Abstract
Background: Patients with mutations of the thyroid hormone receptor alpha (THRA) gene show resistance to thyroid hormone alpha (RTHα). No amendable mouse models are currently available to elucidate deleterious effects of TRα1 mutants during early development. Zebrafish with transient suppressed expression by morpholino knockdown and ectopic expression of TRα1 mutants in the embryos have been reported. However, zebrafish with germline transmittable mutations have not been reported. The stable expression of thra mutants from embryos to adulthood facilitated the study of molecular actions of TRα1 mutants during development.
Methods: In contrast to human and mice, the thra gene is duplicated in zebrafish, thraa, and thrab. Using CRISPR/Cas9-mediated targeted mutagenesis, we created dominant negative mutations in the two duplicated thra genes. We comprehensively analyzed the molecular and phenotypic characteristics of mutant fish during development.
Results: Adult and juvenile homozygous thrab 1-bp ins (m/m) mutants exhibited severe growth retardation, but adult homozygous thraa 8-bp ins (m/m) mutants had very mild growth impairment. Expression of the growth hormone (gh1) and insulin-like growth factor 1 was markedly suppressed in homozygous thrab 1-bp ins (m/m) mutants. Decreased messenger RNA and protein levels of triiodothyronine-regulated keratin genes and inhibited keratinocyte proliferation resulted in hypoplasia of the epidermis in adult and juvenile homozygous thrab 1-bp ins (m/m) mutants, but not homozygous thraa 8-bp ins (m/m) mutants. RNA-seq analysis showed that homozygous thrab 1-bp ins (m/m) mutation had global impact on the functions of the adult pituitary. However, no morphological defects nor any changes in the expression of gh1 and keratin genes were observed in the embryos and early larvae. Thus, mutations of either the thraa or thrab gene did not affect initiation of embryogenesis. But the mutation of the thrab gene, but not the thraa gene, is detrimental in postlarval growth and skin development.
Conclusions: The thra duplicated genes are essential to control temporal coordination in postlarval growth and development in a tissue-specific manner. We uncovered novel functions of the duplicated thra genes in zebrafish in development. These mutant zebrafish could be used as a model for further analysis of TRα1 mutant actions and for rapid screening of therapeutics for RTHα.
Keywords: zebrafish, TRα mutations, growth retardation, epidermis hypoplasia, pituitary defects, RNA-seq
Introduction
Thyroid hormone nuclear receptors (TRs) are transcription factors that mediate the biological activities of the thyroid hormone triiodothyronine (T3). In humans, the two TR genes, thyroid hormone receptor alpha (THRA) and thyroid hormone receptor beta (THRB), encode TRα1 and TRβ, respectively. Given the critical roles of TR in growth, differentiation, and development, mutations of the TR genes are deleterious, resulting in diseases. In the late 1980s, mutation of the THRB gene was reported to cause a genetic disease known as resistance to thyroid hormone (RTH)β with hallmarks of dysregulation of the pituitary–thyroid axis (1). However, no patients with mutation of the THRA gene were discovered until 2012–2013 (2–4). The patients reported so far were found to have the mutation sites clustered at the C-terminal region of the T3-binding domain (2–6). Many mutations are frameshift mutations, ending with truncations, with amino acids shorter than the wild-type (WT) TRα1. Patients have a slightly higher T3 and lower l-thyroxine (T4), but the thyrotropin (TSH) is mostly normal, or mildly elevated. However, patients show RTHα by exhibiting debilitating symptoms of growth retardation, delayed bone development, neurological abnormalities, cognitive defects, and anemia (2–6). The molecular basis underlying these disorders has yet to be fully elucidated.
Our laboratory previously created a mouse model (Thra1PV/+ mice) to elucidate the in vivo pathogenic actions of TRα1 mutants (7). Thra1PV/+ mice express a mutant TRα1, known as PV, which has a C-terminal frameshift mutated sequence similar to the mutant TRα1 identified in two patients. The Thra1PV/+ mouse faithfully reproduces growth retardation (7), delayed bone development (8,9), and anemia (10) as reported for patients. While much has been learned about the molecular actions of TRα1PV mutants from using adult Thra1PV/+ mice, little is known about how these mutants exert their deleterious effects during early development. This is because of the difficulty in obtaining sufficient embryos at the early stage of development in Thra1PV/+ mice. The fertility of Thra1PV/+ mice is severely deficient, yielding a very small litter size with only two or three pups, frequently with no pups having the Thra1PV mice genotype. Clearly, alternative animal models that can mitigate these shortcomings are needed.
We chose zebrafish (Danio rerio) to study the molecular actions of TRα in development because zebrafish have high fecundity, rapid external embryonic development, and easy visualization of transparent embryos. The zebrafish has two duplicated thra genes, the thraa and thrab genes. The thraa gene encodes two TRα1 isoforms, the short (TRα1S) and the long form with an additional 14 amino acids at the C-terminus (TRα1L). The thrab gene encodes one single TRαB receptor. The DNA- and T3-binding domains of TRα1S, TRα1L, and TRαB share 90–95% sequence homology with the human and mouse TRα1 (11,12). The effects of transient suppressed expression of the thraa gene on the embryonic development and larva transition in zebrafish were reported by morpholino knockdown (13). The functional consequences of ectopic expression of several human TRα1 mutants in zebrafish embryos have been evaluated (14). However, in contrast to these studies in which the effects were transient, we aimed to generate novel zebrafish RTHα models in which the mutations are germline transmittable with stable expression of TRα1 mutants controlled by endogenous promoters and transcription regulatory mechanisms. Such stable gene expression will allow us to assess the molecular actions of TRα1 mutants from the embryonic stage to adulthood.
In this study, we report the generation of zebrafish thraa and thrab mutants with C-terminal truncation mutations similar to those found in human RTHα patients. No discernible embryonic defects were detected in fish expressing either thraa or thrab mutations. However, both the thraa and thrab mutations caused growth retardation as in patients, with a stronger deleterious effect induced by the thrab mutant. The onset of the impaired growth occurred during the larva–juvenile transition, when thyroid hormones were at their peak. Furthermore, the thrab mutant, but not the thraa mutant, caused a hypoplastic epidermis and aplastic musculature, beginning at the larva–juvenile transition and persisting to adults. Thus, the fact that the duplicated genes evoke differential responses suggested the thrab gene product, the TRαB receptor, plays a predominant role during postembryonic/larval development. Furthermore, the retarded growth commonly observed in thra mutant fish, as in humans and mice, indicates that the deleterious effects of mutations of TRα are conserved from mammals to teleost fish.
Materials and Methods
Ethics statement
All zebrafish experiments were performed in compliance with the guidelines for animal handling and approved animal study protocols under the National Cancer Institute's and the National Human Genome Research Institute's Animal Care and Use Committees.
Zebrafish husbandry
The zebrafish mutant lines in the TAB5 background were used for studies. Zebrafish embryos were collected by natural crosses for each thra mutant line using heterozygous adults and staged according to hours or days postfertilization (hpf or dpf) as described (15). In all cases, fish were anesthetized in MS222 before an experiment began. Mutant fish after F4 generation were used in the phenotypic characterization.
Generation of thraa and thrab mutant fish and genotyping
Single guide RNAs (sgRNAs) targeting thraa (Ensembl transcript ID: ENSDART00000000160.10) exon 9 (GgTCCCGCCGCTCTTCCTGG) and thrab (Ensembl transcript ID: ENSDART00000153187.2) exon 10 (GgGGGGGAAAGAGTTCAGTG) were designed using the “ZebrafishGenomics” track on the UCSC Genome Browser. Synthesis of target oligonucleotides (Integrated DNA Technologies), preparation of messenger RNA (mRNA), microinjections, evaluation of sgRNA activities by CRISPR-STAT, and generation of mutant lines were carried out as described previously (16). In brief, WT embryos (TAB5) were injected with sgRNAs (50 pg) and Cas9 mRNA (300 pg) and grown to adulthood. Screening for germline transmission of indels was carried out by analysis of eight embryos from the progeny of each founder fish at 24 hpf by fluorescent PCR using the following primer sets: thraa-E9-Forward (5′-TCTGGACTGACATGTGTGG) and thraa-E9-Reverse (5′-TTTTGCCGCTGTGTCTCTGG); thrab-E10-Forward (5′-CTACATCAACTATCGCAAGC), and thrab-E10-Reverse (5′-ACGTTCCTGATTCTCTTGCC). A M13F adapter (5′-TGTAAAACGACGGCCAGT) was added to the 5′ end of each forward primer, and a PIG-tail (5′-GTGTCTT) was added to the 5′ end of each reverse primer as described (17). Progeny of founder fish for selected mutations were grown to adulthood and genotyped by fluorescent PCR followed by sequencing to determine its predicted effect on the encoded protein. The same primer sets were used for all subsequent genotyping of embryos and adults during phenotype analysis of the mutant fish.
Analysis of body length, width, and weight of zebrafish
Mutant fish of the same cohort that were growing together were used in the analysis of body length, width, and weight. The larval fish at 14–15 dpf, juvenile fish at 1 month postfertilization (mpf), and adult fish at 3–6 mpf were euthanized and we performed standard body length (centimeters) and width (centimeters) measurements using digital calipers (VWR international) and weighed (grams) (Mettler Toledo XS64). They were genotyped by tissue collection for larval and juvenile fish, and by fin clipping for adult fish as described earlier. Body length, width, and weight data were grouped based on their genotype and statistical analyses were performed using GraphPad Prism 7.7 software (GraphPad Software, Inc.). For statistical tests, two-tail unpaired t-test were used (p-adjusted <0.05).
Zebrafish sample collection
Fish were euthanized with MS222 before all tissue collections. For RNA-seq analysis, zebrafish pituitaries were isolated from WT (triplicates, pooled pituitaries from 10 to 12 fish for each run) and homozygous thrab 1-bp insertion (m/m) mutant adult females (quadruplicates, pooled pituitaries from 10 to 12 fish for each run). The age of fish was 4.5–5.7 mpf. For real-time quantitative PCR (RT-qPCR) analysis, ∼85 pituitaries were obtained from juveniles at 1 mpf and from adult male and female zebrafish at 3–6 mpf. Muscle tissues were collected by transecting the fish from the anterior border of the anal fin to the posterior border of the dorsal fin, excluding the fins themselves. The skin on the belly of fish was collected by transecting the fish from the pectoral fin to the anal fin, without muscle, digestive organs, and fins. The skin on the belly was collected from female zebrafish (4.9 mpf), from both female and male juveniles (1 mpf), and from larvae at 14 or 15 dpf. In all cases, sample collection was performed with a LEICA GZ4 microscope.
Histological analyses
Zebrafish were euthanized and fixed in 4% formaldehyde at 4°C for a minimum of 24 hours followed by decalcification in 1:1 ratio formic acid/sodium citrate for 24 hours at room temperature. The fish were then dehydrated through a series of ethanol, then xylenes, and finally embedded in paraffin. Five-micrometer sections were prepared and stained with hematoxylin and eosin (Histoserv, Germantown). Histological section images were captured with an Olympus BX41 light microscope.
Determination of whole-body content of total thyroid hormones in zebrafish
The concentrations of total T4 (TT4) and total T3 (TT3) were performed as described (18). To measure the concentrations of TT3 and TT4 by enzyme-linked immunosorbent assay (ELISA), larvae, juvenile, and adult fish were collected postfertilization of day 3, day 7, day 15, day 30, and 3 months. All samples were weighed and homogenized in methanol containing 1 mM 6-propyl-2-thiouracil (Sigma) on ice, then frozen at −80°C until used. Homogenized samples were centrifuged at 6723 × g for 30 minutes at 4°C, supernatant was collected, dried by Speed Vac system (AES1010) for 7 hours, and resuspended in phosphate-buffered saline (PBS) for 24 hours at 4°C (body weight 0.6 g/250 μL PBS) and centrifuged at 14,000 rpm for 5 minutes at 4°C, after which the supernatants were collected for thyroid hormone measurements. Samples were analyzed using a T4 ELISA Kit (Cat. No. 3149-18; Diagnostic Automation) for TT4 concentration and T3 ELISA Kit (Cat. No. 3144-18; Diagnostic Automation) as indicated by the manufacturer's protocol. Standard curves, concentrations of TT3 and TT4 were calculated using online analysis software (www.elisanalysis.com). All standard curves showed R2 > 0.94. Statistical analyses were performed using GraphPad Prism 7.7 software (GraphPad Software, Inc.). The number of fish used in the determination are as follows: 3 and 7 dpf larvae (N = 800–950 per sample, in triplicates), 15 dpf larvae (N = 140–180 per sample, in triplicates), 30 dpf juveniles (N = 6–9 per sample, in triplicates), and adult fish (N = 3 per sample in triplicates). To measure the relative concentration of TT3 and TT4 in 30 dpf juveniles between WT and homozygous thrab 1-bp ins (m/m) mutant fish, the number of fish used were in the range of 6–9, 20–31 per sample, each in triplicates for WT fish and 16–19 or 36 fish per sample, each in triplicates for mutant fish. For 100 dpf adult fish, the number of fish used were 3 per sample, each in triplicates for WT fish and 8–9 or 7–9 fish per sample, each in triplicates for homozygous thrab 1-bp ins (m/m) mutants.
Transcriptome analysis of the pituitary of female homozygous thrab 1-bp insertion mutant fish
The libraries were prepared according to the Illumina TruSeq mRNA Prep protocol for paired-end sequencing and then sequenced on an Illumina HiSeq 2500 sequencer. After confirmed good sequence quality, the raw reads of the samples were processed with common RNA-Seq processing procedure, including trimming reads for removing adapters and low-quality bases using Trimmomatic software, aligning with the reference genome (Ensembl GRCz11) using STAR aligner version 2.5.1. from Cold Spring Harbor Laboratory (PMID: 23104886), marking duplicated reads using Picard's MarkDuplicate utility, calculating raw read counts using RSEM software package version 1.2.22 from University of Wisconsin-Madison (19). Normalization and differential expression analysis between mutant and WT groups was done with edgeR Bioconductor package (20). Mainly, linear models (glmFit and glmLRT) were applied to the normalized counts and significant genes were selected by fold change ≥2 and false discovery rate (FDR) ≤0.05.
RNA isolation and RT-qPCR
Total RNA from pituitaries, tail muscle, and skin on the belly were isolated using TRIzol (Invitrogen) as indicated by the manufacturer's protocol. RT-qPCR was performed with one step SYBR Green RT-qPCR Master Mix (Qiagen, Valencia, CA) on an ABI 7900HT system. In each genotype, samples with triplicates were tested on the target genes. Data were analyzed using Prism 7.7 software (GraphPad Software, Inc.). For statistical tests, two-tail unpaired t-test were used (p-adjusted <0.05). Primer sequences are shown in Supplementary Table S1. Ef1a was used as the housekeeping gene for controls.
Western blot analysis
The Western blot analyses were performed as described (21). Primary antibodies for p-AKT (Ser-473) (Cat. No. 4060), AKT (Cat. No. 9272), p-p70S6 (Thr-389) (Cat. No. 9205), p70S6 (Cat. No. 9202), p-S6 (Ser-240/244) (Cat. No. 2215), S6 (Cat. No. 2217), p-STAT3 (Cat. No. 9131), STAT3 (Cat. No. 4904), p-Rb (Ser-780) (Cat. No. 9307), and GAPDH (Cat. No. 2118) were purchased from Cell Signaling Technology. The keratin-5 primary antibody (Cat. No. MS-1814-S0) and keratin-17 (Cat. No. MS-489-S0), keratin-18 (Cat. Nos. MS-743-S0 and MS-1850-S0), and cyclinD1 (Cat. No. 9041-P1) were purchased from NeoMakers (Fremont, CA). The primary antibody CDK6 (sc-7961) and Rb (sc-50) were purchased from Santa Cruz Biotechnology. Antibodies were used at the manufacturer's recommended concentration. For control of protein loading, the blot was probed with the antibody against GAPDH. The species specificity and other relevant information about the antibodies used in this study are listed in Supplementary Table S2.
Statistical analysis
All data are expressed as mean ± standard deviation. All tests were two-tail unpaired t-test and p < 0.05 was considered significant. GraphPad Prism version 7.7 for Mac OS X was used to perform analyses of variances.
Results
Generation of thraa and thrab mutants by CRISPR/Cas9-mediated targeted mutagenesis
The mutation sites identified in RTHα patients so far are all clustered at the C-terminus of TRα1 (22). In contrast to one single THRA gene in humans, zebrafish have duplicated thra genes: thraa and thrab (11,12,23). The thraa gene, located on chromosome 3, encodes two TRα1 receptors: the short form (TRα1S) and the long form (TRα1L). The thrab gene, located on chromosome 12, encodes one single receptor protein, TRαB. To model RTHα in zebrafish, we needed to mutate both the thraa and thrab genes. These two duplicated genes share 90–95% sequence homology in the functional DNA-binding and ligand-binding domains with the human THRA gene (11–13). We used CRISPR/Cas9-mediated targeted mutagenesis to generate four lines of mutant fish (Table 1). A 4-base pair (bp) deletion in exon 9 of the thraa gene (thraa 4-bp del) resulted in frame-shift truncated ThraaPhe404Leufs*22 and ThraaPhe404Leufs*10, when aligned with the WT TRα1L or TRα1S, respectively (Table 1). An 8-bp insertion in the exon 9 of the thraa gene (thraa 8-bp ins) resulted in a single frame-shift truncated ThraaLeu405Glufs*6 protein when aligned with the WT TRα1L or TRα1S (Table 1). A 4-bp deletion or a 1-bp insertion in exon 9 of the thrab gene (thrab 4-bp del or thrab 1-bp ins) led to a frame-shift mutant ThrabThr393Profs*31 or a truncated ThrabGlu394*, respectively (Table 1).
Table 1.
Comparison of the C-Terminal Amino Acid Sequences of Wild-Type and Mutant Thyroid Hormone Receptor α Generated Through CRISPR/CAS9-Mediated Targeted Mutagenesis
| Protein | C-terminal amino acid sequence |
|---|---|
| The thraa gene | |
| Wild-type TRα1L | -FPPLFLEVFEDQEGSTGVAAQEDGSCLR*-428 |
| 4-bp deletion ThraaPhe404Leufs*22 |
-FPPLLRSSRIRREALEWQHRKTVPA*-425 |
| 8-bp insertion ThraaLeu405Glufs*6 |
-FPPLFEDQEV*-410 |
| Wild-type TRα1S | -FPPLFLEVFEDQEV*-414 |
| 4-bp deletion ThraaPhe404Leufs*10 |
-FPPLLRSSRIRRC*-412 |
| 8-bp insertion ThraaLeu405Glufs*6 |
-FPPLFEDQEV*-410 |
| The thrab gene | |
| Wild-type TRαB | -HASRFLHMKVECPTELFPPLFLEVFEDQDV*-409 |
| 4-bp deletion ThrabThr393Profs*31 |
-HASRFLHMKVECPPSFPHFSWRSSRIRTCDVPANCGKRIRNVS*-422 |
| 1-bp insertion ThrabGlu394* |
-HASRFLHMKVECPT*-394 |
bp, base pair.
Functional characterization of T3-binding (Supplementary Fig. S1; see Supplementary Materials and Methods) and transcriptional activity (Supplementary Fig. S2; see Supplementary Materials and Methods) showed that ThraaPhe404Leufs*22, ThraaLeu405Glufs*6, and ThrabGlu394* had totally lost T3-binding activity and transcriptional capacity (Table 2). Moreover, these three mutants all exhibited strong dominant negative activity by interfering with the transcription activity of WT receptors (Supplementary Fig. S2; Table 2). However, to our surprise, we found that ThrabThr393Profs*31 derived from a 4-bp deletion in exon 9 of the thrab gene functioned as a WT receptor. This C-terminal frame-shift mutant with an additional 13 amino acids longer than TRαB receptor bound to T3 (Supplementary Fig. S1D; see Supplementary Materials and Methods) and exhibited transcriptional activity (Supplementary Fig. S2C; Table 2). On the basis of functional characteristics, we focused our studies on the fish lines derived from an 8-bp insertion of the thraa gene and a 1-bp insertion of the thrab gene that encoded ThraaLeu405Glufs*6 and ThrabGlu394*, respectively (Tables 1 and 2). The C-terminal truncation mutation in ThraaLeu405Glufs*6 is similar to the truncation mutation identified in one patient (TRα1F397fs406X; Supplementary Fig. S3A; see Supplementary Materials and Methods) (3) and the truncation mutation in ThrabGlu394* is similar to the mutations identified in patients (TRα1E403X and TRα1C392X; Supplementary Fig. S3B; see Supplementary Materials and Methods) (2,5).
Table 2.
Summary of Functional Characteristics of Four Mutants
| Genotypes | Mutant proteins | T3 binding | Transcription activity | Dominant negative activity |
|---|---|---|---|---|
| thraa 4-bp deletion | ThraaPhe404Leufs*22 | No | No | Yes |
| thraa 8-bp insertion | ThraaLeu405Glufs*6 | No | No | Yes |
| thrab 1-bp insertion | ThrabGlu394* | No | No | Yes |
| thrab 4-bp deletion | ThrabThr393Profs*31 | Yes | Yes | No |
T3, triiodothyronine.
Differential growth regulatory functions of the thraa and thrab genes
In vitro studies have shown that the ThraaLeu405Glufs*6 mutant and the ThrabGlu394* mutant exhibit dominant negative effect by interfering with the transcription activity of WT TRα1 (Supplementary Fig. S2; Table 2). It is known that TRα1 mutants exerted their dominant negative effects by upregulating the negatively regulated genes such as the thyrotropin beta (TSHβ) subunit gene shown in mutant mice with TRα1R438C mutation (24). In humans, the pituitary responds to serum TSH levels through the pituitary–thyroid axis negative feedback loop. TSH consists of two subunits, α and β. The TSHβ subunit gene is a known thyroid hormone (T3)/TR negatively regulated gene. Mutations of TRα1 was expected to upregulate T3-negatively regulated gene (24). Indeed, we found that the tshba gene was upregulated in the pituitaries of male and female homozygous adult thraa 8-bp insertion (m/m) and thrab 1-bp insertion (m/m) mutant fish (Supplementary Fig. S4; see Supplementary Materials and Methods). Thus, the functions of both mutant receptors were validated in the two lines of mutant fish in vivo.
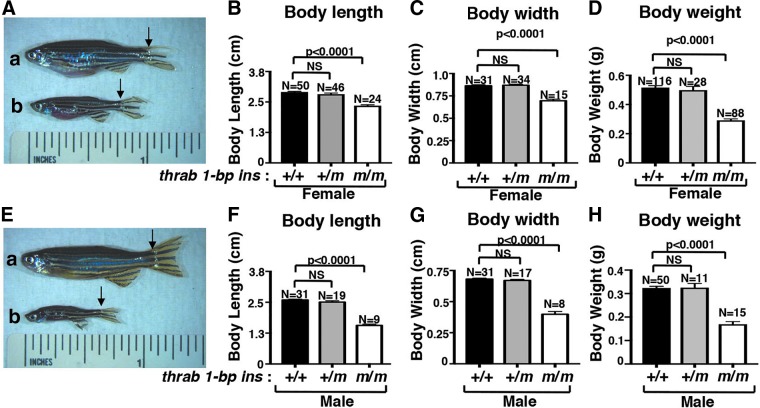
What caught our attention in adult fish was the growth retardation exhibited by homozygous thrab 1-bp insertion (m/m) mutant fish as shown in Figure 1A-b (females) and 1E-b (males). Both females and males showed decreased body length (Fig. 1B, F), body width (Fig. 1C, G), and body weight (Fig. 1D, H). Interestingly, male homozygous thrab 1-bp insertion (m/m) mutant fish showed a more severe growth retardation in that a greater reduction was detected in body length (43% reduction in males and 19% reduction in females; Fig. 1F, B), body width (42% reduction in males vs. 19% reduction in females; Fig. 1G, C), and body weight (48% reduction in males vs. 44% reduction in females; Fig. 1H, D). However, no significant changes were detected in heterozygous fish of either sex in the body length, width, and weight. These data indicate that the growth of males was more affected by the mutations of the thrab gene.
FIG. 1.
Impaired growth in adult homozygous thrab 1-bp insertion (m/m) mutant fish. (A) Representative images of a female WT (a) and homozygous mutant fish (b) and (E) male fish (3.4 mpf) with black arrows to indicate the beginning of caudal fin used in the length measurements. Body length (B, F), body width (C, G), and body weight (D, H) were measured for both females and males of WT, heterozygous, and homozygous mutant fish. The number of fish (N) measured are indicated. The data are shown as mean ± SE with p-values to indicate significant changes. Two-tail unpaired t-test, p-adjusted <0.05 was used for statistical analysis. mpf, month postfertilization; NS, not significant; SE, standard error; WT, wild-type.
The homozygous thraa 8-bp insertion (m/m) mutant fish also exhibited retarded growth. However, the deleterious effect of the thraa 8-bp insertion (m/m) mutation on growth was much weaker than that of the thrab 1-bp insertion (m/m) mutation (Supplementary Fig. S5; see Supplementary Materials and Methods). Only small decreases in body length (∼6%), body width (∼6%), and body weight (∼12%) were detected in homozygous male thraa 8-bp insertion (m/m) mutants, but not females (Supplementary Fig. S5). Thus, similar to thrab 1-bp insertion (m/m) mutants, there was a preponderance in males of growth defects in thraa 8-bp insertion (m/m) mutants. Since the thraa 8-bp insertion (m/m) mutants exhibits very mild growth abnormalities, we focused our studies on the molecular actions of the thrab 1-bp insertion (m/m). We analyzed the expression of growth-related genes in the pituitary to understand how the thrab 1-bp insertion (m/m) mutant led to growth retardation. The growth hormone (gh) gene is a direct T3/TR target gene (25–27). Figure 2A shows that the expression of the gh1 gene was reduced (77%), as was the growth hormone-related smtl gene (28,29) (smtla and smtlb had 99% and 92% reduction, respectively). It is of interest to note that the expression of smtla and smtlb was similarly suppressed in the pituitary of the thraa 8-bp insertion (m/m) mutation fish as in thrab 1-bp insertion (m/m) mutant fish. However, the expression of the gh1 gene was not suppressed, but elevated, in the thraa 8-bp insertion (m/m) mutation fish (Supplementary Fig. S6; see Supplementary Materials and Methods).
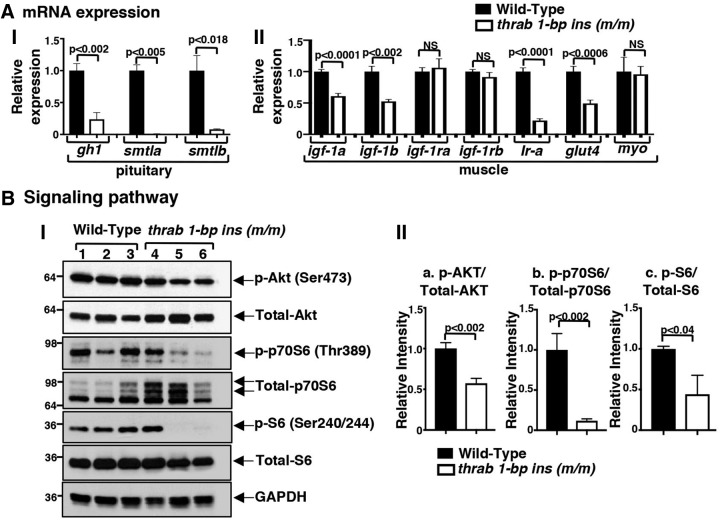
FIG. 2.
Altered gene expression (A) and key regulatory proteins in growth signaling pathway (B) in adult female homozygous thrab 1-bp insertion (m/m) mutant fish. The mRNA expression of gh1, smtla, and smtlb in pituitary (A-I) and mRNA expression of igf-1a, igf-1b, igf-1ra, igf-1rb, Ir-a, glut4, and myo in the muscles (A-II) of WT (solid bars) and sibling homozygous thrab 1-bp insertion (m/m) mutant fish (open bars) were determined by RT-PCR as described in Materials and Methods section. Number of fish = 10–15 for pituitaries, 5–3 for muscles. (B-I) Western blot analysis was carried out for p-AKT (Ser473), total AKT, p-p70S6 (Thr389), total p70S6, p-S6 (Ser240/244), total S6, and GAPDH, using muscle as described in Materials and Methods section. (B-II) Quantitative analysis of relative protein expression levels of the ratios of p-AKT to total AKT (a), p-p70S6 to total p70S6 (b), and p-S6 to total S6 (c) using GAPDH as a loading control. The data are shown as mean ± SE (n = 3; the p-values are indicated). Two-tail unpaired t-test, p-adjusted <0.05 was used for statistical analysis. mRNA, messenger RNA; RT-PCR, real-time PCR.
The growth-promoting effects of the GH are mainly mediated by insulin-like growth factor 1 (IGF-1) signaling. GH stimulates the synthesis of IGF-1 in the muscles (30). IGF-1 increases muscle mass mainly through IGF-1 receptors and also through insulin receptor (Ir). Since it is known that fish growth is primarily dependent on an increase in muscle mass (31), we analyzed the IGF actions in the muscle. We found igf-1a and -1b mRNA was reduced (39–49%). However, the expression of the Igf-1ra and Igf-1rb genes was not affected. But the expression of the Ir-a and glut4 genes was decreased (78% and 51%, respectively; Fig. 2A-II). However, myostatin (myo), which acts as a negative regulator of the GH (32), was not changed in its expression (Fig. 2A-II) such that it did not further affect the gh1 expression.
We next analyzed the IGF-1 downstream AKT-p70S6 pathway (30,33). The activity of AKT was reduced as indicated by the decreased abundance of p-AKT (Fig. 2B-I, B-II, panel a), leading to decreased phosphorylation of p70S6 and 40S ribosomal protein S6 (Fig. 2B-I), a major component of the machinery involved in protein synthesis. Quantitation data show a 56–88% reduction in the ratios of p-p70S6/total p70S6 and p-S6/total S6 (Fig. 2B-II, panels b and c). Taken together, these data indicate that the homozygous thrab 1-bp insertion (m/m) mutant could reduce growth through decreasing IGF-1 signaling in the zebrafish. These findings are consistent with those reported in RTHα patients in that serum IGF-1 was decreased (2,22,34,35).
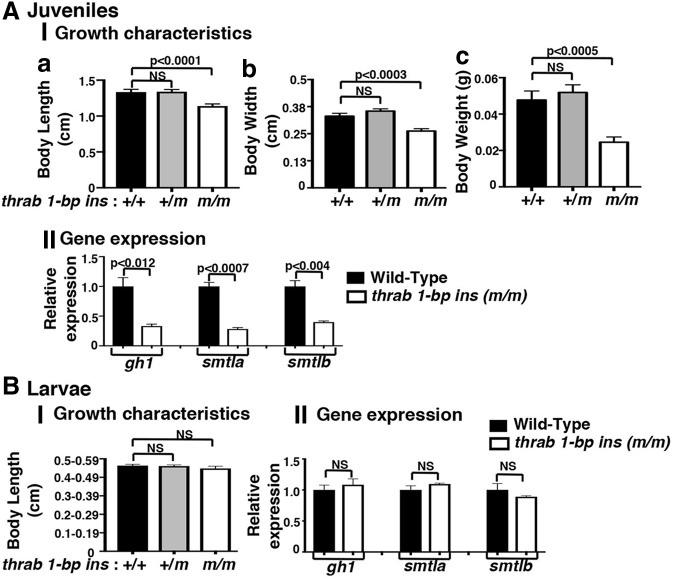
We next asked how early in development the homozygous thrab 1-bp insertion (m/m) mutant exerts its deleterious effects. We evaluated the growth characteristics of juveniles at 28 dpf (Fig. 3A). The body length was decreased by 15% (Fig. 3A-I, panel a), body width by 21% (panel b), and weight by 49% (panel c) in homozygous ThrabGlu394* juveniles as compared with WT siblings. No significant changes in growth characteristics were detected in the heterozygous juveniles. Gene expression analysis showed that the expression of the gh1, smtla, and smtlb genes was decreased 67%, 72%, and 60%, respectively, as compared with WT siblings (Fig. 3A-II). Interestingly, in larvae at the earlier age of 14–15 dpf, no apparent growth defects or altered expression of growth-related genes were detected (Fig. 3B-I, B-II).
FIG. 3.
Impaired growth occurred at larva to juvenile transitory phase of homozygous thrab 1-bp insertion (m/m) mutant fish. (A) Growth characteristics and gene expression of thrab 1-bp ins mutant fish for juveniles (28 dpf) and (B) larvae (15 dpf). Body length (a), body width (b), and body weight (c) were measured for juveniles (A-I) (number of fish = 22–29) and for larvae (B-I) (number of fish = 23–71). Relative mRNA expression of gh1, smtla, and smtlb was determined through RT-qPCR using the primers listed in Supplementary Table S1 for juveniles in the pituitary (A-II) and for larvae in head (B-II). The genotypes are indicated. The data are expressed as mean ± SE (n = 3; the p-values are indicated). Two-tail unpaired t-test, p-adjusted <0.05. dpf, days postfertilization; RT-qPCR, real-time quantitative PCR.
Homozygous thrab 1-bp insertion (m/m) mutant fish exhibit a hypoplastic epidermis and aplastic musculature
Adult homozygous thrab 1-bp insertion (m/m) mutant fish showed the appearance of a bulging “red belly,” as indicated by arrows in Figure 4A-b (also visible in the mutant fish shown in Fig. 1A-b and E-b). Supplementary Figure S7 (see Supplementary Materials and Methods) shows an enlarged image of a homozygous thrab 1-bp insertion (m/m) mutant fish with clearly visible “red belly.” Analysis of WT skin showed normal epidermis, scales, and abdominal wall (Fig. 4B-a, female, and B-c, male). However, in the homozygous thrab 1-bp insertion (m/m) mutant fish, the ventral body wall musculature was thinned or absent in serial sections with only thin skin separating the heart, liver, and intestinal tract from the environment (Fig. 4B-b, B-d). The mutant fish had both hypoplasia (thinning) of the epidermis and aplasia (lack) of scales, and the ventral body wall musculature was hypoplastic to aplastic (for comparison see panels b and d with panels a and c, Fig. 4B). The thinning/loss of these structures led to the fish being more transparent, thus allowing the internal organs to be visible more clearly with the appearance of having reddening of the gill/body cavity (“red belly”; Fig. 4A-b). The scales are present, but in fewer numbers and of smaller size than in WT zebrafish.
FIG. 4.
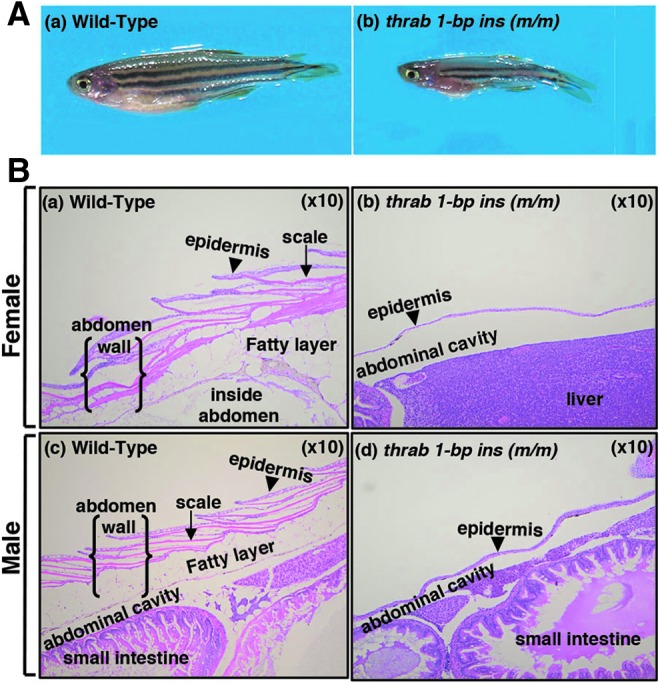
Adult homozygous thrab 1-bp insertion (m/m) mutant fish exhibit hypoplastic epidermis and aplastic musculatures. (A) Representative images of WT fish (a) and homozygous thrab 1-bp insertion (m/m) mutant fish (b) with arrows marking the thin and red belly (denoted as “red belly”). (B) Histology features of hematoxylin and eosin stained adult skin on the belly of WT female (a) and male (c) fish, and female homozygous thrab 1-bp insertion (m/m) mutant fish (b) and male (d) fish (10 × objective). The arrow heads point to epidermis. Homozygous thrab 1-bp insertion (m/m) mutant fish of both sexes show very thin epidermis, lack of scales, and a true abdominal wall.
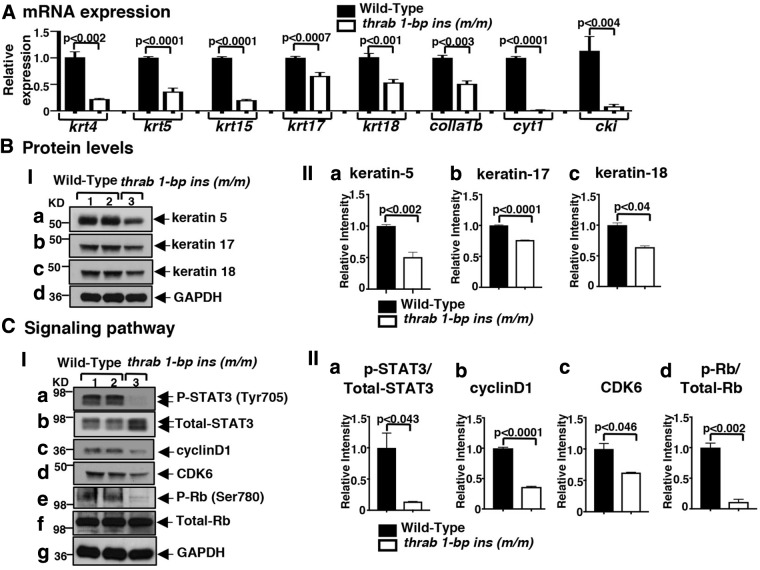
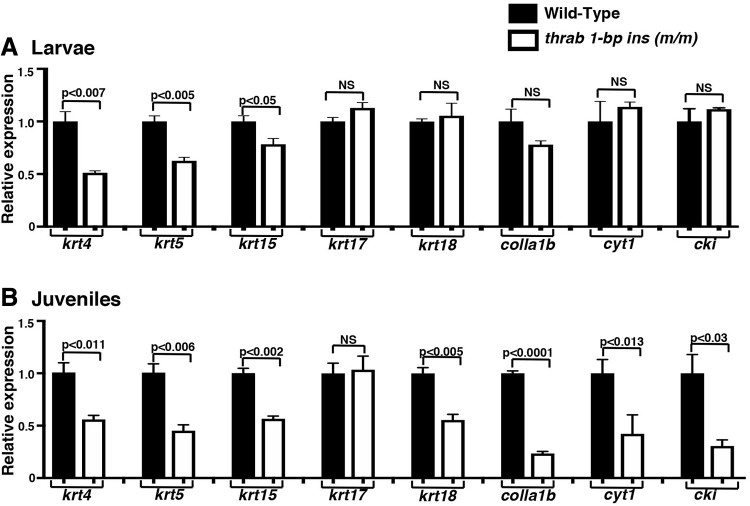
It is generally recognized that thyroid hormone signaling is central to maintaining skin homeostasis. In humans, thyroid dysfunction is associated with alterations in skin architecture and physiology (36). The fact that homozygous thrab 1-bp insertion (m/m) mutant fish display a hypoplastic epidermis provides a model to understand the actions of TRα1 mutants in the skin. The majority of cell types in the epidermis are keratinocytes that express keratins. We determined the mRNA expression of keratin-related genes that are known to be regulated by thyroid hormones/TRs (37). Figure 5A shows that the expressions of krt4, krt5, krt15, krt17, krt18, colla1b, cyt1, and cki were all suppressed in the range of 49% (colla1b) to 92% (cki) in the skin of homozygous thrab 1-bp insertion (m/m) mutant fish. Consistent with the reduced mRNA expression, the protein levels of keratin 5 (Fig. 5B-I, panel a), keratin 17 (panel b), and keratin 18 (panel c) were also decreased, ranging from 24% to 49% (see quantitation in Fig. 5B-II, panels a–c).
FIG. 5.
Decreased keratin gene expression (A), protein abundance (B), and attenuated STAT3 signaling pathway (C) in the skin of adult homozygous thrab 1-bp insertion (m/m) mutant fish. (A) The expression of keratin genes (krt4, krt5, krt15, krt17, and krt18) and skin-regulated genes (col1a1b, cyt1, and cki) from the belly skin of fish with genotypes indicated was determined by RT-qPCR as described in Materials and Methods section (number of fish = 22–25). (B-I) Total protein extracts were prepared from skin on the belly and Western blot analysis for keratin-5, -17, -18 and GAPDH as described in Materials and Methods section. (B-II) Quantitative analysis of relative protein abundance of keratin-5 (a), keratin-17 (b), and keratin-18 (c) from the belly skin of fish with genotypes indicated (number of fish = 10). (C-I) Western blot analysis of p-STAT3 (Tyr705), total STAT3, and downstream STAT3 target proteins [cyclinD1, CDK6, p-Rb (Ser 780), total-Rb] and GAPDH from the belly skin of fish with genotypes indicated. (C-II) Quantitative analysis of relative protein expression levels of the ratio of p-STAT3 to total STAT3 (a), p-Rb to total Rb (d), and relative level of cyclin D1 (b) and CDK6 (c) using GAPDH as a loading control (number of fish = 10). The data are expressed as mean ± SE (n = 3–6; the p-values are indicated). Two-tail unpaired t-test, p-adjusted <0.05 was used for statistical analysis.
The hypoplastic epidermis suggested that proliferation of epidermal cells was inhibited in homozygous thrab 1-bp insertion (m/m) mutant fish. We searched for key cellular regulators that play critical roles in the proliferation of epidermal cells. Signal transducer and activator of transcription 3 (Stat3), upon activation by phosphorylation in the cytoplasm, enters into the nucleus to bind to DNA to stimulate transcription of target genes involved in cell proliferation, survival, migration, and oncogenesis (38,39). Stat3 has been reported to contribute to skin wound healing and keratinocyte migration (40). We, therefore, compared the Stat3 protein levels between WT and homozygous thrab 1-bp insertion (m/m) mutant fish. We found that p-Stat3(Tyr705) levels were markedly lowered in the mutant fish than the WT fish (lane 3 vs. lanes 1 and 2; Fig. 5C-I, panel a). After quantification of the intensities of the bands, the ratio of p-Stat3 versus total Stat3 in mutant fish was found to be reduced by 86% as compared with the WT (Fig. 5C-II, panel a). Cyclin D1, a key cell cycle regulator, is a known Stat3 downstream target gene (41). The protein levels of cyclin D1 together with another key cell cycle regulator, CDK6, were decreased by 64% and 38%, respectively, in the homozygous thrab 1-bp insertion (m/m) mutant fish (lane 3 vs. lanes 1 and 2; Fig. 5C-I, panels c and d, respectively; quantitation, Fig. 5C-II, panels b and c). Decreased cyclin D1/CDK6 led to decreased phosphorylation of retinoblastoma (Rb) (Fig. 5C-I, panel e; the ratios of p-Rb vs. total Rb was 89% lower in mutant fish than WT, Fig. 5C-II, panel d) to delay the cell cycle progression in the epidermis of homozygous thrab 1-bp insertion (m/m) mutant fish.
We next ascertained how early the thinning of the epidermis (“red belly”) became visible. We found that the thinning of the epidermis was apparent as early as 28 dpf. Analysis of the skin of juveniles shows that except krt17, the expression of the keratin-related genes was similarly suppressed (Fig. 6B). These results indicate that the homozygous thrab 1-bp insertion (m/m) mutant acts to suppress these keratin genes, leading to visibly defective epidermis as early as 28 dpf. While the “red belly” was not detectable in the larvae, gene expression indicates that the expression of krt4, krt5, and krt15 is partially suppressed, but this is not the case for krt7, krt18, colla1b, cyt1, and cki (Fig. 6A), suggesting that partial suppression of the three krt genes (i.e., krt4, krt5, and krt15) is not sufficient to result in the hypoplastic phenotype on dpf 28.
FIG. 6.
Suppression of keratin expression program occurs during larva to juvenile transitory phase of homozygous thrab 1-bp insertion (m/m) mutant fish. (A) The expression of keratin genes (krt4, krt5, krt15, krt17, and krt18) and skin-regulated genes (col1a1b, cyt1, and cki) from dissected skin tissue on the belly of WT and homozygous thrab 1-bp insertion (m/m) mutant fish at larvae (15 dpf) (number of fish = 64–46) and (B) at juvenile (28 dpf) stage. The expression of the keratin genes was measured by RT-qPCR as described in Materials and Methods section. The data are expressed as mean ± SE (n = 3–6; the p-values are indicated). Two-tail unpaired t-test, p-adjusted <0.05 was used for statistical analysis.
Temporal expression of the thra and thrb genes and thyroid hormone levels during zebrafish development
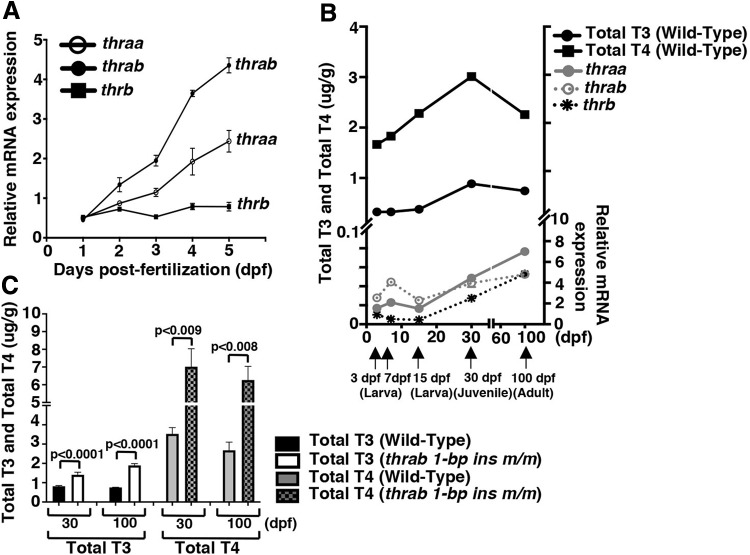
The observations that homozygous thrab 1-bp insertion (m/m) mutation exhibit growth and skin abnormalities beginning at the larva–juvenile transition prompted us to ascertain the temporal expression of thra and thrb gene expression. Figure 7A shows that the expression of the thrab, thraa, and thrb genes is detectable after 1 dpf and gradually increases to day 5, with thrab reaching the highest level among the three genes. Figure 7B shows that the expression of these three genes continues to increase from 7 dpf larvae, reaching higher levels in adulthood.
FIG. 7.
The whole-body content of thyroid hormones and relative expression patterns of thyroid hormone receptor genes at embryonic, larvae, juvenile, and adult stages. (A) Total RNA of pooled embryos or larvae (N = 25–30 at each time point from 24 to 120 hpf) were extracted for RT-qPCR analysis. The relative expression of thyroid hormone receptor genes (thraa, thrab, and thrb) were measured and normalized against the housekeeping gene efla as described in Materials and Methods section. (B) Whole-body content of total l-T4 (TT4) and total l-T3 (TT3) in WT zebrafish were determined from larva to adult at the time point (indicated by the arrows) described in Materials and Methods section. Total RNA of larvae (pooled from 3–7 embryos) or total RNA from skin on the belly of 15-dps larvae, 30-day juveniles or 100-day adults were extracted by RT-qPCR analysis. The relative expression of thyroid hormone receptor genes (thraa, thrab, and thrb) were measured and normalized against the housekeeping gene efla as described in Materials and Methods section. For RT-qPCR, the number of whole larvae at 3 and 7 dpf (N = 25–30 each), skin on the belly were dissected and extracted at 15 dpf (N = 35), at 30 dpf (N = 15), and 100 dpf (N = 10). The data are shown as mean ± SE (n = 3). Whole-body content of TT3 and TT4 are shown as ± SE (n = 3). (C) Whole-body content of TT3 and TT4 were determined by ELISA in WT and homozygous thrab 1-bp insertion (m/m) mutant juvenile at 30 dpf and adult at 100 dpf as described in Materials and Methods section. For 30 dpf juveniles, the number of fish used were in the range of 6–9, or 20–31 per sample, each in triplicates for WT fish and 16–19 or 36 fish per sample, each in triplicates for homozygous thrab 1-bp ins (m/m) mutant fish. For 100 dpf adult fish, the number of fish used were 3 per sample, each in triplicates for WT fish and 8–9 or 7–9 fish per sample, each in triplicates for homozygous thrab 1-bp ins (m/m) mutants. The data are shown mean ± SE (n = 6). Two-tail unpaired t-test, p-adjusted <0.05 was used for statistical analysis. ELISA, enzyme-linked immunosorbent assay; T3, triiodothyronine, ; T4, thyroxine; TT3, total T3; TT4, total T4.
We further determined TT4 and TT3 from embryos at 3 dpf, larvae at 7 dpf, and 15 dpf, juveniles at 30 dpf and adults (100 dpf). Figure 7B shows that TT3 and TT4 were barely detectable until 15 dpf, at which time, TT4 and TT3 began to rise, reaching a peak at juvenile age (30 dpf). Shortly after 30 dpf, both TT4 and TT3 slowly decrease. These findings are consistent with the observations that in WT mice, serum TT3 and TT4 are not detectable on the day of birth and rise to reach a peak on P15 (42). Figure 7C shows that TT3 and TT4 were 1.7- to 2.5-fold higher in homozygous thrab 1-bp insertion (m/m) mutant than WT fish in juveniles and in adults.
The homozygous thrab 1-bp insertion (m/m) mutation has broad impact on the biology and functions of the pituitary
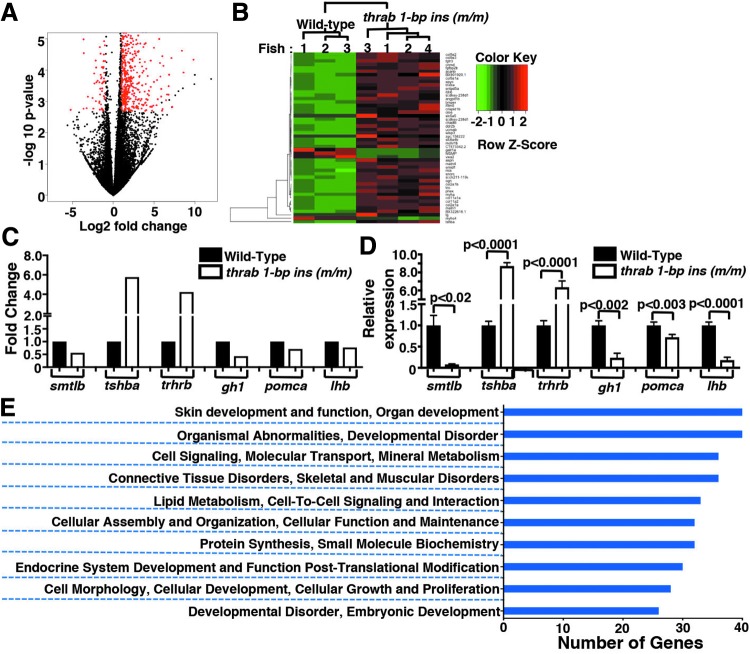
Because the expression of gh1, smtla, and smtlb genes in the pituitary is affected by the homozygous thrab 1-bp insertion (m/m) mutant prompted us to further probe how mutations of the thrab gene could affect the expression of other genes in the pituitary. We, therefore, isolated the pituitaries from adult female fish and compared the global gene expression profiles in the pituitary between WT and homozygous thrab 1-bp insertion (m/m) mutant fish by RNA-seq. The number of genes differentially expressed with more than twofold changes (with FDR <0.05) was 723 (marked in red in this volcano plot; Fig. 8A). Interestingly, 85% of these differentially expressed genes were upregulated (n = 642), and only 15% were downregulated (n = 81). Hierarchical clustering of the top 50 differentially expressed genes clearly showed that the gene expression patterns in the pituitary of WT and mutant fish were clearly distinct (Fig. 8B). Figure 8C shows the major pituitary hormones identified by RNA-seq with FDR <0.05 (Supplementary Table S3; see Supplementary Materials and Methods). The expression of smtlb, gh1, pomca, and lhb were downregulated and the expression of tshba and trhrb were upregulated (Fig. 8C). We validated the changes in the expression of six genes by RT-qPCR (Fig. 8D). The results were found to be in total agreement with the RNA-seq data. Using RT/qPCR, we also found that the thraa, thrab, and thrb genes were all expressed in the pituitary of WT fish (Supplementary Fig. S8; see Supplementary Materials and Methods). We found that thraa mRNA was more abundantly expressed than thrab mRNA. Consistent with reports by others, we also found that thrb mRNA is the major TR isoform expressed in the pituitary (Supplementary Fig. S8).
FIG. 8.
Global impact on the gene expression profile and biological processes of the pituitary by female homozygous thrab 1-bp insertion (m/m) mutant fish. Total RNA was isolated from pituitaries of WT (n = 3) and homozygous thrab 1-bp insertion (m/m) mutant fish (n = 4) for transcriptome analysis. (A) The genes marked in red in this volcano plot were significantly expressed genes with fold changes ≥2 and FDR ≤0.05. The total number of differentially expressed genes was 723, with 642 genes upregulated and 81 genes downregulated. (B) A heatmap presentation of hierarchical clustering analysis of top 50 expressed genes selected by FDR from the significant gene list (fold changes ≥2 and FDR ≤0.05). (C) Comparison of the gene expression profiles of major pituitary hormone transcripts (smtlb, tshba, trhrb, gh1, pomca, and lhb) in the pituitary of WT and homozygous thrab 1-bp insertion (m/m) mutant fish. (D) The mRNA expression levels of the major pituitary hormone transcripts (smtlb, tshba, trhrb, gh1, pomca, and lhb) were measured by RT-qPCR in pituitaries of WT and homozygous thrab 1-bp insertion (m/m) mutant fish (number of fish = 46–62, number of determinations = 3–6). The data are shown as mean ± SE with p-values to indicate significant changes. Two-tail unpaired t-test, p-adjusted <0.05 was used for statistical analysis. (E) Graphical representation of clusters of biological processes with Gene Ontology analysis for the significantly expressed genes. FDR, false discovery rate.
Further Gene Ontology analysis of the differentially expressed genes identified the top 10 clusters of biological processes as shown in Figure 8E. These biological processes were involved in skin development and function, organ development, cell signaling, cellular assembly, and protein synthesis, endocrine system development and function, cellular growth and proliferation, and developmental disorders. The changes in biological processes were mostly affected by the known TRα1 upregulated genes. The Gene Ontology analysis suggested that the homozygous mutation of the thrab gene has broad impact on the biology and functions of the pituitary, as exemplified by decreased GH synthesis, resulting in retarded growth.
Discussion
In this study, we generated zebrafish in which the thraa or thrab genes were mutated to understand the deleterious actions of mutant TRα during development. The two C-terminal truncation mutants, ThraaLeu405Glufs*6 or ThrabGlu394*, are similar to those C-terminal truncation mutants identified in patients (Supplementary Fig. S3; see Supplementary Materials and Methods). These two mutants had completely lost T3-binding activity and transcription capacity, and they exhibited dominant negative activity by interfering with the transcription activity of WT TRα1 (Table 2). Furthermore, in vivo, the mutants suppressed genes positively regulated by T3 such as the gh1 gene, and upregulated T3-negatively regulated genes such as the tshba gene in the pituitary of homozygous thrab 1-bp insertion (m/m) mutant fish. Mutant fish exhibit growth retardation as shown for patients and mice (2,4,5,7,34,35,43). Thus, we have generated a model of RTHα that can be used to interrogate the pathogenic actions of TRα1 mutants during development.
In contrast to humans and mice, in zebrafish the thra gene is duplicated. The thraa gene encodes two TRα1 isoforms, the long and the short forms. The thraa 8-bp insertion site is at nucleotide position 1233 and yields the same truncation mutant protein, ThraaLeu405Glufs*6, for both the long and the short forms (Supplementary Fig. S9A; see Supplementary Materials and Methods). Therefore, for the analysis of the phenotypic expression of thraa 8-bp ins fish, one would not need to consider the contribution from the possible actions of the thraa 8-bp ins long form variant. The thrab 1-bp insertion mutation site was at nucleotide 1180, yielding one single ThrabGlu394* mutant receptor. Therefore, the phenotypic manifestations in these two mutant fish is mediated by either the ThraaLeu405Glufs*6 or the ThrabGlu394* mutant receptor, allowing the comparison of consequences of the mutations in the thraa and thrab genes. We found that there were both common and distinct actions of these two mutant receptors. Both mutants impaired growth, although the extent of impairment by the homozygous thrab 1-bp insertion (m/m) mutant was about fivefold more severe than by the homozygous thraa 8-bp insertion (m/m) mutant (Fig. 1; Supplementary Fig. S5; see Supplementary Materials and Methods). The strikingly distinct phenotype in homozygous thrab 1-bp insertion (m/m) mutant fish, in contrast to homozygous thraa 8-bp insertion (m/m) mutant fish was the appearance of a “red belly” due to epidermal hypoplasia (Fig. 4). The homozygous thrab 1-bp insertion (m/m) mutant suppresses the expression of a panel of keratin genes at the mRNA and protein levels and inhibits keratinocyte proliferation by attenuating STAT3 signaling to decrease the protein abundance of cell cycle key regulators (Fig. 5C).
At present, it remains unclear how homozygous thraa 8-bp insertion (m/m) and homozygous thrab 1-bp insertion (m/m) mutants exert differential effects in a tissue-specific manner. Comparison of the primary amino acid sequences shows 96% homology in the DNA-binding domain and 94% in the ligand-binding domain between ThraaLeu405Glufs*6 and ThrabGlu394* mutants (Supplementary Fig. S9A, B; see Supplementary Materials and Methods). The amino A/B domain showed less homology (52%) between these two mutants and furthermore, the thrab 1-bp insertion mutant is 16 amino acids shorter at the C-terminus than the thraa 8-bp insertion mutant (Supplementary Fig. S9B; see Supplementary Materials and Methods). Previously, using the RTHα mouse model of Thra1PV/+ mice, we showed the aberrant interaction of NCOR1 with TRα1 mutants underlying the dominant actions of TRα1 mutants (44). Furthermore, Bochukova et al. reported that a patient's mutant was affected by NCOR1 (2), further supporting that constitutive association of NCOR1 with TRα1 mutants could lead to clinical manifestations of RTHα. Therefore, it is reasonable to speculate that the thrab 1-bp insertion mutant in which the C-terminal T3-binding domain is 16-amino acids shorter, could lead to a stronger interaction than the thraa 8-bp insertion mutant with the co-repressor complexes, leading to distinct and more severe phenotypes. However, it is not possible to fully understand the molecular basis of the observed differential activity, especially in a target tissue-dependent manner of these duplicated gene mutants, owing to the lack of detailed crystal structure information. Nevertheless, this question warrants further studies in the future.
Previously, using a morpholino approach, Marelli et al. demonstrated that morphants expressing dominant negative thraa exhibit severe developmental abnormalities in embryos and in the embryo–larva transition (13). The phenotypes of thraa-morphants showed brain and cardiac defects, but with normal tshba expression. Furthermore, embryos injected with several human THRA mutants to express the mutant receptors ectopically also manifested variable defects, including cerebral and cardiac edema, incomplete formation of the vascular network, and abnormal motoneurons and craniofacial development (14). In contrast to these findings, we did not detect any discernible morphological abnormalities in the embryos and early larvae in homozygous thrab 1-bp insertion (m/m) and homozygous thraa 8-bp insertion (m/m) mutant fish. Our close evaluation found that mutant embryos developed similarly as in sibling WT embryos. The first visible abnormal development was a hypoplastic epidermis at the larva–juvenile transition (28 dpf) of homozygous thrab 1-bp insertion (m/m) fish with the appearance of a “red belly,” which persisted to adulthood. We detected growth impairment of homozygous thrab 1-bp insertion (m/m) mutant fish only in the larva–juvenile transition beginning at 28 dpf, which persisted to adulthood. It is not immediately clear what may account for the difference between the findings reported by Marelli et al. (13,14) and the findings presented in this study. One possibility could be the different methodologies used to induce the expression of mutants in the embryos of zebrafish, and possibly differences in the expression levels of the mutants.
In this study, we used CRISPR/Cas9-mediated targeted mutagenesis to create the mutations of the two thra duplicated genes. This method allowed us to study the expression of mutant receptors by using endogenous promoters and transcription regulatory systems in the physiological context. The stable expression of mutant receptors facilitated the analysis of the actions of mutant receptors from embryos to adulthood. Consistent with reports by others (13,45,46), we found that thraa, thrab, and thrb are expressed in the embryos (Fig. 7A, B). While all TR genes are expressed, the thrab gene is the most abundant among the three genes during embryonic development (Fig. 7A, B). However, we found that homozygous mutations of either the thraa or thrab gene did not affect embryonic/larva development and larva transition in the mutant fish. One possible explanation for a lack of abnormalities in the embryonic development by mutation of the thraa or thrab gene could be the compensatory functions of other unaffected receptors to overcome the deleterious actions of mutant receptors. However, such compensatory mechanisms could not fully explain the phenotypes of impaired growth and hypoplastic epidermis emerging at the larva–juvenile transition and persisting to adulthood. One plausible alternative mechanism could be the temporal signaling mediated by thyroid hormones. As shown in Figure 7B, TT3 and TT4 were relatively low in the embryonic stage, suggesting that the WT TRs were most likely unliganded. Thus, there was not sufficient ligand-dependent transcription activity of WT receptors to be interfered/antagonized by mutant receptors, which cannot bind thyroid hormones. As the levels of TT3 and TT4 began to rise on day 15 and peaked at the larva–juvenile transition at day 30, more WT TRs are expected to be bound by thyroid hormones in WT fish, particularly in homozygous thrab 1-bp insertion (m/m) mutant fish, in which TT3 and TT4 were higher than WT fish at 30 and 100 dpf (elevated 1.7- to 2.5-fold; Fig. 7C). However, the homozygous thrab 1-bp insertion (m/m) mutant, which cannot bind thyroid hormone, would act in a dominant negative manner to interfere with the transcriptional activity on the T3-target genes. This possibility was supported by the findings that the expression of keratin-4, keratin-17, and keratin-18 was not affected as shown by whole mount in situ hybridization (WISH) between days 1 and 5 during in embryos and the embryo–larva transition (Supplementary Fig. S10B–D; see Supplementary Materials and Methods), but was suppressed in juveniles (Fig. 6) and adults (Fig. 5), thus leading to epidermal hypoplasia. Similarly, there were no changes in the expression of the gh1 gene as shown by WISH in Day 2 to Day 5 embryos and the embryo–larva transition (Supplementary Fig. S10A; see Supplementary Materials and Methods; no gh1 expression was detected on day 1), but the gh1 gene expression was suppressed in juveniles (Fig. 3) and adults (Fig. 2), thus leading to growth impairment. These results underscore the critical role of thyroid hormone in directing the developmental program of zebrafish. Indeed, the demonstration that the rise of TT3 and TT4 at the larva–juvenile transition is critical for postlarval growth shown in this study (Fig. 7B) is consistent with the findings in mice and Xenopus tropicalis. In WT mice, serum TT3 and TT4 are not detectable on the day of birth and rise to reach a peak on P15 (42). In athyroid Pax8−/− mice, the fetus develops and pups are born at term, but if without treatment with thyroid hormone postnatally, the pups die around weaning (47). In X. tropicalis, plasma T3 and T4 are not detectable in early tadpoles, and begin to rise at the premetamorphosis stages 55 and 57, respectively, and reach a peak at metamorphosis climax (stages 61 and 62 for T3 and T4, respectively) for transition and for postmetamorphosis growth (48).
The observation that TT3 and TT4 are relatively low in the embryos and early larvae (3–7 dpf; Fig. 7B) suggests that liganded TRs may not be critical in the initiation of embryogenesis of zebrafish. This notion is supported by the observations that the total absence of thyroid hormone in athyroid Pax8−/− mice did not prevent the development of pups to term, although the pups die shortly after weaning unless supplemented with thyroid hormones (47). In athyroid Pax8−/− mice, TRs are expressed, but mice develop to term, suggesting that liganded TR is not required in the initiation of development. Furthermore, Gothe et al. reported that mice devoid of all known TRs develop to term and remain viable long enough to reach adulthood. Mice deficient in all known TRs, however, exhibit postnatal disorders in several target organs, such as retarded growth and delayed bone maturation (49). Given the observations that pups were born at term in athyroid Pax8−/− mice, as well as in mice deficient in all known TRs, we suggest that TRs might not be required to initiate the development program in zebrafish as mutations of the two duplicated thra genes do not result in any discernable abnormalities during embryogenesis. However, the thrab gene is clearly essential for postlarval growth and the development of certain specific tissues such as the skin. Recently, Yuki et al. (pers. comm.) found that X. tropicalis tadpoles deficient in both TRα and TRβ (double knockout tadpoles) are able to initiate metamorphosis and accomplish many metamorphic changes, such as limb development. But double knockout tadpoles stall and eventually die soon after reaching stage 61, the climax of metamorphosis. The authors concluded that TRs are not required for the initiation of metamorphosis but are essential for the completion of metamorphosis and subsequent survival. Unlike in mice, the transparent zebrafish embryos that develop outside of the body allowed us to clearly visualize possible defects during development, thus providing direct evidence in support of the notion that TRs might not be required in initiating development of zebrafish embryos. Thus, our findings demonstrate the conserved role of TRs in postembryonic development and homeostasis in mice, Xenopus, and zebrafish.
This study shows that the expression of the tshba gene is elevated at the mRNA level (Fig. 8C, D) accompanied by elevated TT3 and TT4 levels (Fig. 7C) in homozygous 1-bp insertion (m/m) mutant fish. Currently, it is not possible to determine the serum TSH concentrations in zebrafish. However, if the elevated tshba mRNA were to be linearly translated into TSH polypeptide in the pituitary and secreted out of the pituitary, it would seem that homozygous 1-bp insertion (m/m) mutant fish would exhibit elevated T3, T4, and TSH profiles. In RTHα patients, serum T3 can be high-normal to high, T4 normal to low, while TSH is normal or mildly raised (22). Thus, except for T4, it would seem that T3 and TSH levels would be in concordance with those observed in RTHα patients. At present, the molecular basis for the minor hormonal profile differences between RTHα patients and mutant zebrafish is not clear.
As in patients and mice with mutations of TRα1, zebrafish expressing mutated thraa and thrab genes exhibit postembryonic growth retardation, indicating the conserved role of TRα1 in growth regulation among these three species. Interestingly, patients with mutations of TRα1 were reported to exhibit a skin disorder with skin tags (34). At present, it is not clear how the skin tags found in patients with mutations of TRα1 may be related to the hypoplastic epidermis found in homozygous thrab 1-bp insertion (m/m) mutant fish. Nonetheless, the skin tags found in patients would suggest that TRα1 mutations would impact skin biology in patients. Indeed, skin is the largest organ of the human body and serves as a barrier to protect the individual from external insults. Thyroid hormone is known to directly affect cutaneous biology, including the epidermis, dermis, and hair. All three T3-binding TR isoforms have been identified in skin tissues (50,51). TRs were shown to directly regulate gene expression in the skin (37). Hypothyroid patients have pale, cold, dry, and xerotic skin with thin epidermis (52). Hypothyroidism at the tissue level is either caused by decreased cellular levels of thyroid hormone or caused by resistance of target tissues to hormone action. This study shows that mutations of the thrab gene lead to epidermal hypoplasia with an easily recognizable phenotype (“red belly”) as early as 4 weeks postfertilization. Thus, this zebrafish model is an excellent tool to further elucidate the actions of TRs, and for rapid screening of potential therapeutics for treating skin disorders.
Supplementary Material
Acknowledgments
We thank Dr. Luca Persani (Universita degli Studi di Milano, Italy) for the generous gift of zthraaL plasmid. To make probes for WISH analyses, we are grateful to Dr. Driever Wolfgang (Albert-Ludwigs-University Freiburg, Germany) for keratin-4, keratin-5, and keratin-17 plasmids; Dr. Benzamin Feldman (NIH/NICHD, USA) for keratin-4, keratin-8, and keratin-18 plasmids; Dr. Eric C. Liao (Harvard University, USA) for keratin-4 and keratin-18 plasmids; Dr. Hammerschmidt Matthias (University of Cologne, Germany) and Dr. David Kimelman (University of Washington, USA) for the p63 plasmid; and Dr. Alberto Rissone (NIH/NHGRI, USA) for the gh1 probe. We thank Dr. Alberto Rissone and Dr. Erica Bresciani (NIH/NHGRI, USA) for valuable discussions and for teaching us WISH, and Dr. Peter McPhie for the analysis of T3-binding data. We also thank Ross Lake (NIH/LGCP Microscopy Core Facility) for discussion of and suggestions for our study. We are indebted to Dr. Victoria Hoffmann (Division of Veterinary Resources, Diagnostic and Research Services Branch, NIH) for showing us how to dissect the tiny pituitary from zebrafish.
Author Disclosure Statement
The authors declare no conflicts of interest.
Funding Information
This research was supported by the Intramural Research Program (InnovationAward) of the Center for Cancer Research, National Cancer Institute, and National Institutes of Health.
Supplementary Material
References
- 1. Concolino P, Costella A, Paragliola RM. 2019. Mutational landscape of resistance to thyroid hormone beta (RTHbeta). Mol Diagn Ther 23:353–368 [DOI] [PubMed] [Google Scholar]
- 2. Bochukova E, Schoenmakers N, Agostini M, Schoenmakers E, Rajanayagam O, Keogh JM, Henning E, Reinemund J, Gevers E, Sarri M, Downes K, Offiah A, Albanese A, Halsall D, Schwabe JW, Bain M, Lindley K, Muntoni F, Vargha-Khadem F, Dattani M, Farooqi IS, Gurnell M, Chatterjee K. 2012. A mutation in the thyroid hormone receptor alpha gene. N Engl J Med 366:243–249 [DOI] [PubMed] [Google Scholar]
- 3. van Mullem AA, Chrysis D, Eythimiadou A, Chroni E, Tsatsoulis A, de Rijke YB, Visser WE, Visser TJ, Peeters RP. 2013. Clinical phenotype of a new type of thyroid hormone resistance caused by a mutation of the TRalpha1 receptor: consequences of LT4 treatment. J Clin Endocrinol Metab 98:3029–3038 [DOI] [PubMed] [Google Scholar]
- 4. Moran C, Schoenmakers N, Agostini M, Schoenmakers E, Offiah A, Kydd A, Kahaly G, Mohr-Kahaly S, Rajanayagam O, Lyons G, Wareham N, Halsall D, Dattani M, Hughes S, Gurnell M, Park SM, Chatterjee K. 2013. An adult female with resistance to thyroid hormone mediated by defective thyroid hormone receptor alpha. J Clin Endocrinol Metab 98:4254–4261 [DOI] [PubMed] [Google Scholar]
- 5. Tylki-Szymanska A, Acuna-Hidalgo R, Krajewska-Walasek M, Lecka-Ambroziak A, Steehouwer M, Gilissen C, Brunner HG, Jurecka A, Rozdzynska-Swiatkowska A, Hoischen A, Chrzanowska KH. 2015. Thyroid hormone resistance syndrome due to mutations in the thyroid hormone receptor alpha gene (THRA). J Med Genet 52:312–316 [DOI] [PubMed] [Google Scholar]
- 6. Moran C, Agostini M, McGowan A, Schoenmakers E, Fairall L, Lyons G, Rajanayagam O, Watson L, Offiah A, Barton J, Price S, Schwabe J, Chatterjee K. 2017. Contrasting phenotypes in resistance to thyroid hormone alpha correlate with divergent properties of thyroid hormone receptor alpha1 mutant proteins. Thyroid 27:973–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kaneshige M, Suzuki H, Kaneshige K, Cheng J, Wimbrow H, Barlow C, Willingham MC, Cheng S. 2001. A targeted dominant negative mutation of the thyroid hormone alpha 1 receptor causes increased mortality, infertility, and dwarfism in mice. Proc Natl Acad Sci U S A 98:15095–15100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. O'Shea PJ, Harvey CB, Suzuki H, Kaneshige M, Kaneshige K, Cheng SY, Williams GR. 2003. A thyrotoxic skeletal phenotype of advanced bone formation in mice with resistance to thyroid hormone. Mol Endocrinol 17:1410–1424 [DOI] [PubMed] [Google Scholar]
- 9. Bassett JH, Boyde A, Zikmund T, Evans H, Croucher PI, Zhu X, Park JW, Cheng SY, Williams GR. 2014. Thyroid hormone receptor alpha mutation causes a severe and thyroxine-resistant skeletal dysplasia in female mice. Endocrinology 155:3699–3712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Park S, Han CR, Park JW, Zhao L, Zhu X, Willingham M, Bodine DM, Cheng SY. 2017. Defective erythropoiesis caused by mutations of the thyroid hormone receptor alpha gene. PLoS Genet 13:e1006991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Darras VM, Van Herck SL, Heijlen M, De Groef B. 2011. Thyroid hormone receptors in two model species for vertebrate embryonic development: chicken and zebrafish. J Thyroid Res 2011:402320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Takayama S, Hostick U, Haendel M, Eisen J, Darimont B. 2008. An F-domain introduced by alternative splicing regulates activity of the zebrafish thyroid hormone receptor alpha. Gen Comp Endocrinol 155:176–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Marelli F, Carra S, Agostini M, Cotelli F, Peeters R, Chatterjee K, Persani L. 2016. Patterns of thyroid hormone receptor expression in zebrafish and generation of a novel model of resistance to thyroid hormone action. Mol Cell Endocrinol 424:102–117 [DOI] [PubMed] [Google Scholar]
- 14. Marelli F, Carra S, Rurale G, Cotelli F, Persani L. 2017. In vivo functional consequences of human THRA variants expressed in the zebrafish. Thyroid 27:279–291 [DOI] [PubMed] [Google Scholar]
- 15. Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. 1995. Stages of embryonic development of the zebrafish. Dev Dyn 203:253–310 [DOI] [PubMed] [Google Scholar]
- 16. Varshney GK, Carrington B, Pei W, Bishop K, Chen Z, Fan C, Xu L, Jones M, LaFave MC, Ledin J, Sood R, Burgess SM. 2016. A high-throughput functional genomics workflow based on CRISPR/Cas9-mediated targeted mutagenesis in zebrafish. Nat Protoc 11:2357–2375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sood R, Carrington B, Bishop K, Jones M, Rissone A, Candotti F, Chandrasekharappa SC, Liu P. 2013. Efficient methods for targeted mutagenesis in zebrafish using zinc-finger nucleases: data from targeting of nine genes using CompoZr or CoDA ZFNs. PLoS One 8:e57239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McMenamin SK, Bain EJ, McCann AE, Patterson LB, Eom DS, Waller ZP, Hamill JC, Kuhlman JA, Eisen JS, Parichy DM. 2014. Thyroid hormone-dependent adult pigment cell lineage and pattern in zebrafish. Science 345:1358–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li B, Dewey CN. 2011. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics 12:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Robinson MD, McCarthy DJ, Smyth GK. 2010. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26:139–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhu X, Zhu YJ, Kim DW, Meltzer P, Cheng SY. 2014. Activation of integrin-ERBB2 signaling in undifferentiated thyroid cancer. Am J Cancer Res 4:776–788 [PMC free article] [PubMed] [Google Scholar]
- 22. van Gucht ALM, Moran C, Meima ME, Visser WE, Chatterjee K, Visser TJ, Peeters RP. 2017. Resistance to thyroid hormone due to heterozygous mutations in thyroid hormone receptor alpha. Curr Top Dev Biol 125:337–355 [DOI] [PubMed] [Google Scholar]
- 23. Marelli F, Persani L. 2018. Role of TRs in zebrafish development. Methods Mol Biol 1801:287–298 [DOI] [PubMed] [Google Scholar]
- 24. Tinnikov A, Nordstrom K, Thoren P, Kindblom JM, Malin S, Rozell B, Adams M, Rajanayagam O, Pettersson S, Ohlsson C, Chatterjee K, Vennstrom B. 2002. Retardation of post-natal development caused by a negatively acting thyroid hormone receptor alpha1. Embo J 21:5079–5087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wood DF, Franklyn JA, Docherty K, Ramsden DB, Sheppard MC. 1987. The effect of thyroid hormones on growth hormone gene expression in vivo in rats. J Endocrinol 112:459–463 [DOI] [PubMed] [Google Scholar]
- 26. Kamegai J, Tamura H, Ishii S, Sugihara H, Wakabayashi I. 2001. Thyroid hormones regulate pituitary growth hormone secretagogue receptor gene expression. J Neuroendocrinol 13:275–278 [DOI] [PubMed] [Google Scholar]
- 27. Marchand O, Duffraisse M, Triqueneaux G, Safi R, Laudet V. 2004. Molecular cloning and developmental expression patterns of thyroid hormone receptors and T3 target genes in the turbot (Scophtalmus maximus) during post-embryonic development. Gen Comp Endocrinol 135:345–357 [DOI] [PubMed] [Google Scholar]
- 28. Fukamachi S, Yada T, Mitani H. 2005. Medaka receptors for somatolactin and growth hormone: phylogenetic paradox among fish growth hormone receptors. Genetics 171:1875–1883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Astola A, Pendon C, Ortiz M, Valdivia MM. 1996. Cloning and expression of somatolactin, a pituitary hormone related to growth hormone and prolactin from gilthead seabream, Sparus aurata. Gen Comp Endocrinol 104:330–336 [DOI] [PubMed] [Google Scholar]
- 30. Velloso CP. 2008. Regulation of muscle mass by growth hormone and IGF-I. Br J Pharmacol 154:557–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fuentes EN, Valdes JA, Molina A, Bjornsson BT. 2013. Regulation of skeletal muscle growth in fish by the growth hormone—insulin-like growth factor system. Gen Comp Endocrinol 192:136–148 [DOI] [PubMed] [Google Scholar]
- 32. Roberts SB, McCauley LA, Devlin RH, Goetz FW. 2004. Transgenic salmon overexpressing growth hormone exhibit decreased myostatin transcript and protein expression. J Exp Biol 207:3741–3748 [DOI] [PubMed] [Google Scholar]
- 33. Schiaffino S, Mammucari C. 2011. Regulation of skeletal muscle growth by the IGF1-Akt/PKB pathway: insights from genetic models. Skelet Muscle 1:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Moran C, Agostini M, Visser WE, Schoenmakers E, Schoenmakers N, Offiah AC, Poole K, Rajanayagam O, Lyons G, Halsall D, Gurnell M, Chrysis D, Efthymiadou A, Buchanan C, Aylwin S, Chatterjee KK. 2014. Resistance to thyroid hormone caused by a mutation in thyroid hormone receptor (TR)alpha1 and TRalpha2: clinical, biochemical, and genetic analyses of three related patients. Lancet Diabetes Endocrinol 2:619–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Demir K, van Gucht AL, Buyukinan M, Catli G, Ayhan Y, Bas VN, Dundar B, Ozkan B, Meima ME, Visser WE, Peeters RP, Visser TJ. 2016. Diverse genotypes and phenotypes of three novel thyroid hormone receptor-alpha mutations. J Clin Endocrinol Metab 101:2945–2954 [DOI] [PubMed] [Google Scholar]
- 36. Slominski A, Wortsman J. 2000. Neuroendocrinology of the skin. Endocr Rev 21:457–487 [DOI] [PubMed] [Google Scholar]
- 37. Antonini D, Sibilio A, Dentice M, Missero C. 2013. An intimate relationship between thyroid hormone and skin: regulation of gene expression. Front Endocrinol (Lausanne) 4:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Macias E, Rao D, Digiovanni J. 2013. Role of stat3 in skin carcinogenesis: insights gained from relevant mouse models. J Skin Cancer 2013:684050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Miyoshi K, Takaishi M, Nakajima K, Ikeda M, Kanda T, Tarutani M, Iiyama T, Asao N, DiGiovanni J, Sano S. 2011. Stat3 as a therapeutic target for the treatment of psoriasis: a clinical feasibility study with STA-21, a Stat3 inhibitor. J Invest Dermatol 131:108–117 [DOI] [PubMed] [Google Scholar]
- 40. Sano S, Chan KS, DiGiovanni J. 2008. Impact of Stat3 activation upon skin biology: a dichotomy of its role between homeostasis and diseases. J Dermatol Sci 50:1–14 [DOI] [PubMed] [Google Scholar]
- 41. Buettner R, Mora LB, Jove R. 2002. Activated STAT signaling in human tumors provides novel molecular targets for therapeutic intervention. Clin Cancer Res 8:945–954 [PubMed] [Google Scholar]
- 42. Friedrichsen S, Christ S, Heuer H, Schafer MK, Mansouri A, Bauer K, Visser TJ. 2003. Regulation of iodothyronine deiodinases in the Pax8-/- mouse model of congenital hypothyroidism. Endocrinology 144:777–784 [DOI] [PubMed] [Google Scholar]
- 43. Espiard S, Savagner F, Flamant F, Vlaeminck-Guillem V, Guyot R, Munier M, d'Herbomez M, Bourguet W, Pinto G, Rose C, Rodien P, Wemeau JL. 2015. A novel mutation in THRA gene associated with an atypical phenotype of resistance to thyroid hormone. J Clin Endocrinol Metab 100:2841–2848 [DOI] [PubMed] [Google Scholar]
- 44. Fozzatti L, Kim DW, Park JW, Willingham MC, Hollenberg AN, Cheng SY. 2013. Nuclear receptor corepressor (NCOR1) regulates in vivo actions of a mutated thyroid hormone receptor alpha. Proc Natl Acad Sci U S A 110:7850–7855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lazcano I, Rodriguez-Ortiz R, Villalobos P, Martinez-Torres A, Solis-Sainz JC, Orozco A. 2019. Knock-down of specific thyroid hormone receptor isoforms impairs body plan development in zebrafish. Front Endocrinol (Lausanne) 10:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Liu YW, Chan WK. 2002. Thyroid hormones are important for embryonic to larval transitory phase in zebrafish. Differentiation 70:36–45 [DOI] [PubMed] [Google Scholar]
- 47. Mansouri A, Chowdhury K, Gruss P. 1998. Follicular cells of the thyroid gland require Pax8 gene function. Nat Genet 19:87–90 [DOI] [PubMed] [Google Scholar]
- 48. Leloup J, Buscaglia M. 1977. La triiodothyronine: hormone de la metamorphose desamphibiens. C R Acad Sci 284:2261–2263 [Google Scholar]
- 49. Gothe S, Wang Z, Ng L, Kindblom JM, Barros AC, Ohlsson C, Vennstrom B, Forrest D. 1999. Mice devoid of all known thyroid hormone receptors are viable but exhibit disorders of the pituitary-thyroid axis, growth, and bone maturation. Genes Dev 13:1329–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ahsan MK, Urano Y, Kato S, Oura H, Arase S. 1998. Immunohistochemical localization of thyroid hormone nuclear receptors in human hair follicles and in vitro effect of L-triiodothyronine on cultured cells of hair follicles and skin. J Med Invest 44:179–184 [PubMed] [Google Scholar]
- 51. Billoni N, Buan B, Gautier B, Gaillard O, Mahe YF, Bernard BA. 2000. Thyroid hormone receptor beta1 is expressed in the human hair follicle. Br J Dermatol 142:645–652 [DOI] [PubMed] [Google Scholar]
- 52. Kasumagic-Halilovic E. 2014. Thyroid disease and the skin. Ann Thyroid Res 1:27–31 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.