Abstract
Stem cell therapy is a promising strategy to treat muscle diseases such as Duchenne muscular dystrophy (DMD). To avoid immune rejection of donor cells or donor-derived muscle, autologous cells, which have been genetically modified to express dystrophin, are preferable to cells derived from healthy donors. Restoration of full-length dystrophin (FL-dys) using viral vectors is extremely challenging, due to the limited packaging capacity of the vectors, but we have recently shown that either a foamy viral or lentiviral vector is able to package FL-dys open-reading frame and transduce myoblasts derived from a DMD patient. Differentiated myotubes derived from these transduced cells produced FL-dys. Here, we transplanted the foamy viral dystrophin-corrected DMD myoblasts intramuscularly into mdx nude mice, and showed that the transduced cells contributed to muscle regeneration, expressing FL-dys in nearly all the muscle fibers of donor origin. Furthermore, we showed that the restored FL-dys recruited members of the dystrophin-associated protein complex and neuronal nitric oxide synthase within donor-derived muscle fibers, evidence that the restored dystrophin protein is functional. Dystrophin-expressing donor-derived muscle fibers expressed lower levels of utrophin than host muscle fibers, providing additional evidence of functional improvement of donor-derived myofibers. This is the first in vivo evidence that foamy virus vector-transduced DMD myoblasts can contribute to muscle regeneration and mediate functional dystrophin restoration following their intramuscular transplantation, representing a promising therapeutic strategy for individual small muscles in DMD.
Keywords: Duchenne muscular dystrophy, foamy virus, codon-optimized full-length dystrophin, mdx nude mice, intramuscular transplantation
Introduction
Duchenne muscular dystrophy (DMD) is an X-linked disorder affecting 1:5,000 boys worldwide.1 The disease is caused by lack of dystrophin, leading to muscle fiber necrosis and progressive weakness. In mouse models of DMD, transplanted normal muscle stem cells contribute to muscle regeneration and reconstitute the muscle stem cell pool,2,3 evidence that stem cell transplantation might be a promising therapeutic strategy to treat DMD. To avoid immune rejection, autologous, genetically modified stem cells4 would be preferable to allogeneic stem cells. To correct the dystrophin mutation in patient cells, one could use clustered, regularly interspaced, short palindromic repeats-associated Cas9 (CRISPR-Cas9)-mediated gene editing,5–7 but this method is highly mutation dependent. Different gene editing strategies need to be designed and tested for patients with different dystrophin mutations. This is time-consuming and editing efficiency is not always guaranteed. In contrast, viral vectors, in particular integrating retroviral or lentiviral vectors that correct the autologous stem cells by introducing extra copies of the dystrophin coding sequence into the genome, are applicable to all patients, regardless of mutation. For therapeutic purposes, full-length dystrophin (FL-dys) is preferable to mini-dystrophin, as only the former contains all of the functional motifs and should fully restore the muscle fiber function. However, the delivery of FL-dys to DMD stem cells using viral vectors has been challenging due to the large size of the FL-dys coding sequence (around 11 kb) and the relatively limited packaging capacity of the therapeutic virus vectors.
Foamy virus (FV) is one of the largest retroviruses, having a packaging capacity of 12–13 kb, which could accommodate the FL-dys open-reading frame (ORF, 11 kb). FV is not endemic in humans.8 High-throughput9 or large-scale10 integration site analysis on FV-transduced human fibroblasts or CD34+ hematopoietic cells showed that FV seldom integrated within genes, changed gene expression, or integrated into active oncogenes, suggesting a unique highly desirable safety profile over that of the lentivirus (LV) and gamma-retrovirus.9,10 FV-transduced hematopoietic stem cells (HSCs) have been used in preclinical animal models to treat dogs with severe erythroid disease.11 The fact that FV can infect both dividing and quiescent cells, and is able to persist in quiescent cells,8 makes it an attractive vector for transducing muscle stem cells, which, following transplantation, give rise to satellite cells (most of which are quiescent in normal adult muscle) in vivo.
We have recently shown that the 11 kb insert of FL-dys could be packaged in either an LV12 or an FV vector13 and both effectively transduced DMD myoblasts, suggesting that such a cell-mediated gene therapy might be applicable to treat DMD. However, the efficiency of this approach and the functionality of the restored FL-dys need to be tested in animal models before moving to clinical application. To efficiently deliver the FL-dys into DMD patient muscle cells, a vector leading to a high level of transgene expression would be ideal. In our recent LV work, the FL-dys we used was the native form of the dystrophin coding sequence12; while in the FV study,13 we used codon-optimized full-length dystrophin (coFLDys), which may result in higher protein expression than the native form of dystrophin.14,15 The LV-dystrophin vector contained a green fluorescent protein (GFP) cassette, which facilitated the enrichment of the transduced cells via fluorescence-activated cell sorting (FACS) and also limited its clinical application due to potential toxicity caused by the expression of GFP.16 On the contrary, the FV-dystrophin vector is devoid of such a selection cassette, making it more clinically applicable.
Here, we transplanted DMD myoblasts, which had been transduced with FV coding for coFLDys, into cryodamaged muscles of immunodeficient, dystrophin-deficient mdx mice, and determined their transplantation efficiency, restoration of FL-dys, and the functionality of the restored dystrophin within muscle fibers of donor origin. Our study provides evidence that restoration of FL-dys in skeletal muscle mediated by virally transduced DMD myoblasts is feasible and might be a promising therapeutic strategy for DMD.
Materials and Methods
Ethics
The work was performed under the NHS National Research Ethics: Setting up of a rare diseases biological samples bank (biobank) for research to facilitate pharmacological, gene and cell therapy trials in neuromuscular disorders (REC Reference No. 06/Q0406/33); and the use of cells as a model system to study pathogenesis and therapeutic strategies for neuromuscular disorders (REC reference 13/LO/1826).
Mice were bred and experimental procedures were carried out in the Biological Services Unit, University College London Great Ormond Street Institute of Child Health, in accordance with the Animals (Scientific Procedures) Act 1986. Experiments were performed under Home Office license numbers 70/7086 and 8389. Experiments were approved by the local University College London Ethics Committee before the license being granted.
Mice were fed Envigo diet 2918. Envigo diet 2919 was used for damp diet, given to the mice after surgery. Both diets were irradiated. Mice were kept in individually ventilated Tecniplast Green Line cages, on a 12-h light/12-h dark cycle.
FV vector production
FV-coFLDys is a self-inactivating FV vector that encodes the FL-dys protein under the control of the SFFV promoter.13 The production, concentration, and titration of FV were performed as described previously.13,17 Briefly, FV was produced in HEK-293T cells transfected with a four-plasmid system using PEImax (Park Scientific Ltd., Northampton, United Kingdom) at a ratio of 3:1 PEI:DNA. A vector containing a supernatant was concentrated by ultracentrifugation in an SW28 rotor at 19,000 rpm for 2 h at 20°C. Pellets were resuspended in phosphate-buffered saline (PBS) containing 5% vol/vol DMSO and aliquots were stored at −80°C. Vector titer was determined by quantitative polymerase chain reaction on genomic DNA extracted from transduced HT1080 cells using primers/probes targeting the FV LTR and albumin as a reference gene as previously described.13 All multiplicities of infection (MOIs) are given in HT1080 transducing units per target cell.
Cell culture and transduction of human muscle-derived stem cells with FV
Normal human skeletal muscle-derived CD133+ cells18 and DMD myoblasts (with a mutation in dystrophin exon 52) were used for FV vector transduction. Cells were maintained in M10 medium, consisting of MegaCell Dulbecco's modified Eagle's medium (DMEM) (Sigma) supplemented with 10% fetal bovine serum (FBS; Thermo Fisher), 2 mM glutamine (Thermo Fisher), and 5 ng/mL basic fibroblast growth factor (Peprotech). Different amounts of vector were added to 1 × 105 cells and the six-well plates were spinoculated at 30°C at 1,200 g for 90 min. Cells were changed into a fresh medium 6 h after adding the vector. For FV-1-GFP, normal CD133+ cells were transduced at MOI 0, 1, 10, 20, 30, and 50; for coFLDys-expressing FV, DMD myoblasts were transduced with vector at MOI 2, 4, 8, and 16. The transduced cells were then expanded in M10 medium for subsequent experiments.
FACS analysis
For GFP-expressing FV-transduced cells, the percentage of GFP+ cells and their mean fluorescent intensity (MFI) were measured 6 days after transduction, using an FACS Calibur machine (BD). The data were analyzed using FlowJo software.
Growth curve assay
Cells transduced with FV-coFLDys were maintained in M10 medium for expansion. Cells were passaged once they reached around 70% confluency and cell number recorded. The growth curve was generated by plotting the number of population doublings the cells underwent (Y axis) over the time (days, X axis) in culture. The number of population doublings of the cells was calculated using the formula: Ln (number of total cells at harvesting/number of cells at seeding)/Ln2.
Immunofluorescent staining
Cultured cells were plated onto Matrigel (0.1 mg/mL; BD Bioscience)-coated eight-well chamber slides at a density of 5 × 104 cells/well in M10 medium. Cells were changed into MegaCell DMEM containing 2% FBS to induce their myogenic differentiation. Cells fixed by 4% paraformaldehyde (PFA) before or 5 days after the onset of differentiation were immunostained using antibodies against dystrophin (1:1,000; Fisher Scientific) alone for staining of nondifferentiated cells, or together with an antibody against myosin (MF20; DSHB) for differentiated cells.
Western blot
FV-transduced cells were induced to undergo myogenic differentiation for 5 days before being lysed with the radioimmunoprecipitation assay buffer (Sigma), supplemented with protease inhibitor (Roche) on ice for 15 min. The cell lysate was collected and boiled for 5 min before being centrifuged at 14,000 g for 10 min at 4°C. Thirty microliters of each sample was loaded/well onto NuPAGE Novex 3–8% Tris-acetate gel, and run at a constant voltage of 150 V for 1.5 h, before being transferred to a PVDF membrane using the Trans-Blot Turbo Transfer system (Bio-Rad). The membrane was blocked with the Odyssey block solution (LI-COR Biosciences, Cambridge, United Kingdom) for 1 h, before being incubated with primary antibodies against dystrophin (1:2,000; Fisher Scientific) and tubulin 2.1 (1:1,000; Santa Cruz) overnight at 4°C. After washing with PBS containing 0.1% Tween 20 for 15 min × 3 times at room temperature, the membrane was incubated with the biotinylated anti-rabbit secondary antibody (1:1,000) for 2 h, followed by IRDye 680RD Streptavidin and IRDye 800CW goat anti-mouse secondary antibodies (1:15,000; LI-COR Biosciences) for 1 h at room temperature. The image of the blotted membrane was acquired by the Odyssey Clx infrared imaging system (LI-COR Biosciences) using Image Studio software.
Intramuscular transplantation
Four- to 8-week-old male Rag2−/γ chain−/C5− mice19,20 or male mdx nude mice4,21 were used as recipients for cell transplantation. On the day of transplantation, mice were anesthetized with isoflurane in the afternoon (during the light cycle) and tibialis anterior (TA) muscles were cryodamaged with three freeze/thaw cycles using a cryoprobe prechilled in liquid nitrogen, to promote engraftment.21–23 Cryoinjury of mouse muscle mimics the degeneration that occurs in DMD patient muscles.
Cells, 5 × 105, in 5 μL medium were injected into each TA with a Hamilton syringe. Grafted muscles were dissected 4 weeks after transplantation and snap-frozen in isopentane chilled in liquid nitrogen. The n value is for the number of TA muscles. We use both hind limb muscles, as they can be considered independent units; cells transplanted intramuscularly into one TA muscle have no effect on the contralateral TA muscle. This is in accordance with the 3Rs guidelines (National Centre for the Replacement, Refinement and Reduction of Animals in Research).
Immunofluorescent staining on muscle sections
Ten-micrometer transverse cryosections were cut throughout the muscle, air-dried, blocked with the mouse IgG blocking reagent for 1 h, and stained with the following combination of antibodies: (i) antibodies against human spectrin (mouse IgG 2b, 1:100, VP-S283; Vector Labs, Peterborough, United Kingdom), human lamin A/C (mouse IgG2b, 1:500, VP-L550; Vector Labs), and human dystrophin (Mandys 106, mouse IgG2a, 1:200; Millipore). The number of human lamin A/C+ nuclei, human spectrin+ fibers, and human spectrin+ fibers containing at least one human lamin A/C+ nucleus (as a confirmation that the spectrin+ fibers were of donor origin)24 were counted in representative transverse sections. The number of dystrophin+/hSpectrin+ fibers was also quantified to evaluate the percentage of dystrophin-expressing fibers of donor origin. The sections were scored by one observer. (ii) Dystrophin (Mandys 106, mouse IgG2a) and α-sarcoglycan (mouse IgG1, 1:100; Leica Biosystems). (iii) Dystrophin (Mandys 106, mouse IgG2a; Millipore) and γ-sarcoglycan (rabbit polyclonal, 1:500; Santa Cruz). (iv) Dystrophin (Mandys 106, mouse IgG2a, 1:100; Millipore) and neuronal nitric oxide synthase (nNOS) (rabbit polyclonal; Santa Cruz). (v) Dystrophin (Mandys 106, mouse IgG2a) and utrophin (mouse IgG1, 1:200; Leica Biosystems). The results were acquired using the Axio Scan slide scanner, and the intensity of α-sarcoglycan, γ-sarcoglycan, nNOS, or utrophin on dystrophin+ or dystrophin− fibers was quantified using MetaMorph software. The correlation between the intensity of these proteins and the dystrophin intensity was analyzed using GraphPad Prism 5.0 software.
Results
FV-transduced human muscle CD133+ cells contribute to muscle regeneration
We have previously shown that FV can transduce myoblasts in vitro13 resulting in transgene expression in transduced myoblasts and the myotubes derived from these cells.
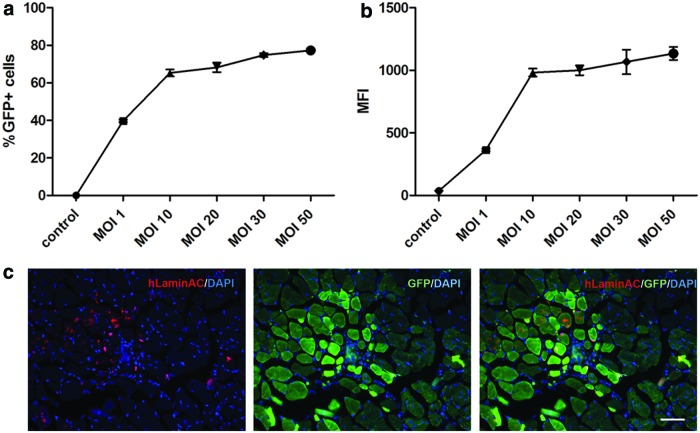
To determine whether FV-modified human muscle CD133+ cells can contribute to muscle regeneration in vivo, we transduced normal CD133+ cells18 with the FV vector coding for GFP under the control of the phosphoglycerate kinase 1 promoter at 1, 10, 20, 30, and 50 MOIs. Six days post-transduction, cells expressed GFP in a dose-dependent manner (Fig. 1a) at low MOI, but the percentage of GFP+ cells reached a plateau (around 76–80%) at an MOI of 10. MFI of the transduced cells followed a similar trend. To avoid potential toxicity caused by the vector,25 we selected cells that were transduced with an MOI of 10 for subsequent experiments.
Figure 1.
Human skeletal muscle CD133+ cells retain their in vivo myogenic capacity post-transduction with FV. The percentage of GFP+ cells (a) and the mean fluorescence intensity (b) of cells transduced with different MOIs of FV coding for GFP, 6 days after transduction. (c) The contribution of transduced cells (MOI 10) to muscle regeneration after their intramuscular transplantation into immunodeficient mice. Donor-derived nuclei expressed human lamin AC+ (red), and donor muscle fibers were identified by their GFP expression. Scale bar = 25 μm. FV, foamy virus; GFP, green fluorescent protein; MOI, multiplicity of infection. Color images are available online.
Transduced CD133+ cells were transplanted intramuscularly into cryodamaged TA muscles (n = 8) of immunodeficient C5−/γ chain−/Rag2− mice.19 Mice were perfused with 4% PFA (under terminal anesthesia) 4 weeks after transplantation, and transverse sections from the TA muscles were analyzed by immunostaining of human lamin A/C and GFP. The human lamin A/C antibody recognizes the nuclear protein lamin A/C specifically from human, not mouse, cells and has been widely used as a human-specific marker to distinguish donor cells from host tissue in human to mouse xenograft experiments.20,26,27 We found GFP+ muscle fibers within the transplanted muscles, and their donor cell origin was confirmed by the presence of human lamin A/C+ nuclei within the GFP+ fibers (Fig. 1). These data demonstrate that FV can deliver a transgene into muscle CD133+ cells, without compromising their ability to contribute to muscle regeneration after in vivo transplantation.
Transduction of FV encoding coFLDys into DMD myoblasts
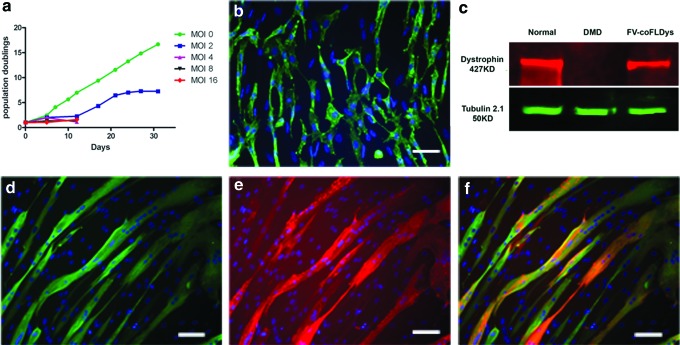
We then investigated delivery of FL-dys into myoblasts using FV vector. We previously reported13 that FV encoding coFLDys under the control of SFFV promoter transduced DMD myoblasts, resulting in FL-dys expression in differentiated myotubes derived from these myoblasts. Using the same FV vector, we transduced 1 × 105 DMD myoblasts with concentrated FV-coFLDys at MOIs of 2, 4, 8, and 16, and determined the proliferation rate of transduced cells, by plotting the population doublings of the cells over the days in culture. The vector elicited a dose-dependent toxicity when high MOIs (≥4) were used, as indicated by the cessation of proliferation of transduced cells (Fig. 2a). Only cells transduced with MOI 2 FV-coFLDys survived and proliferated, but with a compromised proliferation rate in comparison with the nontransduced cells. Nevertheless, these transduced cells could still proliferate to generate sufficient cells for the in vivo transplantation study.
Figure 2.
In vitro transduction of human DMD myoblasts with FV-coFLDys. (a) In vitro proliferation curve of cells transduced with different amounts of FVs, for 30 days after their transduction. (b) The expression of dystrophin in cells with FV of MOI 2. (c) Western blot indicating the correct molecular weight of the transgene in differentiated myotubes derived from the transduced cells. (d–f) Immunostaining of myosin heavy chain (d, green), dystrophin (e, red), and merged images (f). Nuclei were counterstained with DAPI. Scale bar = 25 μm. coFLDys, codon-optimized full-length dystrophin; DMD, Duchenne muscular dystrophy. Color images are available online.
We then examined the transgene expression of the MOI 2 FV-coFLDys-transduced cells. We found that 42.19% ± 6.91% of transduced myoblasts were dystrophin+ (Fig. 2b); after inducing these cells to differentiate into myotubes, 55.22% ± 8.73% of differentiated myotubes expressed dystrophin.
To confirm that the dystrophin was of the expected molecular weight, we performed Western blotting using myotubes differentiated from either nontransduced or transduced cells; myotubes differentiated from normal skeletal muscle-derived CD133+ cells were included as a positive control. There was a clear 427kD dystrophin band in samples from both positive controls and transduced cells, in comparison with the absence of such a band in nontransduced cells (Fig. 2c).
Transduced DMD myoblasts contribute to muscle regeneration and express FL-dys in all donor-derived muscle fibers
Transduced cells were transplanted into cryodamaged TA muscles of mdx nude mice4,21 to determine their contribution to muscle regeneration and restoration of dystrophin in vivo.
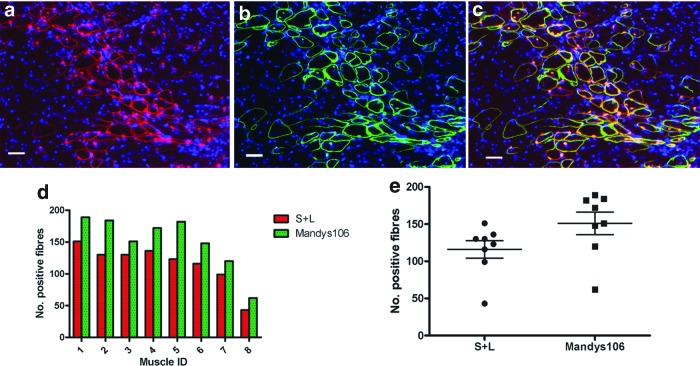
Twenty-eight days after transplantation, the muscles were removed and cryosections stained for antibodies against human lamin A/C (to determine the number of cells of donor origin within host muscle), human spectrin (to determine the number of muscle fibers of donor origin; the combination of both antibodies confirmed the donor origin of the muscle fibers),24 and human dystrophin (Mandys 106) (to determine the number of muscle fibers of donor origin that express dystrophin). The ratio of fibers containing dystrophin to those containing human spectrin and a human lamin A/C+ nucleus indicates the efficiency of dystrophin restoration in donor-derived muscle fibers.
All transplanted muscles contained donor-derived muscle fibers (Fig. 3). There were significantly more myofibers expressing human dystrophin (131.3 ± 4.129, mean ± SEM, n = 8) than human spectrin and human lamin A/C (116 ± 11.71, mean ± SEM, n = 8), suggesting that dystrophin had been restored in all of the donor-derived muscle fibers.
Figure 3.
FV-coFLDys-transduced DMD myoblasts contribute to muscle regeneration after intramuscular transplantation into immunodeficient mdx nude mice. (a–c) Immunostaining of human lamin AC/human spectrin (a, both red), human dystrophin (green, b), and (c) merged image of (a, b). (d, e) Quantification of the number of human spectrin+ fibers, which contained at least one human lamin A/C+ nucleus (S+L); and dystrophin+ fibers in each transplanted muscle. Color images are available online.
Transplanted dystrophin-expressing fibers recruit α-sarcoglycan and γ-sarcoglycan to the sarcolemma
When dystrophin is not present within a myofiber, other members of the dystrophin-associated protein complex (DAPC) are also absent or severely reduced.28 To determine if the restored dystrophin was functional, we investigated whether it recruited members of the DAPC at the sarcolemma of donor-derived myofibers.4,29
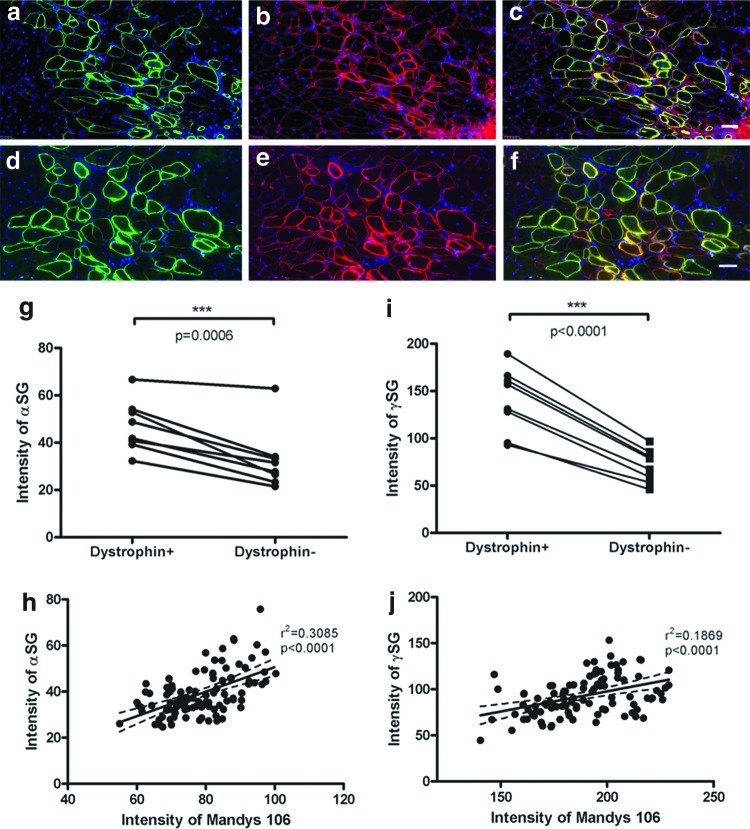
We carried out double immunostaining with antibodies to human dystrophin (Mandys 106) and members of dystrophin glycoprotein complex (α-sarcoglycan or γ-sarcoglycan), to examine their colocalization on muscle fibers.
Human dystrophin+ muscle fibers expressed higher levels of α-sarcoglycan (Fig. 4a–c) or γ-sarcoglycan (Fig. 4d–f), than did human dystrophin− muscle fibers in the same muscle section. Within the eight transplanted muscles, the intensity of the α-sarcoglycan or γ-sarcoglycan in human dystrophin+ fibers is significantly higher than in dystrophin− fibers (Fig. 4g, i, and Supplementary Figs. S1 and S2), as determined by paired t-test (p = 0.0006 for α-sarcoglycan and p < 0.0001 for γ-sarcoglycan). Linear regression analysis of the intensity of dystrophin and α-sarcoglycan demonstrated a significant positive correlation between the two proteins at the sarcolemma of all the donor-derived muscles fibers (Fig. 4h shows data from one representative transplanted muscle; and Supplementary Fig. S1). Similarly, linear regression analysis of the intensity of dystrophin and γ-sarcoglycan also showed a significant positive correlation within all transplanted muscles (Fig. 4j, data from one representative muscle; Supplementary Fig. S2).
Figure 4.
Restored full-length dystrophin recruits members of the DAPC at the sarcolemma of donor-derived myofibers in vivo. (a–c) Immunostaining of dystrophin (green, a) and α-sarcoglycan (red, b) on donor fibers. (c) Merged image of (a, b). (d–f) Immunostaining of dystrophin (green, d) and γ-sarcoglycan (red, e). (f) Merged image of (d, e). (g) Intensity of α-sarcoglycan on dystrophin+ fibers and dystrophin− fibers within the transplanted muscle. Data were compared using paired t-test. (h) Linear regression of the intensity of α-sarcoglycan and dystrophin in a transverse section of a representative muscle. (i) Intensity of γ-sarcoglycan on dystrophin+ fibers and dystrophin− fibers within transplanted muscles; data were compared using paired t-test. (j) Linear regression of the intensity of γ-sarcoglycan and dystrophin in a transverse section of a representative muscle. Scale bar = 50 μm. DAPC, dystrophin-associated protein complex. Color images are available online.
Restored dystrophin recruits nNOS to the sarcolemma of the donor fibers
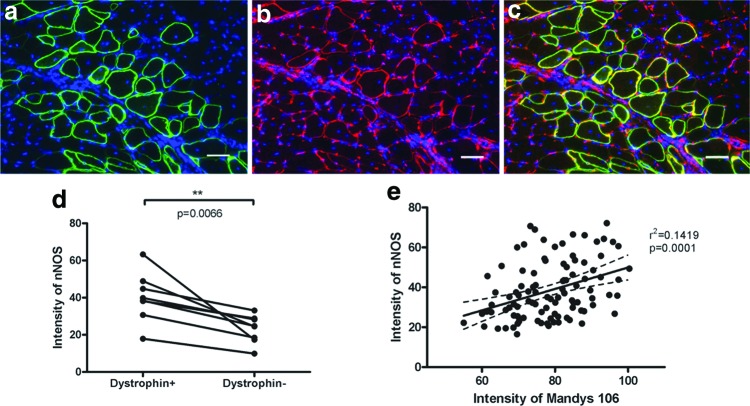
nNOS is an important signaling molecule in skeletal muscle whose sarcolemma location relies on its binding with dystrophin at R16–R17 of the spectrin-like repeats of dystrophin.30,31 To determine whether the restored FL-dys was capable of recruiting nNOS at the sarcolemma, double immunostaining with Mandys 106 and an antibody to nNOS was carried out on transplanted muscle sections. In dystrophin− muscle fibers, there were either no or very low levels of nNOS expression at the sarcolemma, while there was clear expression of nNOS on dystrophin+ muscle fibers (Fig. 5a–c). The intensity of nNOS at the sarcolemma of the dystrophin+ fibers was significantly higher than dystrophin− muscle fibers, as shown by the paired t-test of the eight transplanted muscles (Fig. 5d and Supplementary Fig. S3). Linear regression analysis of the intensity of nNOS and dystrophin showed a significant positive correlation of the expression of the two proteins at the sarcolemma of the dystrophin+ fibers (Fig. 5e and Supplementary Fig. S3).
Figure 5.
Upregulation of nNOS on dystrophin+ donor-derived myofibers within transplanted muscles. (a–c) Immunostaining of dystrophin (green, a) and nNOS (red, b). (c) Merged image of (a, b). Scale bar = 50 μm. (d) Quantification of the intensity of nNOS on dystrophin+ and dystrophin− fibers; data were compared using paired t-test. (e) Linear regression analysis of the intensity of nNOS and dystrophin in a transverse cryosection of a representative muscle showed positive correlation between the two proteins in donor-derived muscle fibers. nNOS, neuronal nitric oxide synthase. Color images are available online.
Donor-derived dystrophin-expressing muscle fibers express lower levels of utrophin than host muscle fibers
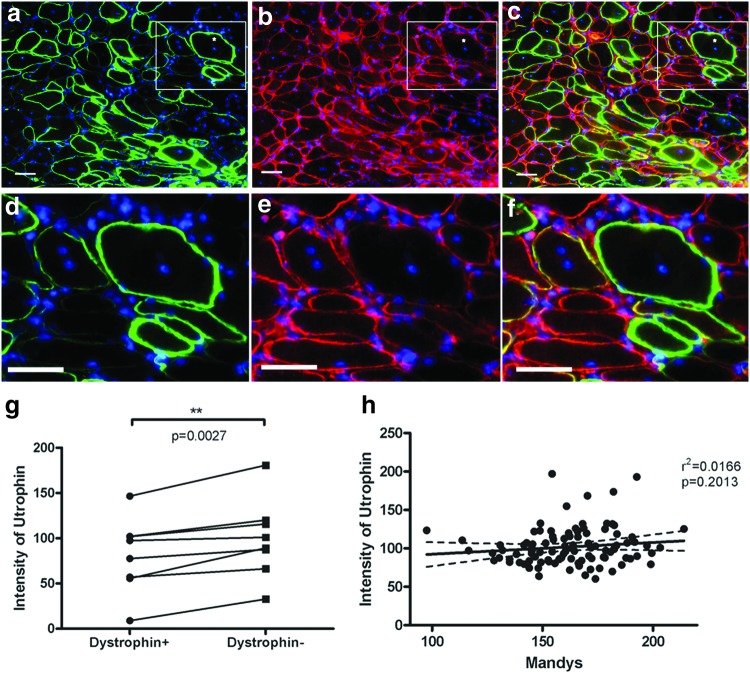
Utrophin is an autosomal homologue of dystrophin, which is upregulated in dystrophin-deficient mouse models and in DMD patients, partially compensating for the lack of dystrophin.32,33 Double immunostaining of dystrophin and utrophin on sections of transplanted muscles showed that the sarcolemmal utrophin expression was significantly lower on dystrophin+ than dystrophin− fibers, compared by paired t-test of the eight transplanted muscles (Fig. 6). Interestingly, unlike the DAPC and nNOS, there was no correlation between utrophin and dystrophin intensity at the sarcolemma of the same muscle fibers (Fig. 6 and Supplementary Fig. S4).
Figure 6.
Restoration of dystrophin decreases the utrophin expression on donor-derived fibers. (a–f) Immunostaining of dystrophin (green, a, d) and utrophin (red, b, e) in transplanted muscles. (c, f) Merged image of (a, b) or (d, e), respectively. (d–f) Enlarged images of the square in (a–c). Scale bar = 50 μm. (g) Paired t-test of the intensity of utrophin in dystrophin+ and dystrophin− fibers. (h) Linear regression analysis of the intensity of the utrophin and dystrophin in dystrophin+ donor fibers from one of the representative muscles suggested no correlation of the expression of the two proteins. Color images are available online.
Discussion
DMD is a genetic disorder characterized by muscle wasting and progressive muscle weakness, caused by loss of dystrophin. There is no cure for DMD, although several therapeutic approaches are in preclinical, or clinical, development.34–36
Viral vectors have been developed to restore dystrophin expression, the most promising being AAV.37,38 In animal models of DMD, AAV vectors coding for micro-dystrophin transduced the majority of muscle fibers following systemic delivery, leading to widespread dystrophin restoration.39–41 However, as AAV has a negligible integration rate in vivo, and immunity to the virus capsid occurs,40 it can only be delivered once and dystrophin expression may decline with time and as a consequence of cell turnover. Moreover, AAV has a small packaging capacity, so it can only restore small, partially functional dystrophin isoforms. After treatment, the muscles of DMD patient are expected to be partially protected from further deterioration, in a similar way to the relative protection conferred in patients with Becker muscular dystrophy by the internally deleted proteins these patients typically produce, rather than a normal phenotype. To fully restore dystrophin function, it is necessary to deliver full-length, rather than mini, dystrophin; however, this is challenging to package into viral vectors. We have already shown that either FV13 or LV12 can package FL-dys and transduce DMD myoblasts in vitro, suggesting that both vectors would be suitable to restore fully functional dystrophin. However, the efficacy of these viral vectors needs to be tested in vivo.
Direct delivery of FV or LV via i.v. injection in vivo is limited by the relatively low titer that can be achieved for these vectors. The alternative is to use them for cell-mediated gene therapy. This would require autologous stem cells, which is an advantage in avoiding or reducing immunological rejection of the transplanted cells.
FV vector has been used for cell-mediated gene therapy in animal models. In a canine model of pyruvate kinase deficiency, following transplantation with HSCs transduced ex vivo using a tricistronic FV vector that expressed EGFP, R-type pyruvate kinase, and MGMTP140K, the myeloid long-term repopulating cells increased from ∼3.5% to 33%, resulting in a functional cure.11 A longer term follow-up study in canine models of leukocyte adhesion deficiency (CLAD),42 which were treated with autologous, CD34+ HSCs transduced by an FV vector expressing canine CD18, showed disease correction/amelioration of disease in CLAD without significant adverse events, supporting the use of an FV vector to treat children with leukocyte adhesion deficiency type 1 in a human gene therapy clinical trial. However, this strategy has not been tested before on models of muscle diseases.
First, we showed that FV can transduce CD133+ cells and that these cells give rise to transgene (GFP)-expressing myofibers after their intramuscular transplantation in an immunodeficient mouse model (Fig. 1). These data suggested that the FV-transduced autologous cells could be potentially used to treat diseases such as muscular dystrophy.
As a tracking marker, GFP is frequently used in basic research or preclinical studies to generate proof-of-principle data. However, GFP is toxic and immunogenic in vivo,16 which is not suitable for clinical application. Thus, a simple and efficient delivery system without the need of FACS after transduction is necessary if contemplating future clinical applications. To meet this requirement, we chose to use a FV-coFLDys vector that did not contain a GFP selection cassette, to transduce DMD myoblasts at early mean population doublings. Transduced cells were then transplanted into mdx nude mice without either FACS or extensive expansion in vitro that reduces muscle stem cell engraftment efficiency.18,43,44 Our results provide the first evidence that the coFLDys ORF could be delivered to DMD myoblasts via FV, and the transduction efficiency is high enough to ensure the downstream in vivo restoration of FL-dys protein by donor cells, without the need of in vitro selection.
In transverse sections of grafted muscles, there were more fibers expressing human dystrophin than fibers expressing human spectrin and containing a human lamin A/C+ nucleus, evidence of highly effective dystrophin restoration. This is likely to be because the FL-dys sequence is codon-optimized, which results in higher protein expression than native cDNA.13–15 Transduced cells containing the integrated virus may fuse with either nontransduced cells (in vitro) or dystrophin-deficient host muscle fibers (in vivo), resulting in mosaic myotubes or myofibers. Such mosaic fibers might contain myonuclei derived from human FV-transduced cells that would produce dystrophin, or myonuclei of mouse origin, and/or derived from nontransduced human cells, that would not produce dystrophin. Myonuclei derived from coFLDys-transduced cells seem to produce sufficient dystrophin to spread a reasonable distance (further than human spectrin) along the fiber.
We examined the muscles at 4 weeks after transplantation, giving sufficient time for the muscle to regenerate postinjury. We did not examine muscles later, which, although of clinical relevance, would be challenging to do, as immunodeficient mice have a shorter life span than nonimmunodeficient mice45 and xenografts may undergo some degree of immunological rejection even within immunodeficient mouse hosts.
Utrophin is an autosomally encoded homologue of dystrophin,46 which in normal muscle, is located at the neuromuscular junction and is absent at the sarcolemma of muscle fibers. It is upregulated at the sarcolemma in dystrophin-negative myofibers to partially compensate for the lack of dystrophin.33,47–49 We examined the expression intensity of utrophin in treated muscle, focusing on the sarcolemma of muscle fibers. When FL-dys was restored, the expression of utrophin decreased at the myofiber sarcolemma. As our transplanted muscles will have regenerated as a response to cryoinjury, we expect that the utrophin distribution at the neuromuscular junction of myofibers would be dysregulated.50 Our findings suggest that the coFLDys outcompetes utrophin and suppresses its expression. However, unlike the DAPC proteins in serial sections, there was no correlation between utrophin and dystrophin intensity on the same muscle fibers (Fig. 6 and Supplementary Fig. S4). This is understandable, as the majority of the dystrophin+ fibers do not have utrophin at the sarcolemma.
We did not perform any physiological studies on our transplanted mouse muscles. It has been shown that 20% of myofibers in a mouse TA muscle need to express dystrophin to give a significant protection against contraction-induced injury51 and that 20–30% of endogenous dystrophin levels are required to reduce the mdx muscle pathology and to avoid muscular dystrophy in humans.52–54 In our study, we obtained a mean of 130 fibers expressing dystrophin in a representative transverse section (Fig. 3). A mouse mdx TA muscle contains ∼2,200 myofibers.55–57 Therefore, we estimate that ∼6% of the myofibers contain human dystrophin. Dystrophin expression derived from cells that have contributed to degenerating mouse myofibers is likely to be segmental,43,58 and so, dystrophin would not be present along the whole fiber and thus would be unlikely to be of physiological benefit to the muscle.59–61 Reaching this level of engraftment remains a major challenge in the muscle stem cell therapy field, but this might be achievable when human cells are transplanted into patients, particularly if they contribute to the satellite cell pool.
One big concern in applying viral vectors for gene therapy is possible mutagenesis or oncogenesis caused by the random integration of the vectors. There is evidence that FV would be sufficiently low risk for clinical application. While the wild-type FV has not been associated with disease in any animal, large-scale integration site analyses on FV-transduced human fibroblasts, CD34+ cells,10 and 293 cells9 suggest that FV has a favorable integration profile compared with other retroviral vectors. In contrast to LV vectors, FV vectors have no preference for integration within genes. Similarly, FV vectors have only a weak preference for integration near CpG islands and transcription start sites when compared with the strong bias exhibited by MLV vectors.62 Moreover, FVs have been found to contain a strong chromatin insulator within their LTR that significantly limits genotoxicity.63 Consistent with this, an analysis in human induced pluripotent stem cells64 suggested that integrated FV proviruses do not impact the expression of chromosomal genes in pluripotent human stem cells or their differentiated derivatives even when integrating near or within genes. Although FV vectors appear to be the safest integrating vector available, integration site analysis in human muscle progenitor cells would be appropriate for clinical use.
In summary, our work demonstrated for the first time the efficacy of a novel potential therapeutic approach for DMD treatment, combining the advantages of autologous cell therapy and FL-dys restoration in vivo. The use of autologous rather than donor-derived stem cells will reduce the risk of immunological rejection and the need for lifelong immunosuppression. These stem cells contain a dystrophin construct that will express dystrophin once they have formed muscle fibers following their local delivery to patient muscles, hence eliminating the underlying cause of muscle fiber loss. Also, in contrast to exon skipping, or gene editing, this cell-mediated FL-dys therapy is applicable to all DMD patients, regardless of their mutation.
Given that systemic delivery of skeletal muscle stem cells remains challenging,65 autologous skeletal muscle stem cells transduced with FLdys could be injected intramuscularly to treat small muscles of a patient. For example, the direct injection of stem cells into the thenar muscles should reduce the progression of the disease in hand muscles, leading to an improvement of function in the hand, which would make a significant difference to nonambulant DMD patients for maintaining independent daily life activities. For this kind of local injection, both obtaining sufficient cells from a muscle biopsy to treat such a small muscle, and sufficient FL-Dys vector to transduce the cells, would be feasible. There are muscles in DMD patients, for example, the extensor digitorum brevis muscle in the foot, that are relatively well-preserved in DMD,66 so this muscle would be a good candidate from which to prepare muscle precursor cells for therapeutic application.67 With this strategy, only a single application of transduced stem cells is required for a long-lasting effect. Unlike AAV gene therapy that requires viable muscle fibers (and would therefore be unsuitable for older DMD patients, who would have already lost a significant proportion of their muscle mass), stem cell therapy should replenish the stem cell pool, repair degenerating muscle fibers, and make new muscle fibers. In the future, local stem cell therapy (delivering FL-Dys) combined with systemic AAV therapy (delivering mini-dystrophin) may be explored, to take advantage of the two separate approaches.
Supplementary Material
Acknowledgments
The support of the MRC Centre for Neuromuscular Diseases Biobank is gratefully acknowledged. We would like to thank Dominic Scaglioni for his help with image capture.
Author Disclosure
No competing financial interests exist.
Funding Information
This work was funded by the MRC (Grant No. G0900872). Nathan Paul Sweeney was supported by Wellcome Trust PhD studentship (093610/Z/10/A) and Jefferiss Research Trust Fellowship. Jennifer Morgan was supported by Great Ormond Street Hospital Children's Charity. The support of MDUK and the MRC Centre for Neuromuscular Diseases is also gratefully acknowledged. Nathan Paul Sweeney and Myra McClure are grateful to the NIHR BRC at Imperial NHS Trust for its support. Part of this research—using DMD patients' cells—was supported by the NIHR Great Ormond Street Hospital Biomedical Research Centre. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health.
Supplementary Material
References
- 1. Mendell JR, Shilling C, Leslie ND, et al. . Evidence-based path to newborn screening for Duchenne muscular dystrophy. Ann Neurol 2012;71:304–313 [DOI] [PubMed] [Google Scholar]
- 2. Sacco A, Doyonnas R, Kraft P, et al. . Self-renewal and expansion of single transplanted muscle stem cells. Nature 2008;456:502–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Collins CA, Olsen I, Zammit PS, et al. . Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell 2005;122:289–301 [DOI] [PubMed] [Google Scholar]
- 4. Meng J, Counsell JR, Reza M, et al. . Autologous skeletal muscle derived cells expressing a novel functional dystrophin provide a potential therapy for Duchenne Muscular Dystrophy. Sci Rep 2016;6:19750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Young CS, Hicks MR, Ermolova NV, et al. . A single CRISPR-Cas9 deletion strategy that targets the majority of DMD patients restores dystrophin function in hiPSC-derived muscle cells. Cell Stem Cell 2016;18:533–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Amoasii L, Long C, Li H, et al. . Single-cut genome editing restores dystrophin expression in a new mouse model of muscular dystrophy. Sci Transl Med 2018;10:eaat0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bengtsson NE, Hall JK, Odom GL, et al. . Muscle-specific CRISPR/Cas9 dystrophin gene editing ameliorates pathophysiology in a mouse model for Duchenne muscular dystrophy. Nat Commun 2017;8:14454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Trobridge GD. Foamy virus vectors for gene transfer. Expert Opin Biol Ther 2009;9:1427–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nowrouzi A, Dittrich M, Klanke C, et al. . Genome-wide mapping of foamy virus vector integrations into a human cell line. J Gen Virol 2006;87(Pt 5):1339–1347 [DOI] [PubMed] [Google Scholar]
- 10. Trobridge GD, Miller DG, Jacobs MA, et al. . Foamy virus vector integration sites in normal human cells. Proc Natl Acad Sci U S A 2006;103:1498–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Trobridge GD, Beard BC, Wu RA, et al. . Stem cell selection in vivo using foamy vectors cures canine pyruvate kinase deficiency. PLoS One 2012;7:e45173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Counsell JR, Asgarian Z, Meng J, et al. . Lentiviral vectors can be used for full-length dystrophin gene therapy. Sci Rep 2017;7:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sweeney NP, Meng J, Patterson H, et al. . Delivery of large transgene cassettes by foamy virus vector. Sci Rep 2017;7:8085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Foster H, Sharp PS, Athanasopoulos T, et al. . Codon and mRNA sequence optimization of microdystrophin transgenes improves expression and physiological outcome in dystrophic mdx mice following AAV2/8 gene transfer. Mol Ther 2008;16:1825–1832 [DOI] [PubMed] [Google Scholar]
- 15. Inouye S, Sahara-Miura Y, Sato J, et al. . Codon optimization of genes for efficient protein expression in mammalian cells by selection of only preferred human codons. Protein Expr Purif 2015;109:47–54 [DOI] [PubMed] [Google Scholar]
- 16. Ansari AM, Ahmed AK, Matsangos AE, et al. . Cellular GFP toxicity and immunogenicity: potential confounders in in vivo cell tracking experiments. Stem Cell Rev 2016;12:553–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sweeney NP, Regan C, Liu J, et al. . Rapid and efficient stable gene transfer to mesenchymal stromal cells using a modified foamy virus vector. Mol Ther 2016;24:1227–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Meng J, Chun S, Asfahani R, et al. . Human skeletal muscle-derived CD133(+) cells form functional satellite cells after intramuscular transplantation in immunodeficient host mice. Mol Ther 2014;22:1008–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ehrhardt J, Brimah K, Adkin C, et al. . Human muscle precursor cells give rise to functional satellite cells in vivo. Neuromuscul Disord 2007;17:631–638 [DOI] [PubMed] [Google Scholar]
- 20. Silva-Barbosa SD, Butler-Browne GS, Di Santo JP, et al. . Comparative analysis of genetically engineered immunodeficient mouse strains as recipients for human myoblast transplantation. Cell Transplant 2005;14:457–467 [DOI] [PubMed] [Google Scholar]
- 21. Meng J, Bencze M, Asfahani R, et al. . The effect of the muscle environment on the regenerative capacity of human skeletal muscle stem cells. Skelet Muscle 2015;5:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Irintchev A, Zweyer M, Wernig A. Impaired functional and structural recovery after muscle injury in dystrophic mdx mice. Neuromuscul Disord 1997;7:117–125 [DOI] [PubMed] [Google Scholar]
- 23. Mueller AL, Bloch RJ. Skeletal muscle cell transplantation: models and methods. J Muscle Res Cell Motil 2019. August 7 [Epub ahead of print]; DOI: 10.1007/s10974-019-09550-w [DOI] [PubMed] [Google Scholar]
- 24. Rozkalne A, Adkin C, Meng J, et al. . Mouse regenerating myofibers detected as false-positive donor myofibers with anti-human spectrin. Hum Gene Ther 2014;25:73–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nasimuzzaman M, Lynn D, Ernst R, et al. . Production and purification of high-titer foamy virus vector for the treatment of leukocyte adhesion deficiency. Mol Ther Methods Clin Dev 2016;3:16004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lorant J, Saury C, Schleder C, et al. . Skeletal muscle regenerative potential of human MuStem cells following transplantation into injured mice muscle. Mol Ther 2018;26:618–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xu X, Wilschut KJ, Kouklis G, et al. . Human satellite cell transplantation and regeneration from diverse skeletal muscles. Stem Cell Rep 2015;5:419–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ohlendieck K, Matsumura K, Ionasescu VV, et al. . Duchenne muscular dystrophy: deficiency of dystrophin-associated proteins in the sarcolemma. Neurology 1993;43:795–800 [DOI] [PubMed] [Google Scholar]
- 29. Cirak S, Feng L, Anthony K, et al. . Restoration of the dystrophin-associated glycoprotein complex after exon skipping therapy in Duchenne muscular dystrophy. Mol Ther 2012;20:462–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Molza AE, Mangat K, Le RE, et al. . Structural basis of neuronal nitric-oxide synthase interaction with dystrophin repeats 16 and 17. J Biol Chem 2015;290:29531–29541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lai Y, Zhao J, Yue Y, et al. . Alpha2 and alpha3 helices of dystrophin R16 and R17 frame a microdomain in the alpha1 helix of dystrophin R17 for neuronal NOS binding. Proc Natl Acad Sci U S A 2013;110:525–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Amenta AR, Yilmaz A, Bogdanovich S, et al. . Biglycan recruits utrophin to the sarcolemma and counters dystrophic pathology in mdx mice. Proc Natl Acad Sci U S A 2011;108:762–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Banks GB, Combs AC, Odom GL, et al. . Muscle structure influences utrophin expression in mdx mice. PLoS Genet 2014;10:e1004431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Messina S, Vita GL. Clinical management of Duchenne muscular dystrophy: the state of the art. Neurol Sci 2018;39:1837–1845 [DOI] [PubMed] [Google Scholar]
- 35. Chamberlain JR, Chamberlain JS. Progress toward gene therapy for Duchenne muscular dystrophy. Mol Ther 2017;25:1125–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Reinig AM, Mirzaei S, Berlau DJ. Advances in the treatment of Duchenne muscular dystrophy: new and emerging pharmacotherapies. Pharmacotherapy 2017;37:492–499 [DOI] [PubMed] [Google Scholar]
- 37. Duan D. Systemic AAV micro-dystrophin gene therapy for Duchenne muscular dystrophy. Mol Ther 2018;26:2337–2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kawecka K, Theodoulides M, Hasoglu Y, et al. . Adeno-associated virus (AAV) mediated dystrophin gene transfer studies and exon skipping strategies for Duchenne muscular dystrophy (DMD). Curr Gene Ther 2015;15:395–415 [DOI] [PubMed] [Google Scholar]
- 39. Kodippili K, Hakim CH, Pan X, et al. . Dual AAV gene therapy for Duchenne muscular dystrophy with a 7-kb mini-dystrophin gene in the canine model. Hum Gene Ther 2018;29:299–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang Z, Kuhr CS, Allen JM, et al. . Sustained AAV-mediated dystrophin expression in a canine model of Duchenne muscular dystrophy with a brief course of immunosuppression. Mol Ther 2007;15:1160–1166 [DOI] [PubMed] [Google Scholar]
- 41. Le GC, Servais L, Montus M, et al. . Long-term microdystrophin gene therapy is effective in a canine model of Duchenne muscular dystrophy. Nat Commun 2017;8:16105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bauer TR Jr., Tuschong LM, Calvo KR, et al. . Long-term follow-up of foamy viral vector-mediated gene therapy for canine leukocyte adhesion deficiency. Mol Ther 2013;21:964–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Brimah K, Ehrhardt J, Mouly V, et al. . Human muscle precursor cell regeneration in the mouse host is enhanced by growth factors. Hum Gene Ther 2004;15:1109–1124 [DOI] [PubMed] [Google Scholar]
- 44. Cooper RN, Thiesson D, Furling D, et al. . Extended amplification in vitro and replicative senescence: key factors implicated in the success of human myoblast transplantation. Hum Gene Ther 2003;14:1169–1179 [DOI] [PubMed] [Google Scholar]
- 45. Boldrin L, Zammit PS, Muntoni F, et al. . Mature adult dystrophic mouse muscle environment does not impede efficient engrafted satellite cell regeneration and self-renewal. Stem Cells 2009;27:2478–2487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Blake DJ, Tinsley JM, Davies KE. Utrophin: a structural and functional comparison to dystrophin. Brain Pathol 1996;6:37–47 [DOI] [PubMed] [Google Scholar]
- 47. Guiraud S, Edwards B, Babbs A, et al. . The potential of utrophin and dystrophin combination therapies for Duchenne muscular dystrophy. Hum Mol Genet 2019;28:2189–2200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pons F, Robert A, Marini JF, et al. . Does utrophin expression in muscles of mdx mice during postnatal development functionally compensate for dystrophin deficiency? J Neurol Sci 1994;122:162–170 [DOI] [PubMed] [Google Scholar]
- 49. Weir AP, Morgan JE, Davies KE. A-utrophin up-regulation in mdx skeletal muscle is independent of regeneration. Neuromuscul Disord 2004;14:19–23 [DOI] [PubMed] [Google Scholar]
- 50. Ferretti R, Neto HS, Marques MJ. Expression of utrophin at dystrophin-deficient neuromuscular synapses of mdx mice: a study of protected and affected muscles. Anat Rec (Hoboken) 2011;294:283–286 [DOI] [PubMed] [Google Scholar]
- 51. Sharp PS, Jee H, Wells DJ. Physiological characterization of muscle strength with variable levels of dystrophin restoration in mdx mice following local antisense therapy. Mol Ther 2011;19:165–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Neri M, Torelli S, Brown S, et al. . Dystrophin levels as low as 30% are sufficient to avoid muscular dystrophy in the human. Neuromuscul Disord 2007;17:913–918 [DOI] [PubMed] [Google Scholar]
- 53. Phelps SF, Hauser MA, Cole NM, et al. . Expression of full-length and truncated dystrophin mini-genes in transgenic mdx mice. Hum Mol Genet 1995;4:1251–1258 [DOI] [PubMed] [Google Scholar]
- 54. Wells DJ, Wells KE, Asante EA, et al. . Expression of human full-length and minidystrophin in transgenic mdx mice: implications for gene therapy of Duchenne muscular dystrophy. Hum Mol Genet 1995;4:1245–1250 [DOI] [PubMed] [Google Scholar]
- 55. Vitiello L, Bassi N, Campagnolo P, et al. . In vivo delivery of naked antisense oligos in aged mdx mice: analysis of dystrophin restoration in skeletal and cardiac muscle. Neuromuscul Disord 2008;18:597–605 [DOI] [PubMed] [Google Scholar]
- 56. Williams JH, Schray RC, Sirsi SR, et al. . Nanopolymers improve delivery of exon skipping oligonucleotides and concomitant dystrophin expression in skeletal muscle of mdx mice. BMC Biotechnol 2008;8:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yin H, Moulton HM, Seow Y, et al. . Cell-penetrating peptide-conjugated antisense oligonucleotides restore systemic muscle and cardiac dystrophin expression and function. Hum Mol Genet 2008;17:3909–3918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Blaveri K, Heslop L, Yu DS, et al. . Patterns of repair of dystrophic mouse muscle: studies on isolated fibers. Dev Dyn 1999;216:244–256 [DOI] [PubMed] [Google Scholar]
- 59. Aartsma-Rus A, Morgan J, Lonkar P, et al. . Report of a TREAT-NMD/World Duchenne Organisation Meeting on Dystrophin Quantification Methodology. J Neuromuscul Dis 2019;6:147–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Godfrey C, Muses S, McClorey G, et al. . How much dystrophin is enough: the physiological consequences of different levels of dystrophin in the mdx mouse. Hum Mol Genet 2015;24:4225–4237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wells DJ. What is the level of dystrophin expression required for effective therapy of Duchenne muscular dystrophy? J Muscle Res Cell Motil 2019;40:141–150 [DOI] [PubMed] [Google Scholar]
- 62. Nasimuzzaman M, Kim YS, Wang YD, et al. . High-titer foamy virus vector transduction and integration sites of human CD34(+) cell-derived SCID-repopulating cells. Mol Ther Methods Clin Dev 2014;1:14020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Goodman MA, Arumugam P, Pillis DM, et al. . Foamy virus vector carries a strong insulator in its long terminal repeat which reduces its genotoxic potential. J Virol 2018;92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Deyle DR, Khan IF, Ren G, et al. . Lack of genotoxicity due to foamy virus vector integration in human iPSCs. Gene Ther 2013;20:868–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Cossu G, Previtali SC, Napolitano S, et al. . Intra-arterial transplantation of HLA-matched donor mesoangioblasts in Duchenne muscular dystrophy. EMBO Mol Med 2015;7:1513–1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kinali M, Arechavala-Gomeza V, Cirak S, et al. . Muscle histology vs MRI in Duchenne muscular dystrophy. Neurology 2011;76:346–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Meng J, Adkin CF, Xu SW, et al. . Contribution of human muscle-derived cells to skeletal muscle regeneration in dystrophic host mice. PLoS One 2011;6:e17454. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.