Abstract
Depressive symptoms among HIV-positive (HIV+) women may negatively impact their health and possibly that of their young children through risk of compromised caregiving. We evaluated how depression symptoms in predominantly (97%) female caregivers relate to neurodevelopmental outcomes in their HIV affected children. Data come from the IMPAACT P1104s Study, an observational cohort across six sites in four countries: Zimbabwe, South Africa, Uganda and Malawi. Participants (n=611) were 5-11 year old children with HIV (HIV), HIV exposed uninfected (HEU), or HIV unexposed uninfected (HUU). Primary caregivers were assessed for depression with the Hopkins Symptom Checklist (HSCL) and children with Behavior Rating Inventory for Executive Function (BRIEF) parent-report, Kauffman Assessment Battery for Children II (KABC), Bruininks-Oseretsky Test of Motor Proficiency 2nd Ed. (BOT-2), Test of Variables of Attention (TOVA), Multiple Indicators Cluster Survey, Child Disability and Development scales (MICS-4). Caregivers with higher depression scores (> 1.75 mean HSCL score) reported more executive function problems in their children, regardless of HIV status. All executive function scores were significantly (p<0.001) associated with depressive symptomatology at baseline and across time. Caregiver depressive symptomatology was not associated with other assessed neurocognitive outcomes. These results highlight the potential impact of caregiver depression on child behavioral outcomes.
Keywords: Executive functioning, child neuropsychology, HIV/AIDS, Sub-Saharan Africa
Introduction
Globally, HIV+ women are more likely to experience depressive symptoms then their uninfected peers (19.4% vs. 4.8%) (Morrison et al., 2002). Recently, a global survey conveyed that up to 82% of HIV+ women reported symptoms of depression (Orza et al., 2015). Substantial evidence from observational and longitudinal studies in low- and middle-income countries (LMIC) suggest that depression in mothers or primary caregivers can contribute to multiple early child growth and developmental problems including nutritional status, health and socio-emotional behaviour (Bennett, Schott, Krutikova, & Behrman, 2016; Black et al., 2007; Grantham-McGregor et al., 2007; Surkan, Kennedy, Hurley, & Black, 2011). In high-income countries, longitudinal studies show that persistent and severe depression symptoms in caregivers are associated with behavioral problems (Cho, Kim, Lim, Lee, & Shin, 2015; Hughes, Ensor, Wilson, & Graham, 2009; Netsi et al., 2018), and lower school performance in young children (Grant, McMahon, & Austin, 2008).
One of the mechanisms linking caregiver depression and child development are family processes such as parenting. In particular, studies from mostly high-income countries have shown an association between maternal depression and suboptimal parenting practices, resulting in less secure mother-child attachments (Goodman et al., 2011; Santona et al., 2015). These studies describe how depressed mothers are more likely to be inconsistent, withdrawn or intrusive, increasing the risk for maladjustment in their children (Goodman, 2007). Although the underlying mechanisms associating maternal psychological well-being and child development might be similar in LMIC (Herba, Glover, Ramchandani, & Rondon, 2016), depression in caregivers is frequently compounded in contexts of high HIV/AIDS with other risk-factors for compromised child development such as trauma, poverty and poor access to basic services (Wekesa, 2000).
Environmental factors frequently encountered in low-income settings and related to HIV disease in mothers such as psychological (e.g. depression) (Surkan, et al., 2011), behavioral (e.g. compromised caregiving) (Cooper et al., 1999), and socioeconomic factors can adversely impact neurodevelopment in young children. In a previous trial conducted in Uganda by our group, high-quality caregiver-child interactions (defined as number of opportunities where mediated learning and praise occurs between caregiver and child) resulting from a directed intervention were shown to partially act as buffers in HIV affected children, moderating negative environmental effects such as poverty and adversity (Bass et al., 2016; Familiar et al., 2018). Therefore, understanding the relationship between caregiver’s depressive symptoms and child neurodevelopment is critical as HIV-affected children are at particular risk for cognitive deficits and developmental delays (Leis, Heron, Stuart, & Mendelson, 2014).
In this paper we sought to assess the association between caregiver’s depressive symptoms and child neuropsychological outcomes in HIV+ and HIV unexposed young children in Sub-Saharan Africa. Based on the available literature, we hypothesize that more depressive symptomatology in caregivers will be associated with poorer neurodevelopment in their children.
Materials and Methods
This is a secondary analysis using data from IMPAACT P1104S, an observational longitudinal study of three cohorts of children enrolled between five and eleven years of age of varying HIV status and conducted at six research sites: South Africa (Wits RHI Shandukani Research Centre in Johannesburg, Perinatal HIV Research Unit in Soweto, and Family Clinical Research Unit in Cape Town), Malawi (University of North Carolina Project at Kamuzu Central Hospital in Lilongwe), Uganda (Makerere University – Johns Hopkins University Clinic Mulago National Referral Hospital in Kampala), and Zimbabwe (Parirenyatwa General Hospital in Harare). IMPAACT P1104s was designed primarily to evaluate the relationship between HIV infection and neurodevelopmental outcomes in children.
Participants and procedures
Details on the study design, objectives and outcome measures can be found elsewhere (M. J. Boivin et al., 2018). Briefly, children and their caregivers were invited to participate if they met the following eligibility criteria: (1) HIV+ children participating in P1060 Version 5.0, (2) HEU children born to HIV+ mothers recruited from the same households, extended families, or neighbourhoods/communities as the P1060 participants, and (3) HUU children attending vaccination and outpatient treatment clinics at each of the P1060 study sites. Only HUU children were medically screened for prior hospitalizations that could involve brain injury (e.g., cerebral malaria, meningitis, head trauma) or severe malnutrition, and excluded if they screened positive for any developmental disability. Six hundred and fifteen children (246 HIV, 185 HEU, 184 HUU) and their caregivers were enrolled in the P1104s study across the six research sites and 611 completed assessments at 3 time points (0, 48 and 96 weeks after baseline). Research assistants from the six study sites were trained by the study PI during a one-week workshop on the administration of study assessments. Most assessments were conducted in the local language (Sesotho, Setswana, Zulu, Xhosa, Afrikaans, English, Chichewa, Luganda, and Shona), after translation and back-translation.
The institutional review board (IRB) at each study site, where applicable the corresponding ministry of health in the host country, and the university partner in the United States for each study site approved this study. Informed consent was obtained from parents or primary caregivers with additional assent from children 7 years and older based on country regulations.
Measures
Child demographics at baseline included age, gender, schooling status, race, height, age of ARV initiation, ARV regimen, HIV disease stage, CD4 percent and HIV RNA copies. Caregiver demographics included HIV status, education, employment, and current residential zone. A socioeconomic index developed ad hoc was calculated based on seven characteristics: fuel, water, refrigerator, caregiver’s work status, social grant as main source of income, caregiver education and sufficiency of household income to meet basic needs. Each item was scored from 0-10, and a mean score was computed with 10 reflecting the highest socio-economic status.
Behavior Rating Inventory of Executive Function–School age (BRIEF).
The BRIEF evaluates behavior, attention and cognitive problems related to disruption of executive function (EF) as reported in a series of questions to the principal caregiver (Gioia and Isquith, 2011). Indices used in this analysis include Behavior Regulation Index (BRI) Metacognition Index (MI), and a combined Global Executive Composite (GEC) score. The BRIEF was previously adapted for use in Uganda (Familiar et al., 2016; Familiar et al., 2015), translated into local languages at all study sites (with permission of the publisher including approval of the back translation).
Kaufman Assessment Battery for Children, second edition (KABC-II).
The KABC-II is a standardized test of cognitive functioning/ abilities, intelligence and achievement, for children ages 3-18 years previously adapted (Bangirana et al., 2009) and validated in the present study sites (Chernoff, 2018). The principal outcome variable used was the Mental Processing Index (MPI, global score).
Multiple Indicators Cluster Survey, 4th round (MICS4) Questionnaire for Child Development and Disability.
At baseline, caregivers responded to the Early Childhood Development Questionnaire for Children Under 5 (17 items), and the Child Disability Questionnaire of the Multiple Indicators Cluster Survey (4th round) (MICS4). Item responses were added and scaled for a total score ranging from 0-100.
Test of Variables of Attention (TOVA).
The TOVA is a computerized visual continuous performance test used in the diagnosis and monitoring of children with attention deficit disorders (Greenberg, 1993) adapted for pediatric HIV research in Uganda. (M. J. Boivin, Busman, et al., 2010; M. J. Boivin, Nakasujja, Sikorskii, Opoka, & Giordani, 2016; M. J. Boivin, Ruel, et al., 2010; Giordani et al., 2015; Ruel et al., 2012). An attention deficit – hyperactivity disorder (ADHD) index score is computed with ADHD scores more negative than −1.8 considered suggestive of ADHD for USA norms.
Bruininks-Oseretsky Test of Motor Proficiency, 2nd edition (BOT-2).
The BOT-2 is a comprehensive motor assessment (Bruininks and Bruininks, 2005) previously adapted for pediatric HIV assessment in Uganda (M. J. Boivin, Ruel, et al., 2010; Ruel, et al., 2012). Three items are combined into total composite score of motor proficiency, standardized by age and gender using American norms.
Hopkins Symptom Checklist (HSCL-25).
Caregiver’s depression symptoms were assessed with the mean of the fifteen depression item scores from the Hopkins Symptom Checklist (HSCL-25), where each item was scored on a Likert scale from “1” (not at all) to “4” (often). Mean depression scores were categorized using the standard cut off of 1.75 (0-1.75, >1.75)(Kinyanda, Hoskins, Nakku, Nawaz, & Patel, 2011; Tsai et al., 2015) to derive clinically meaningful categories (i.e. low and high symptomatology).
Statistical analyses
Socio-demographic and HIV disease characteristics at baseline were summarized and compared across cohorts using chi-square, Kruskal-Wallis, and analysis of variance F-tests.
Unadjusted and adjusted linear regression models were performed with generalized estimating equations (GEE models) to separately assess associations between caregiver depression (as continuous and dichotomous measure) and neuropsychological test scores at each study visit. Adjusted analyses included covariates for design (child HIV status, research site), child (age, gender, WHO height for age z-score), caregiver (biological mother) and household characteristics (residential zone, socio-economic index).
Longitudinal linear GEE regression models explored pairwise and three-way interactions of child HIV group, assessment time point (week 0, 48, or 96) and level of caregiver depression (low vs. high), adjusting for all potential confounders noted above. Only interaction effects with p<0.05 were included in final analyses. Least squares (adjusted) means or slope estimates and their 95% confidence intervals were estimated for each set of regressions. Tests of statistical significance were two-sided and, unless noted, 5% error rates were used for hypothesis testing. SAS v9.4 software was used (SAS Institute Inc., 2013). Adjustments were not made for multiple testing.
Results
Data were available for 611 dyads at the baseline assessment and Table 1 shows their characteristics. HIV+ children had lower height for age z-scores (−1.04, SD=0.97, p<0.001) and higher disability scores (8.78, SD= 11.51, p<0.001) compared to HEU and HUU children and a higher proportion of their caregivers were not biological mothers. Most caregivers were female (97% overall, 93.5% among HIV+ children). In almost all HIV+ children; CD4 percentages were above 25% and HIV RNA viral loads below 400 for over 96% of participants.
Table 1.
Child and caregiver characteristics at baseline by child HIV status
| Cohort | ||||||
|---|---|---|---|---|---|---|
| Characteristic | HIV+ (N=246) |
HEU (N=183) |
HUU (N=182) |
Total (N=611) |
P- Value |
|
| Gender | Male | 111 (45%) | 95 (52%) | 84 (46%) | 290 (47%) | 0.35 (a) |
| Race | Black African | 242 (98%) | 176 (96%) | 150 (82%) | 568 (93%) | <.001 (a) |
| Coloured/White/Other | 4 (2%) | 7 (4%) | 32 (18%) | 43 (7%) | ||
| Age (years) | Mean (s.d.) | 7.1 (1.2) | 7.3 (1.6) | 7.3 (1.5) | 7.2 (1.4) | 0.46 (b) |
| <6 yrs | 41 (17%) | 36 (20%) | 35 (19%) | 112 (18%) | 0.87 (a) | |
| 6-<7 yrs | 83 (34%) | 64 (35%) | 64 (35%) | 211 (35%) | ||
| >=7 yrs | 122 (50%) | 83 (45%) | 83 (46%) | 288 (47%) | ||
| 166 (71%) | 119 (66%) | 121 (70%) | 406 (69%) | 0.65 (a) | ||
| WHO height z-score | Mean (s.d.) | −1.04 (0.97) | −0.38 (1.03) | −0.43 (1.06) | −0.66 (1.06) | <.001 (b) |
| MICS development score | Mean (s.d.) | 73.04 (18.02) | 76.87 (16.39) | 74.87 (16.48) | 74.73 (17.14) | 0.15 (c) |
| MICS disability score | Mean (s.d.) | 8.78 (11.51) | 3.50 (6.61) | 3.57 (6.56) | 5.65 (9.25) | <.001 (c) |
| Caregiver is biological mother | No | 37 (15%) | 2 (1%) | 0 (0%) | 39 (6%) | <.001 (a) |
| Yes | 209 (85%) | 181 (99%) | 182 (100%) | 572 (94%) | ||
| HIV Status of primary caregiver | HIV-uninfected | 10 (4%) | 1 (1%) | 182 (100%) | 193 (32%) | <.001 (a) |
| HIV-infected | 230 (96%) | 182 (99%) | 0 (0%) | 412 (68%) | ||
| Not reported | 6 | 0 | 0 | 6 | ||
| Mean HSCL Depression score | Mean (s.d.) | 1.81 (0.64) | 1.75 (0.64) | 1.75 (0.63) | 1.78 (0.63) | 0.48 (c) |
| Low (0-1.75) | 139 (57%) | 104 (57%) | 108 (60%) | 351 (58%) | 0.79 (a) | |
| High (>1.75+) | 107 (43%) | 79 (43%) | 73 (40%) | 259 (42%) | ||
| Current residential zone | Rural | 51 (21%) | 29 (16%) | 29 (16%) | 109 (18%) | 0.63 (a) |
| Peri-urban | 103 (42%) | 81 (44%) | 84 (46%) | 268 (44%) | ||
| Urban | 92 (37%) | 73 (40%) | 69 (38%) | 234 (38%) | ||
| Missing | 0 | 1 | 0 | 1 | ||
| Socio-economic index | Median (Q1, Q3) | 6.0 (4.4, 7.6) | 5.7 (4.8, 7.4) | 6.2 (4.7, 7.9) | 6.0 (4.6, 7.6) | 0.33 (c) |
Chi-Square Test
Analysis of Variance
Kruskal-Wallis Test
Forty-two percent of caregivers reported depression symptoms above 1.75 in the HSCL and 18% did so at all assessment time-points. Mean depression score at baseline was comparable between caregivers of HIV+ children (mean= 1.81) and of HEU or HUU children (mean=1.75; p=0.48). As can be seen from group means, KABC MPI, BOT and TOVA D Prime scores were significantly lower among HIV+ children compared to HEU and HUU children (Table 2).
Table 2.
Neuropsychological test scores at baseline by child HIV status
| Cohort | |||||
|---|---|---|---|---|---|
| Mean (SD) scores | HIV (N=246) |
HEU (N=183) |
HUU (N=182) |
Total (N=611) |
P-Value (a) |
| KABC Mental Processing Index | 73.2 (10.5) | 79.1 (11.4) | 80.9 (11.7) | 77.3 (11.6) | <.001 |
| BOT Total | 48.3 (8.8) | 52.5 (7.6) | 52.6 (7.8) | 50.8 (8.4) | <.001 |
| BRIEF Metacognition Index | 52.2 (13.0) | 51.9 (12.1) | 49.8 (10.5) | 51.4 (12.1) | 0.11 |
| BRIEF Behavioral Regulation Index | 53.1 (13.0) | 51.2 (12.3) | 51.9 (11.2) | 52.2 (12.3) | 0.27 |
| BRIEF Global Executive Composite | 53.2 (13.3) | 51.5 (12.0) | 50.6 (10.5) | 51.9 (12.2) | 0.09 |
| TOVA ADHD score | −0.51 (3.13) | 0.51 (2.63) | 0.28 (2.59) | 0.03 (2.86) | <.001 |
| TOVA D Prime standard score | 82.5 (14.0) | 88.3 (12.3) | 87.9 (11.7) | 85.9 (13.1) | <.001 |
Analysis of Variance
HIV+: HIV infected; HEU: HIV exposed uninfected; HUU: HIV unexposed uninfected
Number of observations varied by test: KABC= 606, BOT-2= 607, BRIEF= 608, TOVA= 581, MICS= 610
Adjusted regression models showed that higher caregiver’s mean depression score was associated with higher BRI, MI and GEC indices of EF (p < 0.001) at study baseline; for each additional point on the mean caregiver depression score, all BRIEF scores increased by six to seven points, indicative of more EF problems (Table 3). The MICS disability score increased by about 1.5 points (p=0.02) and the MICS developmental score decreased by 3.0 points (p=0.002) for each additional point on mean caregiver depression score.
Table 3.
Association of caregiver depression with neuropsychological test scores at baseline
| Depression score | ||||
|---|---|---|---|---|
| Unadjusted | Adjusted | |||
| Outcome | Slope (SE) | P-val | Slope (SE) | P-val |
| KABC Mental processing index | −1.30 (0.71) | 0.07 | −0.44 (0.68) | 0.52 |
| BOT-2: Total score | 0.46 (0.51) | 0.37 | −0.57 (0.53) | 0.28 |
| BRIEF BRI | 7.29 (0.76) | <0.001 | 7.08 (0.81) | <0.001 |
| BRIEF MI | 5.12 (0.77) | <0.001 | 6.02 (0.84) | <0.001 |
| BRIEF GEC | 6.66 (0.77) | <0.001 | 6.98 (0.84) | <0.001 |
| TOVA ADHD | 0.23 (0.18) | 0.20 | −0.13 (0.19) | 0.49 |
| TOVA D-prime standard score | −0.83 (0.80) | 0.30 | −0.73 (0.89) | 0.41 |
| MICS Disability (%) | 0.79 (0.61) | 0.20 | 1.52 (0.65) | 0.02 |
| MICS Development (%) | 3.27 (1.16) | 0.005 | −2.97 (0.93) | 0.002 |
Kauffman Assessment Battery Test for Children II (KABC -II); Bruininks-Oseretsky Test of Motor Proficiency 2 (BOT-2); Behavior Rating Inventory of Executive Function (BRIEF) Behavioral Regulation Index (BRI), Metacognition Index (MI), Global Executive Composite (GEC); Test of Variables of Attention (TOVA), Attention-Deficit/Hyperactivity Disorder (ADHD).
Note: Results were adjusted for cohort, site, gender, age at baseline, caregiver relationship to child, residential zone, WHO height for age z-score, and socio-economic index
Adjusted regression analyses comparing the association between low and high levels of caregiver depression symptoms with BRIEF scores at baseline showed similar results (data not shown). Interactions between child HIV status and level of caregiver depression (with a cut-off of 1.75) were largely non-significant (p>0.05, results not shown). Mean KABC MPI scores were marginally lower (p=0.05 unadjusted, p=0.12 adjusted analysis) among HIV-infected children (meanadj =76.0) compared with HEU (meanadj = 80.2) and HUU (meanadj = 81.3) at low levels of caregiver depression but even lower for HIV-infected children of caregivers with high levels of depression (meanadj = 73.4).
Table 4 presents the main effects of depression level and study week, as well as the interaction between depression level and study week. Overall, results indicated small but statistically significant and differing changes over time for children of caregivers with differing levels of depression (p<0.001 for all but one depression × week interaction effect).
Table 4.
Adjusted least squares means at each study visit by level of caregiver depression
| Low depression (0-1.75) |
High depression (>1.75) |
P values | ||||
|---|---|---|---|---|---|---|
| Outcome | Week | Adjusted mean (95% CI) |
Adjusted mean (95% CI) |
Depression | Week | Depression × Week |
| KABC Mental Processing Index | 0 | 78.94 (77.48,80.39) | 76.26 (74.76,77.76) | 0.41 | <0.001 | <0.001 |
| 48 | 77.50 (76.14,78.87) | 76.96 (75.45,78.46) | ||||
| 96 | 78.08 (76.66,79.51) | 80.28 (78.71,81.85) | ||||
| BOT-2 Total | 0 | 50.94 (49.87,52.02) | 51.33 (50.21,52.45) | 0.81 | <0.001 | <0.001 |
| 48 | 52.34 (51.33,53.34) | 51.12 (50.04,52.21) | ||||
| 96 | 50.51 (49.54,51.48) | 51.12 (50.07,52.17) | ||||
| BRIEF BRI | 0 | 50.49 (48.75,52.23) | 55.39 (53.56,57.21) | <0.001 | <0.001 | <0.001 |
| 48 | 47.94 (46.26,49.61) | 54.23 (52.37,56.08) | ||||
| 96 | 46.12 (44.58,47.66) | 55.67 (53.80,57.54) | ||||
| BRIEF MI | 0 | 49.95 (48.19,51.71) | 53.43 (51.57,55.29) | <0.001 | <0.001 | <0.001 |
| 48 | 46.64 (44.99,48.28) | 51.15 (49.38,52.93) | ||||
| 96 | 44.46 (42.90,46.02) | 52.66 (50.87,54.45) | ||||
| BRIEF GEC | 0 | 50.09 (48.29,51.89) | 54.58 (52.68,56.48) | <0.001 | <0.001 | <0.001 |
| 48 | 46.99 (45.29,48.70) | 52.45 (50.57,54.33) | ||||
| 96 | 44.85 (43.25,46.46) | 54.07 (52.22,55.91) | ||||
| TOVA ADHD | 0 | −0.19 (−0.64,0.26) | 0.15 (−0.34,0.64) | 0.39 | <0.001 | 0.002 |
| 48 | 0.83 (0.37,1.29) | 0.33 (−0.14,0.81) | ||||
| 96 | 1.00 (0.56,1.45) | 0.77 (0.27,1.27) | ||||
| TOVA D-Prime | 0 | 86.41 (84.46,88.35) | 86.20 (84.08,88.33) | 0.14 | <0.001 | <0.001 |
| 48 | 91.24 (89.24,93.23) | 87.72 (85.61,89.84) | ||||
| 96 | 90.46 (88.56,92.35) | 91.20 (89.05,93.34) | ||||
Kauffman Assessment Battery Test for Children II (KABC -II); Bruininks-Oseretsky Test of Motor Proficiency 2 (BOT-2); Behavior Rating Inventory of Executive Function (BRIEF) Behavioral Regulation Index (BRI), Metacognition Index (MI), Global Executive Composite (GEC); Test of Variables of Attention (TOVA), Attention-Deficit/Hyperactivity Disorder (ADHD).
Number of observations varied by test and assessment time point. In week 48: KABC= 598, BOT-2= 600, BRIEF= 601, T0VA= 592. In week 96: KABC= 583, BOT-2= 585, BRIEF= 585, TOVA= 580.
At all time points, BRIEF scores were lower (indicative of better EF) in children whose mothers had lower levels of depression (Figure 1). All the BRIEF scores tended to decrease over successive assessments in children of caregivers with low levels of depression compared to those children of caregivers with high levels of depression.
Figure 1.
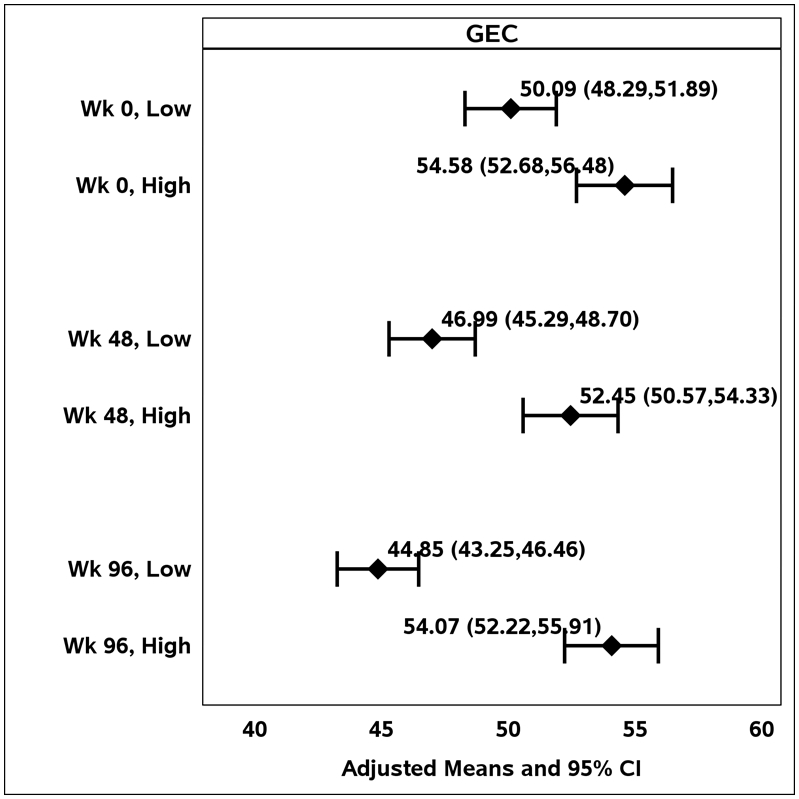
Adjusted means for the Behavior Rating Inventory of Executive Function Global Executive Composite (GEC) score, by caregiver level of depression (high vs. low) over time.
Discussion
Results presented here suggest that EF in HIV-affected children is associated with caregiver’s depression symptoms. Findings help characterize EF among children living in the world region with the greatest burden of HIV-illness. In the present study, all EF domains (eg. BRIEF scores) were significantly associated with caregiver’s depression symptoms, suggesting there is no particular area of EF that is associated with depressive symptomatology. The association between BRIEF scores and depression symptomatology was observed at all time points assessed in this study, suggesting that this cognitive domain may be particularly vulnerable to maternal psychological well-being.
Executive function is a collection of goal-directed behaviors that emerge during early childhood, when children develop the ability to ignore distraction, inhibit inappropriate responses, shift between different tasks, and integrate these abilities to solve more complex problems (Garon, Piccinin, & Smith, 2016). EF is related to problem-solving and decision-making, and a growing body of research highlights the importance of executive functioning in key development areas such as social cognition (Hughes, et al., 2009), academic achievement (Best, Miller, & Naglieri, 2011), and emotion regulation (Ferrier, Bassett, & Denham, 2014). Although the exact mechanism is still elusive, studies in high income countries have shown that depressed mothers frequently display hostile and unresponsive behaviors that can affect the development of basic cognitive skills in children, including EF (Baker, 2018; Network, 2005) (Gueron-Sela, Camerota, Willoughby, Vernon-Feagans, & Cox, 2018). As maternal depression symptoms increase, parenting behaviors promoting children’s sustained attention and EF tend to decline (Gueron-Sela, et al., 2018). Exposure to maternal depressive symptoms in the first 3 years of life has been found to be particularly critical for EF development (Gueron-Sela, et al., 2018), coinciding with the developmental stage when it is thought that EF circuitry is formed in the brain.
Given that depression symptoms and EF behaviors were both reported by the caregiver, one has to consider the possibility of a reporting bias. Previous studies (Najman et al., 2001), including one from our group (Familiar, et al., 2016), have reported that depressed mothers can negatively overstate their children’s EF behaviors in parent-report measures. However, we also found some evidence of poorer EF among children exposed to higher caregiver depressive symptomatology in the KABC test, where there is direct observation of the child performing specific tasks. It should be noted that the design of our previous study (Familiar, et al., 2016) contrasts significantly and could account for differing results; children in the present sample are older (school age vs. pre-school) and the neuropsychological test used is different (KABC vs. Mullen Scales of Early Learning). Collectively, results suggest that the psychological well-being of the caregiver, especially in the presence of depressive symptoms, must be taken into account when assessing child executive behavior.
HIV+ children had slightly lower KABC MPI scores when compared to their non-infected peers, as previously reported in other studies (Brahmbhatt et al., 2014; Sherr, Mueller, & Varrall, 2009) (McHenry et al., 2018) including younger (<2 years) samples of children. Reports hypothesize that symptomatic HIV disease in caregivers can result in compromised cognitive development among their HIV+ children through a disruption in the nurturing capacity of the mother (Van Rie et al., 2008), and diminished quality of caregiving (Bass, et al., 2016).
Overall, cognitive tests used in this study (TOVA ADHD, BOT, and KABC) had small and statistically significant variations at each assessment time point that were in different directions among children exposed to differing levels of caregiver depression. Standard interpretation of T-scores for the KABC proposes that any scores at or −1.5 to −2 standard deviations below the average indicate significant delay. Clinically meaningful differences are accepted at 6 to 8 points. However, without specific norms for African populations, it is difficult to establish cut-off scores. The KABC scores observed in this sample are within those reported among HIV-affected Ugandan children aged 6-12(Ruel, et al., 2012). Given that mean scores for KABC, BOT and TOVA varied in magnitude and direction, their interpretation is limited and warrants caution.
Evidence from high-income countries has associated maternal depression with negative child development outcomes, but research from LMIC has been more inconsistent. Studies conducted in India, Bangladesh (Black, et al., 2007), South Africa (Avan, Richter, Ramchandani, Norris, & Stein, 2010) and Brazil (Quevedo et al., 2012) have found that perinatal depression is associated with clinical and developmental outcomes in children. However, other studies have found no association (Bhat et al., 2015; Familiar, et al., 2016). Important methodological differences may account for these inconsistencies and include different depression assessment instruments, varying cut-offs used to determine associations, variations in cognitive assessments employed, small samples and different age ranges.
Although the proportion of women with clinically meaningful depression symptomatology (e.g. mean depression score > 1.75) was lower than what has been reported in other studies of women living with HIV in semi-urban Uganda (Kinyanda, et al., 2011) and in a clinical setting in Tanzania (Sudfeld et al., 2017), they are still concerning. This high prevalence (42%) of depressive symptoms suggests that HIV+ women living Sub-Saharan Africa may lack access to mental health and psychosocial support services that are urgently needed.
A few limitations must be noted. This is an exploratory and does not address causal relations. We have postulated some interpretations to the associations we observed based on existing literature. Although American norms were used to standardize KABC-II scores, Chernoff et al (2018) obtained consistent results for the factor structure of our KABC-II subtests. The relatively large sample size may have resulted in significant but perhaps small and not clinically meaningful effects, especially when looking at interactions between depression level and time. Although multiple testing may also have led to spurious significance findings, we based our conclusion on the consistency of our findings. Although a small proportion of caregivers in this study were men, we feel the findings would not have changed substantially were we to have excluded them from the analysis. We retained those participants with male caregivers to maximize our sample and obtain an overview of all caregivers’ behaviors. Depression symptoms were collected by self-report and thus social desirability bias may have led to underreporting, or similarly worded items may have influenced women to respond consistently regardless of actual experience. However, this effect is likely to have remained consistent over time and thus provided acceptable assessment of symptom change.
Even with limitations, results presented here highlight the importance of caregiver’s psychological well-being for EF development in their children across several Sub-Saharan African countries. Depression symptoms in caregivers can be a potentially modifiable component of child-development interventions aimed toward at-risk children in LMICs. Integrated programs combining early childhood development curriculums with caregiver psychological well-being support or interventions have been successful in reducing depression symptoms and bolstering child neurodevelopment in LMIC (Michael J Boivin et al., 2017; Singla, Kumbakumba, & Aboud, 2015). Understanding the type of depression treatment that is most helpful to mothers may be an effective strategy to bolster early cognitive development in this context.
Figure 2.
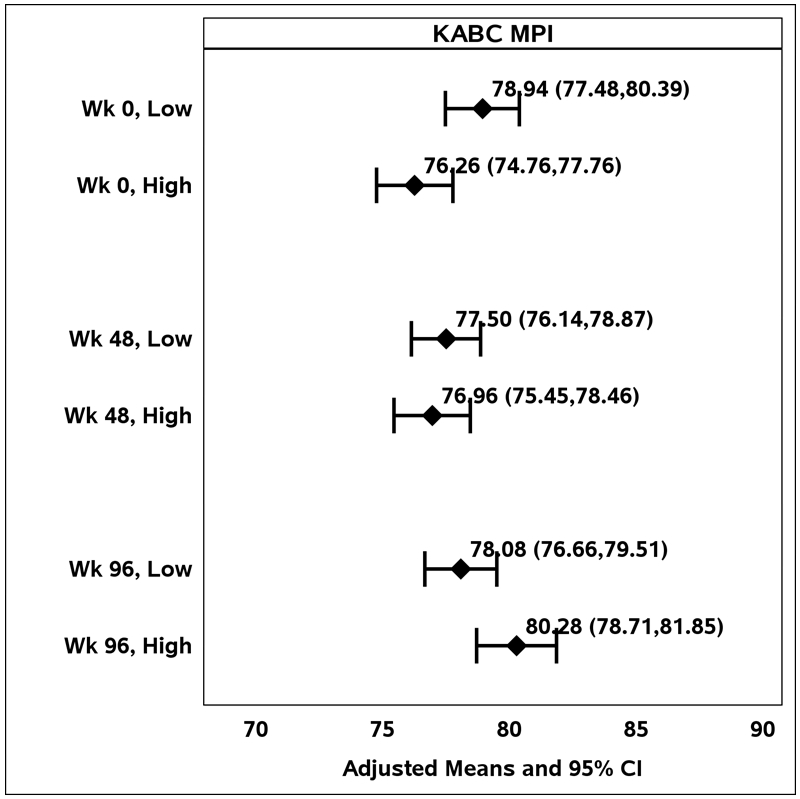
Adjusted means for the Kauffman Assessment Battery for Children II Mental Processing Index score by level of caregiver depression, over time.
Acknowledgements:
Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases (NIAID) with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health (NIMH), all components of the National Institutes of Health (NIH), under Award Numbers UM1AI068632 (IMPAACT LOC), UM1AI068616 (IMPAACT SDMC) and UM1AI106716 (IMPAACT LC), and by NICHD contract number HHSN275201800001I. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Drs. E. Pim Brouwers (NIH/NIMH) and Sonia Lee (NIH/NICHD) served as protocol advisors to the research leadership team for P1104s for their respective NIH institutes. We gratefully acknowledge their expertise and counsel during the study. We would like to acknowledge Jane Lindsey for key contributions to study design, implementation and analysis, to Meredith Warsaw for critical review of study design, and to both Meredith Warshaw and Heather Ribaudo for advice on study analysis. We extend our gratitude to members of the research staff at each site: Mary Nyakato and Agatha Kuteesa (Uganda), Janet Grab and Mmule Ratswana (Zimbabwe), Patricia Thinda, Noel Mumba (Malawi), Haseena Cassim and Given Leshabane (Soweto, RSA), Joan Coetzee and Thandiwe Hamana (Stellenboch, RSA), Sukunema J Maturure and Mary N Tichareva (Zimbabwe).
Footnotes
Declaration of interest
We declare we have no conflicts of interest to disclose
Contributor Information
Itziar Familiar, Department of Psychiatry, Michigan State University, East Lansing, USA.
Miriam Chernoff, Center for Biostatistics in AIDS Research, Harvard University, Boston, USA.
Horacio Ruisenor-Escudero, Department of Psychiatry, Michigan State University, East Lansing, USA.
Barbara Laughton, Department of Pediatrics and Child Health, Stellenboch University, Tygerberg, RSA.
Celeste Joyce, Perinatal HIV Research Unit (PHRU), Chris Hani Baragwanath Hospital, Johannesburg, RSA.
Lee Fairlie, Wits Reproductive Health and HIV Institute, Shandukani Clinic, Johannesburg, RSA.
Tichaona Vhembo, Harare Family Care CRS, University of Zimbabwe, Harare, Zimbabwe.
Portia Kamthunzi, University of North Carolina-Lilongwe Clinical Research Institute, Lilongwe, Malawi.
Linda Barlow, Makerere University-Johns Hopkins University Research Collaboration, Kampala, Uganda.
Bonnie Zimmer, Frontier Science Foundation, Amherst, USA.
Katie McCarthy, FHI 360, Durham, USA.
Michael J. Boivin, Departments of Psychiatry and Neurology, Michigan State University, East Lansing, USA
References
- Avan B, Richter LM, Ramchandani PG, Norris SA, & Stein A (2010). Maternal postnatal depression and children’s growth and behaviour during the early years of life: exploring the interaction between physical and mental health. Arch Dis Child, 95(9), pp. 690–695. [DOI] [PubMed] [Google Scholar]
- Baker CE (2018). Maternal depression and the development of executive function and behavior and behavior problems in Head Start: indirect effects through parenting.. Infant Ment Health J, 39(2), pp. 134–144. doi: 10.1002/imhj.21698 [DOI] [PubMed] [Google Scholar]
- Bangirana P, Musisi S, Allebeck P, Giordani B, John C, Opoka R, … Boivin M (2009). A preliminary investigation of the construct validity of the KABC-II in Ugandan children with prior cerebral insult. African health sciences, 9(3), pp. 186–192. [PMC free article] [PubMed] [Google Scholar]
- Bass JK, Nakasujja N, Familiar-Lopez I, Sikorskii A, Murray SM, Opoka R, … Boivin MJ (2016). Association of caregiver quality of care with neurocognitive outcomes in HIV-affected children aged 2-5 years in Uganda. AIDS Care, 28 Suppl 1, pp. 76–83. doi: 10.1080/09540121.2016.1146215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett IM, Schott W, Krutikova S, & Behrman JR (2016). Maternal mental health, and child growth and development, in four low-income and middle-income countries. J Epidemiol Community Health, 70(2), pp. 168–173. doi: 10.1136/jech-2014-205311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best JR, Miller PH, & Naglieri JA (2011). Relations between executive function and academic achievement from ages 5 to 17 in a large, representative national sample. Learning and individual differences, 21(4), pp. 327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat A, Chowdayya R, Selvam S, Khan A, Kolts R, & Srinivasan K (2015). Maternal prenatal psychological distress and temperament in 1-4 month old infants - A study in a non-western population. Infant Behav Dev, 39, pp. 35–41. doi: 10.1016/j.infbeh.2014.12.002 [DOI] [PubMed] [Google Scholar]
- Black MM, Baqui AH, Zaman K, McNary SW, Le K, Arifeen SE, … Black RE (2007). Depressive symptoms among rural Bangladeshi mothers: implications for infant development. J Child Psychol Psychiatry, 48(8), pp. 764–772. doi: 10.1111/j.1469-7610.2007.01752.x [DOI] [PubMed] [Google Scholar]
- Boivin MJ, Barlow-Mosha L, Chernoff MC, Laughton B, Zimmer B, Joyce C, … Palumbo PE (2018). Neuropsychological performance in African children with HIV enrolled in a multisite antiretroviral clinical trial. AIDS, 32(2), pp. 189–204. doi: 10.1097/qad.0000000000001683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin MJ, Busman RA, Parikh SM, Bangirana P, Page CF, Opoka RO, & Giordani B (2010). A pilot study of the neuropsychological benefits of computerized cognitive rehabilitation in Ugandan children with HIV. Neuropsychology, 24(5), pp. 667–673. doi:2010-17509-013 [pii] 10.1037/a0019312 [DOI] [PubMed] [Google Scholar]
- Boivin MJ, Nakasujja N, Familiar-Lopez I, Murray SM, Sikorskii A, Awadu J, … Schut EE (2017). Effect of Caregiver Training on the Neurodevelopment of HIV-Exposed Uninfected Children and Caregiver Mental Health: A Ugandan Cluster-Randomized Controlled Trial. Journal of Developmental & Behavioral Pediatrics, 38(9), pp. 753–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin MJ, Nakasujja N, Sikorskii A, Opoka RO, & Giordani B (2016). A Randomized Controlled Trial to Evaluate if Computerized Cognitive Rehabilitation Improves Neurocognition in Ugandan Children with HIV. AIDS Res Hum Retrovirusesdoi: 10.1089/AID.2016.0026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin MJ, Ruel TD, Boal HE, Bangirana P, Cao H, Eller LA, … Wong JK (2010). HIV-subtype A is associated with poorer neuropsychological performance compared with subtype D in antiretroviral therapy-naive Ugandan children. Aids, 24(8), pp. 1163–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmbhatt H, Boivin M, Ssempijja V, Kigozi G, Kagaayi J, Serwadda D, & Gray RH (2014). Neurodevelopmental benefits of Anti-Retroviral Therapy in Ugandan children 0–6 Years of age with HIV. Journal of acquired immune deficiency syndromes (1999), 67(3), p 316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruininks RH, & Bruininks BD (2005). BOT2: Bruininks-Oseretsky Test of Motor Proficiency Second Edition Minneapolis, MN: Pearson Assessments. [Google Scholar]
- Chernoff ML,B; Ratswana M; Familiar I; Fairlie L; Vhembo T; Kamthunzi P; Kabugho E; Joyce C; Zimmer B; Ariansen JL; Jean-Philippe P; Boivin M (2018). Validity of Neuropsychological Testing in Young African Children Affected by HIV. Journal of Pediatric Infectious Diseases(01), pp. 1–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SM, Kim E, Lim KY, Lee JW, & Shin YM (2015). The effects of maternal depression on child mental health problems based on gender of the child. Community Ment Health J, 51(3), pp. 354–358. doi: 10.1007/s10597-014-9824-6 [DOI] [PubMed] [Google Scholar]
- Cooper PJ, Tomlinson M, Swartz L, Woolgar M, Murray L, & Molteno C (1999). Post-partum depression and the mother-infant relationship in a South African peri-urban settlement. The British Journal of Psychiatry, 175(6), pp. 554–558. [DOI] [PubMed] [Google Scholar]
- Familiar I, Collins SM, Sikorskii A, Ruisenor-Escudero H, Natamba B, Bangirana P, … Young SL (2018). Quality of Caregiving is Positively Associated With Neurodevelopment During the First Year of Life Among HIV-Exposed Uninfected Children in Uganda. J Acquir Immune Defic Syndr, 77(3), pp. 235–242. doi: 10.1097/qai.0000000000001599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Familiar I, Nakasujja N, Bass J, Sikorskii A, Murray S, Ruisenor-Escudero H, … Boivin MJ (2016). Caregivers’ depressive symptoms and parent-report of child executive function among young children in Uganda. Learn Individ Differ, 46, pp. 17–24. doi: 10.1016/j.lindif.2015.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Familiar I, Ruisenor-Escudero H, Giordani B, Bangirana P, Nakasujja N, Opoka R, & Boivin M (2015). Use of the Behavior Rating Inventory of Executive Function and Child Behavior Checklist in Ugandan children with HIV or a history of severe malaria. J Dev Behav Pediatr, 36(4), pp. 277–284. doi: 10.1097/DBP.0000000000000149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrier DE, Bassett HH, & Denham SA (2014). Relations between executive function and emotionality in preschoolers: Exploring a transitive cognition–emotion linkage. Frontiers in psychology, 5, p 487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garon NM, Piccinin C, & Smith IM (2016). Does the BRIEF-P predict specific executive function components in preschoolers? Applied Neuropsychology: Child, 5(2), pp. 110–118. [DOI] [PubMed] [Google Scholar]
- Gioia GA, & Isquith PK (2011). Behavior Rating Inventory for Executive Functions Encyclopedia of Clinical Neuropsychology (pp. 372–376): Springer. [Google Scholar]
- Giordani B, Novak B, Sikorskii A, Bangirana P, Nakasujja N, Winn BW, & Boivin MJ (2015). Designing and evaluating Brain Powered Games for cognitive training and rehabilitation in at-risk African children. Global Mental Health, 2(e6), pp. 1–14. doi:doi: 10.1017/gmh.2015.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman SH (2007). Depression in mothers. Annu Rev Clin Psychol, 3, pp. 107–135. doi: 10.1146/annurev.clinpsy.3.022806.091401 [DOI] [PubMed] [Google Scholar]
- Goodman SH, Rouse MH, Connell AM, Broth MR, Hall CM, & Heyward D (2011). Maternal depression and child psychopathology: a meta-analytic review. Clin Child Fam Psychol Rev, 14(1), pp. 1–27. doi: 10.1007/s10567-010-0080-1 [DOI] [PubMed] [Google Scholar]
- Grant KA, McMahon C, & Austin MP (2008). Maternal anxiety during the transition to parenthood: a prospective study. J Affect Disord, 108(1-2), pp. 101–111. doi: 10.1016/j.jad.2007.10.002 [DOI] [PubMed] [Google Scholar]
- Grantham-McGregor S, Cheung YB, Cueto S, Glewwe P, Richter L, & Strupp B (2007). Developmental potential in the first 5 years for children in developing countries. Lancet, 369(9555), pp. 60–70. doi: 10.1016/s0140-6736(07)60032-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg LM (1993). The T.O.V.A. (Version 6.X) (Computer Program). Los Alamitos, CA. [Google Scholar]
- Gueron-Sela N, Camerota M, Willoughby MT, Vernon-Feagans L, & Cox MJ (2018). Maternal depressive symptoms, mother-child interactions, and children’s executive function. Dev Psychol, 54(1), pp. 71–82. doi: 10.1037/dev0000389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herba CM, Glover V, Ramchandani PG, & Rondon MB (2016). Maternal depression and mental health in early childhood: an examination of underlying mechanisms in low-income and middle-income countries. Lancet Psychiatry, 3(10), pp. 983–992. doi: 10.1016/s2215-0366(16)30148-1 [DOI] [PubMed] [Google Scholar]
- Hughes C, Ensor R, Wilson A, & Graham A (2009). Tracking executive function across the transition to school: A latent variable approach. Developmental Neuropsychology, 35(1), pp. 20–36. [DOI] [PubMed] [Google Scholar]
- Kinyanda E, Hoskins S, Nakku J, Nawaz S, & Patel V (2011). Prevalence and risk factors of major depressive disorder in HIV/AIDS as seen in semi-urban Entebbe district, Uganda. BMC psychiatry, 11, p 205. doi: 10.1186/1471-244x-11-205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leis JA, Heron J, Stuart EA, & Mendelson T (2014). Associations between maternal mental health and child emotional and behavioral problems: does prenatal mental health matter? Journal of abnormal child psychology, 42(1), pp. 161–171. [DOI] [PubMed] [Google Scholar]
- McHenry MS, McAteer CI, Oyungu E, McDonald BC, Bosma CB, Mpofu PB, … Vreeman RC (2018). Neurodevelopment in Young Children Born to HIV-Infected Mothers: A Meta-analysis. Pediatrics, 141(2), p e20172888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison MF, Petitto JM, Have TT, Gettes DR, Chiappini MS, Weber AL, … Evans DL (2002). Depressive and anxiety disorders in women with HIV infection. American Journal of Psychiatry, 159(5), pp. 789–796. [DOI] [PubMed] [Google Scholar]
- Najman JM, Williams GM, Nikles J, Spence S, Bor W, O’Callaghan M, … Shuttlewood GJ (2001). Bias influencing maternal reports of child behaviour and emotional state. Soc Psychiatry Psychiatr Epidemiol, 36(4), pp. 186–194. [DOI] [PubMed] [Google Scholar]
- Netsi E, Pearson RM, Murray L, Cooper P, Craske MG, & Stein A (2018). Association of persistent and severe postnatal depression with child outcomes. JAMA psychiatry, 75(3), pp. 247–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Network NECCR (2005). Predicting individual differences in attention, memory, and planning in first graders from experiences at home, child care, and school. Developmental psychology, 41(1), p 99. [DOI] [PubMed] [Google Scholar]
- Orza L, Bewley S, Logie CH, Crone ET, Moroz S, Strachan S, … Welbourn A (2015). How does living with HIV impact on women’s mental health? Voices from a global survey. J Int AIDS Soc, 18(Suppl 5), p 20289. doi: 10.7448/ias.18.6.20289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quevedo LA, Silva RA, Godoy R, Jansen K, Matos MB, Tavares Pinheiro KA, & Pinheiro RT (2012). The impact of maternal post-partum depression on the language development of children at 12 months. Child Care Health Dev, 38(3), pp. 420–424. doi: 10.1111/j.1365-2214.2011.01251.x [DOI] [PubMed] [Google Scholar]
- Ruel TD, Boivin MJ, Boal HE, Bangirana P, Charlebois E, Havlir DV, … Wong JK (2012). Neurocognitive and motor deficits in HIV-infected Ugandan children with high CD4 cell counts. Clin Infect Dis, 54(7), pp. 1001–1009. doi: 10.1093/cid/cir1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santona A, Tagini A, Sarracino D, De Carli P, Pace CS, Parolin L, & Terrone G (2015). Maternal depression and attachment: the evaluation of mother-child interactions during feeding practice. Front Psychol, 6, p 1235. doi: 10.3389/fpsyg.2015.01235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAS Institute Inc., S. v. (2013). Base SAS® 9.4 Procedures Guide:Statistical Procedures (Second Edition ed.) Cary, NC, USA: SAS Institute Inc. [Google Scholar]
- Sherr L, Mueller J, & Varrall R (2009). A systematic review of cognitive development and child human immunodeficiency virus infection. Psychology, Health & Medicine, 14(4), pp. 387–404. [DOI] [PubMed] [Google Scholar]
- Singla DR, Kumbakumba E, & Aboud FE (2015). Effects of a parenting intervention to address both maternal psychological wellbeing and child development and growth in rural Uganda: a community-based, cluster randomised trial. Lancet Glob Health, 3(8), pp. e458–469. doi: 10.1016/s2214-109x(15)00099-6 [DOI] [PubMed] [Google Scholar]
- Sudfeld CR, Kaaya S, Gunaratna NS, Mugusi F, Fawzi WW, Aboud S, & Smithfawzi MC (2017). Depression at antiretroviral therapy initiation and clinical outcomes among a cohort of Tanzanian women living with HIV. AIDS (London, England), 31(2), p 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surkan PJ, Kennedy CE, Hurley KM, & Black MM (2011). Maternal depression and early childhood growth in developing countries: systematic review and meta-analysis. Bull World Health Organ, 89(8), pp. 608–615. doi: 10.2471/blt.11.088187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai AC, Wolfe WR, Kumbakumba E, Kawuma A, Hunt PW, Martin JN, … Weiser SD (2015). Prospective Study of the Mental Health Consequences of Sexual Violence Among Women Living With HIV in Rural Uganda. J Interpers Violencedoi: 10.1177/0886260514567966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rie A, Sengupta S, Pungrassami P, Balthip Q, Choonuan S, Kasetjaroen Y, … Chongsuvivatwong V (2008). Measuring stigma associated with tuberculosis and HIV/AIDS in southern Thailand: exploratory and confirmatory factor analyses of two new scales. Tropical medicine & international health, 13(1), pp. 21–30. [DOI] [PubMed] [Google Scholar]
- Wekesa E (2000). The impact of HIV / AIDS on child survival and development in Kenya. AIDS Anal Afr, 10(4), pp. 12–14. [PubMed] [Google Scholar]


