Abstract
The adeno-associated virus (AAV) vector is an efficient tool for gene delivery in skeletal muscle. AAV-based therapies show promising results for treatment of various genetic disorders, including muscular dystrophy. These dystrophies represent a heterogeneous group of diseases affecting muscles and typically characterized by progressive skeletal muscle wasting and weakness and the development of fibrosis. The tropism of each AAV serotype has been extensively studied using systemic delivery routes, but very few studies have compared their transduction efficiency through direct intramuscular injection. Yet, in some muscular dystrophies, where only a few muscles are primarily affected, a local intramuscular injection to target these muscles would be the most appropriate route. A comprehensive comparison between different recombinant AAV (rAAV) serotypes is therefore needed. In this study, we investigated the transduction efficiency of rAAV serotypes 1–10 by local injection in skeletal muscle of control C57BL/6 mice. We used a CMV-nls-LacZ reporter cassette allowing nuclear expression of LacZ to easily localize targeted cells. Detection of β-galactosidase activity on muscle cryosections demonstrated that rAAV serotypes 1, 7, 8, 9, and 10 were more efficient than the others, with rAAV9 being the most efficient in mice. Furthermore, using a model of human muscle xenograft in immunodeficient mice, we observed that in human muscle, rAAV8 and rAAV9 had similar transduction efficiency. These findings demonstrate for the first time that the human muscle xenograft can be used to evaluate AAV-based therapeutical approaches in a human context.
Keywords: AAV, tropism, xenograft, skeletal muscle, fibrosis, preclinical
Introduction
Adeno-associated virus (AAV) recombinant vectors (rAAVs) are tools of choice for a wide range of gene therapy applications in humans due to their good safety profile in clinical trials, including an ability to transduce both dividing and nondividing cells, to elicit a limited cytotoxicity,1 and to allow long-term transgene expression.2 Many serotypes of AAV from human and primate sources have been identified, including AAV1,3 AAV2,4 AAV3,5 AAV4,6 AAV5,7 AAV6,8 AAV7,9 AAV8,9 AAV9,10 and AAV10,11 each with a specific transduction and tropism profile. rAAVs have been shown to efficiently infect many tissues, including the liver,12,13 lung,14 eye,15 and skeletal muscle,16,17 their tissue transduction targeting being dependent on both capsid properties18 and the route of administration.2
Skeletal muscles constitute 40–50% of the body mass in adult humans and encompass a broad range of different muscles. Each of these muscles comprises contractile muscle fibers, postmitotic multinucleated cells, surrounded by a specialized plasma membrane—the sarcolemma—and by a layer of extracellular matrix (ECM) known as the basement membrane. Tropism evaluation of rAAV serotypes in muscle has mainly been studied by systemic administration: rAAV serotypes 1, 6, 7, 8, and 9 showed efficient transduction of skeletal muscle in most animal models.19–21 However, to our knowledge, no study has provided a comprehensive side-by-side comparison of the transduction efficiency and pattern of serotypes 1–10 after a direct, in vivo, local intramuscular injection, a route of delivery needed in some dystrophies where discrete muscles need to be targeted.
Muscular dystrophy comprises a heterogeneous group of genetic disorders characterized by progressive muscle wasting and weakness. These diseases are often associated with excessive accumulation of ECM leading to muscle fibrosis. This is, for example, the case in Duchenne muscular dystrophy (DMD), where mutations in the dystrophin gene lead to cycles of degeneration/regeneration and the eventual development of fatty infiltration and fibrosis. This is also the case for a much rarer disease called oculopharyngeal muscular dystrophy (OPMD) where an abnormal triplet expansion in the PABPN1 gene22 leads primarily to pharyngeal and eyelid muscle weakness and exacerbated fibrosis in these affected muscles.23 Regarding OPMD, promising, preclinical, AAV-based, gene replacement therapy data have been obtained through intramuscular injection in an OPMD mouse model24 and the AAV gene replacement product has now received orphan drug designation from European Medicines Agency and the Food and Drug Administration. Whereas a whole-body transduction efficiency has to be reached for DMD—three AAV trials for DMD are ongoing in the United States based on the delivery of various engineered microdystrophins through systemic delivery using rAAV9 and rAAVrh7425 (NCT03368742 Solid Biosciences; NCT03375164 Nationwide Children's Hospital; NCT03362502 Pfizer),2 in OPMD patients, a local intramuscular injection would probably be preferred since a restricted number of muscles are primarily affected.
Fibrotic muscular substitution is a complex process characterized by excessive accumulation of collagens and other components of the ECM26 surrounding muscle fibers and fibroblasts that have an increasingly appreciated role as an autocrine source of profibrotic stimuli.27 The capacity to target fibrotic interstitial cells with rAAV has never been evaluated.
In this study, we evaluated the transduction efficiency of rAAV serotypes following intramuscular injection. Furthermore, we investigated the transduction translatability into human skeletal muscle using a model of human muscle xenograft into immunodeficient mice, allowing a side-by-side comparison in mouse and human contexts, an essential step for preclinical therapeutic studies.
Materials and Methods
AAV production
AAV pseudotyped vectors containing a CMV-nls-LacZ expression cassette were produced using a three-plasmid transfection in HEK-293 cells, as described previously,28 using pAAV-CMV-LacZ plasmids with pXX6 plasmid coding for the Ad helper genes essential for AAV production and pRepCap plasmid coding for AAV capsids. Vector particles were purified on an iodixanol gradient and concentrated on Amicon Ultra-15 100 K columns (Merck Millipore). The titer corresponding to the number of vector genomes per milliliter was determined by quantitative real-time PCR on a StepOnePlus (Applied Biosystems) using the following primers and probe: CTCCATCACTAGGGGTTCCTTG (forward) and GTAGATAAGTAGCATGGC (reverse) and TAGTTAATGATTAACCC (TaqMan MGB probe; Life Technologies) amplifying a region between the ITR and the promoter. The pAAV plasmid was used as a control to establish the standard curve for absolute quantification.
In vivo injection
Three-month-old, male C57BL/6J (Janvier Labs) mice were injected in the tibialis anterior (TA) with the different AAV serotypes (containing 3.5 × 1010 vector genomes per injection) diluted in 25 μL of phosphate-buffered saline (PBS) using a Hamilton syringe. Injections were carried out under general anesthesia using xylazine (80 mg/kg)/ketamine (10 mg/kg). Animal studies conform to the French laws and regulations concerning the use of animals for research and were approved by an external ethics committee (approval no. 16650-2018090514008520 delivered by the French Ministry of Higher Education and Scientific Research).
Muscle analysis
Four weeks postinjection, mice were sacrificed by cervical dislocation; TA muscles were collected and snap-frozen in liquid nitrogen-cooled isopentane. Transverse 5-μm sections were made with a cryostat (Leica CM1850) throughout the whole muscle with 500 μm between each section (Supplementary Fig. S1A). Additional tissue between the sections was collected for DNA and/or protein extraction, ensuring representation of the whole muscle during data collection.
Xenografts
Three- to four-month-old, male or female, immunodeficient Rag2−/− Il2rb−/− mice were used as recipients of human xenografts. Control donor human tensor fascia lata muscle was cut into strips (8 × 3 × 1 mm). Mice were anesthetized with an intraperitoneal injection of ketamine hydrochloride (80 mg/kg) and xylazine (10 mg/kg) (Sigma-Aldrich, St Louis, MO) in accordance with the French and European Community legislation (approval no. 4165-2016021715164682 delivered by the French Ministry of Higher Education and Scientific Research). The TA and extensor digitorum longus were removed. Human muscle samples were obtained as surgical waste during routine surgical procedures from the MYOBANK, a tissue bank affiliated to EuroBioBank (authorization from the French Ministry ref 2013-1968), after all subjects had signed informed consent. The strip of human muscle was placed in the empty anterior compartment and tied, at both ends, with nonabsorbable sutures (Ethicon) to the tendons of the peroneus longus. Skin was sutured with absorbable sutures (Ethicon). Buprenorphine (0.1 mg/kg) was given subcutaneously at the beginning of the surgery and every 12 h for the first 48 h after surgery for pain control. Four months after surgery, when the human muscle was fully regenerated, the grafts were injected with different AAV serotypes, as described previously.
β-Galactosidase histochemical and histological analysis
Muscle sections were fixed with 0.25% glutaraldehyde for 5 min and stained for several hours with a mixture of 500 μg/mL X-Gal (5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside), 5 mM potassium hexacyanoferrate (III), 5 mM potassium hexacyanoferrate (II) trihydrate, and 2 mM magnesium chloride in PBS at 37°C. Laminin staining was performed by incubating the sections in hydrogen peroxide (H2O2) 3% for 5 min, then blocking in fetal bovine serum (FBS) 2% for 30 min, and incubating with the anti-laminin antibody (1:400 diluted in PBS, Z0097, Dako; Agilent Technologies France, Les Ulis, France) and the biotinylated secondary antibody (1:200 diluted in PBS, E0432, Dako). Laminin staining was revealed with diaminobenzidine with 0.01% H2O2. For assessment of tissue morphology and visualization of fibrosis and connective tissue, muscle sections were stained with hematoxylin and eosin and Sirius red for light microscopic examination. Pictures were taken with a slide scanner (Hamamatsu). For each muscle, we selected the largest cross-sectional area among all the muscle sections of a TA muscle, and on this section, we counted the total number of β-gal–positive nuclei. The relative transduction efficiency was calculated as β-gal-positive nuclei/mm2/genome copy (gc), with the gc number calculated as per microgram of DNA (see Vector genome quantification section).
Western blot analysis
Proteins were extracted from muscle sections by sonicating the cells in RIPA buffer (0.15 M NaCl, 0.1% sodium dodecyl sulfate, 50 mM Tris-HCl [pH 8], 2 mM ethylenediaminetetraacetic acid, and 10% Triton X-100) with the protease inhibitor cocktail (Complete; Roche Diagnostics) and phosphatase inhibitor cocktail (Santa Cruz; sc-45064). Protein concentration was determined by the colorimetric detection method (Pierce BCA Protein Assay; Thermo Fisher). Proteins were separated on 4–12% Bis-Tris gels (Invitrogen) and transferred onto a polyvinylidene fluoride membrane for 1 h at constant 250 mA at 4°C. Membranes were blocked by incubation in 5% bovine serum albumin in 1 × tris-buffered saline and 0.1% Tween-20 (TBST) for 1h at room temperature under agitation. Membranes were stained with the anti-β-galactosidase antibody (Thermo Fisher A-11132; 1:1,000; 1 h) and tubulin antibody (Sigma T5168; 1:1,000; 1 h). Membranes were washed in TBST and incubated with appropriate secondary antibodies. The G:Box system (Syngene) was used to detect signals from the membranes.
Immunofluorescence staining
Muscle sections were blocked with 2% FBS in 1 × PBS for 30 min. The sections were incubated with primary antibodies against human-specific spectrin (1:50 mouse monoclonal IgG2b NCL-spec1, Novocastra; Leica Biosystems, Wetzlar, Germany) human-specific lamin A/C (1:300 mouse monoclonal IgG1 Ab40567; Abcam, Cambridge, UK), or laminin (1:400 diluted, Z0097, Dako) for 1 h and further incubated with appropriate secondary antibodies for 1 h. When necessary, β-gal detection was performed using a chicken antibody (Invitrogen AB9361; 1:1,000; 1 h). Pax7 staining was performed using a mouse monoclonal antibody (1:20; Developmental Studies Hybridoma Bank, Iowa City, IA) after a 10-min fixation with 4% paraformaldehyde. Fiber type composition was obtained using an anti-MYHC-I antibody (A4.840, 1:50; Developmental Studies Hybridoma Bank) and an anti-MYHC-II antibody (A4.74, 1:50; Developmental Studies Hybridoma Bank). Macrophages and neutrophils were stained with the F4/80 antibody (MCA497BB, 1:50; Bio-Rad) and Ly6g antibody (Ab25377, 1:100; Abcam), respectively, after a 10-min fixation with 4% paraformaldehyde. Finally, the sections were incubated for 10 min with Hoechst (0.5 μg/mL, Hoechst No. 33258; Sigma-Aldrich) and mounted with a mounting medium (Cytomation fluorescent mounting medium, S3023, Dako; Agilent Technologies, France).
Vector genome quantification
Transduction efficacy was evaluated by quantification of vector genomes in injected muscles. Genomic DNA was extracted from mouse muscles using a Puregene Blood kit (Qiagen, Courtaboeuf, France) according to the manufacturer's protocol. Copy numbers of AAV genome were measured on 100 ng of genomic DNA by absolute, quantitative real-time PCR on a StepOnePlus™ (Applied Biosystems) using a TaqMan probe and the TaqMan® Universal Master Mix (Applied Biosystems).
To specifically amplify the viral genome sequence, we used the same primers as the one for AAV titration (see AAV production section). A control pAAV plasmid was 10-fold serially diluted (from 107 to 101 copies) and used as a control to establish the standard curve for absolute quantification. All genomic DNA samples were analyzed in duplicates.
Statistical analyses
All data are mean values ± standard errors of the mean. Statistical analyses were performed using Student's t-test or one-way analysis of variance with the Bonferroni post hoc analysis, as indicated. GraphPad Prism (version 4.0b; GraphPad Software, San Diego, CA) was used for the analyses. A difference was considered to be significant at *p < 0.05, **p < 0.01, or ***p < 0.001.
Results
Transduction efficiency in mice
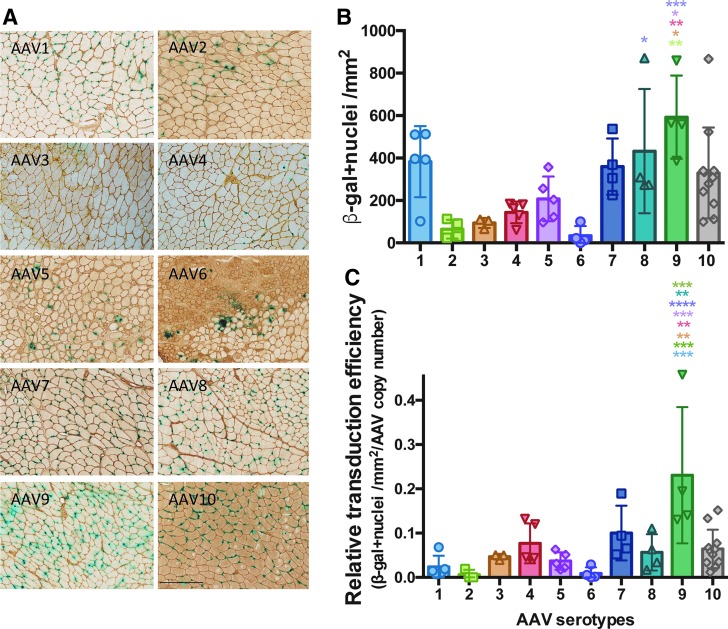
We first investigated the transduction efficiency of single-stranded rAAV serotypes by local injection in skeletal muscle of control C57BL/6 mice. We used a CMV-nls-LacZ reporter cassette to precisely localize transduced nuclei. Ten different serotypes of rAAV-CMV-nls-LacZ (rAAV1–rAAV10), each at 3.5 × 1010 vector genomes, were injected in the TA muscles of control C57BL/6 mice. Four weeks after injection, muscles were harvested and frozen for histological and biochemical analysis (Fig. 1A). For each transduced TA, reporter nls-LacZ expression was quantified on the largest cross-sectional area of the muscle by manual counting of all LacZ-positive nuclei (Fig. 1B; Supplementary Fig. S1A, B) and by Western blot (Supplementary Fig. S2A). The number of vector genomes was quantified by quantitative PCR (Supplementary Fig. S2B). Four weeks postinjection, serotype 9 showed the most robust transduction (Fig. 1C). All serotypes, except rAAV6, showed a limited inflammatory response. It should be noted that all of the rAAV6-injected TA muscles presented an intense inflammatory response with large areas of regenerating myofibers (Supplementary Fig. S2C), thus maybe impairing the quantification of transduction efficiency. This was observed in independent experiments.
Figure 1.
Intramuscular AAV transduction efficiency in control C57BL/6 mice. rAAV serotypes 1–10 were injected into the TA (3.5 × 1010 vg/TA). Mice were sacrificed 4 weeks postinjection and TA muscles were analyzed (n = 4–10 TA). (A) Cryostat sections of TA muscles of C57BL/6 mice injected with rAAV serotypes 1–10 carrying the nls-LacZ gene reporter and stained for β-galactosidase activity and laminin. Scale bar = 250 μm. (B) Quantification of cross-sectional β-galactosidase-positive nuclei by mm2 (n = 4–10 TA). (C) Transduction efficiency is expressed as the β-gal-positive nuclei/mm2 relative to the AAV genome copy number quantified by absolute TaqMan qPCR. *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001 ANOVA Tukey test. AAV, adeno-associated virus; ANOVA, analysis of variance; qPCR, quantitative PCR; rAAV, recombinant AAV; TA, tibialis anterior; vg, vector genome.
Transduction efficiency in human muscle using the xenograft model
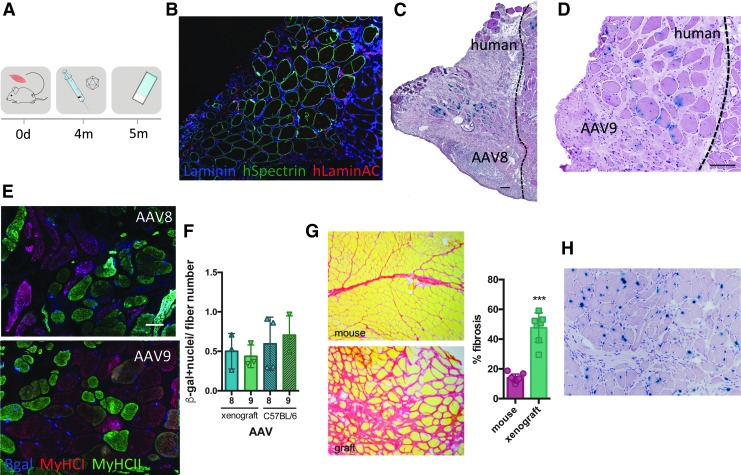
To evaluate intramuscular AAV transduction in the human context, we used a human muscle xenograft model: we transplanted fresh human skeletal muscle into the empty tibial compartment of Rag2−/− Il2rb−/− immunodeficient mice. Four months post-transplant, the engrafted human muscle regenerated as previously described.29 Xenografts comprised entirely of human myofibers (expressing spectrin) (Fig. 2B) with comparable fiber type composition compared with the original donor biopsy (Supplementary Fig. S3A, B). rAAV8 and rAAV9 vectors expressing CMV-nls-LacZ were injected into these xenografts, and mice were analyzed 4 weeks later (Fig. 2A). These AAVs were selected as they showed good efficiency in mice and are currently being tested in several clinical trials for muscular dystrophy in patients. The position of the human xenografts was confirmed by immunofluorescence staining using anti-human spectrin and anti-human lamin A/C antibodies to stain specifically human muscle fiber membranes and human nuclei, respectively (Fig. 2B; Supplementary Fig. S3C). In human muscle xenografts, rAAV8 and rAAV9 gave comparable transduction efficiency (Fig. 2C, D) and transduced both slow and fast fibers (Fig. 2E). When compared with the mouse, we observed a similar level of transduction for both rAAV serotypes (Fig. 2F) even though there is significant muscle fibrosis in the human xenograft (Fig. 2G). Interestingly, we never observed LacZ expression in the human interstitial cells surrounding the muscle fibers (Fig. 2H).
Figure 2.
Intramuscular AAV transduction efficiency in human xenografts. (A) Experimental scheme depicting the xenograft time course. Strips of human biopsies were xenografted into the empty tibial compartment of immunodeficient Rag2−/− Il2rb−/− mice. Four months later, rAAV serotypes 8 and 9 (3 × 1010 vg/TA) were injected into the graft. Mice were sacrificed 4 weeks postinjection and muscles were analyzed. (B) Immunofluorescent staining of human spectrin (green), human lamin a/c (red), and laminin (blue) on a cryosection of human xenograft. (C) Cryosection of xenograft injected with AAV8, stained for β-galactosidase, and counterstained with H&E. Scale bar = 100 μm. (D) Cryosection of xenograft injected with AAV9 and stained for β-galactosidase and colored with H&E. Scale bar = 100 μm. (E) Fiber type (MyHCI-red and MyHCII-green) and β-galactosidase (blue) immunostaining on rAAV8- and rAAV9-injected xenograft. (F) Quantification of cross-sectional xenograft β-galactosidase-positive nuclei related to fiber number on a cross-sectional human xenograft and compared with a C57BL/6 mouse injected with AAV8 and AAV9 (n = 3). ANOVA Tukey test. (G) Sirius red coloration of mouse muscle versus xenograft. Fibrosis was quantified with ImageJ software. ***p < 0.0001, t test. (H) Cryosection of xenograft injected with AAV9, stained for β-galactosidase (blue), and stained with H&E. No β-gal+ interstitial cells are observed. H&E, hematoxylin and eosin.
Discussion
Achieving efficient transduction of rAAV in diseased skeletal muscle, notably in the presence of fibrosis, is a major challenge for treatment of muscular dystrophy. For many dystrophies, a systemic administration of the vector is required; however, for some more localized diseases, local intramuscular rAAV delivery might be sufficient. Whereas the comparison of serotypes has been largely described through systemic delivery, to our knowledge, very few studies have compared their efficiency after a local intramuscular injection. In addition, while it is known that the potency of the rAAV gene delivery can be impacted by several host factors, such as molecular interactions between the capsid and target cell surface receptors30 or the interaction with serum proteins in the circulation,31 the impact of fibrosis by ECM deposition around muscle fibers on rAAV transduction efficiency and transduction of interstitial fibrosis cells has not been evaluated.
In this study, we therefore tested the efficiency of rAAV serotypes 1–10 after direct intramuscular injection into the TA muscles of mice and human xenografted muscle.
When comparing the rAAVs in mouse, we found that rAAV9 was the most efficient vector. Serotypes 1, 7, and 8 were also efficient, although less so following intramuscular injection. rAAV1 gave a high number of vector genome copies not correlated with the high level of expression, suggesting impaired disassembly in the nucleus as previously shown in Hela cells.32 In the human xenograft model, rAAV8 and rAAV9 were found to be equally efficient at transducing both slow and fast human muscle fibers. It should be noted that rAAV9 and rAAV8 are already used in several ongoing clinical trials using systemic delivery in different myopathies: a phase I/II trial with Solid Biosciences (NCT03368742) and one with Pfizer (NCT03362502) for DMD as well as a trial for spinal muscular atrophy type 1 (NCT02122952) and a trial for X-linked myotubular myopathy (NCT03199469).
In our study, rAAV6-injected muscles systematically presented an acute inflammatory response with degeneration and regeneration of majority of the muscle fibers. Such a phenotype has been described previously for other transgenes33–35 even though rAAV6 has also been used to transduce skeletal muscle.36–38 As suggested by Winbanks et al., discrepancies in rAAV6 transduction and related inflammatory response could be due either to the nature of the transgene or to the dose used.38 Regarding the nature of the transgene, the absence of an inflammatory response with all of the other rAAV serotypes used in our study suggests that the LacZ reporter gene is not itself deleterious. Regarding the dose, the question remains as to whether a lower dose of AAV6 would have allowed a better level of expression without any related inflammatory response. We can only conclude here that at this same dose, rAAV6 induces an inflammatory response, whereas other serotypes do not, and this is not related to the quality of rAAV6 production (data not shown).
AAVs use glycan receptors and coreceptors, which play a role in cell attachment, and these interactions have been well characterized for a number of serotypes30: α2–3 and α2–6 N-linked sialic acid for AAV1; heparan sulfate proteoglycan for AAV2 and AAV3; α2–3 O-linked and α2–3 N-linked sialic acid for AAV4 and AAV5; heparan sulfate and α2–3 and α2–6 N-linked sialic acid for AAV6; and N-linked galactose for AAV9. A new cellular AAV receptor (also named KIAA0319L) has also recently been described as essential for AAV cell entry following attachment.39,40 In our study, none of the vectors were able to induce the expression of LacZ in interstitial cells (fibroblasts and inflammatory cells) (Fig. 2H) or satellite cells (Supplementary Fig. S4), as previously described for rAAV636 and rAAV9.41,42 We cannot exclude that these cells are transduced, but unable to express the transgene, maybe due to an uncoating issue. Otherwise, this could be due to differences in cell surface receptors or mitotic status of myogenic and nonmyogenic cells, respectively. Further studies should include the analysis of the expression of these receptors on nonmyogenic/fibrotic cells compared with myogenic cells. Although we do not see any satellite cells targeted in our study, other studies have shown an efficient AAV transduction after systemic delivery with a CMV promoter.43 An important feature of our study is the observation that the fibrosis present in the muscle xenograft does not impair rAAV8 and rAAV9 transduction of muscle fibers, which is promising for future gene therapy strategies. Interestingly, rAAV transduction does not allow expression in interstitial muscle cells, and further studies should look at other vectors that could target these nonmyogenic cells with a potential antifibrotic gene therapy purpose. Elucidating tropism and transduction efficiency is critical to implement targeted and specific applications in a dynamic environment such as pathological muscle.
It is always a challenge to translate results obtained in mice into larger species. For example, different profiles have been observed between transduction efficiency in mice and dogs.44 We used for the first time a muscle xenograft model to evaluate AAV transduction efficiency in a human context. We did not observe any difference between rAAV8 and rAAV9, confirming their efficient transduction profile in human muscle. Interestingly, while different transduction efficiency levels have been observed on different fiber types in mice,33,45 we observed transduction of both slow and fast fiber types in the xenograft model. While bearing in mind that the human xenograft corresponds to a regenerated human muscle, we believe that this xenograft model is an ideal tool for preclinical studies to translate results found in mice into an in vivo human context and to further validate therapeutic strategies. The presence of a high degree of fibrosis in these xenografts could be a very useful model to evaluate therapeutic gene-based strategies in a fibrotic environment, similar to that observed in most muscular dystrophies.
Supplementary Material
Author Disclosure
No competing financial interests exist.
Funding Information
The authors thank Françoise Balter, Véronique Blouin, and the vector core CPV at UMR 1089 (Nantes, France) for AAV production. The authors also thank the Penn Vector Core (Gene Therapy Program, University of Pennsylvania, Philadelphia) for providing the pAAV-1 (p0001), pAAV2 (p5E18), pAAV-5 (p0050-R), pAAV-6 (P0004-R), pAAV-7 (p5E18-VD27), pAAV-8 (p0007-R), pAAV-9 (p5E18-VD29), and pAAV-rh10 (P0009-8) plasmids and Laura Julien from the MyoVector platform of the Centre of Research in Myology-UMRS974 (Paris, France) for AAV production. The authors thank Cécile Peccate and Alison Oliver for technical assistance and thank the MYOBANK-AFM of the Institut de Myologie (BB-0033-00012) for access to human muscle samples. The Pax7, MYHC-I (A4.840), and MYHC-II (A4.74) antibodies were obtained from the Developmental Studies Hybridoma Bank, created by the NICHD of the NIH and maintained at The University of Iowa, Department of Biology, Iowa City, IA. The authors acknowledge funding from the Association Française contre les Myopathies (AFM Telethon), Inserm, Sorbonne Université, Fondation pour la Recherche Médicale (FRM), and the Fondation Maladie Rare (AP-RM-16-035).
Supplementary Material
References
- 1. Colella P, Ronzitti G, Mingozzi F. Emerging issues in AAV-mediated in vivo gene therapy. Mol Ther 2018;8:87–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang D, Tai PWL, Gao G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat Rev Drug Discov 2019;18:358–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xiao W, Chirmule N, Berta SC, et al. Gene therapy vectors based on adeno-associated virus type 1. J Virol 1999;73:3994–4003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Srivastava A, Lusby EW, Berns KI. Nucleotide sequence and organization of the adeno-associated virus 2 genome. J Virol 1983;45:555–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Muramatsu SI, Mizukami H, Young NS, et al. Nucleotide sequencing and generation of an infectious clone of adeno-associated virus 3. Virology 1996;221:208–217 [DOI] [PubMed] [Google Scholar]
- 6. Chiorini JA, Yang L, Liu Y, et al. Cloning of adeno-associated virus type 4 (AAV4) and generation of recombinant AAV4 particles. J Virol 1997;71:6823–6833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chiorini JA, Kim F, Yang L, et al. Cloning and characterization of adeno-associated virus type 5. J Virol 1999;73:1309–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rutledge EA, Halbert CL, Russell DW. Infectious clones and vectors derived from adeno-associated virus (AAV) serotypes other than AAV type 2. J Virol 1998;72:309–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gao G-P, Alvira MR, Wang L, et al. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci USA 2002;99:11854–11859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gao G, Vandenberghe LH, Alvira MR, et al. Clades of adeno-associated viruses are widely disseminated in human tissues. J Virol 2004;78:6381–6388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mori S, Wang L, Takeuchi T, et al. Two novel adeno-associated viruses from cynomolgus monkey: pseudotyping characterization of capsid protein. Virology 2004;330:375–383 [DOI] [PubMed] [Google Scholar]
- 12. Grimm D, Zhou S, Nakai H, et al. Preclinical in vivo evaluation of pseudotyped adeno-associated virus vectors for liver gene therapy. Blood 2003;102:2412–2419 [DOI] [PubMed] [Google Scholar]
- 13. Paulk NK, Pekrun K, Zhu E, et al. Bioengineered AAV capsids with combined high human liver transduction in vivo and unique humoral seroreactivity. Mol Ther 2018;26:289–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Halbert CL, Allen JM, Miller AD. Adeno-associated virus type 6 (AAV6) vectors mediate efficient transduction of airway epithelial cells in mouse lungs compared to that of AAV2 vectors. J Virol 2001;75:6615–6624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Auricchio A, Smith AJ, Ali RR. The future looks brighter after 25 years of retinal gene therapy. Hum Gene Ther 2017;28:982–987 [DOI] [PubMed] [Google Scholar]
- 16. Harper SQ, Hauser MA, DelloRusso C, et al. Modular flexibility of dystrophin: implications for gene therapy of Duchenne muscular dystrophy. Nat Med 2002;8:253–261 [DOI] [PubMed] [Google Scholar]
- 17. Greelish JP, Su LT, Lankford EB, et al. Stable restoration of the sarcoglycan complex in dystrophic muscle perfused with histamine and a recombinant adeno-associated viral vector. Nat Med 1999;5:439–443 [DOI] [PubMed] [Google Scholar]
- 18. Ellis BL, Hirsch ML, Barker JC, et al. A survey of ex vivo/in vitro transduction efficiency of mammalian primary cells and cell lines with Nine natural adeno-associated virus (AAV1–9) and one engineered adeno-associated virus serotype. Virol J 2013;10:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gregorevic P, Blankinship MJ, Allen JM, et al. Systemic delivery of genes to striated muscles using adeno-associated viral vectors. Nat Med 2004;10:828–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang Z, Zhu T, Qiao C, et al. Adeno-associated virus serotype 8 efficiently delivers genes to muscle and heart. Nat Biotechnol 2005;23:321–328 [DOI] [PubMed] [Google Scholar]
- 21. Zincarelli C, Soltys S, Rengo G, et al. Analysis of AAV serotypes 1–9 mediated gene expression and tropism in mice after systemic injection. Mol Ther 2008;16:1073–1080 [DOI] [PubMed] [Google Scholar]
- 22. Brais B, Bouchard JP, Xie YG, et al. Short GCG expansions in the PABP2 gene cause oculopharyngeal muscular dystrophy. Nat Genet 1998;18:164–167 [DOI] [PubMed] [Google Scholar]
- 23. Gidaro T, Negroni E, Perié S, et al. Atrophy, fibrosis, and increased PAX7-positive cells in pharyngeal muscles of oculopharyngeal muscular dystrophy patients. J Neuropathol Exp Neurol 2013;72:234–243 [DOI] [PubMed] [Google Scholar]
- 24. Malerba A, Klein P, Bachtarzi H, et al. PABPN1 gene therapy for oculopharyngeal muscular dystrophy. Nat Commun 2017;8:14848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Duan D. Systemic AAV micro-dystrophin gene therapy for duchenne muscular dystrophy. Mol Ther 2018;26:2337–2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Serrano AL, Muñoz-Cánoves P. Regulation and dysregulation of fibrosis in skeletal muscle. Exp Cell Res 2010;316:3050–3058 [DOI] [PubMed] [Google Scholar]
- 27. Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol 2008;214:199–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Grieger JC, Choi VW, Samulski RJ. Production and characterization of adeno-associated viral vectors. Nat Protoc 2006;1:1412–1428 [DOI] [PubMed] [Google Scholar]
- 29. Zhang Y, King OD, Rahimov F, et al. Human skeletal muscle xenograft as a new preclinical model for muscle disorders. Hum Mol Genet 2014;23:3180–3188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Huang L-Y, Halder S, Agbandje-McKenna M. Parvovirus glycan interactions. Curr Opin Virol 2014;7:108–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Denard J, Rouillon J, Leger T, et al. AAV-8 and AAV-9 vectors cooperate with serum proteins differently than AAV-1 and AAV-6. Mol Ther 2018;10:291–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Keiser NW, Yan Z, Zhang Y, et al. Unique characteristics of AAV1, 2, and 5 viral entry, intracellular trafficking, and nuclear import define transduction efficiency in HeLa cells. Hum Gene Ther 2011;22:1433–1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Riaz M, Raz Y, Moloney EB, et al. Differential myofiber-type transduction preference of adeno-associated virus serotypes 6 and 9. Skelet Muscle 2015;5:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang Z, Tapscott SJ, Chamberlain JS, et al. Immunity and AAV-mediated gene therapy for muscular dystrophies in large animal models and human trials. Front Microbiol 2011;2:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Arnett ALH, Garikipati D, Wang Z, et al. Immune responses to rAAV6: the influence of canine parvovirus vaccination and neonatal administration of viral vector. Front Microbiol 2011;2:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Arnett AL, Konieczny P, Ramos JN, et al. Adeno-associated viral vectors do not efficiently target muscle satellite cells. Mol Ther 2014;1:14038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Qiao C, Zhang W, Yuan Z, et al. Adeno-associated virus serotype 6 capsid tyrosine-to-phenylalanine mutations improve gene transfer to skeletal muscle. Hum Gene Ther 2010;21:1343–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Winbanks CE, Beyer C, Qian H, et al. Transduction of skeletal muscles with common reporter genes can promote muscle fiber degeneration and inflammation. PLoS One 2012;7:e51627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pillay S, Zou W, Cheng F, et al. Adeno-associated virus (AAV) serotypes have distinctive interactions with domains of the cellular AAV receptor. J Virol 2017;91:e00391-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pillay S, Meyer NL, Puschnik AS, et al. An essential receptor for adeno-associated virus infection. Nature 2016;530:108–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stitelman DH, Brazelton T, Bora A, et al. Developmental stage determines efficiency of gene transfer to muscle satellite cells by in utero delivery of adeno-associated virus vector serotype 2/9. Mol Ther 2014;1:14040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nance ME, Shi R, Hakim CH, et al. AAV9 edits muscle stem cells in normal and dystrophic adult mice. Mol Ther 2019;27:1568–1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Goldstein JM, Tabebordbar M, Zhu K, et al. In situ modification of tissue stem and progenitor cell genomes. Cell Rep 2019;27:1254.e7–1264.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pan X, Yue Y, Zhang K, et al. AAV-8 is more efficient than AAV-9 in transducing neonatal dog heart. Hum Gene Ther Methods 2015;26:54–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bostick B, Ghosh A, Yue Y, et al. Systemic AAV-9 transduction in mice is influenced by animal age but not by the route of administration. Gene Ther 2007;14:1605–1609 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.