Abstract
Today schistosomiasis, caused mainly by the three major schistosome species (S. mansoni, S. haematobium and S. japonicum), has for many decades and still continues to be on a rapid and swift rise globally, claiming thousands of lives every year and leaving 800 million people at the risk of infection. Due to the high prevalence of this disease and the steady increase in the infection rates, praziquantel (PZQ) remains the only effective drug against this acute disease although it has no effect on the juvenile schistosome parasite. However, no significant approaches have been made in recent years in the discovery of new or alternative drugs and unfortunately, resistance to this drug has been reported in some parts of the world. Therefore, it is imperative to develop a new drug for this debilitating disease. In this review, a brief history of past, present, and new promising anti-schistosomal drugs is presented.
Keywords: Drugs, praziquantel, schistosomiasis, Schistosoma mansoni, Schistosoma haematobium, schistosome
1. INTRODUCTION
Schistosomiasis continues to be one of the most significant water and vector-borne diseases from a universal public health disposition [1]. This disease is said to follow hookworm infections as the second most prevalent Neglected Tropical Disease (NTD) in sub-Saharan Africa [2]. Currently, schistosomiasis is a debilitating disease that is transmitted in 78 developing countries and affects over 200 million people globally [3, 4]. In the sub-Saharan African region alone, 193 million cases occur mostly due to bad sanitation, poor treatment, and very few control programs. The disease is responsible for the loss of 10.4 million disability adjusted life years (DALYs) and accounts for over 280,000 deaths annually [5]. Additionally, over 800 million people are still vulnerable to this disease [6].
Schistosomiasis, also referred to as bilharziasis [7], is caused by parasitic trematode flatworms found in fresh water belonging to the genus Schistosoma [8]. The lifecycle starts asexually once schistosome eggs are voided into freshwater with the urine or faeces of the definitive host (human) and miracidia are produced from the eggs. These swim freely in water and find their way into the tissue of the intermediate host (snail). In the snail, the miracidia transform into sporocysts that produce many cercariae through asexual reproduction. The infection starts when humans are exposed to waters infested with cercariae, which have the ability to penetrate human skin. Once this takes place, the cercariae lose their bifurcated tails and metamorphose into schistosomulae, which migrate to various tissues such as the heart, liver and lungs. In the liver, the schistosomulae undergo rapid growth and mature into adult worms. Eggs are then produced from mating adults, which are either shed in human excreta, or lodged in various tissues that ultimately lead to various complications of the disease such as genital, pulmonary, hepato-intestinal or neuro-schistosomiasis and other clinical outcomes such as gastrointestinal and hepatic pathologies, anaemia, caloric malnutrition as well as a heightened risk to HIV/AIDS and cancer of the bladder. The worms are able to produce several hundred eggs daily which are all capable of developing into schistosomes. S. mansoni adults lay about 200-300 laterally-spined ovoid eggs daily while S. haematobium and S. japonicum parasites lay 20-200 round, terminally-spined eggs and 500-3500 small and round laterally-spined eggs respectively. As discussed by Hsieh and Mentink-Kane [9], the parasite can stay in host blood vessels for many years and this parasitic ability highlights the Schistosoma species’ aptitude for evasion and prolonged existence in the host immune system.
Not until 1984 when the World Health Organisation Expert Committee proposed chemotherapy, elimination of snails was often used in tackling this chronic debilitating disease [10]. Chemotherapy remains the only means for schistosomiasis control but this has relied solely on one single effective treatment, praziquantel (PZQ), which is now deemed unsatisfactory [11-13]. In recent times, not much approach has been made to develop new drugs for this disease because pharmaceutical companies snub diseases affecting poor nations hence, schistosomiasis was referred to as one neglected tropical diseases. Over the last few decades, many drugs have been used in the treatment of schistosomiasis. In this article, past, recent and currently-used schistosomicides are reviewed and the question of how close we are in the development of effective treatment against this debilitating disease is addressed.
2. PRAZIQUANTEL (PZQ)
Praziquantel-(2-cyclohexylcarbonyl)-1,2,3,6,7,11b-hexa-hydro-4H-pyrazino-(2,1-α), (Fig. 1), marketed under the brand name Biltricide, is a bitter-tasting white crystalline powder. Under normal storage conditions, this drug is stable and practically insoluble in water but soluble in some organic solvents. PZQ was first selected for its action against helminths in the mid-1970s, and was initially used in treating veterinary cestode and trematode infections; subsequently, it was and continues to be used in treating various trematode infections in humans [14]. Over the years, PZQ has remained the best mono-therapeutic agent and drug of choice for all forms of schistosomiasis due to its ready accessibility, inexpensiveness, safety, and high efficacy [11, 15, 16]. However, its cure rate of only 60% to 95%, inability to hinder re-infection [17, 18], ineffectiveness against the juvenile stage of the parasite [19], and resistance have raised concerns.
Fig. (1).

Chemical structure of Praziquantel.
To date, the mechanism of action of PZQ still remains a mystery, thus many researchers have suggested ways in which the drug may be responsible for the parasite’s death. In 2001, Kohn and colleagues [20] hypothesized the drug has a negative effect on the Ca2+ homeostasis of the worm. They speculated that PZQ allows the opening of several channels that lead to the disruption of the interface between the α1/β inside Ca2+ voltage gated channel. Other reports have suggested that PZQ induces muscle contraction and disruption of the tegument system resulting in antigen presentation [21, 22]. PZQ is lipophilic and its action on worm-antigen during exposure may be associated with its interaction on the hydrophobic areas of the tegumental outer membranes [23].
The cure rate for S. mansoni ranges between 60% and 99%. For instance, 25mg/kg bodyweight in two oral doses every 4 hours achieves a cure rate between 63% and 97%. A single oral dose of 40mg/kg bodyweight attains a 72% to 100% cure rate, while 20mg/kg of three divided oral doses every 4 hours kill 71%-99% of the parasites. Yet, a 78.6% to a 90% cure rate may be attained with a single dose of 40mg/kg, while an 84.6% to 98% decrease in egg output among non-cured individuals may be achieved [24]. Intramuscular, oral and intradermal administration of this drug is said to be effective. It is generally a well-tolerated and non-toxic drug. However, nausea, vomiting, hepatomegaly and headache are some of the known negative side effects [25].
3. PAST AND PRESENT DRUGS USED IN THE TREATMENT OF SCHISTOMIASIS
3.1. Metrifonate
Historically, metrifonate (Fig. 2) was used in treating urinary schistosomiasis but the administration of multiple doses in the course of treatment made the drug to lose public approval and acceptance [26]. Metrifonate is an organophos-phorous, 0,0-dimethyl-2,2,2-trichloro-hydroxyethyl-phos-phonate, previously known as trichlorphone and trichlorfone. This compound has variable and selective anti-Schistosoma haematobium activity, due to its incomplete metabolism as an effective acetylcholinesterase inhibitor and organophosphate (dichlorvos). In 1991, Shekhar [27] reported that this drug was the best in treating urinary schistosomiasis caused by S. haematobium but sadly, it showed no activity against other species parasitizing humans [28].
Fig. (2).

Chemical structure of Metrifonate.
The mechanism of action behind this drug exhibiting anti-S. haematobium action is still a mystery. However, an early study by James and colleagues [29] suggested that the inhibition of acetylcholinesterase by metrifonate leads to sweeping of the worm to the lungs where they cannot develop. The results showed that 40% of S. haematobium worms were found in the lungs and 60% in the liver but there was an increase in the quantity of the S. haematobium in the lungs after metrifonate exposure for 2 or 3 days. It was concluded that after 5 days of metrifonate treatment, there was a significant decrease in the quantity of S. haematobium. But, the death of the pharmacologically damaged parasite could also be assumed to be a result of physical factors, inadequate supply of nutrients or by possible cell-mediated mechanisms of cytotoxicity due to the abundance of immunocompetent cells like alveolar macrophages and eosinophils presence in the lungs [30]. One study suggested that metrifonate can cause a stunning effect on the adult parasite. According to Shekhar [27], the S. haematobium worms are stuck, enclosed and killed in the arterioles of the lungs after being stunned.
Moreover, the prescribed oral dosage of metrifonate is 7.5mg/kg to 10mg/kg thrice and it must be administered two weeks apart [30]. In the course of treatment, metrifonate roughly decreases 90-95% of the parasite eggs and a 44% to 93% cure rate is attainable in treated individuals [31]. Metrifonate use can result in minor side effects such as diarrhoea, vomiting, colic, and nausea. Other effects include tiredness, vertigo, syncope, headache, myasthenia, bronchial spasms, sweating and muscular tremor. To date, this drug has been withdrawn from the market as a result of economic, operational and medical standards [32, 33]. However, with optimization, it can be reassessed as the medication or alternative drug for urinary schistosomiasis [34].
3.2. Oltipraz
Oltipraz (OPZ), (Fig. 3), is a synthetic dithiolthione (C8H6N2S3; 5-(2-pyrazinyl)-4-methyl-1,2-dithiol-3-thione) which is similar in structure to the dithiolthiones found in cruciferous vegetables. In humans, OPZ can be used against infections caused by S. intercalatum, S. mansoni, and S. haematobium. Nare and colleagues [35] reported that the action of this drug against schistosomiasis is very slow, taking approximately 2 months to cure. Although the mode of action of this drug is poorly understood, it is assumed that after nine days of use it causes a hepatic shift that moves the parasite to the liver from the mesenteric veins [36]. Exposure of the worm to OPZ causes a reduction in glutathione synthetase (GSS) levels [36, 37], which is assumed to interfere with the metabolism and possible elimination of the worm by the host immune system, probably by decreasing protection against the reactive oxygen intermediates [38]. Other studies have documented that OPZ assists the host to increase its detoxification ability [36, 39]. According to Shekhar [27], an oral dose of OPZ, 3.0 g to 4.5 g three times a day for curative treatment and a dose of 35 mg/kg twice a day only produces 90% cure in S. mansoni infected individuals. Gentilini and colleagues [40] stated that a dose of 1.25 to 4.50g should be administered for S. intercalatum infection for 3 days to achieve a cure rate of 76.5% to 92%. While on the other hand, a dose of 25mg/kg should be administered for 1 or 2 days to achieve a cure rate of 86% to 94% in S. haematobium infected persons [40]. Nausea, vomiting, weakness, stomach pain and insomnia are the frequent side effects of OPZ. At present, OPZ is currently not available in the market for schistosomiasis treatment again due to its induced photosensitivity [39].
Fig. (3).

Chemical structure of Oltipraz.
3.3. Niridazole
Niridazole (1-(5-nitro-1,3-thiazol-2-yl)imidazolidin-2-one (Fig. 4), is an orally administered anti-schistosomal agent, which shows activity against all the three major schistosome species [41]. This drug is shown to be an effective long-lasting suppressor of delayed intolerance [42]. In addition to its anti-schistosome effects, Moczon and Swiderski [43] documented that this drug destroys schistosomes by decreasing glycogen levels of the parasite, thereby inhibiting glucose and lactate uptake and is also responsible for the degeneration of the female reproductive system. This drug acts by taking up [14C]-niridazole which binds covalently and leads to nitro-reduction and subsequent bond formation with the parasite macromolecules [44, 45, 46].
Fig. (4).

Chemical structure of Niridazole.
The prescribed dose is 25mg/kg each day for a week or 35mg/kg daily for 5 days [45]. It shows more activity against S. haematobium infections with 80%-100% cure rate mostly in children and approximately 50% and 30%-70% cure rate in S. japonicum and S. mansoni infections respectively. Niridazole side effects include vertigo, skin rashes, non-specific destruction of the T waves in the electrocardiogram (ECG), nausea, diarrhoea, brown urine and vomiting. Other unpleasant effects include the central nervous system (CNS) and renal toxicity besides alteration of digestive and cardiac functions [47]. An in vivo study by Urman and co-workers [48] showed that this drug is carcinogen. Therefore, due to the many adverse effects attributed to this drug, the populace and medical practitioners have abandoned it in the treatment of schistosomiasis.
3.4. Oxamniquine
Oxamniquine (6-hydroxymethyl-2-isopropyl-aminomet-hyl-7-nitro-1,2,3,4 tetrahydroquinoline), Fig. (5) is the only drug active against S. mansoni worms, particularly the male parasite, but has no effect on S. haematobim or S. japonicum worms [49]. This is due to the fact that the conversion of this drug to its active form requires the activity of sulfotransferase [50], which is only available in the S. mansoni parasite. When converted to its active state (sulfate ester), it dissociates non-enzymatically and alkylates schistosome DNA leading to the inhibition of nucleic acid production, disruption of protein synthesis, delayed destruction and death of the parasites [46, 51]. Over the last two decades, this drug has been the main drug for treating S. mansoni infection in South America [52]. However, resistance to oxaminiquine has been reported in Brazil [53], which may be as a result of alteration in the schistosome gene that encodes the esterifying enzyme [54]. Currently, praziquantel has replaced oxaminiquine as a schistosomicide, not only because of its effectiveness but also largely due to its cost effectiveness [52]. For three consecutive days, oral doses of 20mg/kg bodyweight of oxaminiquine can be administered and depending on the geographic area, this dose provides a cure rate of 80-90%. Some of the negative side effects of this drug include fever, headache, drowsiness, dizziness, convulsions and occasional orange-red urine discoloration.
Fig. (5).

Chemical structure of Oxamniquine.
3.5. Mefloquine
Mefloquine (Fig. 6), an aryl-amino-quinoline used in the treatment of malaria, has been reported to show effective activity against various stages of the schistosome parasite in vivo [55]. In 2008, this antimalarial was used for the first time to treat schistosomiasis by Van Nassauw and colleagues [56] who documented that a dose of 150 mg/kg caused a significant reduction in S. mansoni eggs in infected mice [56]. In vivo investigations further revealed that mefloquine shows good activity against S. mansoni at a single dose of 200 mg/kg resulting in 72.3% total worm burden reduction [57]. On the contrary, oral administration of a higher dose at 400 mg/kg achieved 86.7% and 95.1% worm burden reduction of both immature and mature female worms in infected mice [58]. Although the mechanism of its anti-schistosomal activity has not been investigated, interference with the digestion of hemoglobin and raising intravacuolar pH, have both been suggested to play a role in its mechanism of action against Plasmodium [59]. In addition, mefloquine possesses a wide range of antimicrobial activity as it shows activity against larval and adult stages of Brugia malayi and Brugai patei [58]. It has a high tolerability rate in children and adults with dose-dependent negative effects like gastrointestinal disorders and neuropsychiatric side effects [60]. Therefore, the efficacy of mefloquine in the treatment of schistosomiasis deserves further and extensive study.
Fig. (6).
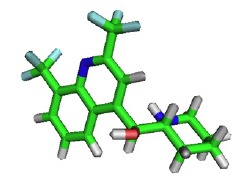
Chemical structure of Mefloquine.
3.6. Artemisinin
Artemisinin (qinghaosu), (Fig. 7), is a key ingredient extracted from Artemisia annua plant leaves, a plant endemic to China, USA, Argentina and Central Europe [61]. It is a sesquiterpene lactone possessing a peroxide bridge that is considered to be the active pharmacophore. This compound constitutes a class of potent drugs used in the treatment of malaria which is known for their good safety profile and tolerance [62, 63]. Thus far, artemisinin‐based combination therapies (ACTs) have been documented as the most potent antimalarial drugs [64]. Interestingly, artemisinin derivatives such as artemether, artesunate, dihydroartemisinin and arteether have been reported in several studies to possess anti-schistosomal activity, both in human and animal experiments [65-67]. In areas of highly endemicity such as in China and Cote d’Ivoire, S. japonicum and S. mansoni infections have been effectively controlled with the administration of artemether in human trials [68].
Fig. (7).

Chemical structure of Artemisinin.
In contrast to PZQ, which shows the highest activity against adult worms [69, 70], artemether shows the highest activity against the juvenile worms of the three main species affecting humans, while leaving the invasive and adult stages of the worm less vulnerable [71, 72]. Thus, joint treatment with PZQ and artemether would cover the entire lifetime of the parasite in its definitive host [73] because once PZQ kills the adult worms, artemether will then subsequently kill the surviving schistosomula, which would have repopulated the host resulting in the abolishment of reinfection. Therefore, it was concluded that ACTs stimulate the destruction of both the juvenile parasite tegument and adult parasite [74].
Moreover, artemisinin derivatives can affect the gut heme in the parasite resulting in heme alteration to an unstable species which can generate reactive oxygen species (ROS) with subsequent worm death [75]. A dose of 6mg/kg is given once in every 2-3 weeks [76].
3.7. Hycanthone
Hycanthone, 1-(2-(diethylamino) ethylamino)-4(hydro-xymethyl)-thioxanthen-9-one (Fig. 8), is a hydroxylated version of lucanthone. Although this drug is no longer in clinical use, studies have documented that when administered in a single intramuscular dose of 3 mg/kg, hycanthone is effective against S. mansoni and S. haematobium parasites [77] but shows no activity against S. japonicum worms [78]. According to Cioli and Knopf [79], male parasites are more susceptible to this treatment than females. In mice, the hepatic shift hits the highest point at approximately 6 days post-treatment [80]. It is believed that this drug irreversibly binds to acetylcholine receptors, therefore paralyzing the digestive system of the parasite, leading to starvation, followed by the death of S. mansoni worms [81]. During hycanthone therapy, Senft and co-workers [82] observed loss of hemoglobin pigment from the gastrointestinal tract, weakening of the tegument, and a decrease in blood volume, as well as body size in S. mansoni worms. However, it was also observed that this drug can be carcinogenic and can induce liver damage [83, 45].
Fig. (8).
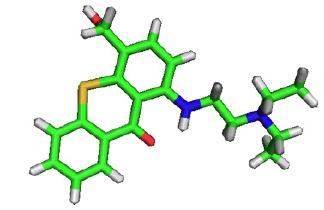
Chemical structure of Hycanthone.
3.8. Combined Chemotherapy for Schistosomiasis
Combination therapy was first used in the treatment of tuberculosis and subsequently it has been adapted for cancer, AIDs, malaria and Schistosomiasis; basically to delay the development of drug resistance and accomplish an additive/synergism therapeutic effect [32, 85]. This is imperative because once an addictive/synergism effect is presented, getting similar or higher efficacy at lower doses with transient side effect can be attainable. According to Gouveia et al. [85], when combining drugs for schistosomiasis, the drugs should possess a different mode of action to PZQ and target the juvenile worm to reduce egg burden and enhance cure. Rational combination of an artemisinin derivative with another anti-malarial drug with contrasting mechanism of action was employed in effective prevention of appearance and spread of drug resistance in Southeast Asia, which might have influenced the stoppage of falciparum malaria transmission in the area [86, 87].
Plethora researchers have combined PZQ and OXA to alleviate schistosomiasis. A study carried out in Malawi showed that combination of PZQ (15 – 20 mg/kg) and OXA (7.5 – 10 mg/kg) achieved significant reduction (about 93% to 99%) in the total number of schistosome eggs laid in 102 schoolchildren infected with S. mansoni. Similarly, a markedly reduction (97% to 99.2%) in eggs laid was recorded in 56 schoolchildren infected with S. haematobium [88]. This finding is somewhat surprising because OXA has been well-known to show no activity against S. haematobium. The combination was safe with only a few negative effects which were self-limiting. Equally, another study was conducted in Zimbabwe among 58 school aged children (7 to 16 years) concurrently infected with S. mansoni and S. haematobium. Co-administration of these drugs was done in three doses (PZQ 8 mg/kg + OXA 4 mg/kg; PZQ 15 mg/kg + OXA 7.5 mg/kg and PZQ 20 mg/kg + OXA10 mg/kg), notably, PZQ 20 mg/kg + OXA10 mg/kg achieved a high cure rate of about 89% in children suffering from S. mansoni infection. Interestingly, among the schoolchildren infected with S. haematobium treated with the varying doses of PZQ-OXA combined therapy, there was no curative evidence although there was a high reduction in egg count for both Schistosoma species (S. mansoni and S. haematobium) in schoolchildren in this study. Hence, it was concluded that the PZQ-OXA combination for the treatment of Schistosoma infection in the Zimbabwean children does not have curative advantage over the use of PZQ alone [89].
In the early 1980s, artemisinin and its derivatives were discovered to show activity against schistosomes, particularly the larval stage and this compound and its derivatives have assisted crucially in the prevention and treatment of human S. japonicum in China [90]. Thus, the combination therapy involving PZQ and artemisinin derivatives may be effective against all stages of the worm development, this treatment regimen may ultimately overcome the emergence of drug resistance recently experienced in the treatment of some cases of Schistosoma infection. Different host-parasite models have been used to test the efficacy of PZQ and artemether (ART) combined therapy. In rabbits infected with schistosomula and adult schistosomes, treatment with 50 mg/kg of PZQ and 15 mg/kg of ART led to reduction in total worm burden (82%) which is significantly higher than results achieved from PZQ (66%) and ART (44%) monotherapies [91]. Adult rabbits infected with S. japonicum were used to confirm these results and PZQ-ART combination achieved 96% - 99% total worm burden reduction and 99% - 100% female worm burden reduction [92]. Therefore, combination of PZQ and ART is safe at lower doses and more effective than PZQ and ART monotherapies. Administration of PZQ-ART combined therapy in another study using mice experimentally infected with different developmental stages of S. mansoni, recorded over 90% worm reduction with a remarkable total absence of eggs from tissues and insignificant changes in the histopathology of the liver [93].
In another study to ascertain the efficacy of PZQ and artesunate (AS), animal models were used. Remarkably, AS rendered the female worms sterile by impairing their fecundity. Co-administration of PZQ and AS was found to significantly reduce total worm count, total eradication of female worms and egg count on tissue [85]. Similarly, a non-blinded open-label treatment trial in Senegal enrolling 110 patients who were positive for S. mansoni infection. Patients in the age-range between aged 1 and 60 years was used to determine the efficacy of PZQ-AS combined therapy. Each person was treated with either a single dose of PZQ (40 mg/kg), AS endorsed dose for malaria treatment (4 mg/kg followed by 2 mg/kg × 4 daily dose), or a co-administration of these two drugs. At 5, 12 and 24 weeks after treatment, cure and egg reduction rates were assessed by examining two Kato-Katz thick smears taken from a single stool sample. Northern Senegal is considered as one of the severe transmission areas of S. mansoni, hence, the rate of re-infection is high and rapid. In this regard, the curative efficacy achieved at 5weeks following treatment will be considered here. The PZQ-AS administration resulted in cure and decrease in the number of eggs (69% and 89%), which is higher than cure and egg reduction rate achieved with monotherapies of PZQ and AS [94].
Another study carried out in Nigeria by Inyang-Etoh and co-workers [95] where they administered PZQ-AS to treat urogenital schistosomiasis. Randomly, they selected schoolchildren aged 4 to 20 years and were treated with PZQ-placebo, AS-placebo, PZQ (40 mg/kg), AS (4 mg/kg) or both 40 mg/kg of PZQ and 4 mg/kg of AS. Co-administration achieved high cure (88.6%) and egg reduction rate (93.6%), conversely PZQ achieved cure rate of 72.7% while AS achieved 70.5% [95].
Furthermore, the efficacy of combined treatments using PZQ-mefloquine (MFQ) and MFQ-artemisinin derivatives have been investigated both in vitro and in vivo and in human clinical trials. El-Lakkany et al [96] reported the pharmacodynamics of PZQ-MFQ in mice infected with S. mansoni. They showed that 200 mg/kg dose of PZQ and 200 mg/kg of MFQ boosted therapeutic efficacy over PZQ dose alone by causing a great reduction in the total numbers of adult worms (PZQ-MFQ = 95%, PZQ alone = 49%), juvenile worms (PZQ-MFQ = 96%, PZQ alone = 29%) and a nearly complete elimination of immature eggs, juvenile females and adult females. The decrease in worm burden aided in repairing lesions on the hepatic granulomatous, restoration of liver histology and normalization of the liver function. In mice experimentally infected with S. japonicum, the efficacy of MFQ at a single dose, multiple doses or concomitant administration with AS, ART or PZQ was examined 4 weeks following treatment [97]. Combined administration of 50 mg/kg or 100 mg/kg of MFQ with either ART or AS (100 mg/kg) completely eradicated female parasite with MFQ-ART showing more curative efficacy. Better results were achieved with 100 mg/kg combined chemotherapy than sole chemotherapy. Combined therapy caused a significant reduction of total worm; 100 mg/kg each of MFQ and AS achieved 76.7% and 100 mg/kg each of MFQ and ART achieved 87.8% while sole therapies achieved; 100 mg/kg AS (59.8%) versus 100 mg/kg MFQ (67.9%) and 100 mg/kg ART (55.6%). The result proposes a synergistic effect between artemisinin and MFQ [97] (Table 1).
Table 1. Different anti-schistosomal drugs and their mechanisms of action.
| Drug Tested | Parasite | Study Type (in vitro/in vivo) | Dose | Route | Mechanism | Reference |
|---|---|---|---|---|---|---|
| Metrifonate | Schistosoma haematobium | Hamster (in vivo) |
7.5mg/kg to 10mg/kg × 3 for 2 wks | Oral | Inhibition of acetylcholinesterase | [29, 30] |
| Oltipraz | S. intercalatum, S. mansoni and S. haematobium | Mice (in vivo) |
3.0 - 4.5 g/day × 3. Curative treatment: 35 mg/kg/day × 2 = 90% cure in S. mansoni. 1.25 - 4.50g for 3 days in S. intercalatum. S. haematobium, 25mg/kg × 1 or 2 days | Oral | Causes hepatic shift | [40, 27] |
| Niridazole | S. japonicum, S. mansoni and S. haematobium | Mice (in vivo) |
25mg/kg each day × 1week or 35mg/kg daily × 5 days. | Oral | Takes up [14C]-niridazole, binds covalently leading to nitroreduction and subsequent bond formation with the parasite macromolecules | [44, 45, 46] |
| Oxamniquine | S. mansoni | Mice (in vivo) |
20mg/kg × 3days | Oral | Converts to its active type sulfate ester which alkylate the schistosome DNA | [46, 51] |
| Artemisnin | S. japonicum, S. mansoni and S. haematobium | Human (in vivo) |
6mg/kg once every 2–3 weeks | Oral | Affect the gut heme in the parasite resulting in heme alteration to an unstable species which can generate ROS with subsequent worm death | [75]. |
| Praziquantel | S. japonicum, S. mansoni and S. haematobium | Human (in vivo) |
Single dose of 40 mg/kg | Oral | Disruption worm Ca2+ homeostasis Impairment of the worm tegument |
[84, 24, 20] |
4. OTHER DRUGS USED AGAINST SCHISTOSO-MIASIS IN THE PAST AND POTENTIAL FUTURE DRUGS
Mirazid is a commercial product extracted from myrrh, an aromatic gum resin. This extract was proposed as an alternative to PZQ as it elicits anti-schistosomal activity by extravasation and uncoupling of the parasite [98]. Earlier studies have shown it to be a promising emerging drug with low toxicity relative to PZQ. For example, Sheir and colleagues [99] showed an initial 91.7% cure rate in S. mansoni at a dose of 10mg/kg three times daily and a 98.1% cure rate after 2 months of initial treatment (6mg/kg × 6 daily). However, throughout the years, results from various studies on this drug have been inconsistent, which has led to its use being stopped by the World Health Organisation (WHO) [100].
Meclonazepam (3-methylclonazepam), a derivative of benzodiazepine developed and patented in 1977 by Hoffman-La Roche, has also been demonstrated by experimental investigations to possess anti-schistosomal activity and relatively long plasma half-life of approximately 40 hours [101]. Studies have established that this drug is very effective against S. mansoni strains and its potency is similar to that of oxamniquine, hycanthone and niridazole [102]. A single dose of 0.3 mg/kg will successfully cure parasitic infections with harsh side-effects like drowsiness, dizziness, slurred speech, ataxia, muscle weakness, reduced mental alertness, and lateral nystagmus [103].
5. THE USE OF NATURAL PRODUCTS AGAINST SCHISTOSOMIASIS
For centuries, plants have not only been used to cater for the basic needs and livelihood of man such as food, shelter, clothing, biologically active products, fertilizers, flavours, fragrances, but also for curative purposes when used as medicines [104, 105]. Steadily old medicines are now becoming the modern and state-of-the-art drugs of today. It has been documented that more than 80% of Africa, Asia, and Latin America’s medicinal needs have depended heavily on traditional and herbal medicines [106]. However, modern technology and orthodox medicine are currently raising interest and getting involved in these sources of alternative health care. However, in the absence of vaccine treatment for schistosomiasis, investigators are now considering natural products as new, inexpensive and effective alternatives to PZQ.
Several plants have been used in traditional African medicine as molluscicides and for curing schistosomiasis [107]. Most of the plants that have been documented for their anti-schistosomal activities and potentials have mostly been in the form of indigenous knowledge passed down through numerous generations, mostly by traditional healers. A study by Ndamba and colleagues [108] successfully interviewed 286 traditional healers from five provinces in Zimbabwe, with 85% of them registered with the largest association of traditional healers in the country– the Zimbabwe National Traditional Healers’ Association (ZINATHA) [108]. The traditional healers reported 47 anti-schistosomal plants, of which 8 were identified as those most commonly used for treatment based on the healers’ knowledge of the urinary form of schistosomiasis. These include Ximenia caffra (Olacaceae), Dicoma anomala (Compositae), Phaseolas vulgaris (Leguminosae), Lannea edulis (Anacardiaceae), Elephantorrhiza goetzei (Leguminosae), Abrus precatorious (Leguminosae), Ozoroa insignis (Anacardiaceae), and Pterocarpus angolensis (Leguminosae).
Furthermore, animal studies using extracts from these eight plants, showed the latter three plants presenting positive lethal activity against adult schistosomes. Further studies by Molgaard and co-workers [109] investigated 23 of these plants by testing their leaf, stem, root, fruit and bark extracts in vitro. Arbus precatorius and Elephantorrhiza goetzei exhibited the best results against schistosomulae and this was attributed to the presence of natural compounds such as tannins, steroids, terpenes, flavonoids and indole alkaloids in the Arbus species.
Terpenoids e.g. (+)-limonene epoxide (extract from Citrus sinensis), Tashinones (cryptotashinone, tanshinone I and IIA), alkaloids (e.g. imidazole alkaloid epiisopiloturine from Pilocarpus microphyllus), quinoline methanols (e.g. quinidine), flavanoids (alpinum isoflavone from Millettia thonningii), arachidonic acid (e.g. oils from Mortierella alpine) Quinones (e.g. plumbagin from Plumbago scandens) and other natural products such as Aspidin, Desaspidin, Flavaspidic acid and Anisomycin are a few examples of plant products that have shown activity against schistosomes [110].
The schistosomicidal activity of some of these compounds has been shown to disrupt the mating process, diminish egg production, and increases the chances of the worms dying as well as affect the parasite’s motor activity and tegument. Other potential anti-schistosomal natural products not only take into account the ability of the worm to migrate to different parts of the body and resist host immune responses, but also consider the ability of the compound itself to kill the worms, the duration taken to do so and any reversible effects once the drug has ceased from being used [111]. These natural products include Epiisopilotulorine (from Pilocarpus microphylus), Piplartine (Piper tubercalatum), Phytol (found in chlorophyll), Phloroglucinols (Dryopteris species), Cinchona Alkaloids, Vernonia amygdalina (Asteraceae), Emetine (from Cephaelis ipecacuanha), Mevinolin (Lovastatin), Plumbagin and Sangunarine.
Familiar plants that have been used for several years as spices or general ingredients in food have also been tested and shown to exhibit anti-schistosomal properties. These include Allium sativum (garlic), pumpkin seeds, peppermint, olive leaves, wormwood, thyme, black walnut, berberine and endod, among others [112]. Garlic has shown activity against schistosomes by causing wrinkling and detrimental effects to the tegument of the worm and severe damage to the parasite tubercles by causing shortness and loss of the spines and thorns [98, 100]. Ginger (Zingiber officinal) on the other hand has been shown to not only kill adult worms, but to also interfere with the production of eggs and worm recovery [98]. Recently, it has been demonstrated that the plant can also ameliorate oxidative stress by increasing chloram-phenicol acetyltransferase (CAT) activity in the liver of infected mice as well as cause an increase in glutathione (GSH) and superoxide dismutase (SOD) antioxidant levels just like PZQ [113]. Curcumin, the principal curcuminoid of turmeric from the Zingiberaceae, has been documented with other compounds such as vernodali, piplartina, artesunate, artemether, artemisina and avocado and soybean oils, to induce the separation of the male from the female and to disrupt the release of eggs [111, 114]. Other effects include the death of adult worms and a decrease in motor activity [110].
Other compounds such as propolis (a glue-like substance that bees collect from plants and tree barks) have also been suggested to be effective in treating schistosomiasis when in synergy with each other natural products or with PZQ. Studies have shown administration of this substance to infected mice significantly reduced schistosomula and the number of eggs in the liver and intestines [115]. However, incomplete eradication was also observed and hence, it has been suggested that propolis in conjunction with PZQ, could result in an effective anti-schistosomal agent. Added to this, a mefloquine-artesunate combination has shown effective anti-schistosomal properties against S. haematobium infected children, especially since both compounds each exhibit anti-schistosomal properties [116]. Additionally, this drug combination can clear malaria infection and reduce schistosomiasis-related illnesses.
6. EMERGING DRUGS IN FIGHTING SCHISTOSO-MISIS
Knowing that chemotherapy for schistosomiasis is still fragmentary; drug repurposing can be an alternative strategy to finding a cure for this acute disease. Drug repositioning involves investigating existing drugs and its application in treating non-related diseases for which they were not originally designed [117]. Although several researchers have studied various classes of drugs for anti-schistosomal effects, drugs used in treating cancers have also shown promising effects. For instance, miltefosine, an alkylphosphocholine with anticancer activities, showed significant activity against the larval and adult stages of the S. mansoni worm [118]. An in vivo study by Eissa and co-workers [119], showed miltefosine interfered with the S. mansoni lifecycle. A dose of 20 mg/kg orally administered daily for five days in invasive, immature or mature S. mansoni infected mice, showed a significant decrease in hepatic granulomata size and a reduction in worm burden [119]. Additionally, a related study showed that using miltefosine and lipid nanocapsules (LNCs) as oral nanovectors resulted in a potent anti-schistosomal effect and a significant worm burden decrease in invasive and immature S. mansoni infected mice [120].
More so, imatinib (Gleevec®) is a kinase inhibitor employed in treating chronic myeloid leukemia; a disease caused by constitutive expression of gastrointestinal stromal tumour and active c-Kit kinases or deregulated Abi kinase activity [121]. Lately, imatinib has attracted attention in schistosomicide drug discovery as a result of its in vitro time and dose-dependent effect in S. mansoni morphology and physiology [122, 123]. Beckmann and Grevelding [122] showed that this drug causes pathological changes in the gonad and gastrodermis in both male and female worms, which leads to the death of the parasite. Additionally, it has been shown to have a remarkable effect on both the ovary and testes. Thus, further research on kinases is indispensable because their inhibitors have the ability to interfere with schistosome biology, thereby making them an attractive compound in new schistosomicide discovery.
Chlorambucil is another anticancer drug that has exhibited anti-schistosomal activity. It is an alkylating agent used in the treatment of chronic lymphocytic leukemia, low-grade non-Hodgkins lymphoma and Hodgkin’s disease [124]. According to Eissa and co-workers [125], this drug has negative and favourable in vivo and in vitro activity on schistosomes. In an in vivo experiment, this drug expressed a significant decrease in all worm burdens, achieving the best results against the juvenile worm where it attained a decrease in intestinal egg count, hepatic egg count and total worm load of 89.2%, 86.7% and 75.8% respectively [125]. Furthermore, a progressive decrease was also displayed in the parasite’s viability in a dose-dependent manner [125].
7. UNIVERSAL STRESS PROTEINS (USPS)
USPs are a group of proteins present in various organisms that include fungi, archaea, bacteria, yeast, protists, and plants [126, 127]. Their up-regulation enables schistosomes to tolerate diverse and mainly harsh ambiences such as high salinity, toxic chemicals and high temperatures during its developmental cycle. However, in the human genome, the genes encoding USPs have not been characterized, therefore making USPs a potential drug target against schistosomiasis [128, 129]. Additionally, their absence from the human host makes them an interesting vaccine target for the disease. It has been suggested that the schistosomulum parasitic stage, which is more prone to oxidative stress than other parasitic stages due to the production of nitric oxide and hydrogen peroxide by the human host, voids the human immune response through the action of USPs and goes through a transition in morphology and adaptation to a different environment, which are both needed for the survival of the parasite.
8. ANTIMICROBIAL PEPTIDES (AMPS)
AMPs are a subset of proteins that forms part of the innate immune system [130, 131]. They serve as the primary defence line in many organisms and can act as signalling molecule, immunomodulatory agent, antitumor agent and drug delivery vector [132, 133]. One of the mechanisms schistosomes use is to reduce the efficacy of the host immune system. However, AMPs have the ability of stimulating the immune system, thereby causing resistant to the disease. In addition, AMPs can scavenge the ROS produced by the schistosomes. In respect to their various mechanisms of actions and characteristics, AMPs was proposed as novel drug targets in drug design and discovery [7].
CONCLUSION
Schistosomiasis is a disease that affects underprivileged rural communities particularly those located in areas where fishing and farming activities are the major occupations. Sadly, pharmaceutical companies have ignored developing drugs for this disease because it usually affects poor people from rural areas who cannot afford highly-priced drugs and good health systems. Therefore, it is important to develop new and affordable anti-schistosomal drugs that are structurally and functionally different from PZQ so as to alleviate the pressure of existing drug resistance by the worm. Moreover, combined chemotherapy based on merging compounds such as artemether and PZQ would serve as an advantage as both would cover all developmental stages of the schistosome worm and the rapid development of PZQ resistance would be tackled. Overall, more detailed knowledge and understanding of the schistosome biology is needed in order to speed up the design and development of new drugs.
AUTHORS CONTRIBUTION
Raphael Taiwo Aruleba and Abidemi Paul Kappo conceived and designed the study. RTA wrote the first draft of the manuscript. Tayo Alex Adekiya and Babatunji Emmanuel Oyinloye enriched, proofread and edited the first draft. Raphael Taiwo Aruleba, Tayo Alex Adekiya, Priscilla Masamba and Londiwe Simphiwe Mbatha did second proofreading. Babatunji Emmanuel Oyinloye, Ashley Pretorius and Abidemi Paul Kappo provided administrative support and critically revised the manuscript. All authors read and approved the final manuscript.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
ACKNOWLEDGEMENTS
The support of the University of Zululand Research Committee to the corresponding author is greatly acknowledged.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Steinmann P., Keiser J., Bos R., Tanner M., Utzinger J. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect. Dis. 2006;6(7):411–425. doi: 10.1016/S1473-3099(06)70521-7. [DOI] [PubMed] [Google Scholar]
- 2.Adenowo A.F., Oyinloye B.E., Ogunyinka B.I., Kappo A.P. Impact of human schistosomiasis in sub-Saharan Africa. Braz. J. Infect. Dis. 2015;19(2):196–205. doi: 10.1016/j.bjid.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lai Y.S., Biedermann P., Ekpo U.F., Garba A., Mathieu E., Midzi N., Mwinzi P., N’Goran E.K., Raso G., Assaré R.K., Sacko M., Schur N., Talla I., Tchuenté L.A., Touré S., Winkler M.S., Utzinger J., Vounatsou P. Spatial distribution of schistosomiasis and treatment needs in sub-Saharan Africa: a systematic review and geostatistical analysis. Lancet Infect. Dis. 2015;15(8):927–940. doi: 10.1016/S1473-3099(15)00066-3. [DOI] [PubMed] [Google Scholar]
- 4.Sokolow S.H., Wood C.L., Jones I.J., Swartz S.J., Lopez M., Hsieh M.H., Lafferty K.D., Kuris A.M., Rickards C., De Leo G.A. Global Assessment of Schistosomiasis Control Over the Past Century Shows Targeting the Snail Intermediate Host Works Best. PLoS Negl. Trop. Dis. 2016;10(7):e0004794. doi: 10.1371/journal.pntd.0004794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Werf M.J., de Vlas S.J., Brooker S., Looman C.W.N., Nagelkerke N.J.D., Habbema J.D.F., Engels D. Quantification of clinical morbidity associated with schistosome infection in sub-Saharan Africa. Acta Trop. 2003;86(2-3):125–139. doi: 10.1016/S0001-706X(03)00029-9. [DOI] [PubMed] [Google Scholar]
- 6.Mutengo M.M., Mwansa J.C.L., Mduluza T., Sianongo S., Chipeta J. High Schistosoma mansoni disease burden in a rural district of western Zambia. Am. J. Trop. Med. Hyg. 2014;91(5):965–972. doi: 10.4269/ajtmh.13-0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oyinloye B., Adenowo F., Gxaba N., Kappo A. The promise of antimicrobial peptides for treatment of human schistosomiasis. Curr. Drug Targets. 2014;15(9):852–859. doi: 10.2174/1389450115666140807154810. [DOI] [PubMed] [Google Scholar]
- 8.Merrifield M., Hotez P.J., Beaumier C.M., Gillespie P., Strych U., Hayward T., Bottazzi M.E. Advancing a vaccine to prevent human schistosomiasis. Vaccine. 2016;34(26):2988–2991. doi: 10.1016/j.vaccine.2016.03.079. [DOI] [PubMed] [Google Scholar]
- 9.Hsieh M.H., Mentink-Kane M.M. Smallpox and dracunculiasis: the scientific value of infectious diseases that have been eradicated or targeted for eradication. Is schistosomiasis next? PLoS Pathog. 2016;12(1):e1005298. doi: 10.1371/journal.ppat.1005298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colon C.P. Schistosomiasis. Medicine. 2005;33:64–67. [Google Scholar]
- 11.Fenwick A., Savioli L., Engels D., Robert Bergquist N., Todd M.H. Drugs for the control of parasitic diseases: current status and development in schistosomiasis. Trends Parasitol. 2003;19(11):509–515. doi: 10.1016/j.pt.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 12.Caffrey C.R. Chemotherapy of schistosomiasis: present and future. Curr. Opin. Chem. Biol. 2007;11(4):433–439. doi: 10.1016/j.cbpa.2007.05.031. [DOI] [PubMed] [Google Scholar]
- 13.Doenhoff M.J., Cioli D., Utzinger J. Praziquantel: mechanisms of action, resistance and new derivatives for schistosomiasis. Curr. Opin. Infect. Dis. 2008;21(6):659–667. doi: 10.1097/QCO.0b013e328318978f. [DOI] [PubMed] [Google Scholar]
- 14.Aragon A.D., Imani R.A., Blackburn V.R., Cupit P.M., Melman S.D., Goronga T., Webb T., Loker E.S., Cunningham C. Towards an understanding of the mechanism of action of praziquantel. Mol. Biochem. Parasitol. 2009;164(1):57–65. doi: 10.1016/j.molbiopara.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hagan P., Appleton C.C., Coles G.C., Kusel J.R., Tchuem-Tchuenté L.A. Schistosomiasis control: keep taking the tablets. Trends Parasitol. 2004;20(2):92–97. doi: 10.1016/j.pt.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 16.Vale N., Gouveia M.J., Rinaldi G., Brindley P.J., Gärtner F., Correia da Costa J.M. Praziquantel for schistosomiasis: single-drug metabolism revisited, mode of action, and resistance. Antimicrob. Agents Chemother. 2017;61(5):e02582–e16. doi: 10.1128/AAC.02582-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gryseels B., Nkulikyinka L., Coosemans M.H. Field trials of praziquantel and oxamniquine for the treatment of schistosomiasis mansoni in Burundi. Trans. R. Soc. Trop. Med. Hyg. 1987;81(4):641–644. doi: 10.1016/0035-9203(87)90439-1. [DOI] [PubMed] [Google Scholar]
- 18.Magnussen P. Treatment and re-treatment strategies for schistosomiasis control in different epidemiological settings: a review of 10 years’ experiences. Acta Trop. 2003;86(2-3):243–254. doi: 10.1016/S0001-706X(03)00045-7. [DOI] [PubMed] [Google Scholar]
- 19.Cioli D., Pica-Mattoccia L., Basso A., Guidi A. Schistosomiasis control: praziquantel forever? Mol. Biochem. Parasitol. 2014;195(1):23–29. doi: 10.1016/j.molbiopara.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 20.Kohn A.B., Anderson P.A., Roberts-Misterly J.M., Greenberg R.M. Schistosome calcium channel beta subunits. Unusual modulatory effects and potential role in the action of the antischistosomal drug praziquantel. J. Biol. Chem. 2001;276(40):36873–36876. doi: 10.1074/jbc.C100273200. [DOI] [PubMed] [Google Scholar]
- 21.Harder A., Goossens J., Andrews P. Influence of praziquantel and Ca2+ on the bilayer-isotropic-hexagonal transition of model membranes. Mol. Biochem. Parasitol. 1988;29(1):55–59. doi: 10.1016/0166-6851(88)90119-3. [DOI] [PubMed] [Google Scholar]
- 22.Ribeiro-dos-Santos G., Verjovski-Almeida S., Leite L.C. Schistosomiasis - A century searching for chemotherapeutic drugs. Parasitol. Res. 2006;99(5):505–521. doi: 10.1007/s00436-006-0175-2. [DOI] [PubMed] [Google Scholar]
- 23.Harnett W., Kusel J.R. Increased exposure of parasite antigens at the surface of adult male Schistosoma mansoni exposed to praziquantel in vitro. Parasitology. 1986;93(Pt 2):401–405. doi: 10.1017/S0031182000051568. [DOI] [PubMed] [Google Scholar]
- 24.Friis H., Byskov J. The effect of praziquantel against Schistosoma mansoni-infections in Botswana. Trop. Geogr. Med. 1989;41(1):49–51. [PubMed] [Google Scholar]
- 25.Montero R., Ostrosky P. Genotoxic activity of praziquantel. Mutat. Res. 1997;387(3):123–139. doi: 10.1016/S1383-5742(97)00027-6. [DOI] [PubMed] [Google Scholar]
- 26.Utzinger J., Xiao S., Keiser J., Chen M., Zheng J., Tanner M. Current progress in the development and use of artemether for chemoprophylaxis of major human schistosome parasites. Curr. Med. Chem. 2001;8(15):1841–1860. doi: 10.2174/0929867013371581. [DOI] [PubMed] [Google Scholar]
- 27.Shekhar K.C. Schistosomiasis drug therapy and treatment considerations. Drugs. 1991;42(3):379–405. doi: 10.2165/00003495-199142030-00004. [DOI] [PubMed] [Google Scholar]
- 28.Borrmann S., Szlezák N., Faucher J.F., Matsiegui P.B., Neubauer R., Binder R.K., Lell B., Kremsner P.G. Artesunate and praziquantel for the treatment of Schistosoma haematobium infections: a double-blind, randomized, placebo-controlled study. J. Infect. Dis. 2001;184(10):1363–1366. doi: 10.1086/324004. [DOI] [PubMed] [Google Scholar]
- 29.James C., Webbe G., Preston J.M. A comparison of the susceptibility to metrifonate of Schistosoma haematobium, S. mattheei and S. mansoni in hamsters. Ann. Trop. Med. Parasitol. 1972;66(4):467–474. doi: 10.1080/00034983.1972.11686848. [DOI] [PubMed] [Google Scholar]
- 30.Feldmeier H., Chitsulo L. Therapeutic and operational profiles of metrifonate and praziquantel in Schistosoma haematobium infection. Arzneimittelforschung. 1999;49(7):557–565. doi: 10.1055/s-0031-1300462. [DOI] [PubMed] [Google Scholar]
- 31.King C.H., Lombardi G., Lombardi C., Greenblatt R., Hodder S., Kinyanjui H., Ouma J., Odiambo O., Bryan P.J., Muruka J. Chemotherapy-based control of schistosomiasis haematobia. I. Metrifonate versus praziquantel in control of intensity and prevalence of infection. Am. J. Trop. Med. Hyg. 1988;39(3):295–305. doi: 10.4269/ajtmh.1988.39.295. [DOI] [PubMed] [Google Scholar]
- 32.Utzinger J., Keiser J., Shuhua X., Tanner M., Singer B.H. Combination chemotherapy of schistosomiasis in laboratory studies and clinical trials. Antimicrob. Agents Chemother. 2003;47(5):1487–1495. doi: 10.1128/AAC.47.5.1487-1495.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khan M.O., Keiser J., Amoyaw P.N., Hossain M.F., Vargas M., Le J.G., Simpson N.C., Roewe K.D., Freeman T.N., Hasley T.R., Maples R.D., Archibald S.J., Hubin T.J. Discovery of anti-schistosomal drug leads based on tetraazamacrocyclic derivatives and their metal complexes. Antimicrob. Agents Chemother. 2016;60(9):5331–5336. doi: 10.1128/AAC.00778-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Danso-Appiah A., Garner P., Olliaro P.L., Utzinger J. Treatment of urinary schistosomiasis: methodological issues and research needs identified through a Cochrane systematic review. Parasitology. 2009;136(13):1837–1849. doi: 10.1017/S0031182009005939. [DOI] [PubMed] [Google Scholar]
- 35.Nare B., Smith J.M., Prichard R.K. Mechanisms of inactivation of and mammalian glutathione -transferase activity by the anti-schistosomal drug oltipraz. Biochem. Pharmacol. 1992;43(6):1345–1351. doi: 10.1016/0006-2952(92)90512-H. [DOI] [PubMed] [Google Scholar]
- 36.Bueding E., Dolan P., Leroy J.P. The antischistosomal activity of oltipraz. Res. Commun. Chem. Pathol. Pharmacol. 1982;37(2):293–303. [PubMed] [Google Scholar]
- 37.Morrison D.D., Thompson D.P., Semeyn D.R., Bennett J.L. Acute effects of oltipraz on adult Schistosoma mansoni and its antagonism in vitro. Biochem. Pharmacol. 1987;36(7):1169–1171. doi: 10.1016/0006-2952(87)90429-1. [DOI] [PubMed] [Google Scholar]
- 38.Mkoji G.M., Smith J.M., Prichard R.K. Glutathione redox state, lipid peroxide levels, and activities of glutathione enzymes in oltipraz-treated adult Schistosoma mansoni. Biochem. Pharmacol. 1989;38(23):4307–4313. doi: 10.1016/0006-2952(89)90530-3. [DOI] [PubMed] [Google Scholar]
- 39.Clapper M.L. Chemopreventive activity of oltipraz. Pharmacol. Ther. 1998;78(1):17–27. doi: 10.1016/S0163-7258(97)00164-2. [DOI] [PubMed] [Google Scholar]
- 40.Gentilini M., Duflo B., Richard-Lenoble D., Brücker G., Danis M., Niel G., Meunier Y. Assessment of 35972 RP (oltipraz) a new antischistosomal drug against Schistosoma haematobium, Schistosoma mansoni, and Schistosoma intercalatum. Acta Trop. 1980;37(3):271–274. [PubMed] [Google Scholar]
- 41.Katz M. Anthelmintics. Drugs. 1977;13(2):124–136. doi: 10.2165/00003495-197713020-00002. [DOI] [PubMed] [Google Scholar]
- 42.Mahmoud A.A., Mandel A., Warren K., Webster L.T. Jr Niridazole. II. A potent long-acting suppressant of cellular hypersensitivity. J. Immunol. 1975;114(1 Pt 2):279–283. [PubMed] [Google Scholar]
- 43.Moczon T., Swiderski Z. Schistosoma haematobium: oxidoreductase histochemistry and ultrastructure of niridazole-treated females. Int. J. Parasitol. 1983;13(2):225–232. doi: 10.1016/0020-7519(83)90017-6. [DOI] [PubMed] [Google Scholar]
- 44.Tracy J.W., Catto B.A., Webster L.T. Jr Reductive metabolism of niridazole by adult Schistosoma mansoni. Correlation with covalent drug binding to parasite macromolecules. Mol. Pharmacol. 1983;24(2):291–299. [PubMed] [Google Scholar]
- 45.Cioli D., Pica-Mattoccia L., Archer S. Antischistosomal drugs: past, present... and future? Pharmacol. Ther. 1995;68(1):35–85. doi: 10.1016/0163-7258(95)00026-7. [DOI] [PubMed] [Google Scholar]
- 46.Harder A. Chemotherapeutic approaches to schistosomes: current knowledge and outlook. Parasitol. Res. 2002;88(5):395–397. doi: 10.1007/s00436-001-0588-x. [DOI] [PubMed] [Google Scholar]
- 47.Thétiot-Laurent S.A.L., Boissier J., Robert A., Meunier B. Schistosomiasis chemotherapy. Angew. Chem. Int. Ed. Engl. 2013;52(31):7936–7956. doi: 10.1002/anie.201208390. [DOI] [PubMed] [Google Scholar]
- 48.Urman H.K., Bulay O., Clayson D.B., Shubik P. Carcinogenic effects of niridazole. Cancer Lett. 1975;1(2):69–74. doi: 10.1016/S0304-3835(75)95362-8. [DOI] [PubMed] [Google Scholar]
- 49.Morgan J.A., Dejong R.J., Snyder S.D., Mkoji G.M., Loker E.S. Schistosoma mansoni and Biomphalaria: past history and future trends. Parasitology. 2001;123(Suppl.):S211–S228. doi: 10.1017/S0031182001007703. [DOI] [PubMed] [Google Scholar]
- 50.Pica-Mattoccia L., Carlini D., Guidi A., Cimica V., Vigorosi F., Cioli D. The schistosome enzyme that activates oxamniquine has the characteristics of a sulfotransferase. Mem. Inst. Oswaldo Cruz. 2006;101(Suppl. 1):307–312. doi: 10.1590/S0074-02762006000900048. [DOI] [PubMed] [Google Scholar]
- 51.Trainor-Moss S., Mutapi F. Schistosomiasis therapeutics: whats in the pipeline? Expert Rev. Clin. Pharmacol. 2016;9(2):157–160. doi: 10.1586/17512433.2015.1102051. [DOI] [PubMed] [Google Scholar]
- 52.Richter J. The impact of chemotherapy on morbidity due to schistosomiasis. Acta Trop. 2003;86(2-3):161–183. doi: 10.1016/S0001-706X(03)00032-9. [DOI] [PubMed] [Google Scholar]
- 53.Saconato H., Atallah A. Interventions for treating Schistosomiasi mansoni. Cochrane Data System Review; 2000. [DOI] [PubMed] [Google Scholar]
- 54.El Ridi R.A.F., Tallima H.A.M. Novel therapeutic and prevention approaches for schistosomiasis review. J. Adv. Res. 2013;4(5):467–478. doi: 10.1016/j.jare.2012.05.002. [review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xiao S.H., Mei J.Y., Jiao P.Y. The in vitro effect of mefloquine and praziquantel against juvenile and adult Schistosoma japonicum. Parasitol. Res. 2009;106(1):237–246. doi: 10.1007/s00436-009-1656-x. [DOI] [PubMed] [Google Scholar]
- 56.Van Nassauw L., Toovey S., Van Op den Bosch J., Timmermans J.P., Vercruysse J., Vercruysse J. Schistosomicidal activity of the antimalarial drug, mefloquine, in Schistosoma mansoni-infected mice. Travel Med. Infect. Dis. 2008;6(5):253–258. doi: 10.1016/j.tmaid.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 57.Ingram K., Ellis W., Keiser J. Antischistosomal activities of mefloquine-related arylmethanols. Antimicrob. Agents Chemother. 2012;56(6):3207–3215. doi: 10.1128/AAC.06177-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Keiser J., Chollet J., Xiao S.H., Mei J.Y., Jiao P.Y., Utzinger J., Tanner M. Mefloquine--an aminoalcohol with promising antischistosomal properties in mice. PLoS Negl. Trop. Dis. 2009;3(1):e350. doi: 10.1371/journal.pntd.0000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brocks D.R., Mehvar R. Stereoselectivity in the pharmacodynamics and pharmacokinetics of the chiral antimalarial drugs. Clin. Pharmacokinet. 2003;42(15):1359–1382. doi: 10.2165/00003088-200342150-00004. [DOI] [PubMed] [Google Scholar]
- 60.Basra A., Mombo-Ngoma G., Melser M.C., Diop D.A., Würbel H., Mackanga J.R., Fürstenau M., Zoleko R.M., Adegnika A.A., Gonzalez R., Menendez C., Kremsner P.G., Ramharter M. Efficacy of mefloquine intermittent preventive treatment in pregnancy against Schistosoma haematobium infection in Gabon: a nested randomized controlled assessor-blinded clinical trial. Clin. Infect. Dis. 2013;56(6):e68–e75. doi: 10.1093/cid/cis976. [DOI] [PubMed] [Google Scholar]
- 61.Seif el-Din S.H., Al-Hroob A.M., Ebeid F.A. Schistosoma mansoni: N-acetylcysteine downregulates oxidative stress and enhances the antischistosomal activity of artemether in mice. Exp. Parasitol. 2011;128(3):230–235. doi: 10.1016/j.exppara.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 62.McIntosh H.M., Olliaro P. Artemisinin derivatives for treating severe malaria. Cochrane Database Syst. Rev. 2000;(2):CD000527. doi: 10.1002/14651858.CD000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Utzinger J., Keiser J. Schistosomiasis and soil-transmitted helminthiasis: common drugs for treatment and control. Expert Opin. Pharmacother. 2004;5(2):263–285. doi: 10.1517/14656566.5.2.263. [DOI] [PubMed] [Google Scholar]
- 64.Doenhoff M.J., Cioli D., Utzinger J. Praziquantel: Mechanisms of action, resistance and new derivatives for schistosomiasis. Curr. Opin. Infect. Dis. 2008;21:659–667. doi: 10.1097/QCO.0b013e328318978f. [DOI] [PubMed] [Google Scholar]
- 65.Utzinger J., N’Goran E.K., N’Dri A., Lengeler C., Xiao S., Tanner M. Oral artemether for prevention of Schistosoma mansoni infection: randomised controlled trial. Lancet. 2000;355(9212):1320–1325. doi: 10.1016/S0140-6736(00)02114-0. [DOI] [PubMed] [Google Scholar]
- 66.Li Y.S., Chen H.G., He H.B., Hou X.Y., Ellis M., McManus D.P. A double-blind field trial on the effects of artemether on Schistosoma japonicum infection in a highly endemic focus in southern China. Acta Trop. 2005;96(2-3):184–190. doi: 10.1016/j.actatropica.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 67.Xiao S.H. Development of antischistosomal drugs in China, with particular consideration to praziquantel and the artemisinins. Acta Trop. 2005;96(2-3):153–167. doi: 10.1016/j.actatropica.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 68.De Clercq D., Vercruysse J., Kongs A., Verlé P., Dompnier J.P., Faye P.C. Efficacy of artesunate and praziquantel in Schistosoma haematobium infected school children. Acta Trop. 2002;82(1):61–66. doi: 10.1016/S0001-706X(02)00003-7. [DOI] [PubMed] [Google Scholar]
- 69.Sabah A.A., Fletcher C., Webbe G., Doenhoff M.J. Schistosoma mansoni: chemotherapy of infections of different ages. Exp. Parasitol. 1986;61(3):294–303. doi: 10.1016/0014-4894(86)90184-0. [DOI] [PubMed] [Google Scholar]
- 70.Xiao S.H., Yue W.J., Yang Y.Q., You J.Q. Susceptibility of Schistosoma japonicum to different developmental stages to praziquantel. Chin. Med. J. (Engl.) 1987;100(9):759–768. [PubMed] [Google Scholar]
- 71.Xiao S., Shen B., Chollet J., Utzinger J., Tanner M. Tegumental changes in adult Schistosoma mansoni harbored in mice treated with artemether. J. Parasitol. 2000;86(5):1125–1132. doi: 10.1645/0022-3395(2000)086[1125:TCIASM]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 72.Utzinger J., Chollet J., Tu Z., Xiao S., Tanner M. Comparative study of the effects of artemether and artesunate on juvenile and adult Schistosoma mansoni in experimentally infected mice. Trans. R. Soc. Trop. Med. Hyg. 2002;96(3):318–323. doi: 10.1016/S0035-9203(02)90110-0. [DOI] [PubMed] [Google Scholar]
- 73.Xiao S., Tanner M., N’Goran E.K., Utzinger J., Chollet J., Bergquist R., Chen M., Zheng J. Recent investigations of artemether, a novel agent for the prevention of schistosomiasis japonica, mansoni and haematobia. Acta Trop. 2002;82(2):175–181. doi: 10.1016/S0001-706X(02)00009-8. [DOI] [PubMed] [Google Scholar]
- 74.Xiao S.H., Booth M., Tanner M. The prophylactic effects of artemether against Schistosoma japonicum infections. Parasitol. Today (Regul. Ed.) 2000;16(3):122–126. doi: 10.1016/S0169-4758(99)01601-4]. [DOI] [PubMed] [Google Scholar]
- 75.Xiao S.H., Wu Y.L., Tanner M., Wu W.M., Utzinger J., Mei J.Y., Scorneaux B., Chollet J., Zhai Z. Schistosoma japonicum: In vitro effects of artemether combined with haemin depend on cultivation media and appraisal of artemether products appearing in the media. Parasitol. Res. 2003;89(6):459–466. doi: 10.1007/s00436-002-0786-1. [DOI] [PubMed] [Google Scholar]
- 76.Adam I., Elhardello O.A., Elhadi M.O., Abdalla E., Elmardi K.A., Jansen F.H. The antischistosomal efficacies of artesunate-sulfamethoxypyrazine-pyrimethamine and artemether-lumefantrine administered as treatment for uncomplicated, Plasmodium falciparum malaria. Ann. Trop. Med. Parasitol. 2008;102(1):39–44. doi: 10.1179/136485908X252214. [DOI] [PubMed] [Google Scholar]
- 77.Archer S. The chemotherapy of schistosomiasis. Annu. Rev. Pharmacol. Toxicol. 1985;25:485–508. doi: 10.1146/annurev.pa.25.040185.002413. [DOI] [PubMed] [Google Scholar]
- 78.Yarinsky A., Hernandez P., Ferrari R.A., Freele H.W. Effects of hycanthone against two strains of Schistosoma japonicum in mice. Kiseichugaku Zasshi. 1972;21:101–108. [Google Scholar]
- 79.Cioli D., Knopf P.M. A study of the mode of action of hycanthone against Schistosoma mansoni in vivo and in vitro. Am. J. Trop. Med. Hyg. 1980;29(2):220–226. doi: 10.4269/ajtmh.1980.29.220. [DOI] [PubMed] [Google Scholar]
- 80.Jewsbury J.M., Homewood C.A., Gibson J. The effect of hycanthone on the distribution of Schistosoma mansoni in the mouse. Ann. Trop. Med. Parasitol. 1974;68(3):365–366. doi: 10.1080/00034983.1974.11686961. [DOI] [PubMed] [Google Scholar]
- 81.Hillman G.R., Senft A.W., Gibler W.B. The mode of action of hycanthone revisited. J. Parasitol. 1978;64(4):754–756. doi: 10.2307/3279978. [DOI] [PubMed] [Google Scholar]
- 82.Senft A.W., Senft D.G., Hillman G.R., Polk D., Kryger S. Influence of hycanthone on morphology and serotonin uptake of Schistosome mansoni. Am. J. Trop. Med. Hyg. 1976;25(6):832–840. doi: 10.4269/ajtmh.1976.25.832. [DOI] [PubMed] [Google Scholar]
- 83.Hartman P.E., Levine K., Hartman Z., Berger H. Hycanthone: a frameshift mutagen. Science. 1971;172(3987):1058–1060. doi: 10.1126/science.172.3987.1058. [DOI] [PubMed] [Google Scholar]
- 84.Pax R., Fetterer R., Bennett J.L. Effects of fluoxetine and imipramine on male Schistosoma mansoni. Comp. Biochem. Physiol. C Comp. Pharmacol. 1979;64(1):123–127. doi: 10.1016/0306-4492(79)90036-4. [DOI] [PubMed] [Google Scholar]
- 85.Gouveia M.J., Brindley P.J., Gärtner F., Costa J.M.C.D., Vale N. Drug repurposing for schistosomiasis: combinations of drugs or biomolecules. Pharmaceuticals (Basel) 2018;11(1):15. doi: 10.3390/ph11010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.White N.J., Nosten F., Looareesuwan S., Watkins W.M., Marsh K., Snow R.W., Kokwaro G., Ouma J., Hien T.T., Molyneux M.E., Taylor T.E., Newbold C.I., Ruebush T.K., II, Danis M., Greenwood B.M., Anderson R.M., Olliaro P. Averting a malaria disaster. Lancet. 1999;353(9168):1965–1967. doi: 10.1016/S0140-6736(98)07367-X. [DOI] [PubMed] [Google Scholar]
- 87.Nosten F., Brasseur P. Combination therapy for malaria: the way forward? Drugs. 2002;62(9):1315–1329. doi: 10.2165/00003495-200262090-00003. [DOI] [PubMed] [Google Scholar]
- 88.Pugh R.N., Teesdale C.H. Synergy of concurrent low dose oxamniquine and praziquantel in schistosomiasis. Br. Med. J. (Clin. Res. Ed.) 1983;287(6396):877–878. doi: 10.1136/bmj.287.6396.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Creasey A.M., Taylor P., Thomas J.E.P. Dosage trial of a combination of oxamniquine and praziquantel in the treatment of schistosomiasis in Zimbabwean school children. Cent. Afr. J. Med. 1986;32(7):165–167. [PubMed] [Google Scholar]
- 90.Liu Y.X., Wu W., Liang Y.J., Jie Z.L., Wang H., Wang W., Huang Y.X. New uses for old drugs: the tale of artemisinin derivatives in the elimination of schistosomiasis japonica in China. Molecules. 2014;19(9):15058–15074. doi: 10.3390/molecules190915058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shuhua X., Jiqing Y., Jinying M., Huifang G., Peiying J., Tanner M. Effect of praziquantel together with artemether on Schistosoma japonicum parasites of different ages in rabbits. Parasitol. Int. 2000;49(1):25–30. doi: 10.1016/S1383-5769(00)00029-5. [DOI] [PubMed] [Google Scholar]
- 92.Utzinger J., Chollet J., You J., Mei J., Tanner M., Xiao S. Effect of combined treatment with praziquantel and artemether on Schistosoma japonicum and Schistosoma mansoni in experimentally infected animals. Acta Trop. 2001;80(1):9–18. doi: 10.1016/S0001-706X(01)00138-3. [DOI] [PubMed] [Google Scholar]
- 93.Mahmoud M.R., Botros S.S. Artemether as adjuvant therapy to praziquantel in murine Egyptian schistosomiasis mansoni. J. Parasitol. 2005;91(1):175–178. doi: 10.1645/GE-322R. [DOI] [PubMed] [Google Scholar]
- 94.De Clercq D., Vercruysse J., Verlé P., Kongs A., Diop M. What is the effect of combining artesunate and praziquantel in the treatment of Schistosoma mansoni infections? Trop. Med. Int. Health. 2000;5(10):744–746. doi: 10.1046/j.1365-3156.2000.00628.x. [DOI] [PubMed] [Google Scholar]
- 95.Inyang-Etoh P.C., Ejezie G.C., Useh M.F., Inyang-Etoh E.C. Efficacy of a combination of praziquantel and artesunate in the treatment of urinary schistosomiasis in Nigeria. Trans. R. Soc. Trop. Med. Hyg. 2009;103(1):38–44. doi: 10.1016/j.trstmh.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 96.el-Lakkany N.M., el-Din S.H.S., Sabra A.N.A.A., Hammam O.A. Pharmacodynamics of mefloquine and praziquantel combination therapy in mice harbouring juvenile and adult Schistosoma mansoni. Mem. Inst. Oswaldo Cruz. 2011;106(7):814–822. doi: 10.1590/S0074-02762011000700006. [DOI] [PubMed] [Google Scholar]
- 97.Xiao S.H., Mei J.Y., Jiao P.Y. Effect of mefloquine administered orally at single, multiple, or combined with artemether, artesunate, or praziquantel in treatment of mice infected with Schistosoma japonicum. Parasitol. Res. 2011;108(2):399–406. doi: 10.1007/s00436-010-2080-y. [DOI] [PubMed] [Google Scholar]
- 98.Badria F., Abou-Mohamed G., El-Mowafy A., Masoud A., Salama O. Mirazid: a new schistosomicidal drug. Pharmaceu Bio. 2001;39(2):127–131. doi: 10.1076/phbi.39.2.127.6253. [DOI] [Google Scholar]
- 99.Sheir Z., Nasr A.A., Massoud A., Salama O., Badra G.A., El-Shennawy H., Hassan N., Hammad S.M. A safe, effective, herbal antischistosomal therapy derived from myrrh. Am. J. Trop. Med. Hyg. 2001;65(6):700–704. doi: 10.4269/ajtmh.2001.65.700. [DOI] [PubMed] [Google Scholar]
- 100.Okwute S.K. In: Plants as Potential Sources of Pesticidal Agents: A Review, Pesticides Advances in Chemical and Botanical Pesticides, Dr. Soundararajan R.P., editor. InTech; 2012. [Google Scholar]
- 101.Huppertz L.M., Bisel P., Westphal F., Franz F., Auwärter V., Moosmann B. Characterization of the four designer benzodiazepines clonazolam, deschloroetizolam, flubromazolam, and meclonazepam, and identification of their in vitro metabolites. Forensic Toxicol. 2015;33:388–395. doi: 10.1007/s11419-015-0277-6. [DOI] [Google Scholar]
- 102.Abdul-Ghani R.A., Loutfy N., Hassan A. Experimentally promising antischistosomal drugs: a review of some drug candidates not reaching the clinical use. Parasitol. Res. 2009;105(4):899–906. doi: 10.1007/s00436-009-1546-2. [DOI] [PubMed] [Google Scholar]
- 103.Vikingsson S., Wohlfarth A., Andersson M., Gréen H., Roman M., Josefsson M., Kugelberg F.C., Kronstrand R. Identifying metabolites of meclonazepam by high-resolution mass spectrometry using human liver microsomes, hepatocytes, a mouse model, and authentic urine samples. AAPS J. 2017;19(3):736–742. doi: 10.1208/s12248-016-0040-x. [DOI] [PubMed] [Google Scholar]
- 104.Borges M.H., Alves D.L.F., Raslan D.S., Piló-Veloso D., Rodrigues V.M., Homsi-Brandeburgo M.I., de Lima M.E. Neutralizing properties of Musa paradisiaca L. (Musaceae) juice on phospholipase A2, myotoxic, hemorrhagic and lethal activities of crotalidae venoms. J. Ethnopharmacol. 2005;98(1-2):21–29. doi: 10.1016/j.jep.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 105.Gurib-Fakim A. Medicinal plants: traditions of yesterday and drugs of tomorrow. Mol. Aspects Med. 2006;27(1):1–93. doi: 10.1016/j.mam.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 106.Ekeopara C.A., Azubuike U. The contribution of african traditional medicine to nigeria’s health care delivery system. IOSR-JHSS. 2017;22:32–43. doi: 10.9790/0837-2205043243. [DOI] [Google Scholar]
- 107.Hostettmann K. On the use of plants and plant-derived compounds for the control of schistosomiasis. Naturwissenschaften. 1984;71(5):247–251. doi: 10.1007/BF00441334. [DOI] [PubMed] [Google Scholar]
- 108.Ndamba J., Nyazema N., Makaza N., Anderson C., Kaondera K.C. Traditional herbal remedies used for the treatment of urinary schistosomiasis in Zimbabwe. J. Ethnopharmacol. 1994;42(2):125–132. doi: 10.1016/0378-8741(94)90106-6. [DOI] [PubMed] [Google Scholar]
- 109.Mølgaard P., Nielsen S.B., Rasmussen D.E., Drummond R.B., Makaza N., Andreassen J. Anthelmintic screening of Zimbabwean plants traditionally used against schistosomiasis. J. Ethnopharmacol. 2001;74(3):257–264. doi: 10.1016/S0378-8741(00)00377-9. [DOI] [PubMed] [Google Scholar]
- 110.Neves B.J., Andrade C.H., Cravo P.V. Natural products as leads in schistosome drug discovery. Molecules. 2015;20(2):1872–1903. doi: 10.3390/molecules20021872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cheuka P.M., Mayoka G., Mutai P., Chibale K. The Role of Natural Products in Drug Discovery and Development against Neglected Tropical Diseases. Molecules. 2016;22(1):58. doi: 10.3390/molecules22010058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ali S.A. Natural products as therapeutic agents for schistosomiasis. J. Med. Plants Res. 2011;5(1):1–20. doi: 10.3923/rjmp.2011.1.20. [DOI] [Google Scholar]
- 113.Ndjonka D., Rapado L.N., Silber A.M., Liebau E., Wrenger C. Natural products as a source for treating neglected parasitic diseases. Int. J. Mol. Sci. 2013;14(2):3395–3439. doi: 10.3390/ijms14023395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hassan F.A.M., Abed G.H., Abdel-Samii M.A.Z., Omar H.M. Anti-schistosomal activity of ginger aqueous extract against experimental Schistosoma mansoni infection in mice. Biom. J. 2016;2(2):1–5. [Google Scholar]
- 115.Issa R.M. Schistosoma mansoni: The prophylactic and curative effects of propolis in experimentally infected mice. Rawal Med. J. 2007;32(2):94–98. [Google Scholar]
- 116.Keiser J., N’Guessan N.A., Adoubryn K.D., Silué K.D., Vounatsou P., Hatz C., Utzinger J., N’Goran E.K. Efficacy and safety of mefloquine, artesunate, mefloquine-artesunate, and praziquantel against Schistosoma haematobium: randomized, exploratory open-label trial. Clin. Infect. Dis. 2010;50(9):1205–1213. doi: 10.1086/651682. [DOI] [PubMed] [Google Scholar]
- 117.Mehndiratta M.M., Wadhai S.A., Tyagi B.K., Gulati N.S., Sinha M. Drug repositioning. Intl J Epilepsy. 2016;3:91–94. doi: 10.1016/j.ijep.2016.09.002. [DOI] [Google Scholar]
- 118.Bergquist R., Utzinger J., Keiser J. Controlling schistosomiasis with praziquantel: How much longer without a viable alternative? Infect. Dis. Poverty. 2017;6(1):74. doi: 10.1186/s40249-017-0286-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Eissa M.M., El-Azzouni M.Z., Amer E.I., Baddour N.M. Miltefosine, a promising novel agent for schistosomiasis mansoni. Int. J. Parasitol. 2011;41(2):235–242. doi: 10.1016/j.ijpara.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 120.El-Moslemany R.M., Eissa M.M., Ramadan A.A., El-Khordagui L.K., El-Azzouni M.Z. Miltefosine lipid nanocapsules: Intersection of drug repurposing and nanotechnology for single dose oral treatment of pre-patent schistosomiasis mansoni. Acta Trop. 2016;159:142–148. doi: 10.1016/j.actatropica.2016.03.038. [DOI] [PubMed] [Google Scholar]
- 121.Dissous C., Grevelding C.G. Piggy-backing the concept of cancer drugs for schistosomiasis treatment: a tangible perspective? Trends Parasitol. 2011;27(2):59–66. doi: 10.1016/j.pt.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 122.Beckmann S., Grevelding C.G. Imatinib has a fatal impact on morphology, pairing stability and survival of adult Schistosoma mansoni in vitro. Int. J. Parasitol. 2010;40(5):521–526. doi: 10.1016/j.ijpara.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 123.Cowan N., Keiser J. Repurposing of anticancer drugs: in vitro and in vivo activities against Schistosoma mansoni. Parasit. Vectors. 2015;8:417. doi: 10.1186/s13071-015-1023-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.British Columbia (BC) Cancer Agency. Cancer Drug Manual: Chlorambucil. 2013.
- 125.Eissa M.M., Mossallam S.F., Amer E.I., Younis L.K., Rashed H.A. Repositioning of chlorambucil as a potential anti-schistosomal agent. Acta Trop. 2017;166:58–66. doi: 10.1016/j.actatropica.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 126.Kvint K., Nachin L., Diez A., Nyström T. The bacterial universal stress protein: function and regulation. Curr. Opin. Microbiol. 2003;6(2):140–145. doi: 10.1016/S1369-5274(03)00025-0. [DOI] [PubMed] [Google Scholar]
- 127.Kim D.J., Bitto E., Bingman C.A., Kim H.J., Han B.W., Phillips G.N. Jr Crystal structure of the protein At3g01520, a eukaryotic universal stress protein-like protein from Arabidopsis thaliana in complex with AMP. Proteins. 2015;83(7):1368–1373. doi: 10.1002/prot.24821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hingley-Wilson S.M., Lougheed K.E., Ferguson K., Leiva S., Williams H.D. Individual Mycobacterium tuberculosis universal stress protein homologues are dispensable in vitro. Tuberculosis (Edinb.) 2010;90(4):236–244. doi: 10.1016/j.tube.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mbah A.N., Mahmud O., Awofolu O.R., Isokpehi R.D. Inferences on the biochemical and environmental regulation of universal stress proteins from Schistosomiasis parasites. Adv. Appl. Bioinform. Chem. 2013;6:15–27. doi: 10.2147/AABC.S37191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Giuliani A., Pirri G., Nicolleto S. Antimicrobial peptides: an overview of a promising class of therapeutics. Cent. Eur. J. Biol. 2007;1:1–33. [Google Scholar]
- 131.Williams M.E., Tincho M., Gabere M., Uys A., Meyer M., Pretorius A. Molecular validation of putative antimicrobial peptides for improved human immunodeficiency virus diagnostics via HIV protein p24. J. AIDS Clin. Res. 2016;7:571. [Google Scholar]
- 132.Aruleba R.T., Adekiya T.A., Oyinloye B.E., Kappo A.P. Structural studies of predicted ligand binding sites and molecular docking analysis of Slc2a4 as a therapeutic target for the treatment of cancer. Int. J. Mol. Sci. 2018;19(2):386. doi: 10.3390/ijms19020386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Brogden K.A. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005;3(3):238–250. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]


