Abstract
Background and Objectives:
Urinary tract infections are common health problem affecting millions worldwide. Antibiotic resistance among uropathogens (Ups) is prevalent in many countries. In the absence of any available data in the region, this hospital-based study investigated the pattern, frequency and susceptibility of Ups at Prince Mutaib Bin Abdulaziz Hospital, Aljouf Region, Saudi Arabia.
Materials and Methods:
A retrospective assessment of UPs and their antibiotics susceptibility was conducted from January 2017 to December 2017 using the fully automated Vitek2 system (BioMérieux, France).
Results:
Among the 415 uropathogens isolates, the most prevalent bacteria were Gram-negatives comprising 137 (51%) E. coli; 46 (17.2%) Klebsiella spp.; 30 (11.2%) Pseudomonas spp.; 25 (9.3%) Proteus spp.; 14 (5.2%) Acinetobacter baumanii and 16 (5.9%) others. On the other hand, Enterococcus spp. were predominant among Gram-positive isolates representing 54 (36.7%), 47 (32.0%) Staphylococcus spp., 22 (15.1%) Streptococcus spp., and 13 (8.8%) S. aureus, and 11 (7.5%) others. Gram-negative Ups showed multidrug resistance towards the majority of the tested antimicrobials (ampicillins, cephalosporins, fluoroquinolones, trimethoprim-sulfamethoxazole, fosfomycin, aztreonam, and nitrofurantoin). While high resistance patterns by Gram-positives was also seen against cephalosporins, penicillins, amoxicillin-clavulanic acid, trimethoprim-sulfamethoxazole, clindamycin, erythromycin and tetracycline.
Conclusion:
The observed widespread multidrug resistance clearly warrant implementing stricter control measures, local guidelines of antimicrobials usage, and continuous epidemiological surveys at hospitals and communities.
Keywords: Urinary tract infection, Gram-negative bacteria, Gram-positive bacteria, Antimicrobial susceptibility
INTRODUCTION
Urinary tract infections (UTIs) are very common wide-spread affecting about 150 million people yearly (1). Gram-negative bacteria cause most UTIs, with E. coli being the most commonly encountered followed by other Gram negatives (e.g. Proteus, Klebsiella, Pseudomonas, Enterococcus), and Gram-positives such as: Group B Streptococci, and Staphylococcus saprophyticus (2, 3). A wide range of antibiotics resistance patterns have been reported in different parts of the world, and the empirical use of antimicrobials has proved to be an important predictor of resistance against the antimicrobial drugs (AMDs) used (4). The increased incidence of UTIs and the need by physicians to start patients’ care before carrying out any microbiological investigations had often led to empirical use of broad-spectrum antibiotics in most communities worldwide (5). This has widely been practiced despite the fact that urine specimens can easily be obtained, and urine culture is a relatively straightforward technique.
The easy access to affordable AMDs results in rapid selection of antimicrobial drug resistance (AMR), which is a difficult one to strike (6). Therefore, a continuous surveillance of the usage of these drugs is always required. In return, this can guide decision makers; AMDs stewardship programs; guidelines for empirical treatment that allow the monitoring of trends in AMR and the potential impact of interventions in reducing its development (7). Unfortunately, this may prove to be difficult to achieve particularly in low income developing countries, due to limited financial and human resources and the poor quality of microbiology laboratories (8).
AMR amongst UPs in different geographical regions of the world is recognized as a serious global health problem (9, 10). Regional surveillance studies to accurately identify UPs and determining their resistance to antibiotics are of paramount importance for the efficient management of patients, leading to clinical and financial benefits such as reducing mortality rates and hospitalization costs (11).
Earlier studies have shown increased resistance patterns among UPs against commonly used AMDs in other regions of Saudi Arabia (12–15). The most commonly encountered UPs were E. coli, Klebsiella spp., Pseudomonas spp., and others. Multiple AMR has widely been reported against ampicillin, trimethoprim-sulfamethoxazole, cephalosporins, gentamicin, and ciprofloxacin (12–14). ESBLs production by E. coli and K. pneumoniae in addition to other risk factors, such as diabetes, recurrent UTI, previous use of antibiotics, previous hospitalization, underlying renal disease and renal transplantation, have all been significantly associated with the wide-spread of antibiotic resistance amongst such pathogens (13, 14).
Although there are available data concerning AMR in other regions in Saudi Arabia, no study was conducted at Aljouf region. Therefore, the present hospital-based study aimed to investigate the pattern, frequency and susceptibility of UPs at Prince Mutaib Hospital at Aljouf Region in northern Saudi Arabia.
MATERIAlS AND METHODS
Study design.
A retrospective hospital-based study of UPs and their susceptibility patterns to antibiotics were carried out during January 2017 to December 2017 at Prince Mutaib Hospital, Sakaka, Aljouf, Saudi Arabia. The study included 415 patients of whom 245 (59.0%) were females and 170 (41.0%) males; 376 (90.6%) were Saudis and the other 39 (9.4%) were non-Saudis. The mean ± SD age of patients was 47.0 ± 24.4 years (range: 55 days to 100 years).
Ethical approval.
The work has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki, amended version 2013). An informed consent was obtained from all subjects, and their privacy rights were observed. The study was approved by the Research Ethics and Advisory Committees of the College of Medicine, Jouf University.
Sampling procedure.
During the study period all urine samples were collected in boric acid containers and sent to the Microbiology Laboratory of Prince Mutaib Hospital. The indication of urine analysis was suspected or having UTIs, presence of urinary symptoms, as well as urine cultures taken preoperatively from asymptomatic patients not having UTIs whether they are inpatients or outpatients.
Identification and determination of antimicrobial susceptibility.
All the urine specimens were cultured on Cystine Lactose Electrolyte Deficient agar (CLED) plates (Oxoid, Basingstoke, UK) and incubated for 24–48 hours at 37°C. All the isolates were identified and tested for their antibiotic sensitivity profiles using an automated VITEK-2 Machine (BioMérieux, Marcy-I’Étoile, France) which was the only available system in the hospital. The Vitek-2 is a fully automated system that depends on the microbial growth technology. The reagent cards used contain 64 wells with different test substrates that are used to measure metabolic activities e.g. enzyme hydrolysis, alkalinization, acidification, and growth in the presence of inhibitory substances. The bacterial suspensions were prepared from pure cultures using 0.5% sterile NaCl and cards were inoculated and incubated accordingly. All bacterial suspensions were prepared to be at a concentration of 0.5–0.63 McFarland using the VITEK DensiCHEK colorimeter (BioMérieux).
The AST-GN72 and AST-GP71 cards were used for both Gram-negative and Gram-positive bacteria respectively. Results of AMR were interpreted according to the recommendation of the Clinical and Laboratory Standards Institute (19). The VITEK-2 system manufacturer’s guidelines were followed in order to determine both the extended-spectrum-β-lactamase (ESBL) and the methicillin-resistant Staphylococcus aureus (MRSA) activities. For quality control purposes E. coli ATCC 25922 and S. aureus ATCC 29213 were used.
Statistical analyses.
Data were initially collected in a pre-formed Data Collection Form prior to being entered in Microsoft Excel Spreadsheet. A descriptive and analytical statistical analysis was performed using the Statistical Package for Social Sciences (SPSS Inc., Chicago, IL, USA) version 22. A p value <0.05 was considered statistically significant. Discrete variables were expressed as frequencies and percentages or mean ± standard deviation (SD) as appropriate. Comparisons between groups were done using Chi-Square or Fisher’s exact test or Mann Whitney-U test as appropriate.
RESUlTS
Of the total urine samples processed over a period of one year; 1640 (76.7%) yielded significant microbial growth of which 960 (58.5%) were Gram-negative bacteria, 671 (40.9%) Gram-positive, and only 9 (0.5%) were Candida spp. However, we are presenting the results of 415 samples which we have managed to retrieve. Out of these 147 (35.4%) and 268 (64.6%) were Gram-positive and Gram-negatives respectively.
Inpatients and outpatients represented 228 (54.9%) and 187 (45.1%) respectively (Table 1). In comparison, patients infected with Gram-negative isolates were found to be significantly older than those with Gram-positive (50.2 ± 25.7 versus 41.1 ± 20.5; p = 0.001) regardless of being inpatient or outpatient (p = 0.000, Table 1). However, there was no significant differences between patients with either isolates regarding gender and nationality, p = 0.233 and p = 0.703 respectively (Table 1). Diagnoses on admission included a wide spectrum of diagnoses that are encountered in both the outpatient and inpatient Departments of a secondary healthcare hospital including chronic renal, metabolic, chest, heart, neurological disorders, and acute illnesses, with suspected UTIs.
Table 1.
Demographic characteristics of patients
| Variable | Unit/Category | Patients with Bacterial Isolates | P value* | ||
|---|---|---|---|---|---|
| All (n=415) | Gram −ve (n=268) | Gram +ve (n=147) | |||
| Age | Years | 46.8 ± 24.5 | 50.2 ± 25.7 | 41.1 ± 20.5 | 0.001 |
| Gender | Male | 170 (41.0) | 117 (43.7) | 53 (36.1) | 0.233 |
| Female | 245 (59.0) | 151 (56.3) | 94 (63.9) | ||
| Nationality | Saudi | 376 (90.4) | 244 (91.0) | 132 (89.8) | 0.703 |
| Non Saudi | 39 (09.6) | 24 (09.0) | 15 (10.2) | ||
| Setting | Inpatient | 228 (66.7) | 100 (37.3) | 87 (59.2) | 0.000 |
| Outpatient | 187 (33.3) | 168 (62.7) | 60 (40.8) | ||
Data are expressed as mean SD or n (%) as appropriate.
Gram −ve versus Gram +ve.
These isolates were distributed as follows: 137 (51.1%) E. coli; 46 (17.2%) Klebsiella spp.; 30 (11.2%) Pseudomonas spp.; 25 (9.3%) Proteus spp.; 14 (5.2%) Acinetobacter baumannii, and 16 (6.0%) other Gram-negative organisms (Table 2). In comparison, the Gram-positive isolates were 54 (36.7%) Enterococcus spp.; 47 (32.0%) Coagulase-negative Staphylococci (CNS); 22 (15.1%) Streptococcus spp., 13(8.8%) S. aureus; and 11 (7.5%) other Gram positive organisms (Table 2).
Table 2.
Frequency and percentages of the different isolated uropathogens (n=415)
| Gram stain | Organism | Frequency |
|---|---|---|
| Gram-negative | All | 268/415 (64.6) |
| E. coli | 137 (51.1) # | |
| Klebsiella spp. | 46 (17.2) # | |
| Proteus spp. | 25 (9.3) # | |
| Pseudomonas spp. | 30 (11.2) # | |
| Acinetobacter baumanii | 14 (5.2) # | |
| Others | 16 (6.0) # | |
| Gram-positive | All | 147/415 (35.4) |
| Staphylococcus spp. | 47 (32.0) * | |
| Staphylococcus aureus | 13 (8.8) * | |
| Enterococcus spp. | 54 (36.7) * | |
| Streptococcus spp. | 22 (15.1) * | |
| Others | 11 (7.5) * | |
Data are expressed as n (%).
= % from all Gram negative isolates.
= % from all Gram positive isolates.
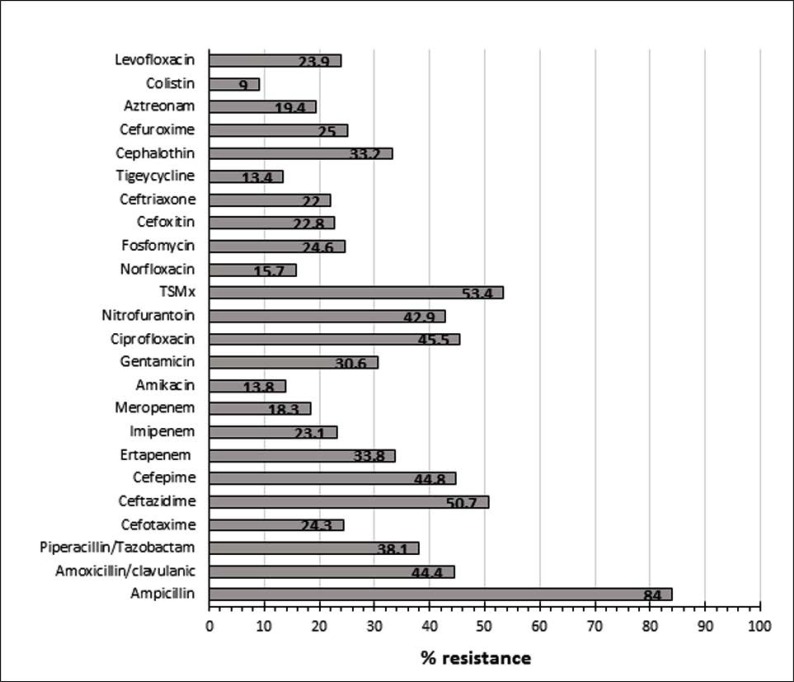
Table 3 and Fig. 1 summarized the AMDs susceptibility patterns of all Gram-negative isolates. It shows a high resistance rate against ampicillin (84.0%), trimethoprim-sulphamethoxazole (53.4%), (50.7%), ciprofloxacin (45.5%) and nitrofurantoin (42.9%). Lower rates were recorded towards colistin, amikacin, norfloxacin, meropenem and ceftriaxone with an overall resistance rate of 9.0%; 18.513.8%, 15.7%, 18.3% and 22.0% respectively. Of the 137 E. coli isolates, 57 (41.6%) were designated as extended spectrum β-lactamase (ESBL) producers compared with 8 out 46 (17.4%) among Klebsiella spp., 3 out of 25 (12.0%) Proteus spp., and none of the Pseudomonas spp., and Acinetobacter spp., (p<0.001) as confirmed by the Vitek 2 System.
Table 3.
Percentage resistance pattern of Gram-negative uropathogens (n=136).
| Drug | E. coli | Klebsiella spp. | Pseudomonas spp. | Proteus spp. | Acinetobacter spp. |
|---|---|---|---|---|---|
| Ampicillin | 85.7 | 90.9 | 100 | 95.4 | 100 |
| Amoxicillin/clavulanic | 30.2 | 50.0 | 95.8 | 55.0 | 100 |
| Piperacillin/Tazobactam | 29.9 | 35.6 | 46.7 | 56.0 | 82.7 |
| Cefotaxime | 62.9 | 57.1 | F | F | F |
| Ceftazidime | 37.5 | 46.7 | 80.0 | 64.0 | 82.7 |
| Cefepime | 37.5 | 41.3 | 51.7 | 68.0 | 82.7 |
| Ertapenem | 31.5 | 41.0 | 86.4 | 47.1 | 100 |
| Imipenem | 16.3 | 13.6 | 50.0 | 24.0 | 85.7 |
| Meropenem | 07.4 | 23.9 | 43.3 | 12.0 | 82.7 |
| Amikacin | 9.50 | 10.9 | 20.0 | 12.0 | 82.7 |
| Gentamicin | 18.2 | 23.9 | 36.7 | 64.0 | 75.0 |
| Ciprofloxacin | 37.5 | 45.6 | 43.3 | 68.0 | 78.6 |
| Nitrofurantoin | 32.0 | 43.6 | 60.9 | 95.0 | 100 |
| TSMx | 40.0 | 46.7 | 96.6 | 80.0 | 57.1 |
| Norfloxacin | 23.2 | 46.7 | 72.7 | 100 | F |
| Fosfomycin | 58.1 | 35.3 | 90.1 | 66.7 | F |
| Cefoxitin | 27.4 | 31.0 | 100 | 33.3 | 100 |
| Ceftriaxone | 46.0 | 54.5 | 100 | 50.0 | 70.0 |
| Tigecycline | 13.3 | 5.60 | 90.0 | 100 | F |
| Cephalothin | 62.3. | 59.1 | 100 | 72.3 | 100 |
| Cefuroxime | 45.5 | 56.5 | 100 | F | 100 |
| Aztreonam | 40.0 | 55.0 | 64.3 | F | 100 |
| Colistin | F | 08.3 | 44.4 | 100 | 0 |
| Levofloxacin | 44.4 | 44.0 | 68.4 | 77.8 | 80.0 |
F= very few to report. TSMx = Trimethoprim/sulfamethoxazole.
Fig. 1.
Percentage resistance pattern of Gram-negative isolates to the studies antimicrobial drugs. TSMx = Trimethoprim-sulphamethoxazole.
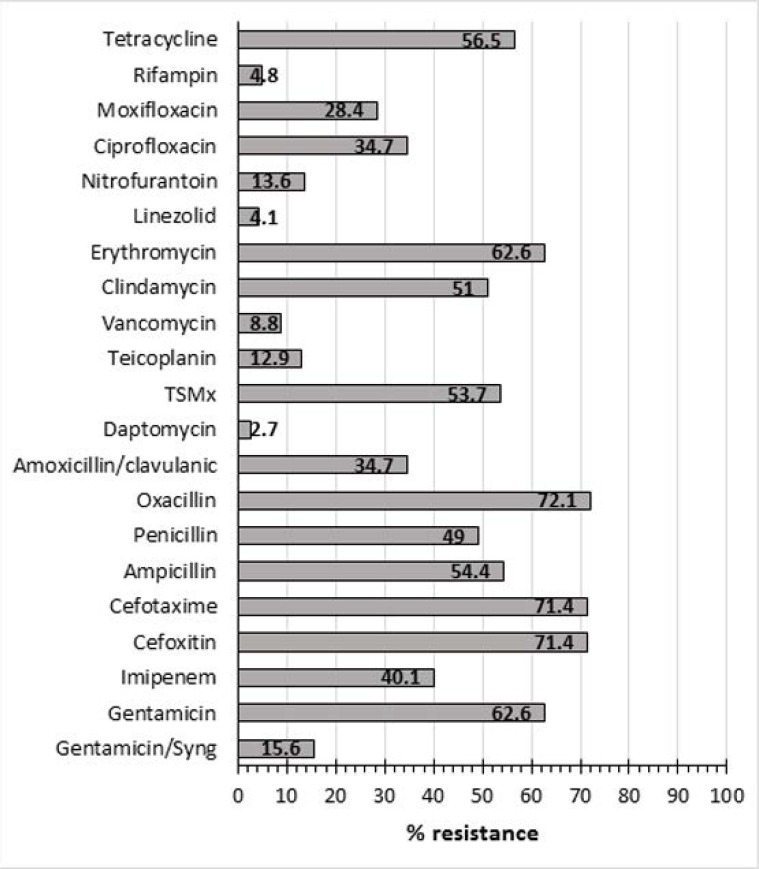
Table 4 and Fig. 2 show the drug resistance pattern of the Gram-positive bacteria against the AMDs tested. A high AMDs resistance pattern was observed towards oxacillin (72.1%), cefoxitin (71.4%), cefotaxime (71.4%), erythromycin (62.6), gentamicin (62.6%), tetracycline (56.5%), trimethoprim-sulphamethoxazole (53.7%) and clindamycin (51.0%). However, lower resistance rates by Gram-positive UPs were recorded for daptomycin (2.7%), linezolid (4.1%), vancomycin (8.8%) and rifampicin (4.8%). Only 2 of the 13 S. aureus isolates (15.4%) were marked as methicillin-resistant S. aureus (MRSA) and 16 out of 26 S. haemolyticus (61.5%) were found to be methicillin-resistant S. haemolyticus (MRSH)
Table 4.
Percentage resistance pattern of Gram-positive uropathogens (n=136)
| Drug |
Staphylococcus spp. n=60 |
Streptococcus
spp. n=22 |
Enterococcus spp. n=54 |
|---|---|---|---|
| Gentamicin/Syng | 56.6 | 20.0 | 38.3 |
| Gentamicin | 0 | 71.4 | 90.6 |
| Imipenem | 78.9 | 33.3 | 30.8 |
| Cefoxitin | 97.6 | 90.9 | 100 |
| Cefotaxime | 78.4 | 83.3 | 100 |
| Ampicillin | 100 | 42.9 | 29.2 |
| Penicillin | 98.0 | 29.4 | 77.8 |
| Oxacillin | 80.0 | 83.3 | 100 |
| Amoxicillin/clavulanic | 69.8 | F | 71.4 |
| Daptomycin | 02.1 | 05.9 | 04.7 |
| TSMx | 35.6 | 66.7 | 90.2 |
| Teicoplanin | 11.9 | 12.5 | 16.7 |
| Vancomycin | 06.8 | 05.6 | 13.0 |
| Clindamycin | 96.6 | 40.0 | 92.9 |
| Erythromycin | 75.5 | 31.3 | 79.2 |
| Linezolid | 01.9 | 05.0 | 07.5 |
| Nitrofurantoin | 13.6 | 08.3 | 17.0 |
| Ciprofloxacin | 36.4 | 09.1 | 51.1 |
| Moxifloxacin | 09.4 | 33.3 | 32.0 |
| Rifampin | 07.5 | 66.7 | F |
| Tetracycline | 47.4 | 33.3 | 77.4 |
F= very few to report. TSMx = Trimethoprim/sulfamethoxazole.
Fig. 2.
Percentage resistance pattern of Gram-positive isolates to the studies antimicrobial drugs. TSMx = Trimethoprim-sulphamethoxazole.
DISCUSSION
UTIs are mostly treated empirically, and the criterion for the selection of AMDs is most commonly based on the most likely pathogen and its expected AMR pattern in a given locality (16). In the present study, E. coli was the most frequently isolated UP among Gram-negative isolates followed by Klebsiella spp., Pseudomonas spp., and others. This is somewhat similar to the findings of many previous studies in that 75–90% of UTIs were due to E. coli; whereas, Staphylococcus spp., were the most common Gram-positive isolates (17–20).
Elderly women are known to be prone to develop asymptomatic bacteriuria and recurrent UTIs, which have been linked with risk factors such as diabetes among this age and gender category (18). Likewise, our result showed similar findings to other studies in that 59% of UPs encountered in this study were among female patients. This is probably due to women-related anatomical and physiological changes in addition to other likely risk factors (2, 21–23). In addition, due to the likely recurrent attacks of UTIs among this group leading to frequent use of AMDs, taking the wrong AMDs for asymptomatic bacteriuria, or treatment of others infections (24) could all play part in the occurrence of UTIs as well as the development of AMR. Similarly, previous hospitalization and history of previous intake of AMDs may well affect the pattern and the sensitivity profile of the UPs in these patients.
As the etiological agents of UTIs and their susceptibility/resistance patterns vary according to geographical locations (1, 24), there is a continuous need for periodic monitoring of the UPs and antibiotic resistance patterns. This will, consequently, give updated recommendation for the optimal empirical therapy of UTIs (25). Our Gram-negative UPs showed an overall AMR rates ranging from 9.0% for colistin to 84.0% for ampicillin. A high resistance rate was recorded against second, third and fourth generation cephalosporins ranging from 22.0% to 50.7%. A similar resistance pattern was also shown against the majority of the tested drugs including fluoroquinolones, fosfomycin, amoxicillin/clavulanic acid, trimethoprim/sulfamethoxazole, nitrofurantoin, and others. In general, over 40% of our G-negative UPs (E. coli, K. pneumoniae, Pseudomonas spp., Proteus spp., and Acinetobacter spp.) were resistant to cephalosporins. Similar studies in Saudi Arabia, have shown that bacterial UPs were highly resistant to the commonly used AMDs such as: ampicillin, third-generation cephalosporins, ciprofloxacin, and trimethoprim-sulfamethoxazole (12–14). In Canada, Europe, and Africa, the resistance rates for ampicillin have also been found to be increasing hitting 45, 50 and 100%, respectively (5, 26–27). A range of 42.5–49.4% resistance rate towards cephalosporins has also been reported (28), whereas, Kalal and Nagaraj (20), showed 79.3% resistance for ampicillin, and 60% against cephalosporins. In the UAE, a lower resistance rate has been documented at 16.7% and 31% against expanded-spectrum cephalosporins among community and hospital-acquired UPs, respectively (29, 30).
Although trimethoprim/sulfamethoxazole has been widely used for the empirical treatment of UTI; the results of the present study showed that 40–97% and 35.6–90.2% of Gram-negative and Gram-positive isolates respectively were resistant to this drug (Tables 3–4). These figures are one of the highest reported for individual UPs. In comparison, the highest resistance rates towards this drug were 26.3% for E. coli; 23.3% for Klebsiella spp. and 16.7% for Proteus spp. (31). In another study from Latin America (32), the highest resistance rate was seen for E. coli (63%). A similar picture was seen in the case of nitrofurantoin with high resistance to this drug was observed for our Gram-negative UPs, namely: Acinetobacter spp. (100%); Proteus spp. (95%); Pseudomonas spp. (61%) and to a lesser extent by Klebsiella (43.6%) and E. coli (32%) (Table 3). These data are in contrast with various clinical recommendations and guidelines regarding the empirical use of trimethoprim-sulfamethoxazole and nitrofurantoin as first-line drugs of choice in the treatment of uncomplicated UTIs (32, 33).
Other widely used antibiotics for the treatment of UTIs are fluoroquinolones (34). The resistance rates among our isolates are comparable to those reported by Choe et al. (28), who also reported a very high resistance rate against fluoroquinolones among UPs isolated in a number of Asian countries with 54.9% resistance rate against ciprofloxacin, and 39% against levofloxacin. Nonetheless, our findings are much higher than those reported in several recent studies in the region and other European and North American countries (35). These high resistance levels are likely to be driven by previous exposure to fluoroquinolones, or renal transplantations which have been recently acknowledged as an independent risk factor for ciprofloxacin-resistant E. coli (23, 36).
Both of our Proteus and Acinetobacter spp. isolates were highly resistant to the aminoglycosides tested (gentamicin and amikacin) with 64, and 75% resistance to gentamicin respectively (Table 4). E. coli yielded the lowest rates of resistance towards amikacin and gentamicin with 9.5% and 18.2%, respectively which coincides with the findings of Kalal and Nagaraj (20) and Choe et al. (28). Nonetheless, these rates are once again higher than some of the published figures in other countries (32). Carbapenems (imipenem, meropenem, and ertapenem) resistance was also evident in our study with all the Acinetobacter spp. being resistant to ertapenem and 82–85% resistant to imipenem and meropenem too. Proteus and Pseudomonas spp. showed variable resistance patterns towards these drugs. On the contrary, E. coli was the least resistant UPs to the carbapenems which is in line with some other reported studies (20, 28). Other than Klebsiella spp., all other Enterobacteriaceae were susceptible to carbapenems (93%) as reported by Kalal and Nagaraj (20). In the absence of culture and sensitivity results, some consider carbapenems as an appropriate choice for the empirical treatment of UTIs (37).
As shown in Table 4, our Gram-positive isolates showed high resistance rates against the majority of the antibiotics tested. This multiple resistance spread was evident against cephalosporins, penicillin’s, aminoglycosides, erythromycin, tetracycline, clindamycin, trimethoprim-sulfamethoxazole, and imipenem. Nonetheless, 86%–96% of these isolates were susceptible to daptomycin, linezolid, vancomycin, rifampicin, and teicoplanin. These results are echoed by the findings of Bitew et al. (17) who showed that their Gram-positive UPs were highly susceptible to daptomycin, nitrofurantoin, gentamicin, vancomycin, and linezolid. The high level resistance observed against the vast majority of the commonly used antibiotics in the empirical treatment of UTIs is overwhelming. Part of this problem could be partially attributed to the irrational use of antimicrobial drugs in this locality, and the abuse of drugs by the public where patients indulge in antibiotic self-medication commonly to treat all kinds of infections has been recorded as one significant way of promoting antibiotic resistance (38). Bin Abdulhak et al. (39) reported on the non-prescription sale of antibiotics in 327 pharmacies in the capital city of Saudi Arabia and showed that 77.6% of antibiotics were dispensed without a medical prescription. Of the commonly prescribed drugs were metronidazole and ciprofloxacin being prescribed in 89% and 86% of cases with diarrhea and UTI respectively. Fortunately, a new regulation has been recently introduced in KSA to restrict the release of antibiotics without authenticated prescriptions by authorized physicians only.
In terms of individual Gram-positive bacterial isolates, the highest rates of resistance by S. aureus were against the penicillins. Lower resistance rates (7–15%) were recorded against trimethoprim-sulfamethoxazole, teicoplanin, vancomycin, and nitrofurantoin. However, no or low resistance rates towards daptomycin, linezolid, ciprofloxacin, or moxifloxacin by S. aureus was noted. In comparison, the CNS showed higher resistance patterns against the majority of the tested antimicrobial drugs. Very low resistance rates were seen against linezolid (2.4%), daptomycin (2.7%), vancomycin (6.4%) and moxifloxacin (7.7%). Of the Streptococcus species isolated in this study, multiple resistance patterns were also evident against cephalosporins, oxacillin, gentamicin, trimethoprim-sulfamethoxazole, clindamycin, tetracycline, and moxifloxacin. As expected the highest level of resistance patterns were recorded for Enterococcus species. The lowest rates of resistance were recorded for daptomycin (4.7%), and linezolid (7.5%), teicoplanin (16.7%), and 13% were designated as vancomycin resistant enterococci (VRE). In a recent study, Yang et al. (40) reported that all of their Enterococcus and Staphylococcus spp. were sensitive to linezolid, vancomycin, and teicoplanin, and suggested that fosfomycin might be an excellent treatment option for outpatients with UTIs.
Of note, the potential limitation of this study was its retrospective nature. However, the similarity of our results to those of other studies performed elsewhere confirms that such nature may unlikely affected our results. In addition, the absence of correlating AMR pattern with the clinical diagnoses cannot be considered as an important limitation of this study as this was beyond its scope and such issue need to be investigated separately.
In conclusion, this study clearly demonstrated that both Gram-positive and Gram-negative Ups in northern Saudi Arabia were highly resistant to a vast majority of the commonly used antimicrobial drugs. This indicates that it is imperative to rationalize the use of antimicrobials in hospitals and the community, and the need for countrywide campaigns for awareness to public and antimicrobial stewardship for health-care workers. Additionally, the wider and indiscriminate use of such agents by people in the community to treat these infections could also play an important role in the high resistance observed; therefore, physicians should be very careful when considering first drugs of choice for empirical treatment of UTIs in the absence of any microbiological laboratory results.
ACKNOWlEDGEMENTS
The authors thank all the staff in the Microbiology Laboratory at Prince Mutaib Bin Abdulaziz Hospital, Sakaka, Aljouf, Saudi Arabia for their cooperation.
REFERENCES
- 1.Cunha MA, Assunção GL, Medeiros IM, Freitas MR. Antibiotic resistance patterns of urinary tract infections in a northeastern Brazilian capital. Rev Inst Med Trop Sao Paulo 2016; 58:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akram M, Shahid M, Khan AU. The etiology and the antibiotic resistance patterns of community-acquired urinary tract infections in the JNMC Hospital Aligarh, India. Ann Clin Microbiol Antimicrob 2007;6:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braoios A, Turatti TF, Meredija LCS, Fields TRS, Denadai FHM. Urinary tract infections in non hospitalized patients: etiology and antibiotic resistance patterns. J Bras Patol Med Lab 2009; 45:449–456. [Google Scholar]
- 4.Kiffer CR, Camargo EC, Shimakura SE, Ribeiro PJ, Jr, Bailey TC, Pignatari AC, et al. A spatial approach for the epidemiology of antibiotic use and resistance in community-based studies: the emergence of urban clusters of Escherichia coli quinolone resistance in Sao Paulo, Brazil. Int J Health Geogr 2011; 10:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rocha JL, Tuon FF, Johnson JR. Sex, drugs, bugs, and age: rational selection of empirical therapy for outpatient urinary tract infection in an era of extensive antimicrobial resistance. Braz J Infect Dis 2012; 16:115–121. [DOI] [PubMed] [Google Scholar]
- 6.Habte TM, Dube S, Ismail N, Hoosen AA. Hospital and community isolates of uropathogens at a tertiary hospital in South Africa. S Afr Med J 2009; 99:584–587. [PubMed] [Google Scholar]
- 7.Sugianli AK, Ginting F, Kusumawati RL, Pranggono EH, Pasaribu AP, Gronthoud F, et al. Antimicrobial resistance in uropathogens and appropriateness of empirical treatment: a population-based surveillance study in Indonesia. J Antimicrob Chemother 2017; 72:1469–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Magale A, Kassim SA, Odera MJ, Omolo H, Jaoko WG, Jolly PE. Antibiotic susceptibility of organisms causing urinary tract infection in patients presenting at Kenyatta national hospital, Nairobi. East Afr Med J 2015; 92:333–337. [PMC free article] [PubMed] [Google Scholar]
- 9.Miragliotta G, Di Pierro MN, Miragliotta L, Mosca A. Antimicrobial resistance among uropathogens responsible for community-acquired urinary tract infections in an Italian community. J Chemother 2008; 20:721–727. [DOI] [PubMed] [Google Scholar]
- 10.WHO. Antimicrobial Resistance: Global Report on Surveillance 2014. https://www.who.int/drugresistance/documents/surveillancereport/en/
- 11.Wagenlehner FME, Naber KG. Current challenges in the treatment of complicated urinary tract infections and prostatitis. Clin Microbiol Infect 2006; 12 Suppl 3:67–80. [DOI] [PubMed] [Google Scholar]
- 12.Al-Tawfiq JA, Anani AA. Antimicrobial susceptibility pattern of bacterial pathogens causing urinary tract infections in a Saudi Arabian hospital. Chemotherapy 2009; 55:127–131. [DOI] [PubMed] [Google Scholar]
- 13.Al Yousef SA. Surveillance of antibiotic-resistant bacteria in King Khalid Hospital, Hafr Al-Batin, Saudi Arabia, during 2013. Jundishapur J Microbiol 2016;9(9): e19552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Otaibi FE, Bukhari EE. Clinical and laboratory profiles of urinary tract infections caused by extended-spectrum beta-lactamase-producing Escherichia coli in a tertiary care center in central Saudi Arabia. Saudi Med J 2013; 34:171–176. [PubMed] [Google Scholar]
- 15.Al-Rubeaan KA, Moharram O, Al-Naqeb D, Hassan A, Rafiullah MR. Prevalence of urinary tract infection and risk factors among Saudi patients with diabetes. World J Urol 2013; 31:573–578. [DOI] [PubMed] [Google Scholar]
- 16.Ekwealor PA, Ugwu MC, Ezeobi I, Amalukwe G, Ugwu BC, Okezie U, et al. Antimicrobial evaluation of bacterial isolates from urine specimen of patients with complaints of urinary tract infections in Awka, Nigeria. Int J Microbiol 2016; 2016:9740273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bitew A, Tamirat M, Meseret C. Species distribution and antibiotic susceptibility profile of bacterial uropathogens among patients complaining urinary tract infections. BMC Infect Dis 2017;17:654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oladeinde BH, Omoregie R, Olley M, Anunibe JA. Urinary tract infections in a rural community of Nigeria. N Am J Med Sci 2011; 3:75–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mody L, Juthani-Mehta M. Urinary tract infections in older women: a clinical review. JAMA 2014; 311:844–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalal BS, Nagaraj S. Urinary tract infections: a retrospective, descriptive study of causative organisms and antimicrobial pattern of samples received for culture, from a tertiary care setting. Germs 2016; 6:132–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manjunath GN, Prakash R, Annam V, Shetty K. Changing trends in the spectrum of antimicrobial drug resistance pattern of uropathogens isolated from hospitals and community patients with urinary tract infections in tumkur and bangalore. Int J Biol Med Res 2011; 2:504–507. [Google Scholar]
- 22.Barate DL, Ukesh CS. Bacterial profile and antibiotic resistance pattern of urinary tract infections. DAVIJS 2012; 1:21–24. [Google Scholar]
- 23.Nerurkar A, Solanky P, Naik SS. Bacterial pathogens in urinary tract infection and antibiotic susceptibility pattern. JPBS 2012; 21:1–3. [Google Scholar]
- 24.de Francesco MA, Giuseppe R, Laura P, Riccardo N, Nino M. Urinary tract infections in Brescia, Italy: Etiology of uropathogens and antimicrobial resistance of common uropathogens. Med Sci Monit 2007; 13:BR136–144. [PubMed] [Google Scholar]
- 25.Adeep M, Nima T, Kezang W, Tshokey T. A retrospective analysis of the etiologic agents and antibiotic susceptibility pattern of uropathogens isolated in the Jigme Dorji Wangchuck National Referral Hospital, Thimphu, Bhutan. BMC Res Notes 2016;9:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haller M, Brandis M, Berner R. Antibiotic resistance of urinary tract pathogens and rationale for empirical intravenous therapy. Pediatr Nephrol 2004; 19:982–986. [DOI] [PubMed] [Google Scholar]
- 27.Prais D, Straussberg R, Avitzur Y, Nussinovitch M, Harel L, Amir J. Bacterial susceptibility to oral antibiotics in community-acquired urinary tract infection. Arch Dis Child 2003; 88:215–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choe HS, Lee SJ, Cho YH, Çek M, Tandoğdu Z, Wagenlehner F, et al. Aspects of urinary tract infections and antimicrobial resistance in hospitalized urology patients in Asia: 10-Year results of the Global Prevalence Study of Infections in Urology (GPIU). J Infect Chemother 2018:24; 278–283. [DOI] [PubMed] [Google Scholar]
- 29.Narchi H, Al-Hamdan MA. Antibiotic resistance trends in paediatric community-acquired first urinary tract infections in the United Arab Emirates. East Mediterr Health J 2010; 16:45–50. [PubMed] [Google Scholar]
- 30.Dash N, Mansour ALZ, Al-Kous N, Al-Shehhi F, Al-Najjar J, Senok A, et al. Distribution and resistance trends of community-associated urinary tract pathogens in Sharjah, UAE. Microbiol Insights 2008; 1:41–45. [Google Scholar]
- 31.Wagenlehner FM, Pilatz A, Naber KG, Weidner W. Therapeutic challenges of urosepsis. Eur J Clin Invest 2008;38 Suppl 2:45–49. [DOI] [PubMed] [Google Scholar]
- 32.Bours PH, Polak R, Hoepelman AI, Delgado E, Jarquin A, Matute AJ. Increasing resistance in community-acquired urinary tract infections in Latin America, five years after the implementation of national therapeutic guidelines. Int J Infect Dis 2010; 14(9):e770–774. [DOI] [PubMed] [Google Scholar]
- 33.McKinnell JA, Stollenwerk NS, Jung CW, Miller LG. Nitrofurantoin compares favorably to recommended agents as empirical treatment of uncomplicated urinary tract infections in a decision and cost analysis. Mayo Clin Proc 2011; 86:480–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kader AA, Kumar A. Prevalence and antimicrobial susceptibility of extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae in a general hospital. Ann Saudi Med 2005; 25:239–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kehinde EO, Rotimi VO, Al-Hunayan A, Abdul-Halim H, Boland F, Al-Awadi KA. Bacteriology of urinary tract infection associated with indwelling ureteral J stent. J Endourol 2004; 18:891–896. [DOI] [PubMed] [Google Scholar]
- 36.Hitzenbichler F, Simon M, Holzmann T, Iberer M, Zimmermann M, Salzberger B, et al. Antibiotic resistance in E. coli isolates from patients with urinary tract infections presenting to the emergency department. Infection 2018; 46:325–331. [DOI] [PubMed] [Google Scholar]
- 37.Patel MH, Trivedi GR, Patel SM, Vegad MM. Antibiotic susceptibility pattern in urinary isolates of gram-negative bacilli with special reference to AmpC β-lactamase in a tertiary care hospital. Urol Ann 2010; 2:7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ventola CL. The antibiotic resistance crisis: part 1: causes and threats. P T 2015; 40:277–283. [PMC free article] [PubMed] [Google Scholar]
- 39.Bin Abdulhak AA, Altannir MA, Almansor MA, Almohaya MS, Onazi AS, Marei MA, et al. Non prescribed sale of antibiotics in Riyadh, Saudi Arabia: A Cross-Sectional Study. BMC Public Health 2011;11:538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang B, Yang F, Wang S, Wang Q, Liu Z, Feng W, et al. Analysis of the spectrum and antibiotic resistance of uropathogens in outpatients at a tertiary hospital. J Chemother 2018; 30:145–149. [DOI] [PubMed] [Google Scholar]