Abstract
Background
Delirium is a very common condition associated with significant morbidity, mortality, and costs. Current critical care guidelines recommend first and foremost the use of nonpharmacological strategies in both the prevention and treatment of delirium. Pharmacological interventions may augment these approaches and they are currently used widely in clinical practice to manage the symptoms of delirium. Benzodiazepines are currently used in clinical practice to treat behavioural disturbances associated with delirium but current guidelines do not recommend their use for this indication. The use of these medicines is controversial because there is uncertainty about whether they are effective for patients or have the potential to harm them.
Objectives
To assess the effectiveness and safety of benzodiazepines in the treatment of delirium (excluding delirium related to withdrawal from alcohol or benzodiazepines) in any healthcare settings other than intensive care units (ICU).
Search methods
We searched ALOIS: the Cochrane Dementia and Cognitive Improvement Group's Specialized Register up to 10 April 2019. ALOIS contains records of clinical trials identified from monthly searches of a number of major healthcare databases (including MEDLINE, Embase, PsycINFO, CINAHL, LILACS), numerous trial registries (including national, international and pharmaceutical registries), and grey literature sources.
Selection criteria
We included randomised controlled trials (RCTs) conducted in healthcare settings that ranged from nursing homes and long‐term care facilities to any hospital setting except for ICUs, involving adult patients with delirium excluding those with delirium related to alcohol or benzodiazepine withdrawal. Included RCTs had to assess the effect of benzodiazepines, at any dose and given by any route, compared with placebo or another drug intended to treat delirium.
Data collection and analysis
Two review authors independently assessed study eligibility, extracted data, and assessed the risk of bias of included studies. We decided whether or not to pool data on the basis of clinical heterogeneity between studies. We used GRADE (Grades of Recommendation, Assessment, Development and Evaluation) methods to assess the quality of evidence.
Main results
We identified only two trials that satisfied the selection criteria. We did not pool the data because of the substantial clinical differences between the trials.
In one trial, participants (n = 58) were patients in an acute palliative care unit with advanced cancer who had a mean age of 64 years. All of the participants had delirium, were treated with haloperidol, and were randomised to receive either lorazepam or placebo in combination with it. Due to very serious imprecision, all evidence was of low certainty. We were unable to determine whether there were clinically important differences in the severity of delirium (mean difference (MD) 2.10, 95% CI ‐0.96 to 5.16; n = 50), length of hospital admission (MD 0.00, 95% CI ‐3.45 to 3.45; n = 58), mortality from all causes (risk ratio (RR) 0.33, 95% CI 0.04 to 3.02; participants = 58) or any of a number of adverse events. Important effects could not be confirmed or excluded. The study authors did not report the length of the delirium episode.
In the other trial, participants (n = 30) were patients in general medical wards with acquired immune deficiency syndrome (AIDS) who had a mean age of 39.2 years. Investigators compared three drug treatments: all participants had delirium, and were randomised to receive lorazepam, chlorpromazine, or haloperidol. Very low‐certainty evidence was identified, and we could not determine whether lorazepam differed from either of the other treatments in the effect on severity of delirium, any adverse event, or mortality from all causes. The study authors did not report the length of the delirium episode or the length of hospital admission.
Authors' conclusions
There is no enough evidence to determine whether benzodiazepines are effective when used to treat patients with delirium who are cared for in non‐ICU settings. The available evidence does not support their routine use for this indication. Because of the scarcity of data from randomised controlled trials, further research is required to determine whether or not there is a role for benzodiazepines in the treatment of delirium in non‐ICU settings.
Plain language summary
The use of benzodiazepines to treat adults with delirium, excluding patients being cared for in intensive care units (ICU)
Background: Delirium is a serious complication of many illnesses, which occurs most commonly in young children and older adults. It usually presents as a sudden change in a patient's behaviour or mental state. Another name for it is ‘acute confusional state.’ Patients with delirium may not know where they are, what time it is, or what is happening to them. They may have frightening experiences, such as vivid hallucinations. They may become either restless or lethargic and inactive. Delirium can be very distressing for patients and for those who are caring for them. Studies show that about a third of patients on general medical wards develop delirium. It is a frequent complication after surgery (e.g. it happens in up to 60% of people who have surgery for a hip fracture). The effects of delirium can last for a long time. For older people, it can lead to longer hospital stays and it has been associated with increased risks of death, disability, loss of independence, and later dementia. It adds significantly to healthcare costs.
Benzodiazepines are medicines that are often used as sedatives. Sometimes, healthcare workers prescribe them to treat delirium when other strategies have not helped. Currently, it is not clear if benzodiazepines are an effective treatment for patients with delirium or whether they can harm them.
Review question: Patients need more and better treatment options for delirium. We wanted to know if benzodiazepines are a helpful treatment option for delirium in any healthcare setting except ICU (patients in ICU are very sick and they may need different kinds of treatment). To find the best answer, we looked for studies where the investigators compared any benzodiazepine to another medicine, or to a dummy medicine that does not contain any active ingredients (placebo). To make the comparison fair, patients in the studies must all have had the same random chance (like the flip of a coin) to receive the benzodiazepine or the other treatment.
Search date: We searched the medical literature up to 10 April 2019.
Study characteristics: We found only two small studies which were suitable to include in our review. In one study, the 58 patients who took part all had advanced cancer. They were treated in a specialist palliative care unit. The study compared lorazepam (a benzodiazepine) to placebo. In the second study, the 30 patients all had AIDS (acquired immune deficiency syndrome). They were treated in general medical wards. This study compared lorazepam to two different drugs that are sometimes used to treat delirium.
Key results: We did not find any important benefits for patients who took lorazepam instead of the other treatment in these two studies. Patients who took it did not have better outcomes. We do not have any definite evidence that lorazepam was more harmful than the other treatment, but, in the study of patients with AIDS, the researchers stopped treating people with lorazepam after the first six people who took it all had serious side effects. Because there were only two suitable studies and both had small numbers of patients in them, we cannot draw any firm conclusions. Currently, there is no good evidence to tell us whether or not benzodiazepines should be used to treat patients with delirium. Clinicians, patients, and carers should be aware of the lack of evidence. We think there is a need for more research, and particularly for studies that involve older patients in general medical and surgical settings, where most delirium is treated.
Summary of findings
Background
Description of the condition
Delirium is a clinical syndrome characterised by the rapid onset of fluctuating confusion, inattention and reduced awareness of the environment, with an underlying organic or metabolic cause. Different areas of cognition can be affected, e.g. memory, orientation, language, and perception (APA 2013). There are three major types of delirium: hypoactive, hyperactive, and mixed. Hypoactive delirium is characterised by decreased responsiveness, withdrawal, and apathy, whereas hyperactive delirium is characterised by agitation, restlessness, and emotional lability (excessive emotional reactivity associated with frequent changes or swings in emotions and mood) (Meagher 2000). Delirium occurs across healthcare settings and populations, but is especially common in medical and surgical patients, with even higher rates in intensive care units (ICUs) and palliative care services. A systematic review by Siddiqi 2006 found delirium was present in 10% to 30% of general hospital admissions, rising to over 33% among general medical patients. Following coronary artery bypass grafting in the elderly, the incidence has been reported as 33.6% (Santos 2004), and following hip fracture the overall prevalence is 43% to 61% (Holmes 2000).
The diagnosis of delirium is usually based on observation of the patient and on information obtained from the nursing staff or caregivers. The American Psychiatric Association recommends that delirium assessment in clinical practice is best achieved when medical diagnosis is supplemented with observational assessment tools (Maldonado 2008). More than 24 delirium instruments have been used in published studies (Inouye 2014). The Diagnostic and Statistical Manual of Mental Disorders (DSM) criteria remain the diagnostic gold standard for a diagnosis of delirium (Radtke 2008).
Risk factors for delirium include older age, pre‐existing cognitive impairment, major surgery, disruption of the circadian rhythm, malnutrition, sleep deprivation, social isolation, physical restraint, dehydration, sensory deprivation, and use of certain medications (NICE 2010). The mechanisms and risk factors for delirium differ between ICU and non‐ICU patients. ICU patients have a greater number of risk factors for delirium (e.g. sedatives and analgesics to facilitate mechanical ventilation (Girard 2009; Pandharipande 2006; Pandharipande 2008). However, age is a stronger predictor of delirium in non‐ICU than in ICU patients (Van Rompaey 2008). Hence, the most effective prevention and treatment strategies may differ between ICU and non‐ICU settings.
Delirium has been linked to poor outcomes, including increased hospital mortality and length of stay, leading to a considerable burden on caregivers or healthcare services, a higher likelihood of death, functional disability, and dementia after discharge (Buss 2007; Ely 2004; Leslie 2008; Lin 2004; Milbrandt 2004; Pisani 2009; Shankar 2014). Among non‐ICU patients, hyperactive delirium has been associated with a better prognosis than hypoactive delirium (O'Keeffe 1999).
It is important to try to prevent delirium by addressing modifiable risk factors. A recent Cochrane Review of interventions to prevent delirium in non‐ICU settings found evidence, based on a meta‐analysis of seven randomised controlled trials (RCTs), that nonpharmacological, multi‐component interventions can reduce delirium incidence, with an overall reduction in the risk of delirium of about 30% compared with usual care (Siddiqi 2016). Once delirium is established, its management should address both the underlying causes and the symptoms. Identification and treatment of the precipitating cause is of prime importance because treatment and reversal of that cause will help in early resolution of delirium, leading to a better outcome (Meagher 2011). Current critical care guidelines recommend, first and foremost, the use of nonpharmacological strategies in both the prevention and treatment of delirium (Barr 2013). Nonpharmacological approaches involve addressing multiple risk factors in a systematic manner together with education and environmental manipulation. They typically involve a multidisciplinary team of nurses, therapists, trained volunteers, and geriatricians. Nonpharmacological strategies for preventing and treating delirium may include: early mobilisation and re‐orientation of the patient; ensuring effective communication and considering involving family, friends, and carers to help with this; engagement in social activities; normalisation of the sleep‐wake cycle; establishment of a good diet and hydration; and adequate oxygen delivery (Bucerius 2004; NICE 2010; O'Mahony 2011; Siddiqi 2007).
Pharmacological interventions may augment these approaches and they are currently used widely in clinical practice to manage the symptoms of delirium. However, the evidence to support this is limited and practice varies. Medications currently used in clinical practice are mainly benzodiazepines and antipsychotic drugs (AGS 2015; Young 2010), but their use is controversial because of the lack of evidence of their effectiveness and potential for harm (Neufeld 2016; Schrijver 2015; Siddiqi 2016). Current guidelines from the National Institute for Health and Care Excellence (NICE) do not support use of benzodiazepines because of an absence of evidence (NICE 2010). This was also the conclusion of an earlier systematic review, which found no adequately controlled trials to support the use of benzodiazepines in the treatment of delirium not related to alcohol withdrawal in hospitalised patients (Lonergan 2009). A recent meta‐analysis found that antipsychotic medications were effective for the treatment of delirium in ICU or non‐ICU patients (Kishi 2016). The Clinical Practice Guideline for Postoperative Delirium in Older Adults recommends that antipsychotics are used at the lowest effective dose for the shortest possible duration to treat patients who are severely agitated or distressed, and are threatening substantial harm to self or others, or both. It also recommends that, in these circumstances, benzodiazepines should not be used as a first‐line treatment, except when they are specifically indicated (including, but not limited to, treatment of alcohol or benzodiazepine withdrawal) (AGS 2015). Some reports have stated that benzodiazepines may actually contribute to the development of delirium in ICU patients (Barr 2013; Pandharipande 2006). Current guidelines also associate use of benzodiazepines with increased postoperative delirium (AGS 2015).
Description of the intervention
Benzodiazepines are a class of psychoactive drugs that enhance the effect of the neurotransmitter (a chemical substance, such as norepinephrine, acetylcholine, or dopamine, that transmits nerve impulses across a synapse) gamma‐aminobutyric acid (GABA) at the GABA‐A receptor, resulting in sedative, hypnotic (sleep‐inducing), anxiolytic (anti‐anxiety), anticonvulsant, and muscle relaxant effects. They are used for the treatment of anxiety disorders, sleep disorders, and seizures (Dold 2012). They are also recommended for controlling severely agitated behaviour in the hospital emergency department or in psychiatric inpatient settings, where evidence suggests that they are at least as effective as antipsychotic drugs (NICE 2005). Benzodiazepines have been effective in treating delirium due to alcohol withdrawal (Mayo‐Smith 1997). One systematic review reported that benzodiazepines exercised a protective function against alcohol withdrawal symptoms, but their efficacy for non‐alcohol withdrawal‐related delirium has not been established (Amato 2010).
Most benzodiazepines are administered orally; however, they can also be given intravenously, intramuscularly, or rectally. The benzodiazepine family is large and includes drugs with different metabolic characteristics. Benzodiazepines may be categorised as short‐, intermediate‐, or long‐acting (e.g. short‐acting with an elimination half‐life of less than six hours and long‐acting with an elimination half‐life of more than 24 hours) (Dold 2012). Long‐acting benzodiazepines or those with long‐acting active metabolites, such as diazepam and chlordiazepoxide, are often prescribed for alcohol withdrawal or for anxiety, where constant dose levels are required throughout the day. Short‐acting and intermediate‐acting benzodiazepines are often preferred for treatment of insomnia (Page 2002; Shorter 2005).
The adverse effects experienced most frequently are drowsiness, dizziness, and problems with concentration. 'Paradoxical effects' may occur, including irritability, impulsivity, and seizures. Respiratory depression is a rare but very severe adverse effect of benzodiazepines in short‐term treatment (Dold 2012; Woods 1992). Importantly, benzodiazepines themselves can actually cause or worsen delirium. For example, benzodiazepine use may be a risk factor for the development of delirium in adult ICU patients (Barr 2013).
How the intervention might work
The mechanism of action of benzodiazepine mainly involves enhancement of the effect of the inhibiting neurotransmitter GABA, which results in sedative, anti‐anxiety effects. The usefulness of benzodiazepines in the management of symptoms of delirium may be greatest in those patients who require significant sedation, are undergoing alcohol or benzodiazepine withdrawal, or where antipsychotics are contraindicated (e.g. in Parkinson's disease or neuroleptic malignant syndrome ‐ a rare but dramatic condition that occurs in severely ill patients being treated with high‐potency antipsychotics (neuroleptics); symptoms include diaphoresis, muscle rigidity, and hyperpyrexia) (Inouye 2006; Kostas 2013).
Why it is important to do this review
Delirium is a very common condition associated with significant morbidity, mortality, and costs. There is uncertainty about the efficacy of pharmacological treatment strategies. Benzodiazepines have been effective in treating delirium due to alcohol withdrawal and, in practice, are prescribed for patients with delirium due to other causes. This Cochrane Review aims to find the best evidence related to the efficacy and safety of benzodiazepines for the treatment of non‐alcohol withdrawal‐related delirium in non‐ICU settings.
Objectives
To determine the effectiveness and safety of benzodiazepines in the treatment of delirium (excluding delirium related to alcohol or benzodiazepines withdrawal) in all settings other than ICUs.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) and quasi‐RCTs (those in which the method of allocation to treatment is known but is not strictly random, e.g. sequence generated by alternation, date of birth, or case record number), including those that used an open‐label study design.
Types of participants
We included studies of adult patients (aged 18 or older) with delirium due to causes other than benzodiazepine or alcohol withdrawal. The diagnosis of delirium must have been made using any edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM) (APA 1980; APA 1987; APA 1994; APA 2000; APA 2013), or the International Statistical Classification of Diseases and Related Health Problems (ICD)‐10 criteria (WHO 1993), or a diagnostic tool validated against these, e.g. confusion assessment method (CAM) (Inouye 1990), or delirium rating scale (DRS) (Trzepacz 1988). Participants could have been treated in any setting other than ICUs, including medical and surgical wards, palliative care facilities, nursing homes, and other long‐term care facilities.
Types of interventions
We included trials that assessed the effect of benzodiazepines, of any dosage and administered by any route, compared with placebo.
We also included head‐to‐head comparisons of benzodiazepines with another drug intended to treat delirium (e.g. antipsychotic, cholinesterase inhibitor).
Included trials could have involved nonpharmacological management strategies, provided we could extract data from groups that differed only in exposure to benzodiazepines and placebo or other comparator medication.
Types of outcome measures
Primary outcomes
The length of delirium episode (defined as the time from which it was first identified to when it was first resolved, measured in days).
Severity of delirium (we anticipated that this may have been measured differently in different trials. Where possible, we used the highest severity recorded. If this was not available, other measures of severity were used. Symptom severity may have been measured using any validated scale, e.g. the DRS (Trzepacz 1988), the Memorial Delirium Assessment Scale (Breitbart 1997), or the Delirium Index (McCusker 1988)).
Any adverse event (counted as the number of participants who experienced at least one adverse event).
Secondary outcomes
Length of hospital admission.
Mortality from all causes (e.g. 15‐day, 30‐day, and other based on reports by study authors).
Discharge (e.g. to care home).
Readmission to hospital.
Use of physical restraints.
Individual side effects, such as falls and injuries, pressure sores, depression, disinhibition, hypotension, suppressed breathing, nausea and changes in appetite, blurred vision.
Search methods for identification of studies
To identify studies for inclusion, we developed detailed search strategies for each electronic database.
Electronic searches
We searched ALOIS (www.medicine.ox.ac.uk/alois), which is the Cochrane Dementia and Cognitive Improvement Group's Specialized Register on 10 April 2019.
The Information Specialists of the Cochrane Dementia and Cognitive Improvement Group maintain ALOIS, which contains dementia and cognitive improvement studies identified from the following sources.
Monthly searches of a number of major healthcare databases: MEDLINE, Embase, CINAHL, PsycINFO, and LILACS.
Monthly searches of a number of trial registers: the metaRegister of Controlled Trials; the Umin Japan Trial Register; the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) portal (which covers ClinicalTrials.gov; ISRCTN; the Chinese Clinical Trials Register; the German Clinical Trials Register; the Iranian Registry of Clinical Trials; and the Netherlands National Trials Register, plus others).
Quarterly search of the Cochrane Library’s Central Register of Controlled Trials (CENTRAL).
Six‐monthly searches of a number of grey literature sources: ISI Web of Knowledge Conference Proceedings;
We ran additional separate searches in many of the above sources to ensure that the most up‐to‐date results were retrieved. The sources searched and the search strategies used can be seen in Appendix 1.
Searching other resources
We checked the reference lists of all included studies for further potentially eligible studies.
Data collection and analysis
Selection of studies
Two review authors (YL and RZ) independently screened the titles and abstracts of all citations identified by the search strategy, and coded studies as either 'retrieve' or 'do not retrieve'. We obtained the full text of any citation that might have been potentially eligible for inclusion. After we excluded duplicate articles, we independently examined all full‐text articles to identify which met the inclusion criteria. We independently recorded the reason for exclusion of articles after full‐text assessment in a Characteristics of excluded studies table. We resolved disagreements by a consensus meeting between the three review authors (YL, RZ, and JM). We presented the study selection process in a PRISMA diagram (Figure 1).
1.
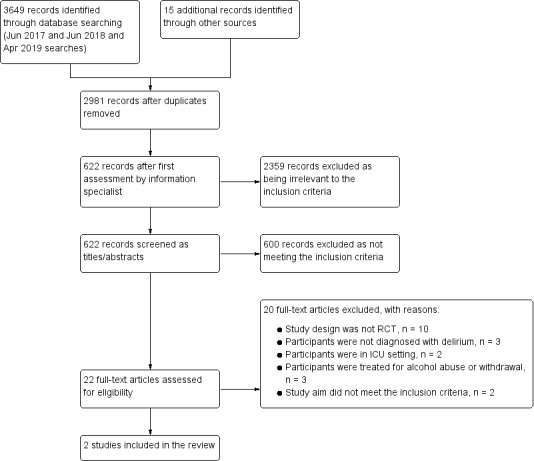
Study flow diagram
Data extraction and management
We used an electronic data extraction form to extract information on source, eligibility, methods, participants, intervention, comparator, outcomes, results, and miscellaneous notes, according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Additionally, we extracted details of the funding source, declarations of interest of the primary investigators, and the methods used to control possible conflicts of interests. Two review authors (NL and WM) pre‐tested the form using two studies. We adapted it thereafter, if necessary.
Two review authors (YL and NL) independently assessed each included study and extracted data. We resolved disagreements by consensus or by involving a third review author (JM). One review author (YL) transferred data into Review Manager 5 (RevMan 5) (RevMan 2014). Another review author (JM) double‐checked that study characteristics and outcome data were entered correctly by comparing the data presented in the systematic review with the study reports.
Assessment of risk of bias in included studies
Two review authors (YL and NL) independently examined the methodological quality of the included trials using the criteria as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved disagreements by discussion. We consulted a third review author (JM) to make a final consensus decision.
We assessed the risk of bias separately for different domains, including the following.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessors.
Incomplete outcome data.
Selective reporting.
Other biases.
We classified the risk of bias in each domain as either low, high, or unclear risk of bias and also assigned an overall risk of bias to each study.
Low risk: describes studies where all domains are considered to be at low risk of bias.
High risk: describes studies where one or more domains are considered to be at high risk of bias.
Unclear risk: describes studies where one or more domain(s) have unclear risk of bias.
Upon completion of the 'Risk of bias' assessments, we generated a 'Risk of bias' graph and 'Risk of bias' summary figure using RevMan 5 (RevMan 2014).
Measures of treatment effect
We used risk ratios (RRs) with 95% confidence intervals (CIs) as measures of treatment effect for dichotomous outcomes. We expressed findings for continuous outcomes in terms of mean differences (MDs) and 95% CI or standardised mean differences (SMDs) for continuous outcomes if the included studies used different scales to measure the same outcome. We planned to use the hazard ratio (HR) for time‐to‐event data.
Unit of analysis issues
Individual participants were the unit of analysis. One trial (Breitbart 1996) had two active comparators. In this case, we compared the benzodiazepine to each comparator intervention separately (see Differences between protocol and review).
Dealing with missing data
As far as possible, we tried to analyse data on an intention‐to‐treat basis in which all randomised participants were analysed in the groups to which they were originally assigned. If the authors of the primary study had imputed missing data, then we planned to analyse the imputation method to establish if it was likely to lead to serious bias. If fewer than 50% of the data had been imputed, we would present and use these data and report the imputation method used. When assessing the risk of bias due to missing data, we considered the amount of missing data, its distribution across intervention groups, and its causes.
Assessment of heterogeneity
We explored the clinical heterogeneity across studies based on differences in the characteristics of participants, interventions, comparators and outcomes. In addition, we looked for diversity across studies regarding variability of study design, risk of bias, or methods and frequency of rating delirium. Where we considered data suitable for pooling, we planned to evaluate statistical heterogeneity using the I2 statistic and the Chi2 test of homogeneity with P < 0.05 indicative of heterogeneity.
Assessment of reporting biases
We did not use a funnel plot to assess publication bias because of the insufficient number of studies.
Data synthesis
We conducted separate data analyses for comparisons of benzodiazepines with placebo and with other drugs individually. We were unable to conduct any meta‐analyses because no comparison was made in more than one study.
Subgroup analysis and investigation of heterogeneity
Data were available for two studies that were not combined in a meta‐analysis. As a result, investigations of heterogeneity and subgroup analysis were not feasible.
Sensitivity analysis
We planned to perform sensitivity analyses to test the robustness of our conclusions throughout the review process by performing the following:
1. Excluding trials at high risk of bias on any one domain;
2. Contrasting the pooled effects between studies that used different validated scales, where SMD was used for continuous outcomes.
However, a sensitivity analysis was not feasible due to insufficient data.
'Summary of findings' table
We used the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach to assess the certainty of the supporting evidence behind each estimate of treatment effect (Schünemann 2011). Certainty is defined as the degree of confidence that can be placed in the estimates of treatment benefits and harms. There are four possible ratings: high, moderate, low, and very low. Rating evidence as high‐certainty implies that we are very confident that the true effect lies close to that of the estimate of the effect. A rating of very low‐certainty implies that we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. The GRADE approach rates evidence from RCTs that do not have serious limitations as high‐certainty. However, several factors can lead to the downgrading of the evidence to moderate, low, or very low. The degree of downgrading is determined by the seriousness of these factors: study limitations (risk of bias); inconsistency; indirectness of evidence; imprecision; and publication bias (Guyatt 2008; Higgins 2011). We presented the following results in the 'Summary of findings' tables, which were created using GRADEproGDT software (GRADEpro GDT 2014).
Length of delirium episode
Severity of delirium
Any adverse event
Length of hospital admission
Mortality from all causes
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
The results of the search are outlined in a PRISMA diagram (Figure 1). The initial search resulted in the identification of a total of 3649 references from databases. We identified 15 additional references through other sources. After de‐duplication, 2981 unique references remained. A total of 2359 references were excluded during a first assessment performed by the Cochrane Dementia and Cognitive Improvement Group information specialist. We excluded an additional 600 references upon inspection of the titles and abstracts. We retrieved 22 full‐text articles describing 22 studies, 20 of which were excluded (see Excluded studies), leaving two eligible studies for inclusion (see Included studies). We identified no eligible ongoing trials.
Included studies
In this review, we included two randomised controlled trials (not industry‐funded), with a total of 88 participants, which were published in 1996 (Breitbart 1996) and 2017 (Hui 2017) and which were conducted in New York and Texas USA, respectively (Characteristics of included studies).
Participants
In Breitbart 1996, the 30 participants were medically hospitalised patients with a diagnosis of acquired immune deficiency syndrome (AIDS). All participants also had delirium according to DSM‐III‐R criteria, with a score of 13 or above on the DRS. The participants had a mean age of 39.2 years (SD = 8.8, range = 23 to 56). Patients in this trial had multiple medical complications, and the context and course of the delirium episode varied between patients. The most common comorbidities were haematologic and metabolic disorders and infectious diseases; there were no differences between the groups in the number of medical complications or in the severity of these complications. There were also no clear differences between the groups in terms of age, education, or Karnofsky scores (estimates of functional ability, ranging from the ability to perform normal activities to total dependence).
In Hui 2017, the 58 participants were in an acute palliative care unit due to advanced cancer, had a DSM‐IV‐TR diagnosis of delirium, and were receiving scheduled treatment with haloperidol. The mean age of the participants was 65 years (range 43 to 90) in the lorazepam group and 63 years (range 30 to 88) in the placebo group. Participants’ mean score was 1.6 points (SD = 0.6) on the Richmond Agitation‐Sedation Scale (RASS) prior to medication administration, meaning that the participants in this trial all had hyperactive or mixed delirium. All participants were receiving scheduled haloperidol dosages of 1 mg to 8 mg per day. Haloperidol was initiated at a dosage of 2 mg intravenously every four hours with an additional 2 mg every hour as needed for agitation. Participants who developed an episode of agitation requiring rescue medication were randomised to receive lorazepam or placebo as an adjuvant to haloperidol.
Interventions
In Breitbart 1996, hospitalised patients diagnosed with AIDS were assigned to treatment with haloperidol, chlorpromazine, or lorazepam, administered either orally or intramuscularly. The initial dosages were haloperidol 0.25 mg/hour orally or 0.125 mg/hour intramuscularly, chlorpromazine 10 mg/hour orally or 5 mg/hour intramuscularly, and lorazepam 0.50 mg/hour orally or 0.20 mg/hour intramuscularly. Each participant with delirium was evaluated hourly with the DRS and the Extrapyramidal Symptom Rating Scale. If the participant's score on the DRS was still 13 or greater, the next level dose of the study drug was administered. If the participant was asleep, calm, and not hallucinating or had scored 12 or below on the DRS, a maintenance dose was started on day 2, which was equal to one‐half of the first 24‐hour dose requirement and was given twice a day. The treatment protocol was up to 6 days. The study authors became 'sufficiently concerned with the occurrence of adverse effects' to terminate the lorazepam arm of the trial before the trial was complete. Participants recruited subsequently were randomised to either the haloperidol or chlorpromazine treatment groups.
In Hui 2017, patients with advanced cancer were randomised to receive either lorazepam or placebo in the event of an episode of agitation, defined as a Richmond Agitation‐Sedation Scale (RASS) score of 2 or more over the past 24 hours, despite receiving scheduled haloperidol. A single dose of 3 mg of lorazepam in 25 mL of 0.9% normal saline solution or identically appearing placebo was infused intravenously over 1.5 minutes. Participants in both groups also received 2 mg of haloperidol intravenously immediately afterwards. The timing of the primary outcome was 8 hours from when the blinded study medication was administered. The median overall survival was 73 hours (95% CI 49 to 106 hours), with a median follow‐up of 164 hours (95% CI 92 to 195 hours).
Outcomes
Both trials reported some of our outcomes of interest. Breitbart 1996 monitored efficacy using the DRS, cognitive status based on the Mini‐Mental State Examination (MMSE), and early terminations and side effects, including oversedation, disinhibition, ataxia, increased confusion and extrapyramidal side effects measured with the Extrapyramidal Symptom Rating Scale (ESRS) at baseline, on day 2 and at the end of treatment. In Hui 2017, the severity of delirium was assessed with the Memorial Delirium Assessment Scale (MDAS) at baseline, 2, 4, and 8 hours. This trial also reported several secondary outcomes included in this review, including length of hospital admission, mortality from all causes, number of participants discharged alive from the acute palliative care unit, and individual side effects assessed by the Udvalg for Kliniske Undersøgelser (UKU) or the Edmonton Symptom Assessment System (ESAS).
Excluded studies
We excluded 20 studies (see the Characteristics of excluded studies tables):
Ten studies were not randomised controlled trials (Chantelau 1980; Gouffier 1980; Menza 1988; Mittal 2017; Pourcher 1994; Sobcyzk 1980; Sroczynski 1974; Stachowiak 1981; Toselli 1969; Wasilewski 1995).
In three studies, the participants were not diagnosed with delirium (Ferraz Gonçalves 2016; Modell 1985; Yu 2017).
In two studies, the participants were in an ICU setting (Linev 2017; Thompson 1975)
In three studies, the participants were treated for alcohol abuse or withdrawal (Brown 1972; Kramp 1978; Welbel 1982)
The other two studies did not meet the inclusion criteria of this review: Rydzyński 1979 was a research study on drug mechanism of action; Tahir 2012 was a letter to the editor commenting on an article that was not relevant to our review.
Risk of bias in included studies
For details of the 'Risk of bias' assessments for each study and the reasons for each rating, please see the Characteristics of included studies tables. A summary of our 'Risk of bias' judgments by study and domain (sequence generation, allocation concealment, blinding, incomplete data, and selective reporting) is presented in Figure 2 and Figure 3.
2.
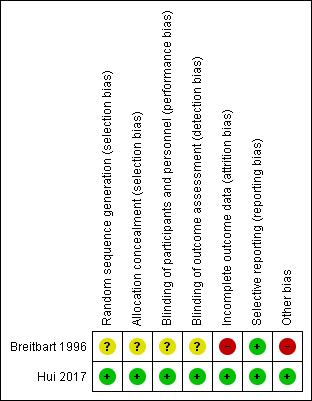
Figure 2: Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Breitbart 1996 did not provide any detailed information about the randomisation method or allocation concealment and was rated as unclear risk of selection bias.
Hui 2017 used a web‐based simple randomisation procedure and allocation was concealed by using a secured web site that was only accessible to the study pharmacist. We therefore considered the risk of selection bias to be low.
Blinding
Breitbart 1996 stated that the trial was "double‐blind". However, we did not find detailed information describing which participants were blinded or the manner in which they were blinded. Therefore, we rated this trial as being at unclear risk of performance and detection bias. Hui 2017 reported that the research staff conducting study assessments, bedside nurses, attending physicians, participants, and caregivers were blinded to the allocation of the study medication and study outcomes. Furthermore, to ensure proper blinding, the study also reported that a separate clinical nurse administered the study medication rather than the bedside nurse who conducted the RASS score assessment. Therefore, we considered this trial to be at low risk of performance and detection bias.
Incomplete outcome data
Breitbart 1996 reported that five participants died (two in the haloperidol group, two in the chlorpromazine group, one in the lorazepam group) within eight days of initiation of the protocol, without reporting the cause of death. The authors reported that some cases of delirium occurred within the context of major organ system failure or during the dying process, which limited the effectiveness of treatment. In addition, due to the different attrition numbers from an already limited number of trial subjects, the study was rated at high risk of attrition bias.
Hui 2017 reported that a modified intention‐to‐treat analysis including only participants who had begun the study intervention was specified a priori because of the nature of the study population. The trial transparently reported the numbers of participants leaving the study as well as the reasons (10.3% were lost to follow‐up prior to completion of administration of the study medication). Outcome data were missing for both intervention groups, and the reasons for these absent data were reported and balanced across the groups. Therefore, we considered this trial to be at low risk of attrition bias.
Selective reporting
We rated both trials as having a low risk of bias on this domain because both presented all outcomes specified in the methods sections of the paper. We also checked the outcomes reported by Hui 2017 against the trial registry entry (https://www.clinicaltrials.gov/ct2/show/NCT01949662?term=NCT01949662&rank=1#outcome measures).
Other potential sources of bias
The randomisation procedure of Breitbart 1996 changed midway through the study. Namely, the lorazepam group was removed from the study due to treatment‐limiting adverse side effects. Therefore, we considered this trial to be at high risk of other bias.
Hui 2017 declared that the authors had no conflicts of interest. We did not find any other obvious bias and therefore rated this domain at low risk of bias.
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings for the main comparison. Benzodiazepines (lorazepam) vs placebo for treatment of delirium in non‐ICU settings.
| Benzodiazepines (lorazepam) vs placebo for treatment of delirium in non‐ICU settings | ||||||
| Patient or population: end‐of‐life patients with advanced cancer and an episode of agitated delirium Settings: acute palliative care unit Intervention: benzodiazepine (lorazepam) + haloperidol Comparison: placebo + haloperidol | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with Placebo | Risk with Benzodiazepines (lorazepam) | |||||
| Length of delirium episode | See comment | See comment | See comment | See comment | See comment | Outcome not reported |
| Severity of delirium change in MDAS, high = worse Follow‐up: baseline to 8 hours | Mean was 0.4 | MD 2.10 higher (0.96 lower to 5.16 higher) | ‐ | 50 (1 study) | ⊕⊕⊝⊝ low1 | |
| Any adverse event | See comment | See comment | See comment | See comment | See comment | Outcome not reported |
| Length of hospital admission (days) Follow‐up: 164 hours | Mean was 6 | MD 0.00 higher (3.45 lower to 3.45 higher) | ‐ | 58 (1 study) | ⊕⊕⊝⊝ low1 | |
| Mortality from all causes Follow‐up: 8 hours | 103 per 1000 |
34 per 1000 (4 to 312) |
RR 0.33 (0.04 to 3.02) |
58 (1 study) | ⊕⊕⊝⊝ low1 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; ESAS: Edmonton symptom assessment system ICU: intensive care unit; MD: mean difference; MDAS: memorial delirium assessment scale; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgraded by two levels due to imprecision: very small sample size and very wide CIs
Summary of findings 2. Benzodiazepines (lorazepam) vs antipsychotics (chlorpromazine) for treatment of delirium in non‐ICU settings.
| Benzodiazepines (lorazepam) vs antipsychotics (chlorpromazine) for treatment of delirium in non‐ICU settings | ||||||
| Patient or population: adult AIDS patients with delirium Settings: general medical wards Intervention: benzodiazepine (lorazepam) Comparison: antipsychotic (chlorpromazine) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with antipsychotics (chlorpromazine) | Risk with Benzodiazepines (lorazepam) | |||||
| Length of delirium episode | See comment | See comment | See comment | See comment | See comment | Outcome not reported |
| Severity of delirium DRS, high = worse Follow‐up: end of treatment | Mean was 11.85 | MD 5.15 higher (0.26 lower to 10.56 higher) | ‐ | 19 (1 study) | ⊕⊝⊝⊝ very low1,2 | |
|
Any adverse event
ESRS, higher = worse Follow‐up: baseline to maintain (no details reported) |
Mean was 5.08 | MD 7.12 higher (0.43 lower to 14.67 higher) | ‐ | 19 (1 study) | ⊕⊝⊝⊝ very low1,2 | ESRS: subjective questionnaire of parkinsonian symptoms; an objective examination of parkinsonism, dystonia, and dyskinetic movements; and a clinical global impression of tardive dyskinesia. The objective parkinsonism subscale includes ratings of tremor, akathisia, expressive automatic movements, bradykinesia, rigidity, gait and posture, and sialorrhoea. The maximum total score for this subscale is 108. |
| Length of hospital admission | See comment | See comment | See comment | See comment | See comment | No studies reported this outcome, so there is no evidence to support or refute benefits of the intervention. |
| Mortality from all causes Follow‐up: 1 week after completing the protocol | 385 per 1000 |
335 per 1000 (88 to 1000) |
RR 0.87 (0.23 to 3.26) |
19 (1 study) | ⊕⊝⊝⊝ very low1,2 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AIDS: acquired immune deficiency syndrome; CI: confidence interval; DRS: delirium rating scale; ESRS: extrapyramidal symptom rating scale; ICU: intensive care unit; MD: mean difference; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgraded by one level due to risk of bias: high risk for attrition and other bias
2 Downgraded by two levels due to imprecision: very small sample size and wide CIs
Summary of findings 3. Benzodiazepines (lorazepam) vs antipsychotics (haloperidol) for treatment of delirium in non‐ICU settings.
| Benzodiazepines (lorazepam) vs antipsychotics (haloperidol) for treatment of delirium in non‐ICU settings | ||||||
| Patient or population: adult AIDS patients with delirium Settings: general medical wards Intervention: benzodiazepine (lorazepam) Comparison: antipsychotic (haloperidol) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with antipsychotics (haloperidol) | Risk with Benzodiazepines (lorazepam) | |||||
| Length of delirium episode | See comment | See comment | See comment | See comment | See comment | Outcome not reported |
| Severity of delirium DRS, higher = worse Follow‐up: end of treatment | Mean was 11.64 | MD 5.36 higher (0.01 lower to 10.73 higher) | ‐ | 17 (1 study) | ⊕⊝⊝⊝ very low1,2 | |
|
Any adverse event
ESRS, higher = worse Follow‐up: baseline to maintain (no details reported) |
Mean was5.54 | MD 6.66 higher (1.53 lower to 14.85 higher) | ‐ | 17 (1 study) | ⊕⊝⊝⊝ very low1,2 | ESRS: subjective questionnaire of parkinsonian symptoms; an objective examination of parkinsonism, dystonia, and dyskinetic movements; and a clinical global impression of tardive dyskinesia. The objective parkinsonism subscale includes ratings of tremor, akathisia, expressive automatic movements, bradykinesia, rigidity, gait and posture, and sialorrhoea. The maximum total score for this subscale is 108. |
| Length of hospital admission | See comment | See comment | See comment | See comment | See comment | No studies reported this outcome, so there is no evidence to support or refute benefits of the intervention. |
| Mortality from all causes Follow‐up: 1 week after completing the protocol | 182 per 1000 |
333 per 1000 (62 to 1000) |
RR 1.83 (0.34 to 9.92) |
17 (1 study) | ⊕⊝⊝⊝ very low1,2 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AIDS: acquired immune deficiency syndrome CI: confidence interval; DRS: delirium rating scale; ICU: intensive care unit; MD: mean difference; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgraded by one level due to risk of bias: high risk for attrition and other bias
2 Downgraded by two levels due to imprecision: very small sample size and wide CIs
1. Benzodiazepines (lorazepam) versus placebo
Only one study provided data for this comparison (Hui 2017). The study was conducted in a very specific patient population (patients with advanced cancer receiving terminal care). Using GRADE methods, we considered the certainty of evidence for all outcomes to low due to very serious concern about imprecision (the sample size was very small and confidence intervals around all results were wide) (see Table 1).
Participants began the study receiving haloperidol and were subsequently randomised to either lorazepam or placebo, administered in addition to haloperidol.
1.1 Severity of delirium (assessed with MDAS, higher = worse)
Due to imprecision in the result, we are unable to determine whether there is a clinically important difference between groups in this outcome measured within a time period of eight hours (MD 2.10, 95% CI ‐0.96 to 5.16; participants = 50; Analysis 1.1; low‐certainty evidence).
1.1. Analysis.
Comparison 1 Benzodiazepines (lorazepam) vs placebo, Outcome 1 Severity of delirium (assessed by MDAS, higher = worse).
1.2 Length of hospital admission (days)
Due to imprecision in the result, we are unable to determine whether there is a clinically important difference between groups in this outcome (MD 0.00, 95% CI ‐3.45 to 3.45; participants = 58; Analysis 1.2; low‐certainty evidence).
1.2. Analysis.
Comparison 1 Benzodiazepines (lorazepam) vs placebo, Outcome 2 Length of hospital admission (days).
1.3 Mortality from all causes
Due to imprecision in the result, we are unable to determine whether there is a clinically important difference in this outcome measured within a time period of eight hours (RR 0.33, 95% CI 0.04 to 3.02; participants = 58; Analysis 1.3; low‐certainty evidence).
1.3. Analysis.
Comparison 1 Benzodiazepines (lorazepam) vs placebo, Outcome 3 Mortality from all causes.
1.4 Discharged (alive from the acute palliative care unit)
Due to imprecision in the result, we are unable to determine whether there is a clinically important difference in this outcome (RR 1.29, 95% CI 0.55 to 2.99; participants = 58; Analysis 1.4; low‐certainty evidence).
1.4. Analysis.
Comparison 1 Benzodiazepines (lorazepam) vs placebo, Outcome 4 Discharged (alive from the acute palliative care unit).
1.5 Individual side effects (measured with UKU, higher = worse)
Eight neurologic symptoms (akathisia, dystonia, epileptic seizures, hyperkinesia, hypokinesia or akinesia, paraesthesia, rigidity, tremor) were documented using the UKU adverse effects rating scale at baseline and on day 3. Each item was assigned a score from 0 (absent) to 3 (most severe) based on symptom severity of the last three days. The number of participants with an increased level of symptom severity on day 3 versus baseline were reported.
No participants reported any worsening of dystonia, epileptic seizure, paraesthesia, rigidity, or tremor in either treatment group. Some participants in both groups reported worsening of akathisia, hyperkinesia and hypokinesia/akinesia, but due to imprecision, we were unable to determine whether there were between‐group differences (akathisia: RR 2.81(95% CI 0.33 to 24.16; participants = 31); hyperkinesia: RR 0.47 (95% CI 0.05 to 4.65; participants = 31); hypokinesia or akinesia: RR 0.70 (95% CI 0.19 to 2.63; participants = 31); all low‐certainty evidence (Analysis 1.5).
1.5. Analysis.
Comparison 1 Benzodiazepines (lorazepam) vs placebo, Outcome 5 Individual side effects (measured with UKU, higher = worse).
1.6 Individual side effects (measured with ESAS, higher = worse)
Individual side effects measured with ESAS were anxiety, appetite, depression, drowsiness, fatigue, feeling of well‐being, nausea, pain, shortness of breath and sleep. For all outcomes, results were imprecise and certainty in effect estimates was low; we were unable to identify any clinically important differences between groups (Analysis 1.6).
1.6. Analysis.
Comparison 1 Benzodiazepines (lorazepam) vs placebo, Outcome 6 Individual side effects (measured with ESAS, higher = worse).
Hui 2017 did not report other outcomes of interest to this review, including the length of the episode of delirium, any adverse event, readmission to the hospital, and use of physical restraints.
2. Benzodiazepines (lorazepam) versus chlorpromazine
Only one study provided data for this comparison (Breitbart 1996). The certainty of evidence for the reported outcomes was very low due to study limitations and imprecision (very small sample size and wide CIs) (see Table 2).
2.1 Severity of delirium (assessed with DRS, higher = worse)
The certainty of the evidence is very low. Therefore, it is uncertain whether there is a difference between groups on this outcome (day two after treatment: MD 5.22, 95% CI 0.35 to 10.09; at the study endpoint: MD 5.15, 95% CI ‐0.26 to 10.56; participants = 19; Analysis 2.1).
2.1. Analysis.
Comparison 2 Benzodiazepines (lorazepam) vs chlorpromazine, Outcome 1 Severity of delirium (assessed by DRS, higher = worse).
2.2 Any adverse events (measured with ESRS, higher = worse)
The certainty of the evidence is very low. Therefore, it is uncertain whether lorazepam and chlorpromazine differ on this outcome (MD 7.12, 95% CI ‐0.43 to 14.67; participants = 19; Analysis 2.2). However, Breitbart 1996 found that all six participants who received lorazepam developed treatment‐limiting side effects, including oversedation, disinhibition, ataxia, and increased confusion, leading to either a refusal to take the drug or a requirement to discontinue the drug.
2.2. Analysis.
Comparison 2 Benzodiazepines (lorazepam) vs chlorpromazine, Outcome 2 Any adverse events (measures with ESRS, higher = worse).
2.3 Mortality from all causes
The certainty of the evidence is very low. Therefore, it is uncertain whether there is a difference between groups on this outcome from the initiation of the protocol to one week after its completion (RR 0.87, 95% CI 0.23 to 3.26; participants = 19; Analysis 2.3).
2.3. Analysis.
Comparison 2 Benzodiazepines (lorazepam) vs chlorpromazine, Outcome 3 Mortality from all causes.
For this comparison, Breitbart 1996 did not report other predefined outcomes in this review, including the length of the episode of delirium, length of hospital admission, discharge, readmission to the hospital, use of physical restraints, and individual side effects.
3. Benzodiazepines (lorazepam) versus haloperidol
Only one study provided data for this comparison (Breitbart 1996). The certainty of evidence for the reported outcomes was very low due to study limitations and imprecision (very small sample size and wide CIs) (see Table 3).
3.1 Severity of delirium (assessed with DRS, higher = worse)
The certainty of the evidence is very low. Therefore, it is uncertain whether there is a difference between groups on this outcome (day two after treatment: MD 4.85, 95% CI 0.03 to 9.67; at the study endpoint: MD 5.36, 95% CI ‐0.01 to 10.73; participants = 17; Analysis 3.1).
3.1. Analysis.
Comparison 3 Benzodiazepines (lorazepam) vs haloperidol, Outcome 1 Severity of delirium (assessed by DRS, higher = worse).
3.2 Any adverse events (measured with ESRS, higher = worse)
The certainty of the evidence is very low. Therefore, it is uncertain whether lorazepam and haloperidol differ on this outcome (MD 6.66, 95% CI ‐1.53 to 14.85; participants = 17; Analysis 3.2). However, Breitbart 1996 found that all six participants who received lorazepam developed treatment‐limiting side effects, including oversedation, disinhibition, ataxia, and increased confusion, leading to either a refusal to take the drug or a requirement to discontinue the drug.
3.2. Analysis.
Comparison 3 Benzodiazepines (lorazepam) vs haloperidol, Outcome 2 Any adverse events (measures with ESRS, higher = worse).
3.3 Mortality from all causes
The certainty of the evidence is very low. Therefore, it is uncertain whether there is a difference between groups on this outcome from the initiation of the protocol to one week after its completion (RR 1.83, 95% CI 0.34 to 9.92; participants = 17; Analysis 3.3).
3.3. Analysis.
Comparison 3 Benzodiazepines (lorazepam) vs haloperidol, Outcome 3 Mortality from all causes.
For this comparison, Breitbart 1996 did not report other predefined outcomes in this review, including the length of an episode of delirium, length of hospital admission, discharge, readmission to the hospital, use of physical restraints, and individual side effects.
Discussion
Summary of main results
We identified two small studies to include in this review. One study (Breitbart 1996) examined the efficacy and side effects of benzodiazepines (lorazepam) for the treatment of delirium in adult participants diagnosed with AIDS. The other study (Hui 2017) focused on drug treatment of delirium in a palliative care setting, comparing lorazepam with the antipsychotics chlorpromazine and haloperidol. Agitated delirium is a common complication encountered in patients undergoing end‐of‐ life care for advanced cancer and the focus of treatment is often the control of symptoms.
Because the two included studies differed in terms of the setting, participants, interventions, scales used to assess outcomes, and study designs (e.g., duration, time points), we did not pool any data, but presented results from each trial separately. The certainty of evidence was rated as low or very low, and there was very serious imprecision associated with effect estimates for all reported outcomes (severity of delirium, any adverse events, length of hospital admission, mortality from all causes, discharges, individual side effects). However, Breitbart 1996 found that all six participants who received lorazepam developed treatment‐limiting side effects, including oversedation, disinhibition, ataxia, and increased confusion, leading to either a refusal to take the drug or a requirement to discontinue the drug.
Overall completeness and applicability of evidence
Delirium occurs in many different patient populations across most healthcare settings. Especially high rates have been identified in patients undergoing palliative care, in elderly patients following coronary artery bypass grafting, and in elderly patients diagnosed with hip fractures. The two included studies in this review were conducted in very specific populations which were not in general medical or surgical settings. In one, the participants were patients with advanced cancer close to the end of life, and in the other, they were adult patients with AIDS (but excluding patients in whom delirium appeared to be part of a terminal event, i.e. death expected within 24 hours). We cannot assume that results would apply to other populations at risk for delirium (such as post‐surgical patients, or geriatric patients). In addition, considering that treatment advances for HIV since the 1990s have changed the course of this disease, our findings are probably not applicable to patients with AIDS today.
Both studies used lorazepam; no other benzodiazepines were evaluated.
Most of our predefined review outcomes were not reported, including the length of the episode of delirium, which was one of our two primary efficacy outcomes.
In summary, the current evidence base is small, incomplete and of very limited applicability.
Quality of the evidence
The certainty of evidence was rated as low or very low across the key outcomes of interest, including the severity of delirium, any adverse event, length of hospital admission, and mortality. Downgrading of the certainty of evidence was mainly due to imprecision (very small sample sizes and very imprecise effect estimates) and, in the case of Breitbart 1996, also due to risks of bias. In addition, the end of treatment time point was not well reported.
Potential biases in the review process
Although we endeavoured to include unpublished studies, we cannot be sure whether other randomised studies have been carried out or whether they have been published or disseminated publicly. However, other potential biases in the review process were minimised. Two review authors independently screened and extracted data using prespecified data extraction forms, a process that is intended to reduce bias in the review process.
Agreements and disagreements with other studies or reviews
Current guidelines do not recommend the use of benzodiazepines for the treatment of delirium (AGS 2015, NICE 2010). The National Comprehensive Cancer Network clinical practice guideline recommends a trial of benzodiazepines in patients whose agitation does adequately respond to haloperidol (Levy 2017). Our findings confirm a lack of evidence to support the administration of benzodiazepines for the treatment of delirium in a non‐ICU setting.
Authors' conclusions
Implications for practice.
There is insufficient or no evidence either to support or reject the routine use of benzodiazepines to treat delirium in a non‐ICU setting. There are no certain benefits and possible harms of lorazepam compared to placebo, chlorpromazine or haloperidol. Clinicians treating patients with benzodiazepines in this context should be aware of the lack of evidence.
Implications for research.
Further research should include older patients in general medical and surgical settings, where delirium is most often treated.
It may be difficult to convince human subjects committees to approve such studies and for participants to consent to random assignment, though well‐conducted and adequately‐powered RCTs of benzodiazepines are the best choices for this area. There is also a need for high‐quality prospective cohort studies of benzodiazepines to treat delirium. Outcome measures should be standardised by creating a core outcome set for delirium treatment research, especially including safety data of specific adverse events, and financial estimates of the costs of delirium to health and social care systems, individuals and families.
Acknowledgements
We thank Sue Marcus, Managing Editor of the Cochrane Dementia and Cognitive Improvement Group (CDCIG); the CDCIG Information Specialists who ran the searches for this Cochrane Review and the peer review editors of the CDCIG for their help in developing this review. Stephanie Sampson and Margueritte Mabry White (MD, Global Community Writer) provided proofreading and editing services for this review.
We would like to thank peer reviewers Kenneth Boockvar and Eamonn Eeles and consumer reviewer Stella O'Brien for their feedback and comments.
Appendices
Appendix 1. Sources searched and search strategies
| Source | Search strategy | Hits retrieved |
| ALOIS (CDCIG specialised register) (http://www.medicine.ox.ac.uk/alois) [Most recent search: 10 April 2019] |
adinazolam or alprazolam or bentazepam or benzodiazepine* or bromazepan or brotizolam or camazepam or chlordiazepoxide or clobazam or clotiazepam or cloxazolam or diazepam or etizolam or flunitrazepam or flurazepam or flutoprazepam or halazepam or ketazolam or loflazepate or loprazolam or lormetazepam or metaclazepam or midazolam or nitrazepam or oxzepam or prazepam or propazepam or ripazepam or serazepine or temazepan or tofisopam or triazolam | Jun 2017: 42 Jun 2018: 0 Apr 2019: 0 |
| CENTRAL (The Cochrane Library) http://crso.cochrane.org [Most recent search: 10 April 2019] |
#1 MESH DESCRIPTOR delirium #2 deliri*:TI,AB,KY #3 ("acute confusion*"):TI,AB,KY #4 ( "acute organic psychosyndrome"):TI,AB,KY #5 ("acute brain syndrome"):TI,AB,KY #6 ("metabolic encephalopathy"):TI,AB,KY #7 ("acute psycho‐organic syndrome"):TI,AB,KY #8 ("clouded state"):TI,AB,KY #9 ("clouding of consciousness"):TI,AB,KY #10 ("exogenous psychosis"):TI,AB,KY #11 ("toxic psychosis"):TI,AB,KY #12 ("toxic confusion"):TI,AB,KY #13 obnubilat*:TI,AB,KY #14 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 #15 MESH DESCRIPTOR benzodiazepines EXPLODE ALL TREES #16 MESH DESCRIPTOR anti‐anxiety agents #17 adinazolam:TI,AB,KY #18 alprazolam:TI,AB,KY #19 bentazepam:TI,AB,KY #20 benzodiazepine*:TI,AB,KY #21 bromazepan:TI,AB,KY #22 brotizolam:TI,AB,KY #23 camazepam:TI,AB,KY #24 chlordiazepoxide:TI,AB,KY #25 clobazam:TI,AB,KY #26 clotiazepam:TI,AB,KY #27 cloxazolam:TI,AB,KY #28 diazepam:TI,AB,KY #29 etizolam:TI,AB,KY #30 flunitrazepam:TI,AB,KY #31 flurazepam:TI,AB,KY #32 flutoprazepam:TI,AB,KY #33 halazepam:TI,AB,KY #34 ketazolam:TI,AB,KY #35 loflazepate:TI,AB,KY #36 loprazolam:TI,AB,KY #37 lormetazepam:TI,AB,KY #38 metaclazepam:TI,AB,KY #39 midazolam:TI,AB,KY #40 nitrazepam:TI,AB,KY #41 oxzepam:TI,AB,KY 0 #42 prazepam:TI,AB,KY 73 #43 propazepam:TI,AB,KY #44 ripazepam:TI,AB,KY #45 serazepine:TI,AB,KY #46 temazepan:TI,AB,KY #47 tofisopam:TI,AB,KY #48 triazolam:TI,AB,KY #49 #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 #50 #14 AND #49 |
Jun 2017: 240 Jun 2018: 13 Apr 2019: 92 |
| MEDLINE In‐process and other non‐indexed citations and MEDLINE 1950‐present (Ovid SP) [Most recent search: 10 April 2019] |
1 exp benzodiazepines/ 2 exp anti‐anxiety agents/ 3 adinazolam.ti,ab. 4 alprazolam.ti,ab. 5 bentazepam.ti,ab. 6 benzodiazepine*.ti,ab. 7 bromazepan.ti,ab. 8 brotizolam.ti,ab. 9 camazepam.ti,ab. 10 chlordiazepoxide.ti,ab. 11 clobazam.ti,ab. 12 clotiazepam.ti,ab. 13 cloxazolam.ti,ab. 14 diazepam.ti,ab. 15 etizolam.ti,ab. 16 flunitrazepam.ti,ab. 17 flurazepam.ti,ab. 18 flutoprazepam.ti,ab. 19 halazepam.ti,ab. 20 ketazolam.ti,ab. 21 loflazepate.ti,ab. 22 loprazolam.ti,ab. 23 lormetazepam.ti,ab. 24 metaclazepam.ti,ab. 25 midazolam.ti,ab. 26 nitrazepam.ti,ab. 27 oxzepam.ti,ab. 28 prazepam.ti,ab. 29 propazepam.ti,ab. 30 ripazepam.ti,ab. 31 serazepine.ti,ab. 32 temazepan.ti,ab. 33 tofisopam.ti,ab. 34 triazolam.ti,ab. 35 or/1‐34 36 exp Delirium/ 37 deliri*.ti,ab. 38 "acute confusion*".ti,ab. 39 "acute organic psychosyndrome".ti,ab. 40 "acute brain syndrome".ti,ab. 41 "metabolic encephalopathy".ti,ab. 42 "acute psycho‐organic syndrome".ti,ab. 43 "clouded state".ti,ab. 44 "clouding of consciousness".ti,ab. 45 "exogenous psychosis".ti,ab. 46 "toxic psychosis".ti,ab. 47 "toxic confusion".ti,ab. 48 obnubilat*.ti,ab. 49 or/36‐48 50 (randomized controlled trial or controlled clinical trial).pt. 51 randomized.ab. 52 placebo.ab. 53 drug therapy.fs. 54 randomly.ab. 55 trial.ab. 56 groups.ab. 57 or/50‐56 58 35 and 49 and 57 59 exp animals/ not humans.sh. 60 58 not 59 |
Jun 2017: 816 Jun 2018: 54 Apr 2019: 57 |
| Embase (Ovid SP) 1974 to 9 April 2019 [Most recent search: 10 April 2019] |
1 exp benzodiazepines/ 2 exp anti‐anxiety agents/ 3 adinazolam.ti,ab. 4 alprazolam.ti,ab. 5 bentazepam.ti,ab. 6 benzodiazepine*.ti,ab. 7 bromazepan.ti,ab. 8 brotizolam.ti,ab. 9 camazepam.ti,ab. 10 chlordiazepoxide.ti,ab. 11 clobazam.ti,ab. 12 clotiazepam.ti,ab. 13 cloxazolam.ti,ab. 14 diazepam.ti,ab. 15 etizolam.ti,ab. 16 flunitrazepam.ti,ab. 17 flurazepam.ti,ab. 18 flutoprazepam.ti,ab. 19 halazepam.ti,ab. 20 ketazolam.ti,ab. 21 loflazepate.ti,ab. 22 loprazolam.ti,ab. 23 lormetazepam.ti,ab. 24 metaclazepam.ti,ab. 25 midazolam.ti,ab. 26 nitrazepam.ti,ab. 27 oxzepam.ti,ab. 28 prazepam.ti,ab. 29 propazepam.ti,ab. 30 ripazepam.ti,ab. 31 serazepine.ti,ab. 32 temazepan.ti,ab. 33 tofisopam.ti,ab. 34 triazolam.ti,ab. 35 or/1‐34 36 exp Delirium/ 37 deliri*.ti,ab. 38 "acute confusion*".ti,ab. 39 "acute organic psychosyndrome".ti,ab. 40 "acute brain syndrome".ti,ab. 41 "metabolic encephalopathy".ti,ab. 42 "acute psycho‐organic syndrome".ti,ab. 43 "clouded state".ti,ab. 44 "clouding of consciousness".ti,ab. 45 "exogenous psychosis".ti,ab. 46 "toxic psychosis".ti,ab. 47 "toxic confusion".ti,ab. 48 obnubilat*.ti,ab. 49 or/36‐48 50 randomized controlled trial/ 51 controlled clinical trial/ 52 random$.ti,ab. 53 randomization/ 54 intermethod comparison/ 55 placebo.ti,ab. 56 (compare or compared or comparison).ti. 57 ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. 58 (open adj label).ti,ab. 59 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. 60 double blind procedure/ 61 parallel group$1.ti,ab. 62 (crossover or cross over).ti,ab. 63 ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. 64 (assigned or allocated).ti,ab. 65 (controlled adj7 (study or design or trial)).ti,ab. 66 (volunteer or volunteers).ti,ab. 67 trial.ti. 68 or/50‐67 69 35 and 49 and 68 |
Jun 2017: 836 Jun 2018: 227 Apr 2019: 154 |
| PsycINFO (Ovid SP) [Most recent search: 10 April 2019] |
1. exp benzodiazepines/ 2. adinazolam.ti,ab. 3. alprazolam.ti,ab. 4. bentazepam.ti,ab. 5. benzodiazepine*.ti,ab. 6. bromazepan.ti,ab. 7. brotizolam.ti,ab. 8. camazepam.ti,ab. 9. chlordiazepoxide.ti,ab. 10. clobazam.ti,ab. 11. clotiazepam.ti,ab. 12. cloxazolam.ti,ab. 13. diazepam.ti,ab. 14. etizolam.ti,ab. 15. flunitrazepam.ti,ab. 16. flurazepam.ti,ab. 17. flutoprazepam.ti,ab. 18. halazepam.ti,ab. 19. ketazolam.ti,ab. 20. loflazepate.ti,ab. 21. loprazolam.ti,ab. 22. lormetazepam.ti,ab. 23. metaclazepam.ti,ab. 24. midazolam.ti,ab. 25. nitrazepam.ti,ab. 26. oxzepam.ti,ab. 27. prazepam.ti,ab. 28. propazepam.ti,ab. 29. ripazepam.ti,ab. 30. Serazepine.ti,ab. 31. temazepan.ti,ab. 32. tofisopam.ti,ab. 33. triazolam.ti,ab. 34. or/1‐33 35. exp Delirium/ 36. deliri*.ti,ab. 37. "acute confusion*".ti,ab. 38. "acute organic psychosyndrome".ti,ab. 39. "acute brain syndrome".ti,ab. 40. "metabolic encephalopathy".ti,ab. 41. "acute psycho‐organic syndrome".ti,ab. 42. "clouded state".ti,ab. 43. "clouding of consciousness".ti,ab. 44. "exogenous psychosis".ti,ab. 45. "toxic psychosis".ti,ab. 46. "toxic confusion".ti,ab. 47. obnubilat*.ti,ab. 48. or/35‐47 49. exp Clinical Trials/ 50. randomly.ab. 51. randomi?ed.ti,ab. 52. placebo.ti,ab. 53. groups.ab. 54. "double‐blind*".ti,ab. 55. "single‐blind*".ti,ab. 56. RCT.ti,ab. 57. or/49‐56 58. 34 and 48 and 57 |
Jun 2017: 37 Jun 2018: 70 Apr 2019: 1 |
| CINAHL (EBSCOhost) [Most recent search: 10 April 2019] |
1 (MH "antianxiety agents, benzodiazepine+") 2 TX adinazolam 3 TX alprazolam 4 TX bentazepam 5 TX benzodiazepine* 6 TX bromazepan 7 TX brotizolam 8 TX camazepam 9 TX chlordiazepoxide 10 TX clobazam 11 TX clotiazepam 12 TX cloxazolam 13 TX diazepam 14 TX etizolam 15 TX flunitrazepam 16 TX flurazepam 17 TX flutoprazepam 18 TX halazepam 19 TX ketazolam 20 TX loflazepate 21 TX loprazolam 22 TX lormetazepam 23 TX metaclazepam 24 TX midazolam 25 TX nitrazepam 26 TX oxzepam 27 TX prazepam 28 TX propazepam 29 TX ripazepam 30 TX serazepine 31 TX temazepan 32 TX tofisopam 33 TX triazolam 34 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 35 (MM "delirium") 36 TX deliri* 37 TX "acute confusion*" 38 TX "acute organic psychosyndrome" 39 TX "acute brain syndrome" 40 TX "metabolic encephalopathy" 41 TX "acute psycho‐organic syndrome" 42 TX "clouded state" 43 TX "clouding of consciousness" 44 TX "exogenous psychosis" 45 TX "toxic psychosis" 46 TX "toxic confusion" 47 TX obnubilat* 48 S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43 OR S44 OR S45 OR S46 OR S47 49 MH "Clinical Trials" 50 TX trial 51 TX "single‐blind*" 52 TX "double‐blind*" 53 TX "treatment as usual" 54 TX randomly 55 S49 OR S50 OR S51 OR S52 OR S53 OR S54 56 S34 AND S48 AND S55 |
Jun 2017: 158 Jun 2018: 16 Apr 2019: 21 |
| ISI Web of Science ‐ all databases [includes: Web of Science (1945‐present); BIOSIS Previews (1926‐present); MEDLINE (1950‐present); Journal Citation Reports] [Most recent search: 10 April 2019] |
(benzodiazepine* OR diazepam OR temazepan OR lorazepam OR alprazolam OR bromazepam OR nitrazepam OR clonazepam) AND TOPIC: (deliri* OR "acute confusion*" OR "acute organic psychosyndrome" OR "acute brain syndrome" OR "metabolic encephalopathy" OR "acute psycho‐organic syndrome" OR "clouded state" OR "clouding of consciousness" OR "exogenous psychosis" OR "toxic psychosis" OR "toxic confusion" OR obnubilat*) ANDTOPIC: (random* or placebo or "double‐blind" or trial OR groups OR "controlled study" OR "time series" OR "comparative study" OR "pretest‐posttest design") | Jun 2017: 641 Jun 2018: 60 Apr 2019: 62 |
| LILACS (BIREME) [Most recent search: 10 April 2019] |
benzodiazepine* OR diazepam OR temazepan OR lorazepam OR alprazolam OR bromazepam OR nitrazepam OR clonazepam [Words] and and delirium OR delious OR deliria OR delirio OR loucura [Words] | Jun 2017: 0 Jun 2018: 0 Apr 2019: 0 |
| ClinicalTrials.gov (www.clinicaltrials.gov) [Most recent search: 10 April 2019] |
delirium OR toxic psychosis OR toxic confusion OR metabolic encephalopathy OR clouded state OR exogenous psychosis | benzodiazepine* OR diazepam OR temazepan OR lorazepam OR alprazolam OR bromazepam OR nitrazepam OR clonazepam | Jun 2017: 15 Jun 2018: 1 Apr 2019: 6 |
| ICTRP (http://apps.who.int/trialsearch) [Most recent search: 10 April 2019] |
delirium OR toxic psychosis OR toxic confusion OR metabolic encephalopathy OR clouded state OR exogenous psychosis | benzodiazepine* OR diazepam OR temazepan OR lorazepam OR alprazolam OR bromazepam OR nitrazepam OR clonazepam | Jun 2017: 7 Jun 2018: 1 Apr 2019: 2 |
| TOTAL before de‐duplication | Jun 2017: 2792 Jun 2018: 442 Apr 2019: 415 TOTAL: 3649 |
|
| TOTAL after de‐duplication | Jun 2017: 2256 Jun 2018: 390 Apr 2019: 335 TOTAL: 2981 |
|
| TOTAL after first assessment by information specialist | Jun 2017: 466 Jun 2018: 61 Apr 2019: 95 TOTAL: 622 |
|
Data and analyses
Comparison 1. Benzodiazepines (lorazepam) vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Severity of delirium (assessed by MDAS, higher = worse) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 2.1 [‐0.96, 5.16] |
| 2 Length of hospital admission (days) | 1 | 58 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐3.45, 3.45] |
| 3 Mortality from all causes | 1 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.04, 3.02] |
| 4 Discharged (alive from the acute palliative care unit) | 1 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.55, 2.99] |
| 5 Individual side effects (measured with UKU, higher = worse) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Akathasia | 1 | 31 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.81 [0.33, 24.16] |
| 5.2 Dystonia | 1 | 31 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 Epileptic seizure | 1 | 31 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.4 Hyperkinesia | 1 | 31 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.05, 4.65] |
| 5.5 Hypokinesia or akinesia | 1 | 31 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.19, 2.63] |
| 5.6 Paresthesia | 1 | 31 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.7 Rigidity | 1 | 31 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.8 Tremor | 1 | 31 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Individual side effects (measured with ESAS, higher = worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 anxiety | 1 | 25 | Mean Difference (IV, Fixed, 95% CI) | ‐1.30 [‐10.17, 7.57] |
| 6.2 appetite | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐1.5 [‐7.46, 4.46] |
| 6.3 depression | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | ‐1.60 [‐8.56, 5.36] |
| 6.4 drowsiness | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 3.9 [‐2.13, 9.93] |
| 6.5 fatigue | 1 | 17 | Mean Difference (IV, Fixed, 95% CI) | 1.90 [‐5.53, 9.33] |
| 6.6 feeling of well‐being | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐0.80 [‐8.42, 6.82] |
| 6.7 nausea | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [‐6.93, 10.93] |
| 6.8 pain | 1 | 25 | Mean Difference (IV, Fixed, 95% CI) | ‐0.7 [‐8.46, 7.06] |
| 6.9 shortness of breath | 1 | 25 | Mean Difference (IV, Fixed, 95% CI) | ‐0.6 [‐8.89, 7.69] |
| 6.10 sleep | 1 | 25 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐7.88, 6.88] |
Comparison 2. Benzodiazepines (lorazepam) vs chlorpromazine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Severity of delirium (assessed by DRS, higher = worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Day 2 after treatment | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | 5.22 [0.35, 10.09] |
| 1.2 End of treatment | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | 5.15 [‐0.26, 10.56] |
| 2 Any adverse events (measures with ESRS, higher = worse) | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | 7.12 [‐0.43, 14.67] |
| 3 Mortality from all causes | 1 | 19 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.23, 3.26] |
Comparison 3. Benzodiazepines (lorazepam) vs haloperidol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Severity of delirium (assessed by DRS, higher = worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Day 2 after treatment | 1 | 17 | Mean Difference (IV, Fixed, 95% CI) | 4.85 [0.03, 9.67] |
| 1.2 End of treatment | 1 | 17 | Mean Difference (IV, Fixed, 95% CI) | 5.36 [‐0.01, 10.73] |
| 2 Any adverse events (measures with ESRS, higher = worse) | 1 | 17 | Mean Difference (IV, Fixed, 95% CI) | 6.66 [‐1.53, 14.85] |
| 3 Mortality from all causes | 1 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [0.34, 9.92] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Breitbart 1996.
| Methods | Design: randomised, three‐arm trial Blinding: double‐blind Duration: duration was not specified. Authors reported the outcomes respectively based on the time of baseline, day two and end of treatment. Setting: St Luke's/ Roosevelt Hospital Center, New York, USA |
|
| Participants | Diagnosis: adult patients who met the case definition for AIDS of the Centers for Disease Control and were undergoing treatment for acquired immune deficiency syndrome (AIDS)‐related medical problems N = 30. Age: mean 39.2 years old (range 23 to 56). Sex: 7 female, 23 male. Race/ethnicity: 17 black, 8 Hispanic, 4 white, 1 Asian. Inclusion criteria: medically hospitalised patients diagnosed with AIDS and who met DSM‐III‐R criteria for delirium scoring 13 or greater on the Delirium Rating Scale (DRS) Exclusion criteria: included known hypersensitivity to neuroleptics or benzodiazepines; presence of neuroleptic malignant syndrome; concurrent treatment with neuroleptic malignant syndrome; concurrent treatment with neuroleptic drugs; seizure disorder; concurrent systemic chemotherapy for Kaposi's sarcoma; withdrawal syndrome of anticholinergic delirium for which a more specific treatment was indicated; current or past diagnosis of schizophrenia, schizoaffective disorder, or bipolar disorder; patients in whom delirium appeared to be part of a terminal event |
|
| Interventions | 1. Lorazepam (n = 6)*: initial dose was lorazepam 0.50 mg/hour for oral and 0.20 mg/hour for intramuscular. Mean drug dose administered during the first 24 hours of treatment was lorazepam 3.0 mg. Average maintenance dosage was lorazepam 4.6 mg. Note: that this arm of the trial was terminated half‐way through due to 'treatment limiting' adverse events, and all remaining participants in this arm were randomised to either chlorpromazine or haloperidol. 2. Chlorpromazine (n = 13): initial dose was chlorpromazine 10 mg/hour for oral and 5 mg/hour for intramuscular. Mean drug dose administered during the first 24 hours of treatment was chlorpromazine 50.0 mg. Average maintenance dosage was chlorpromazine 36 mg. 3. Haloperidol (n = 11): initial dose was haloperidol 0.25 mg/hour for oral and 0.125 mg/hour for intramuscular. Mean drug dose administered during the first 24 hours of treatment was haloperidol 2.8 mg. Average maintenance dosage was haloperidol 1.4 mg. *Each participant with delirium was evaluated hourly with the DRS and the Extrapyramidal Symptom Rating Scale (ESRS). If the patient's score on the DRS was still 13 or greater, the next level dose of study drug was administered. If the patient was asleep, calm, and not hallucinating or had scored 12 or below on the DRS, a maintenance dose was started on day two, and the maintenance dose was equal to one half of the first 24‐hour dose requirement, given in a twice a day regimen. |
|
| Outcomes | Severity of delirium measured by DRS (a 10‐item scale integrating DSM‐III criteria designed to be used by clinicians to identify delirium in the medically ill; items are rated on a Likert‐type scale of 0 to 3 (absent ‐ mild ‐ moderate ‐ severe)); Mortality from all causes; Extrapyramidal side effects (measured using Extrapyramidal Symptom Rating Scale, ESRS) — unable to use (not predefined in this review protocol); Cognitive status measured by the Mini‐Mental State (a score of 28 to 30 was rated as 0 (no deficits) on item 6 of the Delirium Rating Scale, a score of 25 to 28 was rated as 1 (very mild deficits), a score of 20 to 24 was rated as 2 (focal deficits), a score of 15 to 19 was rated as 3 (significant deficits), and a score of 15 or less was rated as 4 (severe deficits)); Early termination/side effects (symptom checklist) |
|
| Notes | Enrolment: quote: "patients were enrolled onto the study when they became delirious and met criteria for treatment in the protocol during the 28‐month data collection period." Consent: quote: "since informed consent could not ethically be obtained from subjects once delirium had developed, we devised a prospective research strategy in which only medically stable patients who had the capacity to give informed consent were recruited. While many patients exhibited clinical evidence of AIDS‐related dementia, only those who lacked the capacity to give informed consent were not approached." *When we performed the analyses, we created separate comparisons to compare lorazepam with each of the other controls (chlorpromazine, haloperidol). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients meeting criteria for delirium treatment were randomised by the hospital pharmacy and treated in a double‐blind fashion with one of the three study drugs." The paper mentioned "randomly assigned", but there was a lack of description about the randomisation method. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote "in a double‐blind fashion", but no further information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote "in a double‐blind fashion", but no further information |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Five participants (two in the haloperidol group ‐ 2/11, 18.1%; two in the chlorpromazine group ‐ 2/13, 15.4%; and one in the lorazepam group ‐ 1/6, 16.7%) lost to follow‐up because they died within eight days of initiation of the protocol. |
| Selective reporting (reporting bias) | Low risk | Results were reported for all stated outcomes. |
| Other bias | High risk | Quote "Midway through the study, it became apparent that all the patients who received one of the study drugs developed treatment‐limiting adverse side effects. Consistent with hospital policy, the study pharmacist determined which drug had been used to treat these patients. All of these patients had been treated with lorazepam. Lonazepam was removed from the study at that point. All subsequent patients were treated with the two remaining study drugs in a randomized, double‐blind procedure." The randomisation procedure changed midway through the study. Funding: The trial was supported by NIMH grant MH‐45664. The paper reported that "some of the authors of this publication are also working on these related projects: meaning‐centred group psychotherapy for cancer survivors". Declarations of interest: authors did not provide information regarding conflict of interest. |
Hui 2017.
| Methods | Design: randomised, single‐centre Blinding: double‐blind Duration: the timing of the primary outcome was eight hours from when the blinded study medication was administered. Setting: inpatient palliative care unit, University of Texas MD Anderson Cancer Center, Houston, Texas, USA |
|
| Participants | Diagnosis: cancer (including head and neck, breast, gastrointestinal, genitourinary, gynaecological, haematological, respiratory). Most participants had metastatic cancer stage (n = 46), others locally advanced (n = 1) and recurrent or persistent (n = 11). N = 58 (90 randomised patients*, 58 received the study medication) Age: mean 65 years old (range 30 to 90) Sex: 27 female, 31 male Race/ethnicity: 44 white, 8 black, 2 Hispanic, 4 'other' Inclusion criteria: adult patients who were 18 years or older with a diagnosis of advanced cancer in the acute palliative care unit, and having a diagnosis of delirium according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM‐IV‐TR), and had a history of agitation according to a Richmond Agitation‐Sedation Scale (RASS) score of two or more higher in the past 24 hours, despite receiving scheduled haloperidol of 1 mg to 8 mg per day (patients with hyperactive or mixed delirium) Exclusion criteria: patients with dementia; use of benzodiazepines or chlorpromazine within the past 48 hours; contraindications to neuroleptics; contraindications to benzodiazepines |
|
| Interventions | 1. Lorazepam + haloperidol (n = 29): lorazepam (3 mg) given in addition to haloperidol (2 mg) intravenously upon the onset of an agitation episode 2. Placebo + haloperidol (n = 29): placebo given in addition to haloperidol (2 mg) intravenously upon the onset of an agitation episode All enroled participants immediately initiated a standardised open‐label regimen with haloperidol (2 mg) every 4 hours intravenously and another 2 mg every hour as needed for agitation. Once the participant met the threshold with RASS score two or higher, a single dose of 3 mg of lorazepam in 25 mL of 0.9% normal saline solution or identically appearing placebo (25 mL of 0.9% normal saline) was infused intravenously over 1.5 minutes. Participants in both groups then received 2 mg of haloperidol intravenously afterwards. "All patients had at least two days of delirium with documentation of agitation before starting the study intervention. The use of other medications and withholding of scheduled haloperidol were permissible as per standard of practice according to the clinical judgment of the attending physician and bedside nurse." |
|
| Outcomes | The severity of delirium assessed with Memorial Delirium Assessment Scale (MDAS, range, 0 to 30; higher scores indicates greater severity); Length of hospital admission: duration of stay in acute palliative care unit; Mortality from all causes; Discharged (alive from the acute palliative care unit); Individual side effects: adverse symptoms measured using the Edmonton Symptom Assessment System (ESAS range, 0 to 10; higher scores indicate greater severity); adverse effects related to use of benzodiazepines and antipsychotics measured using Udvalg for Kliniske Undersøgelser assessment (range 0 to 3; higher scores indicate greater severity); Unable to use (not predefined in this review protocol) RASS (Richmond Agitation‐Sedation Scale) score, a validated 10‐point numeric rating scale that ranges from ‐5: unarousable to 4: combative); the use of any additional psychotropic agents during the first 8 hours after study medication administration and then daily until discharge; patient comfort perceived by caregivers and bedside nurses daily (5‐point Likert scale ranging from "strongly agree" to "strongly disagree"); the recalled frequency of 6 delirium symptoms; the communication capacity perceived by caregivers and bedside nurses assessed daily; overall survival from the time of study medication administration |
|
| Notes | "Because of the nature of the study population, 32 patients died or were discharged before requiring the study medication." Enrolment: occurred from February 11, 2014, to Jun 30, 2016. Data collection was completed in October 2016. Consent: it was stated that "written surrogate consent was obtained from the medical power of attorney or legal representative of included participants". |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 1:1 ratio using web‐based simple randomisation |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocation was concealed by using a secured web site that was only accessible to the study pharmacist, who then assigned patients to the study intervention." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Research staff conducting the study assessments, bedside nurses, attending physicians, patients, and caregivers were blinded to the allocation of the study medication and study outcomes throughout the entire study. To ensure proper blinding, a separate clinical nurse administered the study medication instead of the bedside nurse who conducted the RASS score assessments." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | As above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data available for n = 58 (n = 29 in each group from originally n = 90 randomised). Because of the nature of the study population, many participants died or were discharged before requiring the study medication. For these 58 participants in the primary analysis, six participants (6/58, 10.3%; three in each group) were lost to follow‐up prior to completion of 8 hours of study medication administration. |
| Selective reporting (reporting bias) | Low risk | Results were reported for all stated outcomes. Authors detailed protocol changes relating to study objectives, eligibility criteria and statistical analysis. These have been provided fully in the supplementary section of their published paper. |
| Other bias | Low risk | Funding: This study was supported by grant R21CA186000‐01A1 from the National Cancer Institute; a Mentored Research Scholar Grant in Applied and Clinical Research (MRSG‐14‐1418‐01‐CCE) from the American Cancer Society and the Andrew Sabin Family Foundation; grant P30CA016672 from the National Institutes of Health Cancer Center; and grant R01CA200867 from the National Institutes of Health. It was stated that "the funding sources were not involved in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; the preparation, review, and approval of the manuscript, and the decision to submit for publication". Declarations of interest: Quote: "All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and none were reported." |
AIDS: Acquired Immune Deficiency Syndrome DRS: Delirium Rating Scale DSM‐III‐R: Diagnostic and Statistical Manual of Mental Disorders, Third Edition, Revision DSM‐IV‐TR: Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision ESAS: Edmonton Symptom Asessment System ESRS: Extrapyramidal Symptom Rating Scale MDAS: Memorial Delirium Assessment Scale RASS: Richmond Agitation‐Sedation Scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Brown 1972 | Patients treated for alcohol abuse or withdrawal |
| Chantelau 1980 | Not randomised All participants were given benzodiazepines. |
| Ferraz Gonçalves 2016 | Patients were not diagnosed with delirium. Study aim was to investigate the effect of midazolam for agitation control. |
| Gouffier 1980 | Not randomised Only one group in study |
| Kramp 1978 | Patients treated for alcohol abuse or withdrawal |
| Linev 2017 | ICU patients |
| Menza 1988 | Not randomised |
| Mittal 2017 | Not randomised Retrospective study |
| Modell 1985 | Patients were not diagnosed with delirium. |
| Pourcher 1994 | Not randomised Case report |
| Rydzyński 1979 | The study of mechanism about action of flunitrazepam, not a clinical trial |
| Sobcyzk 1980 | Not randomised Retrospective study |
| Sroczynski 1974 | Not randomised Case‐series report |
| Stachowiak 1981 | Not randomised Case‐series report |
| Tahir 2012 | A letter to editor. Not relevant interventions |
| Thompson 1975 | ICU patients |
| Toselli 1969 | Not randomised Case‐series report |
| Wasilewski 1995 | Not randomised |
| Welbel 1982 | Patients treated for alcohol abuse or withdrawal |
| Yu 2017 | Patients were not diagnosed with delirium. Study aim was delirium prevention. |
ICU: Intensive Care Unit
Differences between protocol and review
We made the following changes to the published protocol (Jin 2017).
The protocol states that when studies had more than one intervention group (e.g. different doses of benzodiazepines), we planned to combine all relevant experimental intervention groups of the study into a single group or select one pair of interventions and exclude the others depending on specific circumstances, for example, size of difference in drug doses. We would only use the data for each group of participants once in the meta‐analysis. In the full review, no included studies with the above mentioned issues were identified. Only one RCT had two active comparators. Therefore, we removed previous statements and added the sentence: "In this case, we compared the benzodiazepine to each comparator intervention separately".
Because of the insufficient number of included studies, we did not perform meta‐analysis, assessment of reporting biases, subgroup analysis, investigation of heterogeneity, and sensitivity analysis.
-
We improved the presentation structure of the 'Summary of findings' table section with a few minor changes made to the content. We list these changes here.
we updated the expression of evidence ratings: "Rating evidence as high quality implies that we are very confident that the true effect lies close to that of the estimate of the effect. A rating of very low quality implies that we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect."
we removed the sentence "We will present all outcomes of the review, including a summary of the amount of data, the magnitude of the effect size, and the overall quality of the evidence..." in the previous protocol and updated it as follows:
We presented the following results in the 'Summary of findings' tables, which were created using GRADEproGDT software (GRADEpro GDT 2014).
The length of delirium episode;
Severity of delirium;
Any adverse event;
Length of hospital admission;
Mortality from all causes.
Contributions of authors
Yan Li (YL): data screening after 2019 new search, data extraction, data entry, and data analysis after searching was updated, writing first draft of full review, revising the final full review
Jun Ma (JM): data screening after 2019 new search, data checking, writing first draft of full review, reviewing and revising the final full review
Yinghui Jin (YJ): protocol development
Nan Li (NL): initial data extraction before 2019 new search
Rui Zheng (RZ): data screening before 2019 new search
Wei Mu (WM): initial data extraction before 2019 new search
Jiaying Wang (JW): initial data extraction before 2019 new search
Jin Hua Si (JHS): data screening before 2019 new search
Jing Chen (JC): revising the protocol
Hong Cai Shang (HCS): protocol development and revising the protocol
Sources of support
Internal sources
No sources of support supplied
External sources
-
NIHR, UK.
This review was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Dementia and Cognitive Improvement group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service or the Department of Health
-
Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support, China.
Code: ZYLX201810
Declarations of interest
Yan Li: none known
Jun Ma: none known
Yinghui Jin: none known
Nan Li: none known
Rui Zheng: none known
Wei Mu: none known
Jiaying Wang: none known
Jin Hua Si: none known
Jing Chen: none known
Hong Cai Shang: none known
New
References
References to studies included in this review
Breitbart 1996 {published data only}
- Breitbart W, Marotta R, Platt MM, Weisman H, Derevenco M, Grau C, et al. A double‐blind trial of haloperidol, chlorpromazine, and lorazepam in the treatment of delirium in hospitalized AIDS patients. American Journal of Psychiatry 1996;153(2):231‐7. [DOI] [PubMed] [Google Scholar]
Hui 2017 {published data only}
- Hui D, Frisbee‐Hume S, Wilson A, Dibaj S, Nguyen T, Cruz MDL, et al. Effect of lorazepam with haloperidol vs haloperidol alone on agitated delirium in patients with advanced cancer receiving palliative care: a randomized clinical trial. JAMA 2017;318(11):1047‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Brown 1972 {published data only}
- Brown JH, Moggey DE, Shane FH. Delirium tremens: a comparison of intravenous treatment with diazepam and chlordiazepoxide. Scottish Medical Journal 1972;17(1):9. [DOI] [PubMed] [Google Scholar]
Chantelau 1980 {published data only}
- Chantelau EA, Schneider J. Favorable effect of clonazepam in the treatment of delirium tremens. Die Medizinische Welt 1980;31(12):451‐4. [PubMed] [Google Scholar]
Ferraz Gonçalves 2016 {published data only}
- Ferraz Gonçalves JA, Almeida A, Costa L, Silva P, Carneiro R. Comparison of haloperidol alone and in combination with midazolam for the treatment of acute agitation in an inpatient palliative care service. Journal of Pain & Palliative Care Pharmacotherapy 2016;30(4):284‐8. [DOI] [PubMed] [Google Scholar]
Gouffier 1980 {published data only}
- Gouffier E, Monterio I, Schnurman D, Voiry AM. Treatment of delirium tremens by means of intravenous administration of large doses of diazepam. La Nouvelle Presse Médicale 1980;9(37):2748. [PubMed] [Google Scholar]
Kramp 1978 {published data only}
- Kramp P, Rafaelsen OJ. Delirium tremens: a double‐blind comparison of diazepam and barbital treatment. Acta Psychiatrica Scandinavica 1978;58(2):174‐90. [DOI] [PubMed] [Google Scholar]
Linev 2017 {published data only}
- Linev DV, Yaroshetskiy Andrey I, Protsenko DN, Gelfand BR. The efficacy and the safety of dexmedetomidine, haloperidol and diazepam for treatment of delirium: a comparative study. Anesteziologiya I Reanimatologiya 2017;62(2):442‐8. [Google Scholar]
Menza 1988 {published data only}
- Menza MA, Murray GB, Holmes VF, Rafuls WA. Controlled study of extrapyramidal reactions in the management of delirious, medically ill patients: intravenous haloperidol versus intravenous haloperidol plus benzodiazepines. Heart & Lung 1988;17(3):238‐41. [PubMed] [Google Scholar]
Mittal 2017 {published data only}
- Mittal DB, Urmi DM, Ravikiran LS, Kashyap RV. Post operative delirium in elderly patients undergoing hip surgery: diazepam versus haloperidol. International Journal of Pharmaceutical Sciences and Research 2017;8(7):2965‐7. [Google Scholar]
Modell 1985 {published data only}
- Modell JG, Lenox RH, Weiner S. Inpatient clinical trial of lorazepam for the management of manic agitation. Journal of Clinical Psychopharmacology 1985;5(2):109‐13. [DOI] [PubMed] [Google Scholar]
Pourcher 1994 {published data only}
- Pourcher E, Filteau MJ, Bouchard RH, Baruch P. Efficacy of the combination of buspirone and carbamazepine in early posttraumatic delirium. American Journal of Psychiatry 1994;151(1):150‐1. [DOI] [PubMed] [Google Scholar]
Rydzyński 1979 {published data only}
- Rydzyński Z, Araszkiewicz A, Gozdalska J, Gruszczyński W. Flunitrazepam in the treatment of delirium tremens. ‐‐ preliminary report. Activitas Nervosa Superior 1979;21(3):161. [PubMed] [Google Scholar]
Sobcyzk 1980 {published data only}
- Sobcyzk P, Jena K, Klir T. Comparative evaluation of the effectiveness of various methods of treating delirium tremens. Psychiatria Polska 1980;44(3):481‐6. [PubMed] [Google Scholar]
Sroczynski 1974 {published data only}
- Sroczynski Z, Lunding M. Acute respiratory insufficiency following treatment with chlordiazepoxide in patients with delirium tremens. Ugeskrift for Laeger 1974;136(26):1444. [PubMed] [Google Scholar]
Stachowiak 1981 {published data only}
- Stachowiak J, Szyburski M, Tronczyński K. Flunitrazepam in the treatment of delirium tremens. Psychiatria Polska 1981;15(4‐6):343. [PubMed] [Google Scholar]
Tahir 2012 {published data only}
- Tahir TA, Farewell D, Bisson J. Randomised control trials for delirium: current evidence and statistical methods. Journal of Psychosomatic Research 2012;72(1):84‐5. [DOI] [PubMed] [Google Scholar]
Thompson 1975 {published data only}
- Thompson WL, Johnson AD, Maddrey WL. Diazepam and paraldehyde for treatment of severe delirium tremens. Annals of Internal Medicine 1975;82(2):175‐80. [DOI] [PubMed] [Google Scholar]
Toselli 1969 {published data only}
- Toselli E. Preliminary observations on the use of valium in intravenous injections in delirium tremens. Minerva Medica 1969;60(50):2503. [PubMed] [Google Scholar]
Wasilewski 1995 {published data only}
- Wasilewski D, Matsumoto H, Kur E, Dziklińska A, Woźny E, Stençka K, et al. Treatment of delirium tremens with quick administration of diazepam. Psychiatria Polska 1995;29(5):675‐86. [PubMed] [Google Scholar]
Welbel 1982 {published data only}
- Welbel L, Zareba J, Kowalewska I. Clomethiazole (chloromethiazole) in the treatment of delirium tremens. Psychiatria Polska 1982;16(1‐2):19. [PubMed] [Google Scholar]
Yu 2017 {published data only}
- Yu DN, Zhu Y, Ma J, Sun Q. Comparison of post‐anesthesia delirium in elderly patients treated with dexmedetomidine and midazolam maleate after thoracic surgery. Biomedical Research (India) 2017;28(15):6852‐5. [Google Scholar]
Additional references
AGS 2015
- American Geriatrics Society Expert Panel on Postoperative Delirium in Older Adults. American Geriatrics Society abstracted clinical practice guideline for postoperative delirium in older adults. Journal of the American Geriatrics Society 2015;63(1):142‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Amato 2010
- Amato L, Minozzi S, Vecchi S, Davoli M. Benzodiazepines for alcohol withdrawal. Cochrane Database of Systematic Reviews 2010, Issue 3. [DOI: 10.1002/14651858.CD005063.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
APA 1980
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM‐3). 3rd Edition. Washington, DC: American Psychiatric Association Publishing, 1980. [Google Scholar]
APA 1987
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM‐3R). 3rd Edition. Washington, DC: American Psychiatric Association Publishing, 1987. [Google Scholar]
APA 1994
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Discorders (DSM‐4). 4th Edition. Washington, DC: American Psychiatric Association Publishing, 1994. [Google Scholar]
APA 2000
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, text revised (DSM‐IV‐TR). 4th Edition. Washington DC: American Psychiatric Association Publishing, 2000. [Google Scholar]
APA 2013
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM‐5). 5th Edition. Arlington: American Psychiatric Association Publishing, 2013. [Google Scholar]
Barr 2013
- Barr J, Fraser GL, Puntillo K, Ely EW, Gélinas C, Dasta JF, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Critical Care Medicine 2013;41(1):263–306. [DOI] [PubMed] [Google Scholar]
Breitbart 1997
- Breitbart W, Rosenfeld B, Roth A, Smith MJ, Cohen K, Passik S. The Memorial Delirium Assessment Scale. Journal of Pain and Symptom Management 1997;13(3):128‐37. [DOI] [PubMed] [Google Scholar]
Bucerius 2004
- Bucerius J, Gummert JF, Borger MA, Walther T, Doll N, Falk V, et al. Predictors of delirium after cardiac surgery delirium: effect of beating‐heart (off‐pump) surgery. Journal of Thoracic and Cardiovascular Surgery 2004;127(1):57–64. [DOI] [PubMed] [Google Scholar]
Buss 2007
- Buss MK, Vanderwerker LC, Inouye SK, Zhang B, Block SD, Prigerson HG. Associations between caregiver‐perceived delirium in patients with cancer and generalized anxiety in their caregivers. Journal of Palliative Medicine 2007;10(5):1083‐92. [DOI] [PubMed] [Google Scholar]
Dold 2012
- Dold M, Li C, Tardy M, Khorsand V, Gillies D, Leucht S. Benzodiazepines for schizophrenia. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD006391.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ely 2004
- Ely EW, Shintani A, Truman B, Speroff T, Gordon SM, Harrell FE Jr, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. Journal of the American Medical Association 2004;291(14):1753–62. [DOI] [PubMed] [Google Scholar]
Girard 2009
- Girard TD, Pandharipande PP, Ely EW. Delirium in the intensive care unit. International Review of Psychiatry 2009;21(1):43‐58. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2014 [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed 08 March 2017. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Holmes 2000
- Holmes JD, House AO. Psychiatric illness in hip fracture. Age and Ageing 2000;29(6):537–46. [DOI] [PubMed] [Google Scholar]
Inouye 2006
- Inouye SK. Delirium in older persons. New England Journal of Medicine 2006;354(11):1157–65. [DOI] [PubMed] [Google Scholar]
Inouye 2014
- Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet 2014;383(9920):911–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jin 2017
- Jin YH, Li N, Zheng R, Mu W, Lei X, Si JH, et al. Benzodiazepines for treatment of delirium in non‐ICU settings. Cochrane Database of Systematic Reviews 2017, Issue 5. [DOI: 10.1002/14651858.CD012670] [DOI] [Google Scholar]
Kishi 2016
- Kishi T, Hirota T, Matsunaga S, Iwata N. Antipsychotic medications for the treatment of delirium: a systematic review and meta‐analysis of randomised controlled trials. Journal of Neurology, Neurosurgery, and Psychiatry 2016;87(7):764‐74. [DOI] [PubMed] [Google Scholar]
Kostas 2013
- Kostas TR, Zimmerman KM, Rudolph JL. Improving delirium care: prevention, monitoring, and assessment. Neurohospitalist 2013;3(4):194‐202. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leslie 2008
- Leslie DL, Marcantonio ER, Zhang Y, Leo‐Summers L, Inouye SK. One year health‐care costs associated with delirium in the elderly population. Archives of Internal Medicine 2008;168(1):27‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Levy 2017
- Levy MH, Smith T, Alvarez‐Perez A, et al. NCCN clinical practice guidelines in oncology: palliative care. nccn.org/professionals/physician_gls/f_guidelines.asp (accessed April 19, 2017).
Lin 2004
- Lin SM, Liu CY, Wang CH, Lin HC, Huang CD, Huang PY, et al. The impact of delirium on the survival of mechanically ventilated patients. Critical Care Medicine 2004;32(11):2254‐9. [DOI] [PubMed] [Google Scholar]
Lonergan 2009
- Lonergan E. Benzodiazepines for delirium. International Journal of Evidence‐based Healthcare 2009;8(2):103‐4. [PubMed] [Google Scholar]
Maldonado 2008
- Maldonado JR. Delirium in the acute care setting: characteristics, diagnosis and treatment. Critical Care Clinics 2008;24(4):657‐722. [DOI] [PubMed] [Google Scholar]
Mayo‐Smith 1997
- Mayo‐Smith MF, American Society of Addiction Medicine Working Group on Pharmacological Management of Alcohol Withdrawal. Pharmacological treatment of alcohol withdrawal: a meta‐analysis and evidence‐based practice guidelines. Journal of the American Medical Association 1997;278(2):144–51. [DOI] [PubMed] [Google Scholar]
McCusker 1988
- McCusker J, Cole M, Bellavance F, Primeau F. Reliability and validity of a new measure of severity of delirium. International Psychogeriatrics 1988;10(4):421‐33. [DOI] [PubMed] [Google Scholar]
Meagher 2000
- Meagher DJ, Trzepacz PT. Motoric subtypes of delirium. Seminars in Clinical Neuropsychiatry 2000;5(2):75‐85. [DOI] [PubMed] [Google Scholar]
Meagher 2011
- Meagher DJ, Leonard M, Donnelly S, Conroy M, Adamis D, Trzepacz PT. A longitudinal study of motor subtypes in delirium: relationship with other phenomenology, etiology, medication exposure and prognosis. Journal of Psychosomatic Research 2011;71(6):395‐403. [DOI] [PubMed] [Google Scholar]
Milbrandt 2004
- Milbrandt EB, Deppen S, Harrison PL, Shintani AK, Speroff T, Stiles RA, et al. Costs associated with delirium in mechanically ventilated patients. Critical Care Medicine 2004;32(4):955‐62. [DOI] [PubMed] [Google Scholar]
Neufeld 2016
- Neufeld KJ, Yue J, Robinson TN, Inouye SK, Needham DM. Antipsychotic medication for prevention and treatment of delirium in hospitalized adults: a systematic review and meta‐analysis. Journal of the American Geriatrics Society 2016;64(4):705. [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2005
- National Institute for Health and Care Excellence. Violence: the short‐term management of disturbed/violent behaviour in psychiatric in‐patient settings and emergency departments. Clinical guideline [CG25]. nice.org.uk/guidance/cg25 (accessed 12 January 2016).
NICE 2010
- National Institute for Health and Care Excellence. Delirium: Diagnosis, prevention and management. Clinical guideline [CG103]. nice.org.uk/guidance/cg103 (accessed 12 January 2016).
O'Keeffe 1999
- O'Keeffe ST, Lavan JN. Clinical significance of delirium subtypes in older people. Age and Ageing 1999;28(2):115‐9. [DOI] [PubMed] [Google Scholar]
O'Mahony 2011
- O'Mahony R, Murthy L, Akunne A, Young J, Guideline Development Group. Synopsis of the National Institute for Health and Clinical Excellence guideline for prevention of delirium. Annals of Internal Medicine 2011;154(11):746‐51. [DOI] [PubMed] [Google Scholar]
Page 2002
- Page C, Curtis M, Sutter MC, Walker M, Hoffman B. Integrated Pharmacology. 2nd Edition. St Louis, Missouri: Mosby CV, 2002. [Google Scholar]
Pandharipande 2006
- Pandharipande P, Shintani A, Peterson J, Pun BT, Wilkinson GR, Dittus RS, et al. Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology 2006;104(1):21‐6. [DOI] [PubMed] [Google Scholar]
Pandharipande 2008
- Pandharipande P, Cotton BA, Shintani A, Thompson J, Pun BT, Morris JA Jr, et al. Prevalence and risk factors for development of delirium in surgical and trauma intensive care unit patients. Journal of Trauma 2008;65(1):34‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pisani 2009
- Pisani MA, Kong SY, Kasl SV, Murphy TE, Araujo KL, Ness PH. Days of delirium are associated with 1‐year mortality in an older intensive care unit population. American Journal of Respiratory and Critical Care Medicine 2009;180(11):1092‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Radtke 2008
- Radtke FM, Franck M, Schneider M, Luetz A, Seeling M, Heinz A, et al. Comparison of three scores to screen for delirium in the recovery room. British Journal of Anaesthesia 2008;101(3):338‐43. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Santos 2004
- Santos FS, Velasco IT, Fráguas R Jr. Risk factors for delirium in the elderly after coronary artery bypass graft surgery. International Psychogeriatrics 2004;16(2):175–93. [PubMed] [Google Scholar]
Schrijver 2015
- Schrijver EJ, Graaf K, Vries OJ, Maier AB, Nanayakkara PW. Efficacy and safety of haloperidol for in‐hospital delirium prevention and treatment: a systematic review of current evidence. European Journal of Internal Medicine 2015;27:14‐23. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and ’Summary of findings’ tables. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Shankar 2014
- Shankar KN, Hirschman KB, Hanlon AL, Naylor MD. Burden in caregivers of cognitively impaired elderly adults at time of hospitalization: a cross‐sectional analysis. Journal of the American Geriatric Society 2014;62(2):276‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shorter 2005
- Shorter E. "Benzodiazepines". A Historical Dictionary of Psychiatry. Oxford: Oxford University Press, 2005. [Google Scholar]
Siddiqi 2006
- Siddiqi N, House AO, Holmes JD. Occurrence and outcome of delirium in medical in‐patients: a systematic literature review. Age and Ageing 2006;35(4):350‐64. [DOI] [PubMed] [Google Scholar]
Siddiqi 2007
- Siddiqi N, Stockdale R, Britton AM, Holmes J. Interventions for preventing delirium in hospitalised patients. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD005563.pub2] [DOI] [PubMed] [Google Scholar]
Siddiqi 2016
- Siddiqi N, Harrison JK, Clegg A, Teale EA, Young J, Taylor J, et al. Interventions for preventing delirium in hospitalised non‐ICU patients. Cochrane Database of Systematic Reviews 2016, Issue 3. [DOI: 10.1002/14651858.CD005563.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Trzepacz 1988
- Trzepacz PT, Baker RW, Greenhouse J. A symptom rating scale for delirium. Psychiatry Research 1988;23(1):89–97. [DOI] [PubMed] [Google Scholar]
Van Rompaey 2008
- Rompaey B, Schuurmans MJ, Shortridge‐Baggett LM, Truijen S, Bossaert L. Risk factors for intensive care delirium: a systematic review. Intensive Critical Care Nursing 2008;24(2):98–107. [DOI] [PubMed] [Google Scholar]
WHO 1993
- World Health Organization. The ICD‐10 Classification of Mental and Behavioral Disorders: Diagnostic Criteria for Research. Geneva: World Health Organization, 1993. [Google Scholar]
Woods 1992
- Woods JH, Katz JL, Winger G. Benzodiazepines: use, abuse, and consequences. Pharmacological Reviews 1992;44(2):151‐347. [PubMed] [Google Scholar]
Young 2010
- Young J, Murthy L, Westby M, Akunne A, O'Mahony R, Guideline Development Group. Diagnosis, prevention, and management of delirium: summary of NICE guidance. BMJ 2010;341:c3704. [DOI] [PubMed] [Google Scholar]


