Abstract
We performed a pooled analysis to evaluate the efficacy and adverse events (AEs) of olanzapine combined with dexamethasone plus 5-hydroxytryptamine type 3 receptor antagonist (5-HT3 RA) compared with 5-HT3 RA plus dexamethasone for the prevention and treatment of chemotherapy-induced nausea and vomiting (CINV) in high and moderate emetogenic chemotherapy based on randomised controlled trials (RCTs). PubMed, EMBASE, Web of Science, the Cochrane Library, China Biomedical Literature database (CBM), WanFang Database, China National Knowledge Infrastructure (CNKI), and Chinese Science and Technology Periodical Database (VIP) (from their inception to April 2019) were searched to capture relevant articles. Relative risk with 95% confidence intervals for CINV and AEs were all extracted or calculated. Eleven studies with 1107 cancer patients were involved in this review. The pooled RR of delayed CINV (RR 0.50, 95% CI 0.38 to 0.66; p<0.01) were significantly decreased in the olanzapine group. The occurrence of insomnia was also statistically decreased, as was the rate of acute CINV (RR 0.60, 95% CI 0.48 to 0.75; p<0.01). However, only the percentages of CINV III and CINV IV were significantly decreased in the acute and delayed phases. Subgroup analysis demonstrated that the efficacy was not statistically significantly different between 5 mg and 10 mg olanzapine. Olanzapine significantly decreased the occurrence of CINV III and IV and insomnia in high and moderately emetogenic chemotherapy. Compared with 10 mg per day, 5 mg oral olanzapine may be more appropriate for patients with cancer.
Keywords: olanzapine, 5-HT3 RA, dexamethasone, chemotherapy-induced nausea and Vomiting, meta-analysis
Introduction
Chemotherapy-induced nausea and vomiting (CINV) is a common adverse effect (AE) in the treatment of cancer, which can significantly impair the patient’s quality of life, resulting in poor compliance and malnutrition.1 The incidence of acute CINV (occurrence within 24 hours of administration of chemotherapy) was found to be 36% and for delayed CINV it was 59% (2–5 days after the administration of chemotherapy).2 While 5-hydroxytryptamine type 3 receptor antagonist (5-HT3 RA),3 dexamethasone, and neurokinin-1 receptor antagonist (NK-1 RA)4 have significantly decreased the incidence of CINV, 54.7% of patients still experience nausea when these agents are used.5
The US Food and Drug Administration (FDA) approved olanzapine as an antipsychotic agent that blocks α1 adrenergic receptors, serotonin 5-HT2a, 5-HT2c, 5-HT3, 5-HT6 receptors, muscarinic receptors, dopamine D1, D2, D3 receptors, and histamine H1 receptors.5 Randomised controlled trials (RCTs)6 7 and meta-analyses8–10 have suggested that olanzapine is useful for patients with cancer who receive chemotherapy. The 2016 Multinational Association of Supportive Care in Cancer/European Society for Medical Oncology (MASCC/ESMO) guidelines11 recommend olanzapine combined with 5-HT3 RA plus dexamethasone to prevent CINV, but the level of recommendation was graded low. The National Comprehensive Cancer Network (NCCN) guidelines for antiemesis (version 1.2019) also recommend olanzapine combined with a 5-HT3 RA plus dexamethasone after NK-1 RA for high and moderate emetogenic chemotherapy; however, 5 mg per day was considered only when 10 mg per day caused serious sedation. RCTs12 13 demonstrated 5 mg oral olanzapine per day to be effective, and a randomised phase 2 dose-finding study14 even revealed that 5 mg had a higher complete control in delayed CINV compared with 10 mg (83.1% vs 77.6%). Meta-analysis15 also found that both 5 mg and 10 mg olanzapine exhibited similar efficacy. However, three (olanzapine, 5-HT3 RA and dexamethasone) or four (olanzapine, 5-HT3 RA, NK-1 RA and dexamethasone) drugs were used and the level of CINV was not reported. Therefore, we designed this review to evaluate the efficacy and AEs of olanzapine combined with 5-HT3 RA plus dexamethasone compared with 5-HT3 RA plus dexamethasone for the prevention and treatment of CINV in high and moderately emetogenic chemotherapy based on currently available studies.
Methods
This review was performed following the Cochrane Handbook for Systematic Reviews of Interventions. Moreover, we reported pooled results by the recommendations listed in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) harms checklist16 statement (online supplementary data 1).
esmoopen-2019-000621supp001.pdf (127.5KB, pdf)
Search strategy
PubMed, Web of Science, the Cochrane Library, EMBASE, WanFang Database, China National Knowledge Infrastructure (CNKI), Chinese Science and Technology Periodical Database (VIP) and China Biomedical Literature database (CBM) (from corresponding inception to April 2019) were searched using the following terms: “olanzapine”, “chemotherapy-induced nausea and vomiting”, “CINV”, “emesis”, “nausea”. The search strategy for PubMed is documented in online supplementary data 2. Language or date restrictions were not imposed. The bibliographies of relevant trials and reviews were also browsed. Two researchers (LH and CX) conducted this part independently, and any inconsistency was solved by consulting a third researcher (HM).
esmoopen-2019-000621supp002.pdf (32.8KB, pdf)
Study inclusion
Two reviewers (J-GZ and LH) independently screened the studies based on inclusion and exclusion criteria. Any inconsistency was resolved by the third investigator (HM). Eligible studies had to meet the following criteria: (1) population—cancer patients who had received high and moderately emetogenic chemotherapy classified by MASCC/ESMO guidelines11; (2) intervention—olanzapine combined with 5-HT3 RA plus dexamethasone for the prevention and treatment of CINV; (3) comparison—dexamethasone plus 5-HT3 RA for the prevention and treatment of CINV; (4) outcome—primary endpoint: the percentage delay of CINV; secondary endpoint: AEs; (5) study type—only RCTs were considered.
Exclusion criterion
Exclusion criteria were: (1) patients with no solid tumours; (2) use of other antiemetic drugs (ie, NK-1 RA); (3) letters to the editor or meeting abstracts.
Data extraction
Two researchers (S-HJ and CX) evaluated the eligibility of all identified studies by checking abstracts and titles fitting the inclusion and exclusion criteria, and full-text were carefully checked. Data involved authors, country, population, publication year, drug dosage and duration, the percentage of acute and delayed CINV, and AEs. All divergences about eligibility assessment and data extraction were resolved by consulting a third senior investigator (HM).
Assessment of methodologic quality
The methodologic quality was appraised using the Cochrane Collaboration’s Risk Bias assessment tool for assessing the risk of bias17 by two researchers (J-GZ and CX), and RevMan (version 5.3) was used in this part. Seven domains (blinding of outcome assessment, blinding of participants and personnel, selective reporting, allocation concealment, random-sequence generation, incomplete outcome data, and other bias) were assessed for each study, and all of them were labelled as “high risk”, “unclear risk” or “low risk”.
Statistical analysis
The dichotomous outcomes were estimated using relative risk (RR) with 95% confidence intervals (95% CI). I2 statistic and p value were used to estimate the level of heterogeneity of involved studies.18 We considered heterogeneity substantial if I 2 ≥50% or p<0.10.19 A fixed model was used when I 2 <50% or p>0.10; if significant heterogeneity was conducted, a random model was used. If noticeable differences were found in clinical characteristics and methodology, regardless of I 2 statistic or p value, a belief of qualitative analysis was conducted. An approximate standard error of the log rate ratio is given by SE of ln rate ratio=. A correction of 0.5 might be added to each count in the case of zero events (section 9.4.8 of the Cochrane Handbook version 5.1.0). Subgroup analysis of olanzapine dosage (5 mg vs 10 mg) was also conducted. Begg, Egger regression20 21 and LFK index22 (using MetaXL, www.epigear.com) were used to detect potential publication bias. All analyses used R (version 3.3.0) and the M eta package.
Results
Literature research and characteristics of studies
As shown in online supplementary data 3, a total of 787 studies of olanzapine for the prevention and treatment CINV were identified, and 11 articles12 13 23–31 were evaluated in our review according to the set criteria.
esmoopen-2019-000621supp003.pdf (600.2KB, pdf)
All involved studies were published from 2009 to 2018; 1107 patients with cancer were included in this review, with 561 patients in the olanzapine group and 546 patients in the control group, respectively. Except for Mukhopadhyay et al,23 all studies were from China. All patients with solid tumours received high or moderated emetogenic chemotherapy. Olanzapine combined with 5-HT3 RA plus dexamethasone was compared with 5-HT3 RA plus dexamethasone for the prevention and treatment of CINV. Two different olanzapine dosages were used in the intervention group: six studies23–25 28 30 31 used 10 mg oral once a day, and four studies12 13 26 29 used 5 mg oral once a day; Meng et al 27 used 2.5 mg oral twice a day. Six studies12 13 25–28 reported CINV according to the WHO classification standards for gastrointestinal reactions of anticancer drugs: CINV I (nausea without vomiting), CINV II (vomiting 1–2 times a day, does not affect eating), CINV III (nausea and vomiting 3–5 times daily, affects eating, needed to treat), and CINV IV (uncontrollable nausea and vomiting, more than five times) were reported in the articles. Only Wang et a l,28 Guo and Lin13 and Meng et a l 27 used National Cancer Institute Common Terminology Criteria for Adverse Events (NCICTCAE) 4.0 to evaluate the AEs; the others did not provide the definition of all related AEs. The main characteristics of the involved studies are presented in table 1.
Table 1.
Main characteristics of the included studies
| Studies | Year | Sample size | Intervention | Comparison |
| Zhao | 2018 | 54 | OLA 10 mg oral once daily d1–5, granisetron 3 mg iv once daily d1–3 DEX 10 mg iv once daily d1–3 |
Granisetron 3 mg iv once daily d1–3 DEX 10 mg iv once daily d1–3 |
| He | 2018 | 101 | OLA 5 mg oral once daily d1–7, tropisetron 5 mg iv once daily d1–7 DEX 10 mg iv once daily d1–7 |
Tropisetron 5 mg iv once daily d1–7 DEX 10 mg iv once daily d1–7 |
| Guo | 2018 | 60 | OLA 5 mg oral, tropisetron 5 mg iv, DEX 10 mg iv | Tropisetron 5 mg iv, DEX 10 mg iv |
| Tian | 2017 | 100 | OLA 10 mg oral once daily d1–2, tropisetron 5 mg once daily d1–2 DEX 10 mg oral once daily d1–2 |
Tropisetron 5 mg once daily d1–2 DEX 10 mg oral once daily d1–2 |
| Liu | 2017 | 117 | OLA 5 mg oral once daily d1–4, tropisetron 5 mg iv once daily d1 DEX 5 mg iv once daily d1 |
Tropisetron 5 mg iv once daily d1 DEX 5 mg iv once daily d1 |
| Sandip | 2017 | 100 | OLA 10 mg oral once daily d1–5, palonosetron 0.25 mg iv once daily d1, DEX 8 mg or 16 mg iv once daily d1, DEX 8 mg oral once or twice daily d2–5 | Palonosetron 0.25 mg iv once daily d1, DEX 8 mg or 16 mg iv once daily d1, DEX 8 mg oral once or twice daily d2–5 |
| Wang | 2016 | 60 | OLA 10 mg oral once daily d1–4, granisetron 5 mg iv, DEX 5 mg iv | Granisetron 5 mg iv, DEX 5 mg iv |
| Meng | 2016 | 120 | OLA 2.5 mg oral twice daily d1–5, granisetron 5 mg iv, DEX 5 mg iv | Granisetron 5 mg iv, DEX 5 mg iv |
| Li | 2015 | 80 | OLA 5 mg oral once daily, tropisetron 5 mg once daily, DEX 10 mg oral once daily | Tropisetron 5 mg iv once daily DEX 5 mg iv once daily |
| Chen | 2015 | 86 | OLA 10 mg oral once daily d1–5, tropisetron 5 mg iv once daily d1–5, DEX 10 mg iv once daily d1–5 | Tropisetron 5 mg iv once daily d1–5, DEX 10 mg iv once daily d1–5 |
| Tian | 2009 | 229 | OLA 10 mg oral once daily d1–5, azasetron 5 mg iv once daily d1–5, DEX 10 mg iv once daily d1–5 | Azasetron 5 mg iv once daily d1–5, DEX 10 mg iv once daily d1–5 |
d, day(s); DEX, dexamethasone; iv, intravenously; OLA, olanzapine.
Assessing risk of methodologic quality
The detail of the risk-of-bias assessment is summarised in online supplementary data 4. Except for Sandip et al,23 all studies were open-label randomised studies and assessed as high risk in allocation concealment. Four studies12 23 24 31 reported the detail of the randomised method, and the low risk was designated. Incomplete outcome data and selective reporting for all studies were assessed as low risk. For all studies the overall methodologic quality was accepted.
esmoopen-2019-000621supp004.pdf (1MB, pdf)
Efficacy of olanzapine for prevention and treatment of delayed CINV
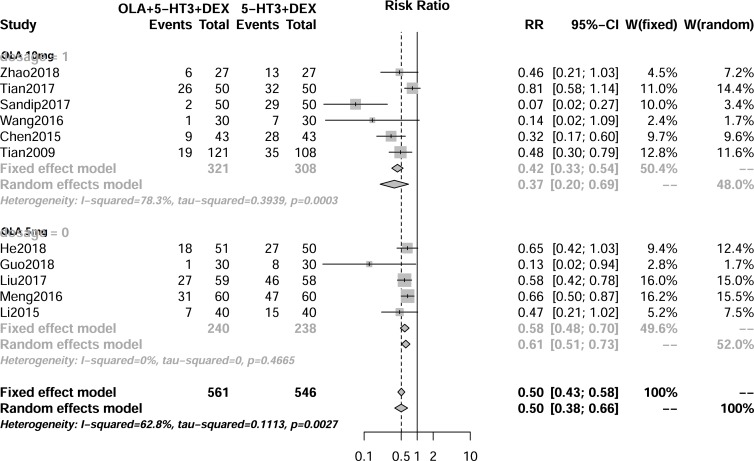
As shown in figure 1, 11 RCTs (1107 cancer patients) reported delayed CINV, and significant heterogeneity (I 2=68%, p<0.1) was conducted. Therefore, a random model was used. There was a significantly lower rate of delayed CINV (RR 0.50, 95% CI 0.38 to 0.66; p<0.01) when olanzapine was added to 5-HT3 RA and dexamethasone. Six articles (table 2) reported delayed CINV. CINV III (RR 0.27, 95% CI 0.14 to 0.52; p<0.01) and CINV IV (RR 0.16, 95% CI 0.04 to 0.60; p<0.01) were significantly decreased in the olanzapine group. Subgroup analysis (table 3) showed that there was no statistical difference between 5 mg and 10 mg olanzapine for delayed CINV.
Figure 1.
Result of the meta-analysis of the relative risk of delayed chemotherapy-induced nausea and vomiting. DEX, dexamethasone; 5-HT3 RA, 5-hydroxytryptamine type 3 receptor antagonist; OLA, olanzapine.
Table 2.
Results of the meta-analysis of the relative risk of the level of acute and delayed chemotherapy-induced nausea and vomiting (CINV)
| CINV | No. of studies | No. of patients | Mode | RR and 95% CI | Heterogeneity | P value for RR | |
| P value | I2 (%) | ||||||
| Acute CINV | 10 | 502/488 | Fixed | 0.60 (0.48 to 0.75) | 0.90 | 0.0 | <0.01 |
| Acute CINVI | 6 | 254/253 | Fixed | 1.19 (0.76 to 1.87) | 0.43 | 0.0 | 0.44 |
| Acute CINVII | 6 | 254/253 | Fixed | 0.66 (0.38 to 1.17) | 0.99 | 0.0 | 0.16 |
| Acute CINV III | 6 | 254/253 | Fixed | 0.26 (0.13 to 0.54) | 0.64 | 0.00 | <0.01 |
| Acute CINV IV | 3 | 151/150 | Fixed | 0.17 (0.04 to 0.73) | 0.63 | 0.0 | 0.02 |
| Delayed CINV | 11 | 561/546 | Random | 0.50 (0.38 to 0.66) | <0.01 | 62.8 | <0.01 |
| Delayed CINVI | 6 | 254/253 | Random | 0.79 (0.43 to 1.45) | 0.09 | 47.8 | 0.44 |
| Delayed CINVII | 6 | 254/253 | Fixed | 0.61 (0.35 to 1.05) | 0.88 | 0.0 | 0.07 |
| Delayed CINV III | 6 | 254/253 | Fixed | 0.27 (0.14 to 0.52) | 0.99 | 0.0 | <0.01 |
| Delayed CINV IV | 5 | 211/210 | Fixed | 0.16 (0.04 to 0.60) | 0.91 | 0.0 | <0.01 |
Table 3.
Results of subgroup analysis about dose of olanzapine
| Olanzapine dose | No. of studies | No. of patients | Mode | RR and 95% CI | Heterogeneity | P value for two groups | |
| P value | I2 (%) | ||||||
| Acute CINV | |||||||
| 10 mg | 6 | 321/308 | Fixed | 0.65 (0.45 to 0.95) | 0.95 | 0.0 | 0.53 |
| 5 mg | 4 | 181/180 | Fixed | 0.56 (0.43 to 0.74) | 0.45 | 0.0 | |
| Delayed CINV | |||||||
| 10 mg | 6 | 321/308 | Random | 0.37 (0.20 to 0.69) | 0.00 | 78.3 | 0.13 |
| 5 mg | 5 | 240/238 | Fixed | 0.58 (0.48 to 0.70) | 0.47 | 0.0 | |
CINV, chemotherapy-induced nausea and vomiting.
AEs of olanzapine used for prevention and treatment of CINV
There were seven AEs (dizziness, constipation, somnolence, anorexia, fatigue, insomnia, thirst) included in this systematic review for quantitative analysis. Anorexia and thirst were found to be heterogeneous. Therefore, a random model was used. For the rest of the AEs no significant heterogeneity was detected, and a fixed model was therefore used in this review. As shown in table 4, except for insomnia, the RR is 0.12 (95% CI 0.06 to 0.26; p<0.01). Taking all AEs together, no statistically significant difference was found between the two groups. The results of subgroup analysis were as follows:
Table 4.
Result of the meta-analysis of the relative risk of adverse events
| Adverse events | No. of studies | No. of patients | Mode | RR and 95% CI | P valve for RR | Heterogeneity | |
| P value | I2 (%) | ||||||
| Dizziness | 5 | 208/207 | Fixed | 1.14 (0.66 to 1.98) | 0.65 | 0.97 | 0.0 |
| Constipation | 7 | 317/315 | Fixed | 1.13 (0.87 to 1.46) | 0.35 | 0.55 | 0.0 |
| Anorexia | 2 | 101/100 | Random | 1.73 (0.13 to 23.60) | 0.68 | 0.08 | 68.1 |
| Somnolence | 4 | 206/205 | Fixed | 1.20(0.63 to 2.27) | 0.58 | 0.61 | 0.0 |
| Fatigue | 4 | 210/208 | Fixed | 0.94 (0.61 to 1.43) | 0.76 | 0.85 | 42.2 |
| Insomnia | 2 | 90/90 | Fixed | 0.12 (0.06 to 0.26) | <0.01 | 0.45 | 0.0 |
| Thirst | 2 | 119/118 | Random | 1.24 (0.65 to 2.12) | 0.60 | 0.16 | 50.5 |
Dizziness
Five studies reported the occurrence of dizziness. Since no significant heterogeneity (I 2=0.0%, p=0.97) was conducted, a fixed model was used. As shown in table 4, no significant difference was found between the two groups (RR 1.14, 95% CI 0.66 to 1.98; p=0.65). The subgroup analysis revealed that no statistically significant difference was observed between the 10 mg and 5 mg olanzapine doses (table 3).
Constipation
Seven studies reported on constipation and a fixed model was used (I 2=0.0%, p=0.25). The rate of constipation was comparable (RR 1.13, 95% CI 0.87 to 1.46; p=0.35) between the two groups. No significant difference was detected by the subgroup analysis (table 3).
Anorexia
Two studies reported data on anorexia and significant heterogeneity (I 2=68.1%, p=0.08) was found. Therefore, a random model was used. The RR of anorexia was 1.73 (95% CI 0.13 to 23.60; p=0.68). A subgroup analysis was not conducted.
Somnolence
Four studies reported data on somnolence and a fixed model (I 2=0.0%, p=0.61) was used. No significant difference was found between the two groups (RR 1.20, 95% CI 0.63 to 2.77; p=0.58). Furthermore, subgroup analysis did not detect a significant difference between the 10 mg and 5 mg olanzapine doses (table 3).
Fatigue
Four studies reported the occurrence of fatigue. Since no significant heterogeneity (I 2=42.2%, p=0.85) was seen, a fixed model was used. As shown in table 4, no significant difference was found between the groups (RR 0.94, 95% CI 0.61 to 1.43; p=0.76). All studies used 5 mg olanzapine per day and hence subgroup analysis was not conducted.
Insomnia
Two studies were included in this review and a fixed model was used (I 2=0.0%, p=0.45). As shown in table 4, the percentage of insomnia was significantly lower in the olanzapine group (RR 0.12, 95% CI 0.06 to 0.26; p<0.01). Because only two studies were involved, subgroup analysis was not conducted.
Thirst
Two studies were included and a random model was used (I 2=50.5%, p=0.16). No significant difference was found (RR 1.24, 95% CI 0.65 to 2.12; p=0.60). Because only two studies were involved, subgroup analysis was not conducted.
Efficacy of olanzapine for prevention and treatment of acute CINV
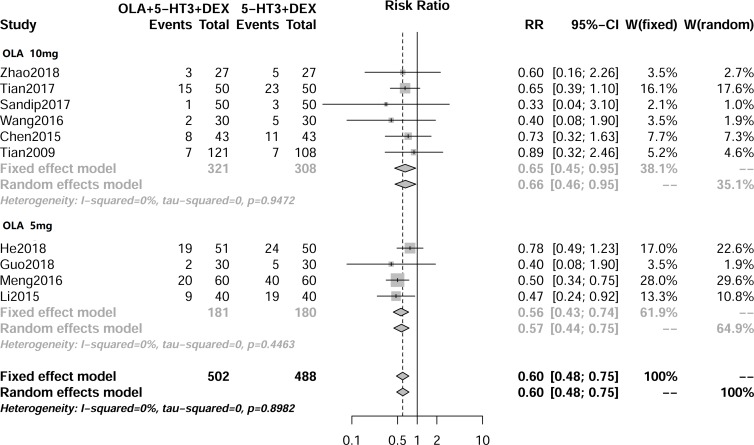
As shown in figure 2, 10 RCTs (990 cancer patients) provided data on acute CINV. Since no statistically significant difference was found, a fixed model was used. The overall pooled RR of acute CINV was 0.60 (95% CI 0.48 to 0.75; p<0.01). Six articles (table 2) reported the level of acute CINV, while only CINV III (RR 0.26, 95% CI 0.13 to 0.54; p<0.01) and CINV IV (RR 0.17, 95% CI 0.04 to 0.73; p=0.02) revealed a statistically significant difference. Subgroup analysis (table 3) showed that there was no statistically significant difference between the 5 mg and 10 mg doses of olanzapine for acute CINV.
Figure 2.
Result of the meta-analysis of the relative risk of acute chemotherapy-induced nausea and vomiting. DEX, dexamethasone; 5-HT3 RA, 5-hydroxytryptamine type 3 receptor antagonist; OLA, olanzapine.
Publication bias
The publication bias of our meta-analysis was assessed using Begg, Egger regression and LFK index. Evidence of publication bias was found from the use of these statistical tests (Begg’s test, p=0.02, Egger’s test, p<0.01, LFK=−4.90).
Discussion
Eleven studies with 1107 cancer patients were involved in this review. The key finding is that olanzapine combined with 5-HT3 RA plus dexamethasone is more effective and induces less insomnia compared with 5-HT3 RA plus dexamethasone for the prevention and treatment of CINV in high and moderately emetogenic chemotherapy. It has to be noted that, compared with 10 mg per day, 5 mg oral olanzapine may be more appropriate for cancer patients.
CINV III and CINV IV greatly reduce the quality of life of patients, and medical treatment is needed. Six studies included in our review reported the level of CINV, and the rates of CINV III and CINV IV were significantly less in the olanzapine groups. Olanzapine was still found to be effective when aprepitant failed to control CINV in patients receiving highly emetogenic chemotherapy.32 One can conclude that olanzapine is an excellent alternative for the prophylaxis of CINV. The percentage of acute and delayed CINV in the olanzapine group was lower compared with the study of Rudolph et al 5 (acute CINV: 17.1% vs 26.2%; delayed CINV: 26.2% vs 57.6%). Possible reasons may be: (1) our review involved high and moderately emetogenic chemotherapy, and Rudolph et al only included high emetogenic chemotherapy; (2) compared with Rudolph et al, the time periods of 5-HT3 RA and dexamethasone application were longer (4–5 days vs 1 day). This calls for more studies in the future.
Two different olanzapine dosages were used in this review, and subgroup analysis shows that both doses (10 mg and 5 mg olanzapine) provided comparably significant improvement in acute and delayed CINV. The phase 2 trial by Yanai et al 14 found that 5 mg of olanzapine had a higher complete control in delayed CINV compared with 10 mg (83.1% vs 77.6%). Because olanzapine may lead to central nervous system depression, especially in elderly and debilitated patients,33 5 mg per day of olanzapine should be more suitable for cancer patients.
Polled analysis was conducted with seven AEs (dizziness, constipation, somnolence, anorexia, fatigue, insomnia, thirst) in this systematic review, and the incidence of insomnia (RR 0.12, 95% CI 0.06 to 0.26; p<0.01) was found to be significantly lower in the olanzapine groups. Insomnia is the most common AE of dexamethasone34 and olanzapine is an atypical antipsychotic agent that may cause drowsiness.35 The patients with cancer had lower insomnia when olanzapine was combined with NK-1 RA and dexamethasone. At the same time, olanzapine did not increase the incidence of somnolence, while no studies reported the severity and duration of AEs. Our current RCT about olanzapine combined with tropisetron and dexamethasone for the prevention and treatment of nausea and vomiting induced by chemotherapy of patients with lung cancer (NCT03571126) concentrates on the level of AEs, and the aforementioned concern may be resolved. Subgroup analysis showed that there is no significant difference between 5 mg and 10 mg doses of olanzapine in regard to dizziness, constipation and somnolence. Therefore, 5 mg may be more appropriate. A previous phase 3 trial5 found that there were no serious adverse events related to olanzapine, and no patient discontinued olanzapine because of toxic effects. Therefore olanzapine can be regarded as being safe to use in the control of CINV.
For the first time, we analysed the level of CINV that is most important for patients and doctors. The result showed that CINV III and CINV IV were significantly decreased when olanzapine was used. This review is less biased compared with previous studies9 10 15 36 because the intervention measure is more consistent in all involved articles. Furthermore, we analysed the efficacy and AEs of olanzapine combined with 5-HT3 RA plus dexamethasone compared with 5-HT3 RA plus dexamethasone. Finally, our review involves the largest sample numbers (1107 cancer patients) based on RCTs; therefore a higher level of evidence may be expected. Nevertheless, our review also has limitations. Not all included studies reported the whole range of AEs and the grade of AEs was not reported. The above mentioned ongoing RCT conducted by our team will report relevant data about that in the future. Also, different types and dosages of 5-HT3 RA were used in this review, and the efficacy and AEs were not all comparable between the different types of 5-HT3 RA37; therefore potential bias may exist. Finally, the period of use of the antiemetic drugs and the use of dexamethasone were not all consistent, which could affect the results to some degree.
In conclusion, olanzapine significantly decreases the occurrence of CINV III and CINV IV and insomnia in high and moderately emetogenic chemotherapy. Compared with 10 mg per day, 5 mg olanzapine orally may be more appropriate for cancer patients. Nevertheless, there is still uncertainty about the severity and duration of AEs.
Footnotes
J-GZ, LH and S-HJ contributed equally.
Contributors: LH and J-GZ contributed equally to this work as first authors. LH and CX conducted the "search strategy", J-GZ and LH screened the studies, S-HJ and CX extracted the data, J-GZ and CX assessed the methodologic quality, and any inconsistencies were resolved by consulting with HM.
Funding: This work was supported by the National Natural Science Foundation of China (Grant No. 81660512), Science and Technology Planning Project of Guizhou Province (Grant No. QKHCG 2019-4440), the Research Programs of Science and Technology Commission Foundation of Zunyi City (Grant No. HZ2019-11, HZ2019-07), and the Research Programs of Health Commission Foundation of Guizhou Province (Grant No. gzwjkj2019-1-073, gzwjkj2019-1-172). This research was presented orally in the fifth edition of the ESMO Asia Congress [38] (Singapore, 22 Nov 2019).
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Sommariva S, Pongiglione B, Tarricone R. Impact of chemotherapy-induced nausea and vomiting on health-related quality of life and resource utilization: a systematic review. Crit Rev Oncol Hematol 2016;99:13–36. 10.1016/j.critrevonc.2015.12.001 [DOI] [PubMed] [Google Scholar]
- 2. Cohen L, de Moor CA, Eisenberg P, et al. Chemotherapy-Induced nausea and vomiting: incidence and impact on patient quality of life at community oncology settings. Support Care Cancer 2007;15:497–503. 10.1007/s00520-006-0173-z [DOI] [PubMed] [Google Scholar]
- 3. Navari RM. Management of chemotherapy-induced nausea and vomiting : focus on newer agents and new uses for older agents. Drugs 2013;73:249–62. 10.1007/s40265-013-0019-1 [DOI] [PubMed] [Google Scholar]
- 4. Aapro M, Carides A, Rapoport BL, et al. Aprepitant and fosaprepitant: a 10-year review of efficacy and safety. Oncologist 2015;20:450–8. 10.1634/theoncologist.2014-0229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Navari RM, Qin R, Ruddy KJ, et al. Olanzapine for the prevention of chemotherapy-induced nausea and vomiting. N Engl J Med 2016;375:134–42. 10.1056/NEJMoa1515725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Abe M, Hirashima Y, Kasamatsu Y, et al. Efficacy and safety of olanzapine combined with aprepitant, palonosetron, and dexamethasone for preventing nausea and vomiting induced by cisplatin-based chemotherapy in gynecological cancer: KCOG-G1301 phase II trial. Support Care Cancer 2016;24:675–82. 10.1007/s00520-015-2829-z [DOI] [PubMed] [Google Scholar]
- 7. Nakagaki M, Barras M, Curley C, et al. A randomized trial of olanzapine versus palonosetron versus infused ondansetron for the treatment of breakthrough chemotherapy-induced nausea and vomiting in patients undergoing hematopoietic stem cell transplantation. Support Care Cancer 2017;25:607–13. 10.1007/s00520-016-3445-2 [DOI] [PubMed] [Google Scholar]
- 8. Yokoe T, Hayashida T, Nagayama A, et al. Effectiveness of antiemetic regimens for highly emetogenic chemotherapy-induced nausea and vomiting: a systematic review and network meta-analysis. Oncologist 2019;24:e347–57. 10.1634/theoncologist.2018-0140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sutherland A, Naessens K, Plugge E, et al. Olanzapine for the prevention and treatment of cancer-related nausea and vomiting in adults. Cochrane Database Syst Rev 2018;9:Cd012555 10.1002/14651858.CD012555.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yoodee J, Permsuwan U, Nimworapan M. Efficacy and safety of olanzapine for the prevention of chemotherapy-induced nausea and vomiting: a systematic review and meta-analysis. Crit Rev Oncol Hematol 2017;112:113–25. 10.1016/j.critrevonc.2017.02.017 [DOI] [PubMed] [Google Scholar]
- 11. Roila F, Molassiotis A, Herrstedt J, et al. 2016 MASCC and ESMO guideline update for the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting and of nausea and vomiting in advanced cancer patients. Ann Oncol 2016;27:v119–33. 10.1093/annonc/mdw270 [DOI] [PubMed] [Google Scholar]
- 12. He Y, Xi X, He F. The efficacy and safety of olanzapine in the treatment of highly and moderately emetogenic chemotherapy induced nausea and vomiting. Med J West China 2018;30:1315–8. [Google Scholar]
- 13. Guo X, Lin X. Observation on the curative effect of olanzapine combined with conventional antiemetic drugs on chemotherapy-associated vomiting. China Continuing Medical Education 2018;10:124–6. [Google Scholar]
- 14. Yanai T, Iwasa S, Hashimoto H, et al. A double-blind randomized phase II dose-finding study of olanzapine 10 mg or 5 mg for the prophylaxis of emesis induced by highly emetogenic cisplatin-based chemotherapy. Int J Clin Oncol 2018;23:382–8. 10.1007/s10147-017-1200-4 [DOI] [PubMed] [Google Scholar]
- 15. Chiu L, Chow R, Popovic M, et al. Efficacy of olanzapine for the prophylaxis and rescue of chemotherapy-induced nausea and vomiting (CINV): a systematic review and meta-analysis. Support Care Cancer 2016;24:2381–92. 10.1007/s00520-016-3075-8 [DOI] [PubMed] [Google Scholar]
- 16. Zorzela L, Loke YK, Ioannidis JP, et al. PRISMA harms checklist: improving harms reporting in systematic reviews. BMJ 2016;352:i157 10.1136/bmj.i157 [DOI] [PubMed] [Google Scholar]
- 17. Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen B, Benedetti A. Quantifying heterogeneity in individual participant data meta-analysis with binary outcomes. Syst Rev 2017;6:243 10.1186/s13643-017-0630-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ma H, Liu Y, Huang L, et al. The adverse events of oxycodone in cancer-related pain: a systematic review and meta-analysis of randomized controlled trials. Medicine 2016;95:e3341 10.1097/MD.0000000000003341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lin L, Chu H. Quantifying publication bias in meta-analysis. Biometrics 2018;74:785–94. 10.1111/biom.12817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huang L, Zhou J-G, Zhang Y, et al. Opioid-Induced constipation relief from fixed-ratio combination prolonged-release oxycodone/naloxone compared with oxycodone and morphine for chronic nonmalignant pain: a systematic review and meta-analysis of randomized controlled trials. J Pain Symptom Manage 2017;54:737–48. 10.1016/j.jpainsymman.2017.07.025 [DOI] [PubMed] [Google Scholar]
- 22. Furuya-Kanamori L, Xu C, Lin L, et al. P value-driven methods were underpowered to detect publication bias: analysis of Cochrane review meta-analyses. J Clin Epidemiol 2019;118:86–92. 10.1016/j.jclinepi.2019.11.011 [DOI] [PubMed] [Google Scholar]
- 23. Mukhopadhyay S, Kwatra G, Alice K P, et al. Role of olanzapine in chemotherapy-induced nausea and vomiting on platinum-based chemotherapy patients: a randomized controlled study. Support Care Cancer 2017;25:145–54. 10.1007/s00520-016-3386-9 [DOI] [PubMed] [Google Scholar]
- 24. Tan L, Liu J, Liu X, et al. Clinical research of olanzapine for prevention of chemotherapy-induced nausea and vomiting. J Exp Clin Cancer Res 2009;28:131 10.1186/1756-9966-28-131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen L. The effect of tropisetron combina dexamethasone plus olanzapine in preventing nausea and vomiting in lung cancer patients received chemotherapy. Chin J Mod Drug Appli 2015;9:2 10.14164/j.cnki.cn11-5581/r.2015.19.104 [DOI] [Google Scholar]
- 26. LI J, Wei F, Zhang M, et al. The clinical observation of small dose of olanzapine to prevent and control nausea and vomiting after chemotherapy. Modern Oncology 2015;23:2673–5. [Google Scholar]
- 27. Meng Q, Chen G-H, Guo P-M. Olanzapine combined with normal antiemetic drugs in patients on solid tumor chemotherapy: antiemetic effect and impact on quality of life. World Chinese Journal of Digestology 2016;24:1117–23. 10.11569/wcjd.v24.i7.1117 [DOI] [Google Scholar]
- 28. Wang Y, Hu J, Wei R. To explore the effects of olanzapine combined with granisetron and dexamethasone in the prevention of chemotherapy-induced emesis nausea and the sleep disorder in cancer patients. Guide of China Medicine 2016;14:1–3. [Google Scholar]
- 29. Liu L, Ding T, Wang J, et al. Low dose olanzapine alleviates delayed nausea and vomiting induced by highly emetogenic hemotherapy in female lung cancer patient. Journal of Chinese Ontology 2017;23:251–3. [Google Scholar]
- 30. Tian R, Zheng Z, Chen X, et al. Antiemetic efficacy of olanzapine in breast cancer neoadjuvant chemotherapy. China & Foreign Medical Treatment 2017;36:118–20. [Google Scholar]
- 31. Zhao Q, Shen H. The effect of olanzapine for prevention of nausea and vomiting in medium and high emetogenic chemotherapy. Journal of Clinical Medical 2018;5:156–7. [Google Scholar]
- 32. Mehra N, Ganesan P, Ganesan TS, et al. Effectiveness of olanzapine in patients who fail therapy with aprepitant while receiving highly emetogenic chemotherapy. Med Oncol 2018;35:12 10.1007/s12032-017-1074-3 [DOI] [PubMed] [Google Scholar]
- 33. Morita T, Tei Y, Shishido H, et al. Olanzapine-Induced delirium in a terminally ill cancer patient. J Pain Symptom Manage 2004;28:102–3. 10.1016/j.jpainsymman.2004.04.008 [DOI] [PubMed] [Google Scholar]
- 34. Kirchner V, Aapro M, Terrey JP, et al. A double-blind crossover study comparing prophylactic intravenous granisetron alone or in combination with dexamethasone as antiemetic treatment in controlling nausea and vomiting associated with chemotherapy. Eur J Cancer 1997;33:1605–10. 10.1016/S0959-8049(97)00160-3 [DOI] [PubMed] [Google Scholar]
- 35. Callaghan JT, Bergstrom RF, Ptak LR, et al. Olanzapine. pharmacokinetic and pharmacodynamic profile. Clin Pharmacokinet 1999;37:177–93. 10.2165/00003088-199937030-00001 [DOI] [PubMed] [Google Scholar]
- 36. Chow R, Chiu L, Navari R, et al. Efficacy and safety of olanzapine for the prophylaxis of chemotherapy-induced nausea and vomiting (CINV) as reported in phase I and II studies: a systematic review. Support Care Cancer 2016;24:1001–8. 10.1007/s00520-015-3000-6 [DOI] [PubMed] [Google Scholar]
- 37. Saito M, Aogi K, Sekine I, et al. Palonosetron plus dexamethasone versus granisetron plus dexamethasone for prevention of nausea and vomiting during chemotherapy: a double-blind, double-dummy, randomised, comparative phase III trial. Lancet Oncol 2009;10:115–24. 10.1016/S1470-2045(08)70313-9 [DOI] [PubMed] [Google Scholar]
- 38. Zhou J-G. Olanzapine combined with 5-HT3 RA plus dexamethasone for prevention and treatment of chemotherapy-induced nausea and vomiting in high and moderate emetogenic chemotherapy: a systematic review and meta-analysis of randomized controlled trials. Ann Oncol 2019;30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
esmoopen-2019-000621supp001.pdf (127.5KB, pdf)
esmoopen-2019-000621supp002.pdf (32.8KB, pdf)
esmoopen-2019-000621supp003.pdf (600.2KB, pdf)
esmoopen-2019-000621supp004.pdf (1MB, pdf)