Abstract
Background
Oral 5‐aminosalicylic acid (5‐ASA) preparations were intended to avoid the adverse effects of sulfasalazine (SASP) while maintaining its therapeutic benefits. Previously, it was found that 5‐ASA drugs in doses of at least 2 g/day, were more effective than placebo but no more effective than SASP for inducing remission in ulcerative colitis. This updated review includes more recent studies and evaluates the efficacy and safety of 5‐ASA preparations used for the treatment of mild to moderately active ulcerative colitis.
Objectives
The primary objectives were to assess the efficacy, dose‐responsiveness and safety of oral 5‐ASA compared to placebo, SASP, or 5‐ASA comparators for induction of remission in active ulcerative colitis. A secondary objective of this systematic review was to compare the efficacy and safety of once daily dosing of oral 5‐ASA with conventional (two or three times daily) dosing regimens.
Search methods
A computer‐assisted literature search for relevant studies (inception to July 9, 2015) was performed using MEDLINE, EMBASE and the Cochrane Library. Review articles and conference proceedings were also searched to identify additional studies.
Selection criteria
Studies were accepted for analysis if they were randomized controlled clinical trials of parallel design, with a minimum treatment duration of four weeks. Studies of oral 5‐ASA therapy for treatment of patients with active ulcerative colitis compared with placebo, SASP or other formulations of 5‐ASA were considered for inclusion. Studies that compared once daily 5‐ASA treatment with conventional dosing of 5‐ASA (two or three times daily) and 5‐ASA dose ranging studies were also considered for inclusion.
Data collection and analysis
The outcomes of interest were the failure to induce global/clinical remission, global/clinical improvement, endoscopic remission, endoscopic improvement, adherence, adverse events, withdrawals due to adverse events, and withdrawals or exclusions after entry. Trials were separated into five comparison groups: 5‐ASA versus placebo, 5‐ASA versus sulfasalazine, once daily dosing versus conventional dosing, 5‐ASA versus comparator 5‐ASA, and 5‐ASA dose‐ranging. Placebo‐controlled trials were subgrouped by dosage. SASP‐controlled trials were subgrouped by 5‐ASA/SASP mass ratios. Once daily versus conventional dosing studies were subgrouped by formulation. 5‐ASA‐controlled trials were subgrouped by common 5‐ASA comparators (e.g. Asacol, Claversal, Salofalk and Pentasa). Dose‐ranging studies were subgrouped by 5‐ASA formulation. We calculated the relative risk (RR) and 95% confidence intervals (95% CI) for each outcome. Data were analyzed on an intention‐to‐treat basis.
Main results
Fifty‐three studies (8548 patients) were included. The majority of included studies were rated as low risk of bias. 5‐ASA was significantly superior to placebo with regard to all measured outcome variables. Seventy‐one per cent of 5‐ASA patients failed to enter clinical remission compared to 83% of placebo patients (RR 0.86, 95% CI 0.82 to 0.89). A dose‐response trend for 5‐ASA was also observed. No statistically significant differences in efficacy were found between 5‐ASA and SASP. Fifty‐four per cent of 5‐ASA patients failed to enter remission compared to 58% of SASP patients (RR 0.90, 95% CI 0.77 to 1.04). No statistically significant differences in efficacy or adherence were found between once daily and conventionally dosed 5‐ASA. Forty‐five per cent of once daily patients failed to enter clinical remission compared to 48% of conventionally dosed patients (RR 0.94, 95% CI 0.83 to 1.07). Eight per cent of patients dosed once daily failed to adhere to their medication regimen compared to 6% of conventionally dosed patients (RR 1.36, 95% CI 0.64 to 2.86). There does not appear to be any difference in efficacy among the various 5‐ASA formulations. Fifty per cent of patients in the 5‐ASA group failed to enter remission compared to 52% of patients in the 5‐ASA comparator group (RR 0.94, 95% CI 0.86 to 1.02). A pooled analysis of 3 studies (n = 1459 patients) studies found no statistically significant difference in clinical improvement between Asacol 4.8 g/day and 2.4 g/day used for the treatment of moderately active ulcerative colitis. Thirty‐seven per cent of patients in the 4.8 g/day group failed to improve clinically compared to 41% of patients in the 2.4 g/day group (RR 0.89; 95% CI 0.78 to 1.01). Subgroup analysis indicated that patients with moderate disease may benefit from the higher dose of 4.8 g/day. One study compared (n = 123 patients) Pentasa 4 g/day to 2.25 g/day in patients with moderate disease. Twenty‐five per cent of patients in the 4 g/day group failed to improve clinically compared to 57% of patients in the 2.25 g/day group (RR 0.44; 95% CI 0.27 to 0.71). A pooled analysis of two studies comparing MMX mesalamine 4.8 g/day to 2.4 g/day found no statistically significant difference in efficacy (RR 1.03, 95% CI 0.82 to 1.29). There were no statistically significant differences in the incidence of adverse events between 5‐ASA and placebo, once daily and conventionally dosed 5‐ASA, 5‐ASA and comparator 5‐ASA formulation and 5‐ASA dose ranging (high dose versus low dose) studies. Common adverse events included flatulence, abdominal pain, nausea, diarrhea, headache and worsening ulcerative colitis. SASP was not as well tolerated as 5‐ASA. Twenty‐nine percent of SASP patients experienced an adverse event compared to 15% of 5‐ASA patients (RR 0.48, 95% CI 0.37 to 0.63).
Authors' conclusions
5‐ASA was superior to placebo and no more effective than SASP. Considering their relative costs, a clinical advantage to using oral 5‐ASA in place of SASP appears unlikely. 5‐ASA dosed once daily appears to be as efficacious and safe as conventionally dosed 5‐ASA. Adherence does not appear to be enhanced by once daily dosing in the clinical trial setting. It is unknown if once daily dosing of 5‐ASA improves adherence in a community‐based setting. There do not appear to be any differences in efficacy or safety among the various 5‐ASA formulations. A daily dosage of 2.4 g appears to be a safe and effective induction therapy for patients with mild to moderately active ulcerative colitis. Patients with moderate disease may benefit from an initial dose of 4.8 g/day.
Plain language summary
Oral 5‐aminosalicylic acid for the treatment of active ulcerative colitis
Sulfasalazine (SASP) has been used for treating ulcerative colitis for decades. SASP is made up of 5‐aminosalicylic acid (5‐ASA) linked to a sulfur molecule. Up to a third of patients treated with SASP have reported side effects, which are thought to be related to the sulfur part of the molecule. Common side effects associated with SASP include nausea, indigestion, headache, vomiting and abdominal pain. 5‐ASA drugs were developed to avoid the side effects associated with SASP. This review includes 53 randomized trials with a total of 8548 participants. Oral 5‐ASA was found to be more effective than placebo (fake drug). Although oral 5‐ASA drugs are effective for treating active ulcerative colitis, they are no more effective than SASP therapy. Patients taking 5‐ASA are less likely to experience side effects than patients taking SASP. Side effects associated with 5‐ASA are generally mild in nature, and common side effects include gastrointestinal symptoms (e.g. flatulence, abdominal pain, nausea, and diarrhea), headache and worsening ulcerative colitis. Male infertility is associated with SASP and not with 5‐ASA, so 5‐ASA may be preferred for patients concerned about fertility. 5‐ASA compounds are more expensive than SASP, so SASP may be the preferred option where cost is an important factor. 5‐ASA dosed once daily appears to be as effective and safe as conventionally dosed (two or three times daily) 5‐ASA. There do not appear to be any differences in effectiveness or safety among the various 5‐ASA formulations. A daily dosage of 2.4 g appears to be a safe and effective therapy for patients with mild to moderately active ulcerative colitis. Patients with moderate disease may benefit from an initial dose of 4.8 g/day.
Summary of findings
Background
The successful management of ulcerative colitis was greatly facilitated after the introduction of sulfasalazine (SASP) by Svartz (Svartz 1942). SASP is composed of 5‐aminosalicylic acid (5‐ASA) linked to sulfapyridine via a diazo bond. This bond is readily cleaved by bacterial azoreductases in the colon (Peppercorn 1972), to yield the two components. Of these, 5‐ASA has been found to be the therapeutically active component, while sulfapyridine, which is primarily absorbed into systemic circulation, is assumed to function solely as a carrier molecule (Azad Khan 1977; Klotz 1980; Van Hees 1980).
Administration of unbound or uncoated 5‐ASA revealed that it was readily absorbed in the upper jejunum and was unable to reach the colon in therapeutic concentrations (Schroeder 1972; Nielsen 1983; Myers 1987). Ingested SASP largely resists such premature absorption and thus is able to serve as a delivery system that transports the 5‐ASA to the affected regions of the lower intestinal tract (Schroeder 1972). While corticosteroid therapy is more effective for the treatment of severe ulcerative colitis (Truelove 1955; Truelove 1959) the use of SASP in maintaining remission has been well established (Misiewitz 1965; Sutherland 2006a).
Despite its benefits, up to 30% of patients receiving SASP have reported adverse events (Nielsen 1982). It was concluded that many were due to the sulfapyridine moiety, especially those effects found to be dose‐dependent (Das 1973; Myers 1987). This discovery spawned more than a decade of research aimed at finding alternative 5‐ASA delivery systems.
Asacol® (Proctor and Gamble) consists of a pellet of 5‐ASA destined for release in the terminal ileum or colon due to a coating known as Eudragit‐S, a resin that dissolves at a pH greater than 7 (Dew 1982). Claversal®/Mesasal® (Smith, Kline and French), Salofalk® (Axcan Pharma, Falk Foundation), and Rowasa® (Reid‐Rowell) are similar delayed‐release preparations of 5‐ASA pellets coated with Eudragit L, a resin that dissolves at a pH greater than 6 (the approximate pH of the ileum/colon) (Hardy 1987; Myers 1987). Pentasa® (Marion‐Merrell‐Dow) is a microsphere formulation that consists of 5‐ASA microgranules enclosed within a semi‐permeable membrane of ethylcellulose. It is designed for controlled release that begins in the duodenum and continues into the affected regions of the lower bowel (Rasmussen 1982). Olsalazine/Dipentum® (Pharmacia & Upjohn) consists of two 5‐ASA molecules linked by a diazo bond (Willoughby 1982; Staerk Laursen 1990). Other formulations, such as benzalazine, balsalazide/Colazide® (Astra Zeneca), and balsalazide disodium/Colazal® (Salix Pharmaceuticals) are composed of 5‐ASA molecules azo‐bonded to various benzoic acid derivatives (Chan 1983; Fleig 1988). Like SASP, these compounds are poorly absorbed in the upper digestive tract but are readily metabolized by the intestinal flora in the lower bowel. MMX mesalamine (Lialdaa® or Mezavant®) uses MMX Multi Matrix System (MMX) technology to delay and extend delivery of active drug throughout the colon (Kamm 2007; Lichtenstein 2007).
The newer 5‐ASA preparations were intended to avoid the adverse effects of SASP while maintaining its therapeutic benefits; however they are more costly and have also been shown to cause adverse effects in some patients (Rao 1987). The efficacy and safety of 5‐ASA preparations have been evaluated in numerous clinical trials that have often lacked sufficient statistical power to arrive at definitive conclusions. Previous systematic reviews (Sutherland 1993; Sutherland 1997; Sutherland 2006b; Feagan 2012), found that oral 5‐ASA, in doses of at least 2 g/day, was more effective than placebo yet no more effective than SASP for induction of remission in ulcerative colitis. We proceeded with this updated review in order to include more recent studies as well as to evaluate the efficacy, dose‐responsiveness (including dose‐ranging studies of various 5‐ASA formulations), and safety of oral 5‐ASA preparations compared to placebo or SASP. We also aimed to investigate any differences in efficacy and safety between various formulations of oral 5‐ASA.
Many patients are non‐adherent with conventional multi‐dose (two or three times daily) treatment regimens which may result in reduced efficacy and can lead to an increased risk of relapse in patients with quiescent disease (Kane 2001; Kane 2003), a poorer long‐term prognosis (Kane 2008; Kruis 2009) and increased healthcare costs (Kane 2008; Beaulieu 2009). Poor adherence may be particularly problematic in quiescent disease (Kane 2001; Kane 2003), since patients lack continuing symptoms that incentivize them to take medication. Although multiple factors have been shown to influence medication adherence in patients with ulcerative colitis it is commonly believed that a high pill burden and multi‐dose regimens are major determinants (Ediger 2007; Kane 2008). Other factors affecting adherence in ulcerative colitis patients include disease extent and duration, medication costs, fear of side effects, individual psychosocial characteristics and the patient‐physician relationship (Kane 2008). Mesalamine formulations that involve once daily dosing may improve adherence and outcomes.
The efficacy and safety of once daily oral dosing of mesalamine compared to conventional dosing for the treatment of ulcerative colitis has been evaluated in numerous clinical trials. These trials have investigated the efficacy of once daily dosing of various formulations of mesalamine compared to conventional dosing schedules of the same drugs or different formulations. Many of these trials were small in size and lacked sufficient statistical power to arrive at definitive conclusions. A secondary objective of this systematic review was to investigate the efficacy and safety of once daily dosing of mesalamine compared to conventional dosing for the treatment of active ulcerative colitis.This systematic review is an update of a previously published Cochrane review (Feagan 2012).
Objectives
The primary objectives were to assess the efficacy, dose‐responsiveness, and safety of oral 5‐aminosalicylic acid (5‐ASA) compared to placebo, sulfasalazine (SASP), or 5‐ASA comparators (i.e. other formulations of 5‐ASA) for induction of remission in active ulcerative colitis. A secondary objective of this systematic review was to compare the efficacy and safety of once daily dosing of oral 5‐ASA with conventional dosing regimens.
Methods
Criteria for considering studies for this review
Types of studies
Prospective, randomized controlled clinical trials of parallel design, with minimum treatment duration of four weeks were considered for inclusion.
Types of participants
Adult patients (> 18 years) with active mild‐to‐moderate ulcerative colitis as defined by Truelove and Witts were considered for inclusion (Truelove 1955).
Types of interventions
Studies of oral 5‐ASA therapy for treatment of patients with active ulcerative colitis compared with placebo, SASP or other formulations of 5‐ASA were considered for inclusion. Studies that compared once daily 5‐ASA treatment with conventional dosing of 5‐ASA (two or three times daily) and 5‐ASA dose ranging studies were also considered for inclusion.
Types of outcome measures
Outcome measures included endoscopic, global or clinical measures of improvement or complete remission as defined by the authors of each study.
Primary outcomes
The primary outcome was the proportion of patients who failed to enter complete global or clinical remission as defined by the authors of each study and expressed as a percentage of total patients randomized (intention‐to‐treat analysis).
Secondary outcomes
Secondary outcomes included:
the proportion of patients who failed to improve clinically;
the proportion of patients who failed to enter endoscopic remission;
the proportion of patients who failed to improve endoscopically;
the proportion of patients who failed to adhere with their medication regimen;
the proportion of patients who experienced at least one adverse event;
the proportion of patients who withdrew due to adverse events; and
the proportion of patients excluded or withdrawn after entry.
Search methods for identification of studies
MEDLINE (OvidSP), EMBASE (Ovid SP), and the Cochrane Library were searched from inception to March 19, 2014. No language or document type restrictions were applied. The multipurpose search command for the Ovid SP interface (.mp.) was used to search both text and database subject heading fields. Review articles and conference proceedings were also searched to identify additional studies. The search strategies are listed in Appendix 1.
Data collection and analysis
Study Selection
Two authors (YW or JKM or CEP) independently selected relevant studies for analysis on the basis of the inclusion criteria described above. When necessary, the original investigators were contacted to clarify points regarding trial methodology. The reasons for exclusion were indicated for each study deemed ineligible.
Data Collection
Two authors (YW or JKM or CEP) independently extracted data using a standard data extraction form. We recorded results on an intention‐to‐treat basis, regardless of whether or not the original authors had done so. Any discrepancies between authors were settled by consensus.
Risk of bias assessment
Two authors (YW or JKM or CEP) independently assessed the risk of bias in the included studies using the Cochrane risk of bias tool (Higgins 2011). Factors assessed included:
sequence generation (i.e. was the allocation sequence adequately generated?);
allocation sequence concealment (i.e. was allocation adequately concealed?);
blinding (i.e. was knowledge of the allocated intervention adequately prevented during the study?);
incomplete outcome data (i.e. were incomplete outcome data adequately addressed?);
selective outcome reporting (i.e. are reports of the study free of suggestion of selective outcome reporting?); and
other potential sources of bias (i.e. was the study apparently free of other problems that could put it at a high risk of bias?).
A judgement of 'Yes' indicates low risk of bias, 'No' indicates high risk of bias, and 'Unclear' indicates unclear or unknown risk of bias. Disagreements were resolved by consensus. Study authors were contacted when insufficient information was provided to determine risk of bias.
We used the GRADE approach for rating the overall quality of evidence for the primary outcomes and selected secondary outcomes of interest. Randomized trials start as high quality evidence, but may be downgraded due to: (1) limitations in design and implementation (risk of bias), (2) indirectness of evidence, (3) inconsistency (unexplained heterogeneity), (4) imprecision (sparse data), and (5) reporting bias (publication bias). The overall quality of evidence for each outcome was determined after considering each of these elements, and categorized as high quality (i.e. further research is very unlikely to change our confidence in the estimate of effect); moderate quality (i.e. further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate); low quality (i.e. further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate); and very low quality (i.e. we are very uncertain about the estimate) (Guyatt 2008; Schünemann 2011).
Statistical Methods
Trials were separated into five comparison groups: 5‐ASA versus placebo, 5‐ASA versus sulfasalazine, once daily dosing versus conventional dosing, 5‐ASA versus comparator 5‐ASA, and 5‐ASA dose‐ranging. Within each group, raw data for every measured outcome were extracted and converted into individual 2x2 tables. The tables for placebo‐controlled trials were further subgrouped according to the dose of 5‐ASA. The tables for SASP‐controlled trials were subgrouped by 5‐ASA/SASP mass ratios. The tables for the once daily versus conventional dosing studies were subgrouped by formulation. The tables for 5‐ASA‐controlled trials were subgrouped by common 5‐ASA comparators (e.g. Asacol, Claversal, Salofalk and Pentasa). The tables for dose‐ranging studies were subgrouped by 5‐ASA formulation. For dichotomous outcomes, we calculated the risk ratio (RR) and corresponding 95% confidence interval (95% CI). The results for each comparison group were pooled to determine the RR and 95% CI for each outcome resulting from 5‐ASA therapy relative to either placebo, SASP or 5‐ASA comparator and once daily 5‐ASA therapy relative to conventional dosing. A fixed‐effect model was used. Studies were pooled for analysis if patients, outcomes and interventions were similar (determined by consensus among authors). Studies comparing 5‐ASA formulations were pooled for analysis if they compared equimolar doses of oral 5‐ASA.
Dose‐responsiveness was analyzed using a Chi2 test for trend. Trials were also subgrouped according to the specific 5‐ASA preparation for those outcomes for which there were two or more studies that used a similar drug. Tests for homogeneity among trials within each comparison group were performed. The presence of heterogeneity among studies was assessed using the Chi2 test (a P value of 0.10 was regarded as statistically significant) and the I2 statistic (Higgins 2003). If statistically significant heterogeneity was identified, the RR and 95% CI were calculated using a random‐effects model. Data were not pooled for meta‐analysis if a high degree of heterogeneity was identified (e.g. I2 > 75%). We conducted sensitivity analyses as appropriate to investigate heterogeneity. We also conducted sensitivity analyses excluding studies with a high risk of bias. All statistical analyses were performed using the Cochrane Collaboration RevMan 5 software package.
Results
Description of studies
A literature search conducted on July 9, 2015 identified 2525 studies. Five additional studies were identified through searching of references. After duplicates were removed a total of 1613 reports remained for review of titles and abstracts. Two authors independently reviewed the titles and abstracts of these studies and 100 reports of oral 5‐ASA for treatment of active ulcerative colitis were selected for full text review (See Figure 1). Eleven of these studies were excluded (See Characteristics of excluded studies).
1.
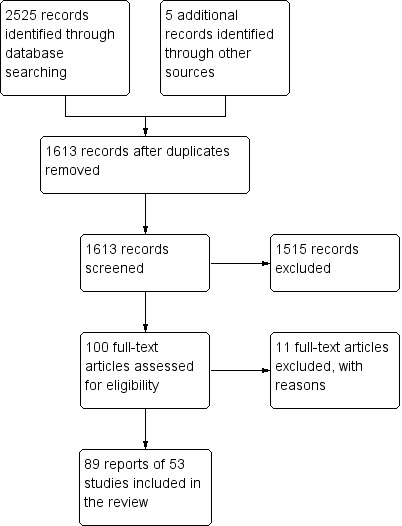
Study flow diagram.
Eighty‐nine reports of fifty‐three studies involving a total of 8548 patients, were selected for inclusion (See Characteristics of included studies). Sixteen studies were placebo‐controlled (Hetzel 1986; Schroeder 1987; Robinson 1988; Feurle 1989; Sutherland 1990; Zinberg 1990; Sninsky 1991; Hanauer 1993; Hanauer 1996; Kamm 2007; Lichtenstein 2007; Scherl 2009; Ito 2010; Sandborn 2012; Feagan 2013; Pontes 2014). Eighteen studies compared 5‐ASA to SASP (Maier 1985; Andreoli 1987; Ewe 1988; Fleig 1988; Mihas 1988; Riley 1988; Willoughby 1988; Rachmilewitz 1989; Rao 1989; Bresci 1990; Rijk 1991; Good 1992; Munakata 1995; Cai 2001; Green 2002; Mansfield 2002; Jiang 2004; Qian 2004). Four studies compared once daily dosing of mesalamine with conventional dosing (Kamm 2007; Kruis 2009; Lichtenstein 2007; Flourie 2013). The Kamm 2007 study had four treatment arms including placebo, Asacol 2.4 g/day (dosed 3 times daily) and two different doses of once daily MMX mesalamine (2.4 g and 4.8 g per day). Kruis 2009 was a formal non‐inferiority study comparing mesalazine (Salofalk granules) 3.0 g dosed once daily with 1 g dosed three times daily. The Lichtenstein 2007 study had three treatment arms including placebo, MMX mesalamine 2.4 g dosed twice daily and MMX mesalamine 4.8 g dosed once daily. In Flourie 2013 patients received 4.0 g of mesalazine once daily or 2.0 g of meslazine twice daily for a total of 8 weeks. Ten trials were dose‐ranging studies of oral 5‐ASA (Schroeder 1987; Miglioli 1990; Sninsky 1991; Kruis 2003; Hanauer 2005; D'Haens 2006; Hanauer 2007; Kamm 2007; Sandborn 2009; Hiwatashi 2011). Twelve trials compared the efficacy and safety of various formulations of oral 5‐ASA to other formulations of oral 5‐ASA (Green 1998; Kruis 1998; Farup 2001; Levine 2002; Pruitt 2002; Raedler 2004; Tursi 2004; Forbes 2005; Marakhouski 2005; Gibson 2006; Kamm 2007; Ito 2010).
Risk of bias in included studies
A summary of the risk of bias assessment is provided in Figure 2. Most of the included studies were of high methodological quality. Five studies were rated at high risk of bias due to incomplete outcome data (Green 1998; Kruis 2003) and lack of blinding (Farup 2001; Tursi 2004; Flourie 2013). Thirty‐two of 53 included studies did not describe the method used for randomization and were rated as unclear for this item. Twenty‐six studies did not describe methods used for allocation concealment and were rated as unclear for this item. The methods used for blinding were not described in five studies, and these studies were rated as unclear. Twenty studies were rated as unclear for incomplete outcome data because reasons for withdrawal were either not described or were not attributed to intervention groups. Six studies were rated as unclear for selective reporting.
2.
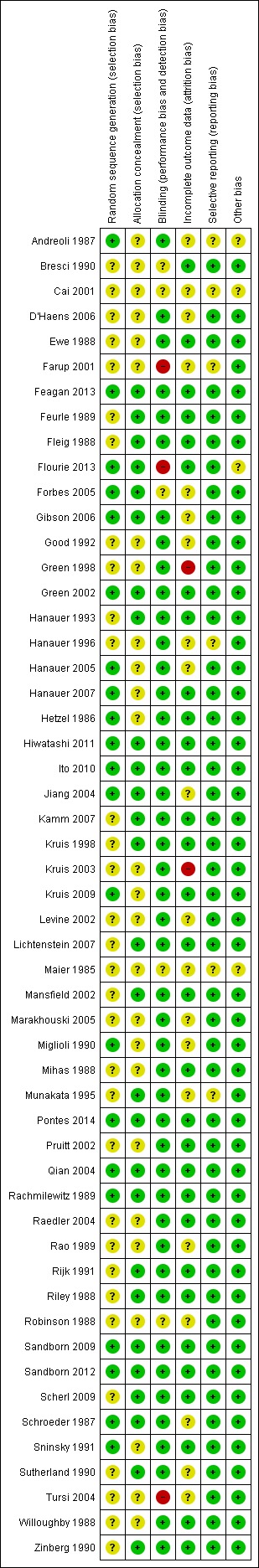
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5
Summary of findings for the main comparison. Oral 5‐ASA versus placebo for induction of remission in ulcerative colitis.
| Oral 5‐ASA versus placebo for induction of remission in ulcerative colitis | ||||||
| Patient or population: Patients with active mild to moderate ulcerative colitis Settings: Outpatients Intervention: Oral 5‐ASA versus placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Oral 5‐ASA versus placebo | |||||
|
Failure to induce global or clinical remission |
830 per 10001 | 714 per 1000 (681 to 739) | RR 0.86 (0.82 to 0.89) | 2,387 (11 studies) | ⊕⊕⊕⊕ high | |
|
Failure to induce clinical improvement |
651 per 10001 | 443 per 1000 (397 to 488) | RR 0.68 (0.61 to 0.75) | 2,256 (15 studies) | ⊕⊕⊕⊝ moderate2 | |
| Adverse events | 486 per 10001 | 462 per 1000 (413 to 520) | RR 0.95 (0.85 to 1.07) | 1,218 (8 studies) | ⊕⊕⊕⊕ high | |
| Withdrawal due to adverse events | 62 per 10001 | 55 per 1000 (38 to 77) | RR 0.88 (0.62 to 1.24) | 2,091 (12 studies) | ⊕⊕⊕⊝ moderate3 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Relative risk | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Control group risk estimates come from control arm of meta‐analysis, based on included trials. 2 Downgraded one level due to heterogeneity I2 = 47%. 3 Downgraded one level due to sparse data (122 events).
Summary of findings 2. Oral 5‐ASA versus SASP for induction of remission in ulcerative colitis.
| Oral 5‐ASA versus SASP for induction of remission in ulcerative colitis | ||||||
| Patient or population: Patients with active mild to moderate ulcerative colitis Settings: Outpatients Intervention: Oral 5‐ASA versus SASP | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Oral 5‐ASA versus SASP | |||||
|
Failure to induce global or clinical remission |
583 per 10001 | 525 per 1000 (449 to 606) | RR 0.90 (0.77 to 1.04) | 526 (8 studies) | ⊕⊕⊕⊝ moderate2 | |
|
Failure to induce clinical improvement |
467 per 10001 | 411 per 1000 (355 to 472) | RR 0.88 (0.76 to 1.01) | 1,053 (14 studies) | ⊕⊕⊕⊕ high | |
| Adverse events | 287 per 10001 | 138 per 1000 (103 to 181) | RR 0.48 (0.36 to 0.63) | 909 (12 studies) | ⊕⊕⊕⊝ moderate3 | |
| Withdrawal due to adverse events | 129 per 10001 | 52 per 1000 (31 to 88) | RR 0.40 (0.24 to 0.68) | 640 (10 studies) | ⊕⊕⊕⊝ moderate4 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Relative risk | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Control group risk estimates come from control arm of meta‐analysis, based on included trials. 2 Downgraded one level due to sparse data (294 events). 3 Downgraded one level due to sparse data (190 events). 4 Downgraded one level due to sparse data (54 events).
Summary of findings 3. Once daily dosing versus conventional dosing for induction of remission in ulcerative colitis.
| Once daily dosing versus conventional dosing for induction of remission in ulcerative colitis | ||||||
| Patient or population: Patients with active mild to moderate ulcerative colitis Settings: Outpatients Intervention: Once daily dosing versus conventional dosing | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | OD versus conventional dosing | |||||
|
Failure to induce global or clinical remission |
477 per 10001 | 448 per 1000 (396 to 510) | RR 0.94 (0.83 to 1.07) | 944 (4 studies) | ⊕⊕⊕⊕ high | |
|
Failure to induce clinical improvement |
458 per 10001 | 398 per 1000 (311 to 504) | RR 0.87 (0.68 to 1.10) | 358 (2 studies) | ⊕⊕⊕⊝ moderate2 | |
| Failure to adhere to medication regimen | 139 per 10001 | 189 per 1000 (89 to 398) | RR 1.36 (0.64 to 2.86) | 358 (2 studies) | ⊕⊕⊝⊝ low3 | |
| Adverse events | 374 per 10001 | 329 per 1000 (273 to 400) | RR 0.88 (0.73 to 1.07) | 769 (3 studies) | ⊕⊕⊕⊝ moderate4 | |
| Withdrawal due to adverse events | 24 per 10001 | 14 per 1000 (6 to 35) | RR 0.58 (0.23 to 1.44) | 940 (4 studies) | ⊕⊕⊝⊝ low5 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Relative risk | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Control group risk estimates come from control arm of meta‐analysis, based on included trials. 2 Downgraded one level due to sparse data (153 events). 3 Downgraded two levels due to very sparse data (26 events). 4 Downgraded one level due to sparse data (271 events). 5 Downgraded two levels due to very sparse data (9 events).
Summary of findings 4. Oral 5‐ASA versus comparator 5‐ASA for induction of remission in ulcerative colitis.
| Oral 5‐ASA versus comparator 5‐ASA for induction of remission in ulcerative colitis | ||||||
| Patient or population: Patients with active mild to moderate ulcerative colitis Settings: Outpatients Intervention: Oral 5‐ASA versus 5‐ASA (different formulations) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Oral 5‐ASA versus comparator 5‐ASA | |||||
|
Failure to induce global or clinical remission |
519 per 10001 | 488 per 1000 (446 to 529) | RR 0.94 (0.86 to 1.02) | 1,968 (11 studies) |
⊕⊕⊕⊝ moderate2 | A sensitivity analysis excluding two high risk of bias studies produced similar results (RR 0.95; 95% CI 0.87 to 1.04; P = 0.28) |
|
Failure to induce clinical improvement |
346 per 10001 | 308 per 1000 (266 to 350) | RR 0.89 (0.77 to 1.01) | 1,647 (8 studies) |
⊕⊕⊕⊝ moderate3 | A sensitivity analysis excluding one high risk of bias study produced similar results (RR 0.91; 95% CI 0.79 to 1.05; P = 0.20) |
| Adverse events | 457 per 10001 | 462 per 1000 (420 to 512) | RR 1.01 (0.92 to 1.12) | 1,576 (9 studies) |
⊕⊕⊕⊝ moderate4 | |
| Withdrawal due to adverse events | 39 per 10001 | 37 per 1000 (20 to 60) | RR 0.94 (0.57 to 1.54) | 1,489 (9 studies) |
⊕⊕⊕⊝ moderate5 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Relative risk | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Control group risk estimates come from control arm of meta‐analysis, based on included trials. 2 Downgraded one level due to high risk of bias in two studies in the pooled analysis (both due to lack of blinding). 3 Downgraded one level due to high risk of bias in one study in the pooled analysis (lack of blinding). 4 Downgraded one level due to high risk of bias in one study in the pooled analysis (lack of blinding). 5 Downgraded one level due to sparse data (57 events).
Summary of findings 5. High dose oral 5‐ASA versus low dose 5‐ASA for induction of remission in ulcerative colitis.
| High dose oral 5‐ASA versus low dose 5‐ASA for induction of remission in ulcerative colitis | ||||||
| Patient or population: Patients with active mild to moderate ulcerative colitis Settings: Outpatients Intervention: High dose oral 5‐ASA versus low dose 5‐ASA | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | High dose 5‐ASA versus low dose 5‐ASA | |||||
|
Failure to induce global or clinical remission |
602 per 10001 | 620 per 1000 (494 to 777) | RR 1.03 (0.82 to 1.29) | 194 (2 studies) | ⊕⊕⊕⊝ moderate2 | MMX mesalazine 4.8 g/day OD versus 2.4 g/day OD |
|
Failure to induce global or clinical remission |
495 per 10001 | 337 per 1000 (243 to 470) | RR 0.68 (0.49 to 0.95) | 210 (1 study) | ⊕⊝⊝⊝ low3,4 | Salofalk 3 g/day versus 1.5 g/day |
|
Failure to induce clinical improvement |
413 per 10001 | 368 per 1000 (322 to 417) | RR 0.89 (0.78 to 1.01) | 1,459 (3 studies) | ⊕⊕⊕⊕ high | Asacol 4.8 g/day versus 2.4 g/day (Ascend I, II and III) in patients with moderate ulcerative colitis |
|
Failure to induce clinical improvement |
727 per 10001 | 262 per 1000 (138 to 501) | RR 0.36 (0.19 to 0.69) | 49 (1 study) | ⊕⊝⊝⊝ low5 | Asacol 4.8 g/day versus 1.6 g/day |
|
Failure to induce clinical improvement |
571 per 10001 | 251 per 1000 (154 to 405) | RR 0.44 (0.27 to 0.71) | 123 (1 study) |
⊕⊕⊕⊝ moderate6 | Pentasa 4 g/day versus 2.25 g/day |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Relative risk | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Control group risk estimates come from control arm of meta‐analysis, based on included trials. 2 Downgraded one level due to sparse data (118 events). 3 Downgraded one level due to high risk of bias (incomplete outcome data). 4 Downgraded one level due to sparse data (87 events). 5 Downgraded two levels due to very sparse data (18 events). 6 Downgraded one level due to sparse data (51 events).
EFFICACY
5‐ASA versus Placebo Eleven trials (n = 2387 patients) reported treatment outcomes in terms of the failure to induce complete global or clinical remission (Schroeder 1987; Sninsky 1991; Hanauer 1993; Hanauer 1996; Kamm 2007; Lichtenstein 2007; Scherl 2009; Ito 2010; Sandborn 2012; Feagan 2013; Pontes 2014). Seventy‐one per cent of 5‐ASA patients failed to enter remission compared to 83% of placebo patients. The pooled relative risk (RR) of failure to induce complete global or clinical remission for all trials was 0.86 (95% CI 0.82 to 0.89; I2 = 25%; P < 0.00001) using a fixed‐effect model. There was a trend towards greater efficacy with higher doses of 5‐ASA with a statistically significant benefit for the 2 to 2.9 g/day (RR 0.88; 95% CI 0.82 to 0.94; I2 = 27%; P = 0.0001) and the > 3 g/day subgroups (RR 0.83; 95% CI 0.77 to 0.88; I2 = 25%; P < 0.00001). The five trials that involved Asacol® (Schroeder 1987; Sninsky 1991; Kamm 2007; Ito 2010; Feagan 2013), had a pooled RR of 0.84 (95% CI 0.79 to 0.90). Two trials using MMX mesalazine (Kamm 2007; Lichtenstein 2007), had a pooled RR of 0.81 (95% CI 0.73 to 0.90). The GRADE analysis indicated that the overall quality of the evidence for the primary outcome for the placebo‐controlled studies (failure to induce complete global or clinical remission) was high (See Table 1).
Fourteen trials (Hetzel 1986; Schroeder 1987; Robinson 1988; Feurle 1989; Sutherland 1990; Zinberg 1990; Sninsky 1991; Hanauer 1993; Kamm 2007; Lichtenstein 2007; Scherl 2009; Ito 2010; Feagan 2013; Pontes 2014), comprised of 2169 patients, provided data regarding the failure to induce global or clinical improvement (including remission). Forty‐two per cent of 5‐ASA patients failed to improve clinically compared to 65% of placebo patients. The pooled RR for all trials was 0.68 (95% CI 0.61 to 0.75; I2 = 47%; P < 0.00001) using a random‐effects model. There was a trend towards greater efficacy with higher doses of 5‐ASA (P = 0.003) with a statistically significant benefit for all dosage subgroups: < 2 g/day (RR 0.79; 95% CI 0.64 to 0.97; I2 = 0%; P = 0.49); 2 to 2.9 g/day (RR 0.77; 95% CI 0.67 to 0.88; I2 = 32%; P = 0.0002); > 3 g/day (RR 0.57; 95% CI 0.51 to 0.65; I2 = 5%; P < 0.00001). Five trials involving Asacol® (Schroeder 1987; Sninsky 1991; Kamm 2007; Ito 2010; Feagan 2013), had a pooled RR of 0.68 (95% CI 0.58 to 0.80). Four studies involved olsalazine (Hetzel 1986; Robinson 1988; Feurle 1989; Zinberg 1990), and resulted in a pooled RR of 0.80 (95% CI 0.65 to 0.97). Two trials using MMX mesalazine (Kamm 2007; Lichtenstein 2007), had a pooled RR of 0.61 (95% CI 0.51 to 0.72). The GRADE analysis indicated that the overall quality of the evidence for this outcome for the placebo‐controlled studies (failure to induce global or clinical improvement) was moderate due to heterogeneity I2 = 47% (See Table 1). Four studies (Hanauer 1993; Hanauer 1996; Kamm 2007; Scherl 2009), with a total of 1154 patients, reported on failure to induce complete endoscopic remission. Fifty per cent of 5‐ASA patients failed to enter endoscopic remission compared to 66% of placebo patients. The superiority of 5‐ASA over placebo was demonstrated by a pooled RR of 0.77 (95% CI 0.67 to 0.89; I2 = 42%; P = 0.0003) using a random‐effects model. Within the dosage subgroups, the superiority of 5‐ASA only reached statistical significance for treatment arms involving doses equal to or greater than 3 g (RR 0.70; 95% CI 0.56 to 0.87; I2 = 51%; P = 0.001).
Four trials (Hanauer 1996; Hetzel 1986; Robinson 1988; Zinberg 1990), with a total of 416 patients, all involving olsalazine, reported the failure to induce endoscopic remission or improvement. Forty‐four per cent of 5‐ASA patients failed to improve endoscopically compared to 63% of placebo patients. The pooled RR was 0.71 (95% CI 0.59 to 0.86; I2 = 43%; P = 0.0005) using a fixed‐effect model.
5‐ASA versus Sulfasalazine
The failure to induce complete global or clinical remission was reported in eight studies with a total of 526 patients (Maier 1985; Andreoli 1987; Riley 1988; Rachmilewitz 1989; Rijk 1991; Green 2002; Mansfield 2002; Jiang 2004). Fifty‐four per cent of 5‐ASA patients failed to enter remission compared to 58% of SASP patients. A statistically significant difference between 5‐ASA and SASP was not observed, pooled RR 0.90 (95% CI 0.77 to 1.04; I2 = 0%; P = 0.15). Two studies involving Claversal® (Andreoli 1987; Rachmilewitz 1989), had a pooled RR of 1.00 (95% CI 0.83 to 1.21). Two studies involving balsalazide (Green 2002; Mansfield 2002), had a pooled RR of 0.66 (95% CI 0.43 to 1.02). Two studies involving olsalazine (Jiang 2004), had a pooled RR of 0.93 (95% CI 0.57 to 1.51). The GRADE analysis indicated that the overall quality of the evidence for the primary outcome for the SASP‐controlled studies (failure to induce complete global or clinical remission) was moderate due to imprecision (sparse data, 294 events; See Table 2).
Thirteen trials (Maier 1985; Ewe 1988; Fleig 1988; Mihas 1988; Riley 1988; Willoughby 1988; Rachmilewitz 1989; Rao 1989; Bresci 1990; Good 1992; Munakata 1995; Jiang 2004; Qian 2004), with a total of 1053 patients reported the failure to induce global/clinical remission or improvement. Thirty‐seven per cent of 5‐ASA patients failed to improve compared to 47% of SASP patients. A statistically significant difference between 5‐ASA and SASP was not observed, The pooled RR was 0.88 (95% CI 0.76 to 1.01; I2 = 0%; P = 0.06). Six olsalazine trials (Ewe 1988; Willoughby 1988; Rao 1989; Cai 2001; Jiang 2004; Qian 2004), had a pooled RR of 0.76 (95% CI 0.57 to 1.00). The GRADE analysis indicated that the overall quality of the evidence for this outcome for the SASP‐controlled studies (failure to induce global or clinical improvement) was high (See Table 2).
Since only two trials (Jiang 2004; Rachmilewitz 1989), reported the failure to induce complete endoscopic remission, this outcome was not considered in our analysis. A pooled RR for complete endoscopic remission was not calculated, as the two studies used different indices to measure endoscopic remission. Neither study showed statistically significant differences in complete endoscopic remission between 5‐ASA and SASP. However, six studies (Fleig 1988; Riley 1988; Willoughby 1988; Rao 1989; Rijk 1991; Munakata 1995), with a total of 362 patients provided data regarding the failure to induce endoscopic improvement (including remission). Forty‐one per cent of 5‐ASA patients failed to improve endoscopically compared to 45% of SASP patients. The pooled RR of 0.82 (95% CI 0.65 to 1.02; I2 = 0%; P = 0.07) indicated a non‐significant trend towards the superiority of 5‐ASA over SASP. Three trials involving olsalazine (Rao 1989; Rijk 1991; Willoughby 1988), had a pooled RR of 0.88 (95% CI 0.46 to 1.71).
Once Daily Dosing versus Conventional Dosing
Four trials (n = 944 patients) reported treatment outcomes in terms of the failure to induce complete global or clinical remission (Kamm 2007; Lichtenstein 2007; Kruis 2009; Flourie 2013). Forty‐eight per cent of conventionally dosed 5‐ASA patients failed to enter remission compared to 45% of patients who were dosed once daily. The pooled RR was 0.94 (95% CI 0.83 to 1.07; I2 = 0%) showing no statistically significant difference between once daily dosing and conventional dosing for induction of remission (P = 0.34). None of the subgroup comparisons by formulation showed any differences in efficacy between once daily dosing and conventional dosing. However, only four formulations were evaluated in this pooled analysis. The GRADE analysis indicated that the overall quality of the evidence for the primary outcome (failure to induce complete global or clinical remission) was high (See Table 3).
Three trials (n = 564 patients) reported treatment outcomes in terms of the failure to induce global or clinical improvement including remission (Kamm 2007; Lichtenstein 2007; Flourie 2013). Thirty‐seven per cent of conventionally dosed 5‐ASA patients failed to improve clinically compared to 28% of patients who were dosed once daily. The pooled RR was 0.74 (95% CI 0.49 to 1.10) showing no statistically significant difference between once daily dosing and conventional dosing for induction of remission or clinical improvement (P = 0.13). A fair amount of heterogeneity (I2 = 59%) was detected for this comparison. A visual inspection of the forest plot indicated that the Flourie 2013 study was the likely source of the heterogeneity. When we performed a sensitivity analysis excluding this high risk of bias study the I2 value dropped to 0%. Forty‐six per cent of conventionally dosed 5‐ASA patients failed to improve clinically compared to 40% of patients who were dosed once daily (RR 0.87, 95% CI 0.68 to 1.10). A GRADE analysis indicated that the overall quality of the evidence for this outcome (failure to improve clinically) was moderate due to sparse data (153 events; See Table 3).
Two studies provided dichotomous data regarding the failure to adhere to medication regimen at study endpoint (Kamm 2007; Lichtenstein 2007). The pooled analysis of the ITT population for the two studies included 358 patients. The pooled RR was 1.36 (95% CI 0.64 to 2.86; I2 = 34%) showing no statistically significant difference in medication adherence between once daily dosing and conventional dosing at eight weeks (P = 0.42). The GRADE analysis indicated that the overall quality of the evidence for this outcome (failure to improve clinically) was low due to very sparse data (26 events; See Table 3). Flourie 2013 reported on a continuous outcome for compliance with medication. There was no statistically significant difference in compliance with medication (MD ‐4.00, 95% CI ‐17.38 to 9.38).
5‐ASA versus Comparator 5‐ASA
Eleven studies (n = 1968 patients) reported treatment outcomes in terms of the failure to induce complete global or clinical remission (Kruis 1998; Farup 2001; Levine 2002; Pruitt 2002; Raedler 2004; Tursi 2004; Forbes 2005; Marakhouski 2005; Gibson 2006; Kamm 2007; Ito 2010). The Green 1998 study was not included in the pooled analysis because it enrolled patients with moderate to severe disease whereas the other studies in the pooled analysis enrolled patients with mild to moderately active ulcerative colitis. The Green 1998 study also allowed the use of rectal steroid foam to relieve active symptoms which was not allowed in the other 5‐ASA controlled studies. The overall pooled risk ratio showed no statistically significant difference in failure to enter global or clinical remission between various formulations of 5‐ASA (including Balsalazide, Pentasa, Olsalazine, MMX mesalazine; Ipocol and 5‐ASA micropellets) and comparator formulations of 5‐ASA (including Asacol, Claversal and Salofalk). Fifty per cent of patients in the 5‐ASA group failed to enter remission compared to 52% of patients in the 5‐ASA comparator group. The pooled RR of failure to induce complete global or clinical remission for all trials was 0.94 (95% CI 0.86 to 1.02; I2 = 0%; P = 0.11) using a fixed‐effect model. The GRADE analysis indicated that the overall quality of the evidence for the primary outcome (failure to induce complete global or clinical remission) was moderate due to a high risk of bias (lack of blinding) in two studies in the pooled analysis (See Table 4). However, a sensitivity analysis excluding the two high risk of bias studies (Farup 2001; Tursi 2004) produced similar results (9 studies; n = 1681). Forty‐eight per cent of patients in the 5‐ASA group failed to enter remission compared to 50% of patients in the 5‐ASA comparator group. The pooled RR for failure to induce complete global or clinical remission was 0.95 (95% CI 0.87 to 1.04; I2 = 0%; P = 0.28) using a fixed‐effect model. The Green 1998 study compared Balsalazide 6.75 g/day (n = 50) to Asacol 2.4 g/day (n = 49). At eight weeks 22% of patients in the Balsalazide group failed to enter remission compared to 45% of patients in the Asacol group (RR 0.49; 95% CI 0.27 to 0.90).
Eight studies (n = 1647 patients) reported treatment outcomes in terms of failure to induce global/clinical improvement including remission (Kruis 1998; Farup 2001; Levine 2002; Pruitt 2002; Raedler 2004; Marakhouski 2005; Gibson 2006; Kamm 2007; Ito 2010). The overall pooled RR showed no statistically significant difference in failure to improve clinically between various formulations of 5‐ASA (including Balsalazide, Pentasa, Olsalazine, MMX mesalazine; and 5‐ASA micropellets) and comparator formulations of 5‐ASA (including Asacol, Claversal, Salofalk and Pentasa). Thirty per cent of patients in the 5‐ASA group failed to improve clinically compared to 35% of patients in the 5‐ASA comparator group. The pooled RR for failure to improve clinically for all trials was 0.89 (95% CI 0.77 to 1.01; I2 = 0%; P = 0.08) using a fixed‐effect model. The GRADE analysis indicated that the overall quality of the evidence for this outcome (failure to induce clinical improvement) was moderate due to a high risk of bias (lack of blinding) in one study in the pooled analysis (See Table 4). However, a sensitivity analysis excluding the high risk of bias study (Farup 2001) produced similar results (7 studies; n = 1420). Thirty‐two per cent of patients in the 5‐ASA group failed to improve clinically compared to 35% of patients in the 5‐ASA comparator group. The pooled RR for failure to improve clinically was 0.91 (95% CI 0.79 to 1.05; I2 = 0%; P = 0.20) using a fixed‐effect model.
5‐ASA Dose Ranging
Several randomized trials have looked at dose‐ranging for various formulations of 5‐ASA (e.g. Asacol, Salofalk, Pentasa, MMX mesalamine). Two studies examined the efficacy of various doses of Salofalk or Pentasa for induction of global or clinical remission in patients with mild or moderately active ulcerative colitis (Kruis 2003; Hiwatashi 2011). Kruis 2003 found no statistically significant difference in efficacy between Salofalk 4.5 g/day compared to 3 g/day (213 patients; RR 1.35; 95% CI 0.96 to 1.89) or 1.5 g/day (212 patients; RR 0.91 (95% CI 0.69 to 1.22). Kruis 2003 found a statistically significant difference between Salofalk 3 g/day compared to 1.5 g/day. Thirty‐four per cent of patients in the 3 g/day group failed to enter remission compared to 50% of patients in the 1.5 g/day group. The pooled RR was 0.68 (95% CI 0.49 to 0.95; P = 0.02). The GRADE analysis indicated that the overall quality of the evidence for this outcome (failure to induce global or clinical remission) was low due to a high risk of bias (incomplete outcome data) and sparse data (87 events: See Table 5). Hiwatashi 2011 examined the efficacy of Pentasa 4 g/day compared to 2.25 g/day in patients with moderately active ulcerative colitis. No statistically significant difference in failure to induce remission was found between Pentasa 4 g/day and 2.25 g/day (RR 0.91; 95% CI 0.77 to 1.08).
D'Haens 2006 and Kamm 2007 investigated the efficacy of MMX mesalamine 2.4 g/day dosed once daily versus 4.8 g/day dosed once daily for induction of remission in active ulcerative colitis. The pooled analysis of the ITT population included 194 patients. Sixty‐one per cent of patients in the 4.8 g/day group failed to enter remission compared to 60% of patients in the 2.4 g/day group. The pooled RR was 1.03 (95% CI 0.82 to 1.29; I2 = 0%; P = 0.80) showing no statistically significant difference between the 4.8 g and 2.4 g/day groups. The GRADE analysis indicated that the overall quality of the evidence for this outcome (failure to induce global or clinical remission) was moderate due to sparse data (118 events: See Table 5).
Six studies examined the efficacy of various doses of Asacol for global/clinical improvement including remission in patients with mild or moderately active ulcerative colitis (Schroeder 1987; Miglioli 1990; Sninsky 1991; Hanauer 2005; Hanauer 2007; Sandborn 2009). Schroeder 1987 found 4.8 g/day Asacol to be significantly more effective than 1.6 g/day for induction of clinical improvement (49 patients; RR 0.36; 95% CI 0.19 to 0.69). The GRADE analysis indicated that the overall quality of the evidence for this outcome (failure to induce clinical improvement) was low due sparse data (18 events from one small study: See Table 5). Miglioli 1990 found no statistically significant difference in efficacy between Asacol 3.6 g/day compared to 2.4 g/day (48 patients; RR 0.70; 95% CI 0.32 to 1.53) or 1.2 g/day (49 patients; RR 0.61 (95% CI 0.29 to 1.28). A pooled analysis of two studies (Miglioli 1990; Sninsky 1991: n = 155 patients) found no statistically significant difference between Asacol 2.4 g/day and 1.6g or 1.2 g/day (RR0.92; 95% CI 0.70 to 1.21). A pooled analysis of the ASCEND (I, II and III, n = 1459 patients) studies found no statistically significant difference in clinical improvement between Asacol 4.8 g/day and 2.4 g/day. Thirty‐seven per cent of patients in the 4.8 g/day group failed to improve clinically compared to 41% of patients in the 2.4 g/day group (RR 0.89; 95% CI 0.78 to 1.01; I2 = 0%; P = 0.08). The GRADE analysis indicated that the overall quality of the evidence for this outcome (failure to induce clinical improvement) was high (See Table 5). Subgroup analyses indicated that patients with moderate disease may benefit from the higher dose of 4.8 g/day (Hanauer 2005; Hanauer 2007), particularly among patients previously treated with corticosteroids, oral 5‐ASA, rectal therapies or multiple ulcerative colitis medications (Hanauer 2005; Hanauer 2007; Sandborn 2009).
Kamm 2007 provided data regarding the failure to induce global/clinical remission or improvement. The ITT population included 169 patients. Thirty‐five per cent of patients in the 4.8 g/day group failed to improve clinically compared to 39% of patients in the 2.4 g/day group. The RR was 0.90 (95% CI 0.61 to 1.33; P = 0.59) showing no statistically significant difference between the 4.8 g and 2.4 g/day groups.
Hiwatashi 2011 examined the efficacy of Pentasa 4 g/day compared to 2.25 g/day in patients with moderately active ulcerative colitis. Twenty‐five per cent of patients in the 4 g/day group failed to improve clinically compared to 57% of patients in the 2.25 g/day group (RR 0.44; 95% CI 0.27 to 0.71; P < .001). The GRADE analysis indicated that the overall quality of the evidence for this outcome (failure to induce clinical improvement) was moderate due sparse data (51 events; See Table 5).
SAFETY
Three different outcome measures were used to evaluate safety: the proportion of patients with adverse events, the proportion of patients withdrawing due to adverse events, and the total number of patients excluded or withdrawn before completion of the study. Since many studies only reported the total number of adverse events rather than the number of patients who experienced an event, we were often unable to include such data in the analysis.
5‐ASA versus Placebo
Eight studies (n = 1218 patients) reported the proportion of patients who experienced at least one adverse event (Hetzel 1986; Schroeder 1987; Feurle 1989; Lichtenstein 2007; Scherl 2009; Ito 2010; Feagan 2013; Pontes 2014). There was no statistically significant difference in the incidence of adverse events between 5‐ASA and placebo patients. Fifty‐two per cent of 5‐ASA patients experienced at least one adverse event compared to 49% of placebo patients (RR 0.95; 95% CI 0.85 to 1.07; I2 = 0%; P = 0.43). Three trials that involved Asacol® (Schroeder 1987: Ito 2010; Feagan 2013), had a pooled RR of 1.03 (95% CI 0.87 to 1.21). Two studies that involved Olsalazine (Hetzel 1986; Feurle 1989), had a pooled RR of 1.09 (95% CI 0.55 to 2.15). The GRADE analysis indicated that the overall quality of the evidence for this outcome for the placebo‐controlled studies (the proportion of patients who experienced at least one adverse event) was high (See Table 1).
Thirteen studies (n = 2372 patients) reported the proportion of patients withdrawn due to adverse events (Hetzel 1986; Schroeder 1987; Robinson 1988; Feurle 1989; Zinberg 1990; Sninsky 1991; Hanauer 1993; Hanauer 1996; Kamm 2007; Lichtenstein 2007; Scherl 2009; Ito 2010; Feagan 2013). There was a statistically significant difference in withdrawal due to adverse events favoring 5‐ASA over placebo patients. Withdrawals due to adverse events were reported for 6% of 5‐ASA patients compared to 9% of placebo patients (RR 0.72; 95% CI 0.54 to 0.97; I2 = 13%; P = 0.03). The pooled analysis of five Asacol® trials (Schroeder 1987; Sninsky 1991; Kamm 2007; Ito 2010; Feagan 2013) showed that a significantly higher proportion of placebo patients (9.7%) were withdrawn due to adverse events compared to Asacol® patients (3.5%) (RR 0.50; 95% CI 0.30 to 0.84) . However, when five olsalazine studies (Hetzel 1986; Robinson 1988; Feurle 1989; Zinberg 1990; Hanauer 1996) were pooled a significantly higher proportion of olsalazine patients (8.8%) were withdrawn due to adverse events compared to placebo (3.3%) (RR 2.58; 95% CI 1.16 to 5.70). When two MMX mesalamine studies were pooled (Lichtenstein 2007; Kamm 2007) a significantly higher proportion of placebo patients (8.8%) were withdrawn due to adverse events compared to MMX mesalamine (2.6%) (RR 0.30; 95% CI 0.12 to 0.72). An inspection of the forest plot showed that the statistically significant difference in withdrawals favoring 5‐ASA over placebo was driven by the large Feagan 2013 study, which reported that worsening of ulcerative colitis was the most common adverse event leading to withdrawal. Worsening of ulcerative colitis leading to withdrawal was reported for 10 of 12 withdrawals in the 5‐ASA group compared to 30 of 30 withdrawals in the placebo group (Feagan 2013). A sensitivity analysis excluding the Feagan 2013 study showed no statistically significant difference in withdrawals due to adverse events between 5‐ASA and placebo. Withdrawals due to adverse events occurred in 5.6% of 5‐ASA patients compared to 6% of placebo patients (RR 0.88; 95% CI 0.62 to 1.24; I2 = 5%; P = 0.46). A GRADE analysis indicated that the overall quality of the evidence for this outcome for the placebo‐controlled studies (the proportion of patients withdrawn due to adverse events) was moderate due to sparse data (122 events; See Table 1).
Fifteen studies (n = 2529 patients) reported the proportion of patients excluded or withdrawn after entry (Hetzel 1986; Schroeder 1987; Robinson 1988; Feurle 1989; Sutherland 1990; Zinberg 1990; Sninsky 1991; Hanauer 1993; Hanauer 1996; Kamm 2007; Lichtenstein 2007; Scherl 2009; Ito 2010; Feagan 2013; Pontes 2014). Significantly fewer 5‐ASA patients were withdrawn or excluded after entry than placebo patients. Twenty‐four per cent of 5‐ASA patients were withdrawn or excluded after entry compared to 37% of placebo patients (RR 0.61; 95% CI 0.51 to 0.72; I2 = 37%; P < 0.00001; See Analysis 1.8). However, the studies were heterogeneous (P = 0.04; I2 = 37%) and this result should be interpreted with caution.
1.8. Analysis.
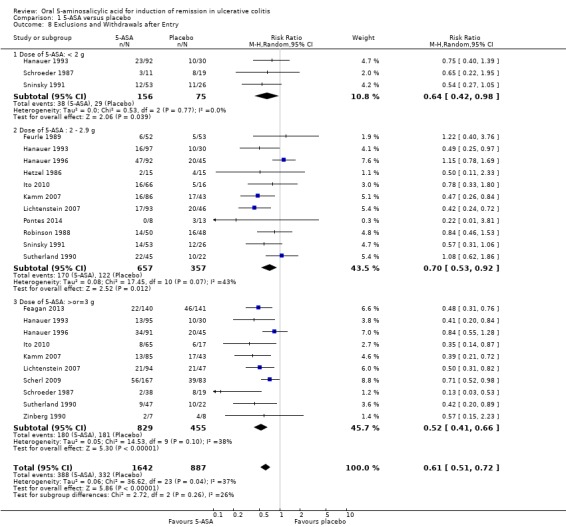
Comparison 1 5‐ASA versus placebo, Outcome 8 Exclusions and Withdrawals after Entry.
Commonly reported adverse events included: headache (Hetzel 1986; Schroeder 1987; Sutherland 1990; Sninsky 1991; Hanauer 1993; Kamm 2007; Scherl 2009; Pontes 2014), nausea (Hetzel 1986; Schroeder 1987; Feurle 1989; Hanauer 1993; Kamm 2007; Scherl 2009), abdominal pain or cramps (Schroeder 1987; Feurle 1989; Sutherland 1990; Hanauer 1993; Kamm 2007; Pontes 2014), nasopharyngitis or symptoms of upper respiratory infection (Sutherland 1990; Kamm 2007; Scherl 2009; Ito 2010), rash (Hetzel 1986; Zinberg 1990; Sninsky 1991; Hanauer 1993), anorexia or loss of appetite (Hetzel 1986; Feurle 1989; Hanauer 1993), flatulence or gas ( Schroeder 1987; Sninsky 1991; Kamm 2007), dizziness (Schroeder 1987; Kamm 2007), gastrointestinal disorders (Feagan 2013) and fever (Schroeder 1987; Hanauer 1993). Diarrhea was reported in four studies involving Olsalazine (Robinson 1988; Feurle 1989; Zinberg 1990; Hanauer 1996) and one study of Pentasa (Hanauer 1993).
5‐ASA versus Sulfasalazine Twelve studies (n = 909 patients) reported the proportion of patients who experienced at least one adverse event (Ewe 1988; Fleig 1988; Mihas 1988; Rachmilewitz 1989; Rao 1989; Bresci 1990; Rijk 1991; Munakata 1995; Cai 2001; Green 2002; Mansfield 2002; Qian 2004). It should be noted that with two exceptions (Mihas 1988; Rao 1989), the inclusion criteria for entry included tolerance of SASP. Nevertheless, SASP patients were significantly more likely than 5‐ASA patients to experience an adverse event. Fifteen per cent of 5‐ASA patients experienced at least one adverse event compared to 29% of SASP patients (RR 0.48; 95% CI 0.36 to 0.63; I2 = 0%; P < 0.00001). Five olsalazine trials (Ewe 1988; Rao 1989; Rijk 1991; Cai 2001; Qian 2004) had a combined RR of 0.48 (95% CI 0.32 to 0.71) and two balsalazide trials (Green 2002; Mansfield 2002) had a combined RR of 0.16 (95% CI 0.05 to 0.52).The GRADE analysis indicated that the overall quality of the evidence for this outcome for the SASP‐controlled studies (the proportion of patients who experienced at least one adverse event) was moderate due to sparse data (188 events; See Table 2).
Ten studies (n = 640 patients) reported the proportion of patients withdrawn due to adverse events (Ewe 1988; Fleig 1988; Mihas 1988; Riley 1988; Willoughby 1988; Rachmilewitz 1989; Rao 1989; Green 2002; Mansfield 2002; Qian 2004). SASP resulted in a significantly higher proportion of patients withdrawn due to adverse events. Thirteen per cent of SASP patients were withdrawn due to adverse events compared to 5% of 5‐ASA patients (RR 0.40; 95% CI 0.24 to 0.68; I2 = 0%; P = 0.0006). When four olsalazine trials were combined (Ewe 1988; Willoughby 1988; Rao 1989; Qian 2004), the RR was 0.63 (95% CI 0.24 to 1.66). The pooling of two balsalazide trials (Green 2002; Mansfield 2002) had a combined RR of 0.16 (95% CI 0.05 to 0.52). The GRADE analysis indicated that the overall quality of the evidence for this outcome for the SASP‐controlled studies (the proportion of patients withdrawn due to adverse events) was moderate due to sparse data (52 events; See Table 2).
Ten studies (n = 701 patients) reported the proportion of patients excluded or withdrawn after entry (Andreoli 1987; Fleig 1988; Riley 1988; Willoughby 1988; Rachmilewitz 1989; Rao 1989; Rijk 1991; Munakata 1995; Green 2002; Mansfield 2002). Twenty‐six per cent of SASP patients were withdrawn or excluded after entry compared to 19% of 5‐ASA patients (RR 0.76; 95% CI 0.58 to 0.99; I2 = 28%; P = 0.04).
Commonly reported adverse events included: nausea (Ewe 1988; Fleig 1988; Riley 1988; Willoughby 1988; Rachmilewitz 1989; Rao 1989; Good 1992; Green 2002; Mansfield 2002; Jiang 2004), headache (Ewe 1988; Riley 1988; Willoughby 1988; Rachmilewitz 1989; Green 2002; Mansfield 2002), dyspepsia (Riley 1988; Rao 1989; Bresci 1990; Green 2002; Mansfield 2002; Jiang 2004), vomiting (Fleig 1988; Riley 1988; Rachmilewitz 1989; Mansfield 2002), abdominal pain (Rachmilewitz 1989; Green 2002; Mansfield 2002), and rash (Willoughby 1988; Rachmilewitz 1989; Mansfield 2002), Diarrhea was reported in three studies involving olsalazine (Ewe 1988; Willoughby 1988; Jiang 2004).
Once Daily Dosing versus Conventional Dosing
Three studies (n = 769 patients) reported the proportion of patients who experienced at least one adverse event (Lichtenstein 2007; Kruis 2009; Flourie 2013). There was no statistically significant difference in the incidence of adverse events between once daily and conventionally dosed patients. Thirty‐three per cent of patients who were dosed once daily experienced at least one adverse event compared to 37% of conventionally dosed patients (RR 0.88; 95% CI 0.73 to 1.07; I2 = 0%; P = 0.20). The GRADE analysis indicated that the overall quality of the evidence for this outcome (the proportion of patients who experienced at least one adverse event) was moderate due to sparse data (271 events; See Table 3).
Four studies (n = 940 patients) reported the proportion of patients withdrawn due to adverse events (Kamm 2007; Lichtenstein 2007; Kruis 2009). There was no statistically significant difference in the proportion of patients withdrawn due to adverse events between once daily and conventionally dosed patients. Two per cent of conventionally dosed patients were withdrawn due to adverse events compared to 1% of patients dosed once daily (RR 0.58; 95% CI 0.23 to 1.44; I2 = 0%; P = 0.24). The GRADE analysis indicated that the overall quality of the evidence for this outcome (the proportion of patients withdrawn due to adverse events) was low due to very sparse data (9 events; See Table 3).
Four studies (n = 738 patients) reported on the proportion of patients excluded or withdrawn after entry (Kamm 2007; Lichtenstein 2007; Kruis 2009; Flourie 2013). There was no statistically significant difference in the proportion of patients excluded or withdrawn after entry between once daily and conventionally dosed patients. Fourteen per cent of patients dosed once daily patients were excluded or withdrawn after entry compared to 14% of conventionally dosed patients (RR 1.02; 95% CI 0.74 to 1.39; I2 = 0%; P = 0.92). Common adverse events included flatulence (Kamm 2007; Lichtenstein 2007), abdominal pain (Kamm 2007; Flourie 2013), nausea (Kamm 2007; Lichtenstein 2007; Flourie 2013), diarrhea (Lichtenstein 2007), nasopharyngitis (Kruis 2009), dyspepsia (Lichtenstein 2007), headache (Kamm 2007; Lichtenstein 2007; Kruis 2009; Flourie 2013) and worsening ulcerative colitis (Lichtenstein 2007; Kruis 2009; Flourie 2013).
5‐ASA versus Comparator 5‐ASA
Nine studies (n = 1576 patients) reported the proportion of patients who experienced at least one adverse event (Kruis 1998; Levine 2002; Pruitt 2002; Raedler 2004; Tursi 2004; Forbes 2005; Marakhouski 2005; Gibson 2006; Ito 2010). The overall pooled relative risk showed no difference in the incidence of adverse events between various formulations of 5‐ASA (including Balsalazide, Pentasa, Olsalazine, Ipocol and 5‐ASA micropellets) and comparator formulations of 5‐ASA (including Asacol, Claversal and Salolafk). Forty‐six per cent of patients in the 5‐ASA group experienced at least one adverse event compared to 46% of patients in the 5‐ASA comparator group (RR 1.01; 95% CI 0.92 to 1.12; I2 = 10%; P = 0.81). The GRADE analysis indicated that the overall quality of the evidence for this outcome (the proportion of patients who experienced at least one adverse event) was moderate due to a high risk of bias (lack of blinding) in one study in the pooled analysis (See Table 4).
Nine studies (n = 1489 patients) reported the proportion of patients withdrawn due to adverse event (Kruis 1998; Levine 2002; Pruitt 2002; Raedler 2004; Tursi 2004; Forbes 2005; Marakhouski 2005; Kamm 2007; Ito 2010). The overall pooled relative risk showed no difference in withdrawal due to adverse events between various formulations of 5‐ASA (including Balsalazide, Pentasa, Olsalazine, MMX mesalazine; Ipocol and 5‐ASA micropellets) and comparator formulations of 5‐ASA (including Asacol, Claversal and Salolafk). Four per cent of patients in the 5‐ASA group were withdrawn due to adverse events compared to 4% of patients in the 5‐ASA comparator group (RR 0.94: 95% CI 0.57 to 1.54; I2 = 15%; P = 0.79). The GRADE analysis indicated that the overall quality of the evidence for this outcome (the proportion of patients withdrawn due to adverse events) was moderate due to sparse data (57 events; See Table 4).
Ten studies (n = 1574 patients) reported the proportion of patients excluded or withdrawn after entry (Kruis 1998; Levine 2002; Pruitt 2002; Raedler 2004; Tursi 2004; Forbes 2005; Marakhouski 2005; Gibson 2006; Kamm 2007; Ito 2010). The overall pooled relative risk showed no difference in exclusions or withdrawals after entry between various formulations of 5‐ASA (including Balsalazide, Pentasa, Olsalazine, MMX mesalazine; Ipocol and 5‐ASA micropellets) and comparator formulations of 5‐ASA (including Asacol, Claversal and Salolafk). Eighteen per cent of patients in the 5‐ASA group were excluded or withdrawn after entry compared to 18% of patients in the 5‐ASA comparator group (RR 0.99: 95% CI 0.80 to 1.22; I2 = 0%; P = 0.91)
Common adverse events included headache (Green 1998; Levine 2002; Pruitt 2002; Raedler 2004; Gibson 2006; Kamm 2007), abdominal pain (Green 1998; Kruis 1998; Levine 2002; Pruitt 2002; Raedler 2004; Tursi 2004; Gibson 2006; Kamm 2007), nausea (Green 1998; Kruis 1998; Levine 2002; Pruitt 2002; Raedler 2004; Gibson 2006; Kamm 2007), flatulence (Kruis 1998; Pruitt 2002; Raedler 2004; Kamm 2007) diarrhea (Kruis 1998), nasopharyngitis (Gibson 2006; Ito 2010), dyspepsia (Green 1998; Kruis 1998), vomiting (Green 1998; Kruis 1998; Pruitt 2002) and worsening ulcerative colitis (Levine 2002).
5‐ASA Dose Ranging
Three dose‐ranging studies reported the proportion of patients who experienced at least one adverse event (Schroeder 1987; Kruis 2003; Hiwatashi 2011). Kruis 2003 found no statistically significant difference in the proportion of patients who experienced at least one adverse event between Salofalk 4.5 g/day compared to 3 g/day (213 patients; RR 0.96; 95% CI 0.78 to 1.20) 1.5 g/day (212 patients; RR 0.96 (95% CI 0.77 to 1.19) or between 3 g and 1.5 g/day (RR 1.04; 95% CI 0.84 to 1.29). Hiwatashi 2011 found no statistically significant difference in the proportion of patients who experienced at least one adverse event between Pentasa 4 g/day and 2.25 g/day (RR 0.93; 95% CI 0.78 to 1.11). Schroeder 1987 found no statistically significant difference in the proportion of patients who experienced at least one adverse event between Asacol 4.8 g/day and 1.6 g/day (RR 0.76; 95% CI 0.48 to 1.21).
Five dose‐ranging studies reported the proportion of patients who were withdrawn due to adverse events (Schroeder 1987; Sninsky 1991; Kruis 2003; Hanauer 2005; Hiwatashi 2011). No statistically significant differences in withdrawal due to adverse events were found between Asacol 4.8 g/day and 2.4 g/day (RR 0.93; 95% CI 0.24 to 3.63); Asacol 4.8 g/day and 1.6 g/day (RR 0.29; 95% CI 0.02 to 4.26); Asacol 2.4 g/day and 1.6 g/day (RR 5.00; 95% CI 0.25 to 101.73); Salofalk 4.5 g/day and 3 g/day (RR 1.30; 95% CI 0.50 to 3.36); Salofalk 4.5 g/day and 1.5 g/day (RR 0.80; 95% CI 0.34 to 1.84); Salofalk 3 g/day and 1.5 g/day (RR 0.61; 95% CI 0.25 to 1.52); and Pentasa 4 g/day and 2.25 g/day (RR 0.21; 95% CI 0.01 to 4.28).
Six dose‐ranging studies reported the proportion of patients who were excluded or withdrawn after entry (Schroeder 1987; Miglioli 1990; Sninsky 1991; Kruis 2003; Hanauer 2005; Hiwatashi 2011). A statistically significant difference was found between Salofalk 3 g/day and 1.5 g/day (RR 0.61; 95% CI 0.38 to 0.99). No other statistically significant differences were found in exclusions or withdrawals after entry between Asacol 4.8 g/day and 2.4 g/day (RR 0.68; 95% CI 0.40 to 1.16); Asacol 4.8 g/day and 1.6 g/day (RR 0.19; 95% CI 0.04 to 1.01); Asacol 3.6 g/day and 2.4 g/day (RR 0.50; 95% CI 0.10 to 2.48); Asacol 3.6 g/day and 1.2 g/day (RR 0.42; 95% CI 0.09 to 1.95) Asacol 2.4 g/day and 1.6 or 1.2 g/day (RR 1.07; 95% CI 0.60 to 1.92); Salofalk 4.5 g/day and 3 g/day (RR 1.01; 95% CI 0.59 to 1.74); Salofalk 4.5 g/day and 1.5 g/day (RR 0.62; 95% CI 0.38 to 0.99); and Pentasa 4 g/day and 2.25 g/day (RR 0.53; 95% CI 0.24 to 1.14).
The most common adverse event reported in the D'Haens 2006 study was headache. Other less frequent adverse events included diarrhea, nausea and abdominal pain. Adverse events for the Kamm 2007 study which included two different dose groups for once daily MMX mesalamine (2.4 g/day and 4.8 g/day), an Asacol reference arm and a placebo group are reported above.
Discussion
This systematic review largely confirms the results of previous meta‐analyses (Sutherland 1993; Sutherland 1997; Sutherland 2006b; Feagan 2012), but differs from the previous work in a variety of aspects. The 2006 version of this review included 21 studies and 2124 patients. The 2012 version of this review included 48 studies and 7776 patients. This updated review includes new data from five studies. Three of the new studies compared 5‐ASA to placebo, one study compared SASP to 5‐ASA and one study compared once daily 5‐ASA to a conventional dosing regimen. The updated review includes 53 studies and 8548 patients which greatly increases statistical power. Whenever possible the data concerning complete remission versus improvement/remission were separated. Different quality assessment criteria (i.e. the Cochrane risk of bias tool) were used in the current and 2012 version of the review. The current and the 2012 version of the review also utilized the GRADE criteria (Guyatt 2008; Schünemann 2011) to assess the overall quality of the data obtained from the randomized studies included in the review.
Unfortunately, there are some limitations to making general conclusions. Almost every study utilized a unique clinical or endoscopic index. Unlike Crohn's disease, the lack of standard indices in ulcerative colitis prevented the collection of consistent treatment efficacy data and makes comparisons across clinical studies difficult. The use of endoscopic remission as an outcome would provide a more rigorous assessment of treatment efficacy in clinical trials. Clinicians should use a standardized approach to assess endoscopic appearance to allow for comparisons across trials. Most of the included studies were not of sufficient duration to permit documentation of endoscopic healing. As well, results were periodically obscured in several studies that failed to specify the treatment arm to which certain excluded patients were initially randomised. Despite these and other common factors that must be considered when interpreting meta‐analyses, the data provided strong evidence that pointed towards a number of conclusions.
The effectiveness of oral 5‐ASA preparations for the treatment of mild‐to‐moderate active ulcerative colitis was confirmed. Oral 5‐ASA is superior to placebo for induction of remission and clinical improvement in patients with active mild to moderate ulcerative colitis.The number needed to treat in order for one patient to benefit from treatment is nine patients. The quality of the placebo‐controlled trials was assessed using the Cochrane risk of bias tool and the possibility of bias was rated as low for these studies. The outcomes induction of remission and clinical improvement were rated as 'high' and 'moderate' respectively using the GRADE criteria indicating that further research is unlikely to change our confidence in the point estimates of effect. In support of our previous conclusions, we observed the dose‐responsiveness of 5‐ASA when compared to placebo. The efficacy of oral 5‐ASA increases with dose. The trend was significant in terms of global/clinical improvement (including remission), but only marginally significant when the rate of complete global/clinical remission was evaluated.
As was found in our previous meta‐analysis, there was a non‐significant trend in favour of a slight benefit for the newer 5‐ASA preparations over SASP for the induction of global/clinical and endoscopic improvement (including remission). There are several points to be considered. It is possible that larger sample populations would confirm the significance of this finding, but the clinical relevance of such a difference would be debatable. Another possible explanation for the difference may be related to our use of the intention‐to‐treat principle which should benefit medications with lower dropout rates, in this case, 5‐ASA. The quality of the SASP‐controlled trials was assessed using the Cochrane risk of bias tool and the possibility of bias was rated as low for these studies. The outcomes induction of remission and clinical improvement were rated as 'moderate' (due to sparse data) and 'high' respectively using the GRADE criteria indicating that further research is unlikely to change our confidence in the point estimates of effect.
The assumption that SASP serves only as a pro‐drug to deliver 5‐ASA to its site of action has been questioned in light of the observation that increasing doses of 5‐ASA, within the dose‐response range of SASP, fail to enhance its efficacy beyond that of the standard 2 to 4 g therapeutic doses of SASP (Hayllar 1991). In active disease, a variety of 5‐ASA to SASP mass ratios were studied; doses of 5‐ASA corresponding to up to 10 g of SASP were commonly prescribed while just 2 to 4 g/day of SASP were used as controls. Despite this discrepancy, a significant superiority of 5‐ASA could not be confirmed. Furthermore, when trial arms were subdivided according to their 5‐ASA/SASP mass ratios, r (r<1/2, 1/1>r>or=1/2, r>or=1/1), no general dose trends could be detected (data not shown). It has been suggested that if an increase in the colonic concentration of 5‐ASA within the range of SASP dose‐dependence does not parallel an enhanced efficacy, then 5‐ASA is unlikely to be the only mediator of therapeutic activity (Hayllar 1991). Elucidation of the mechanisms of action of 5‐ASA, sulfapyridine, and SASP (reviewed by Greenfield 1993), corroborated with their individual clinical effects, may explain this curious finding as well as facilitate the determination of the currently unknown etiology of ulcerative colitis.
It was apparent that the newer 5‐ASA preparations were not entirely innocent of causing adverse effects in a number of patients. However, the incidence of adverse events and withdrawals due to the 5‐ASA formulations did not significantly differ from that associated with placebo. Furthermore, there were significantly more withdrawals due to adverse events with SASP than 5‐ASA.
Olsalazine caused a significantly higher proportion of withdrawals due to adverse events relative to placebo, but lower than the proportion caused by SASP. The most common adverse event attributed to olsalazine was diarrhea, an effect previously observed to occur in approximately 10% of patients receiving the drug (Ireland 1987). It should be noted that there may have been a bias in favour of SASP since many of the studies involved patients who were known to have tolerated SASP in the past. It has been suggested that protocol alterations may reduce the withdrawal rates in future trials, since encouraging patients to take olsalazine with meals appears to reduce the incidence of diarrhea to approximately 3% of patients (Jarnerot 1996); of the included olsalazine trials, only two (Hetzel 1986; Zinberg 1990) reported that patients were instructed to take their medication with meals. Mesalamine‐induced interstitial nephritis is a serious but rare adverse event (Elseviers 2004). Although there have been case reports of interstitial nephritis in IBD patients treated with 5‐ASA (Maeda 2001; Frandsen 2002; Arend 2004), there were no reports of interstitial nephritis in the studies included in this systematic review.
This meta‐analysis indicates that oral 5‐ASA administered once daily is as effective as conventional dosing (twice or three times daily) for induction therapy in mild to moderately active ulcerative colitis. The pooled analyses of induction trials showed no significant differences between once daily and conventional dosing for induction of remission (RR 0.94; 95% CI 0.83 to 1.07; P = 0.34) or clinical improvement (RR 0.74; 95% CI 0.49 to 1.10; P = 0.13). Furthermore, subgroup analyses by drug formulation (MMX mesalazine, Salofalk, Asacol and Pentasa) showed no differences in efficacy between once daily and conventional dosing for induction of remission. However, the latter results should be interpreted cautiously since only four formulations were evaluated in this analysis. We believe that the methodological basis for these conclusions is relatively sound. The quality of the trials comparing once daily to conventional dosing was assessed using the Cochrane risk of bias tool and the possibility of bias was judged to be low for these studies. The overall quality of the evidence using the GRADE approach was rated as high for the primary outcome (clinical remission) and moderate for secondary outcomes clinical improvement and adverse events due to sparse data in the pooled analyses indicating that further research might have an impact on our confidence in the estimate of effect and may change the estimate.
Furthermore, no differences between once daily and conventionally dosed oral 5‐ASA were observed for safety outcomes including the overall incidence of adverse events, withdrawal from treatment due to an adverse event or exclusions or withdrawals after entry. In keeping with the well‐established safety profile of oral 5‐ASA, most of the adverse events reported in the studies were mild to moderate in intensity. Common adverse events were gastrointestinal symptoms (e.g. flatulence, abdominal pain, nausea, and diarrhea), headache and worsening ulcerative colitis.
Important patient preference and adherence differences may exist between dosing regimens. In the study that measured patient preference the majority of patients preferred once daily dosing to conventional dosing (Kruis 2009). Although it is generally believed that administration of fewer tablets and less frequent dosing improves both efficacy and adherence, we could not demonstrate the superiority of once daily dosing for either of these outcomes. This result suggests that patient adherence does not appear to be enhanced by once daily dosing in the clinical trial setting. Several possible explanations exist for these observations, however the most plausible one concerns the unique aspects of the clinical trial environment. It is noteworthy that adherence was remarkably high in the studies that measured this outcome (Kamm 2007; Lichtenstein 2007). The pooled adherence rate was 92% in the once daily dosing group compared to 94% in the conventional dosing group. These rates likely reflect the highly supervised environment in which the studies were conducted. Adherence with medication in clinical trials is generally greater than in clinical practice, since participants are highly selected volunteers who are more likely, in general, to be adherent with drug regimens (Andrade 1995; Kane 2001; Kane 2006; Kane 2008). In addition, adherence is continuously reinforced during the clinical trial process. Thus, it may be difficult to detect differences in adherence between once daily and multiple dose regimens in this setting. Accordingly, a need exists to compare dosing regimens in large scale community‐based studies.In this regard reported adherence rates in community based studies range from 40 to 60% and are especially poor among patients in remission (Levy 1999; Kane 2001; Kane 2003; Shale 2003). However, whether once daily dosing regimens improve adherence in the community remains unknown.
Experience from other indications suggest that factors other than the dosing regimen are important for long‐term compliance (Brixner 2007; Kane 2008). Long‐term observations in ulcerative colitis patients as well as in other indications indicate that patients' and physicians' behaviors play a dominant role in adherence (Magowan 2006; Beaulieu 2009). The patient‐physician relationship should reinforce adherence through education, open communication and mutual agreement regarding the value of treatment (Kane 2008).
There does not appear to be any difference in efficacy between the various formulations of oral 5‐ASA. The overall pooled risk ratio (11 studies; n = 1968 patients) showed no statistically significant difference in failure to enter global or clinical remission between various formulations of 5‐ASA (including Balsalazide, Pentasa, Olsalazine, MMX mesalazine; Ipocol and 5‐ASA micropellets) and comparator formulations of 5‐ASA (including Asacol, Claversal and Salofalk). Forty‐eight per cent of patients in the 5‐ASA group failed to enter remission compared to 50% of patients in the 5‐ASA comparator group. The pooled risk ratio for failure to induce complete global or clinical remission for all trials was 0.94 (95% CI 0.86 to 1.02; I2 = 0%; P = 0.19) using a fixed‐effect model. The GRADE analysis indicated that the overall quality of the evidence for the primary outcome (failure to induce complete global or clinical remission) was moderate due to a high risk of bias (lack of blinding) in two studies in the pooled analysis (See Table 4). However, a sensitivity analysis excluding the two high risk of bias studies (Farup 2001; Tursi 2004) produced similar results (9 studies; n = 1681). Forty‐eight per cent of patients in the 5‐ASA group failed to enter remission compared to 50% of patients in the 5‐ASA comparator group. The pooled risk ratio for failure to induce complete global or clinical remission for all trials was 0.95 (95% CI 0.87 to 1.04; I2 = 0%; P = 0.28) using a fixed‐effect model.
To further support the conclusion that there is no difference in efficacy between 5‐ASA formulations, it should be noted that only one induction study (Green 1998) reported a difference in efficacy between two different formulations of 5‐ASA. Green 1998 reported that Balsalazide 6.75 g/day was superior to Asacol 2.4 g/day for induction of complete remission (none or mild symptoms and sigmoidoscopy score of 0 or 1) at 12 weeks. However, two similar trials did not support these findings (Levine 2002; Pruitt 2002).
Pharmacokinetic studies suggest that systemic exposure to 5‐ASA is similar for all oral 5‐ASA formulations and 5‐ASA prodrugs (Sandborn 2002a; Sandborn 2002b; Sandborn 2002c; Sandborn 2003). With the exception of olsalazine‐related diarrhea (Robinson 1988; Feurle 1989; Zinberg 1990; Hanauer 1996), there does not appear to be any difference in safety between the various formulations of oral 5‐ASA.The overall pooled relative risks showed no statistically significant differences in the incidence of adverse events, withdrawal due to adverse events or exclusions or withdrawals after entry. Thus, all of the 5‐ASA formulations can be considered safe and effective for the treatment of active ulcerative colitis, and from a practical standpoint, they can be considered therapeutically equivalent at equimolar doses (Sandborn 2002a). Treatment with sulfasalazine and olsalazine may not be preferable due to the high frequency of adverse events. When selecting among the remaining 5‐ASA formulations, physicians and patients should consider dose‐response data for 5‐ASA doses up to 4 to 4.8 g/day of 5‐ASA, adherence issues related to dose forms (size of dose form and total number of tablets or capsules per day), and price, when deciding what formulations to use ( Sandborn 2002a).
The ASCEND I, ASCEND II and ASCEND III studies compared Asacol 4.8 g/day to Asacol 2.4 g/day in patients with mild to moderately active ulcerative colitis (Hanauer 2005; Hanauer 2007), or in patients with moderately active disease (Sandborn 2009). A pooled analysis of the three studies (n = 1459 patients) showed no statistically significant difference between the dose groups in failure to induce clinical improvement. However, subgroup analyses indicated that patients with moderate disease may benefit from the higher dose of 4.8 g/day (Hanauer 2005; Hanauer 2007), particularly among patients previously treated with corticosteroids, oral 5‐ASA, rectal therapies or multiple ulcerative colitis medications (Hanauer 2005; Hanauer 2007; Sandborn 2009). Both doses appear to have similar efficacy in patients with mild disease which suggests that a dose of 2.4 g/day may be preferred for patients with mildly active disease. Hiwatashi 2011 compared Pentasa 4 g/day to Pentasa 2.25 g/day in patients with moderate disease and found a statistically significant difference in favour of the higher dose group for clinical improvement which appears to confirm the results of the ASCEND studies. Hiwatashi 2011 concluded that patients with severe symptoms such as relapse‐remitting and moderately active disease should be treated initially with 4 g/day.
A pooled analysis of two studies (n = 194 patients) comparing MMX mesalazine 4.8 g to 2.4 g day showed no statistically significant difference between the dose groups in failure to induce clinical remission or improvement suggesting that both dosage groups are efficacious in patients with mild to moderately active ulcerative colitis (D'Haens 2006; Kamm 2007). A subgroup analysis by severity did not show any advantage for the higher dose (4.8 g/day) in patients with moderate disease (Kamm 2007). However, further research may be necessary to identify patients who will benefit from varying doses of MMX mesalamine (Kamm 2007). Kruis 2003 evaluated the efficacy of three doses of Salofalk mesalamine pellets (1.5, 3.0, and 4.5 g/day) in patients with active ulcerative colitis, and found no statistically significant difference in remission rates between 4.5 g/day and 3 g/day and a statistically significant difference in remission rates between 3 g and 1.5 g/day. Kruis 2003 concluded that there was no dose response between the three dose groups and recommended the lowest effective dose (1.5 g/day) for treatment of patients with mild to moderate ulcerative colitis. Patients failing this dose might benefit from an increase to 3 g/day, but doses higher than this amount do not appear to provide any additional benefit (Kruis 2003).
Authors' conclusions
Implications for practice.
5‐ASA was superior to placebo and no more effective than SASP. It is possible that special populations of patients can benefit from 5‐ASA therapy. For example, the seminal fluid abnormalities associated with SASP can be reversed with the substitution of a 5‐ASA preparation in lieu of SASP (Riley 1987; Kjaergaard 1989). Nonetheless, it is clear that the newer 5‐ASA preparations have yet to be proven to be more clinically beneficial than SASP for the treatment of ulcerative colitis. The cost of oral 5‐ASA formulations exceeds that of SASP by three to four times. The decision to use 5‐ASA or SASP should consider tolerance to SASP and cost. Oral 5‐ASA administered once daily is as effective and safe as conventional dosing (twice or three times daily) for induction therapy in mild to moderately active ulcerative colitis. There do not appear to be any differences in efficacy or safety between the various formulations of 5‐ASA. Among patients with mildly active ulcerative colitis a dosage of 4 to 4.8 g/day does not appear to provide any additional benefit over a dosage of 2 to 2.4 g/day. Patients with severe symptoms and moderately active disease may benefit from an initial dosage of 4 to 4.8 g/day. When selecting among the various 5‐ASA formulations, physicians and patients should consider dose‐response data, adherence issues related to dose forms (size of dose form and total number of tablets or capsules per day), and price (Sandborn 2002a).
Implications for research.
Future trials comparing the efficacy of oral 5‐ASA with placebo or SASP do not appear to be justified. There is little evidence to suggest that there is a difference in efficacy between the oral 5‐ASA drugs. Future trials should look at enhancing patient adherence with medication. Adherence to therapy is important for treatment success and may be an important predictor of relapse (Kane 2003; Kane 2001). Future trials could assess whether once daily dosing regimens improve adherence in the community. Future trials may be necessary to identify patients who will benefit from varying doses of MMX mesalamine or Salofalk.
What's new
| Date | Event | Description |
|---|---|---|
| 14 June 2016 | Amended | Correction of minor error in study flow diagram |
History
Review first published: Issue 4, 1997
| Date | Event | Description |
|---|---|---|
| 9 July 2015 | New search has been performed | A new literature was conducted on 9 July 2015. New studies added |
| 9 July 2015 | New citation required but conclusions have not changed | Updated review with new authors |
Acknowledgements
Partial funding for the Cochrane IBD Group (April 1, 2016 ‐ March 31, 2018) has been provided by Crohn's and Colitis Canada (CCC).
We would like to thank the following investigators for providing additional information about their studies: Nobuo Hiwatashi, Peter Gibson
Appendices
Appendix 1. Search strategies
MEDLINE Search Strategy:
1. random$.tw.
2. factorial$.tw.
3. (crossover$ or cross over$ or cross‐over$).tw.
4. placebo$.tw.
5. single blind.mp.
6. double blind.mp.
7. triple blind.mp.
8. (singl$ adj blind$).tw.
9. (double$ adj blind$).tw.
10. (tripl$ adj blind$).tw.
11. assign$.tw.
12. allocat$.tw.
13. crossover procedure/
14. double blind procedure/
15. single blind procedure/
16. triple blind procedure/
17. randomized controlled trial/
18. or/1‐17
19. (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.)
20. 18 not 19
21. (colitis and ulcerat*).mp.
22. ulcerative colitis.mp. or exp ulcerative colitis/
23. (inflammatory bowel disease* or IBD).mp.
24. 21 or 22 or 23
25. 20 and 24
26 5‐aminosalicylic acid.mp. or exp Mesalamine/
27. Mesalazine.mp. or exp Mesalamine/
28. Sulfasalazine.mp. or exp Sulfasalazine/
29. sulphasalazine.mp. or exp Sulfasalazine/
30. 26 or 27 or 28 or 29
8. 25 and 30
EMBASE Search Strategy:
1. random$.tw.
2. factorial$.tw.
3. (crossover$ or cross over$ or cross‐over$).tw.
4. placebo$.tw.
5. single blind.mp.
6. double blind.mp.
7. triple blind.mp.
8. (singl$ adj blind$).tw.
9. (double$ adj blind$).tw.
10. (tripl$ adj blind$).tw.
11. assign$.tw.
12. allocat$.tw.
13. crossover procedure/
14. double blind procedure/
15. single blind procedure/
16. triple blind procedure/
17. randomized controlled trial/
18. or/1‐17
19. (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.)
20. 18 not 19
21. (colitis and ulcerat*).mp.
22. ulcerative colitis.mp. or exp ulcerative colitis/
23. (inflammatory bowel disease* or IBD).mp.
24. 21 or 22 or 23
25. 20 and 24
26 5‐aminosalicylic acid.mp. or exp Mesalamine/
27. Mesalazine.mp. or exp Mesalamine/
28. Sulfasalazine.mp. or exp Sulfasalazine/
29. sulphasalazine.mp. or exp Sulfasalazine/
30. 26 or 27 or 28 or 29
8. 25 and 30
Cochrane Library Search Strategy:
1. MeSH descriptor: [Colitis, Ulcerative] explode all trees
2. colitis
3. #1 or #2
4. 5‐ASA
5. 5‐aminosalicylic acid
6. Mesalamine
7. Sulfasalazine
8. Salazosulfapyridine
9. Sulphasalazine
10. #4 or #5 or #6 or #7 or #8 or #9
11. #3 and #10
Cochrane IBD Specialized Register:
1. 5‐ASA (ab/ti)
2. 5‐Amino* (ab/ti)
3. Mesala* (ab/ti)
4. Sulfa* (ab/ti)
5. Sulpha* (ab/ti)
6. 1 or 2 or 3 or 4 or 5
7. Colitis (ab/ti)
8. 6 and 7
Data and analyses
Comparison 1. 5‐ASA versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to Induce Global/Clinical Remission | 11 | 2387 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.82, 0.89] |
| 1.1 Dose of 5‐ASA: < 2 g | 3 | 231 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.84, 1.02] |
| 1.2 Dose of 5‐ASA : 2 ‐ 2.9 g | 8 | 956 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.82, 0.94] |
| 1.3 Dose of 5‐ASA: >or=3 g | 8 | 1200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.77, 0.88] |
| 2 Failure to Induce Global/Clinical Remission or Improvement | 14 | 2256 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.61, 0.75] |
| 2.1 Dose of 5‐ASA: < 2 g | 3 | 231 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.64, 0.97] |
| 2.2 Dose of 5‐ASA: 2 ‐ 2.9 g | 10 | 877 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.67, 0.88] |
| 2.3 Dose of 5‐ASA: >or=3 g | 9 | 1148 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.51, 0.65] |
| 3 Failure to Induce Endoscopic Remission | 4 | 1154 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.67, 0.89] |
| 3.1 Dose of 5‐ASA: < 2 g | 1 | 122 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.64, 1.14] |
| 3.2 Dose of 5‐ASA : 2 ‐ 2.9 g | 3 | 393 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.70, 1.05] |
| 3.3 Dose of 5‐ASA: >or=3 g | 4 | 639 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.56, 0.87] |
| 4 Failure to Induce Endoscopic Remission or Improvement | 4 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.59, 0.86] |
| 4.1 Dose of 5‐ASA: < 2 g | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Dose of 5‐ASA : 2 ‐ 2.9 g | 3 | 265 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.58, 0.92] |
| 4.3 Dose of 5‐ASA: >or=3 g | 2 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.49, 0.96] |
| 5 Development of Any Adverse Event | 8 | 1218 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.85, 1.07] |
| 5.1 Dose of 5‐ASA: < 2 g | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.74, 2.13] |
| 5.2 Dose of 5‐ASA : 2 ‐ 2.9 g | 5 | 377 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.82, 1.33] |
| 5.3 Dose of 5‐ASA: >or=3 g | 5 | 811 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.80, 1.05] |
| 6 Withdrawal from Study due to Adverse Event | 13 | 2372 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.54, 0.97] |
| 6.1 Dose of 5‐ASA: < 2 g | 3 | 231 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.19, 1.63] |
| 6.2 Dose of 5‐ASA : 2 ‐ 2.9 g | 9 | 926 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.65, 1.94] |
| 6.3 Dose of 5‐ASA: >or=3 g | 9 | 1215 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.41, 0.87] |
| 7 Withdrawal from Study due to Adverse Event (sensitivity analysis) | 12 | 2091 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.62, 1.24] |
| 7.1 Dose of 5‐ASA: < 2 g | 3 | 231 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.19, 1.63] |
| 7.2 Dose of 5‐ASA : 2 ‐ 2.9 g | 9 | 926 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.65, 1.94] |
| 7.3 Dose of 5‐ASA: >or=3 g | 8 | 934 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.47, 1.26] |
| 8 Exclusions and Withdrawals after Entry | 15 | 2529 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.51, 0.72] |
| 8.1 Dose of 5‐ASA: < 2 g | 3 | 231 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.42, 0.98] |
| 8.2 Dose of 5‐ASA : 2 ‐ 2.9 g | 11 | 1014 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.53, 0.92] |
| 8.3 Dose of 5‐ASA: >or=3 g | 10 | 1284 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.41, 0.66] |
1.1. Analysis.
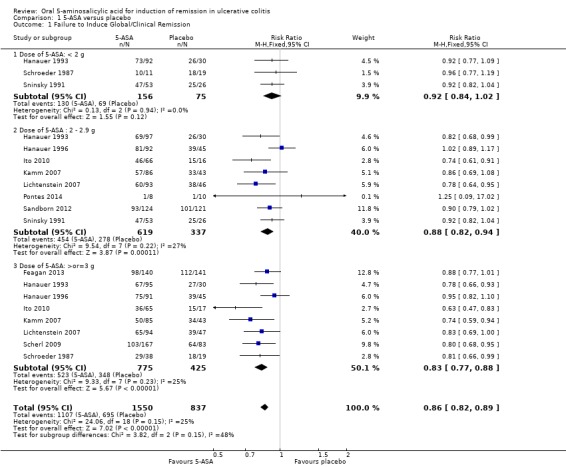
Comparison 1 5‐ASA versus placebo, Outcome 1 Failure to Induce Global/Clinical Remission.
1.2. Analysis.
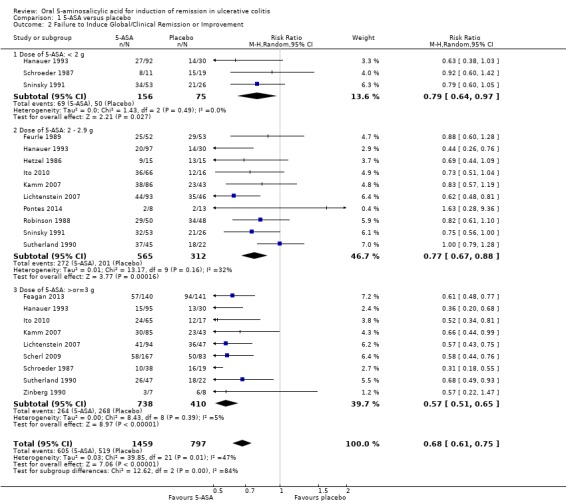
Comparison 1 5‐ASA versus placebo, Outcome 2 Failure to Induce Global/Clinical Remission or Improvement.
1.3. Analysis.
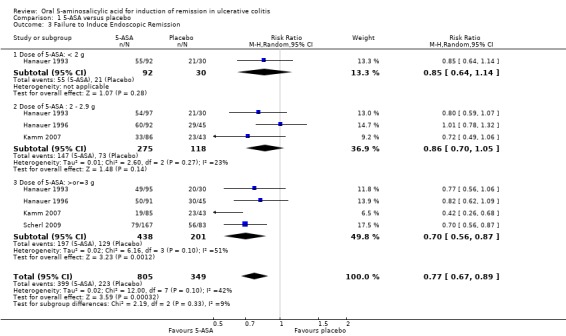
Comparison 1 5‐ASA versus placebo, Outcome 3 Failure to Induce Endoscopic Remission.
1.4. Analysis.
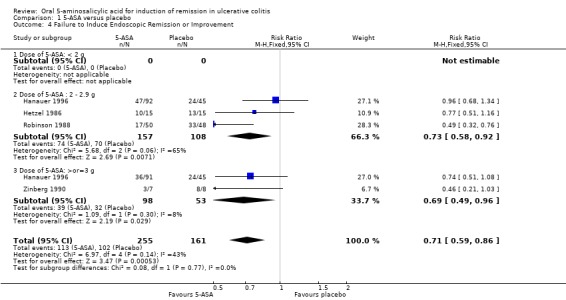
Comparison 1 5‐ASA versus placebo, Outcome 4 Failure to Induce Endoscopic Remission or Improvement.
1.5. Analysis.
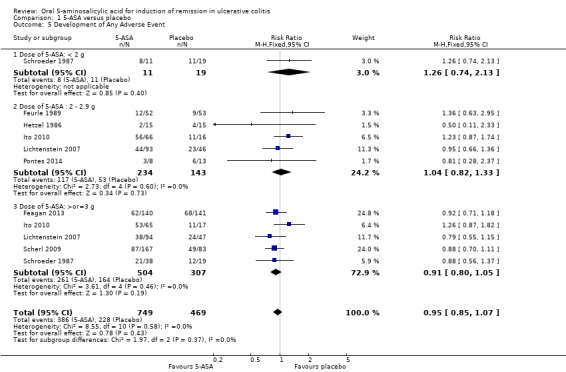
Comparison 1 5‐ASA versus placebo, Outcome 5 Development of Any Adverse Event.
1.6. Analysis.
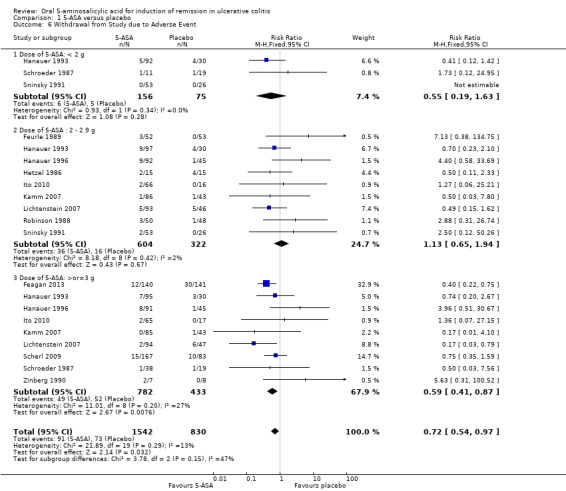
Comparison 1 5‐ASA versus placebo, Outcome 6 Withdrawal from Study due to Adverse Event.
1.7. Analysis.
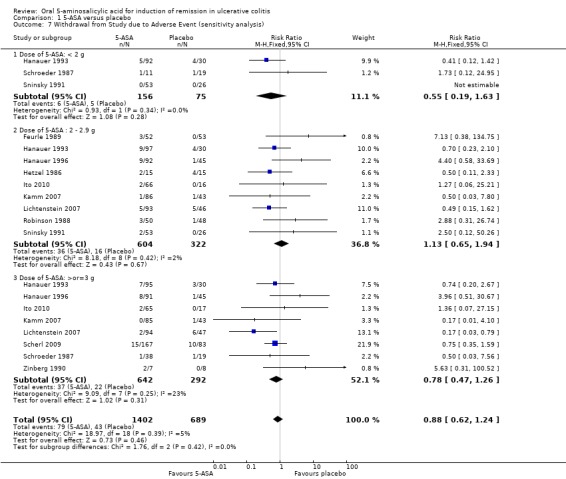
Comparison 1 5‐ASA versus placebo, Outcome 7 Withdrawal from Study due to Adverse Event (sensitivity analysis).
Comparison 2. 5‐ASA versus sulfasalazine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to Induce Global/Clinical Remission | 8 | 526 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.77, 1.04] |
| 1.1 5‐ASA / SASP <1/2 | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.9 [0.57, 1.41] |
| 1.2 1/1 > 5‐ASA / SASP >or= 1/2 | 5 | 359 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.77, 1.08] |
| 1.3 5‐ASA / SASP >or= 1/1 | 3 | 138 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.59, 1.23] |
| 2 Failure to Induce Global/Clinical Remission or Improvement | 14 | 1053 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.76, 1.01] |
| 2.1 5‐ASA / SASP <1/2 | 3 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.47, 1.27] |
| 2.2 1/1 > 5‐ASA / SASP >or= 1/2 | 11 | 804 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.77, 1.05] |
| 2.3 5‐ASA / SASP >or= 1/1 | 3 | 126 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.54, 1.22] |
| 3 Failure to Induce Endoscopic Remission | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 5‐ASA / SASP <1/2 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 1/1 > 5‐ASA / SASP >or= 1/2 | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 5‐ASA / SASP >or= 1/1 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Failure to Induce Endoscopic Remission or Improvement | 6 | 362 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.65, 1.02] |
| 4.1 5‐ASA / SASP <1/2 | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.58, 1.04] |
| 4.2 1/1 > 5‐ASA / SASP >or= 1/2 | 4 | 246 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.53, 1.02] |
| 4.3 5‐ASA / SASP >or= 1/1 | 2 | 87 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.72, 1.57] |
| 5 Development of Any Adverse Event | 12 | 909 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.36, 0.63] |
| 5.1 5‐ASA / SASP <1/2 | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.10, 1.20] |
| 5.2 1/1 > 5‐ASA / SASP >or= 1/2 | 9 | 746 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.41, 0.73] |
| 5.3 5‐ASA / SASP >or= 1/1 | 2 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.05, 0.52] |
| 6 Withdrawal from Study due to Adverse Event | 10 | 640 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.24, 0.68] |
| 6.1 5‐ASA / SASP <1/2 | 2 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.02, 1.41] |
| 6.2 1/1 > 5‐ASA / SASP >or= 1/2 | 5 | 361 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.32, 1.39] |
| 6.3 5‐ASA / SASP >or= 1/1 | 4 | 194 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.10, 0.60] |
| 7 Exclusions and Withdrawals after Entry | 10 | 701 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.58, 0.99] |
| 7.1 5‐ASA / SASP <1/2 | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 1.80] |
| 7.2 1/1 > 5‐ASA / SASP >or= 1/2 | 6 | 478 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.71, 1.34] |
| 7.3 5‐ASA / SASP >or= 1/1 | 4 | 194 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.25, 0.77] |
2.1. Analysis.
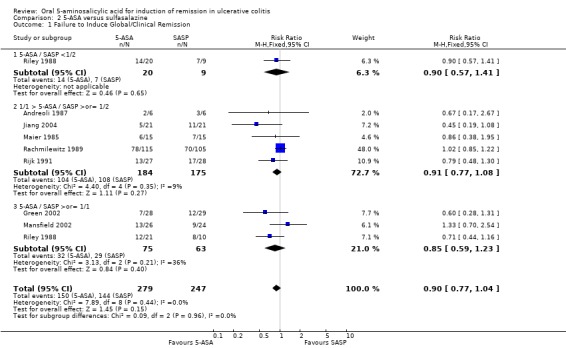
Comparison 2 5‐ASA versus sulfasalazine, Outcome 1 Failure to Induce Global/Clinical Remission.
2.2. Analysis.
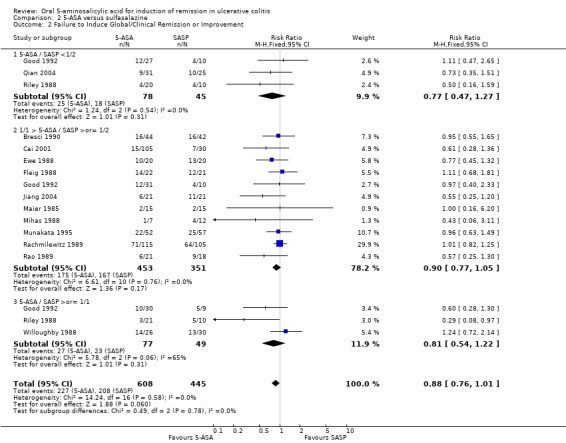
Comparison 2 5‐ASA versus sulfasalazine, Outcome 2 Failure to Induce Global/Clinical Remission or Improvement.
2.3. Analysis.
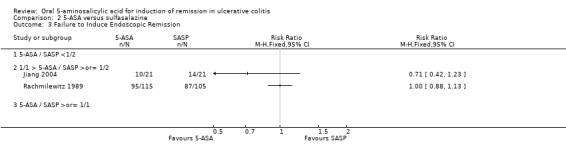
Comparison 2 5‐ASA versus sulfasalazine, Outcome 3 Failure to Induce Endoscopic Remission.
2.4. Analysis.
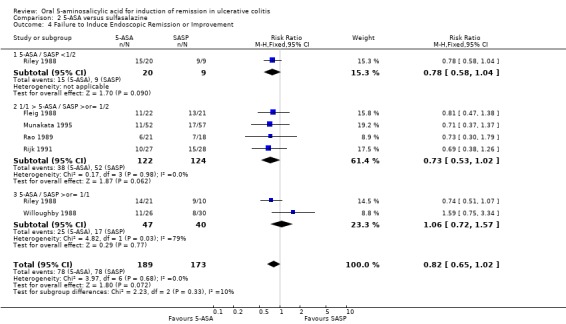
Comparison 2 5‐ASA versus sulfasalazine, Outcome 4 Failure to Induce Endoscopic Remission or Improvement.
2.5. Analysis.
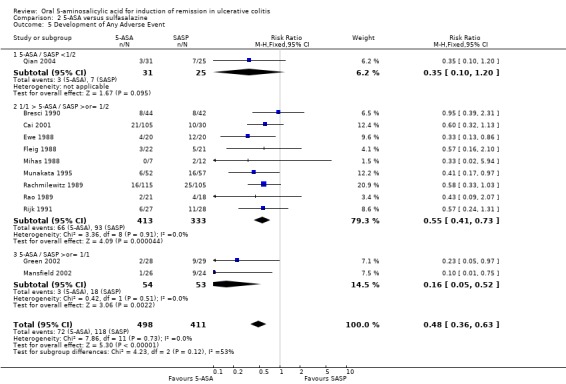
Comparison 2 5‐ASA versus sulfasalazine, Outcome 5 Development of Any Adverse Event.
2.6. Analysis.
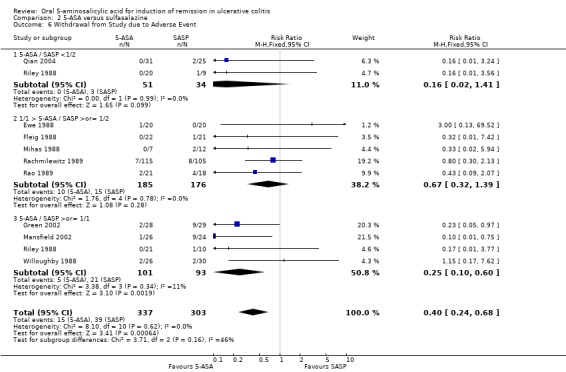
Comparison 2 5‐ASA versus sulfasalazine, Outcome 6 Withdrawal from Study due to Adverse Event.
2.7. Analysis.
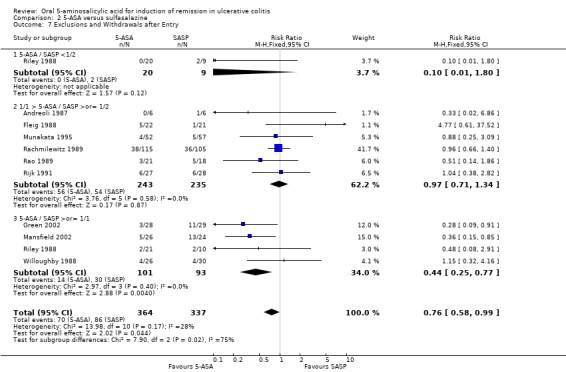
Comparison 2 5‐ASA versus sulfasalazine, Outcome 7 Exclusions and Withdrawals after Entry.
Comparison 3. Once daily dosing versus conventional dosing.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to Induce Global/Clinical Remission | 4 | 944 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.83, 1.07] |
| 1.1 MMX (OD versus BID) | 1 | 187 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.88, 1.31] |
| 1.2 Salofalk granules (OD versus TID) | 1 | 380 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.59, 1.25] |
| 1.3 MMX (OD) versus Asacol (TID) | 1 | 171 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.70, 1.12] |
| 1.4 Pentasa (OD versus BID) | 1 | 206 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.73, 1.17] |
| 2 Failure to Induce Global/Clinical Remission or Improvement | 3 | 564 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.49, 1.10] |
| 2.1 MMX (OD versus BID) | 1 | 187 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.67, 1.26] |
| 2.2 MMX (OD) versus Asacol (TID) | 1 | 171 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.55, 1.16] |
| 2.3 Pentasa (OD versus BID) | 1 | 206 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.17, 0.79] |
| 3 Failure to Induce Global/Clinical Remission or Improvement (sensitivity analysis) | 2 | 358 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.68, 1.10] |
| 3.1 MMX (OD versus BID) | 1 | 187 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.67, 1.26] |
| 3.2 MMX (OD) versus Asacol (TID) | 1 | 171 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.55, 1.16] |
| 4 Failure to adhere to medication regimen | 2 | 358 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.64, 2.86] |
| 5 Compliance | 1 | 206 | Mean Difference (IV, Fixed, 95% CI) | ‐4.0 [‐17.38, 9.38] |
| 6 Development of Any Adverse Event | 3 | 769 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.73, 1.07] |
| 7 Withdrawal from Study due to Adverse Event | 4 | 940 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.23, 1.44] |
| 8 Exclusions and Withdrawals after Entry | 4 | 944 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.74, 1.39] |
3.1. Analysis.
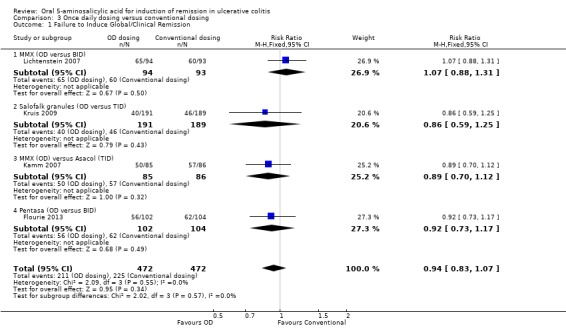
Comparison 3 Once daily dosing versus conventional dosing, Outcome 1 Failure to Induce Global/Clinical Remission.
3.2. Analysis.
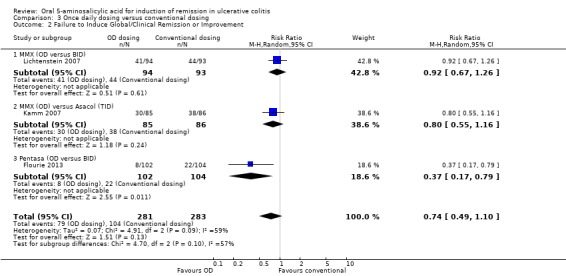
Comparison 3 Once daily dosing versus conventional dosing, Outcome 2 Failure to Induce Global/Clinical Remission or Improvement.
3.3. Analysis.
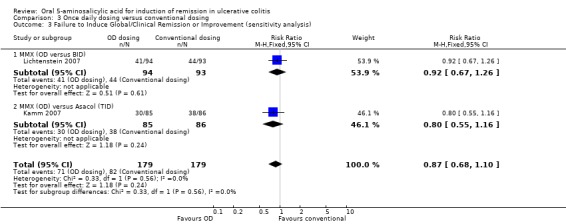
Comparison 3 Once daily dosing versus conventional dosing, Outcome 3 Failure to Induce Global/Clinical Remission or Improvement (sensitivity analysis).
3.4. Analysis.
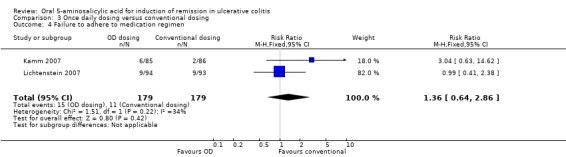
Comparison 3 Once daily dosing versus conventional dosing, Outcome 4 Failure to adhere to medication regimen.
3.5. Analysis.
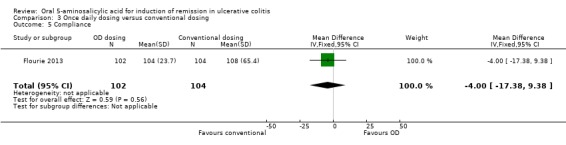
Comparison 3 Once daily dosing versus conventional dosing, Outcome 5 Compliance.
3.6. Analysis.
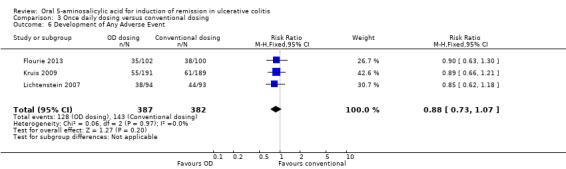
Comparison 3 Once daily dosing versus conventional dosing, Outcome 6 Development of Any Adverse Event.
3.7. Analysis.
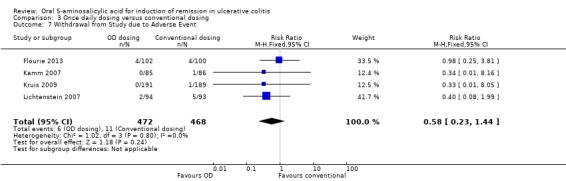
Comparison 3 Once daily dosing versus conventional dosing, Outcome 7 Withdrawal from Study due to Adverse Event.
3.8. Analysis.
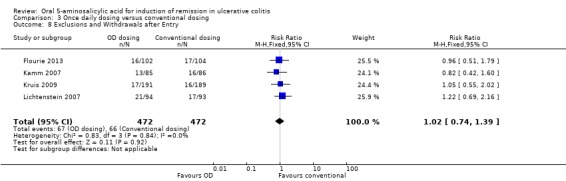
Comparison 3 Once daily dosing versus conventional dosing, Outcome 8 Exclusions and Withdrawals after Entry.
Comparison 4. 5‐ASA versus comparator 5‐ASA.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to Induce Global/Clinical Remission | 11 | 1968 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.86, 1.02] |
| 1.1 Asacol comparator | 6 | 720 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.85, 1.04] |
| 1.2 Claversal comparator | 2 | 530 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.78, 1.17] |
| 1.3 Salofalk comparator | 2 | 491 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.72, 1.18] |
| 1.4 Pentasa comparator | 1 | 227 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.74, 1.10] |
| 2 Failure to Induce Global/Clinical Remission (sensitivity analysis) | 9 | 1681 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.87, 1.04] |
| 2.1 Asacol comparator | 5 | 660 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.86, 1.07] |
| 2.2 Claversal comparator | 2 | 530 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.78, 1.17] |
| 2.3 Salofalk comparator | 2 | 491 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.72, 1.18] |
| 3 Failure to Induce Global/Clinical Remission or Improvement | 8 | 1647 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.77, 1.01] |
| 3.1 Asacol comparator | 3 | 399 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.73, 1.11] |
| 3.2 Claversal comparator | 2 | 530 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.67, 1.10] |
| 3.3 Salofalk comparator | 2 | 491 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.74, 1.36] |
| 3.4 Pentasa comparator | 1 | 227 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.45, 1.08] |
| 4 Failure to Induce Global/Clinical Remission or Improvement (sensitivity analysis) | 7 | 1420 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.79, 1.05] |
| 4.1 Asacol comparator | 3 | 399 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.73, 1.11] |
| 4.2 Claversal comparator | 2 | 530 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.67, 1.10] |
| 4.3 Salofalk comparator | 2 | 491 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.74, 1.36] |
| 5 Development of Any Adverse Event | 9 | 1576 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.92, 1.12] |
| 5.1 Asacol comparator | 5 | 556 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.80, 1.03] |
| 5.2 Claversal comparator | 2 | 530 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [1.01, 1.66] |
| 5.3 Salofalk comparator | 2 | 490 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.81, 1.20] |
| 6 Withdrawal due to adverse event | 9 | 1489 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.57, 1.54] |
| 6.1 Asacol comparator | 6 | 726 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.22, 1.04] |
| 6.2 Claversal comparator | 2 | 530 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.70, 3.14] |
| 6.3 Salofalk comparator | 1 | 233 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.90 [0.44, 34.35] |
| 7 Exclusions and withdrawals after entry | 9 | 1574 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.80, 1.22] |
| 7.1 Asacol comparator | 5 | 553 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.67, 1.24] |
| 7.2 Claversal comparator | 2 | 530 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.74, 1.63] |
| 7.3 Salofalk comparator | 2 | 491 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.67, 1.51] |
4.1. Analysis.
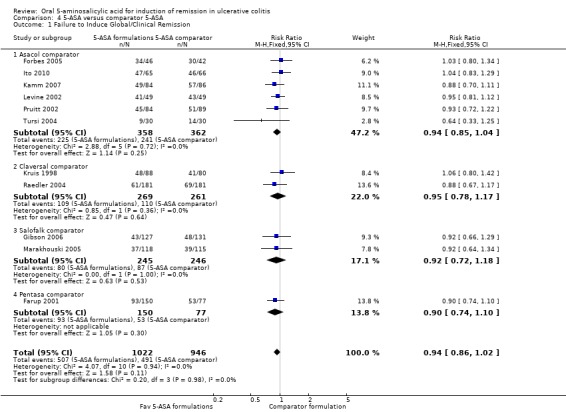
Comparison 4 5‐ASA versus comparator 5‐ASA, Outcome 1 Failure to Induce Global/Clinical Remission.
4.2. Analysis.
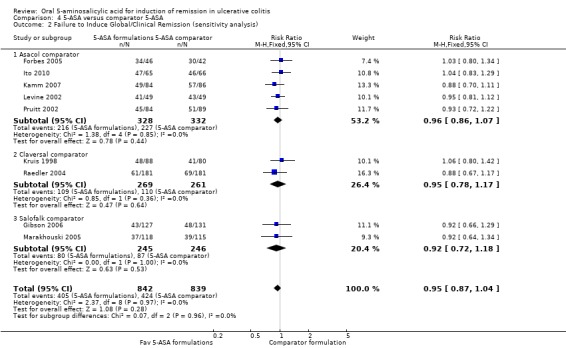
Comparison 4 5‐ASA versus comparator 5‐ASA, Outcome 2 Failure to Induce Global/Clinical Remission (sensitivity analysis).
4.3. Analysis.
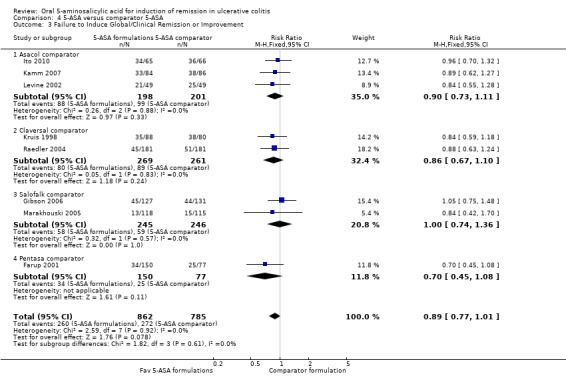
Comparison 4 5‐ASA versus comparator 5‐ASA, Outcome 3 Failure to Induce Global/Clinical Remission or Improvement.
4.4. Analysis.
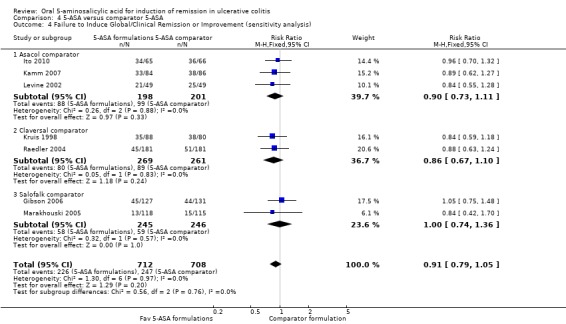
Comparison 4 5‐ASA versus comparator 5‐ASA, Outcome 4 Failure to Induce Global/Clinical Remission or Improvement (sensitivity analysis).
4.5. Analysis.
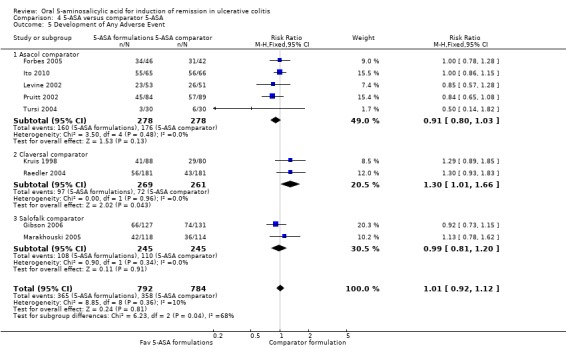
Comparison 4 5‐ASA versus comparator 5‐ASA, Outcome 5 Development of Any Adverse Event.
4.6. Analysis.
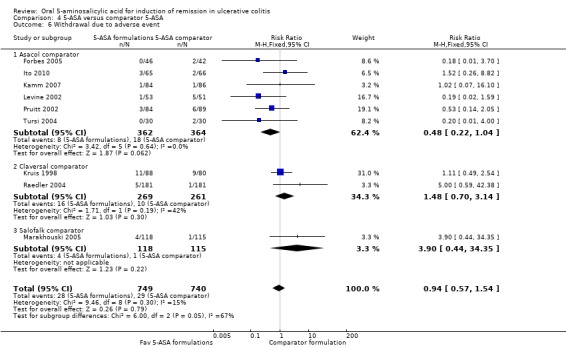
Comparison 4 5‐ASA versus comparator 5‐ASA, Outcome 6 Withdrawal due to adverse event.
4.7. Analysis.
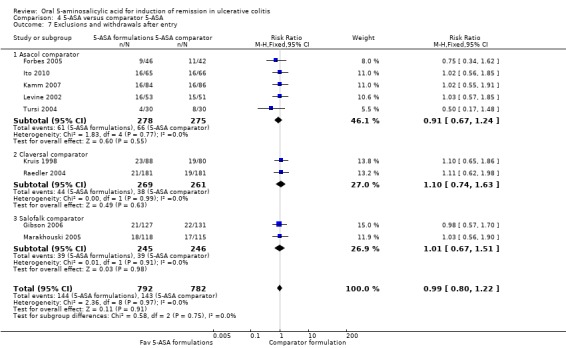
Comparison 4 5‐ASA versus comparator 5‐ASA, Outcome 7 Exclusions and withdrawals after entry.
Comparison 5. 5‐ASA dose ranging.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to Induce Global/Clinical Remission | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 MMX mesalazine 4.8 g versus 2.4 g/day | 2 | 194 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.82, 1.29] |
| 1.2 Salofalk 4.5 g versus 3 g/day | 1 | 213 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.96, 1.89] |
| 1.3 Salofalk 4.5 g versus 1.5 g/day | 1 | 209 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.69, 1.22] |
| 1.4 Salofalk 3 g versus 1.5 g/day | 1 | 210 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.49, 0.95] |
| 1.5 Pentasa 4 g versus 2.25 g/day | 1 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.77, 1.08] |
| 1.6 Asacol 3.6 g versus 2.4 g/day | 1 | 131 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.61, 1.04] |
| 2 Failure to Induce Global/Clinical Remission or Improvement | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Asacol 4.8 g versus 2.4 g/day | 3 | 1459 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.78, 1.01] |
| 2.2 MMX mesalazine 4.8 g versus 2.4 g/day | 1 | 169 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.61, 1.33] |
| 2.3 Asacol 4.8 g versus 1.6 g/day | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.19, 0.69] |
| 2.4 Asacol 3.6 g versus 2.4 g/day | 2 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.48, 0.97] |
| 2.5 Asacol 3.6 g versus 1.2 g/day | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.29, 1.28] |
| 2.6 Asacol 2.4 g versus 1.6 or 1.2 g/day | 2 | 155 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.70, 1.21] |
| 2.7 Pentasa 4 g versus 2.25 g/day | 1 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.27, 0.71] |
| 3 Development of any adverse event | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Asacol 4.8 g versus 1.6 g/day | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.48, 1.21] |
| 3.2 Salofalk 4.5 g versus 3 g/day | 1 | 213 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.78, 1.20] |
| 3.3 Salofalk 4.5 g versus 1.5 g/day | 1 | 209 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.77, 1.19] |
| 3.4 Salofalk 3 g versus 1.5 g/day | 1 | 213 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.84, 1.29] |
| 3.5 Pentasa 4 g versus 2.25 g/day | 1 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.78, 1.11] |
| 4 Withdrawal from study due to adverse event | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Asacol 4.8 g versus 2.4 g/day | 1 | 268 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.24, 3.63] |
| 4.2 Asacol 4.8 g versus 1.6 g/day | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.02, 4.26] |
| 4.3 Asacol 2.4 g versus 1.6 g/day | 1 | 106 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.25, 101.73] |
| 4.4 Salofalk 4.5 g versus 3 g/day | 1 | 213 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.50, 3.36] |
| 4.5 Salofalk 4.5 g versus 1.5 g/day | 1 | 209 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.34, 1.84] |
| 4.6 Salofalk 3 g versus 1.5 g/day | 1 | 210 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.25, 1.52] |
| 4.7 Pentasa 4 g versus 2.25 g/day | 1 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.28] |
| 5 Exclusions and withdrawals after entry | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Asacol 4.8 g versus 2.4 g/day | 1 | 386 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.40, 1.16] |
| 5.2 Asacol 4.8 g versus 1.6 g/day | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.04, 1.01] |
| 5.3 Asacol 3.6 g versus 2.4 g/day | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.10, 2.48] |
| 5.4 Asacol 3.6 g versus 1.2 g/day | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.09, 1.95] |
| 5.5 Asacol 2.4 g versus 1.6 or 1.2 g/day | 2 | 155 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.60, 1.92] |
| 5.6 Salofalk 4.5 g versus 3 g/day | 1 | 213 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.59, 1.74] |
| 5.7 Salofalk 4.5 g versus 1.5 g/day | 1 | 209 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.38, 0.99] |
| 5.8 Salofalk 3 g versus 1.5 g/day | 1 | 210 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.38, 0.99] |
| 5.9 Pentasa 4 g versus 2.25 g/day | 1 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.24, 1.14] |
5.1. Analysis.
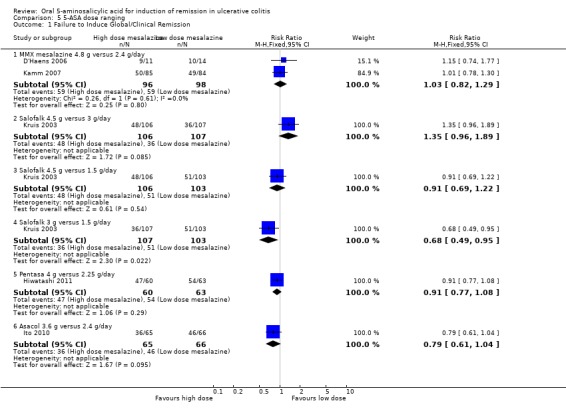
Comparison 5 5‐ASA dose ranging, Outcome 1 Failure to Induce Global/Clinical Remission.
5.2. Analysis.
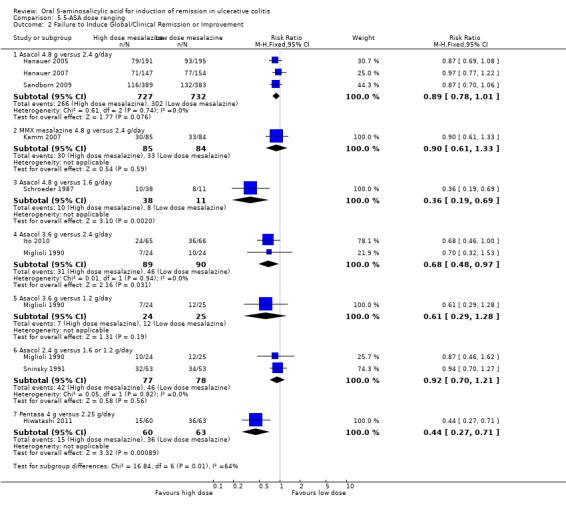
Comparison 5 5‐ASA dose ranging, Outcome 2 Failure to Induce Global/Clinical Remission or Improvement.
5.3. Analysis.
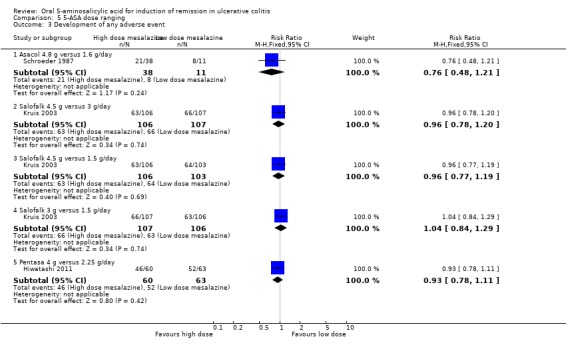
Comparison 5 5‐ASA dose ranging, Outcome 3 Development of any adverse event.
5.4. Analysis.
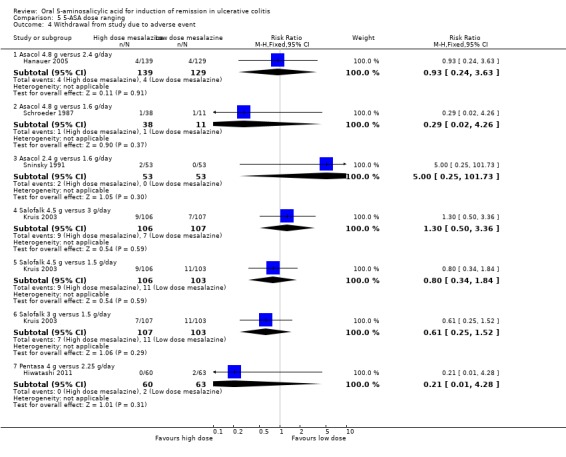
Comparison 5 5‐ASA dose ranging, Outcome 4 Withdrawal from study due to adverse event.
5.5. Analysis.
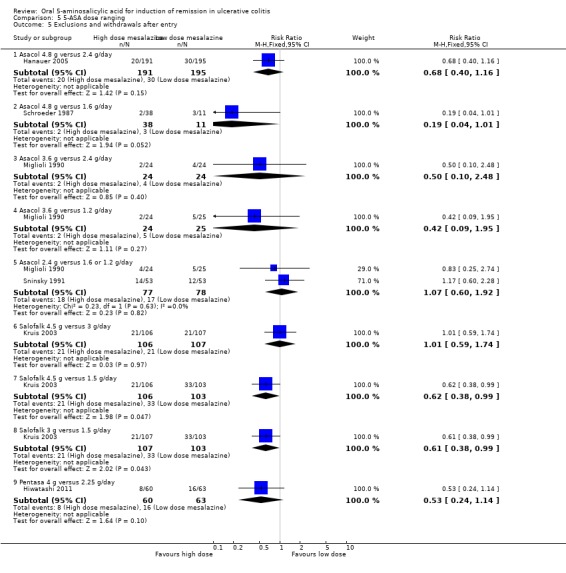
Comparison 5 5‐ASA dose ranging, Outcome 5 Exclusions and withdrawals after entry.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Andreoli 1987.
| Methods | Randomized, double‐blind trial comparing 5‐aminosalicylic acid and SASP. Allocation of drugs was performed using a table of random numbers | |
| Participants | Male and female patients, ages 19 to 63 years, with acute ulcerative colitis (N = 12) | |
| Interventions | 1.5 g/day 5‐ASA or 3 g/day SASP for 2 months | |
| Outcomes | Clinical endoscopic remission within 2 months of start of therapy was considered as a positive indication of remission induction | |
| Notes | Abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
| Selective reporting (reporting bias) | Unclear risk | Not described |
| Other bias | Unclear risk | Not described |
Bresci 1990.
| Methods | Randomized trial comparing 5‐aminosalicylic acid and SASP | |
| Participants | Adult patients with ulcerative colitis of at least two years duration with mild to moderate relapse (N = 86) | |
| Interventions | 2.4 g/day 5‐ASA (n = 44) or 3 g/day SASP (n = 42) for 6 weeks | |
| Outcomes | Clinical improvement, endoscopic and histologic appearance, indexes of phlogosis, haematic crasis, hepatic and renal functionality, and adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No drop‐outs were reported |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Cai 2001.
| Methods | Randomized controlled trial | |
| Participants | Adult patients (aged 18 to 65 years) with active ulcerative colitis (N = 135) | |
| Interventions | Olsalazine 3 g/day (n = 105) or SASP 4 g/day (n = 30) | |
| Outcomes | Clinical improvement and adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
| Selective reporting (reporting bias) | Unclear risk | Not described |
| Other bias | Unclear risk | Not described |
D'Haens 2006.
| Methods | Randomized, multicenter, double‐blind, parallel‐group, dose‐ranging study | |
| Participants | Adult patients (aged > 18 years) with histologically confirmed, newly diagnosed or relapsing mild‐to‐moderately active ulcerative colitis (N = 38) | |
| Interventions | MMX mesalazine (SPD476) 1.2 (n = 13), 2.4 (n = 14) or 4.8 g/day (n = 11) given once daily for 8 weeks | |
| Outcomes | Primary outcome: remission defined as a UC‐DAI score < 1 with a score of 0 for rectal bleeding and stool frequency, and at least a 1‐point reduction from baseline in sigmoidoscopy score. Secondary outcomes: change in UC‐DAI score, sigmoidoscopic appearance and histology from baseline to week 8, and the change in symptoms (rectal bleeding and stool frequency) from baseline to weeks 2, 4 and 8 for the three dose groups | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind: MMX mesalazine and placebo tablets were identical in appearance |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The 1.2 g/day group had 6 withdrawals (6/13) compared to 3 (3/14) in the 2.4 g/day and 1 (1/11) in the 4.8 g/day groups. LOCF was used to address incomplete outcome data |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Ewe 1988.
| Methods | Randomized, double‐blind trial comparing 5‐aminosalicylic acid (olsalazine) and SASP | |
| Participants | Adult patients with mild to moderate active chronic ulcerative colitis (N = 40) | |
| Interventions | 1.5 g/day 5‐ASA (Olsalazine) for 14 days, and followed by 3 g/day SASP for a further 14 days (n = 20), or vice versa (n = 20) | |
| Outcomes | Clinical improvement: at each study visit a physical examination was performed and a detailed history was taken. In addition, a diary completed daily by the patient was evaluated. The diary was designed to record stool frequency and consistency, and blood staining of stools. Based on these variables investigators rated the efficacy of treatment as “improved”, ”no change” or “worse” | |
| Notes | Cross‐over trial. Data for outcomes were available before crossover | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind, double‐dummy |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data available for 40 of 41 patients entered in the study |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Farup 2001.
| Methods | Randomized, open‐label, non‐inferiority study | |
| Participants | Adult patients with confirmed diagnosis of active mild to moderate ulcerative colitis (N = 227). Patients with proctitis were excluded | |
| Interventions | Pentasa sachet prolonged‐release granules two 1 g packets twice daily (n = 74), 1 packet four times daily (n = 76) or Pentasa prolonged‐release 500 mg tablets ‐ 2 tablets four times daily (n = 77) for 8 weeks | |
| Outcomes | Primary outcome: mean improvement in UC‐DAI. Secondary outcomes: remission (UC‐DAI 0 or 1), improvement (reduction in UC‐DAI of > 2 from baseline), satisfaction with regimen, adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 80 patients did not complete the study. Reasons are provided but are not attributed to individual treatment groups |
| Selective reporting (reporting bias) | Unclear risk | Expected outcomes were reported but reporting for withdrawals and adverse events was inadequate |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Feagan 2013.
| Methods | Randomized, double‐blind, placebo‐controlled, multicenter, phase 3 study | |
| Participants | Adult patients (18 years or older) with a documented diagnosis of mild to moderate UC, defined by a modified Ulcerative Colitis Disease Activity Index (UCDAI) (N = 281) | |
| Interventions | Asacol 4.8 g/day (n = 140) or placebo (n = 141) | |
| Outcomes | Primary outcome: proportion of patients in clinical remission, defined as a score of 0 for stool frequency and rectal bleeding, and absence of fecal urgency at week 6 Secondary outcomes: clinical remission at weeks 6 and 10, endoscopic remission (defined as a sigmoidoscopic score of < 1) at week 6, endoscopic remission at week 10, improvement (defined as a decrease of at least 3 points from baseline in the modified UCDAI score) at week 6, improvement at week 10, mean changes in the modified UCDAI and UCCS from baseline to week 10 and adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Generated in permutated blocks by computer |
| Allocation concealment (selection bias) | Low risk | An interactive voice/web response system managed the randomization procedure and dispensed the study drug |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Patients and central readers were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients involved in the trial were accounted for with reasons |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported in the published study |
| Other bias | Low risk | The study appears to be free of other sources biases |
Feurle 1989.
| Methods | Double‐blinded, placebo‐controlled, and centrally‐randomized with stratification in blocks of 10 for each of the 12 centres. Clinical and laboratory examinations were performed at recruitment, after 2 weeks, and at the end of 4 weeks. Endoscopy and biopsy were performed on days 0 and 28. Clinical observations were made on days 0, 14, and 28 | |
| Participants | Outpatients with mild to moderate ulcerative colitis recruited in West Germany between 1984 and 1986 (N = 105) | |
| Interventions | Olsalazine 2 g/day (4 doses of 2 gelatin capsules each; n = 52) or 8 placebo capsules with identical appearance (n = 53). Patients were advised to start with less than 8 pills and reach complete dosage by the third or fourth day and continue for 4 weeks. Compliance was verified by laboratory tests | |
| Outcomes | Endoscopic score was the mean of redness/hyperemia, contact bleeding, spontaneous bleeding and erosions each graded on a 3‐point scale. Clinical status was based on number of stools, presence of blood in stool, stool consistency, and mucous in stool. The clinical score was considered improved when at least 3 of the 4 parameters increased. Occurrence of withdrawals and side effects were also tabulated | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Centralized randomization |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind: identical placebo capsule |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐outs balanced across intervention groups with similar reasons for withdrawal |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Fleig 1988.
| Methods | Prospective, randomized, double‐blind comparison of benzalazine (SAB) and SASP. Consecutive patients were randomized. Laboratory and clinical evaluations were performed once per week, in addition to patient diaries to record number and consistency of stools, and occurrence of rectal bleeding. Endoscopy was performed at entry and after 6 weeks to determine severity of inflammation and to obtain a biopsy which was evaluated on a 4‐point scale | |
| Participants | Patients, ages 18 to 75 years, with histologically and endoscopically diagnosed ulcerative colitis for 16 months with an acute episode defined as the occurrence of diarrhea with at least 5 stools daily for at least 3 days. Endoscopic appearance was graded according to a 4‐point scale (N = 43) | |
| Interventions | Equimolar, identical‐appearing doses of either SASP (2 tablets, 3 times/day; 0.5 g per tablet; n = 21) or SAB (2 tablets, 3 x/day; 0.36 g per tablet; n = 22) for 6 weeks, except for the first week when dosage of either was 2 tablets, 4 times daily | |
| Outcomes | Efficacy was evaluated in terms of positive changes in major clinical (number and consistency of stools), sigmoidoscopic, and morphological (histologic grading of inflammation) criteria. Occurrence of side effects and withdrawals were also reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Patients received the medication assigned to their patient number according to the sequence of entry into the trial. Treatment was assigned to patient numbers by random |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind: tablets of identical appearance. Assignment was blind to both patients and treating physicians |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two patients in 5‐ASA group were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Flourie 2013.
| Methods | Multicentre, controlled, randomised, investigator‐blinded, comparative, non‐inferiority study | |
| Participants | Adult patients (18 years or older) with newly diagnosed or relapsing mild‐to‐moderate ulcerative colitis, with disease extension beyond the rectum (N = 206) | |
| Interventions | Mesalazine (4 g/day), either once daily with two sachets of 2 g mesalazine granules in the morning (n = 102) or twice daily with one 2 g sachet in the morning and one in the evening (n = 104) for 8 weeks | |
| Outcomes | Primary outcome: percentage of patients in clinical and endoscopic remission after 8 weeks (defined as UC‐DAI score < 1) Secondary outcomes: complete remission at week 8 (clinical and endoscopic UC‐DAI = 0), clinical and endoscopic improvement at week 8 (decrease in UC‐DAI by at least 2 points), clinical remission at weeks 4, 8 and 12, determined by normal stool frequency, no bloody stools and no active disease by physician’s assessment, time to remission (based on patient’s diary with normal stool frequency and cessation of bleeding; estimated using Kaplan–Meier methodology), mucosal healing at 8 weeks (defined as an UC‐DAI endoscopic sub‐score of 0 or 1, or alternatively a Rachmilewitz endoscopic index of < 4), adherence, global patient’s acceptability and adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomised centrally via a computer‐generated randomisation system |
| Allocation concealment (selection bias) | Low risk | To maintain the investigator‐blind trial design, sealed treatment boxes were identical in size and weight, and contained written instruction about the dosing arm to which the patient was assigned; investigators were unaware of this information |
| Blinding (performance bias and detection bias) All outcomes | High risk | Only Investigators were blinded in this trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients involved with the study are accounted for |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | Unclear risk | The study appeared to be free of other sources of biases |
Forbes 2005.
| Methods | Randomized non‐inferiority trial | |
| Participants | Adult ulcerative colitis patients with mild to moderate relapse (N = 88) | |
| Interventions | Asacol two 400 mg tablets 3 times/day (2.4 g/day, n = 42) or Ipocol two 400 mg tablets 3 times/day (2.4 g/day, n = 46) for 8 weeks | |
| Outcomes | Outcomes included clinical remission (investigator’s overall clinical assessment), modified St Mark’s Colitis Activity score, macroscopic and microscopic appearance of the rectum, and adverse events. Outcomes were evaluated at entry and weeks 2, 4, and 8. Tablet counts were performed by pharmacy departments to check compliance | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated randomization |
| Allocation concealment (selection bias) | Low risk | Centralized telephone randomization by Lagap Pharmaceuticals Ltd |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Study drug was provided in an anonymous blister package with instructions to take two 400 mg tablets three times a day. The tablets themselves were not identical as they are somewhat different in shape. Patients were advised that they might find that they were prescribed a tablet shaped differently from those they had received before, but not that this was or was not Asacol or Ipocol. Clinical investigators took care neither to see nor to enquire of the nature of the tablets |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | During the course of the study, 11 patients withdrew from the Asacol group, and nine withdrew from the Ipocol group: reasons for withdrawal were not provided |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Gibson 2006.
| Methods | Multicenter, randomized, double‐blind, double‐dummy parallel group trial | |
| Participants | Adult patients (19 to 70 years) with mild to moderately active ulcerative colitis confirmed by standard endoscopic and histopathological criteria (N = 258) | |
| Interventions | Eudragit‐L‐coated mesalazine tablets (Salofalk 3 g/day, n = 131) or ethylcellulose‐coated mesalazine tablets (Pentasa 3 g/day, n = 127) for 8 weeks | |
| Outcomes | Primary outcome: clinical remission (CAI < 4). Secondary outcomes: CAI; clinical improvement (clinical remission or improved CAI of > 3 from baseline), # stools; # bloody stools; time to first symptomatic remission; endoscopic remission (EI < 4); endoscopic improvement; histological remission; histological improvement; physician's global assessment; and adverse events | |
| Notes | LOCF if patients withdrew early. Patients were assumed to be treatment failures if no CAI score was available. Adherence checked by tablet count. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated using the program "Rancode +" |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, sealed, non‐transparent envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind, double‐dummy |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 43 patients were excluded from the per protocol analysis but it is not clear what groups these patients came from. ITT analysis was presented for the primary outcome |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Good 1992.
| Methods | Multicenter, double‐blind, randomized comparison of SASP and mesalamine. Each site was independently randomized in blocks of six. Clinical assessments were performed at entry, 4 weeks, and at 8 weeks | |
| Participants | Patients with endoscopically confirmed active ulcerative colitis (N = 117) | |
| Interventions | Mesalamine, 1 g/day (n = 27), 2 g/day (n = 31) or 4 g/day (n = 30) or SASP, 4 g/day (n = 29). Drugs were dispensed in blister packs according to a double‐dummy technique | |
| Outcomes | Efficacy was rated according to positive changes in disease activity index and a physician's overall assessment | |
| Notes | Abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind, double‐dummy |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Green 1998.
| Methods | Multi‐centre, randomised, double‐blind, double‐dummy, parallel group study | |
| Participants | Adult patients (18 to 80 years) with moderate to severely active ulcerative colitis confirmed by flexible sigmoidoscopy (N = 99) | |
| Interventions | Balsalazide (2.25 g three times daily: 6.75 g/day, n = 50) or Asacol (0.8 g three times daily: 2.4 g/day, n = 49) for 12 weeks | |
| Outcomes | The primary outcome was the proportion of patients achieving complete remission (based on diary card) by 12 weeks. Patients left the study at weeks 4 or 8 if they achieved complete remission. Complete remission was defined as none or mild symptoms sigmoidoscopic grade of 0 or 1 and no use of rectal steroid foam. Other outcomes included patient and investigator satisfaction, laboratory assessments, median time to relief of symptoms, cumulative days free of symptoms, study dropouts, dropouts due to treatment failure and adverse events. Outcomes were evaluated at entry and weeks 2, 4, 8 and 12. Adherence was assessed at follow‐up visits | |
| Notes | Patients were provided with rectal steroid foam as relief medication for use as required | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind, double‐dummy: Placebos of identical appearance to the balsalazide capsules and mesalamine tablets were provided. Patients received three capsules (balsalazide/placebo) and two tablets (mesalamine/placebo) three times daily |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Thirty‐eight percent of the patients (38 of 101) did not complete the study (15 balsalazide; 23 mesalamine), the main reason being treatment failure, which was more common in the mesalamine group (6 balsalazide; 16 mesalamine; P = 0.015). Other reasons for withdrawal included noncompliance with the study protocol (6 balsalazide, 3 mesalamine), unacceptable adverse events (1 balsalazide, 1 mesalamine), and treatment with excluded medication (1 balsalazide, 1 mesalamine). Three patients (1 balsalazide, 2 mesalamine) who were erroneously included into the study were also withdrawn; 1 patient receiving balsalazide did not have UC, 1 patient receiving mesalamine was not using adequate contraception, and 1 patient receiving mesalamine was included into the study after the recruitment deadline had passed |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Green 2002.
| Methods | Double‐blind and randomized using a random number table | |
| Participants | Patients with acute relapse of ulcerative colitis and newly diagnosed patients (N = 57) | |
| Interventions | Sulfasalazine, 3 g daily (n = 29), or balsalazide, 6.75 g daily (n = 28), according to a double‐dummy protocol for 12 weeks. Some patients were receiving concomitant oral or topical steroids | |
| Outcomes | Efficacy was graded according to clinical, sigmoidoscopic and histological criteria. Tolerance was also evaluated | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Low risk | Centralized randomization |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind, double‐dummy |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One patient from the SASP group was lost to follow up |
| Selective reporting (reporting bias) | Low risk | Expected outcome were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Hanauer 1993.
| Methods | Multicenter, double‐blind, placebo‐controlled, randomized, dose‐response trial conducted at 20 sites. In addition to daily patient diaries, clinical assessments and sigmoidoscopy were performed at weeks 1, 4, 8 or upon withdrawal | |
| Participants | Patients, over 18 years old, with mild to moderate active ulcerative colitis confirmed by clinical and colonoscopic evidence with a score of 5 or greater on a 15‐point index, were selected from March 6, 1987 to August 4, 1988. Patients were stratified according to extent of disease. Therapies of steroids, SASP, or other mesalamine formulations were stopped at least 7 days before trial. Immunosuppressives were stopped at least 90‐days before study (N = 374) | |
| Interventions | Mesalamine (Pentasa) 1 (n = 92), 2 (n = 97) or 4 g per day (n = 95), or placebo (n = 90), in 250 mg capsules in identical blister cards for 8 weeks. Loperamide (2 mg) was dispensed to patients when absolutely necessary for control of diarrhea | |
| Outcomes | Clinical improvement was assessed using the physician's global assessment, assessment of treatment failure, sigmoidoscopic index, biopsy score, patients' perceptions, and trips to the toilet. Induction of remission was assessed by more stringent criteria for physician's assessment, sigmoidoscopic index and biopsy score | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered drug containers of identical appearance |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind: study drug was supplied in 250 mg capsules in identical blister cards to ensure blinding of both the investigator and the patient |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Four patients were lost to follow‐up. More patients withdrew from the placebo group due to insufficient therapeutic effect |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Hanauer 1996.
| Methods | Multicenter, double‐blind, placebo‐controlled, randomized, dose‐ranging trial. Assessments were performed at entry, 6 and 12 weeks (or upon termination) | |
| Participants | Patients from 24 centers with mild to moderately active ulcerative colitis. No anti‐diarrheals were allowed (N = 273) | |
| Interventions | Olsalazine, 2 (n = 92) or 3 g per day (n = 91), or placebo (n = 90) for 12 weeks. Full dosage was reached after 1 week | |
| Outcomes | End‐points included induction of clinical remission (according to number of bowel movements and amount of blood in stool) and induction of endoscopic remission or endoscopic improvement (evaluated on a 5‐pt. scale, where 0 or 1 indicated remission) | |
| Notes | Abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
| Selective reporting (reporting bias) | Unclear risk | Expected outcomes were reported, Post hoc re‐scoring of endoscopic reports were reported for endoscopic remission |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Hanauer 2005.
| Methods | Multicenter, randomized, double‐blind, double‐dummy, parallel group study (ASCEND II) | |
| Participants | Adult patients (aged 18 to 75 years) with moderately active ulcerative colitis confirmed by endoscopy or radiography (N = 386) | |
| Interventions | Asacol 2.4 g/day (400 mg tablet; n = 139) or 4.8 g/day of mesalamine (Asacol 800 mg tablet; n = 129) for 6 weeks | |
| Outcomes | Primary outcome: treatment success at 6 weeks defined as either complete remission or a clinical response to therapy. Complete remission was defined as complete resolution of: (i) stool frequency (normal stool frequency); (ii) rectal bleeding (no rectal bleeding); (iii) PFA score (generally well); (iv) endoscopy findings (normal), and a PGA score of 0. A clinical response to therapy was defined as improvement in the baseline PGA score and improvement in at least one other clinical assessment (stool frequency, rectal bleeding, PFA, endoscopy findings) and no worsening in any other clinical assessment. Secondary outcomes: overall improvement at week 3, improvement from baseline in each of the clinical assessment subscores at weeks 3 and 6, overall improvement at week 6 in the subgroup of patients with ulcerative colitis limited to the left side of the colon (proctitis, proctosigmoiditis, or left‐sided colitis), time to normalization of stool frequency (based on the patient’s daily diary), time to resolution of rectal bleeding (based on the patient’s daily diary), and change from baseline in the Ulcerative Colitis Disease Activity Index (UCDAI) and adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Permutated block randomization scheme |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind, double‐dummy: identical placebos were used. Both patients and investigative staff were blinded to treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 18.7% of patients in 2.4 g/day group withdrew (26/139) compared to 12.4% of the 4.8 g/day group (16/129). More patients withdrew from the 2.4 g/day group due to lack of treatment effect |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Hanauer 2007.
| Methods | Multicenter, randomized, double‐blind, double‐dummy, parallel group study (ASCEND I) | |
| Participants | Adult patients (aged 18 to 75 years) with mild to moderately active ulcerative colitis confirmed by endoscopy or radiography (N = 301) | |
| Interventions | Asacol 2.4 g/day (400 mg tablet; n = 154) or 4.8 g/day of Asacol (800 mg tablet; n = 147) for 6 weeks | |
| Outcomes | Primary outcome: treatment success at week 6. Secondary efficacy end points included the proportion of patients who improved from baseline at week 3 and the percentage of patients whose clinical assessment scores (stool frequency, rectal bleeding, sigmoidoscopy scores, PFA scores and PGA scores) improved from baseline scores at weeks 3 and 6, improvement in QOL from baseline to weeks 3 and 6, and time to symptom relief (stool frequency, rectal bleeding or both) and adverse events. Overall improvement or treatment success was defined as either complete remission or a clinical response to therapy. Complete remission was defined as normal stool frequency, no rectal bleeding, a PFA score of 0 (generally healthy), normal endoscopy findings and a PGA score of 0 (quiescent disease activity). A clinical response to therapy was defined as a decrease in the PGA score of at least one point from baseline, plus improvement in at least one other clinical assessment parameter (stool frequency, rectal bleeding, PFA or endoscopy findings) and no worsening in any of the other clinical assessments | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Permutated block randomization scheme |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind, double‐dummy: identical placebos were used. Both investigators and patients were blinded to treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐outs balanced across intervention groups with similar reasons for withdrawal |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Hetzel 1986.
| Methods | Random, double‐blinded allocation of placebo or ADS by number code. Patients were seen 1 week before trial, and weekly during treatment, and 6 weeks after completion of treatment | |
| Participants | Patients with mild‐to‐moderate exacerbation of ulcerative proctitis or left‐sided colitis (N = 30). None had evidence of a severe attack of colitis (i.e. no fever, tachycardia, haemoglobin less than 10 g/l or ESR greater than 30 mm/h). Diagnosis confirmed by sigmoidoscopy, histology of rectal biopsies, radiological or colonoscopic appearance, and negative stool samples (for Salmonella, Shigella, campylobacter, Clostridium difficile). Patients known to be intolerant of SASP were included to determine whether their sensitivity extended to Olsalazine sodium (ADS) | |
| Interventions | Disodium azodisalicylate (ADS, Olsalazine sodium; n = 15), 2 g/day (1 g b.i.d.; four gelatin capsules; n = 15), or matching placebo with meals for 6 weeks | |
| Outcomes | Sigmoidoscopic appearances at weeks 0 and 6 were graded according to a four point scale (Grade 0‐ normal mucosa; grade 1‐ mild mucosal hyperemia; grade 2 ‐moderately severe proctitis with granularity of mucosa; grade 3‐ severe proctitis with spontaneous bleeding and/or ulceration and/or pus). Rectal biopsies (also at weeks 0 and 6) were assessed by a single experienced observer. Comparisons between samples were classified as 'much improved', 'improved', 'unchanged' or 'worse' | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number code |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind: matching placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐outs balanced across intervention groups with similar reasons for withdrawal |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Hiwatashi 2011.
| Methods | Multicenter, randomized, double‐blind, parallel group study | |
| Participants | Patients (aged 15 to 64 years) with moderately active ulcerative colitis (modified Mayo score 6 to 8 points) (N = 123) | |
| Interventions | 2.25 g/day mesalazine (3 round 250 mg tablets, 3 times per day; n = 63) or 4.0 g/day mesalazine (4 oval 500 mg tablets, 2 times per day; n = 60) | |
| Outcomes | Primary outcome: mean change in UC‐DAI. Secondary outcomes: mean change in each UC‐DAI variable (stool frequency, rectal bleeding, mucosal appearance, and physician’s overall assessment of disease), clinical remission, clinical improvement and adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Biased‐coin minimization algorithm |
| Allocation concealment (selection bias) | Low risk | Centralized randomization by independent CRO |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind, double‐dummy: Study medication consisted of a round tablet containing 250 mg of mesalazine, an oval tablet containing 500 mg of mesalazine and placebo tablets identical in size and appearance to the study drugs |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 patients dropped out from the 2.25 g/day group and 1 patient dropped out from the 4.0 gh/day group |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Ito 2010.
| Methods | Multi‐centre, randomized, double‐blind, double‐dummy, placebo‐controlled trial. Patients were evaluated at baseline and week 8 or at early withdrawal | |
| Participants | Patients (aged > 16 to < 65 years) with mild to moderately active ulcerative colitis. Disease activity was assessed using the ulcerative colitis disease activity index (UC‐DAI; Sutherland 1987). Patients with mild to moderate active ulcerative colitis who had a score of 3 to 8 on the UC‐DAI with a bloody stool score of > 1 were eligible for the study (N = 229) | |
| Interventions | The objective of the study was to demonstrate the superiority of Asacol 3.6 g/day and non‐inferiority of Asacol 2.4 g/day against Pentasa 2.25 g/day. Patients were randomized to Asacol 3.6 g/day (n = 65), Asacol 2.4 g/day (n = 66), Pentasa 2.25 g/day (n = 65) or placebo (n = 33) for eight weeks | |
| Outcomes | The primary outcome was reduction in UC‐DAI score from baseline. Secondary outcomes included reduction in each UC‐DAI item score, the proportion of patients achieving remission (a UC‐DAI score of < 2 and zero points for bloody stool score); the proportion of patients achieving efficacy (remission or patient who did not achieve remission but whose reduction of UC‐DAI score is > 2) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Biased‐coin minimization algorithm. |
| Allocation concealment (selection bias) | Low risk | Centralized randomization: A person independent from the study was in charge of the random allocation. The randomization code was sealed and stored until the blind was removed |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind, double‐dummy: the appearance of the medication was identical |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐outs balanced across intervention groups with similar reasons for withdrawal |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Jiang 2004.
| Methods | Randomized, double‐blind, double‐dummy comparison of olsalazine and SASP. Allocation of drugs was performed using a table of random numbers. Clinical and laboratory examinations were performed at entry and after 1, 2, 4, 6 and 8 weeks of treatment. Colonoscopy and biopsy were performed 3 days before treatment and within 3 days of completion | |
| Participants | Male and female patients (average age 32.6 years) with acute relapse of ulcerative colitis (N = 42) | |
| Interventions | Olsalazine 2 g/day (n = 21) or SASP 4 g/day (n = 21) for 8 weeks. Lopermide (1 to 2 pills/day) was given to patients unable to tolerate diarrhea but not for more than 10 days | |
| Outcomes | Outcomes included induction of complete remission (subsidence of clinical symptoms with a relatively normal mucous membrane on colonoscopy), induction of clinical remission (0 to 2 stools per day with no gross blood or red cells in stool), colonoscopic remission (evaluated on a 2 or 5 point scale) and histological remission (evaluated on a 5 point scale) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers |
| Allocation concealment (selection bias) | Low risk | Centralized randomization |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind, double‐dummy |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The number of patients who completed the trial was not reported |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Kamm 2007.
| Methods | Multi‐centre, randomized, double‐blind, double‐dummy, placebo‐controlled trial with an Asacol reference group. Outcomes were evaluated at entry and week 8 or at early withdrawal. | |
| Participants | Patients (aged > 18 years) with mild to moderately active ulcerative colitis (N = 341). New or relapsing cases of ulcerative colitis were included in the study. Ulcerative colitis was defined by symptomatic, radiographic and endoscopic criteria. Disease activity was assessed using a modified ulcerative colitis disease activity index (UC‐DAI; Sutherland 1987). Patients with mild to moderate active ulcerative colitis who had a score of 4 to 10 on the UC‐DAI with a sigmoidoscopy score > 1 and a physician’s global assessment score < 2 with comparable histology were eligible for the study. To increase stringency, patients showing any mucosal friability were given a sigmoidoscopy score of at least 2. During the screening period patients were permitted to continue receiving a stable dose of mesalamine (< 2.0 g/day) if they were receiving this treatment prior to screening. This was withdrawn at baseline if the patient was found to be eligible for inclusion | |
| Interventions | MMX mesalamine 2.4 g/day (n = 84) or 4.8 g/day (n = 85) given once daily, Asacol 2.4 g/day (n = 86) given in three divided doses, or placebo (n = 86) | |
| Outcomes | The primary outcome was the proportion of patients at week 8 in clinical and endoscopic remission (modified UC‐DAI of < 1 with rectal bleeding and stool frequency scores of 0, no mucosal friability, and a > 1 point reduction in sigmoidoscopy score from baseline). Secondary outcomes included the proportion of patients achieving clinical remission (a score of zero points for stool frequency and rectal bleeding); clinical improvement (a decrease > 3 points from baseline in modified UC‐DAI), changes in modified UC‐DAI score (baseline to week 8); changes in sigmoidoscopic appearance (baseline to week 8); and changes in rectal bleeding and stool frequency (from baseline to any study visit). Other secondary outcomes included an analysis of treatment failure rate, a comparison of time to withdrawal and adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Centralized randomization: Patients were randomized centrally via an interactive voice response system |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind, double‐dummy |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐outs balanced across intervention groups with similar reasons for withdrawal |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Kruis 1998.
| Methods | Multi‐centre, randomised, double‐blind, double‐dummy, parallel group study | |
| Participants | Adult patients (18 to 75 years) with a mild to moderate (less than endoscopic score of 4) attack of ulcerative colitis (N = 168) | |
| Interventions | Olsalazine 3 g/day (n = 88) or mesalazine (Claversal) 3 g/day (n = 80) for 12 weeks | |
| Outcomes | The primary outcome was endoscopic remission (defined as a score of 0 or 1 on the Rachmilewitz index). Secondary outcomes included clinical remission (< 1 on modified Rachmilewitz index), physician's global assessment on four‐point scale | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Centralized randomization |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind, double‐dummy |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Twenty‐five per cent drop out rate. However, drop‐outs balanced across intervention groups with similar reasons for withdrawal |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Kruis 2003.
| Methods | Multicenter, randomized, double‐blind trial | |
| Participants | Adult patients (aged 18 to 70 years) with mild to moderate (CAI 6 to 12; EI > 4) attack of UC with at least 1 previous episode or persistently bloody diarrhea at least 14 days preceding entry (N = 316) | |
| Interventions | Mesalamine (Salofalk pellets) 1.5 g/day (0.5 g three times daily; n = 103); 3.0 g/day (1.0 g three times daily; n = 107) or 4.5 g/day (1.5 g three times daily; n = 106) for 8 weeks | |
| Outcomes | Primary outcome: clinical remission (CAI < 4). Secondary outcomes: endoscopic remission (EI < 4); endoscopic improvement (reduction of EI by at least 1 point); clinical improvement (CAI decreased by at least 3 points), life quality index; physician's global assessment; and adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The pellets were dispensed by sachets containing mesalamine pellets or a mixture of mesalamine and placebo pellets. The pellets with active drug and placebo pellets were identical in outward appearance. To ensure blindness, the sachets of the 3 different dose groups contained the same number and volume of pellets. In the sachets with the highest dose all pellets consisted of the active drug |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Drop out rate in 1.5 g/day group was 32.0% (33/103) compared to 19.6% (21/107) in the 3.0 g/day group and 19.8% (21/106) in the 4.5 g/day group. The most frequent reason for premature termination was inefficiency of treatment (23%, 17%, and 13%, respectively). No other reasons for withdrawal were provided |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Kruis 2009.
| Methods | Randomized, double‐blind, double‐dummy, parallel group, multi‐centre, phase III non‐inferiority study assessing the efficacy and safety of mesalazine (Salofalk granules) 3.0 g once daily dosing versus 1 g three times daily dosing for the treatment of active ulcerative colitis. Adherence with study medication was checked by counting the medication returned at study visits | |
| Participants | Adult patients (aged 18 to 75 years) with active ulcerative colitis (CAI ≥ 6 and EI ≥ 4; Rachmilewitz criteria) were recruited from 54 centers in 13 countries for an eight week induction trial (N = 380) | |
| Interventions | Mesalazine 3.0 g once daily (n = 191) or 1 g three times daily (n = 189) | |
| Outcomes | The primary outcome was the percentage of patients achieving clinical remission at the end of the study (defined by CAI < 4). Secondary outcomes included clinical improvement (decrease in CAI by at least 1 point baseline), disease activity index (DAI) , endoscopic index, histological index (HI, based on Riley), time to first resolution of clinical symptoms, physician’s global assessment (PGA) and patient preference | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind, double‐dummy |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐outs balanced across intervention groups with similar reasons for withdrawal |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Levine 2002.
| Methods | Multi‐centre, randomised, double‐blind, double‐dummy, dose response, parallel group study | |
| Participants | Adult patients (aged 18 to 80 years) with mild to moderately active ulcerative colitis confirmed by flexible sigmoidoscopy (N = 154) | |
| Interventions | Balsalazide 6.75 g/day (n = 35), Balsalazide 2.25 g/day (n = 35) or Asacol 2.4 g/day (n = 36) for 8 weeks | |
| Outcomes | The primary outcome was a significant difference between treatment groups in rectal bleeding and in at least one other symptom. Improvement was defined as improvement in at least one category of the disease activity scale (i.e. normal, mild, moderate, severe). Secondary outcomes included remission status (normal stool frequency and no blood in stool for 48 hours before visit, physician’s global assessment score of quiescent and a sigmoidoscopy score of mild or normal), rectal biopsy score, and IBDQ score | |
| Notes | For the purposes of this review only the comparison between Balsalazide 6.75 g and Asacol 2.4 g (i.e. equimolar doses) was utilized | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind, double‐dummy: Placebos were identical in appearance to the balsalazide capsules and mesalamine (Asacol) tablets |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 31% drop‐out rate. Drop‐outs appear to be balanced across intervention groups. More patients withdrew from the low dose balsalazide and mesalamine groups due to lack of therapeutic effect than the high dose balsalazide group |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Lichtenstein 2007.
| Methods | Multi‐centre, randomized, double‐blind, double‐dummy, placebo‐controlled trial. Outcomes were evaluated at the screening visit (week –1) baseline (week 0), week 2, week 4 and week 8 or at early withdrawal | |
| Participants | Patients with newly diagnosed or relapsing (relapsed < 6 weeks prior to entry) mild to moderately active ulcerative colitis (modified UC‐DAI score of 4‐10, with a sigmoidoscopy score > 1 and a PGA score < 2 with compatible histology) (N = 262) | |
| Interventions | Patients were randomised to MMX mesalamine 4.8 g/day (n = 94) given once daily, 2.4 g twice daily (n = 93), or placebo (n = 93) for eight weeks | |
| Outcomes | The primary outcome was the proportion of patients at week 8 in clinical and endoscopic remission (modified UC‐DAI of < 1 with rectal bleeding and stool frequency scores of 0, no mucosal friability, and a > 1 point reduction in sigmoidoscopy score from baseline). Secondary outcomes included the proportion of patients achieving clinical remission (a score of zero points for stool frequency and rectal bleeding); clinical improvement (a decrease > 3 points from baseline in modified UC‐DAI), changes in modified UC‐DAI score (baseline to week 8); changes in sigmoidoscopic appearance (baseline to week 8); and changes in rectal bleeding and stool frequency (from baseline to any study visit). Other secondary outcomes included an analysis of treatment failure rate, a comparison of time to withdrawal and adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Patients were randomized centrally via an interactive voice response system |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind, double‐dummy. MMX mesalamine and placebo tablets were identical in appearance |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐outs balanced across intervention groups with similar reasons for withdrawal. There were a higher number of withdrawals in the placebo group due to lack of efficacy |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Maier 1985.
| Methods | Randomized controlled trial | |
| Participants | Patients with active inflammatory bowel disease (ulcerative colitis N = 30, or Crohn's disease N = 30) | |
| Interventions | Oral 5‐ASA, 0.5 g three times daily (n = 15) or oral SASP, 1.0 g three times daily (n = 15) for 8 weeks | |
| Outcomes | Remission and clinical improvement | |
| Notes | Study also enrolled 30 patients with Crohn's disease | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
| Selective reporting (reporting bias) | Unclear risk | Not described |
| Other bias | Unclear risk | Not described |
Mansfield 2002.
| Methods | Randomized, multicenter, double‐blind, parallel group study. Clinical and laboratory examinations were performed at recruitment, and weeks 2, 4 and 8 | |
| Participants | Adults with newly diagnosed or recently relapsed ulcerative colitis confirmed by sigmoidoscopy in conjunction with a negative stool culture (N = 50) | |
| Interventions | Sulfasalazine, 3 g daily (n = 24), or balsalazide, 6.75 g daily (n = 26) according to a double‐dummy protocol for 8 weeks | |
| Outcomes | Remission was defined as a stool frequency of two or less per day without blood and with a sigmoidoscopic appearance of normal rectal mucosa or minimal erythema | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Centralized randomization |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind, double‐dummy, identical gelatine capsules |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were lost to follow‐up. More patients were withdrawn from the SASP due to adverse events than the balsalazide group. Othe drop‐outs were balanced across intervention groups with similar reasons for withdrawal |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Marakhouski 2005.
| Methods | Multicenter, randomized, double‐blind, double‐dummy, parallel‐group study | |
| Participants | Adult patients (18 to 70 years) with mild to moderately active ulcerative colitis (N = 233) | |
| Interventions | Mesalazine pellets (Salofalk; n = 115) or mesalazine tablets (n = 118) at an initial dose of 1.5 g/day. In case of inadequate response the dose could be increased up to 3 g/day after the first follow‐up visit at 2 weeks. Patients were treated for 8 weeks | |
| Outcomes | The primary outcome was complete response (clinical remission) defined as CAI < 4 at individual study end. Secondary outcomes: time to first response; endoscopic remission (defined as EI < 4) and improvement; histological improvement; and physician’s global assessment | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind, double‐dummy: Placebos of identical appearance to 5‐ASA tablets and pellets were used to ensure double‐blind performance of the trial |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 13.5% drop‐out rate. Drop‐outs were balanced across groups. Reasons for dropping out were summarized across both groups |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Miglioli 1990.
| Methods | Multicenter, randomized, double‐blind, double‐dummy, parallel dose‐response study | |
| Participants | Adult patients (aged 18 to 65 years) with clinically mild active ulcerative colitis based on Truelove and Witts criteria (N = 73) | |
| Interventions | Mesalazine (Asacol 400 mg tablets) at daily doses of 1.2 g (n = 25), 2.4 g (n = 24) or 3.6 g (n = 24) for 4 weeks | |
| Outcomes | Clinical remission or improvement, endoscopic and histological improvement. Clinical remission was defined as no more than two bowel movements per day with no visible blood in the stool in the symptom less patient. Clinical improvement defined as a clear decrease in severity of symptoms and signs not satisfying remission criteria | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind, double‐dummy: identical placebo tablets |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Eleven patients did not complete the study (5 in 1.2 g/day group; 4 in 2.4 g/day group; and 2 in 3.6 g/day group because of worsening of disease in five, lack of improvement in 4 and loss to follow‐up and intercurrent disease in one). It is not clear which reasons apply to each group |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Mihas 1988.
| Methods | A prospective, controlled, double‐blind trial | |
| Participants | Adult patients (18 year or older) with exacerbated ulcerative colitis (N = 19) | |
| Interventions | Oral 5‐ASA 0.8g TID (2.4g/day, n = 7) vs. sulfasalazine 1g TID (3g/day, n = 12) for 4 weeks | |
| Outcomes | Response to treatment was based on endoscopic appearance, subjective symptoms, objective criteria and laboratory findings | |
| Notes | Abstract publication only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | A prospective double‐blind trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two of 12 patients from sulfasalazine group were unable to complete the study because of adverse events |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Munakata 1995.
| Methods | Multicenter, double‐blind, double‐dummy comparison of SASP and mesalazine. Randomisation was under the direction of a central controller. Clinical and endoscopic assessment was performed at entry, and after 2 and 4 weeks | |
| Participants | Patients, 16 years and older, with mild to moderately active ulcerative colitis were enrolled from July 1992 to March 1994 (N = 109) | |
| Interventions | Controlled‐release mesalazine, 1.5 g/day plus SASP‐matched placebo (n = 52) or active SASP, 3 g/day, with mesalazine‐matched placebo (n = 57), for 4 weeks. | |
| Outcomes | Improvement was assessed in terms of changes in clinical status based on disease activity and severity of symptoms, compared to baseline findings. Improvement was also measured in terms of endoscopic findings | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Centralized randomization |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind, double‐dummy |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 9 patients dropped out of the study |
| Selective reporting (reporting bias) | Unclear risk | Not described |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Pontes 2014.
| Methods | Randomized, double‐blind, placebo‐ and active‐controlled proof of concept study | |
| Participants | Adult patients (18 to 65 years) with mild‐to‐moderate active ulcerative colitis (Total Mayo score (TMS) >5 and <10, ) confirmed by endoscopy (N = 34) | |
| Interventions | Dersalazine 3 x 400 mg BID (2.4 g/day, n = 13), mesalazine 3 x 400 mg BID (2.4 g/day, n = 8), or placebo (n = 13) for 4 weeks | |
| Outcomes | The primary safety outcome: proportion of patients with adverse events (AE) of severe intensity or treatment withdrawal
Secondary efficacy outcomes: change in TMS from baseline to week 4, change in partial mayo score (PMS) from baseline to weeks 2 and 4, complete remission, clinical remission, TMS clinical response and mucosal healing rates by week 4, and partial Mayo score (PMS) clinical response by weeks 2 and 4 Secondary safety outcomes: proportion of patients with AEs, AEs with suspected relationship to study medication, and with clinically relevant abnormalities in laboratory tests or physical examination |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization list in blocks of 4 with a ratio of 2:1:1 (dersalazine sodium:mesalazine:placebo) |
| Allocation concealment (selection bias) | Low risk | Centrally randomized |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The treatments had undistinguishable appearance and were uniquely identified with a randomization number according to a computer‐generated randomization list |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Five patients did not complete the 4‐week treatment (3 from placebo group, and 2 from dersalazine group) |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | They study appears to be free of other sources of bias |
Pruitt 2002.
| Methods | Multi‐centre, randomised, double‐blind, double‐dummy, parallel group study | |
| Participants | Patients (aged 12 to 80 years) with mild to moderately active ulcerative colitis confirmed by flexible sigmoidoscopy (N= 173) | |
| Interventions | Balsalazide 6.75 g/day (n = 84) or Asacol 2.4 g/day (n = 89) for 8 weeks | |
| Outcomes | The primary outcome: proportion of patients in symptomatic remission (based on diary card) at the end of week 8 or at early completion of treatment. Symptomatic remission was defined as patient functional assessment rating of normal or mild and absence of rectal bleeding. Secondary outcomes: time to symptomatic remission, proportion of patients in complete remission (symptomatic remission plus sigmoidoscopic evaluation score of normal or mild), improvement in sigmoidoscopic evaluation score, change from baseline in physician’s global assessment of disease activity at week 8 or early completion and adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind, double‐dummy: each study drug treatment was administered three times daily as three capsules (balsalazide active drug or placebo) and two tablets (Asacol active drug or placebo) to maintain blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐outs balanced across intervention groups with similar reasons for withdrawal |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Qian 2004.
| Methods | Randomised, double‐blind, double‐dummy, parallel group study | |
| Participants | Adult patients (aged 18 to 70 years) with active ulcerative colitis (N = 56) | |
| Interventions | Olsalazine (250 mg capsules: 4 capsules twice daily; n = 31) or SASP (250 mg tabletss, 4 tablets 4 times daily; n = 25) for 8 weeks | |
| Outcomes | Clinical improvement and adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer stratified randomization |
| Allocation concealment (selection bias) | Low risk | Pharmaceuticals were packed and encoded according to random numbers. The encoding process was monitored by the staff from Shanghai Pharmaceutical Affairs Bureau |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind, double‐dummy |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 patients from SASP group were unable to complete the study |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Rachmilewitz 1989.
| Methods | Randomised, double‐blind parallel group comparison of mesalazine versus SASP. Drugs were centrally packaged and labelled. Patients were randomised in groups of 4 according to a predetermined list generated by a computer. Entry assessment involved physical exam, history, colonoscopy, and lab tests. In addition to patient diaries, assessments, including lab test, urine analysis, blood counts and liver/kidney function tests, were performed at bi‐weekly follow‐ups. Mandatory repeat colonoscopy was performed after week 8 | |
| Participants | Out‐patients, aged 18 to 70 years, at 46 centres in seven countries, with active mild to moderate ulcerative colitis (N = 220) | |
| Interventions | Coated mesalazine (Mesasal), 1.5 g/day (n = 115), or SASP 3 g/day (n = 105) for 8 weeks in a double‐dummy manner. Compliance was monitored by pill counts | |
| Outcomes | Clinical/endoscopic remission was defined as a clinical/endoscopic activity index score < 4. Improvement was also assessed in terms of changes in frequency and consistency of stools, and blood in stools. The incidence of adverse effects was also tabulated | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Centralized randomization |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind, double‐dummy |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐outs balanced across intervention groups with similar reasons for withdrawal |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Raedler 2004.
| Methods | Phase 2, multicenter, randomized, double‐blind, double‐dummy, parallel‐group study | |
| Participants | Adult patients (18 to 75 years) with recurrent mild to moderately active ulcerative colitis (N =362) | |
| Interventions | 3 g/day mesalazine in sachets of micropellets (1.5 g sachet taken twice daily with liquid, n = 181) or tablets (Claversal 500 mg; 2 tablets taken three times daily, n = 181) for 8 weeks | |
| Outcomes | The primary outcome was clinical remission (sum of CAI components 1 to 4 based on Rachmilewitz was CAI < 2) within 8 weeks of treatment. Secondary outcomes included: complete clinical remission (sum of CAI components 1 to 7 was < 4) endoscopic remission (EI based on Rachmilewitz was < 2) | |
| Notes | Adherence assessed by tablet and sachet counts | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind, double‐dummy: active drug and the matching placebo were identical in appearance, form, smell and taste. Medication labels were identical for both treatments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐outs balanced across intervention groups with similar reasons for withdrawal |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Rao 1989.
| Methods | Randomised, double‐blind, double‐dummy, multicenter comparison of olsalazine and SASP. At entry and at 4 weeks, patients were assessed clinically, by sigmoidoscopy, rectal biopsy, blood tests, stool samples and urine analysis. As well, patients kept stool diary records | |
| Participants | Out‐patients with a first attack of mild to moderately severe ulcerative colitis, confirmed by sigmoidoscopic and histologic evidence and negative stool cultures (N = 37) | |
| Interventions | Olsalazine, 2 g/day (n = 20), or enteric‐coated SASP, 3 g/day (n = 17), provided in sealed blister packs, administered 4 x per day. Full dosage was reached after 7 days and continued for 4 weeks. Double‐dummy technique required each patient to take a physically indistinguishable dummy containing mainly potato starch. Compliance was confirmed by pill counts | |
| Outcomes | Changes in daily stool frequency and consistency, sigmoidoscopic and histological appearance, and clinical assessments were defined as 'improved' (an increase by at least one point), 'unchanged' or 'worsened'. Remission was defined as the lack of blood in stool, no more than 2 bowel movements per day, and no systemic disturbance. Overall improvement was defined as a positive change in at least two of the above criteria | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind, double‐dummy. Patients received olsalazine or sulphasalazine along with physically indistinguishable dummies. The drugs were provided in sealed blister packs |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 3 patients in the olsalazine group did not complete the study compared to 4 patients in the SASP group. Reasons for withdrawal were not described |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Rijk 1991.
| Methods | Prospective, double‐blinded, multi‐center trial comparing olsalazine and sulfasalazine. Patients were centrally randomized | |
| Participants | Patients with active ulcerative colitis (N = 55) | |
| Interventions | 6 g/day SASP (n = 28) or 3 g/day olsalazine (n = 27) in externally‐indistinguishable capsules, for 6 weeks | |
| Outcomes | Remission was assessed on the basis of clinical and endoscopic criteria. Withdrawals and occurrence of adverse side‐effects were also measured | |
| Notes | Abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Centralized randomization |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind: externally‐indistinguishable capsules |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Six patients from each group were withdrawn because of adverse events or increasing severity of disease |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Riley 1988.
| Methods | Randomised, double‐blind, double‐dummy comparison of mesalamine and SASP. Meds were centrally prepackaged and randomly distributed to each centre. History, physical, blood counts, urine samples, sigmoidoscopy and biopsy were performed upon entry. In addition to daily diaries, patients were assessed at 2 and 4 weeks and any other time they wished. At 4 weeks, clinical assessment, biopsy and sigmoidoscopy were repeated | |
| Participants | Adult out‐patients with mild to moderate ulcerative colitis relapse or first attack, recruited from 3 hospitals in close geographical proximity. All were passing blood at least once per day and all had hemorrhagic rectal mucosa (N = 60) | |
| Interventions | SASP 2 g/day (n = 20), delayed‐release mesalazine (Asacol), 800 mg/day (n = 20), or Asacol, 2.4 g/day (n = 21). Each patient received 3 sets of tablets (two placebo and one active) as per a double‐dummy method | |
| Outcomes | Stool frequency, rectal bleeding, sigmoidoscopic, and histologic measures were used for comparison of groups. Withdrawals and adverse side‐effects were also measured | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Centralized randomization |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind, double‐dummy |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One patient dropped out of the SASP group |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Robinson 1988.
| Methods | Double‐blinded, randomized, single‐center trial. Patient evaluations were performed at days 14 and 28 for clinical and laboratory parameters | |
| Participants | Patients with acute attacks of mild to moderate ulcerative colitis. No concomitant medications for UC were allowed (N = 98) | |
| Interventions | Olsalazine, 3 g/day, or placebo, for 28 days | |
| Outcomes | Efficacy was based on evaluations of diarrhea, rectal bleeding, mucorrhea, sigmoidoscopic score, nausea, abdominal tenderness, stool consistency, and global disease severity rating as compared to baseline status | |
| Notes | Abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Sandborn 2009.
| Methods | Multicenter, randomized, double‐blind, double‐dummy, active‐controlled trial (ASCEND III) | |
| Participants | Adult patients (aged 18 to 75 years) with moderately active ulcerative colitis that extended proximally beyond 15 cm from the anal verge, as confirmed by flexible sigmoidoscopy or colonoscopy (N = 772) |
|
| Interventions | Asacol 2.4 g/day (400 mg tablet; n = 383) or 4.8 g/day of mesalamine (Asacol 800 mg tablet; n = 389) for 6 weeks | |
| Outcomes | Primary outcome: treatment success (overall improvement) at week 6, defined as improvement in the Physician’s Global Assessment (based on clinical assessments of rectal bleeding, stool frequency, and sigmoidoscopy), with no worsening in any individual clinical assessment. Secondary outcomes: clinical remission at weeks 3 and 6; improvement in stool frequency, rectal bleeding, and PFA assessments at weeks 3 and 6; improvement in the sigmoidoscopy with CFT, PGA, and UCDAI assessments at week 6; and treatment success in patients with left‐sided disease at week 6 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigator or designated representative telephoned the Interactive Voice Response System for patient randomization and allocation of study medication once the patient was determined to be eligible for the study |
| Allocation concealment (selection bias) | Low risk | Interactive Telephone Voice Response System |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind, double‐dummy, identical placebos |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐outs balanced across intervention groups with similar reasons for withdrawal |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Sandborn 2012.
| Methods | Phase 3, multicenter, randomized, double‐blind, double dummy, placebo‐controlled trial | |
| Participants | Adult patients (18 to 75 years of age) with active, mild to moderate ulcerative colitis for at least 6 months, with an ulcerative colitis disease activity index (UCDAI) score of 4‐10 points (N = 489). | |
| Interventions | Budesonide MMX 9 mg/day (n = 123), budesonide MMX 6 mg/day (n = 121), mesalamine (Asacol 2.4 g/day, as reference, n = 124), or placebo (n = 121) for 8 weeks | |
| Outcomes | Primary outcome: combined clinical and endoscopic remission at week 8. Remission was defined as combined clinical and endoscopic remission with a UCDAI score < 1 point, with subscores of 0 for both rectal bleeding and stool frequency, no mucosal friability on colonoscopy, and a > 1‐point reduction from baseline in the endoscopic index score Secondary outcomes: clinical improvement (> 3‐point reduction in UCDAI), endoscopic improvement (> 1‐point reduction in the UCDAI mucosal appearance subscore), symptom resolution (score of 0 for both rectal bleeding and stool frequency subscores from the UCDAI), histologic healing (histologic score of < 1 (corresponding to a histologic activity grade of 0) according to the Saverymuttu scale and adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization for this study was developed by an external contractor and administered centrally |
| Allocation concealment (selection bias) | Low risk | The interactive voice response system was used to centrally randomize patients to study drug |
| Blinding (performance bias and detection bias) All outcomes | Low risk | A double‐dummy procedure was used to maintain blinding, with patients in each treatment group receiving their blinded study drug 3 times daily. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 140 patients were unable to complete the study (34 from budesonide 9mg QD, 32 from budesonide 6mg QD, 29 from Asacol 2.4 g/day, and 45 from placebo group) |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported in the published study |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Scherl 2009.
| Methods | Multicenter, randomized, double‐blind, placebo‐controlled trial. Patients were assessed at screening visit, baseline, day 7, day 14 day 28 and day 56 and follow‐up. Patients assessment included MMDAI (deletion of friability from endoscopy score equal to 1), and physical exam, laboratory tests and patient diary cards | |
| Participants | Acute are of mild‐to‐moderate active UC; baseline Modified Mayo Disease Activity Index (MMDAI) score between 6 and 10 (Table 1), inclusive (e.g., mild‐to moderately active UC) with an individual subscale score = 2 for rectal bleeding and mucosal appearance; disease extending at least 20 cm from the rectum on screening endoscopy /sigmoidoscopy; had not taken = 6.75 g / day of balsalazide, or greater than 2.4 g / day of mesalamine or equivalent daily dose of any other 5‐ASA product during the 14 days before the initiation of study medication (N = 250) | |
| Interventions | Balsalazide 3.3 g/day (n = 167) or matching placebo (n = 83) | |
| Outcomes | The primary efficacy end point was the proportion of patients in the intention‐to‐treat (ITT) population that achieved clinical improvement and improvement in the rectal bleeding subscale of the MMDAI at week 8 or end of treatment. Clinical improvement was defined as a ≥ 3 point improvement from baseline in the total MMDAI score and a ≥ 1 point improvement from baseline in the rectal bleeding subscale of the MMDAI. Secondary efficacy end points included the proportion of patients in clinical remission, defined as a score of 0 for rectal bleeding and a combined score of ≤ 2 for bowel frequency and physician's assessment using the MMDAI subscales, at week 8 or end of treatment; proportion of patients who experienced mucosal healing, defined as an endoscopy or sigmoidoscopy score of 0 or 1 at week 8 or end of treatment; proportion of patients with improvement ( ≥ 1 point improvement) from baseline to week 8 or end of treatment in the MMDAI subscale of mucosal appearance, bowel frequency, rectal bleeding, and physician's assessment; proportion of patients achieving complete remission, defined as a MMDAI score of ≤ 1, at week 8 or end of treatment; and mean change from baseline to week 8 or end of treatment for the MMDAI score | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Centralized, automated, validated interactive voice response system |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind: All tablets were identical in appearance. Both the investigator and patient were blinded to assigned treatment throughout the study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 patients lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Schroeder 1987.
| Methods | Placebo‐controlled, double‐blinded, and randomized according to a sequence used by the dispensing pharmacist. Patient population was stratified into four strata: 1‐ previous treatment, left‐sided disease; 2‐ previous treatment, universal disease; 3‐ no previous treatment, left‐sided disease; 4‐ no previous treatment, universal disease. Evaluation occurred at 3 weeks and 6 weeks | |
| Participants | Patients, ages 15 to 70 years, with mild to moderate ulcerative colitis seen at the Mayo Clinic (Rochester, Minn.) from September 1, 1984 to February 28, 1986 (N = 87). UC was defined by symptomatic, radiographic, endoscopic criteria. Colonic involvement was determined by flexible proctosigmoidoscopy with double‐contrast x‐ray films of colon or complete colonoscopy, or both. Newly or previously diagnosed cases were included. Patients receiving corticosteroids or SASP were required to stop such therapy at least 1 week prior to start of study. Pre‐entry evaluations included history, physical, blood count, chemistry screening, urinalysis, stool sample (had to be negative for ova, parasites, enteric pathogens) | |
| Interventions | Asacol tablets (400 mg of 5‐ASA, coated with pH‐sensitive polymer Eudragit‐S which dissolves at pH 7 or higher) or matching placebo (500 mg microcellulose with identical pH‐sensitive coating, n = 38) 4.8 g/day (n = 38) or 1.6 g/day (latter dose only used in stratum 1, n = 11), 12 tablets daily for 6 weeks. No pill count, but patients were asked about compliance | |
| Outcomes | Clinical response, described as 'complete', 'partial', or 'no response', was determined on the basis of stool frequency, amount of rectal bleeding, and physician's global assessment (which included sigmoidoscopic appearance) on 4‐point scales, compared to baseline data. 'Complete response' indicated resolution of all symptoms. Occurences of adverse reactions were also tabulated | |
| Notes | Early termination of treatment for any reason was deemed to constitute treatment failure | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization sequence was developed by the Section of Medical Research Statistics, Rochester Methodist Hospital |
| Allocation concealment (selection bias) | Low risk | Centralized randomization by pharmacist |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind: matching placebo |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | More placebo patients (n= 16) did not complete the study than 5‐ASA patients (n = 5). Placebo patients were more likely to drop out do to flare of UC or no improvement |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Sninsky 1991.
| Methods | Multicenter, double‐blind, placebo‐controlled, computer‐randomized trial involving 5 university‐based medical centres, one inflammatory bowel disease center, and 3 private practice sites. Patients were not stratified according to clinical characteristics. Initial patient evaluation and follow‐up exams consisted of lab tests, flexible proctosigmoidoscopy and radiographic films or colonoscopy at entry, followed by sigmoidoscopy at 3 and 6 weeks | |
| Participants | Patients, ages 18 to 75 years, with mildly to moderately active ulcerative colitis were enrolled from November 1988 to June 1989 (N = 158). Diagnosis by symptomatic, radiographic, and endoscopic criteria had to have been confirmed by colonoscopy, proctosigmoidoscopy or barium enema within 24 months of start of study. Cases of both newly and previously diagnosed disease showing continued active signs, despite SASP therapy were included. Steroid therapy had to be stopped at least one month before start of study; SASP and topical rectal therapies were discontinued at least 1 week before start. Concomitant use of corticosteroids, aspirin, NSAIDs, metronidazole, 6‐mercaptopurine, azathioprine, cyclosporine, or other investigational drugs was not permitted | |
| Interventions | 1.6 g/day (n = 53) or 2.4 g/day (n = 53) oral mesalamine (Asacol) in 400 mg tablets coated with pH‐sensitive polymer (Eudragit‐S) or matching placebo tablets (n = 52) containing microcellulose. Compliance was checked by pill count at each visit and by review of patient diaries | |
| Outcomes | Clinical grading was based on stool frequency, rectal bleeding, sigmoidoscopic findings, and patient's functional assessment, each on 4‐point scale, which together gave the 'physician's global assessment', also on a 4‐point scale. The change in this clinical grade was indicated by classifying each patient as being 'in remission', 'improved', 'maintained', or 'worsened'. Withdrawals and adverse side effects were also reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind: matching placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐outs balanced across intervention groups with similar reasons for withdrawal |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Sutherland 1990.
| Methods | Double‐blind, placebo‐controlled, multicenter, parallel trial with random allocation of placebo or drug. Patients were initially screened with a baseline history, physical exam, and flexible sigmoidoscopy or colonoscopy in order to calculate the activity index (see 'Participants'). Follow‐up was assessed by telephone contact at end of week 1, 2, 4 and 5 and by clinical exam at the ends of weeks 3 and 6. Each clinic visit included flexible sigmoidoscopy and a physician's global assessment | |
| Participants | Male and non‐pregnant female patients, at least 18 years of age, with ulcerative colitis of variable extent, from five American and two Canadian centres and all enrolled between July 1985 and September 1986 (N = 136). Ulceration had to extend at least 20 cm proximal to the anus. Patients had to have a minimum score of 4 measured by Disease Activity Index (four subgroups for each of bowel frequency, presence of blood, sigmoidoscopic appearance, and physician's assessment of severity for a maximum score of 12) | |
| Interventions | Random allocation of Rowasa (250 mg tablets) taken as four tablets, four times per day, for a total of either 4 g/day (n = 47) or 2 g/day (n = 45), and an identical‐appearing placebo (n = 44) for 6 weeks. Compliance was measured by pill counts | |
| Outcomes | Efficacy was assessed by changes in the disease activity index and physician's global assessment. The change in physician's global assessment was described as 'much or somewhat improved', 'unchanged', or 'somewhat worse or much worse'. The change in the disease activity index score was evaluated in terms of end of study score minus 'baseline' | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | All assignments to treatment and subsequent assessments of response to treatment were under double‐blind conditions |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind: identical placebo |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 34% drop‐out rate, however drop‐outs appear to be balanced across intervention groups with similar reasons for withdrawal |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Tursi 2004.
| Methods | Multicenter, randomized trial | |
| Participants | Adult patients (19 to 69 years) with mild to moderate active ulcerative colitis confirmed by endoscopic evaluation (N = 90) | |
| Interventions | Balsalazide 4.5 g/day (n = 30) or Balsalazide 2.25 g/day + VSL#3 (n = 30) or Asacol 2.4 g/day (n = 30) for 8 weeks | |
| Outcomes | The primary outcome was the proportion of patients in symptomatic remission based on clinical evaluation and diary card at 2, 4 and 8 weeks. Symptomatic remission was defined as patient functional assessment ratings of normal bowel movements and absence of rectal bleeding. Secondary outcomes included time to symptomatic remission, the proportion of patients achieving improvement in endoscopic evaluation score at 8 weeks, change in CAI from baseline at 8 weeks, improvement in histology at 8 weeks, and adverse events | |
| Notes | For the purposes of this review only the comparison between Balsalazide 4.5 g/day and Asacol 2.4 g/day was utilized (N = 60) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label. Physicians and patients were not blinded. Histological specimens were examined and graded for inflammation by one histopathologist blind to the treatment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 4 patients withdrew from the Balsalazide group (13%) compared to 8 in the Asacol group (26%). Reasons for withdrawal are similar expect that 2 patients from the Asacol group withdrew for adverse events |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Willoughby 1988.
| Methods | Randomized, double‐dummy, multicenter comparison of SASP and olsalazine. Randomization was restricted in blocks of four to ensure approximately equal numbers of patients allocated to each form of treatment. In addition to diary cards, patients were clinically assessed upon entry, after 2 weeks, and after 5 weeks. Biopsy, sigmoidoscopy, and lab tests were performed at entry and after week 5 | |
| Participants | Out‐patients with mild to moderately active ulcerative colitis, either first attack or relapse (N = 56) | |
| Interventions | Oral sulphasalazine, 3 g/day (n = 30), or oral olsalazine, 3 g/day (n = 26), each in divided doses. Dose escalation schedule was used for first week of treatment after which full‐dose therapy continued for further 4 weeks. Tablets were counted to monitor compliance | |
| Outcomes | Clinical response was evaluated in terms of changes in stool frequency and loss of blood and mucus from stools. Sigmoidoscopic and histological assessments were considered to have improved if score on a standard scale increased by at least 1 point (Grayson, Carpenter, Dick, & Petrie 1964, as cited in Willougby 1988). Withdrawals and adverse effects were also tabulated | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind, double‐dummy |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐outs appear to be balanced across intervention groups with similar reasons for withdrawal |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Zinberg 1990.
| Methods | Double‐blind, placebo‐controlled trial. Randomization was on an alternate basis between drug and placebo and allocated by pharmaceutical manufacturer. At initial patient interview, history and physical exam were performed including baseline laboratory studies. Urine analysis for enteric pathogens was also performed. Evaluations were performed at the end of the 2nd and 4th weeks. Endoscopic evaluation was performed at entry and after 4 weeks | |
| Participants | Male and female patients, 18 to 75 years of age, with mild to moderate ulcerative colitis ‐ visible blood in the stool and disease involvement of 15 cm or more above the anal verge as defined by flexible sigmoidoscopy or colonoscopy (N = 15). The exacerbation could be a first instance or relapse of established disease. At least 3 days prior to participation, SASP, antidiarrheal agents, antispasmodics, and anticholinergics were discontinued. Oral or rectal steroids were not permitted within 1 week of study entry and other immunosuppressants were not permitted within 1 month of study. Concomitant medications not permitted during the study included NSAIDs, salicylates, digitalis derivatives, tranquilizers, and anti‐depressants | |
| Interventions | Olsalazine (Pharmacia) in opaque gelatin capsules, each of 250 mg (n = 7) or indistinguishable placebo capsules (n = 8) in identical containers, 12 capsules/day (3 with each meal and 3 at bedtime) for 28 days. Compliance was assessed by interview as well as by pill count | |
| Outcomes | Clinical evaluation included patient recordings of number of daily bowel movements, stool consistency, presence of blood and mucus, urgency, and incontinence. Endoscopic evaluation assessed the severity of ulceration, friability, erythema, and exudate, each on a 3‐point scale. The sum of these three scores gave a total endoscopic score. Improvement was assessed in terms of the changes in both clinical and endoscopic evaluations | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Centralized randomization |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind: physically indistinguishable placebo capsules were provided in identical containers |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐outs balanced across intervention groups with similar reasons for withdrawal |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adrizzone 2006 | Trial does not have a placebo, SASP or other formulation of 5‐ASA comparison group. Trial compares 5‐ASA versus azathioprine |
| Ahluwalia 1992 | Dose‐ranging study. The study does not provide details on pre‐specified outcomes |
| Gross 2011 | Trial does not have a placebo, SASP or other formulation of 5‐ASA comparison group. Trial compared once daily dosing of mesalazine (Salofalk) with once daily budesonide |
| Irvine 2008 | Pooled quality of life data from two RCTs (ASCEND I and ASCEND II) |
| Kamm 2009 | Not a RCT ‐ open‐label extension study |
| Mahmood 2005 | Oral 5‐ASA combined with trefoil factor 3 enema versus oral 5‐ASA combined with placebo enema |
| Paoluzi 2002 | Trial looks at 4 weeks of combined oral and topical 5‐ASA (mesalazine) versus 8 weeks of combined oral and topical 5‐ASA (mesalazine) |
| Pruitt 1991 | Single‐centre report abstracted from a larger multicenter trial (Sninsky 1991) |
| Safdi 1997 | Trial compared oral mesalamine (Asacol) to mesalamine enema (Rowasa) to combination of oral mesalamine and enema |
| Vecchi 2001 | Trial compared oral 5‐ASA (Salofalk) + placebo enema to oral 5‐ASA + 5‐ASA enema (Salofalk) |
| Vernia 2000 | Trial compared oral mesalazine to combination of oral mesalazine + oral sodium butyrate |
Declarations of interest
Yongjun Wang: None known.
Claire E Parker: None known.
Tania Bhanji: None known.
Brian G Feagan has received fees from Abbott/AbbVie, Amgen, Astra Zeneca, Avaxia Biologics Inc., Bristol‐Myers Squibb, Celgene, Centocor Inc., Elan/Biogen, Ferring, JnJ/Janssen, Merck, Nestles, Novartis, Novonordisk, Pfizer, Prometheus Laboratories, Protagonist, Salix Pharma, Takeda, Teva, TiGenix, Tillotts Pharma AG and UCB Pharma for Scientific Advisory Board membership; fees from Abbott/AbbVie, Actogenix, Akros, Albireo Pharma, Amgen, Astra Zeneca, Avaxia Biologics Inc., Avir Pharma, Axcan, Baxter Healthcare Corp., Biogen Idec, Boehringer‐Ingelheim, Bristol‐Myers Squibb, Calypso Biotech, Celgene, Elan/Biogen, EnGene, Ferring Pharma, Roche/Genentech, GiCare Pharma, Gilead, Given Imaging Inc., GSK, Ironwood Pharma, Janssen Biotech (Centocor), JnJ/Janssen, Kyowa Kakko Kirin Co Ltd., Lexicon, Lilly, Lycera BioTech, Merck, Mesoblast Pharma, Millennium, Nektar, Nestles, Novonordisk, Pfizer, Prometheus Therapeutics and Diagnostics, Protagonist, Receptos, Salix Pharma, Serono, Shire, Sigmoid Pharma, Synergy Pharma Inc., Takeda, Teva Pharma, TiGenix, Tillotts, UCB Pharma, Vertex Pharma, VHsquared Ltd., Warner‐Chilcott, Wyeth, Zealand, and Zyngenia for consultancy; payment for lectures from Abbott/AbbVie, JnJ/Janssen, Takeda, Warner‐Chilcott, UCB Pharma; his institution has received grants/grants pending from Abbott/AbbVie, Amgen, Astra Zeneca, Bristol‐Myers Squibb (BMS), Janssen Biotech (Centocor), JnJ/Janssen, Roche/Genentech, Millennium, Pfizer, Receptos, Santarus, Sanofi, Tillotts, and UCB Pharma. Dr Feagan was the author of one manuscript that was included in this review.
John K MacDonald: None known.
Edited (no change to conclusions)
References
References to studies included in this review
Andreoli 1987 {published data only}
- Andreoli A, Cosintino R, Trotti R, Berri F, Prantera C. 5‐aminosalicylic acid versus salazopirin (SASP) in the oral treatment of active ulcerative colitis (UC) and in remission. Clinical Controversies in Inflammatory Bowel Disease 1987:170. [Google Scholar]
Bresci 1990 {published data only}
- Bresci G, Carrai M, Venturini G, Gambardella L. Therapeutic effectiveness and tolerance of 5‐aminosalicylic acid in short term treatment of patients with ulcerative colitis at a low or medium phase of activity. International Journal of Tissue Reactions 1990;12:243‐6. [PubMed] [Google Scholar]
Cai 2001 {published data only}
- Cai JT, Wu LF, Du Q, Qian KD. Olsalazine versus sulfasalazine in the treatment of ulcerative colitis: randomized controlled clinical trial. Chinese Journal of Digestion 2001;21(10):593‐5. [Google Scholar]
D'Haens 2006 {published data only}
- D'Haens G, Hommes D, Engels L, Baert F, Waaij L, Connor P, et al. Once daily MMX mesalazine for the treatment of mild‐to‐moderate ulcerative colitis: a phase II, dose‐ranging study. Alimentary Pharmacology and Therapeutics 2006;24(7):1087‐97. [DOI] [PubMed] [Google Scholar]
Ewe 1988 {published data only}
- Ewe K, Eckhardt V, Kanzler G. Treatment of ulcerative colitis with olsalazine and sulfasalazine: efficacy and side effects. Scandinavian Journal of Gastroenterology 1988;23(Suppl 148):70‐5. [DOI] [PubMed] [Google Scholar]
Farup 2001 {published data only}
- Farup PG, Hinterleitner TA, Lukás M, Hébuterne X, Rachmilewitz D, Campieri M, et al. Mesalazine 4 g daily given as prolonged‐release granules twice daily and four times daily is at least as effective as prolonged‐release tablets four times daily in patients with ulcerative colitis. Inflammatory Bowel Diseases 2001;7(3):237‐42. [DOI] [PubMed] [Google Scholar]
- Farup PG, Oddsson E, Hinterleitner T. Mesalamine 4 g prolonged release granules BID and QID versus tablets QID for mild/moderate UC. Gastroenterology 1999;116(4 Part 2):A713. [Google Scholar]
Feagan 2013 {published data only}
- Feagan B, Sandborn W, D'Haens G, McDonald J, Rutgeerts P, Munkholm P, et al. The value of a central image management system (CIMS) in the conduct of randomized controlled trials of therapy for ulcerative colitis (UC). American Journal of Gastroenterology 2012;107:S579‐80. [Google Scholar]
- Feagan BG, Sandborn WJ, D’Haens G, Pola S, McDonald JWD, Rutgeerts P, et al. The role of centralized reading of endoscopy in a randomized controlled trial of mesalamine for ulcerative colitis. Gastroenterology 2013;145(1):149‐57. [DOI] [PubMed] [Google Scholar]
Feurle 1989 {published data only}
- Feurle GE, Theuer D, Velasco S, Barry BA, Wordehoff D, Sommer A, et al. Olsalazine versus placebo in the treatment of mild to moderate ulcerative colitis: a randomised double‐blind trial. Gut 1989;10:1354‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feurle GE, Theuer D, Velasco S, Barry BA, Wordehoff D, Sommer A, et al. Olsalazine versus placebo in the treatment of mild to moderate ulcerative colitis: a randomized double‐blind trial. Gastroenterology 1988;94(5 Part 2):A126. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fleig 1988 {published data only}
- Fleig WE, Laudage G, Sommer H, Wellman W, Stange EF, Riemann J. Prospective, randomized, double‐blind comparison of benzalazine and sulfasalazine in the treatment of active ulcerative colitis. Digestion 1988;40:173‐80. [DOI] [PubMed] [Google Scholar]
Flourie 2013 {published data only}
- Flourie B, Hagege H, Tucat G, Masclee A, Dewit O, Probert C, et al. Once‐daily versus twice‐daily mesalazine for active ulcerative colitis: Efficacy results from MOTUS, a multicentre, controlled, randomised, investigator‐blinded study. Journal of Crohn's and Colitis 2012;6:S82. [Google Scholar]
- Flourie B, Hagege H, Tucat G, Masclee A, Dewit O, Probert C, et al. Once‐daily versus twice‐daily mesalazine for active ulcerative colitis: efficacy results from motus, a multicentre, controlled, randomised, investigator‐blinded study. Gastroenterology 2012;142(5 suppl 1):S197. [Google Scholar]
- Flourie B, Hagège H, Tucat G, Maetz D, Hébuterne X, Kuyvenhoven JP, et al. Randomised clinical trial: once‐ vs. twice‐daily prolonged‐release mesalazine for active ulcerative colitis. Alimentary Pharmacology and Therapeutics 2013;37(8):767‐75. [DOI] [PubMed] [Google Scholar]
- Flourie B, Kuyvenhoven J, Probert C, Dewit O. Comparing the efficacy of once‐daily or twice‐daily mesalazine dosing in the treatment of left‐sided ulcerative colitis versus the overall MOTUS study population. Journal of Crohn's and Colitis 2013;7:S236. [Google Scholar]
- Pierik M, Hagege H, Tucat G, Masclee A, Dewit O, Probert C, et al. Once‐daily versus twice‐daily mesalazine for mild to moderately active ulcerative colitis: Mucosal healing and early response data from MOTUS, a multicentre, controlled, randomised, investigator‐blinded study. Journal of Crohn's and Colitis 2012;6:S82‐3. [Google Scholar]
- Pierik M, Hagege H, Tucat G, Masclee A, Dewit O, Probert C, et al. Once‐daily versus twice‐daily mesalazine for mild to moderately active ulcerative colitis: mucosal healing and early response data from MOTUS, a multicentre, controlled, randomised, investigator‐blinded study. Journal of Crohn's and Colitis 2012;6:S82‐3. [Google Scholar]
Forbes 2005 {published data only}
- Forbes A, Al‐Damluji A, Ashworth S, Bramble M, Herbert K, Ho J, et al. Multicentre randomized‐controlled clinical trial of Ipocol, a new enteric‐coated form of mesalazine, in comparison with Asacol in the treatment of ulcerative colitis. Alimentary Pharmacology and Therapeutics 2005;21(9):1099‐104. [DOI] [PubMed] [Google Scholar]
Gibson 2006 {published data only}
- Gibson PR, Fixa B, Pekárková B, Bátovský M, Radford‐Smith G, Tibitanzl J, et al. Comparison of the efficacy and safety of Eudragit‐L‐coated mesalazine tablets with ethylcellulose‐coated mesalazine tablets in patients with mild to moderately active ulcerative colitis. Alimentary Pharmacology and Therapeutics 2006;23(7):1017‐26. [DOI] [PubMed] [Google Scholar]
Good 1992 {published data only}
- Good L, Nester T, Borgen L. A double‐blind comparison of controlled release mesalamine tablets and sulfasalazine in the treatment of ulcerative colitis. Gastroenterology 1992;102:A630. [Google Scholar]
Green 1998 {published data only}
- Green JRB, Holdsworth CD, Lobo AJ, Leicester R, Gibson JA, Kerr GD, et al. Balsalazide is more effective and better tolerated than mesalazine in acute ulcerative colitis. Digestive Disease Week Abstract Book. AbstractsOnDisk. 1997. [Google Scholar]
- Green JRB, Lobo AJ, Holdsworth CD, Leicester RJ, Gibson JA, Kerr GD, et al. Balsalazide is more effective and better tolerated than mesalazine in the treatment of acute ulcerative colitis. Gastroenterology 1998;114(1):15‐22. [DOI] [PubMed] [Google Scholar]
Green 2002 {published data only}
- Green JRB, Mansfield JC, Gibson JA, Kerr GD, Thornton PC. A double‐blind comparison of balsalazide, 6.75 g daily, and sulfasalazine, 3g daily, in patients with newly diagnosed or relapsed active ulcerative colitis. Alimentary Pharmacology and Therapeutics 2002;16(1):61‐8. [DOI] [PubMed] [Google Scholar]
- Green JRB, Swan CHJ, Rowlinson AE, Gibson JA, Brown P, Kerr GD, et al. Sulphasalazine or high dose balsalazide to treat acute relapse in ulcerative colitis? Results of a randomized trial. Gastroenterology 1993;104(4 Part 2):709. [Google Scholar]
Hanauer 1993 {published data only}
- Hanauer S, Beshears L, Wilkinson C. Induction of remission in a dose‐ranging study of oral mesalamine capsules (Pentasa). Gastroenterology 1990;98(5 Part 2):A174. [Google Scholar]
- Hanauer S, Schwartz J, Robinson M, Roufail W, Arora S, Cello J, et al. Mesalamine capsules for treatment of active ulcerative colitis: results of a controlled trial. American Journal of Gastroenterology 1993;88:1188‐97. [PubMed] [Google Scholar]
- Hanauer S, Schwartz J, Roufail W, Robinson M, Cello J, Safdi M, et al. Dose‐ranging study of oral mesalamine capsule (Pentasa) for active ulcerative colitis. Gastroenterology 1989;96:A195. [PubMed] [Google Scholar]
- Miner P, Nostrant T, Wruble L, Hines C, Johnson S, Wilkinson C, et al. Multicenter trial of Pentasa for active ulcerative colitis. Gastroenterology 1991;100(5 Part 2):A231. [Google Scholar]
- Robinson M, Cello J, Safdi M, Schwartz J, Roufail W, Hoop R, et al. Mesalamine (Pentasa) enhances quality of life (QofL) for ulcerative colitis (UC) patients. Gastroenterology 1991;100(5 Part 2):A243. [Google Scholar]
- Robinson M, Hanauer S, Hoop R, Zbrozek A, Wilkinson C. Mesalamine capsules enhance the quality of life for patients with ulcerative colitis. Alimentary Pharmacology and Therapeutics 1994;8(1):27‐34. [DOI] [PubMed] [Google Scholar]
Hanauer 1996 {published data only}
- Hanauer SB, Barish C, Pambianco D, Sigmon R, Gannan R, Koval G, et al. A multi‐center, double‐blind, placebo‐controlled, dose‐ranging trial of olsalazine for mild‐moderately active ulcerative colitis. Gastroenterology 1996;110:A921. [Google Scholar]
Hanauer 2005 {published data only}
- Hanauer SB, Sandborn WJ, Kornbluth A, Katz S, Safdi M, Woogen S, et al. Delayed‐release oral mesalamine at 4.8 g/day (800 mg tablet) for the treatment of moderately active ulcerative colitis: the ASCEND II trial. American Journal of Gastroenterology 2005;100(11):2478‐85. [DOI] [PubMed] [Google Scholar]
Hanauer 2007 {published data only}
- Hanauer SB, Sandborn WJ, Dallaire C, Archambault A, Yacyshyn B, Yeh C, et al. Delayed‐release oral mesalamine 4.8 g/day (800 mg tablets) compared to 2.4 g/day (400 mg tablets) for the treatment of mildly to moderately active ulcerative colitis: The ASCEND I trial. Canadian Journal of Gastroenterology 2007;21(12):827‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hetzel 1986 {published data only}
- Hetzel DJ, Bochner F, Imhoff DM, Gibson GE, Fitch RJ, Hecker R, et al. Azodisacylate (ADS) in the treatment of ulcerative colitis (UC): a controlled trial and assessment of drug disposition. Gastroenterology 1985;88:A1418. [DOI] [PubMed] [Google Scholar]
- Hetzel DJ, Shearman DJC, Bochner F, Imhoff DM, Gibson GE, Fitch RJ, et al. Azodisalicylate (Olsalazine) in the treatment of active ulcerative colitis. A placebo controlled clinical trial and assessment of drug disposition. Journal of Gastroenterology and Hepatology 1986;1:257‐66. [DOI] [PubMed] [Google Scholar]
- Hetzel DJ, Shearman DJC, Labrooy J, Bochner F, Imhoff DM, Gibson GE, et al. Olsalazine in the treatment of active ulcerative colitis: a placebo controlled clinical trial and assessment of drug disposition. Scandinavian Journal of Gastroenterology Supplement 1988;23(148):61‐9. [DOI] [PubMed] [Google Scholar]
Hiwatashi 2011 {published data only}
- Hiwatashi N, Suzuki Y, Mitsuyama K, Munakata A, Hibi T. Clinical trial: Effects of an oral preparation of mesalazine at 4 g/day on moderately active ulcerative colitis. A phase III parallel‐dosing study. Journal of Gastroenterology 2011;46(1):46‐56. [DOI] [PubMed] [Google Scholar]
Ito 2010 {published data only}
- Ito H, Iida M, Matsumoto T, Suzuki Y, Koyama H, Yoshida T, et al. A direct comparative study of two different mesalamine formulations revealed appropriate use of mesalamine for patients with active ulcerative colitis depending on the characteristics of disease. Gastroenterology 2010;1:S166. [Google Scholar]
- Ito H, Iida M, Matsumoto T, Suzuki Y, Sasaki H, Yoshida T, et al. Direct comparison of two different mesalamine formulations for the induction of remission in patients with ulcerative colitis: a double‐blind, randomized study. Inflammatory Bowel Diseases 2010;16(9):1567‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jiang 2004 {published data only}
- Jiang XL, Cui HF. Different therapy for different types of ulcerative colitis. World Journal of Gastroenterology 2004;10(10):1513‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kamm 2007 {published data only}
- Hanauer S, Sandborn W, Lichtenstein G, Kamm M, Barrett K, Joseph R. MMX mesalamine for providing remission of active mild‐to‐moderate ulcerative colitis: an evidence‐based medicine analysis. Inflammatory Bowel Diseases 2007;13(S5):663. [Google Scholar]
- Kamm MA, Sandborn WJ, Gassull M, Schreiber S, Jackowski L, Butler T, et al. Once‐daily, high‐concentration MMX mesalamine in active ulcerative colitis. Gastroenterology 2007;132(1):66‐75. [DOI] [PubMed] [Google Scholar]
Kruis 1998 {published data only}
- Kruis W, Brandes JW, Schreiber S, Theuer D, Krakamp B, Schutz E, et al. Olsalazine versus mesalazine in the treatment of mild to moderate ulcerative colitis. Alimentary Pharmacology and Therapeutics 1998;12(8):707‐15. [DOI] [PubMed] [Google Scholar]
- Kruis W, Schreiber S, Theuer D, Schutz E, Krakamp B, Otto P, et al. Comparison of an azoboundamino salicylate (olsalazine) vs. a coated aminosalicylate (mesalamine) for active ulcerative colitis. Gastroenterology 1996;110(4):942. [Google Scholar]
Kruis 2003 {published data only}
- Kruis W, Bar‐Meir S, Feher J, Mickisch O, Mlitz H, Faszczyk M, et al. The optimal dose of 5‐aminosalicylic acid in active ulcerative colitis: a dose‐finding study with newly developed mesalamine. Clinical Gastroenterology and Hepatology 2003;1(1):36‐43. [DOI] [PubMed] [Google Scholar]
- Kruis W, Meir SB, Feher J, Stolte M. Dose finding study of the efficacy and safety of newly developed 5‐ASA containing pellets in patients with active ulcerative colitis. Gastroenterology 2000;118(4 Suppl 2):A780. [Google Scholar]
Kruis 2009 {published data only}
- Kruis W, Gorelov A, Kiudelis G, Rácz I, Pokrotnieks J, Horynski M, et al. Once daily dosing of 3g mesalamine (Salofalk® Granules) is therapeutic equivalent to a three‐times daily dosing of 1g mesalamine for the treatment of active ulcerative colitis. Gastroenterology 2007;132(4 Suppl 1):A130‐1. [Google Scholar]
- Kruis W, Kiudelis G, Rácz I, Gorelov IA, Pokrotnieks J, Horynski M, et al. Once daily versus three times daily mesalazine granules in active ulcerative colitis: a double‐blind, double‐dummy, randomised, non‐inferiority trial. Gut 2009;58(2):233‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Levine 2002 {published data only}
- Levine DS, Pruitt R, Riff D, Koval G, Sales D, Wruble L, et al. A multi‐center, double‐blind dose‐response trial of Colazide (balsalazide disodium) and Asacol (mesalamine) for mild‐moderately active ulcerative colitis. Gastroenterology 1997;112:A1026. [Google Scholar]
- Levine DS, Riff DS, Pruitt R, Wruble L, Koval G, Sales D, et al. A randomized, double blind, dose‐response comparison of balsalazide (6.75 g), balsalazide (2.25 g), and mesalamine (2.4 g) in the treatment of active, mild‐to‐moderate ulcerative colitis. American Journal of Gastroenterology 2002;97(6):1398‐407. [DOI] [PubMed] [Google Scholar]
Lichtenstein 2007 {published data only}
- Lichtenstein GR, Kamm MA, Boddu P, Gubergrits N, Lyne A, Butler T, et al. Effect of once‐ or twice‐daily MMX mesalamine (SPD476) for the induction of remission of mild to moderately active ulcerative colitis. Clinical Gastroenterology and Hepatology 2007;5(1):95‐102. [DOI] [PubMed] [Google Scholar]
Maier 1985 {published data only}
- Maier K, Fruhmorgen P, Bode JC, Heller T, Gaisberg U, Klotz U. Successful acute treatment of chronic inflammatory intestinal diseases with oral 5‐aminosalicylic acid [Erfolgreiche akutbehandlung chronisch‐entzundlicher darmerkrankungen mit oraler 5‐aminosalicylsaure]. Deutsche medizinische Wochenschrift 1985;110(10):363‐8. [DOI] [PubMed] [Google Scholar]
Mansfield 2002 {published data only}
- Mansfield JC, Giaffer MH, Cann PA, McKenna D, Thornton PC. A double‐blind comparison of balsalazide, 6.75 g, and sulfasalazine, 3 g, as sole therapy in the management of ulcerative colitis. Alimentary Pharmacology and Therapeutics 2002;16(1):69‐77. [DOI] [PubMed] [Google Scholar]
Marakhouski 2005 {published data only}
- Marakhouski Y, Fixa B, Holoman J. Erratum: a double‐blind dose‐escalating trial comparing novel mesalazine pellets with mesalazine tablets in active ulcerative colitis. Alimentary Pharmacology and Therapeutics 2005;21(6):793. [DOI] [PubMed] [Google Scholar]
- Marakhouski Y, Fixa B, Holoman J, Hulek P, Lukas M, Batovsky M, et al. A double‐blind dose‐escalating trial comparing novel mesalazine pellets with mesalazine tablets in active ulcerative colitis. Alimentary Pharmacology and Therapeutics 2005;21(2):133‐40. [DOI] [PubMed] [Google Scholar]
Miglioli 1990 {published data only}
- Miglioli M, Bianchi Porro G, Brunetti G, Sturniolo GC, and the Italian IBD group. Oral delayed‐release mesalazine in the treatment of mild ulcerative colitis: a dose ranging study. European Journal of Gastroenterology and Hepatology 1990;2:229‐34. [Google Scholar]
- Miglioli M, Brunetti G, Sturniolo GC, Bianchi Porro G, Campieri M, Cottone M, et al. Oral 5‐ASA (Asacol) in mild ulcerative colitis. a randomized double blind dose ranging trial. Italian Journal of Gastroenterology 1989;21(1 Suppl):7‐8. [Google Scholar]
Mihas 1988 {published data only}
- Mihas AA, Xynopoulos D, Mihas TA. A prospective trial of oral 5‐aminosalicylic acid vs sulfasalazine in ulcerative colitis.. Gastroenterology 1988;94(5 Part 2):A303. [Google Scholar]
Munakata 1995 {published data only}
- Munakata A, Yoshida Y, Muto T. Clinical efficacy of oral controlled‐release mesalazine, N‐5ASA on ulcerative colitis: double‐blind comparative study in comparison with salazosulphapyridineu. Yakuri to Chiryo (Japanese Pharmacology and Therapeutics) 1994;22(Suppl 10):S2555‐83. [Google Scholar]
- Munakata A, Yoshida Y, Muto T, Tsuchiya S, Fukushima T, Hiwatashi N, et al. Double‐blind comparative study of sulfasalazine and controlled‐release mesalazine tablets in the treatment of active ulcerative colitis. Journal of Gastroenterology 1995;30(Suppl 8):108‐11. [PubMed] [Google Scholar]
Pontes 2014 {published data only}
- Pontes C, Vives R, Torres F, Panes J. Safety and activity of dersalazine sodium in patients with mild‐to‐moderate active colitis: double‐blind randomized proof of concept study. Inflammatory Bowel Diseases 2014;20(11):2004‐12. [DOI] [PubMed] [Google Scholar]
Pruitt 2002 {published data only}
- Pruitt R, Hanson J, Safdi M, Wruble L, Hardi R, Johanson J, et al. Balsalazide is superior to mesalamine in the time to improvement of signs and symptoms of acute mild‐to‐moderate ulcerative colitis. American Journal of Gastroenterology 2002;97(12):3078‐86. [DOI] [PubMed] [Google Scholar]
- Pruitt R, Hanson J, Safdi M, Wruble L, Hardi R, Johanson JF, et al. Balsalazide is superior to mesalamine in the time to improvement of signs and symptoms of acute ulcerative colitis. Gastroenterology 2000;118(4 Suppl 2):A120‐1. [DOI] [PubMed] [Google Scholar]
Qian 2004 {published data only}
- Qian LP, Lin GJ, Xu SR, Ding WQ. Clinical effect of olsalazine sodium capsule in the treatment of ulcerative colitis. Fudan University Journal of Medical Sciences 2004;31(4):421‐4. [Google Scholar]
Rachmilewitz 1989 {published data only}
- Rachmelewitz D. Mesalazine (5‐ASA) is as effective as sulfasalazine (SZ) in the treatment of active ulcerative colitis (UC). Gastroenterology 1988;94(5 Part 2):A362. [Google Scholar]
- Rachmilewitz D. Coated mesalazine (5‐aminosalicylic acid) versus sulphasalazine in the treatment of active ulcerative colitis: a randomised trial. BMJ 1989;298:82‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Raedler 2004 {published data only}
- Behrens C, Bias P, Malchow H, Raedler A. Mesalazine (5‐ASA) micropellets show comparable efficacy and tolerability as mesalazine tablets in patients with ulcerative colitis. A prospective, multi‐national, randomised, double‐blind, active‐controlled clinical phase II study. Gastroenterology 2003;124(4 Suppl 1):A379. [Google Scholar]
- Raedler A, Behrens C, Bias P. Mesalazine (5‐aminosalicylic acid) micropellets show similar efficacy and tolerability to mesalazine tablets in patients with ulcerative colitis ‐‐ results from a randomized‐controlled trial. Alimentary Pharmacology and Therapeutics 2004;20(11‐12):1353‐63. [DOI] [PubMed] [Google Scholar]
Rao 1989 {published data only}
- Rao SS, Dundas SA, Holdsworth CD, Cann PA, Palmer KR, Corbett CL. Olsalazine or sulphasalazine in first attacks of ulcerative colitis? A double‐blind study. Gut 1989;30(5):675‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SS, Holdsworth CD, Palmer KL, Cann PA, Dundas SA, Corbett CL. Olsalazine versus sulphasalazine (SASP) in first attacks of ulcerative colitis (UC): a double blind study. Gut 1988;29:A705. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rijk 1991 {published data only}
- Rijk MCM, Tongerson JHM. The efficacy and safety of sulphasalazine and olsalazine in patients with active ulcerative colitis. Gastroenterology 1991;100:A243. [Google Scholar]
Riley 1988 {published data only}
- Riley SA, Mani V, Goodman MJ, Herd ME, Dutt S, Turnberg A. Comparison of delayed release 5‐aminosalicylic acid (mesalazine) and sulphasalazine in the treatment of mild to moderate ulcerative colitis relapse. Gut 1988;29:669‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley SA, Mani V, Goodman MJ, Turnberg LA. Delayed‐release 5‐aminosalicylic acid (5‐ASA) and sulphasalazine (SSZ) in the treatment of mild to moderate ulcerative colitis (UC) relapse. Gut 1987;28:A1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
Robinson 1988 {published data only}
- Robinson M, Gitnick G, Balart L, Das K, Turkin D. Olsalazine in the treatment of mild to moderate ulcerative colitis. Gastroenterology 1988;84:A381. [Google Scholar]
Sandborn 2009 {published data only}
- Sandborn WJ, Regula J, Feagan B, Belousova EA, Jojic NV, Lukas M, et al. Efficacy and safety of delayed‐release oral mesalamine at 4.8g/d (800mg tablet) in the treatment of moderately active ulcerative colitis: results of the ASCEND III study. Gastroenterology 2008;134(4 Suppl 1):A99. [Google Scholar]
- Sandborn WJ, Regula J, Feagan BG, Belousova E, Jojic N, Lukas M, et al. Delayed‐release oral mesalamine 4.8 g/day (800‐mg tablet) is effective for patients with moderately active ulcerative colitis. Gastroenterology 2009;137(6):1934‐43. [DOI] [PubMed] [Google Scholar]
Sandborn 2012 {published data only}
- Sandborn WJ, Travis S, Moro L, Jones R, Gautille T, Bagin R, et al. Once‐daily budesonide MMX® extended‐release tablets induce remission in patients with mild to moderate ulcerative colitis: results from the CORE I study. Gastroenterology 2012;143(5):1218‐26. [DOI] [PubMed] [Google Scholar]
Scherl 2009 {published data only}
- Pruitt RE, Rosen AA, Wruble L, Sedghi S, Shepard RD, Mareya SM, et al. Safety and tolerability of twice‐daily balsalazide tablets: results of a phase 3, randomized, double‐blind, placebo‐controlled, multicenter study. Gastroenterology 2008;134(4 Suppl 1):A494. [Google Scholar]
- Rubin DT, Rosen AA, Sedghi S, Shepard RD, Mareya SM, Huang S, et al. Twice‐daily balsalazide tablets improve patient quality of life after 2 and 8 weeks of treatment: results of a phase 3, randomized, double‐blind, placebo‐controlled, multicenter study. Gastroenterology 2008;134(4 Suppl 1):A494. [Google Scholar]
- Scherl EJ, Pruitt R, Gordon GL, Lamet M, Shaw A, Huang S, et al. Safety and efficacy of a new 3.3 g b.i.d. tablet formulation in patients with mild‐to‐moderately‐active ulcerative colitis: a multicenter, randomized, double‐blind, placebo‐controlled study. American Journal of Gastroenterology 2009;104(6):1452‐9. [DOI] [PubMed] [Google Scholar]
- Scherl EJ, Pruitt RE, Gordon GL, Lamet M, Shaw AL, Huang S, et al. Twice‐daily dosing of balsalazide tablets 3.3 g is safe and effective in the treatment of mild‐to‐moderate ulcerative colitis. Gastroenterology 2009;1:A520. [Google Scholar]
Schroeder 1987 {published data only}
- Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5‐aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. New England Journal of Medicine 1987;317:1625‐9. [DOI] [PubMed] [Google Scholar]
Sninsky 1991 {published data only}
- Sninsky CA, Cort DH, Shanahan F, Powers BJ, Sessions JT, Pruitt RE, et al. Oral mesalamine (Asacol) for mildly to moderately active ulcerative colitis. A multicenter study. Annals of Internal Medicine 1991;115:350‐5. [DOI] [PubMed] [Google Scholar]
Sutherland 1990 {published data only}
- Sutherland LR, Robinson M, Onstad G, Peppercorn M, Greenberger N, Goodman M, et al. A double‐blind, placebo‐controlled, multicentre study of the efficacy and safety of 5‐aminosalicylic acid tablets in the treatment of ulcerative colitis. Canadian Journal of Gastroenterology 1990;4:463‐7. [Google Scholar]
Tursi 2004 {published data only}
- Tursi A, Brandimarte G, Giorgetti GM, Forti G, Modeo ME, Gigliobianco A. Low‐dose balsalazide plus a high‐potency probiotic preparation is more effective than balsalazide alone or mesalazine in the treatment of acute mild‐to‐moderate ulcerative colitis. Medical Science Monitor 2004;10(11):PI126‐31. [PubMed] [Google Scholar]
Willoughby 1988 {published data only}
- Willoughby CP, Cowan RE, Gould SR, Machell RJ, Stewart JB. Double‐blind comparison of olsalazine and sulfasalazine in active ulcerative colitis. Scandinavian Journal of Gastroenterology 1988;148:40‐4. [DOI] [PubMed] [Google Scholar]
Zinberg 1990 {published data only}
- Zinberg J, Molinas S, Das KM. A double‐blinded, placebo‐controlled clinical study of azodisalicylate sodium (olsalazine) in the treatment of ulcerative colitis. Gastroenterology 1987;92:A1711. [PubMed] [Google Scholar]
- Zinberg J, Molinas S, Das KM. Double‐blind placebo‐controlled study of olsalazine in the treatment of ulcerative colitis. American Journal of Gastroenterology 1990;85:562‐6. [PubMed] [Google Scholar]
References to studies excluded from this review
Adrizzone 2006 {published data only}
- Ardizzone S, Maconi G, Russo A, Imbesi V, Colombo E, Bianchi Porro G. Randomised controlled trial of azathioprine and 5‐aminosalicylic acid for treatment of steroid dependent ulcerative colitis. Gut 2006;55(1):47‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ahluwalia 1992 {published data only}
- Ahluwalia NK, Thompson DG, Goodman MJ, Mani V, McIntyre J, Dane G. Double‐blind randomized trial of 4.8 g vs. 2.4 g of mesalazine for 4 weeks in the treatment of acute ulcerative colitis. Gastroenterology 1992;102:A588. [Google Scholar]
Gross 2011 {published data only}
- Gross V, Bunganic I, Belousova EA, Mikhailova TL, Kupcinskas L, Kiudelis G, et al. 1.3g mesalazine granules are superior to 9mg budesonide for achieving remission in active ulcerative colitis: a double‐blind, double‐dummy, randomised trial. Journal of Crohn's and Colitis 2011;5(2):129‐38. [DOI] [PubMed] [Google Scholar]
Irvine 2008 {published data only}
- Irvine EJ, Yeh CH, Ramsey D, Stirling AL, Higgins PD. The effect of mesalazine therapy on quality of life in patients with mildly and moderately active ulcerative colitis. Alimentary Pharmacology and Therapeutics 2008;28(11‐12):1278‐86. [DOI] [PubMed] [Google Scholar]
Kamm 2009 {published data only}
- Kamm MA, Lichtenstein GR, Sandborn WJ, Schreiber S, Lees K, Barrett K, et al. Effect of extended MMX mesalamine therapy for acute, mild‐to‐moderate ulcerative colitis. Inflammatory Bowel Diseases 2009;15(1):1‐8. [DOI] [PubMed] [Google Scholar]
Mahmood 2005 {published data only}
- Mahmood A, Melley L, Fitzgerald AJ, Ghosh S, Playford RJ. Trial of trefoil factor 3 enemas, in combination with oral 5‐aminosalicylic acid, for the treatment of mild‐to‐moderate left‐sided ulcerative colitis. Alimentary Pharmacology and Therapeutics 2005;21(11):1357‐64. [DOI] [PubMed] [Google Scholar]
Paoluzi 2002 {published data only}
- Paoluzi P, D'Albasio G, Pera A, Bianchi Porro G, Paoluzi OA, Pica R, et al. Oral and topical 5‐aminosalicylic acid (mesalazine) in inducing and maintaining remission in mild‐moderate relapse of ulcerative colitis: one‐year randomised multicentre trial. Digestive and Liver Disease 2002;34(11):787‐93. [DOI] [PubMed] [Google Scholar]
Pruitt 1991 {published data only}
- Pruitt RE, Gremillion DE, Herring RW, Bailey AH, Faust TW, Potter M, et al. Oral Asacol in the treatment of mild to moderate ulcerative colitis: the Nashville experience. Journal of the Tennessee Medical Association 1991;84:237. [PubMed] [Google Scholar]
Safdi 1997 {published data only}
- Safdi M, DeMicco M, Sninsky C, Banks P, Wruble L, Deren J, et al. A double‐blind comparison of oral versus rectal mesalamine versus combination therapy in the treatment of distal ulcerative colitis. American Journal of Gastroenterology 1997;92(10):1867‐71. [PubMed] [Google Scholar]
Vecchi 2001 {published data only}
- Vecchi M, Meucci G, Gionchetti P, Beltrami M, Maurizio P, Beretta L, et al. Oral versus combination mesalazine therapy in active ulcerative colitis: A double‐blind, double‐dummy, randomized multicentre study. Alimentary Pharmacology and Therapeutics 2001;15:251‐6. [DOI] [PubMed] [Google Scholar]
Vernia 2000 {published data only}
- Vernia P, Monteleone G, Grandinetti G, Villotti G, Giulio E, Frieri G, et al. Combined oral sodium butyrate and mesalazine treatment compared to oral mesalazine alone in ulcerative colitis: randomized, double‐blind, placebo‐controlled pilot study. Digestive Diseases and Sciences 2000;45(5):976‐81. [DOI] [PubMed] [Google Scholar]
Additional references
Andrade 1995
- Andrade SE, Walker AM, Gottlieb LK, Hollenberg NK, Testa MA, Saperia GM, et al. Discontinuation of antihyperlipidemic drugs – do rates reported in clinical trials reflect rates in primary care settings?. New England Journal of Medicine 1995;332(17):1125‐31. [DOI] [PubMed] [Google Scholar]
Arend 2004
- Arend LJ, Springate JE. Interstitial nephritis from mesalazine: case report and literature review. Pediatric Nephrology 2004;19(5):550‐3. [DOI] [PubMed] [Google Scholar]
Azad Khan 1977
- Azad Khan AK, Piris J, Truelove SC. An experiment to determine the active therapeutic moiety of sulfasalazine. Lancet 1977;2(8044):892‐5. [DOI] [PubMed] [Google Scholar]
Beaulieu 2009
- Beaulieu DB, Schwartz DA. Medication persistence in patients with ulcerative colitis: meeting the challenges and improving patient outcomes. Medscape CME Gastroenterology 2009. Available from http://www.medscape.org/viewarticle/713895.
Brixner 2007
- Brixner D, Magowan S, Accortt N. Evaluation of prescription refill patterns based on daily dosing regimen and pill load for calcium channel blockers. Academy of Managed Care Pharmacy Annual Meeting. San Diego, CA, 2007.
Chan 1983
- Chan RP, Pope DJ, Gilbert AP, Sacra PJ, Baron JH, Lennard‐Jones JE. Studies of two novel sulfasalazine analogs, ipsalazide and balsalazide. Digestive Diseases and Sciences 1983;28:609‐15. [DOI] [PubMed] [Google Scholar]
Das 1973
- Das KM, Eastwood MA, McManus JPA, Sircus W. Adverse reactions during salicylazosulfapyridine therapy and the relation with drug metabolism and acetylator phenotype. New England Journal of Medicine 1973;289:491‐5. [DOI] [PubMed] [Google Scholar]
Dew 1982
- Dew MJ, Hughes PJ, Lee MG, Evans BK, Rhodes J. An oral preparation to release drugs in the human colon. British Journal of Clinical Pharmacology 1982;14:405‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ediger 2007
- Ediger JP, Walker JR, Graff L, Lix L, Clara I, Rawsthorne P, et al. Predictors of medication adherence in inflammatory bowel disease. American Journal of Gastroenterology 2007;102(7):1417‐26. [DOI] [PubMed] [Google Scholar]
Elseviers 2004
- Elseviers MM, D'Haens G, Lerebours E, Plane C, Stolear JC, Riegler G, et al. Renal impairment in patients with inflammatory bowel disease: association with aminosalicylate therapy?. Clinical Nephrology 2004;61(2):83‐9. [DOI] [PubMed] [Google Scholar]
Frandsen 2002
- Frandsen NE, Saugmann S, Marcussen N. Acute interstitial nephritis associated with the use of mesalazine in inflammatory bowel disease. Nephron 2002;92(1):200‐2. [DOI] [PubMed] [Google Scholar]
Greenfield 1993
- Greenfield SM, Punchard NA, Teare JP, Thompson RP. Review article: the mode of action of the aminosalicylates in inflammatory bowel disease. Alimentary Pharmacology and Therapeutics 1993;7:369‐83. [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hardy 1987
- Hardy JG, Healey JNC, Reynolds JR. Evaluation of an enteric‐coated delayed‐release 5‐aminosalicylic acid tablet in patients with inflammatory bowel disease. Alimentary Pharmacology and Therapeutics 1987;1:273‐80. [DOI] [PubMed] [Google Scholar]
Hayllar 1991
- Hayllar J, Bjarnason I. Sulphasalazine in ulcerative colitis: in memoriam?. Gut 1991;32:462‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Altman DG. Measuring inconsistency in meta‐analysis. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Ireland 1987
- Ireland A, Jewell DP. Olsalazine in patients intolerant of sulphasalazine. Scandinavian Journal of Gastroenterology 1987;22(9):1038‐40. [DOI] [PubMed] [Google Scholar]
Kane 2001
- Kane SV, Cohen RD, Aikens JE, Hanauer SB. Prevalence of nonadherence with maintenance mesalamine in quiescent ulcerative colitis. American Journal of Gastroenterology 2001;96(10):2929‐33. [DOI] [PubMed] [Google Scholar]
Kane 2003
- Kane S, Huo D, Aikens J, Hanauer S. Medication nonadherence and the outcomes of patients with quiescent ulcerative colitis. American Journal of Medicine 2003;114(1):39‐43. [DOI] [PubMed] [Google Scholar]
Kane 2006
- Kane SV. Systematic review: adherence issues in the treatment of ulcerative colitis. Alimentary Pharmacology and Therapeutics 2006;23(5):577‐85. [DOI] [PubMed] [Google Scholar]
Kane 2008
- Kane SV, Brixner D, Rubin DT, Sewitch MJ. The challenge of compliance and persistence: focus on ulcerative colitis. Journal of Managed Care Pharmacy 2008;14(1 Suppl A):s2‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kjaergaard 1989
- Kjaergaard N, Ambrosius Christensen L, Lauritsen JG, Rasmussen N, Honore Hansen S. Effects of mesalazine substitution on salicylazosulfapyridine‐induced seminal abnormalities in men with ulcerative colitis. Scandinavian Journal of Gastroenterology 1989;24:891‐6. [DOI] [PubMed] [Google Scholar]
Klotz 1980
- Klotz U, Maier K, Fischer C, Heinkel K. Therapeutic efficacy of sulfasalazine and its metabolites in patients with ulcerative colitis and Crohn's disease. New England Journal of Medicine 1980;303:1499‐502. [DOI] [PubMed] [Google Scholar]
Levy 1999
- Levy RL, Feld AD. Increasing patient adherence to gastroenterology treatment and prevention regimens. American Journal of Gastroenterology 1999;94(7):1733‐42. [DOI] [PubMed] [Google Scholar]
Maeda 2001
- Maeda S, Nomura S, Tahara M, Haneda M, Kikkawa R. Interstitial nephritis after treatment with mesalazine in the patient with ulcerative colitis. Nihon Naika Gakkai Zasshi 2001;90(5):872‐3. [DOI] [PubMed] [Google Scholar]
Magowan 2006
- Magowan S, Kane S, Lange JL. 5‐ASA prescription refill rates for ulcerative colitis are independent of formulation and dosing regimens. American Journal of Gastroenterology 2006;101:S447. Abstract 1144. [Google Scholar]
Misiewitz 1965
- Misiewitz JJ, Lennard‐Jones JE, Connell AM, Baron JH, Jones FA. Controlled trial of sulfasalazine in maintenance therapy for ulcerative colitis. Lancet 1965;1:185‐8. [Google Scholar]
Myers 1987
- Myers B, Evans DNW, Rhodes J, Evans BK, Hughes BR, Lee MG, et al. Metabolism and urinary excretion of 5‐aminosalicylic acid in healthy volunteers when given intravenously or released for absorption at different sites in the gastrointestinal tract. Gut 1987;28:196‐200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nielsen 1982
- Nielsen OH. Sulfasalazine intolerance: a retrospective study of the reasons for discontinuing treatment with sulfasalazine in patients with chronic inflammatory bowel disease. Scandinavian Journal of Gastroenterology 1982;17:389‐93. [DOI] [PubMed] [Google Scholar]
Nielsen 1983
- Nielsen OH, Bondesen S. Kinetics of 5‐aminosalicylic acid after jejunal instillation in man. British Journal of Clinical Pharmacology 1983;16:738‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Peppercorn 1972
- Peppercorn MA, Goldman P. The role of intestinal bacteria in the metabolism of salicylazo‐sulfapyridine. Journal of Pharmacology and Experimental Therapeutics 1972;181:555‐62. [PubMed] [Google Scholar]
Rao 1987
- Rao SS, Cann PA, Holdsworth CD. Clinical experience of the tolerance of mesalazine and olsalazine in patients intolerant of sulphasalazine. Scandinavian Journal of Gastroenterology 1987;22(3):332‐6. [DOI] [PubMed] [Google Scholar]
Rasmussen 1982
- Rasmussen SN, Bondesen S, Hvidberg EF, Hansen SH, Binder V, Halskou S, et al. 5‐aminosalicylic acid in a slow‐release preparation: bioavailability, plasma level, and excretion in humans. Gastroenterology 1982;83:1062‐70. [PubMed] [Google Scholar]
Riley 1987
- Riley SA, Lecarpentier J, Mini V, Goodman JJ, Mandal BK, Turnberg LA. Sulfasalazine induced seminal abnormalities in ulcerative colitis: result of mesalazine substitution. Gut 1987;28:1008‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sandborn 2002a
- Sandborn WJ. Rational selection of oral 5‐aminosalicylate formulations and prodrugs for the treatment of ulcerative colitis. American Journal of Gastroenterology 2002;97(12):2939‐41. [DOI] [PubMed] [Google Scholar]
Sandborn 2002b
- Sandborn WJ, Hanauer SB, Buch A. Comparable systemic absorption of 5‐ASA and N‐AC‐5‐ASA from U.S. Asacol and Colazal. American Journal of Gastroenterology 2002;97:S263. [Google Scholar]
Sandborn 2002c
- Sandborn WJ, Hanauer SB. The pharmacokinetic profiles of oral 5ASA formulations used in the management of ulcerative colitis: A systematic review. American Journal of Gastroenterology 2002;97:S269. [Google Scholar]
Sandborn 2003
- Sandborn WJ, Hanauer SB. Systematic review: the pharmacokinetic profiles of oral mesalazine formulations and mesalazine pro‐drugs used in the management of ulcerative colitis. Alimentary Pharmacology and Therapeutics 2003;17(1):29‐42. [DOI] [PubMed] [Google Scholar]
Schroeder 1972
- Schroeder H, Campbell DES. Absorption, metabolism, and excretion of salicylazosulfapyridine in man. Clinical Pharmacology and Therapeutics 1972;13:539‐51. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Shale 2003
- Shale MJ, Riley SA. Studies of compliance with delayed‐release mesalazine therapy in patients with inflammatory bowel disease. Alimentary Pharmacology and Therapeutics 2003;18(2):191‐8. [DOI] [PubMed] [Google Scholar]
Staerk Laursen 1990
- Staerk Laursen L, Stokholm M, Bukhave K, Rask‐Madsen J, Lauritsen K. Disposition of 5‐aminosalicylic acid by olsalazine and three mesalazine preparations in patients with ulcerative colitis: comparison of intraluminal colonic concentrations, serum values, and urinary excretion. Gut 1990;31:1271‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sutherland 2006a
- Sutherland LR, MacDonald JK. Oral 5‐aminosalicylic acid for maintenance of remission in ulcerative colitis. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD000544.pub2] [DOI] [PubMed] [Google Scholar]
Svartz 1942
- Svartz N. Salazyopyrin, a new sulfanilamide preparation: A. Therapeutic results in rheumatic polyarthritis. B. Therapeutic results in ulcerative colitis. C. Toxic manifestations in treatment with sulfanilamide preparation. Acta Medica Scandinavia 1942;110:557‐90. [Google Scholar]
Truelove 1955
- Truelove SC, Witts LJ. Cortisone in ulcerative colitis: final report on a therapeutic trial. British Medical Journal 1955;4947:1041‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Truelove 1959
- Truelove SC, Witts LJ. Cortisone and corticotrophin in ulcerative colitis. British Medical Journal 1959;i:387‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Hees 1980
- Hees PAM, Bakker JH, Tongeren JHM. Effect of sulfapyridine, 5‐aminosalicylic acid, and placebo in patients with idiopathic proctitis: a study to determine the active therapeutic moiety of sulfasalazine. Gut 1980;21:632‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Willoughby 1982
- Willoughby CP, Aronson JK, Agback H, Bodin NO, Truelove SC. Distribution and metabolism in healthy volunteers of disodium azodisalicylate, a potential therapeutic agent for ulcerative colitis. Gut 1982;23:1081‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Feagan 2012
- Feagan BG, MacDonald JK. Oral 5‐aminosalicylic acid for induction of remission in ulcerative colitis. Cochrane Database of Systematic Reviews 2012, Issue 10. [DOI: 10.1002/14651858.CD000543.pub3] [DOI] [PubMed] [Google Scholar]
Sutherland 1993
- Sutherland LR, May GR, Shaffer EA. Sulfasalazine revisited: a meta‐analysis of 5‐aminosalicylic acid in the treatment of ulcerative colitis. Annals of Internal Medicine 1993;118:540‐9. [DOI] [PubMed] [Google Scholar]
Sutherland 1997
- Sutherland LR, Roth DE, Beck PL. Alternatives to sulfasalazine: A meta‐analysis of 5‐ASA in the treatment of ulcerative colitis. Inflammatory Bowel Diseases 1997;3:65‐78. [PubMed] [Google Scholar]
Sutherland 2006b
- Sutherland LR, MacDonald JK. Oral 5‐aminosalicylic acid for induction of remission in ulcerative colitis. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD000543.pub2] [DOI] [PubMed] [Google Scholar]


