Summary
In rheumatoid arthritis (RA), breakdown of self-tolerance and onset of clinical disease are separated in time and space, supporting a multi-hit model in which emergence of autoreactive T cells is a pinnacle pathogenic event. Determining factors in T cell differentiation and survival include antigen recognition, but also the metabolic machinery that provides energy and biosynthetic molecules for cell building. Studies in patients with RA have yielded a disease-specific metabolic signature, which enables naïve CD4 T cells to differentiate into pro-inflammatory helper T cells that are prone to invade into tissue and elicit inflammation through immunogenic cell death. A typifying property of RA CD4 T cells is the shunting of glucose away from glycolytic breakdown and mitochondrial processing towards the pentose phosphate pathway, favoring anabolic over catabolic reactions. Key defects have been localized to the mitochondria and the lysosome; including instability of mitochondrial DNA due to the lack of the DNA repair nuclease MRE11A and inefficient lysosomal tethering of AMPK due to deficiency of N-myristoyltransferase 1 (NMT1). The molecular taxonomy of the metabolically reprogrammed RA T cells includes glycolytic enzymes (glucose-6-phosphate dehydrogenase, phosphofructo-kinase), DNA repair molecules (MRE11A, ATM), regulators of protein trafficking (NMT1) and the membrane adaptor protein Tks5. As the mechanisms determining abnormal T cell behavior in RA are unraveled, opportunities will emerge to interject autoimmune T cells by targeting their metabolic checkpoints.
Keywords: rheumatoid arthritis, T cell, macrophage, glycolysis, mitochondria, autoimmunity, DNA damage, DNA repair, cell cycle, telomere, myristoylation, protein trafficking
Introduction
The classical theory of autoimmune disease holds that immunity designed to protect against “danger”, such as pathogens, foreign bodies, and dead tissues can deviate to attack healthy host cells. From a clinical perspective, autoimmune diseases differ in their at-risk populations, their preferred target organs, their age at onset, their course and their response to immunosuppressive therapy. It is therefore unlikely that a single unifying hypothesis can explain the broad spectrum of autoimmune conditions. Rather, for each autoimmune disease, disease-specific immune abnormalities will need to be discovered and defined. In the case of rheumatoid arthritis (RA), several features of this autoimmune condition provide clues as to what initially goes wrong and which disease pathways mediate the final steps of tissue inflammation, rendering an individual susceptible to a symmetrical destructive polyarthritis. Two features of RA predict immune system abnormalities of critical importance in the pathogenesis: (1) autoantibody production, the proof of a tolerance defect, precedes clinically relevant disease by years to decades;1–6 and (2) drug-free remission is rare. Withdrawal of immunosuppressive therapy, even in patients with drug-induced remission, in most cases prompts recurrence of disease. These properties indicate a multi-hit disease process with the tolerance breakdown separated from induction of inflammation in time and space and permanent memorization of the abnormal behavior. Analysis of the inflammatory joint lesions will allow studying mediators and pathways of tissue inflammation but may not be informative in the search for the tolerance defect, which appears deeply engrained into the immune system, “memorized” by fundamental rewiring of the memory T cell compartment.
Work from the last 10 years has identified a series of molecules mechanistically involved in redirecting differentiating CD4 T cells away from transitioning into protective memory T cells and instead forcing such T cells to become cytokine-producing, tissue-invasive, hypermigratory effector T cells that that are highly efficient in driving synovial membrane inflammation.7–10 These molecules are presented in Figure 1. The common denominator of how these molecules affect CD4 T cell function lies in the regulation of the cell cycle and the programming of metabolic cascades. Here, we will review the evidence for how these molecules alter immunity to promote the emergence and persistence of auto-aggressive T cells.
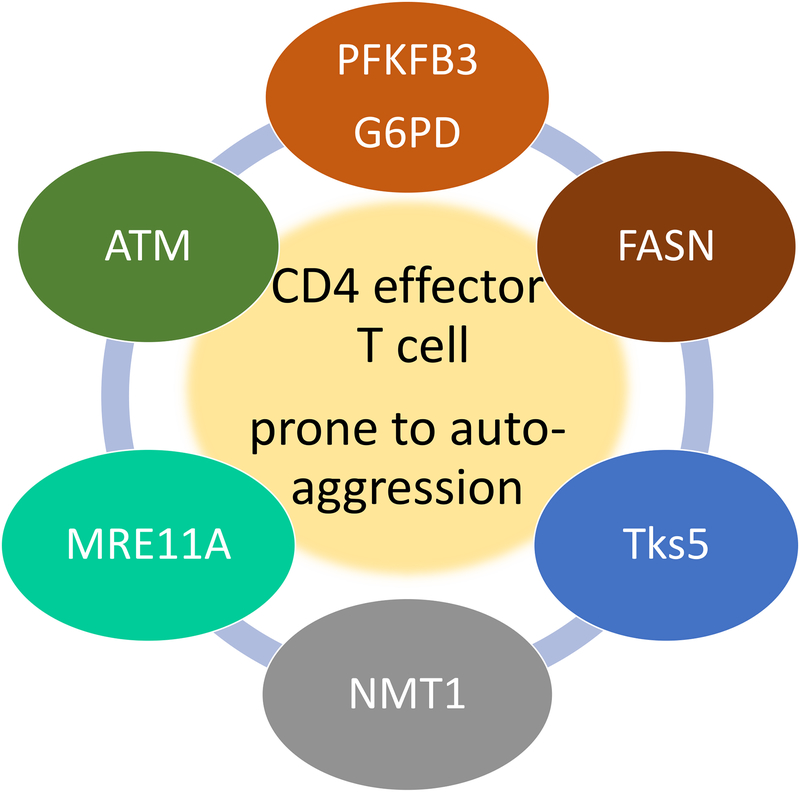
Figure 1. Metabolic checkpoints in pro-inflammatory and auto-aggressive T cells.
Studies in CD4 T cells from patients with RA have identified a series of molecules that deviate T cell function towards pro-inflammatory capabilities. All molecules identified have in common that they regulate or are regulated by the cell’s metabolic machinery. PFKFB3, G6PD and FASN directly regulate cytosolic glycolysis and lipogenesis. The cell cycle kinase ATM senses metabolic activity through reactive oxygen species to coordinate cell cycle passage to nutrient supply. The DNA repair nuclease MRE11A maintains metabolic competence by protecting mitochondrial DNA. The transferase NMT1 enables trafficking of the energy sensor AMPK to the lysosomal surface. Metabolic intermediates regulate expression of the membrane adaptor molecule Tks5, thereby rendering T cells tissue-invasive.
How to examine the auto-aggressive potential of human CD4 T cells
Several inherent properties of T cells dictate their demand for energy and their utilization of different nutrient sources: (1) they are long-lived, persisting in the host many decades; (2) they have enormous proliferative capacity, requiring the ability to build millions of daughter cells; (3) they differentiate into either short-lived effector cells or long-lived memory cells; (4) they need to be highly mobile; (5) they access food sources in very different tissue microenvironments.11–13 For most of the experiments cited below, CD4 T cells were harvested from patients with RA and from age/gender-matched controls and separated into naïve and memory populations. To examine their responsiveness to antigenic stimulation, the T cell receptor was crosslinked with CD3/CD28-coated beads for 72–96 h, before the metabolic machinery and the functional behavior was analyzed.
Metabolic signatures defined in patient-derived T cells could be subject to the inflammatory environment in the donor patient. To control for this variable, studies have utilized CD4 T cells from patients with psoriatic arthritis (PsA) as an “inflammatory” control. PsA is a systemic polyarthritis with high inflammatory capacity, typically lacking the autoantibodies encountered in RA.14 In vivo T cell behavior has been assessed by adoptively transferring human T cell populations into immunodeficient NSG mice. To examine the role of T cells in synovitis, the mice receive a graft of human synovial tissue prior to the adoptive transfer. This synovial tissue graft is then harvested and used for tissue transcriptome analysis, immunohistochemical staining or isolation of tissue-residing T cells.15
RA CD4 T cells shunt glucose towards the pentose phosphate pathway
Glucose is a critical energy carrier for proliferating T cells. Triggering of the TCR is coupled to the induction of a metabolic program that enhances mitochondrial function, but also upregulates extramitochondrial glycolysis to generate fast ATP and deliver glucose to the pentose phosphate pathway (PPP) for generation of biosynthetic precursor molecules. Naïve CD4 T cells from RA patients fundamentally change the assignment of glucose to the different pathways, minimizing breakdown into pyruvate and lactate and maximizing shunting into the PPP15–17 (Figure 2). The observation that RA T cells fail to upregulate the key glycolytic enzyme 6-Phosphofructo-2-Kinase/Fructose-2,6-Bisphosphatase 3 (PFKFB3) was one of the first hints that patient-derived cells utilize glucose differently.18 PFKFB3 is a bifunctional enzyme that catalyzes the synthesis and the degradation of fructose-2,6-bisphosphate. PFKFB3 is recognized as a key metabolic driver in cancer cells and the molecule is considered a therapeutic target to inhibit cell proliferation and impair survival.19, 20 CD4 T cells from healthy individuals upregulate PFKFB3 upon stimulation, with a maximum expression 72 h poststimulation. In activated T cells, the enzyme stays elevated for a prolonged period, supportive of its role in securing T cell survival during clonal expansion. The transcriptional repression of PFKFB3 transcripts in patient-derived T cells results in reduced production of ATP, pyruvate and lactate.
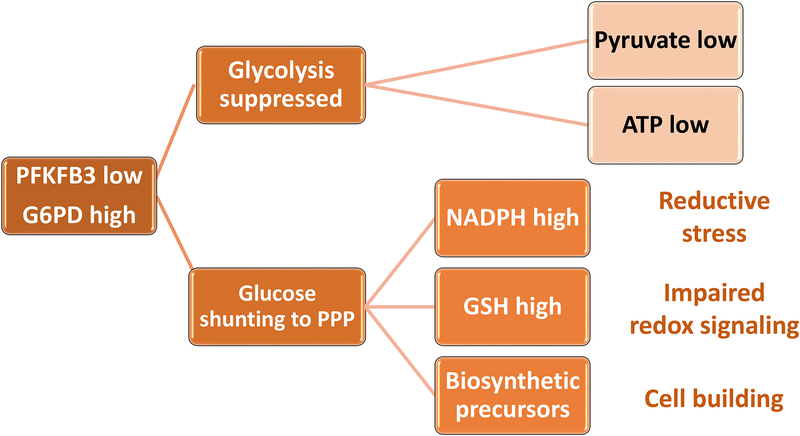
Figure 2. Glucose shunting to the pentose phosphate pathway enables anabolic processes.
In RA CD4 T cells, glucose is shunted from glycolytic breakdown towards the pentose phosphate pathway (PPP). Transcriptional repression of phosphofructokinase/fructose biphosphatase 3 (PFKFB3) results in reduced ATP and pyruvate production. Upregulation of Glucose-6-phosphate dehydrogenase (G6PD) funnels glucose towards the PPP, supplying NADPH, reduced glutathione (GSH) and biosynthetic precursors. As an outcome, the cell’s redox status shifts towards reductive stress, impairing redox-dependent signaling. Also, the cell has access to biosynthetic precursors, enabling a cell building program.
Expression of the GLUT-1 glucose transporter is intact in RA T cells; indicative for the intactness of the T cell receptor signaling machinery. Also, GLUT-1 expression will secure unrestricted access of RA T cells to glucose. Instead of breaking down the 6-carbon energy carrier into two 3-carbon pyruvate molecules, glucose is shifted towards the PPP. Glucose-6-phophate dehydrogenase (G6PD) acts as gate keeper to the PPP and this enzyme is upregulated in protein expression and function in the patients’ CD4 T cells.15 G6PD’s major function lies in supplying NADPH, the key electron donor in the cytosol; required for reductive biosynthetic reactions, such as lipogenesis, but also as a counter agent to reactive oxygen species (ROS).21 NADPH reduces oxidized glutathione (GSSG), thus maintaining the cell’s reductive state.22
Under physiologic conditions, the expression of PFKFB3 and G6PD is balanced in CD4 T cells.15, 23 In RA patients, this balance is shifted towards G6PD and the ratio of the two enzymes is correlated to disease activity; strongly suggestive for a direct role of these two enzymes in regulating the pro-inflammatory propensity of T cells in RA.
Based on the biochemistry of glucose catabolism, several predictions can be made, if CD4 T cells preferentially supply carbon to the PPP; including, excess availability of NADPH, reductive pressure, lack of metabolites for mitochondria and preference for reductive biosynthetic reactions, such as fatty acid synthesis. All of these predictions hold for patient-derived CD4 T cells: they favor lipogenesis and disfavor mitochondrial activity23, 24 and they have low ROS concentrations, imposing reductive instead of oxidative stress.
The loss-of-function of PFKFB3 has in vivo relevance. In a human tissue-mouse chimera model, selective knockdown of PFKFB3 in adoptively transferred healthy T cells was sufficient to induce robust synovial inflammation, generating a transcriptome pattern similar to rheumatoid synovitis.25 Conversely, repair of PFKFB3 expression in adoptively transferred RA T cells was highly effective in dampening synovial tissue inflammation. In essence, by shifting the ratio of PFKFB3/G6PD, RA T cells divert glucose from ATP production towards biosynthesis, generating inflammation-inducing effector cells.
RA T cells bypass the G2/M cell cycle checkpoint
Redirecting glucose catabolism to the synthesis of NADPH and the synthesis of biomolecules prepares RA T cells to produce cellular offspring.26 Clonal expansion is a prerequisite in generating effector T cells that leave lymph nodes and bone marrow storage sites and enter peripheral tissues to orchestrate anti-cancer and anti-pathogen immunity. RA T cells have shortened telomeres and display a pre-aged phenotype;27–29 implying that they have entered a state of senescence, defined as an irreversible cell cycle block. However, senescence-imposed cell cycle block, well described for such cell types as fibroblasts, is not a feature of RA T cells.13, 30, 31 To the opposite, observations made almost 15 years ago indicated that cell cycle passage is accelerated in patient-derived T cells.32
With metabolic conditions favoring the effector state, accelerated cell building and clonal expansion, the question arises whether cell cycle control is intact in RA T cells. Studies have demonstrated that RA T cells spend less time in the G2/M phase of the cell cycle and passage through cell division at a higher speed than healthy control T cells.15 Cell cycle arrest in G2/M is required for double-strand break repair by homologous recombination, as chromosomal pairs are available during this period.33 Molecular analysis of RA T cells has identified the DNA damage sensing kinase ATM as being repressed.15, 34 ATMlow T cells bypass the G2/M cell cycle checkpoint and hyperproliferate. They also accumulate DNA double strand breaks and are highly susceptible to radiation-induced cell death.35 ATM deficiency is typical for the progeroid syndrome Ataxia telangiectasia, an inherited disease manifesting with neurodegeneration, premature aging, increased risk of malignancy and accelerated cardiovascular disease.36 In RA T cells, ATM-specific transcripts, ATM protein and phosphorylated ATM are all downregulated15 (Figure 3). ATM deficiency as a risk factor for autoimmune disease is shared between RA and other autoimmune diseases, such as colitis.37
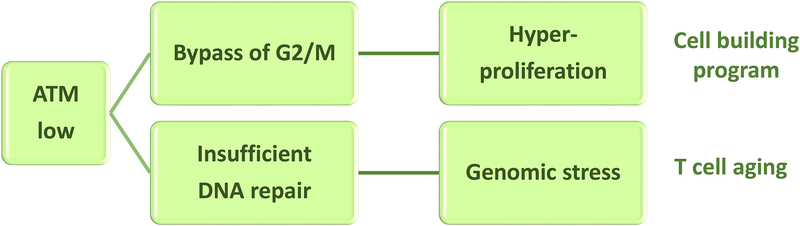
Figure 3. Dysfunctional cell cycle control results in T cell hyperproliferation.
In RA CD4 T cells, the DNA damage sensing kinase Ataxia telangiectasia mutated (ATM) is impaired. ATM is required for the initiation of double-strand break repair by homologous recombination and functions by slowing down the G2/M phase of the cell cycle. In patient-derived CD4 T cells, ATM protein and activity are diminished. ATM function requires dimerization, which is dependent on redox signaling. Low abundance of reactive oxygen species in RA T cells results in insufficient ATM activation. The outcome includes a high DNA damage burden, bypassing of the G2/M cell cycle checkpoint and T cell hyperproliferation.
The ATMlow phenotype, and the functional consequences of ATM deficiency are directly linked to the altered metabolic conditions in the cells (Figure 3). Specifically, ATM is a redox sensor and senses the cell’s entry into heightened metabolic activity associated with cellular proliferation. Redox sensing induces dimerization of ATM, a prerequisite for phosphorylation of the kinase.38 Surplus NADPH production and accumulation of reduced glutathione set the stage for impaired ROS sensing, thus interfering with ATM activation.
The finding of cell cycle checkpoint failure as a direct consequence of glucose catabolism raises the possibility of redox manipulation as a means to modify the pro-inflammatory propensity of RA T cells. Opposite to the long-held paradigm that ROS induce the cell’s demise, ATM inactivation due to ROS deficiency redirects attention away from oxidative stress towards reductive stress.10, 23, 39 Accordingly, pro-oxidants have been tested as anti-inflammatory reagents in T cell-induced synovitis.15 Both, menadione, a redox-cycling agent, and buthionine sulphoximine, an inhibitor of gamma-glutamyl cysteine synthase had powerful anti-inflammatory effects in human synovium NSG chimeric mice.
These data mechanistically link metabolic conditioning and redox homeostasis to ATM activation and cell checkpoint intactness, which ultimately determine the potential of a T cell to trigger synovial inflammation.
RA T cells lose the mitochondrial protector MRE11A
A cardinal feature of RA T cells is a state of premature aging. Original observations leading to the recognition of accelerated T cell aging in RA included the enrichment of CD28neg CD4 T cells in the synovial lesions.40 CD4+CD28neg T cells are end-differentiated effector T cells (TEMRA) with a prolonged proliferative history.41 Synovial CD4+CD28neg T cells are typically autoreactive and have a low threshold setting to produce pro-inflammatory effector cytokines.42 The accumulation of clonally expanded CD4+CD28neg T cells is shared between patients with RA and coronary artery disease43–45 and has been cited as a possible mechanism of enhanced cardiovascular risk in RA patient populations.
The aging phenotype of RA T cells is easily captured by the shortening of telomeres. Studies analyzing the lengths of T cell telomeres suggested that accelerated T cell aging begins during the second decade of life. Besides cellular turnover imposing the loss of telomeric sequences, chromosomal ends are also subject to DNA damage and a phenotype of telomeric fragility in RA T cells has been described.46 Structural instability of telomeres may well lead to a short-telomere presentation, which is unrelated to proliferative pressure.
In the case of RA T cells, telomeric shortening was related to the loss of the DNA repair nuclease MRE11A7, 46 (Figure 4). MRE11A has 3’ to 5’ exonuclease and endonuclease activity and is required for DNA double-strand break repair by homologous recombination.47, 48 The nuclease partners with RAD50 and ATM to form the MRN complex and hypomorphic mutations of MRE11A cause ataxia-telangiectasia-like disorders.
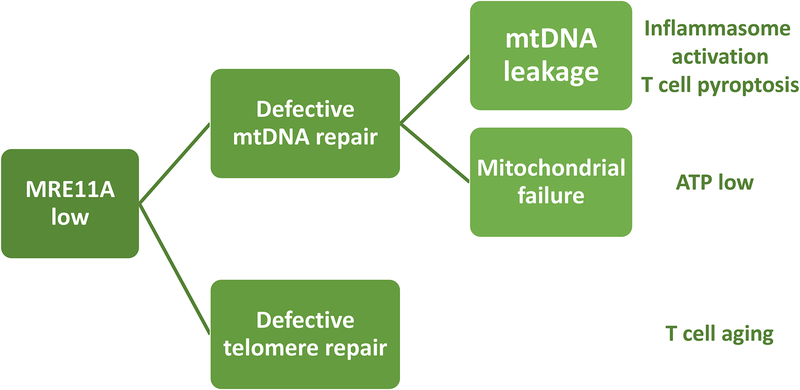
Figure 4. DNA repair failure in the mitochondria and at the telomere causes metabolic maladaptation and premature T cell aging.
In RA CD4 T cells, the DNA repair is inefficient, affecting both the mitochondrial genome and the telomeric ends. The nuclease MRE11A is a limiting factor in double strand break repair and is transcriptionally repressed in RA CD4 T cells. Lack of MRE11A’s nucleolytic activity in the mitochondria leads to instability of mitochondrial DNA (mtDNA), leakage of mtDNA into the cytosol, activation of the inflammasome and induction of immunogenic T cell death (pyroptosis). Lack of mitochondrial MRE11A leads to diminished oxygen consumption and ATP generation. Insufficiency of MRE11A at the telomeric ends results in telomere fragility and T cell aging.
The protein content of MRE11A in CD4 T cells declines with age and is low in T cells from RA patients even through the 2nd-5th decade of life. In the nucleus, MRE11A is predominantly localized at the chromosomal ends and genetic or pharmacologic inhibition of MRE11A induces telomeric instability and T cell aging7 (Figure 4). T cells with low MRE11A protein content have a propensity to induce synovial inflammation. Vice versa, correction of the MRE11Alow phenotype in RA T cells by forced overexpression is highly effective in suppressing synovitis.
MRE11A loss-of-function results in a metabolic phenotype, with marked reduction of mitochondrial oxygen consumption and ATP generation49 (Figure 4). How can the double-strand break repair nuclease interfere with metabolic competence of T cells? MRE11A is a nuclear protein but is also present in the cytoplasm and in the mitochondria.50 Cytoplasmic MRE11A has been implicated in DNA sensing.51 Mitochondrial MRE11A binds to mitochondrial DNA (mtDNA) and genetic or pharmacologic inhibition of MRE11A causes leakage of mtDNA into the cytoplasm.49 Leaked mtDNA is then recognized by the NLRP3 and the AIM2 inflammasome to trigger procaspase-1 cleavage. MRE11A-dependent inflammasome assembly and caspase-1 activation lead to IL-1β release, but more importantly, give rise to pyroptotic T cell death.
These data place MRE11A at the pinnacle of a signaling cascade that connects genome stability to tissue inflammation (Figure 4); including fundamental biologic processes, such as DNA damage sensing, DNA repair, mitochondrial fidelity, ATP generation, mtDNA containment and inflammasome activation. The mechanistic link between genome stability, bioenergetic competence and tissue inflammation centers on pyroptotic T cell death, which is strongly pro-inflammatory. In in vivo experiments, MRE11Alow T cells are powerful inducers of synovial tissue inflammation. T cells death in the synovial tissue is associated with mtDNA deposition into the extracellular space. Two aspects of these studies will impact the conceptual understanding of RA immunopathology; (1) the association of synovial inflammation with dying T cells, emphasizing that functional studies of lesional T cells may miss the most important subset; and, (2) T cell death as a consequence of metabolic failure. Notably, unprovoked activation of caspase-1 activation is also a feature of CD4 T cells in patients with human immunodeficiency virus (HIV) infection.52 Indeed, immunostaining for cleaved caspase-1 in T cells was strongly positive in lymph node biopsies of patients with HIV but was equally prominent in lymph nodes harvested from RA patients.49 Commonalities of T cell abnormalities in RA and HIV have been discussed as possible explanations for the shared immune aging phenotype53, 54 and the combination between insufficient adaptive immunity and chronic-smoldering inflammation seen in both RA and AIDS patients. Overall, recognizing lymph nodes as a site of T cell abnormalities provides opportunities to capture defects in immune tolerance that precede inflammation of the synovial tissue.
RA T cells favor lipogenesis and accumulate cytoplasmic lipid droplets
Suppression of mitochondrial activity due to insufficient repair of mtDNA has implications beyond the reduced generation of ATP and ROS; damaged mitochondria in RA T cells are no longer available for β-oxidation and catabolism of acetyl-CoA (Figure 5). Gene expression studies have revealed a bias for lipogenesis instead of lipolysis in patient-derived T cells.25 RA T cells, both from the circulation and within the synovial infiltrates, contain cytoplasmic lipid droplets.23, 55 Under physiologic conditions, cells utilize lipid droplets as a rapidly accessible source of fatty acids needed during periods of starvation. Recent work has identified lipid droplets as a sink for lipids released by autophagic breakdown of membranous organelles when cells are entering periods of prolong nutrient deprivation.56–58 Thus, lipid droplets may predominantly be a sign of cellular stress and serve as a storage organelle to avoid lipotoxicity, especially derived from acylcarnitines, that threaten integrity of mitochondrial membranes. Notably, RA T cells, despite the low production of ATP, do not activate autophagic recycling of cellular content.59, 60 Mechanistic studies have identified PFKFB3, the marker enzyme that is repressed in RA T cells, as an activator of autophagic flux.59 Just as they accumulate DNA damage, RA T cells also appear to accumulate damaged membranes, organelles etc. It is not surprising that such stressed and damaged T cells are culled by pyroptosis.
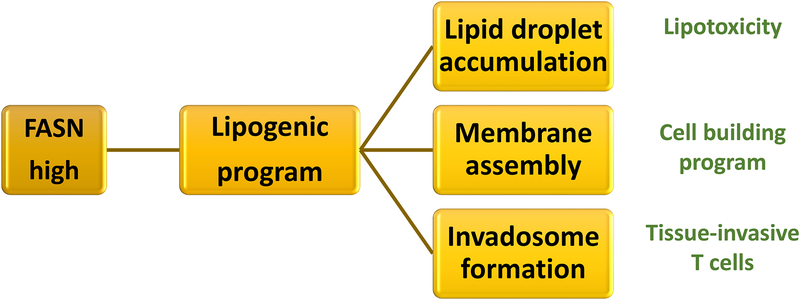
Figure 5. Disproportional lipogenesis fuels tissue invasiveness of RA T cells.
RA CD4 T cells favor lipogenesis over lipolysis. The cytosol is rich in reducing equivalents (NADPH), mitochondrial b-oxidation is impaired and the lipogenic gene signature, e.g. fatty acid synthase (FASN) is upregulated; all supporting the synthesis and not the breakdown of fatty acids. Excess lipogenesis leads to the deposition of cytosolic lipid droplets, availability of lipid precursors to build membranes and the formation of invasive membranes structures resembling invadosomes. As a result, T cells enter a cell building program and become tissue-invasive.
Cytoplasmic lipid droplets in RA T cells are not only an indicator of cellular stress. Rather, the availability of neutral lipids stored as cytoplasmic lipid droplets is a necessary element in a cell building program;61 e.g. during times of effector T cell differentiation. Thus, favoring lipogenesis over lipolysis supports the RA T cell’s switch from catabolism to anabolism and from ATP production to building cellular offspring. How membrane generation and organelle generation are affected by the shift in neutral lipid consumption versus storage is unknown. Also, it has not been examined whether the membrane quality is affected by metabolic rewiring.
Notably, no abnormalities in the mevalonic acid pathway have been described for patients with RA. Sterol regulatory element-binding proteins (SREBPs) are an essential element in generating protective CD8 T cell immunity, mostly so by supplying lipids to meet the requirements for membrane synthesis during clonal expansion.62 Modulating the cholesterol metabolism to enhance the cytotoxicity of CD8 T cells has been reported for anti-tumor immune responses.63 Whether metabolic interference with lipid utilization in membranes could be applied to weaken adaptive immunity in autoimmune disease is unexplored.
RA T cells assemble tissue-invasive membrane ruffles
The efficiency of pro-inflammatory effector T cells depends on priming of such cells by antigen-presenting cells, the journey of such cells from central lymphoid storage sites (lymph nodes, bone marrow etc) to peripheral tissue, the transmigration of T cells from the bloodstream to the perivascular space and the maneuvering of T cells through extra-cellular matrix. Migration of lymphocytes and fibroblasts within the tissue involves fundamentally different strategies. Fibroblasts and other mesenchymal cells employ proteolytic degradation of matrix proteins by serine proteases, matrix metalloproteinases, and cathepsins to overcome barriers in sites of tissue inflammation.64 T lymphocytes have to be fast and mobile to reach their goal, and rely on non-proteolytic migration, which does not require matrix proteolysis but rather involves a shape change to enable cells to glide and squeeze through preexisting matrix defects.65, 66 T cell locomotion generates high energy demands, but equally important are rearrangements of the cellular membranes to allow cells to slip through matrix gaps and trail through connective tissues.67
A key finding in the examination of abnormal T cell behavior was the recognition that RA T cells are prone to form invasive membrane structures25 (Figure 6), resembling podosomes and invadosomes utilized by cancer cells when they metastasize.68, 69 Membrane-proximal colocalization of cortactin and actin filaments identifies podosomal structures in T cells. Formation and motility of such membrane compartments imposes needs for biosynthetic activity; and, accordingly, podosome generation in RA T cells is under metabolic control. A cassette of motility genes appears to monitor the cell’s glycolytic flux. Transcription of the T cell motility genes is highly dependent on fatty acid synthesis.25 T cell motility, and the propensity to function as arthritogenic effector cells, are mechanistically linked to the divergence of glucose away from glycolysis and the shunting towards the PPP. A critical element in permitting RA T cells to form invadasomes and penetrate synovial tissue is the adaptor protein, Tks5, encoded by the SH3PXD2A gene (Figure 6). Equipped with protein-binding and lipid-binding modules, Tks5 is ideally suited to facilitate scaffolding and focalizing of actin polymerization.70 Tks5 transcripts are strongly induced in RA T cells, and, within a cohort of RA patients, amounts of SH3PXD2A transcripts correlated closely with the inflammatory burden in individual patients.25 Metabolically controlled abnormalities in T cell invasiveness appear to be disease-specific for RA. In a different autoimmune disease, giant cell arteritis, T cells invasion into the inflammatory lesions within the walls of large arteries is entirely dependent on MMP-9-producing macrophages, which first have to digest the basal membrane before T cells can leave the circulation and transmigrate into the tissue.71, 72
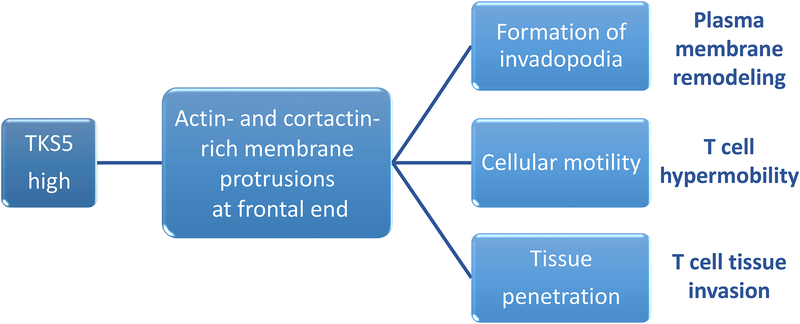
Figure 6. Membrane ruffling enables T cell tissue invasiveness.
CD4 T cells from RA patients have high abundance of Tks5 (encoded by the SH3PXD2A gene), a SH3- and PX-domain containing scaffolding protein. Tks5 localizes to the podosmal membrane extrusions on the frontal end of T cells. Formation of membrane ruffles is part of a metabolically-controlled T cell motility program, and high expression of Tks5 renders T cells highly mobile, permitting transition from blood vessels into the extravascular space and fast maneuvering in extracellular matrix.
RA T cells misplace AMP-activated protein kinase (AMPK) to non-lysosomal sites and unleash mTORC1
The metabolic signature of RA T cells favors biosynthetic activity over catabolic processes, diverting energy resources towards offspring building instead of supporting long-term persistence of memory T cells. The suppression of mitochondrial activity and glucose catabolism in favor of the PPP must expose these T cells to considerable stress. One would expect that upstream signals that misguide the cell into an energy crisis finally leading to lytic death should elicit countermeasures, protecting T cells from bioenergetic failure and pyroptosis. A critical mechanism directing cells towards appropriate adaptations lies in the continuous monitoring of cellular ATP availability through the kinase AMPK. AMPK, composed of a catalytic α subunit and regulatory βand γ subunits, functions as the cell’s fuel gauge that is activated when AMP:ATP or ADP:ATP ratios increase. AMPK activation modifies a series of target molecules, all aimed at stimulating energy production while reducing energy consumption. To promote energy production and coordinate nutrient supply with energy demand and consumption, AMPK plays a critical role in driving mitochondrial biogenesis, mitochondrial fusion and fission, autophagic flux and non-immunogenic cell death.73, 74 While mitochondrial oxygen consumption is markedly repressed in RA T cells, mitochondrial mass is maintained and is indistinguishable from that in healthy T cells. However, stability and containment of mtDNA is compromised in the patient-derived T cells.49
Despite the low ATP generation and the high AMP:ATP ratio in RA T cells, AMPK is not activated, crippling a fundamental pathway to secure energy homeostasis. The underlying molecular defect, namely dismantling of the cellular fuel gauge, has been unraveled75, 76 (Figure 7). Specifically, for AMPK to sense ATP and glucose availability it needs to be placed on the cytoplasmic face of the lysosome. Here, AMPK colocalizes with mTORC1, an equally important sensor of energy sources required for all steps of cellular proliferation. If there is ample nutrient supply, mTORC1 promotes cell growth by stimulating biosynthetic pathways, including synthesis of proteins, lipids and nucleotides, and by inhibiting cellular catabolism through repression of the autophagic pathway.77, 78 Colocalization of AMPK and mTORC1 on the lysosomal surface allows for direct cross-regulation of AMPK and mTORC1,79, 80 which share the lysosomal v-ATPase-Ragulator complex as an activator.
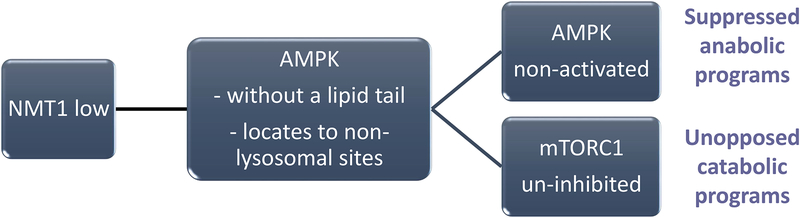
Figure 7. Compromised myristoylation alters subcellular trafficking of AMP-activated protein kinase (AMPK).
In RA T cells, posttranslational lipidation modification of proteins is compromised due to impaired function of the N-myristoyltransferase 1 (NMT1). Lack of NMT1 function affects the subcellular distribution of the energy sensor AMPK, which is normally recruited to the cytosolic face of the lysosome to monitor the AMP/ATP ratio. Energy deficiency prompts AMPK activation, triggers a multitude of anabolic programs and inhibits energy-utilizing catabolic processes by inactivating mTORC1. In RA CD4 T cells, AMPK lacks a lipid tail, fails to relocate to the lysosomal surface and malfunctions as an mTORC1 inhibitor. AMPK deficiency promotes mitochondrial restraint and confers continuous mTORC1 activity.
Analysis of AMPK recruitment to the lysosomal membrane has demonstrated low amounts of lysosomal AMPK in RA T cells, explaining the persistent mitochondrial underperformance despite energy deficiency (Figure 7). Lysosomal anchoring of AMPK requires the addition of the C14-fatty acid myristic acid to the kinase’s beta chain, accomplished by N-myristoyltransferase 1 (NMT1).81, 82 In RA T cells, transcription of the NMT1 gene is intact, but the enzyme functions insufficiently, leaving the cell with a myristoylation defect. Downstream consequences include mistrafficking of proteins, especially proteins en route to membrane sites. In vivo experiments have confirmed the necessity for AMPK routing to the lysosome to prevent unopposed mTORC1 activity. Experiments with forced overexpression of NMT1 could bring AMPK to the lysosomal membrane, avoid mTORC1 activation and suppress tissue inflammation.75 The anti-diabetic drug metformin functions as an AMPK activator.83 However, treating RA T cells with metformin failed to restore AMPK activation and left mTORC1 activation unaffected. A769662 is a small molecule that activates extra-lysosomal AMPK. Treatment of human synovium mouse chimeras with A769662 had strong anti-inflammatory effects, similar to the immunosuppressive action of rapamycin.75
These data support the notion that protein trafficking is a critical mechanism in determining the metabolic status of T cells and that lysosomal placement of AMPK is a key event in controlling the balance between pro- and anti-inflammatory signaling pathways.
Macrophages from RA patients are hypermetabolic
Many of the environmental cues and cell-intrinsic abnormalities that direct the metabolic machinery of RA T cells remain undefined. The preference of RA T cells for anabolic over catabolic metabolic settings appears to be cell-type specific, affecting naïve as well as memory T cell populations, but not monocytic cells. Thus, altered metabolic cascades in RA T cells do not reflect an organismal response to a change in nutrient supply. Macrophages, which colocalize with T cells in the synovial tissue lesions, have undergone a different set of metabolic adaptations. While their metabolic status is less well investigated, available data suggest that RA macrophages commit to a hypermetabolic state and that the metabolic imprinting is already present in undifferentiated monocytes8, 84 (Figure 8). Monocytes and macrophages derived from RA patients share their metabolic wiring with monocytes and macrophages isolated from patients with coronary artery disease.85 Molecular analysis has identified GSK-3β as an upstream regulator of highly active mitochondria. The protein kinase GSK-3β is constitutively active and is deactivated by serine-phosphorylation. Monocytes and macrophages from RA and CAD patients accumulate pGSK-3β-Ser9, both in tissue culture and in tissue lesions. The inactivated kinase is physically bound to mitochondria and mitochondrial respiration under resting and stressed deconditions is markedly higher than in healthy control cells.84 In patient-derived macrophages, mitochondria undergo structural changes, with formation of mitochondria-associated membranes and increased calcium transfer between endoplasmic reticulum and mitochondria. Heightened mitochondrial activity is linked to changes in effector functions; specifically, the production of pro-inflammatory cytokines and cathepsin K (Figure 8). Indeed, the severity of RA and the number of affected coronary arteries in CAD are directly correlated to the activity of cathepsin K. Pharmacologic inhibition of GSK-3β reproduces the phenotype of highly activated cathepsin K in patient-derived macrophages.
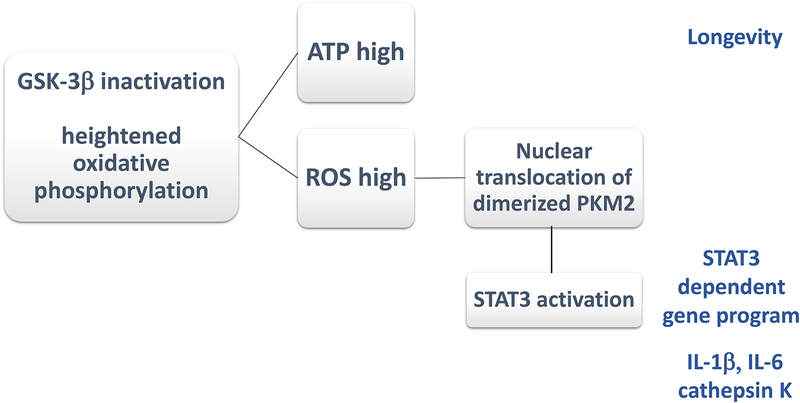
Figure 8. Hypermetabolic macrophages in rheumatoid arthritis.
In RA macrophages, Glycogen Synthase Kinase 3β (GSK-3β) is inactivated, enhancing the activity of the mitochondrial electron transport chain. GSK-3β is a constitutively active protein kinase that negatively regulates glucose homeostasis. Inactivation of GSK3β results in the activation of oxidative phosphorylation, enhanced ATP production and increased ROS release. Functional consequences include longevity of highly activated macrophages. ROS facilitate the dimerization of the cytosolic enzyme pyruvate kinase M2 (PKM2) and nuclear translocation of the enzyme, where it activates STAT3. Several pro-inflammatory activities of macrophages are dependent on GSK-3β inactivation and enhanced ROS release; such as the production of cathepsin K, IL-1β, and IL-6.
The mechanism of how hypermetabolic macrophages sustain the production of the pro-inflammatory cytokines IL-1β and IL-6 has been defined.85 Posttranslational modifications of the glycolytic enzyme pyruvate kinase M2 (PKM2), precisely the dimerization of the enzyme under oxidative stress conditions, causes cytoplasm-to-nucleus translocation. The enzyme is most active in glycolysis when in tetrameric formation.86 The ratio of dimeric/tetrameric PKM2 depends on the cell’s ROS concentrations. Hyper-metabolic macrophages from CAD patients release more ROS, particularly in the peri-mitochondrial space; resulting in the translocation of the cytoplasmic enzyme into the nucleus. Nuclear PKM2 functions as a kinase activating STAT3. The result is the induction of IL-1β and IL-6 gene transcripts. This mechanism directly connects the cell’s metabolic status, precisely the activity of mitochondrial respiration, with pro-inflammatory effector functions.
Conclusions
The loss of self-tolerance in patients with RA precedes joint inflammation by decades and is firmly engraved into the innate and adaptive immune system. The early immunopathogenesis must include maldifferentiation of T cells, which provide help for autoantibody production. Naive CD4 T cells from RA patients have two fundamental abnormalities: they have accelerated aging and have reprogrammed their metabolic machinery. Upon stimulation, these RA T cells differentiate into hyperproliferative and tissue-invasive effector cells that initiate and sustain aggressive tissue inflammation in the synovium.
Molecular studies have identified defects in the DNA repair machinery and in the utilization of glucose, which are linked to changes in T cell behavior:
RA T cells fail to upregulate the glycolytic enzyme PFKFB3 and instead shunt glucose into the pentose phosphate pathway (PPP).
Overutilization of the PPP generates excess NADPH and biosynthetic precursor molecules; resulting in a reductive environment and in cell building.
Surplus of NADPH prevents the activation of ATM, enabling RA T cells to bypass the G2/M cell cycle checkpoint and hyperproliferate.
RA T cells underperform in ATP generation due to a mitochondrial defect.
Mitochondrial insufficiency results from failed repair of mitochondrial DNA, caused by the loss of the DNA repair nuclease MRE11A.
Damaged mitochondrial DNA leaks into the cytoplasm and triggers inflammasome activation. RA T cells are therefore highly pro-inflammatory.
Mitochondrial underperformance prevents fatty acid oxidation and promotes deposition of cytoplasmic lipid droplets.
The bias to lipogenesis supplies membrane lipids for the cell building program and fosters the generation of front-end membrane protrusions, rendering the T cell tissue invasive.
RA T cells are maladaptive and fail to counterbalance the metabolic commitment towards anabolic pathways. Specifically, they fail to activate the energy sensor AMPK and continue to favor catabolic processes despite low ATP concentrations. In RA T cells, lysosomal recruitment of AMPK is insufficient, preventing AMPK activation and mTORC1 inactivation. The diminution of lysosomal AMPK leaves the T cell with lack of anabolic activity and unopposed activation of mTORC1. The underlying defect has been localized to the failure of NMT1, a transferase that adds a lipid tail to AMPK and thus determines its intracellular trafficking and distribution.
Metabolically rewired RA T cells partner with macrophages that have unleashed mitochondrial activity. Hypermetabolic RA macrophages respond to mitochondrial stress with translocation of the glycolytic enzyme PKM2 into the nucleus, where the kinase drives a STAT3-dependent gene program, promoting IL-1β and IL-6 production.
Acknowledgements
This work was supported by the National Institutes of Health (R01AR042527, R01HL117913, R01AI108906, P01HL129941, R01AI108891, R01AG045779, U19AI057266, R01AI129191) and the Encrantz Family Discovery Fund. The authors have no conflicts to declare.
References
- 1.Martins P, Fonseca JE. How to investigate: Pre-clinical rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2019;101438. [DOI] [PubMed] [Google Scholar]
- 2.Kalinkovich A, Gabdulina G, Livshits G. Autoimmunity, inflammation, and dysbiosis mutually govern the transition from the preclinical to the clinical stage of rheumatoid arthritis. Immunol Res. 2018;66(6):696–709. [DOI] [PubMed] [Google Scholar]
- 3.Ma WT, Chang C, Gershwin ME, Lian ZX. Development of autoantibodies precedes clinical manifestations of autoimmune diseases: A comprehensive review. J Autoimmun. 2017;8395–112. [DOI] [PubMed] [Google Scholar]
- 4.Deane KD, Demoruelle MK, Kelmenson LB, Kuhn KA, Norris JM, Holers VM. Genetic and environmental risk factors for rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2017;31(1):3–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagy G, van Vollenhoven RF. Sustained biologic-free and drug-free remission in rheumatoid arthritis, where are we now? Arthritis Res Ther. 2015;17181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Woude D, van der Helm-van Mil AHM. Update on the epidemiology, risk factors, and disease outcomes of rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2018;32(2):174–187. [DOI] [PubMed] [Google Scholar]
- 7.Li Y, Goronzy JJ, Weyand CM. DNA damage, metabolism and aging in pro-inflammatory T cells: Rheumatoid arthritis as a model system. Exp Gerontol. 2018;105118–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weyand CM, Zeisbrich M, Goronzy JJ. Metabolic signatures of T-cells and macrophages in rheumatoid arthritis. Curr Opin Immunol. 2017;46112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weyand CM, Goronzy JJ. Immunometabolism in early and late stages of rheumatoid arthritis. Nat Rev Rheumatol. 2017;13(5):291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang Z, Matteson EL, Goronzy JJ, Weyand CM. T-cell metabolism in autoimmune disease. Arthritis Res Ther. 2015;1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vignali PDA, Barbi J, Pan F. Metabolic Regulation of T Cell Immunity. Adv Exp Med Biol. 2017;101187–130. [DOI] [PubMed] [Google Scholar]
- 12.Kim C, Fang F, Weyand CM, Goronzy JJ. The life cycle of a T cell after vaccination - where does immune ageing strike? Clin Exp Immunol. 2017;187(1):71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goronzy JJ, Weyand CM. Mechanisms underlying T cell ageing. Nat Rev Immunol. 2019;19(9):573–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ocampo DV, Gladman D. Psoriatic arthritis. F1000Res. 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang Z, Shen Y, Oishi H, et al. Restoring oxidant signaling suppresses proarthritogenic T cell effector functions in rheumatoid arthritis. Sci Transl Med. 2016;8(331):331ra338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abboud G, Choi SC, Kanda N, Zeumer-Spataro L, Roopenian DC, Morel L. Inhibition of Glycolysis Reduces Disease Severity in an Autoimmune Model of Rheumatoid Arthritis. Front Immunol. 2018;91973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsokos GC. Metabolic control of arthritis: Switch pathways to treat. Sci Transl Med. 2016;8(331):331fs338. [DOI] [PubMed] [Google Scholar]
- 18.Yang Z, Fujii H, Mohan SV, Goronzy JJ, Weyand CM. Phosphofructokinase deficiency impairs ATP generation, autophagy, and redox balance in rheumatoid arthritis T cells. J Exp Med. 2013;210(10):2119–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bartrons R, Rodriguez-Garcia A, Simon-Molas H, Castano E, Manzano A, Navarro-Sabate A. The potential utility of PFKFB3 as a therapeutic target. Expert Opin Ther Targets. 2018;22(8):659–674. [DOI] [PubMed] [Google Scholar]
- 20.Yalcin A, Telang S, Clem B, Chesney J. Regulation of glucose metabolism by 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatases in cancer. Exp Mol Pathol. 2009;86(3):174–179. [DOI] [PubMed] [Google Scholar]
- 21.Yang HC, Wu YH, Liu HY, Stern A, Chiu DT. What has passed is prolog: new cellular and physiological roles of G6PD. Free Radic Res. 2016;50(10):1047–1064. [DOI] [PubMed] [Google Scholar]
- 22.Miller CG, Holmgren A, Arner ESJ, Schmidt EE. NADPH-dependent and -independent disulfide reductase systems. Free Radic Biol Med. 2018;127248–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weyand CM, Shen Y, Goronzy JJ. Redox-sensitive signaling in inflammatory T cells and in autoimmune disease. Free Radic Biol Med. 2018;12536–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weyand CM, Wu B, Goronzy JJ. The metabolic signature of T cells in rheumatoid arthritis. Curr Opin Rheumatol. 2019;In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen Y, Wen Z, Li Y, et al. Metabolic control of the scaffold protein TKS5 in tissue-invasive, proinflammatory T cells. Nat Immunol. 2017;18(9):1025–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perl A, Gergely P Jr., Puskas F, Banki K. Metabolic switches of T-cell activation and apoptosis. Antioxid Redox Signal. 2002;4(3):427–443. [DOI] [PubMed] [Google Scholar]
- 27.Koetz K, Bryl E, Spickschen K, O’Fallon WM, Goronzy JJ, Weyand CM. T cell homeostasis in patients with rheumatoid arthritis. Proc Natl Acad Sci U S A. 2000;97(16):9203–9208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schonland SO, Lopez C, Widmann T, et al. Premature telomeric loss in rheumatoid arthritis is genetically determined and involves both myeloid and lymphoid cell lineages. Proc Natl Acad Sci U S A. 2003;100(23):13471–13476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goronzy JJ, Shao L, Weyand CM. Immune aging and rheumatoid arthritis. Rheum Dis Clin North Am. 2010;36(2):297–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gire V, Dulic V. Senescence from G2 arrest, revisited. Cell Cycle. 2015;14(3):297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goronzy JJ, Weyand CM. Understanding immunosenescence to improve responses to vaccines. Nat Immunol. 2013;14(5):428–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fujii H, Shao L, Colmegna I, Goronzy JJ, Weyand CM. Telomerase insufficiency in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2009;106(11):4360–4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iyama T, Wilson DM 3rd. DNA repair mechanisms in dividing and non-dividing cells. DNA Repair (Amst). 2013;12(8):620–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shao L, Fujii H, Colmegna I, Oishi H, Goronzy JJ, Weyand CM. Deficiency of the DNA repair enzyme ATM in rheumatoid arthritis. J Exp Med. 2009;206(6):1435–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Biton S, Barzilai A, Shiloh Y. The neurological phenotype of ataxia-telangiectasia: solving a persistent puzzle. DNA Repair (Amst). 2008;7(7):1028–1038. [DOI] [PubMed] [Google Scholar]
- 36.Amirifar P, Ranjouri MR, Yazdani R, Abolhassani H, Aghamohammadi A. Ataxia-telangiectasia: A review of clinical features and molecular pathology. Pediatr Allergy Immunol. 2019;30(3):277–288. [DOI] [PubMed] [Google Scholar]
- 37.Westbrook AM, Schiestl RH. Atm-deficient mice exhibit increased sensitivity to dextran sulfate sodium-induced colitis characterized by elevated DNA damage and persistent immune activation. Cancer Res. 2010;70(5):1875–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kruger A, Ralser M. ATM is a redox sensor linking genome stability and carbon metabolism. Sci Signal. 2011;4(167):pe17. [DOI] [PubMed] [Google Scholar]
- 39.Weyand CM, Yang Z, Goronzy JJ. T-cell aging in rheumatoid arthritis. Curr Opin Rheumatol. 2014;26(1):93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmidt D, Goronzy JJ, Weyand CM. CD4+ CD7- CD28- T cells are expanded in rheumatoid arthritis and are characterized by autoreactivity. J Clin Invest. 1996;97(9):2027–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weyand CM, Brandes JC, Schmidt D, Fulbright JW, Goronzy JJ. Functional properties of CD4+ CD28- T cells in the aging immune system. Mech Ageing Dev. 1998;102(2–3):131–147. [DOI] [PubMed] [Google Scholar]
- 42.Schmidt D, Martens PB, Weyand CM, Goronzy JJ. The repertoire of CD4+ CD28- T cells in rheumatoid arthritis. Mol Med. 1996;2(5):608–618. [PMC free article] [PubMed] [Google Scholar]
- 43.Liuzzo G, Kopecky SL, Frye RL, et al. Perturbation of the T-cell repertoire in patients with unstable angina. Circulation. 1999;100(21):2135–2139. [DOI] [PubMed] [Google Scholar]
- 44.Liuzzo G, Goronzy JJ, Yang H, et al. Monoclonal T-cell proliferation and plaque instability in acute coronary syndromes. Circulation. 2000;101(25):2883–2888. [DOI] [PubMed] [Google Scholar]
- 45.Liuzzo G, Vallejo AN, Kopecky SL, et al. Molecular fingerprint of interferon-gamma signaling in unstable angina. Circulation. 2001;103(11):1509–1514. [DOI] [PubMed] [Google Scholar]
- 46.Li Y, Shen Y, Hohensinner P, et al. Deficient Activity of the Nuclease MRE11A Induces T Cell Aging and Promotes Arthritogenic Effector Functions in Patients with Rheumatoid Arthritis. Immunity. 2016;45(4):903–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oh J, Symington LS. Role of the Mre11 Complex in Preserving Genome Integrity. Genes (Basel). 2018;9(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Casari E, Rinaldi C, Marsella A, et al. Processing of DNA Double-Strand Breaks by the MRX Complex in a Chromatin Context. Front Mol Biosci. 2019;643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li Y, Shen Y, Jin K, et al. The DNA Repair Nuclease MRE11A Functions as a Mitochondrial Protector and Prevents T Cell Pyroptosis and Tissue Inflammation. Cell Metab. 2019;30(3):477–492 e476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Syed A, Tainer JA. The MRE11-RAD50-NBS1 Complex Conducts the Orchestration of Damage Signaling and Outcomes to Stress in DNA Replication and Repair. Annu Rev Biochem. 2018;87263–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kondo T, Kobayashi J, Saitoh T, et al. DNA damage sensor MRE11 recognizes cytosolic double-stranded DNA and induces type I interferon by regulating STING trafficking. Proc Natl Acad Sci U S A. 2013;110(8):2969–2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Doitsh G, Galloway NL, Geng X, et al. Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature. 2014;505(7484):509–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sokoya T, Steel HC, Nieuwoudt M, Rossouw TM. HIV as a Cause of Immune Activation and Immunosenescence. Mediators Inflamm. 2017;20176825493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Appay V, Sauce D. Assessing immune aging in HIV-infected patients. Virulence. 2017;8(5):529–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nguyen TB, Olzmann JA. Lipid droplets and lipotoxicity during autophagy. Autophagy. 2017;13(11):2002–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Henne M And three’s a party: lysosomes, lipid droplets, and the ER in lipid trafficking and cell homeostasis. Curr Opin Cell Biol. 2019;5940–49. [DOI] [PubMed] [Google Scholar]
- 57.Cohen S Lipid Droplets as Organelles. Int Rev Cell Mol Biol. 2018;33783–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saka HA, Valdivia R. Emerging roles for lipid droplets in immunity and host-pathogen interactions. Annu Rev Cell Dev Biol. 2012;28411–437. [DOI] [PubMed] [Google Scholar]
- 59.Yang Z, Goronzy JJ, Weyand CM. The glycolytic enzyme PFKFB3/phosphofructokinase regulates autophagy. Autophagy. 2014;10(2):382–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang Z, Goronzy JJ, Weyand CM. Autophagy in autoimmune disease. J Mol Med (Berl). 2015;93(7):707–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Henne M, Goodman JM, Hariri H. Spatial compartmentalization of lipid droplet biogenesis. Biochim Biophys Acta Mol Cell Biol Lipids. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kidani Y, Elsaesser H, Hock MB, et al. Sterol regulatory element-binding proteins are essential for the metabolic programming of effector T cells and adaptive immunity. Nat Immunol. 2013;14(5):489–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang W, Bai Y, Xiong Y, et al. Potentiating the antitumour response of CD8(+) T cells by modulating cholesterol metabolism. Nature. 2016;531(7596):651–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sanz-Moreno V, Gadea G, Ahn J, et al. Rac activation and inactivation control plasticity of tumor cell movement. Cell. 2008;135(3):510–523. [DOI] [PubMed] [Google Scholar]
- 65.Korpos E, Wu C, Song J, Hallmann R, Sorokin L. Role of the extracellular matrix in lymphocyte migration. Cell Tissue Res. 2010;339(1):47–57. [DOI] [PubMed] [Google Scholar]
- 66.Gaylo A, Schrock DC, Fernandes NR, Fowell DJ. T Cell Interstitial Migration: Motility Cues from the Inflamed Tissue for Micro- and Macro-Positioning. Front Immunol. 2016;7428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hind LE, Vincent WJ, Huttenlocher A. Leading from the Back: The Role of the Uropod in Neutrophil Polarization and Migration. Dev Cell. 2016;38(2):161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alonso F, Spuul P, Daubon T, Kramer I, Genot E. Variations on the theme of podosomes: A matter of context. Biochim Biophys Acta Mol Cell Res. 2019;1866(4):545–553. [DOI] [PubMed] [Google Scholar]
- 69.Eddy RJ, Weidmann MD, Sharma VP, Condeelis JS. Tumor Cell Invadopodia: Invasive Protrusions that Orchestrate Metastasis. Trends Cell Biol. 2017;27(8):595–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Courtneidge SA. Cell migration and invasion in human disease: the Tks adaptor proteins. Biochem Soc Trans. 2012;40(1):129–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Watanabe R, Maeda T, Zhang H, et al. MMP (Matrix Metalloprotease)-9-Producing Monocytes Enable T Cells to Invade the Vessel Wall and Cause Vasculitis. Circ Res. 2018;123(6):700–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nosalski R, Maffia P, Guzik TJ. Monocytes M(MP)aking Way for T-Cell Vascular Infiltration. Circ Res. 2018;123(6):638–640. [DOI] [PubMed] [Google Scholar]
- 73.Herzig S, Shaw RJ. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol. 2018;19(2):121–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hardie DG. AMP-activated protein kinase: an energy sensor that regulates all aspects of cell function. Genes Dev. 2011;25(18):1895–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wen Z, Jin K, Shen Y, et al. N-myristoyltransferase deficiency impairs activation of kinase AMPK and promotes synovial tissue inflammation. Nat Immunol. 2019;20(3):313–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Finlay DK. N-myristoylation of AMPK controls T cell inflammatory function. Nat Immunol. 2019;20(3):252–254. [DOI] [PubMed] [Google Scholar]
- 77.Kim J, Guan KL. mTOR as a central hub of nutrient signalling and cell growth. Nat Cell Biol. 2019;21(1):63–71. [DOI] [PubMed] [Google Scholar]
- 78.Lin SC, Hardie DG. AMPK: Sensing Glucose as well as Cellular Energy Status. Cell Metab. 2018;27(2):299–313. [DOI] [PubMed] [Google Scholar]
- 79.Lamming DW, Bar-Peled L. Lysosome: The metabolic signaling hub. Traffic. 2019;20(1):27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wolfson RL, Sabatini DM. The Dawn of the Age of Amino Acid Sensors for the mTORC1 Pathway. Cell Metab. 2017;26(2):301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Oakhill JS, Chen ZP, Scott JW, et al. beta-Subunit myristoylation is the gatekeeper for initiating metabolic stress sensing by AMP-activated protein kinase (AMPK). Proc Natl Acad Sci U S A. 2010;107(45):19237–19241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liang J, Xu ZX, Ding Z, et al. Myristoylation confers noncanonical AMPK functions in autophagy selectivity and mitochondrial surveillance. Nat Commun. 2015;67926. [DOI] [PubMed] [Google Scholar]
- 83.Foretz M, Guigas B, Viollet B. Understanding the glucoregulatory mechanisms of metformin in type 2 diabetes mellitus. Nat Rev Endocrinol. 2019;15(10):569–589. [DOI] [PubMed] [Google Scholar]
- 84.Zeisbrich M, Yanes RE, Zhang H, et al. Hypermetabolic macrophages in rheumatoid arthritis and coronary artery disease due to glycogen synthase kinase 3b inactivation. Ann Rheum Dis. 2018;77(7):1053–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shirai T, Nazarewicz RR, Wallis BB, et al. The glycolytic enzyme PKM2 bridges metabolic and inflammatory dysfunction in coronary artery disease. J Exp Med. 2016;213(3):337–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Alquraishi M, Puckett DL, Alani DS, et al. Pyruvate kinase M2: A simple molecule with complex functions. Free Radic Biol Med. 2019;143176–192. [DOI] [PMC free article] [PubMed] [Google Scholar]