Abstract
Many studies have examined the impact of dispersal on local adaptation, but much less attention has been paid to how local adaptation influences range shifts. The aim of this study was to test how local adaptation might affect climate-driven range shifts in plants, and if this might differ between plants with different life histories. Simulated range shift dynamics were compared for hypothetical annual, perennial and tree species, each comprised of either one plastic genotype or six locally adapted genotypes. The landscape consists of shifting climate bands made up of 20 × 20 m patches containing multiple individuals. Effects of seed dispersal, breadth of the plastic species’ tolerance, steepness of the climate gradient and rate of the climate shift are also examined. Local adaptation increased the equilibrium range size and aided range shifts by boosting fitness near range edges. However, when the rate of climate change was doubled on a steep gradient, locally adapted trees exhibited a higher percent loss of range during the climate shift. The plastic annual species with short dispersal was unable to recover its range size even after the climate stabilized, while the locally adapted annuals tracked climate change well. The results suggest that in most situations local adaptation and longer dispersal distances will be advantageous, though not necessarily sufficient, for tracking suitable climates. However, local adaptation might put species with long generation times at greater risk when climate shifts are very rapid. If confirmed by empirical tests, these results suggest that identifying variation between species in how fitness varies along climate gradients and in these key demographic rates might aid in prioritizing management actions.
Keywords: Climate change, dispersal, life history, local adaptation, range shift, simulation
This paper uses a simulation model to explore how local adaptation in plants may shape responses to climate shifts. When local adaptation improved survival and reproduction at range edges, this increased the area occupied when climate is stable and usually improved tracking of climate shifts. Local adaptation yielded the biggest benefit for low-dispersal annual species. However, the area occupied by the species can still lag behind climate change even when the period of climate change and the distance to reach newly suitable areas are both short. This is especially true of trees; locally adapted trees sometimes exhibited greater lags.
Introduction
Considerable research effort has focused on the impact of gene flow on local adaptation (Kirkpatrick and Barton 1997; Lenormand 2002; Holt et al. 2004; Bridle and Vines 2006; Garant et al. 2007; Kimbrell and Holt 2007; Kawecki 2008; Räsänen and Hendry 2008; Bell and Gonzales 2009; Bridle et al. 2009, 2010; Sexton et al. 2009, 2011; Dawson et al. 2010; North et al. 2010; Kremer et al. 2012; Polechová and Barton 2015). Less attention has been paid to how local adaptation affects the potential for range shifts under climate change. In addition, it is not clear whether effects would be the same across life history types.
Researchers have defined and tested the degree of local adaptation in plants in two ways. The first approach, generally based on a small number of reciprocal transplants, classifies populations as locally adapted if they either perform better in their home site than in other sites or if, within a site, local genotypes perform better than foreign ones (Kawecki and Ebert 2004). The second approach, used most commonly in provenance studies of trees in which many seed sources are planted in multiple common gardens (not including all source sites), defines a population as being locally adapted if it performs best in sites that are more environmentally similar to its home (Aitken and Whitlock 2013; Browne et al. 2019). This sense is commonly used in studies focusing on performance along environmental gradients (Angert et al. 2011; Leites et al. 2012; Moran et al. 2017), variation in traits or specific genetic markers relative to null expectations (Keller et al. 2011; Csilléry et al. 2014) or responses to changing environmental conditions (Atkins and Travis 2010; Bocedi et al. 2013; Browne et al. 2019). This definition has the benefit of specifying what a species is adapted to, and is therefore how ‘locally adapted’ will be used here.
Local adaptation is common but not universal in plants (Leimu and Fischer 2008; Hereford 2009; Browne et al. 2019) and results from the interplay of gene flow with natural selection (Garant et al. 2007; Kawecki 2008; Räsänen and Hendry 2008; Sexton et al. 2009). Gene flow can increase local genetic variation and population size, which boost adaptive potential (Holt et al. 2004; Kimbrell and Holt 2007; North et al. 2010; Kremer et al. 2012), but excessive immigration from environmentally distinct areas may also ‘swamp’ local adaptation (Kirkpatrick and Barton 1997; Lenormand 2002; Bridle and Vines 2006; Kawecki 2008). Strong selection can, however, create local adaptation in the presence of high gene flow (Gonzalo-Turpin and Hazard 2009; Csilléry et al. 2014; Eckert et al. 2015; Moran et al. 2017), which can result in a pattern of ‘isolation by environment’ (IBE) at loci relevant for fitness but little differentiation at neutral loci (Sexton et al. 2014).
Several studies have examined how the existence of locally adapted subdivisions within widespread species may affect habitat suitability as climate changes. Most have focused on trees, as provenance experiments in the 20th century yielded much information on local adaptation to climate (Leites et al. 2012). Taking population subdivisions into account often results in less negative predictions of climate change impacts on species ranges (Garzon et al. 2011; Oney et al. 2013; Maguire et al. 2018). Few studies have examined this in herbaceous plants. One exception was a study of Mimulus cardinalis that found that population-level mechanistic models predicted greater range size following climate change than a mechanistic model fit to species-level average temperature responses (Angert et al. 2011). However, most such studies have not examined the species’ ability to fill suitable habitat through dispersal.
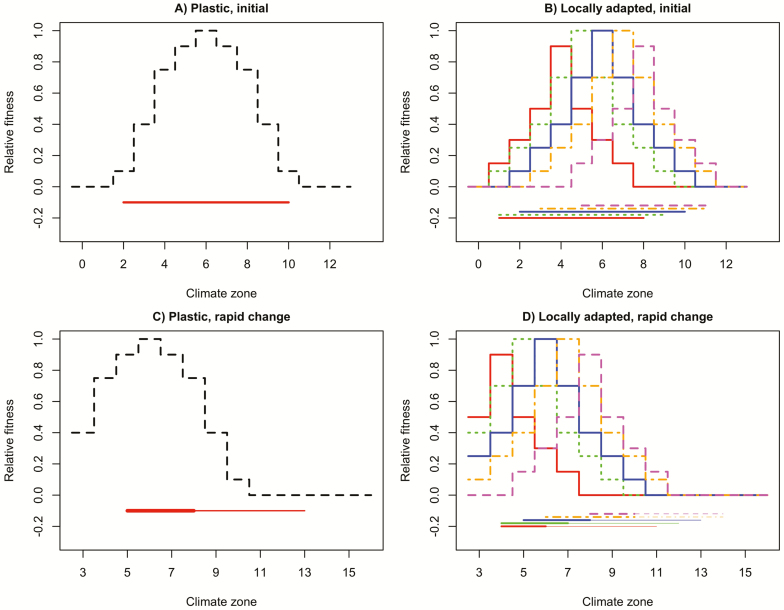
Local adaptation may reduce fitness differences between populations across the range, though such specialization can have costs. For instance, in the invasive plant Lythrum salicaria northern populations evolved earlier flowering, which increased their reproductive output there, but associated decreases in vegetative growth decreased the fitness of these genotypes in the south (Colautti and Barrett 2013). Figure 1 depicts two hypothetical species, one made up of a single population or genotype with wide climatic tolerances (Fig. 1A), and one made up of several populations or genotypes, each with narrower climatic tolerances (Fig. 1B). If the climate gradient shifts, species 1 will likely see a greater change in habitat suitability overall (Fig. 1C and D). However, each narrowly adapted population/genotype of species 2, if it stays in place and climate change continues, will more rapidly find itself in conditions to which it is maladapted. Equalization of fitness across the range is important, as the furthest-forward individuals are the most likely source for seeds dispersing beyond the current range edge (Clark 1998; Clark et al. 2001). Conversely, a lack of seed produced near the range edge could slow expansion (Kroiss and HilleRisLambers 2015).
Figure 1.
(A) Dashed black curve depicts fitness across climate zones for a hypothetical plastic species, horizontal red line the area that species might occupy on the landscape. (B) Coloured curves depict fitness across climate zones for each of five populations in a locally adapted species. Horizontal coloured lines depict area of landscape each population could occupy in the absence of competition from other populations. (C) Dashed black curve depicts fitness across climate zones for plastic species after a period of rapid climate change. Horizontal red line depicts the original range, with areas where fitness increases drawn thick and areas where fitness decreases drawn thin. (D) Coloured curves depict fitness across climate zones for each of five populations after rapid climate change. Horizontal coloured lines depict the original range, with areas where fitness increases drawn thick and areas where fitness decreases drawn thin.
Simulation model studies disagree about the impact of local adaptation on range shift ability. Some of this disagreement stems from details of how the models are constructed, particularly what assumptions they make about the structure of the landscape and population. For instance, Moran and Ormond (2015), Bocedi et al. (2013) and Atkins and Travis (2010) all considered species spreading along a linear climate gradient that shifts by a fixed amount each year, in which climate effects on fitness are determined by the genotype at a diploid locus. Atkins and Travis found that cold-adapted genotypes tended to block the spread of warm-adapted genotypes, but this is likely because only one individual could occupy each patch/cell in the landscape, mortality was independent of climate and the landscape was small (200 × 200 patches). Models that allow multiple individuals to occupy a patch and that considered larger landscapes have not found this effect (Bocedi et al. 2013; Moran and Ormond 2015). Bocedi et al. (2013) found that cold-adapted alleles could ‘surf’ backwards and displace warm-adapted alleles, and that ranges could become disjointed, with a loss of central alleles. These patterns may have been affected by the fact that only 20 % of the landscape was treated as suitable habitat, making spread of all genotypes more difficult. The Moran and Ormond model (2015) treated habitat as continuous and found that local adaptation in a low-elevation species aided spread to higher-elevation habitats, while local adaptation of the high-elevation species slowed spread of the low-elevation species.
It is possible that life history and dispersal characteristics might also influence the effect of local adaptation on range shifts. Species with shorter generation times could exhibit more rapid responses to changing conditions. However, plant species that take longer to mature often have greater height and larger seed crops, which increases the probability of long-distance dispersal. In an analysis of 80 plant species, we found that groups with very different generation times had similar predicted average spread rates (around 5.3 m per year), with higher seed production and dispersal in long-lived plants more than balancing out their longer lifespans (Lustenhower et al. 2017). There was substantial variation within life history groups due to variation in fecundity and dispersal. On the other hand, species with longer lifespans and generation times might exhibit substantial lags in spread or adaptation as the rate of change increases (Kuparinen et al. 2010). However, no previous simulation or empirical study has explicitly examined how life history and local adaptation may interact to affect range shifts under climate change.
In this study, I compare simulations of three basic plant life history types: a wind-pollinated tree with moderately shade-intolerant seedlings, a clump-forming perennial and an annual wildflower. Dispersal distances tend to be positively affected by plant height, and maximum seed crop by overall size, and so are assumed to increase from annual to perennial to tree (Herrera 1991; Dodd and Silvertown 2000; Nathan et al. 2011; Lustenhower et al. 2017), but I consider several values for seed dispersal parameters that overlap across types. Each life history type may consist of either a single broadly adapted genotype (‘plastic’) or several narrowly adapted genotypes that differ in abundance across the elevation gradient (‘locally adapted’). It should be noted that none of the simulated ‘species’ are meant to represent a single real plant species, or the classes of ‘annual’, ‘perennial’ and ‘tree’ as a whole. Rather, in recognition of the fact that features like dispersal distance and time to maturity do not vary independently (Lustenhower et al. 2017), they are meant to represent plausible combinations of demographic rates within these categories.
In part 1, I compare range shift responses along an elevation gradient during and after a short period of moderately rapid climate change for plastic or locally adapted versions of each of these three types. In part 2, I test how the rate of decline in fitness at the range margins affects spread independent of number of genotypes. This is done by making the maximum fitness of the plastic perennial genotype across the range match that of the locally adapted perennial species overall. In part 3, I test the cumulative impact of annual climate effects on range size for species that live for >1 year. To do this, I weaken the impact of climate on annual demographic rates of the perennial species such that the lifetime effects more closely resemble those of the annual species. Finally, in part 4, I test whether the long generation times of trees change the effects seen in part 1 if climate change is more rapid or climate bands are broader.
I hypothesized that:
1) If a species is locally adapted such that close-to-maximum fitness is maintained near the climatic limits of the species, this will result in a wider equilibrium range and less lag in responding to a climate shift than in an equivalent plastic species with fitness that declines more gradually.
2) If the plastic species has a single population/genotype with the same maximum fitness across environments as all the subpopulations/genotypes of the locally adapted species, it will have a broader equilibrium range and lower lag in climate response than either the narrowly plastic or locally adapted species.
3) Species with high seed movement and/or short generation times will exhibit lower lags in range shifts.
4) If annual survival or reproduction is affected equally by climate for all life histories, the species with the longer generation times will exhibit a narrower geographic range, as multiple years of selection will eliminate them from marginal habitats before or soon after they begin to reproduce.
Methods
This model is based on Moran and Ormond (2015). The main changes are to the dispersal calculations, to make them more easily adjustable to different grid sizes, and the inclusion of life history options other than trees. The landscape is also smaller and finer-grained and there are six genotypes per species instead of three. The finer-grained landscape was introduced because a 50 m × 50 m patch would be too large to capture the dynamics of plants that disperse 20 m or less on average. Similarly, including three alleles that result in six genotypes results in a subtler and hopefully more realistic species response to climate gradients than the two allele/three genotype model.
Demographic rates
Size classes
The annuals have two life stages, seed and adult. The perennials have four size classes in addition to seed: seedlings plus small, medium and large adults. Perennial seedlings are non-reproductive. The trees have an additional non-reproductive size category (saplings) between seedlings and small adults. In both perennials and trees, larger adults produce more seed and pollen (Table 1).
Table 1.
Demographic parameters. ‘K’ indicates thousands. Maximum numbers of seed or pollen and maximum survival and transition rates are given for each size category, where applicable.
| Annual | Perennial | Tree | |
|---|---|---|---|
| Mean seed dispersal (m) | 1, 20 or 50 | 20 or 80 | 40 or 80 |
| Mean pollen dispersal (m) | 60 | 80 | 180 |
| Maximum germination rate | 0.3 | 0.45 | 0.6 |
| Maximum seeds/pollen | 300/8K | 0/0, 150/800, 1K/10K, 5K/100K | 0/0, 0/0, 150/800, 1K/10K, 5K/100K |
| Maximum survival | 0 | 0.3, 0.5, 0.65, 0.7 | 0.6, 0.85, 0.96, 0.98, 0.99 |
| Maximum transition rate | 0 | 0.3, 0.15, 0.1, 0 | 0.15, 0.11, 0.06, 0.02, 0 |
| Individual size | NA | 0.004, 0.06, 0.25, 2 (m2) | 0.0001, 0.003, 0.07, 0.14, 0.5 (m2 BA) |
| Competitive effects on germination | NA | 0.003 × total area occupied | 0.08 × total BA |
| Competitive effects on survival or transition | Based on number of individuals | 0.005, 0.003, 0.001 or 0.0005 × total area of larger individuals | 0.1, 0.07, 0.02, 0.01 or 0 × total BA of larger individuals |
| Standard equilibration period | 20 years | 100 years | 200 years |
Dispersal
Dispersal in all species follows a fat-tailed 2Dt kernel (Clark et al. 1999). I compared two or three different average seed dispersal distances for each species (Table 1). In a previous study, we found that seed dispersal distances varied widely within life history classes but tended to be higher for trees (mean 4.2–100 m) than for perennials (mean 0.2–33.3 m) or annuals (mean 0.1–20 m) (Lustenhower et al. 2017). However, due to various constraints and what could be included in the spread rate model, that study assumed negative exponential dispersal kernels. In reality, seed dispersal kernels tend to have more long-distance events that increase the mean than expected under normal or exponential distributions (Clark et al. 2005; Cousens et al. 2008). Therefore, I included a 50 m average version for the annual, and an 80 m average version for the perennial, since I was envisioning the latter as a tall, wind-dispersed clump-former such as Solidago or Asclepias. The tree averages were put near but not beyond the top of the range estimated for exponential kernels, as these hypothetical species were meant to represent a moderately heavy-seeded type such as Pinus or Quercus. 2Dt dispersal kernels estimated for Quercus rubra based on genetic markers indicated a mean dispersal distance of 15 m at one site and 125 m at another (Moran and Clark 2012).
Data on pollen dispersal distances across different plant life history classes are sparse. For simplicity, I assumed that the trees are wind-pollinated, with an average dispersal distance of 180 m, while the herbaceous plants are pollinated by insects with a moderately long flight distance: 80 m for the taller perennial, 60 m for the shorter and less visible annual (Table 1). Because of the fat tail of the probability distribution many of the seeds or pollen grains stay in their patch of origin, but some disperse much further than the mean distance [seeSupporting Information—Fig. S1.1]. Dispersal calculations are explained in Supporting Information—Appendix S1.
Initialization and sequence of events
The initial numbers and sizes of each genotype are given in Supporting Information—Appendix S1. These numbers are arbitrary but large enough to avoid stochastic extinction and each genotype is distributed across the landscape somewhat more broadly than the expected equilibrium distribution. Annual plants were initiated as seeds, perennials as small adults and trees as saplings. In each simulation, there is an initialization period to allow the species to equilibrate with the climate that corresponds roughly to 20 generations (Table 1).
Each year of the simulation starts with the germination of seeds (if present). Non-germinating seeds are removed, meaning there is no persistent seed bank. Survival of each size class for the year is then calculated, followed by pollen production and dispersal, seed production and dispersal, and transition to larger size classes.
Demographic rates given climate and competition
Demographic rates for all three life history types are given in Table 1. Again, it should be noted that none of the simulated ‘species’ are meant to represent a ‘type’ rather than single real plant species. Like dispersal, fecundity (seeds per year) differs substantially between real species within a particular life history class (Lustenhower et al. 2017). Because the simulated annual species was envisioned as a relatively small-bodied plant, its annual fecundity was set closer to the low end of the observed range (3–82 000). The Solidago and Asclepias species included in this previous analysis had estimated mean fecundities of 450 and 600 seeds per year, but Solidago canadensis (formerly altissima) can produce over 20 000 seeds per year (Meyer and Schmid 1999), so the maximum fecundity for the simulated perennial species was set at an intermediate value of 5000. For Pinus and Quercus annual fecundities included in the previous study ranged from 216 to 12 000, so a maximum value of 5000 was considered reasonable for the simulated tree species as well. Annuals by definition mature within a year and then die. For the other species, mortality and growth rates were set such that average time to maturity fell within the range observed in the previous study for perennials (1–17 years) and trees (9–40 years) (Lustenhower et al. 2017).
Production of seed and pollen is affected by climate, with fecundity at one or more climate steps from the optimum being a fraction of the maximum. Thus:
where ClimEff[C.steps] is the climate effect corresponding to a certain number of steps away from the optimum.
For all life history classes, the number of individuals germinating, surviving or transitioning for each genotype in each size class in each patch is generated by drawing from a Bernoulli distribution, such that:
where N0 is the number of individuals before the event.
All demographic rates in the tree and perennial are affected by both climate and a competitive effect (CompEff) that depends either on the basal area (BA) of larger trees or the surface area occupied by larger perennials in the patch (TA) and the size of the target individual (Table 1). Thus:
where Max.Rate is the maximum possible rate and CA is the surface or BA of competitors in the patch.
In annuals, germination is affected only by climate, as no plants exist on the landscape prior to this step:
However, because competitive interactions might become important as annuals grow, if the number of seedlings in a patch exceeds 7000, the probability of survival to reproduction = 7000/(initial seedling density). Annuals live only 1 year and do not transition.
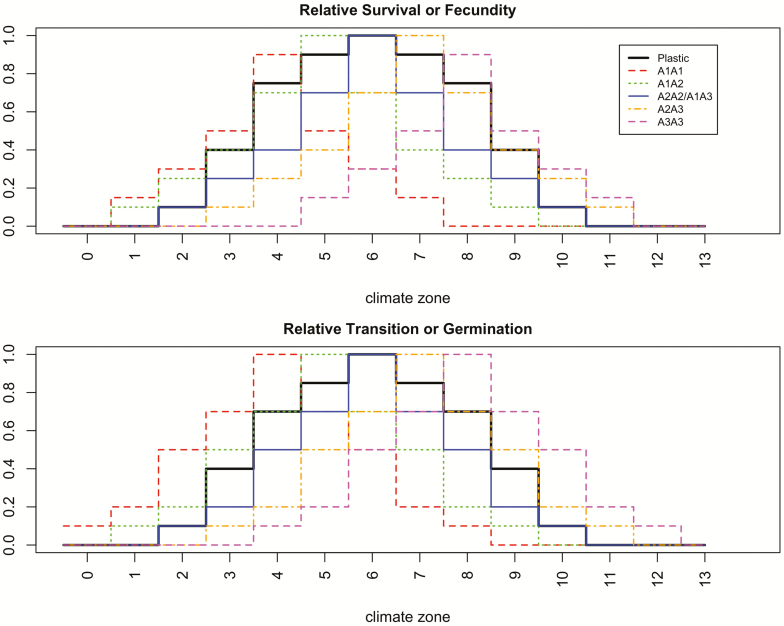
In all cases, if the calculated rate is less than zero, the rate is set to zero. Maximum demographic rates and competitive effects on demographic rates are given in Table 1. Standard climate effects on demographic rates for each genotype are illustrated in Fig. 2 and described further in Supporting Information—Appendix S1. Note that in the species with multiple genotypes (the initially locally adapted species), each genotype has a different optimum climate, with the optima for heterozygotes being intermediate between the optima for the homozygotes. For the widely plastic single genotype (part 2), the response curve traces the maximum species-level fitness of the locally adapted species [seeSupporting Information—Fig. S1.2]. In part 3, to make the lifetime fitness effects of climate more similar for perennials and annuals, I reduced the negative impacts of each step away from the optimum climate by half [seeSupporting Information—Fig. S1.2].
Figure 2.
Demographic rates relative to maximum in each climate zone for either the single plastic genotype (dark black line), or individual genotypes/populations in the variable species (coloured lines).
Landscape and climate
In a mountainous landscape, a shift in elevation of 1000 m (3280 ft) often represents a major shift in vegetation. For instance, in Sequoia National Park, plots at 1500 m in elevation contain a diverse mix of white fir, incense cedar, sugar and ponderosa pines, and black oak, while at around 2500 m they are dominated by red fir and lodgepole or western white pine (Moran et al. 2019). A shift of 1 km along the surface of a mountain slope can represent a shift of 745–913 m in elevation for slopes of 20–40 degrees. The ‘standard’ landscape therefore consisted of a grid 1 km wide × 2 km representing a ‘slice’ of a species’ range an elevation gradient, with climate bands 200 m wide ranging from warmer at the left to cooler at the right. The 2 km low-high axis thus represents a 1490 to 1826 m change in elevation. The exact relationship between temperature and elevation can vary, and this landscape is not meant to replicate any real landscape. However, for reference, an elevation gradient of this size the Sequoia National Park example would represent at least a 7.3 °C difference in mean annual temperature and a 10.6 °C difference in July maximum temperature (Moran et al. 2019). Each patch within the landscape represents a 20 × 20 m area and can accommodate multiple individuals.
After the initialization period, the climate bands shift at a rate of 20 m (one patch width) per year for 20 years, such that the range of available climates ranges from 10 (warmest) to 1 (coldest) at the beginning and from 12 to 3 at the end, the two coldest climates disappearing entirely. This rate is close to the mean rates estimated by Loarie et al. (2009) for many montane environments under an A1B emission scenario. The populations were then allowed to adjust to the new climate gradient for a length of time equal to the initial equilibration period. The number of patches occupied by adults of each genotype and by the species as a whole were recorded at the end of the 20-year climate change period and at the end of the re-equilibration period, and compared to occupancy at the start. Actual contemporary climate change is of course projected continue at these rates for far beyond 20 years (Loarie et al. 2009; IPCC 2013). The limited time period was chosen so that loss in range would be mostly due to lags in climate tracking rather than loss of suitable habitat from the landscape; over longer time periods, both sources of range loss would be more severe.
Variations used include:
- Allowing 10× as long for restabilization when the species appeared far from equilibrium with climate after the standard restabilization period.
- Increasing the rate of the climate shift from 20 to 40 m per year to assess the impact on trees (the category with the longest generation times).
- A landscape in which the coldest climate bands was three times wider. This was used where the species would otherwise lose total suitable habitat, and allowed me to check whether a reduction in occupied range was due to the loss of suitable habitat or to failure to fill suitable habitat.
- A much longer landscape (8 km) with a shallower gradient, in which all climate bands are four times as wide as in the standard landscape. The climate shifted at a rate of four patch widths (80 m) per year so that the colder climate bands disappear at the same rate as in the standard scenario. This was used to assess the effect of a less steep gradient when seeds have to travel farther to reach a cooler climate.
Results
Part 1—Standard model
Initial occupancy
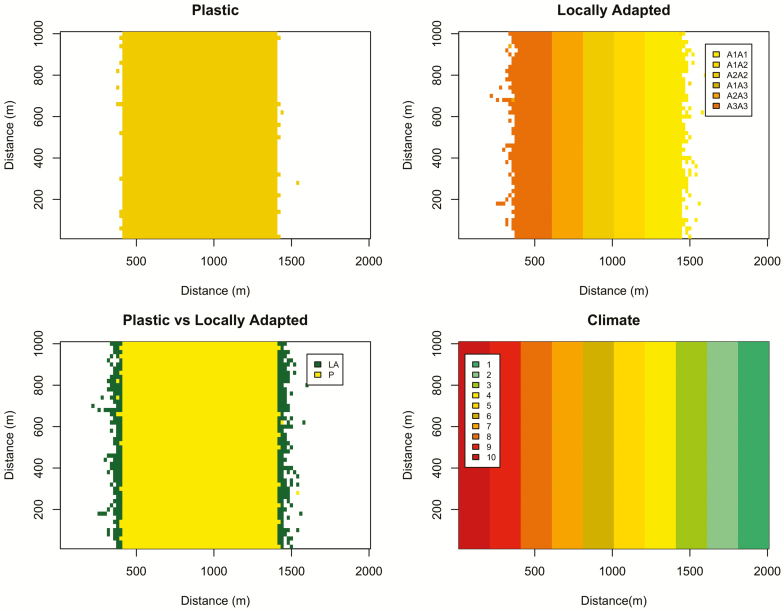
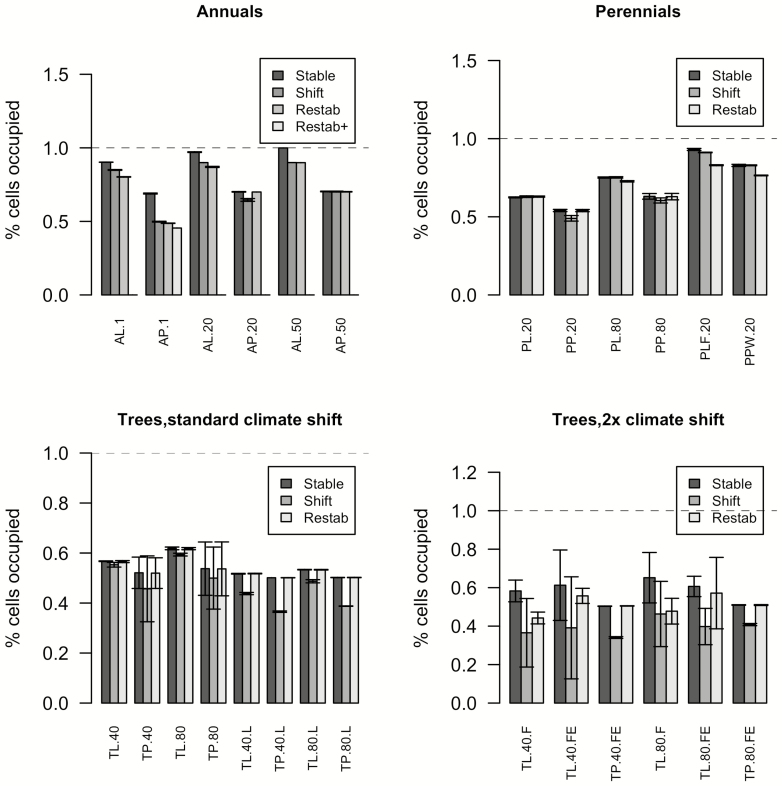
During the establishment period, the abundance of the species across the landscape changes and genotypes sort themselves into bands corresponding to their areas of highest fitness (Fig. 3). Note that the populations in these bands would thus meet either the ‘home vs. away’ or ‘local vs. foreign’ definitions of being locally adapted (Kawecki and Ebert 2004). For example, if one examines Fig. 2, one can see that if the most common climate zone 5 genotype (A1A2) were moved to zone 7, it would have lower survival, fecundity, germination and growth than the local genotype (A2A3), and vice versa. As hypothesized, occupancy of the landscape under the initial climate conditions was greater for the locally adapted species than the plastic one, due to higher fitness near the range edges (Figs 3 and 4). Initial occupancy was also higher for longer dispersal distances (Fig. 4). Due to the cumulative impacts of climate on yearly demographic rates, the geographical distributions are smaller for longer-lived species (Fig. 4).
Figure 3.
Example pre-shift geographic range for a tree with 40 m average dispersal. The top two panels show the patches occupied by the plastic species (single genotype, optimum climate = 6), and the locally adapted species (colour corresponding to most common genotype and its favoured climate). The plastic vs. locally adapted panel illustrates that the locally adapted species (LA) has a slightly wider geographic range than the plastic species (P).
Figure 4.
Percent of landscape occupied after initial stabilization period (‘Stable’), at the end of the period of climate change (‘shift’) and after the restabilization period (‘Restab’). AL = annual, locally adapted; AP = annual, plastic; PL = perennial, locally adapted; PP = perennial, plastic; TL = tree, locally adapted; TP = tree, plastic. Numbers following these codes indicate average dispersal distance in meters. (Top left panel) For AP.1, the plastic annual with 1 m dispersal, a restabilization period 10× as long was tested in one run (‘Restab+’). (Top right panel) Variants for perennial species include PLF (a locally adapted with half the annual fitness impacts of climate) and PPW (widely plastic). (Bottom left) Trees on standard landscape or on landscape where climate bands are 4× as wide (.L variants), in which the two coldest climate bands disappear by the end of the simulation. (Bottom right) Trees exposed to a faster climate shift on the steeper gradient landscape with either the standard setup (.F variants) or an extended landscape that ensures suitable climates do not run off the edge (.FE variants). Error bars show SD over four model runs.
Lags during and after climate shift
As the climate gradient shifts, most species exhibit a reduction in range occupancy due to extinctions at the trailing edge, lags at the leading edge or both (Fig. 4; seeSupporting Information—Table S2.1). This loss was highest for the plastic annual species with a mean seed dispersal distance of 1 m, which lost 27.8 % of its range due to increasing unsuitability of the ‘rear edge’ coupled with a failure to colonize newly suitable territory. This species never extended its range during the simulation, even when 200 years were allowed for restabilization. By contrast, the low-dispersal but locally adapted annual species tracked the climate shift well due to some individuals already being present in the areas that were becoming more suitable. In perennials and trees the low-dispersal plastic species also experienced the greatest lags during the climate shift: 9.3 and 4 % losses for the perennial and the tree, respectively, compared to a 0.7 % gain and 2.5 % loss for their locally adapted counterparts. However, their seed dispersal distances were long enough to make up this gap after climate stabilized. Similarly, the tree with 80 m dispersal exhibited less of a lag when it was locally adapted. The locally adapted annuals with 50 m dispersal and the locally adapted perennials all tracked climate change closely. Occupancy was more variable for plastic than for locally adapted tree species (Fig. 4). After restabilization, species that had very broad initial ranges experienced some range loss due to the loss of climates 1 and 2. All other species except for the short-dispersing plastic annual established a post-climate-change range of similar size to their initial range.
Part 2—Effects of equalizing range-margin fitness for plastic and locally adapted species
The widely plastic perennial species was able to occupy more territory initially and track climate shifts more closely than either narrowly plastic or locally adapted species with the same dispersal distance. In Fig. 4, this can be seen by comparing PPW.20 to PP.20 and PL.20. This seems to be because it combines the benefits of higher fitness near the range edge with broad climate tolerances for all individuals.
Part 3—Effects of reducing climate effects on annual demographic rates
Reducing by half the negative effects on demographic rates of climatic mismatch on the perennial species resulted in behaviour that more closely resembled the annual species with the same dispersal distance. This can be seen in Fig. 3 by comparing PLF.20 to PL.20 and AL.20. Initial occupancy was 4637.3 patches (vs. 4854.5 for the annual), with 1.8 % of this initial range being lost during the climate shift (annual lost 7.3 %) and 10.54 % lost after restabilization (annual lost 10.4 %). The initial range was smaller for the perennial than the annual, most likely because the proportion of perennials that reach reproductive age is still smaller. The lag was smaller for the perennial, most likely because fecundity is higher for perennials.
Part 4—Range shifts with different temporal and spatial scales
When the rate of climate change was doubled, climates 1–4 disappear and the trees lost a large portion of their potential range. This resulted in a reduction in range size post-climate change of 23–26 %. However, when the initial width of the coldest climate band is adjusted so that only climates 1 and 2 are lost, all trees re-equilibrated with the new climate gradient within 200 years. The lags increased substantially with more rapid climate change (Fig. 4; seeSupporting Information—Table S2.1). Although the plastic species still had a smaller equilibrium range size than the locally adapted species, it lost less of this original range during the more rapid climate shift: 42.42 % vs. 32.36 % for the 40 m disperser, and 31.94 % vs. 19.97 % for the 80 m disperser. In the locally adapted species, the narrowly warm-adapted genotype at the rear edge lost more habitat patches. The narrowly cold-adapted genotype at the leading edge was slow to respond. In the locally adapted 40 m dispersal scenario few patches at the end of the climate shift contain the A1 allele (at frequencies <50 %), indicating that this cold-tolerant allele was nearly lost. In a reverse of part 1, occupancy was more variable for the locally adapted trees.
When these trees are given a climate gradient in which each climate band is four times as wide, trees occupy a lower proportion of the total landscape than in the standard scenario, likely because fewer seeds reach marginal areas and form sink populations. The lag in climate tracking is greater than in the standard scenario, but less extreme than for the scenario with doubled range shift speed (Fig. 4; seeSupporting Information—Table S2.1). The lag is greater for the plastic species than the locally adapted one whether the trees have 40 m dispersal (27.07 % vs. 15.34 % loss) or 80 m dispersal (22.77 % vs. 8.7 % loss). As with the other scenarios, the trees re-equilibrated with the new climate gradient within 200 years.
Discussion
The results of this analysis suggest that local adaptation is likely to be more of a help than a hindrance to plant species in tracking climate shifts, at if locally adapted species usually have higher reproductive success at the leading range edge than species that are less locally adapted. Local adaptation nearly always increased equilibrium range size and the ability to track climate shifts across life history types. This was most notable in the case of the annual with 1 m average dispersal; the locally adapted species was able to occupy newly suitable territory, while the plastic one was not. The negative effects of local adaptation seen in some previous models are likely due to the structure of the simulated landscape. For instance, a small landscape with single-occupancy patches (Atkins and Travis 2010) can more easily lead to established genotypes blocking the spread of warm-adapted ones. However, if the landscape is highly fragmented, this blocking effect could potentially be seen in a larger landscape.
The one scenario in which this simulation found a negative effect of local adaptation was in trees when the climate shift over 20 years (~1 tree generation) was 10–20 times greater than the average seed dispersal distance. In this scenario, climate change ‘outran’ narrowly adapted genotypes before they could spread. Interestingly, although the rates of climate change in the ‘shallow gradient’ scenario were twice that of the rapid shift scenario, locally adapted species still did better. This may be because the larger population sizes within each band provided more seeds, aiding the spread of all genotypes. However, if any of these climate change scenarios persisted for multiple tree generations—as they are expected to do in reality—the tree populations would likely be badly impacted due to cumulative lags in response combined with loss of potentially suitable habitat.
Contrary to my hypothesis, shorter generation times did not always help track climate shifts. Among plastic species with similar dispersal ability, lags were greater for those with longer generation times. However, among locally adapted species, the perennial experienced no lag while the annual and the tree both did. This may be because the perennial always had greater fecundity than the annual and a shorter generation time than the tree. This ‘perennial advantage’ would not necessarily hold consistently in the real world; as noted in the methods all such plant life history categories contain considerable variation in dispersal ability and fecundity, and generation time in perennials can vary from 1 year to several decades (Lustenhower et al. 2017).
How much these results say about relative range shift potential in the real world will depend on how well the assumptions of the model capture the conditions under which plants are spreading in four regards: (i) the qualities of the landscape and climate gradient and how individual plants fill it, (ii) how well the treatment of genotypes captures the process of gene flow and local adaptation, (iii) how local adaptation influences fitness near range edges and (iv) how far seed and pollen disperse.
As mentioned briefly above, I consider that allowing multiple individuals to occupy a patch is more realistic in most cases than restricting occupancy to one individual per patch. In forests, for example, it is not uncommon to find smaller individuals growing below the adult canopy. Even when a species is extremely shade-intolerant, a patch that might hold only one adult can still hold multiple small individuals. The landscape itself in many ways is not realistic but rather represents a best-case scenario: only climate restricts the suitability of patches (e.g. no inappropriate soil types or competition from other species) and the distances to newly suitable climates are shorter than for many latitudinal climate gradients (Loarie et al. 2009). Most such complications would be likely to increase lags in range shifts, especially for species with poor dispersal. An exception might be topographical complexity, which can create ‘micro-refugia’ in otherwise unsuitable landscapes. Such refugia might prevent total local extinction at the trailing edge (Franklin et al. 2013) or speed up spread if they exist close to the leading range edge (McLachlan et al. 2005). Moreover, the 1 × 2 km elevation modelled here would not represent the complete range of most species. In more widely distributed species, latitudinal and elevational shifts might be happening simultaneously, and larger, more complex modelled landscapes could be used to explore the implications of this.
This model, like many others (Atkins and Travis 2010; Bocedi et al. 2013; Kubisch et al. 2013), uses a single diploid genetic locus governing adaptation to climate. This is an oversimplification, as most traits involved in climate responses are affected by multiple genes (Neale and Savolainen 2004; Weinig et al. 2014). It is perhaps best to think of each genotype as a population—for example, that A1A1 represents a cold-adapted population, A3A3 a heat-adapted population and A1A3 an intermediate hybrid. In the case of the plastic species, no evolution could take place because it was represented by a single genotype with a fixed response to the environment. This was done to provide a simple contrast with the initially locally adapted, multi-genotype species. However, plasticity itself is a trait that can evolve, and which can have interesting interactions with local adaption, as other models have explored (e.g. Via and Lande 1985; Tufto 2000; Lande 2009; Gomez-Mestre and Jovani 2013).
The scale of the response curves to the environment was, in these simulations, assumed to be symmetrical for each genotype and the same breadth regardless of whether the species was an annual, a perennial or a tree. This assumption was made in the interest of focusing on the difference between the locally adapted and plastic scenarios. However, it would be worthwhile examining the implications of these assumptions in future studies. While response curves in provenance studies are often fit using symmetrical functions, these may not be ideal in all scenarios (Leites et al. 2012; Browne et al. 2019; Sebastian-Azcona et al. 2019). In terms of the breadth of the response curves, local adaptation has been detected in herbaceous plants over 1000–2000 m elevation gradients (e.g. Halbritter et al. 2015). We know that in trees fitness-relevant traits such as onset of cold hardiness, early-life relative growth rate or height at a particular age can, for a particular provenance, vary substantially between sites differing in temperature by 5–10 °C (e.g. Leites et al. 2012; Browne et al. 2019; Sebastian-Azcona et al. 2019). Genetic association analyses have detected differences in allele frequencies linked to environmental gradients in wind-pollinated pines in mountainous environments based on samples taken 5–45 km apart (Eckert et al. 2015; Kolb et al. 2016). The breadth of each genotype’s tolerance in the locally adapted tree scenarios might therefore be a bit narrow. This would likely serve to increase the apparent difference between the locally adapted and plastic scenarios for this life history category.
In this analysis, the number of alleles was fixed. Other similar models have allowed for mutation, but this might lead to overly optimistic estimates of adaptive potential, as the effect of one new allele in a single-locus model can be quite strong. For instance, Kubisch et al. (2013) found that allowing for mutation in the climate response alleles could lead to complete occupation of the landscape. Moreover, adaptation from standing variation is generally considered more likely, especially when generation times are long and the rate of environmental change is rapid (Barrett and Schluter 2008). Some studies have modelled individual traits such as budburst timing with explicit multi-allelic genetic models and examined the effect on range occupancy or fit of traits to the environment (Kramer et al. 2010; Kuparinen et al. 2010; Cotto et al. 2017). In such models, the presence of genetic variation and the possibility of local adaptation also tend to ameliorate, though not eliminate, negative climate change effects on populations.
The results of this simulation suggest that local adaptation in plants tends to decrease lags in tracking climate change if it boosts fitness near the advancing range edge. While local adaptation is common, less is known about how fitness changes across a species range relative to environmental gradients. Several recent analyses have found that, contrary to the ‘abundant centre’ hypothesis, most species examined do not have higher population sizes in the centre of their geographic or climatic ranges, though edge populations may be more patchy on the landscape (Abeli et al. 2014; Dallas et al. 2017; Pironon et al. 2017). Seed production was similar across the range in ~50 % of plants examined (Abeli et al. 2014). Over a wide range of species, including plants, Pironon et al. (2017) found no consistent centre vs. edge pattern to any vital rates, including survival and fecundity. Few studies have sampled extensively enough to reveal how sharply demographic rates change as one approaches a range boundary. Some exceptions include Jump and Woodward (2003), Samis and Eckert (2007), Vaupel and Mattheis (2012) and Moeller et al. (2012). Of the demographic rates examined, many show no pattern while those that do often show linear declines with some measure of ‘marginality’. However, it would be useful to re-analyse such data sets in terms of environmental distance rather than physical distance.
Finally, are the pollen and seed dispersal kernels used in the model realistic? The seed dispersal kernels were inspired by a previous spread analysis based on actual species traits (Lustenhower et al. 2017). As that paper noted, there are multiple ways that seed dispersal has been measured, not all of which are directly comparable, and many plant species lack accessible data on this and other relevant parameters. Pollen dispersal distances are even less reported on. While this analysis held pollen dispersal for a life history class constant, there is likely variation in pollen dispersal within such groups. Both can also vary due to changes in biotic or abiotic dispersal vectors. For instance, pollinator behaviour can be one of the factors limiting plant reproduction at range boundaries (Moeller et al. 2012). However, all else being equal, longer seed dispersal distances should aid species in tracking climate shifts, while very limited dispersal will hamper them. The effects of pollen dispersal are likely to be more complicated, as extensive pollen movement may hamper local adaptation and would not directly aid spread, since pollen cannot ‘outrun’ the leading edge of the population.
Based on the results of these simulations and the unknowns outlined above, I would propose several avenues of research that may aid in predicting how well plant species will track climate change, and which may be at risk.
1) Examination of local adaptation in annual species that have poor dispersal and/or highly fragmented ranges, and comparison of observed range shifts in such species to co-occurring species with more effective dispersal mechanisms.
2) Comparison of predicted shifts in climate gradients to generation time and average dispersal distances of tree species. Species in which the predicted climate shift over a generation is greater than 10 times the average dispersal distance may be most at risk, and could be targeted for further study.
3) Simulation analyses of predicted spread rates based on trait values through actual landscapes—including patchy habitat suitability and competition—that can be compared to observed shifts (such as those documented in Lenoir et al. (2008) or Beckage et al. (2008)). This would help identify the most important factors that interact with dispersal, time to maturity and local adaptation to speed up or slow down shifts.
Supporting Information
The following additional information is available in the online version of this article—
Appendix S1. Model details.
Appendix S2. Additional model results.
Data
Model code is available on GitHub: https://github.com/emoran5/LocalAdaptation_RangeShifts
Sources of Funding
Models were run on the MERCED computer cluster at the University of California Merced (supported by NSF Award ACI-1429783).
Conflict of Interest
None declared.
Acknowledgements
Thanks to Jason Sexton, Mengjun Shu, Dean Wu and Jeffrey Lauder for their comments on the manuscript.
Literature Cited
- Abeli T, Gentili R, Mondoni A, Orsenigo S, Rossi G. 2014. Effects of marginality on plant population performance. Journal of Biogeography 41:239–249. [Google Scholar]
- Aitken SN, Whitlock MC. 2013. Assisted gene flow to facilitate local adaptation to climate change. Annual Review of Ecology, Evolution, and Systematics 44:367–388. [Google Scholar]
- Angert AL, Sheth SN, Paul JR. 2011. Incorporating population-level variation in thermal performance into predictions of geographic range shifts. Integrative and Comparative Biology 51:733–750. [DOI] [PubMed] [Google Scholar]
- Atkins KE, Travis JM. 2010. Local adaptation and the evolution of species’ ranges under climate change. Journal of Theoretical Biology 266:449–457. [DOI] [PubMed] [Google Scholar]
- Barrett RD, Schluter D. 2008. Adaptation from standing genetic variation. Trends in Ecology & Evolution 23:38–44. [DOI] [PubMed] [Google Scholar]
- Beckage B, Osborne B, Gavin DG, Pucko C, Siccama T, Perkins T. 2008. A rapid upward shift of a forest ecotone during 40 years of warming in the Green Mountains of Vermont. Proceedings of the National Academy of Sciences of the United States of America 105:4197–4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell G, Gonzalez A. 2009. Evolutionary rescue can prevent extinction following environmental change. Ecology Letters 12:942–948. [DOI] [PubMed] [Google Scholar]
- Bocedi G, Atkins KE, Liao J, Henry RC, Travis JM, Hellmann JJ. 2013. Effects of local adaptation and interspecific competition on species’ responses to climate change. Annals of the New York Academy of Sciences 1297:83–97. [DOI] [PubMed] [Google Scholar]
- Bridle JR, Gavaz S, Kennington WJ. 2009. Testing limits to adaptation along altitudinal gradients in rainforest Drosophila. Proceedings of the Royal Society B: Biological Sciences 276:1507–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridle JR, Polechová J, Kawata M, Butlin RK. 2010. Why is adaptation prevented at ecological margins? New insights from individual-based simulations. Ecology Letters 13:485–494. [DOI] [PubMed] [Google Scholar]
- Bridle JR, Vines TH. 2006. Limits to evolution at range margins: when and why does adaptation fail? Trends in Ecology & Evolution 22:140–147. [DOI] [PubMed] [Google Scholar]
- Browne L, Wright JW, Fitz-Gibbon S, Gugger PF, Sork VL. 2019. Adaptational lag to temperature in valley oak (Quercus lobata) can be mitigated by genome-informed assisted gene flow. Proceedings of the National Academy of Sciences of the United States of America 116:25179–25185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark JS. 1998. Why trees migrate so fast: confronting theory with dispersal biology and the paleorecord. The American Naturalist 152:204–224. [DOI] [PubMed] [Google Scholar]
- Clark JS, Lewis M, Horvath L. 2001. Invasion by extremes: population spread with variation in dispersal and reproduction. The American Naturalist 157:537–554. [DOI] [PubMed] [Google Scholar]
- Clark CJ, Poulsen JR, Bolker BM, Connor EF, Parker VT. 2005. Comparative seed shadows of bird-, monkey-, and wind-dispersed trees. Ecology 86:2684–2694. [Google Scholar]
- Clark JS, Silman M, Kern R, Macklin E, Lambers JHR. 1999. Seed dispersal near and far: patterns across temperate and tropical forests. Ecology 80:1475–1494. [Google Scholar]
- Colautti RI, Barrett SC. 2013. Rapid adaptation to climate facilitates range expansion of an invasive plant. Science 342:364–366. [DOI] [PubMed] [Google Scholar]
- Cotto O, Wessely J, Georges D, Klonner G, Schmid M, Dullinger S, Thuiller W, Guillaume F. 2017. A dynamic eco-evolutionary model predicts slow response of alpine plants to climate warming. Nature Communications 8:15399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousens R, Dytham C, Law R.. 2008. Dispersal in plants: a population perspective. New York: Oxford University Press. [Google Scholar]
- Csilléry K, Lalagüe H, Vendramin GG, González-Martínez SC, Fady B, Oddou-Muratorio S. 2014. Detecting short spatial scale local adaptation and epistatic selection in climate-related candidate genes in European beech (Fagus sylvatica) populations. Molecular Ecology 23:4696–4708. [DOI] [PubMed] [Google Scholar]
- Dallas T, Decker RR, Hastings A. 2017. Species are not most abundant in the centre of their geographic range or climatic niche. Ecology Letters 20:1526–1533. [DOI] [PubMed] [Google Scholar]
- Dawson MN, Grosberg RK, Stuart YE, Sanford E. 2010. Population genetic analysis of a recent range expansion: mechanisms regulating the poleward range limit in the volcano barnacle Tetraclita rubescens. Molecular Ecology 19:1585–1605. [DOI] [PubMed] [Google Scholar]
- Dodd ME, Silvertown J. 2000. Size-specific fecundity and the influence of lifetime size variation upon effective population size in Abies balsamea. Heredity 85:604–609. [DOI] [PubMed] [Google Scholar]
- Eckert AJ, Maloney PE, Vogler DR, Jensen CE, Mix AD, Neale DB. 2015. Local adaptation at fine spatial scales: an example from sugar pine (Pinus lambertiana, Pinaceae). Tree Genetics & Genomes 11:42–58. [Google Scholar]
- Franklin J, Davis FW, Ikegami M, Syphard AD, Flint LE, Flint AL, Hannah L. 2013. Modeling plant species distributions under future climates: how fine scale do climate projections need to be? Global Change Biology 19:473–483. [DOI] [PubMed] [Google Scholar]
- Garant D, Forde SE, Hendry AP. 2007. The multifarious effects of dispersal and gene flow on contemporary adaptation. Functional Ecology 21:434–443. [Google Scholar]
- Garzon BM, Alia R, Robson TM, Zavala MA. 2011. Intra-specific variability and plasticity influence potential tree species distributions under climate change. Global Ecology & Biogeography 20:766–778. [Google Scholar]
- Gomez-Mestre I, Jovani R. 2013. A heuristic model on the role of plasticity in adaptive evolution: plasticity increases adaptation, population viability, and genetic variation. Proceedings of the Royal Society B: Biological Sciences 280:20131869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalo-Turpin H, Hazard L. 2009. Local adaptation occurs along altitudinal gradient despite the existence of gene flow in the alpine plant species Festuca eskia. Journal of Ecology 97:742–751. [Google Scholar]
- Halbritter AH, Billeter R, Edwards PJ, Alexander JM. 2015. Local adaptation at range edges: comparing elevation and latitudinal gradients. Journal of Evolutionary Biology 28:1849–1860. [DOI] [PubMed] [Google Scholar]
- Hereford J. 2009. A quantitative survey of local adaptation and fitness trade-offs. The American Naturalist 173:579–588. [DOI] [PubMed] [Google Scholar]
- Herrera CM. 1991. Dissecting factors responsible for individual variation in plant fecundity. Ecology 72:1436–1448. [Google Scholar]
- Holt RD, Knight TM, Barfield M. 2004. Allee effects, immigration, and the evolution of species’ niches. The American Naturalist 163:253–262. [DOI] [PubMed] [Google Scholar]
- IPCC 2013. Climate change 2013: the physical science basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge and New York: Cambridge University Press. [Google Scholar]
- Jump AS, Woodward FI. 2003. Seed production and population density decline approaching the range-edge of Cirsium species. The New Phytologist 160:349–358. [DOI] [PubMed] [Google Scholar]
- Kawecki TJ. 2008. Adaptation to marginal habitats. Annual Review of Ecology, Evolution, and Systematics 39:321–342. [Google Scholar]
- Kawecki TJ, Ebert D. 2004. Conceptual issues in local adaptation. Ecology Letters 7:1225–1241. [Google Scholar]
- Keller SR, Soolanayakanahally RY, Guy RD, Silim SN, Olson MS, Tiffin P. 2011. Climate-driven local adaptation of ecophysiology and phenology in balsam poplar, Populus balsamifera L. (Salicaceae). American Journal of Botany 98:99–108. [DOI] [PubMed] [Google Scholar]
- Kimbrell T, Holt RD. 2007. Canalization breakdown and evolution in a source-sink system. The American Naturalist 169:370–382. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick M, Barton NH. 1997. Evolution of a species’ range. The American Naturalist 150:1–23. [DOI] [PubMed] [Google Scholar]
- Kolb TE, Grady KC, McEttrick MP, Herrero A. 2016. Local-scale drought adaptation of ponderosa pine seedlings at habitat ecotones. Forest Science 62:641–651. [Google Scholar]
- Kramer K, Degen B, Buschbom J, Hickler T, Thuiller W, Sykes MT, de Winter W. 2010. Modelling exploration of the future of European beech (Fagus sylvatica L.) under climate change - range, abundance, genetic diversity and adaptive response. Forest Ecology and Management 259:2213–2222. [Google Scholar]
- Kremer A, Ronce O, Robledo-Arnuncio JJ, Guillaume F, Bohrer G, Nathan R, Bridle JR, Gomulkiewicz R, Klein EK, Ritland K, Kuparinen A, Gerber S, Schueler S. 2012. Long-distance gene flow and adaptation of forest trees to rapid climate change. Ecology Letters 15:378–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroiss SJ, HilleRisLambers J. 2015. Recruitment limitation of long-lived conifers: implications for climate change responses. Ecology 96:1286–1297. [DOI] [PubMed] [Google Scholar]
- Kubisch A, Degen T, Hovestadt T, Poethke HJ. 2013. Predicting range shifts under global change: the balance between local adaptation and dispersal. Ecography 36:873–882. [Google Scholar]
- Kuparinen A, Savolainen O, Schurr FM. 2010. Increased mortality can promote evolutionary adaptation of forest trees to climate change. Forest Ecology and Management 259:1003–1008. [Google Scholar]
- Lande R. 2009. Adaptation to an extraordinary environment by evolution of phenotypic plasticity and genetic assimilation. Journal of Evolutionary Biology 22:1435–1446. [DOI] [PubMed] [Google Scholar]
- Leimu R, Fischer M. 2008. A meta-analysis of local adaptation in plants. PLoS One 3:e4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leites LP, Rehfeldt GE, Robinson AP, Crookston NL, Jaquish B. 2012. Possibilities and limitations of using historic provenance tests to infer forest species growth responses to climate change. Natural Resource Modeling 25:409–433. [Google Scholar]
- Lenoir J, Gégout JC, Marquet PA, de Ruffray P, Brisse H. 2008. A significant upward shift in plant species optimum elevation during the 20th century. Science 320:1768–1771. [DOI] [PubMed] [Google Scholar]
- Lenormand T. 2002. Gene flow and the limits to natural selection. Trends in Ecology & Evolution 17:183–189. [Google Scholar]
- Loarie SR, Duffy PB, Hamilton H, Asner GP, Field CB, Ackerly DD. 2009. The velocity of climate change. Nature 462:1052–1055. [DOI] [PubMed] [Google Scholar]
- Lustenhower N, Moran EV, Levine JM. 2017. Trait correlations equalize spread rates across plant life histories. Global Ecology & Biogeography 26:1398–1407. [Google Scholar]
- Maguire KC, Shinneman DJ, Potter KM, Hipkins VD. 2018. Intraspecific niche models for ponderosa pine (Pinus ponderosa) suggest potential variability in population-level response to climate change. Systematic Biology 67:965–978. [DOI] [PubMed] [Google Scholar]
- McLachlan JS, Clark JS, Manos PS. 2005. Molecular indicators of tree migration capacity under rapid climate change. Ecology 86:2088–2098. [Google Scholar]
- Meyer AH, Schmid B. 1999. Seed dynamics and seedling establishment in the invading perennial Solidago altissima under different experimental treatments. Journal of Ecology 87:28–41. [Google Scholar]
- Moeller DA, Geber MA, Eckhart VM, Tiffin P. 2012. Reduced pollinator service and elevated pollen limitation at the geographic range limit of an annual plant. Ecology 93:1036–1048. [DOI] [PubMed] [Google Scholar]
- Moran EV, Clark JS. 2012. Between-site differences in the scale of dispersal and gene flow in red oak. PLoS One 7:e36492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran EV, Das AJ, Keeley J, Stephenson NL. 2019. Negative impacts of summer heat on Sierra Nevada tree seedlings. Ecosphere 10:e02776. [Google Scholar]
- Moran EV, Ormond RA. 2015. Simulating the interacting effects of intraspecific variation, disturbance, and competition on climate-driven range shifts in trees. PLoS One 10:e0142369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran EV, Reid A, Levine JM. 2017. Population genetics and adaptation to climate along elevation gradients in invasive Solidago canadensis. PLoS One 12:e0185539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan R, Horvitz N, He Y, Kuparinen A, Schurr FM, Katul GG. 2011. Spread of North American wind-dispersed trees in future environments. Ecology Letters 14:211–219. [DOI] [PubMed] [Google Scholar]
- Neale DB, Savolainen O. 2004. Association genetics of complex traits in conifers. Trends in Plant Science 9:325–330. [DOI] [PubMed] [Google Scholar]
- North A, Pennanen J, Ovaskainen O, Laine AL. 2010. Local adaptation in a changing world: the roles of gene-flow, mutation, and sexual reproduction. Evolution 65:79–89. [DOI] [PubMed] [Google Scholar]
- Oney B, Reineking B, O’Neill G, Kreyling J. 2013. Intraspecific variation buffers projected climate change impacts on Pinus contorta. Ecology and Evolution 3:437–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pironon S, Papuga G, Villellas J, Angert AL, García MB, Thompson JD. 2017. Geographic variation in genetic and demographic performance: new insights from an old biogeographical paradigm. Biological Reviews of the Cambridge Philosophical Society 92:1877–1909. [DOI] [PubMed] [Google Scholar]
- Polechová J, Barton NH. 2015. Limits to adaptation along environmental gradients. Proceedings of the National Academy of Sciences of the United States of America 112:6401–6406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Räsänen K, Hendry AP. 2008. Disentangling interactions between adaptive divergence and gene flow when ecology drives diversification: adaptive divergence and gene flow. Ecology Letters 11:624–636. [DOI] [PubMed] [Google Scholar]
- Samis KE, Eckert CG. 2007. Testing the abundant center model using range-wide demographic surveys of two coastal dune plants. Ecology 88:1747–1758. [DOI] [PubMed] [Google Scholar]
- Sebastian-Azcona J, Hamann A, Hacke UG, Rweyongeza D. 2019. Survival, growth and cold hardiness tradeoffs in white spruce populations: implications for assisted migration. Forest Ecology and Management 433:544–552. [Google Scholar]
- Sexton JP, Hangartner SB, Hoffmann AA. 2014. Genetic isolation by environment or distance: which pattern of gene flow is most common? Evolution 68:1–15. [DOI] [PubMed] [Google Scholar]
- Sexton JP, McIntyre PJ, Angert AL, Rice KJ. 2009. Evolution and ecology of species range limits. Annual Review of Ecology, Evolution, and Systematics 40:415–436. [Google Scholar]
- Sexton JP, Strauss SY, Rice KJ. 2011. Gene flow increases fitness at the warm edge of a species’ range. Proceedings of the National Academy of Sciences of the United States of America 108:11704–11709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tufto J. 2000. The evolution of plasticity and nonplastic spatial and temporal adaptations in the presence of imperfect environmental cues. The American Naturalist 156:121–130. [DOI] [PubMed] [Google Scholar]
- Vaupel A, Matthies D. 2012. Abundance, reproduction, and seed predation of an alpine plant decrease from the center toward the range limit. Ecology 93:2253–2262. [DOI] [PubMed] [Google Scholar]
- Via S, Lande R. 1985. Genotype-environment interaction and the evolution of phenotypic plasticity. Evolution 39:505–522. [DOI] [PubMed] [Google Scholar]
- Weinig C, Ewers BE, Welch SM. 2014. Ecological genomics and process modeling of local adaptation to climate. Current Opinion in Plant Biology 18:66–72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.