Abstract
Meghalaya, (in India), in the region of the mega-biodiversity hotspots, is home to a plethora of wild mushrooms. The present study concerns the exploration of the order Agaricales, which includes rare gilled mushrooms considered endangered under IUCN A4c criteria, due to the declining habitat. Electron microscopy of the gill sections revealed an abundance of clamp connections, hyphal cell walls, cystidia, and basidia. This rare species which belongs to the family Cyphellaceae, exhibits morphological and molecular differences from the Cyphella spp. Phylogenetic analysis revealed that it formed a clade under the genus Campanophyllum of the order Agaricales, confirmed by both Neighbor Joining (NJ) and Bayesian phylogenetic analysis. Being nutritionally potent along with its efficient antioxidant value, the fungal extract shows significant rise of two-fold in the antimicrobial activity along with the commercial antibiotics. The compound, Phenol, 2, 4-bis (1, 1-Dimethylethyl) (2, 4-DTBP) showed in ample range in the fungal extract along with aliphatic hydrocarbons, terpene, alcohol and volatile organic compounds on further characterization in GCMS. The present study indicates the endangered Campanophyllum proboscideum could be a rich source of natural antioxidants and an effective pharmaceutical agent.
Keywords: Campanophyllum, microscopic, molecular, phylogeny, nutritional
1. Introduction
Meghalaya, extending across an area of approximately 22,549 km2, is located between the coordinates of 25°47′–26°10′N latitude and 89°45′–92°47′E longitude. The region is noted for high rainfall during the summer receiving an annual average of above 12,000 mm and temperature touching a high of 28 °C in the summer to a low of 2 °C in the winter, with a periodic deviation to below freezing temperature. The high humidity prevailing in the area provides atmospheric conditions conducive for the growth of several saprophytes, including mushrooms growing in the forest tier or mycorrhiza associated with the plant life. Although the region is rich in specimens belonging to Boletaceae, Russulaceae, Clavariaceae, Gomphaceae, and Tricholmataceae, specific knowledge regarding the importance of these mushrooms is limited to the old and elderly villagers and the region continues to be scientifically explored for its mushroom flora [1]. The identification of the mushrooms in these regions is completely based on ethnic knowledge, which includes their colors and smells, with no information regarding their additional properties. Locals collect these mushrooms for their livelihood, without any accurate knowledge in terms of their toxic essence, which sometimes leads to fatalities among them. This occurs because of the consumption of the toxic species which mimic their edible counterparts. Researchers have demonstrated that cryptic species are a very common occurrence among these fungi. Accuracy based on the morphological traits can prove nonreliable and hence the DNA barcode has become an authentic tool in identification [2,3].
The diversity of the gilled cyphelloid polyporous fungus, Campanophyllum proboscideum once reported is common among the “reduced series” members of Campanella, Cyphella, Rimbachia, and Skepperiella, which were earlier predominant in Mesoamerica [4]. The genus was limited in 2003 to include the species termed Lentinus proboscoides, the name applied to the mushroom, which had been collected by Örsted (more than 150 years ago) in Costa Rica, but later was restricted to genus Campanophyllum related to the “Gloeostereae” clade [4,5]. The study of the rDNA sequence has given valuable insight into its evolutionary relationships with the other basidiomycetes group. The species was also considered endangered under IUCN A4c criteria due to its declining habitat and low population. As the genus features among the “reduced series”, no records regarding its polyphasic approach or bioactivity have been reported from the foothills of the Eastern Himalayas, in India.
At present, there are vast records of a variety of bioactive metabolites drawn from mushrooms which have fascinated many and kindled great scientific interest for their biological behavior as antioxidants, antimicrobials, anti-inflammatory, and anti-tumor agents [6,7]. High traces of phenolic compounds are present in mushrooms. Many in vitro and epidemiological studies have contributed toward the use of individual phenolic compounds possessing different antioxidant and antagonistic activities with the potential of being free radical polymerization inhibitors (chain breaker), peroxide decomposers, and oxygen scavengers [8].
In the present study, the specimen was collected from the East Khasi Hill district of Meghalaya, nestled in the foothills of the Eastern Himalayas in India and is illustrated and described. The total soluble phenol and its bioactive components in some commercial mushrooms have been researched by several authors, but no reports on detailed profiling of the chemical and nutritional compounds of the Mexican endangered mushroom, which can be utilized later as a potential pharmacological substance, are available in the database [9–11].
2. Materials and methods
2.1. Sample collection
Fresh mushroom was collected from the forest of Meghalaya (25°26.7′N and 91°44.92′E) at an altitude of 1788 m with 75% humidity. Collected sample was brought to the laboratory condition in a sterile bag. The sample was washed in luke warm water to remove the dirt, while maintaining safety using sterile gloves and mask. Macro-morphological traits were recorded at the site of collection for the fresh voucher. Field photograph of the fresh voucher was taken with a Canon camera. A unique laboratory identification number has been assigned against the collected sample, MBSRJ38.
2.2. Microscopic analysis
Transverse sections of the mushroom cap along with the gills were viewed in Leica Microsystems (CH-9435; Leica, Heerbrugg, Germany) using Melzer’s solution [12].
Scanning electron microscope (SEM) analysis of the sample was carried as described earlier where the processed tissues were mounted on an aluminum stubs using double sided adhesive tape and was layered with gold by sputtering in a vacuum sputter (JFC-1100; Jeol, Tokyo, Japan) at various magnification to obtain surface ornamentation at 20 kV of accelerating voltage [13].
2.3. Molecular analysis
The fungal tissue was considered for extraction of genomic nuclear DNA using HiPurA fungal DNA isolation kit (Himedia, Mumbai, India) and was amplified by two primers ITS1F (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS4R (5′-TCCTCCGCTTATTGATATGC-3′) [14]. Amplification was carried out in a GeneAmp 9700 Thermal Cycler (Applied Biosystems, Foster City, CA, USA) under the following conditions: initial denaturation at 95 °C for 5 min, 30 cycles of denaturation at 94 °C for 1 min, annealing at 52 °C for 30 s, extension at 72 °C for 1 min and final extension at 72 °C for 10 min. The amplified nucrDNAITS1-5.8S- ITS2 (ITS barcode) ribotypes were purified using QIA quick® gel extraction kit (Qiagen, Hilden, Germany) and was sequenced (Xcelris Lab, Gujarat, India).
2.4. Sequence and phylogenetic analysis
The nucleotide sequence coding for the ITS gene was computed for their alignment along with its homology sequence using ClustalW (https://www.ebi.ac.uk/Tools/msa/clustalo/). Electropherograms was manually edited to omit the gap using Chromas Lite (Technelysium, Queensland, Australia) and deposited to NCBI to obtain the accession number.
Neighbor joining (NJ) and Bayesian inference were used to analyze the evolutionary distance matrix between the query sequence and its homologous. NJ tree was computed using MEGA 6 software [15]. Robustness of NJ was calculated by analyzing the bootstrap of 1000 replicates (Kimura-2 parameter). Bayesian analysis was performed with MrBayes 3.2 and was run by specifying a general-time reversible (GTR) model with gamma-distributed variations across site [16]. Four Markov chains (3 heated and one cold chain) with incremental heating temperature of 0.05 were run for one million generations and sampled every 10 generations. The first 25% of the trees were discarded as burn-in. Clade posterior probabilities (PP) were determined for the combined sets of trees from two simultaneous runs.
2.5. Nutritional analysis
The mushroom sample was analyzed for its presence of nutritional composite using AOAC protocol for moisture, fat, fiber, and ash [17].
Total crude protein analysis of the examined sample was evaluated by Bradford test [18]. The tests were performed in three replicas to reduce the possibility of error.
2.6. Extraction of crude metabolite
The sample was dried and was extracted in methanolic solvent using the earlier protocol as described earlier [13].
2.7. Qualitative analysis for phytochemicals
The crude methanolic extract was further diluted at a concentration of 5 mg/mL for its various phytochemical assays. The presence of alkaloids, flavonoid, phenolic, carbohydrates, cardiac glycosides, terpenoids, and saponins were determined qualitatively [19]. Meixner test was performed to test the presence of deadly amanitin and other hallucinic alkaloids [20].
2.8. Determination of antioxidant assay
The free radical scavenging activity of the methanolic extract of the mushroom was performed [21]. Absorbance was measured at 517 nm in UV spectrophotometer. A low absorbance value indicated a high free-radical-scavenging activity. Butylated hydroxytoluene (BHT) was considered as a positive control for the test. The percentage free radical scavenging activity was analyzed using the following equation
where AC is the absorbance of the reaction with test sample and AD is the absorbance of the 2, 2-diphenyl-1-picrylhydrazyl (DPPH) reagent without the test sample.
Reducing power assay was performed using the protocol as described by Oyaizu [22]. To various concentrations of the methanolic extract (2.5 mL) were mixed the reagents and the absorbance was measured spectrophotometrically at 700 nm. Higher absorbance indicates higher reducing powers. BHT was considered as a standard.
2.9. Determination of bioactive compounds
For flavonoid content, the methanolic extract (5 mL) was treated with 10% of aluminum nitrate (1 mL) and 1 M aqueous potassium acetate (1 mL) and absorbance was noted at 415 nm. Quercitin was used as a standard for the test [23].
Folin-Ciocalteu calorimetric method was used to determine the total soluble phenolic matter of the crude extract [24]. Results were expressed in terms of mg of gallic acid equivalents per gram (mg GAE g−1) of the test mushroom.
β-carotene and lycopene in the metabolite was determined and was calculated with the following formula [25]
2.10. GCMS analysis
GC–MS analysis was performed using a GC-MS spectrometer (Clarus 680/600 EI; Perkin-Elmer, Norwalk, CT, USA) with a capillary column (30 m × 0.25 mm × 0.25 microm). The Clarus 680 GC used purified helium as the carrier gas, at a constant flow rate of 1 mL/min. One microliter of samples were injected and oven temperature was programmed from 60 °C to 300 °C for 2 min at the rate of 10 °C/min and then isothermally held for 6 min until the analysis was completed. Later the compounds were analyzed in mass spectrophotometer. Both the structure and molecular weight of the compounds were interpreted by comparing with the database of National Institute Standard and Technology (NIST) compound database.
2.11. Antimicrobial and synergistic assay
In vitro antimicrobial assay was carried out by well diffusion method against clinical pathogens [13]. The test bacterial strains, Escherichia coli (MTCC730), Staphylococcus aureus (MTCC96), Streptococcus pyogenes (MTCC1925), and Klebsiella pneumonia (MTCC109) were considered. The bacterial strains were grown on brain-heart infusion broth at temperature of 37 °C for 18–24 h until it reached a turbidity of 0.5 McFarland standards (106 colony forming units/mL). The antimicrobial assay was performed on Mueller Hinton Agar plates. The crude extract at a concentration of 1 mg/mL (40 µL) was used for the assay along with the pure extraction solvent (DMSO) as a negative control. The plates were incubated at 37 °C for 24 h. Chloramphenicol (30 µg) was used as a positive control to determine the sensitivity of the strains. Antimicrobial activity of the crude extract was determined by measuring the zone of inhibition around the well.
Synergistic activity of the metabolite was analyzed by calculating the mean surface area of zone of inhibition by antibiotic and to that of metabolite (1 mg/mL, 40 µL) in combination with commercial antibiotic chloramphenicol. The percentage in fold increase area was determined by the following relation
where A is zone of inhibition for antibiotic and B is the metabolite in combination with antibiotic, respectively.
3. Results and discussion
3.1. Etymology
This label refers to the polyphasic similarity of the mushroom to Campanophyllum proboscideum.
3.2. Taxonomic analysis
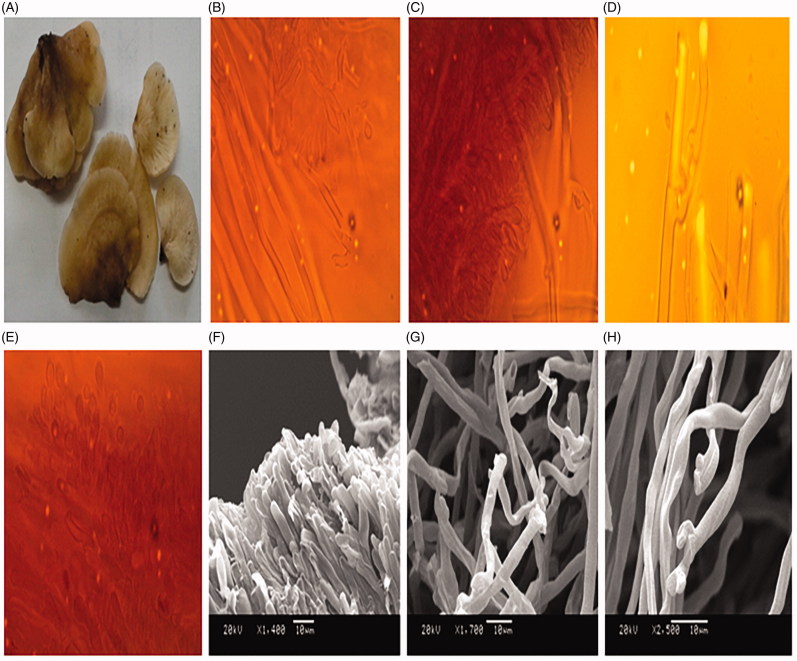
Pileus: Pileus span from 2.5 to 3.9 cm diameter, asci with wavy and lobe-shaped margin to somewhat in-rolled, and curved. Slightly umbonated surface, sticky, moist, and fibrillose in texture. Lamellae sinuate, crowded, whitish in color when young, pale yellow at maturity, but becomes slightly darker brown during storage or drying. Stipe is absent. The asci-shaped pileus attaches on to the tree bark (Figure 1(A)).
Figure 1.
(A) Fresh basidiomycota of Campanophyllum proboscideum; (B) mycelia morphology showing cystidia with tapering end; (C) cystidia with globular end; (D) clamp connection; (E) Globular basidia formation observed in the transverse section of gills; (F) Scanning electron micrograph of gills showing globular end; (G) Scanning electron micrograph of gills with hymenium; (H) Scanning electron micrograph of gills showing clamp connection.
3.3. Microscopic analysis
The asci-shaped C. proboscideum differs from the sac silhouette of Cyphella digitalis in its morphological trait, despite being a member of the same family, Cyphellaceae. Among the subicular hymenium observed, the cystidium is the biggest cell, with an average length of 6–8.83 µm and is cylindrical or globular to subclavate (Figure 1(B,C)). The subhymenia are mainly generative with a slightly double-thickened wall, leaving the cytoplasmic components in some parts of the hymenia. The hyphal system appears monomitic in form, having a width of 2.77 µm. The hymenium is narrow and clavate and frequently shows clamp connections, with the clamp ranging in diameter from 2.94 µm to 4.95 µm (Figure 1(D)). The hyphae have been observed to be swallowed up in some regions. The cheilocystidia are noted to have short necks present at the lamellar edge, which are similar to pleuramacrocystidia (Figure 1(D)) [26].
The cheilocystidia are crystalline in the center. The lamellae are observed to become mature with the basidia formation having a diameter width of 2.42 µm to 2.65 µm at the apex (Figure 1(E)). Some basidia appear to have a clavate structure. The lower parts of the basidia are noted to have swallow cells. Sterigmata and basidiospores are absent. The lamellar trama, which is seen to acquire a yellowish color with Melzer’s reagent, possess a hollow tube like structure at its apex. The pileipellis is somewhat thick. Marginal cells appear cylindrical to short-ellipsoidal in shape.
Most of the hyphal trama appear to be having a clear club-shaped apex with globular basidia in the juvenile stage; they lack sterigmata and basidiospores, as well as the basidioles in the SEM image. The hymenial cells were observed to be short in length (Figure 1(F)). The hyphae were thick-walled, loosely interwoven, and distantly aerial. The apex was somewhat enveloped. Clamp connections were observed and were often branched at the junctions, with wide angles (Figure 1(G)). Microscopically, abundant fusiform to cystidia cylindrical in structure were present, often bulbous and tapering at the apex (Figure 1(H)). The unique feature of C. proboscideum was the long thick hyphal cell wall showing the presence of clamp connections, together with the cheilocystidia along the lamellar edge, which were evident in the section of the gills under the optical microscope.
3.4. Molecular analysis
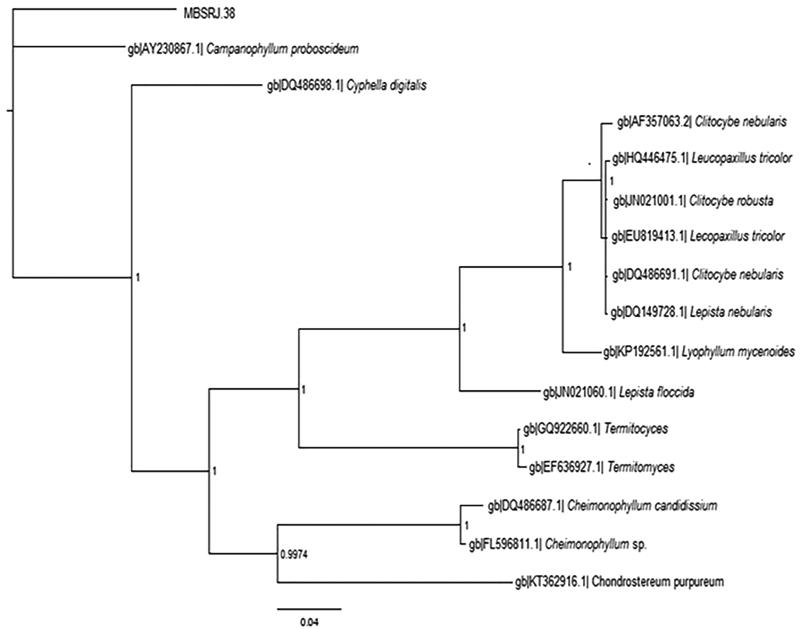
The macrofungi rRNA-ITS region was considered the confirmatory identification, in which the sequence data were aligned using BLAST and deposited on to the NCBI to obtain the accession number (KP843881). Both the Neighbor Joining (NJ) and Bayesian analysis were performed to confirm the relationship clade. The tree based on the ITS rRNA revealed the sequence to be included in the clade with C. proboscideum, having 93% sequence homology with a strong supported bootstrap of 1000 replicates while computing Neighbor joining which was later confirmed with the Bayesian topology. The Bayesian topology was analyzed to verify the second hypothetical interference of the concatenated file. The topology test specified that the sequence data (MBSRJ38) needed to be in same clade with C. proboscideum with one posterior probability, which supports the best-scoring NJ analysis (Figure 2). The species is identified as Campanophyllum based on its morphological and microscopic structure, including basidia, cystidia, and hyphal cell wall, and is authenticated by molecular characterization using ITS. The fungal sequence showed hits of 83% identity to C. proboscideum with a query cover of 94% in the NCBI BLAST. Being monotypic, the sequence supports 93% bootstrap and forms a clade with C. proboscideum identified in Costa Rica in 2003 (NCBI Accession: AY230867) [4].
Figure 2.
Bayesian tree inferred from ITS nucleotide sequence with 1 posterior probability.
3.5. Nutritional analysis
The nutritional analysis of the fruiting body varies significantly in its content of protein, fiber, moisture, ash, and fat. The protein content was found to be significantly higher. The protein content was found to be significantly higher. The sample was analyzed for total moisture, fiber content in its dried mass, fat content, and crude ash (Table 1). The crude protein content of the mushroom in this experiment was found to be appreciably good. According to a few reports, the crude protein present in mushrooms ranges from 19 to 39 g in 100 g of dry weight [27–29]. The low fat content and higher protein level reveal it to be a source of treasured dietary compounds, indicating that the mushroom in this study is essentially highly beneficial in terms of human health.
Table 1.
Nutritional profiling of Campanophyllum proboscidium (g/100g).
| Moisture (%) | Fiber | Fat | Ash | Protein |
|---|---|---|---|---|
| 56.78 ± 0.13 | 5.78 ± 0.43 | 0.7 ± 0.03 | 2.87 ± 0.18 | 21.67 ± 0.67 |
Result considered was the mean ± SEM of 3 trials (one-way ANOVA).
3.6. Phytochemical analysis
The experimental mushroom was analyzed for the presence of phyto-constituents which was found to be positive for alkaloids, flavonoids, terpenes, saponins, cardiac glycosides, phenolics, and carbohydrates. From the Meixner test, the metabolite was analyzed to be negative for both deadly amanitin and hallucinogenic alkaloid psilocybin, as the extract did not show any color change when treated with high-lignin content newspaper and hydrochloric acid, making it safe for human consumption.
3.7. Antioxidant assay
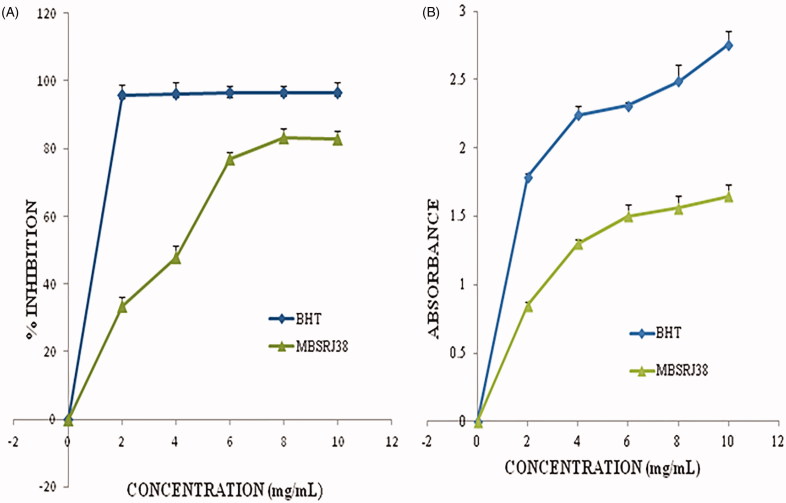
The DPPH forms a free radical, which when it reacts with the active antioxidant can donate its hydrogen or electron, and get reduced from dark violet in color to pale yellow. The reduction or removal of the hydrogen ion from DPPH is determined by its lowered absorption when determined at 517 nm. Figure 3(A) shows the scavenging activity (%) on the DPPH radical by the experimental mushroom metabolite in terms of the commercially synthesized BTH as the positive control. The % inhibition by the methanolic extract of C. proboscideum ranges between 33.52 ± 0.27% and 82.94 ± 0.22%. The free radical scavenging effect was found to be in an exponential phase with the increase in the concentration, whereas the IC50 value obtained from the regression graph was around 4.6537 mg/mL. The data obtained from this study are much higher than a report on a few wild mushrooms as recorded earlier, when the scavenging effect by the methanolic extract of the wild mushroom was noted to be 84.4–85.8% at a concentration of 30 mg/mL for both the stipe and pileus, individually [29].
Figure 3.
(A) Free radical scavenging activity of the methanolic extract at various concentrations; (B) Reducing power activity of the mushroom methanolic extract at various concentrations.
The reducing power assay is based on the ability of the metabolite to reduce the yellow ferric iron to the blue ferrous form by donating an electron. The reducing power of the experimental mushroom was found to be clearly manifested (p < 0.05), where the BHT was accepted as the standard. The higher the absorbance the higher the reducing power (Figure 3(B)), and this might be due to the high phenolic or flavonoid content which participates in electron donation and stabilizes the free radical reaction [22]. The reducing power of the methanolic extract of C. proboscideum ranges from 0.85 ± 0.13 to 1.648 ± 0.14. The commercially synthesized antioxidant BHT reveals a higher range of reducing activity, with mean absorbance ranging from 1.79 ± 0.1 to 2.76 ± 0.14 from 2 mg/mL to 10 mg/mL concentration. However, a good reducing power was reported at a concentration of 1–5 mg/mL for ranges from 0.73 to 0.84 [30].
3.8. Determination of the bioactive compounds
Flavonoids are known to possess strong antioxidant activities to scavenge the free radicals. The total flavonoid content in the experimental mushrooms was estimated in terms of Quercetin (Table 2). The flavonoid level is a little lowers than that of the other wild mushrooms, which ranges from 115.16 mg/100 g–134.31 mg/100 g in the literature and which may be the result of several epigenetic factors [31].
Table 2.
Bioactive compound profiling of Campanophyllum proboscidium.
| Total flavonoids (mg QE/g dry weight) | Total phenolics (mg GAE/g dry weight) | β-carotene (µg/g) | Lycopene (µg/g) |
|---|---|---|---|
| 0.94 ± 0.037 | 0.5127 ± 0.020 | 7.42 ± 0.04 | 3.84 ± 0.02 |
Result considered was the mean ± SEM of 3 trials (one way ANOVA).
The total phenolic content of the mushroom sample examined was estimated with standard curve equation: y = 0.9138x + 0.0064, R2 = 0.9801. Soluble phenolic compounds have been studied to facilitate the consumption of edible mushroom by the tribal communities, and which may also be the source of effective anti-inflammatory, anti-tumor, antibacterial, antioxidant, and antiviral agents [9]. The total phenol found in our experimental species was much less than that of some wild mushrooms, which could be due to certain genetic factors (species-to-species variations), epigenetic factors, solvent concentration, solvent-to-solid ratio, solvent polarity, or moisture content of the mushroom [32–34]. A similar study on tea, catechin, and caffeine has also reported much higher total phenol content in the ethanolic extract than that of methanol or water extract, which may be due to the inability of the solvent to permeate into the tissue [33].
β-carotenes are known as the precursors of Vitamin A and the presence of lycopene is considered the most efficient supply of an oxygen quencher [31]. In this study, the presence of carotenoid in the mushroom species makes them significant and on par with vegetables. The β-carotene and lycopene content was estimated from the studied mushroom (Table 2). Various reports have suggested the presence of high quantities of β-carotene and lycopene in the wild mushroom, including Russula delica, Lentinus squarrosulus, and L. sajor-caju [35,36].
3.9. Chromatographic analysis of the active components
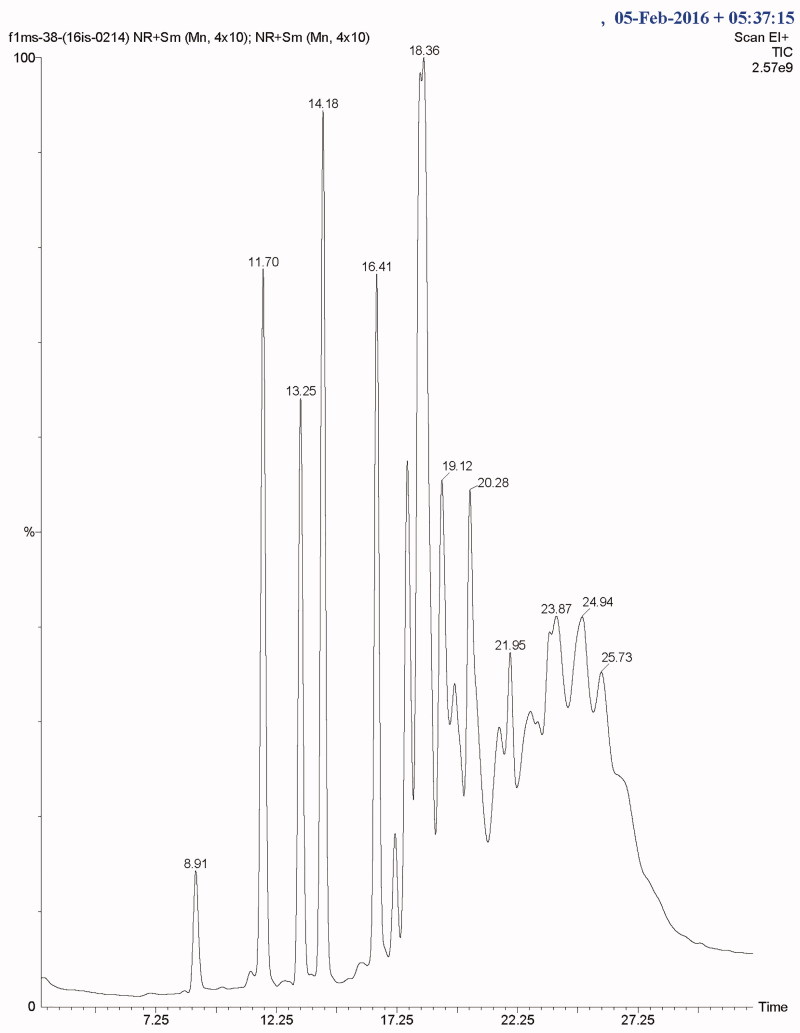
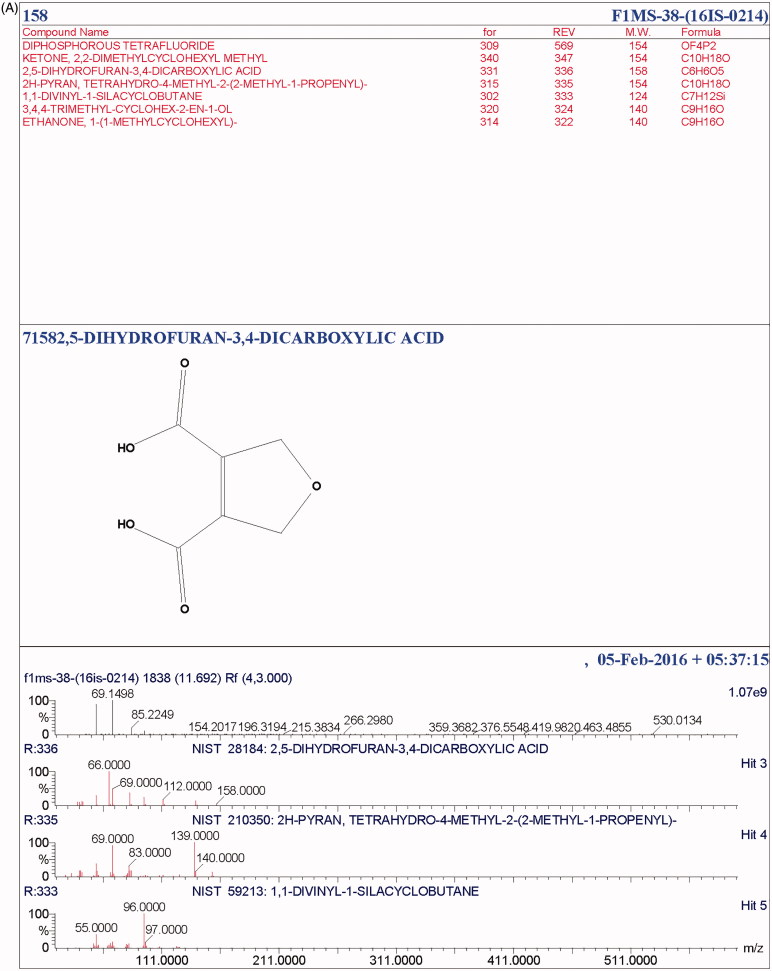
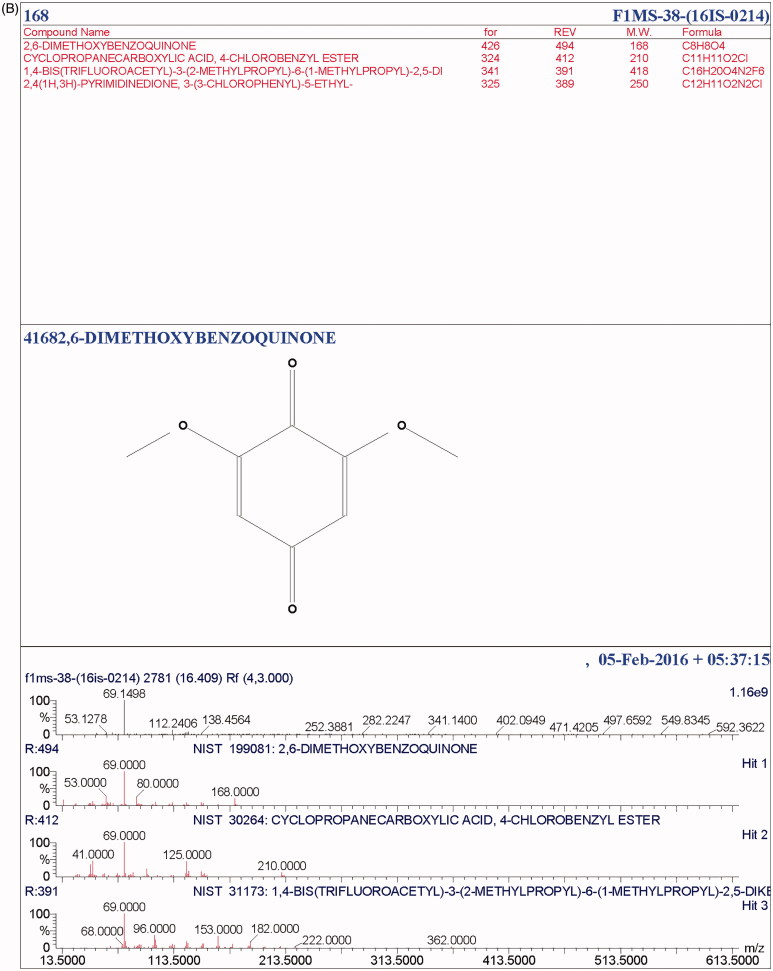
The total ionic count from the chromatographic analysis is shown in Figure 4. The alcoholic, long-chain aliphatic hydrocarbon, phenolics and benzoquinone, as well as the terpenes, esters, ethyl group, carboxylic group, and volatile organic compounds were detected. At a retention time of 11.6, a terpene, 2H-pyran, tetrahydro-4-methyl-2-(2-methyl-1-propenyl)- was present, a well-recognized flavor-enhancer. Terpenoids like ergosta-5, 7, 22-trien-3-ol, 22-tetraen-3.β.-ol, D-xenialactol, gosterol, ergothionene, dimethylhydrazine were earlier reported in various higher fungi [37,38]. Terpenoids are known to possess antioxidant, anticancer, anti-inflammatory, anticholesterol properties [38]. The phenolic compound, 2, 6-bis (1, 1-dimethylethyl) was present at 13.25 retention time with peak abundance touching as high as 12.5. This compound is commonly known as a precursor of the complex compound utilized as an antioxidant. At 13.27 retention time, with peak abundance of 7.5%, Phosphetane, 1, 1-bis (1, 1-dimethylethyl)-1fluoro-1, 1-dihydro was observed. Toxic benzoquinone, 2, 6-dimethoxybenzoquinone (2, 6-DMBQ) was present at a retention time of 16.4. The aliphatic hydrocarbon, 1-Eicosene (m/z of 280) was observed at a retention time of 18.3, with peak abundance of 2.5% (Table 3). Aliphatic hydrocarbons are known to indirectly express metabolic activity by stimulating alcohol and ester formation, which further facilitates the growth of the cellular biomass by metabolizing the Kreb cycle. A few of these compounds have been reported for their potential in pharmaco-activities. Some compounds like alcoholic, volatile organic compounds, and ester components have been known to play a substantial roles either as a flavoring component in the pharmaceutical industries or as a defoaming agent and many more. In addition, esters are recognized as a major fraction in various edible mushrooms, in several fruits (apple, apricot, pineapple) as reported, and are known to enhance the fragrance [39–42]. The presence of cyclohexadecane, a heterocyclic metabolite, and a few pyrrole derivatives are known to protect the mushroom from grazing animals. The peaks obtained in the chromatogram spectra were compared with the database from the NIST libraries (Figure 5(A,B)).
Figure 4.
Total ionic count of the mushroom metabolic by GCMS.
Table 3.
GCMS profiling of Campanophyllum proboscidium.
| RT (min) | Compounds (Identified) | Mol. formula | m/z | Peak abundance (%) | Activity |
|---|---|---|---|---|---|
| 11.6 | 2H-Pyran, Tetrahydro-4-methyl-2-(2-methyl-1-propenyl)- | C10H18O | 154 | 1.5 | Flavoring agent |
| 13.25 | 2-methylsulfanyl-1-(thiomorpholin-4-yl) Ethanone | C7H13NOS2 | 191.30 | 100 | – |
| 13.25 | Phenol, 2, 6bis (1, 1-Dimethylethyl) - | C14H22O | 206 | 12.5 | Anti-inflammatory |
| 13.27 | Phosphetane, 1, 1-bis (1, 1-dimethyethyl) 1-fluoro-1, 1-dihydro | C11H24FP | 206 | 7.5 | – |
| 14.18 | Cyclohexadecane | C16H32 | 224 | 2.0 | – |
| 16.4 | 2, 6-dimethoxybenzoquinone | C8H8O4 | 168 | 1.5 | Toxic (mutagenic, cytotoxic, hepatotoxic) |
| 17.04 | 3-methyl-2-(2-oxopropyl) furan | C8H10O2 | 138 | 1.02 | Anti-inflammatory, antipyretic, hepatoprotective |
| 17.6 | 2-hydroxy-4-methylthiobutanoate | C5H9O3S | 149.18 100 | – | |
| 18.3 | 1-Eicosene | C20H40 | 280 | 2.5 | Anti-diabetic, anti-cancer |
| 19.12 | S-propyl N-butyl-N-ethylcarbamothiote | C10H21NOS | 203.13 | 0.5 | – |
| 19.12 | 1-Methyl-1-histidine, trimethylsilyl ester | C10H19N3O2Si | 241 | 0.8 | – |
| 19.12 | Sarcosine, n-pentafluoropropionyl-, propyl ester | C9H12F5NO3 | 277 | 0.3 | – |
| 20.29 | - (–) Epicatechin | C5H14O6 | 207.1043 | 3.25 | Antioxidant |
Figure 5.
(A) NIST library analysis of active compound 71582. (B) NIST library analysis of an active compound 41682.
3.10. Antimicrobial and synergistic efficiency
The mushroom metabolite showed a strong antimicrobial assay in terms of the commercial antibiotic chloramphenicol (Table 4). The observed zone of inhibition, together with its synergistic effect implies its bactericidal properties. The increased fold area of the mushroom metabolite was significantly higher (with 26.56% in Streptococcus pyogenes) than that of the other test pathogens, which may differ in their cell wall compositions.
Table 4.
Antimicrobial and synergistic activity of the metabolite against pathogens Escherichia coli, Staphylococcus aureus, Streptococcus pyogenes and Klebsiella pneumoniae.
| Clinical pathogens | Zone of inhibition (in mm) | Antibiotic [Chloramphenicol] A | Metabolite + Antibiotic Zone of inhibition (in mm) B |
Fold area increase (%) (B2−A2)/ A2 × 100 A-Antibiotic (ZOI) B-Antibiotic + metabolite |
|---|---|---|---|---|
| Escherichia coli | 10 mm | 32 | 33 | 6.34 |
| Staphylococcus aureus | 12 mm | 31 | 32 | 6.55 |
| Streptococcus pyogenes | >10 | 32 | 36 | 26.56 |
| Klebsiellapneumoniae | >10 | 31 | 32 | 6.55 |
4. Conclusion
Ecologically, C. proboscideum grows in the areas of highest rainfall. It was initially reported from Costa Rica, where the climatic variations are dependent upon the quantity of rainfall received. It is well known that the region receives the highest rainfall between May and November, with the annual rainfall exceeding 5000 mm in some areas. Due to a few climatic similarities, the sample studied was collected twice, from one of the preserved forests of Meghalaya (Mawphlang), which receives the highest rainfall (as high as 12,000 mm) throughout the year, with the humidity higher than 85%. The findings suggest that the biogeographic features have played a unique role in determining the evolutionary traits of this fungus. The specimen was given due attention, and its ultrastructure and molecular characterization were analyzed using the Internal Transcribed Spacer (ITS) markers. However, the morphological evidence of Campanophyllum suggests an evolutionary clade in the basidiomycetes, based on the presence of the basidia. Besides, much attention was paid to the molecular structure of the specimen and its bioactive metabolites. Terpenes, esters, and heterocyclic metabolites were very frequently found in the dried extract. The presence of flavonoids was rather scarce when compared with the wild mushroom reported, which may show variations due to different genetic and epigenetic factors. However, it has the capacity to scavenge the free radicals and can be a powerful antioxidant source. It has shown dynamic synergistic activity and its various bactericidal properties can be used in the pharmaceutical industry. Further investigations will uncover greater details regarding their distribution, availability and toxicity perspective.
Funding Statement
Authors acknowledge the financial support received from Department of Science and Technology [SERB/SR/SO/PS/49/2012], Government of India to carry out the present work. Authors also acknowledge Sophisticated Analytical Instrument Facility (SAIF), NEHU, Shillong for providing the SEM facility.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Khaund P, Joshi SR. Enzymatic profiling of wild edible mushrooms consumed by the ethnic tribes of India. J Kor Soc Appl Biol Chem. 2014;57(2):263–271. [Google Scholar]
- 2.Maba DL, Guelly AK, Yorou NS, et al. The genus Lactarius s str. (Basdiomycota, Russulales) in Togo (West Africa): phylogeny and a new species described. IMA Fungus. 2014;5(1):39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khaund P, Joshi SR. DNA barcoding of wild edible mushrooms consumed by the ethnic tribes of India. Gene. 2014;550(1):123–130. [DOI] [PubMed] [Google Scholar]
- 4.Cifuentes J, Petersen RH, Hughes K. Campanophyllum: a new genus for an old species name. Mycol Prog. 2003;2:85–295. [Google Scholar]
- 5.Moncalvo JM, Vilgalys R, Redhead SA, et al. One hundred and seventeen clades of Euagarics. Mol Phylogenet Evol. 2002;23(3):357–400. [DOI] [PubMed] [Google Scholar]
- 6.Kim MY, Seguin P, Ahn JK, et al. Phenolic compound concentration and antioxidant activities of edible and medicinal mushrooms from Korea. J Agric Food Chem. 2008;56(16):7265–7270. [DOI] [PubMed] [Google Scholar]
- 7.Barros L, Duenas M, Ferreira I, et al. Phenolic acids determination by HPLC–DAD–ESI/MS in sixteen different Portuguese wild mushrooms species. Food Chem Toxicol. 2009;47(6):1076–1079. [DOI] [PubMed] [Google Scholar]
- 8.Palacios IL, Moro M, D’Arrigo C, et al. Antioxidant properties of phenolic compounds occurring in edible mushrooms. Food Chem. 2011;128(3):674–678. [Google Scholar]
- 9.Ferreira I, Barros L, Abreu R. Antioxidants in wild mushrooms. Curr Med Chem. 2009;16(12):1543–1560. [DOI] [PubMed] [Google Scholar]
- 10.Barros L, Ferreira M-J, Queirós B, et al. Total phenols, ascorbic acid, β-carotene and lycopene in Portuguese wild edible mushrooms and their antioxidant activities. Food Chem. 2007;103(2):413–419. [Google Scholar]
- 11.Barros L, Venturini B, Baptista P, et al. Chemical composition and biological properties of Portuguese wild mushrooms: a comprehensive study. J Agric Food Chem. 2008;56(10):3856–3862. [DOI] [PubMed] [Google Scholar]
- 12.Tsujikawa K, Kanamori T, Iwata Y, et al. Morphological and chemical analysis of magic mushrooms in Japan. Forensic Sci Int. 2003;138(1–3):85–90. [DOI] [PubMed] [Google Scholar]
- 13.Borthakur M, Joshi SR. Micrographical analysis of growth deformities in common pathogens induced by voucher fungi from India. J Microsc Ultrastruct. 2016;4:203–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.White TJ, Bruns T, Lee S, et al. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols: a guide to methods and applications. New York (NY): Academic Press; 1990. p. 315–322. [Google Scholar]
- 15.Tamura K, Stecher G, Peterson D, et al. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30(12):2725–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ronquist F, Teslenko M, Mark P, et al. Mr Bayes3.2: efficient bayesian phylogenetic inference and model choice across a large model space. Syst Biol. 2012;61(3):539–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Association of Official Analytical Chemists. Official methods of analysis. 15th ed Arlington (TX): AOAC; 1990. [Google Scholar]
- 18.Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1–2):248–254. [DOI] [PubMed] [Google Scholar]
- 19.Adebayo EA, Oloke JK, Ayandele AA, et al. Phytochemical, antioxidant and antimicrobial assay of mushroom metabolite from Pleurotus pulmonarius-LAU 09(JF736658). J Microbiol Biotech Res. 2012;2(2):366–374. [Google Scholar]
- 20.Beuhler M, Lee DC, Gerkin R. The Meixner test in the detection of alpha-amanitin and false-positive reactions caused by psilocin and 5-substituted tryptamines. Ann Emerg Med. 2004;44(2):114–120. [DOI] [PubMed] [Google Scholar]
- 21.Devi KP, Suganthy N, Kesika P, et al. Bioprotective properties of seaweeds: in vitro evaluation of antioxidant activity and antimicrobial activity against food borne bacteria in relation to polyphenolic content. BMC Complement Altern Med. 2008;8(1):38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oyaizu M. Studies on products of browning reactions: antioxidative activities of products of browning reaction prepared from glucosamine. Jpn J Nutr Diet. 1986;44(6):307–315. [Google Scholar]
- 23.Park YS, Jung ST, Kang SG, et al. Antioxidants and proteins in ethylene-treated kiwifruits. Food Chem. 2008;107(2):640–648. [Google Scholar]
- 24.Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999;299:152–178. [Google Scholar]
- 25.Nagata M, Yamashita I. Simple method for simultaneous determination of chlorophyll and carotenoids in tomato fruit. J Food Sci Technol. 1992;39(10):925–928. [Google Scholar]
- 26.Kuo M. Using a microscope to study mushrooms; 2006. Available from: www.mushroomexpert.com.
- 27.Breene WM. Nutritional and medicinal value of specialty mushrooms. J Food Protect. 1990;53(10):883–894. [DOI] [PubMed] [Google Scholar]
- 28.Ço_Kuner Y, Özdemir Y. Acid and EDTA blanching effects on the essential element content of mushrooms (Agaricus bisporus). J Sci Food Agric. 2000;80:2074–2076. [Google Scholar]
- 29.Chen XH, Xia LX, Z HB, et al. Chemical composition and antioxidant activities of Russula griseocarnosa sp. nov. J Agric Food Chem. 2010;58(11):6966–6971. [DOI] [PubMed] [Google Scholar]
- 30.Mao JL, Chao GR, Wu KT. Antioxidant properties of methanolic extracts from several ear mushrooms. J Agric Food Chem. 2001;49:5461–5467. [DOI] [PubMed] [Google Scholar]
- 31.Tibuhwa DD. A comparative study of antioxidant activities between fresh and dry mushrooms in the genera Cantharellus and Afrocantharellus from Tanzania. Food Nutr Sci. 2014;5:212–221. [Google Scholar]
- 32.Khaund P, Joshi SR. Functional nutraceutical profiling of wild edible and medicinal mushrooms consumed by ethnic tribes in India. Int J Med Mushrooms. 2015;17(2):187–197. [DOI] [PubMed] [Google Scholar]
- 33.Tomsone L, Kruma Z, Galoburda R. Comparison of different solvents and extraction methods for isolation of phenolic compounds from horseradish roots (Armoracia rusticana). World Acad Sci Eng Technol. 2012;64:903–908. [Google Scholar]
- 34.Abugri DA, McElhenney WH. Extraction of total phenolic and flavonoids from edible wild and cultivated medicinal mushrooms as affected by different solvents. J Nat Prod Plant Resour. 2013;3(3):37–42. [Google Scholar]
- 35.Yaltirak T, Aslim B, Ozturk S, et al. Antimicrobial and antioxidant activities of Russula delica Fr. Food Chem Toxicol. 2009;47(8):2052–2056. [DOI] [PubMed] [Google Scholar]
- 36.Hussein JM, Tibuhwa DD, Mshandete AM, et al. Antioxidant properties of seven wild edible mushrooms from Tanzania. Afr J Food Sci. 2015;9(9):471–479. [Google Scholar]
- 37.Patel Y, Naraian R, Singh VK. Medicinal properties of Pleurotus species oyster mushroom-a review. World J Fungal Plant Biol. 2012;3:1–12. [Google Scholar]
- 38.Mohamed EM, Farghaly FA. Bioactive compounds of fresh and dried Pleurotus ostreatus mushroom. Int J Biotech Well Indus. 2014;3(1):4–14 [Google Scholar]
- 39.Daigle P, Gélinas P, Leblanc D, et al. Production of aroma compounds by Geotrichum candidum on waste breadcrumb. Food Microbiol. 1999;16(5):517–522. [Google Scholar]
- 40.Dastager SG. Aroma compounds In: Nigam PS, Pandey A, editors. Biotechnology for agro-industrial residues utilization. Dordrecht: Springer; 2009. p. 105–127. [Google Scholar]
- 41.Lanciotti R, Gianotti A, Patrignani F, et al. Use of natural aroma compounds to improve shelf life and safety of minimally processed fruits. Trends Food Sci Technol. 2004;15(3–4):201–208. [Google Scholar]
- 42.Zawirska-Wojtasiak R, Siwulski M, Mildner-Szkudlarz S, et al. Studies on the aroma of different species and strains of Pleurotus measured by GCMS, sensory analysis and electronic nose. Acta Sci Pol Technol Aliment. 2009;8:47–61. [Google Scholar]