Abstract
Chondrocyte mechanotransduction is not well understood, but recently, it has been proposed that mechanically activated ion channels such as transient receptor potential vanilloid 4 (TRPV4), Piezo1, and Piezo2 are of functional importance in chondrocyte mechanotransduction. The aim of this study was to distinguish the potential contributions of TRPV4, Piezo1, and Piezo2 in transducing different intensities of repetitive mechanical stimulus in chondrocytes. To study this, TRPV4-, Piezo1-, or Piezo2-specific siRNAs were transfected into cultured primary chondrocytes to knock down (KD) TRPV4, Piezo1, or Piezo2 expression, designated TRPV4-KD, Piezo1-KD, or Piezo2-KD cells. Then we used Flexcell® Tension System to apply cyclic tensile strains (CTS) of 3% to 18% at 0.5 Hz for 8 h to the knockdown and control siRNA-treated cells. Finally, using a Ca2+ imaging system, stretch-evoked intracellular Ca2+ ([Ca2+]i) influx in chondrocytes was examined to investigate the roles of TRPV4, Piezo1, and Piezo2 in Ca2+ signaling in response to different intensities of repetitive mechanical stretch stimulation. The characteristics of [Ca2+]i in chondrocytes evoked by stretch stimulation were stretch intensity dependent when comparing unstretched cells. In addition, stretch-evoked [Ca2+]i changes were significantly suppressed in TRPV4-KD, Piezo1-KD, or Piezo2-KD cells compared with control siRNA-treated cells, indicating that any channel essential for Ca2+ signaling induced by stretch stimulation in chondrocytes. Of note, they played different roles in calcium oscillation induced by different intensities of stretch stimulation. More specifically, TRPV4-mediated Ca2+ signaling played a central role in the response of chondrocytes to physiologic levels of strain (3% and 8% of strain), while Piezo2-mediated Ca2+ signaling played a central role in the response of chondrocytes to injurious levels of strain (18% of strain). These results provide a basis for further examination of mechanotransduction in cartilage and raise a possibility of therapeutically targeting Piezo2-mediated mechanotransduction for the treatment of cartilage disease induced by repetitive mechanical forces.
Impact statement
Chondrocytes in cartilage are constantly subjected to load-induced stimuli and regulate their metabolic activities in order to maintain cartilage homeostasis. Therefore, mechanotransduction is important in chondrocytes and is vital for their role in cartilage function. Our results indicate that chondrocytes might sense and distinguish the different intensities of repetitive mechanical stimulus by using different mechanosensitive ion channels. Specifically, TRPV4 is mainly responsible for sensing physiologic levels of repetitive CTS stimulus, while Piezo2 mainly contributes to chondrocyte sensing noxious levels of repetitive CTS loading. These results provide a basis for further examination of mechanotransduction in cartilage and raise the possibility of therapeutically targeting Piezo2-mediated mechanotransduction for the treatment of OA which is induced by injurious and repetitive mechanical stimulation.
Keywords: Chondrocyte, cyclical mechanical stretch, calcium signaling, mechanotransduction, mechanosensitive ion channel
Introduction
Articular cartilage, the load-bearing surface in diarthrodial joints, is subjected to millions of cycles of mechanical loads of several times body weight per year, in humans for decades of life.1,2 During the joint bearing loading, chondrocytes, as the only cell type in the articular cartilage, are constantly exposed to a combination of different forces, in which tensile strain plays a critical role.3,4 As mechanosensitive cells, chondrocytes perceive and respond to load-induced stimuli throughout life. Thus, the resulting mechanical signals acting on chondrocytes represent critical regulators of tissue adaptation, structure, and function.4–6
However, the ion channels involved in chondrocyte mechanotransduction pathways by which the cell senses and responds to repetitive mechanical stimulation, such as tensile strain, have not been unambiguously identified. Notably, there are several candidates including transient receptor potential vanilloid 4 (TRPV4), Piezo1, and Piezo2 channels in articular chondrocytes.7–9 The mechanosensitive ion channels TRPV4 and Piezos are appreciably expressed and active in articular chondrocytes.10,11 TRPV4 has been linked to mechanotransduction processes in different cell types as well as chondrocytes.9,12 Piezo1 and Piezo2 are transmembrane pore-forming cation channels that have recently been identified as genuine mechanosensors in numerous eukaryotic cell types and shown to be involved in numerous physiological and pathophysiological processes.8,11,13 Recent researches have indicated that TRPV4, Piezo1, and Piezo2 are of functional importance in chondrocyte mechanotransduction.7,9,11,14 While TRPV4 and Piezos channels have been associated to transduction of biophysical stimulation, their roles in transducing repetitive stimulations are not clear.
Ca2+ are recognized as an important second messenger with crucial roles in many cellular processes and multiple cell types, and chondrocytes are no exception.15–17 Numerous studies have shown that Ca2+ regulation is vital for chondrocyte behavior and function to display an adequate response to mechanical stimulation.18–20 Intracellular calcium ([Ca2+]i) oscillation is among the earliest and most fundamental molecular responses of chondrocytes to most physical stimuli.18,21 For example, experiments performed with in situ chondrocytes using fluorescent Ca2+ indicators showed that an increase in [Ca2+]i is one of the earliest events in the cascade induced by cyclical compression of the cartilage explant.22
Therefore, understanding the roles of TRPV4 and Piezo1/2 channels in chondrocytes in the mechanisms by which repetitive mechanical stimulation of these cells can be converted into cellular calcium signaling is a rational path toward understanding cartilage mechanobiology and associated diseases, particularly osteoarthritis (OA) induced by repetitive mechanical factors. In this study, we used different intensities of cyclic strain to simulate the mechanical microenvironment that chondrocytes encounter in the body and studied the roles of TRPV4, Piezo1, and Piezo2 in Ca2+ signaling in response to repetitive mechanical stretch stimulation. Our results give insight into the potential contributions of TRPV4 and Piezos in transducing repetitive mechanical stimuli and provide new targets for the prevention and treatment of joint injuries and diseases induced by repetitive mechanical forces.
Materials and methods
Cell isolation and culture
Primary chondrocytes were isolated from the full-depth cartilage taken from the femoral heads, femoral condyles, and tibial plateaux of six-day-old mice (Experimental Animal Center, Shanxi Medical University, Taiyuan, China) by sequential enzyme digestion with collagenase D (Roche, Mannheim, Germany) as previously described.23 All experiments were performed according to protocols approved by the Animal Ethics Committee of Shanxi Medical University. The cell suspension was mixed thoroughly to disperse any cell aggregates, filtered through a 40-µm cell strainer (BD Falcon, USA), and then centrifuged at 1500 rmp for 5 min. The chondrocytes thus obtained were washed twice with PBS, resuspended in DMEM containing 10% fetal calf serum (FCS, Sigma, St Louis, USA) and 1% penicillin/streptomycin, and counted with a hemocytometer. Chondrocytes which were plated on collagen І-coated six-well BioFlex® plates in passages 2–3 were used for experiments, where the vast majority of them exhibited the typical chondrocyte morphology, with a rounded or polygonal shape (Figures S1 and S2).
Application of cyclic tensile strain
For the cell-tensile experiment, chondrocytes were seeded onto collagen І-coated six-well BioFlex® plates (Flexcell Int. Corp., Hillsborough, NC, USA) at an initial density of 5 × 104/well. After the cells reached 70 to 80% confluence, the culture medium was replaced with DMEM containing 1% FCS. Cells were then subjected to CTS at various magnitudes (3, 8, 13, or 18%) and frequency of 0.5 Hz for 8 h using a Flexcell® Tension Plus™ FX-4000™ system (Flexcell Int. Corp., Hillsborough, NC, USA) at 37°C in a humidified incubator with an atmosphere containing 5% CO2. Cells plated on BioFlex® plates but not subjected to stretch served as controls. Four different treatment regimens were assigned: (i) non-stretched controls, (ii) cells treated with CTS, (iii) TRPV4, Piezo1, or Piezo2 knockdown (TRPV4-KD, Piezo1-KD, or Piezo2-KD) cells, (iv) TRPV4-KD, Piezo1-KD, or Piezo2-KD cells treated with CTS.
Chondrocyte siRNA treatment
siRNAs against mouse TRPV4, Piezo1, Piezo2, and an siRNA negative control (NC) were designed and chemically synthesized by Shanghai Sangon Biotechnology Co., Ltd (Shanghai, China). The designed TRPV4-targeting siRNA (si-TRPV4) sequences were 5′-GCAACAUGCGUGAAUUCAUTT-3′ (sense) and 5′-AUGAAUUCACGCAUGUUGCTT-3′ (antisense); the designed Piezo1-targeting siRNA (si-Piezo1) sequences were 5′-GCUGGUCUAUUUGCUGUUUTT-3′ (sense) and 5′-AAACAGCAAAUAGACCAGCTT-3′ (antisense); the designed Piezo2-targeting siRNA (si-Piezo2) sequences were 5′-GCUCAGAAAUGGUGUGCUATT-3′ (sense) and 5’-UAGCACACCAUUUCUGAGCTT-3′ (antisense). Chondrocytes plated in six-well BioFlex® plates were transfected with 40 nM siRNAs by using Lipofectamine 2000 (Thermo Scientific), according to the manufacturer’s instructions. The expression of mRNA was measured by real-time PCR after 48 h of cultivation, and protein expression levels were assessed by Western blot analysis after 60 h of cultivation. Repetitive mechanical stretch experiments were performed after 48–60 h of cultivation.
Real-time polymerase chain reaction (RT-PCR)
The procedure used for real-time polymerase chain reaction (RT-PCR) was similar to that reported previously.24 Briefly, total RNA was extracted by TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to manufacturer-recommended procedures. One microgram of RNA was converted to cDNA with the PrimeScript™ RT reagent Kit (TaKaRa Biotechnology Co., Dalian, China) by gradient PCR device (Eppendorf, Germany). Reverse transcription was performed for 2 min at 42°C, 15 min at 37°C, and 5 s at 85°C, followed by cooling to 4°C. Then, two microliters of 30-fold-diluted cDNA products were amplified with SYBR® Premix Ex Taq™ II (TaKaRa Biotechnology Co.) by the StepOnePlus™ RT-PCR System (Applied biosystems, USA) using the following gene-specific primers (Table 1) designed by Shanghai Sangon Biotechnology Co., Ltd (Shanghai, China).
Table 1.
Primers for the target gene.
| Primer | 5′-3′ sequence (forward; reverse) |
|---|---|
| TRPV4 | For: TACGACCTGCTGCTTCTCAA Rev: TCCTCATCTGTCACCTCACG |
| Piezo1 | For: ATCCTGCTGTATGGGCTGACRev: AAGGGTAGCGTGTGTGTTCC |
| Piezo2 | For: CGCTCAGAAATGGTGTGCTARev: AGATCAAGATGGGCAACAGG |
| GAPDH | For: CACAATTTCCATCCCAGACCRev: GTGGGTGCAGCGAACTTTAT |
TRPV4: transient receptor potential vanilloid 4; GAPDH: glyceraldehyde 3-phosphate dehydrogenase.
For quantification, all target mRNA expressions were normalized to the expressed housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH). RT-PCR conditions were as follows: 95°C for 30 s and then 40 cycles at 95°C for 5 s, 60°C for 30 s, and 72°C for 1 min, followed by 72°C for 10 min. The relative quantity of mRNA was calculated using the 2−ΔΔCт method, in which Cт is the threshold cycle. The resulting data were expressed as a ratio to the control value denoted as one.
Western blot
For semiquantitative estimation of proteins synthesis, Western blot analysis was used as described previously.25 Briefly, cells exposed to various regimens were lysed in ice-cold RIPA buffer (Applygen Technologies Inc., Beijing, China) containing PMSF protease inhibitor, and the concentrations of the extracted proteins were determined by a BCA protein assay kit (Applygen Technologies Inc., Beijing, China). Equal amounts of protein (25 µg) from each sample were loaded into a 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Protein samples were mixed with loading buffer (20% glycerol, 10% 2-mercaptoethanol, 4% SDS, and 0.2 mg/mL bromophenol blue in 0.1 mol/L Tris-HCl (pH 6.8)), boiled and then separated by SDS-PAGE. The proteins were transferred to polyvinylidene difluoride (PVDF) membrane (Millipore, Billerica, MA). After blocking and washing, membranes were incubated overnight at 4°C with rabbit anti-Piezo1 antibody (1:1000 dilution; NBP1-78537, Novus Biologicals, Littleton, CO, USA), rabbit anti-Piezo2 antibody (1:2000 dilution; NBP1-78624, Novus Biologicals, Littleton, CO, USA), or rabbit anti-TRPV4 antibody (1:2000 dilution; ACC-034, Alomone Labs, Jerusalem, Israel). Monoclonal rabbit anti-α-tubulin antibody (1:1000 dilution; ab7291, Abcam, Cambridge, MA) was used to reprobe the same blots as an internal standard. Membranes were then incubated with a 1:6000 dilution of goat anti-rabbit IgG (ZB-2301, Zhongshan Biotechnology, Beijing, China) for 2 h at room temperature. The membranes with protein bands were visualized by enhanced chemiluminescence detection reagents (Applygen Technologies Inc., Beijing, China) and exposed to X-ray film. Finally, the semiquantitative analysis of luminescent bands was carried out with Image-Pro Plus 5.1 analysis software.
Ca2+ imaging
Chondrocytes were plated on six-well BioFlex® plates and subjected to CTS for 8 h, then loaded with cells with the fluorescent Ca2+ indicator Fluo-4 AM (5.12 µM for 30 min; Invitrogen). Ca2+ images were taken at 488 nm using laser scanning confocal fluorescence microscope (FV1000, Olympus, Japan) at 37°C and fluorescent images were acquired every 3 s. The background fluorescence (baseline value) was acquired for three cycles and used to normalize the fluorescence of the whole experiment. Fluorescence values were calculated and plotted according to the formula ΔF/F0 = (F−F0)/F0, where F0 is baseline fluorescence and F is the fluorescence intensity at a determined time point. Cellular Ca2+ responses rate and the amplitude and frequency of [Ca2+]i oscillations were chosen as characteristic parameters of Ca2+ signaling evoked by repetitive CTS stimulation in our experiment. Among them, the amplitude and frequency of [Ca2+]i oscillations were measured using a custom written MATLAB program.
Statistical analysis
All results were presented as mean ± standard deviation of three independent experiments and statistically performed by using SPSS v.19.0 software and one-way analysis of variance (ANOVA) analysis. A P value < 0.05 was considered to denote a statistically significant difference.
Results
Effect of CTS on TRPV4, Piezo1, and Piezo2 channel expression
TRPV4 channel
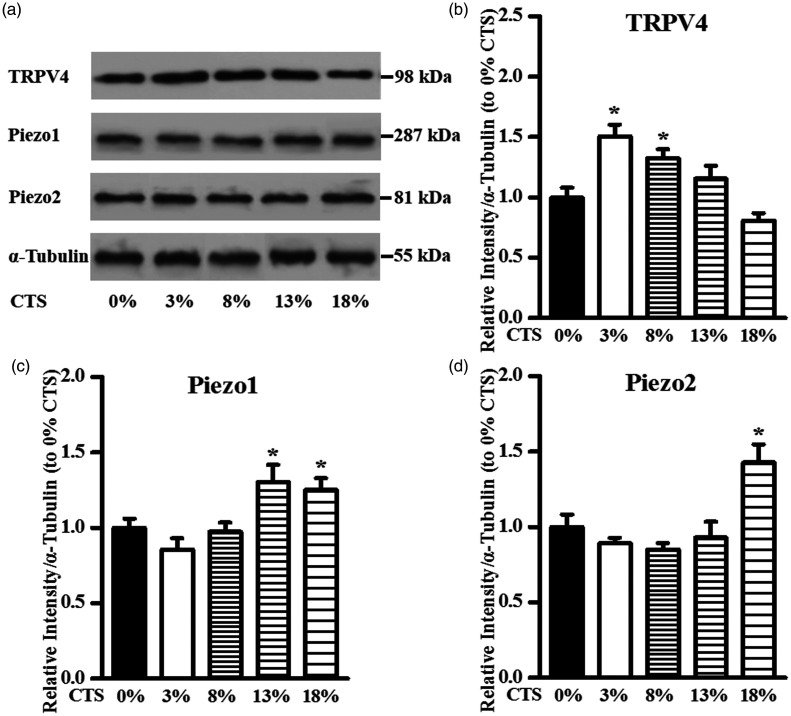
The expression level of the TRPV4 channel in chondrocytes following exposure to CTS was monitored at different intensities (Figure 1(a)). The ratios of stretch to non-stretched control values of TRPV4 were 1.51 ± 0.09 at 3% strain level (P < 0.05), 1.32 ± 0.07 at 8% strain level (P < 0.05), 1.15 ± 0.11 at 13% strain level (P > 0.05), and 0.81 ± 0.06 at 18% strain level (P > 0.05), respectively (Figure 1(b)). As depicted in Figure 1(b), CTS significantly induced TRPV4 expression at a low strain level of 3% and 8%, whereas no significant effects were observed at a high strain level 13% and 18% compared with the unstretched control.
Figure 1.
Effect of CTS on TRPV4/Piezo1/Piezo2 protein levels in chondrocytes. (a) Expression of TRPV4/Piezo1/Piezo2 channels in articular chondrocytes following exposure to CTS for 8 h. The fold change in the expression of (b) TRPV4/(c) Piezo1/(d) Piezo2 channels based on densitometric analysis of Western blot using Image-Pro Plus by comparing to the housekeeping protein α-tubulin. Data are presented as the mean ± standard deviation of three independent experiments. *P < 0.05, stretched vs. non-stretched control.
TRPV4: transient receptor potential vanilloid 4; CTS: cyclic tensile strains.
Piezo1 channel
The influence of CTS with different intensities on TRPV4 expression in chondrocytes was investigated at the protein level (Figure 1(a)). The ratios of stretch to non-stretched control values of Piezo1 were 0.86 ± 0.07 at 3% strain level (P > 0.05), 0.97 ± 0.07 at 8% strain level (P > 0.05), 1.30 ± 0.11 at 13% strain level (P < 0.05), and 1.25 ± 0.08 at 18% strain level (P < 0.05), respectively (Figure 1(c)). Figure 1(c) shows that CTS significantly induced Piezo1 expression at a high strain level of 13% and 18%, whereas no significant effects were observed at a low strain level 3% and 8% compared with the unstretched control.
Piezo2 channel
The Piezo2 channel protein level in chondrocytes following exposure to CTS was monitored at different intensities (Figure 1(a)). The ratios of stretch to non-stretched control values of Piezo2 were 0.89 ± 0.04 at 3% strain level (P > 0.05), 0.85 ± 0.04 at 8% strain level (P > 0.05), 0.93 ± 0.10 at 13% strain level (P > 0.05), and 1.42 ± 0.12 at 18% strain level (P < 0.05), respectively (Figure 1(d)). As shown in Figure 1(d), Piezo2 expression was enhanced by CTS only at a high strain level of 18%, whereas no significant changes were observed at a strain level 3%, 8%, and 13% compared to unstretched cells.
Effects of CTS on [Ca2+]i response
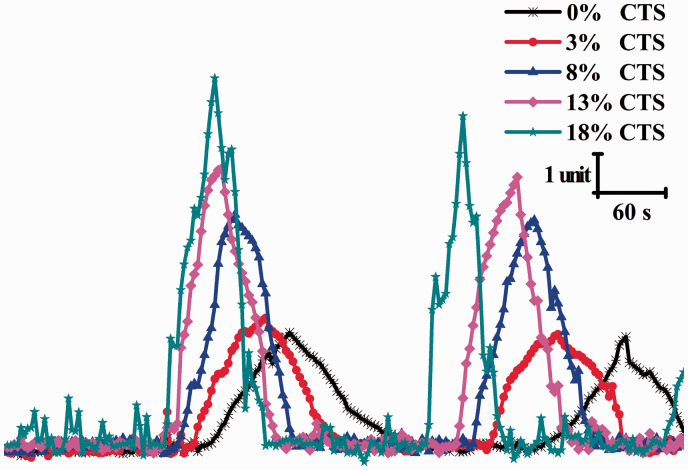
Chondrocytes were cultivated on six-well BioFlex® plates, and [Ca2+]i changes in response to stretch stimulation at different strain magnitudes were examined. Figure 2 illustrates the representative [Ca2+]i oscillations occurring in cells in six-well BioFlex® plate samples. As shown in Figure 2, the CTS stimulation had a significant effect on the characteristics of [Ca2+]i oscillation in chondrocytes, dependent of applied strain magnitude. Furthermore, [Ca2+]i oscillation characteristics including peak amplitude, oscillation frequency (peaks/min), and cellular Ca2+ response rates (%) were quantified. As shown in Table 2, together with increasing strain, the average cellular Ca2+ response rates increased with increasing strain from 18.54 ± 1.43 in 0% CTS, 18.97 ± 1.35 in 3% CTS, 20.86 ± 1.17 in 8% CTS, 22.88 ± 1.82 in 13% CTS, and 24.67 ± 2.01 in 18% CTS, respectively. Obviously, CTS stimulation markedly increased the average cellular Ca2+ response rates in a strain magnitude-dependent manner. Similarly, oscillation peak [Ca2+]i amplitude significantly increased with CTS stimulation compared to non-stretched control (P < 0.05). Moreover, the oscillation peak [Ca2+]i amplitude increased in a strain magnitude-dependent manner in chondrocytes. Additionally, cells in 18% CTS group had significantly more frequent oscillations compared to the non-stretched control (P < 0.05). While the oscillation frequency of the 3%, 8%, or 13% CTS group was higher than that of the non-stretched group, no statistical difference was observed.
Figure 2.
Representative [Ca2+]i oscillation profiles response to CTS stimulation in chondrocytes at different strain magnitudes (3%, 8%, 13%, and 18%). CTS: cyclic tensile strains. (A color version of this figure is available in the online journal.)
Table 2.
Average properties of [Ca2+]i oscillation for chondrocytes stimulated with CTS stimulation at different strain magnitudes (mean ± standard deviation).
| 0% CTS (n = 38) | 3% CTS (n = 41) | 8% CTS (n = 39) | 13% CTS (n = 35) | 18% CTS (n = 43) | |
|---|---|---|---|---|---|
| [Ca2+]i response rates (%) | 18.54 ± 1.43 | 18.97 ± 1.35 | 20.86 ± 1.17 | 22.88 ± 1.82 | 24.67 ± 2.01 |
| Amplitude | 3.81 ± 0.25 | 4.43 ± 0.31 | 6.82 ± 0.44 | 8.05 ± 0.51 | 9.85 ± 0.48 |
| Frequency (Peaks/min) | 0.18 ± 0.01 | 0.19 ± 0.01 | 0.20 ± 0.01 | 0.20 ± 0.01 | 0.21 ± 0.01 |
Note: Data were collected from n = 38 cells, n = 41 cells, n = 39 cells, n = 35 cells, and n = 43 cells for 3%, 8%, 13%, and 18% strain magnitude, respectively. Data are represented as the mean ± standard deviation of three independent experiments.
CTS: cyclic tensile strains.
siRNA knockdown of TRPV4, Piezo1, and Piezo2 in chondrocytes
To better understand the roles of TRPV4, Piezo1, and Piezo2 in the responses to cell CTS stimulation, we knocked down the TRPV4/Piezo1/Piezo2 expression in cultured primary chondrocytes by using specific TRPV4/Piezo1/Piezo2 siRNAs, designated TRPV4-KD, Piezo1-KD, or Piezo2-KD cells, respectively.
TRPV4-KD cells
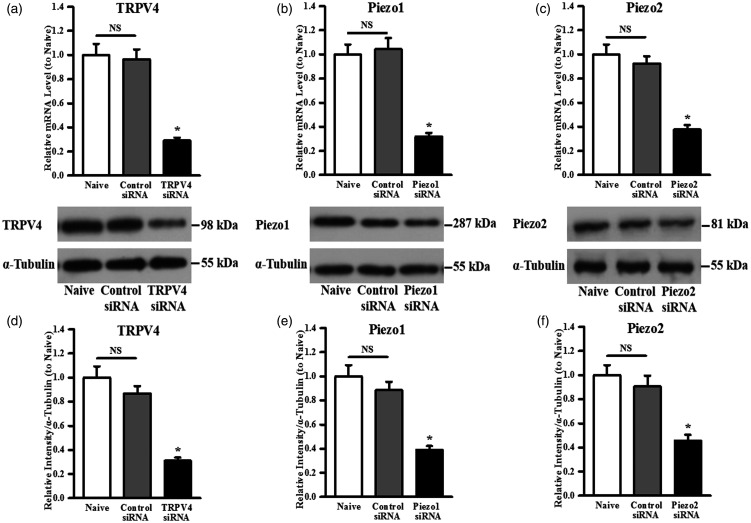
This intervention reduced TRPV4 mRNA levels by ∼71%, whereas treatment with control siRNA did not (Figure 3(a)). Western blotting analyses revealed that TRPV4 siRNA treatment also suppressed TRPV4 protein expression, respectively (Figure 3(d)). Specifically, Figure 3(g) shows the transfection of TRPV4 specific siRNA resulted in ∼62% knockdown of TRPV4 protein.
Figure 3.
Quantitative analysis of (a) TRPV4/(b) Piezo1/(c) Piezo2 mRNA levels by real-time PCR in untreated cells (naive) and cells treated with control siRNA or TRPV4/Piezo1/Piezo2 siRNA, respectively. Representative Western blot image for (d) TRPV4/(e) Piezo1/(f) Piezo2 channel protein expression. Quantitative analysis based on densitometry using Image-Pro Plus by comparing to the housekeeping protein α-tubulin showing the specific knockdown of the expression of (g) TRPV4/(h) Piezo1/(i) Piezo2. Data are presented as percentage of naive cells and the mean ± standard deviation of three independent experiments. *P < 0.05, TRPV4, Piezo1, or Piezo2 siRNA vs. control siRNA.
NS: non-significant; TRPV4: transient receptor potential vanilloid 4.
Piezo1-KD cells
This intervention reduced Piezo1 mRNA levels by ∼68%, whereas treatment with control siRNA did not (Figure 3(b)). Western blotting analyses revealed that Piezo1 siRNA treatment also suppressed Piezo1 protein expression, respectively (Figure 3(e)). Specifically, Figure 3(h) shows that the transfection of Piezo1-specific siRNA resulted in ∼56% knockdown of Piezo1 protein.
Piezo2-KD cells
This intervention reduced Piezo2 mRNA levels by ∼58%, whereas treatment with control siRNA did not (Figure 3(c)). Western blotting analyses revealed that Piezo2 siRNA treatment also suppressed Piezo2 protein expression, respectively (Figure 3(f)). Specifically, Figure 3(i) shows that the transfection of Piezo2-specific siRNA resulted in ∼49% knockdown of Piezo2 protein.
Effects of TRPV4/Piezo1/Piezo2 knockdown on [Ca2+]i response evoked by CTS stimulation
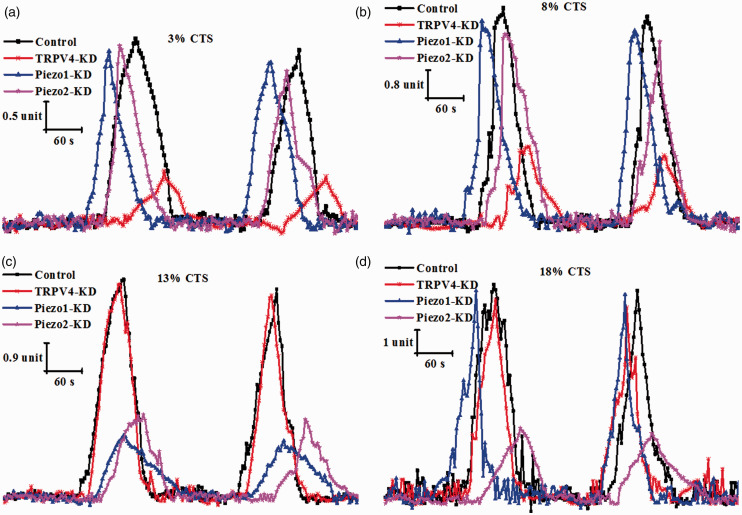
To explore the functional roles of TRPV4, Piezo1, and Piezo2 channels, we evaluated the stretch-evoked changes in [Ca2+]i response in control cells, TRPV4-KD cells, Piezo1-KD cells, and Piezo2-KD cells with four different intensities of repetitive mechanical stimulus (3%, 8%, 13%, and 18% CTS). At 3% CTS, the stretch-evoked [Ca2+]i response was significantly suppressed in TRPV4-KD cells compared with control cells (Figure 4(a)). For example, the average [Ca2+]i response rates, amplitude and frequency in response to 3% CTS stimulation showed decrease in TRPV4-KD cells relative to control group by 38%, 55%, and 20%, respectively (P < 0.05; Table 3). However, no significant change in [Ca2+]i response was observed in either the Piezo1-KD or Piezo2-KD group relative to control group (Table 3). Notably, the same result also occured at 8% CTS (Figure 4(b) and Table 4). In contrast, at 13% CTS, the stretch-evoked [Ca2+]i responses in Piezo1-KD cells and Piezo2-KD cells were significantly lower than that in control cells, but the [Ca2+]i response in TRPV4-KD cells was not significantly different from that in control cells (Figure 4(c) and Table 5). More specifically, the average [Ca2+]i response rates, amplitude and frequency in response to 13% CTS stimulation showed decrease in Piezo1-KD cells and Piezo2-KD cells compared to control cells by 28% and 30%, 62% and 53%, and 20% and 24%, respectively (P < 0.05; Table 5). At 18% CTS, the stretch-evoked [Ca2+]i response was significantly reduced in Piezo2-KD cells relative to control cells, but the [Ca2+]i response in TRPV4-KD or Piezo1 cells was not significantly different from that in control cells (Figure 4(d) and Table 6). Data in Table 6 show that the average [Ca2+]i response rates, oscillation amplitude, and frequency in response to 18% CTS stimulation showed a decrease in Piezo2-KD cells relative to control group by 35%, 59%, and 30%, respectively (P < 0.05).
Figure 4.
[Ca2+]i oscillation evoked by CTS stimulation at different strain magnitudes of (a) 3%, (b) 8%, (c) 13%, or (d) 18% in control siRNA-treated chondrocytes (control), TRPV4-KD cells (TRPV4-KD), Piezo1-KD cells (Piezo1-KD), and Piezo2-KD cells (Piezo2-KD). TRPV4: transient receptor potential vanilloid 4; CTS: cyclic tensile strains; KD: knockdown. (A color version of this figure is available in the online journal.)
Table 3.
[Ca2+]i oscillation for Control, TRPV4-KD, Piezo1-KD, and Piezo2-KD stimulated with 3% CTS stimulation (mean ± standard deviation).
| 3% CTS control (n = 41) | 3% CTS TRPV4-KD (n = 36) | 3% CTS Piezo1-KD (n = 43) | 3% CTS Piezo2-KD (n = 39) | |
|---|---|---|---|---|
| [Ca2+]i response rates (%) | 19.37 ± 1.64 | 12.02 ± 1.03 ↓ | 20.14 ± 1.71 | 19.59 ± 1.68 |
| Amplitude | 4.24 ± 0.33 | 1.91 ± 0.14 ↓ | 4.02 ± 0.29 | 3.95 ± 0.38 |
| Frequency (Peaks/min) | 0.20 ± 0.01 | 0.16 ± 0.01 ↓ | 0.19 ± 0.01 | 0.19 ± 0.02 |
Note: Cell numbers examined for [Ca2+]i oscillation were 41 cells (control), 36 cells (TRPV4-KD), 43 cells (Piezo1-KD), and 39 cells (Piezo2-KD) for 3% CTS, respectively. Data are represented as the mean ± standard deviation of three independent experiments.
TRPV4: transient receptor potential vanilloid 4; CTS: cyclic tensile strains; KD: knockdown.
Table 4.
[Ca2+]i oscillation for control, TRPV4-KD, Piezo1-KD, and Piezo2-KD stimulated with 8% CTS stimulation (mean ± standard deviation).
| 8% CTS control (n = 47) | 8% CTS TRPV4-KD (n = 38) | 8% CTS Piezo1-KD (n = 41) | 8% CTS Piezo2-KD (n = 42) | |
|---|---|---|---|---|
| [Ca2+]i response rates (%) | 20.12 ± 1.72 | 13.04 ± 1.16 ↓ | 21.45 ± 1.87 | 20.18 ± 1.99 |
| Amplitude | 7.07 ± 0.51 | 3.01 ± 0.27 ↓ | 6.64 ± 0.67 | 6.42 ± 0.59 |
| Frequency (Peaks/min) | 0.20 ± 0.01 | 0.17 ± 0.01 ↓ | 0.20 ± 0.02 | 0.19 ± 0.02 |
Note: Cell numbers examined for [Ca2+]i oscillation were 47 cells (control), 38 cells (TRPV4-KD), 41 cells (Piezo1-KD), and 42 cells (Piezo2-KD) for 8% CTS, respectively. Data are represented as the mean ± standard deviation of three independent experiments.
TRPV4: transient receptor potential vanilloid 4; CTS: cyclic tensile strains; KD: knockdown.
Table 5.
[Ca2+]i oscillation for control, TRPV4-KD, Piezo1-KD, and Piezo2-KD stimulated with 13% CTS stimulation (mean ± standard deviation).
| 13% CTS control (n = 37) | 13% CTS TRPV4-KD (n = 40) | 13% CTS Piezo1-KD (n = 33) | 13% CTS Piezo2-KD (n = 34) | |
|---|---|---|---|---|
| [Ca2+]i response rates (%) | 21.47 ± 1.82 | 20.85 ± 1.86 | 15.33 ± 1.22 ↓ | 14.96 ± 1.37 ↓ |
| Amplitude | 7.86 ± 0.66 | 7.69 ± 0.71 | 2.87 ± 0.24 ↓ | 3.63 ± 0.31 ↓ |
| Frequency (Peaks/min) | 0.20 ± 0.01 | 0.19 ± 0.01 | 0.16 ± 0.01 ↓ | 0.15 ± 0.01 ↓ |
Note: Cell numbers examined for [Ca2+]i oscillation were 37 cells (control), 40 cells (TRPV4-KD), 33 cells (Piezo1-KD), and 34 cells (Piezo2-KD) for 13% CTS, respectively. Data are represented as the mean ± standard deviation of three independent experiments.
TRPV4: transient receptor potential vanilloid 4; CTS: cyclic tensile strains; KD: knockdown.
Table 6.
[Ca2+]i oscillation for control, TRPV4-KD, Piezo1-KD, and Piezo2-KD stimulated with 18% CTS stimulation (mean ± standard deviation).
| 18% CTS control (n = 37) | 18% CTS TRPV4-KD (n = 42) | 18% CTS Piezo1-KD (n = 45) | 18% CTS Piezo2-KD (n = 38) | |
|---|---|---|---|---|
| [Ca2+]i response rates (%) | 23.55 ± 2.14 | 24.16 ± 2.37 | 24.03 ± 2.19 | 15.21 ± 1.28 ↓ |
| Amplitude | 10.06 ± 0.96 | 9.84 ± 0.87 | 9.35 ± 0.91 | 3.96 ± 0.34 ↓ |
| Frequency (Peaks/min) | 0.20 ± 0.02 | 0.21 ± 0.02 | 0.21 ± 0.02 | 0.14 ± 0.01 ↓ |
Note: Cell numbers examined for [Ca2+]i oscillation were 37 cells (control), 42 cells (TRPV4-KD), 45 cells (Piezo1-KD), and 38 cells (Piezo2-KD) for 18% CTS, respectively. Data are represented as the mean ± standard deviation of three independent experiments.
TRPV4: transient receptor potential vanilloid 4; CTS: cyclic tensile strains; KD: knockdown.
[Ca2+]i oscillations in control, TRPV4-KD, Piezo1-KD, and Piezo2-KD cells were also presented to study the effects of TRPV4, Piezo1, or Piezo2 knockdown on spontaneous [Ca2+]i oscillation in chondrocyte (Table S1). The data in Table S1 show that knockdown of any of the three channels could weaken the [Ca2+]i oscillation. Only a relatively small change in [Ca2+]i oscillation before and after knockdown of any of the three channels was observed. In sharp contrast, TRPV4, Piezo1, or Piezo2 knockdown could significantly reduce some specific mechanical stretch-evoked [Ca2+]i response (Tables 3 to 6).
Discussion
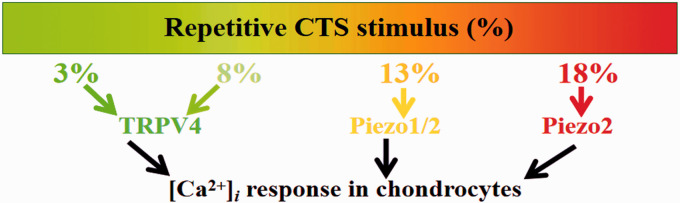
The objective of this study was to investigate the roles of TRPV4, Piezo1, and Piezo2 in transducing different intensities of repetitive mechanical stimulus (Physiologic and Injurious levels of strain) in chondrocytes. For this purpose, different intensities of CTS were used to simulate the load-induced stimuli that chondrocytes encounter in the body, and the potential contributions of TRPV4, Piezo1, and Piezo2 in the repetitive mechanical stretch-induced [Ca2+]i response in chondrocytes were explored. Findings from this work showed, firstly, that TRPV4, Piezo1, and Piezo2 expression in response to CTS showed significant differences, which depend on the strain magnitudes of CTS stimulation. Specifically, TRPV4, Piezo1, and Piezo2 expression reached its highest level at 3%, 13%, and 18% CTS, respectively (Figure 1). Secondly, repetitive CTS stimulation evoked [Ca2+]i response in a strain magnitude-dependent manner in chondrocytes (Figure 2 and Table 2). Lastly, and most importantly, TRPV4 contributed to the 3% and 8% CTS-induced [Ca2+]i responses in chondrocytes; both Piezo1 and Piezo2 contributed to the 13% CTS-induced [Ca2+]i response in chondrocytes, while Piezo2 contributed to the 18% CTS-induced [Ca2+]i response in chondrocytes (Figure 5). These findings indicate that there is an overlap in the sensing ranges of Piezo1 and Piezo2, or in addition, this overlap phenomenon may disappear at ranges that were not tested in our experiment. Overall, TRPV4-mediated calcium signals were shown to respond to low magnitudes of repetitive CTS stimulus, while Piezo2 regulated the chondrocyte calcium response to high magnitudes of repetitive CTS loading. One of the earliest responses of chondrocytes to mechanical stimuli is [Ca2+]i oscillation.18,26 Our results provide useful information to the field of chondrocyte mechanobiology because Ca2+ signaling induced by stretch stimulation has been known to promote both cartilage cell health and death; the channels involved remain elusive. Taking the results together, the chondrocytes might sense and distinguish the different intensities of repetitive mechanical stimulus by different mechanosensitive ion channels.
Figure 5.
TRPV4 and Piezo-mediated CTS induced [Ca2+]i oscillation in chondrocyte. CTS: cyclic tensile strains. (A color version of this figure is available in the online journal.)
TRPV4 knockout mice were proved to have impaired pressure and osmotic sensation as well as impaired stretch sensitivity in the bladder wall.27–29 In addition, OA is more likely to develop in mice lacking TRPV4, and moreover, the risk of developing OA increases with age and high body weight.30,31 Inhibition of TRPV4 impaired cyclical mechanical loading-induced cartilage ECM secretion.7 Atomic force microscope cantilever-induced Ca2+ transient was significantly weakened in Piezo1- or Piezo2-KD porcine articular chondrocytes.32 Additionally, the differences between TRPV4 and Piezo1 channels were also observed in mechanosensation in the urothelium, and the sensitivity of Piezo1 to mechanical stretch stimulation was higher than that of TRPV4 in the urothelium.33 Recent researches have reported that Piezo2 plays a critical role not only in somatosensory mechanotransduction in response to injurious stimulation but also in non-noxious tactile mechanotransduction.32,34–36 Thus, it can be concluded that TRPV4 is mainly responsible for sensing physiologic levels of repetitive CTS stimulus, while Piezo2 mainly contributes to chondrocyte sensing noxious levels of repetitive CTS loading, which is similar to previous reports for TRPV4 and Piezo, respectively.32 Our findings provide strong support for the previous hypothesis of the Lee W group reporting that the anabolic response of chondrocytes to gentle stimuli, is regulated by a TRPV4-based mechanism, and the catabolic response of chondrocytes to injurious stimuli is regulated by a Piezo-based mechanism, respectively.32 Mechanotransduction is important in chondrocytes and is vital for their role in cartilage function and maintenance.4,6 Since high-intensity exercise is associated with an increase in the risk of knee OA, thus, our results have significant implications for mechanobiology of load-bearing articular cartilage that are prone to injury and degeneration. It is possible that inhibiting Piezo2 may prevent or delay the development of OA which is induced by injurious and repetitive mechanical factors. In addition, it is worth mentioning that a Piezo2 antagonist such as Grammostola spatulata mechanotoxin-4 (GsMTx4) has been developed and proved to have a very benign safety profile.37 A recent report has found that GsMTx4 pre-treatment prevented chondrocyte apoptosis following mechanical impact to cartilage.32 Although it is premature at this stage to describe GsMTx4 as a drug for treating knee OA, our findings, that Piezo2 mainly contributes to the injurious levels of repetitive CTS-induced [Ca2+]i response in chondrocytes, provide further support for the hypothesis that Piezo2 inhibition by drugs or molecular knockdown represent an alternative approach for the treatment of OA which is induced by injurious and repetitive mechanical stimulation.
In fact, passaged chondrocytes may have undergone a degree of dedifferentiation. The majority of cultured cells did not show significant dedifferentiation in terms of morphology in our study. This may be related to our special cultivation environment (collagen type I-coated six-well BioFlex® plates). Culturing chondrocytes on collagen type I-coated substrate could significantly inhibit chondrocyte dedifferentiation and enhance preservation of the chondrocyte phenotype.38–40 It should be noted that a recent study indicated that chondrocytes and dedifferentiated cells displayed distinct mechanosensitivity. Specifically, compared with chondrocytes, dedifferentiated cells were more sensitive to substrate deflections, and there was no significant difference in tensile mechanical stimulation-induced currents.41 As with the previous the findings in the Servin-vences MR eLife 2017 paper, our research showed that TRPV4 might be a more sensitive mechanosensor than Piezo for chondrocyte exposed to repetitive CTS stimulus. However, we still need to continue to study the potential mechanism of the difference in sensitivity of TRPV4 and Piezo.
The specific features of a repetitive mechanical stimulus include not only the amplitude but also the frequency. In addition, mechanosensitive cells are exposed to repetitive mechanical stimulation that varies over a wide range of frequencies.42,43 Lewis group examined how Piezo1 and Piezo2 mechanosensitive ion channels in HEK293t cells responded to repetitive mechanical stimulations by electrophysiological recordings and found that Piezo1 and Piezo2 acted as pronounced frequency filters whose transduction efficiencies vary with stimulus frequency.44 The mechanical inputs experienced by Piezos in vivo are arguably more complex and repetitive in nature.44 However, only the amplitude of repetitive mechanical stimulus is currently considered in our cell-tensile experiment. Our results give an insight into the potential difference at the contributions of TRPV4 and Piezos in transducing repetitive mechanical stimuli with different stretch amplitudes. The chondrocyte biological response depended on subtle changes of mechanical stimulation characteristics such as frequency and intensity.4 Therefore, we have every reason to believe that TRPV4 and Piezos-mediated chondrocyte mechanotransduction processes may also be related to the frequency of repetitive mechanical stimulation. Therefore, in the future study, we will jointly consider the load-induced repetitive mechanical stretch stimulation in both the amplitude and frequency for more realistic simulation of the mechanical microenvironment of chondrocytes encounter in the body.
In conclusion, in the present study conducted on primary mouse articular chondrocytes, we show that TRPV4 mainly mediates physiologic levels of repetitive CTS stimulus-induced [Ca2+]i responses and Piezo2 mainly mediates injurious levels of repetitive CTS loading-induced [Ca2+]i responses. Our findings have significant implications for understanding how repetitive mechanical forces in articular chondrocytes are coupled to down-stream [Ca2+]i responses and give insight into the potential contributions of TRPV4 and Piezos in transducing repetitive mechanical stimuli.
Supplemental Material
Supplemental material, EBM892601 Supplemental Material for Roles of TRPV4 and piezo channels in stretch-evoked Ca2+ response in chondrocytes by Genlai Du, Li Li, Xinwang Zhang, Jianbing Liu, Jianqing Hao, Jianjun Zhu, Hao Wu, Weiyi Chen and Quanyou Zhang in Experimental Biology and Medicine
Authors’ contributions
W-YC, Q-YZ and G-LD contributed to the design and review of the research. G-LD and Q-YZ conducted the experiments. Q-YZ, LL, J-BL, J-QH, J-JZ, X-WZ and HW analyzed the data. G-LD wrote the article. G-LD, Q-YZ, X-WZ, J-JZ and W-YC revised the article. All authors approved the final version of the article for publication.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
This study was financially supported by the National Natural Science Foundation of China (grant no. 11872263, 11632013), Science Research Start-up Fund for Doctor of Shanxi Medical University (XD1813), Science Research Start-up Fund for Doctor of Shanxi Province (SD1813), and Scientific and Technological Innovation Programs of Higher Education Institutions in Shanxi (2019L0404).
ORCID iD
Genlai Du https://orcid.org/0000-0001-7069-937X
References
- 1.Fregly BJ, Besier TF, Lloyd DG, Delp SL, Banks SA, Pandy MG, D'Lima DD. Grand challenge competition to predict in vivo knee loads. J Orthop Res 2012; 30:503–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller RH. Joint loading in runners does not initiate knee osteoarthritis. Exerc Sport Sci Rev 2017; 45:87–95 [DOI] [PubMed] [Google Scholar]
- 3.Saxby D, Lloyd D. Osteoarthritis year in review 2016: mechanics. Osteoarthr Cartilage 2017; 25:190–8 [DOI] [PubMed] [Google Scholar]
- 4.Bleuel J, Zaucke F, Brüggemann GP, Niehoff A. Effects of cyclic tensile strain on chondrocyte metabolism: a systematic review. Plos One 2015; 10:e0119816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Responte DJ, Lee JK, Hu JC, Athanasiou KA. Biomechanics-driven chondrogenesis: from embryo to adult. FASEB J 2012; 26:3614–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Athanasiou KA, Responte DJ, Brown WE, Hu JC. Harnessing biomechanics to develop cartilage regeneration strategies. J Biomech Eng 2014; 137:020901–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Conor CJ, Leddy HA, Benefield HC, Liedtke WB, Guilak F. TRPV4-mediated mechanotransduction regulates the metabolic response of chondrocytes to dynamic loading. P Natl Acad Sci USA 2014; 111:1316–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gottlieb P. A tour de force: the discovery, properties, and function of piezo channels. Curr Top Membr 2017; 79:1–36 [DOI] [PubMed] [Google Scholar]
- 9.Boettner B. PIEZO de resistance. SciBX 2014; 7:1368–9 [Google Scholar]
- 10.Asmar A, Barrett-Jolley R, Werner A, Kelly R, Jr, Stacey M. Membrane channel gene expression in human costal and articular chondrocytes. Organogenesis 2016; 12:94–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moroni M, Servin-Vences MR, Fleischer R, Sánchez-Carranza O, Lewin GR. Voltage gating of mechanosensitive PIEZO channels. Nat Commun 2018; 9:1096–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mamenko M, Zaika O, Boukelmoune N, O'Neil RG, Pochynyuk O. Deciphering physiological role of the mechanosensitive TRPV4 channel in the distal nephron. Am J Physiol-Renal 2014; 308:F275–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soattin L, Fiore M, Gavazzo P, Viti F, Facci P, Raiteri R, Difato F, Pusch M, Vassalli M. The biophysics of piezo1 and piezo2 mechanosensitive channels. Biophys Chem 2016; 208:26–33 [DOI] [PubMed] [Google Scholar]
- 14.Servin-Vences MR, Richardson J, Lewin GR, Poole K. Mechanoelectrical transduction in chondrocytes. Clin Exp Pharmacol Physiol 2018; 45:481–8 [DOI] [PubMed] [Google Scholar]
- 15.Steward A, Kelly D, Wagner D. The role of calcium signalling in the chondrogenic response of mesenchymal stem cells to hydrostatic pressure. eCM 2014; 28:358–71 [DOI] [PubMed] [Google Scholar]
- 16.Beggs MR, Alexander RT. Intestinal absorption and renal reabsorption of calcium throughout postnatal development. Exp Biol Med (Maywood) 2017; 242:840–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Madden RM, Han SK, Herzog W. The effect of compressive loading magnitude on in situ chondrocyte calcium signaling. Biomech Model Mechanobiol 2015; 14:135–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han SK, Wouters W, Clark A, Herzog W. Mechanically induced calcium signaling in chondrocytes in situ. J Orthop Res 2012; 30:475–81 [DOI] [PubMed] [Google Scholar]
- 19.Farnsworth NL, Mead BE, Antunez LR, Palmer AE, Bryant SJ. Ionic osmolytes and intracellular calcium regulate tissue production in chondrocytes cultured in a 3D charged hydrogel. Matrix Biol 2014; 40:17–26 [DOI] [PubMed] [Google Scholar]
- 20.Zhou Y, David MA, Chen X, Wan LQ, Duncan RL, Wang L, Lucas Lu X. Effects of osmolarity on the spontaneous calcium signaling of in situ juvenile and adult articular chondrocytes. Ann Biomed Eng 2016; 44:1138–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raizman I, De Croos JA, Pilliar R, Kandel RA. Calcium regulates cyclic compression-induced early changes in chondrocytes during in vitro cartilage tissue formation. Cell Calcium 2010; 48:232–42 [DOI] [PubMed] [Google Scholar]
- 22.Lv M, Zhou Y, Chen X, Han L, Wang L, Lu XL. Calcium signaling of in situ chondrocytes in articular cartilage under compressive loading: roles of calcium sources and cell membrane ion channels. J Orthop Res 2018; 36:730–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gosset M, Berenbaum F, Thirion S, Jacques C. Primary culture and phenotyping of murine chondrocytes. Nat Protoc 2008; 3:1253–60 [DOI] [PubMed] [Google Scholar]
- 24.Hua P, Liu LB, Liu JL, Wang M, Jiang HQ, Zeng K, Yang YQ, Yang SR. Inhibition of apoptosis by knockdown of caspase-3 with siRNA in rat bone marrow mesenchymal stem cells. Exp Biol Med (Maywood) 2013; 238:991–8 [DOI] [PubMed] [Google Scholar]
- 25.Lu J, Liu F, Liu D, Du H, Hao J, Yang X, Cui W. Amlodipine and atorvastatin improved hypertensive cardiac hypertrophy through regulation of receptor activator of nuclear factor kappa B ligand/receptor activator of nuclear factor kappa B/osteoprotegerin system in spontaneous hypertension rats. Exp Biol Med (Maywood) 2016; 241:1237–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kono T, Nishikori T, Kataoka H, Uchio Y, Ochi M, Enomoto K. Spontaneous oscillation and mechanically induced calcium waves in chondrocytes. Cell Biochem Funct 2006; 24:103–11 [DOI] [PubMed] [Google Scholar]
- 27.Liedtke W, Friedman JM. Abnormal osmotic regulation in TRPV4-/-mice. P Natl Acad Sci USA 2003; 100:13698–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mizuno A, Matsumoto N, Imai M, Suzuki M. Impaired osmotic sensation in mice lacking TRPV4. Am J Physiol Cell Physiol 2003; 285:C96–C101 [DOI] [PubMed] [Google Scholar]
- 29.Lechner SG, Markworth S, Poole K, Smith ESJ, Lapatsina L, Frahm S, May M, Pischke S, Suzuki M, Ibanez-Tallon I, Luft FC, Jordan J, Lewin GR. The molecular and cellular identity of peripheral osmoreceptors. Neuron 2011; 69:332–44 [DOI] [PubMed] [Google Scholar]
- 30.O’Conor CJ, Case N, Guilak F. Mechanical regulation of chondrogenesis. Stem Cell Res Ther 2013; 4:61–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’Conor CJ, Ramalingam S, Zelenski NA, Benefield HC, Rigo I, Little D, Wu C, Chen D, Liedtke W, McNulty AL, Guilak F. Cartilage-specific knockout of the mechanosensory ion channel TRPV4 decreases age-related osteoarthritis. Sci Rep 2016; 6:29053–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee W, Leddy HA, Chen Y, Lee SH, Zelenski NA, McNulty AL, Wu J, Beicker KN, Coles J, Zauscher S, Grandl J, Sachs F, Guilak F, Liedtke WB. Synergy between Piezo1 and Piezo2 channels confers high-strain mechanosensitivity to articular cartilage. Proc Natl Acad Sci USA 2014; 111:E5114–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miyamoto T, Mochizuki T, Nakagomi H, Kira S, Watanabe M, Takayama Y, Suzuki Y, Koizumi S, Takeda M, Tominaga M. Functional role for Piezo1 in stretch-evoked Ca2+ influx and ATP release in urothelial cell cultures. J Biol Chem 2014; 289:16565–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ranade SS, Woo SH, Dubin AE, Moshourab RA, Wetzel C, Petrus M, Mathur J, Bégay V, Coste B, Mainquist J, Wilson AJ, Francisco AG, Reddy K, Qiu Z, Wood JN, Lewin GR, Patapoutian A. Piezo2 is the major transducer of mechanical forces for touch sensation in mice. Nature 2014; 516:121–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Woo SH, Ranade S, Weyer AD, Dubin AE, Baba Y, Qiu Z, Petrus M, Miyamoto T, Reddy K, Lumpkin EA, Stucky CL, Patapoutian A. Piezo2 is required for Merkel-cell mechanotransduction. Nature 2014; 509:622–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nonomura K, Woo SH, Chang RB, Gillich A, Qiu Z, Francisco AG, Ranade SS, Liberles SD, Patapoutian A. Piezo2 senses airway stretch and mediates lung inflation-induced apnoea. Nature 2017; 541:176–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gottlieb PA, Suchyna TM, Sachs F. Properties and mechanism of the mechanosensitive ion channel inhibitor GsMTx4, a therapeutic peptide derived from tarantula venom . Curr Top Memb 2007; 59:81–109 [DOI] [PubMed] [Google Scholar]
- 38.Rosenzweig DH, Solar-Cafaggi S, Quinn TM. Functionalization of dynamic culture surfaces with a cartilage extracellular matrix extract enhances chondrocyte phenotype against dedifferentiation. Acta Biomater 2012; 8:3333–41 [DOI] [PubMed] [Google Scholar]
- 39.Rutgers M, Saris DB, Vonk LA, van Rijen MH, Akrum V, Langeveld D, Boxtel Av Dhert WJ, Creemers LB. Effect of collagen type I or type II on chondrogenesis by cultured human articular chondrocytes. Tissue Eng Part A 2012; 19:59–65 [DOI] [PubMed] [Google Scholar]
- 40.Kino-Oka M, Yashiki S, Ota Y, Mushiaki Y, Sugawara K, Yamamoto T, Takezawa T, Taya M. Subculture of chondrocytes on a collagen type I-coated substrate with suppressed cellular dedifferentiation. Tissue Eng 2005; 11:597–608 [DOI] [PubMed] [Google Scholar]
- 41.Servin-Vences MR, Moroni M, Lewin GR, Poole K. Direct measurement of TRPV4 and PIEZO1 activity reveals multiple mechanotransduction pathways in chondrocytes. Elife 2017; 6:e21074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Robles L, Ruggero MA. Mechanics of the mammalian cochlea. Physiol Rev 2001; 81:1305–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eastwood AL, Sanzeni A, Petzold BC, Park SJ, Vergassola M, Pruitt BL, Goodman MB. Tissue mechanics govern the rapidly adapting and symmetrical response to touch. Proc Natl Acad Sci USA 2015; 112:E6955–E63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lewis AH, Cui AF, McDonald MF, Grandl J. Transduction of repetitive mechanical stimuli by Piezo1 and Piezo2 ion channels. Cell Rep 2017; 19:2572–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, EBM892601 Supplemental Material for Roles of TRPV4 and piezo channels in stretch-evoked Ca2+ response in chondrocytes by Genlai Du, Li Li, Xinwang Zhang, Jianbing Liu, Jianqing Hao, Jianjun Zhu, Hao Wu, Weiyi Chen and Quanyou Zhang in Experimental Biology and Medicine