Abstract
This article is the second part of a literature review concerning diabetic foot ulcers (DFUs) and the use of antimicrobial photodynamic therapy (PDT). PDT involves the topical application of a photosensitiser into the tissue, followed by illumination that induces the formation of reactive oxygen species (ROS). PDT provides bacterial inactivation and promotes wound healing, and it can be used to manage the infection and microbial colonisation of DFUs. It has pivotal advantages in comparison with chemotherapeutics, such as no potential to induce resistance, and a wide spectrum of activity. Tetracationic Zn(II) phthalocyanine derivatives have been developed for PDT. Among these, we would like to focus on RLP068, whose antimicrobial activity has been widely demonstrated in preclinical studies and in a clinical trial. This article reports previously published evidence and presents four unpublished clinical cases of DFUs treated in the real-life setting with PDT.
Keywords: antimicrobial therapy, diabetes, photodynamic therapy, RLP068, ulcer
Background
Foot ulcers are a prevalent complication of diabetes mellitus and account for significant morbidity, mortality, and healthcare expenditures.1,2 Furthermore, patients living with diabetic foot ulcers (DFUs) suffer low health-related quality of life, poor psychosocial adjustment and need frequent healthcare interactions.3
Management of foot ulcers aims at promoting wound healing, but ever-present microbial colonisation/infection often hinders wound healing, thereby increasing morbidity associated with the ulcer and possibly mortality. The resistance of bacteria to antibiotics is a growing issue that could be overcome through the introduction of new alternative treatments. The efficacy of chemotherapeutic antibiotics in DFUs is limited, due to the frequent occurrence of resistance and difficulties with the local delivery of drugs because of low blood flow. Therefore, infection and microbial colonisation are two of the main problems to face in the management of DFUs.
This article is the second part of a literature review concerning diabetic foot ulcers (DFUs) and the use of antimicrobial photodynamic therapy (PDT).4 This review presents the rationale for the use of photodynamic therapy (PDT) in DFUs, taking advantage of its bacterial inactivation activity (photodynamic antimicrobial chemotherapy) and promotion of the wound healing process, with a special focus on a tetracationic Zn(II) phthalocyanine, RLP068, as a photosensitiser.5,6 Recent clinical experience of the authors with RLP068 is also reported to familiarise readers with this procedure.
Methods
A narrative review of the literature was written after retrieving relevant articles in PubMed; the following keywords were used for searches: diabetic ulcer, diabetes, antimicrobial PDT, and RLP068. In addition, the authors report four exemplary cases recently treated following the reported evidence and obtaining good results, as expected. These case reports are presented to the reader as tutorial examples.
Rationale of photodynamic antimicrobial chemotherapy
PDT is based on the application of a nontoxic dye or photosensitiser (PS) to the lesion area, that is then exposed to light. Red or near-infrared light is most often used because they have a high potential to penetrate tissues. Reactive oxygen species (ROS) are generated when energy or electrons are transferred from the light-excited PS to ambient oxygen. Following ROS production, the increased concentration of oxidisation kills cells.7 This approach was first applied to tumour cells, and anticancer PDT is currently used in hospitals and dermatology clinics worldwide – actinic keratosis and basal cell carcinoma are often treated by PDT.8 The first report concerning a photodynamic antimicrobial effect was published in 1900. The inactivation of Paramecium caudatum was achieved by exposure to the dyes acridine or eosin and then sunlight. Antimicrobial PDT is increasingly appreciated by clinicians and health systems because it may provide an answer to the challenging issues facing local infection management.
The antimicrobial use of PDT is based on the observation that the cytotoxic action obtained from PS can be selectively directed against microorganisms, with little or no effect on host cells. Various chemical structures have been investigated for use in PDT, and procedures producing effectively selective activity have been identified. PDT has two main advantages over pharmacological antibiotic therapy: it does not induce microorganism resistance and it reduces the need of systemic drugs.9
ROS such as singlet oxygen, superoxide anions, and hydroxyl radicals act on many different molecules (e.g. proteins, lipids, and nucleic acids) so that the development of resistance by microbes is very unlikely.10,11 In addition, the agent is not necessarily internalised by the cell to kill it; so, another resistance mechanism is skipped.12 This mechanism of action neither selects for photo-antimicrobial resistance nor does it alter sensitivity to conventional antibacterial drugs, which makes it possible to use PDT as an add-on to the pharmacologic antibiotic treatment.13
Photo-antimicrobials act on many targets: Gram-positive and Gram-negative bacteria, fungi, viruses, and protozoa, and this may facilitate empirical use. The killing effects are rapid, whilst conventional antibiotics may require long periods of time to become effective.13
Moreover, photo-antimicrobial action degrades and oxidises factors such as protein toxins, proteases, α-haemolysin, sphingomyelinase, and lipopolysaccharide.13
Several PS have been investigated. Typically, porphyrins-7,8 and phthalocyanines have been shown to photosensitise when irradiated with visible light, a variety of microbial pathogens including Gram-positive and Gram-negative bacteria, fungi, yeasts, and mycoplasmas.14
A widespread clinical application of PDT obviously requires that the photocytotoxic action against microbial cells takes place with minimal damage to the host tissues. In fact, photoactivated porphyrin derivatives promote the efficient inactivation of mammalian cells both in vitro and in vivo.15 Therefore, it appears necessary to define phototherapeutic protocols and to produce photosensitisers which lead to an extensive and highly preferential decrease in the survival of microbial cells. The study of relationships between the chemical structure and the antimicrobial photoactivity of phthalocyanines has identified a restricted number of molecules which exhibit a very high photosensitising efficiency against both wild and antibiotic-resistant strains of bacteria and yeasts, with lower selectivity for fibroblasts and keratinocytes (i.e. amongst the main constituents of host tissues, such as skin and mucosa).16
MRSA inactivation
Staphylococcus aureus is the most important representative of the staphylococcal group which causes clinically relevant infections within immunocompetent patients, and methicillin-resistant S. aureus (MRSA) remains one of the most prevalent multidrug-resistant organisms causing healthcare-associated infections.17 Staphylococcal infections have been associated with key multidrug resistance phenotypes due to the possession or acquisition of unique antibiotic resistance and virulence traits that are generally attributed to a variety of mobile genetic elements (MGE) as well as chromosomal determinants.18
MRSA growth was inhibited in an in vitro study by PDT. A 15-minute exposure to a 632.8 nm HeNe laser in the presence of 50 μg/mL toluidine blue O as a PS completely eradicated MRSA.19 In addition, 16 epidemic MRSA strains could be inactivated by PDT with a light-activated antimicrobial agent, aluminium disulfonated phthalocyanine (AlPcS2).20
It was also demonstrated that depending on the PS used the same bacterial isolate could be categorised as highly resistant or highly sensitive to PDT. Additionally, the same PS could totally eradicate some bacterial strains and could be noneffective in the case of other strains.18
A single treatment of antimicrobial PDT with RLP068 was effective on infection induced with MRSA in a mouse model of a surgical wound. On day 2 from infection, a strong reduction of bacterial counts (about 3 logs) was observed in mice treated with tetracationic Zn(II) phthalocyanine derivative in comparison with infected untreated mice. On day 9 from infection, reduction of bacterial counts was about 2 logs in mice treated with RLP068.6
PDT efficacy on wound healing process
A systematic review of the literature demonstrated that PDT contributes in several ways to the wound healing process leading to cellular death, reducing or increasing inflammation, stimulating fibroblasts proliferation (and consequently, collagen and elastin deposition), and raising transforming growth factor beta and metalloproteinases.21
In a mouse model, histological examinations of wounds infected with MRSA and treated by PDT with RLP068 showed, on day 9, in addition to reduced infection, a complete re-epithelialisation with a continuous epithelial lining.6
Clinical studies on PDT for colonised or infected diabetic foot ulcers
Few clinical studies or case reports on the use of PDT in wound healing and treatment of DFUs have been published, despite the high number of experimental studies. A systematic review of the literature on the treatment of DFUs with PDT was performed. It included eight randomised trials (with 316 participants) and suggested that phototherapy when compared to no phototherapy/placebo, may increase the proportion of wounds completely healed during follow-up and may reduce wound size in people with diabetes, but there was no evidence that phototherapy improved quality of life.22
A phase IIa randomised, blinded, placebo-controlled study evaluated the efficacy of antimicrobial PDT in 16 patients with chronic leg ulcers colonised by bacteria and in 16 subjects with DFU, and demonstrated that bacterial load was reduced after PDT; in addition, a trend towards accelerated wound healing was observed.23 At baseline, all patients had ulcer duration >3 months, and bacterially colonised ulcers with >1×104 colony-forming units/cm2. A phenothiazinium derivative or placebo was applied topically to wounds for 15 minutes, followed immediately by 50 J/cm2 of red light. Treatment was well tolerated with no reports of pain or other safety issues. In contrast to placebo, patients on active treatment showed a reduction in bacterial load immediately post-treatment (p<0.001). After 3 months, 50% (4/8) of patients with actively treated chronic leg ulcers showed complete healing, compared with 12% (1/8) of patients on placebo.23
The effect of repeated applications of PDT with RLP068, twice or thrice a week, on bacterial load and ulcer healing was investigated in a series of 64 patients with diabetes who had infected DFUs. Patients were treated according to clinical practice, and samples for bacteriological culture were collected before the first application, 1 hour after the first application, and 14 days after treatment initiation (samples were not collected at the end of treatment for 9 patients). Bacterial load decreased significantly after a single PDT treatment with RLP068 and the benefit persisted for 2 weeks, both in patients who received PDT twice a week and in those who received it three times a week. The ulcer area showed a significant reduction during follow-up, even in patients with ulcers infected with Gram-negative bacteria or with exposed bone.24
These results are in agreement with a recently published pilot study of RLP068 PDT for infected venous ulcers of different pathophysiology amongst 36 patients with diabetes.25 Two sessions of RLP068 PDT were performed without systemic or topical antibiotic treatment. After the second PDT session, bacterial swab results were negative in all but two ulcers.25
Case series
Based on previously published evidence, the authors of this article treated some patients with DFUs using PDT; four exemplary cases are reported here, showing that results in the real-life setting agreed with those obtained in the clinical study by Mannucci and colleagues.26
In this experience with patients affected by DFUs, the photosensitiser RLP068 (VULNOFAST plus; Molteni Therapeutics S.R.L., Italy) was used for PDT. Two applications/week were performed, according to the manufacturer’s instructions. RLP068 is a tetracationic Zn(II) phthalocyanine derivative that has been developed for PDT and is actually indicated for the treatment of ulcers and skin wounds. RLP068-PDT efficacy was evaluated in vitro on S. aureus, Pseudomonas aeruginosa, and Candida albicans, and more than 3 log10 for all microbial strains were killed.5 After PDT with RLP068 bacteria were killed and regrowth was inhibited, resulting in significantly accelerated wound healing, in a mouse skin abrasion model. Infection with MRSA was reduced by 2.9 log units, as measured in terms of bioluminescence. Bacterial regrowth after PDT with RLP068 was fourfold lower than in the control group, and bioluminescence (infection) was not detectable after 3 days.27
In each of the cases, a single dose of RLP068 was applied using a sterile syringe on the wound, which was covered with a Tegaderm™ patch and a nontransparent bandage. Thirty minutes after the application, the ulcer was illuminated for 8 minutes by a portable LED light device (VULNOLIGHT, Molteni Therapeutics S.R.L., Italy) with a red light at 630 nm wavelength, providing a total energy of 60 J/cm2. The ulcer was then washed with a saline solution to remove the residues of the photosensitiser and covered with a dressing.
Patients were chosen based on clinical evaluation, as eligible to gain benefit from the treatment. In accordance with the World Medical Association Declaration of Helsinki, all the data referring to the patients are published in an anonymous way, without any details allowing the re-identification of the patient.
Case report 1
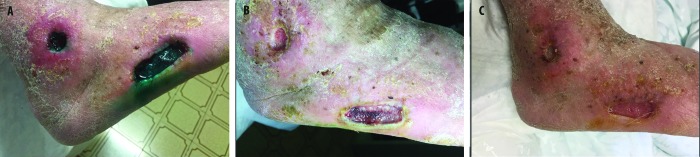
A 61-year-old male, affected with type 2 diabetes mellitus for 20 years, was treated with oral antidiabetics and insulin, but metabolic control was not reached (HbA1c=7.9%). He had the second and distal phalanges of the third toe and all phalanges of the fourth toe of the left foot amputated because of ulcers. At presentation, pes cavus and flicking first toe were observed on both feet; in addition, hyperkeratosis, onychomycosis, and nail hypertrophy were present. Walking function was reduced, and the patient needed orthotics and special shoes. The Winsor index was calculated, and the presence of neuropathy was assessed. An ulcer was observed in the last phalanx of the second toe of left foot, with bone exposition. Pus was present, and the bacteriological examination of the lesion found levofloxacin-susceptible S. aureus infection. An Rx examination, joined to the clinical observation, ruled out the presence of osteomyelitis. The ulcer was scored as Texas grade 2, B stage. Levofloxacin 500 mg/day was initiated, with debridement, and the antidiabetic therapy was changed to improve glycaemic control. Nevertheless, the ulcer condition was not changed after 2 weeks. Exudate and bone exposure persisted (Figure 1A). Antimicrobial PDT was initiated with RLP068, in addition to levofloxacin, to prevent the risk of further amputation. Granulation tissue was present in the ulcer after five applications of PDT. Ulcer size was reduced by 50% after seven applications; oedema was reduced, granulation was increased, and bone exposition was no longer evident. The ulcer was cured in 2.5 months, with 16 applications of RLP068-PDT (Figure 1B).
Figure 1.
Antimicrobial PDT with RLP068, in addition to levofloxacin. (A) Patient condition at the beginning of treatment. (B) Patient condition after 16 applications of RLP068-PDT.
Case report 2
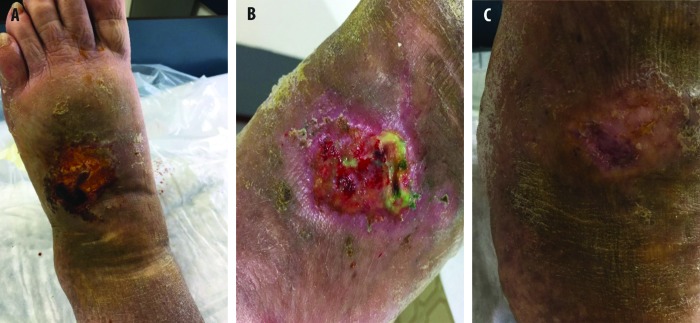
A 63-year-old man had been affected with type 2 diabetes mellitus for 10 years, was receiving oral antidiabetics and a GLP-1 analogue at the time of presentation. His glycaemic metabolism was controlled (HbA1c=6.9%). An ulcer was present on the lateral aspect of the right foot, within a hyperkeratotic area. The ulcer was graded Texas 1, B stage (Figure 2A). The Winsor index was within normal values; sensory neuropathy was detected. Surgical debridement was performed, after a bacteriological examination that showed infection with Klebsiella pneumoniae. Due to this serious infection, ciprofloxacin 500 mg twice/day was initiated, with antimicrobial RLP068-PDT. The antibiotic was changed into amoxicillin + clavulanic acid 1 g twice/day, due to intolerance. Improvement of the ulcer, with the presence of granulation tissue was observed after 10 PDT applications. The ulcer was cured after 16 applications (Figure 2B). An orthotic was adopted to obtain the offloading of the cured area.
Figure 2.
Antimicrobial PDT with RLP068, in addition to amoxicillin/clavulanic acid. (A) Patient condition before treatments. (B) Patient condition after 16 applications of RLP068-PDT.
Case report 3
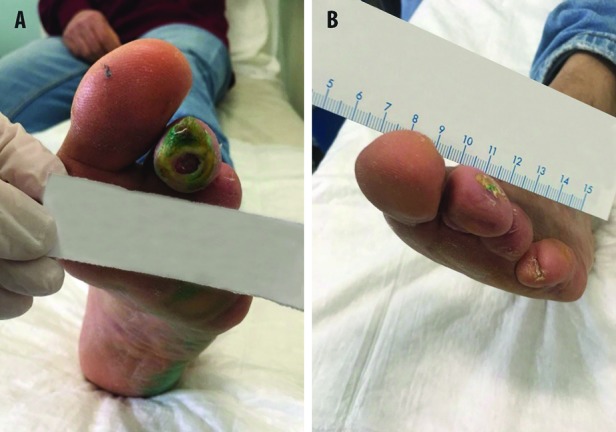
A 78-year-old male patient was affected by type 2 diabetes. He received insulin and the glycaemic metabolism was controlled. In April 2018, an ulcer of the lateral malleolus and a second ulcer at the metatarsophalangeal joint of the right foot occurred. An echocolordoppler of lower limbs showed stenosis of anterior and posterior tibial arteries of the right leg. Percutaneous transluminal angioplasty of two tibial arteries was performed in May 2018. The ulcers persisted in November 2018 and were classified at grade IIB according to Texas Wound Classification (Figure 3A). A bacteriological examination found the presence of P. aeruginosa, and antimicrobial treatment was initiated. The patient received ceftriaxone and RLP068-PDT. After eight PDT applications, granulation tissue was apparent in the ulcers (Figure 3B). Ten days after the antimicrobial treatment, the bacteriological examination was negative and the ulcer size was reduced (Figure 3C)
Figure 3.
Antimicrobial PDT with RLP068, in addition to ceftriaxone. (A) Patient condition before treatments. Patient condition after 8 (B) and 16 (C) applications of RLP068-PDT.
Case report 4
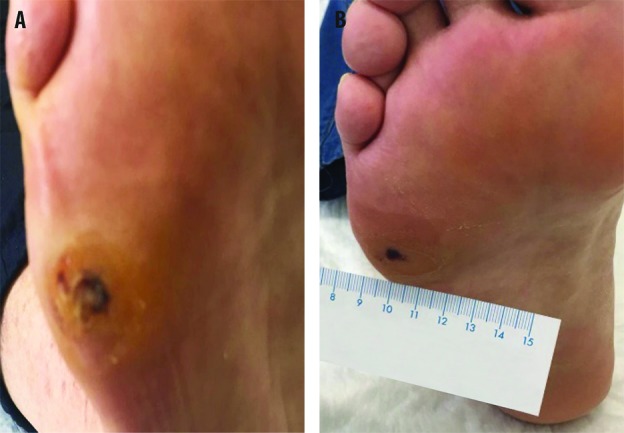
A 55-year old female, obese (BMI 47.2), was affected by blood hypertension, mixed type dyslipidaemia, and type 2 diabetes (treated with metformin). She had undergone bilateral stripping of the saphenous vein some years before. An ulcer of the dorsum of the left foot had been present for a month. An echocolordoppler of lower limbs found no signs of stenosis. At presentation, the ulcer was classified as grade IIB according to Texas Wound Classification (Figure 4A). The patient reported serious pain. The bacteriological examination showed an infection with amoxicillin + clavulanic acid-sensitive S. aureus. Antimicrobial treatment with the combination of antibiotics + RLP068-PDT was performed twice a week for 2 weeks (Figure 4B). One month after the therapy, the bacteriological examination was negative and the ulcer diameters were reduced (Figure 4C). Pain was improved.
Figure 4.
Antimicrobial PDT with RLP068, in addition to amoxicillin/clavulanic acid. (A) Patient condition before treatments. Patient condition after 4 (B) and 16 (C) applications of RLP068-PDT.
Conclusions
PDT is an established procedure as an antitumour treatment, and its antimicrobial efficacy has also been widely assessed by experimental studies. Antimicrobial PDT has pivotal advantages in comparison with chemotherapeutics, such as no potential to induce resistance, and a wide spectrum of activity extended to saprophytes, pathogen bacteria, yeasts, viruses, and protozoa. RLP068-PDT has been observed to reduce the microbial load in DFUs and has shown a trend towards accelerated wound healing. The clinical experience of the authors, as shown by the four exemplary cases reported here, is in agreement with previously published findings and supports the use of RLP068-PDT as an add-on to antibiotic therapy. This procedure helped to prevent resistance, accelerated infection eradication, and promoted ulcer healing. Experience showed that antimicrobial effects of PDT with RLP068-PDT start immediately after the first application and persist in the following weeks. Wound healing improvement is evident after eight applications and further improvements, even up to complete healing, were observed in long-term follow-up. The authors propose this treatment to improve DFU management in serious cases.
Acknowledgments
Content Ed Net provided editorial assistance, with the helpful support of Laura Brogelli, PhD, in the drafting of the manuscript and of Mr Bilal Bham and Hussein Hijazi in the final reading of the manuscript for English language improvement.
Footnotes
Contributions: All authors contributed equally to the preparation of this review. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosure and potential conflicts of interest: The authors declare that they have no conflicts of interest relevant to this manuscript. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors is available for download at https://www.drugsincontext.com/wp-content/uploads/2020/01/dic.2019-10-3-COI.pdf
Funding declaration: Editorial assistance was funded by Molteni Farmaceutici, Scandicci FI, Italy.
Correct attribution: Copyright © 2020 Pantò F, Adamo L, Giordano C, Licciardello C. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 4.0.
Provenance: submitted; externally peer reviewed.
Drugs in Context is published by BioExcel Publishing Ltd. Registered office: Plaza Building, Lee High Road, London, England, SE13 5PT.
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252 7720 07.
For all manuscript and submissions enquiries, contact the Editor-in-Chief gordon.mallarkey@bioexcelpublishing.com
For all permissions, rights and reprints, contact David Hughes david.hughes@bioexcelpublishing.com
Peer review comments to author: 21 November 2019
References
- 1.Boulton AJ, Armstrong DG, Albert SF, et al. Comprehensive foot examination and risk assessment. A report of the Task Force of the Foot Care Interest Group of the American Diabetes Association, with endorsement by the American Association of Clinical Endocrinologists. Phys Ther. 2008;88:1436–1443. doi: 10.2337/dc08-9021. [DOI] [PubMed] [Google Scholar]
- 2.Walsh JW, Hoffstad OJ, Sullivan MO, et al. Association of diabetic foot ulcer and death in a population-based cohort from the United Kingdom. Diabet Med. 2016;33:1493–1498. doi: 10.1111/dme.13054. [DOI] [PubMed] [Google Scholar]
- 3.Driver VR, Fabbi M, Lavery LA, et al. The costs of diabetic foot: The economic case for the limb salvage team. J Vasc Surg. 2010;52:17s–22s. doi: 10.1016/j.jvs.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Martinelli N, Curci V, Quarantiello A, Saldalamacchia G. The benefits of antimicrobial photodynamic therapy with RLP068 in the management of diabetic foot ulcers. Drugs Context. 2019;8:212610. doi: 10.7573/dic.212610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giuliani F, Martinelli M, Cocchi A, et al. In vitro resistance selection studies of RLP068/Cl, a new Zn(II) phthalocyanine suitable for antimicrobial photodynamic therapy. Antimicrob Agents Chemother. 2010;54:637–642. doi: 10.1128/AAC.00603-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simonetti O, Cirioni O, Orlando F, et al. Effectiveness of antimicrobial photodynamic therapy with a single treatment of RLP068/Cl in an experimental model of Staphylococcus aureus wound infection. Br J Dermatol. 2011;164:987–995. doi: 10.1111/j.1365-2133.2011.10232.x. [DOI] [PubMed] [Google Scholar]
- 7.Demidova TN, Hamblin MR. Photodynamic therapy targeted to pathogens. Int J Immunopathol Pharmacol. 2004;17:245–254. doi: 10.1177/039463200401700304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agostinis P, Berg K, Cengel KA, et al. Photodynamic therapy of cancer: an update for clinicians. CA Cancer J Clin. 2011;61:250–281. doi: 10.3322/caac.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dei D, Chiti G, De Filippis MP, et al. Phthalocyanines as photodynamic agents for the inactivation of microbial pathogens. J Porphyrins Phthalocyanines. 2006;10:147–159. doi: 10.1142/S1088424606000181. [DOI] [Google Scholar]
- 10.Hamblin M, Hassan T. Photodynamic therapy: a new antimicrobial approach to infectious disease? Photochem Photobiol Sci. 2004;3:436–450. doi: 10.1039/b311900a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vatansever F, de Melo WC, Avci P, et al. Antimicrobial strategies centered around reactive oxygen species-bactericidal antibiotics, photodynamic therapy, and beyond. FEMS Microbiol Rev. 2013;37:955–989. doi: 10.1111/1574-6976.12026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wainwright M, Crossley KB. Photosensitising agents-circumventing resistance and breaking down biofilms: a review. Int Biodeterior Biodegradation. 2004;53:119–126. doi: 10.1016/j.ibiod.2003.11.006. [DOI] [Google Scholar]
- 13.Wainwright M, Maisch T, Nonell S, et al. Photoantimicrobials-are we afraid of the light? Lancet Infect Dis. 2017;17:e49–e55. doi: 10.1016/S1473-3099(16)30268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Minnock A, Vernon DI, Schofield J, et al. Photoinactivation of bacteria. Use of a cationic watersoluble zinc-phthalocyanine to photoinactivate both Gram-negative and Gram-positive bacteria. J Photochem Photobiol B. 1996;32:159–164. doi: 10.1016/1011-1344(95)07148-2. [DOI] [PubMed] [Google Scholar]
- 15.Berg K. Mechanisms of cell damage in photodynamic therapy. In: Honigsmann H, Jori G, Young AE, editors. The Fundamental Basis of Phototherapy. Milan: OEMF; 1996. pp. 181–207. [Google Scholar]
- 16.Wilson M. Photolysis of oral bacteria and its potential use in the treatment of caries and periodontal diseases. J Appl Bacteriol. 1993;78:299–306. doi: 10.1111/j.1365-2672.1993.tb02780.x. [DOI] [PubMed] [Google Scholar]
- 17.Jean S-S, Hsueh P-R. High burden of antimicrobial resistance in Asia. Int J Antimicrob Agents. 2011;37:291–295. doi: 10.1016/j.ijantimicag.2011.01.009. https://doi.org/10.1039/b311900a doi.org/10.1016/j.ijantimicag.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 18.Agrawal T, Avci P, Gupta GK, et al. Harnessing the power of light to treat staphylococcal infections focusing on MRSA. Curr Pharm Des. 2015;21:2109–2121. doi: 10.2174/1381612821666150310102318. [DOI] [PubMed] [Google Scholar]
- 19.Hajim KI, Salih DS, Rassam YZ. Laser light combined with a photosensitizer may eliminate methicillin-resistant strains of Staphylococcus aureus. Lasers Med Sci. 2010;25:743–748. doi: 10.1007/s10103-010-0803-z. [DOI] [PubMed] [Google Scholar]
- 20.Griffiths MA, Wren BW, Wilson M. Killing of methicillin-resistant Staphylococcus aureus in vitro using aluminium disulphonated phthalocyanine, a light-activated antimicrobial agent. J Antimicrob Chemother. 1997;40:873–876. doi: 10.1093/jac/40.6.873. [DOI] [PubMed] [Google Scholar]
- 21.Nesi-Reis V, Lera-Nonose DSSL, Oyama J, et al. Contribution of photodynamic therapy in wound healing: a systematic review. Photodiagnosis Photodyn Ther. 2018;21:294–305. doi: 10.1016/j.pdpdt.2017.12.015. [DOI] [PubMed] [Google Scholar]
- 22.Wang H, Yuan JQ, Zhang B, et al. Phototherapy for treating foot ulcers in people with diabetes. Cochrane Database Syst Rev. 2017;6:CD011979. doi: 10.1002/14651858.CD011979.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morley S, Griffiths J, Philips G, et al. Phase IIa randomized, placebo-controlled study of antimicrobial photodynamic therapy in bacterially colonized, chronic leg ulcers and diabetic foot ulcers: a new approach to antimicrobial therapy. Br J Dermatol. 2013;168:617–624. doi: 10.1111/bjd.12098. [DOI] [PubMed] [Google Scholar]
- 24.Monami M, Scatena A, Schlecht M, et al. Antimicrobial photodynamic therapy in infected diabetic foot ulcers: a multicenter preliminary experience. J Am Podiatr Med Assoc. doi: 10.7547/18-069. [DOI] [PubMed] [Google Scholar]
- 25.Mosti G, Picerni P, Licau M, Mattaliano V. Photodynamic therapy in infected venous and mixed leg ulcers: a pilot experience. J Wound Care. 2018;27:816–821. doi: 10.12968/jowc.2018.27.12.816. [DOI] [PubMed] [Google Scholar]
- 26.Mannucci E, Genovese S, Monami M, et al. Photodynamic topical antimicrobial therapy for infected foot ulcers in patients with diabetes: a randomized, double-blind, placebo-controlled study-the D.A.N.T.E (Diabeticulcer Antimicrobial New Topical treatment Evaluation) study. Acta Diabetol. 2014;51:435–440. doi: 10.1007/s00592-013-0533-3. http://doi:10.1007/s00592-013-0533-3. [DOI] [PubMed] [Google Scholar]
- 27.Vecchio D, Dai T, Huang L, Fantetti L, et al. Antimicrobial photodynamic therapy with RLP068 kills methicillin-resistant Staphylococcus aureus and improves wound healing in a mouse model of infected skin abrasion PDT with RLP068/Cl in infected mouse skin abrasion. J Biophotonics. 2013;6:733–742. doi: 10.1002/jbio.201200121. [DOI] [PMC free article] [PubMed] [Google Scholar]