Abstract
Stroke is a major cause of morbidity, mortality and disability in systemic lupus erythematosus (SLE). Patients with SLE have a two-fold increase in the risk of stroke with younger patients (ie, less than 50 years of age) having an ever-higher risk (up to 10-fold). Although the prognosis of SLE has improved, mortality due to cerebrovascular events (CVE) remains unchanged. Cerebrovascular disease may be directly attributed to the disease per se, as a manifestation of neuropsychiatric SLE, or be the result of traditional cardiovascular risk factors accompanying the disease. Elucidation of the underlying mechanism(s) of CVE is essential as it may guide the type of therapy (ie, antithrombotic or anticoagulant therapy versus immunosuppressive). Strokes attributed to lupus usually occur early in the course of the disease and are often accompanied by evidence of activity in other organs; those related to antiphospholipid antibodies can occur at any time, in patients with either active or inactive SLE. In this review, we discuss the epidemiology, work-up, management and primary prevention of CVE in patients with lupus. In view of the effectiveness of thrombolysis, physicians need to educate lupus patients and their families for the early recognition of the signs of stroke and the need to seek prompt attention. To this end acronyms, such as FAST (Facial drooping, Arm weakness, Speech difficulties and Time to call emergency service) can be used as a mnemonic to help detect and enhance responsiveness to the needs of a person having a stroke.
Keywords: Cerebrovascular Events, Systemic Lupus Erythematosus, Brain Imaging, Drug Therapy
INTRODUCTION
Neuropsychiatric systemic lupus erythematosus (NPSLE) may involve the central and peripheral nervous system in patients with SLE.1 The range of neuropsychiatric (NP) manifestations varies from serious events, such as myelitis, seizures and stroke, to more subtle events such as headache, mood disorders and mild cognitive dysfunction.2 Approximately 40% of patients experience some type of neuropsychiatric manifestations, with less than half of them directly attributed to SLE (primary NPSLE).3 The gold standard of NPSLE diagnosis remains physician judgment. Risk factors for NPSLE are prior major NP manifestation, generalized disease activity, and anti-phospholipid antibody (aPL) positivity.4,5
Cerebrovascular events (CVE) represent one of the most common and severe NP manifestations.6 Their prevalence varies from 3% to 20%, accounting for up to 15% of deaths in SLE.7,8 Strokes attributed to SLE tend to occur close to disease diagnosis and may be explained by systemic inflammation, endothelial activation or a prothrombotic state due to aPL.9,10 On the other hand, strokes unrelated to the disease usually occur at late stages and are caused by atherosclerosis due to traditional cardiovascular risk factors such as hypertension, hyperlipidemia and diabetes mellitus, which represent common comorbidities in SLE.11 Attribution of a CVE to SLE remains a challenge because, in the former case, immunosuppressive therapy should be considered.4
Herein, we review the epidemiology and discuss the issue of attribution of CVEs in SLE. We also propose a work-up in presenting with CVE and provide recommendations for primary prevention and treatment.
EPIDEMIOLOGY
The majority of strokes related to SLE occur within the first year after diagnosis;9 thus the true prevalence of primary CV events can be captured even by epidemiological studies of short duration of follow-up. Strokes related to atherosclerosis and traditional factors tend to appear in late stages and thus need longitudinal cohort studies with prolonged follow-up.
The prevalence of stroke in SLE ranges from a little over 2% to as high as 19%,6,9,12–15 while the incidence ranges from 5.8 to 25.3 new cases per 1000 person-years.6,9,12,13,15 The large discrepancies between studies can be explained by various factors, such as duration of follow-up, race, age, and study design. A recent meta-analysis16 found a two-fold increased risk of ischemic stroke in SLE subjects compared to the general population, together with a three-fold increased risk of intracerebral haemorrhage, although individual studies have questioned the increased risk of intracranial hemorrhage.12,13 The relative risk is higher in younger ages,17 as young patients with SLE (age < 50 years) are almost 10 times more prone to develop CVE compared to subjects of similar age without SLE.17 The epidemiology of stroke also shows racial/ethnic as well as socioeconomic variations.13,18 An elevated relative risk of stroke has been observed among Hispanics and blacks compared to whites (11% and 36%, respectively). Low educational level and income is also associated with increased frequency of traditional cardiovascular factors and cardiovascular events in SLE patients.18 Shaharir et al.19 reported that males manifest cardiovascular events (including CVE) more frequently than females. A detailed presentation of epidemiologic data is depicted in Table 1. Of note, although survival of SLE patients has improved over the last decades,20 mortality rates due to complications of CVE have not decreased accordingly.21
Table 1.
Studies reporting epidemiology of CVE in patients with SLE.
| Study | Incidence (1000pt/yrs) | Prevalence | RR | Median follow-up (years) | Comments |
|---|---|---|---|---|---|
| Mok et al.12 | 6.45 | 4.08% | 2.02 | 8 | Duration of hospitalization and mortality rate similar between SLE and non-SLE patients. |
| Hanly et al.6 | 5.8 | 4.49% | NR | 5.6 | Fourth most frequent NP event in SLE, the majority attributable to lupus |
| Barbhaiya et al.13 | 5.88 | 2.19% | NR | 3.7 | Increased stroke risk among Blacks and Hispanics compared to Caucasians |
| Arkema et al.9 | 7.70 | 3.71% | 2.2 | NA | aPL carriers excluded |
| Chiu et al.14 | NR | 2.22% | 1.67 | 7 | 11,637 newly diagnosed SLE patients and 58,185 subjects without SLE, matched for age, gender, and comorbidities (very large cohort) |
| Mikdashi et al. al.15 | 25.3 | 19% | NR | 8 | Higher prevalence and incidence compared to other studies, but small sample size (n= 238) |
RR: Relative risk compared to the general population; NR: Not reported; NP: Neuropsychiatric; NA: Not applicable; aPL: Antiphospholipid antibodies.
In our experience from two lupus cohorts in Athens and Heraklion, Greece,22,23 50 patients from a total of 1331 patients have suffered ischemic stroke, corresponding to an overall prevalence of 3.75%. Of them, 26 patients (52%) developed stroke in the context of secondary APS with only two having documented central nervous system (CNS) vasculitis (4%) (Table 2).
Table 2.
Characteristics of CVE in SLE patients in “Attikon” and “Leto” SLE cohorts (n=1331).
| Diagnostic work-up | |
|---|---|
| Number of patients with stroke | 50 |
| Ischemic stroke, n (%) | 50 (100) |
| APS-related stroke, n (%) | 26 (52) |
| CNS vasculitis, n (%) | 2 (4) |
| Generalized lupus activity at the time of stroke, n (%) | 30 (60) |
| Treatment | |
| Antiplatelets, n (%) | 50 (100) |
| Anticoagulation, n (%) | 17 (34%, all with APS) |
| Immunosuppressive Therapy, n (%) | 24 (48) |
| ✓ Cyclophosphamide, n (%) | ✓ 17 (70.8%) |
| ✓ Azathioprine, n (%) | ✓ 7 (29.2) |
CLINICAL PRESENTATION OF STROKE
Acute stroke should be considered in any patient with SLE presenting with sudden onset neurological deficit and/or alteration in level of consciousness. Clinical presentation varies based on the type of stroke and the arterial territory involved. Typical symptoms include, but are not limited to: abrupt onset of hemiparesis or monoparesis, hemisensory deficits, visual disturbances, dysarthria, facial droop, ataxia, aphasia and sudden decrease in level of consciousness. These symptoms may occur isolated or, more likely, in various combinations. Differentiation between ischemic and haemorrhagic stroke is not possible on the basis of history/physical examination, although headache, vomiting, nausea, and abrupt loss of consciousness are more commonly observed in haemorrhagic strokes, highlighting the vital role of emergency brain imaging.
Stroke as Initial Manifestation of Lupus
Stroke may be the presenting manifestation of lupus, and patients with SLE may suffer a stroke at a young age. Albeit rare, CVEs due to lupus in patients below 50 are common cause of referral to stroke units.24 A hospital-based study showed that the yearly incidence is 2.4 per 100.000 people (aged 20–24), 4.5 (aged 30–34) and 32.9 (aged 45–49).25
In a patient presenting with stroke, several conditions need to be sought for and ruled out. Extracranial arterial disease, mainly carotid dissection, but also vasculitis and other vasculopathies, may present with stroke. In such cases, contrast-enhanced magnetic resonance angiography (MRA) combined with carotid Doppler are recommended for the identification of carotid dissection.26,27 MRA with fat suppression is the best modality for carotid dissection if ultrasound is negative, while catheter angiography should be used in case of high suspicion of vasculitis or rare vasculopathies.24 Cardioembolism is another major cause that should be ruled out. Patent foramen ovale is one of the most common sources of unexplained stroke in young adults, thus transoesophageal echocardiography is recommended in every young patient without known cause of CVA. Holter electrocardiograms are also recommended as part of the initial work-up.28,29 Screening for hereditary thrombophilic disorders is not routinely recommended, as the latter are mainly associated with venous thromboembolic events. Less frequent causes of strokes are summarized in Table 3.
Table 3.
Most common causes of stroke in patients < 50 years old.
| Causes of strokes in young patients |
| More common |
| Cryptogenic (up to 30%) |
| Congenital and acquired heart disease |
| Drugs (Cocaine, methamphetamine) |
| Less common |
| Endocarditis |
| Arterial dissection |
| Traumatic |
| Fibromuscular dysplasia |
| CNS vasculitis |
| Large vessel vasculitis |
| Pregnancy |
| Oral contraceptives |
| Inherited prothrombotic states |
| SLE/APS |
| Systemic autoimmune diseases |
| Leukaemia and other malignancies |
| Metabolic disorders |
| Protein-losing enteropathy |
| Nephrotic syndrome |
Among autoimmune diseases, the main diagnosis that should be considered in young patients presenting with stroke are SLE and/or APS. Young patients with unknown cause of stroke should be screened for antinuclear antibodies (ANA) and aPL (anti-cardiolipin [aCL], anti β2-glycoprotein I [β2-GPI] and lupus anticoagulant [LA]). If positive, aPL should be repeated after 3 months to diagnose definite APS.30 A systematic review showed that aPL were detected in 17.2% (2–56%) of patients with stroke and 11.7% (2–45%) of transient ischemic attack (TIA); conversely, the presence of aPL increases the risk for stroke approximately 5-fold.31
Stroke in a Patient with Established SLE – The Challenge of Attribution
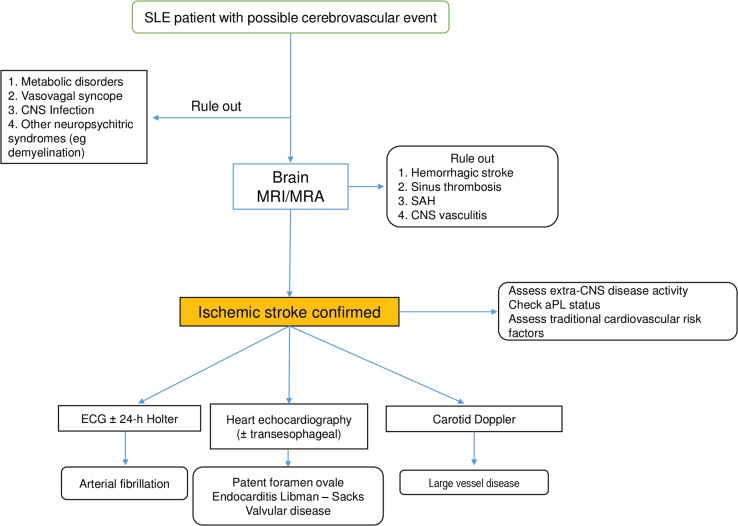
In a patient with known SLE, the physician should distinguish whether the stroke represents true primary NPSLE or is unrelated to the disease. Apart from the essential work-up outlined above, patients should be assessed for the presence of extra-neurological disease activity (eg, nephritis, arthritis, serositis, skin rashes, and hematologic abnormalities) (Figure 1). Use of a validated index of disease activity, such as the SLE Disease Activity Index (SLEDAI) is useful.32 Timing of CVE occurrence is also important, as most primary NP events tend to manifest near or early after the diagnosis of SLE. History of a previous major NP manifestation also constitutes a risk factor for primary NPSLE, and stroke in particular.33 Brain MRI/MRA is mandatory; the latter provides information for a possible vasculitic process, although documentation of frank vasculitis in SLE-related stroke is very rare in clinical practice. On the contrary, patients with long-standing lupus and presence of cardiovascular risk factors tend to experience strokes in the absence of generalized disease activity, due to atherosclerosis as a late sequel of the disease.34 The presence of sudden, severe headache in a lupus patient should raise the suspicion of cerebral sinus venous thrombosis (CSVT), subarachnoid haemorrhage (SAH) or CNS vasculitis.35
Figure 1.
Initial work-up of an SLE patient presenting with stroke.
SAH, subarachnoid haemorrhage; ECG, electrocardiogram; CNS, central nervous system; MRI/MRA, magnetic resonance imaging/angiography; aPL, antiphospholipid antibodies.
White matter hyperintense lesions (WMHs) in SLE
Unidentified bright objects (UBOs) on MRI are hyperintense lesions seen on T2-weighted/fluid attenuated inversion recovery (FLAIR) images commonly identified in the subcortical white matter and other areas of the brain.36 These lesions are formed slowly due to vascular injury (small vessel disease, SVD) representing a “silent” process and are a common finding among older subjects. They have been proposed as a neuroimaging marker of “brain frailty” and have been associated with CV events and worse outcomes.37 The prevalence of these white matter hyperintensities (WMHs) increases with age and accrual of cardiovascular risk factors,38–40 but can also be seen in ∼5% of healthy young individuals.41
SVD is well recognized in SLE, confirmed also in autopsy studies,42 due to both inflammatory and non-inflammatory factors (small clots due to aPL, complement activation/immune complexes, plasma factors and endothelial cell adhesion molecules).43 WMHs have long been associated with NPSLE, being present in up to 60% of patients with CNS involvement, but with no correlation with a specific NP event.44,45 Approximately 18–40% of SLE patients without any NP manifestation also have such lesions on brain MRI.46 They are usually located subcortically and periventricularly in the frontal and parietal lobes (70–80%). Although there are no studies on association between WMHs and stroke in SLE, there are many studies on the clinical significance of WMHs in subjects without lupus.36 A meta-analysis of 22 longitudinal studies revealed a 3-fold increased risk of stroke in subjects with WMHs.47 Similarly, a large study in 1884 individuals with an average follow-up of 14.5 found that the relative risk for stroke and stroke-related mortality was 3.5 and 3, respectively, among subjects with WMHs ≤ 3mm; in those with both WMH ≥ 3mm and ≤ 3mm, the relative risk was 8.6 and 7, respectively. Patients with WMHs tend also to develop larger infracts with worse clinical outcomes.48 Although there are no studies on the management of asymptomatic WMH in SLE patients, physicians should consider these findings as evidence of cerebral vasculopathy and a risk factor for cerebrovascular disease; accordingly, an aggressive strategy against traditional cardiovascular factors should be adopted. Regarding immunosuppressive therapy, we do not routinely titrate immunosuppressive therapy, rather follow an individualized approach, based on number and size of lesions, as well as presence of symptoms. Finally, in asymptomatic patients, repeat MRI is not always warranted, but this should also be decided on a patient-by-patient basis.
MANAGEMENT OF CVEs
Primary prevention
Patients with SLE carry an increased risk of stroke, starting from the time of disease diagnosis, thus primary prevention is of utmost importance. Lupus-related risk factors include uncontrolled disease activity and aPL positivity.4 Moreover, SLE individuals with long-standing disease accumulate comorbidities, such as hypertension, diabetes mellitus and dyslipidaemia. When brain MRI is available, the presence of WMHs should be viewed as an additional risk factor. Smoking cessation is mandatory and modifiable cardiovascular risk factors should be treated appropriately, following a 10-year cardiovascular risk stratification using one of the available algorithms.49 Attainment of remission or -at least- low disease activity with immunosuppressive treatment should be the target of SLE therapy.50
Various imaging surrogate markers for atherosclerosis, including flow-mediated dilatation of the brachial artery, carotid intima media thickness and pulse wave velocity, have been tested in patients with SLE.51 Albeit these studies have added valuable information, no single surrogate marker (molecular or imaging) has yet shown a clear association with “hard” cardiovascular endpoints in lupus, ie, vascular events or cardiovascular mortality. Thus, outside the context of research studies, we do not routinely advise for screening with non-invasive modalities in our patients.
Although several studies have shown the beneficial effects of statins in the management of lupus,52–56 their use in SLE subjects with normal lipid status remains controversial. Two randomized controlled trials in a paediatric and an adult population failed to demonstrate a positive effect of statins on subclinical atherosclerosis progression over three and two years, respectively,57, 58 but follow-up may have been too short to detect an effect.
There is no clear recommendation for the use of aspirin as thrombophylactic agent in aPL-positive adults.59 Aspirin administration at low dose was recently recommended for all patients with juvenile onset lupus and positive aPL, by a recent initiative of a Task Force.60 In adults, a meta-analysis found a significant protective role of low-dose aspirin against thrombotic events in lupus patients with positive aPL.61 Thus, SLE patients with high-risk aPL profile (persistently positive medium/high titers or multiple positivity) may receive primary prophylaxis with antiplatelet agents, especially if other atherosclerotic/thrombophilic factors are present, after balancing for the bleeding hazard. In case of negative aPL, SLE patients may be candidates for preventative strategies as in the general population, including low-dose aspirin and/or lipid-lowering agents, based on their individual cardiovascular risk profile. A 10-year risk stratification should always be performed, although the risk may be underestimated in patients with lupus.49 In general, scepticism has recently been raised regarding the use of aspirin for primary prevention in high-risk populations (elderly and diabetics), after the publication of studies which showed that protection from cardiovascular events conferred by low-dose aspirin was largely counterbalanced by a significantly higher risk of major bleeding.62,63 However, one has to keep on mind that SLE patients represent a special group of individuals with chronic systemic inflammation and proven increased CVD risk.
Hydroxychloroquine (HCQ) is the cornerstone of lupus therapy due to its multiple beneficial effects.64 HCQ has additional antithrombotic actions; indeed, several prospective and retrospective studies have shown that HCQ reduces the relative risk of arterial thrombosis in general, and CVE, in particular, with hazard ratios ranging from 0.15 to 0.28 for cerebrovascular disease.65,66,67 Combination of HCQ with low-dose aspirin has been suggested to provide additive protection against stroke,68 but this needs to be further validated.
Treatment and secondary prevention
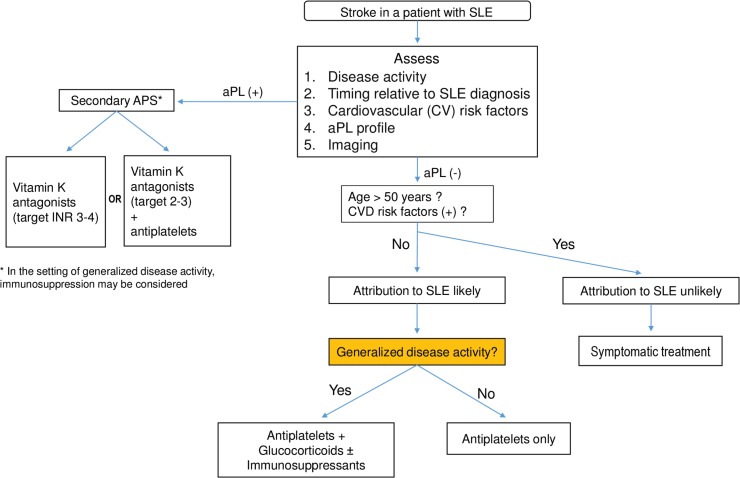
Treatment decisions in a SLE patient presenting with stroke should be based on whether the latter is considered directly related to the disease or not, and whether there is a coexisting APS or not. In the acute phase, the initial work-up and management does not differ between SLE patients and the general population.4 Symptomatic therapy similar to non-lupus patients should be provided in individuals with stroke occurring in the absence of generalized disease activity and/or APS. In particular, thrombolysis has been used successfully in lupus patients with stroke.69,70 In the setting of definite APS-related stroke, patients should be treated either with vitamin K antagonists with target INR 2–3 plus low-dose aspirin or vitamin K antagonists with target INR 3–4 long-term.71 In the first scenario, INR should be upgraded at 3–4, in case of stroke recurrence. Up to two-thirds of strokes in lupus occur in the setting of generalized disease activity,23 suggesting the role of systemic inflammation in the pathogenesis of CVE. In such cases, lupus-related stroke should be managed with high doses of glucocorticoids and/or major immunosuppressive therapy such as cyclophosphamide or rituximab, in order to suppress systemic inflammation.5 A recommended algorithm is depicted in Figure 2.
Figure 2.
Management of stroke in SLE.
APS, antiphospholipid syndrome; aPL, antiphospholipid antibodies; CVD, cardiovascular disease.
In our experience, 60% of CVE (30/50) occurred in the presence of generalized disease activity (SLEDAI≥6) and immunosuppressive therapy was instituted in 21/30 (70%). Specifically, 17 patients were treated with cyclophosphamide (CYC), 7 with azathioprine (AZA). Three patients were treated sequentially with AZA, CYC and finally with rituximab due to ongoing disease activity and severity of CVE. All patients (21/21, 100%) who received combined immunosuppression/antithrombotic treatment had a favourable outcome at 6 months and no recurrence was observed after at least three years of median follow-up (Table 2).
CONCLUSIONS
Stroke represents one of the most devastating complications of lupus, as it is accompanied by significant disability, impaired quality of life and increased mortality. Over the last decades, the management of stroke in lupus has followed a “yin and yang” course, with all patients initially treated with high-dose glucocorticoids and/or cyclophosphamide, assuming that stroke was driven by vasculitis. With the recognition of the APS and the accelerated atherosclerosis in SLE, the balance tilted heavily towards anticoagulation and antiplatelet therapy. However, in more recent years, the realization that inflammation promotes thrombosis and the emergence of studies showing evidence for an increased frequency of stroke in the first years after the disease, has prompted discussions about combination therapy (anticoagulation and immunosuppression), especially in patients with generalized disease activity. WMHs, a common finding on brain MRI of SLE subjects, may represent an additional risk factor for CVE in lupus, but longitudinal studies are needed to confirm this hypothesis. In the meantime, increased vigilance and modification of atherosclerotic risk factors is essential. In view of the effectiveness of thrombolysis, physicians need to educate lupus patients and their families to the early recognition of the signs of stroke and the need to seek prompt attention. To this end the stroke association has proposed the FAST acronym used as a mnemonic to help detect and enhance responsiveness to the needs of a person having a stroke. The acronym stands for Facial drooping, Arm weakness, Speech difficulties and Time to call emergency service.
ACKNOWLEDGEMENTS
We are indebted to the patients and nurses of the Rheumatology and Clinical Immunology Units in Athens and Heraklion and to Drs G Bertsias, P Sidiropoulos, I Gergianaki, C Adamichou, P Katsimbri, G Tsivgoulis, M Papathanasiou, E Papadaki, A Pieta, D Tseronis, and T Karageorgas for care of the patients and helpful discussions.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Fanouriakis A, Boumpas DT, Bertsias GK. Pathogenesis and treatment of CNS lupus. Curr Opin Rheumatol 2013;25:577–83. [ 10.1097/BOR.0b013e328363eaf1] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 2.Bertsias GK, Boumpas DT. Pathogenesis, diagnosis and management of neuropsychiatric SLE manifestations. Nat Rev Rheumatol 2010;6:358–67. [ 10.1038/nrrheum.2010.62] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 3.Unterman A, Nolte JE, Boaz M, Abady M, Shoenfeld Y, Zandman-Goddard G. Neuropsychiatric syndromes in systemic lupus erythematosus: a meta-analysis. Semin Arthritis Rheum 2011;41:1–11. [ 10.1016/j.semarthrit.2010.08.001] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 4.Bertsias GK, Ioannidis JP, Aringer M, Bollen E, Bombardieri S, Bruce IN, et al. EULAR recommendations for the management of systemic lupus erythematosus with neuropsychiatric manifestations: report of a task force of the EULAR standing committee for clinical affairs. Ann Rheum Dis 2010;69:2074–82. [ 10.1136/ard.2010.130476] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 5.Fanouriakis A, Pamfil C, Rednic S, Sidiropoulos P, Bertsias G, Boumpas DT. Is it primary neuropsychiatric systemic lupus erythematosus? Performance of existing attribution models using physician judgment as the gold standard. Clin Exp Rheumatol 2016;34:910–7. [PMID: ] [PubMed] [Google Scholar]
- 6.Hanly JG, Li Q, Su L, Urowitz MB, Gordon C, Bae SC, et al. Cerebrovascular Events in Systemic Lupus Erythematosus. Arthritis Care Res (Hoboken) 2018;70:1478–87. [ 10.1002/acr.23509] [PMID: ] [PMCID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cervera R, Khamashta MA, Font J, Sebastiani GD, Gil A, Lavilla P, et al. Morbidity and mortality in systemic lupus erythematosus during a 10-year period: a comparison of early and late manifestations in a cohort of 1,000 patients. Medicine (Baltimore) 2003;82:299–308. [ 10.1097/01.md.0000091181.93122.55] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 8.Saadatnia M, Sayed-Bonakdar Z, Mohammad-Sharifi G, Sarrami AH. The necessity of stroke prevention in patients with systemic lupus erythematosus. J Res Med Sci 2012;17: 894–5. [PMID: ] [PMCID: ] [PMC free article] [PubMed] [Google Scholar]
- 9.Arkema EV, Svenungsson E, Von Euler M, Sjowall C, Simard JF. Stroke in systemic lupus erythematosus: a Swedish population-based cohort study. Ann Rheum Dis 2017;76: 1544–9. [ 10.1136/annrheumdis-2016-210973] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 10.Pons-Estel GJ, Andreoli L, Scanzi F, Cervera R, Tincani A. The antiphospholipid syndrome in patients with systemic lupus erythematosus. J Autoimmun 2017;76:10–20. [ 10.1016/j.jaut.2016.10.004] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 11.Schoenfeld SR, Kasturi S, Costenbader KH. The epidemiology of atherosclerotic cardiovascular disease among patients with SLE: a systematic review. Semin Arthritis Rheum 2013;43:77–95. [ 10.1016/j.semarthrit.2012.12.002] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 12.Mok CC, Ho LY, To CH. Annual incidence and standardized incidence ratio of cerebrovascular accidents in patients with systemic lupus erythematosus. Scand J Rheumatol 2009;38:362–8. [ 10.1080/03009740902776927] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 13.Barbhaiya M, Feldman CH, Guan H, Chen SK, Fischer MA, Solomon DH, et al. Racial/ethnic variation in stroke rates and risks among patients with systemic lupus erythematosus. Semin Arthritis Rheum 2018;S0049-0172(18)30059-3. [ 10.1016/j.semarthrit.2018.07.012] [PMID: ] [PMCID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiu CC, Huang CC, Chan WL, Chung CM, Huang PH, Lin SJ, et al. Increased risk of ischemic stroke in patients with systemic lupus erythematosus: a nationwide population-based study. Intern Med 2012;51:17–21. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 15.Mikdashi J, Handwerger B, Langenberg P, Miller M, Kittner S. Baseline disease activity, hyperlipidemia, and hypertension are predictive factors for ischemic stroke and stroke severity in systemic lupus erythematosus. Stroke 2007;38:281–5. [ 10.1161/01.STR.0000254476.05620.14] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 16.Holmqvist M, Simard JF, Asplund K, Arkema EV. Stroke in systemic lupus erythematosus: a meta-analysis of population-based cohort studies. RMD Open 2015;1:e000168. [ 10.1136/rmdopen-2015-000168] [PMID: ] [PMCID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ward MM. Premature morbidity from cardiovascular and cerebrovascular diseases in women with systemic lupus erythematosus. Arthritis Rheum 1999;42:338–46. [] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 18.Maynard JW, Fang H, Petri M. Low socioeconomic status is associated with cardiovascular risk factors and outcomes in systemic lupus erythematosus. J Rheumatol 2012;39:777–83. [ 10.3899/jrheum.110724] [PMID: ] [PMCID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shaharir SS, Kadir WDA, Nordin F, Bakar FA, Ting MWH, Jamil A, et al. Systemic lupus erythematosus among male patients in Malaysia: how are we different from other geographical regions? Lupus 2018;28(1):137–44. [ 10.1177/0961203318812676] [DOI] [PubMed] [Google Scholar]
- 20.Tektonidou MG, Lewandowski LB, Hu J, Dasgupta A, Ward MM. Survival in adults and children with systemic lupus erythematosus: a systematic review and Bayesian meta-analysis of studies from 1950 to 2016. Ann Rheum Dis 2017;76: 2009–16. [ 10.1136/annrheumdis-2017-211663] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 21.Bjornadal L, Yin L, Granath F, Klareskog L, Ekbom A. Cardiovascular disease a hazard despite improved prognosis in patients with systemic lupus erythematosus: results from a Swedish population based study 1964–95. J Rheumatol 2004;31:713–9. [PMID: ] [PubMed] [Google Scholar]
- 22.Gergianaki I, Fanouriakis A, Repa A, Tzanakakis M, Adamichou C, Pompieri A, et al. Epidemiology and burden of systemic lupus erythematosus in a Southern European population: data from the community-based lupus registry of Crete, Greece. Ann Rheum Dis 2017;76:1992–2000. [ 10.1136/annrheumdis-2017-211206] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 23.Pamfil C, Fanouriakis A, Damian L, Rinzis M, Sidiropoulos P, Tsivgoulis G, et al. EULAR recommendations for neuropsychiatric systemic lupus erythematosus vs usual care: results from two European centres. Rheumatology (Oxford) 2015;54:1270–8. [ 10.1093/rheumatology/keu482] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 24.Ferro JM, Massaro AR, Mas JL. Aetiological diagnosis of ischaemic stroke in young adults. Lancet Neurol 2010;9:1085–96. [ 10.1016/S1474-4422(10)70251-9] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 25.Putaala J, Metso AJ, Metso TM, Konkola N, Kraemer Y, Haapaniemi E, et al. Analysis of 1008 consecutive patients aged 15 to 49 with first-ever ischemic stroke: the Helsinki young stroke registry. Stroke 2009;40:1195–203. [ 10.1161/STROKEAHA.108.529883] [DOI] [PubMed] [Google Scholar]
- 26.Khan S, Cloud GC, Kerry S, Markus HS. Imaging of vertebral artery stenosis: a systematic review. J Neurol Neurosurg Psychiatry 2007;78:1218–25. [ 10.1136/jnnp.2006.111716] [PMID: ] [PMCID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wardlaw JM, Chappell FM, Best JJ, Wartolowska K, Berry E, Research NHS, et al. Non-invasive imaging compared with intra-arterial angiography in the diagnosis of symptomatic carotid stenosis: a meta-analysis. Lancet 2006;367:1503–12. [ 10.1016/S0140-6736(06)68650-9] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 28.de Bruijn SF, Agema WR, Lammers GJ, van der Wall EE, Wolterbeek R, Holman ER, et al. Transesophageal echocardiography is superior to transthoracic echocardiography in management of patients of any age with transient ischemic attack or stroke. Stroke 2006;37:2531–4. [ 10.1161/01.STR.0000241064.46659.69] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 29.Liao J, Khalid Z, Scallan C, Morillo C, O’Donnell M. Noninvasive cardiac monitoring for detecting paroxysmal atrial fibrillation or flutter after acute ischemic stroke: a systematic review. Stroke 2007;38:2935–40. [ 10.1161/STROKEAHA.106.478685] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 30.Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006;4:295–306. [ 10.1111/j.1538-7836.2006.01753.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 31.Sciascia S, Sanna G, Khamashta MA, Cuadrado MJ, Erkan D, Andreoli L, et al. The estimated frequency of antiphospholipid antibodies in young adults with cerebrovascular events: a systematic review. Ann Rheum Dis 2015;74:2028–33. [ 10.1136/annrheumdis-2014-205663] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 32.Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum 1992;35:630–40. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 33.Govoni M, Bombardieri S, Bortoluzzi A, Caniatti L, Casu C, Conti F, et al. Factors and comorbidities associated with first neuropsychiatric event in systemic lupus erythematosus: does a risk profile exist? A large multicentre retrospective cross-sectional study on 959 Italian patients. Rheumatology (Oxford) 2012;51:157–68. [ 10.1093/rheumatology/ker310] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 34.Roman MJ, Shanker BA, Davis A, Lockshin MD, Sammaritano L, Simantov R, et al. Prevalence and correlates of accelerated atherosclerosis in systemic lupus erythematosus. N Engl J Med 2003;349:2399–406. [ 10.1056/NEJMoa035471] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 35.de Amorim LC, Maia FM, Rodrigues CE. Stroke in systemic lupus erythematosus and antiphospholipid syndrome: risk factors, clinical manifestations, neuroimaging, and treatment. Lupus 2017;26:529–36. [ 10.1177/0961203316688784] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 36.Wardlaw JM, Valdes Hernandez MC, Munoz-Maniega S. What are white matter hyperintensities made of? Relevance to vascular cognitive impairment. J Am Heart Assoc 2015;4:001140. [ 10.1161/JAHA.114.001140] [PMID: ] [PMCID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005;173:489–95. [ 10.1503/cmaj.050051] [PMID: ] [PMCID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dufouil C, de Kersaint-Gilly A, Besancon V, Levy C, Auffray E, Brunnereau L, et al. Longitudinal study of blood pressure and white matter hyperintensities: the EVA MRI Cohort. Neurology 2001;56:921–6. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 39.Ferguson SC, Blane A, Perros P, McCrimmon RJ, Best JJ, Wardlaw J, et al. Cognitive ability and brain structure in type 1 diabetes: relation to microangiopathy and preceding severe hypoglycemia. Diabetes 2003;52:149–56. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 40.Gons RA, van Norden AG, de Laat KF, van Oudheusden LJ, van Uden IW, Zwiers MP, et al. Cigarette smoking is associated with reduced microstructural integrity of cerebral white matter. Brain 2011;134:2116–24. [ 10.1093/brain/awr145] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 41.Hopkins RO, Beck CJ, Burnett DL, Weaver LK, Victoroff J, Bigler ED. Prevalence of white matter hyperintensities in a young healthy population. J Neuroimaging 2006;16:243–51. [ 10.1111/j.1552-6569.2006.00047.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 42.Cohen D, Rijnink EC, Nabuurs RJ, Steup-Beekman GM, Versluis MJ, Emmer BJ, et al. Brain histopathology in patients with systemic lupus erythematosus: identification of lesions associated with clinical neuropsychiatric lupus syndromes and the role of complement. Rheumatology (Oxford) 2017;56:77–86. [ 10.1093/rheumatology/kew341] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 43.Cavallaro M, Barbaro U, Caragliano A, Longo M, Cicero G, Granata F, Racchiusa S. Stroke and Systemic Lupus Erythematosus: A Review. EMJ Rheumatol 2018;5:100–07. [Google Scholar]
- 44.Luyendijk J, Steens SC, Ouwendijk WJ, Steup-Beekman GM, Bollen EL, van der Grond J, et al. Neuropsychiatric systemic lupus erythematosus: lessons learned from magnetic resonance imaging. Arthritis Rheum 2011;63:722–72. [ 10.1002/art.30157] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 45.Toledano P, Sarbu N, Espinosa G, Bargallo N, Cervera R. Neuropsychiatric systemic lupus erythematosus: magnetic resonance imaging findings and correlation with clinical and immunological features. Autoimmun Rev 2013;12:1166–70. [ 10.1016/j.autrev.2013.07.004] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 46.Appenzeller S, Vasconcelos Faria A, Li LM, Costallat LT, Cendes F. Quantitative magnetic resonance imaging analyses and clinical significance of hyperintense white matter lesions in systemic lupus erythematosus patients. Ann Neurol 2008;64:635–43. [ 10.1002/ana.21483] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 47.Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ 2010;341:c3666. [ 10.1136/bmj.c3666] [PMID: ] [PMCID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ay H, Arsava EM, Rosand J, Furie KL, Singhal AB, Schaefer PW, et al. Severity of leukoaraiosis and susceptibility to infarct growth in acute stroke. Stroke 2008;39:1409–13. [ 10.1161/STROKEAHA.107.501932] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 49.Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J 2016;37: 2315–81. [ 10.1093/eurheartj/ehw106] [PMID: ] [PMCID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ugarte-Gil MF, Wojdyla D, Pons-Estel GJ, Pons-Estel BA, Alarcon GS, Gladel Remission or low disease activity as a target in systemic lupus erythematosus. Ann Rheum Dis 2019;78:e3. [ 10.1136/annrheumdis-2017-212876] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 51.Teixeira V, Lam RS. Novel Insights in Systemic Lupus Erythematosus and Atherosclerosis. Front Med (Lausanne) 2017;4:262. [ 10.3389/fmed.2017.00262] [PMID: ] [PMCID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Artola RT, Mihos CG, Santana O. Effects of Statin Therapy in Patients with Systemic Lupus Erythematosus. South Med J 2016;109:705–11. [ 10.14423/SMJ.0000000000000561] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 53.Watanabe T, Oku K, Amengual O, Hisada R, Ohmura K, Nakagawa I, et al. Effects of statins on thrombosis development in patients with systemic lupus erythematosus and anti-phospholipid antibodies. Lupus 2018;27:225–34. [ 10.1177/0961203317716787] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 54.Jorge AM, Lu N, Keller SF, Rai SK, Zhang Y, Choi HK. The Effect of Statin Use on Mortality in Systemic Autoimmune Rheumatic Diseases. J Rheumatol 2018;45:1689–95. [ 10.3899/jrheum.171389] [PMID: ] [PMCID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sahebkar A, Rathouska J, Derosa G, Maffioli P, Nachtigal P. Statin impact on disease activity and C-reactive protein concentrations in systemic lupus erythematosus patients: A systematic review and meta-analysis of controlled trials. Autoimmun Rev 2016;15:344–53. [ 10.1016/j.autrev.2015.12.007] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 56.Vera-Lastra O, Mendez-Flores S, Cruz-Dominguez MP, Medina G, Calderon-Aranda E, Jara LJ. Effect of ezetimibe plus pravastatin on endothelial dysfunction in patients with systemic lupus erythematosus. Lupus 2016;25:741–8. [ 10.1177/0961203316631631] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 57.Petri MA, Kiani AN, Post W, Christopher-Stine L, Magder LS. Lupus Atherosclerosis Prevention Study (LAPS). Ann Rheum Dis 2011;70:760–5. [ 10.1136/ard.2010.136762] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 58.Schanberg LE, Sandborg C, Barnhart HX, Ardoin SP, Yow E, Evans GW, et al. Use of atorvastatin in systemic lupus erythematosus in children and adolescents. Arthritis Rheum 2012;64:285–96. [ 10.1002/art.30645] [PMID: ] [PMCID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bertsias G, Ioannidis JP, Boletis J, Bombardieri S, Cervera R, Dostal C, et al. EULAR recommendations for the management of systemic lupus erythematosus. Report of a Task Force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics. Ann Rheum Dis 2008;67:195–205. [ 10.1136/ard.2007.070367] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 60.Groot N, de Graeff N, Marks SD, Brogan P, Avcin T, Bader-Meunier B, et al. European evidence-based recommendations for the diagnosis and treatment of childhood-onset lupus nephritis: the SHARE initiative. Ann Rheum Dis 2017;76:1965–73. [ 10.1136/annrheumdis-2017-211898] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 61.Arnaud L, Mathian A, Ruffatti A, Erkan D, Tektonidou M, Cervera R, et al. Efficacy of aspirin for the primary prevention of thrombosis in patients with antiphospholipid antibodies: an international and collaborative meta-analysis. Autoimmun Rev 2014;13: 281–91. [ 10.1016/j.autrev.2013.10.014] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 62.Gaziano JM, Brotons C, Coppolecchia R, Cricelli C, Darius H, Gorelick PB, et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomised, double-blind, placebo-controlled trial. Lancet 2018;392:1036–46. [ 10.1016/S0140-6736(18)31924-X] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Group ASC. Bowman L, Mafham M, Wallendszus K, Stevens W, Buck G, et al. Effects of Aspirin for Primary Prevention in Persons with Diabetes Mellitus. N Engl J Med 2018;379: 1529–39. [ 10.1056/NEJMoa1804988] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 64.Ruiz-Irastorza G, Ramos-Casals M, Brito-Zeron P, Khamashta MA. Clinical efficacy and side effects of antimalarials in systemic lupus erythematosus: a systematic review. Ann Rheum Dis 2010;69:20–8. [ 10.1136/ard.2008.101766] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 65.Petri M. Hydroxychloroquine use in the Baltimore Lupus Cohort: effects on lipids, glucose and thrombosis. Lupus 1996;5 Suppl 1:S16–22. [PMID: ] [PubMed] [Google Scholar]
- 66.Ruiz-Irastorza G, Egurbide MV, Pijoan JI, Garmendia M, Villar I, Martinez-Berriotxoa A, et al. Effect of antimalarials on thrombosis and survival in patients with systemic lupus erythematosus. Lupus 2006;15:577–83. [ 10.1177/0961203306071872] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 67.Tektonidou MG, Laskari K, Panagiotakos DB, Moutsopoulos HM. Risk factors for thrombosis and primary thrombosis prevention in patients with systemic lupus erythematosus with or without anti-phospholipid antibodies. Arthritis Rheum 2009;61:29–36. [ 10.1002/art.24232] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 68.Fasano S, Pierro L, Pantano I, Iudici M, Valentini G. Longterm Hydroxychloroquine Therapy and Low-dose Aspirin May Have an Additive Effectiveness in the Primary Prevention of Cardiovascular Events in Patients with Systemic Lupus Erythematosus. J Rheumatol 2017;44:1032–8. [ 10.3899/jrheum.161351] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 69.Camara-Lemarroy CR, Infante-Valenzuela A, Andrade-Vazquez CJ, Enriquez-Noyola RV, Garcia-Valadez EA, Gongora-Rivera F. Successful intravenous thrombolysis in a patient with antiphospholipid syndrome, acute ischemic stroke and severe thrombocytopenia. Blood Coagul Fibrinolysis 2016;27:354–6. [ 10.3389/fneur.2018.01001] [PMID: ] [PMCID: ] [DOI] [PubMed] [Google Scholar]
- 70.Chen X, Xu G. Intravenous thrombolysis in SLE-related stroke: a case report and literature review. Neurol Sci 2018;39:155–9. [ 10.1007/s10072-017-3158-4] [PMID: ] [DOI] [PubMed] [Google Scholar]
- 71.Schreiber K, Sciascia S, de Groot PG, Devreese K, Jacobsen S, Ruiz-Irastorza G, et al. Antiphospholipid syndrome. Nat Rev Dis Primers 2018;4:17103. [ 10.1038/nrdp.2017.103] [PMID: ] [DOI] [PubMed] [Google Scholar]