Abstract
Background
People with heart failure report various symptoms and show a trajectory of periodic exacerbations and recoveries, where each exacerbation event may lead to death. Current clinical practice guidelines indicate the importance of discussing future care strategies with people with heart failure. Advance care planning (ACP) is the process of discussing an individual's future care plan according to their values and preferences, and involves the person with heart failure, their family members or surrogate decision‐makers, and healthcare providers. Although it is shown that ACP may improve discussion about end‐of‐life care and documentation of an individual's preferences, the effects of ACP for people with heart failure are uncertain.
Objectives
To assess the effects of advance care planning (ACP) in people with heart failure compared to usual care strategies that do not have any components promoting ACP.
Search methods
We searched CENTRAL, MEDLINE, Embase, CINAHL, Social Work Abstracts, and two clinical trials registers in October 2019. We checked the reference lists of included studies. There were no restrictions on language or publication status.
Selection criteria
We included randomised controlled trials (RCTs) that compared ACP with usual care in people with heart failure. Trials could have parallel group, cluster‐randomised, or cross‐over designs. We included interventions that implemented ACP, such as discussing and considering values, wishes, life goals, and preferences for future medical care. The study participants comprised adults (18 years of age or older) with heart failure.
Data collection and analysis
Two review authors independently extracted outcome data from the included studies, and assessed their risk of bias. We contacted trial authors when we needed to obtain missing information.
Main results
We included nine RCTs (1242 participants and 426 surrogate decision‐makers) in this review. The meta‐analysis included seven studies (876 participants). Participants' mean ages ranged from 62 to 82 years, and 53% to 100% of the studies' participants were men. All included studies took place in the US or the UK.
Only one study reported concordance between participants' preferences and end‐of‐life care, and it enrolled people with heart failure or renal disease. Owing to one study with small sample size, the effects of ACP on concordance between participants' preferences and end‐of‐life care were uncertain (risk ratio (RR) 1.19, 95% confidence interval (CI) 0.91 to 1.55; participants = 110; studies = 1; very low‐quality evidence). It corresponded to an assumed risk of 625 per 1000 participants receiving usual care and a corresponding risk of 744 per 1000 (95% CI 569 to 969) for ACP. There was no evidence of a difference in quality of life between groups (standardised mean difference (SMD) 0.06, 95% CI –0.26 to 0.38; participants = 156; studies = 3; low‐quality evidence). However, one study, which was not included in the meta‐analysis, showed that the quality of life score improved by 14.86 points in the ACP group compared with 11.80 points in the usual care group.
Completion of documentation by medical staff regarding discussions with participants about ACP processes may have increased (RR 1.68. 95% CI 1.23 to 2.29; participants = 92; studies = 2; low‐quality evidence). This corresponded to an assumed risk of 489 per 1000 participants with usual care and a corresponding risk of 822 per 1000 (95% CI 602 to 1000) for ACP. One study, which was not included in the meta‐analysis, also showed that ACP helped to improve documentation of the ACP process (hazard ratio (HR) 2.87, 95% CI 1.09 to 7.59; participants = 232).
Three studies reported that implementation of ACP led to an improvement of participants' depression (SMD –0.58, 95% CI –0.82 to –0.34; participants = 278; studies = 3; low‐quality evidence). We were uncertain about the effects of ACP on the quality of communication when compared to the usual care group (MD –0.40, 95% CI –1.61 to 0.81; participants = 9; studies = 1; very low‐quality evidence). We also noted an increase in all‐cause mortality in the ACP group (RR 1.32, 95% CI 1.04 to 1.67; participants = 795; studies = 5).
The studies did not report participants' satisfaction with care/treatment and caregivers' satisfaction with care/treatment.
Authors' conclusions
ACP may help to increase documentation by medical staff regarding discussions with participants about ACP processes, and may improve an individual's depression. However, the quality of the evidence about these outcomes was low. The quality of the evidence for each outcome was low to very low due to the small number of studies and participants included in this review. Additionally, the follow‐up periods and types of ACP intervention were varied. Therefore, further studies are needed to explore the effects of ACP that consider these differences carefully.
Plain language summary
Advance care planning for adults with heart failure
Review question
To investigate how strategies of advance care planning (ACP) affect people with heart failure.
Background
People with heart failure present with various symptoms, such as breathlessness and fatigue, and symptoms are often complicated with periodic exacerbations and recoveries. Current clinical guidelines emphasise the importance of discussing future care with people with heart failure as well as their families. Advance care planning (ACP) is the process of discussing an individual's future care plan (e.g. the type of care/treatment that the individual prefers to receive, the environment or setting where the care/treatment takes place) according to their values and preferences. This interaction involves the individual, the responsible healthcare providers, family members, and other immediate carers/supporters. ACP may improve discussion about a patient's future care and documentation of an individual's preferences regarding care. However, it is unclear whether ACP is beneficial in improving quality of life, depression, and overall satisfaction with care among people with heart failure.
Study characteristics
In October 2019, we searched for studies assessing the effects of ACP in people with heart failure. We included studies that delivered ACP, which included different methods such as discussion and consideration of individuals' values and preferences on future care and medical treatment compared to usual care strategies.
Key results
This review included nine studies involving 1242 participants and 426 families/carers. Data from seven studies (876 participants) showed that ACP may increase completion of documentation by medical staff regarding discussions with participants about ACP processes and may improve depression of participants. All‐cause mortality might be increased in participants receiving ACP. The effects of ACP on quality of life remained uncertain due to the inclusion of small studies in our meta‐analysis. This is further illustrated by the fact that only one study reported whether the received end‐of‐life care met participants' preferences. Similarly, only one study reported the quality of communication during ACP between participants and healthcare providers. Therefore, the effects of ACP on whether end‐of‐life care met participants' preferences and on quality of communication were uncertain. None of the studies evaluated satisfaction with care.
Quality of the evidence
The quality of evidence was low/very low. Since the number of studies and patients in this review was small, the effects of ACP were limited. There is clearly a need for high‐quality evidence from large studies to fully explore the effects of ACP for people with heart failure, in particular their quality of life and whether end‐of‐life care received after ACP actually meet their preferences.
Summary of findings
Summary of findings for the main comparison. Advance care planning compared with usual care for patients with heart failure.
| Advance care planning compared with usual care for patients with heart failure | ||||||
|
Patient or population: people with heart failure with or without their surrogate decision‐makers/carers Settings: inpatient and outpatient hospitals and clinics Intervention: ACP Comparison: usual care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Usual care | ACP | |||||
|
Concordance between participants' preferences and end‐of‐life care (yes/no) Post‐death data: mean days to death, ACP group (388.8 ± 255.7); usual care group (362.2 ± 288.4) |
625 per 1000 | 744 per 1000 (569 to 969) | RR 1.19 (0.91 to 1.55) | 110 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | — |
|
Participants' quality of life Measured by EQ‐5D and KCCQ. Higher scores indicate high‐quality of life. Follow‐up: 2 weeks to 6 months |
The quality of life score in the ACP groups was on average 0.06 SDs higher (0.26 lower to 0.38 higher) than in the usual care groups. | — | 156 (3 RCTs) | ⊕⊕⊝⊝ Lowc,d | 1 additional study reported quality of life using the MLHF Questionnaire. The study showed that the quality of life score was improved by 14.86 points in the intervention group compared with 11.80 points in the usual care group at 3 months. Generally, 0.2 SD represents a small difference, 0.5 moderate, and 0.8 large. |
|
| Patients' satisfaction with care/treatment (yes/no) | — | — | — | — | — | Outcome not reported. |
|
Completion of documentation by medical staff regarding discussions with participants about ACP processes (yes/no) Follow‐up: 3–6 months |
489 per 1000 | 822 per 1000 (602 to 1000) | RR 1.68 (1.23 to 2.29) | 92 (2 RCTs) | ⊕⊕⊝⊝ Lowc,e | 1 additional study reported completion of documentation with HR (HR 2.87, 95% CI 1.09 to 7.59; P = 0.033). |
|
Participants' depression Measured on PHQ‐8, PHQ‐9, and HADS. Higher scores indicate high depression Follow‐up: 2 weeks to 6 months |
The depression score in the ACP groups was on average 0.58 SDs (0.82 to 0.34) lower than in the usual care groups. | — | 278 (3 RCTs) | ⊕⊕⊝⊝ Lowc,e |
Generally, 0.2 SD represents a small difference, 0.5 moderate, and 0.8 large. | |
| Caregivers' satisfaction with care/treatment (yes/no) | — | — | — | — | — | Outcome not reported. |
|
Quality of communication Measured on Quality of Patient‐Clinician Communication About End‐of‐Life Care. Higher score indicates high satisfaction with the quality of communication Assessed after intervention |
11.2 ± 0.8 (mean ± SD) | MD 0.4 lower (1.61 lower to 0.81 higher) |
— | 9 (1 RCT) | ⊕⊝⊝⊝ Very lowb,f | — |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ACP: advanced care planning; CI: confidence interval; EQ‐5D: EuroQol‐5D; HADS: Hospital Anxiety and Depression Survey; HR: hazard ratio; KCCQ: Kansas City Cardiomyopathy Questionnaire; MD: mean difference; MLHF: Minnesota Living with Heart Failure; PHQ: Patient Health Questionnaire; RCT: randomised controlled trial; RR: risk ratio; SD: standard deviation; SMD: standardised mean difference. | ||||||
| GRADE Working Group grades of evidence High‐quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate‐quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low‐quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low‐quality: we are very uncertain about the estimate. | ||||||
aDowngraded one level for indirectness because the study included participants other than people with heart failure.
bSince the outcome included only one study, the sample size was too small, and had wide confidence intervals. Therefore, we downgraded two levels for imprecision.
cDowngraded one level for risk of bias because most included studies showed unclear selection bias and high attrition bias.
dDowngraded one level for imprecision due to small sample size and wide confidence intervals.
eDowngraded one level for imprecision due to small sample size.
fDowngraded one level for risk of bias due to high risk of selection bias.
Background
Description of the condition
Heart failure is a complex clinical syndrome that is associated with various symptoms including dyspnoea, fatigue, peripheral oedema, and depression (Falk 2013; Ponikowski 2016). The condition is caused by any structural cardiac disorder, functional cardiac disorder, or both, affecting the ability of the ventricles to pump blood (Yancy 2013). Coronary heart disease, heart valve disease, arrhythmias, familial cardiomyopathy, toxin‐induced cardiomyopathy, and hypertension are all linked to heart failure (Ponikowski 2016; Yancy 2013). Heart failure is classified using the American College of Cardiology Foundation/American Heart Association (ACCF/AHA) stages or the New York Heart Association (NYHA) functional classification system (Ponikowski 2016; Yancy 2013). The ACCF/AHA stages are based on structural heart changes, and focus on the development and progression of disease (Ponikowski 2016; Yancy 2013). In contrast, the NYHA functional classification describes the severity of symptoms and exercise capacity (Ponikowski 2016; Yancy 2013).
Incidence of heart failure is more common with increasing age (Redfield 2003; Yancy 2013), and is a worldwide public health problem. In 2015, the worldwide prevalence of heart failure was estimated to be approximately 40 million people (GBD 2016). In 2013, approximately 17.3 million people died from cardiovascular disease globally (Benjamin 2017). People with heart failure are often linked to a trajectory of periodic exacerbations and recoveries, where each exacerbation event may lead to death (Lynn 2001). The mortality rate is high for such people, however, and estimating a prognosis is challenging. When a person's medical condition worsens, in many cases, they require urgent and intensive management (Allen 2012). In this situation, people with heart failure and their families might not be able to contemplate treatment and care options that take into account the person's values and care preferences. Therefore, the AHA has emphasised the importance of discussing with patients via advance care planning (ACP) to better co‐ordinate future healthcare management based on the patient's values and preferences, as well as their current clinical status such as symptom burden and quality of life, potential treatment options, and prognosis, as part of an annual heart failure review (Allen 2012). Currently, the 2013 ACCF/AHA Guideline for the Management of Heart Failure recommends palliative and supportive care for people with advanced heart failure to improve quality of life (class I recommendation; level of evidence B) (Yancy 2013). Some systematic reviews have collectively shown that ACP may lead to improvement of communication between patients and healthcare providers, satisfaction with care, and concordance between a patient's preferences of care and received care (Houben 2014; Martin 2016; Weathers 2016).
Description of the intervention
ACP is the process of discussing an individual's future care plan between the person, their family members, surrogate decision‐makers, and healthcare providers while considering the person's values, concerns, wishes, life goals, and preferences for future medical care (NCPC 2007; Rietjens 2017; Sudore 2017). This generally occurs while the person is still able to communicate their wishes independently and is in a stable symptoms and clinical condition of heart failure (Stevenson 2015). In hospital, ACP may start when individuals are diagnosed with heart failure or make an outpatient visit (Mullick 2013; Stevenson 2015). Additionally, the topic of ACP can be broached when patients undergo repeat hospitalisations for exacerbated comorbidities and move into extended care facilities (Stevenson 2015). The discussion component of ACP may include an individual's family members, surrogate decision‐makers (such as friends and neighbours), and key people who are involved in their care (NCPC 2007; Sudore 2017), since they best understand the person's values, goals, and care preferences (Sudore 2017). In the event that the person loses their capacity to make informed decisions, it becomes the responsibility of the surrogate decision‐makers to direct medical care in keeping with the person's wishes, and with an understanding of their illness and prognosis (NCPC 2007; Sudore 2010; Sudore 2017). The aim of ACP, therefore, is to ensure that the care a person receives is consistent with their goals, values, and preferences (Sudore 2017).
In 1990, the Patient Self‐Determination Act was enacted in the USA, and highlighted the rights of patients in the shared decision‐making of their own medical treatment (Allen 2012; Prendergast 2001; Stevenson 2015). In 2005, the Mental Capacity Act was enacted in the UK, and it also emphasised the importance of patients' rights in decision‐making (Hayhoe 2011; NCPC 2007). Discussion about ACP has since been facilitated worldwide. Initially, ACP focused on specific treatment decisions (Prendergast 2001), as well as formal documents. For example, advance directives, shared decision‐making, living wills, power of attorney, physician orders for life‐sustaining treatment, and do‐not‐resuscitate orders are commonly used to legally appoint a spokesperson for the patient and record their wishes about future medical treatment. Although these documents continue to be a part of ACP, the process has evolved to emphasise the importance of communication and understanding between the patient, their family members, and healthcare providers regarding the individual's values, wishes, and preferences for future care (Prendergast 2001; Rietjens 2017; Sudore 2017).
Although heart failure is sometimes stable for a long time, it may change suddenly (Mullick 2013). It is, therefore, important to discuss ACP not only when heart failure is exacerbated, but early in the disease process (Mullick 2013). In addition, because a person's values and preferences can change over time, it is also important to regularly review advance care plans (Sudore 2010).
How the intervention might work
People with heart failure report various symptoms, however, they often have a poor understanding of their disease, and they rarely realise the terminal nature of their condition (Browne 2014). An ACP approach that involves communicating and understanding an individual's values, life goals, and wishes can lead to agreement between patients and their surrogate decision‐makers concerning future care preferences (Lorenz 2008). In addition, supportive physician behaviours and shared decision‐making are associated with patient and family satisfaction with care (Fine 2010). ACP can also help to resolve differences of opinion among family members (Rhee 2013), assist families to face end‐of‐life scenarios, and reduce their psychological and physical distress (Rhee 2013). In people with cancer and their families, ACP is associated with fewer patients receiving aggressive medical interventions as end‐of‐life treatment (Wright 2008). Such aggressive medical interventions can result in lower quality of life for patients in end‐of‐life care and can intensify depressive disorders among distressed family members (Wright 2008). Therefore, implementation of ACP and effective communication among patients, family members, and healthcare providers might lead to or contribute to positive outcomes.
Why it is important to do this review
Healthcare providers can play a vital role in the care of patients with advanced heart failure by discussing a patient's values, preferences, and planning for future care (Allen 2012). The current guideline from the ACCF/AHA recommends the implementation of palliative and supportive care in advanced heart failure (class I recommendation; level of evidence B) (Yancy 2013). The European Society of Cardiology (ESC) guideline recommends that, as a key component of palliative and end‐of‐life care, healthcare providers should focus on improving or maintaining quality of life of patients and their carers until death (Ponikowski 2016). Despite both the USA and European guidelines' recommendations of ACP, many people with heart failure do not have documented ACP (Butler 2015).
Barriers to ACP for healthcare providers include the unpredictable trajectory of heart failure, the difficulty in determining when to initiate ACP, and patients' poor understanding of their disease (Browne 2014; De Vleminck 2014; Hjelmfors 2014). However, despite the unpredictable prognosis of disease, healthcare providers need to understand and discuss with patients the patients' own values and preferences for future care, while revising their expectations of the disease course (Allen 2012). Moreover, patients and their families emphasise that effective communication, in particular focusing on the patient's treatments and future care options, are important elements (Virdun 2015).
An existing Cochrane Review explores the effects of ACP for participants receiving haemodialysis (Lim 2016). This review suggested that indepth discussion regarding end‐of‐life care did not lead to unnecessary discomfort or anxiety (Lim 2016). The review's included studies also reported that the surrogate decision‐makers' understanding of the patient's goals and preferences for future medical treatment increased after the implementation of ACP. Additionally, ACP intervention improves the proportion of patients completing advance care directives (Lim 2016). Two other reviews, one of people aged 65 years of age and older (Weathers 2016), and another of adults with various diseases such as cancer, cardiac diseases, and chronic renal failure (Houben 2014), suggested that ACP interventions might improve documentation of end‐of‐life care preferences and increased discussion about end‐of‐life care between patients and healthcare providers.
Although the aforementioned systematic reviews investigated the effects of ACP, none studied the effects of ACP in people with heart failure. Little is known about the impact of ACP on the physical and psychological conditions, quality of life, and care satisfaction of people with heart failure, their families, and carers. Therefore, a review aiming to reveal the effects of ACP for people with heart failure is warranted.
Objectives
To assess the effects of advance care planning (ACP) in people with heart failure compared to usual care strategies that do not have any components promoting ACP.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs), including randomised parallel‐group trials, cross‐over trials, and cluster‐randomised trials. For cross‐over studies, we included only the first‐phase treatment. We imposed no restrictions on publication status or language.
Types of participants
We included adults (18 years of age or older) with a clinical diagnosis of heart failure. We included participants with all types of heart failure (e.g. preserved ejection fraction (diastolic heart failure) or reduced ejection fraction (systolic heart failure)), who were recruited to the trials without a carer, as well as participants who were enrolled with their surrogate decision‐makers/carers by study investigators. If participants of various diagnoses (i.e. besides heart failure) were included, we contacted authors and extracted the data of only participants with heart failure. If we were unable to obtain the data of only participants with heart failure, we included studies if the majority (when the number of participants with heart failure in the study was highest, and it accounted for almost half of participants) of participants had a diagnosis of heart failure and we pursued a sensitivity analysis to assess the impact of mixed diagnoses on the effectiveness of ACP (Sensitivity analysis).
Types of interventions
We included trials that implement ACP practices, such as discussing and considering participants' values, wishes, and life goals; understanding participants' illness and prognosis; and their preferences about future medical care. We compared the ACP intervention with usual care that did not involve any components to promote ACP. Although the current guidelines recommended the implementation of palliative and supportive care including ACP, there was no standardised approach of ACP. Therefore, we included trials that aimed to assess the effectiveness of interventions to promote, improve, or strengthen ACP. We included interventions that involved video or websites to promote ACP. These tools may provide information required for ACP such as resuscitation measures, and may educate patients and their families about the importance of communication about a patient's own values, life goals, and preferences about future medical care between patients, their families, surrogate decision‐makers, and healthcare providers. The eligibility criterion was any study that described an ACP intervention as defined by the trial investigators.
Types of outcome measures
Primary outcomes
Concordance between participants' preferences and end‐of‐life care (measurement defined by trial authors, e.g. comparing end‐of‐life care that participants received with participant‐stated preferences) (yes/no).
Participants' quality of life (e.g. Minnesota Living with Heart Failure Questionnaire (MLHFQ), the Kansas City Cardiomyopathy Questionnaire (KCCQ), the 36‐Item Short Form Health Survey (SF‐36), or other measurement scales, as defined by trial authors).
Participants' satisfaction with care/treatment (measurement defined by trial authors) (yes/no).
Secondary outcomes
Completion of documentation by medical staff regarding discussions with participants about ACP processes (yes/no).
Participants' depression (measurement defined by trial authors, e.g. Hospital Anxiety and Depression Scale (HADS), the Patient Health Questionnaire (PHQ‐9)).
Caregivers' satisfaction with care/treatment (measurement defined by trial authors) (yes/no).
Quality of communication (measurement defined by trial authors, e.g. Quality of Communication (QOC) questionnaire).
Use of life‐sustaining treatment, such as intubation (yes/no).
Participants' decisional conflict (measurement defined by trial authors, e.g. Decisional Conflict Scale).
Use of hospice services (yes/no).
All‐cause mortality.
We were interested in effects of the intervention from the longest follow‐up duration; however, the numerous outcome measures were obtained at different time points in patients with serious illness, and thus we also considered the effects of ACP at shorter follow‐up time points where possible and performed subgroup analyses accordingly (Subgroup analysis and investigation of heterogeneity).
We did not anticipate ACP to be associated with any adverse effects and thus we decided not to consider adverse effects as a prespecified outcome measure of interest. If adverse effects were reported in the included studies, we decided to describe the findings narratively.
Reporting one or more of the outcomes listed here in the trial was not an inclusion criterion for the review.
Search methods for identification of studies
Electronic searches
We identified trials through systematic searches of the following bibliographic databases:
Cochrane Central Register of Controlled Trials (CENTRAL), in the Cochrane Library (2019, Issue 10);
Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, MEDLINE Daily and MEDLINE (Ovid, 1946 to 8 October 2019; searched 10 October 2019);
Embase (Ovid, 1980 to 2019 week 40; searched 10 October 2019);
CINAHL Plus (EBSCOhost, 1937 to 10 October 2019; searched 10 October 2019);
Social Work Abstracts (Ovid, 1977 to 24 October 2019; searched 24 October 2019).
We adapted the preliminary search strategy for MEDLINE (Ovid) for use in the other databases (Appendix 1). We applied the Cochrane sensitivity‐maximising RCT filter to MEDLINE (Ovid) and adapted it for Embase and CINAHL Plus (Lefebvre 2011).
We conducted a search of the following trial registers for ongoing or unpublished trials:
ClinicalTrials.gov (www.clinicaltrials.gov; to 21 October 2019);
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp; to 21 October 2019).
We imposed no restrictions on language of publication or publication status.
We did not anticipate this type of intervention to be associated with any adverse effects and thus did not perform a separate search for adverse effects of ACP.
Searching other resources
We checked reference lists of all included studies and any relevant systematic reviews identified for additional references to trials. We also examined any relevant retraction statements and errata for included studies.
In the case of unpublished or incomplete data, we contacted the original study authors for further information.
Data collection and analysis
Selection of studies
Two review authors (YN screened all titles and abstracts; NH and AM screened by sharing titles and abstracts) independently screened titles and abstracts for inclusion of all the potential studies identified by the search, and coded them 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve'. In case of disagreements, a third review author (HF) arbitrated. We retrieved the full‐text study reports/publication and two review authors (YN screened all full‐text; NH, HF, and AM screened by sharing the full text) independently screened the full‐text and identified studies for inclusion, and identified and recorded reasons for exclusion of the ineligible studies. We resolved any disagreement through discussion or consulted a third review author (EO). We identified and excluded duplicates and collated multiple reports of the same study so that each study, rather than each report, was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) flow diagram and Characteristics of excluded studies table (Liberati 2009).
Data extraction and management
We designed a data extraction form specifically for this Cochrane Review in order to extract relevant information regarding study and population characteristics and outcome data. For consistency during the review development process and to reduce bias and improve validity and reliability, five review authors (YN, NH, HF, AM, MM) first designed the data extraction form by extracting data from a random sample of two studies. We refined/amended the form according to comments or suggestions that arose from the pilot stage before finalising the data extraction form for the full review development process. Two review authors (YN extracted study data from all included studies; NH, HF, AM, and MM extracted study data by sharing the included studies) independently extracted study characteristics and outcome data from the remaining included studies.
We extracted the following study characteristics and reported them in the Characteristics of included studies table.
Methods: study design, total duration of study, number of study centres and location, study setting (community centres, hospices, hospitals, etc.), and date of study.
Participants: number randomised, number lost to follow‐up/withdrawn from studies, number analysed, mean age, age range, gender, severity of condition (such as the commonly used classification system, the NYHA Functional Classification; or the ACC/AHA stages of heart failure) and diagnostic criteria (e.g. according to the ESC guideline diagnostic algorithm for diagnosis of heart failure (Ponikowski 2016)), study inclusion and exclusion criteria.
Interventions: intervention and comparison.
Outcomes: primary and secondary outcomes specified and collected, and time points reported. Although adverse effects were not our outcome measure of interest, for completeness we also extracted any participant‐ or physician‐reported adverse outcomes in the study publications (Types of outcome measures).
Notes: funding for trial, and notable conflicts of interest of trial authors.
We resolved disagreements by consensus or by involving a third review author (EO). One review author (YN) transferred data into the Review Manager 5 (Review Manager 2014). We double‐checked that data were entered correctly by comparing the data presented in the systematic review with the study reports. A second review author (NH) spot‐checked study characteristics for accuracy against the trial report.
Assessment of risk of bias in included studies
Two review authors (YN assessed risk of bias for all of included studies; NH, HF, AM, and MM assessed risk of bias by sharing the included studies) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreements by discussion or by involving another review author (EO). We assessed the risk of bias of the included RCTs and cluster‐RCTs according to the following domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias (e.g. industry funding, lack of individual randomisation).
We graded each potential source of bias as high, low, or unclear and provided a quote from the study report together with a justification for our judgement in the 'Risk of bias' table. We summarised the risk of bias judgements across different studies for each of the domains listed. Where information on risk of bias related to unpublished data or correspondence with a trialist, we noted this in the 'Risk of bias' table.
Although we had planned to include cluster‐RCTs, our systematic literature search did not identify any eligible ones. Should cluster‐RCTs be included in future updates of this review, we would pay attention to the following sources of bias: recruitment bias; baseline imbalance; loss of clusters; incorrect analysis; and comparability with individually randomised trials (Higgins 2011).
We reported results of our 'Risk of bias' assessment using a 'Risk of bias' summary and a 'Risk of bias' graph, with detailed descriptions of our observations in the Characteristics of included studies table.
When considering treatment effects, we took into account the risk of bias for the studies that contributed to that outcome.
Assessment of bias in conducting the systematic review
We conducted the review according to the published protocol (Nishikawa 2018), and reported any deviations in the 'Differences between protocol and review' section of the systematic review.
Measures of treatment effect
For dichotomous outcome measures, we used risk ratios (RRs) with 95% confidence intervals (CIs). For continuous endpoints, we used mean differences (MDs) (or standardised mean differences (SMDs) studies used different measuring scales, such as in the case of quality‐of‐life scores) with 95% CIs. We interpreted SMDs based on the approach as illustrated in Cohen 1988.
We stratified analyses by pooling data from measuring scales/scoring systems with a consistent direction of effect. We had planned to analyse the results of the study with the opposite scale (i.e. quality‐of‐life data) separately. However, as we only had one study with a scale in the opposite direction, we reported it narratively.
Unit of analysis issues
For studies with repeated outcome measurements at different follow‐up durations, we included data from the longest follow‐up available. We conducted a subgroup analysis using the longest follow‐up time points for each study, which was divided into short‐term and long‐term follow‐up time points. We planned to include cluster‐randomised trials but did not identify any eligible ones. Should cluster‐randomised trials be included in updates of this review, we plan to analyse their reported data along with those from individually randomised trials. If adjustment for the cluster design effects are missing, we would adjust the relevant summary statistics (such as sample sizes and standard deviations) according to the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), using an estimate of the intraclass correlation coefficient (ICC) derived from the trial (if possible), from a similar trial, or from a study of a similar population. If we decide to use ICCs from other sources in updates, we would perform sensitivity analyses to test the effects of variation in the ICC.
Dealing with missing data
We contacted investigators or study sponsors in order to verify key study characteristics and obtained missing numerical outcome data where possible (e.g. when a study was identified as abstract only). Where this was not possible, and the missing data were thought to introduce serious bias, we explored the impact of including such studies in the overall assessment of results by sensitivity analysis.
Assessment of heterogeneity
We used the Chi2 test and the I2 statistic to measure heterogeneity among the trials in each analysis. We used a P value of 0.10 for the Chi2 test for statistical significance and interpreted the I2 statistic according to the following guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
0% to 40%: might not be important.
30% to 60%: may represent moderate heterogeneity.
50% to 90%: may represent substantial heterogeneity.
75% to 100%: considerable heterogeneity.
If we had identified substantial heterogeneity (I2 greater than 50%), we would have reported it and explored possible causes by prespecified subgroup analysis. However, none of our analyses had substantial heterogeneity.
We assessed the degree of heterogeneity by visually inspecting forest plots.
Assessment of reporting biases
If we were able to include more than 10 trials, we planned to create and examine a funnel plot to explore possible small‐study biases for the primary outcomes (Higgins 2011). However, since only a small number of studies were eventually included, we did not pursue assessment of reporting bias.
Data synthesis
We conducted meta‐analyses only if the treatments, participants, and underlying clinical question were similar enough for data pooling. We first used a random‐effects model to pool the studies, and measured the degree of heterogeneity using Tau2 and the I2 statistics. As these results did not indicate substantial heterogeneity, we decided to use the fixed‐effect model.
'Summary of findings' table
We created a 'Summary of findings' table for the following outcomes (Types of outcome measures).
Concordance between participants' preferences and end‐of‐life care.
Participants' quality of life.
Participants' satisfaction with care/treatment.
Completion of documentation by medical staff regarding discussions with participants about ACP processes.
Participants' depression.
Caregivers' satisfaction with care/treatment.
Quality of communication.
Two review authors (YN, NH) independently assessed the overall quality of the evidence and resolved disagreements by discussion or by involving a third review author (EO). We documented and incorporated our corresponding judgements into the reporting of results for each outcome based on the GRADE approach to evaluate the overall quality of evidence and considered the following five GRADE criteria: study limitations, consistency of effect, imprecision, indirectness, and publication bias. We followed methods and recommendations described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), using GRADEpro GDT software (GRADEpro GDT 2015). Our decisions to downgrade the quality of studies are explained in the footnotes of the 'Summary of findings' table and we made comments to aid the reader's understanding of the review where necessary.
Subgroup analysis and investigation of heterogeneity
We planned to carry out the following subgroup analyses for our primary outcomes, if sufficient (stratified) data from included studies allowed for such analyses (Types of outcome measures).
Mean age 70 years or older versus under the age of 70 years.
Gender.
Trial registration status (preregistered in a clinical trial registry, had a published protocol, provided ethics approval, or a combination of these): trials with the above compared to trials without.
Study design (cluster‐RCTs versus parallel RCTs).
Follow‐up periods (defined by trial authors).
We used the formal test for subgroup interactions in Review Manager 5 (Review Manager 2014).
The incidence and prevalence of heart failure is more common with increasing age (Redfield 2003; Yancy 2013). Since the prevalence of heart failure gradually increases at 70 years of age and older (Gottdiener 2000; Ponikowski 2016; Yancy 2013), we decided a cut‐off at 70 years old.
We conducted a subgroup analysis regarding follow‐up periods for participants' quality of life and participants' depression. The other subgroup analysis which we had planned were not conducted because of lack of data.
Sensitivity analysis
Given that a number of included studies enrolled mixed populations of varied diagnoses, of which only a subset had the diagnosis of interest (heart failure), in addition to the main analysis where we included all participants, we also conducted a sensitivity analysis in which we analysed data from studies that enrolled only participants with the diagnosis of interest, in order to examine the influence of such baseline clinical characteristics on the overall effectiveness of ACP. For updates, we would also consider the possibility of performing a sensitivity analysis by including only studies with an overall low risk of bias (we defined low risk of bias for studies in which we judged at least four domains to be 'low').
Reaching conclusions
We based our conclusions only on findings from the quantitative or narrative synthesis of included studies for this review. We avoided making recommendations for practice and our implications for research suggested priorities for future research and outlined what the remaining uncertainties are in the area.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; Characteristics of ongoing studies tables.
Results of the search
We identified 2273 records through an electronic database search. We also checked reference lists of all included studies and identified 308 additional records. After removing duplicates, 2466 records remained. We assessed the titles and abstracts of these records and excluded 2383. We assessed the remaining 83 records for eligibility, and 29 studies (38 references) were excluded, 10 studies (11 references) were identified as awaiting classification, and 10 studies (12 references) were classified as ongoing. Nine studies (22 references) met the review inclusion criteria, of which seven studies (18 references) were included in the meta‐analyses (Figure 1).
1.
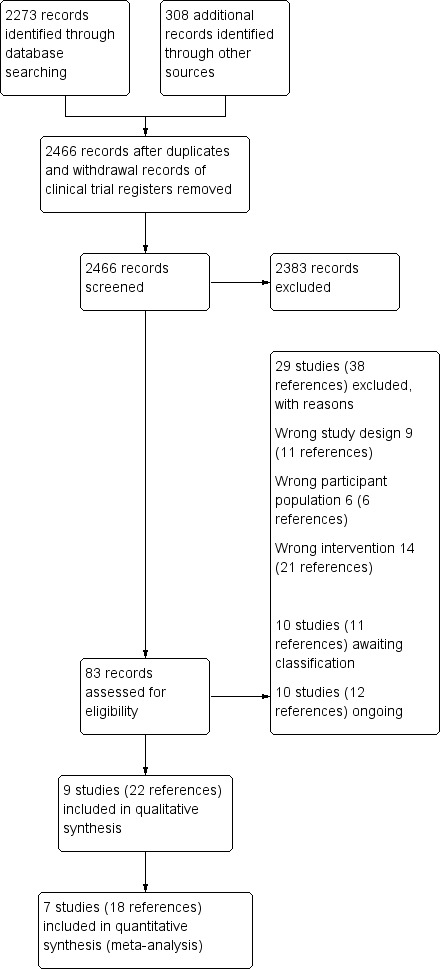
Study flow diagram.
Included studies
We included nine studies involving 1242 patients and 426 patients' surrogate decision‐makers in this systematic review; 1016 people had heart failure. Three of nine studies enrolled participants other than people with heart failure, including people with end‐stage renal disease and chronic obstructive pulmonary disease (COPD) (Briggs 2004; Kirchhoff 2010; Menon 2016). Of these three studies, Menon 2016 did not report any of our prespecified outcomes. For the other two studies, we contacted the trial authors to obtain the outcome data of only people with heart failure. We were able to obtain relevant data from people with heart failure from Briggs 2004; however, although we contacted the trialists of the Kirchhoff 2010 study, we were unable to extract the desired data. Participants of heart failure included in Kirchhoff 2010 accounted for 58.3% of all participants. Therefore, we assessed this study to be eligible for inclusion in the qualitative synthesis, and moreover, we used sensitivity analysis to investigate the effect of including a study with a proportion of non‐eligible participants.
We had planned to include cluster‐randomised trials but did not identify any eligible ones.
Participants' mean age ranged from 62.3 to 81.9 years. The proportion of men in each study ranged from 52.7% to 100%. The main inclusion criteria across the included studies were NYHA class III or IV, those who had previous hospitalisation for heart failure within one year, or a predicted poor prognosis. One study focused on participants with left ventricular assist devices (Metzger 2016). Eight studies were implemented in the US (Briggs 2004; El‐Jawahri 2016; Kirchhoff 2010; Menon 2016; Metzger 2016; O'Donnell 2018; Rogers 2017; Sidebottom 2015), and one study was conducted in the UK (Denvir 2016).
The ACP intervention applied in the included studies consisted of structured discussion, use of tools including video and the Values Inventory, and ACP as a part of palliative care by a multidisciplinary team. Five studies conducted a structured discussion of participants' current clinical condition, prognosis, predictable complications or future treatment, participants' preferences and goals of care, values, concerns, and documentation of preferences (Briggs 2004; Denvir 2016; Kirchhoff 2010; Metzger 2016; O'Donnell 2018). Of these, four studies conducted the structured discussions with patients and their surrogate decision‐makers or carers (Briggs 2004; Denvir 2016; Kirchhoff 2010; Metzger 2016). Trained cardiologists, nurses, social workers, and chaplains facilitated structured discussions. The intervention consisted of one‐time discussions (Briggs 2004; Kirchhoff 2010; Metzger 2016), and multiple times with a follow‐up duration of three to six months (Denvir 2016; O'Donnell 2018). Two studies delivered the intervention via video and the Values Inventory (El‐Jawahri 2016; Menon 2016). The video included an explanation regarding goals of care and treatment about life‐prolonging care, limited medical care, and comfort care. The Values Inventory was used as an ACP discussion aid and to assess participants' own values. Two studies conducted ACP as a part of palliative care by a multidisciplinary team (Rogers 2017; Sidebottom 2015). These participants received palliative care including symptom and psychosocial assessment and management, and they also participated in discussions about ACP. The participants of comparators in the included studies received information about advance directives, ACP materials, or usual care, the details of which were not described.
Four studies assessed the quality of life of participants (Denvir 2016; O'Donnell 2018; Rogers 2017; Sidebottom 2015), and three studies assessed depression (O'Donnell 2018; Rogers 2017; Sidebottom 2015). The studies used different tools to assess participants' quality of life and depression (e.g. quality of life was measured using the EuroQol‐5D (EQ5D) instrument, the KCCQ and the MLHFQ.
Although we had planned to describe information on adverse effects and the economic costs associated with ACP narratively, none of the included studies reported such information.
Excluded studies
We excluded 29 studies following the assessment of the full text (see Characteristics of excluded studies table). Fourteen studies' interventions did not meet our criteria regarding types of ACP (Allen 2018; Blue 2001; Doorenbos 2016; Harter 2016; Hjelmfors 2018; Hua 2017; Hughes 2000; McIlvennan 2016; Molloy 2000; NCT01817686; NCT01917188; Ng 2018; Sahlen 2016; Wells 2018). Nine studies were not RCTs (Anonymous 2007; Johnson 2010; Johnson 2018; Miller 1971; Nenner 2012; Newton 2009; Rabow 2004; Svendsen 2006; UMIN000029805). Six studies did not meet our criteria of participant (Detering 2010; Gutheil 2005; Pearlman 2005; Radwany 2014; Skorstengaard 2019; Thoonsen 2015 ). Three studies did not enrol any participants with diagnosed heart failure (Gutheil 2005; Pearlman 2005; Thoonsen 2015). The remaining three studies were excluded owing to ineligibility of participants (Detering 2010; Radwany 2014; Skorstengaard 2019). Detering 2010 included participants of various diagnoses such as respiratory disease; since only a minority of the study population had a diagnosis of heart failure (30%), this study was eventually excluded. Radwany 2014 included various participants other than those with heart failure such as COPD and diabetes with renal disease; there was a lack of information on the rate of each diagnosis among participants. Skorstengaard 2019 included various diagnoses such as cancer and lung disease, and only 17% of participants were patients with heart failure.
Studies awaiting classification
We identified 10 studies as studies awaiting classification (ACTRN12613001377729; ACTRN12618001045202; Hughes 2015; NCT00105599; NCT02463162; NCT03128060; NCT03539510; NCT03649191; O'Riordan 2014; Skorstengaard 2017; see Characteristics of studies awaiting classification table). Eight studies have not yet been published (ACTRN12613001377729; ACTRN12618001045202; Hughes 2015; NCT00105599; NCT02463162; NCT03128060; NCT03539510; NCT03649191). One study reported in its abstract about the baseline survey of the RCT, however the full‐text has not yet been published (O'Riordan 2014). Further papers are to follow the published Skorstengaard 2017 study. This first paper was a cross‐sectional study describing the place of care and place of death preferred by participants, and their level of anxiety. The second paper was an RCT including a high proportion of patients with conditions other than heart failure. Skorstengaard 2017 plans to publish one more article.
Ongoing studies
We identified 10 ongoing studies (ACTRN12614000590662; ACTRN12617001040358; Ejem 2019; Graney 2019; Malhotra 2016; NCT00489021; NCT02429479; NCT02612688; NCT03170466; NCT03516994; see Characteristics of ongoing studies table). Two studies restricted the study population to participants with diagnosed heart failure (Malhotra 2016; NCT03170466), while the remaining eight studies included participants with different diagnoses such as cancer, COPD, or renal disease.
Risk of bias in included studies
The results of our assessment of risk of bias in the included studies are illustrated in Figure 2 and Figure 3.
2.
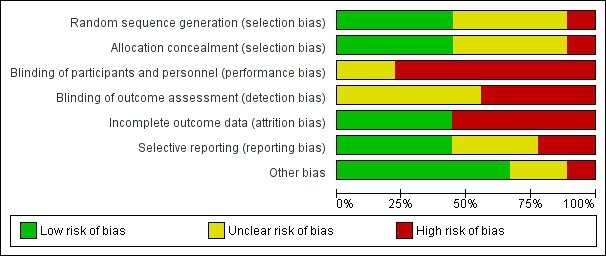
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
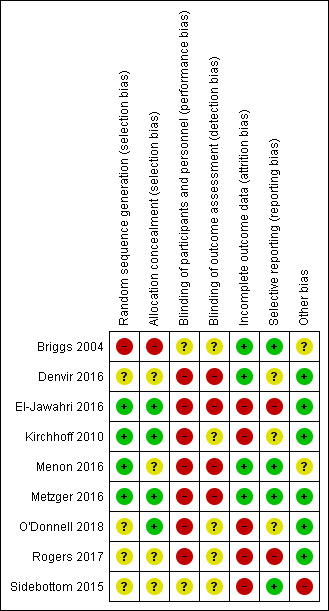
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
For random sequence generation, four studies were at low risk (El‐Jawahri 2016; Kirchhoff 2010; Menon 2016; Metzger 2016). We judged four studies at unclear risk of selection bias due to a lack of sufficient information (Denvir 2016; O'Donnell 2018; Rogers 2017; Sidebottom 2015). One study reported the use of alternating group assignment, and we rated it at high risk of selection bias (Briggs 2004).
Four studies reported adequate methods of allocation concealment and we rated them at low risk of bias (El‐Jawahri 2016; Kirchhoff 2010; Metzger 2016; O'Donnell 2018). We judged four studies at unclear risk of bias due to insufficient study information (Denvir 2016; Menon 2016; Rogers 2017; Sidebottom 2015). One study was at high risk because of predictable allocation (Briggs 2004).
Blinding
As the nature of the ACP intervention involves facilitated discussion with participants, six studies were at high risk of performance bias regarding the blinding of participants and personnel (Denvir 2016; Kirchhoff 2010; Menon 2016; Metzger 2016; O'Donnell 2018; Rogers 2017). El‐Jawahri 2016 was also assessed at high risk of performance bias because the research assistants showed a video on goals of care to participants as part of the intervention. The other two studies were at unclear risk of performance bias due to insufficient information (Briggs 2004; Sidebottom 2015).
We rated five studies at unclear risk of detection bias in terms of the blinding of outcome assessors due to insufficient information (Briggs 2004; Kirchhoff 2010; O'Donnell 2018; Rogers 2017; Sidebottom 2015). We judged four studies at high risk (Denvir 2016; El‐Jawahri 2016; Menon 2016; Metzger 2016): Denvir 2016 and Metzger 2016 both employed a structured discussion approach where trial personnel and outcomes were assessed using subjective self‐reported questionnaires; two studies collected outcomes using unblinded research assistants (El‐Jawahri 2016; Metzger 2016), and one study assessed qualitative data about discussions between participants and their physicians (Menon 2016). Metzger 2016 conducted a structured discussion with trial personnel, and outcomes were assessed by a research assistant via telephone.
Incomplete outcome data
We assessed four studies at low risk of attrition bias (Briggs 2004; Denvir 2016; Menon 2016; Metzger 2016). We classified the remaining five studies at high risk of attrition bias because of the high rate of loss to follow‐up due to death, withdrawal, refusal by participants' surrogate decision‐makers, or unclear reasons (El‐Jawahri 2016; Kirchhoff 2010; O'Donnell 2018; Rogers 2017; Sidebottom 2015).
Selective reporting
We rated four studies at low risk of reporting bias because the reported outcomes in the 'Methods' section were described in the 'Results' section of the published papers, or the outcomes that were preregistered in the trial register were reported in the published papers (Briggs 2004; Menon 2016; Metzger 2016; Sidebottom 2015). We judged three studies at unclear risk of reporting bias (Denvir 2016; Kirchhoff 2010; O'Donnell 2018). Although all outcomes of the two studies that were mentioned in the trial register were reported in the published papers, participants' recruitment and enrolment were completed before entry into the trial registry (Denvir 2016; O'Donnell 2018). Kirchhoff 2010 did not report the details of outcome measures in the trial register. We judged two studies at high risk of reporting bias because not all outcomes that were preregistered in the trial register or in the design paper were reported in the published paper (El‐Jawahri 2016; Rogers 2017).
Other potential sources of bias
We rated six studies at low risk of other bias (Denvir 2016; El‐Jawahri 2016; Kirchhoff 2010; Metzger 2016; O'Donnell 2018; Rogers 2017). We judged two studies at unclear risk of other bias (Briggs 2004; Menon 2016). Briggs 2004 showed an imbalance in participants' gender between groups at baseline. However, the effect of gender imbalance on outcomes was uncertain. In Menon 2016, participants were encouraged to have discussions about end‐of‐life care with their physician using the Values Inventory tool, and the contents of the discussions were analysed. Although physicians' characteristics may affect end‐of‐life discussions, the details of physicians' characteristics were not reported. We judged one study at high risk of other bias, because the study showed statistically significant differences between groups at baseline about age (Sidebottom 2015).
Effects of interventions
See: Table 1
See: Table 1.
Advance care planning versus usual care
Nine studies compared advance care planning with usual care; of these, two studies did not report our prespecified outcomes and thus were not included in our meta‐analyses.
Concordance between participants' preferences and end‐of‐life care
One study reported concordance between participants' preferences and end‐of‐life care (Kirchhoff 2010). The study included people with heart failure (n = 179; 57.2%) and renal disease (n = 134; 42.8%). Of the 313 participants with heart failure and renal disease, 110 died before the end of the study and their data were included in the analysis of this outcome. Of the 110 decedents, 62 were in the intervention group and 48 were in the control group. In the situation of a low chance of survival, 46/62 (74.1%) participants in the intervention group were consistent with their preferences and received care compared with 30/48 (62%) participants in the control group (RR 1.19, 95% CI 0.91 to 1.55; participants = 110; very low‐quality evidence). This corresponded to an assumed risk of 625 per 1000 participants with usual care and a corresponding risk of 744 per 1000 participants (95% CI 569 to 969) for ACP.
Participants' quality of life
Four studies reported participants' quality of life (Denvir 2016; O'Donnell 2018; Rogers 2017; Sidebottom 2015). Three studies reported quality of life using the EQ5D and the KCCQ (Denvir 2016; O'Donnell 2018; Rogers 2017). Sidebottom 2015 reported quality of life using the MLHF Questionnaire. The MLHF instrument implies that a lower score indicates better quality of life and vice versa, which is opposite to the direction of effect of other quality‐of‐life measuring scales such as the EQ5D and the KCCQ. Although it is possible to align the direction of the MLHF score with EQ5D and KCCQ scores by multiplying by –1 and obtain the consistent direction of effect, we had only one study with a scale in the opposite direction (Sidebottom 2015). In addition, the MLHF has significant differences in the range of the score and the grading policy. Therefore, we decided to conduct a meta‐analysis of three studies (Denvir 2016; O'Donnell 2018; Rogers 2017), and reported findings of Sidebottom 2015 narratively.
Pooled data analysis on the three studies showed that there was no evidence of a difference in quality of life between ACP and the usual care group (SMD 0.06, 95% CI –0.26 to 0.38; participants = 156; low‐quality evidence; Analysis 1.1). The scores were calculated as the degree of changes from the baseline score.
1.1. Analysis.
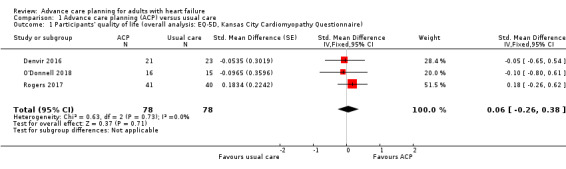
Comparison 1 Advance care planning (ACP) versus usual care, Outcome 1 Participants' quality of life (overall analysis: EQ‐5D, Kansas City Cardiomyopathy Questionnaire).
Sidebottom 2015 showed that the mean change of the score in the intervention group was 12.92 points between baseline and one month, and 14.86 points between baseline and three months. In the control group, the mean change was 8.00 points between baseline and one month, and 11.80 points between baseline and three months. The MLHF in the intervention group was improved 4.92 points higher than the control group at one month (P = 0.001), and 3.06 points higher at three months (P = 0.001).
We conducted a subgroup analysis according to the follow‐up duration (three months or less and more than three months). For participants' quality of life, there was no difference by length of follow‐up (test for differences between subgroups P = 0.66; follow‐up duration three months or less: SMD –0.05, 95% CI –0.65 to 0.54; participants = 44; more than three months: SMD 0.11, 95% CI –0.27 to 0.48; participants = 112; Analysis 1.2).
1.2. Analysis.
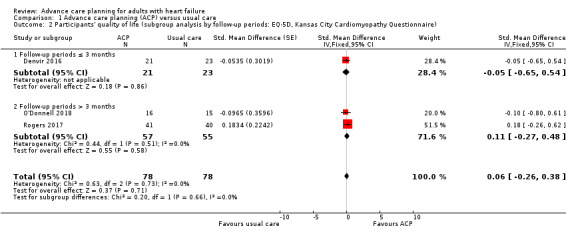
Comparison 1 Advance care planning (ACP) versus usual care, Outcome 2 Participants' quality of life (subgroup analysis by follow‐up periods: EQ‐5D, Kansas City Cardiomyopathy Questionnaire).
Participants' satisfaction with care/treatment
None of the included studies reported on participants' satisfaction with care/treatment.
Completion of documentation by medical staff regarding discussions with participants about advance care planning processes
Three studies reported completion of documentation by medical staff regarding discussions with participants about ACP processes (Denvir 2016; O'Donnell 2018; Sidebottom 2015). In particular, Sidebottom 2015 assessed the "documented completion of the disease‐specific ACP process in the record within 6 months of the study hospitalization" and calculated the hazard ratio (HR), which was adjusted for age, sex, and marital status (HR 2.87, 95% CI 1.09 to 7.59; P = 0.033); however, the study failed to clearly explain why HR was chosen as an effect measure for completion of ACP documentation.
Pooled data analysis of the other two studies showed that documentation about the ACP process was more often completed in the intervention group compared with the control group (RR 1.68, 95% CI 1.23 to 2.29; participants = 92; low‐quality evidence; Analysis 1.3). This corresponded to an assumed risk of 489 per 1000 participants with usual care and a corresponding risk of 822 per 1000 participants (95% CI 602 to 1000) for ACP.
1.3. Analysis.

Comparison 1 Advance care planning (ACP) versus usual care, Outcome 3 Completion of documentation by medical staff regarding discussions with participants about ACP processes.
Participants' depression
Three studies reported on participants' depression (O'Donnell 2018; Rogers 2017; Sidebottom 2015). Depression was reported using the PHQ‐8, the HADS with depression subscale, and the PHQ‐9. Pooled data analysis on the three studies showed that ACP intervention may have improved depression when compared with usual care (SMD –0.58, 95% CI –0.82 to –0.34; participants = 278; low‐quality evidence; Analysis 1.4). The scores were calculated as the degree of changes from the baseline score.
1.4. Analysis.

Comparison 1 Advance care planning (ACP) versus usual care, Outcome 4 Participants' depression (overall analysis).
We conducted a subgroup analysis according to the follow‐up duration (three months or less and more than three months). Analysis for participants' depression demonstrated that ACP led to improved outcomes in both subgroups, and the test for subgroup differences indicated no difference (three months or less months: SMD –0.69, 95% CI –1.01 to –0.38; participants = 167; more than three months: SMD –0.41, 95% CI –0.79 to –0.03; participants = 111; P = 0.26; Analysis 1.5).
1.5. Analysis.
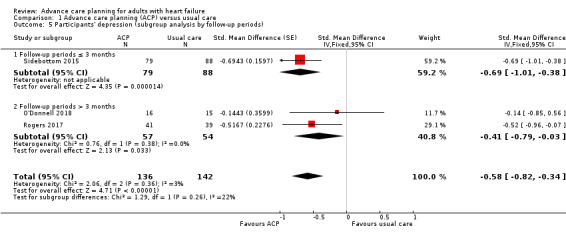
Comparison 1 Advance care planning (ACP) versus usual care, Outcome 5 Participants' depression (subgroup analysis by follow‐up periods).
Caregivers' satisfaction with care/treatment
We identified no studies reporting caregivers' satisfaction with care/treatment.
Quality of communication
One study reported quality of communication which was measured using the Quality of Patient‐Clinician Communication About End‐of‐Life Care (Briggs 2004). We were uncertain about the effects of ACP on the quality of communication when compared to the usual care group (MD –0.40, 95% CI –1.61 to 0.81; participants = 9; very low‐quality evidence).
Use of life‐sustaining treatment
None of the included studies reported use of life‐sustaining treatment, such as intubation.
Participants' decisional conflict
Two studies reported participants' decisional conflict using the Decisional Conflict Scale (Briggs 2004; Metzger 2016). Pooled data analysis on the two studies showed no evidence of a difference in participants' decisional conflict between the ACP intervention and usual care group (MD –0.26, 95% CI –0.55 to 0.02; participants = 38; Analysis 1.6).
1.6. Analysis.
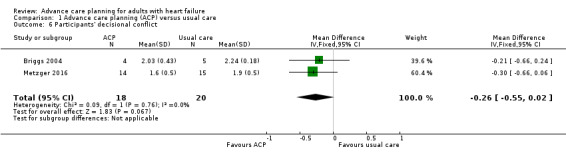
Comparison 1 Advance care planning (ACP) versus usual care, Outcome 6 Participants' decisional conflict.
Use of hospice services
One study reported use of hospice services (Sidebottom 2015). Sidebottom 2015 reported the use of hospice services with HR adjusted for age, sex, and marital status (HR 1.60, 95% CI 0.58 to 4.38; participants = 232). It is unclear why Sidebottom 2015 used an HR as an effect measure for the use of hospice services.
All‐cause mortality
Five studies reported all‐cause mortality (Denvir 2016; Kirchhoff 2010; O'Donnell 2018; Rogers 2017; Sidebottom 2015). Pooled data analysis on the five studies showed that all‐cause mortality might have been increased in participants who received ACP intervention compared with usual care (RR 1.32, 95% CI 1.04 to 1.67; participants = 795; Analysis 1.7).
1.7. Analysis.
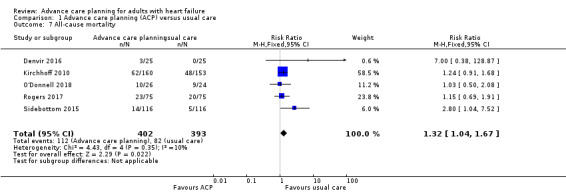
Comparison 1 Advance care planning (ACP) versus usual care, Outcome 7 All‐cause mortality.
Of these five studies, four studies included only participants with heart failure (Denvir 2016; O'Donnell 2018; Rogers 2017; Sidebottom 2015), and one study enrolled participants with heart failure and participants with renal disease (Kirchhoff 2010). A sensitivity analysis excluding Kirchhoff 2010 was pursued to explore the influence of clinical diagnosis status of study participants on the overall results. Pooled analysis of the four studies enrolling only participants with heart‐failure still showed a higher risk of all‐cause mortality among people who received ACP (RR 1.44, 95% CI 0.99 to 2.09; participants = 482; Analysis 1.8).
1.8. Analysis.
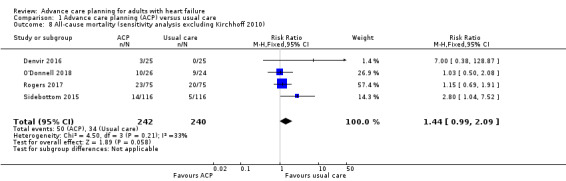
Comparison 1 Advance care planning (ACP) versus usual care, Outcome 8 All‐cause mortality (sensitivity analysis excluding Kirchhoff 2010).
Discussion
Summary of main results
The main results of this review revealed that the ACP intervention may increase the completion of documentation by medical staff regarding discussions with participants about ACP processes and may improve individuals' depression compared with usual care. However, the quality of the evidence was low due to small sample sizes, unclear selection bias, and high attrition bias. For depression, there was a statistically significant difference between ACP and usual care (P < 0.00001), with an effect size of –0.58. Although the included evidence was of low quality, due to relatively narrow CIs (–0.82 to –0.34) and a lack of statistical heterogeneity (I2 = 3%; P = 0.36 for Chi2), we are confident that this statistically significant finding could also be interpreted as a clinically significant and relevant finding, suggesting that implementation of ACP could lead to at least a moderate level of improvement in depression. The ACP intervention showed no evidence of a difference on participants' quality of life and decisional conflict, but the sample sizes of each study were relatively small and the quality of evidence about quality of life was low.
We are uncertain about the effects of ACP on concordance between participants' preferences and end‐of‐life care and quality of communication. Each outcome included only one small study, and the quality of the evidence was very low. The ACP intervention might be associated with a higher risk of all‐cause mortality; our sensitivity analysis by excluding one study that enrolled participants with both heart failure and renal diseases produced a consistently higher risk of all‐cause mortality in the ACP intervention group compared with usual care group. The included studies did not report the outcomes of participants' and caregivers' satisfaction with care/treatment and use of life‐sustaining treatment.
The majority of the included studies conducted structured discussions as the ACP intervention, but employed various types of interventions and follow‐up periods: some studies used tools such as video and the Values Inventory to support decision‐making, and other studies had three‐ to six‐month follow‐up periods. Therefore, there were limitations in evaluating the effect of each type of ACP.
Overall completeness and applicability of evidence
Many participants in this review were NYHA class III or IV, had previous heart failure hospitalisation within one year, or a predicted poor prognosis. It is important to discuss ACP early in the disease process and to review ACP regularly (Mullick 2013; Sudore 2010). The AHA also recommended that ACP needs to be discussed and documented (Allen 2012). Since the participants in this review included people with severe heart failure, there were limitations in applying the findings of this review to people with mild heart failure.
Eight out of nine included studies were conducted in the USA (Briggs 2004; El‐Jawahri 2016; Kirchhoff 2010; Menon 2016; Metzger 2016; O'Donnell 2018; Rogers 2017; Sidebottom 2015), and one study was conducted in the UK (Denvir 2016). Previous studies suggested that cultural values and ethnicity may affect general awareness of ACP and acceptability of talking about death (Hong 2018; Ohr 2017). Therefore, careful attention should be paid to this point when applying the findings of this review to a wider range of people with heart failure.
The ACP intervention included structured discussion, the use of tools to understand treatment and care or to promote discussion about ACP, and ACP as a part of palliative care. Therefore, the effect of ACP interventions that use tools such as video and the Values Inventory was uncertain. Furthermore, structured discussion included discussions implemented only once and those with a long‐term follow‐up range of three to six months, including multiple discussions. As each study had a small sample size and the included studies in this review were small, we were unable to show the effect of the ACP intervention based on differences in the number of discussions and each type of ACP. Therefore, there were limitations in generalising the effect of the intervention.
Quality of the evidence
We assessed the quality of the evidence for the main outcomes using the GRADE approach. Since all outcomes had a small sample size, the quality of the evidence for concordance between participants' preferences and end‐of‐life care, quality of life, completion of documentation by medical staff regarding discussions with participants about ACP processes, depression, and quality of communication were downgraded one level. Concordance between participants' preferences and end‐of‐life care included only one study (Kirchhoff 2010), and this study included people with congestive heart failure and people with end‐stage renal disease. Therefore, we downgraded the quality of evidence one level due to the indirectness domain, and judged the quality of the evidence to be very low. We judged the quality of the evidence for participants' quality of life, completion of documentation by medical staff regarding discussions with participants about ACP processes, and participants' depression as low, because the sample size was small and approximately two‐thirds of selection bias and attrition bias was unclear or high. Only one study included quality of communication (Briggs 2004). Since this study had an extremely small sample size of nine participants and a high risk of selection bias, we judged the quality of the evidence as very low. None of the included studies reported patients' satisfaction with care/treatment and caregivers' satisfaction with care/treatment.
Potential biases in the review process
We contacted trial authors if there was missing information in the published and unpublished studies. However, we were only able to obtain limited information.
Owing to the nature of ACP and outcomes, the interventions and assessment of outcomes were not blinded to participants and personnel in many trials. Therefore, we considered performance and detection bias as high. Attrition biases were also high because five of nine included trials had incomplete data (El‐Jawahri 2016; Kirchhoff 2010; O'Donnell 2018; Rogers 2017; Sidebottom 2015).
In our review, only nine studies met the inclusion criteria. All trials were small and our primary outcomes were scarcely reported. Therefore, we could not conduct meta‐analyses for two of our three primary outcomes. The power to detect the effect of ACP is currently inadequate.
One source of bias could be the differences in the contents of the intervention to promote ACP among trials. For example, Briggs 2004 and Denvir 2016 mainly provided the scheduled one‐hour interview as the intervention, while El‐Jawahri 2016 utilised a six‐minute video. Furthermore, there were differences in the number of discussion about ACP and the follow‐up duration. Long‐term involvement with patients and healthcare providers might enhance their relationship. It was showed that the therapeutic relationship between patients and healthcare providers may impact on the satisfaction and comfort of discussion about ACP (Miller 2019). The diversity of ACP interventions may affect the effectiveness of the intervention and heterogeneity in future reviews.
Since Kirchhoff 2010 included the study centre which conducted ACP education in the community, effects of intervention might have diminished. Additionally, if colleagues and departments in study centres were interested in ACP, it might have affected the effects of intervention.
The fact that almost all of trials were conducted in the USA could be another source of bias and the effect of ACP should be culturally sensitive. Future trials in other countries or cultural settings could potentially contribute to our review.
Agreements and disagreements with other studies or reviews
Our findings showed that the effects of the ACP intervention were uncertain because of low‐ or very low‐quality evidence. One previous systematic review and narrative synthesis reported that the use of hospice services increased with ACP intervention (Kernick 2018). We were unable to conduct a meta‐analysis because the outcome of hospice use was included in only one study in our review (Sidebottom 2015). Sidebottom 2015 indicated that the use of hospice services did not show a significant increase with the ACP intervention. However, since the studies included in Kernick 2018 were part of a cohort study, we were unable to show the effectiveness of the ACP intervention in relation to the use of hospices.
Another systematic review that included participants with various diagnoses besides heart failure reported that the ACP intervention may increase the completion of advance directives (odds ratio 3.26, 95% CI 2.00 to 5.32; participants = 12,012; Houben 2014). The interventions included in Houben 2014 were classified into two types, one focusing on the completion of advance directives and the other focusing on communication about ACP. Completion of advance directives increased in both intervention types. In our review, three studies showed that documentation about ACP processes was more often completed in the intervention group compared with the control group (Denvir 2016; O'Donnell 2018; Sidebottom 2015). Although Houben 2014 included participants with other conditions besides heart failure, it showed findings similar to our review. The ACP intervention may increase ACP documentation for patients with a wide range of diseases.
Houben 2014 also reported that the ACP intervention was able to improve the quality of communication. However, the outcome included only two studies. In our review, only one study reported the quality of communication with a very small sample size (Briggs 2004).
Authors' conclusions
Implications for practice.
The USA and European guidelines recommend advanced care planning (ACP). In this review, although the quality of evidence was low, we found that the implementation of ACP may promote documentation by medical workers about ACP processes and may lead to improvement in participants' depression. The effect of ACP on concordance between participants' preferences and end‐of‐life care, which was one of the main objectives of ACP, quality of life, quality of communication, and use of hospice services remains unclear. In our review, we were unable to show the effect of the ACP intervention in relation to participants' and caregivers' satisfaction with care/treatment and use of life‐sustaining treatment, because the included studies did not report these outcomes.
Although the sample size of each study was small, our meta‐analysis showed that ACP intervention may increase all‐cause mortality. Patients in the ACP intervention group whose preferences and care plans to not receive invasive and life‐sustaining treatment were followed may have subsequently experienced increased rates of all‐cause mortality. Although we prespecified the use of life‐sustaining treatment as an outcome, none of the included studies reported it. In addition, these studies did not report the reasons for death and types of care received during the end‐of‐life phase. Therefore, the precise rationale behind the effects on all‐cause mortality remains uncertain because it is currently unclear as to the types of medical treatment modalities delivered to the study participants and if participants' preferences of care/treatment were noted and subsequently followed.
The included studies consisted of various ACP interventions, such as structured discussion or ACP as a part of palliative care and different follow‐up periods. The ACP is a process of discussion over time (Stevenson 2015; Sudore 2017). The extent of the relationship between patients and healthcare providers which is related to the follow‐up duration might affect the effects of ACP. Since the follow‐up durations of included studies in this review varied, the effects of long‐term follow‐up interventions remained uncertain.
Some qualitative systematic reviews about ACP for people with cancer and patients with life‐threatening disease showed that patients feel benefit as well as unpleasant feeling on ACP, their perception may change in the ACP process, and relationship with families or therapeutic relationship may also have an effect on the implementation of ACP (Johnson 2016; Zwakman 2018). Therefore, it is necessary to accumulate qualitative studies about patients' and families' experiences of the ACP process as well as quantitative studies about the effects of ACP using effective ACP based on these findings of studies.
Implications for research.
The studies included in this review had small sample sizes. In addition, since the quality of evidence for the main outcomes in this review was relatively low and limited, we need to pay more attention to interpreting the results, and we need further studies to support our conclusion.
It is necessary to confirm whether differences in the severity of heart failure, type of intervention, and follow‐up periods will influence the effect of ACP interventions. Future studies need to
measure the effect of ACP interventions on participants with mild heart failure;
measure the effect of ACP interventions in different ethnic groups;
collect intervention data about each type of intervention, such as structured discussion, use of tools, and ACP as a part of palliative care; and
collect data on ACP interventions that differ in the number of discussions and follow‐up periods.
Furthermore, since the aim of ACP is to ensure that the care a person receives is consistent with their goals, values, and preferences (Sudore 2017), it is necessary to assess the effects of ACP in people with heart failure by collecting data on concordance between participants' preferences and end‐of‐life care and on what type of care/treatment was received at the end of life.
Acknowledgements
We are grateful to contact editor Mariann Gyongyosi; to peer reviewers Shyh Poh Teo, Maral A'arab‐Amini, Noemi Pavo, and Kit Byatt; and to consumer reviewer Richard Fitzgerald for providing helpful comments on earlier version of this review.
Appendices
Appendix 1. Search strategies
CENTRAL
#1 MeSH descriptor: [Advance Care Planning] explode all trees
#2 advance* care near plan* (Word variations have been searched)
#3 advance* near (medical plan* or statement*) (Word variations have been searched)
#4 "ACP" (Word variations have been searched)
#5 Statement of wishes (Word variations have been searched)
#6 MeSH descriptor: [Terminal Care] explode all trees
#7 terminal care (Word variations have been searched)
#8 ((end of life or EOL) near/5 (care or discuss* or decision* or plan* or preference*)) (Word variations have been searched)
#9 MeSH descriptor: [Treatment Refusal] this term only
#10 MeSH descriptor: [Withholding Treatment] explode all trees
#11 (treatment near/3 (refus* or withhold* or withdraw*)) (Word variations have been searched)
#12 MeSH descriptor: [Living Wills] this term only
#13 living will* (Word variations have been searched)
#14 ((shared or sharing) near/2 decision*) (Word variations have been searched)
#15 SDM (Word variations have been searched)
#16 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15
#17 MeSH descriptor: [Heart Failure] explode all trees
#18 (cardi* or heart* or myocard*) near/2 (fail* or incompet* or insufficien* or decomp*) (Word variations have been searched)
#19 #17 or #18
#20 #16 and #19
MEDLINE (Ovid)
1. exp Advance Care Planning/
2. (advance* care adj plan*).tw.
3. (advance* adj (medical plan* or statement*)).tw.
4. acp.tw.
5. Statement of wishes.tw.
6. Terminal Care/
7. terminal care.tw.
8. ((end of life or EOL) adj5 (care or discuss* or decision* or plan* or preference*)).tw.
9. Treatment Refusal/
10. exp Withholding Treatment/
11. (treatment adj3 (refus* or withhold* or withdraw*)).tw.
12. Living Wills/
13. living will*.tw.
14. ((shared or sharing) adj2 decision*).tw.
15. SDM.tw.
16. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15
17. exp Heart Failure/
18. ((cardi* or heart* or myocard*) adj2 (fail* or incompet* or insufficien* or decomp*)).tw.
19. 17 or 18
20. 16 and 19
21. randomized controlled trial.pt.
22. controlled clinical trial.pt.
23. randomized.ab.
24. placebo.ab.
25. drug therapy.fs.
26. randomly.ab.
27. trial.ab.
28. groups.ab.
29. 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28
30. exp animals/ not humans.sh.
31. 29 not 30
32. 20 and 31
Embase (Ovid)
1 terminal care/
2 advance care planning/
3 living will/
4 (advance* care adj plan*).tw.
5 (advance* adj (medical plan* or statement*)).tw.
6 acp.tw.
7 statement of wishes.tw.
8 terminal care.tw.
9 ((end of life or EOL) adj5 (care or discuss* or decision* or plan* or preference*)).tw.
10 treatment refusal/
11 treatment withdrawal/
12 withholding treatment.tw.
13 (treatment adj3 (refus* or withhold* or withdraw*)).tw.
14 "living will*".tw.
15 shared decision making/
16 ((shared or sharing) adj2 decision*).tw.
17 SDM.tw.
18 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17
19 exp heart failure/
20 ((cardi* or heart* or myocard*) adj2 (fail* or incompet* or insufficien* or decomp*)).tw.
21 19 or 20
22 18 and 21
23 "random*".tw. 24 "factorial*".tw.
25 "crossover*".tw.
26 "cross over*".tw.
27 "cross‐over*".tw.
28 "placebo*".tw.
29 (doubl* adj blind*).tw.
30 (singl* adj blind*).tw.
31 "assign*".tw.
32 "allocat*".tw.
33 "volunteer*".tw.
34 crossover procedure/
35 double blind procedure/
36 randomized controlled trial/
37 single blind procedure/
38 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37
39 (animal/ or nonhuman/) not human/
40 38 not 39
41 22 and 40
CINAHL
S33 S20 AND S32
S32 S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31
S31 TX allocat* random*
S30 (MH "Quantitative Studies")
S29 (MH "Placebos")
S28 TX placebo*
S27 TX random* allocat*
S26 (MH "Random Assignment")
S25 TX randomi* control* trial*
S24 TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) )
S23 TX clinic* n1 trial*
S22 PT Clinical trial
S21 (MH "Clinical Trials+")
S20 S16 AND S19
S19 S17 OR S18
S18 TX ((cardi* or heart* or myocard*) N2 (fail* or incompet* or insufficien* or decomp*))
S17 (MH "Heart Failure+")
S16 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15
S15 TX SDM
S14 TX ((shared or sharing) N2 decision*)
S13 TX living will*
S12 (MH "Living Wills")
S11 TX (treatment N3 (refus* or withhold* or withdraw*))
S10 (MH "Euthanasia, Passive")
S9 (MH "Treatment Refusal")
S8 TX ((end of life or EOL) N5 (care or discuss* or decision* or plan* or preference*))
S7 TX terminal care
S6 (MH "Terminal Care")
S5 TX Statement of wishes
S4 TX acp
S3 TX advance* N1 (medical plan* or statement*)
S2 TX advance* care N1 plan*
S1 (MH "Advance Care Planning")
Social Work Abstracts
heart failure AND ("advance care planning" OR "living will" OR "acp" OR "Statement of wishes" OR "shared decision making" OR "SDM" OR "terminal care")
ClinicalTrials.gov
heart failure AND ("advance care planning" OR "living will" OR "acp" OR "Statement of wishes" OR "shared decision making" OR "SDM" OR "terminal care")
WHO International Clinical Trials Registry Platform (ICTRP) Search Portal
heart failure AND (advance care planning OR living will OR acp OR Statement of wishes OR shared decision making OR SDM OR terminal care)
Data and analyses
Comparison 1. Advance care planning (ACP) versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants' quality of life (overall analysis: EQ‐5D, Kansas City Cardiomyopathy Questionnaire) | 3 | 156 | Std. Mean Difference (Fixed, 95% CI) | 0.06 [‐0.26, 0.38] |
| 2 Participants' quality of life (subgroup analysis by follow‐up periods: EQ‐5D, Kansas City Cardiomyopathy Questionnaire) | 3 | 156 | Std. Mean Difference (Fixed, 95% CI) | 0.06 [‐0.26, 0.38] |
| 2.1 Follow‐up periods ≤ 3 months | 1 | 44 | Std. Mean Difference (Fixed, 95% CI) | ‐0.05 [‐0.65, 0.54] |
| 2.2 Follow‐up periods > 3 months | 2 | 112 | Std. Mean Difference (Fixed, 95% CI) | 0.11 [‐0.27, 0.48] |
| 3 Completion of documentation by medical staff regarding discussions with participants about ACP processes | 2 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.68 [1.23, 2.29] |
| 4 Participants' depression (overall analysis) | 3 | 278 | Std. Mean Difference (Fixed, 95% CI) | ‐0.58 [‐0.82, ‐0.34] |
| 5 Participants' depression (subgroup analysis by follow‐up periods) | 3 | 278 | Std. Mean Difference (Fixed, 95% CI) | ‐0.58 [‐0.82, ‐0.34] |
| 5.1 Follow‐up periods ≤ 3 months | 1 | 167 | Std. Mean Difference (Fixed, 95% CI) | ‐0.69 [‐1.01, ‐0.38] |
| 5.2 Follow‐up periods > 3 months | 2 | 111 | Std. Mean Difference (Fixed, 95% CI) | ‐0.41 [‐0.79, ‐0.03] |
| 6 Participants' decisional conflict | 2 | 38 | Mean Difference (IV, Fixed, 95% CI) | ‐0.26 [‐0.55, 0.02] |
| 7 All‐cause mortality | 5 | 795 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [1.04, 1.67] |
| 8 All‐cause mortality (sensitivity analysis excluding Kirchhoff 2010) | 4 | 482 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.99, 2.09] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Briggs 2004.
| Methods |
Study design: individual RCT (pair of patients and surrogates) Total duration of study: not reported Number of study centres and location: 1 centre (2 clinics and 1 dialysis unit)/La Crosse, WI Study setting: clinic and renal dialysis unit, a 325‐bed clinically affiliated organisation Date of study: not reported |
|
| Participants |
Randomised (n): 27 pairs of patients and surrogates (intervention: 13 pairs (CHF: 4; ESRD: 5; pre‐OHS: 4); control: 14 pairs (CHF: 5; ESRD: 5; OHS: 4)) Lost to follow‐up/withdrawn from studies (n): 0 withdrawals Analysed (n): intervention: 13 pairs (CHF: 4; ESRD: 5; OHS: 4); control: 14 pairs (CHF: 5; ESRD: 5; OHS: 4) Mean age (years): all patients: 68.7; all surrogates: 50 Age range: not reported Gender (n and % men): intervention: 5 (38.5%) in patients, 3 (23.1%) in surrogates; control: 11 (78.6%) in patients, 4 (28.6%) in surrogates Severity of condition and diagnostic criteria: not reported Inclusion criteria:
Exclusion criteria: not reported |
|
| Interventions |
Intervention: patients and their surrogates participated in scheduled 1‐hour PC‐ACP interview implemented by experienced advance care planning facilitator. Interview consisted of 5 stages.
Stage 1: assessed patient's understanding of his or her current medical condition, prognosis, and potential complications
Stage 2: explored misconceptions the patient might have regarding planning for future medical decision‐making
Stage 3: briefly reviewed rationale for future medical decisions patient would want chosen surrogate to understand and act on. Goal was to prepare surrogate to be able to fully represent patient's wishes
Stage 4: Statement of Treatment Preferences survey used to introduce replacement information: it describes real clinical situations patient could experience and related treatment choices that surrogate might need to make
Stage 5: summarised value of previous discussion for patient and surrogate as well as need for future discussions as situations and preferences change Comparison: participants were approached on admission and asked if they had an advance directive or if they would like more information (or both). They were given an information card describing their right to have an advance directive and materials that explained the process of advance care planning and completion of an advance directive if they desired. Referrals were made to trained advance care planning facilitators for additional discussion and assistance. Completed advance directive documents were placed in a specific place in the medical record as determined by organisational policy. |
|
| Outcomes |
Primary outcomes and time points: planned outcomes not reported Secondary outcomes and time points:
Adverse outcomes: not reported |
|
| Notes |
Funding for trial: not reported Notable conflicts of interest of trial authors: not reported We contacted the trialists for further details of study data, and received the data regarding quality of communication. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "…Systematic assignment was used within disease categories by alternating group assignment after assignment of the first patient‐surrogate pair was randomly determined by flipping a coin…" Comment: study generated random sequence generation by alternating group assignment. Only allocation of the first patient–surrogate pair was determined by coin tossing. |
| Allocation concealment (selection bias) | High risk | Quote: "alternating group assignment." Personnel may have been able to predict participants' allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Intervention was PC‐ACP interview, and participants may have been able to know their allocation. However, details of interventionist were not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome measures included self‐reported outcome, and participants may have been able to know allocation. However, uncertain whether investigators were aware of assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals in either groups. |
| Selective reporting (reporting bias) | Low risk | Study protocol and trial register were not reported; all outcomes listed in the 'Methods' section of the study were reported in the 'Results' section. |
| Other bias | Unclear risk | There were more women (61.5%) than men (38.5%) in the intervention group. In the control group, 21.4% were women and 78.6% were men. Unclear whether gender imbalance would affect intervention effects. |
Denvir 2016.
| Methods |
Study design: cross‐over RCT Total duration of study: not reported Number of study centres and location: not reported/UK Study setting: participant recruitment undertaken in cardiology, medical and care of hospital wards for elderly people Date of study: screened between 1 October 2013 and 31 September 2014 |
|
| Participants |
Randomised (n): patients: 50 (intervention: 25; control: 25); carers: 32 (intervention: 19; control: 13). Patients were randomised in 1:1 ratio based on cross‐over design using random permuted blocks to receive either 12 weeks of an FCP at discharge followed by crossover to 12 weeks of usual care (early group) or to receive 12 weeks of usual care followed by crossover to 12 weeks of FCP (delayed group). Lost to follow‐up/withdrawn from studies (n): intervention: 2 participants died after allocation and did not receive intervention, 2 participants lost to follow‐up (1 died, 1 withdrew) at 12 weeks' follow‐up; control: 2 participants withdrew at 12 weeks' follow‐up Analysed (n): intervention: 21 participants were analysed at 12 weeks' follow‐up; control: 23 participants were analysed at 12 weeks' follow‐up Mean age (years): all participants: 81.1 (SD 8.6); intervention: 81.9 (SD 7.1); control: 80.2 (SD 10.0) Age range: not reported Gender (n and % men): intervention: 17 (68%) men; control: 13 (52%) men Severity of condition and diagnostic criteria: not reported (quote: "an unscheduled hospital admission with HF and or ACS based on European Society of Cardiology guidelines"). Inclusion criteria: predicted 12‐month mortality risk ≥ 20% estimated using the GRACE score for ACS and the EFFECT score for HF; people with aortic stenosis who presented with HF Exclusion criteria: moderate/severe dementia; prognosis < 30 days and people already on a palliative care register |
|
| Interventions |
Intervention: 1. Initial, 1‐hour semi‐structured meeting with trial cardiologist and trial nurse specialists involving patient and their carer; followed by 2 × 1‐hour meetings with trial nurse in patient's home at 6 and 12 weeks. 2. Discussion and documentation of an agreed personal FCP which was sent to each patient and uploaded by general practitioner using electronic KIS (used in Scotland to routinely communicate with out‐of‐hours and hospital services) 3. Ongoing telephone support (available Monday to Friday, 9 am–5 pm) from trial nurse for 12 weeks offering advice, support, and information about their healthcare and social needs. First interview/meeting focused on information needs, key concerns, and priorities of patient and carer using a semi‐structured interview guide to provide a consistent framework. Key topics discussed were: exploring their experiences of being in hospital, what treatments had been given or changed, their understanding and expectations about their condition, nominating a Power of Attorney or a surrogate decision maker, preferences for place of care should they deteriorate in the future (including place of care when dying), and likely outcomes of treatment; specifically discontinuing life‐prolonging treatments and CPR. Comparison: usual care |
|
| Outcomes |
Primary outcome and time point:
Secondary outcome and time point:
Adverse outcomes: not reported |
|
| Notes |
Funding for trial: Marie Curie Research Notable conflicts of interest of trial authors: authors declared no competing financial interests. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "1:1 ratio using random permuted blocks" Additional information about block size or how permuted blocks were generated was not described. |
| Allocation concealment (selection bias) | Unclear risk | Details not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Trial cardiologist and trial nurse specialists implemented semi‐structured meeting with participants and their carer. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Since the intervention was semi‐structured discussion with trial cardiologist and trial nurse, participants may have been aware of their allocation. Primary outcome, QoL, was subjective self‐reported outcome. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intervention: 4/25 (16%) participants discontinued at 12 weeks (3 died and 1 withdrew consent); control: 2/25 (8%) participants discontinued at 12 weeks (2 withdrew due to progressive illness). |
| Selective reporting (reporting bias) | Unclear risk | Planned outcomes as prespecified in the trial register record (NCT02302014) were reported. However, we noted that participants' screening and enrolment commenced before trial registry entry. |
| Other bias | Low risk | None identified. |
El‐Jawahri 2016.
| Methods |
Study design: individual RCT Total duration of study: not reported Number of study centres and location: 7 centres/USA Study setting: teaching hospitals Date of study: enrolment 28 June 2012 to 7 February 2015 |
|
| Participants |
Randomised (n): 246 (intervention: 123; control: 123) Lost to follow‐up/withdrawn from studies (n): intervention: 50 participants withdrawn at 1 month, and 7 at 3 months; control: 60 participants withdrawn at 1 month, and 11 at 3 months Analysed (n): intervention: 73 participants analysed at 1 month, and 66 at 3 months; control: 63 participants analysed at 1 month, and 52 at 3 months Mean age (years): intervention: 81 (SD 8) at baseline; control: 81 (SD 9) at baseline Age range: not reported Gender (n and % women): intervention: 50 (41%); control: 47 (38%) Severity of condition and diagnostic criteria: intervention: NYHA class II: 2 (2%); NYHA class III: 111 (90%); NYHA class IV: 10 (8%); control: NYHA class II: 0 (0%); NYHA class III: 112 (91%); NYHA class IV: 11 (9%); diagnostic criteria not reported Inclusion criteria:
Exclusion criteria: not an established HF patient, candidate for transplant or mechanical circulatory support, been referred to or enrolled in hospice care, psychiatric illness as determined by physician that would make this study inappropriate; any patient that had been excluded for transplant or circulatory support for psychological or psychiatric comorbidities |
|
| Interventions |
Intervention:
Comparison: participants listened to a description of the 3 goals of care used in the intervention arm read out loud by the research assistants. |
|
| Outcomes |
Primary outcomes and time points: planned outcomes that we prespecified for this review not reported Secondary outcomes and time points: planned outcomes that we prespecified for this review not reported Adverse outcomes: not reported |
|
| Notes |
Funding for trial: the National Heart, Lung, and Blood Institute Notable conflicts of interest of trial authors: Dr Paasche‐Orlow received compensation as a consultant to Nous Foundation, Inc, a not‐for‐profit foundation that disseminates educational videos. Dr Volandes was the President of the not‐for‐profit foundation. Dr Volandes had financial interests in the not‐for‐profit foundation, which were reviewed and managed by Massachusetts General Hospital and Partners HealthCare in accordance with their conflict of interest policies. The other authors reported no conflicts. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐generated 2:2 block randomization design stratified on the basis of use of an implantable cardioverter‐defibrillator at each institution." |
| Allocation concealment (selection bias) | Low risk | Quote: "assignments concealed in numbered envelopes." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The trained research assistants explained goals of care, and showed a goals‐of‐care video to participants. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Data collection was implemented by trained research assistants. Quote: "data collectors were not blinded to the randomization." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | In the follow‐up interview at 1 and 3 months after intervention, participants were asked whether they had discussed goals of care with their clinician. In the intervention group, 50 participants (40.7%) lost to follow‐up at 1 month and 57 participants (46.3%) at 3 months. In the control group, 60 participants (48.8%) lost to follow‐up at 1 month and 71 participants (57.7%) at 3 months. Other self‐reported outcomes (patients' goals‐of‐care preferences, CPR and intubation preferences, and patients' knowledge of goals of care) were assessed postintervention, and nobody lost to follow‐up. |
| Selective reporting (reporting bias) | High risk | Not all outcomes that were mentioned in the trial register (NCT01589120) were reported. The outcomes of decisional conflict, QoL, referral to hospice, place of death, and carer bereavement score were not reported. |
| Other bias | Low risk | None identified. |
Kirchhoff 2010.
| Methods |
Study design: individual RCT (pair of patients and surrogates) Total duration of study: not reported Number of study centres and location: 2 centres (6 clinics)/La Crosse, Madison and Milwaukee, Wisconsin Study setting: clinics and dialysis units Date of study: recruited from 1 January 2004 to 31 July 2007 |
|
| Participants |
Randomised (n): all participants: 338 (intervention: 164 pairs of patients and surrogates; control: 174 pairs of patients and surrogates); CHF: 197 pairs (intervention: 93 pairs; control: 104 pairs) Lost to follow‐up/withdrawn from studies (n): all participants (intervention: 2 patients died, 2 participants withdrawn; control: 21 participants withdrawn), CHF (intervention: 2 patients died, 1 participant withdrawn; control: 15 participants withdrawn) Analysed (n): all participants (intervention:160 pairs; control: 153 pairs), CHF (intervention: 90 pairs; control: 89 pairs) Mean age (years): intervention: 71.4 (all patients), 59.5 (surrogates); control: 70.6 (all patients), 57.4 (surrogates) Age range (years): 37–93 (all patients) Gender (n and % men): intervention: 96 (60.0%) patients, and 43 (26.9%) surrogates; control: 89 (58.2%) patients, 41 (26.8%) surrogates (all participants) Severity of condition and diagnostic criteria: not reported Inclusion criteria: patients receiving medical care but had clinical symptoms and signs that indicated a risk of serious complication or death in next 2 years; NYHA II, III, or IV; patients with ESRD had serum albumin < 3.7 g/dL and comorbidity such as diabetes mellitus, CHF, COPD, history of acute myocardial infarction, or above‐the‐knee amputation; aged ≥ 18 years; had decision‐making capacity; able to speak and understand English Exclusion criteria: not reported |
|
| Interventions |
Intervention: trained facilitator implemented PC‐ACP interview. Purposes of interview were to assess patient's and surrogate's understanding of patient's illness, experiences, hopes, fears, and concerns; to provide individualised information about disease‐specific treatment choices and their benefit and burdens; to assist in documentation of disease‐specific goals of care; and to prepare surrogate to understand the patient's choices and make future decisions to honour these choices. Interview consisted of 5 stages. Stage 1: assess patient's understanding of current medical condition, potential complications, hopes, fears, and perception of living well Stage 2: explore experiences patient may have had that affected their goals for future medical decision‐making Stage 3: assist patient and surrogate in appreciating value of discussing specific treatment choices patient is likely to experience in the future and how these discussions will prepare the surrogate to make substitute decisions based on a more‐thorough understanding of the patients' goals and preferences Stage 4: use a disease‐specific STP document to help patient and surrogate understand real scenarios that may occur because of illness complications and to assist patient in verbalising goals and values related to acceptable and unacceptable burdens and outcomes Stage 5: summarise the value of discussion for patients and surrogates, need for future discussions as situations and preferences change, and expectation that patients are more likely to have their wishes honoured in the future Comparison: all patients received care provided by their local health organisation for the completion of ADs. For the La Crosse centre, usual care included ACP facilitation with patient but not the indepth, patient‐centred intervention in the presence of the surrogate decision‐maker. For all sites, usual care included standard AD counselling, assessment of an AD on admission to the organisation, and questions as to whether they would like more information |
|
| Outcomes |
Primary outcomes and time points:
2 reviewers blinded to group allocation determined what treatment the patient received. Patients identified previously whether they want to receive treatment in the situation of a low chance of survival. Then, the treatment was compared with patients' preferences of treatment. Secondary outcomes and time points:
Adverse outcomes: not reported |
|
| Notes |
Funding for trial: Agency of Health Care Research and Quality, the Clinical and Translational Science Award (CTSA) program of the National Center for Research Resources, National Institutes of Health Notable conflicts of interest of trial authors: Dr Kirchhoff received Agency of Health Care Research and Quality award. Dr Hammes and Ms Briggs were employed by the Gundersen Lutheran Medical Foundation, Inc. which owns the rights to the Respecting Choices programme, of which the intervention used in the current study, Disease‐Specific Advance Care Planning, was part. Dr Kehl was supported by the Clinical and Translational Science Award program of the National Center for Research Resources, National Institutes of Health, during the final year of this project. Dr Brown had no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the allocation sequence using a computerized random number generator." |
| Allocation concealment (selection bias) | Low risk | Quote: "using the sealed‐envelope method within each setting and disease condition." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "the PC‐ACP facilitator was not blinded to group assignment." Intervention was PC‐ACP interview, therefore, participants were not blinded to investigators. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Since the intervention was structured interview, participants may have been aware of their allocation. For outcomes of concordance between participants' preferences and end‐of‐life care after patients' death (quote): "two reviewers blinded to group determined what treatment the patient received". Other details of outcome assessors not reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | For participants with HF in the intervention group, 2 participants died and there was 1 withdrawal/refusal by surrogate carers. In the control group, 15 participants withdrew or refused by surrogate carers. More participants in the control group withdrew or refused, and there was an imbalance of the reason and the number of lost to follow‐up between groups. |
| Selective reporting (reporting bias) | Unclear risk | The efficacy of PC‐ACP was mentioned in the trial register (NCT00204802) as the primary outcome. The details of outcome measures were not mentioned in the trial register. |
| Other bias | Low risk | None identified |
Menon 2016.
| Methods |
Study design: individual RCT Total duration of study: not reported Number of study centres and location: not reported/Houston (TX) Study setting: Veterans Affairs Medical Center Date of study: not reported |
|
| Participants |
Randomised (n): all participants: 120 (intervention: 60; control: 60) Lost to follow‐up/withdrawn from studies (n): intervention: 3 participants; control: 0 withdrawn Analysed (n): all participants: 117 (intervention: 57; control: 60); CHF: 53 (intervention: 28; control: 25) Mean age (years): intervention: 66.4; control: 68.4 Age range (years): intervention: 55–85; control: 56–84 Gender: all men Severity of condition and diagnostic criteria: CHF; ejection fraction < 25%/diagnostic criteria not reported Inclusion criteria: CHF with an ejection fraction < 25% and ≥ 1 previous hospitalisation; COPD/emphysema, requiring mechanical ventilation, with ≥ 2 previous hospitalisations; chronic liver disease with cirrhosis and ascites; any metastatic solid tumour (e.g. colon carcinoma with liver metastases) and non‐small‐cell lung cancer, stage IIIb or IV; ESRD on haemodialysis Exclusion criteria: dementia (Mini‐Mental State Examination score ≤ 24, diagnosis of dementia listed in the patients' chart) |
|
| Interventions |
Intervention: received explicit verbal instructions to use the values inventory as a starting point for future care planning with their physician during their visit and explicit instructions to discuss the values inventory at the beginning of the visit. A values inventory (VI) is a tool used in self‐assessment that allows patients to rate different values according to their relative importance. It gives the treating physician information about what is important to the patient and can thus be used as a discussion aid about ACP Comparison: usual care |
|
| Outcomes |
Primary outcomes and time points: planned outcomes that we prespecified for this review not reported Secondary outcomes and time points: planned outcomes that we prespecified for this review not reported Adverse outcomes: not reported |
|
| Notes |
Funding for trial: Department of Veterans Affairs, Veterans Health Administration, Health Services Research and Development Service, grant IIR‐02‐224 Notable conflicts of interest of trial authors: quote: "Neither the principal investigator nor any coauthors have any conflicts of interest." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 6 participants in each clinical department were randomised using a simple random number table, and 120 participants were randomised as a whole. |
| Allocation concealment (selection bias) | Unclear risk | Details not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Physicians were not blinded to patients' assignments, but they did not know a priori if the patient had been assigned to the VI [value inventory] or usual‐care group at the outset of the encounter." A value inventory (VI) which intervention group used was a self‐assessment tool, and it was used as a discussion about ACP. Participants was supported to show their physician the VI and discuss ACP. Therefore, personnel were able to aware which participants allocated eventually. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "All patient–physician encounters were audiotaped with a small and unobtrusive boundary microphone." This audiotaped data was analysed. Therefore, investigators will be able to anticipate which group participants were allocated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | In the intervention group, 3/60 (5%) participants withdrew. In the control group, 0 withdrew. |
| Selective reporting (reporting bias) | Low risk | Outcomes mentioned in trial register (NCT00122135) were reported. |
| Other bias | Unclear risk | Physicians' characteristics and experience who provided end‐of‐life discussion not reported. Physicians' characteristics may have affected the contents of end‐of life discussion. |
Metzger 2016.
| Methods |
Study design: individual RCT (pair of patients and surrogates) Total duration of study: not reported Number of study centres and location: 1 centre/North Carolina Study setting: academic medical centre Date of study: recruited from November 2014 to June 2015 |
|
| Participants |
Randomised (n): 29 pairs of patients and surrogates (intervention: 14 pairs; control: 15 pairs) Lost to follow‐up/withdrawn from studies (n): 0 lost to follow‐up Analysed (n): 29 pairs (intervention: 14 pairs; control: 15 pairs) Mean age (years): intervention: 62.6 (SD 7.6) (patients), 56.2 (SD 12.4) (surrogates); control: 62.3 (SD 12.3) (patients), 56.5 (SD 17.6) (surrogates) Age range (years): intervention: 44–74 (patients), surrogates not reported; control: 43–85 (patients), 28–85 (surrogates) Gender( n and % men): intervention: 11 (78.6%) patients, 2 (14.3%) surrogates; control: 9 (60.0%) patients, 2 (13.3%) surrogates Severity of condition and diagnostic criteria: not reported (intervention: 3 were bridge to transplant, 11 were destination therapy; control: 5 were bridge to transplant, 10 were destination therapy) Inclusion criteria: English‐speaking adults, ≥ 30 days post‐LVAD placement and medically stable; had access to telephone, and had a willing surrogate to participate in study with the patient. Surrogates were English‐speaking adults with access to a telephone Exclusion criteria: people hospitalised in critical condition |
|
| Interventions |
Intervention: usual care + SPIRIT‐HF. SPIRIT‐HF was a structured, guided discussion, delivered by a trained interventionist in a single, approximately 1‐hour session. 5 steps: 1. assessing representations; 2. identifying gaps and concerns; 3. creating conditions for conceptual change; 4. introducing replacement information; 5. setting goals, planning, and summarising. During SPIRIT‐HF, interventionist gained an understanding of the patient's experiences, thoughts, attitudes, and beliefs, related to his/her HF and LVAD, which in turn, facilitated the delivery of targeted, individualised information. Patient was given opportunity to consider his/her values and preferences related to end‐of‐life care. The inclusion of the surrogate ensured that surrogate had an opportunity to learn about patient's illness experiences, and end‐of‐life concerns, and to prepare for the role of surrogate decision‐maker. Participants were provided a copy of the Goals of Care used during the discussion, a written summary of the discussion, and information on resources regarding advance directives. Comparison: usual care, which consisted of a pre‐VAD evaluation by clinicians in psychology, social work, nutrition, nursing, cardiology, and cardiac surgery. Information about advance directives was provided during the LVAD evaluation. However, palliative care consultations and ACP discussions were not routinely part of usual care |
|
| Outcomes |
Primary outcomes and time points: planned outcomes that we prespecified for this review were not reported Secondary outcomes and time points:
Adverse outcomes: not reported |
|
| Notes |
Funding for trial: Sigma Theta Tau International/Hospice and Palliative Nurses 2014 Foundation End of Life Nursing Care Research Grant; National Center for Advancing Translational Sciences (NCATS), National Institutes of Health, through Grant Award; Sigma Theta Tau International, Alpha Chapter Postdoc Award Notable conflicts of interest of trial authors: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "using a computer‐generated random scheme." |
| Allocation concealment (selection bias) | Low risk | Quote: "The interventionist was blinded to group assignment until she opened a sequentially numbered opaque envelope containing the enrolled dyad's assignment." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The interventionist was blinded to group assignment until she opened a sequentially numbered opaque envelope containing the enrolled dyad's assignment." The intervention was a 5‐step discussion between interventionist and participants. Neither the participants nor the study personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "A trained research assistant (not the interventionist) assessed all outcomes by telephone." Since the intervention was a structured interview delivered by interventionist and outcomes were assessed by research assistant, it is likely that they were aware of the type of intervention allocation. Participants' decisional conflict assessed using subjective self‐reported questionnaire. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data in either group. |
| Selective reporting (reporting bias) | Low risk | Study protocol and trial register were not reported; all outcomes listed in the 'Methods' section of the study were reported in the 'Results' section. |
| Other bias | Low risk | None identified |
O'Donnell 2018.
| Methods |
Study design: individual RCT Total duration of study: not reported Number of study centres and location: 1 centre/Boston (MA) Study setting: hospital Date of study: recruited from September 2014 to December 2015, analyses began in July 2016 |
|
| Participants |
Randomised (n): 50 (intervention: 26; control: 24) Lost to follow‐up/withdrawn from studies (n): intervention: 10 participants died; control: 9 participants died Analysed (n): outcome of documentation of AC preferences (intervention: 26; control: 24); outcomes of QoL and depression (intervention: 16; control: 15) Mean age (years): intervention: 74.7 (SD 11.2); control: 69.2 (SD 10.2) Age range: not reported Gender (n and % men): intervention: 14 (53.9%); control: 15 (62.5%) Severity of condition (n and %) and diagnostic criteria: intervention: 16 (61.5%) were NYHA III or IV; control: 16 (66.7%) were NYHA III or IV/diagnostic criteria not reported Inclusion criteria: symptomatic HF (NYHA II‐IV); ≥ 1 risk factor for poor prognosis from following: prior hospitalisation for HF within 1 year, aged ≥ 80 years, chronic kidney disease (estimated GFR ≤ 45 mL/min/m2), systolic blood pressure ≤ 100 mmHg, serum sodium ≤ 130 meq/L, cardiogenic shock (Cardiac Index ≤ 2.0), serious non‐cardiovascular Illness (e.g. advanced stage cancer, COPD); ability to provide informed consent; permission of attending physician Exclusion criteria: anticipated major cardiac surgery, including VAD or transplant, within 3 months of enrolment; already enrolled in hospice or receiving outpatient palliative care |
|
| Interventions |
Intervention: social worker‐led intervention began as a structured goals of care conversation. Conversation prepared the patient for future decisions and solicit permission to proceed; assessed prognostic understanding and preferences regarding receipt of prognostic information; discussed prognosis and its implications for decision‐making; explored key topics including patient goals and priorities, fears and worries, sources of strength, perceived critical abilities, tradeoffs between length and QoL, and family awareness of preferences and prognosis; and summarised impressions and recommendations. All patients were reviewed with a palliative care physician who provided guidance regarding strategies for facilitation of further discussions and directed specific interventions (formal palliative care physician consultation, Medical Orders for Life Sustaining Treatment, hospice referral, etc.) where indicated. Social worker then contacted the patient by telephone or during subsequent scheduled clinic visits over the 6‐month follow‐up period to further develop the conversation begun with the patient during the initial visit. Comparison: all patients received printed materials containing information about ACP as provided routinely by Brigham and Women's Hospital and the Brigham and Women's Heart Failure guide. Palliative care and advanced planning discussions in these patients were initiated at discretion of treating team. Results of the questionnaires detailed symptoms burden, anxiety, and depression were available to the care team so that additional consultative resources (social work, psychiatry, palliative care) could be deployed as appropriate. |
|
| Outcomes |
Primary outcome and time points:
Secondary outcomes and time points:
Adverse outcomes: not reported |
|
| Notes |
Funding for trial: Watkins Discovery Award from Brigham and Women's Hospital, made possible through a philanthropic gift from the E. G. Watkins Family Foundation Notable conflicts of interest of trial authors: no conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly allocated 1:1 to a structured, social worker–led palliative care intervention or usual care using a permuted block randomization scheme" Comment: additional information about block size or how permuted blocks were generated was not described. |
| Allocation concealment (selection bias) | Low risk | Quote: "All patients were identified and enrolled by the study coordinator, who was blinded to the allocation sequence." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Patients allocated to the intervention group received a structured goals of care discussion based on the framework of the Serious Illness Conversation Guide conducted by a social worker (A.E.O.)" Comment: participants were provided the intervention by investigator. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Since the intervention was a structured discussion, participants may have been aware of their allocation. Documentation about advance care preferences were assessed by (quote) "blinded review of the electronic health record by 2 nonstudy clinicians." Other details of outcome assessors not reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Outcome of documentation of –1 preferences was analysed all participants (n = 50). However, when outcomes of QoL and depression were analysed, 10/26 (38.5%) participants in the intervention group and 9/24 (37.5%) participants in the control group were lost to follow‐up due to participants' death. Although the reasons were balanced across groups, the rate of losses were high. |
| Selective reporting (reporting bias) | Unclear risk | Planned outcomes as indicated in the trial register entry (NCT02805712) were reported. However, participants' recruitment commenced before the trial was registered. |
| Other bias | Low risk | None identified. |
Rogers 2017.
| Methods |
Study design: RCT Total duration of study: duration of intervention phase 6 months, but patients in both groups were followed until death or end of the study Number of study centres and location: 1 centre/US Study setting: not reported Date of study: 15 August 2012 to 25 June 2015 |
|
| Participants |
Randomised (n): 150 (intervention: 75; control: 75) Lost to follow‐up/withdrawals (n): QoL measurement: intervention: 34 lost to follow‐up (23 died) at 6 months; control: 35 lost to follow‐up (20 died) at 6 months. Depressive measurement: intervention: 34 lost to follow‐up (23 died) at 6 months; control: 36 lost to follow‐up (20 died) 6 months Analysed (n): QoL measurement at: 2 weeks: 123 (intervention: 63; control: 60); 6 weeks: 110 (intervention: 53; control: 57); 3 months: 90 (intervention: 47; control: 43); 6 months 81 (intervention: 41; control: 40). Depressive measurement at: 2 weeks: 113 (intervention: 59; control: 54); 3 months: 89 (intervention: 46; control: 43); 6 months: 80 (intervention: 41; control: 39) Mean age (years): intervention: 71.9 (SD 12.4); control: 69.8 (SD 13.4) Age range: not reported Gender (n and % men): intervention: 42 (56.0%); control: 37 (49.3%) Severity of condition (n and %) and diagnostic criteria: intervention: 54 (72.0%) were NYHA III, 15 (20.0%) were NYHA IV; control: 58 (77.3%) were NYHA III, 5 (6.7%) were NYHA IV/diagnostic criteria not reported Inclusion criteria: aged > 18 years, hospitalisation for acute HF (either systolic HF or HF with preserved ejection fraction) or within 2 weeks of discharge of a hospitalisation for acute HF, dyspnoea at rest or minimal exertion plus ≥ 1 sign of volume overload, previous HF hospitalisation within the past 1 year, ESCAPE risk score ≥ 4 indicating 50% predicted 6‐month mortality, anticipated discharge from hospital with anticipated ability to return to outpatient follow‐up appointments, hospitalised with acute HF with signs/symptoms of volume overload who did not meet all other eligibility criteria may also be considered for enrolment if they could be categorised into 1 of the following high‐risk groups.
Exclusion criteria: ACS within 30 days, cardiac resynchronisation therapy within the past 3 months or current plan to implant, active myocarditis, constrictive pericarditis, severe stenotic valvular disease amendable to surgical intervention, anticipated heart transplant or VAD within 6 months, renal replacement therapy, non‐cardiac terminal illness, pregnant or planning to become pregnant, inability to comply with study protocol |
|
| Interventions |
Intervention: Palliative Care in Heart Failure (PAL‐HF). Study team assessed and managed the multiple domains of QoL for patients with advanced HF, including physical symptoms, psychosocial and spiritual concerns, and advance care planning Intervention co‐ordinated by a certified palliative care nurse practitioner. Performed in collaboration with each patient's clinical cardiology team and focused on shared goal‐setting to combine HF symptom amelioration with palliative care goals. After hospital discharge, the palliative nurse practitioner actively participated in the ongoing management of patients in outpatient environment. Comparison: managed by a cardiologist‐directed team with HF expertise. Inpatient care was focused on symptom relief and use of evidence‐based therapies as detailed in current guidelines. After discharge, patients received outpatient follow‐up with their general practitioners and an HF cardiologist or nurse practitioner with care focused on guidelines‐based medication titration and serial monitoring of end‐organ function. |
|
| Outcomes |
Primary outcomes and time points:
Secondary outcomes and time points:
Adverse outcomes: not reported |
|
| Notes |
Funding for trial: National Institute of Nursing Research (NINR) Notable conflicts of interest of trial authors: "Dr. Mentz has received research support from the National Institutes of Health (U10HL110312 and R01AG045551‐01A1), Amgen, AstraZeneca, Bristol‐Myers Squibb, GlaxoSmithKline, Gilead, Medtronic, Novartis, Otsuka, and ResMed; honoraria from HeartWare, Janssen, Luitpold Pharmaceuticals, Novartis, ResMed, and Thoratec/St. Jude; and has served on an advisory board for Luitpold Pharmaceuticals, Inc., and Boehringer Ingelheim. Dr. Granger has received research funding from Novartis, Sanofi, Daiichi, Boe[h]ringer Ingelheim, and AstraZeneca. Dr. Johnson has received research support from projects funded by the National Institute on Aging (RO1AG042130; K08AG028975). Dr. Krishnamoorthy has worked on projects funded by research grants to the Duke Clinical Research Institute from the NIH, Novartis, Daiichi‐Sankyo, and Eli Lilly; and has received support to attend educational conferences from HeartWare, Thoratec, and Medtronic. Dr. Mark has received consulting fees from Medtronic; and has received research funding from Eli Lilly, Bristol‐Myers Squibb, Pfizer, AstraZeneca, Merck and Company, Oxygen Therap[e]utics, and Gilead. Dr. Tulsky has received research funding from PCORI (SC 14‐1403‐13975). All other authors have reported that they have no relationships relevant to the contents of this paper to disclose." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described in design paper that participants were assigned using a complete randomisation scheme, but details not reported. |
| Allocation concealment (selection bias) | Unclear risk | Described in the design paper that participants were assigned using a complete randomisation scheme, but details were not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The trial was unblinded because blinding of the intervention was not feasible." Comment: study team assessed and managed the participants' multiple domains of QoL. Therefore, participants were not blinded to investigators. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Spiritual concerns were assessed by the study nurse practitioner;" "Goals of care were iteratively assessed by the intervention nurse practitioner." Comment: other details of outcome assessors not reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | QoL measurement (n and %): intervention: 23 (30.7%) died, 11 (14%) lost to follow‐up; control group: 20 (26.7%) died, 15 (20%) lost to follow‐up. Depressive measurement (n and %): intervention: 23 (30.7%) died, 11 (14%) lost to follow‐up; control: 20 (26.7%) died, 16 (21%) lost to follow‐up. |
| Selective reporting (reporting bias) | High risk | Not all outcomes that were described in the design and methods paper were reported. The outcomes of a structured interview with HF patient's carer regarding overall quality of care, and costs and resource utilisation which we prespecified were not reported. |
| Other bias | Low risk | None identified. |
Sidebottom 2015.
| Methods |
Study design: individual RCT Total duration of study: not reported Number of study centres and location: 1 centre/Minnesota Study setting: hospital Date of study: recruited April 2012 to February 2013 |
|
| Participants |
Randomised (n): 232 (intervention: 116; control: 116) Lost to follow‐up/withdrawals (n): Intervention: 4 withdrew at time of intervention, and 11 discharged prior to intervention after randomisation Intervention: 26 lost to follow‐up at 1 month (7 died, 4 withdrew, 15 surveys were not completed for reasons unknown); 22 lost to follow‐up at 3 months (7 died, 1 withdrew, 14 surveys were not completed for reasons unknown) Control: 27 lost to follow‐up at 1 month (4 died, 2 withdrew, 21 surveys were not completed for reasons unknown), 22 lost to follow‐up at 3 months (1 died, 3 withdrew, 18 surveys not completed for reasons unknown) Analysed (n): intervention: 65; control: 78 (completed all surveys) Mean age (years): intervention: 76.0 (SD 13.0); control: 70.9 (SD 13.6) Age range: not reported Gender (n and % men): intervention: 55 (47.4%); control: 67 (57.8%) Severity of condition and diagnostic criteria: not reported Inclusion criteria: adult inpatients with a diagnosis of acute HF Exclusion criteria: in ICU, on a ventilator, undergoing evaluation for a heart transplant or a LVAD, post‐transplant to post‐LVAD, determined to be actively dying, or if they had cognitive impairments such that informed consent and data collection would not be possible or if they spoke limited English; patients who had already had a palliative care order request by their attending physician during hospital stay |
|
| Interventions |
Intervention: order for palliative care immediately entered, and triaged by palliative care team with goal of conducting palliative care consult within 24 hours of order. Providers did an initial consult and then determined whether further appointments were necessary and discussed that with the patient. Baseline study measures of symptom burden, depression, and QoL were available to providers to review at time of consultation. Study paid only for initial palliative care consultation and any subsequent visits were billed to patient's insurance as standard care. Actions of palliative care providers during visits generally included assessment of symptom burdens; emotional, spiritual, and psychosocial aspects of care; co‐ordination of care orders; recommendations for change in current or future treatment; referrals; and future care planning assessment and discussions Comparison: usual care |
|
| Outcomes |
Primary outcome and time points:
Secondary outcomes and time points:
Adverse outcomes: not reported |
|
| Notes |
Funding for trial: Abbott Northwestern Hospital Foundation Notable conflicts of interest of trial authors: "No competing financial interests exist." We contacted the trialists for further details of study data, and received some data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details not reported. |
| Allocation concealment (selection bias) | Unclear risk | Details not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Details not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "questionnaires were administered via mailed surveys at 1 and 3 months after enrollment. Patients who did not return mailed surveys within a week after the 1‐ or 3‐month follow‐up date were called by the research nurse and encouraged to return the survey or were offered the option to complete the survey over the phone." Comment: outcome data collected from self‐reported measures or electronic health record data. Since blinding of participants and personnel was unclear, it was unknown whether the outcomes of QoL and depression were affected. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Intervention group: 65/116 (56%) participants completed all surveys. Control: 78/116 (67.2%) participants completed all surveys. |
| Selective reporting (reporting bias) | Low risk | Study protocol and trial register were not reported; all outcomes listed in the 'Methods' section of the study were reported in the 'Results' section. |
| Other bias | High risk | Quote: "Patients in the intervention group were average 5.1 years older than patients in the control group." Comment: intervention: mean age 76.0 (SD 11.9) years, control: 70.9 (SD 13.6) years. |
AC: advanced care; ACP: advanced care planning; ACS: acute coronary syndrome; AD: advanced directive; CHF: congestive heart failure; COPD: chronic obstructive pulmonary disease; CPR: cardiopulmonary resuscitation; EFFECT: Enhanced Feedback for Effective Cardiac Treatment; EGFR: estimated glomerular filtration rate; EQ5D: EuroQol‐5D; ESCAPE: Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness; ESRD: end‐stage renal disease; FACIT–Pal: Functional Assessment of Chronic Illness Therapy–Palliative Care scale; FCP: Future Care Plan; n: number of participants; GRACE: Global Registry of Acute Coronary Syndrome; HADS: Hospital Anxiety and Depression Survey; HF: heart failure; ICU: intensive care unit; KCCQ: Kansas City Cardiomyopathy Questionnaire; LVAD: left ventricular assist device; NYHA: New York Heart Association; MLHF: Minnesota Living with Heart Failure; NTproBNP: N‐terminal‐pro B‐type natriuretic peptide; OHS: open‐heart surgery; PC‐ACP: Patient‐Centered Advance Care Planning; PHQ‐8: 9‐item Patient Health Questionnaire; QoL: quality of life; RCT: randomised controlled trial; SD: standard deviation; SPIRIT‐HF: SPIRonolactone In the Treatment of Heart Failure; SPMQ: Short Portable Mental Status Questionnaire; STP: Statement of Treatment Preferences; VAD: ventricular assist device.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Allen 2018 | Wrong intervention |
| Anonymous 2007 | Wrong study design |
| Blue 2001 | Wrong intervention |
| Detering 2010 | Ineligible participants. Study included participants of various diagnoses such as respiratory disease. We planned that we would include studies if the majority of participants had a diagnosis of heart failure, but this study included people with heart failure only about 30%. |
| Doorenbos 2016 | Wrong intervention |
| Gutheil 2005 | Wrong participant population |
| Harter 2016 | Wrong intervention |
| Hjelmfors 2018 | Wrong intervention |
| Hua 2017 | Wrong intervention |
| Hughes 2000 | Wrong intervention |
| Johnson 2010 | Wrong study design |
| Johnson 2018 | Wrong study design |
| McIlvennan 2016 | Wrong intervention |
| Miller 1971 | Wrong study design |
| Molloy 2000 | Wrong intervention |
| NCT01817686 | Wrong intervention |
| NCT01917188 | Wrong intervention |
| Nenner 2012 | Wrong study design |
| Newton 2009 | Wrong study design |
| Ng 2018 | Wrong intervention |
| Pearlman 2005 | Wrong participant population |
| Rabow 2004 | Wrong study design |
| Radwany 2014 | Ineligible participants. Study included participants of various diagnoses such as chronic obstructive pulmonary disease and diabetes with renal disease. Study did not indicate the rate of each diagnosis. |
| Sahlen 2016 | Wrong intervention |
| Skorstengaard 2019 | Ineligible participants. Study included participants of various diagnoses such as cancer and lung disease. We planned that we would include studies if the majority of participants had a diagnosis of heart failure, but this study included only about 17% of people with heart disease. Study was published as a series of studies about Skorstengaard 2017 that classified as awaiting studies. |
| Svendsen 2006 | Wrong study design |
| Thoonsen 2015 | Wrong participant population |
| UMIN000029805 | Wrong study design |
| Wells 2018 | Wrong intervention |
Characteristics of studies awaiting assessment [ordered by study ID]
ACTRN12613001377729.
| Methods | RCT |
| Participants |
Inclusion criteria: people with HF or lung disease at risk of dying within 12 months Exclusion criteria: high risk of death with 3 months |
| Interventions |
Intervention: single case conference of 30–40 minutes between general practitioner, palliative care physician, and specialist nurse to develop a care plan for people with life‐limiting heart or lung disease. Case conference provided a comprehensive review of the case from a palliative care perspective, including symptom control, psychosocial issues for patient and carer, ACP, and service delivery, with an emphasis on care co‐ordination between specialist and community‐based care. Comparison: normal care of specialist medical services focusing on maximising function, general practitioner providing day‐to‐day care but not in close liaison with specialists, and nurses providing case management at home, and palliative care not involved at all. |
| Outcomes |
Primary outcome:
Secondary outcomes:
|
| Notes | Last updated: December 2013 |
ACTRN12618001045202.
| Methods | RCT |
| Participants |
Inclusion criteria: aged ≥ 18 years; being 'Supportive and Palliative Care Indicators Tool' positive in accordance to the tools criteria, i.e. positive for section 1 (general indicators of poor or deteriorating health) and positive for an indicator in section 2 (clinical indicators of life‐limiting conditions) of the tool. Exclusion criteria: patient already having been referred to the intervention supportive care clinic |
| Interventions |
Intervention: multidisciplinary supportive care clinic management and ongoing follow‐up as clinically required. Clinic consisted of palliative care, renal medicine, general medicine, cardiac failure medicine, social work, ACP, and complex care nurse practitioners working in a combined clinic using a multidisciplinary clinic paradigm to manage chronic disease and care plan. Face‐to‐face consultations with patients and their carers with a palliative care physician or general medicine physician (or both), nurse practitioner, and social worker. Medical issues will be reviewed, symptoms managed, and care planning issues reviewed. Comparator: standard care includes all usual health care provided in settings other than the interventional clinic, such as the usual outpatient speciality clinics within Monash Health, usual inpatient care and emergency department care, and community care including general practice. |
| Outcomes |
Primary outcomes:
Secondary outcomes:
|
| Notes | Last updated: June 2018 |
Hughes 2015.
| Methods | individual RCT, 2 academic medical centres |
| Participants | 56 dyads Inclusion criteria: dyads of adults with cardiovascular implantable electronic device and NYHA level II or III HF; an adult family member whom they might involve in heart care decision‐making Exclusion criteria: patients or family members with cognitive impairment |
| Interventions |
Intervention: TAILORED intervention, which is a brief, nurse‐facilitated, patient‐family discussion involving assessment of patient preference for family involvement in healthcare decision and support for family members involved in proxy decision‐making. Comparison: unknown |
| Outcomes |
|
| Notes | Trial report available as conference abstract. Although we sent the corresponding trial author an e‐mail requesting further information whether article was published, we did not get a response. |
NCT00105599.
| Methods | RCT |
| Participants |
Estimated enrolment: 140 participants Inclusion criteria: ejection fraction ≤ 35% and NYHA classification III or IV; surrogates of patients meeting this criteria. Exclusion criteria: unknown |
| Interventions |
Intervention: Fair Care Program Comparison: usual care |
| Outcomes |
|
| Notes | Actual study completion date in ClinicalTrials.gov: September 2004. Study results are not available. Although we contacted the trialists for further details whether article was published, we received no response. |
NCT02463162.
| Methods | RCT |
| Participants |
Enrolment: 240 participants Inclusion criteria: diagnosis of either: class III or IV NYHA Functional Classification or Stage D based on American Heart Association/American College of Cardiology Heart Failure Classification; renal failure and on dialysis; or metastatic lung, gastrointestinal (including pancreatic), or gynaecological cancer and receiving chemotherapy, radiotherapy, or palliative care; aged ≥ 19 years; able to communicate in English; reside within 100 km of Edmonton or Calgary; owns a telephone Exclusion criteria: cognitive impairment that would impact ability to give informed consent; in crisis; first visit or in the case of renal patients, in the first month of dialysis; participated in ACP pilot study; visual or hearing impairment (or both) that would impact their ability to see or hear (or both) the videos, or complete the assessments including the telephone follow‐up interviews. |
| Interventions |
Intervention: 2 Conversations Matter videos: ACP and GCD Comparison: usual care (to receive no intervention) |
| Outcomes |
Primary outcomes:
|
| Notes | Actual study completion date in ClinicalTrials.gov: November 2016. Study results not available. Although we contacted the trialists for further details whether article was published, we received no response. |
NCT03128060.
| Methods | RCT |
| Participants |
Estimated enrolment: 1185 participants Inclusion criteria: aged ≥ 18 years; diagnosis of HF, COPD, or advanced cancer; ≥ 1 hospitalisations or emergency department visits in the previous year; Palliative Performance Scale score ≤ 70%; English‐ or Spanish‐speaking; PCP assessment that he/she "would not be surprised" if the patient died within 1 year Exclusion criteria: receiving hospice care; has end‐stage renal disease; lives in a nursing home |
| Interventions |
Intervention: home‐based palliative care consisting of home visits by an interdisciplinary primary care team (physician, nurse, social worker, and chaplain) that provides pain and symptom management, psychosocial support, ACP, disease management education, spiritual and grief counselling, and other services as needed. Comparison: enhanced usual care consisting of usual primary care provided by a PCP who has received special training in the core elements of palliative care. |
| Outcomes |
Primary outcomes:
Secondary outcomes:
|
| Notes | Last updated on ClinicalTrials.gov: April 2017 |
NCT03539510.
| Methods | RCT |
| Participants |
Estimated enrolment: 20 participants Inclusion criteria: aged > 18 years; a regular patient in trialists' clinic; able to read and write English; familiar with the use and have access to a personal computer, e‐mail, and Internet Exclusion criteria: none |
| Interventions |
Intervention: HF‐ACP Website, which leads participants through 4 e‐learning modules. Each module contains 3 core elements: educational content that provides information and support to help patients complete the module, interactive tools for documenting their thoughts and progress, and motivational video clips that encourage behaviour change by validating participants ambivalence, suggesting strategies to help participants complete the task and to encourage and reassure participants that they can do this. Comparison: usual care. The standard of care for ACP at the trialists' institution is the "Speak Up" booklet and the Power of Attorney workbook from the Attorney General's Office – Ontario. Patients randomised to the control arm will be asked to register on a separate research portal where they will have electronic access to both booklets and a link to the Speak Up online Interactive workbook. There is no specific information on HF or HF treatments. They will be asked to complete the ACP using the interactive workbook. They will not receive any additional communication from the research team about their progress. |
| Outcomes |
Primary outcome:
Secondary outcomes:
|
| Notes | Last updated on ClinicalTrials.gov: May 2018 |
NCT03649191.
| Methods | RCT |
| Participants |
Estimated enrolment: 1129 participants Inclusion criteria: residents of participating nursing homes; aged ≥ 65 years; at high risk of death in the next 6–12 months, as indicated by data collected on the Resident Assessment Instrument that is completed quarterly in most Canadian nursing homes. Specifically the high‐risk elements are any of: CHESS score ≥ 3, cancer, congestive H, leave > 25% of their food uneaten; resident and resident's substitute decision‐maker provide informed consent to participate Exclusion criteria: resident and substitute decision‐maker do not speak English or French; residents who are deemed not be competent to make their own medical decisions AND their substitute decision‐maker is a legally assigned public guardian, or they have no substitute decision‐maker; residents who are transferred to a BABEL study home from another BABEL study home, with the date of transfer being after study initiation. Residents who transferred into a study home from a non‐study home are eligible. |
| Interventions |
Intervention: BABEL Approach to Advance Care Planning in Nursing Homes. Eligible residents will: receive the BABEL Approach to Advance Care Planning; after these ACP discussions occur, the resident's PCP will be notified of the residents' ACP wishes; a brightly coloured document will be placed in a standard location of the nursing home chart that identifies the resident's ACP wishes; paramedics will be educated to know about these sheets and where to find them, and that they should be taken with any resident transferred to another care setting. Comparison: control group will receive the prevalent approach to ACP in that nursing home. No elements of the BABEL Approach to Advance Care Planning will be introduced |
| Outcomes |
Primary outcomes:
Secondary outcomes:
|
| Notes | Last updated on ClinicalTrials.gov: September 2018 |
O'Riordan 2014.
| Methods | Baseline survey for RCT |
| Participants | 29 people with HF, further details not reported |
| Interventions | Intervention: in collaboration with HF specialists, the intervention consists of an interdisciplinary palliative care intervention focused on symptom management, ACP, and psychosocial support. The initial palliative care visit is conducted in hospital, followed by ≥ 3 in‐person and 3 telephone consultations over 6 months. |
| Outcomes | Not reported |
| Notes | We contacted the trialists for further details whether article was published. Their article was in the process of submission, and we were unable to get further information on the article. |
Skorstengaard 2017.
| Methods | RCT |
| Participants | 360 participants Inclusion criteria: eligible patients from the departments of oncology, cardiology, and respiratory medicine at Aarhus University Hospital; aged > 18 years; acceptable Danish language skills Exclusion criteria: cognitive impairment; expected to die within the next month; has no relatives |
| Interventions |
Intervention: ACP conversation between a health professional and a patient about end‐of‐life discussions Comparison: usual care |
| Outcomes |
Primary outcomes:
Secondary outcomes:
|
| Notes | Study completed. We contacted the trial authors and were informed that publication of study findings is in progress. Although 2 articles were published, 1 study design was cross‐sectional study and the other had ineligible participants. The trialists are planning to publish 1 more article. Mrs Marianne H Skorstengaard, E‐mail: hsk@ki.au.dk Trial registration: NCT01944813 (first posted: 18 September 2013) |
ACP: advance care planning; AD: advance directive; CHESS: Changes in Health, End‐stage disease and Symptoms and Sign; COPD: chronic obstructive pulmonary disease; GAD‐7: 7‐item Generalized Anxiety Disorder Scale; GCD: Goals of Care Designations; HF: heart failure; NYHA: New York Heart Association; PCP: primary care physician; PHQ‐9: 9‐item Patient Health Questionnaire; QoL: quality of life; RCT: randomised controlled trial; SF‐12: 12‐item Short Form.
Characteristics of ongoing studies [ordered by study ID]
ACTRN12614000590662.
| Trial name or title | A randomised control trial for advance care planning and symptom management for patients identified in the emergency department and followed up at home |
| Methods | RCT |
| Participants |
Estimated enrolment: 500 participants Inclusion criteria: people presenting to the Prince of Wales Hospital Emergency Department with any advanced progressive life‐threatening illness as identified by clinical staff using the iPAL‐EM screening tool guide who do not presently require referral to specialist palliative care. Exclusion criteria: aged < 18 years, prisoners, pregnant women, and people with unstable psychotic illnesses or serious mood disorders |
| Interventions |
Intervention: ACP + Symptom Management and Support, a 2‐pronged intervention. ACP: series of nurse‐lead discussions that explore and identify values and concerns relating to the participant's health care and management of care at EOL. Discussions will include patient and patient's carer if available and willing to participate in discussion. Anticipated that discussions will take place over ≤ 4 visits lasting 1–1.5 hours each. SMS: education component provided to participants' GPs and other healthcare providers. This includes: in‐service style training by a member of research team conducted at baseline and repeated at 6 months; individualised education regarding palliative care; provision of a symptom management resource list; letter to GP with information about Plan Early and ACP, written information about referral to specialist palliative care, and 6‐weekly follow‐up telephone calls to GP and family; education and training of RACF staff and GPs in the SELHD Terminal Care Plan. Comparison: usual care, which is standard evidence‐based care provided at the discretion of patient's GP and other healthcare providers. As health conditions will vary among participants in control group, usual care is expected to vary. |
| Outcomes |
Primary outcomes:
Secondary outcomes:
|
| Starting date | Date registered: June 2014 Date of first enrolment: February 2014 |
| Contact information | Dr Megan Sands, E‐mail: Meg.Sands@sesiahs.health.nsw.gov.au Prince of Wales Hospital Barker Street Randwick NSW 2031, Australia |
| Notes | Last updated: 4 June 2014 |
ACTRN12617001040358.
| Trial name or title | FINnish PALliative care education trial (FINPAL) to improve nursing home residents' quality of life |
| Methods | Cluster RCT |
| Participants |
Estimated enrolment: 300 participants Inclusion criteria: Finnish speaking; permanent resident in a nursing home/assisted‐living facility in city of Helsinki/Vantaa, Finland; ≥ 1 condition evaluated to affect severely health and prognosis (severe dementia, heart failure, COPD, diabetes mellitus, coronary heart disease, cancer, cachexia, chronic inflammation, frailty, disability, malnutrition); volunteer; able to give informed consent Exclusion criteria: aged < 65 years; evaluated prognosis > 1‐year terminal disease; end‐stage dementia; end‐stage COPD; end‐stage heart failure; end‐stage renal failure; malignant cancer; severe stroke; permanent institutionalisation; severe disability |
| Interventions |
Intervention: staff working in units that are randomised to intervention group will be able to take part in educational sessions in palliative and end‐of‐life care over 3 months. Educational sessions will be performed in small groups of 10–20 staff members. Teaching method will be interactive encouraging discussions and questions. Sessions will encourage staff members to share and learn from previous experiences and patient cases in their own units to help them improve teamwork. Comparison: usual care, information leaflet to staff on current care guidelines on terminal care. |
| Outcomes |
Primary outcomes:
Secondary outcomes:
|
| Starting date | Date registered: July 2017 Date of first enrolment: September 2017 |
| Contact information | Prof Kaisu Pitkälä, E‐mail: kaisu.pitkala@helsinki.fi University of Helsinki Department of General Practice, PO Box 20. 00014 University of Helsinki, Finland |
| Notes | URL: www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=372296 Last updated: July 2017 |
Ejem 2019.
| Trial name or title | Reducing disparities in the quality of palliative care for older African Americans through improved advance care planning: study design and protocol |
| Methods |
Study design: RCT Number of study centres and location: 10 clinics in 5 US states/Alabama, Georgia, North Carolina, Texas, and South Carolina Study setting: clinic |
| Participants |
Inclusion criteria: aged ≥ 65 years; non‐Hispanic, African‐American, or white; English speaking; residence in a non‐institutional setting; ≥ 2 visits at 1 of the participating clinics in last year; serious, chronic illness (≥ 1 of metastatic solid tumour or haematological cancer, end‐stage renal disease, advance liver disease or cirrhosis, COPD or interstitial lung disease on home oxygen, or hospitalised for condition in the last year, diabetes with severe complications (ischaemic heart disease, peripheral vascular disease, and renal disease); NYHA Stage III or IV congestive heart failure with hospitalisation in the last year; ≥ 2 unplanned hospitalisations in the last year; dependence in ≥ 1 activities of daily; aged ≥ 80 years; referral by provider at a participating clinic if provider considered patient would die in next 12 months. Exclusion criteria: residence in nursing home or assisted living facility; deafness; blindness; diagnosis of dementia; significant cognitive impairment; EMR documentation or patient report of ACP (living will, health care proxy, MOST form, and provider note); current or prior use of hospice or home‐based palliative care |
| Interventions |
Intervention: 2 ACP approaches for comparison: a structured ACP approach using Respecting Choices First Steps and a patient‐driven, self‐management approach, including a Five Wishes form. Both approaches are delivered by lay ACP facilitators. In addition, all participants receive state advance directive forms and a contact card with telephone number and picture of their assigned lay ACP facilitator. Participants receive Respecting Choices materials that include general information about ACP and choosing a healthcare agent. Within 2 weeks, patient is contacted by a lay ACP facilitator who sets up a meeting with patient and surrogate decision maker for a 60–90‐minute ACP session. Conversation focuses on identifying cultural, spiritual, and personal beliefs that influence treatment preferences, identifying a healthcare agent, and exploring goals for medical care. Meetings occur in person if possible (by telephone if not). Lay ACP facilitator follows up with telephone call 2 weeks after meeting to answer questions. Patients may request 1 additional follow‐up call. Comparison: patients at clinics randomised to the patient‐driven, self‐management ACP approach receive the Five Wishes advance directive form and the Five Wishes Conversation Guide for Individuals and Families. Document allows patients to share their 'wishes' for the person they would like to serve as surrogate decision maker; the type of medical care that they would like to receive; how comfortable they want to be; how they want people to treat them; and what they want their loved ones to know. |
| Outcomes |
Primary outcome:
Secondary outcomes:
|
| Starting date | Not reported |
| Contact information | Deborah B Ejem, E‐mail: tejem@uab.edu Division of Preventive Medicine, School of Medicine, University of Alabama at Birmingham, Birmingham, Alabama |
| Notes |
Graney 2019.
| Trial name or title | Advancing Symptom Alleviation with Palliative Treatment (ADAPT) trial to improve quality of life: a study protocol for a randomized clinical trial |
| Methods |
Study design: RCT Number of study centres and location: 2 Veterans Health Administration facilities/Eastern Colorado, Puget Sound Study setting: community‐based outpatient clinics |
| Participants |
Estimated enrolment: 300 participants Inclusion criteria: diagnosis of CHF or COPD within 2 years prior to enrolment; diagnosis of interstitial lung disease within 2 years prior to enrolment; among those with CHF or COPD, high risk for hospitalisation and death; poor quality of life; symptomatic; primary care or other provider who is willing to facilitate intervention medical recommendations; able to read and understand English; consistent access to and able to use a standard telephone Exclusion criteria: diagnosis of dementia; active substance abuse; comorbid metastatic cancer; diagnosis of obesity hypoventilation syndrome; nursing home resident; heart or lung transplant or LVAD; participation in the intervention arm of the CASA (Collaborative Care to Alleviate Symptoms and Adjust to Illness in Chronic Heart Failure) trial (NCT01739686); enrolled in palliative care, hospice, or home‐based primary care; prisoner; pregnant |
| Interventions |
Intervention: multidisciplinary, team‐based approach to addressing symptoms and psychosocial needs of participants. Personnel include a registered nurse and Master's level social worker. They integrate into a larger collaborative care team that includes a representative primary care provider and palliative care specialist. Specialist support with a cardiologist or pulmonologist is available for the team for additional management recommendations if needed. Each site has a team that meets weekly for 30–60 minutes, integrating palliative symptom management with disease‐specific care plans. Comparison: usual care at the discretion of their care providers. No limitations on care recommendations or referrals, which may include management by subspecialists, mental health providers, or palliative care at the discretion of their primary care providers. |
| Outcomes |
Primary outcomes:
Secondary outcomes:
|
| Starting date | Recruitment began 1 September 2016 |
| Contact information | Bridget A Graney, E‐mail: bridget.graney@ucdenver.edu Division of Pulmonary Sciences and Critical Care Medicine, Department of Medicine, University of Colorado School of Medicine, Anschutz Medical Campus, Aurora, CO, USA University of Colorado Anschutz Medical Campus, Aurora, USA |
| Notes | Trial registration: NCT02713347. Registered 18 March 2016. Last updated 16 August 2019 |
Malhotra 2016.
| Trial name or title | Impact of advance care planning on the care of patients with heart failure: study protocol for a randomized controlled trial |
| Methods |
Study design: RCT Number of study centres and location: 2 centres/Singapore Study setting: hospital |
| Participants | 282 participants Inclusion criteria: people with advanced CHF (NYHA classes III and IV); hospitalised; aged ≥ 21 years; able to give informed consent Exclusion criteria: psychiatric or cognitive disorders; previously documented ACP; people who have undergone ACP facilitation |
| Interventions |
Intervention: ACP facilitator will assist in determining patients' preferences for future medical care, and will provide patients and their families with emotional support to make end‐of‐life decisions. Family members will be encouraged to be present during ACP discussion so that whole family unit will be able to explore goals, values, and beliefs towards patient's medical care. Patients will be encouraged to appoint a substitute healthcare decision‐maker who will make decisions on their behalf when they are no longer able to do so. These patients' preferences will be noted in the ACP document, and copy of ACP document will be provided to patients, their substitute healthcare decision‐makers, and the healthcare team. The ACP document will be recorded electronic medical records in the National IT System, and will be shared with another hospital in Singapore. The ACP facilitator will review patients' preferences regularly. If patients' preferences about future medical care change, the ACP facilitator will discuss patients' preferences with patients and their families or substitute healthcare decision‐makers, and will update the ACP document. Comparison: usual care with no ACP discussions and documentation. |
| Outcomes |
Primary outcome:
Secondary outcomes:
|
| Starting date | February 2015 |
| Contact information | Chetna Malhotra, E‐mail: chetna.malhotra@duke‐nus.edu.sg Duke‐NUS Graduate Medical School, Singapore |
| Notes | Trial registration: NCT02299180. Registered 18 November 2014 |
NCT00489021.
| Trial name or title | Feasibility and outcomes of older patients' hospitalization (official title not provided) |
| Methods | RCT |
| Participants |
Estimated enrolment: 300 participants Inclusion criteria: aged > 65 years; haemodynamic, nutritional, and respiratory stability; eligibility for subacute hospitalisation Exclusion criteria: oncological active disease; uncorrectable hypoxaemia (oxygen saturation 90%); suspected myocardial ischaemia; presence of an acute illness, other than the target illness |
| Interventions | Not known |
| Outcomes | Not known |
| Starting date | July 2007 |
| Contact information | Doron Nezer, E‐mail: doronne@clalit.org.il |
| Notes | Last updated on ClinicalTrials.gov: 21 June 2007 Although we contacted the trialists for further details whether study was completed and article was published, we received no response. |
NCT02429479.
| Trial name or title | Preparing family caregivers to make medical decisions for their loved ones |
| Methods | RCT |
| Participants |
Enrolment: 570 participants Inclusion criteria: aged ≥ 18 years; diagnosis of kidney disease (e.g. chronic kidney disease, end‐stage renal disease) OR advanced cancer (Stage IV disease or having an estimated survival < 2 years) OR severe heart failure (e.g. NYHA class III or IV) OR severe lung disease (e.g. Stage III or IV COPD by modified GOLD Spirometric Classification, idiopathic pulmonary fibrosis); able to read and understand English at 8th grade level (word 26 on either blue or tan version of the Wide Range Achievement Test‐3 reading subtest); neuro‐cognitively able to engage in ACP (Mini‐Mental State Examination score > 23); no active suicidal ideations (i.e. score of 0 or 1 on item 9 of the Beck Depression Inventory‐II) Exclusion criteria: failure on any of the above inclusion criteria |
| Interventions |
Group 1: standard ACP/patient alone Group 2: decision aid/patient alone Group 3: standard ACP/patients and carers together Group 4: decision aid/patients and carers together |
| Outcomes |
Primary outcome:
Secondary outcomes:
|
| Starting date | June 2013 |
| Contact information | Dr Benjamin H Levi, E‐mail: bhl3@psu.edu Milton S. Hershey Medical Center/Penn State College of Medicine, H134 Humanities Hershey, PA, USA |
| Notes | Last updated on ClinicalTrials.gov: November 2017 |
NCT02612688.
| Trial name or title | Pragmatic trial of video education in nursing homes (PROVEN) |
| Methods | RCT |
| Participants |
Inclusion criteria: facilities within Genesis HealthCare or PruittHealth healthcare systems; facilities have facility identifiers that indicate that they are Medicare/Medicaid‐certified nursing facilities in the US; facilities serve both short and long‐stay patients; facilities have > 50 beds; facilities have an electronic medical records system; facilities had ≥ 20 admissions and 20 annual Minimum Data Set assessments (regardless of whether they were discharged alive) in 2013. Exclusion criteria: facilities excluded per corporate leaders in healthcare system because of recent turnover in nursing home administrator or Director of Nursing; facilities excluded per corporate leaders in health care system because of recent bad state or federal quality assurance survey (e.g. restriction on admissions, levied large civil monetary penalty, etc.); facilities excluded per corporate leaders in healthcare system because of current new initiatives/competing demands. |
| Interventions |
Intervention: ACP Video Program, which consists of 5 videos that address ACP decisions: General Goals of Care, Goals of Care for Advanced Dementia, Hospice, Hospitalization, and ACP for Healthy Patients. Nursing home staff will offer videos to patients at these clinical triggers: within 7 days of admission or readmission; every 6 months for long‐stay patients; when there is a significant change in clinical status; when a treatment decision arises for which there is a specific video; and special circumstances when goals of care are being considered (e.g. family visiting). Comparison: usual ACP procedures |
| Outcomes |
Primary outcome:
Secondary outcomes:
|
| Starting date | March 2016 |
| Contact information | Vincent Mor, E‐mail: Vincent_Mor@brown.edu Health Services, Policy & Practice, Brown University, Rhode Island, USA |
| Notes | Last updated on ClinicalTrials.gov: May 2018 |
NCT03170466.
| Trial name or title | Primary palliative care in heart failure: a pilot trial |
| Methods | RCT |
| Participants |
Enrolment: 30 participants Inclusion criteria: NYHA class III or IV heart failure; ≥ 2 hospitalisations in the past year due to heart failure Exclusion criteria: aged < 40 years; currently awaiting a transplant; received outpatient palliative care within the past 12 months; pregnant or intends to be within next 12 months; no regular telephone access; not fluent in English; failed the Callahan 6‐item Screener; does not intent to regularly attend clinic for the next 12 months |
| Interventions |
Intervention: 4 primary mechanisms 1. An existing heart failure nurse will deliver the intervention to patients during regularly scheduled visits. 2. Telephone calls will reinforce topics. 3. Patients will regularly report symptoms through the MyUPMC patient portal. 4. The nurse will act as a liaison to communicate concerns to the patient's cardiologist and primary care physician, as well as facilitating other resources The intervention will span 4 domains: symptom management (e.g. dyspnoea), psychosocial support (e.g. anxiety), ACP (e.g. understanding prognosis and electing a proxy), and care co‐ordination (e.g. communication across providers). Comparison: usual care, which will include standard of high‐quality heart failure care provided to all patients. |
| Outcomes |
Primary outcome:
Secondary outcomes:
|
| Starting date | October 2017 |
| Contact information | Dio Kavalieratos, PhD, Assistant Professor of Medicine, E‐mail: diok@pitt.edu University of Pittsburgh, 230 McKee Place Suite 600 Pittsburgh, PA 15213, USA |
| Notes | Recruitment status: active, not recruiting Last verified: June 2019 Estimated study completion date: 31 May 2020 |
NCT03516994.
| Trial name or title | Reducing disparities in the quality of advance care planning for older adults (EQUALACP) |
| Methods | Cluster randomised trial, 4 locations |
| Participants |
Estimated enrolment: 800 participants Inclusion criteria: African‐American or white people; aged ≥ 65 years; English‐speaking; residing in non‐institutional setting; cognitively able to participate in ACP; serious or chronic illness including: metastatic cancer, end‐stage renal disease, advanced liver disease, heart disease, lung disease, amyotrophic lateral sclerosis, severe Parkinson's disease; ≥ 2 unplanned hospitalisations in last year; requiring assistance with any basic activity of daily living. Exclusion criteria: residence in nursing home or assisted living facility; diagnosis of dementia or unable to consent; documented ACP (living will, health care proxy, MOST form, provider note); current or prior use of hospice; current or prior use of non‐hospice palliative care except inpatient palliative care consultation |
| Interventions |
Intervention: structured ACP. Patients will participate in 60‐ to 90‐minute facilitated ACP conversation with a trained person using Respecting Choices (First Steps) guide and receive a state advance directive form. The ACP facilitator will follow‐up as needed after the session to answer additional questions. Comparison: patient‐driven ACP. Patients will receive a Five Wishes Form (easy to understand advance directive written in plain language), a state advance directive form, and ≥ 2 follow‐up telephone calls with an ACP contact who will answer questions. |
| Outcomes |
Primary outcomes:
Secondary outcomes:
|
| Starting date | January 2019 |
| Contact information | Kimberly S Johnson, E‐mail: kimberly.s.johnson@duke.edu Duke University, Durham, NC, USA |
| Notes | Last updated on ClinicalTrials.gov: October 2018 |
ACP: advance care planning; ALS: advanced life support; CHF: chronic heart failure; COPD: chronic obstructive pulmonary disease; CPR: cardiopulmonary resuscitation; EMR: electronic medical record; EOL: end of life; GOLD: Global Initiative for Chronic Obstructive Lung Disease; GP: general practitioner; iPAL‐EM: Improving Palliative Care in Emergency Medicine; MDS‐RAI: Resident Assessment Instrument‐Minimum Data Set; MOST: Medical Orders for Scope of Treatment; NWAU: National Weighted Activity Unit; NYHA: New York Heart Association; PACE: Programs of All‐Inclusive Care for the Elderly; RACF: residential aged care facilities; RCT: randomised controlled trial; SELHD: South Eastern Sydney Local Health District.
Differences between protocol and review
We changed the title of the review from 'Advance care planning for heart failure' to Advance care planning for adults with heart failure.
Contributions of authors
YN: conceived the concept, developed, and finalised the protocol and the review. NH: developed and finalised the protocol and the review. HF: conceived the concept, developed, and finalised the protocol and the review. EO: developed and finalised the protocol and the review. AM: developed and finalised the protocol and the review. MM: developed and finalised the protocol and the review. DY: developed and finalised the protocol and the review. JK: developed and finalised the protocol and the review.
Sources of support
Internal sources
No sources of support supplied
External sources
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure to the Heart Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service (NHS), or the Department of Health, UK.
Declarations of interest
YN: none. NH: none. HF: none. EO: none. AM: this research is supported by the 'Practical Research Project for Life‐Style related Diseases including Cardiovascular Diseases and Diabetes Mellitus' from the Japan Agency for Medical Research and Development, AMED. MM: none. DY: none. JK: none.
New
References
References to studies included in this review
Briggs 2004 {published data only}
- Briggs LA, Kirchhoff KT, Hammes BJ, Song MK, Colvin ER. Patient‐centered advance care planning in special patient populations: a pilot study. Journal of Professional Nursing 2004;20(1):47‐58. [PUBMED: 15011193] [DOI] [PubMed] [Google Scholar]
- Song MK, Sereika SM. An evaluation of the Decisional Conflict Scale for measuring the quality of end‐of‐life decision making. Patient Education and Counseling 2006;61(3):397‐404. [PUBMED: 15970420] [DOI] [PubMed] [Google Scholar]
Denvir 2016 {published data only}
- Boyd K, Cudmore S, Highet G, Robertson S, Donald L, Weir C, et al. Future care planning in advanced heart disease. Palliative Medicine 2016;30(4):S15. [DOI: 10.1177/0269216316631462] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denvir M, Highet G, Cudmore S, Robertson S, Weir C, Murray S, et al. Future care planning in advanced heart disease; a stepped wedge randomised, controlled trial. Palliative Medicine 2016;30(6):Np57. [DOI: 10.1177/0269216316646056] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denvir MA, Cudmore S, Highet G, Robertson S, Donald L, Stephen J, et al. Phase 2 randomised controlled trial and feasibility study of future care planning in patients with advanced heart disease. Scientific Reports 2016;6:24619. [PUBMED: 27090299] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02302014. Palliative care for patients with advanced heart disease. clinicaltrials.gov/show/nct02302014 (first received 26 November 2014).
El‐Jawahri 2016 {published data only}
- El‐Jawahri A, Paasche‐Orlow MK, Matlock D, Stevenson LW, Lewis EF, Stewart G, et al. Randomized, controlled trial of an advance care planning video decision support tool for patients with advanced heart failure. Circulation 2016;134(1):52‐60. [PUBMED: 27358437] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01589120. Using videos to facilitate advance care planning for patients with heart failure (VIDEO‐HF). clinicaltrials.gov/ct2/show/nct01589120 (first received 1 May 2012).
Kirchhoff 2010 {published data only}
- Kirchhoff KT, Hammes BJ, Kehl KA, Briggs LA, Brown RL. Effect of a disease‐specific advance care planning intervention on end‐of‐life care. Journal of the American Geriatrics Society 2012;60(5):946‐50. [PUBMED: 22458336] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhoff KT, Hammes BJ, Kehl KA, Briggs LA, Brown RL. Effect of a disease‐specific planning intervention on surrogate understanding of patient goals for future medical treatment. Journal of the American Geriatrics Society 2010;58(7):1233‐40. [PUBMED: 20649686] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00204802. Patient‐centered advance care planning. clinicaltrials.gov/ct2/show/nct00204802 (first received 20 September 2005).
Menon 2016 {published data only}
- Menon S, McCullough LB, Beyth RJ, Ford ME, Espadas D, Braun UK. Use of a values inventory as a discussion aid about end‐of‐life care: a pilot randomized controlled trial. Palliative & Supportive Care 2016;14(4):330‐40. [PUBMED: 26458331] [DOI] [PubMed] [Google Scholar]
- NCT00122135. A culturally sensitive values‐guided aid for end of life decision‐making. clinicaltrials.gov/show/nct00122135 (first received 21 July 2005).
Metzger 2016 {published data only}
- Metzger M, Song MK, Ward S, Chang PP, Hanson LC, Lin FC. A randomized controlled pilot trial to improve advance care planning for LVAD patients and their surrogates. Heart & Lung 2016;45(3):186‐92. [PUBMED: 26948697] [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Donnell 2018 {published data only}
- NCT02805712. Focused palliative care intervention in advanced heart failure. clinicaltrials.gov/show/nct02805712 (first received 20 June 2016).
- O'Donnell AE, Schaefer KG, Stevenson LW, DeVoe K, Walsh K, Mehra MR, et al. Social worker‐aided palliative care intervention in high‐risk patients with heart failure (SWAP‐HF): a pilot randomized clinical trial. JAMA Cardiology 2018;3(6):516‐9. [PUBMED: 29641819] [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell AE, Schaefer KG, Stevenson LW, Mehra MR, Desai AS. A randomized controlled trial of a social worker‐aided palliative care intervention in high risk patients with heart failure (SWAP‐HF). Journal of Cardiac Failure 2016;22(11):940. [Google Scholar]
Rogers 2017 {published data only}
- Mentz RJ, Tulsky JA, Granger BB, Anstrom KJ, Adams PA, Dodson GC, et al. The palliative care in heart failure trial: rationale and design. American Heart Journal 2014;168(5):645‐51.e1. [PUBMED: 25440791] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01589601. Palliative care in heart failure (PAL‐HF). clinicaltrials.gov/ct2/show/nct01589601 (first received 2 May 2012).
- Rogers JG, Patel CB, Mentz RJ, Granger BB, Steinhauser KE, Fiuzat M, et al. Palliative care in heart failure: the PAL‐HF randomized, controlled clinical trial. Journal of the American College of Cardiology 2017;70(3):331‐41. [PUBMED: 28705314] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JG, Patel CB, Mentz RJ, Steinhauser KE, Granger BB, Fiuza M, et al. Palliative care in heart failure: results of a randomized, controlled clinical trial. Journal of Cardiac Failure 2016;22(11):940. [Google Scholar]
Sidebottom 2015 {published and unpublished data}
- Sidebottom AC, Jorgenson A, Richards H, Kirven J, Sillah A. Inpatient palliative care for patients with acute heart failure: outcomes from a randomized trial. Journal of Palliative Medicine 2015;18(2):134‐42. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Allen 2018 {published data only}
- Allen LA, McIlvennan CK, Thompson JS, Dunlay SM, LaRue SJ, Lewis EF, et al. Effectiveness of a shared decision making intervention for patients offered a destination therapy left ventricular assist device for end‐stage heart failure: the DECIDE‐LVAD trial. Circulation 2017;136:e461. [DOI: 10.1161/CIR.0000000000000546] [DOI] [Google Scholar]
- Allen LA, McIlvennan CK, Thompson JS, Dunlay SM, LaRue SJ, Lewis EF, et al. Effectiveness of an intervention supporting shared decision making for destination therapy left ventricular assist device: the DECIDE‐LVAD randomized clinical trial. JAMA Internal Medicine 2018;178(4):520‐9. [PUBMED: 29482225] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02344576. PCORI‐1310‐06998 trial of a decision support intervention for patients and caregivers offered destination therapy heart assist device. clinicaltrials.gov/show/nct02344576 (first received 26 January 2015).
Anonymous 2007 {published data only}
- Anonymous. Nurses awarded for end‐of‐life care. Nursing Times 2007; Vol. 103, issue 28:4‐5.
Blue 2001 {published data only}
- Blue L, Lang E, McMurray JJ, Davie AP, McDonagh TA, Murdoch DR, et al. Randomised controlled trial of specialist nurse intervention in heart failure. BMJ (Clinical Research Ed.) 2001;323(7315):715‐8. [PUBMED: 11576977] [DOI] [PMC free article] [PubMed] [Google Scholar]
Detering 2010 {published data only}
- Detering KM, Hancock AD, Reade MC, Silvester W. The impact of advance care planning on end of life care in elderly patients: randomised controlled trial. BMJ (Clinical Research Ed.) 2010;340:c1345. [PUBMED: 20332506] [DOI] [PMC free article] [PubMed] [Google Scholar]
Doorenbos 2016 {published data only}
- Doorenbos AZ, Levy WC, Curtis JR, Dougherty CM. An intervention to enhance goals‐of‐care communication between heart failure patients and heart failure providers. Journal of Pain and Symptom Management 2016;52(3):353‐60. [PUBMED: 27401505] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gutheil 2005 {published data only}
- Gutheil IA, Heyman JC. Communication between older people and their health care agents: results of an intervention. Health & Social Work 2005;30(2):107‐16. [PUBMED: 15974371] [DOI] [PubMed] [Google Scholar]
Harter 2016 {published data only}
- Harter M, Dirmaier J, Dwinger S, Kriston L, Herbarth L, Siegmund‐Schultze E, et al. Effectiveness of telephone‐based health coaching for patients with chronic conditions: a randomised controlled trial. PloS One 2016;11(9):e0161269. [PUBMED: 27632360] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hjelmfors 2018 {published data only}
- Hjelmfors AL, Stromberg AS, Friedrichsen MF, Sandgren AS, Martensson JM, Jaarsma TJ. Using co‐design to develop communication interventions in heart failure care. European Journal of Cardiovascular Nursing 2016;15:S19. [Google Scholar]
- Hjelmfors L, Stromberg A, Friedrichsen M, Sandgren A, Martensson J, Jaarsma T. Using co‐design to develop an intervention to improve communication about the heart failure trajectory and end‐of‐life care. BMC Palliative Care 2018;17(1):85. [PUBMED: 29890974] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hua 2017 {published data only}
- Hua CY, Huang Y, Su YH, Bu JY, Tao HM. Collaborative care model improves self‐care ability, quality of life and cardiac function of patients with chronic heart failure. Brazilian Journal of Medical and Biological Research 2017;50(11):e6355. [PUBMED: 28953989] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hughes 2000 {published data only}
- Hughes SL, Weaver FM, Giobbie‐Hurder A, Manheim L, Henderson W, Kubal JD, et al. Effectiveness of team‐managed home‐based primary care: a randomized multicenter trial. JAMA 2000;284(22):2877‐85. [PUBMED: 11147984] [DOI] [PubMed] [Google Scholar]
Johnson 2010 {published data only}
- Johnson MJ, Booth S. Palliative and end‐of‐life care for patients with chronic heart failure and chronic lung disease. Clinical Medicine (London, England) 2010;10(3):286‐9. [PUBMED: 20726465] [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnson 2018 {published data only}
- Johnson MJ, McSkimming P, Geue C, Millerick Y, Hogg K. Patient reported outcomes, health service utilisation and place of care/death for advanced heart failure patients attending a palliative cardiology clinic or receiving usual care: a feasibility study. European Journal of Heart Failure 2017;19:558‐9. [DOI: 10.1002/ejhf.833] [DOI] [Google Scholar]
- Johnson MJ, McSkimming P, McConnachie A, Geue C, Millerick Y, Briggs A, et al. The feasibility of a randomised controlled trial to compare the cost‐effectiveness of palliative cardiology or usual care in people with advanced heart failure: two exploratory prospective cohorts. Palliative Medicine 2018;32(6):1133‐41. [PUBMED: 29688127] [DOI] [PMC free article] [PubMed] [Google Scholar]
McIlvennan 2016 {published data only}
- McIlvennan CK, Thompson JS, Matlock DD, Cleveland JC Jr, Dunlay SM, LaRue SJ, et al. A multicenter trial of a shared decision support intervention for patients and their caregivers offered destination therapy for advanced heart failure: DECIDE‐LVAD: rationale, design, and pilot data. Journal of Cardiovascular Nursing 2016;31(6):E8‐E20. [PUBMED: 27203272] [DOI] [PubMed] [Google Scholar]
- McIlvennan CK, Thompson JS, Matlock DD, Cleveland JC, Allen LA. An acceptability and feasibility study of decision aids for patients and their caregivers considering destination therapy left ventricular assist device. Journal of Cardiac Failure 2015;21(8):S6‐S7. [Google Scholar]
Miller 1971 {published data only}
- Miller MB. Decision‐making in the death process of the ill aged. Geriatrics 1971;26(5):105‐16. [PUBMED: 5556470] [PubMed] [Google Scholar]
Molloy 2000 {published data only}
- Molloy DW, Guyatt GH, Russo R, Goeree R, O'Brien BJ, Bedard M, et al. Systematic implementation of an advance directive program in nursing homes: a randomized controlled trial. JAMA 2000;283(11):1437‐44. [PUBMED: 10732933] [DOI] [PubMed] [Google Scholar]
NCT01817686 {published data only}
- NCT01817686. Study of default options in advance directives. clinicaltrials.gov/show/nct01817686 (first received 25 March 2013).
NCT01917188 {published data only}
- NCT01917188. Promoting patient‐centered care through a heart failure simulation study. clinicaltrials.gov/show/nct01917188 (first received 6 August 2013).
Nenner 2012 {published data only}
- Nenner F. Getting better. American Journal of Hospice & Palliative Medicine 2012;29(1):80‐1. [DOI: 10.1177/1049909111408862] [DOI] [PubMed] [Google Scholar]
Newton 2009 {published data only}
- Newton J, Clark R, Ahlquist P. Evaluation of the introduction of an advanced care plan into multiple palliative care settings. International Journal of Palliative Nursing 2009;15(11):554‐61. [PUBMED: 20081730] [DOI] [PubMed] [Google Scholar]
Ng 2018 {published data only}
- Ng AY, Wong FK. Effects of a home‐based palliative heart failure program on quality of life, symptom burden, satisfaction and caregiver burden: a randomized controlled trial. Journal of Pain and Symptom Management 2018;55(1):1‐11. [PUBMED: 28801001] [DOI] [PubMed] [Google Scholar]
- Ng AY, Wong FK, Lee PH. Effects of a transitional palliative care model on patients with end‐stage heart failure: study protocol for a randomized controlled trial. Trials 2016;17:173. [PUBMED: 27037096] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pearlman 2005 {published data only}
- Pearlman RA, Starks H, Cain KC, Cole WG. Improvements in advance care planning in the Veterans Affairs System: results of a multifaceted intervention. Archives of Internal Medicine 2005;165(6):667‐74. [PUBMED: 15795344] [DOI] [PubMed] [Google Scholar]
Rabow 2004 {published data only}
- Rabow MW, Dibble SL, Pantilat SZ, McPhee SJ. The comprehensive care team: a controlled trial of outpatient palliative medicine consultation. Archives of Internal Medicine 2004;164(1):83‐91. [PUBMED: 14718327] [DOI] [PubMed] [Google Scholar]
- Rabow MW, Petersen J, Schanche K, Dibble SL, McPhee SJ. The comprehensive care team: a description of a controlled trial of care at the beginning of the end of life. Journal of Palliative Medicine 2003;6(3):489‐99. [PUBMED: 14509498] [DOI] [PubMed] [Google Scholar]
Radwany 2014 {published data only}
- Radwany SM, Hazelett SE, Allen KR, Kropp DJ, Ertle D, Albanese TH, et al. Results of the promoting effective advance care planning for elders (PEACE) randomized pilot study. Population Health Management 2014;17(2):106‐11. [PUBMED: 24156664] [DOI] [PubMed] [Google Scholar]
Sahlen 2016 {published data only}
- Brannstrom M, Boman K. A new model for integrated heart failure and palliative advanced homecare – rationale and design of a prospective randomized study. European Journal of Cardiovascular Nursing 2013;12(3):269‐75. [PUBMED: 22645405] [DOI] [PubMed] [Google Scholar]
- Brannstrom M, Boman K. Effects of person‐centred and integrated chronic heart failure and palliative home care. PREFER: a randomized controlled study. European Journal of Heart Failure 2014;16(10):1142‐51. [PUBMED: 25159126] [DOI] [PubMed] [Google Scholar]
- Sahlen KG, Boman K, Brannstrom M. A cost‐effectiveness study of person‐centered integrated heart failure and palliative home care: based on a randomized controlled trial. Palliative Medicine 2016;30(3):296‐302. [PUBMED: 26603186] [DOI] [PubMed] [Google Scholar]
Skorstengaard 2019 {published data only}
- Skorstengaard MH, Jensen AB, Andreassen P, Brogaard T, Brendstrup E, Lokke A, et al. Advance care planning and place of death, hospitalisation and actual place of death in lung, heart and cancer disease: a randomised controlled trial. BMJ Supportive & Palliative Care Published online first: 11 April 2019. [DOI: 10.1136/bmjspcare-2018-001677] [DOI] [PubMed]
Svendsen 2006 {published data only}
- Svendsen A, Arnold JM, Parker J. Caring for patients with heart failure. Canadian Nurse 2006; Vol. 102, issue 3:14‐7. [PubMed]
Thoonsen 2015 {published data only}
- Thoonsen B, Vissers K, Verhagen S, Prins J, Bor H, Weel C, et al. Training general practitioners in early identification and anticipatory palliative care planning: a randomized controlled trial. BMC Family Practice 2015;16:126. [PUBMED: 26395257] [DOI] [PMC free article] [PubMed] [Google Scholar]
UMIN000029805 {published data only}
- UMIN000029805. Evaluation of the effectiveness of support tool for advance care planning in chronic HEART failure. upload.umin.ac.jp/cgi‐open‐bin/ctr_e/ctr_view.cgi?recptno=R000034054 (first received 2 November 2017).
Wells 2018 {published data only}
- Wells R, Stockdill ML, Dionne‐Odom JN, Ejem D, Burgio KL, Durant RW, et al. Educate, Nurture, Advise, Before Life Ends Comprehensive Heartcare for Patients and Caregivers (ENABLE CHF‐PC): study protocol for a randomized controlled trial. Trials 2018;19(1):422. [PUBMED: 30081933] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
ACTRN12613001377729 {published data only}
- ACTRN12613001377729. Case conferences between general practitioners and specialist teams to plan end of life care of people with end stage heart failure and lung disease: pilot study. apps.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12613001377729 (first received 16 December 2013).
ACTRN12618001045202 {published data only}
- ACTRN12618001045202. Collaborative supportive care for patients with complex chronic disease. apps.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12618001045202 (first received 22 June 2018).
Hughes 2015 {published data only}
- Hughes M, Yenokyan G, Sulmasy D, Lehmann LS, Johnson J, Kub J, et al. Effect of the tailored ICD facilitated discussion on patient and family decision‐making about CIED deactivation at the end of life: a pilot randomized controlled trial. Journal of General Internal Medicine 2015;30(2):S148. [Google Scholar]
NCT00105599 {published data only}
- NCT00105599. Effectiveness of the FairCare system for patients with advanced illness. clinicaltrials.gov/show/nct00105599 (first received 16 March 2005).
NCT02463162 {published data only}
- NCT02463162. Trial of advance care planning (ACP) & goals of care designations (GCD) discussions. clinicaltrials.gov/show/nct02463162 (first received 4 June 2015).
NCT03128060 {published data only}
- NCT03128060. Expanding access to home‐based palliative care. clinicaltrials.gov/ct2/show/nct03128060 (first received 25 April 2017).
NCT03539510 {published data only}
- NCT03539510. Effectiveness of the HF‐ACP website study. clinicaltrials.gov/ct2/show/nct03539510 (first received 28 May 2018).
NCT03649191 {published data only}
- NCT03649191. BABEL advance care planning in long‐term care. clinicaltrials.gov/ct2/show/NCT03649191 (first received 28 August 2018).
O'Riordan 2014 {published data only}
- O'Riordan D, Rathfon M, Dracup K, Rabow M, Pantilat S, Marco T. A randomized clinical trial of palliative care for people with heart failure: baseline characteristics. Journal of Pain and Symptom Management 2014;47:496. [Google Scholar]
Skorstengaard 2017 {published data only}
- NCT01944813. Advance care planning: a way to improve end‐of‐life care. clinicaltrials.gov/show/nct01944813 (first received 18 September 2013).
- Skorstengaard MH, Neergaard MA, Andreassen P, Brogaard T, Bendstrup E, Lokke A, et al. Preferred place of care and death in terminally ill patients with lung and heart disease compared to cancer patients. Journal of Palliative Medicine 2017;20(11):1217‐24. [PUBMED: 28574737] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
ACTRN12614000590662 {published data only}
- ACTRN12614000590662. A randomised control trial for advance care planning and symptom management for patients identified in the emergency department and followed up at home. apps.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12614000590662 (first received 4 June 2014).
ACTRN12617001040358 {published data only}
- ACTRN12617001040358. FINnish PALliative care education trial (FINPAL) to improve nursing home residents' quality of life. apps.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12617001040358 (first received 17 July 2017).
Ejem 2019 {published data only}
- Ejem DB, Barrett N, Rhodes RL, Olsen M, Bakitas M, Durant R, et al. Reducing disparities in the quality of palliative care for older African Americans through improved advance care planning: study design and protocol. Journal of Palliative Medicine 2019;22(S1):90‐100. [PUBMED: 31486728] [DOI] [PubMed] [Google Scholar]
Graney 2019 {published data only}
- Graney BA, Au DH, Baron AE, Cheng A, Combs SA, Glorioso TJ, et al. Advancing Symptom Alleviation with Palliative Treatment (ADAPT) trial to improve quality of life: a study protocol for a randomized clinical trial. Trials 2019;20(1):355. [PUBMED: 31196156] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02713347. Advancing symptom alleviation with palliative treatment. clinicaltrials.gov/ct2/show/nct02713347 (first received 18 March 2016).
Malhotra 2016 {published data only}
- Malhotra C, Sim DK, Jaufeerally F, Vikas NN, Sim GW, Tan BC, et al. Impact of advance care planning on the care of patients with heart failure: study protocol for a randomized controlled trial. Trials 2016;17(1):285. [PUBMED: 27287330] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02299180. Impact of advance care planning on care for patients with advanced heart failure. clinicaltrials.gov/ct2/show/nct02299180 (first received 24 November 2014).
NCT00489021 {published data only}
- NCT00489021. Feasibility and outcomes of older patients hospitalization. clinicaltrials.gov/show/nct00489021 (first received 21 June 2007).
NCT02429479 {published data only}
- NCT02429479. Preparing family caregivers to make medical decisions for their loved ones. clinicaltrials.gov/show/nct02429479 (first received 29 April 2015).
NCT02612688 {published data only}
- NCT02612688. Pragmatic trial of video education in nursing homes. clinicaltrials.gov/show/nct02612688 (first received 24 November 2015).
NCT03170466 {published data only}
- NCT03170466. Primary palliative care in heart failure: a pilot trial. clinicaltrials.gov/ct2/show/nct03170466 (first received 31 May 2017).
NCT03516994 {published data only}
- NCT03516994. Reducing disparities in the quality of advance care planning for older adults. clinicaltrials.gov/show/nct03516994 (first received 7 May 2018).
Additional references
Allen 2012
- Allen LA, Stevenson LW, Grady KL, Goldstein NE, Matlock DD, Arnold RM, et al. Decision making in advanced heart failure: a scientific statement from the American Heart Association. Circulation 2012;125(15):1928‐52. [PUBMED: 22392529] [DOI] [PMC free article] [PubMed] [Google Scholar]
Benjamin 2017
- Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. Heart disease and stroke statistics. 2017 update: a report from the American Heart Association. Circulation 2017;135(10):e146‐603. [PUBMED: 28122885] [DOI] [PMC free article] [PubMed] [Google Scholar]
Browne 2014
- Browne S, Macdonald S, May CR, Macleod U, Mair FS. Patient, carer and professional perspectives on barriers and facilitators to quality care in advanced heart failure. PloS One 2014;9(3):e93288. [PUBMED: 24676421] [DOI] [PMC free article] [PubMed] [Google Scholar]
Butler 2015
- Butler J, Binney Z, Kalogeropoulos A, Owen M, Clevenger C, Gunter D, et al. Advance directives among hospitalized patients with heart failure. JACC. Heart Failure 2015;3(2):112‐21. [PUBMED: 25543976] [DOI] [PubMed] [Google Scholar]
Cohen 1988
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd Edition. Mahwah (NJ): Lawrence Erlbaum Associates, 1988. [Google Scholar]
De Vleminck 2014
- Vleminck A, Pardon K, Beernaert K, Deschepper R, Houttekier D, Audenhove C, et al. Barriers to advance care planning in cancer, heart failure and dementia patients: a focus group study on general practitioners' views and experiences. PloS One 2014;9(1):e84905. [PUBMED: 24465450] [DOI] [PMC free article] [PubMed] [Google Scholar]
Falk 2013
- Falk H, Ekman I, Anderson R, Fu M, Granger B. Older patients' experiences of heart failure‐an integrative literature review. Journal of Nursing Scholarship 2013;45(3):247‐55. [PUBMED: 23617442] [DOI] [PubMed] [Google Scholar]
Fine 2010
- Fine E, Reid MC, Shengelia R, Adelman RD. Directly observed patient‐physician discussions in palliative and end‐of‐life care: a systematic review of the literature. Journal of Palliative Medicine 2010;13(5):595‐603. [PUBMED: 20491550] [DOI] [PMC free article] [PubMed] [Google Scholar]
GBD 2016
- GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990‐2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388(10053):1545‐602. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gottdiener 2000
- Gottdiener JS, Arnold AM, Aurigemma GP, Polak JF, Tracy RP, Kitzman DW, et al. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. Journal of the American College of Cardiology 2000;35(6):1628‐37. [PUBMED: 10807470] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Hayhoe 2011
- Hayhoe B, Howe A. Advance care planning under the Mental Capacity Act 2005 in primary care. British Journal of General Practice 2011;61(589):e537‐41. [PUBMED: 21801576] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hjelmfors 2014
- Hjelmfors L, Stromberg A, Friedrichsen M, Martensson J, Jaarsma T. Communicating prognosis and end‐of‐life care to heart failure patients: a survey of heart failure nurses' perspectives. European Journal of Cardiovascular Nursing 2014;13(2):152‐61. [PUBMED: 24480779] [DOI] [PubMed] [Google Scholar]
Hong 2018
- Hong M, Yi EH, Johnson KJ, Adamek ME. Facilitators and barriers for advance care planning among ethnic and racial minorities in the U.S.: a systematic review of the current literature. Journal of Immigrant and Minority Health 2018;20(5):1277‐87. [PUBMED: 29124502] [DOI] [PubMed] [Google Scholar]
Houben 2014
- Houben CH, Spruit MA, Groenen MT, Wouters EF, Janssen DJ. Efficacy of advance care planning: a systematic review and meta‐analysis. Journal of the American Medical Directors Association 2014;15(7):477‐89. [PUBMED: 24598477] [DOI] [PubMed] [Google Scholar]
Johnson 2016
- Johnson S, Butow P, Kerridge I, Tattersall M. Advance care planning for cancer patients: a systematic review of perceptions and experiences of patients, families, and healthcare providers. Psycho‐oncology 2016;25(4):362‐86. [PUBMED: 26387480] [DOI] [PubMed] [Google Scholar]
Kernick 2018
- Kernick LA, Hogg KJ, Millerick Y, Murtagh FE, Djahit A, Johnson M. Does advance care planning in addition to usual care reduce hospitalisation for patients with advanced heart failure: a systematic review and narrative synthesis. Palliative Medicine 2018;32(10):1539‐51. [PUBMED: 30234421] [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Medicine 2009;6(7):e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lim 2016
- Lim CE, Ng RW, Cheng NC, Cigolini M, Kwok C, Brennan F. Advance care planning for haemodialysis patients. Cochrane Database of Systematic Reviews 2016, Issue 7. [DOI: 10.1002/14651858.CD010737.pub2; PUBMED: 27457661] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lorenz 2008
- Lorenz KA, Lynn J, Dy SM, Shugarman LR, Wilkinson A, Mularski RA, et al. Evidence for improving palliative care at the end of life: a systematic review. Annals of Internal Medicine 2008;148(2):147‐59. [PUBMED: 18195339] [DOI] [PubMed] [Google Scholar]
Lynn 2001
- Lynn J. Perspectives on care at the close of life. Serving patients who may die soon and their families: the role of hospice and other services. JAMA 2001;285(7):925‐32. [PUBMED: 11180736] [DOI] [PubMed] [Google Scholar]
Martin 2016
- Martin RS, Hayes B, Gregorevic K, Lim WK. The effects of advance care planning interventions on nursing home residents: a systematic review. Journal of the American Medical Directors Association 2016;17(4):284‐93. [PUBMED: 26861748] [DOI] [PubMed] [Google Scholar]
Miller 2019
- Miller H, Tan J, Clayton JM, Meller A, Hermiz O, Zwar N, et al. Patient experiences of nurse‐facilitated advance care planning in a general practice setting: a qualitative study. BMC Palliative Care 2019;18(1):25. [PUBMED: 30841925] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mullick 2013
- Mullick A, Martin J, Sallnow L. An introduction to advance care planning in practice. BMJ (Clinical Research Ed.) 2013;347:f6064. [PUBMED: 24144870] [DOI] [PubMed] [Google Scholar]
NCPC 2007
- National Council for Palliative Care. Advance care planning: a guide for health and social care staff. February 2007. www.ncpc.org.uk/publication/advance‐care‐planning‐guide‐health‐and‐social‐care‐staff (accessed 30 August 2017).
Ohr 2017
- Ohr S, Jeong S, Saul P. Cultural and religious beliefs and values, and their impact on preferences for end‐of‐life care among four ethnic groups of community‐dwelling older persons. Journal of Clinical Nursing 2017;26(11‐12):1681‐9. [PUBMED: 27603557] [DOI] [PubMed] [Google Scholar]
Ponikowski 2016
- Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. European Journal of Heart Failure 2016;18(8):891‐975. [PUBMED: 27207191] [DOI] [PubMed] [Google Scholar]
Prendergast 2001
- Prendergast TJ. Advance care planning: pitfalls, progress, promise. Critical Care Medicine 2001;29(2 Suppl):N34‐9. [PUBMED: 11228571] [DOI] [PubMed] [Google Scholar]
Redfield 2003
- Redfield MM, Jacobsen SJ, Burnett JC Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA 2003;289(2):194‐202. [PUBMED: 12517230] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rhee 2013
- Rhee JJ, Zwar NA, Kemp LA. Advance care planning and interpersonal relationships: a two‐way street. Family Practice 2013;30(2):219‐26. [PUBMED: 23028000] [DOI] [PubMed] [Google Scholar]
Rietjens 2017
- Rietjens JA, Sudore RL, Connolly M, Delden JJ, Drickamer MA, Droger M, et al. Definition and recommendations for advance care planning: an international consensus supported by the European Association for Palliative Care. Lancet Oncology 2017;18(9):e543‐51. [PUBMED: 28884703] [DOI] [PubMed] [Google Scholar]
Stevenson 2015
Sudore 2010
- Sudore RL, Fried TR. Redefining the "planning" in advance care planning: preparing for end‐of‐life decision making. Annals of Internal Medicine 2010;153(4):256‐61. [PUBMED: 20713793] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sudore 2017
- Sudore RL, Lum HD, You JJ, Hanson LC, Meier DE, Pantilat SZ, et al. Defining advance care planning for adults: a consensus definition from a multidisciplinary Delphi panel. Journal of Pain and Symptom Management 2017;53(5):821‐32. [PUBMED: 28062339] [DOI] [PMC free article] [PubMed] [Google Scholar]
Virdun 2015
- Virdun C, Luckett T, Davidson PM, Phillips J. Dying in the hospital setting: a systematic review of quantitative studies identifying the elements of end‐of‐life care that patients and their families rank as being most important. Palliative Medicine 2015;29(9):774‐96. [PUBMED: 25921707] [DOI] [PMC free article] [PubMed] [Google Scholar]
Weathers 2016
- Weathers E, O'Caoimh R, Cornally N, Fitzgerald C, Kearns T, Coffey A, et al. Advance care planning: a systematic review of randomised controlled trials conducted with older adults. Maturitas 2016;91:101‐9. [PUBMED: 27451328] [DOI] [PubMed] [Google Scholar]
Wright 2008
- Wright AA, Zhang B, Ray A, Mack JW, Trice E, Balboni T, et al. Associations between end‐of‐life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA 2008;300(14):1665‐73. [PUBMED: 18840840] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yancy 2013
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013;128(16):e240‐327. [PUBMED: 23741058] [DOI] [PubMed] [Google Scholar]
Zwakman 2018
- Zwakman M, Jabbarian LJ, Delden J, Heide A, Korfage IJ, Pollock K, et al. Advance care planning: a systematic review about experiences of patients with a life‐threatening or life‐limiting illness. Palliative Medicine 2018;32(8):1305‐21. [PUBMED: 29956558] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Nishikawa 2018
- Nishikawa Y, Fukahori H, Ota E, Mizuno A, Hiroyama N, Miyashita M, Yoneoka D, Kwong JS. Advance care planning for heart failure. Cochrane Database of Systematic Reviews 2018, Issue 5. [DOI: 10.1002/14651858.CD013022] [DOI] [PMC free article] [PubMed] [Google Scholar]


