Significance
The response of species to climate change is of increasingly urgent importance. Here, we address the specific changes in climate that were associated with recent population extinctions, using data from 538 plant and animal species distributed globally. Surprisingly, extinctions occurred at sites with smaller changes in mean annual temperatures but larger increases in hottest yearly temperatures. We also evaluate whether species may survive climate change by dispersing, shifting their niches to tolerate warmer conditions, or both. Given dispersal alone, many of these species (∼57–70%) may face extinction. However, niche shifts can potentially reduce this to only 30% or less. Overall, our results show the importance of maximum temperatures for causing species extinction and niche shifts for allowing their survival.
Keywords: climate change, disperal, extinction, niche shift
Abstract
Climate change may be a major threat to biodiversity in the next 100 years. Although there has been important work on mechanisms of decline in some species, it generally remains unclear which changes in climate actually cause extinctions, and how many species will likely be lost. Here, we identify the specific changes in climate that are associated with the widespread local extinctions that have already occurred. We then use this information to predict the extent of future biodiversity loss and to identify which processes may forestall extinction. We used data from surveys of 538 plant and animal species over time, 44% of which have already had local extinctions at one or more sites. We found that locations with local extinctions had larger and faster changes in hottest yearly temperatures than those without. Surprisingly, sites with local extinctions had significantly smaller changes in mean annual temperatures, despite the widespread use of mean annual temperatures as proxies for overall climate change. Based on their past rates of dispersal, we estimate that 57–70% of these 538 species will not disperse quickly enough to avoid extinction. However, we show that niche shifts appear to be far more important for avoiding extinction than dispersal, although most studies focus only on dispersal. Specifically, considering both dispersal and niche shifts, we project that only 16–30% of these 538 species may go extinct by 2070. Overall, our results help identify the specific climatic changes that cause extinction and the processes that may help species to survive.
Climate change may be a major threat to global biodiversity in the next 100 years (y) (1–6), with predictions for species loss ranging from as low as 0% to as high as 54% (5). These predictions are generally based on ecological niche modeling of species distributions under future climates, assuming that species’ climatic niches will remain similar over time (where the climatic niche is the set of large-scale temperature and precipitation conditions where the species can and does occur; refs. 7 and 8). Different scenarios for species survival are then based on these projected future distributions, combined with different assumptions about the extent to which species can disperse to track their current climatic niches over space. However, accurately predicting biodiversity loss from climate change may require a more detailed understanding of what aspects of climate change cause extinctions, and of the mechanisms that can allow species to survive. There has now been important work on mechanisms of decline in certain species (9–12). Yet, one of the most basic questions remains largely unanswered: Which changes in climate will actually cause extinctions? For example, will populations and species be driven extinct by shifts in temperature or in precipitation, by changes in annual means or extremes (9), and by overall amounts of change or by rapid rates of change? Similarly, the mechanisms by which species can potentially persist in a changing climate are also unclear. Specifically, will species be able to persist by dispersing to remain within their current climatic niche (2, 13–15), by shifting their niches to accommodate modified climates (16–18), by both, or by neither (19–22)? To our knowledge, these urgent questions have not been addressed empirically at a broad scale (i.e., across many species, taxonomic groups, and regions). Nevertheless, they may be crucial to predicting how many species will likely be lost in a warming world.
One powerful way to approach these questions is to analyze local extinctions that have already happened. Numerous studies have now documented shifts in species geographic ranges that are potentially related to climate change (23–27). These studies typically utilize data from historical surveys, which documented the presence and absence of species at sites along elevational and latitudinal transects. These historical surveys are then combined with more recent resurveys to infer shifts in species ranges over time, shifts that are potentially related to climate change. Many of these studies documented local extinctions (i.e., apparent disappearance of a species from one or more sites; ref. 28). Data from these studies can offer many potential insights into how climate change causes extinction and how species might persist.
Here, we used these data to address the specific climatic changes associated with local extinctions, to infer the mechanisms that may allow species persistence, and to estimate overall levels of species loss. We analyzed data from 10 studies (SI Appendix, Table S1) that provided detailed information on 538 species and 581 sites (Datasets S1 and S2). We focused on terrestrial plant and animal species along elevational gradients. Species sampling was dominated by plants, insects, and birds. Many sites were temperate (87%), but many species were tropical (70%). Plants were surveyed at 323 sites and animals at 258 sites.
We first generated fine-scale climatic data for each site for the time period of each historical survey and recent resurvey (SI Appendix, Table S2 and Dataset S3). We calculated the absolute change in climatic variables between surveys and their rates of change (Dataset S3). We then estimated the climatic drivers of local extinction. We focused on comparing sites with local extinctions to those without, given that most sites (75%) did not have any local extinctions (Dataset S3). We also performed analyses using the frequency of local extinction at each site, which yielded similar results (SI Appendix, Text S1 and Dataset S4). We utilized discriminant analysis of principal components (29) to estimate which climate change variables best differentiated between sites with and without local extinctions. We then tested these climatic variables individually against the occurrence of local extinctions using univariate logistic regression. We also used these data from historical surveys and recent resurveys to infer rates of dispersal and to estimate how much climatic niches can change in local populations without those populations going extinct (especially in those niche variables identified as most important for driving local extinction). We then combined these inferred rates of dispersal and niche change with projections of future climate change to infer whether species can potentially avoid extinction by dispersing or shifting their climatic niches, and which of these mechanisms might be generally most important for species survival. Finally, we estimated the overall amounts of biodiversity loss based on the patterns in these species.
Results and Discussion
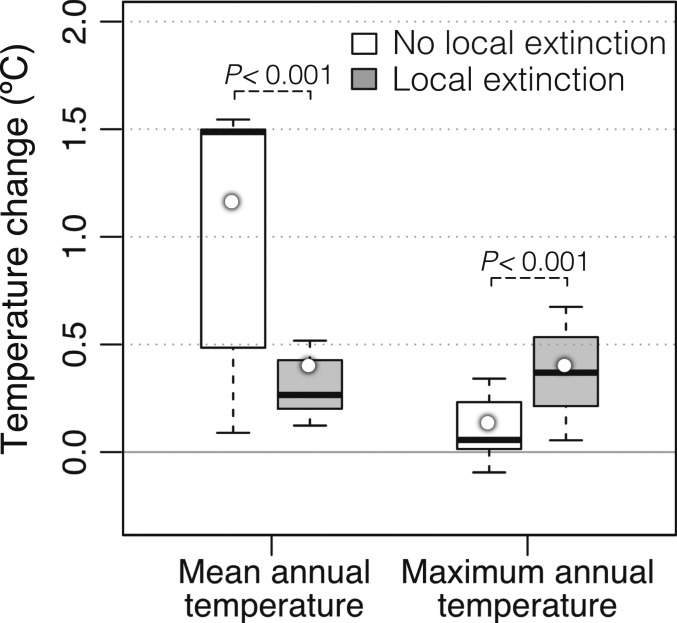
The increase in maximum annual temperatures was the most important variable associated with local extinctions (SI Appendix, Fig. S1 and Table S3), considering both absolute change and rates of change. Maximum temperatures increased roughly three times more at sites with local extinction than those without (Fig. 1; mean increase = 0.413 °C vs. 0.147 °C, respectively; P < 0.001, n = 581 sites) and more than three times as fast (0.018 °C·y−1 vs. 0.005 °C·y−1; P < 0.001). Surprisingly, changes in mean annual temperature were significantly smaller at sites with local extinction (mean change between surveys at sites with local extinction = 0.413 °C; mean change at sites without = 1.174 °C, P < 0.001; Fig. 1). Thus, extinctions generally occurred at sites with larger changes in maximum annual temperatures but smaller changes in mean annual temperatures.
Fig. 1.
Changes in temperature over time at sites with and without local extinction. We illustrate two of the strongest predictors of local extinction across the 581 sites. Quantifying the change in these variables over time (between surveys) at each site shows that those sites with local extinction had significantly larger increases in maximum annual temperatures but significantly smaller changes in mean annual temperatures. Boxes are bounded by the first (25th percentile) and third quartiles (75th percentile). Bottom and top whiskers depict minimum and maximum values. Thick lines within boxes depict median values, and means are circles within boxes. Statistical results are summarized in SI Appendix, Table S3. Data are presented in Dataset S3.
This surprising pattern occurs because changes in maximum temperatures were negatively related to changes in mean temperature among sites (r2 = 0.186, P < 0.001, n = 581). There was a strong positive relationship between changes in mean annual temperature between surveys and absolute latitude of sites (r2 = 0.644, P < 0.001) and a weak negative relationship between changes in maximum temperature and latitude (r2 = 0.042, P < 0.001). In temperate regions, sites with local extinction had greater increases in maximum temperatures than those without (0.456 °C vs. 0.153 °C, P < 0.001, n = 505 sites) and smaller increases in mean temperatures (0.412 °C vs. 1.231 °C, P < 0.001). In tropical regions, sites with local extinction also had greater increases in maximum temperatures (0.316 °C vs. 0.061 °C, P < 0.001, n = 76) but changes in mean temperatures were similar (0.415 °C vs. 0.406 °C, P = 0.897).
Precipitation-related variables were also associated with local extinction but were less important predictors than maximum temperatures (SI Appendix, Fig. S1 and Table S3). Sites with local extinction generally had decreasing precipitation over time (mean change in annual precipitation at sites with local extinction = −29.029 mm; mean at sites without = 80.008 mm, P < 0.001).
We then used the observed relationships between maximum temperatures and local extinction to predict the extent of species loss by 2070, and to estimate the processes that may allow species to survive. For future climates (30), we analyzed up to 19 general circulation models (GCMs) and four representative concentration pathways (RCPs). We present results here for intermediate (RCP4.5) and high (RCP8.5) emission scenarios, with results for each RCP averaged across all available GCMs (complete results in SI Appendix, Tables S4 and S7–S10). We specifically addressed whether species will likely survive within their transects.
First, we addressed whether the current niches of these 538 species will be found along these transects in 2070, focusing on maximum annual temperatures. We found that maximum temperatures will be unsuitable (i.e., values outside the current range) for 78–86% of the 538 species by 2070 (all ranges based on RCP4.5 and RCP8.5; Dataset S5 and SI Appendix, Table S4). On average, maximum temperatures at the coldest site for each species in each transect are expected to be between 1.589 °C (RCP4.5) and 2.625 °C (RCP8.5) warmer by 2070 than the current highest values for these species across their present ranges (Dataset S5). Thus, most species will either need to disperse to remain within their current niche for maximum temperatures or else shift their niches substantially to survive under these warmer conditions.
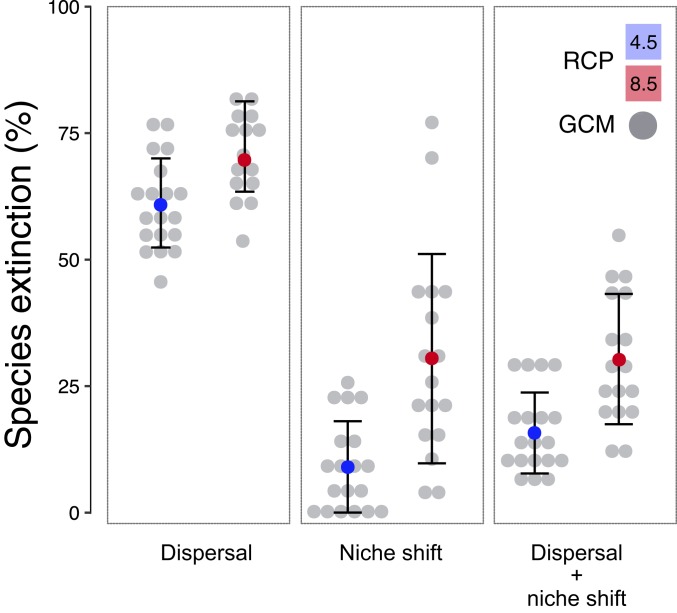
We next investigated whether species are likely to be able to disperse quickly enough to remain within their current niche for maximum annual temperatures. First, for species that dispersed upwards between surveys (n = 185), we evaluated whether the predicted increase in maximum temperatures would be counterbalanced by dispersal at their upper edge (given their past upward dispersal rate, and that upward dispersal is limited by mountain height; SI Appendix, Table S6). We found that 39–60% of these 185 species will not disperse quickly enough (range for RCP4.5 and RCP8.5; SI Appendix, Table S7 and Dataset S6). However, 66% of the 538 species did not disperse upwards at all between surveys (n = 353). Including these species (and assuming they will not disperse quickly enough) suggests that 57–70% of all 538 species will not avoid extinction (Fig. 2 and SI Appendix, Table S8). Alternative scenarios, involving limits on upward dispersal and dispersal in those species that did not disperse upwards between surveys, gave identical extinction estimates (i.e., transect-wide extinctions in 57–70% of the species by 2070; SI Appendix, Table S8). Therefore, the survival of most species may hinge on their ability to tolerate much warmer conditions by shifting their climatic niches, either through plasticity, evolution, or both (20–22).
Fig. 2.
Projected species-level extinction from climate change when species respond by dispersal, niche shifts, or both. We show the percentage of the 538 sampled species that are predicted to go extinct (within their transects) by 2070. These results suggest that niche shifts are far more important for avoiding species-level extinction than dispersal. Different projections of future climates are shown, including the mean across different GCMs (gray data points) for each RCP (blue circle, RCP4.5; red circle, RCP8.5), along with error bars (SD). For dispersal, we consider upward dispersal to be limited by mountaintop height, and we assume that species that did not disperse upwards previously will not disperse upwards in the future. Alternative scenarios give identical results. Niche shifts assume a 95% extinction threshold. Full results are summarized in SI Appendix, Tables S8–S10.
Next, we estimated the absolute change in maximum annual temperatures that populations were able to tolerate without going locally extinct. We used logistic regression to estimate the absolute change in maximum annual temperature at the warmest sites in each species’ range that led to local extinction (Dataset S7). We estimated that 95% of the species underwent local extinction at sites that warmed by >2.860 °C (P < 0.001; n = 538). Thus, many populations survived remarkable temperature increases, but their ability to tolerate higher maximum temperatures was not unlimited. We then evaluated whether all sites in each species’ range are predicted to warm by >2.860 °C (for maximum annual temperatures), potentially leading to extinction at all sites in their transect. Based on this criterion, extinctions within transects are likely for only 9–30% of the 538 species by 2070 (range for RCP4.5–RCP8.5; Fig. 2; SI Appendix, Table S9 and Dataset S7). We also used the change at which 50% of the species experienced local extinction (>0.519 °C) to estimate overall extinction, assuming 50% of the species will go extinct that exceed this threshold. This criterion yielded similar but larger values for the percentage of the 538 species going extinct (35–42%; SI Appendix, Table S9 and Dataset S7). Overall, these results suggest that niche shifts may be far more important for species survival than dispersal, even though niche shifts are rarely included explicitly when predicting impacts of climate change.
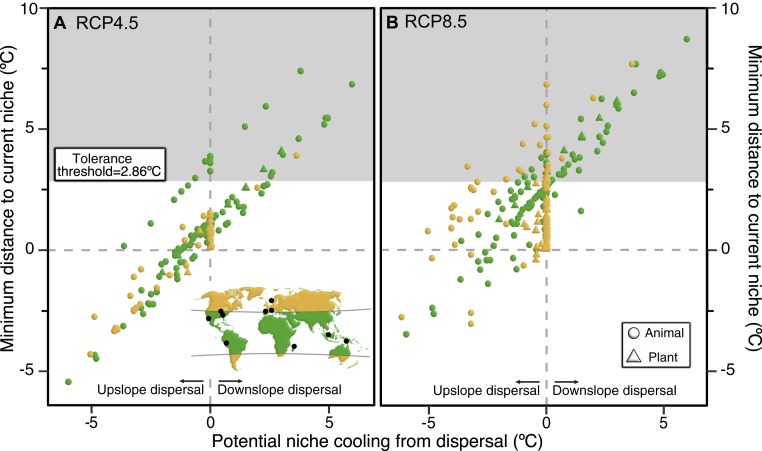
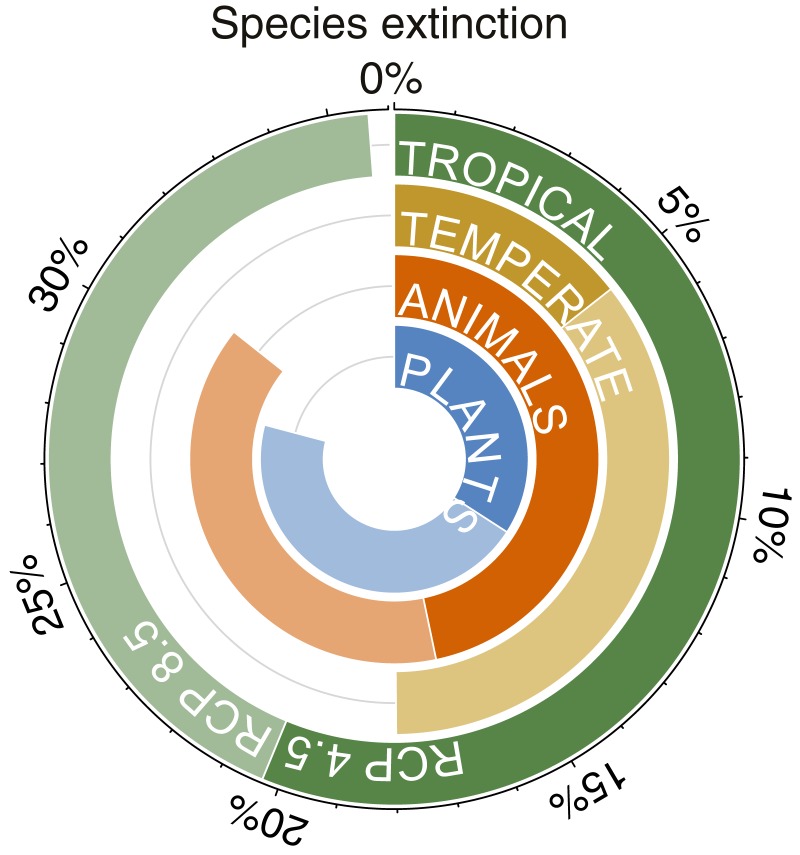
Finally, we asked how many species might avoid extinction through both dispersal and niche shifts. Specifically, we estimated if dispersal could decrease the change in maximum annual temperatures that species experience to below the estimated threshold for local extinction. Based on these values, we project extinction of all populations in their transects in 16–30% of the 538 species by 2070 (RCP4.5–RCP8.5; Figs. 2 and 3, SI Appendix, Table S10 and Dataset S8). Analyses based on alternative assumptions about those species that did not disperse between surveys gave similar estimates (i.e., transect-wide extinctions in 15–30% of species by 2070; SI Appendix, Table S10 and Datasets S9 and S10). Estimates were similar using the 50% temperature threshold (27–35%; SI Appendix, Table S10 and Text S2 and Datasets S8–S10). Our results suggest that extinction may be widespread among both plants and animals, especially in the tropics (Figs. 3 and 4). Importantly, these projections are based on means across warming scenarios, and under the most extreme warming scenarios, 55% of all 538 species could be lost (SI Appendix, Table S10).
Fig. 3.
Projected species-level extinctions among 538 plant and animal species by 2070. Each datapoint (circle, triangle) represents one species. Species are from sites considered subtropical/tropical (green; <35° absolute latitude) or temperate/arctic (yellow). We assume an intermediate level of climate change (RCP4.5) (A) and high level of climate change (RCP8.5) (B). The y axis is the difference between the projected maximum annual temperature at the current coldest site in each species’ range and the current value at the warmest site. Positive values indicate the current niche will not occur in the species current distribution in 2070. The x axis is the cooling gained through upward dispersal, based on species’ past rates of upward dispersal. Many species failed to disperse (zeroes) or moved downslope (positive values). We assumed these species would fail to move upwards in the future, but alternative analyses assumed all species would move upwards (SI Appendix, Table S10). Most species cannot tolerate increases in maximum temperatures >2.860 °C (0.95 threshold). Therefore, species in the gray shaded areas are projected to go extinct, even after upward dispersal. These include 16% (A) and 30% (B) of the 538 species (RCP 4.5, RCP8.5; means across GCMs). Two species with rapid downward dispersal (predicted to go extinct by 2070) are not depicted here. Full results in Datasets S8–S10 and SI Appendix, Table S10.
Fig. 4.
Projections for species-level extinction summarized for different climatic regions and taxonomic groups. We estimated the percentage of tropical, temperate, plant, and animal species in our dataset (n = 538) that are projected to go extinct within their transects by 2070. Extinctions are projected to be especially widespread in tropical regions and among animal species. We summarize results under two alternative RCPs (darker colors, RCP4.5; lighter colors, RCP8.5), based on the means across GCMs for each RCP (note that projected extinction is much more extensive under some GCMs, including up to 55% of all species; Fig. 2). These analyses assumed that species can both disperse (given their past dispersal rates) and shift their climatic niches. The results shown assume that species that did not disperse upwards previously will not disperse upwards in the future, that dispersal is constrained by mountaintop height, and a 95% extinction threshold. Results under alternative assumptions are similar and are given in SI Appendix, Table S10.
These results are not direct estimates of species’ global extinction. Nevertheless, the overall ranges of many of these species presumably consist of similar elevational distributions on these and other mountain slopes. Therefore, species lost from a single transect might be lost from all of them. Furthermore, our results are based only on terrestrial plants and animals on elevational transects. However, most plant and animal species are terrestrial (SI Appendix, Text S2), and most biodiversity hotspots involve montane regions (31). Thus, our results should be relevant to much of Earth’s plant and animal diversity. We acknowledge that many other factors might affect extinction risk beyond those considered here (SI Appendix, Text S2). Importantly, our results suggest that those factors impacting dispersal may not be the most important for species survival.
Conclusions
In summary, our study identifies the specific climatic factors that are associated with the widespread local extinctions that have already occurred due to anthropogenic climate change. We find that the absolute increases in hottest temperatures during the year are most strongly associated with local extinction, more so than changes in precipitation or in other temperature-related variables. Our results also show that mean annual temperatures might be misleading about the impacts of climate change, given that local extinctions were most common at sites where increases in this variable are smaller, not larger. We also estimate the extent of future species-level extinctions, incorporating both dispersal and niche shifts. Our results show that niche shifts have allowed many populations to survive dramatic changes in temperatures. In contrast, dispersal alone may be insufficient to save most species considered here, at least based on their past dispersal rates. These results contrast with the widespread practice of projecting species survival by utilizing species-distribution models that assume no change in species climatic niches over time and by focusing primarily on how dispersal will impact these estimates. Our results strongly support research on incorporating niche shifts into future climate change projections (32, 33) but are agnostic as to whether these niche shifts are primarily evolutionary or not. Finally, we project that 30% or more of these 538 species may go extinct within their transects and possibly globally. Under some climate-change scenarios, more than half of these species might be lost (55%), even after accounting for both dispersal and niche shifts. However, our results also suggest that successful implementation of the Paris Agreement targets (i.e., warming <1.5 °C by 2100, roughly equivalent to RCP4.5; ref. 30) could help reduce extinctions considerably, possibly to 16% or less by 2070.
Methods
Selection of Studies.
We started with 27 studies from a systematic review of climate-related range shifts (28). For greater comparability, we included only terrestrial elevational gradients, excluding the fewer studies of latitudinal gradients and aquatic species. We also excluded studies without data on individual species at individual localities at specific time points. We included 10 studies (SI Appendix, Table S1). Studies were based on surveys of local sites for two time periods (≥10 y apart) and documented whether each species persisted at each locality over time. There was no overlap in species between studies (SI Appendix, Table S1). Additional details are in SI Appendix, Text S1.1.
Locality Data.
We obtained all necessary data directly for some studies. In other cases, authors provided detailed locality data but not coordinates. In these cases, we used Google Earth to estimate coordinates for localities corresponding to these elevations. The main driver of climate among nearby localities along an elevational transect should be elevation (e.g., regression between elevation and mean annual temperature: r2 = 0.99, P < 0.001). We also ensured that localities were on the same slope (i.e., north vs. south facing) as in the original study. Additional details are in SI Appendix, Text S1.2.
Climatic Data.
We obtained climatic data from georeferenced localities using the CRU TS 3.22 (Climate Research Unit Time Series) dataset (34). Climatic variables were downscaled to ∼1 km based on WorldClim raster files (35–37). The resulting dataset included high-resolution climatic data (∼1 km) for each year from 1901 to 2013. When sampling was conducted over multiple years, we selected the oldest year for the historical survey, and the most recent date for the resurvey (SI Appendix, Text S1.3).
We used empirical mode decomposition (EMD) to reduce the stochasticity in the interannual variability for each climatic variable. For each site, we fit an EMD model using the R package EMD (38) based on the entire CRU temporal window (i.e., oldest and modern survey dates). We used default parameters in the R function emd, which are optimized for detrending climatic time series (38, 39). We then calculated the 19 WorldClim variables (SI Appendix, Table S2) following standard definitions (40). These variables are considered important drivers of species distributions (41). We also generated alternative datasets based on mean climatic conditions during the 5-y period and 10-y period before each survey. These datasets yielded similar results to those based on EMD (SI Appendix, Text S3).
Data Analysis.
We generated four datasets (19 variables each) to describe climate at each locality over time: 1) historic (year of the initial survey of the site); 2) modern (resurvey year); 3) absolute change over time (difference between the historic and modern values); and 4) rate of change (absolute change between surveys divided by the time interval between surveys).
We used two approaches to estimate the potential importance of each climatic variable for local extinctions. First, we focused on which climatic variables distinguished between those sites with local extinctions in one or more species, and those sites with no local extinctions. Alternatively, we tested for relationships between climatic variables and the frequency of local extinction among all of the species surveyed at each site. However, local extinctions were absent at most sites (75%), so the main results focused on the occurrence of any local extinction at a site, not frequencies.
Occurrence of local extinctions.
We used discriminant analysis of principal components (DAPC) to determine which variables best differentiated between sites with and without local extinction (see SI Appendix, Text S1.4.1 for additional details). DAPC finds the linear combination of variables that maximizes the difference between groups and minimizes within-group variances. DAPC were fitted independently for each climatic dataset (i.e., historical, absolute change, and rate of change) using the R package adegenet (42), after scaling each variable, and retaining the number of principal components associated with an optimal alpha score (using the optim.a.score function in the same package; ref. 43). The estimated importance of each climatic variable in differentiating between sites with and without local extinction within each dataset is summarized as a DAPC loading (SI Appendix, Table S3). Variables with larger DAPC loadings are better at discriminating between sites with and without local extinction. We focused primarily on variables with loadings in the top 95th percentile for each dataset. The top predictors of local extinction were generally similar across the three datasets (compare SI Appendix, Fig. S1 A–C). No P values are associated with DAPC analyses. Therefore, we used logistic regression models to test for significant effects of each climatic variable on local extinction. We fit univariate generalized lineal models in R version 3.4.2 (37).
Frequency of local extinctions.
For our second approach, we summarized the frequency of local extinctions at each site and then tested which climatic variables were most strongly related to these frequencies (details in SI Appendix, Text S1.4.2). We first used a multivariate approach to estimate the relative importance of each climatic variable. We then fit univariate linear regression models between local extinction frequencies and each climatic variable. Overall, results from frequencies were similar to those based on presence/absence of local extinction and supported the importance of maximum annual temperatures in driving local extinction (SI Appendix, Text S1.4 and Dataset S4).
Projected Climate Change and Extinction.
We explored the effects of projected climate change on extinction within transects for 2070. When we refer to species distributions, extinctions, and persistence here, we specifically mean within the elevational transects studied. Additional details are provided in SI Appendix, Text S1.5.
Projected climatic conditions at each sampled site for 2070 were obtained using the WorldClim raster files at a 0.5′ resolution (∼1 km; ref. 35). Climatic conditions for 2070 were estimated by averaging projected conditions for 2061 and 2080. We analyzed combinations of up to 19 GCMs and four different RCPs (35). Results were based primarily on an intermediate scenario of predicted change (RCP4.5) and a scenario assuming more extensive warming (RCP8.5). For each scenario, we followed standard practice (4, 6) and estimated extinctions for each RCP based on the mean of estimates across all available GCMs (SI Appendix, Tables S4 and S7–S10). The RCP4.5 scenario has been widely used for predicting impacts of future climate change (44–46). However, the RCP8.5 scenario has recently been considered highly likely given increasing greenhouse gas emissions over the past two decades (47, 48). We generated results for all four available RCPs but did not focus on RCP2.6 or RCP6.0 (49, 50).
Based on the different future climate projections (12–19 GCMs and 4 RCPs), we analyzed four aspects of species responses to projected climate change. All four focused on the maximum temperature of the warmest month (shortened here to “maximum annual temperature”; Bio5), given our finding that this variable seems to best predict local extinctions (SI Appendix, Fig. S1 and Table S3 and Dataset S4). First, we estimated the minimum change in Bio5 that species will likely experience by 2070. Second, we analyzed the role of elevational dispersal in potentially allowing species to avoid extinction within transects by moving upwards and tracking their current climatic niche. Third, we examined the change in Bio5 that local populations have tolerated in the past without going extinct (niche shifts). Fourth, we examined the combined effects of dispersal and niche shifts on species persistence.
Minimum temperature increase.
For each species, we evaluated whether the maximum annual temperatures (Bio5) present across their current elevational range (i.e., during the resurvey) will be present in their current elevational range in 2070, or if only higher values will be present. We estimated current Bio5 values for each site across their current distribution. Next, we used the predicted Bio5 values for 2070 to estimate future Bio5 values for these sites. If no overlap was found between the future and current Bio5 across the current distribution, we considered the species to be exposed to unsuitable conditions across their current range (within the transect).
Next, for species predicted to be exposed to unsuitable Bio5 values across their current range, we estimated the minimum difference between current and future Bio5 across their current distribution. Specifically, minimum changes were estimated by subtracting the current value of Bio5 at the species’ current warmest site in their geographic range (i.e., at the time of the resurvey) from the projected Bio5 (for 2070) at the coldest site in their current range.
We assumed that species are potentially able to survive the minimum change in maximum annual temperatures by either dispersing to higher elevations, tolerating higher temperatures (niche shift), or by doing both simultaneously. The analyses below explore each of these possibilities.
Dispersal.
We assessed whether species are likely to be able to disperse fast enough to avoid extinction within their transects by 2070. First, we estimated the absolute change in the upper limit of the elevational range for each species that expanded its upper elevational range between surveys. To do this, we subtracted the historical maximum elevation of the species’ distribution on the transect (i.e., from the time of the initial survey) from the current maximum record (i.e., resurvey). Then, the rate of upward dispersal was estimated by dividing the absolute change in maximum elevation between surveys by the time between surveys. When surveys were conducted over multiple dates, the time between surveys was calculated based on the earliest historical survey and latest resurvey (details in SI Appendix, Text S1.4).
Next, we estimated the amount of cooling that can potentially be gained from upward dispersal by 2070 (see SI Appendix, Text S1.5.2 for details). Specifically, for each species recorded as dispersing upward in the past (between surveys), we multiplied the upward dispersal rate by the mean change in Bio5 with elevation across the species’ elevational transect (see regressions for each transect in SI Appendix, Table S6), and by the number of years between the year of the modern survey and the future date (2070). The final units for potential dispersal-related cooling are in degrees Celsius.
For each upward-dispersing species (n = 185), we evaluated whether cooling gained through upward dispersal could be as large as the change in Bio5 over time. We focused on two alternative scenarios (SI Appendix, Table S7): an unconstrained scenario and one where the height of each mountain range (on which the survey was performed) constrained the maximum cooling gained through upward dispersal. The latter scenario should be more realistic (13).
Unconstrained Scenario: For each upward-dispersing species, we evaluated whether the cooling gained through recent dispersal (between surveys) was larger than the predicted minimum change in Bio5 by 2070. If the cooling gained through upward dispersal was larger than the predicted minimum change, we considered dispersal to be fast enough for the species to remain in their current niche for Bio5.
Constrained Scenario: For each upward-dispersing species, the maximum cooling gained through dispersal was constrained to be equal to the difference between the current Bio5 at the upper limit of their elevational range and the predicted Bio5 at the mountaintop by 2070. We used Google Earth to obtain the coordinates for each mountaintop, and then obtained Bio5 values for this site using projections for 2070.
Finally, we analyzed the potential for dispersal to allow all species to persist in their current climatic niches (n = 538; SI Appendix, Table S8). We analyzed three scenarios that varied in their assumptions about historically nondispersing species. We performed the same set of analyses summarized above for upward-dispersing species. First, a scenario assuming that species that did not previously disperse upwards (at their upper range limits) will not disperse upwards in the future. Second, we assumed that these nondispersing species would instead move upwards at the mean upward rate across all species that dispersed (including downward dispersal as negative values when calculating the mean). Note that downward dispersal (negative changes in maximum elevation) most likely occurred through range contractions at the upper elevational range edge, but this pattern is clearly inconsistent with upward dispersal. Third, we assumed that these nondispersing species would instead move upwards at the mean upward rate across all species (counting nondispersing species as zero when calculating the mean). Extinction frequencies under each of these scenarios were also calculated under constrained and unconstrained dispersal scenarios (based on species current distances to mountaintops).
Niche shift.
For each species, we first estimated the absolute change in maximum annual temperature (Bio5) between surveys at the warmest site in their range where they occurred in the initial survey. Local extinctions generally occurred at the warmest site in a species range on each transect (for 202 of the 239 species with local extinctions), with extinctions at additional sites (usually adjacent ones) in some cases. We then fit a logistic regression model between the occurrence of local extinction and the absolute change in maximum annual temperature at the warmest site in the species’ historical range (i.e., at the time of the initial survey). This model (odds = 3.517, P < 0.001) was then used to estimate the absolute change in maximum annual temperature at which 50% and 95% of the species are predicted to experience local extinction. The main results used the 95% threshold. The full results are presented in SI Appendix, Table S9 and Dataset S7. We calibrated a binomial assay in the dose.p function from the R package MASS (51). These analyses included all 538 species, regardless of whether they experienced extinction at their warmest site.
Finally, we evaluated whether each species was likely to be able to tolerate the minimum change in maximum annual temperatures (Bio5) across their range by 2070. We assumed that species can tolerate shifts in Bio5 across their range that are below the estimated threshold that generally caused local extinctions. Specifically, we compared each threshold (i.e., 50% vs. 95%) to the minimum change in Bio5 each species is projected to experience in their range on their transect. We considered species likely to persist if the minimum change was below the given threshold generally leading to local extinction. For analyses using the 50% threshold, we assumed that only 50% of the species exposed to temperatures above the threshold temperature would go extinct. For the 95% threshold, we assumed all species would go extinct.
Simultaneous effects of dispersal and niche shifts.
We analyzed the extent to which the combined effects of dispersal and niche shifts can potentially reduce species extinctions within transects. Above, we estimated the minimum change in maximum annual temperatures (Bio5) for each species, the potential decrease in temperature caused by upward dispersal (based on past rates of dispersal), and the change in Bio5 at local sites that is likely to cause local extinction (using the 50% and 95% thresholds). For the final set of analyses, we evaluated whether the minimum change in maximum temperatures that species will experience will be below the threshold for local extinction, after incorporating the potential cooling caused by upward dispersal. Again, when using the 50% threshold we assumed that only 50% of the species exceeding this threshold would go extinct, so we divided the raw extinction frequencies under each climatic scenario (Datasets S8–S10) by two (summarized in SI Appendix, Table S10). Note that these extinction frequencies were estimated only for the set of species that did not disperse quickly enough and that exceeded the 50% temperature threshold. Otherwise, we did not estimate which species would go extinct or persist within this set of species.
We performed three sets of analyses, corresponding to different ways of dealing with the large number of species (n = 252) that failed to disperse upwards between surveys in the past (see above). These are 1) species that did not disperse previously will not disperse in the future; 2) nondispersing species will move upwards at the mean rate across all species that dispersed between surveys in the past; and 3) nondispersing species will move upwards at the mean upward rate estimated across all species. These three sets of analyses were performed using both the 50% and 95% thresholds for local extinction.
Finally, for each of these three dispersal scenarios, we considered dispersal to be constrained by the maximum height of the mountains on which surveys were performed. For this constrained scenario, we assumed that maximum cooling for upward-dispersing species is restricted by the predicted temperatures at the maximum elevation on the mountain range by 2070.
General Methodological Issues.
We address eight methodological issues at length in SI Appendix, Text S2, and we briefly mention them here. Major effects of false local extinction events (species persisting but undetected at a site) and of extinctions unrelated to climate both seem unlikely to have impacted our study. Most local extinctions occurred in the warmest part of each species’ range (as predicted under climate change) and were significantly associated with climatic variables. Effects of land use change also seemed unlikely: Most studies were in protected or undeveloped locations. Those studies in areas impacted by humans addressed and ruled out this issue. We also performed reanalyses showing that this factor does not explain our conclusions (SI Appendix, Text S3). We did not identify proximate mechanisms of extinction, but identifying climatic drivers of local extinction is crucial regardless. Similarly, our study does not identify combined effects of multiple variables on extinction but instead sought the most important predictor(s). We did not identify how climatic drivers might vary across taxonomic groups or regions, given limited sampling within regions and groups. Changing rates of upward dispersal are possible but are unlikely to overturn our conclusions given limited mountain heights and since most species did not disperse upwards at all between surveys. We estimated species-level extinction based only on species’ ranges on these transects, but species’ overall distributions presumably consist of similar elevational ranges across mountains. We focused on terrestrial plants and animals on elevational gradients, but most macroscopic organisms are terrestrial plants and animals, and many of Earth’s most diverse regions (e.g., biodiversity hotspots) are montane regions (31).
Data Availability.
All data are available as Datasets S1–S15, in the Supporting Information and on Dryad (http://datadryad.org/stash/dataset/doi:10.5061/dryad.4tmpg4f5w).
Supplementary Material
Acknowledgments
We thank Shea Lambert and Elizabeth Miller for critical discussions, Emilie Ploquin for providing data, and two anonymous reviewers for many helpful comments. Support was provided by US National Science Foundation Grant DEB 1655690 (to J.J.W.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Data deposition: All data are presently included as Supporting Information and are available on Dryad (http://datadryad.org/stash/dataset/doi:10.5061/dryad.4tmpg4f5w).
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1913007117/-/DCSupplemental.
References
- 1.Thomas C. D., et al. , Extinction risk from climate change. Nature 427, 145–148 (2004). [DOI] [PubMed] [Google Scholar]
- 2.Loarie S. R., et al. , The velocity of climate change. Nature 462, 1052–1055 (2009). [DOI] [PubMed] [Google Scholar]
- 3.Pimm S. L., Climate disruption and biodiversity. Curr. Biol. 19, R595–R601 (2009). [DOI] [PubMed] [Google Scholar]
- 4.Warren R., et al. , Quantifying the benefit of early climate change mitigation in avoiding biodiversity loss. Nat. Clim. Chang. 3, 678–682 (2013). [Google Scholar]
- 5.Urban M. C., Accelerating extinction risk from climate change. Science 348, 571–573 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Warren R., Price J., Graham E., Forstenhaeusler N., VanDerWal J., The projected effect on insects, vertebrates, and plants of limiting global warming to 1.5°C rather than 2°C. Science 360, 791–795 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Soberón J., Grinnellian and Eltonian niches and geographic distributions of species. Ecol. Lett. 10, 1115–1123 (2007). [DOI] [PubMed] [Google Scholar]
- 8.Holt R. D., Bringing the Hutchinsonian niche into the 21st century: Ecological and evolutionary perspectives. Proc. Natl. Acad. Sci. U.S.A. 106 (suppl. 2), 19659–19665 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sinervo B., et al. , Erosion of lizard diversity by climate change and altered thermal niches. Science 328, 894–899 (2010). [DOI] [PubMed] [Google Scholar]
- 10.Cahill A. E., et al. , How does climate change cause extinction? Proc. Biol. Sci. 280, 20121890 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ockendon N., et al. , Mechanisms underpinning climatic impacts on natural populations: Altered species interactions are more important than direct effects. Glob. Change Biol. 20, 2221–2229 (2014). [DOI] [PubMed] [Google Scholar]
- 12.Panetta A. M., Stanton M. L., Harte J., Climate warming drives local extinction: Evidence from observation and experimentation. Sci. Adv. 4, eaaq1819 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freeman B. G., Scholer M. N., Ruiz-Gutierrez V., Fitzpatrick J. W., Climate change causes upslope shifts and mountaintop extirpations in a tropical bird community. Proc. Natl. Acad. Sci. U.S.A. 115, 11982–11987 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schloss C. A., Nuñez T. A., Lawler J. J., Dispersal will limit ability of mammals to track climate change in the Western Hemisphere. Proc. Natl. Acad. Sci. U.S.A. 109, 8606–8611 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corlett R. T., Westcott D. A., Will plant movements keep up with climate change? Trends Ecol. Evol. 28, 482–488 (2013). [DOI] [PubMed] [Google Scholar]
- 16.Jump A. S., Peñuelas J., Running to stand still: adaptation and the response of plants to rapid climate change. Ecol. Lett. 8, 1010–1020 (2005). [DOI] [PubMed] [Google Scholar]
- 17.Quintero I., Wiens J. J., Rates of projected climate change dramatically exceed past rates of climatic niche evolution among vertebrate species. Ecol. Lett. 16, 1095–1103 (2013). [DOI] [PubMed] [Google Scholar]
- 18.Jezkova T., Wiens J. J., Rates of change in climatic niches in plant and animal populations are much slower than projected climate change. Proc. Biol. Sci. 283, 20162104 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holt R. D., The microevolutionary consequences of climate change. Trends Ecol. Evol. 5, 311–315 (1990). [DOI] [PubMed] [Google Scholar]
- 20.Berg M. P., et al. , Adapt or disperse: understanding species persistence in a changing world. Glob. Change Biol. 16, 587–598 (2010). [Google Scholar]
- 21.Hoffmann A. A., Sgrò C. M., Climate change and evolutionary adaptation. Nature 470, 479–485 (2011). [DOI] [PubMed] [Google Scholar]
- 22.Norberg J., Urban M. C., Vellend M., Klausmeier C. A., Loeuille N., Eco-evolutionary responses of biodiversity to climate change. Nat. Clim. Chang. 2, 747 (2012). [Google Scholar]
- 23.Chen I.-C., Hill J. K., Ohlemüller R., Roy D. B., Thomas C. D., Rapid range shifts of species associated with high levels of climate warming. Science 333, 1024–1026 (2011). [DOI] [PubMed] [Google Scholar]
- 24.Parmesan C., Yohe G., A globally coherent fingerprint of climate change impacts across natural systems. Nature 421, 37–42 (2003). [DOI] [PubMed] [Google Scholar]
- 25.Root T. L., et al. , Fingerprints of global warming on wild animals and plants. Nature 421, 57–60 (2003). [DOI] [PubMed] [Google Scholar]
- 26.Moritz C., et al. , Impact of a century of climate change on small-mammal communities in Yosemite National Park, USA. Science 322, 261–264 (2008). [DOI] [PubMed] [Google Scholar]
- 27.Lenoir J., Svenning J.-C., Climate-related range shifts–A global multidimensional synthesis and new research directions. Ecography 38, 15–28 (2014). [Google Scholar]
- 28.Wiens J. J., Climate-related local extinctions are already widespread among plant and animal species. PLoS Biol. 14, e2001104 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jombart T., Devillard S., Balloux F., Discriminant analysis of principal components: a new method for the analysis of genetically structured populations. BMC Genet. 11, 94 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.IPCC , Climate Change 2013: The Physical Science Basis (Cambridge University Press, New York, 2013). [Google Scholar]
- 31.Myers N., Mittermeier R. A., Mittermeier C. G., da Fonseca G. A., Kent J., Biodiversity hotspots for conservation priorities. Nature 403, 853–858 (2000). [DOI] [PubMed] [Google Scholar]
- 32.Bush A., et al. , Incorporating evolutionary adaptation in species distribution modelling reduces projected vulnerability to climate change. Ecol. Lett. 19, 1468–1478 (2016). [DOI] [PubMed] [Google Scholar]
- 33.Razgour O., et al. , Considering adaptive genetic variation in climate change vulnerability assessment reduces species range loss projections. Proc. Natl. Acad. Sci. U.S.A. 116, 10418–10423 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harris I., Jones P., Osborn T., Lister D., Updated high-resolution grids of monthly climatic observations–The CRU TS3.10 Dataset. Int. J. Climatol. 34, 623–642 (2013). [Google Scholar]
- 35.Hijmans R. J., Cameron S. E., Parra J. L., Jones P. G., Jarvis A., Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 25, 1965–1978 (2005). [Google Scholar]
- 36.Fick S. E., Hijmans R. J., WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 37, 4302–4315 (2017). [Google Scholar]
- 37.R Core Team , R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna, 2016). [Google Scholar]
- 38.Kim D., Oh H. S., EMD: A package for empirical mode decomposition and Hilbert spectrum. R J. 1, 40–46 (2009). [Google Scholar]
- 39.Wu Z., Huang N. E., Long S. R., Peng C.-K., On the trend, detrending, and variability of nonlinear and nonstationary time series. Proc. Natl. Acad. Sci. U.S.A. 104, 14889–14894 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O’Donnell M. S., Ignizio D. A., “Bioclimatic predictors for supporting ecological applications in the conterminous United States” (U.S. Geological Survey Data Series 691, 2012).
- 41.Booth T. H., Nix H. A., Busby J. R., Hutchinson M. F., Bioclim: the first species distribution modelling package, its early applications and relevance to most current MaxEnt studies. Divers. Distrib. 20, 1–9 (2013). [Google Scholar]
- 42.Jombart T., adegenet: A R package for the multivariate analysis of genetic markers. Bioinformatics 24, 1403–1405 (2008). [DOI] [PubMed] [Google Scholar]
- 43.Jombart T., Collins C., A Tutorial for Discriminant Analysis of Principal Components (DAPC) Using Adegenet 2.0.0 (Imperial College London, MRC Centre for Outbreak Analysis and Modelling, London, 2015). [Google Scholar]
- 44.Goberville E., Beaugrand G., Hautekèete N.-C., Piquot Y., Luczak C., Uncertainties in the projection of species distributions related to general circulation models. Ecol. Evol. 5, 1100–1116 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Makino A., et al. , The effect of applying alternate IPCC climate scenarios to marine reserve design for range changing species. Conserv. Lett. 8, 320–328 (2014). [Google Scholar]
- 46.García Molinos J., et al. , Climate velocity and the future global redistribution of marine biodiversity. Nat. Clim. Chang. 6, 83–88 (2015). [Google Scholar]
- 47.Wang Z., et al. , Scenario dependence of future changes in climate extremes under 1.5 °C and 2 °C global warming. Sci. Rep. 7, 46432 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dyderski M. K., Paź S., Frelich L. E., Jagodziński A. M., How much does climate change threaten European forest tree species distributions? Glob. Change Biol. 24, 1150–1163 (2018). [DOI] [PubMed] [Google Scholar]
- 49.Raftery A. E., Zimmer A., Frierson D. M. W., Startz R., Liu P., Less than 2°C warming by 2100 unlikely. Nat. Clim. Chang. 7, 637–641 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Christensen P., Gillingham K., Nordhaus W., Uncertainty in forecasts of long-run economic growth. Proc. Natl. Acad. Sci. U.S.A. 115, 5409–5414 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Venables W. N., Ripley B. D., Modern Applied Statistics with S (Springer, New York, 2002). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available as Datasets S1–S15, in the Supporting Information and on Dryad (http://datadryad.org/stash/dataset/doi:10.5061/dryad.4tmpg4f5w).