Abstract
Background
It is unknown whether a socio-economic difference exists in the association of diet quality with type 2 diabetes incidence, nor how diet influences the socioeconomic inequality in diabetes burden.
Methods
In 91,025 participants of the population-based Lifelines Cohort (aged ≥30, no diabetes or cardiovascular diseases at baseline), type 2 diabetes incidence was based on self-report, fasting glucose ≥ 7·0 mmol/l and/or HbA1c ≥ 6·5%. The evidence-based Lifelines Diet Score was calculated with data of a 110-item food frequency questionnaire. Socio-economic status (SES) was defined by educational level. Cox proportional hazards models were adjusted for age, gender, smoking, energy intake, alcohol intake and physical activity.
Findings
In 279,796 person-years of follow-up, 1045 diabetes cases were identified. Incidence rate was 5·7, 3·2 and 2·4 cases/1000 person-years in low, middle and high SES, respectively. Diet was associated with greater diabetes risk (HR(95%CI) in Q1 (poor diet quality) vs. Q5 (high diet quality) = 2·11 (1·70–2·62)). SES was a moderator of the association(pINTERACTION = 0·038). HRs for Q1 vs. Q5 were 1·66 (1·22–2·.27) in low, 2·76 (1·86–4·08) in middle and 2·46 (1·53–3·97) in high SES. With population attributable fractions of 14·8%, 40·1% and 37·3%, the expected number of cases/1000 person-years preventable by diet quality improvement was 0·85 in low, 1·28 in middle and 0·90 in high SES.
Interpretation
Diet quality improvement can potentially prevent one in three cases of type 2 diabetes, but because of a smaller impact in low SES, it will not narrow the socioeconomic health gap in diabetes burden.
Funding
None.
Keywords: Socio-economic status, Health inequality, Socio-economic inequality, Diet quality, Type 2 diabetes, Prospective cohort study, Public health
Research in context.
Evidence before this study
In February 2019, we searched PubMed for evidence (no date or language restriction) on the association between diet quality, socio-economic status and type 2 diabetes, or any combination of two of these concepts, by using a combination of MeSH terms and regular search terms. Meta-analyses of prospective cohort studies confirmed the importance of a healthy diet in the prevention of type 2 diabetes (pooled relative risk (95% CI) = 0·82 (0·78–0·85)). Furthermore, it is also clear that socio-economic inequalities exist in the burden of type 2 diabetes. However, it is currently unknown whether a socio-economic difference exists in the association of diet quality and diabetes incidence. Additionally, it is unclear whether diet quality improvement has the potential to narrow the socioeconomic gap in diabetes burden.
Added value of this study
The current study illustrates that a socio-economic disparity exists in the association of diet quality with diabetes incidence (pINTERACTION = 0·038). Although diabetes risk in the poorest diet quality group was higher in low SES than in high SES, the hazard ratios for poorest vs. best diet quality were lower in low SES than in middle and high SES. This difference is driven by a notably elevated risk even in low SES individuals with the highest diet quality. This is likely due to the presence of concurrent risk factors, which can also explain the smaller fraction of cases preventable by diet quality improvement in low SES.
Implications of all the available evidence
This study underlines that poor diet quality is not only a concern in low SES, but in all levels of SES. Although public health initiatives aiming to improve diet quality have considerable health potential across all levels of SES, one should be aware that this may broaden rather than narrow the socio-economic inequality in type 2 diabetes burden. Since the smaller impact of diet quality improvement in low SES is likely related to the remaining presence of concurrent risk factors, interventions targeting multiple risk factors have greater potential to narrow the socioeconomic health gap.
Alt-text: Unlabelled box
1. Introduction
Type 2 diabetes is a worldwide health concern, even more so in populations with low socioeconomic status (SES) [1]. To lower the total burden of disease, and to narrow the socioeconomic health gap, it is of major relevance to elucidate the health potential of risk factor improvement within different levels of SES. Diet quality is a modifiable risk factor for which the importance in the development of type 2 diabetes is well established [2]. However, the degree to which improvement in diet quality can contribute to diabetes prevention in different levels of SES is unknown.
At the food product level, there is evidence from meta-analyses of prospective cohort studies that higher intake of fruits and vegetables [3], whole grains [4] and yoghurt [5] lowers the risk of diabetes, while higher intake of red/processed meat [6] and sugar-sweetened beverages [7] is associated with an a greater diabetes risk. At the food pattern level, diets of the highest quality, as assessed by the Healthy Eating Index, Alternate Healthy Eating Index and DASH Diet, were associated with an 18% lower diabetes risk in a meta-analysis of prospective cohort studies [8]. A randomized controlled trial in a centre of the PREDIMED study, showed that a Mediterranean Diet intervention reduced diabetes incidence by 52% in four years of follow-up [9]. This confirms the importance of diet quality in diabetes prevention.
Poor diet quality is more common in low SES groups [10], and could therefore contribute to the socio-economic inequality in diabetes burden. Indeed, the inverse relationship between SES and diabetes prevalence was in part, but not fully, explained by differences in diet quality in a cohort study of healthy adults [11]. In addition to differences in exposure to risk factors, the associations of risk factors like diet with diabetes may also differ across levels of SES [12]. If diet quality differentially affects diabetes risk over levels of SES, the health potential of diet quality improvement will also vary with SES.
To our knowledge, it has not been previously investigated whether a socioeconomic difference exists in the association between diet quality and diabetes incidence. We aimed to investigate this in adults participating in the contemporary, population-based Lifelines cohort, and to determine how the results will influence the socioeconomic inequality in diabetes burden.
2. Methods
2.1. Cohort design and study population
The Lifelines cohort study is a multi-disciplinary prospective population-based cohort study examining in a unique three-generation design, the health and health-related behaviours of 167,729 persons living in the North of the Netherlands. It employs a broad range of investigative procedures in assessing the biomedical, socio-demographic, behavioural, physical and psychological factors which contribute to health and disease of the general population, with a special focus on multi-morbidity and complex genetics. The overall design and rationale of the study have been described in detail elsewhere [13]. Participants were included in the study between 2006 and 2013, and written informed consent was obtained from all participants. The Lifelines study is conducted according to the principles of the Declaration of Helsinki and approved by the Medical Ethics Committee of the University Medical centre Groningen, The Netherlands.
So far, four assessment rounds took place (T1=baseline, median + IQR of time in months to follow-up rounds: T2=13 [12–14], T3=24 [23–27], T4=44 [35–51]). Participants without follow-up data, under the age of 30, or reporting cardiovascular disease or any type of diabetes at baseline were excluded. Also, women who reported pregnancy at baseline or during follow-up were excluded to prevent misperception of gestational diabetes. Participants with missing or unreliable data on diet quality or covariates were excluded as well. Out of 152,662 adult Lifelines participants, 91,025 met the inclusion criteria (Supplementary Fig. 1).
3. Data collection
3.1. Type 2 diabetes prevalence and incidence
At baseline, participants who had either any type of self-reported diabetes, a fasting glucose ≥ 7·0 mmol/L or HbA1c ≥ 6·5%, or who reported to use prescribed diabetes medication (ATC A10A/A10B), were categorized as prevalent diabetes case and therefore excluded. At T2, T3 and T4, participants were considered an incident case when they answered confirmative to the question whether they were diagnosed with diabetes since the last time they filled out the questionnaire. At T4, we additionally considered participants with a fasting glucose ≥ 7·0 or HbA1c ≥ 6·5 as incident cases. Data on prescribed medication were not available during follow-up.
3.2. Dietary assessment
At baseline, dietary consumption was assessed using a 110-item semi-quantitative FFQ assessing food consumption over the previous month [14]. Energy intake was estimated from the FFQ data by using the 2011 Dutch food composition database [15]. FFQ data was considered unreliable when the ratio between reported energy intake and basal metabolic rate, calculated with the Schofield equation [16], was below 0·50 or above 2·75, or when energy intake was below 800 kcal/day (males) or 500 kcal/day (females) [17].
The Lifelines Diet Score (LLDS) was calculated as a measure of relative diet quality. This score is based on the 2015 Dutch dietary guidelines, summarizing contemporary evidence on diet and chronic disease relations [18]. Since these guidelines are fully based on scientific evidence from international peer-reviewed literature, and not on expert opinions, they are suitable for use in scientific research. The development of this food-based diet score has been described in detail elsewhere [19]. In short, the LLDS ranks the relative intake of nine food groups with proven positive health effects and three food groups with proven negative health effects. For each of the food groups, quintiles of consumption in grams/1000 kcal are determined and awarded zero to four points (Supplementary Table 1). For the positive food groups, that is vegetables, fruit, whole grain products, legumes & nuts, fish, oils & soft margarines, unsweetened dairy, coffee and tea, higher scores are awarded to higher quintiles of consumption. For the negative food groups, that is red & processed meat, butter & hard margarines and sugar-sweetened beverages, higher scores are awarded to lower quintiles of consumption. The sum of these LLDS components varied from zero to 48. The LLDS scores were then categorized into quintiles, with quintile 1 including 20% of participants with the lowest diet quality and quintile 5 including 20% of participants with the highest diet quality (LLDS range Q1: 0–18, Q2: 19–22, Q3: 23–25, Q4: 26–29, Q5: 30–48). The quintiles for each product group were predefined in the total Lifelines cohort. Higher scores in women, individuals of older age and higher education level, support the validity of the LLDS [19].
3.3. Educational level
Educational level was used as an indicator for socioeconomic status, and was assessed at baseline with the following question: “What is the highest level of education you have finished?”. Educational level was categorized as low (no education, primary school, lower vocational or lower general secondary education: ISCED level 0, 1 or 2), middle (intermediate vocational training or higher secondary education: ISCED level 3 or 4) or high (higher vocational or university education: ISCED level 5 or 6) [20].
3.4. Demographics and lifestyle
At baseline, height and body weight without shoes and heavy clothing were measured at 12 local Lifelines research sites, and rounded to the nearest 0·5 cm and 0·1 kg. Self-administered questionnaires at baseline were used to collect data regarding ethnicity and lifestyle (alcohol, smoking, physical activity). The validated short questionnaire to assess health-enhancing physical activity (SQUASH) was used to assess physical activity [21]. From the SQUASH data, leisure time and commuting physical activity, including sports, at moderate (4·0–6·4 MET) to vigorous (≥ 6·5 MET) intensity (non-work moderate to vigorous physical activity (MVPA)) was calculated in minutes per week [21]. Alcohol consumption was estimated based on Lifelines’ Food Frequency Questionnaire (FFQ) data [14]. To resolve missings in MVPA (n = 6045, 6·6%), multiple imputation and Hot Deck imputation were explored. As the results virtually did not differ between both imputation methods, the simpler Hot Deck method was used. With the Hot Deck imputation macro for SPSS [22] missing values were replaced with the value of a participant who was similar in age, gender, smoking status, energy intake and BMI.
4. Data analysis
Cox proportional hazards regression was used to investigate the association of diet quality, and educational level with diabetes incidence. Non-cases with incomplete follow-up were censored at the last time-point for which data was available. Additionally, all participants were censored after 60 months. The assumptions of the Cox proportional hazards model were checked both by inspection of the log-minus-log plots, as well as by performing a Cox regression with time-dependent covariate, to investigate whether ratios differ before vs. after T2 or T3. For analyses including the LLDS in quintiles, quintile 5 representing the highest diet quality was set as the reference group. For educational level, this was high education.
In the first analyses, the independent associations of diet quality and educational level were investigated by including both the LLDS in quintiles, and educational level in 3 categories. Diet quality and educational level were first entered into the model separately (model 1), and then combined (model 2). Subsequently, the model was adjusted for potential confounders (model 3 – gender, age; model 4 – smoking status (current, former, never), energy intake (kcal/day), alcohol intake (g/day), non-work MVPA (min/week)). As we consider BMI as a potential intermediate factor, rather than a confounder, baseline BMI was included in a separate 5th model.
Subsequently, a Cox regression was performed including diet quality, educational level and their interaction, to test whether the association of diet quality with diabetes incidence differs over levels of SES. Ordinal coding was used for diet quality to test this interaction effect. When the interaction term was significant, additional Cox regression analyses were performed stratified by educational level. These analyses followed the same steps of including covariates as described above.
To estimate the fraction of cases preventable through diet quality improvement in the different educational groups, multivariable logistic regression analyses were performed including all participants with complete follow-up. Based on the confounder adjusted odds ratios from the logistic regression analysis in 67,125 participants, adjusted population attributable fractions were calculated using the punaf package, as described by Newson [23].
Data analysis was performed in IBM SPSS 23 (SPSS, Chicago Illinois, USA), except for population attributable fractions, for which STATA 13.0 (StataCorp) was used. P-levels below 0·05 were considered significant.
5. Data statement
The Lifelines Cohort does not enable public data sharing. The cohort's data is only available to scientists who, upon approval of a submitted research proposal, have signed a Data/Material Transfer Agreement.
6. Results
During a total of 279,796 years of follow-up, 1045 type 2 diabetes cases were identified, providing an average incidence rate of 3·73 per 1000 person-years (4·41 in men, 3·25 in women). Incidence rates were lower in higher education groups, irrespective of differences in age and gender (age- and gender-adjusted incidence rates: 4·91 in low, 3·59 in middle, 2·57 in high education). Participants in high vs. low education were, amongst others, younger, more often male, less often smokers, had higher diet quality and physical activity, and lower BMI (Table 1). In total 45·3% of the cases were identified through self-reported questionnaires (48·1% in low, 42·1% in middle, 43·8% in high education), and 54·7% were identified through lab measurements (51·9% in low, 57·9% in middle, 56·2% in high education) (Supplementary Fig. 2).
Table 1.
Characteristics of Lifelines participants over different educational levels.
| Educational level |
||||
|---|---|---|---|---|
| Total sample (N = 91,025) | Low (N = 28,134) | Middle (N = 34,938) | High (N = 27,953) | |
| Gender (%) | ||||
| Male | 42·1 | 40·0 | 40·2 | 46·5 |
| Female | 57·9 | 60·0 | 59·8 | 53·5 |
| Age at baseline | 48 ± 10 | 52 ± 11 | 46 ± 9 | 46 ± 10 |
| White/East & West European ethnicity (%)* | 98·8 | 98·9 | 98·8 | 98·6 |
| Diabetes Incidence rate | ||||
| Total | 3·73 | 5·74 | 3·20 | 2·42 |
| Male | 4·41 | 6·10 | 4·11 | 3·30 |
| Female | 3·25 | 5·50 | 2·58 | 1·64 |
| Smoking status (%) | ||||
| Never | 44·9 | 36·2 | 45·4 | 53·2 |
| Former | 36·3 | 41·2 | 34·7 | 33·4 |
| Current | 18·8 | 22·6 | 19·9 | 13·5 |
| Non-occupational MVPA (minutes/week) | 180 [60–360] | 180 [60–370] | 180 [60–360] | 210 [90–380] |
| LLDS | 24·5 ± 5·9 | 24·1 ± 6·0 | 24·1 ± 5·8 | 25·6 ± 5·8 |
| Alcohol-users (%) | 83·0 | 78·4 | 82·4 | 88·3 |
| Intake among users in g/day | 6·4 [2·5–12·4] | 6·4 [2·5–12·6] | 6·3 [2·4–12·1] | 6·6 [2·7–12·4] |
| Energy intake (kcal/day) | ||||
| Male | 2382 ± 637 | 2418 ± 677 | 2430 ± 651 | 2298 ± 575 |
| Female | 1846 ± 472 | 1819 ± 479 | 1869 ± 478 | 1845 ± 453 |
| Body weight (kg) | ||||
| Male | 88·3 ± 12·7 | 89·2 ± 13·2 | 88·9 ± 12·8 | 87·0 ± 12·1 |
| Female | 74·2 ± 13·4 | 75·0 ± 13·5 | 74·8 ± 13·7 | 72·4 ± 12·5 |
| BMI (kg/m2) | ||||
| Male | 26·5 ± 3·4 | 27·1 ± 3·6 | 26·5 ± 3·4 | 25·8 ± 3·2 |
| Female | 25·9 ± 4·5 | 26·7 ± 4·6 | 26·0 ± 4·6 | 24·9 ± 4·1 |
| Glucose (mmol/L) | 4·9 ± 0·5 | 5·0 ± 0·5 | 4·9 ± 0·5 | 4·9 ± 0·5 |
| HbA1c (%) | 5·5 ± 0·3 | 5·6 ± 0·3 | 5·5 ± 0·3 | 5·5 ± 0·3 |
Values in means ± SD, or median [25th–75th percentile].
based on available data (92.9% of participants).
6.1. LLDS, educational level and diabetes incidence
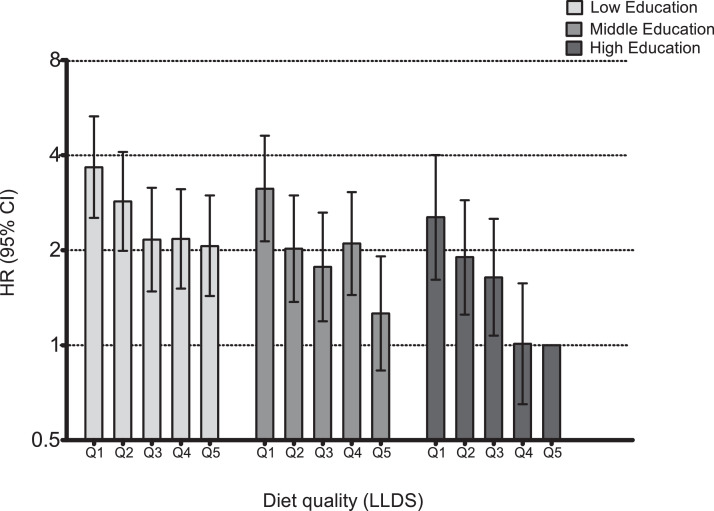
In Cox regression analyses, none of the checks performed found evidence that the assumption of proportional hazards was violated for the exposure of interest. Diet quality and educational level were independently associated with diabetes incidence in crude and adjusted models (Table 2). Incidence rate in Q1 (poor diet quality) vs. Q5 (high diet quality) was 2·11 (HR, 95%CI = 1·70–2·62) times higher. Because of the significant interaction between diet quality and education (pINTERACTION = 0·038), analyses were repeated stratified by educational level (Table 3). The results illustrate that the strength of the association between diet quality and diabetes incidence was lowest at the low level of education. In this group, the rate of diabetes incidence was 1·66 (95%CI: 1·22–2·27) times greater in Q1 of the LLDS (poor diet quality), compared to Q5 (high diet quality). For middle and high educational level, the rate was 2·76 (95%CI: 1·86–4·08) and 2·46 (95%CI: 1·53–3·97) times greater in participants with poor diet quality (Q1). Additional adjustment for BMI attenuated the associations at all three educational levels by 8%–17%, suggesting that a small part of the association of diet quality with diabetes incidence was explained by lower BMI among participants with a higher diet quality. To elucidate the mechanism underlying the weaker association in low SES, the joint associations of diet quality and SES were further investigated in an additional analysis with Q5, high education level as reference category for all groups. The risk for diabetes was higher in both Q1 and Q5 for participants in low SES compared to high SES. That the hazard ratio for Q1 vs. Q5 in low SES was smaller than in high SES, is because the SES difference in risk is larger for Q5. Individuals with a low level of education, adhering to a high quality diet (Q5) have a 2·06 times (95%CI: 1·43–2·98) greater risk of diabetes than highly educated participant with an equally healthy diet. This notably higher risk in the reference group with the healthiest diet (Q5) in low SES makes the difference between good and poor diet quality smaller. To quantify this, for low SES the Q1/Q5 ratio was 3·66/2·06 = 1·78. For high SES, this was 2·54/1·00 = 2.54 (Fig. 1/Supplementary Table 2). The healthfulness of the dietary quintiles across levels of SES was comparable since quintiles were based on the whole population.
Table 2.
Independent associations of diet quality (LLDS) and educational level with diabetes incidence. Hazard ratio's and 95% confidence intervals from Cox proportional hazards regression.
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | |
|---|---|---|---|---|---|
| Diet quality quintile | |||||
| P-value(Cox regression) | < 0·001 | 0·005 | < 0·001 | < 0·001 | < 0·001 |
| Q1 (Poorest) | 1·53 (1·26–1·86) | 1·42 (1·16–1·73) | 2·01 (1·63–2·48) | 2·11 (1·70–2·62) | 1·87 (1·50–2·31) |
| Q2 | 1·22 (1·01–1·48) | 1·18 (0·97–1·43) | 1·49 (1·22–1·81) | 1·54 (1·26–1·88) | 1·40 (1·15–1·71) |
| Q3 | 1·07 (0·87–1·31) | 1·04 (0·85–1·28) | 1·23 (1·00–1·52) | 1·26 (1·02–1·55) | 1·19 (0·96–1·46) |
| Q4 | 1·12 (0·92–1·35) | 1·10 (0·91–1·34) | 1·19 (0·98–1·45) | 1·21 (0·99–1·47) | 1·15 (0·95–1·40) |
| Q5 (Best) | 1 | 1 | 1 | 1 | 1 |
| Educational level | |||||
| P-value(Cox regression) | < 0·001 | < 0·001 | < 0·001 | < 0·001 | 0·002 |
| Low | 2·40 (2·04–2·82) | 2·33 (1·99–2·75) | 1·71 (1·45–2·02) | 1·67 (1·41–1·97) | 1·34 (1·13–1·59) |
| Middle | 1·32 (1·12–1·57) | 1·29 (1·09–1·53) | 1·34 (1·12–1·59) | 1·32 (1·11–1·57) | 1·15 (0·97–1·37) |
| High | 1 | 1 | 1 | 1 | 1 |
Model 1: LLDS in quintiles OR educational level in 3 categories.
Model 2: LLDS in quintiles AND educational level in 3 categories.
Model 3: 2 + age, gender.
Model 4: 3 + smoking status, energy intake, alcohol intake, non-work MVPA.
Model 5: 4 + BMI.
N = 91 025.
Table 3.
Education-level specific associations of diet quality (LLDS) with diabetes incidence. Hazard ratio's and 95% confidence intervals from Cox proportional hazards regression, stratified by education level.
| Q1 | Q2 | Q3 | Q4 | Q5 | PLLDS (Cox regression) | |
|---|---|---|---|---|---|---|
| Low Education | ||||||
| Cases/person-years | 99/15,379 | 113/18,950 | 81/16,418 | 104/18,621 | 96/16,538 | |
| Model 1 | 1·11 (0·84–1·47) | 1·02 (0·78–1·34) | 0·84 (0·63–1·13) | 0·96 (0·72–1·26) | 1 | 0·452 |
| Model 2 | 1·64 (1·22–2·22) | 1·33 (1·00–1·76) | 1·02 (0·75–1·37) | 1·04 (0·78–1·37) | 1 | 0·003 |
| Model 3 | 1·66 (1·22–2·27) | 1·33 (1·00–1·78) | 1·02 (0·75–1·38) | 1·04 (0·79–1·37) | 1 | 0·003 |
| Model 4 | 1·48 (1·09–2·02) | 1·25 (0·94–1·67) | 0·98 (0·72–1·32) | 1·01 (0·76–1·33) | 1 | 0·028 |
| Middle Education | ||||||
| Cases/person-years | 77/18,297 | 73/24,706 | 61/21,309 | 84/23,198 | 47/19,498 | |
| Model 1 | 1·73 (1·20–2·48) | 1·22 (0·84–1·76) | 1·17 (0·80–1·71) | 1·48 (1·03–2·11) | 1 | 0·021 |
| Model 2 | 2·44 (1·67–3·56) | 1·57 (1·08–2·29) | 1·38 (0·94–2·03) | 1·66 (1·16–2·37) | 1 | <0·001 |
| Model 3 | 2·76 (1·86–4·08) | 1·73 (1·18–2·53) | 1·47 (0·99–2·16) | 1·72 (1·20–2·46) | 1 | <0·001 |
| Model 4 | 2·55 (1·73–3·77) | 1·60 (1·09–2·34) | 1·42 (0·96–2·09) | 1·72 (1·20–2·47) | 1 | <0·001 |
| High Education | ||||||
| Cases/person-years | 35/9537 | 50/16,737 | 45/16,814 | 39/21,600 | 41/22,196 | |
| Model 1 | 1·93 (1·23–3·03) | 1·58 (1·05–2·39) | 1·42 (0·93–2·16) | 0·96 (0·62–1·48) | 1 | 0·007 |
| Model 2 | 2·43 (1·52–3·88) | 1·81 (1·18–2·76) | 1·58 (1·03–2·44) | 0·98 (0·63–1·53) | 1 | <0·001 |
| Model 3 | 2·46 (1·53–3·97) | 1·85 (1·20–2·85) | 1·62 (1·05–2·50) | 1·00 (0·64–1·55) | 1 | <0·001 |
| Model 4 | 2·04 (1·26–3·30) | 1·56 (1·01–2·41) | 1·44 (0·93–2·22) | 0·88 (0·56–1·36) | 1 | 0·003 |
Model 1: LLDS in quintiles.
Model 2: 1 + age, gender.
Model 3: 2 + smoking status, energy intake, alcohol intake, non-work MVPA.
Model 4: 3 + BMI.
N = 91 025.
Fig. 1.
Joint associations of diet quality and SES with diabetes incidence. Hazard ratio's for diet quality (LLDS) in quintiles, with Q1 representing poorest, and Q5 representing highest diet quality. High SES, Q5 as reference for all groups. Adjusted for age, gender, smoking status, energy intake, alcohol intake, non-work MVPA.
6.2. Adjusted population attributable fractions per educational level
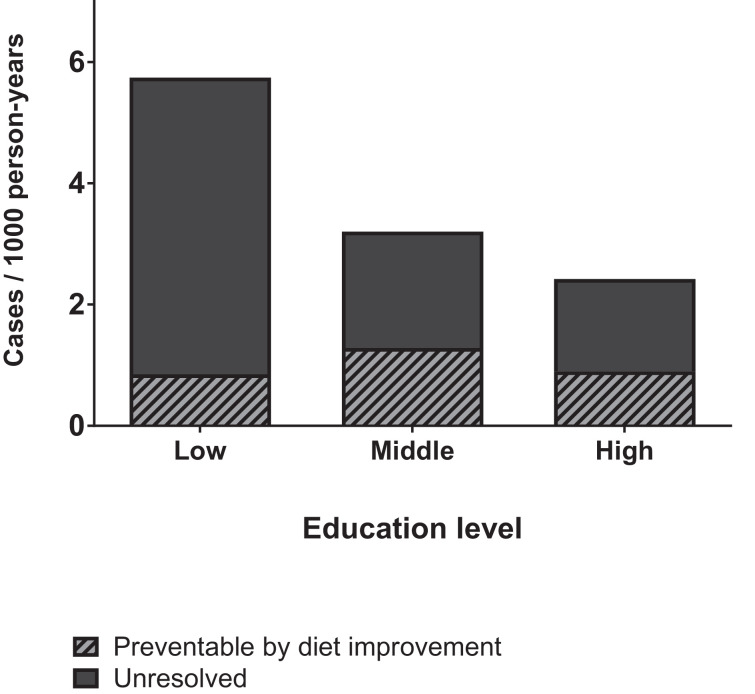
The adjusted population attributable fractions reflect the proportion of the total burden of diabetes that could be eliminated if all participants adhered to a diet as in quintile 5 of the LLDS, while other confounding factors remained the same. The attributable fractions were 14·8% (95CI: −3·8%–30·1%) in low, 40·1% (95CI: 20·0%–55·1%) in middle and 37·3% (95CI: 13·4%–54·6%) in high education. The number of diabetes cases that can be prevented depends on the incidence rate, as well as on the fraction of cases attributed to sub-optimal diet quality. Incidence rate in the low education group is greater than in middle and high education (Table 1). Therefore, the expected number of cases per 1000 person years, preventable by diet quality improvement is 0·85 in low, vs. 1·28 in middle and 0·90 in high education (Fig. 2).
Fig. 2.
Estimated proportion of cases that could be prevented if all participants adhered to a diet of a quality defined by quintile 5 of the LLDS. Based on confounder adjusted population attributable fractions, and incidence rates per educational level.
7. Discussion
Socio-economic inequalities exist in the association of diet quality with diabetes incidence, with stronger associations being present in middle and high SES. The lower relative risk within low SES primarily represented a disproportionally low benefit of a healthy diet. An individual with low level of education, adhering to a high quality diet (Q5) has a two times greater risk of diabetes than a highly educated participant adhering to an equally healthy diet. Despite this lower relative benefit of diet quality improvement in low SES, due to the much higher diabetes incidence in low SES, diet quality improvement has the potential to meaningfully reduce diabetes incidence in all levels of SES.
In the present study we found that diet quality was strongly associated with diabetes incidence, also after adjustment for other lifestyle factors and BMI. The overall, fully adjusted hazard ratio was 1·87 for poor (Q1) vs. high (Q5) diet quality, which equals a HR of 0·53 for Q5 vs. Q1. Similar prospective associations were previously reported in the Nurses Health and Health Professionals Follow-up Study (RR for Q5 vs. Q1 was 0·82 for HEI-2005, 0·67 for AHEI-2010) [24] and Women's Health Initiative (HR for Q5 vs. Q1 of AHEI = 0·76) [25]. However, these studies do not address the primary aim of this study, which was to assess whether a socioeconomic difference exists in the association between diet quality and diabetes.
This study showed that the association between diet and diabetes is significantly modified by SES. In low SES, the lifestyle adjusted HR for poor diet quality (Q1) vs. high diet quality (Q5) was 1·66, whereas this was 2·76 in middle and 2·46 in high SES. A few previous studies investigated the possibility of socio-economic disparities in associations of lifestyle factors with other health outcomes, although not for diet and diabetes. In the UK Biobank, an extended score of unhealthy lifestyle factors had a much stronger association with cardiovascular and all-cause mortality in lower SES groups [26]. In the Scottish Health Surveys, the risk of morbidity or mortality from high alcohol intake was greater for drinkers in deprived areas [27]. Our finding that diabetes risk in Q1 was higher in low SES than middle and high SES is in line with these studies. However, we found weaker, instead of stronger associations of diet quality with diabetes in low SES. We additionally illustrated that this was primarily the result of a two times greater risk in the reference category with high diet quality (Q5) in low SES, compared to high SES. This lower health benefit of adhering to a high quality diet may be one of the mechanisms underlying socioeconomic inequalities with regard to diabetes.
From a policy perspective, improving diet quality has health potential over all levels of SES, despite the weaker association in low SES. The calculations of the population attributable fractions showed that eliminating poor diet quality as a risk factor for diabetes was estimated to prevent 14·8% of cases in low SES, but 40·1% in middle and 37·3% in high SES. First of all, the large proportions of diabetes cases preventable by diet quality improvement in middle and high SES illustrate that poor diet quality is definitely not a problem in low SES alone. Furthermore, despite the smaller relative contribution of diet to diabetes incidence in low SES, the absolute number of cases preventable by diet quality improvement is more comparable over all levels of SES because of the higher diabetes incidence in low SES (Table 2). This underlines that public health interventions aiming to improve diet quality are of importance irrespective of SES, as there is potential health impact over all levels of SES. At the same time, however, this does mean that improvement of diet quality alone will not be sufficient to overcome socioeconomic health inequalities.
As the high diabetes incidence in low SES is likely related to the preponderance of risk factors in that category, multifaceted lifestyle interventions are needed to reduce diabetes burden in low SES and to narrow the socioeconomic health gap. A theory that fits the results of the present study, is that harmful lifestyle factors have lower health effects in low social classes due to the existence of many concurrent risk factors [28]. This implies that even when adhering to an equally healthy diet, a low SES individual remains at higher health risk than its high SES peers. The causal pie model, introduced by Rothman, provides a further illustration of this principle [29]. A combination of component causes (like genetic predisposition, poor diet quality, smoking, lack of physical activity or stress) can add up to a sufficient cause, resulting in disease. By eliminating one component cause, in this case poor diet quality, some cases will not be prevented because the remaining risk factors can still add up to a sufficient cause. This is expected to happen more often in low SES, where more concurrent risk factors exist. The results of the previously discussed UK Biobank study [26] are in line with this reasoning, as this study, investigating a combined lifestyle score instead of a single risk factor, did not find an elevated risk of cardiovascular disease and mortality for the most healthy lifestyle category in low SES compared to high SES individuals. This indicates that when multiple risk factors are targeted, low SES individuals with a healthy lifestyle are at similar health risk as their high SES peers with a comparable lifestyle. In contrast, we showed that targeting diet quality alone will render low SES individuals at higher risk than their high SES peers.
This prospective study was performed in the contemporary Dutch Lifelines Cohort. An advantage of the large sample of 91,025 participants is that it enabled the comparison of diet quality quintiles, stratified by SES. As the cohort is extensively phenotyped, the risk of bias is low, although residual confounding cannot be ruled out. Furthermore, diet quality was assessed with the food-based Lifelines Diet Score, which is fully based on international scientific evidence on associations between diet quality and risk of major chronic diseases, including type 2 diabetes. A limitation is that in two of the three follow-up assessments, only self-reported data on diabetes incidence was available. Therefore, cases may have been unnoticed in the first and second follow-up assessment, because blood glucose and HbA1c measurements were not available. Since data on prescribed diabetes medication was not available, it is possible that more cases have been missed. Despite these limitations, diabetes incidence in our study (4·45 in males, 3·25 in females) is comparable to incidence in the Netherlands as a whole (3·9 in males and 3·2 in females in 2017) [30], which warrants the reliability of our measurements. At T4, the proportion of self-reported diabetes cases was slightly higher in lower levels of SES, which may yield a higher risk of false positives in low SES. However, the majority of cases was identified through laboratory assessment, which lowers the risk of bias by differences in self-report between levels of SES. Finally, since this study was performed in a developed country with a high quality health care system, results may not be generalizable to less developed populations.
To conclude, diet quality improvement can potentially prevent one in three cases of diabetes, but because of a smaller impact in low SES, it will not narrow the socioeconomic inequalities in type 2 diabetes. The smaller impact in low SES is expected to be related to the presence of concurrent risk factors. Therefore, interventions targeting multiple risk factors are needed to lower diabetes burden, in particular in low SES. It is a common perception that poor diet quality is predominantly a problem in lower socioeconomic classes. Nonetheless, the large proportion of diabetes cases preventable by diet quality improvement in middle and high SES, illustrates that there is still lots of room for improvement across all three levels of SES.
Declaration of competing interest
All authors confirm that no conflicts of interest exist.
Acknowledgments
Acknowledgments
The Lifelines Biobank initiative has been made possible by subsidy from the Dutch Ministry of Health, Welfare and Sport, the Dutch Ministry of Economic Affairs, the University Medical centre Groningen (UMCG the Netherlands), University Groningen and the Northern Provinces of the Netherlands. The authors wish to acknowledge the services of the Lifelines Cohort Study, the contributing research centres delivering data to Lifelines, and all study participants.
Funding
None.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2019.100252.
Appendix. Supplementary materials
References
- 1.Agardh E., Allebeck P., Hallqvist J., Moradi T., Sidorchuk A. Type 2 diabetes incidence and socio-economic position: a systematic review and meta-analysis. Int J Epidemiol. 2011;40:804–818. doi: 10.1093/ije/dyr029. [DOI] [PubMed] [Google Scholar]
- 2.Roumen C., Blaak E.E., Corpeleijn E. Lifestyle intervention for prevention of diabetes: determinants of success for future implementation. Nutr Rev. 2009;67:132–146. doi: 10.1111/j.1753-4887.2009.00181.x. [DOI] [PubMed] [Google Scholar]
- 3.Cooper A.J., Forouhi N.G., Ye Z. Fruit and vegetable intake and type 2 diabetes : ePIC-InterAct prospective study and meta-analysis. Eur J Clin Nutr. 2013;66:1082–1092. doi: 10.1038/ejcn.2012.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aune D., Norat T., Romundstad P., Vatten L.J. Whole grain and refined grain consumption and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis of cohort studies. Eur J Epidemiol. 2013;28:845–858. doi: 10.1007/s10654-013-9852-5. [DOI] [PubMed] [Google Scholar]
- 5.Chen M., Sun Q., Giovannucci E., Mozaffarian D., Manson J.E., Willett W.C. Dairy consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. BMC Med. 2014;12:215. doi: 10.1186/s12916-014-0215-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feskens E.J.M., Sluik D., Van Woudenbergh G.J. Meat consumption, diabetes, and its complications. Curr Diab Rep. 2013;13:298–306. doi: 10.1007/s11892-013-0365-0. [DOI] [PubMed] [Google Scholar]
- 7.Greenwood D.C., Threapleton D.E., Evans C.E.L., Cleghorn C.L., Nykjaer C., Woodhead C. Association between sugar-sweetened and artificially sweetened soft drinks and type 2 diabetes: systematic review and dose–response meta-analysis of prospective studies. Br J Nutr. 2014;112:725–734. doi: 10.1017/S0007114514001329. [DOI] [PubMed] [Google Scholar]
- 8.Schwingshackl L., Bogensberger B., Hoffmann G. Diet quality as assessed by the healthy eating index, alternate healthy eating index, dietary approaches to stop hypertension score, and health outcomes: an updated systematic review and meta-analysis of cohort studies. J Acad Nutr Diet. 2018;118:74–100. doi: 10.1016/j.jand.2017.08.024. e11. [DOI] [PubMed] [Google Scholar]
- 9.Salas-Salvadó J., Bulló M., Babio N., Martínez-González M., Ibarrola-Jurado N., Basora J. Reduction in the incidence of type 2 diabetes with the Mediterranean diet: results of the PREDIMED-Reus nutrition intervention randomized trial. Diabetes Care. 2011;34:14–19. doi: 10.2337/dc10-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Darmon N., Drewnowski A. Does social class predict diet quality? Am J Clin Nutr. 2008;87:1107–1117. doi: 10.1093/ajcn/87.5.1107. [DOI] [PubMed] [Google Scholar]
- 11.Panagiotakos D.B., Pitsavos C., Chrysohoou C., Vlismas K., Skoumas Y., Palliou K. Dietary habits mediate the relationship between socio-economic status and CVD factors among healthy adults: the Attica study. Public Health Nutr. 2008;11:1342–1349. doi: 10.1017/S1368980008002978. [DOI] [PubMed] [Google Scholar]
- 12.Diderichsen F., Hallqvist J., Whitehead M. Differential vulnerability and susceptibility: how to make use of recent development in our understanding of mediation and interaction to tackle health inequalities. Int J Epidemiol. 2018;48:268–274. doi: 10.1093/ije/dyy167. [DOI] [PubMed] [Google Scholar]
- 13.Scholtens S., Smidt N., Swertz M.A., Bakker S.J.L., Dotinga A., Vonk J.M. Cohort profile: lifeLines, a three-generation cohort study and biobank. Int J Epidemiol. 2015;44:1172–1180. doi: 10.1093/ije/dyu229. [DOI] [PubMed] [Google Scholar]
- 14.Siebelink E., Geelen A., de Vries J.H.M. Self-reported energy intake by FFQ compared with actual energy intake to maintain body weight in 516 adults. Br J Nutr. 2011;106:274–281. doi: 10.1017/S0007114511000067. [DOI] [PubMed] [Google Scholar]
- 15.NEVO-tabel (2011) Dutch Food Composition Table 2011 - version 3. 2011. http://www.rivm.nl/Documenten_en_publicaties/Algemeen_Actueel/Nieuwsberichten/2011/Nieuwe_NEVO_tabel_2011_beschikbaar.
- 16.Schofield W.N. Predicting basal metabolic rate, new standards and review of previous work. Hum Nutr Clin Nutr. 1985;39(Suppl 1):5–41. [PubMed] [Google Scholar]
- 17.Willett W. 3rd ed. Oxford University Press; 2013. Issues in Analysis and Presentation of Dietary Data; p. 306. [Google Scholar]
- 18.Kromhout D., Spaaij C.J.K., de Goede J., Weggemans R.M. The 2015 Dutch food-based dietary guidelines. Eur J Clin Nutr. 2016;70:869–878. doi: 10.1038/ejcn.2016.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vinke P.C., Corpeleijn E., Dekker L.H., Jacobs D.R., Navis G., Kromhout D. Development of the lifelines diet score (LLDS) and its application in 129,369 lifelines participants. Eur J Clin Nutr. 2018;72:1111–1119. doi: 10.1038/s41430-018-0205-z. [DOI] [PubMed] [Google Scholar]
- 20.International Standard Classification of EducationI S C E D 1997. http://www.unesco.org/education/information/nfsunesco/doc/isced_1997.htm. (accessed May 8, 2019).
- 21.Wendel-Vos G.C.W., Schuit A.J., Saris W.H.M., Kromhout D. Reproducibility and relative validity of the short questionnaire to assess health-enhancing physical activity. J Clin Epidemiol. 2003;56:1163–1169. doi: 10.1016/s0895-4356(03)00220-8. [DOI] [PubMed] [Google Scholar]
- 22.Myers T.A. Goodbye, listwise deletion: presenting hot deck imputation as an easy and effective tool for handling missing data. Commun Methods Meas. 2011;5:297–310. [Google Scholar]
- 23.Newson R.B. Attributable and unattributable risks and fractions and other scenario comparisons. Stata J. 2013;13:672–698. [Google Scholar]
- 24.Chiuve S.E., Fung T.T., Rimm E.B., Hu F.B., McCullough M.L., Wang M. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142:1009–1018. doi: 10.3945/jn.111.157222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qiao Y., Tinker L., Olendzki B.C., Hébert J.R., Balasubramanian R., Rosal M.C. Racial/ethnic disparities in association between dietary quality and incident diabetes in postmenopausal women in the united states: the women’s health initiative 1993-2005. Ethn Health. 2014;19:328–347. doi: 10.1080/13557858.2013.797322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foster H.M.E., Celis-Morales C.A., Nicholl B.I., Petermann-Rocha F., Pell J.P., Gill M.R. The effect of socioeconomic deprivation on the association between an extended measurement of unhealthy lifestyle factors and health outcomes: a prospective analysis of the UK Biobank cohort. Lancet Public Health. 2018;3:e576–e585. doi: 10.1016/S2468-2667(18)30200-7. [DOI] [PubMed] [Google Scholar]
- 27.Katikireddi S.V., Whitley E., Lewsey J., Gray L., Leyland A.H. Socioeconomic status as an effect modifier of alcohol consumption and harm: analysis of linked cohort data. Lancet Public Health. 2017;2:e267–e276. doi: 10.1016/S2468-2667(17)30078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blaxter M. Routledge; London: 1990. Health and lifestyles. [Google Scholar]
- 29.Wensink M., Westendorp R.G.J., Baudisch A. The causal pie model: an epidemiological method applied to evolutionary biology and ecology. Ecol Evol. 2014;4:1924–1930. doi: 10.1002/ece3.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aantal nieuwe gevallen van diabetes mellitus. Niv. Zorgregistraties eerste lijn. 2017. https://www.volksgezondheidenzorg.info/onderwerp/diabetes-mellitus/cijfers-context/huidige-situatie#node-aantal-nieuwe-gevallen-van-diabetes.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.