Abstract
Background
Non-small cell lung carcinoma (NSCLC) incidence and progression is increasing because of genetic and epigenetic changes. The mutations in the Kirsten rat sarcoma (KRAS) are the most frequently oncogene aberrations in lung carcinoma patients. A candidate tumor suppressor gene (TSG) Ras Association Domain Family 1 Isoform A (RASSF1A), is silenced by promoter hypermethylation in several human malignancies including non-small cell lung carcinoma (NSCLC). We hypothesized that RASSF1A methylation and KRAS mutations may play an important role in NSCLC.
Methods
Non-small cell lung carcinoma patients (n = 100) and equal number of healthy controls were assessed for activating KRAS (exon 2) mutations using allele-specific oligonucleotide polymerase chain reaction (ASO-PCR) and promoter hypermethylation of RASSF1A using methylation specific PCR.
Results
The frequency of mutations in Kirsten rat sarcoma (KRAS) were found in 31% of NSCLC patients in the Kashmiri population and occur most commonly, but not exclusively, in adenocarcinoma histology and life-long smokers. The NSCLC patients in advanced stage reported the higher frequency of mutation in KRAS (exon 2). A significant higher frequency of this mutation was reported in patients with NSCLC (29.16%) who are positive for metastasis (P < 0.03). The frequencies of promoter hypermethylation at Ras Association Domain Family 1 Isoform A (RASSF1A) were 41% in cases and 3% in control samples. The frequency of KRAS mutation and RASSF1A promoter methylation were significantly different between adenocarcinomas (ADC) and squamous cell carcinomas (SCC) patients with NSCLC (P < 0.03). In addition, we reported that NSCLC patients having RASSF1A promoter methylation was significantly associated with smoking (P = 0.01). It was identified that NSCLC patients with RASSF1A promoter region hypermethylation had poorer survival and faster disease progression compared with those without hypermethylation of RASSF1A promoter region (P = 0.0001). The Median survivals among with cases containing promoter region hypermethylation of RASSF1A were 17.20 and 42.13 months for patients without promoter region hypermethylation of RASSF1A and the patients with KRAS mutation with or without hypermethylation of the promoter region of RASSF1A a tumor suppressor gene had poorer survival compared with those patients with wild type KRAS gene, with or without hypermethylation of RASSF1A promoter region. These differences were statistically significant based on Log-rank (Mantel-cox) test (P = 0.0001). The median survivals among patients with mutation in KRAS protooncogene were 16 months and 42 months for NSCLC patients with wild type KRAS gene.
Conclusions
The aberrant RASSF1A gene promoter methylation with the subsequent mutation in KRAS gene (exon 2) plays a significant role in the pathogenesis and disease progression of non-small cell lung carcinoma (NSCLC).
Keywords: Cancer research, Cell biology, Adenocarcinoma, KRAS, RASSF1A, Metastasis, Methylation, Squamous cell carcinoma
Cancer research; Cell biology; Adenocarcinoma; KRAS; RASSF1A; Metastasis; Methylation; Squamous cell carcinoma
1. Introduction
Lung malignancy is the most frequent and commonly diagnosed cancer. It is the most common cause of cancer death worldwide and majority of which are having non-small cell lung cancer sub type of lung cancer, accounts for 85% [1, 2]. The most common cancer amongst men in India is lung cancer with approximately 33,000 new cases ever year and constitutes 6.8% of deaths [3]. Arshad et al, [4] recently reported that in Kashmir valley the second most common malignancy in men and third among all is lung cancer is (14.6%). Non-small cell lung carcinoma (NSCLC) arise as a result of molecular, genetic and epigenetic alterations alongside with additional morphological changes that gives rise to neoplastic tissue by the transformation of benign bronchial epithelium. Genetic alterations in tumour suppressor and proto-oncogenes genes have been detected to play important role in all stages of lung tumourigenesis. Hypermethylation of CpG Island causing epigenetic silencing in the promoter has been shown to be an important phenomenon in cancer formation [5]. DNA methylation is an alternative mechanism to mutations or deletions which disrupt the function of tumor suppressor gene. Aberrant gene methylation in the promoter region in the tumor suppressor gene has also been most frequently found in NSCLC [6]. It has have been implicated that Kirsten rat sarcoma (KRAS) gene activating point mutations play an important role in the pathogenesis of human malignancies including those of lung [7]. Histologically, about 80% of lung cancers are non-small cell and 20% are small cell lung cancers [8]. Among the NSCLCs, adenocarcinoma (ADC) and Squamous cell carcinomas (SCC) are the most common histological subtypes. In particular NSCLC patients with lung adenocarcinomas have been shown to harbor most of the KRAS mutations. However, KRAS mutations were rarely detected in lung squamous cell carcinomas [9, 10, 11, 12, 13, 14]. The Ras Association Domain Family 1 (RASSF1) gene located at 3p21.3 that code for more than 7 isoforms resulting from alternative mRNA splicing and promoter usage [15, 16]. Among them, Ras Association Domain Family 1 Isoform A (RASSF1A) and Ras Association Domain Family 1 Isoform C (RASSF1C) are major transcripts. It is predicted that RASSF1A is to encode a 39-kd peptide containing an N-terminal diacylglycerol (DAG)-binding domain and a Ras-association domain [15]. Transcriptional repression by methylation of promoter region is an important means for silencing of number of cancer-associated genes including RASSF1A [15]. RASSF1A conataining a Ras effector domain is ceded inactivated by epigenetics silencing of its promoter in a variety of human cancers including lung, colon, breast, prostate, thyroid, and renal cell carcinomas [16, 17]. The purpose of this study was to examine the association between KRAS mutation and RASSF1A hypermethylation in non-small cell lung carcinoma, and to determine their correlation with clinicopathological characteristics.
2. Material and methods
2.1. Sample collection
3–4 ml of peripheral blood was collected from confirmed primary NSCLC blood samples (n = 100) and equal no. of age matched control samples between December 2016 to August 2018 at Sher-I-Kashmir Institute of Medical Sciences (SKIMS), Soura, Srinagar were included in the study. The study was approved by Institutional ethics committee of Sher-I-Institute of Medical Sciences (SKIMS), Soura, Srinagar, Jammu and Kashmir, India. Informed consent was obtained from all patients for blood sample collection after confirmed diagnosis of NSCLC and blood samples were collected in EDTA vials. We have critically examined prospectively all newly diagnosed patients with NSCLC (n = 100) analysed for KRAS mutations and RASSF1A methylation. Staging of the NSCLC patient were performed according to the guidelines provided by Union for International Cancer Control (UICC, Seventh Edition) [18]. The Demographic characteristics of the cases recruited for this study are shown in Table 1. A total of 62 males and 32 females were included in the study. The patients were presented with average age of 61.18 ± 10 years; most of the patients were found within range of 50–84 years age group. Two age groups were made, patients with age ≤45 years included 13 cases, and >45 years included 87 cases. A good proportion of the NSCLC cases 40 (57.14%) that were recruited for this study had a monthly income of less than 6000 INR.
Table 1.
Clinicopathological characteristics of non-small cell lung carcinoma (NSCLC) patients (n = 100) undertaken for study.
| S.No | Characteristic | Sub group | No. of cases n (%) |
|---|---|---|---|
| 1. | Sex | Male | 68 |
| Female | 32 | ||
| 2. | Age at diagnosis (yr) | ≤45 | 13 |
| >45 | 87 | ||
| 3. | Age (Mean ± SD, yr) | 61.18 ± 10 yr | |
| 4. | Smoking | Non-smokers | 38 |
| Smokers | 62 | ||
| 5. | Dwelling | Urban | 43 |
| Rural | 57 | ||
| 6. | Rural: Urban | 1.32:1 | |
| 7. | Metastasis | Positive | 18 |
| Negative | 82 | ||
| 8. | Histological grade | Well differentiated | 9 |
| Moderately differentiated | 65 | ||
| Poorly differentiated | 24 | ||
| Undifferentiated | 2 | ||
| 9. | Histological type | Adenocarcinoma | 72 |
| Squamous cell carcinoma | 28 | ||
| 10. | Clinical stage (TNM staging) | I & II (early) | 43 |
| III & IV (Advanced) | 57 | ||
| 11. | Smoking level | Mild (<10) | 5 |
| Moderate (<40) | 30 | ||
| Heavy (>40) | 27 | ||
| No smoking | 38 | ||
2.2. DNA extraction
According to the manufacturer's protocol the total genomic (g) DNA was extracted from blood samples using quick gDNA MiniPrep kit (Zymo Research, USA). The quality and integrity of the DNA was determined by the A260/280 ratios and total DNA concentrations were calculated by Nano Drop 2000c Spectrophotometer (Thermo Scientific, Asheville, NC, USA).
2.3. Allele specific oligonucleotide-PCR (ASO-PCR)
ASO-PCR for KRAS gene (exon 2) was performed in a total reaction volume 25 μl containing 3 μl of 100 ng template DNA with a working concentration 25 pmol each Primers (0.3 μl of each primer), 0.6 μl 10 mmol/L dNTPs, 2.2 μl of 20 mmol/L MgCl2, 0.3 μl of 5 U/μl Taq polymerase (Thermo scientific) with 2.5 μl of 10× Taq Buffer (Thermo scientific) and 15.8 μl of nuclease free ddH2O.
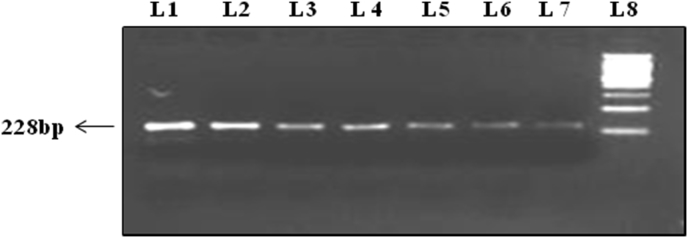
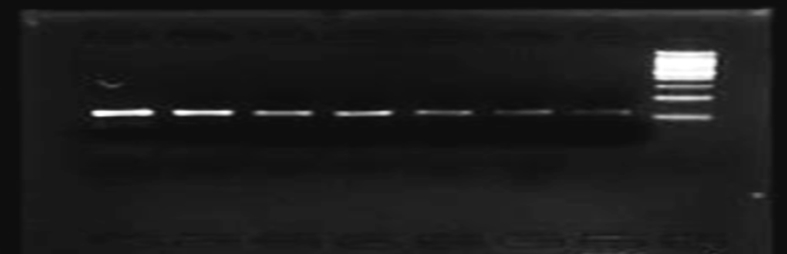
Specific primers designed for KRAS Exon 2 gene amplification are given below: Forward primer KRAS 2F (Exon 2): 5′-TTCCTACAGGAAGCAAGT-3′. Reverse primer KRAS 2R (Exon 2): 5′-CACAAAGAAAGCCCTCCCCA-3′. PCR products were visualized with ethidium bromide on 2.0% agarose gel, under a UV transilluminator. The product obtained for KRAS (exon 2) had a band size of 228 bp (Figure 1).
Figure 1.
Representative gel picture of the KRAS (exon 2) amplified by polymerase chain reaction. Lane no. L1 to L7 represents 228 bp fragment of exon 2 of KRAS gene amplified by PCR, Lane no. L8 represent 200 bp ladder (See also figure S1).
2.4. genomic DNA modifications by Bisulphite treatment
By using BisulFlash DNA Modification Kit (Epigentek, USA) the genomic DNA (~1μg) ranged from the concentration 50–200ng per reaction was modified with sodium bisulphite. The complete conversion of unmethylated cytosine into uracil of whole DNA by Bisulphite was carried out at 95 °C for 20 min. This converted DNA was cleaned-up of and stored at -80 °C.
2.5. Methylation-specific PCR (MSP)
Methylation specific PCR (MS-PCR) were used to determine the methylation status in the CpG islands of RASSF1A promoter region of modified DNA (bisulphite treated DNA) by 2 sets of specific primers, one for determining the methylated DNA sequence one set for unmethylated DNA sequence.
The methylated DNA of RASSF1A was amplified using methylated (M) set of primers,
5′-GGGTTTTGCGAGAGCGCG3′ (sense),
5′-GCTAACAAACGCGAACCG-3′ (antisense),
The Unmethylated DNA of RASSF1A was amplified using unmethylated (U) set of primers,
5′-GGTTTTGTGAGAGTGTGTTTAG-3′ (sense),
5′-CACTAACAAACACAAACCAAA-3′ (anti-sense).
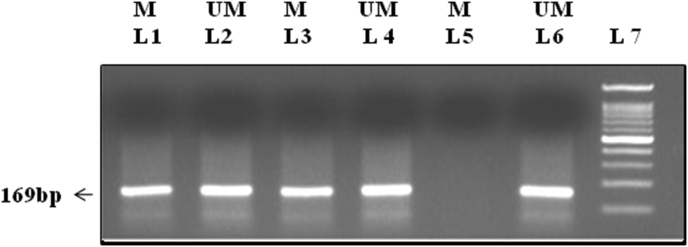
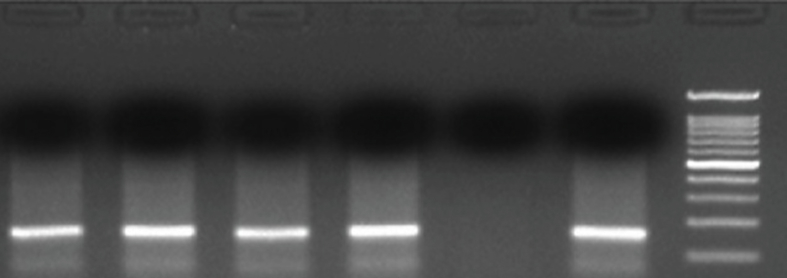
Bisulfite-modified DNA(2μl) was amplified using MS-PCR in a total volume of 25 containing 2.5 μl of 10× PCR buffer (Fermentas, USA), 0.5 μl of 10 mmol/L dNTP, 0.5 μl of 10 μM each primer, and 0.1μl of 5U/mL Taq DNA polymerase (Fermentas, USA) and PCR amplification was programmed at initial denaturation at 95 °C for 5 min, 40 cycles of amplification at 95 °C for 30s, 60 °C for both methylated and Unmethylated primers for 50s, and 72 °C for 45s and final extension of 72 °C for 10 min. 10 μl of each PCR product were resolved by loading onto 1.5% agarose gel, and the bands were visualized under UV trans-illumination by ethidium bromide staining A band size of 169bp was observed for both RASSF1A methylated and unmethylated set (Figure 2).
Figure 2.
Gel electrophoresis band pattern for RASSF1A gene methylation visualized on 1.5% agarose gel under ultraviolet trans-illumination yielding 169-bp reaction product (See also figure S2)
M = represents methylated product.
UM = represents unmethylated product.
Lane 1, 3, 5: represents NSCLC samples amplified by methylated primers.
Lane 2, 4, 6: represents NSCLC samples amplified by unmethylated primers.
Lane 7: 100bp Ladder.
2.6. Statistical analysis
The data were processed for doing all statistical analyses by Statistical Package for Social Sciences, version 20.0 Chicago, IL, USA (SPSS20). The association between the KRAS (exon 2) mutations as well as between methylation status of RASSF1A promoter region and various clinicopathological characteristics were statistically computed using Chi Square test (□2) or Fisher's exact test. The P value was computed using chi square test; odds ratio (OR) and confidence intervals (95% CI) was computed by an unconditional logistic regression method. The level of significance was set to P < 0.05. Kaplan Meier survival analysis plots were used to evaluate the effect of methylation of RASSF1A and KRAS (exon 2) mutations on overall survival of NSCLC patients, and differences in the survival rate between two groups were compared using the Log-rank (Mantel-cox) test.
3. Results
3.1. Demographic characteristics of study population
The patient's demographic characteristics are summarized in Table 1. A total of 68 males and 32 females were included in the study. Two age groups were made, patients with age ≤45 years included 13 cases, and >45 years included 87 cases. The patients were presented with average age 61.18 years; most of the patients were found within range of 50–84 years age group. Moreover, it was found that, of all the ten districts of the Kashmir division, the highest number of NSCLC cases turned out from the central district of Srinagar, with a total no. of 25.72% of NSCLC patients followed by Budgam 11.43% recruited for the study (Table 1). In NSCLC type of Lung cancer, adenocarcinoma was the most common and was found in 72 (72%) patients and squamous cell carcinoma second most common type of non-small cell lungs cancer, found in 28 patients (28%). Majority of patients 57 (57%) were diagnosed in the advanced stage (III and IV) of disease. The majority of patients were having history of smoking.
3.2. KRAS mutation status
Out of hundred NSCLC patients, 31 (31%) were positive for KRAS (exon 2) gene mutations and 69 (69%) were negative. The difference observed was statistically significant (P < 0.001). The KRAS gene (exon 2) mutation status in relation to various clinicopathological features as shown in the Table 2.
Table 2.
Association of KRAS gene mutation and RASSF1A aberrant promoter region methylation with the clinicopathological characteristics of NSCLC patients.
| S.no | Variables | No. of Patients |
KRAS Mutation (Exon 2) |
OR (95%CI) | p value |
RASSF1A methylation status |
OR (95%CI) | p value | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Positive n (%) | Negative n (%) | No. of methylated samples n (%) | No. of Unmethylated samples n (%) | ||||||||
| 1. | Age at Diagnosis | ||||||||||
| ≤45Y | 13 | 3 (23.08) | 10 (76.92) | 0.632 (0.120–3.329) | 0.58 | 3 (23.08) | 10 (76.92) | 0.824 (0.261–2.589) | 0.13 | ||
| >45Y | 87 | 28 (32.18) | 59 (67.82) | 1 | 38 (43.67) | 49 (56.33) | 1 | ||||
| 2. | Gender | ||||||||||
| Male | 68 | 26 (38.23) | 42 (61.77) | 0.970 (0.327–2.873) | 0.95 | 30 (44.11) | 38 (55.89) | 0.540 (0.130–2.145) | 0.60 | ||
| Female | 32 | 5 (15.62) | 27 (84.38) | 1 | 11 (34.37) | 21 (65.63) | 1 | ||||
| 3. | Histological Type | ||||||||||
| Adenocarcinoma | 72 | 21 (29.16) | 51 (70.84) | 0.811 (0.257–2.588) | 0.01∗ | 34 (47.22) | 38 (52.78) | 0.621 (0.157–2.183) | 0.03∗ | ||
| Squamous Cell Carcinoma |
28 | 10 (35.71) | 18 (64.29) | 1 | 7 (25) | 21 (75) | 1 | ||||
| 4. | Smoking Status | ||||||||||
| Smokers | 62 | 25 (40.32) | 37 (59.68) | 4.899 (1.273–18.77) | 0.01∗ | 32 (51.61) | 30 (48.39) | 3.099 (1.167–4.443) | 0.01∗ | ||
| Non smokers | 38 | 6 (15.78) | 32 (84.22) | 1 | 9 (23.68) | 29 (76.32) | 1 | ||||
| Current smokers | 20 | 6 (30) | 14 (70) | 0.182 (0.047–0.697) | 0.01∗ | 12 (60) | 8 (40) | 0.282 (0.036–0.856) | 0.03∗ | ||
| Ex-smokers | 42 | 19 (45.23) | 23 (54.77) | 1 | 20 (47.61) | 22 (52.39) | 1 | ||||
| 5. | TNM Staging | ||||||||||
| Early stage (I and II) | 53 | 11 (20.75) | 42 (79.25) | 0.353 (0.112–1.116) | 0.05∗ | 11 (20.75) | 42 (79.25) | 0.359 (0.114–1.129) | 0.05∗ | ||
| Advanced Stage (III and IV) | 47 | 20 (42.55) | 27 (57.45) | 1 | 30 (63.82) | 17 (36.18) | 1 | ||||
| 6. | Distant Metastasis | ||||||||||
| Positive | 18 | 06 (33.33) | 12 (66.67) | 0.941 (0.319–2.775) | 0.03∗ | 10 (55.55) | 08 (44.45) | 0.821 (0.289–2.786) | 0.02∗ | ||
| Negative | 82 | 25 (30.48) | 57 (69.52) | 1 | 31 (37.80) | 51 (62.20) | 1 | ||||
| 7. | Smoking Level | ||||||||||
| Mild (≤10) | 5 | 03 (60.00) | 02 (40.00) | 1 | 0.64 | 0 (0.00) | 05 (100) | 1 | 0.72 | ||
| Moderate (≤40) | 30 | 12 (40) | 18 (60) | 0.550 (0.043–7.034) | 16 (53.33) | 14 (46.67) | 0.460 (0.023–7.045) | ||||
| Heavy (>40) | 27 | 10 (37.03) | 17 (62.97) | 0.308 (0.024–3.968) | 0.34 | 18 (67.66) | 9 (32.34) | 0.209 (0.014–3.799) | 0.22 | ||
| No-smoking | 38 | 6 (15.78) | 32 (84.22) | 1 | 7 (18.42) | 31 (81.58) | 1 | ||||
∗ significant (p < 0.05).
3.3. Frequency of KRAS (exon 2) mutations with respect to various clinico-pathological characteristics
3.3.1. Frequency of KRAS (exon 2) mutation with respect to histological type, gender and age
KRAS gene mutation was found in higher frequency in patients Adenocarcinoma (ADC) (29.16%) than in squamous cell carcinoma (SCC) (10%) histological types of lung cancer. The difference was statistically significant (OR = 0.81, 95% CI: 0.257–2.588, P < 0.01). There was a much difference in frequency of KRAS gene mutation in NSCLC patients with respect to gender however also the higher frequency of those mutations was reported in higher age group >45 (32.18%) than lower age group ≤45 (23.08%) (Table 2).
3.3.2. Frequency of KRAS (exon 2) mutation with respect to stage
The higher frequency of KRAS (exon 2) mutation among the different stages, NSCLC patients were found in advanced stage (42.55%) than the early stages (20.75%). The difference was statistically significant (OR = 0.353, 95% CI: 0.112–1.116, P < 0.05). Also the development of NSCLC was reported to be faster among the patients in advanced stage with KRAS (exon 2) mutations (Table 2).
3.3.3. Frequency of KRAS (exon 2) mutation with respect to smoking type
We observed a statistically significant correlation (OR = 4.899, 95% CI: 1.273–18.77, P < 0.01) between KRAS mutation and smoking status however a higher frequency of KRAS (exon 2) mutation were found in smoker (40.32%) than non-smokers (15.78%). We observed statistically significant higher frequencies of KRAS gene (exon 2) mutations in NSCLC patients who were ex-smokers (50%) and current smokers (40%) (OR = 0.182, 95% CI: 0.047–0.697, P < 0.01) (Table 2).
3.3.4. Frequency of KRAS (exon 2) mutation with respect to metastasis
The significantly higher frequency of the KRAS gene (exon 2) mutation was reported in NSCLC patients positive for metastasis (33.33%) with metastasis (OR = 0.941, 95% CI: 0.319–2.775, P < 0.03) (Table 2).
3.3.5. Frequency of KRAS (exon 2) mutation with respect to other clinico-pathological feature
There was no statistical significant association were evaluated between level of smoking (mild, moderate and heavy), family history and KRAS gene mutation (Table 2).
3.4. Mutational analysis
As far as the mutation frequency is concerned, 100 patients were investigated through screening by direct sequencing, 31% showed mutation in exon 2 corresponding to codons 12 and 13 of the KRAS proto-oncogene. Conversely 69% showed wild-type (WT) sequence.
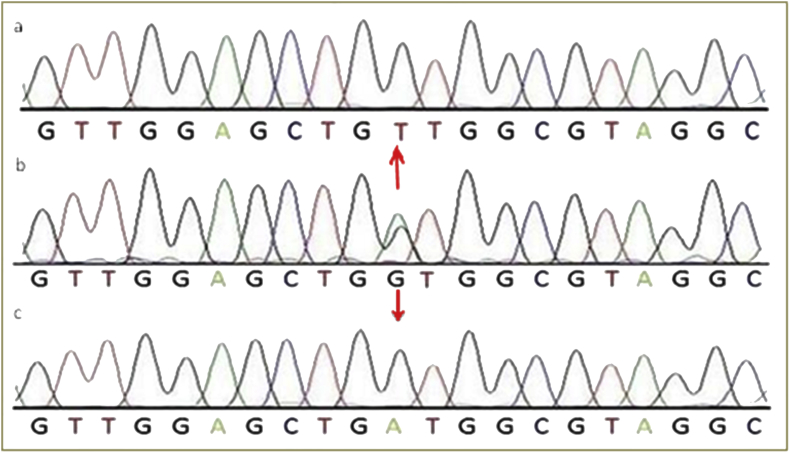
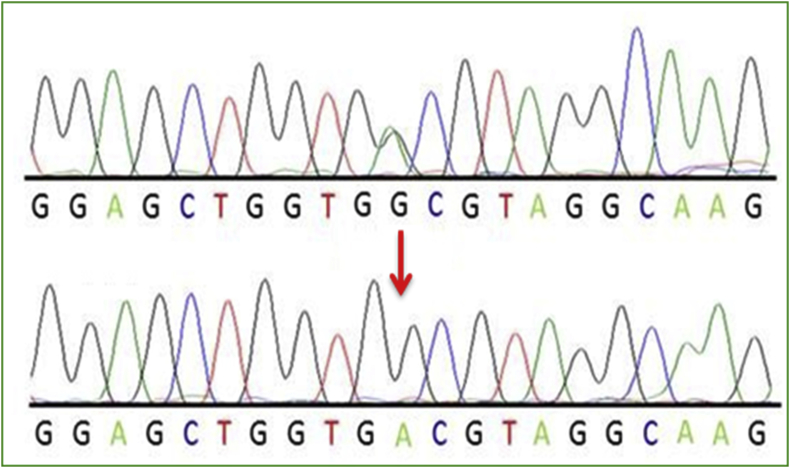
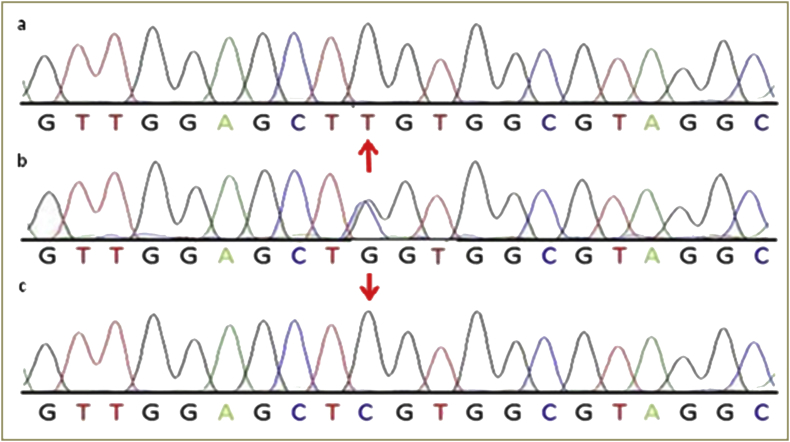
The alignment of all the sequences pertained to DNA samples of various cases was done with respect to normal sequence. Sequencing results for KRAS (exon 2) amplicon for potential mutation for cases shown by chromatograms (Figures 3, 4, and 5). The most common mutations that was found in NSCLC patients are glycine (G) to cysteine (C) on codon 12 (p.G12C, 32.25% of mutated samples; 10 of 31) Figure 4, glycine (G) to valine (V) on codon 12 (p.G12V, 22.58% of mutated samples; 07 of 31) Figure 5, glycine (G) to aspartate (D) on codon 12 (p.G12D, 19.04% of mutated samples; 05 of 31), glycine (G) to aspartate (D) on codon 13 (p.G13D, 9.67% of mutated samples; 03 of 31). The three mutations G12C, G12V, G12D account 70.96% of all the mutations.
Figure 3.
Represents chromatogram of (a) direct sequencing for KRAS c.34 G > T (G12C), (b) normal DNA sequence of KRAS c.34 and (c) direct sequencing for KRAS c.34G > C (G12R), at position 34, on codon 12.
Figure 4.
Represents chromatogram of (a) direct sequencing for KRAS c.35G > T (G12V), (b) normal DNA sequence of KRAS c.35 and (c) direct sequencing for KRAS c.35G > A (G12D), at position 35, on codon 12.
Figure 5.
Representative chromatogram of direct sequencing for KRAS c.38G > A (G13D) mutation in affected individuals containing G to A transition at position 38, on codon13.
3.5. Methylation analysis of RASSF1A tumour suppressor gene
The allocation of RASSF1A gene methylation frequencies in cases and controls is depicted in Table 3. However when compared with healthy controls a statistical significant difference was observed (P = 0.03) with patients having RASSF1A gene methylation. The result illustrated that methylation of RASSF1A promoter region is more frequent (41.0%) in NSCLC cases as against those in healthy controls (3.0%) (Table 3). Our results indicate that NSCLC patients who show hypermethylation of promoter region of RASSF1A gene have a statistical significant increased risk of developing NSCLC and can be a useful candidate biomarker in studying the prognosis of the disease.
Table 3.
Distribution of Methylation of RASSF1A gene amongst cases and controls.
| Gene | No. of methylated samples n (%) | No. of Unmethylated samples n (%) | Chi square | Df1 | P value |
|---|---|---|---|---|---|
|
RASSF1A Cases |
41 (41) | 59 (59) | 4.77 | 1 | 0.031 |
|
RASSF1A Controls |
3 (3) | 97 (97) |
df = degree of freedom.
3.5.1. Association between RASSF1A aberrant gene promoter hypermethylation and age at diagnosis
The association of RASSF1A aberrant gene promoter hypermethylation and age at diagnosis is described in Table 2. In both groups ≤45 years and >45 years, the association was found to be non-significant (OR = 0.824, 95% CI 0.261–2.589, P < 0.13.) although methylation of RASSF1A gene was observed to be more evident in the age group >45 years (43.67%) as compared to the age group ≤45 years (23.08%) (Table 2).
3.5.2. RASSF1A aberrant gene promoter hypermethylation in relation to stage of the disease
RASSF1A gene promoter hypermethylation was found a higher in the advanced stage disease (55.31%) than in NSCLC patients in early stage disease (16.98%). A statistical significant correlation found between methylation frequencies for RASSF1A and stage of the NSCLC (OR = 0.359, 95%CI = 0.114–1.129, P = 0.05) (Table 2).
3.5.3. RASSF1A aberrant gene promoter hypermethylation in relation to different histological types
RASSF1A aberrant gene methylation was found to be the highest in NSCLC patients with Adenocarcinoma (52.77%) as compared to squamous cell carcinoma (25%). A statistical significant association was found between the methylation status of RASSF1A gene with different histological types (OR = 0.621, 95% CI = 0.157–2.183, P < 0.05) (Table 2).
3.6. Survival analysis
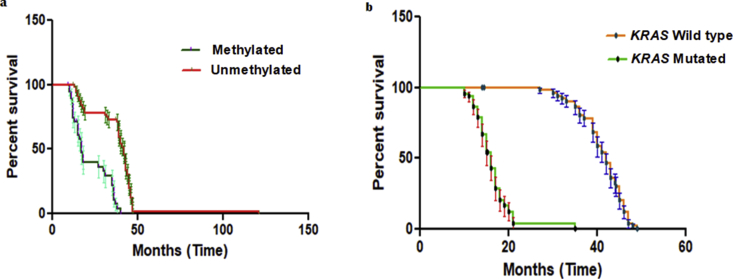
The Follow-up of the 100 primary NSCLCs patients regarding disease progression in terms of survival was performed by using Kaplan-Meier survival plot we observed NSCLCs patients with RASSF1A aberrant gene promoter region hypermethylation had a poorer survival compared with those without hypermethylation of promoter region of RASSF1A gene, however these differences were statistically significant based on Log-rank (Mantel-cox) test (P = 0.0001). Median survivals among with NSCLC patients containing hypermethylation of promoter region of RASSF1A were 17.20 and 42.13 months for NSCLC patients without hypermethylation of RASSF1A promoter Figure 6(a). The NSCLC patients with KRAS (exon 2) mutation with or without RASSF1A promoter region hypermethylation had poorer survival compared with those patients with wild type KRAS gene, with or without RASSF1A promoter region hypermethylation. These differences were statistically significant based on Log-rank (Mantel-cox) test (P = 0.0001). Median survivals among NSCLC patients with KRAS mutation with or without hypermethylation cases were 16 months and 42 months for patients with wild type KRAS gene, with or without hypermethylation of RASSF1A promoter Figure 6(b).
Figure 6.
Kaplan-Meier survival curves for 100 primary NSCLCs. a. Patients with RASSF1A promoter region hypermethylation had poorer survival compared with those without RASSF1A promoter region hypermethylation. b. Patients with KRAS (exon 2) mutation, with or without hypermethylation of the RASSF1A promoter region had poorer survival compared with those NSCLC patients with wild type KRAS gene, with or without hypermethylation of RASSF1A.
3.7. Association between RASSF1A inactivation and KRAS mutation
The analysis of aberrant RASSF1A promoter methylation when combined with KRAS (exon 2) mutation data, we found that only 9 of 72 (12.5%) patients with adenocarcinoma contained both a KRAS mutation and RASSF1A aberrant gene promoter methylation. Patients with squamous cell carcinomas 1 of 28 (3.57%) with promoter region methylation of RASSF1A contained a KRAS mutation (Table 4). Our results indicated that the majority of the NSCLC patients with adenocarcinomas containing KRAS mutations lack RASSF1A aberrant gene promoter methylation. In addition, 13 of 72 (18.05%) adenocarcinomas, and 14 of 28 (50%) squamous cell carcinomas contain neither KRAS mutation nor RASSF1A methylation (Table 4).
Table 4.
Relationship between the KRAS activation and RASSF1A methylation in NSCLCs.
| Characteristic | Sub Group | Patient Number | KRAS Mutation | RASSF1A Methylation | Tumour Contain KRAS Mutations and RASSF1A Methylation | Tumour Contain neither KRAS Mutation nor RASSF1A Methylation |
|---|---|---|---|---|---|---|
| Histological Type | Adenocarcinoma | 72 | 21/72 (29.16%) | 34/72 (52.77%) | 9/72 (12.50%) | 13/72 (18.05%) |
| Squamous Cell Carcinoma | 28 | 10/28 (35.71%) | 7/28 (25%) | 1/28 (3.57%) | 14/28 (50%) | |
| Smoking | Non-smokers | 38 | 6/38 (15.78%) | 9/38 (23.68%) | 3/38 (7.89%) | 12/38 (31.57%) |
| Smokers | 62 | 25/62 (40.32%) | 32/62 (51.61%) | 23/62 (37.09%) | 10/62 (16.12%) | |
| Metastasis | Positive | 18 | 06/18 (33.33%) | 10/18 (55.55%) | 06/18 (33.33%) | 12/18 (66.66%) |
| Negative | 82 | 25/82 (30.48%) | 31/82 (37.80%) | 20/82 (24.39%) | 23/82 (28.04%) | |
| Clinical Stage | I and II (early) | 53 | 11/53 (20.75%) | 11/53 (20.75%) | 6/53 (11.32%) | 2/53 (3.77%) |
| III and IV (advanced) | 47 | 20/47 (42.55%) | 30/47 (63.82%) | 13/47 (27.65%) | 5/47 (10.63%) |
4. Discussion
Non-small cell lung carcinoma (NSCLC) is known as a disease, characterized by manifold genomic variations in proto-oncogenes and in the tumour suppressor genes. The most common molecular events in the NSCLC are mutations in the KRAS gene, suggesting a significant role in lung tumour carcinogenesis [19, 20, 21, 22, 23]. The mutations in codons 12 and 13 in the KRAS gene are the most common in lung cancers [24].
In present study the frequency of KRAS gene (exon 2) mutations in NSCLC patients was 31%. The frequency of KRAS mutation reported in the study of Sameer et al, [25] was 22.64%, 39% in study of Plesec et al, [26], 32.7% in Yunxia et al, study [27] and it was 37% in work of Brink et al, [28]. The ratio of 31% in our study is consistent with these studies.
The significant higher frequency of KRAS (exon 2) gene mutation was seen in non-small cell lung cancer patients with ADC histological type of (21%) than SCC (10%), however the difference between the histological subtypes was statistically significant (OR = 0.81, 95% CI = 0.257–2.588, p < 0.01). We observed higher frequency of KRAS mutations were detected largely in patients with lung adenocarcinomas than NSCLC patients with squamous cell carcinomas of the lung. In fact, in the study conducted by Ju et al, [19]; Herbest et al, [29]; Brando et al, [30] found patients with adenocarcinoma have common mutation in KRAS gene.
There was a much difference in frequency of KRAS gene mutation in NSCLC patients with respect to age however also the higher frequency of those mutations was reported in higher age group >45 (32.18%) than lower age group ≤45 (23.01%). In another study conducted by Shah et al, [14] and in the work of Riely et al, [18] higher frequency of KRAS mutation was seen in higher age groups.
In this study, our results revealed that the higher frequency of KRAS (exon 2) mutations among the different stages was reported in NSCLC patients in advanced stage (III &IV) (42.55%) than the early stages (I & II) (20.75%). The difference was statistically significant (OR = 0.353, 95% CI = 0.112–1.116, p < 0.05), this shows that there is a significant association with increased risk of NSCLC if patients are in advanced stage (III &IV). A similar studies conducted Mascaux et al, [31]; Johnson et al, [32] showed from a clinical perspective, KRAS-mutant lung carcinomas have been generally associated with type of tumours, particularly found mostly in the advanced-stage setting.
In addition it was found that higher frequency of KRAS mutation was reported from the smokers (40.32%) than non-smokers (15.78%); however, this difference was statistically significant (OR = 4.899, 95%CI = 1.273–18.77, p < 0.01). Also a significant association was seen between the frequency of KRAS gene (exon 2) mutations in ex-smokers (45.23%) and current smokers (30%) (OR = 0.182, 95%CI = 0.047–0.697, p < 0.01). A metanalysis of Mao et al. [33] found higher frequency of KRAS gene mutations in patients who are having history of long-life smoking and the study by El Osta et al, [34] found KRAS mutations are typically found more often in the patients who are heavy smokers and KRAS mutations rarely occur in lung cancers in light or never smokers.
Our results showed higher incidence KRAS gene (exon 2) mutations in patients with distant organ metastasis. Wagner et al, [21] found a significant association of K-RAS mutation and metastasis in NSCLC patients.
There was no significant association found in NSCLC patients between smoking level (mild, moderate and heavy), family history and KRAS gene mutation.
In a total of 100 primary NSCLC cases diagnosed, the KRAS (exon 2) mutations were found in 31 of 100 cases (30%). 28 of 31 (90.32%) were located in codon 12, and 3 (9.68%) was located in codon 13. In another studies by Macerellia et al, [35]; Riely et al, [36]; Dogan et al, [37] evaluated most activating KRAS (exon 2) mutations in NSCLC are sited in codons 12 or 13.
Further, mutations in KRAS detected in this study were diverse comprising 31, out of which, 10 were KRAS c.34G > T transversion, and 8 were KRAS c.35G > T transversion, 5 were KRAS c.35G > A transition, 4 were KRAS c.34G > A transition, 2 was KRAS c.34G > C transversion on codon 12 and 2 were KRAS c.38G > A on codon 13. Riely et al, [22] showed NSCLC KRAS mutations mainly occur as transversion mutations.
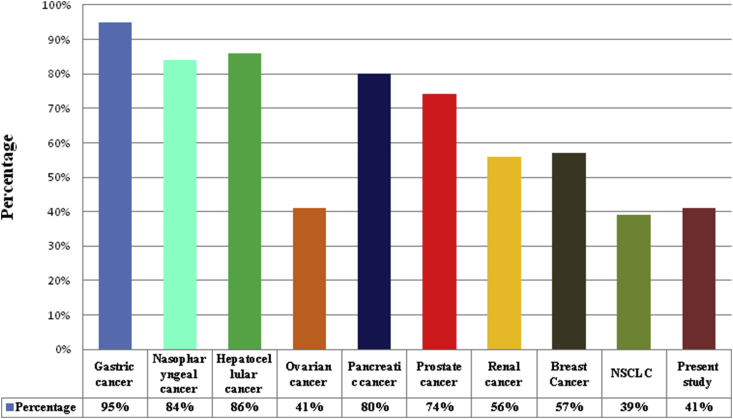
The aberrant promoter hypermethylation of RASSF1A gene has been witnessed in a variety of human carcinomas such as pancreatic endocrine tumor [38], colorectal carcinoma [39], nasopharyngeal carcinoma [40], prostate carcinoma [41], ovary and renal carcinoma [42], hepatocellular carcinoma [43], breast cancer [44], NSCLC [45], gastric cancer [46] as depicted in the Figure 7.
Figure 7.
Distribution of RASSF1A gene methylation in various cancers all over the world.
Most of the recent studies have reported that an epigenetic changes result in many types of carcinomas. The DNA methylation is observed as one of the commonest epigenetic alterations in cancerous cells, methylation of DNA is caused by an addition of methyl group to the carbon 5 position of the cytosine ring catalyzed by an enzyme DNA methyltransferase particularly in the sequence 5′-CG-3′, which is also called as CpG dinucleotide [47, 48]. The transcriptional activation could be blocked by methylation of CpG-rich promoters. The DNA hypomethylation is probable contributors to oncogenesis and are mediated by various mechanisms that include aberrant hyper-methylation of tumor suppressor genes and chromosomal instability in various human cancers [49, 50, 51].
In the present study, MS-PCR was used for scrutiny of the RASSF1A gene promoter region methylation status. The aberrant methylation particularly in CpG islands of promoter regions the RASSF1A gene that may play an important role in the progression and development of lung carcinoma. In this study, we observed that RASSF1A promoter methylation was present in 41% in NSCLC patients. This frequency of RASSF1A aberrant gene promoter region hypermethylation according to the present study is consistent with the findings reported by other groups [52, 53, 54, 55]. The aberrant RASSF1A gene promoter region hypermethylation was found to be significantly associated with different histological types, however higher frequencies were found in adenocarcinoma subtype than squamous cell carcinoma sub type (P = 0.03). The study conducted by Kim et al, [56], and Li et al, [57] shows the results that consistent with our study. We also found that RASSF1A gene aberrant promoter region hypermethylation predominantly occurs in NSCLC patients with advanced stage of disease. Our study is consistent with the studies conduct by Sekido et al, [58]; Toyooka et al, [59]; Belinsky et al, [60] showing the same results, consistent with our findings. In the present study, we observed that an aberrant promoter methylation of the RASSF1A gene and distant organ metastasis status although could be of prognostic significance. These results suggest that aberrant RASSF1A gene methylation in promoter region accompanies the induction and progression toward a more aggressive form of NSCLC genesis process. Oh et al, [61] reported that RASSF1A gene promoter region is mostly methylated in non-small cell lung cancer (NSCLC) patients with distant organ metastasis which is consistent with our study showing the same results. However there was no statistical significant correlation found between RASSF1A gene aberrant promoter region hypermethylation and other various clinicopathological characteristics like age, gender and smoking level (light, moderate and heavy).
This study reveals that smoking is significantly associated with RASSF1A gene promoter region hypermethylation in NSCLC patients (OR = 3.099, 95%CI = 1.167–4.443, P = 0.01) and that hypermethylation of the RASSF1A gene in the promoter region may affect a patient's overall survival in NSCLC. The predisposition of specific genes to hypermethylation in smokers could be attributed to unsystematic changes in the milieu around that specific gene. The methylation status of a gene may be affected by quite a lot of factors, including the activity of enzymes like DNA methyltransferase and the specific transcription factors, such as SP1, structure of chromatin, immediacy to a methylation center, and the pre-existing methylation status in gene specifically in the CpG islands of the promoter region [62].
According to methylation status of RASSF1A gene promoter region in NSCLC patients indicated that the overall survival of NSCLC patients group with methylation in RASSF1A gene promoter region had an obviously shorter survival time than those in the un-methylation group (P = 0.001).
Hypermethylation of the RASSF1A gene promoter and advanced stage (III and IV) disease were also associated with poorer overall survival among NSCLC patients. These results suggest that hypermethylation and staging of the RASSF1A are independent prognostic factors in NSCLC. The study conducted by Buckingham et al, [63] showed the survival difference according to RASSF1A promoter hypermethylation status was greatest in patients with stage I and II of NSCLC which is consistent with our study showing the same results. In conclusion, the data suggests that methylation of RASSF1A gene promoter region in NSCLC patients might be specifically closely associated to males patients, smokers, patients in advanced stage of the disease, patients who have adenocarcinoma histological type of NSCLC, metastatic stage of disease and patients aged >45years. Although aberrant methylation of promoter region of RASSF1A gene located at 3p21is the region frequently methylated in non-small cell lung cancer, and it's also frequently hypermethylated in non-small cell lung cancer with a history of smoking (51.61%) the results were consistent with the studies conducted by Han et al [64] and Huang et al http://clincancerres.aacrjournals.org/content/10/18/6119 - ref-17[65].
In the present study, we systematically evulated the correlation between activating KRAS (exon 2) mutations and RASSF1A aberrant promoter hypermethylation in the NSCLC patients were It was observed that 21 of 72 (29.16%) of the adenocarcinomas (ADC) and 10 of 28 (35.71%) of squamous cell carcinomas (SCC) histological subtypes contained activating KRAS (exon 2) mutations restricted to codon 12 or 13. In addition, we analyzed the methylation status of the promoter region of the RASSF1A gene. Our results showed that aberrant RASSF1A promoter region methylation was observed in 38 of 72 (52.77%) of adenocarcinomas and 3 of 28 (10.71%) of squamous cell carcinomas histological subtypes in NSCLC patients. When KRAS mutations and RASSF1A gene methylation status data were combined, we observed that only 9 of 52 (12.5%) of lung adenocarcinomas and 1 of 28 (3.57%) of squamous cell carcinomas contains both a KRAS mutation and RASSF1A gene methylation (Table 4). In this study, the data evaluated together with previous study [66], specified that the majority of NSCLC patients with adenocarcinomas and squamous cell carcinomas containing activating KRAS gene mutations lack RASSF1A promoter methylation.
Declarations
Author contribution statement
Naseer Ue Din Shah: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Md Niamat Ali, Bashir A Ganai, Malik Tariq Rasool, Aabid Maqbool Lone: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Syed Mudassar, Mosin Saleem Khan: Conceived and designed the experiments.
Jasbir Kour, Ajaz Ahmad Waza: Contributed reagents, materials, analysis tools or data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
Figure S1 final.
Figure s2 final.
References
- 1.Greenlee R.T., Hill-Harmon M.B., Murray T., Thun M. Cancer statistics. CA Cancer J. Clin. 2001;51:15–36. doi: 10.3322/canjclin.51.1.15. [DOI] [PubMed] [Google Scholar]
- 2.Malik S.A., Khan M.S., Dar M., Hussain M.U., Shah M.A., Shafi S.M., Mudassar S. Molecular alterations and expression dynamics of LATS1 and LATS2 genes in non-small-cell lung carcinoma. Pathol. Oncol. Res. 2018;24(2):207–214. doi: 10.1007/s12253-017-0225-3. [DOI] [PubMed] [Google Scholar]
- 3.NandaKumar A. Indian Council of Medical research, National Cancer Registry Program; New Delhi: 2001. Consolidated Report of the Population Based Cancer Registries, Incidence & Distribution of Cancer, 1990–1996. [Google Scholar]
- 4.Arshad A.P. Siddiqi MA Burden of cancers in the valley of Kashmir: 5Year epidemiological study reveals a different scenario. Tumor Biol. 2012;33:1629–1637. doi: 10.1007/s13277-012-0418-z. [DOI] [PubMed] [Google Scholar]
- 5.Baylin S.B., Herman J.G. DNA hypermethylation in tumorigenesis. Trends Genet. 2000;16:168–174. doi: 10.1016/s0168-9525(99)01971-x. [DOI] [PubMed] [Google Scholar]
- 6.Esteller M., Sanchez-Cespedes M., Rosell R. Detection of aberrant promoter hypermethylation of tumor suppressor genes in serum DNA from non-small cell lung cancer patients. Canc. Res. 1999;59:67–70. [PubMed] [Google Scholar]
- 7.Boss J. Ras oncogene in human cancers: a review. Canc. Res. 1989;49:4682–4689. [PubMed] [Google Scholar]
- 8.Schiller J.H., Harrington D., Belani C.P., Langer C., Sandler A., Krook J., Zhu J., Johnson D.H. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N. Engl. J. Med. 2002;346:92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 9.Rodenhuis S., Slebos R.J., Boot A.J., Evers S.G., Mooi W.J., Wagenaar S.S., van Bodegom P.C., Bos J.L. Incidence and possible clinical significance of K-ras oncogene activation in adenocarcinoma of the human lung. Canc. Res. 1988;48:5738–5741. [PubMed] [Google Scholar]
- 10.Reynolds S.H., Anna C.K., Brown K.C., Wiest J.S., Beattie E.J., Pero R.W., Iglehart J.D., Anderson M.W. Activated protooncogenes in human lung tumors from smokers. Proc. Natl. Acad. Sci. 1991;88:1085–1089. doi: 10.1073/pnas.88.4.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suzuki Y., Orita M., Shiraishi M., Hayashi K., Sekiya T. Detection of ras gene of polymerase chain reaction products. Oncogene. 1990;5:1037–1043. [PubMed] [Google Scholar]
- 12.Li S., Rosell R., Urban A., Font A., Ariza A., Armengol P., Abad A., Navas J.J., Monzo M. K-ras gene point mutation: a stable tumor marker in non-small cell lung carcinoma. Lung Canc. 1994;11:19–27. doi: 10.1016/0169-5002(94)90279-8. [DOI] [PubMed] [Google Scholar]
- 13.Mills N.E., Fishman C.L., Rom W.N., Dubin N., Jacobson D.R. Increased prevalence of K-ras oncogene mutations in lung adenocarcinoma. Canc. Res. 1995;55:1444–1447. [PubMed] [Google Scholar]
- 14.Shah N.U.D., Md-Niamat A., Mudassar S., Ganai B.A., Rasool M.T., Mosin S.K., Jasbir K. KRAS gene mutations in relation to development and progression of non-small cell lung carcinoma in Indian Kashmiri population. J. Int. Transl. Med. 2018;6(3):113–120. [Google Scholar]
- 15.Endoh H., Yasushi Y., Shigeki S., Kohei T., Hiroyuki K., Takashi T., Tetsuya M. RASSF1A gene inactivation in non-small cell lung cancer and its clinical implication. Int. J. Canc. 2003;106(1):45–51. doi: 10.1002/ijc.11184. [DOI] [PubMed] [Google Scholar]
- 16.Dammann R., Takahashi T., Pfeifer P.G. The CpG island of the novel tumor suppressor gene RASSF1A is intensely methylated in primary small cell lung carcinomas. Oncogene. 2001;20:3563–3567. doi: 10.1038/sj.onc.1204469. [DOI] [PubMed] [Google Scholar]
- 17.Schagdarsurengin U., Gimm O., Hoang-Vu C., Dralle H., Pfeifer G.P., Dammann R. Frequent epigenetic silencing of the CpG island promoter of RASSF1A in thyroid carcinoma. Canc. Res. 2002;62:3698–3701. [PubMed] [Google Scholar]
- 18.Riely G.J., Kris M.G., Rosenbaum D. Frequency and distinctive spectrum of KRAS mutations in never smokers with lung adenocarcinoma. Clin. Canc. Res. 2008;14(18):5731–5734. doi: 10.1158/1078-0432.CCR-08-0646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ju L., Han M., Zhao C. EGFR, KRAS and ROS1 variants coexist in a lung adenocarcinoma patient. Lung Canc. 2016;95:94–97. doi: 10.1016/j.lungcan.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 20.Sasaki H., Okuda K., Kawano O. Nras and KRAS mutation in Japanese lung cancer patients: genotyping analysis using Light Cycler. Oncol. Rep. 2007;18:623–628. [PubMed] [Google Scholar]
- 21.Wagner P.L., Stied A., Wilbertz T. Frequency and clinicopathologic correlates of KRAS amplification in non-small cell lung carcinoma. Lung Canc. 2011;74(1):118–123. doi: 10.1016/j.lungcan.2011.01.029. [DOI] [PubMed] [Google Scholar]
- 22.Riely G.J., Kris M.G., Rosenbaum D. Frequency and distinctive spectrum of KRAS mutations in never smokers with lung adenocarcinoma. Clin. Canc. Res. 2008;14(18):5731–5734. doi: 10.1158/1078-0432.CCR-08-0646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malvezzi M., Bertuccio P., Levi F. European cancer mortality predictions for the year 2012. Ann. Oncol. 2012;23:1044–1052. doi: 10.1093/annonc/mds024. [DOI] [PubMed] [Google Scholar]
- 24.Uchiyama M., Usami N., Kondo M. Loss of heterozygosity of chromosome 12p does not correlate with KRAS mutation in non-small cell lung cancer. Int. J. Canc. 2003;107(6):962–969. doi: 10.1002/ijc.11493. [DOI] [PubMed] [Google Scholar]
- 25.Sameer A.S., Chowdhri N.A., Abdullah S. Mutation pattern of K-ras gene in colorectal cancer of Kashmir: a report. Indian J. Canc. 2009;46(3):219–225. doi: 10.4103/0019-509X.52956. [DOI] [PubMed] [Google Scholar]
- 26.Plesec T.P., Hunt J.L. KRAS mutation testing in colorectal cancer. Adv. Anat. Pathol. 2009;16(4):196–203. doi: 10.1097/PAP.0b013e3181a9d4ed. [DOI] [PubMed] [Google Scholar]
- 27.Zuo Y.X., Cao J., Zhu G.S. Mutations in epidermal growth factor receptor and K-ras in Chinese patients with colorectal cancer. BMC Med. Genet. 2010;11:p34. doi: 10.1186/1471-2350-11-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brink M., de Goeij A.F.P.M., Weijenberg P.M. K-ras oncogene mutations in sporadic colorectal cancer in The Netherlands cohort study. Carcinogenesis. 2003;24(4):703–710. doi: 10.1093/carcin/bgg009. [DOI] [PubMed] [Google Scholar]
- 29.Herbst R.S., Kelly K., Chansky K. Phase II selection design trial of concurrent chemotherapy and cetuximab versus chemotherapy followed by cetuximab in advanced-stage non-small-cell lung cancer: southwest oncology group study S0342. J. Clin. Oncol. 2010;28(31):4747–4754. doi: 10.1200/JCO.2009.27.9356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brandao G.D.A., Brega E.F., Spatz A. The role of molecular pathology in non-small-cell lung carcinoma-now and in the future. Curr. Oncol. 2012;19(1):24–32. doi: 10.3747/co.19.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mascaux C., Iannino N., Martin B. The role of RAS oncogene in survival of patients with lung cancer: a systematic review of the literature with meta-analysis. Br. J. Canc. 2005;92:131–139. doi: 10.1038/sj.bjc.6602258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson M.L., Sima C.S., Chaft J. Association of KRAS and EGFR mutations with survival in patients with advanced lung adenocarcinomas. Cancer. 2013;119:356–362. doi: 10.1002/cncr.27730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mao C., Qiu L., Liao R.Y. KRAS mutations and resistance to EGFRTKIs treatment in patients with non-small cell lung cancer: a meta-analysis of 22 studies. Lung Canc. 2010;69:272–278. doi: 10.1016/j.lungcan.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 34.El Osta B.E., Behera M., Kim S. Characteristics and outcomes of patients (pts) with metastatic KRAS mutant lung adenocarcinomas: lung Cancer Mutation Consortium (LCMC) database. J. Clin. Oncol. 2017;35:9021. doi: 10.1016/j.jtho.2019.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Macerellia M., Caramella C., Faivre L. Does KRAS mutational status predict chemoresistance in advanced non-small cell lung cancer (NSCLC)? Lung Canc. 2014;83:383–388. doi: 10.1016/j.lungcan.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 36.Riely G.J., Marks J., Pao W. KRAS mutations in Non-small cell lung cancer. Proc. Am. Thorac. Soc. 2009;6(2):201–205. doi: 10.1513/pats.200809-107LC. [DOI] [PubMed] [Google Scholar]
- 37.Dogan S., Shen R., Ang D. Molecular epidemiology of EGFR and KRAS mutations in 3,026 lung adenocarcinomas: higher susceptibility of women to smoking-related KRAS-mutant cancers. Clin. Canc. Res. 2012;18:6169–6177. doi: 10.1158/1078-0432.CCR-11-3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giorgio M., Eliana A., Mario D., Caterina F., Valentina D., Letizia B. Methylation-associated down-regulation of RASSF1A and up-regulation of RASSF1AC in pancreatic endocrine tumors. BMC Canc. 2011;11:p351. doi: 10.1186/1471-2407-11-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Torbjorn K.N., Zarah M.L., Xiao-Feng S. DNA methylation of the p14ARF , RASSF1A and APC1A genes as an independent prognostic factor in colorectal cancer patients. Int. J. Oncol. 2013;42:127–133. doi: 10.3892/ijo.2012.1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Joseph K., Kwok-Wai L., Ka-Fai T. Promoter hypermethylation of multiple genes in nasopharyngeal carcinoma. Clin. Canc. Res. 2002;8:131–137. [PubMed] [Google Scholar]
- 41.Limin L., Jung-Hoon Y., Reinhard D. Frequent hypermethylation of the RASSF1A gene in prostate cancer. Oncogene. 2002;21:6835–6840. doi: 10.1038/sj.onc.1205814. [DOI] [PubMed] [Google Scholar]
- 42.Yoon J.H., Dammann R., Pfeifer G.P. Hypermethylation of the CpG island of the RASSF1A gene in ovarian and renal cell carcinomas. Int. J. Canc. 2001;94(2):212–217. doi: 10.1002/ijc.1466. [DOI] [PubMed] [Google Scholar]
- 43.Saelee Pensri, Wongkham Sopit, Chariyalertsak Sunanta. RASSF1A promoter hypermethylation as a prognostic marker for hepatocellular carcinoma. Asian Pac. J. Cancer Prev. APJCP. 2010;11(6):1677–1681. [PubMed] [Google Scholar]
- 44.Magdalini K., Loukas K., Dimitris M. Prognostic significance of RASSF1A promoter methylation in operable breast cancer. Clin. Biochem. 2009;42:970–997. doi: 10.1016/j.clinbiochem.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 45.Wang J., Lee J.J., Wang L., Liu D.D., Lu C., Fan Y.H., Mao L. Value of p16INK4a and RASSF1A promoter hypermethylation in prognosis of patients with resectable non–small cell lung cancer. Clin. Canc. Res. 2004;10(18):6119–6125. doi: 10.1158/1078-0432.CCR-04-0652. [DOI] [PubMed] [Google Scholar]
- 46.Byun D.S., Lee M.G., Chae K.S., Ryu B.G., Chi S.G. Frequent epigenetic inactivation of RASSF1A by aberrant promoter hypermethylation in human gastric adenocarcinoma. Canc. Res. 2001;61(19):7034–7038. [PubMed] [Google Scholar]
- 47.Singal R., Ginder G.D. DNA methylation. Blood. 1999;93:4059–4070. [PubMed] [Google Scholar]
- 48.Jones P.A., Laird P.W. Cancer epigenetics comes of age. Nat. Genet. 1999;93:163–167. doi: 10.1038/5947. [DOI] [PubMed] [Google Scholar]
- 49.O’Neill R.J.W., O’Neill M.J., Graves J.A.M. Under-methylation associated with retro element activation and chromosome remodelling in an interspecific mammalian hybrid. Nature. 1998;393:68–72. doi: 10.1038/29985. [DOI] [PubMed] [Google Scholar]
- 50.Chen R.Z., Pettersson U., Beard C., Jackson-Grusby L., Jaenisch R. DNA hypomethylation leads to elevated mutation rates. Nature. 1998;395:89–92. doi: 10.1038/25779. [DOI] [PubMed] [Google Scholar]
- 51.Kim D.H., Nelson H.H., Wiencke J.K., Zheng S., Christiani D.C., Wain J.C., Mark E.J., Kelsey K.T. p16INK4a and histology-specific methylation of CpG islands by exposure to tobacco smoke in non-small cell lung cancer. Canc. Res. 2001;61:3419–3424. [PubMed] [Google Scholar]
- 52.Esteller M., Tortola S., Toyota M., Capella G., Peinado M.A., Baylin S.B., Herman J.G. Hypermethylation-associated inactivation of p14ARF is independent of p16INK4a methylation and p53 mutational status. Canc. Res. 2000;60:129–133. [PubMed] [Google Scholar]
- 53.Zöchbauer-Müller S., Fong K.M., Virmani A.K., Geradts J., Gazdar A.F., Minna J.D. Aberrant promoter methylation of multiple genes in non-small cell lung cancers. Canc. Res. 2001;61:249–255. [PubMed] [Google Scholar]
- 54.Dammann R., Li C., Yoon J.H., Chin P.L., Bates S., Pfeifer G.P. Epigenetic inactivation of a RAS association domain family protein from the lung tumor suppressor locus 3p21.3. Nat. Genet. 2000;25:315–319. doi: 10.1038/77083. [DOI] [PubMed] [Google Scholar]
- 55.Burbee D.G., Forgacs E., Zöchbauer-Müller S., Shivakumar L., Fong K., Gao B., Randle D., Kondo M., Virmani A., Scott B., Sekido Y., Latif F., Milchgrub S., Toyooka S., Gadzar A.F., Lerman M.I., Zabarovsky E., White M., Minna J.D. Epigenetic inactivation of RASSF1A in lung and breast cancers and malignant phenotype suppression. J. Natl. Cancer Inst. (Bethesda) 2001;93:691–699. doi: 10.1093/jnci/93.9.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim D.H., Kim J.S., Park J.H., Lee S.K., Ji Y.I., Kwon Y.M. Relationship of Ras association domain family 1 methylation and K-ras mutation in primary non-small cell lung cancer. Canc. Res. 2003;63:6206–6211. [PubMed] [Google Scholar]
- 57.Li J., Zhang Z., Dai Z., Popkie A.P., Plass C., Morrison C. RASSF1A promoter methylation and KRAS2 mutations in non-small cell lung cancer. Neoplasia. 2003;5:362–366. doi: 10.1016/S1476-5586(03)80029-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sekido Y., Fong K.M., Minna J.D. Progress in understanding the molecular pathogenesis of human lung cancer. Biochim. Biophys. Acta. 1998;1378:F21–F59. doi: 10.1016/s0304-419x(98)00010-9. [DOI] [PubMed] [Google Scholar]
- 59.Toyooka S., Toyooka K.O., Maruyama R., Virmani A.K., Girard L., Miyajima K. DNA methylation profiles of lung tumors. Mol. Canc. Therapeut. 2001;1:61–67. [PubMed] [Google Scholar]
- 60.Belinsky S.A., Nikula K.J., Palmisano W.A., Michels R., Saccomanno G., Gabrielson E. Aberrant methylation of p16INK4a is an early event in lung cancer and a potential biomarker for early diagnosis. Proc. Natl. Acad. Sci. 1998;95:11891–11896. doi: 10.1073/pnas.95.20.11891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oh M.H., Lockwood W.W. RASSF1A methylation, YAP1 activation and metastasis: a new role for an old foe in lung cancer. J. Thorac. Dis. 2017;9(5):1165–1167. doi: 10.21037/jtd.2017.04.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vertino P.M., Yen R.W., Gao J., Baylin S.B. De novo methylation of CpG island sequences in human fibroblasts overexpressing DNA (cytosine-5-) methyltransferase. Mol. Cell Biol. 1996;16:4555–4565. doi: 10.1128/mcb.16.8.4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Buckingham L., Penfield-Faber L., Kim A., Liptay M., Barger C., Basu S., Coon J. PTEN, RASSF1 and DAPK site-specific hypermethylation and outcome in surgically treated stage I and II non-small cell lung cancer patients. Int. J. Canc. 2010;126(7):1630–1639. doi: 10.1002/ijc.24896. [DOI] [PubMed] [Google Scholar]
- 64.Han J.C., Xu F., Chen N., Qi G.B., Wei Y.J., Li H.B., Li X.F. Promoter methylations of RASSF1A and p16 is associated with clinicopathological features in lung cancers. J. Canc. Res. Therapeut. 2016;12(1):340–349. doi: 10.4103/0973-1482.154926. [DOI] [PubMed] [Google Scholar]
- 65.Huang T., Chen X., Hong Q., Deng Z., Ma H., Xin Y., Ye M. Meta-analyses of gene methylation and smoking behavior in non-small cell lung cancer patients. Sci. Rep. 2015;5:8897–8902. doi: 10.1038/srep08897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tomizawa Y., Kohno T., Kondo H., Otsuka A., Nishioka M., Niki T., Yamada T., Maeshima A., Yoshimura K., Saito R., Minna J.D., Yokota J. NSCLCs with KRAS2 Mutations and RASSF1A Methylation Li. Clinicopathological significance of epigenetic inactivation of RASSF1A at 3p21.3 in stage I lung adenocarcinoma. Clin. Canc. Res. 2002;8:2362–2368. [PubMed] [Google Scholar]