Abstract
Mesenchymal stromal cells (MSCs) are among of the most studied cell type for cellular therapy thanks to the ease of isolation, cultivation, and the high ex vivo expansion potential. In 2018, the European Medicines Agency finally granted the first marketing authorization for an MSC product. Despite the numerous promising results in preclinical studies, translation into routine practice still lags behind: therapeutic benefits of MSCs are not as satisfactory in clinical trial settings as they appear to be in preclinical models. The bench-to-bedside-and-back approach and careful evaluation of discrepancies between preclinical and clinical results have provided valuable insights into critical components of MSC manufacturing, their mechanisms of action, and how to evaluate and quality-control them. We sum up these past developments in the introductory section (“Mesenchymal stromal cells: name follows function”). From the huge amount of information, we then selected a few examples to illustrate challenges and opportunities to improve MSCs for clinical purposes. These include tissue origin of MSCs, MSC culture conditions, immune compatibility, and route of application and dosing. Finally, we add some information on MSC mechanisms of action and translation into potency assays and give an outlook on future perspectives raising the question of whether the future clinical product may be cell-based or cell-derived.
Keywords: mesenchymal stromal cells, translation, clinical perspectives, secretome, extracellular vesicles
Mesenchymal stromal cells: name follows function
In 2018, the first marketing authorization for a mesenchymal stromal cell (MSC) product was granted by the European Medicines Agency for the treatment of complex perianal fistulas in patients with Crohn’s disease 1. From a regulatory perspective, MSCs are classified as an advanced therapy medicinal product (ATMP) ( https://www.ema.europa.eu/en/human-regulatory/overview/advanced-therapy-medicinal-products-overview). This represents a milestone in the long history of MSCs, which were first described in 1867 by Cohnheim as non-hematopoietic bone marrow–derived cells to migrate through the bloodstream to distant sites of injury and participate in tissue regeneration 2. In the 1970s, Friedenstein et al. characterized them as a minor subpopulation of marrow-derived plastic adherent cells with osteogenic and hematopoietic supportive potential 3. He also established the term colony-forming unit-fibroblast. In the 1990s, Caplan 4, 5 and Pittenger et al. 6 coined the term “mesenchymal stem cells” on the basis of the multi-lineage differentiation potential of these cells. At this time, controversy arose as to whether these cells are stem cells or not. Bianco and Robey and colleagues used the term “skeletal stem cells” for cells residing in the postnatal bone marrow and giving rise to cartilage, bone, hematopoiesis-supportive stroma, and marrow adipocytes in defined in vivo assays 7– 9. In 2006, to put an end to the discussion, the International Society for Cell and Gene Therapy defined the term “mesenchymal stromal cells” and set up minimal criteria defining (bone marrow–derived) MSCs ( Table 1) 10. At that time, it became evident that MSCs (or at least cells with similar characteristics) could be isolated from a variety of different tissues, suggesting a perivascular origin 11, 12. Given the similarity to fibroblasts, Haniffa et al. asked: “Mesenchymal stem cells: The fibroblasts’ new clothes?” 13. However, with the increasing use in (pre)clinical studies, it became evident that apparently not the proposed multi-lineage differentiation potential but rather their secreted bioactive molecules that modulate immune and inflammatory responses were key to exerting therapeutic effects (in fact, only few transplanted cells were found in vivo to be engrafted and differentiated) 14, 15. Thus, Caplan introduced the term “medicinal signaling cells” to illustrate their versatility and flexibility to adapt to the local milieu 16. We interpret the abbreviation “MSCs” as “mesenchymal stromal cells” as, according to our own experimental observations, the cells do not fulfil “stem cell” criteria such as indefinite self-renewal.
Table 1. Definition of mesenchymal stromal cell.
| Adherence to
plastic |
Specific surface
markers |
In vitro multipotent
differentiation potential |
|---|---|---|
| Positive:
CD105 CD73 CD90 Negative: CD45 CD34 CD14 CD11b CD79a CD19 HLA class I |
Osteoblasts
Adipocytes Chondroblasts |
The minimal criteria defining the mesenchymal stromal cell by the International Society for Cell and Gene Therapy (according to 10).
Despite arguments about the most appropriate name, MSCs have emerged as the most intensely studied cell type for experimental cell therapy. Starting with the first use in patients in a hematological transplant setting 17, numerous clinical indications have been investigated, ranging from hematological disorders (including graft-versus-host disease, or GvHD), bone/cartilage defects, diabetes, cardiovascular and neurological diseases (including autoimmune diseases), and liver and renal diseases 18– 20. The ease of isolation, cultivation, and the high ex vivo expansion potential in line with the numerous therapeutic mechanisms (paracrine pro-regenerative, anti-fibrotic, anti-apoptotic, pro-angiogenic, and immunomodulatory functions) have contributed to this broad exploitation.
Challenges and opportunities to improve mesenchymal stromal cells for clinical purposes
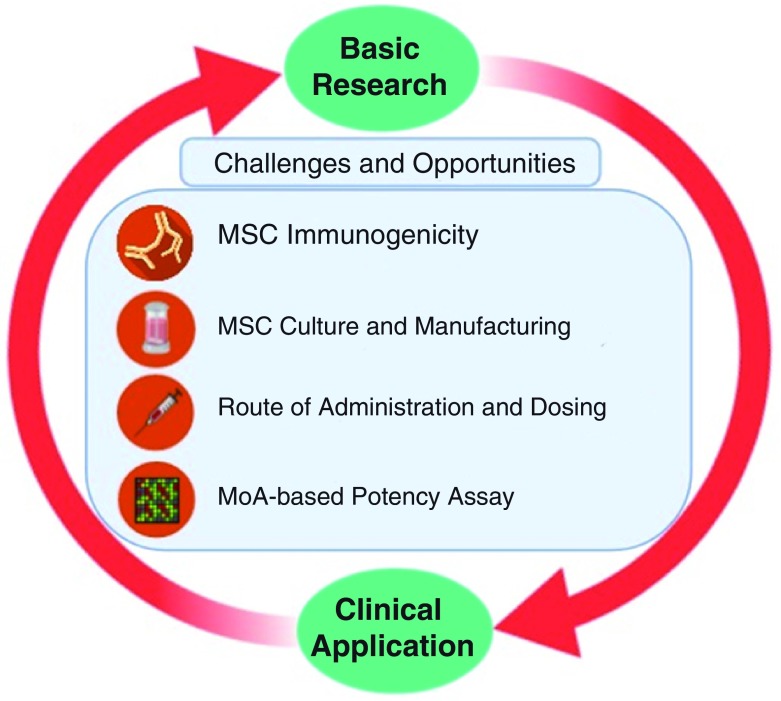
Despite the promising results in preclinical studies, therapeutic benefits of MSCs are not as satisfactory in clinical trial settings 21. This section addresses some factors that might contribute to this disparity and how to improve the therapeutic capacity of MSCs ( Figure 1).
Figure 1. Bench-to-bedside-and-back.
Challenges and opportunities in translating mesenchymal stromal cell (MSC)-based therapy from basic research to clinical practices, including immunogenicity of MSCs, Good Manufacturing Practice–compliant MSC manufacturing as well as determining the route of administration and dosing. MoA, mechanism of action.
Tissue origin of mesenchymal stromal cells
The most prevalent source for MSCs is adult bone marrow 18. Adipose tissue is emerging as an important source, as exemplified by the ATMP granted marketing authorization by the European Medicines Agency (mentioned above). We and others have tried to understand how interchangeable MSCs from different tissue sources are and whether one may be more suitable for certain disease entities than for others. The observed differences suggest an “environmental niche memory”, which could help to select the most appropriate tissue source for a certain clinical indication 12, 22– 26.
Mesenchymal stromal cell culture conditions and cellular fitness
Usage of fetal bovine serum (FBS) as culture supplement has been a major issue in MSC production 27. The growing concern relates to transmission of pathogens such as prions and possible immune reactions against xenogeneic agents 28, 29. Consequently, as a replacement of FBS, other supplements have been introduced. One of the most common is platelet lysate (PL) 29– 31, as it contains growth factors suitable to support MSC ex vivo expansion without causing genomic instability 32. However, the use of PL is not without concerns: batch-to-batch variation and pathogen reduction need to be addressed to standardize PL use in MSC manufacturing 33.
MSC culture conditions differ enormously, hampering comparability of data 34. Cellular “fitness” is considered the most critical parameter and is influenced by cellular/replicative age and potential “cryo-injury” 21, 35, 36.
Expansion of MSCs in vitro, required to achieve clinical doses (see below), ultimately results in replicative senescence that compromises therapeutic efficacy 37, 38. Thus, genomic stability should be addressed as a safety measure before clinical application 39. In addition, the thawing of cryopreserved MSCs just before transplantation may hamper their therapeutic capacity. In most animal experiments, MSCs are harvested freshly before the transplantation, while on the peak of the replicative phase. Meanwhile, in clinical trial, most MSCs are pre-banked and expanded to their proliferative limit, frozen down, and just thawed prior the transplantation 35, 40. Following retrieval from liquid nitrogen, MSCs have been shown to undergo a heat shock response (“cryo stun effect”) leading to cell injury for at least the first 24 hours 40. This has been shown to compromise immune-modulation function, enhance vulnerability to lysis by immune cells and the complement system, and decrease in vivo persistence upon intravenous administration 40. A rescue culture for a few days could eventually reduce this “cryo stun effect”.
As it has become clear that culture conditions can greatly affect MSC function, it also opens a new window for MSC priming to improve their therapeutic efficacy. A growing body of data report a wide array of priming approaches, from usage of cytokines, growth factors, hypoxia, pharmaceutical drugs, and 3D culture using biomaterials 41, 42. For example, MSC priming with interferon-gamma (IFN-γ) is considered key to suppress T-cell proliferation, partly through production of indolamine-2,3-dioxygenase (IDO) and programmed cell death-1 ligand (PDL-1) upregulation 43. Indeed, allogeneic infusion of IFN-γ–primed MSCs to non-obese diabetic/severe combined immunodeficiency (NOD/SCID) mice reduced GvHD symptoms 44. However, MSC priming with IFN-γ should be carried out with caution as it can upregulate the expression of HLA class I and II molecules, which could affect immune compatibility 45.
Lastly, another challenge to bring MSCs to clinical application is upscaling of MSC culture. A number of strategies for upscaling cell, secretome, or extracellular vesicle production have been reported and reviewed extensively 46– 48. However, economically feasible approaches that meet Good Manufacturing Practice compliance have yet to be standardized 49.
Route of application and dosing
Depending on the clinical purposes, MSCs are administered differently, either systemically infused or locally injected. Contrary to the old belief that MSCs migrate to the site of injury and replace the injured tissue once MSCs are injected intravenously, they are mostly trapped in lungs and die within 24 hours 50. Pulmonary embolism and infarct of three related patients have been reported after adipose MSC infusion 51. MSCs express tissue factor, a cell surface glycoprotein that plays an important role in extrinsic coagulation, which by triggering procoagulation has led to thromboembolic events after MSC infusion. Thus, adding an anti-coagulant during the infusion should be considered 52.
The majority of preclinical studies using mice and rats infuse around 50 million and 10 to 20 million MSCs per kilogram of body weight, respectively 53, 54. Meanwhile, the average number of MSCs transfused intravenously is 100 million per patient, corresponding to 1 to 2 million per kilogram of body weight 55. This may in part explain the huge discrepancy of outcome between preclinical and clinical studies, assuming that the therapeutic benefit is dose-dependent 21. Although the notion of increasing MSC dose might be tempting, safety should be assessed carefully for it might, for example, increase the risk for embolism or adverse reactions. In addition, the lack of standardized pharmacodynamics and pharmacokinetics models applied to MSCs represents a limiting factor 56.
Another potential explanation for the translational gap between clinical and preclinical data is that, in patients, the degree of severity might be too high for MSC therapy to be as efficacious as in animal studies. In order to get better clinical outcomes, MSC-based therapy may be considered as prevention treatment together with first-line therapy and not only as salvage or even palliative therapy. However, this notion will require proper risk–benefit evaluation and support from ethics committees.
Hemocompatibility and immune compatibility
For a long time, MSCs have been considered to be immune-privileged, allowing their transplantation across histocompatibility barriers 57. Recent data, however, indicate that MSC transplantation may provoke donors’ humoral and cellular immune responses, especially in allogeneic settings 21, 58. In GvHD, in fact, this immune recognition appears to be fundamental for the therapeutic effect: MSCs recognized by cytotoxic T cells undergo apoptosis and are phagocytosed by macrophages which subsequently elicit immunosuppression via prostaglandin E 2 (PGE 2) and IDO activities 59– 61.
MSCs have also been shown to elicit an instant blood-mediated inflammatory reaction (IBMIR) in both a cell dose- and a donor-dependent manner 62. Non-bone marrow-derived MSCs appear to express higher levels of pro-coagulant tissue factor, which makes them more likely to induce IMBIR 63. Adding anti-coagulants during MSC transplantation may be a good option for clinical application 36, 52. Likewise, the selection of tissue factor–negative or low expressing MSCs has been proposed as a strategy to improve hemocompatibility 63.
Moreover, allogeneic MSC transplantation can provoke an adaptive immune response in mice through increased T-cell memory and allo-antibodies 64, 65. The latter may be associated with complement-mediated cytotoxicity 58. Yet only two clinical trials 1, 67 report the development of donor-specific antibodies in patients who have received allogeneic MSCs 66. But the authors argue that the increased allo-antibodies have no relevance in the clinical outcome or the occurrence of adverse events 1, 67. Moreover, in two other clinical trials, which used allogeneic MSCs to treat type 2 diabetes and diabetic nephropathy, there was no report of patients developing donor-specific HLA antibodies despite donor–recipient mismatching 68, 69. Although the results from clinical studies seem encouraging, the scarcity of studies elucidating possible allo-immune reactions may cause a bias in the observed trend 66. The occurrence of FBS (used as culture supplement for cell expansion)-specific antibodies has prompted the search for alternative and improved culture conditions as described above 70.
Mechanisms of action
Mesenchymal stromal cell secretome
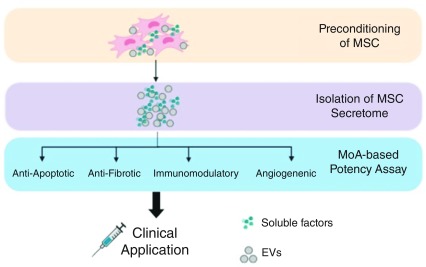
The MSC secretome is composed of different soluble factors, including cytokines, growth factors, chemokines, immunomodulatory molecules, cell organelles, and nucleic acids, which are produced, some of these eventually encapsulated in extracellular vesicles, and secreted or directly transferred to neighboring cells 71, 72. These factors can modulate the immune system, inhibit cell death and fibrosis, stimulate vascularization, and promote tissue remodeling ( Figure 2) 73. MSCs can adapt efficiently to the local milieu and change their secretome 74. On one hand, this significantly hampers the understanding of their mechanisms of action (MoAs) in vivo and the establishment of predictive and quantitative potency assays. On the other hand, it paves the way to potentially improve therapeutic efficacy (for example, the preconditioning with different factors that activate very specific signaling pathways). Treating MSCs in vitro with hypoxia, 3D culture, or soluble factors such as stromal cell–derived factor 1 (SDF-1) or transforming growth factor-beta (TGF-β) triggers Akt, ERK, and p38MAPK signaling pathways. These pathways, at the same time, can induce the production of cytoprotective molecules (catalase, heme oxygenase-1, and so on), pro-regenerative (basic fibroblast growth factor, hepatocyte growth factor, insulin-like growth factor-1, and so on) and pro-angiogenic (vascular endothelial growth factor, or VEGF) factors, and also immunomodulatory cytokines (IDO, PGE 2, interleukin-6, and so on) 42, 71.
Figure 2. Mesenchymal stromal cell (MSC) secretome in clinical application.
Strategies to harness the MSC secretome for clinical purposes include preconditioning/priming of MSCs to manipulate their paracrine factors, isolation of the secretome, and establishing MoA-based potency assay. EV, extracellular vesicle; MoA, mechanism of action.
Extracellular vesicles
Extracellular vesicles (EVs) are further candidates to explain the therapeutic effects of MSCs. EVs are membrane-enclosed particles of different sizes (exosomes, microvesicles, and apoptotic bodies) released by cells in the plasma and other body fluids 75, 76. EVs transport biologically active molecules and genetic information to target cells, influencing their function 77. Thanks to these characteristics, EVs are also emerging as biomarkers for various diseases 78. EVs carry a wide variety of genetic material, in particular microRNAs, which play an important role in the biological function of EVs. These small RNAs regulate the cell cycle and migration (for example, miR-191, miR-222, miR-21, and let-7a), inflammation (for example, miR-204-5p), and angiogenesis (for example, miR-222 and miR-21). In a new therapeutic approach, MSC-derived EVs are being engineered by increasing or modifying their content (proteins or RNA) 77. As an example, an effective drug delivery system for wound healing in diabetes was developed by transfecting non-coding RNA (Lnc-RNA-H19) into EVs 79. Based on these data, some researchers suggest that the conditioned medium or even EVs should be used as drugs rather than MSCs 80.
In some circumstances (for example, GvHD mentioned above), dead or dying cells may contribute to therapeutic efficacy. Thus, an improved understanding on MSC “necrobiology” has been proposed, considering apoptosis, autophagy, mitochondrial transfer, and also vesicles 61. Recognition by the innate immune system in different disease contexts may be key to understand and improve MSC function 60, 81.
Potency assays
For advanced clinical trials, assays that can verify MSC identity and quality and can predict their functionality in vivo are required 82. Owing to the manifold functions of MSCs and their rapid adaptation to the local milieu, which may modify their function at sites of injury, disease, or inflammation, assays to predict these functions in vivo are hard to develop.
Agreed quality-control criteria include the determination of presence and absence of certain surface markers and of MSC differentiation potential, their senescence status, their secretome and immunomodulatory functions. In addition, surrogate assays which more specifically test the proposed therapeutic mechanism of action, for example angiogenesis have been established 83– 87. The group of Galipeau was the first to suggest a combinatorial assay matrix as a platform to integrate different assays 88, 89. In the first study, they employed secretome analysis and quantitative RNA-based array to estimate the immunomodulatory capacity of MSCs and their crosstalk with peripheral blood mononuclear cells (PBMCs), in which CXCL9, CXCL10, VEGF, and CCL2 secretion and expression were correlated with suppression of T-cell proliferation 88. The other study investigated the phosphorylation of signal transducer and activator of transcription (STAT) in MSC-PBMC co-culture settings where STAT1 and STAT3 phosphorylation was associated with MSC immunoinhibitory capacity 89. Moreover, Phinney et al. reported a “Clinical Indications Prediction Scale” that, based on Twist-1 expression levels, could predict therapeutic efficacy: high levels of Twist-1 predict higher angiogenic potential, whereas low levels are in line with improved anti-inflammatory and immunosuppressive actions 90.
We expect rapid progress in the development of combinatorial potency assays based on the increasing knowledge of MSC biology (omics, including single-cell analyses) 91, 92. Integrating this more comprehensive insight into MSC heterogeneity with MSC molecular signatures and their highly complex interaction with the local microenvironment in line with a better understanding of molecular mechanisms of action in various pathological settings will hopefully enable easy-to-perform assays with predictive value.
Future perspectives
The key questions for the future may be, do we need cells? Do we need viable cells, or do apoptotic cells and subcellular components such as EVs, or mitochondria, or just the secretome do a similar job?
The biological properties of the MSC secretome and how it orchestrates MSC immunomodulatory and regenerative capacity in the disease context remain enigmatic, prompting further studies. Moreover, given the possibility of modulating MSCs and their secretome, a disease-specific MSC priming (for instance, with pro-inflammatory cytokines) may improve efficacy.
Lastly, in order to standardize MSC therapy and avoid outcome bias, rigorous potency assays are needed. However, the selection of specific potency assays, whether it is a disease-specific (for example, angiogenesis and immunomodulation) or a more general (for example, proliferation) evaluation of MSC function that can be used regardless of diseases’ pathophysiology, needs further elucidation.
Given the enormous knowledge gain in MSCs over the past years, largely obtained by bench-to-bedside-and-back approaches and recapitulated by the continuous adaptation of the term MSCs (“name follows function”), we expect the development of novel translational strategies. A better understanding of failures, the identification and consequent mitigation of challenges and finally an improved understanding of MoAs, translating into robust potency assays, will be key for a successful translation of MSCs into clinical practice.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Thomas Ritter, Regenerative Medicine Institute (REMEDI), College of Medicine, Nursing and Health Sciences, National University of Ireland, Galway, Ireland
Massimo Dominici, epartment of Medical and Surgical Sciences for Children and Adults, Division of Oncology, University-Hospital of Modena and Reggio Emilia, Modena, Italy
Bettina Wilm, Department of Cellular and Molecular Physiology, University of Liverpool, Liverpool, UK
Funding Statement
We acknowledge funding from the European Union’s Horizon 2020 research and innovation programme under Marie Skłodowska-Curie grant agreement number 813839.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; peer review: 3 approved]
References
- 1. Panés J, García-Olmo D, van Assche G, et al. : Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn's disease: A phase 3 randomised, double-blind controlled trial. Lancet. 2016;388(10051):1281–90. 10.1016/S0140-6736(16)31203-X [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 2. Cohnheim J: Ueber Entzündung und Eiterung. Archiv f pathol Anat. 1867;40:1–79. 10.1007/BF02968135 [DOI] [Google Scholar]
- 3. Friedenstein AJ, Chailakhyan RK, Latsinik NV, et al. : Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation. 1974;17(4):331–40. 10.1097/00007890-197404000-00001 [DOI] [PubMed] [Google Scholar]
- 4. Caplan AI: Mesenchymal stem cells. J Orthop Res. 1991;9(5):641–50. 10.1002/jor.1100090504 [DOI] [PubMed] [Google Scholar]
- 5. Caplan AI: The mesengenic process. Clin Plast Surg. 1994;21(3):429–35. [PubMed] [Google Scholar]
- 6. Pittenger MF, Mackay AM, Beck SC, et al. : Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–7. 10.1126/science.284.5411.143 [DOI] [PubMed] [Google Scholar]
- 7. Bianco P, Robey PG, Simmons PJ: Mesenchymal Stem Cells: Revisiting History, Concepts, and Assays. Cell Stem Cell. 2008;2(4):313–9. 10.1016/j.stem.2008.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kuznetsov SA, Mankani MH, Gronthos S, et al. : Circulating Skeletal Stem Cells. J Cell Biol. 2001;153(5):1133–40. 10.1083/jcb.153.5.1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bianco P, Cao X, Frenette PS: et al: The meaning, the sense and the significance: Translating the science of mesenchymal stem cells into medicine. Nat Med. 2013;19(1):35–42. 10.1038/nm.3028 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 10. Dominici M, Le Blanc K, Mueller I, et al. : Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–7. 10.1080/14653240600855905 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 11. Crisan M, Yap S, Casteilla L, et al. : A Perivascular Origin for Mesenchymal Stem Cells in Multiple Human Organs. Cell Stem Cell. 2008;3(3):301–13. 10.1016/j.stem.2008.07.003 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 12. Kern S, Eichler H, Stoeve J, et al. : Comparative Analysis of Mesenchymal Stem Cells from Bone Marrow, Umbilical Cord Blood, or Adipose Tissue. Stem Cells. 2006;24(5):1294–301. 10.1634/stemcells.2005-0342 [DOI] [PubMed] [Google Scholar]
- 13. Haniffa MA, Collin MP, Buckley CD, et al. : Mesenchymal stem cells: The fibroblasts' new clothes? Haematologica. 2009;94(2):258–63. 10.3324/haematol.13699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fiori A, Terlizzi V, Kremer H, et al. : Mesenchymal stromal/stem cells as potential therapy in diabetic retinopathy. Immunobiology. 2018;223(12):729–43. 10.1016/j.imbio.2018.01.001 [DOI] [PubMed] [Google Scholar]
- 15. Torres Crigna A, Daniele C, Gamez C, et al. : Stem/Stromal Cells for Treatment of Kidney Injuries With Focus on Preclinical Models. Front Med (Lausanne). 2018;5:179. 10.3389/fmed.2018.00179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Caplan AI: MSCs: The Sentinel and Safe-Guards of Injury. J Cell Physiol. 2016;231(7):1413–6. 10.1002/jcp.25255 [DOI] [PubMed] [Google Scholar]
- 17. Lazarus HM, Haynesworth SE, Gerson SL, et al. : Ex vivo expansion and subsequent infusion of human bone marrow-derived stromal progenitor cells (mesenchymal progenitor cells): Implications for therapeutic use. Bone Marrow Transplant. 1995;16(4):557–64. [PubMed] [Google Scholar]
- 18. Sharma RR, Pollock K, Hubel A, et al. : Mesenchymal stem or stromal cells: A review of clinical applications and manufacturing practices. Transfusion. 2014;54(5):1418–37. 10.1111/trf.12421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Andrzejewska A, Lukomska B, Janowski M: Concise Review: Mesenchymal Stem Cells: From Roots to Boost. Stem Cells. 2019;37(7):855–64. 10.1002/stem.3016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schäfer R: Advanced cell therapeutics are changing the clinical landscape: Will mesenchymal stromal cells be a part of it? BMC Med. 2019;17(1):53. 10.1016/j.stem.2018.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Galipeau J, Sensébé L: Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell. 2018;22(6):824–33. 10.1016/j.stem.2018.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mattar P, Bieback K: Comparing the Immunomodulatory Properties of Bone Marrow, Adipose Tissue, and Birth-Associated Tissue Mesenchymal Stromal Cells. Front Immunol. 2015;6:560. 10.3389/fimmu.2015.00560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Paliwal S, Chaudhuri R, Agrawal A, et al. : Human tissue-specific MSCs demonstrate differential mitochondria transfer abilities that may determine their regenerative abilities. Stem Cell Res Ther. 2018;9(1):298. 10.1186/s13287-018-1012-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pittenger MF, Discher DE, Péault BM, et al. : Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen Med. 2019;4:22. 10.1038/s41536-019-0083-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ragni E, Montemurro T, Montelatici E, et al. : Differential microRNA signature of human mesenchymal stem cells from different sources reveals an "environmental-niche memory" for bone marrow stem cells. Exp Cell Res. 2013;319(10):1562–74. 10.1016/j.yexcr.2013.04.002 [DOI] [PubMed] [Google Scholar]
- 26. de Almeida DC, Ferreira MRP, Franzen J, et al. : Epigenetic Classification of Human Mesenchymal Stromal Cells. Stem Cell Reports. 2016;6(2):168–75. 10.1016/j.stemcr.2016.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van der Valk J, Bieback K, Buta C, et al. : Fetal Bovine Serum (FBS): Past - Present - Future. ALTEX. 2018;35(1):99–118. 10.14573/altex.1705101 [DOI] [PubMed] [Google Scholar]
- 28. Bieback K: Platelet lysate as replacement for fetal bovine serum in mesenchymal stromal cell cultures. Transfus Med Hemother. 2013;40(5):326–35. 10.1159/000354061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Burnouf T, Strunk D, Koh MB, et al. : Human platelet lysate: Replacing fetal bovine serum as a gold standard for human cell propagation? Biomaterials. 2016;76:371–87. 10.1016/j.biomaterials.2015.10.065 [DOI] [PubMed] [Google Scholar]
- 30. Phinney DG, Galipeau J: Manufacturing mesenchymal stromal cells for clinical applications: A survey of Good Manufacturing Practices at U.S. academic centers. Cytotherapy. 2019;21(7):782–92. 10.1016/j.jcyt.2019.04.003 [DOI] [PubMed] [Google Scholar]
- 31. Astori G, Amati E, Bambi F, et al. : Platelet lysate as a substitute for animal serum for the ex-vivo expansion of mesenchymal stem/stromal cells: present and future. Stem Cell Res Ther. 2016;7(1):93. 10.1186/s13287-016-0352-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Trojahn Kølle SF, Oliveri RS, Glovinski PV, et al. : Pooled human platelet lysate versus fetal bovine serum-investigating the proliferation rate, chromosome stability and angiogenic potential of human adipose tissue-derived stem cells intended for clinical use. Cytotherapy. 2013;15(9):1086–97. 10.1016/j.jcyt.2013.01.217 [DOI] [PubMed] [Google Scholar]
- 33. Bieback K, Fernandez-Muñoz B, Pati S, et al. : Gaps in the knowledge of human platelet lysate as a cell culture supplement for cell therapy: a joint publication from the AABB and the International Society for Cell & Gene Therapy. Cytotherapy. 2019;21(9):911–24. 10.1016/j.jcyt.2019.06.006 [DOI] [PubMed] [Google Scholar]
- 34. Martin I, de Boer J, Sensebe L, et al. : A relativity concept in mesenchymal stromal cell manufacturing. Cytotherapy. 2016;18(5):613–20. 10.1016/j.jcyt.2016.02.004 [DOI] [PubMed] [Google Scholar]
- 35. Gao F, Chiu SM, Motan DAL, et al. : Mesenchymal stem cells and immunomodulation: current status and future prospects. Cell Death Dis. 2016;7:e2062. 10.1038/cddis.2015.327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hoogduijn MJ, Lombardo E: Mesenchymal Stromal Cells Anno 2019: Dawn of the Therapeutic Era? Concise Review. Stem Cells Transl Med. 2019;8(11):1126–34. 10.1002/sctm.19-0073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bieback K, Hecker A, Schlechter T, et al. : Replicative aging and differentiation potential of human adipose tissue-derived mesenchymal stromal cells expanded in pooled human or fetal bovine serum. Cytotherapy. 2012;14(5):570–83. 10.3109/14653249.2011.652809 [DOI] [PubMed] [Google Scholar]
- 38. Barilani M, Palorini R, Votta G, et al. : Central metabolism of functionally heterogeneous mesenchymal stromal cells. Sci Rep. 2019;9(1):15420. 10.1038/s41598-019-51937-9 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 39. Sensebé L: Beyond genetic stability of mesenchymal stromal cells. Cytotherapy. 2013;15(11):1307–8. 10.1016/j.jcyt.2013.09.001 [DOI] [PubMed] [Google Scholar]
- 40. Moll G, Geißler S, Catar R, et al. : Cryopreserved or Fresh Mesenchymal Stromal Cells: Only a Matter of Taste or Key to Unleash the Full Clinical Potential of MSC Therapy? Adv Exp Med Biol. 2016;951:77–98. 10.1007/978-3-319-45457-3_7 [DOI] [PubMed] [Google Scholar]
- 41. Schäfer R, Spohn G, Baer PC: Mesenchymal Stem/Stromal Cells in Regenerative Medicine: Can Preconditioning Strategies Improve Therapeutic Efficacy? Transfus Med Hemother. 2016;43(4):256–67. 10.1159/000447458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Noronha NC, Mizukami A, Caliári-Oliveira C, et al. : Priming approaches to improve the efficacy of mesenchymal stromal cell-based therapies. Stem Cell Res Ther. 2019;10(1):131. 10.1186/s13287-019-1224-y [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 43. Chinnadurai R, Copland IB, Patel SR, et al. : IDO-independent suppression of T cell effector function by IFN-γ-licensed human mesenchymal stromal cells. J Immunol. 2014;192(4):1491–501. 10.4049/jimmunol.1301828 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 44. Kim DS, Jang IK, Lee MW, et al. : Enhanced Immunosuppressive Properties of Human Mesenchymal Stem Cells Primed by Interferon-γ. EBioMedicine. 2018;28:261–73. 10.1016/j.ebiom.2018.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Burand AJ, Gramlich OW, Brown AJ, et al. : Function of Cryopreserved Mesenchymal Stromal Cells With and Without Interferon-γ Prelicensing is Context Dependent. Stem Cells. 2017;35(5):1437–9. 10.1002/stem.2528 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 46. Robb KP, Fitzgerald JC, Barry F, et al. : Mesenchymal stromal cell therapy: progress in manufacturing and assessments of potency. Cytotherapy. 2019;21(3):289–306. 10.1016/j.jcyt.2018.10.014 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 47. Leber J, Barekzai J, Blumenstock M, et al. : Microcarrier choice and bead-to-bead transfer for human mesenchymal stem cells in serum-containing and chemically defined media. Process Biochem. 2017;59(Part B):255–65. 10.1016/j.procbio.2017.03.017 [DOI] [Google Scholar]
- 48. Pachler K, Lener T, Streif D, et al. : A Good Manufacturing Practice-grade standard protocol for exclusively human mesenchymal stromal cell-derived extracellular vesicles. Cytotherapy. 2017;19(4):458–72. 10.1016/j.jcyt.2017.01.001 [DOI] [PubMed] [Google Scholar]
- 49. Viswanathan S, Shi Y, Galipeau J, et al. : Mesenchymal stem versus stromal cells: International Society for Cell & Gene Therapy (ISCT®) Mesenchymal Stromal Cell committee position statement on nomenclature. Cytotherapy. 2019;21(10):1019–24. 10.1016/j.jcyt.2019.08.002 [DOI] [PubMed] [Google Scholar]
- 50. Prockop DJ: Repair of tissues by adult stem/progenitor cells (MSCs): controversies, myths, and changing paradigms. Mol Ther. 2009;17(6):939–46. 10.1038/mt.2009.62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jung JW, Kwon M, Choi JC, et al. : Familial occurrence of pulmonary embolism after intravenous, adipose tissue-derived stem cell therapy. Yonsei Med J. 2013;54(5):1293–6. 10.3349/ymj.2013.54.5.1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Moll G, Ankrum JA, Kamhieh-Milz J, et al. : Intravascular Mesenchymal Stromal/Stem Cell Therapy Product Diversification: Time for New Clinical Guidelines. Trends Mol Med. 2019;25(2):149–63. 10.1016/j.molmed.2018.12.006 [DOI] [PubMed] [Google Scholar]
- 53. Coppin L, Sokal E, Stéphenne X: Thrombogenic Risk Induced by Intravascular Mesenchymal Stem Cell Therapy: Current Status and Future Perspectives. Cells. 2019;8(10). pii: E1160. 10.3390/cells8101160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wysoczynski M, Khan A, Bolli R: New Paradigms in Cell Therapy: Repeated Dosing, Intravenous Delivery, Immunomodulatory Actions, and New Cell Types. Circ Res. 2018;123(2):138–58. 10.1161/CIRCRESAHA.118.313251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kabat M, Bobkov I, Kumar S, et al. : Trends in mesenchymal stem cell clinical trials 2004-2018: Is efficacy optimal in a narrow dose range? Stem Cells Transl Med. 2020;9(1):17–27. 10.1002/sctm.19-0202 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 56. Salvadori M, Cesari N, Murgia A, et al. : Dissecting the Pharmacodynamics and Pharmacokinetics of MSCs to Overcome Limitations in Their Clinical Translation. Mol Ther Methods Clin Dev. 2019;14:1–15. 10.1016/j.omtm.2019.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 57. Le Blanc K, Davies LC: MSCs-cells with many sides. Cytotherapy. 2018;20(3):273–8. 10.1016/j.jcyt.2018.01.009 [DOI] [PubMed] [Google Scholar]
- 58. Ankrum JA, Ong JF, Karp JM: Mesenchymal stem cells: Immune evasive, not immune privileged. Nat Biotechnol. 2014;32(3):252–60. 10.1038/nbt.2816 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 59. Cheung TS, Galleu A, von Bonin M, et al. : Apoptotic mesenchymal stromal cells induce prostaglandin E2 in monocytes: Implications for the monitoring of mesenchymal stromal cell activity. Haematologica. 2019;104(10):e438–e441. 10.3324/haematol.2018.214767 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 60. Galleu A, Riffo-Vasquez Y, Trento C, et al. : Apoptosis in mesenchymal stromal cells induces in vivo recipient-mediated immunomodulation. Sci Transl Med. 2017;9(416). pii: eaam7828. 10.1126/scitranslmed.aam7828 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 61. Weiss DJ, English K, Krasnodembskaya A, et al. : The Necrobiology of Mesenchymal Stromal Cells Affects Therapeutic Efficacy. Front Immunol. 2019;10:1228. 10.3389/fimmu.2019.01228 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 62. Moll G, Rasmusson-Duprez I, von Bahr L, et al. : Are Therapeutic Human Mesenchymal Stromal Cells Compatible with Human Blood? Stem Cells. 2012;30(7):1565–74. 10.1002/stem.1111 [DOI] [PubMed] [Google Scholar]
- 63. Oeller M, Laner-Plamberger S, Hochmann S, et al. : Selection of Tissue Factor-Deficient Cell Transplants as a Novel Strategy for Improving Hemocompatibility of Human Bone Marrow Stromal Cells. Theranostics. 2018;8(5):1421–34. 10.7150/thno.21906 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 64. Badillo AT, Beggs KJ, Javazon EH, et al. : Murine Bone Marrow Stromal Progenitor Cells Elicit an In Vivo Cellular and Humoral Alloimmune Response. Biol Blood Marrow Transplant. 2007;13(4):412–22. 10.1016/j.bbmt.2006.12.447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zangi L, Margalit R, Reich-Zeliger S, et al. : Direct imaging of immune rejection and memory induction by allogeneic mesenchymal stromal cells. Stem Cells. 2009;27(11):2865–74. 10.1002/stem.217 [DOI] [PubMed] [Google Scholar]
- 66. Lohan P, Treacy O, Griffin MD, et al. : Anti-Donor Immune Responses Elicited by Allogeneic Mesenchymal Stem Cells and Their Extracellular Vesicles: Are We Still Learning? Front Immunol. 2017;8:1626. 10.3389/fimmu.2017.01626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. García-Sancho J, Sánchez A, Vega A, et al. : Influence of HLA Matching on the Efficacy of Allogeneic Mesenchymal Stromal Cell Therapies for Osteoarthritis and Degenerative Disc Disease. Transplant Direct. 2017;3(9):e205. 10.1097/TXD.0000000000000724 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 68. Skyler JS, Fonseca VA, Segal KR, et al. : Allogeneic Mesenchymal Precursor Cells in Type 2 Diabetes: A Randomized, Placebo-Controlled, Dose-Escalation Safety and Tolerability Pilot Study. Diabetes Care. 2015;38(9):1742–9. 10.2337/dc14-2830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Packham DK, Fraser IR, Kerr PG, et al. : Allogeneic Mesenchymal Precursor Cells (MPC) in Diabetic Nephropathy: A Randomized, Placebo-controlled, Dose Escalation Study. EBioMedicine. 2016;12:263–9. 10.1016/j.ebiom.2016.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Horwitz EM, Prockop DJ, Fitzpatrick LA, et al. : Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999;5(3):309–13. 10.1038/6529 [DOI] [PubMed] [Google Scholar]
- 71. Ferreira JR, Teixeira GQ, Santos SG, et al. : Mesenchymal Stromal Cell Secretome: Influencing Therapeutic Potential by Cellular Pre-conditioning. Front Immunol. 2018;9:2837. 10.3389/fimmu.2018.02837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Fontaine MJ, Shih H, Schäfer R, et al. : Unraveling the Mesenchymal Stromal Cells' Paracrine Immunomodulatory Effects. Transfus Med Rev. 2016;30(1):37–43. 10.1016/j.tmrv.2015.11.004 [DOI] [PubMed] [Google Scholar]
- 73. Teng X, Chen L, Chen W, et al. : Mesenchymal Stem Cell-Derived Exosomes Improve the Microenvironment of Infarcted Myocardium Contributing to Angiogenesis and Anti-Inflammation. Cell Physiol Biochem. 2015;37(6):2415–24. 10.1159/000438594 [DOI] [PubMed] [Google Scholar]
- 74. DiMarino AM, Caplan AI, Bonfield TL: Mesenchymal stem cells in tissue repair. Front Immunol. 2013;4:201. 10.3389/fimmu.2013.00201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Théry C, Witwer KW, Aikawa E, et al. : Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1):1535750. 10.1080/20013078.2018.1535750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Jeppesen DK, Fenix AM, Franklin JL, et al. : Reassessment of Exosome Composition. Cell. 2019;177(2):428–445.e18. 10.1016/j.cell.2019.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 77. Grange C, Skovronova R, Marabese F, et al. : Stem Cell-Derived Extracellular Vesicles and Kidney Regeneration. Cells. 2019;8(10): pii: E1240. 10.3390/cells8101240 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 78. Shah R, Patel T, Freedman JE: Circulating Extracellular Vesicles in Human Disease. N Engl J Med. 2018;379(22):2180–1. 10.1056/NEJMc1813170 [DOI] [PubMed] [Google Scholar]
- 79. Tao SC, Rui BY, Wang QY, et al. : Extracellular vesicle-mimetic nanovesicles transport LncRNA-H19 as competing endogenous RNA for the treatment of diabetic wounds. Drug Deliv. 2018;25(1):241–55. 10.1080/10717544.2018.1425774 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 80. Witwer KW, Van Balkom BWM, Bruno S, et al. : Defining mesenchymal stromal cell (MSC)-derived small extracellular vesicles for therapeutic applications. J Extracell Vesicles. 2019;8(1):1609206. 10.1080/20013078.2019.1609206 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 81. Saas P, Daguindau E, Perruche S: Concise Review: Apoptotic Cell-Based Therapies-Rationale, Preclinical Results and Future Clinical Developments. Stem Cells. 2016;34(6):1464–73. 10.1002/stem.2361 [DOI] [PubMed] [Google Scholar]
- 82. Bieback K, Kuçi S, Schäfer R: Production and quality testing of multipotent mesenchymal stromal cell therapeutics for clinical use. Transfusion. 2019;59(6):256. 10.1111/trf.15252 [DOI] [PubMed] [Google Scholar]
- 83. Pachler K, Ketterl N, Desgeorges A, et al. : An In Vitro Potency Assay for Monitoring the Immunomodulatory Potential of Stromal Cell-Derived Extracellular Vesicles. Int J Mol Sci. 2017;18(7): pii: E1413. 10.3390/ijms18071413 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 84. Thej C, Ramadasse B, Walvekar A, et al. : Development of a surrogate potency assay to determine the angiogenic activity of Stempeucel®, a pooled, ex-vivo expanded, allogeneic human bone marrow mesenchymal stromal cell product. Stem Cell Res Ther. 2017;8(1):47. 10.1186/s13287-017-0488-3 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 85. Murgia A, Veronesi E, Candini O, et al. : Potency Biomarker Signature Genes from Multiparametric Osteogenesis Assays: Will cGMP Human Bone Marrow Mesenchymal Stromal Cells Make Bone? PLoS One. 2016;11(10):e0163629. 10.1371/journal.pone.0163629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Bloom D, Bhatia N, Hanley P, et al. : A Reproducible Potency Assay to Measure Differences in MSC-mediated T cell Suppression. Transfusion. 53:64a–64a. Reference Source [Google Scholar]
- 87. Viganò M, Budelli S, Lavazza C, et al. : Tips and Tricks for Validation of Quality Control Analytical Methods in Good Manufacturing Practice Mesenchymal Stromal Cell Production. Stem Cells Int. 2018;2018:3038565. 10.1155/2018/3038565 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 88. Chinnadurai R, Rajan D, Qayed M, et al. : Potency Analysis of Mesenchymal Stromal Cells Using a Combinatorial Assay Matrix Approach. Cell Rep. 2018;22(9):2504–17. 10.1016/j.celrep.2018.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 89. Chinnadurai R, Rajakumar A, Schneider AJ, et al. : Potency Analysis of Mesenchymal Stromal Cells Using a Phospho-STAT Matrix Loop Analytical Approach. Stem Cells. 2019;37(8):1119–25. 10.1002/stem.3035 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 90. Boregowda SV, Krishnappa V, Haga CL, et al. : A Clinical Indications Prediction Scale Based on TWIST1 for Human Mesenchymal Stem Cells. EBioMedicine. 2016;4:62–73. 10.1016/j.ebiom.2015.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kuci S, Kuci Z, Kollet J, et al. : Genetic Signature of Mesenchymal Stromal Cells Derived from Human Bone Marrow Cd271+Mononuclear Cells. Bone Marrow Transpl. 2014;49:S191–S192. [Google Scholar]
- 92. Khong SL, Lee M, Kosaric N, et al. : Single-Cell Transcriptomics of Human Mesenchymal Stem Cells Reveal Age-Related Cellular Subpopulation Depletion and Impaired Regenerative Function. Stem Cells. 2019;37(2):240–6. 10.1002/stem.2934 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation