acquire additional sources of support.8 Although there is compelling evidence for secondary prevention following CAD, a large majority fail to achieve lifestyle changes and therapeutic targets set by the European Society of Cardiology guidelines.9 Therefore, adherence to treatment is also of concern. Non-adherence to medications is common for patients with cardiovascular diseases.10 Taking prescribed antiplatelet and other secondary preventive medication after percutaneous coronary intervention (PCI) is pivotal; however, it is unknown if non-adherence also applies for patients following PCI.
This paper describes a multicentre cohort study, the CONCARDPCI, that seeks to identify bottlenecks and hurdles in the patient journey and suggest the optimal timing of services and alignment with patient preferences for patients with CAD undergoing PCI. Of special interest are challenges with continuity of care, health literacy and self-management, adherence to treatment advice, costs at all care levels and associations with future short and long-term health outcomes.
Uptake to cardiac rehabilitation (CR) is suboptimal,9 11 12 and few sufficiently powered real-world studies have been undertaken with the explicit purpose of investigating continuity of care and pathways of CR in patients after PCI. In addition to investigating factors associated with low referral, participation, health literacy and adherence rates among CR participants, studies are increasingly needed on evaluating alternative modes of providing CR. Follow-up of healthcare use, costs and predictors of costs following PCI in a non-clinical trial setting have been infrequently investigated.13 14 Thus, a large cohort of real-world observations that can ascertain interventions for future clinical trials is needed.15 The CONCARDPCI responds to this challenge. In CONCARDPCI, we hypothesise that continuity of care, Health literacy and self-management, and adherence to treatment in patients are directly associated to outcomes after PCI.
Aim of the research programme
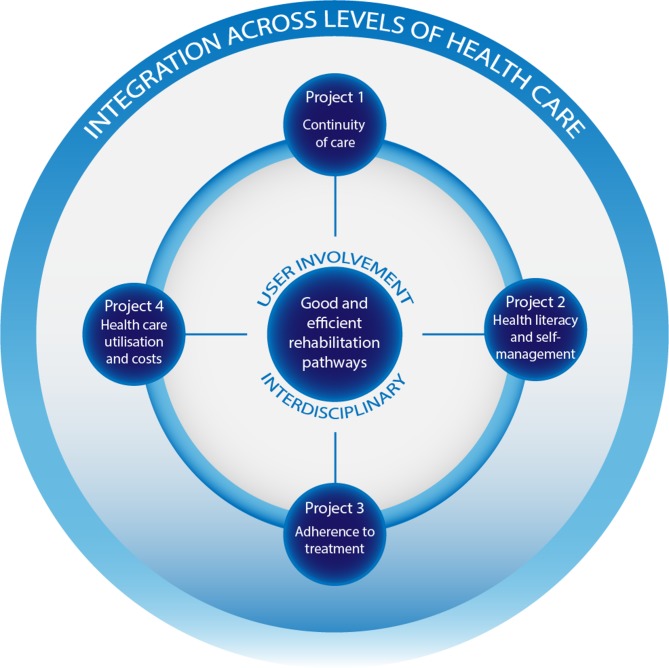
The overall aim of CONCARDPCI is to investigate (1) continuity of care, (2) health literacy and self-management, (3) adherence to treatment, and (4) healthcare utilisation and costs, to determine associations with future short and long-term health outcomes in patients after PCI. CONCARDPCI is organised into four thematic projects on continuity of care; health literacy and self-management; adherence to treatment; and healthcare use and costs (figure 1).
Figure 1.
Projects in CONCARDPCI researching bottlenecks for good and efficient patient pathways across levels of healthcare.
Methods
Study design and setting
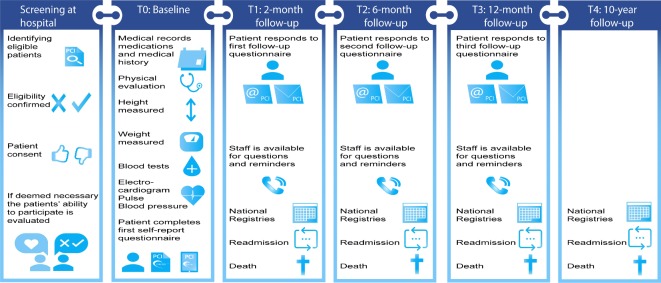
CONCARDPCI is a large-scale multicentre cohort study with serial prospective survey data collection, clinical data and register-based follow-up. We collect data from hospital medical records, patient self-report surveys and national registries (figure 2). Preliminary work has been performed including in-depth interviews on patients’ experiences of healthcare delivery to provide a context for the quantitative data and inform the content of the cohort survey questionnaires. Three follow-up surveys over 1 year are undertaken, and a 10-year follow-up for adverse events.
Figure 2.
Measuring time points and data collection in the cohort study in CONCARDPCI.
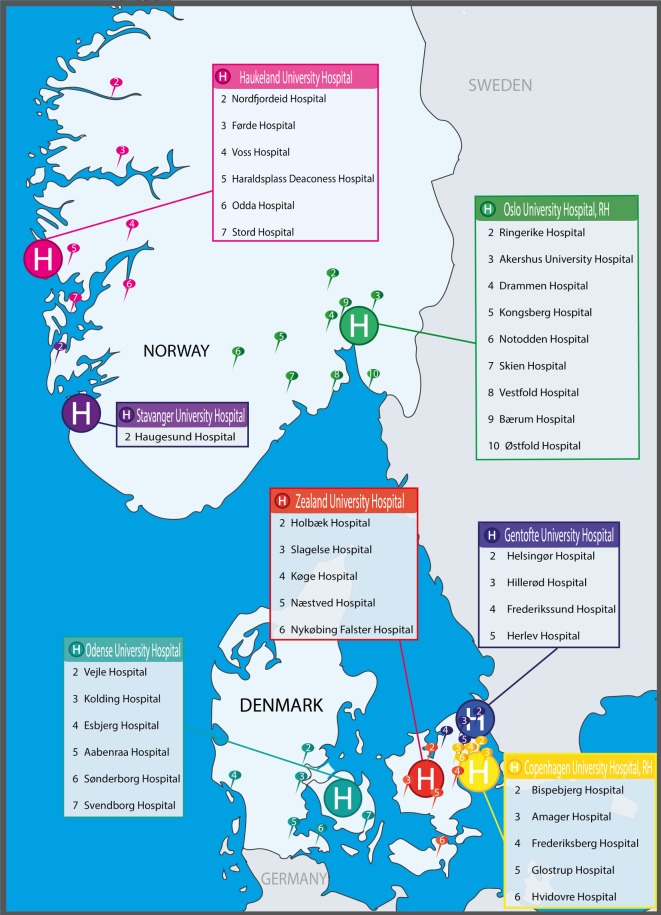
Seven large referral PCI centres in Norway and Denmark were selected based on the following considerations: presence of a committed research team including CONCARDPCI study nurses and a local principal investigator, prior research experience including research infrastructure, geographical location and size. The PCI centres perform from 900 to >2000 (mean 1668) PCI procedures annually, are having 629–1400 beds (mean 943) and are referral centres for coronary angiography and PCI for a total of 37 local hospitals (figure 3, table 1). Haukeland University Hospital is the sponsor centre of this investigator-initiated research programme. For study organisation, see online supplementary appendix.
Figure 3.
H=PCI centres including the local hospitals in their catchment area.
Table 1.
Description of centres participating in CONCARDPCI
| Centre 1 (HUS) |
Centre 2 (SUS) |
Centre 3 (RHOsl) |
Centre 4 (HGH) |
Centre 5 (ZUH) |
Centre 6 (RHCph)* |
Centre 7 (OUH) |
|
| Total hospital beds | 1400 | 482 | 697 | 949 | 629 | 1377 | 1064 |
| PCI procedures per year† | 1565 | 905 | 2124 | 1290 | 921 | 2243 | 2633 |
| Catchment area of number of local hospitals | 7 | 1 | 9 | 4 | 5 | 5 | 6 |
Centre 1 is the Sponsor Coordinating Centre.
*RHCph has regional function for all patients with ST-elevation myocardial infarction affiliated to the capital region and Zealand region.
†Figures from 2017.
HGH, Herlev and Gentofte University Hospital, Copenhagen, Denmark; HUS, Haukeland University Hospital, Bergen, Norway; OUH, Odense University Hospital, Odense, Denmark; PCI, percutaneous coronary intervention; RHCph, Copenhagen University Hospital, Rigshospitalet, Copenhagen, Denmark; RHOsl, Oslo University Hospital, Rikshospitalet, Oslo, Norway; SUS, Stavanger University Hospital, Stavanger, Norway; ZUH, Zealand University Hospital, Roskilde, Denmark.
bmjopen-2019-031995supp001.pdf (136.4KB, pdf)
Study population
All patients undergoing PCI at seven large PCI centres are prospectively screened for eligibility. Screening is performed in the hospital setting by the site coordinator and trained CONCARDPCI study nurses. Daily admission records and the operating programme are reviewed to identify potentially eligible patients. Electronic medical records are reviewed to confirm eligibility according to the inclusion and exclusion criteria (table 2). When cognitive impairment is suspected by clinical or study personnel and there is no medical record of the problem, the Confusion Assessment Method16 and 4AT17 are used to investigate whether the patient must be excluded. Patients who are delirious or too clinically unstable to participate following PCI, who would otherwise be eligible, are reassessed until discharge. During the in-hospital assessment, participants provide informed consent. Because many of the questionnaires are designed for patient self-assessment, patients who need a complete proxy are ineligible. If participants need assistance in filling out the questionnaires, this is registered in the case report form (CRF). Regarding sample size and study power, see the Data analysis and sample size determination section.
Table 2.
Eligibility criteria for CONCARDPCI
| Inclusion criteria |
|
| Exclusion criteria |
|
Measurement and data collection
In CONCARDPCI a broad range of outcomes are measured and data are collected by physical assessment at baseline, review of the medical records, patient self-reported questionnaires (at baseline, 2, 6 and 12 months) and from national registries (table 3 and figure 2). A comprehensive data dictionary and CRF are provided to ensure standardisation of abstracted data. For the Danish centres, eCRFs are used. Patients included in the study undergo a brief physical assessment and complete the self-report questionnaires at baseline after PCI (T0) (table 3 and figure 2). A follow-up with postal or electronic questionnaires is distributed to all patients included in the study, 2 months after discharge (T1). The time interval ensures time for follow-up care to evaluate early postdischarge continuity of care. A consecutive subgroup of patients (n=100) at the Sponsor Coordinating Centre are approached for a retest of the eHealth Literacy Scale (eHEALS)18 and the Heart Continuity of Care Questionnaire (HCCQ)19 as part of the validation process of the instruments. All patients are followed up with postal or electronic questionnaires at 2 (T1), 6 (T2) and 12 (T3) months after discharge. Non-responders receive one reminder. Vital status is identified to avoid sending questionnaires to deceased patients or their family. Patient adverse events are followed through national registers for 10 years or until death (T4) (figure 2). Questionnaire packages are discussed with patient representatives and piloted at every measuring time point (T0–T3) before employed in the large-scale cohort study.
Table 3.
Sociodemographic, clinical and patient-reported measures, and timing of assessments in the CONCARDPCI prospective cohort study
| Measure | Details | Self-report | Hospital medical records | National registry | Time* | Project† |
| Sociodemographic data | Marital status, cohabitation status, education, work status, immigration status, income, rehabilitation participation, available support system, readmission to hospital, time of first meeting with general practitioner. | X | X | T0–T3 | 1–4 | |
| Clinical characteristics | Clinical status at admission (blood pressure, heart rate, laboratory results (haemoglobin, creatinine, troponin, total and high/low-density lipoproteins)), body weight, height, waist circumference, upper arm circumference, medical history including comorbidity and frailty, and previous hospital admissions, procedural and angiographic findings including completeness of revascularisation, complications during hospital stay, additional procedures, length of hospital stay, death. | X | X | X | T0 | 1–4 |
| Medication | Medication at discharge (type and dosage), consumption of prescribed medication during follow-up, side effects from medication, polypharmacy, discontinuation, serum levels of cardiac medications (quantified using liquid chromatography with mass spectrometry). | X | X | X | T0–T3 | 3 |
| Lifestyle | Physical activity (frequency, duration, intensity), sexual activity, tobacco use (current, previous, never), alcohol consumption (frequency, units per week), diet (frequency and amount of intake of different foods, beverages, supplements). | X | T0–T3 | 1–3 | ||
| Healthcare utilisation | Patients’ use of the healthcare system (community vs hospital-based services, specialist vs general provider, urban vs rural setting). | X | X | T1–T3 | 4 | |
| Internet use | Patients’ use of electronic equipment with internet access, use of internet to find health information and use of the web portal helsenorge.no | X | T0–T3 | 2–3 | ||
| Major life events | Comprises three items assessing major life events. | X | T1–T3 | 1–3 | ||
| Beliefs about Medicines Questionnaire (BMQ)42 | Comprises 11 items (the BMQ-Specific) and assesses the key psychological constructs that underpin the core beliefs influencing adherence to medicines. | X | T1-T3 | 3 | ||
| eHealth Literacy Scale (eHEALS)18 | Comprises 10 items and assesses patients’ combined knowledge, comfort, and perceived skills at finding, evaluating and applying electronic health information to health problems. | X | T0, T3 | 2 | ||
| EQ-5D-5L43 | Comprises five items and is widely used for measuring economic preferences for health states. | X | T0–T3 | 4 | ||
| Health Literacy Questionnaire (HLQ)44 | Comprises 20 items measuring four levels of health literacy: appraisal of health information (5 items); social support for health (5 items); ability to find good information (5 items); and understanding health information (5 items). | X | T0, T3 | 2 | ||
| Heart Continuity of Care Questionnaire (HCCQ)19 | Comprises 33 items covering eight topic areas: heart condition explained, communication among providers, preparation for discharge, posthospital review of treatment, receipt of conflicting information, information on medications and on physical and dietary needs. | X | T1 | 1 | ||
| HeartQol45 | Comprises 14 items with 10-item physical and 4-item emotional subscales. | X | T3 | 1–4 | ||
| Medication Adherence Report Scale (MARS-5)46 | Comprises five items and measures self-reported adherence to medicines, and assesses both intentional and unintentional non-adherence. | X | T1–T3 | 3 | ||
| Minimal Insomnia Symptom Scale (MISS)47 48 | Comprises three items assessing major features of insomnia, that is, difficulties initiating sleep, waking at night and not feeling refreshed by sleep. | X | T0–T3 | 1–3 | ||
| Patient Activation Measure (PAM)49 | Comprises 13 items assessing patient knowledge, skill and confidence for self-management. | X | T2 | 2 | ||
| RAND-1250 | Comprises 12 items with 3–5 response levels. It generates two health indices: mental and physical health. | X | T0–T3 | 1–4 | ||
| Sleep Sufficient Index (SSI)48 51 | Comprises two items assessing amount of actual and desired sleep. | X | T0–T3 | 1–3 | ||
| Study of Osteoporotic Fractures (SOF index)52 | Comprises three items and assesses weight loss, inability to rise from a chair five times without using the arms and self-reported poor energy. | X | T0, T3 | 1–3 | ||
| Hospital Anxiety and Depression Scale (HADS)53 | Comprises 14 items and determines the levels of anxiety and depression that a patient is experiencing, and generates two subscales: HADS-D and HADS-A. | X | T0–T3 | 1–4 | ||
| Myocardial Infarction Dimensional Assessment Scale (MIDAS)54 | Comprises 35 items specifically measuring seven different domains of health status and daily life challenges in individuals who have suffered a myocardial infarction: physical activity (12 items), insecurity (9 items), emotional reaction (4 items), dependency (3 items), diet (3 items), concerns over medication (2 items) and side effects (2 items). | X | T1–T3 | 1–3 | ||
| Nordic Patient Experiences Questionnaire (NORPEQ)55 | Comprises eight items and gives a brief measure of patient experiences in evaluation of the quality of healthcare delivery. | X | T1 | 1 | ||
| Seattle Angina Questionnaire (SAQ-7)56 | Comprises seven dimensions of coronary artery disease: physical limitation, angina frequency and quality of life. | X | T0–T3 | 1–3 | ||
| WHOQOL-BREF57 | Comprises one global item on overall quality of life. | X | T0–T3 | 1–4 |
*T0: baseline, T1: 2-month follow-up, T2: 6-month follow-up, T3: 12-month follow-up.
†Project 1: continuity of care. Project 2: health literacy and self-management. Project 3: adherence to treatment. Project 4: healthcare use and costs.
EQ-5D-5L, 5-level version of EuroQol-5 Dimension; RAND-12, RAND-12 Item Health Survey; WHOQOL-BREF, The World Health Organization Quality of Life Instrument Abbreviated.
To objectively assess adherence to therapy, serum levels of a wide panel of cardiac medications are measured. A consecutive subsample of 500 Norwegian patients from two centres will be invited to give a blood sample 1 year after the index procedure. The time is chosen as it corresponds to collection of patient-reported data on adherence. Moreover, adherence tends to diminish over time20; hence, the 1-year contact was chosen. Serum levels are submitted to an accredited clinical pharmacology laboratory, and quantified using liquid chromatography with mass spectrometry. Patients are labelled as non-adherent when serum level of at least one of the evaluated drugs is below the limit of quantification.
Management of cohort and registry data
For the Norwegian centres, baseline (T0) data are transferred to the National Coordinating Centre for data entry and/or review. The forms are reviewed and queries sent to the centre for missing or incomplete items. All follow-up data are collected by postal mail and managed at the National Coordinating Centre. The paper version data are entered into electronic files by trained staff.
For the Danish centres, each centre registers patients who are screened, and either included or excluded in separate Microsoft Excel (V.2016) spreadsheets in a shared secure team site server hosted by the National Coordinating Centre. Data from medical records are entered into a shared SurveyXact (V.12.9) database at each study site and managed by the National Coordinating Centre. Patient self-reports at both baseline (T0) and follow-up are collected either electronically using a tablet via a SurveyXact link or by paper as requested by the patient. Paper version data are entered into the SurveyXact database by trained CONCARDPCI study nurses. All follow-up data are collected and managed by the National Coordinating Centre.
Every resident in Norway and Denmark has a unique personal identifier that allows data sets from national registries to be merged on an individual level. The data sets will be released in a coded and deidentified form, but with a unique identifier common to the data sets making individual merging possible. The heart registries, prescription registries,21 22 cause of death registries23 24 and administrative registries on social security microdata and healthcare utilisation25 26 are mandatory, and legally exempted from requirement of obtaining patient consent. Strict rules on how data can be used or linked are followed to secure privacy protection. Although these data are similar in composition, we are interested in contrasting and comparing Denmark with its high CR uptake to Norway with a lower uptake.
Data analysis and sample size determination
Descriptive statistics of the cohort by nation will be generated using proportions, means and SDs or medians and IQRs as appropriate. Cross-sectional analysis will be used for continuity of care (table 4) using multiple linear regression testing for a random effect for nation. For health literacy, there is a single follow-up and multiple linear regression testing for a random nation effect will be used. The cohort’s longitudinal observations over 1 year will be modelled using generalised linear mixed models (GLMM) that account for within-person correlation for adherence to medications, healthcare utilisation and cost (table 4). We will test whether patients clustered within nation is significant. If so, we will include it as a hierarchy in the GLMMs. For time to readmission, and time to major adverse cardiac event, we will use competing risk models to account for censoring by death. We will construct risk stratification models that predict the probability of each outcome for specific combinations of risk factors. We will establish internal validity by using bootstrapping techniques. We will test whether missing data are at random. If not, we will estimate the probability of missingness and include it as a weight or covariate factor in the models. For psychometric evaluation of translated instruments we evaluate the structural, discriminant and convergent validity, and reliability of the scales. For internal consistency, Cronbach’s alpha is used. Test–retest reliability is evaluated by using intraclass correlation coefficients of patients’ results obtained at a 2-week retest interval. Confirmatory factor analysis is used for evaluating the factor structure of the original eHEALS18 and HCCQ19 instruments.
Table 4.
Definition of outcomes in CONCARDPCI
| Outcome | Definition |
| Continuity of care | As measured by the Heart Continuity of Care Questionnaire (HCCQ).19 |
| Health literacy and eHealth literacy | As measured by the Health Literacy Questionnaire (HLQ)44 and eHealth Literacy Scale (eHEALS) questionnaire.18 |
| Adherence to medication | As measured by the Medication Adherence Report Scale (MARS-5),46 Beliefs about Medicines Questionnaire (BMQ)42 and data related to consumption of prescribed medication identified through national prescription registries, and serum levels of cardiac medication. |
| Healthcare utilisation | As measured by patients’ use of primary care services (general practitioner visits) and secondary care services (inpatient admissions and outpatient visits). |
| Healthcare (associated) costs | As measured by the tariffs of national agreements between the professional associations of medical specialists and the National Health Services, and the tariffs of the national case-mix system of the diagnosis-related groupings (DRG) and the Danish Ambulatory Grouping System (DAGS). |
| Time to readmission | Cardiac and all-cause readmissions. |
| Time to death | Cardiac and all-cause mortality. |
| Time to major adverse cardiac events (MACE) | A composite of cardiac mortality and hospitalisation for cardiovascular disease or chest pain. |
Power calculations for the cohort study are based on time-to-first event outcomes, as these require the most patients. To maintain at family-wise type I error of 0.05% and 80% power using the method of Hsieh and Lavori27 for adjusted Cox regression models 2550 patients are needed. To adjust for losses to follow-up, we increased this estimate by 18% for a total of 3000 patients. Thus, all outcomes will have ≥80% power with alpha ≤0.05.
Ethics approval and consent to participate
The ethical guidelines of the World Medical Association, Declaration of Helsinki and the legislation in Norway and Denmark guide the study (Declaration of Helsinki, 2008). At inclusion, a detailed letter informing the potential participant of the study, and the right to withdraw from the study at any time without any reason is underlined. The identifying key is kept in a separate file from the data. The data are kept in strict confidence in locked files at research servers to protect the participants’ privacy. Approval by the Norwegian Regional Committee for Ethics in Medical Research in Western Norway has been granted (REK 2015/57), and from the Data Protection Agency in the Zealand region for the Danish centres (REG-145-2017). Written agreements between the Sponsor Coordinating Centre, and the local principal investigators and directors of the departments in each participating study centre, are signed before initiation of data collection. The study is registered at ClinicalTrials.gov.
Patient and user involvement
CONCARDPCI involves patients and stakeholders to target aspects of the patient journey to identify bottlenecks and carve out a user-friendly intervention. Patient involvement is carried out in several ways: two patient representatives with a history of CAD, and trained to be patient representatives both in healthcare and research settings,28 provide input to the planning, implementing and reporting of results from the study. Representatives from all healthcare levels will be end users of knowledge from the project and are actively involved in the project through the CONCARDPCI Expert Group (online supplementary appendix). Reporting of patient involvement will follow the GRIPP2 reporting checklists.29
Communication of results and transition of knowledge
The CONCARDPCI has a close-to-practice and clinical approach, which will be an advantage in dissemination and communication with end users. Results will be disseminated to patients through patient organisations, and to healthcare professionals in PCI treatment teams and CR teams, as well as in primary care through seminars and scientific meetings. Due to the comprehensiveness of the outcome measures in the thematic projects (table 4), numerous scientific papers are expected. Long-term follow-up will be reported as data become accessible. Authorship on publications from the study will be allocated using the guidelines for authorship defined by the International Committee of Medical Journal Editors and depends on personal involvement.
Discussion
While medicine has produced large advances in cardiac treatment, there is need for more consistent patient pathways and systematic follow-up care. In order to do so, bottlenecks in the patient journey need to be identified. CONCARDPCI aims to close knowledge gaps related to four main areas: (1) continuity of care, (2) health literacy and self-management, (3) adherence to treatment, and (4) healthcare utilisation and costs of care. Although landmark cohort studies have been carried out to describe the aftercare of patients after acute myocardial infarction (MI), less is described of the patient journey, specifically after PCI, and rarely have these included extensive self-report from patients. In the past decade, an increasing number of studies using patient-reported outcomes have been performed, but in a different setting, with shorter follow-up and targeting subgroups of patients with acute MI.30–34 The US-based SILVER-AMI study focused on older adults,30 the VIRGO study31 concentrated on younger women after acute MI, TRIUMPH32 was designed to examine racial differences after acute MI, Vanderbilt Inpatient Cohort Study33 included both patients after acute MI and patients with heart failure, and NOR-COR34 retrospectively surveyed patients below 80 years of age 2–38 months after the index event including also patients with coronary artery bypass surgery or no intervention. Age is of particular concern as it is documented that invasive strategies benefit clinically stable very old patients with non-ST-elevation acute coronary syndrome.35 In contrast, CONCARDPCI has an extended perspective by prospectively including adult patients with no age limit, engaging stakeholders throughout the study, applying a comprehensive interdisciplinary approach and including data from national registries. One great asset of the participating Nordic countries is infrastructure in research with access to demographics and health information through the national registries. The registries include all citizens, and a personal identifying number ensures no loss to follow-up. In addition to national compulsory registries on death (National Death Registry),23 24 readmission and use of healthcare services (National Patient Registry)25 26 and prescription and medication consumption (National Prescription Registry),21 22 the countries have disease-specific national medical quality registries (eg, the Norwegian Registry of Invasive Cardiology). With establishing national registries, opportunities for nationwide comparisons and quality improvement of healthcare service are created.
While the aforementioned studies30–34 also have detailed data abstracted from medical records and self-report, CONCARDPCI has a timely approach in the four thematic projects—one of which concerns health literacy, and specifically eHealth literacy is of particular relevance in information technology-driven societies. The AHA Scientific Statement on health literacy2 calls for studies examining health literacy and cardiovascular outcomes beyond 30-day readmission. It is suggested that health literacy can be evaluated as part of programmes aiming to improve secondary prevention in that health literacy influences dropout rates in CR. CONCARDPCI responds to this challenge.
Lack of continuity of care and low health literacy are likely to carry increased healthcare utilisation (eg, readmission to hospital) and increased cost.36 The potential need for rethinking CR based on patient preferences and in-built economic analysis is a relevant path to follow. Moving towards a more patient-centred care aims to maximise patients’ self-care abilities. Increased self-care is an overarching goal when healthcare expenditure rises to unaffordable levels. Further, in additional parameters, patient-reported outcomes can potentially identify patients at high risk of adverse outcomes and hospital readmissions,37 38 which is of importance both to patients and society.
The importance of increased patient involvement and shared decision-making at all levels of healthcare is underlined in policy documents at a governmental and regional level.39 Patient involvement is a unique feature of CONCARDPCI scarcely described in comparable large-scale studies. The use of standardised patient-reported outcome measures may provide information that can assist in this decision-making.37 38 In CONCARDPCI, we include patient-reported outcome measures on a global, generic and disease-specific level,40 and pose research questions related to patient pathways that concern a large group of patients. We anticipate that treatment outcome (adherence), safe communication (continuity and health literacy) and self-management will prove important to future healthcare.
However, the study has some limitations. We lack participating hospitals from northern Norway. The remoteness and distance to the PCI centre is a feature of that area and therefore of particular concern. However, travel time to the PCI centre from the most remote fjords in western Norway is also long and this catchment area is included in the study (figure 3). Further, we exclude patients with delirium and dementia due to ethical reasons regarding informed consent and logistical difficulties. Delirious patients and patients too clinically unstable to be included following the PCI procedure, who would otherwise be eligible, are reassessed until discharge. Non-participants will be compared with participants on a limited number of registry variables to account for potential selection bias. Extensive self-report is a feature of CONCARDPCI, and we use validated questionnaires and only a few de novo created questions based on patient interviews. Still, the response rate of follow-up (T1–T3) may be a potential limitation. However, previous methodological work in patients with CAD showed high acceptability of comprehensive questionnaires41 and patient representatives participating in planning of CONCARDPCI ensured relevance of the questionnaires.
Status
Data collection for the cohort study commenced on 12 June 2017 and is expected to continue until July 2020, with a 10-year follow-up until July 2029. The inclusion of patients for the blood sampling for objective medication adherence measurement has started.
Supplementary Material
Acknowledgments
We acknowledge the full group of CONCARD Investigators and our collaborators. A list of institutions and people involved can be found in the online supplementary appendix. The authors are grateful for the assistance provided by Marie Hayes for the development of the figures. We acknowledge the in-house contributions of all the cohort study centres.
Footnotes
Twitter: @TNorekval, @CNRGCam, @Trond Røed Pettersen@EdTrond
Contributors: TMN is the principal investigator of CONCARDPCI and was responsible for study conception, development of the project outline and ethical approval. HGA, GB, NF, TBH, TRP, IV and SR contributed to the development of the project outline. HGA is chairing the Scientific Advisory Board with CD, HH, RS and ADZ as contributing members. NF is the coordinator of the cohort study in CONCARDPCI with TBH as the national coordinator in Denmark, and AIL, BBe, BBo, PP, TBH and TBR are local principal investigators. CB and SI give specific input on transition of care. GB, TRP, TBH and IV are leaders of thematic projects. JS is a major contributor in design of studies on serum levels of cardiac medications and Project 3 in general. TMN wrote the first draft of the manuscript. All authors revised the manuscript critically, and read and approved the final manuscript. A more detailed description of the roles of all authors can be found in the online supplementary appendix.
Funding: The CONCARDPCI is funded by a major grant from the Western Norway Health Authority (Grant No 912184). We also received funding from the Novo Nordisk Foundation (Grant No NNF17OC0030130), Zealand Regional Research Foundation (Grant No 15-000342), Bergen Health Trust grants 2016–2018 and the Copenhagen University Hospital, Rigshospitalet. HGA is supported in part by the NIH/NIA R01 AG047891, R33 AG057806 and P30 AG021342. TMN is supported in part by a Western Norway Health Authority research grant (Grant No 911870). TRP is supported by a Western Norway Health Authority PhD fellow grant for CONCARDPCI (Grant No 912295), and IV by a PhD fellow grant from the Western Norway University of Applied Sciences.
Map disclaimer: The depiction of boundaries on this map does not imply the expression of any opinion whatsoever on the part of BMJ (or any member of its group) concerning the legal status of any country, territory, jurisdiction or area or of its authorities. This map is provided without any warranty of any kind, either express or implied.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Batterham RW, Hawkins M, Collins PA, et al. Health literacy: applying current concepts to improve health services and reduce health inequalities. Public Health 2016;132:3–12. 10.1016/j.puhe.2016.01.001 [DOI] [PubMed] [Google Scholar]
- 2. Magnani JW, Mujahid MS, Aronow HD, et al. Health literacy and cardiovascular disease: fundamental relevance to primary and secondary prevention: a scientific statement from the American heart association. Circulation 2018;138:e48–74. 10.1161/CIR.0000000000000579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Piepoli MF, Corrà U, Dendale P, et al. Challenges in secondary prevention after acute myocardial infarction: a call for action. Eur J Cardiovasc Nurs 2017;16:369–80. 10.1177/1474515117702594 [DOI] [PubMed] [Google Scholar]
- 4. Timmis A, Townsend N, Gale C, et al. European Society of cardiology: cardiovascular disease statistics 2017. Eur Heart J 2018;39:508–79. 10.1093/eurheartj/ehx628 [DOI] [PubMed] [Google Scholar]
- 5. Haggerty JL, Freeman GK, Starfield BH. Continuity of care: a multidisciplinary review. BMJ 2003;327:1219–21. 10.1136/bmj.327.7425.1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Riley DL, Stewart DE, Grace SL. Continuity of cardiac care: cardiac rehabilitation participation and other correlates. Int J Cardiol 2007;119:326–33. 10.1016/j.ijcard.2006.07.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barker I, Steventon A, Deeny SR. Association between continuity of care in general practice and hospital admissions for ambulatory care sensitive conditions: cross sectional study of routinely collected, person level data. BMJ 2017;356:j84 10.1136/bmj.j84 [DOI] [PubMed] [Google Scholar]
- 8. Prvu Bettger J, Alexander KP, Dolor RJ, et al. Transitional care after hospitalization for acute stroke or myocardial infarction: a systematic review. Ann Intern Med 2012;157:407–16. 10.7326/0003-4819-157-6-201209180-00004 [DOI] [PubMed] [Google Scholar]
- 9. Kotseva K, Wood D, De Bacquer D, et al. EUROASPIRE IV: a European Society of cardiology survey on the lifestyle, risk factor and therapeutic management of coronary patients from 24 European countries. Eur J Prev Cardiol 2016;23:636–48. 10.1177/2047487315569401 [DOI] [PubMed] [Google Scholar]
- 10. Naderi SH, Bestwick JP, Wald DS. Adherence to drugs that prevent cardiovascular disease: meta-analysis on 376,162 patients. Am J Med 2012;125:882–7. 10.1016/j.amjmed.2011.12.013 [DOI] [PubMed] [Google Scholar]
- 11. Resurrección DM, Moreno-Peral P, Gómez-Herranz M, et al. Factors associated with non-participation in and dropout from cardiac rehabilitation programmes: a systematic review of prospective cohort studies. Eur J Cardiovasc Nurs 2019;18:38–47. 10.1177/1474515118783157 [DOI] [PubMed] [Google Scholar]
- 12. Aragam KG, Dai D, Neely ML, et al. Gaps in referral to cardiac rehabilitation of patients undergoing percutaneous coronary intervention in the United States. J Am Coll Cardiol 2015;65:2079–88. 10.1016/j.jacc.2015.02.063 [DOI] [PubMed] [Google Scholar]
- 13. Ho PM, O'Donnell CI, Bradley SM, et al. 1-Year risk-adjusted mortality and costs of percutaneous coronary intervention in the Veterans health administration: insights from the Va CART program. J Am Coll Cardiol 2015;65:236–42. 10.1016/j.jacc.2014.10.048 [DOI] [PubMed] [Google Scholar]
- 14. Bakhai A, Ferrieres J, Iñiguez A, et al. Clinical outcomes, resource use, and costs at 1 year in patients with acute coronary syndrome undergoing PCI: results from the multinational APTOR registry. J Interv Cardiol 2012;25:19–27. 10.1111/j.1540-8183.2011.00690.x [DOI] [PubMed] [Google Scholar]
- 15. Swieczkowski D, Mogielnicki M, Cwalina N, et al. Medication adherence in patients after percutaneous coronary intervention due to acute myocardial infarction: from research to clinical implications. Cardiol J 2016. 10.5603/CJ.a2016.0048 [DOI] [PubMed] [Google Scholar]
- 16. Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med 1990;113:941–8. 10.7326/0003-4819-113-12-941 [DOI] [PubMed] [Google Scholar]
- 17. Bellelli G, Morandi A, Davis DHJ, et al. Validation of the 4AT, a new instrument for rapid delirium screening: a study in 234 hospitalised older people. Age Ageing 2014;43:496–502. 10.1093/ageing/afu021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Norman CD, Skinner HA. eHEALS: the eHealth literacy scale. J Med Internet Res 2006;8:e27 10.2196/jmir.8.4.e27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hadjistavropoulos HD, Biem HJ, Kowalyk KM. Measurement of continuity of care in cardiac patients: reliability and validity of an in-person questionnaire. Can J Cardiol 2004;20:883–91. [PubMed] [Google Scholar]
- 20. Ho PM, Bryson CL, Rumsfeld JS. Medication adherence: its importance in cardiovascular outcomes. Circulation 2009;119:3028–35. 10.1161/CIRCULATIONAHA.108.768986 [DOI] [PubMed] [Google Scholar]
- 21. Furu K. Establishment of the nationwide Norwegian prescription database (NorPD) – new opportunities for research in pharmacoepidemiology in Norway. Norsk Epidemiologi 2009;18. [Google Scholar]
- 22. Kildemoes HW, Sørensen HT, Hallas J. The Danish national prescription registry. Scand J Public Health 2011;39:38–41. 10.1177/1403494810394717 [DOI] [PubMed] [Google Scholar]
- 23. Helweg-Larsen K. The Danish register of causes of death. Scand J Public Health 2011;39:26–9. 10.1177/1403494811399958 [DOI] [PubMed] [Google Scholar]
- 24. Gjertsen F. [Cause of death registry--an important data source for medical research]. Tidsskr Nor Laegeforen 2002;122:2551–4. [PubMed] [Google Scholar]
- 25. Bakken IJ, Surén P, Håberg SE, et al. [The Norwegian patient register--an important source for research]. Tidsskr Nor Laegeforen 2014;134:12–13. 10.4045/tidsskr.13.1417 [DOI] [PubMed] [Google Scholar]
- 26. Schmidt M, Schmidt SAJ, Sandegaard JL, et al. The Danish national patient registry: a review of content, data quality, and research potential. Clin Epidemiol 2015;7:449–90. 10.2147/CLEP.S91125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hsieh FY, Lavori PW. Sample-size calculations for the COX proportional hazards regression model with nonbinary covariates. Control Clin Trials 2000;21:552–60. 10.1016/S0197-2456(00)00104-5 [DOI] [PubMed] [Google Scholar]
- 28. National Institute for Health Research Involving users in the research process. A 'how to' guide for researchers, 2010. [Google Scholar]
- 29. Staniszewska S, Brett J, Simera I, et al. GRIPP2 reporting checklists: tools to improve reporting of patient and public involvement in research. BMJ 2017;358:j3453 10.1136/bmj.j3453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dodson JA, Geda M, Krumholz HM, et al. Design and rationale of the comprehensive evaluation of risk factors in older patients with AMI (SILVER-AMI) study. BMC Health Serv Res 2014;14:506 10.1186/s12913-014-0506-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lichtman JH, Lorenze NP, D'Onofrio G, et al. Variation in recovery: role of gender on outcomes of young AMI patients (VIRGO) study design. Circ Cardiovasc Qual Outcomes 2010;3:684–93. 10.1161/CIRCOUTCOMES.109.928713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Arnold SV, Chan PS, Jones PG, et al. Translational research investigating underlying disparities in acute myocardial infarction patients' health status (triumph): design and rationale of a prospective multicenter registry. Circ Cardiovasc Qual Outcomes 2011;4:467–76. 10.1161/CIRCOUTCOMES.110.960468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Meyers AG, Salanitro A, Wallston KA, et al. Determinants of health after hospital discharge: rationale and design of the Vanderbilt inpatient cohort study (VICS). BMC Health Serv Res 2014;14:10 10.1186/1472-6963-14-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Munkhaugen J, Sverre E, Peersen K, et al. The role of medical and psychosocial factors for unfavourable coronary risk factor control. Scand Cardiovasc J 2016;50:1–8. 10.3109/14017431.2015.1111408 [DOI] [PubMed] [Google Scholar]
- 35. Tegn N, Abdelnoor M, Aaberge L, et al. Invasive versus conservative strategy in patients aged 80 years or older with non-ST-elevation myocardial infarction or unstable angina pectoris (after eighty study): an open-label randomised controlled trial. The Lancet 2016;387:1057–65. 10.1016/S0140-6736(15)01166-6 [DOI] [PubMed] [Google Scholar]
- 36. Haun JN, Patel NR, French DD, et al. Association between health literacy and medical care costs in an integrated healthcare system: a regional population based study. BMC Health Serv Res 2015;15:249 10.1186/s12913-015-0887-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rumsfeld JS, Alexander KP, Goff DC, et al. Cardiovascular health: the importance of measuring patient-reported health status: a scientific statement from the American heart association. Circulation 2013;127:2233–49. 10.1161/CIR.0b013e3182949a2e [DOI] [PubMed] [Google Scholar]
- 38. Anker SD, Agewall S, Borggrefe M, et al. The importance of patient-reported outcomes: a call for their comprehensive integration in cardiovascular clinical trials. Eur Heart J 2014;35:2001–9. 10.1093/eurheartj/ehu205 [DOI] [PubMed] [Google Scholar]
- 39. Barry MJ, Edgman-Levitan S. Shared decision making--pinnacle of patient-centered care. N Engl J Med 2012;366:780–1. 10.1056/NEJMp1109283 [DOI] [PubMed] [Google Scholar]
- 40. Norekvål TM, Fålun N, Fridlund B. Patient-Reported outcomes on the agenda in cardiovascular clinical practice. Eur J Cardiovasc Nurs 2016;15:108–11. 10.1177/1474515115614133 [DOI] [PubMed] [Google Scholar]
- 41. Peersen K, Munkhaugen J, Gullestad L, et al. Reproducibility of an extensive self-report questionnaire used in secondary coronary prevention. Scand J Public Health 2017;45:269–76. 10.1177/1403494816688375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Horne R, Weinman J, Hankins M. The beliefs about medicines questionnaire: the development and evaluation of a new method for assessing the cognitive representation of medication. Psychol Health 1999;14:1–24. 10.1080/08870449908407311 [DOI] [Google Scholar]
- 43. Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol group. Ann Med 2001;33:337–43. 10.3109/07853890109002087 [DOI] [PubMed] [Google Scholar]
- 44. Osborne RH, Batterham RW, Elsworth GR, et al. The grounded psychometric development and initial validation of the health literacy questionnaire (HLQ). BMC Public Health 2013;13:658 10.1186/1471-2458-13-658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Oldridge N, Höfer S, McGee H, et al. The HeartQoL: Part II. validation of a new core health-related quality of life questionnaire for patients with ischemic heart disease. Eur J Prev Cardiol 2014;21:98–106. 10.1177/2047487312450545 [DOI] [PubMed] [Google Scholar]
- 46. Horne R, Weinman J. Patients' beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res 1999;47:555–67. 10.1016/S0022-3999(99)00057-4 [DOI] [PubMed] [Google Scholar]
- 47. Hellström A, Hagell P, Fagerström C, et al. Measurement properties of the minimal insomnia symptom scale (miss) in an elderly population in Sweden. BMC Geriatr 2010;10:84 10.1186/1471-2318-10-84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Amofah HA, Broström A, Fridlund B, et al. Sleep in octogenarians during the postoperative phase after transcatheter or surgical aortic valve replacement. Eur J Cardiovasc Nurs 2016;15:168–77. 10.1177/1474515115620992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hibbard JH, Mahoney ER, Stockard J, et al. Development and testing of a short form of the patient activation measure. Health Serv Res 2005;40:1918–30. 10.1111/j.1475-6773.2005.00438.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ware J, Kosinski M, Keller SD. A 12-Item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996;34:220–33. 10.1097/00005650-199603000-00003 [DOI] [PubMed] [Google Scholar]
- 51. Broström A, Hűbbert L, Jakobsson P, et al. Effects of long-term nocturnal oxygen treatment in patients with severe heart failure. J Cardiovasc Nurs 2005;20:385–96. 10.1097/00005082-200511000-00005 [DOI] [PubMed] [Google Scholar]
- 52. Ensrud KE, Ewing SK, Taylor BC, et al. Frailty and risk of falls, fracture, and mortality in older women: the study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci 2007;62:744–51. 10.1093/gerona/62.7.744 [DOI] [PubMed] [Google Scholar]
- 53. Bjelland I, Dahl AA, Haug TT, et al. The validity of the hospital anxiety and depression scale. An updated literature review. J Psychosom Res 2002;52:69–77. 10.1016/s0022-3999(01)00296-3 [DOI] [PubMed] [Google Scholar]
- 54. Thompson DR, Jenkinson C, Roebuck A, et al. Development and validation of a short measure of health status for individuals with acute myocardial infarction: the myocardial infarction dimensional assessment scale (MIDAS). Qual Life Res 2002;11:535–43. 10.1023/A:1016354516168 [DOI] [PubMed] [Google Scholar]
- 55. Skudal KE, Garratt AM, Eriksson B, et al. The Nordic patient experiences questionnaire (NORPEQ): cross-national comparison of data quality, internal consistency and validity in four Nordic countries. BMJ Open 2012;2:e000864 10.1136/bmjopen-2012-000864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chan PS, Jones PG, Arnold SA, et al. Development and validation of a short version of the Seattle angina questionnaire. Circ Cardiovasc Qual Outcomes 2014;7:640–7. 10.1161/CIRCOUTCOMES.114.000967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hanestad BR, Rustøen T, Knudsen O, et al. Psychometric properties of the WHOQOL-BREF questionnaire for the Norwegian general population. J Nurs Meas 2004;12:147–59. 10.1891/jnum.2004.12.2.147 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2019-031995supp001.pdf (136.4KB, pdf)