Significance
People are increasingly affected by social anxiety that includes emotional hypersensitivity and inaccurate interpretation of social encounters, and varies markedly in its subjective manifestations. We searched for insights into the underlying neurocognitive mechanisms of Taijin-kyofusho (TKS), a specific subtype of social-anxiety disorder common in East Asia and dominated by empathic or other-oriented embarrassment. We found TKS to be characterized by enhanced affective and reduced cognitive empathy. Moreover, analysis of functional MRI data—collected while subjects viewed videos of badly singing people—revealed disruption of the cognitive–empathy network, possibly obstructing flexible inference of others’ perspective or augmenting maladaptive feelings of embarrassment. Our findings shed light on how altered affective and cognitive processing can contribute to the development of imaginary fears.
Keywords: social anxiety, empathy, embarrassment, intersubject correlation, functional magnetic resonance imaging
Abstract
Social-anxiety disorder involves a fear of embarrassing oneself in the presence of others. Taijin-kyofusho (TKS), a subtype common in East Asia, additionally includes a fear of embarrassing others. TKS individuals are hypersensitive to others’ feelings and worry that their physical or behavioral defects humiliate others. To explore the underlying neurocognitive mechanisms, we compared TKS ratings with questionnaire-based empathic disposition, cognitive flexibility (set-shifting), and empathy-associated brain activity in 23 Japanese adults. During 3-tesla functional MRI, subjects watched video clips of badly singing people who expressed either authentic embarrassment (EMBAR) or hubristic pride (PRIDE). We expected the EMBAR singers to embarrass the viewers via emotion-sharing involving affective empathy (affEMP), and the PRIDE singers to embarrass via perspective-taking involving cognitive empathy (cogEMP). During affEMP (EMBAR > PRIDE), TKS scores correlated positively with dispositional affEMP (personal-distress dimension) and with amygdala activity. During cogEMP (EMBAR < PRIDE), TKS scores correlated negatively with cognitive flexibility and with activity of the posterior superior temporal sulcus/temporoparietal junction (pSTS/TPJ). Intersubject correlation analysis implied stronger involvement of the anterior insula, inferior frontal gyrus, and premotor cortex during affEMP than cogEMP and stronger involvement of the medial prefrontal cortex, posterior cingulate cortex, and pSTS/TPJ during cogEMP than affEMP. During cogEMP, the whole-brain functional connectivity was weaker the higher the TKS scores. The observed imbalance between affEMP and cogEMP, and the disruption of functional brain connectivity, likely deteriorate cognitive processing during embarrassing situations in persons who suffer from other-oriented social anxiety dominated by empathic embarrassment.
Social-anxiety disorder (SAD), also called social phobia, is one of the most common psychiatric illnesses, with a 15% lifetime prevalence (1). SAD is characterized by avoidance of social interactions (2) due to fear of negative evaluation, such as embarrassing oneself in the presence of others (2, 3). Taijin-kyofusho (TKS), a subtype of SAD, additionally includes fear of embarrassing others (4), for example, making them feel uncomfortable because of the person’s blushing, sweating, or trembling appearance (4). People with TKS overly imagine how they look from the perspective of others and think that their physical defects and/or socially inappropriate behaviors would offend or humiliate others (5).
Although TKS was initially described as a culturally specific SAD subtype prominent in interdependent cultures, particularly in East Asia, similar manifestations are consistently reported in independent cultures. For example, in the United States, 75% of SAD patients exhibit at least one of the five TKS symptoms related to other-oriented fear (6). TKS has recently been described in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) under SAD (7), which reflects its universal relevance and heterogeneous manifestations (e.g., shyness, self-criticism, and submissiveness; refs. 8–10). However, the brain basis and correlates of TKS still remain unclear (11, 12). As the fear of being negatively evaluated by others is the hallmark of social anxiety (9, 13), studying TKS as an exemplar of other-oriented anxiety might add crucial insights into the mechanisms underlying subjective experiences of social anxiety (14).
Given that persons suffering from TKS are hypersensitive to others’ feelings and easily misunderstand others’ perspectives (4, 5), we envisioned that TKS is associated with heightened affective empathy (affEMP; emotion-sharing via self–other overlap or matching) that amplifies perception of others’ negative feelings (15), or with reduced cognitive empathy (cogEMP; perspective-taking via self–other distinction) that hinders flexible inference of others’ views and intentions that differ from one’s own (16, 17). Feelings of embarrassment might excessively capture TKS subjects’ attention (4, 5), and heightened affEMP might further enhance personal distress in response to others’ distress or misfortunes (18). As a result, TKS subjects could readily translate the feelings of embarrassment of other people into personally experienced (empathic) embarrassment that leads to other-oriented fear.
Previous research suggests that affEMP is uniquely supported by fear-related activations in amygdala, insula, and anterior cingulate cortex (ACC) (11, 19, 20). Instead, cogEMP is supported by the ventromedial prefrontal and the orbitofrontal cortex (vmPFC/OFC) as well as by the temporoparietal junction (TPJ) and posterior superior temporal sulcus (pSTS) (15) involved in perspective-taking (via self–other distinction) and attention-shifting (15, 21). These empathy-related regions may also be associated with feelings of embarrassment (22–24). However, how such feelings are triggered and translated into other-oriented social fear warrants further investigation.
To study the neurocognitive basis of other-oriented social anxiety, we first correlated TKS level with scores of affEMP, cogEMP, and cognitive flexibility among 23 Japanese adults. We also applied the Toronto Alexithymia Scale (TAS) as a measure of altered self-awareness and emotionality, reflecting a precursor of empathic abnormalities (25). To measure cognitive flexibility (attentional set-shifting), we used the Wisconsin Card Sorting Test (SI Appendix) that is related to cognitive empathy (26, 27). Subsequently, we examined the relationship between the TKS scores and the strength and distribution of empathy-related functional MRI (fMRI) signals.
During fMRI recording, the subjects watched video clips of people who were singing badly and expressed for their performance either authentic embarrassment (EMBAR) or hubristic pride (PRIDE) (Fig. 1). Based on previous studies suggesting that empathic embarrassment can occur via two processes dominant in affective (bottom-up) and cognitive (top-down) domains (15, 17, 22), we expected that both singers would embarrass the viewers: the EMBAR singers via affEMP and the PRIDE singers via cogEMP. We further hypothesized that socially anxious subjects’ affEMP would increase and cogEMP decrease during the viewing (for details, see the Introduction in SI Appendix).
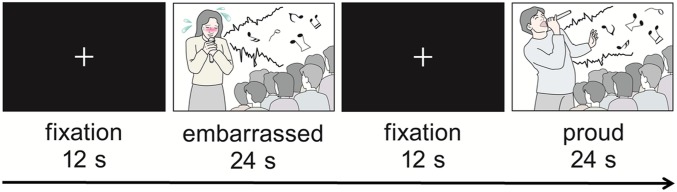
Fig. 1.
fMRI task. Subjects watched video clips of five female and seven male singers singing badly in front of an audience during a singing competition. The singers acted either embarrassed or proud of their singing and expressed this via facial expression and bodily gestures. Altogether 12 blocks of video clips were presented in a pseudorandom order to each subject. Each 24-s block contained two video clips representing the same task condition (12 s each). The same video clips appeared twice through the scanning (in separate blocks). A fixation cross was displayed for 12 s between the blocks.
Social anxiety may be associated with alterations of brain networks (28, 29), but the available brain connectivity findings are inconsistent (11, 28), either because of the variability of subject characteristics (e.g., high comorbidities and medication; refs. 8 and 30) or the diversity of the applied methods (e.g., the use of different anatomical masks). We thus conducted a fully data-driven, whole-brain functional segmentation analysis (FuSeISC) to account for interindividual differences in the brain-activation patterns (31, 32), thereby to better capture brain activity that is synchronized across subjects, while at the same time illustrating intersubject spatial variability in brain networks. We specifically tested the relationship between TKS scores and the strength of whole-brain connectivity (33).
Results
Our subjects’ TKS scores ranged from 45 to 154 (mean ± SD = 80.0 ± 28.1), reflecting low-to-relatively-high TKS intensities (4, 34). The scores statistically significantly correlated positively with affEMP (personal distress: r = 0.45, P = 0.048) but not with cogEMP (perspective-taking: r = −0.23, P = 0.331) and negatively with cognitive flexibility (r = −0.52, P = 0.019; Wisconsin Card Sorting Test; SI Appendix).
The affEMP contrast (EMBAR > PRIDE) in general linear model (GLM)-based fMRI analyses revealed increased activity in the right amygdala and ACC (P < 0.01, corrected for family-wise error [FWE]; Table 1) as well as, contrary to our predictions, in OFC. The cogEMP contrast (EMBAR < PRIDE) revealed increased activity within the right pSTS/TPJ (for whole-brain analysis results, see SI Appendix).
Table 1.
Empathy-related brain activity in affEMP and cogEMP contrasts
| Contrast | Brain region | MNI, x, y, z | z | Cluster size, voxels |
| affEMP | Right amygdala | 32, −4, −28 | 4.41 | 70* |
| ACC | 6, 24, −8 | 4.80 | 684* | |
| OFC | 4, 34, −14 | 4.63 | 459* | |
| cogEMP | Right pSTS/TPJ | 62, −12, 0 | 4.85 | 704* |
P < 0.01 (FWE-corrected).
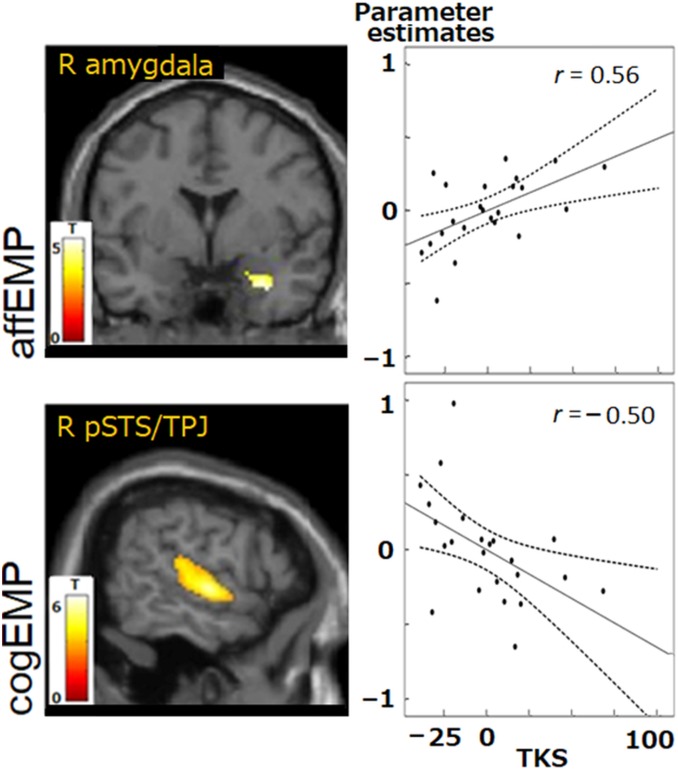
Fig. 2, Upper shows that the activity of the right amygdala, revealed from the affEMP contrast, correlated positively with TKS scores (r = 0.56, P = 0.044; false-discovery rate [FDR] corrected for multiple comparisons), whereas the activity of the right pSTS/TPJ (Fig. 2, Lower), revealed from the cogEMP contrast, correlated negatively with the TKS scores (r = −0.50, P = 0.048).
Fig. 2.
Correlations between hemodynamic activity and TKS scores obtained in GLM-based analyses. During affEMP contrast (Upper), activity of the right amygdala increased as a function of increasing TKS scores (cluster-level P < 0.01, FWE-corrected). During cogEMP contrast (Lower), activation of the right pSTS/TPJ decreased as a function of increasing TKS scores. Dashed lines represent 95% confidence intervals. Pearson’s correlation coefficients r are indicated (P < 0.05 after controlling for age and sex; FDR-corrected for multiple comparisons). R amygdala, right amygdala; R pSTS/TPJ, right pSTS/TPJ.
The strength of ACC activity correlated positively with alexithymia scores (externally/other-oriented thinking: r = 0.57, P = 0.009), and the right pSTS/TPJ activity correlated positively with the postscan ratings of embarrassment (r = 0.45, P = 0.047; subjects watched the same video clips of the singing contest after the fMRI scanning and rated the embarrassment level for each clip; Materials and Methods).
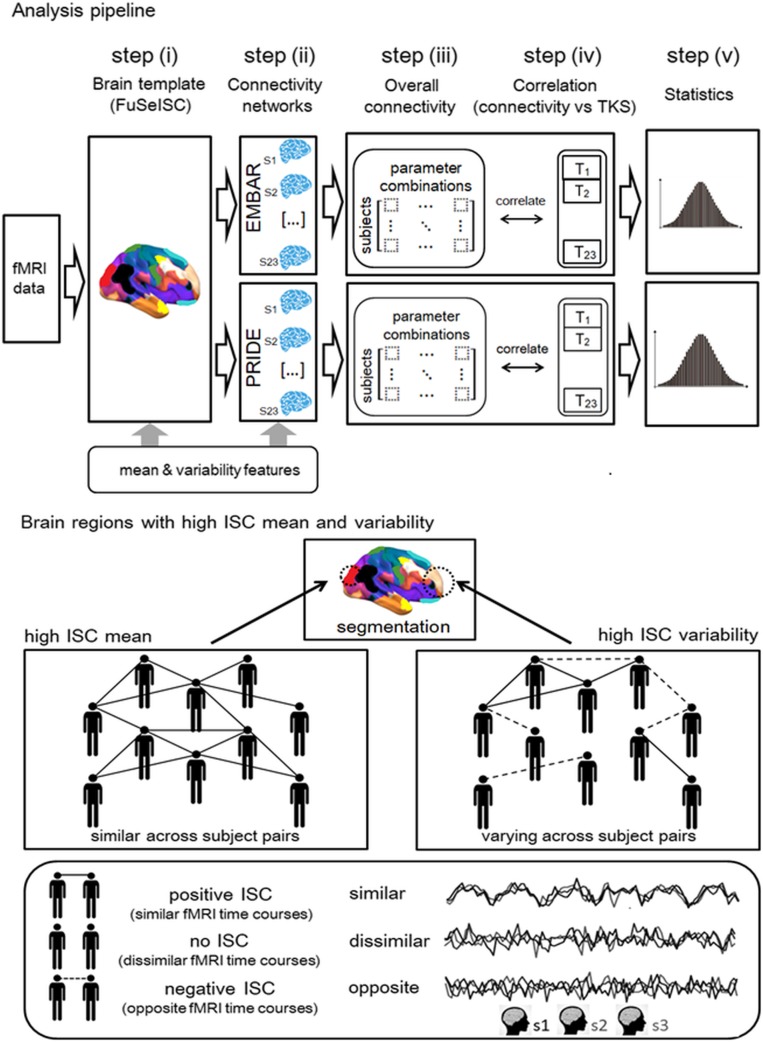
Our fully data-driven, whole-brain functional segmentation analysis (FuSeISC) included computation of both the mean and the variability of intersubject correlations (ISCs) to account for interindividual differences in the brain-activation patterns (31). For the pipeline of the procedure, see Fig. 4 in Materials and Methods.
Fig. 4.
Pipeline for the analysis. (Upper) The analysis steps of associations between TKS scores and overall brain connectivity during the empathic embarrassment task: construction of group-level brain template by FuSeISC (see Lower for explanation of ISC mean and variability) (i); estimation of connectivity networks for EMBAR and PRIDE (ii); computation of overall connectivity strength for each subject for multiple choices of connectivity parameters (SI Appendix) (iii); correlations between overall connectivity strengths and TKS scores, separately for each parameter combination (iv); and statistical evaluation of the average correlations across parameter combinations (v). (Lower) Concepts of ISC mean and ISC variability, used as inherent features to divide brain areas into functional segments. High mean ISC corresponds to similar fMRI time courses across multiple subject pairs (mainly positive ISCs). Meanwhile, high ISC variability corresponds to varying fMRI time courses across subject pairs, that is, similar (positive ISC), dissimilar (no ISC), and opposite (negative ISC). Some brain regions, such as early sensory areas, are typically characterized by high ISC means, whereas, for example, certain higher-order brain areas are potentially characterized by relatively high ISC variability (31).
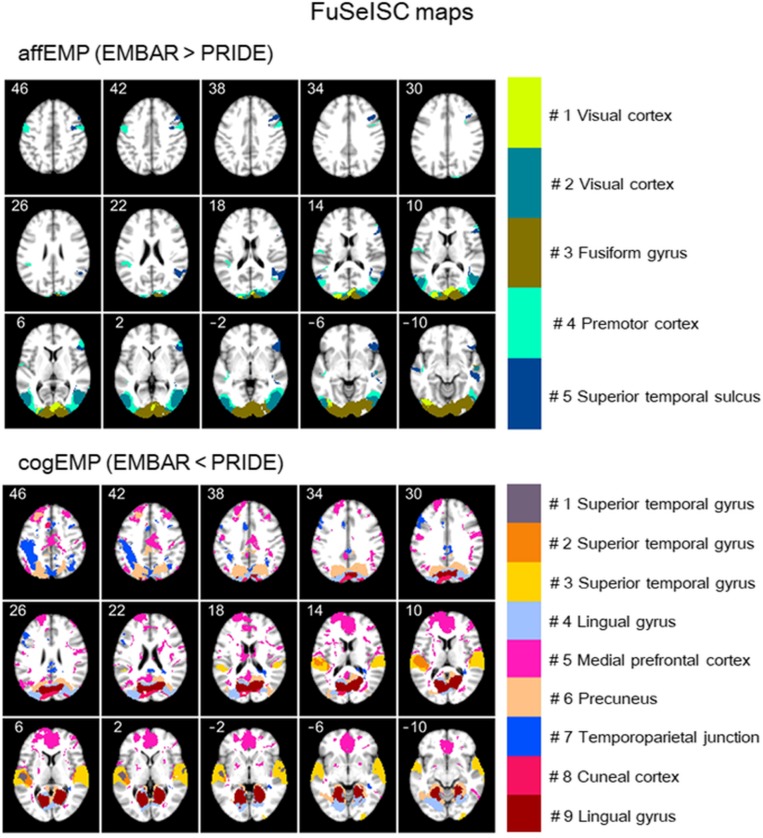
Fig. 3 shows statistically significant FuSeISC contrast maps separately for affEMP (upper images) and cogEMP (lower images) (q < 0.05, FDR-corrected for multiple comparisons). Note that the numbering and coloring of segments differs between the upper and lower images (for details, see SI Appendix). In the affEMP contrast (upper images), brain regions showing statistically significant involvement across subjects include the bilateral occipital cortices (segments 1, 2, and 3), bilateral premotor cortex (segment 4, also including, e.g., right anterior insula, inferior frontal gyrus, and cerebellum; see SI Appendix, Table S1 for a comprehensive list of all activated brain regions), superior temporal sulcus (segment 5, also including, e.g., inferior frontal gyrus and cerebellum).
Fig. 3.
ISC-based whole-brain functional segmentation. Axial FuSeISC maps are shown for the observed segments in the affEMP (Upper) and cogEMP (Lower) contrasts (q < 0.05, FDR-corrected), with the different segments indicated with different colors. Note that the visible (labeled) brain areas are not necessarily the only ones included in a certain segment because spatial constraints were not used in FuSeISC. MNI z coordinates (in millimeters) are indicated for each slice (see SI Appendix, Table S1 for a comprehensive list of brain regions for each segment).
In the cogEMP contrast (Fig. 3, lower images), the brain regions with statistically significant involvement include regions of the bilateral superior temporal gyrus and pSTS/TPJ (segments 1, 2, 3, and 7), lingual gyrus (segments 4 and 9), ventral and dorsal medial prefrontal cortex (segment 5), precuneus (segment 6), and cuneus (segment 8). These segments also included multiple other brain regions, listed in SI Appendix, Table S1. Note that the mPFC region that we predicted to be involved in our task was not visible in the cogEMP contrast of the GLM analysis but was prominent in the FuSecISC analysis (segment 5) potentially because of its high interindividual variability (refs. 31 and 35 and Fig. 4).
In the functional connectivity analyses, the overall connectivity strength—computed in a whole-brain network comprising nodes within each of spatially isolated segment obtained from FuSeISC—correlated negatively with the TKS scores during cogEMP (raverage = −0.23, P = 0.015; corrected for multiple comparisons), whereas during affEMP, the correlation did not reach the statistical significance (raverage = −0.14, P = 0.108); see SI Appendix for the statistical tests.
Discussion
Our results provide both behavioral and brain-level support for the idea that other-oriented social anxiety is associated with enhanced affEMP and reduced cogEMP (5, 12, 36, 37). The negative correlation of the overall network strength with the TKS scores during cogEMP supports decreased cognitive processing in embarrassing situations, likely obstructing flexible inference of others’ perspectives and attention-shifting or augmenting maladaptive feelings of embarrassment. Our results thus suggest that TKS is characterized, besides by an imbalance of affective and cognitive empathy, by disruption of the cogEMP brain network. These findings extend the current understanding of social anxiety, demonstrating how altered affEMP and cogEMP might be associated with experiences of social anxiety dominated by other-oriented imaginary fear (4, 7).
Prior studies on social anxiety have identified neural systems thought to support fear and embarrassment (11, 23), but it has remained unclear whether and how these systems might contribute to other-oriented anxiety. Here, by using naturalistic video stimuli that induced empathic embarrassment, we illuminated the behavioral and neural correlates of other-oriented anxiety in two key ways, showing that 1) TKS scores correlated positively with dispositional affEMP (personal distress) and with amygdala activity during affEMP, and 2) TKS scores correlated negatively with cognitive flexibility (attentional and perspective-shifting; refs. 15 and 38) and pSTS/TPJ activity during cogEMP. Both these results would be in line with enhanced affEMP and reduced cogEMP in other-oriented anxiety.
Stronger pSTS/TPJ activity during cogEMP than affEMP, revealed both in GLM and FuSeISC analyses, is consistent with previous research that has suggested that pSTS/TPJ subserves cogEMP via flexible shifting of attention and perspective (15, 38). This finding also converges with prior ISC studies that have highlighted the role of pSTS/TPJ in moment-to-moment cognitive appraisals via socially attuned attention (39–43). The association between pSTS/TPJ activity and the embarrassment that the viewers were feeling during cogEMP (when the singers sang badly but acted as if they were proud of their singing) also supports involvement of the pSTS/TPJ region in gaining a better understanding of others’ situations in social contexts (38, 44, 45).
The reduced cogEMP in TKS, as reflected by the negative association of TKS scores with pSTS/TPJ activity, suggests that other-oriented anxiety is related to decreased ability to recognize embarrassment in social situations. This view is counterintuitive because people with social anxiety are often argued to be hypersensitive to other’s feelings, especially to others’ negative emotions (3, 9). However, our view aligns with the growing body of literature implying that declined social cognition can coincide with high social sensitivity in people with social anxiety (12). In other words, whereas the socially anxious people may be highly focused on others’ mental states via noticing and sharing emotions of others (affEMP skills), their inferences of the social situations or perspective of others (cogEMP skills) may be highly inaccurate (12).
Accordingly, elevated affEMP (emotional sharing) can obstruct cogEMP (perspective-taking) during highly emotional situations (46). Moreover, social anxiety may be associated with difficulties in cogEMP, especially when discerning complex emotions (47). Indeed, our subjects with high TKS scores exhibited enhanced affEMP and reduced cogEMP, possibly amplifying their attention to feelings of others but hindering flexible understanding of other’s social contexts (14). These subjects therefore were preoccupied with other-oriented, irrational fear (4, 7).
The results of ISC-based brain segmentation further supported the unique roles of affEMP and cogEMP in empathic embarrassment. Along with previous studies (15, 17), the ventral and dorsal mPFC, PCC/precuneus, and pSTS/TPJ were more strongly involved during cogEMP than affEMP, whereas the anterior insula, inferior frontal gyrus, premotor cortex, STS, and cerebellum were more prominently involved during affEMP than cogEMP. One potential explanation for these differences is differential involvement of mentalization (15, 17) and motor-mirroring (48) in cognitive and affective empathy.
The negative correlation between TKS scores and the strength of overall network connectivity during cogEMP further supports the notion that social anxiety may involve disruptions of cognitive, in addition to affective, processing (12, 13, 28). More research is, however, required to investigate the extent to which these alterations can affect flexible distinction and/or balance between self–other perspectives (17, 49) or exaggerate negative perspective bias toward others, as well as toward self (e.g., misinterpreting others’ impression about oneself and distorting self-image; ref. 50).
Our findings on TKS can contribute to a better understanding of the neurocognitive dysfunction of SAD, owing to the shared altered self–other awareness in both disorders (4, 5). Both TKS and SAD individuals excessively focus on others’ perspectives (5). Individuals with SAD are often preoccupied with the likelihood of negative evaluation by others (51), accompanied by heightened self-awareness (12) but blurred experiences of their own emotions (47). Meanwhile, individuals with TKS are afraid of discomforting others by their physical/behavioral features (4).
The empathic embarrassment paradigm, involving self–other merging and distinction, allowed us to reveal that individuals who are more prone to other-oriented social anxiety may show enhanced affective and reduced cognitive empathy. The observed association between TKS and self-awareness scores further supports this view (SI Appendix), underlining the unique self–other representation in social anxiety (3, 50). In this regard, our results support the proposal that social anxiety is represented on a spectrum (9, 13), comprising diverse clinical manifestations from mild to severe and an even wider continuum of social anxiety extending into the general population, thereby affecting a multitude of interpersonal interactions (3, 50).
Limitations.
The limitations of the present study include the correlational nature of our analyses, which does not inform about causal relationships between social anxiety and brain function supporting empathic involvement. Future intervention studies promoting empathy might clarify this issue. Although our study included subjects with subclinical social anxiety, some subjects’ social-anxiety levels were fairly equivalent to those obtained from patients with SAD (52–54). Accordingly, manifestations of social anxiety appear to range widely from the subclinical (e.g., shyness and submissiveness) to clinical level, possibly relying on the same or overlapping dysfunctional brain mechanisms (9, 13). Nevertheless, it is essential to replicate and generalize the current findings in patients with SAD.
Although the unpredicted OFC activity observed in the affEMP contrast of the GLM analysis could reflect emotion-related processing, such as affective perspective-taking (55) and cognitive control of emotion (56), OFC is also known to be involved in cognitive flexibility (57) and cognitive empathy (27). Thus, more studies are required to further examine the role of OFC in TKS, and here event-related analysis might provide additional insights.
Notwithstanding these limitations, the present study enhances our understanding of the neural correlates of social anxiety and illustrates how data-driven brain imaging approaches (e.g., ISC) might illuminate the heterogenetic experiences of social anxiety. As our sample of 23 persons is relatively small, it would be beneficial to replicate our findings with a larger sample. To improve reproducibility and transparency, we provide the analysis code for ISC analysis at https://www.nitrc.org/projects/isc-toolbox/ (58, 59).
Conclusion.
Our findings suggest that other-oriented social anxiety, here studied in subjects suffering from TKS, is characterized by an imbalance of empathy (enhanced affEMP and reduced cogEMP) as well as by disruption of the cogEMP brain network. These aberrations possibly deteriorate cognitive processing during embarrassing situations. Our results shed light on how altered affective and cognitive processing can contribute to the development of social fear.
Materials and Methods
This study was approved by the Committee on Medical Ethics of the Kyoto University and carried out in accordance with the World Medical Association’s International Code of Ethics (60) and Declaration of Helsinki (61). Twenty-three subjects (16 males, 7 females; mean ± SD age, 21.3 ± 1.2 y) were recruited through an advertisement in Kyoto University and participated after written consent. Exclusion criteria included history of neurological disease, major physical/surgical illness, and substance abuse. Subjects were screened for major psychiatric disorders, including depression, schizophrenia, and bipolar disorders, with the Structured Clinical Interview for DSM-IV Axis I diagnoses, administered by experienced psychiatrists attending the Department of Psychiatry of Kyoto University. Based on the previous fMRI studies on empathy and embarrassment using a block designs and region of interest (ROI) analyses (23, 62–64), as well as sample-size determination software G-power (65), 23 subjects were considered sufficient to detect a statistically significant (P < 0.05) difference (dz = 0.9) (66) between conditions on a two-sided test of proportions (difference between two dependent means) with >80% power.
Behavioral Data.
The conventional 31-item TKS questionnaire (4) was administered to find out how the subject differ in their TKS symptom level. This questionnaire assesses the subjects’ concerns that they will do something to offend or embarrass others. The items on the questionnaire are based on clinical experience and are consistent with descriptions of the defined symptoms of TKS. The relationship between TKS scores and empathy was assessed with the Interpersonal Reactivity Index, a measure of affEMP and cogEMP, and the TAS, a measure of altered self-awareness and empathy (25, 64). The subjects’ cognitive flexibility and attentional set-shifting skills (SI Appendix) were assessed with Wisconsin Card Sorting Test.
fMRI Task, Data Acquisition, and Analyses.
Subjects watched video clips of singers who were singing badly in front of an audience during a singing competition (Fig. 1). Singers acted embarrassed or proud of their singing. These performances were designed to embarrass the viewers either via emotion-sharing (affEMP) or via perspective-taking (cogEMP). All singers presented popular Japanese songs that were familiar to our subjects. The empathic embarrassment task included two additional conditions that will be reported in a separate study (for further details, see SI Appendix).
The fMR images were acquired with a 3-tesla magnet equipped with a 32-channel phased-array head coil (Verio, Siemens) located at the Kokoro Research Center in Kyoto University. Functional images were obtained using a T2*-weighted gradient echoplanar imaging sequence with the following parameters: echo time (TE)/repetition time (TR), 29/2,400 ms; flip angle, 90°; field of view (FOV), 192 × 192 mm2; matrix, 64 × 64; 38 interleaved axial slices of 3-mm thickness without gaps; resolution, 3 × 3 × 3 mm3 voxels. Structural scans were also acquired using T1-weighted 3-dimensional magnetization-prepared rapid gradient echo sequences (TE, 3.51 ms; TR, 2,000 ms; inversion time, 990 ms; FOV, 256 × 256 mm2; matrix, 256 × 256; resolution, 1.0 × 1.0 × 1.0 mm3; altogether, 208 total axial sections without gaps). After completing the scanning session, subjects watched the same video clips of the singing contest outside the scanner and rated the intensity of embarrassment using a seven-point Likert scale (representing none to extreme).
Imaging data were preprocessed and analyzed using Statistical Parametric Mapping (SPM) 12 (Wellcome Department of Imaging Neuroscience). All functional brain volumes were realigned to the first volume and spatially normalized into a standard stereotactic space using a template in Montreal Neurological Institute (MNI) space. These images were resampled into 2 × 2 × 2 mm3 voxels during the normalization process. All EPI images were smoothed using an 8-mm Gaussian kernel. Data were high pass-filtered with a time constant of 128 s.
At the single-subject level, we used a GLM in SPM and conducted t tests for the contrasts EMBAR > PRIDE and EMBAR < PRIDE (67). At the group level, we conducted ROI-based random-effects analyses to investigate activity specifically recruited within empathy-related brain regions. Activity within ROI masks was considered statistically significant if it survived FWE correction for multiple comparisons at the cluster level at P < 0.01 (primary threshold at voxel-level uncorrected, P < 0.001). Parameter estimates were extracted as first eigenvariates from statistically significant clusters within these a priori regions. Additionally, we report activity outside these ROIs, thresholded at the voxel-level at P < 0.01, with a minimum cluster extent of 50 contiguous voxels (400 mm3) after whole-brain FWE correction for multiple comparisons. Finally, parameter estimates from the EMBAR > PRIDE and EMBAR < PRIDE contrasts were correlated with behavioral scores (Pearson’s r correlation analyses in SPSS 22.0) after controlling for age and sex. Statistical significance was set at P < 0.05 (two-tailed).
FuSeISC and Connectivity Analyses.
We performed data-driven FuSeISC (31) of brain regions using the ISC toolbox (59) implemented in Matlab. FuSeISC segments the whole brain directly at the group level without utilizing spatial information (such as locations, shapes, and sizes defined in the anatomical masks) but includes computation of both the mean and the variability of ISCs to account for interindividual differences in the brain-activation patterns (31, 32). Each segment is characterized by a unique pattern of ISC (31). In the current study, based on whole-brain FuSeISC, we performed condition-contrast and brain network-connectivity analyses. Fig. 4 shows the pipeline of the FuSeISC analysis from fMRI data to the statistics of correlation between TKS scores and strengths of connectivity via whole-brain segmentation (for details, see SI Appendix, Fig. S1).
In the connectivity analysis, nodes were defined within each of the spatially isolated segment obtained by FuSeISC, and functional networks were estimated by mixed neighborhood selection method (68). This method incorporated a random-effects component into the model, enabling the learning of both group-level and subject-specific connectivities for each node in the network. Two connectivity graphs per each subject were initially built separately, based on the time series of two conditions (EMBAR and PRIDE). From the estimated weighted connectivity graphs, we computed the overall connectivity strength for each subject (33, 69). Overall strength was obtained by calculating the median of the positive, pairwise correlation values between nodes for each subject (33). Subsequently, we examined the association between the overall connectivity strength and TKS scores across subjects during both EMBAR and PRIDE.
Data Deposition.
The data processing and analytical pipeline have been deposited at the University Hospital Medical Information Network (UMIN) Center, Japan (https://upload.umin.ac.jp/cgi-open-bin/icdr_e/ctr_view.cgi?recptno=R000043844). The data discussed in this paper are available to readers upon request. Aggregated forms of data are available from S.T. Matlab code developed during the current study is also available upon reasonable request.
Supplementary Material
Acknowledgments
We thank the research team of the Kokoro Research Center at Kyoto University, Japan, for their skillful assistance in data acquisition. This study was conducted using the MRI scanner and related facilities of the Kokoro Research Center. This study was supported by Grants-in-Aid for Scientific Research A (24243061, to H.T.) and on Innovative Areas (23120009 and 16H06572, to H.T.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan; Grants-in-Aid for Scientific Research C (17K10326, to S.T.) and Young Scientists B (17K16398, to J.F.) from the Japan Society for the Promotion of Science, Graduate Research Opportunities Worldwide Fellowship (a component of National Science Foundation Graduate Research Fellowship 2011122786 to K.F.J.); and the Takeda Science Foundation (H.T.).
Footnotes
The authors declare no competing interest.
Data deposition: The data processing and analytical pipeline discussed in this paper have been deposited at the University Hospital Medical Information Network (UMIN) Center, Japan (https://upload.umin.ac.jp/cgi-open-bin/icdr_e/ctr_view.cgi?recptno=R000043844). Preregistration materials, including a preanalytical plan, can be found in the Clinical Trials Registry of the UMIN Center, Japan (https://upload.umin.ac.jp/cgi-open-bin/icdr_e/ctr_view.cgi?recptno=R000043844).
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1918081117/-/DCSupplemental.
References
- 1.Kessler R. C., et al. , The global burden of mental disorders: An update from the WHO World Mental Health (WMH) surveys. Epidemiol. Psichiatr. Soc. 18, 23–33 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bögels S. M., et al. , Social anxiety disorder: Questions and answers for the DSM-V. Depress. Anxiety 27, 168–189 (2010). [DOI] [PubMed] [Google Scholar]
- 3.Goldin P. R., Manber T., Hakimi S., Canli T., Gross J. J., Neural bases of social anxiety disorder: Emotional reactivity and cognitive regulation during social and physical threat. Arch. Gen. Psychiatry 66, 170–180 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kleinknecht R. A., Dinnel D. L., Kleinknecht E. E., Hiruma N., Harada N., Cultural factors in social anxiety: A comparison of social phobia symptoms and Taijin kyofusho. J. Anxiety Disord. 11, 157–177 (1997). [DOI] [PubMed] [Google Scholar]
- 5.Norasakkunkit V., Kitayama S., Uchida Y., Social anxiety and holistic cognition: Self-focused social anxiety in the united states and other-focused social anxiety in Japan. J. Cross Cult. Psychol. 43, 742–757 (2012). [Google Scholar]
- 6.Choy Y., Schneier F. R., Heimberg R. G., Oh K. S., Liebowitz M. R., Features of the offensive subtype of Taijin-Kyofu-Sho in US and Korean patients with DSM-IV social anxiety disorder. Depress. Anxiety 25, 230–240 (2008). [DOI] [PubMed] [Google Scholar]
- 7.Heimberg R. G., et al. , Social anxiety disorder in DSM-5. Depress. Anxiety 31, 472–479 (2014). [DOI] [PubMed] [Google Scholar]
- 8.Hofmann S. G., Heinrichs N., Moscovitch D. A., The nature and expression of social phobia: Toward a new classification. Clin. Psychol. Rev. 24, 769–797 (2004). [DOI] [PubMed] [Google Scholar]
- 9.Stein M. B., Stein D. J., Social anxiety disorder. Lancet 371, 1115–1125 (2008). [DOI] [PubMed] [Google Scholar]
- 10.Shackman A. J., et al. , Neural mechanisms underlying heterogeneity in the presentation of anxious temperament. Proc. Natl. Acad. Sci. U.S.A. 110, 6145–6150 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brühl A. B., Delsignore A., Komossa K., Weidt S., Neuroimaging in social anxiety disorder—A meta-analytic review resulting in a new neurofunctional model. Neurosci. Biobehav. Rev. 47, 260–280 (2014). [DOI] [PubMed] [Google Scholar]
- 12.Tibi-Elhanany Y., Shamay-Tsoory S. G., Social cognition in social anxiety: First evidence for increased empathic abilities. Isr. J. Psychiatry Relat. Sci. 48, 98–106 (2011). [PubMed] [Google Scholar]
- 13.Kajimura S., Kochiyama T., Nakai R., Abe N., Nomura M., Fear of negative evaluation is associated with altered brain function in nonclinical subjects. Psychiatry Res. 234, 362–368 (2015). [DOI] [PubMed] [Google Scholar]
- 14.Mobbs D., et al. , Viewpoints: Approaches to defining and investigating fear. Nat. Neurosci. 22, 1205–1216 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shamay-Tsoory S. G., Aharon-Peretz J., Perry D., Two systems for empathy: A double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial prefrontal lesions. Brain 132, 617–627 (2009). [DOI] [PubMed] [Google Scholar]
- 16.Lamm C., Bukowski H., Silani G., From shared to distinct self-other representations in empathy: Evidence from neurotypical function and socio-cognitive disorders. Philos. Trans. R. Soc. Lond. B Biol. Sci. 371, 20150083 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singer T., Lamm C., The social neuroscience of empathy. Ann. N. Y. Acad. Sci. 1156, 81–96 (2009). [DOI] [PubMed] [Google Scholar]
- 18.Lebra T., “Shame and guilt in Japan” in Psychological Anthropology: A Reader on Self in Culture., LeVine R. A., Ed. (John Wiley & Sons, 2010), pp. 102–111. [Google Scholar]
- 19.Fujino J., et al. , Altered brain response to others’ pain in major depressive disorder. J. Affect. Disord. 165, 170–175 (2014). [DOI] [PubMed] [Google Scholar]
- 20.Mobbs D., et al. , Neural activity associated with monitoring the oscillating threat value of a tarantula. Proc. Natl. Acad. Sci. U.S.A. 107, 20582–20586 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frith C. D., The social brain? Philos. Trans. R. Soc. Lond. B Biol. Sci. 362, 671–678 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paulus F. M., Müller-Pinzler L., Jansen A., Gazzola V., Krach S., Mentalizing and the role of the posterior superior temporal sulcus in sharing others’ embarrassment. Cereb. Cortex 25, 2065–2075 (2015). [DOI] [PubMed] [Google Scholar]
- 23.Müller-Pinzler L., et al. , Neural pathways of embarrassment and their modulation by social anxiety. Neuroimage 119, 252–261 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takahashi H., et al. , Brain activation associated with evaluative processes of guilt and embarrassment: An fMRI study. Neuroimage 23, 967–974 (2004). [DOI] [PubMed] [Google Scholar]
- 25.Moriguchi Y., et al. , Empathy and judging other’s pain: An fMRI study of alexithymia. Cereb. Cortex 17, 2223–2234 (2007). [DOI] [PubMed] [Google Scholar]
- 26.Grattan L. M., Bloomer R. H., Archambault F. X., Eslinger P. J., Cognitive flexibility and empathy after frontal lobe lesion. Neuropsychiatry Neuropsychol. Behav. Neurol. 7, 251–259 (1994). [Google Scholar]
- 27.Shamay-Tsoory S. G., Tomer R., Goldsher D., Berger B. D., Aharon-Peretz J., Impairment in cognitive and affective empathy in patients with brain lesions: Anatomical and cognitive correlates. J. Clin. Exp. Neuropsychol. 26, 1113–1127 (2004). [DOI] [PubMed] [Google Scholar]
- 28.Sylvester C. M., et al. , Functional network dysfunction in anxiety and anxiety disorders. Trends Neurosci. 35, 527–535 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang X., et al. , Network analysis reveals disrupted functional brain circuitry in drug-naive social anxiety disorder. Neuroimage 190, 213–223 (2019). [DOI] [PubMed] [Google Scholar]
- 30.Freitas-Ferrari M. C., et al. , Neuroimaging in social anxiety disorder: A systematic review of the literature. Prog. Neuropsychopharmacol. Biol. Psychiatry 34, 565–580 (2010). [DOI] [PubMed] [Google Scholar]
- 31.Kauppi J. P., Pajula J., Niemi J., Hari R., Tohka J., Functional brain segmentation using inter-subject correlation in fMRI. Hum. Brain Mapp. 38, 2643–2665 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seghier M. L., Price C. J., Interpreting and utilising intersubject variability in brain function. Trends Cogn. Sci. 22, 517–530 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Servaas M. N., et al. , Connectomics and neuroticism: An altered functional network organization. Neuropsychopharmacology 40, 296–304 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tarumi S., Ichimiya A., Yamada S., Umesue M., Kuroki T., Taijin Kyofusho in university students: Patterns of fear and predispositions to the offensive variant. Transcult. Psychiatry 41, 533–546 (2004). [DOI] [PubMed] [Google Scholar]
- 35.Zilles K., Amunts K., Individual variability is not noise. Trends Cogn. Sci. 17, 153–155 (2013). [DOI] [PubMed] [Google Scholar]
- 36.Gaebler M., Daniels J. K., Lamke J. P., Fydrich T., Walter H., Behavioural and neural correlates of self-focused emotion regulation in social anxiety disorder. J. Psychiatry Neurosci. 39, 249–258 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shu J., Hassell S., Weber J., Ochsner K. N., Mobbs D., The role of empathy in experiencing vicarious anxiety. J. Exp. Psychol. Gen. 146, 1164–1188 (2017). [DOI] [PubMed] [Google Scholar]
- 38.Tei S., et al. , Collaborative roles of temporoparietal junction and dorsolateral prefrontal cortex in different types of behavioural flexibility. Sci. Rep. 7, 6415 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hari R., Kujala M. V., Brain basis of human social interaction: From concepts to brain imaging. Physiol. Rev. 89, 453–479 (2009). [DOI] [PubMed] [Google Scholar]
- 40.Hasson U., Ghazanfar A. A., Galantucci B., Garrod S., Keysers C., Brain-to-brain coupling: A mechanism for creating and sharing a social world. Trends Cogn. Sci. 16, 114–121 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nummenmaa L., et al. , Emotions promote social interaction by synchronizing brain activity across individuals. Proc. Natl. Acad. Sci. U.S.A. 109, 9599–9604 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lahnakoski J. M., et al. , Naturalistic FMRI mapping reveals superior temporal sulcus as the hub for the distributed brain network for social perception. Front. Hum. Neurosci. 6, 233 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tei S., et al. , Inter-subject correlation of temporoparietal junction activity is associated with conflict patterns during flexible decision-making. Neurosci. Res. 144, 67–70 (2019). [DOI] [PubMed] [Google Scholar]
- 44.Pantelis P. C., Byrge L., Tyszka J. M., Adolphs R., Kennedy D. P., A specific hypoactivation of right temporo-parietal junction/posterior superior temporal sulcus in response to socially awkward situations in autism. Soc. Cogn. Affect. Neurosci. 10, 1348–1356 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hill C. A., et al. , A causal account of the brain network computations underlying strategic social behavior. Nat. Neurosci. 20, 1142–1149 (2017). [DOI] [PubMed] [Google Scholar]
- 46.Kanske P., Böckler A., Trautwein F. M., Parianen Lesemann F. H., Singer T., Are strong empathizers better mentalizers? Evidence for independence and interaction between the routes of social cognition. Soc. Cogn. Affect. Neurosci. 11, 1383–1392 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morrison A. S., et al. , Empathy for positive and negative emotions in social anxiety disorder. Behav. Res. Ther. 87, 232–242 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hari R., Sams M., Nummenmaa L., Attending to and neglecting people: Bridging neuroscience, psychology and sociology. Philos. Trans. R. Soc. Lond. B Biol. Sci. 371, 20150365 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tei S., et al. , Egocentric biases and atypical generosity in autistic individuals. Autism Res. 12, 1598–1608 (2019). [DOI] [PubMed] [Google Scholar]
- 50.Bas-Hoogendam J. M., van Steenbergen H., van der Wee N. J. A., Westenberg P. M., Not intended, still embarrassed: Social anxiety is related to increased levels of embarrassment in response to unintentional social norm violations. Eur. Psychiatry 52, 15–21 (2018). [DOI] [PubMed] [Google Scholar]
- 51.Judah M. R., Grant D. M., Carlisle N. B., The effects of self-focus on attentional biases in social anxiety: An ERP study. Cogn. Affect. Behav. Neurosci. 16, 393–405 (2016). [DOI] [PubMed] [Google Scholar]
- 52.Nishikawa Y., Laposa J. M., Regev R., Rector N. A., Social anxiety and fear of causing discomfort to others: Diagnostic specificity, symptom correlates and CBT treatment outcome. Behav. Cogn. Psychother. 45, 382–400 (2017). [DOI] [PubMed] [Google Scholar]
- 53.Kim J., Rapee R. M., Gaston J. E., Symptoms of offensive type Taijin-Kyofusho among Australian social phobics. Depress. Anxiety 25, 601–608 (2008). [DOI] [PubMed] [Google Scholar]
- 54.Peters L., Discriminant validity of the social phobia and anxiety inventory (SPAI), the social phobia scale (SPS) and the social interaction anxiety scale (SIAS). Behav. Res. Ther. 38, 943–950 (2000). [DOI] [PubMed] [Google Scholar]
- 55.Shamay-Tsoory S. G., Harari H., Aharon-Peretz J., Levkovitz Y., The role of the orbitofrontal cortex in affective theory of mind deficits in criminal offenders with psychopathic tendencies. Cortex 46, 668–677 (2010). [DOI] [PubMed] [Google Scholar]
- 56.Ochsner K. N., Gross J. J., The cognitive control of emotion. Trends Cogn. Sci. 9, 242–249 (2005). [DOI] [PubMed] [Google Scholar]
- 57.Clarke H. F., Dalley J. W., Crofts H. S., Robbins T. W., Roberts A. C., Cognitive inflexibility after prefrontal serotonin depletion. Science 304, 878–880 (2004). [DOI] [PubMed] [Google Scholar]
- 58.Kauppi J.-P., Pajula J., Tohka J., isc-toolbox: Inter-subject correlation analysis for fMRI in Matlab. NeuroImaging Tools and Resources Collaboratory. https://www.nitrc.org/projects/isc-toolbox/ (15 June 2015).
- 59.Kauppi J. P., Pajula J., Tohka J., A versatile software package for inter-subject correlation based analyses of fMRI. Front. Neuroinform. 8, 2 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.World Medical Association , WMA Declaration of Helsinki – Ethical principles for medical research involving human subjects (2018). https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/. Accessed 6 November 2018.
- 61.World Medical Association , WMA International Code of Medical Ethics (2018). https://www.wma.net/policies-post/wma-international-code-of-medical-ethics/. Accessed 6 November 2018.
- 62.Zaki J., Weber J., Bolger N., Ochsner K., The neural bases of empathic accuracy. Proc. Natl. Acad. Sci. U.S.A. 106, 11382–11387 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zaki J., Hennigan K., Weber J., Ochsner K. N., Social cognitive conflict resolution: Contributions of domain-general and domain-specific neural systems. J. Neurosci. 30, 8481–8488 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tei S., et al. , Can we predict burnout severity from empathy-related brain activity? Transl. Psychiatry 4, e393 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Faul F., Erdfelder E., Lang A. G., Buchner A., G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 39, 175–191 (2007). [DOI] [PubMed] [Google Scholar]
- 66.Suwabe K., et al. , Rapid stimulation of human dentate gyrus function with acute mild exercise. Proc. Natl. Acad. Sci. U.S.A. 115, 10487–10492 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nummenmaa L., Hirvonen J., Parkkola R., Hietanen J. K., Is emotional contagion special? An fMRI study on neural systems for affective and cognitive empathy. Neuroimage 43, 571–580 (2008). [DOI] [PubMed] [Google Scholar]
- 68.Monti R. P., Anagnostopoulos C., Montana G., Learning population and subject-specific brain connectivity networks via mixed neighborhood selection. Ann. Appl. Stat. 11, 2142–2164 (2017). [Google Scholar]
- 69.Rubinov M., Sporns O., Complex network measures of brain connectivity: Uses and interpretations. Neuroimage 52, 1059–1069 (2010). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.