Abstract
The T cell co-stimulatory molecule OX40 and its cognate ligand OX40L have attracted broad research interest as a therapeutic target in T cell-mediated diseases. Accumulating preclinical evidence highlights the therapeutic efficacy of both agonist and blockade of the OX40–OX40L interaction. Despite this progress, many questions about the immuno-modulator roles of OX40 on T cell function remain unanswered. In this review we summarize the impact of the OX40–OX40L interaction on T cell subsets, including Th1, Th2, Th9, Th17, Th22, Treg, Tfh, and CD8+ T cells, to gain a comprehensive understanding of anti-OX40 mAb-based therapies. The potential therapeutic application of the OX40–OX40L interaction in autoimmunity diseases and cancer immunotherapy are further discussed; OX40–OX40L blockade may ameliorate autoantigen-specific T cell responses and reduce immune activity in autoimmunity diseases. We also explore the rationale of targeting OX40–OX40L interactions in cancer immunotherapy. Ligation of OX40 with targeted agonist anti-OX40 mAbs conveys activating signals to T cells. When combined with other therapeutic treatments, such as anti-PD-1 or anti-CTLA-4 blockade, cytokines, chemotherapy, or radiotherapy, the anti-tumor activity of agonist anti-OX40 treatment will be further enhanced. These data collectively suggest great potential for OX40-mediated therapies.
Key words: OX40, CD4+, CD8+, Treg, Autoimmunity diseases, Cancer immunotherapy
Graphical abstract
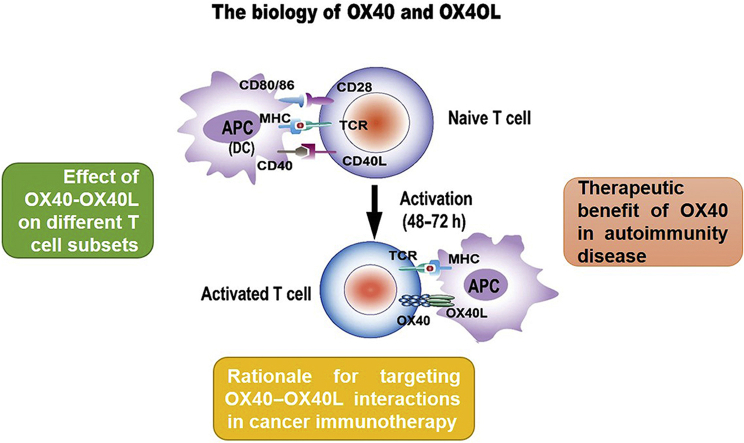
This review discusses the biology of OX40 and OX40L and summarizes the impact of the OX40–OX40L interaction on T cell subsets to provide a comprehensive understanding of anti-OX40 mAbs-based therapy. The potential therapeutic application of OX40–OX40L interaction in autoimmunity diseases and cancer immunotherapy are also discussed.
1. Introduction
The immune system is finely-tuned with various highly differentiated immune cells and organs working together. As one of the major types of lymphocytes, T cells play essential roles in different stages of immune responses. Naïve T cells can quickly proliferate and differentiate into heterogeneous effector T cells after encountering antigens through particular cytokine-environment stimulation. T cells are classified into CD4+ T cells and CD8+ T cells according to the glycoprotein types on their membrane surface. CD4+ T cells, which are also called Th (T helper) cells, can differentiate into different subsets according to the cytokine profiles1. The widely studied subsets of CD4+ T cells are Th1, Th2, Th9, Th17, Th22, Treg (regulatory T cells), and Tfh (follicular helper T cells)1. CD8+ T cells, known as cytotoxic T lymphocytes, play important roles in immune defense against intracellular pathogens or infected cells. They engage and kill their target through several mechanisms, such as secreting cytokines such as tumor necrosis factor-α (TNF-α) and interferon-γ (INF-γ), releasing cytotoxic granules such as perforin and granzymes, and initiating FAS/FASL interaction.
In addition to antigen stimulation through T cell receptors (TCR) and cytokine receptors, T cell activation also requires costimulatory molecular signals, such as constitutive CD27 or CD28 and inductive glucocorticoid-induced tumor necrosis factor receptor (GITR), and induced costimulatory molecules (ICOS) such as OX40 or 4-1BB2. Among those costimulatory molecules, OX40 is widely studied for its beneficial functions. Ligation OX40 signals with agonist anti-OX40 mAbs to convey activating signals to T cells, resulting in cytokine production, survival, expansion, and homeostasis of effector or memory T cells3, 4. OX40 is a member of the tumor necrosis factor receptor (TNFR) family, and it is also known as CD134 or TNFRSF44. OX40 was first discovered on the surface of activated rat CD4+ T cells in 1987. Subsequent studies revealed that OX40 was expressed on both activated CD4+ and CD8+ T cells, neutrophils, and nature killer (NK) cells. OX40 has important co-stimulatory functions during T cell activation, mediating the survival and expansion of both CD4+ and CD8+ T cells in multiple animal models of autoimmunity, infectious disease, and cancer5. OX40 is also involved in controlling effector and memory T cell responses4.
The mechanism by which OX40–OX40L signaling affects T cell function is complicated. Under certain conditions OX40 can strongly promote the induction of Th1, Th2, and Th9 cells, and under other conditions it can inhibit the generation of forkhead box p3 (Foxp3) Tregs6. Previous studies showed that OX40–OX40L signals can augment the immune response in T cells subsets through NF-κB, mitogen-activated protein kinase (MAPK), nuclear factor of activated T cells (NFAT), and BCL-2/XL-dependent anti-apoptotic pathways7, 8. These signaling pathways jointly contribute to the expression of pro-survival molecules and elevated cytokine production and augment the generation of both effector and memory T cell subsets5. Some reports focusing on the immunity role of OX40–OX40L signals on T cell biology considered the CD4+ T cells as a whole9, leaving details of the OX40 influence on different T cell subsets neglected. Here in this review we first discuss the biology of OX40 and OX4OL and then present the detailed impact of OX40–OX40L signals on T cell subsets, including Th1, Th2, Th9, Th17, Th22, Treg, Tfh, and CD8+ T cells, to provide a comprehensive understanding of its biology.
As a crucial T cell activation stimulator, either activation or blockade of OX40–OX40L signals can yield therapeutic efficacy. On one hand, blocking the OX40–OX40L pathway using OX40 or OX40L binding agents seems to inhibit autoreactive T cells and ameliorate autoimmunity disease, as in experimental autoimmune encephalomyelitis (EAE) models8. On the other hand, augmentation of OX40 signaling using OX40 agonist antibodies appears to enhance T cell-mediated anti-tumor immunity, contributing to tumor regression and prolonged survival. Here, OX40 agonists may increase T cell infiltration into tumors and enhance antitumor immune responses in conventional CD4+ and CD8+ T cells, thus improving survival rates in several preclinical cancer models, including B16 melanoma, Lewis lung carcinoma, colon cancer, breast cancer and 4T-1 breast cancer10, 11. Therefore, agonists of the OX40–OX40L pathway potentially can be adopted for cancer immunotherapy.
2. The biology of OX40 and OX40L
OX40 is a type 1 transmembrane glycoprotein composed of about 275 amino acids with three full cysteine-rich domains (CRDs) and a partial C-terminal CRDs7. The apparent molecular weight of OX40 is 50 kDa5. OX40, along with other TNFRSF members such as 4-1BB, CD27, CD30, and CD40, are T cell costimulatory molecules acting at different stages to modulate and control the immune response6. Unlike other constitutive T cell costimulatory receptors, such as CD28 and CD27, OX40 is not expressed on naïve T cells. OX40 is transiently induced by signaling following TCR engagement after antigen (Ag) recognition, peaking 48–72 h in activated T cells, including activated CD4+ and CD8+ T cells, as well as in neutrophils, natural killer cells (NK) and natural killer T cells (NKTs)5. The only exception is that OX40 is constitutive in mouse FOXP3+ Treg cells. Many factors are involved in the kinetics of OX40 expression, including the cytokine milieu, the persistence of antigen, the inflammatory environment, and the influence of other co-stimulation pathways5. OX40-deficient T cells might normally proliferate and differentiate into effector T cells at 2–3 days after TCR engagement, but they showed notable reduction of survival after 12–13 days12, 13. That is, OX40 signaling may not affect the initial activation phase of T cells, but may play an essential role in maintaining late proliferation and survival in the T cell effector phase.
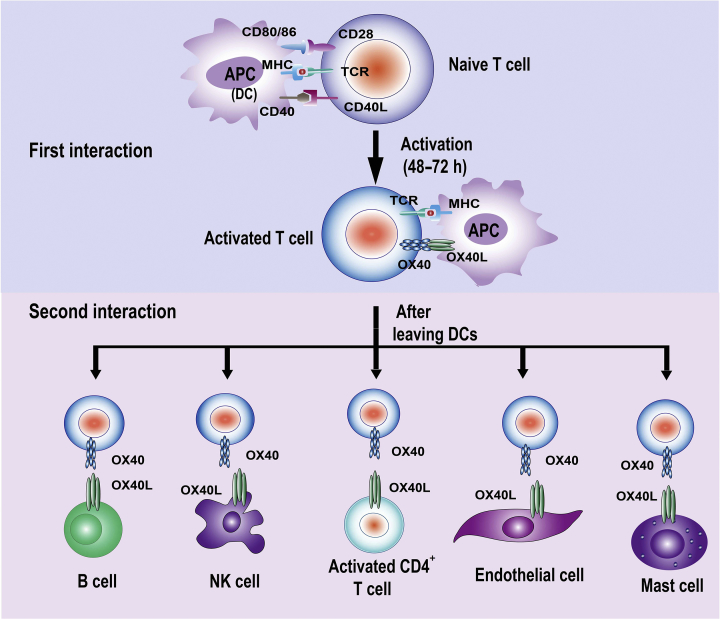
The ligand for OX40 (OX40L, also known as CD252), is also a member of the TNFR superfamily. OX40L is a type II glycoprotein with a 23-amino-acid cytoplasmic tail and a 133-amino-acid extracellular domain7. Murine OX40L can stimulate both human and mouse T cells; however, human OX40L can only stimulate human T cells14. OX40L is mainly expressed on activated antigen-presenting cells (APCs), such as dendritic cells (DCs), activated B cells, and macrophages. The types of cells that can be induced to express OX40L is much broader. Apart from APCs, OX40L also expressed on hematopoietic cells such as activated NK cells, mast cells or the responding CD4+ T cells, and non-hematopoietic cells, such as endothelial cells or smooth muscle cells (summarized in Table 1)4, which provides the foundation for the two-step OX40–OX40L costimulation model (Fig. 1).
Table 1.
A brief summary of the expression of OX40 or OX40L on cells.
| Molecule | Expression level | Expressed by |
|---|---|---|
| OX40 | Inducible | CD4+ and CD8+ T cells Neutrophils, NK cells, NKT cells Mouse Treg cells (constitutive expression) |
| OX40L | Inducible | DCs, macrophages, B cells NK, mast, activated T cells smooth muscle, endothelial cells |
Figure 1.
Schematic diagram of the two-step OX40L costimulation model. The OX40 signals to activated OX40-expressing T cells are first provided by professional APCs during 48–72 h after antigen recognition. After the first interaction the OX40-expressing T cells may leave DCs and interact with other OX40L-expressing cells during the effector phase, such as B cells, nature killer (NK) cells, activated CD4+ T cells, endothelial cells, or mast cells, which results in the multi-channel activation of T cells through OX40–OX40L signaling.
More specifically, the first step of OX40–OX40L signaling is the interaction between OX40 and OX40L after 2–3 days, when activated T cells bind to professional APCs, mainly DCs, since OX40L expression on the APC surface is inducible 1–3 days following initial antigen encounter. After that, the OX40-expressing activated T cells can encounter other OX40L-expressing cells during the effector phase after leaving the initial DCs. For example, OX40L expressed by CD4+ T cells can also bind to OX40 on other CD4+ T cells to enhance T cell survival15. OX40L+ B cells16, OX40L+ NK cells, OX40L+ mast cells17 and OX40L+ endothelial cells can interact with OX40+-activated T cells to support T-cell function during the effector phase, which may result in multi-channel activation of T cells.
3. Effect of the OX40–OX40L interaction on different T cell subsets
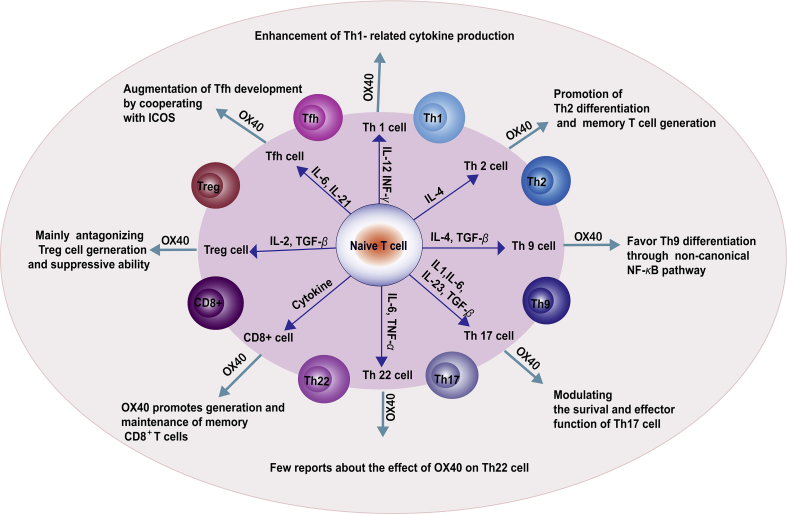
As for the impact of the OX40–OX40L interaction on T cell subsets, Table 2 displays a brief summary and is also illustrated in Fig. 2. Regarding CD4+ T cell subsets, OX40–OX40L signals can enhance the Th1-mediated immune response, promote the generation and maintenance of Th2, favor Th9 differentiation through the non-canonical NF-κB pathway, augment Tfh development, and antagonize Treg generation and Treg-mediated suppression. As for CD8+ T cell subsets, OX40 promotes the survival and expansion of CD8+ T cells and the recall response of CD8+ memory T cells in vivo. Nevertheless, studies on the effect of OX40 on Th22 are relatively rare at present.
Table 2.
Effect of OX40 on different T cell subsets.
| T cell | The impact of OX40 stimulatory signal | Condition | Ref. |
|---|---|---|---|
| CD4+ | Inhibiting FOXP3 expression and Tregs induction via AKT–mTOR pathway | In vitro | 18 |
| Enhancement of helper CD4 T cell activity and humoral immunity | Rodent malaria | 19 | |
| Triggering larger memory Th1 and Tfh CD4 T cell | Plasmodium infections | 20 | |
| Promoting CD4 T cell proliferation and survival | Graves' disease | 21 | |
| Inducing splenic CD4 T cell activation, and splenic Tfh cell accumulation | Systemic lupus erythematosus | 22 | |
| Promoting Th1 cell differentiation and proliferation, and attenuating Treg suppressive activity | Obesity mice | 23 | |
| Orientation of CD4+ cells toward Th1 responses | Glioma-bearing mice | 24 | |
| Enhancement of tumoral CD4+ effector T cell responses | In vitro | 25 | |
| OX40-deficient mice are reduction of both Th1 and Th2 cytokines | In vivo | 26 | |
| Promoting IL-17 production | Rheumatoid synovium | 27 | |
| Th2 | Contribution of OX40L to the development of Th2-mediated pulmonary inflammation | Murine model of asthma | 28 |
| Th9 | Induction of Th9 cells | Airway inflammation | 29 |
| Th17 | Inhibiting IL-17 expression and Th17 cell-mediated autoimmunity | In vitro | 30 |
| Augmentating Th17 cytokine expression | Uveitis | 31 | |
| Tfh | Promoting BLIMP-1 expression and diverting cells away from Tfh cell differentiation | Lymphocytic choriomeningitis virus | 32 |
| Promoting the differentiation of human Th cells toward the Tfh lineage | Systemic lupus erythematosus | 33 | |
| Amplification of Tfh cell development cooperating with ICOS | Vaccinia virus infection | 34 | |
| Treg | OX40 expression in Tregs was greater than in conventional CD4 and CD8 T cells | Head and neck cancer | 35 |
| Reversing the suppressive effects of Tregs | Cutaneous squamous cell carcinoma | 25 | |
| Blockade of OX40L decreased Tregs proliferation | Crescentic glomerulonephritis | 36 | |
| Treg cells suppress mast cell degranulation through OX40–OX40L interaction | Allergies | 37 | |
| Induction of Treg activation and their suppressive function | In vitro | 38 | |
| Supporting Treg cell development, homeostasis, and suppressive activity | Inflammatory bowel disease mice model | 39 | |
| Blocking Tregs inhibitory activity, and restored effector T-cell proliferation | Graft-versus-host disease mice model | 40 | |
| Blocking inducible and natural regulatory T cell function | Human OX40 antibodies | 41 | |
| Tregs | OX40 inhibits TGF-β- and antigen-driven conversion of naive CD4 T cells into CD25+FOXP3+ T cells | In vitro | 42 |
| OX40 ligand shuts down IL-10-producing Treg cells | In vitro | 43 | |
| OX40 costimulation turns off FOXP3+Tregs | In vitro | 44 | |
| OX40 expression by T reg cells was indispensable for suppression colitogenic T cell responses | Mouse models of colitis | 45 | |
| CD8+ | OX40 can control survival of primed CD8 T cells | Adoptive T cell transfer | 46 |
| OX40 supports CD8 T cells expansion and confer CTL-mediated protection against tumor growth. | Adoptive transfer of CD8 cells | 47 | |
| Cooperation between CD4 and CD8 T cells for antitumor activity is enhanced by OX40 signals | Adoptive transfer of CD8 cells | 48 | |
| OX40-deficient mice are competent in generating B cell and CTL responses after virus infection | LCMV and influenza virus | 49 | |
| OX40L costimulated memory CD8 T cell responses largely through indirect effects | In vitro | 50 | |
| OX40 signals directly augment activation, cytokine secretion, proliferation of human CD8+ T cells | In vitro | 51 | |
| Synergy with anti-PD-L1 in the initial reversal of CD8+ T cell exhaustion | In vitro | 52 | |
| OX40 agonism enhance CD8+ memory | In vitro | 53 | |
| CD4−CD8− | Control CD4–CD8– survival by regulation on BCL-2, BCL-XL, and BCL2L11 | In vitro | 54 |
| CD4+CD28− | An alternative costimulator of CD4+CD28− T cells | Autoimmune arthritis | 55 |
Figure 2.
The summary of the impact of the OX40–OX40L interaction on T cell subsets. OX40–OX40L signals can enhance the Th1-mediated immune response, promote generation and maintenance of Th2, favor Th9 differentiation through the non-canonical NF-κB pathway, augment Tfh development, and antagonize Treg cell generation and Treg-mediated suppression. And as for CD8+ T cell subsets, OX40 promotes the survival and expansion of CD8+ T cells and the recall response of CD8+ memory T cells in vivo.
3.1. OX40 enhances the Th1-mediated immune response
T helper 1 (Th1) cells are characterized by the secretion of interferon-γ (IFN-γ), as well as a series of other cytokines including tumor necrosis factor alpha (TNF-α), interleukin-2 (IL-2), granulocyte-macrophage colony-stimulating factor (GM-CSF) and lymphotoxin1. Th1 cells are mainly implicated in cell-mediated antiviral and antimicrobial immunity to intracellular pathogens. As a matter of fact, the effect of Th1 cells on immunology is pleiotropic and the multitude of functions of Th1 is expanding. An excessive Th1 response will promote immunopathology and exacerbate autoimmunity by enhancing the production of pathogenic autoantibodies. However, deficiency in Th1 function would be associated with immunodeficiency.
Since OX40- or OX40L-deficient animals have shown reductions in both Th1 and Th2 cytokine responses in vivo, the role of OX40–OX40L interactions in regulating Th1 responses is unclear. In naive CD4+ T cells, OX40–OX40L signals bias cytokine interleukin-4 (IL-4) production toward T-helper (Th) cell 2 (Th2) generation. With the help of antigen or IL-12 priming, OX40–OX40L signals may overcome this Th2 directive action and regulate the differentiation of CD4+ T cells into Th1 cells56. It also has been demonstrated that in the absence of adjuvant, OX40–OX40L interaction is capable of inducing a Th2 response, while in the presence of adjuvants both Th1 and Th2 responses are augmented. In an OX40L-deficient murine model of allergic asthma, Arestides et al.57 found that blockade of OX40–OX40L signaling failed to support potent CD4 T cell responses, and the levels of allergen-induced Th1 as well as Th2 cytokines were significantly reduced. Thus, OX40 appears to be involved in a variety of mechanisms to control the priming of Th2 and Th1 responses, which appears dependent on the experimental conditions adopted. And engagement of OX40 with OX40L may deliver a strong co-stimulatory signal to newly activated effector T-cells and enhance both Th1 and Th2 responses7.
While further studies proved that OX40–OX40L interactions exerted critical costimulatory effects on the Th1-mediated immune response and contributed to immunopathology in a variety of animal models58 including experimental inflammatory bowel disease59, EAE60, rheumatoid arthritis (RA)61, colitis62 and graft-versus-host-disease. Blockade of OX40–OX40L interactions ameliorated these Th1-specific immune diseases. Using a myelin oligodendrocyte glycoprotein peptide (MOG)-induced EAE murine model, Ndhlovu, et al.60 showed that the severity of EAE was either aggravated or alleviated depending on the expression of OX40L. OX40L-deficient mice or wild-type mice treated with anti-OX40L mAb (MGP-34) showed reduced clinical manifestations of EAE coupled with a reduction in Th1 cytokine production, including IFN-γ and IL-260. Conversely, transgenic overexpression of OX40L in mice lead to a greater severity of EAE with significantly elevated levels of IL-2, IL-6, and IFN-γ60. Although these altered Th1 cytokine responses may have resulted from the inability of differentiation of both Th1 and Th2 effectors; noteworthy, however, is the recognition that EAE is usually regarded as a Th1-mediated disease and OX40–OX40L interactions were indeed involved in the Th1-mediated immune responses.
3.2. OX40 promotes generation and maintenance of Th2
Th2 cells are recognized for the secretion of IL-4, IL-5 and IL-13. Th2 cells are typically involved in immunity defense against extracellular pathogens and multi-cellular parasites, allergies and atopic illnesses1, 63. Th2 cells can exert anti-inflammatory ability by suppressing cell-mediated or Th1-mediated inflammation, since IL-4 possesses strong suppression of Th1 cell generation even in the presence of IFN-γ. However, Th2 cells also play a role in several humorally-associated autoimmune diseases, in that aberrant IL-4 expression can induce activation of autoreactive B cells and thereby promote tissue inflammation and immunopathology.
Under neutral conditions the OX40–OX40L interaction preferentially promotes CD4+ T cells to differentiate into Th2 effector cells through enhanced calcium/nuclear factor-activated T cell (NFATc) signals and up-regulated IL-4 production. A major role for the OX40–OX40L interaction in Th2-mediated allergic asthma64 and Leishmania infection murine models has been substantiated65. An impaired ability to generate a Th2 response in an OX40-deficient murine asthma model has also been demonstrated, and was characterized by high levels of immunoglobulin E, IL-5 and IL-4 due to a reduced number of antigen-specific memory T cells, thus resulting in diminished lung inflammation and attenuated airway hyperreactivity64. On the other hand, Hoshino et al.28 demonstrated a critical contribution of OX40L to the development of Th2-mediated experimental leishmaniasis and pulmonary inflammation. In an OX40L-deficient murine asthma model, all asthmatic responses including increased mucus production, accumulation of eosinophils, and high levels of Th2 cytokines were greatly diminished28. Administration of the neutralizing anti-OX40L mAb to wild-type asthmatic mice also abolished the induction of asthmatic responses during the sensitization period, indicating a pathogenic role of OX40L in differentiation of Th2 cells and in the pathway of Th2 polarization in vivo28. In accordance with several reports, in the absence of OX40–OX40L signals Th2 cells are impaired in clonal expansion resulting in a quite low level of memory T cells, suggesting that the OX40–OX40L interaction was indispensable in Th2 cell differentiation and Th2 polarization66, 67. What's more, the OX40–OX40L interaction was also indispensable in Th2 memory T cell generation and memory recall responses by sustaining expression of the antiapoptotic members of BCL-2 family, such as BCL-2, BCL-XL, or BFL166, 67.
3.3. OX40 favors Th9 differentiation through a non-canonical NF-κB pathway
Th9 cells, preferentially producing IL-9, are a novel CD4+ T cell subset in the immune system with proinflammatory functions1. Th9 cells are featured with potent anticancer ability, as well as properties to promote the development of autoimmune and allergic diseases1. Th9 cells are closely associated with Th2 cells, since the Th2-produced cytokine IL-4 provides one of the key signals for Th9 induction. However, even under optimal polarizing conditions in vitro, the differentiation frequency of Th9 cells is quite low. Their activation not only requires integration of signals from TGF-β and IL-4 cytokines but also needs the combined stimulation of costimulatory molecules, such as OX40.
OX40 ligation on activated CD4+ T cells showed great potency in promoting Th9 cell induction, converting a large number of CD4+ Tconv cells into Th9 cells in vitro, thus emphasizing the importance of OX40–OX40L costimulation67. OX40-mediated Th9 cell enhancement has repercussions in vivo, including contributions to antitumor immunity or regulation of immunopathology in allergic lung and ocular inflammation67. In an allergic murine model, injection of agonist anti-OX40 antibody induced Th9 cell-dependent allergic airway inflammation and increased hyper-proliferation of mucin-producing cells in the airway epithelium67. There was an absence of any IL-9 enhancement in OX40L-deficient T cells, indicating the specific stimulation through the OX40–OX40L pathway.
In addition, Th9 cells induced by OX40 costimulation showed very high expression of IL-9 without expression of Th2 or Th17 cytokines, such as IL-10, which seemed to be distinct from Th9 cells without OX40 ligation68. Subsequent study revealed that the promotion of Th9 through OX40–OX40L signals didn't depend on PU.1, which was a key transcriptional regulator for the induction of Th9, but instead through tumor necrosis factor receptor-associated factor (TRAF) adaptor molecules, particularly TRAF6, which activated an NF-κB transcription factor complex and triggered a wide array of downstream biological functions68. OX40 costimulation could still increase IL-9-producing T cell differentiation under repression of the canonical NF-κB pathway, while mice lacking the non-canonical NF-κB subunit p52 were unresponsive to OX40 L68. Although OX40–OX40L signals can activate both the canonical and non-canonical NF-κB pathway, OX40 may act through the non-canonical NF-κB pathway to favor the potentiation of Th9 differentiation.
3.4. OX40 modulates the survival and function of Th17
Th17 cells are a newly emerged effector T helper cell subset. They can reinforce certain aspects of the adaptive immunity system such as host defense and tissue repair responses. They also can induce local tissue inflammation and promote autoimmune tissue injury via the secretion IL-17 family cytokines (mainly IL17). IL-17 signals can contribute to activation of innate immune cells, enhancement of B cell responses, recruitment of neutrophils, up-regulation of proinflammatory mediators such as TNF-α, IL-1β or intercellular adhesion molecule 1 (ICAM-1)1. In addition to IL-17, Th17 cells can also secrete IL-21, IL-22, IL-251. Th17 cells also possess anti-inflammatory ability through the production of the potent anti-inflammatory cytokines IFN-γ and IL-10, thereby attenuating inflammation and pathology.
Accumulated evidence suggests that OX40 is a crucial co-stimulatory molecule involved in modulating the survival and function of Th17 cell27. In a mouse model of destructive arthritis deficient in IL-1 receptor, the authors showed that IL-17 did not induce OX40 expression, while activation of T cells through OX40 ligation enhanced IL-17 production, and blocking the OX40–OX40L pathway efficiently repressed the development of inflammatory peripheral arthritis, which could be at least partially linked to a significant reduction of IL-17 from Th17 cells in the peripheral synovial joints69. In an ovalbumin-induced uveitis model, Zhang, et al.31 demonstrated that OX40-activating antibody significantly augmented the transfer of OX40-stimulated lymphocytes and elicited a more severe ocular inflammation in vivo, while an IL-17-neutralizing antibody attenuated OX40-mediated uveitis.
However, other studies also reported completely opposite results, with ligation of OX40 with OX40L on human T cells inhibiting IL-17 production, which was mediated through up-regulation of IFN-γ and IL-4, both of which are reported to inhibit the production of IL-1770. Moreover, OX40L still suppressed IL-17 production even in the presence of IL-23, which is a potent stimulator of IL-17 and differentiation factor for Th17 cells70. Therefore, the OX40–OX40L pathway plays a crucial role for enhancing the elaboration of Th17 cells, which may be partially dependent on the conditions.
3.5. Limited studies of the effect of OX40 on Th22
Th22 cells play a complicated role in inflammatory and autoimmune disease. Th22 cells produce predominantly IL-22 cytokine71, 72; IL-22 seems to possess both pathogenic and protective effects according to environmental cues71. On one hand, IL-22 can promote inflammatory and autoimmune conditions in psoriasis, rheumatoid arthritis, Crohn's disease, and atopic dermatitis patients, suggesting its pathogenic role. On the other hand, IL-22 was down-regulated in the serum of patients with sarcoidosis and systemic lupus erythematosus71. However, there is little known about the effect of OX40–OX40L signals on Th22 cells. The influence of OX40–OX40L signals on Th22 cells, and the relationship between Th22 cells and other Th subsets, particularly Th17 cells, needs further investigation.
3.6. OX40 augments Tfh development
Follicular helper T (Tfh) cells are firstly recognized by their residence in B cell secondary lymphoid tissues areas. The anatomical location of Tfh cells allows them to favor the function of B cells, and the formation of germinal centers (GC). Many identifying molecules are involved in those functions, such as CXC chemokine receptor 5 (CXCR5), IL-21 and ICOS. Tfh cells can produce IL-21, and IL-21 serves as a “helper” cytokine to stimulate B cells through interacting with IL-21R. Due to the special role of Tfh cells, disruption of Tfh cell function may result in pathogenesis of either immune deficiencies or autoimmune diseases.
Many studies have explored the possibility that OX40–OX40L signals might have any impact on Tfh development and GC responses. OX40–OX40L signals have been found to promote expression of CXCR5 mRNA in activated mouse T cells in vitro. Soluble OX40L treatment increased the expression of several Tfh-associated molecules on T cells in lupus patients in vitro, including CXCR5, BCL-6 and IL-21. These studies also showed that OX40–OX40L interactions promoted CD4 T cells to accumulate at the T/B border and in B cell follicles in murine models73. There was a reduction of antibody production in OX40L-transgenic mice and mice lacking OX40 or OX40 L73. Based on those findings, it was concluded that OX40–OX40L signals indeed exert an effect on the maintenance of the Tfh function.
Using a vaccinia virus (VacV) infection murine model, Tahiliani et al.34 showed that the number of Tfh cells was about 70% fewer in OX40-deficient mice compared to WT mice on Day 8–15 after VacV infection. A similar decrease was also found in the level of VacV-specific IgG Abs. This study also demonstrated that OX40–OX40L interactions were involved in maintaining the Tfh and GC Tfh pool after the Tfh phenotype was initially acquired. So whether OX40 is expressed on transitional Tfh cells or on mature Tfh cells, it always can play a strong role to maximize the Tfh response. OX40–OX40L interactions were found to co-operate with ICOS–ICOSL interactions to maintain the Tfh response and amplify the ongoing Ab response at a late stage after infection34. Since OX40 was co-expressed with ICOS on both transitional and mature Tfh cells, it may be active in controlling Tfh cells at the same time.
In another study, OX40–OX40L signals contributed to an aberrant Tfh cell response in human systemic lupus erythematosus (SLE)33. OX40–OX40L signals promoted the expression of multiple Tfh-related molecules including CXCR5, IL-21 and CD40L in both naive and memory Tfh cells, and subsequently increased the generation of CXCR5+ Tfh cells while downregulating the transcription repressor PR domain zinc finger protein 1 (PRDM1) which inhibits Tfh generation33. OX40L–OX40 signals were sufficient to render both naive and memory Tfh cells to become efficient B cell helpers, mainly mediated by TCR signal enhancement. One of the hallmarks of autoimmune diseases like SLE is autoreactive antibody production. This may suggest that the pathogenic contribution of Tfh cells in human SLE is via the OX40L–OX40 pathway, thus providing a rationale to target this pathway as a therapeutic modality.
However, there also are some contradictory results on the OX40–OX40L interaction in the regulation of Tfh cell responses. This study showed that the absence of OX40 signals did not affect Tfh differentiation, antibody generation, or even the CXCR5 expression74. Furthermore, Boettler et al.32 showed that agonistic anti-OX40 mAb suppressed the expression of Bcl-6 while enhancing the expression of the Tfh-specific molecule BLIMP-1. As for the dual roles of OX40, one possible reason is that OX40 signals may collaborate with other factors to regulate Tfh differentiation, such as the microenvironment, the APCs, and Th cells themselves.
3.7. OX40 mainly antagonizes Treg-mediated suppression
To prevent autoreactivity, immune responses need to be properly controlled, which is partially dependent on the suppressive ability of Treg cells75. Treg cells are characterized by the expression of FOXP3, CD4, and CD25, and they are composed of two major subtypes, natural thymic derived Tregs (nTregs) and inducible Tregs (iTregs) post thymic maturation. The most commonly associated cytokines with Tregs are transforming growth factor-β (TGF-β) and IL-10. Generally, Treg cells are immunosuppressive and responsible for maintaining tolerance to self-antigens. Treg cells are also known to act as immunoregulators in many inflammatory and autoimmune disease by modulating anti-inflammatory cytokines secretion75.
In physiological immune responses, OX40 supports the survival and proliferation of activated CD4+ T cells. It has been proposed that the potent costimulatory effect of OX40 is due to the blocking of the inhibitory activity of Tregs, although it could also be explained by the stimulatory effect on effector T cells in enhancing their resistant to suppression. Over last half-decade evidence has accumulated that OX40 signals are critical for controlling the function of FOXP3+ Treg T cells. OX40 may act as negative regulator of Treg function, which indirectly boosts effector CD4+ T cells by relieving them from Treg cell-mediated suppression. OX40 signals can counteract Treg-mediated suppression when they are delivered directly to antigen-engaged naive T cells in vivo39.
OX40 is constitutively expressed in mouse Treg cells, and rapidly induced in human Treg cells. T cells were converted into FOXP3+ cells to a greater extent when OX40 was not expressed and, accordingly, an agonist antibody to OX40 also markedly suppressed CD4+ T cell differentiation into FOXP3+ iTregs even in presence of TGF-β42. In addition to antagonizing inducible Treg generation, some studies revealed that OX40 also blocks natural Treg activity in certain situations40. Voo et al.41 developed an anti-human OX40 agonistic mouse mAb that could promote effector CD4+ and CD8+ T cell proliferation while blocking natural Treg-suppressive function. However, Vu et al.44 pointed out that OX40 stimulation did not significantly affect naturally arising CD4+FOXP3+ Tregs, but strongly antagonized the induction of new inducible FOXP3+ Tregs from T effector cells.
Ligation of OX40 on the FOXP3+ Treg cells abrogated their ability to suppress T effector cell proliferation, IFN-γ production, and T effector cell-mediated allograft rejection. Triggering the OX40–OX40L interaction has also been proven to shut down the generation of IL-10-producing type 1 Tregs (Tr1)43, turn off FOXP3+ Treg proliferation44, and inhibit TGF-β- and antigen-driven conversion of naive CD4+ T cells into CD25+FOXP3+ T cells42. Valzasina et al.40 found that agonist anti-OX40 mAb could abrogate Treg-mediated suppression of T-cell proliferation, and restored IL-2 gene transcription, cytokine production, and effector T cell proliferation in a model of graft versus host disease in vivo. Many studies have tried to identify the mechanisms by which OX40 costimulation suppresses Foxp3 expression and iTreg induction8. Possible pathways were through phosphorylation and nuclear exclusion of FOXO1/38. Moreover, OX40 can upregulate basic leucine zipper ATF-like transcription factor 3 (BATF3) in activated CD4+ T cells, which produced a closed chromatin configuration to repress Foxp3 expression in a SIRT1/7-dependent manner18.
Although the OX40–OX40L interaction potently counteracts Treg-mediated suppression in multiple experimental settings, paradoxically, some reports claimed that the OX40–OX40L axis may play a necessary role in the homeostasis of Treg cells, and OX40 agonists can enhance Treg proliferation and potentiate Treg cell suppressive activity. mRNA profiling experiments revealed that compared to non-regulatory T cells, CD25+CD4+ Treg cells had high expression of OX4076. They also reported a reduced number of Treg cells in the spleen of young OX40-deficient mice while there was an elevated number in the thymus of mice that overexpress OX40L, suggesting that abnormal OX40–OX40L signals would disturb Treg cells development76. Griseri et al.45 reported that OX40 was required by Treg cells for their accumulation in the colon under steady-state conditions in an adoptive T cell transfer model of colitis. While under inflammatory conditions, OX40-deficient Treg cells showed reduced accumulation in the colon and peripheral lymphoid organs, resulting in their inability to keep pace with the effector response, indicating that OX40 delivers an important survival signal to Treg cells45.
Consistent with those results, Ikuo Takeda et al. found that Treg cells from OX40L-over-expressed mice showed more effective suppression than those from wild-type mice in vitro, suggesting that the suppressive function of Treg cells could be potentiated by OX40–OX40L interactions39. Valzasina et al.40 did not observe differences in Treg cell number in the OX40-deficient mice described by Takeda et al.39 They speculated that it was possibly obscured by the genetic background difference or environmental factors. OX40-deficient mice only showed a reduced number of Treg cells in their early life, implying that OX40 is either augmented or unnecessary in the homeostasis of Treg cells39, 77.
3.8. OX40 promotes the generation and maintenance of memory CD8+ T cells
CD8+ T cells play an important role in eradication of viruses, intracellular parasites, and suppression of tumors. To differentiate into effector cytotoxic T cells (CTL), CD8+ T cells require both antigenic stimulation and costimulatory signals. In addition to CD28, TNF receptor superfamily molecules also are involved in mediating costimulatory signals for proliferation and differentiation of CD8+ T cells78.
OX40–OX40L signals were initially found to be dispensable in CD8+ T cell functions in a viral-infection murine model49. Although naive CD8+ T cells can initially differentiate into CTL independently of OX40–OX40L interactions in vivo, OX40-deficient CD8+ cells were not maintained at high numbers after they initially expanded. There was a significantly reduced accumulation of CTLs at the peak of primary responses, thus emphasizing the critical involvement of OX4047. Additionally, OX40-deficient CD8+ T cell expansion was impaired in response to antigen in several models, including contact hypersensitivity reactions and allograft rejection26.
Considering that OX40L-deficient mice or agonist anti-OX40 mAb can also influence the function of non-CD8+ T cells, it was doubted whether OX40 signals could influence CD8+ T cell functions directly. Lena Serghides et al. investigated the co-stimulatory effects of OX40L with B7.1 and 4-1BBL50. OX40L co-stimulation increased numbers of antiviral memory CD8+ T cells when cultured with total T cells. However, when cultured with purified CD8+ T cells, OX40L barely activated memory CD8+ T cells, and synergistic co-stimulatory effects were detected when OX40L was combined with B7.1 or 4-1BBL. Thus, OX40L was speculated to promote memory CD8+ T cell responses in an indirect way, likely in co-operation with B7.1 or 4-1BBL50.
Nevertheless, Song et al.48 reported that anti-OX40 agonist treatment was essential for the expansion and anti-tumor activity of CD8+ T cell. As OX40–OX40L signals could not only enhance the expansion of tumor-specific CD4+ and CD8+ cells, but also promote the cooperation between CD8+ T cells and non-CD8+ T cells, mainly the CD4+ T cell, to enhance tumor rejection following adoptive transfer of the in vitro-primed CD8+ cells into tumor-bearing hosts. Accordingly, the tumor suppression effects of OX40 agonist antibody were greatly dependent on the participation of both CD4+ and CD8+ T cells during tumor-specific priming10, 79. Using human CD8+ T cells, Fujita et al.51 demonstrated that OX40 signals directly augmented proliferation of human CD8+ T cells, as well as cytokine secretion, such as INF-γ. Using primed tumor-specific or generated effector CD8+ T cells from OX40-deficient mice, Song et al.46 demonstrated that the OX40–OX40L interaction was indispensable in both the CD8+ priming process and the recall response when primed CD8+ T cells encounter tumor-expressed antigen, which contributed to the survival and expansion of CD8+ T cells and the recall response of CD8+ memory T cells in vivo.
4. Therapeutic benefit of OX40 in autoimmunity disease
Autoimmune disease is typically characterized by autoantibodies reactive against self-antigens due to the failure of the immune system to maintain self-tolerance against autoantigen. There are some well-known autoimmune diseases with high morbidity due to poorly understood pathogenesis. These include experimental autoimmune encephalomyelitis (EAE), systemic lupus erythematosus (SLE), and rheumatoid arthritis (RA), colitis, autoimmune experimental uveitis (AEU), type 1 diabetes mellitus, and multiple sclerosis (MS).
Due to the pivotal role of T cells in controlling the immune response and costimulatory effect of OX40–OX40L interaction with T cells, targeting the OX40–OX40L interaction might ameliorate the autoantigen-specific T-cell responses. Therefore, experiments have been conducted to verify whether targeting the OX40–OX40L interaction by blockade of OX40 or OX40L had the therapeutic benefit for autoimmune diseases, including EAE, SLE, RA, colitis, AEU, type 1 diabetes mellitus, MS, graft-versus-host disease (GVHD) and inflammatory bowel disease (IBD). Table 3 summarizes the effect of the OX40–OX40L interaction on several diseases. Overall, it shows that OX40 is upregulated at the sites of autoimmunity and correlates with disease severity. More specifically, there is no significant increased expression of OX40 in peripheral blood in localized diseases such as colitis, while a typical increase of peripheral OX40+CD4+ T cells can be detected in more systemic conditions like SLE. OX40–OX40L blockade in vivo generally ameliorates autoimmunity, mainly by preventing migration, moderating T cell polarization, altering cytokine production, and preventing proliferation of active CD4+ T cells8. Below we discuss the role of the OX40–OX40L interaction on EAE, SLE, and RA.
Table 3.
Therapeutic effect of OX40–OX40L in autoimmunity disease.
| Autoimmunity | Model | Intervention and effect | Ref. |
|---|---|---|---|
| Experimental autoimmune encephalomyelitis (EAE) | Activated induced EAE murine model | A neutralizing OX40L mAb (RM134L) reduced mononuclear cell infiltration into the spinal cord | 80 |
| Adoptive transfer EAE murine model | OX40 immunotoxin injection resulted in amelioration of EAE | 81 | |
| EAE murine model | Soluble OX-40R treatment ameliorated both actively induced and adoptively transferred EAE disease | 82 | |
| OX40L-deficient mice (OX40L−/−) | Abortive T cell priming greatly reduced EAE clinical manifestations | 60 | |
| OX40L transgenic mice (OX40L-Tg) | OX40L-Tg mice developed a greater severity of EAE despite a delayed onset | 60 | |
| EAE Lewis rats | OX40 antibody enhances the autoantigen specific Vβ8.2+ T cells | 83 | |
| Systemic lupus erythematosus (SLE) | SLE patients | An increased frequency of peripheral CD4+OX40+ T cells in SLE patients | 84 |
| SLE patients | OX40L–OX40 axis contributes to the aberrant Tfh cell response | 33 | |
| SLE patients | The serum level of OX40L or OX40 expression on CD4+ T cells may act as markers of SLE | 85 | |
| SLE patients | Renal biopsies of SLE patients showed infiltration of OX40+ T cells | 86 | |
| SLE patients | OX40 mAb could inhibit expression of perforin and hemolysis activities to ameliorate SLE | 87 | |
| SLE patients | Whole genome association (WGA) studies of OX40L | 88 | |
| Rheumatoid arthritis (RA) | Collagen-induced arthritis (CIA) murine model | OX40 blockade reduced the proinflammatory responses and ameliorated RA development | 55 |
| CIA murine model | Anti-OX40L mAb ameliorated RA disease and suppressed IFN-γ and anti-CII IgG2a production | 61 | |
| CIA murine model and HTLV-I Tg mouse model. | Low OX40 expression on T cells inIL-1-deficient mice resulted in impaired suppression of RA | 89 | |
| RA patients | OX40 plasma levels were higher than control group treatment of methotrexate and adalimumab | 90 | |
| CIA murine model | Antigen inhibition of CIA is associated with induction of OX40 on T cells | 91 | |
| IL-1R deficient (IL-1Ra−/−) mice | Anti-OX40 Ab accelerated the production of IL-17 | 69 | |
| Colitis | Dextran sulfate sodium induced murine model | OX40-IgG treatment resulted in a dose-dependent reduction of intestinal inflammation | 92 |
| T cell transfer model of colitis | OX40 regulated the homeostasis of intestinal FOXP3+ Treg cells and suppressed colitis | 45 | |
| Crohn's disease murine model | Combination of anti-TNF-α and anti-OX40L mAbs improved the therapeutic effect of chronic colitis | 93 | |
| Biopsy specimens of patients | Positive of OX40 staining in all biopsies of patients with ulcerative colitis | 94 | |
| T cell-restored SCID mice | Anti-OX40L mAb completely blocked development of colitis | 62 | |
| Autoimmune experimental uveitis (AEU) | Ovalbumin-induced AEU | Anti-OX40L antibody substantially inhibited the antigen-specificocular inflammation | 31 |
| IRBP161-180 induced AEU | OX40-activating Ab prolonged and exacerbated the disease course of EAU | 95 | |
| Type 1 diabetes mellitus | Type 1 diabetes patients | Co-expression of CD25 and OX40 receptors delineates autoreactive T-cells in type 1 diabetes | 96 |
| Non-obese diabetic (NOD) mice | Inhibiting OX40–OX40L interactions at a late stage prevented diabetes development | 97 | |
| NOD mice | An OX40 agonistic antibody (OX86) treatment reduced type 1 diabetes (T1D) incidence | 98 | |
| Multiple sclerosis (MS) | Systemic sclerosis (SSc) patients | Serum soluble OX40 levels correlate with the early-onset of SSc disease | 99 |
| MS patients | CD4+ OX40+ T cells were not increased in clinically active MS | 100 | |
| MS patients | OX40 is upregulated in the CNS of MS patients | 101 | |
| MS patients | TNFSF4 polymorphisms can affect systemic sclerosis genetic susceptibility | 102 | |
| SSc patients | Polymorphisms in the TNFSF4 gene region are associated with susceptibility to SSc | 103 |
4.1. Blocking OX40–OX40L engagement ameliorates EAE
EAE is a well-established animal model for the human demyelinating disease of the central nervous system (CNS), multiple sclerosis (MS)104. MS is an autoimmune disorder characterized by variable pathological manifestations, including destruction of the myelin sheath, demyelinating plaques and axonal damage. There are several well studied EAE models and they are induced by different myelin-derived antigens: proteolipid protein (PLP)-induced EAE, myelin basic protein (MBP)-induced EAE, myelin oligodendrocyte glycoprotein (MOG)-induced EAE, and adoptively transferred EAE model. They play a crucial role in testing novel treatments for modulating the course of MS105.
The possible therapeutic benefit of OX40 to autoimmune disease was first found in an EAE rat model, as both OX40 and OX40L are expressed on the surface of CNS cells in EAE mice; autoantigen-specific CD4+ T cells from CNS had a significantly higher level of OX40 than those from peripheral blood. OX40 or OX40L deficiency can potentially reduce the ongoing signs of EAE. OX40L-deficient mice showed decreased clinical manifestations of actively induced EAE and decreased production of proinflammatory cytokines IFN-γ, IL-2, and IL-6, which was mainly due to impaired APC capacity and abortive T cell priming60. In contrast, transgenic mice expressing OX40L developed a greater severity of EAE despite a delayed onset, with a markedly enhanced T cell response to protein antigen60.
To further verify the therapeutic effect of the OX40–OX40L interaction blockade on the development of EAE, Nohara et al.80 used PLP 139–151 peptide to build the active EAE model and an adoptively transferred EAE model, and treated those mice with various doses of neutralizing OX40L mAb (RM134L). RM134L treatment ameliorated the clinical signs of EAE in a dose-dependent manner in both actively induced EAE and the adoptively transferred EAE model, thus indicating a substantial contribution of OX40 L87. In this study RM134L treatment greatly inhibited the accumulation of OX40-expressing CD4+ T cells and the migration of pathogenic T cells into the CNS, but had little effect on the proliferation of IFN-γ producing Th1 cells in DLN80.
In another study Weinberg et al.82 also reported an improvement in clinical signs of EAE by blocking OX40–OX40L engagement. The blockade of the OX40–OX40L interaction was achieved by administration of a soluble human OX40 immunoglobulin fusion protein with the ability to bind to murine OX-40L. OX40-blockade in mice inhibited T cell proliferation by 50%–70%, resulting in a marked reduction in the severity of EAE and quicker recovery from EAE disease. However, the frequency and severity of relapses was not improved, suggesting that the effect may be short-lived82. As adoptive transfer of wild-type donor T cells to OX40L-deficient mice efficiently transferred EAE disease, while OX40L-deficient donor T cells failed to transfer EAE disease to wild-type recipient mice, this suggested that OX40–OX40L interaction may be involved in the T cell priming process in the occurrence of EAE disease60.
4.2. OX40 expression may correlate with SLE severity
SLE is a chronic multisystem complex autoimmune disorder. It is characterized by pathogenic autoantibodies in association with the dysfunction of the cellular immune, humoral immune and complement system106. The etiological cause of SLE is still incompletely understood, while the pathogenesis is mainly owing to the formation of immune complexes and the loss of immune tolerance. The defective B cell suppression and excess T cell helper cells are clearly involved in these processes through complex interactions among T cells, B cells, and APCs106.
To seek possible SLE biomarkers, Patschan et al.84 analyzed the expression of CD70, CD80, CD86, CD137, CD137L, OX40, CD152, CD154 and ICOS of CD4+ T cell populations in the peripheral blood of SLE patients. Among all these co-stimulatory markers, CD80, CD86, and OX40 showed obviously elevated levels compared to a healthy group, which also correlated with the systemic lupus erythematosus disease activity index (SLEDAI)84. This was consistent with the results reported by Farres et al.85 They investigated the percentage of CD4+ T cell expressing OX40 and serum OX40L levels in SLE patients with lupus nephritis (LN). They found that there was a significantly elevated percentage of OX40 expression CD4+ T cells in SLE patients. The significantly increased percentage of OX40 expression CD4+ T cells can also be found in SLE patients with LN. The same increase also can be noted in the serum OX40L levels. They suggested that OX40 expression CD4+ T cells and serum OX40L levels are an indicator of disease activity in SLE85.
Some studies also investigated the pathogenesis of SLE through the identification of genetic components88. Multiple predisposing genetic factors are implicated in SLE pathogenesis, including OX40L which showed genetic linkage with SLE and also acts as components of B cell pathways107. Cunninghame Graham et al.107 pointed out OX40L as a lupus susceptibility gene, as they showed the upstream region of OX40L contained a single risk haplotype for SLE. An over-expressed haplotype is correlated with increased expression of OX40L, which may influence the functional consequences of T cell activation via OX40 and destabilize peripheral tolerance107. In another study it was found that the OX40–OX40L interaction is bidirectional. The reverse signaling pathway via OX40L may augment the B cell differentiation and hyperactivity to aggravate SLE pathology, which is in keeping with the hallmark of SLE disease, autoreactive antibody88.
The frequency of blood active-phenotype Tfh cells is increased in active SLE patients. Jacquemin et al.33 reported that OX40L could promote SLE disease through promoting a Tfh cell response. As Tfh cells were specialized for provision of help to B cells, there may be certain interactions between autoantibody-producing B cells and pathogenic Tfh cells through the OX40–OX40L axis. The OX40L signals in SLE patients can induce the proliferation of Tfh cells from Th cells and assist them to become functional B cell helpers33. Although the role of the OX40–OX40L axis in SLE disease is widely investigated, the mechanisms of the OX40–OX40L axis involved in SLE patients still needs further study. In the future, OX40–OX40L blockade may developed into a possible therapeutic approach to ameliorate SLE disease and alleviate autoantibody production, like the well-characterized T cell activation pathway, CD28–CD80/CD86 interaction.
4.3. OX40 blockade may ameliorate RA development
RA is a symmetric poly-articular autoimmune disorder. It is characterized by joint pain and progressive damage to the synovial tissue, especially in the hands, feet, and wrist, and may eventually result in joint destruction and bone erosion89. Although the etiological cause of RA is not totally understood, T cell-mediated autoimmune responses are definitely involved in the pathogenesis of RA. Administration of either anti-T cell antibodies or immunosuppressive drug targeting to T cells was effective in suppression of the progression of established RA murine disease. Saijo et al.89 found that either IL-1α- or IL-1β-deficient mice correlated with lower expression of OX40 and CD40L, and showed reduced incidence of RA, suggesting that those factors collectively contributed to the lower responsiveness of T cells. The existence of OX40L can be detected on activated synovial dendritic cells or macrophages in synovial tissue and inflamed joints from RA patients. Additionally, the expression of OX40 on infiltrating activated T cells could be enhanced by locally produced pro-inflammatory cytokines in RA synovium, such as TNF-α or IL-1β. Therefore, it is likely that OX40–OX40L signaling acts through cellular contact-dependent interaction between infiltrating activated T cells and their nearby APC cells in the RA synovium under the dysregulated pro-inflammatory cytokine environment in the rheumatoid synovium89.
Yoshioka et al.61 investigated the contribution of the OX40–OX40L interaction on the pathogenesis of RA by administration of a neutralizing anti-OX40L mAb to a type II collagen (CII)-induced arthritis (CIA) murine model. Interestingly, they found that administration of anti-OX40L mAb had the potential to ameliorate the severity of RA through suppression of the production of IFN-γ and anti-CII IgG2a; however, it did not suppress the activation of CII-reactive T cells. They speculated that the OX40–OX40L interaction may exert its contribution in subsequent steps after CII-reactive T cell activation61. That is, OX40 may act as an accelerator, but not an initiator, in the pathological development of RA.
Jiang et al.55 reported the pathogenic role of CD4+CD28– OX40+ T cells in RA pathogenesis. Abnormal accumulation of CD4+CD28– OX40+ T cells was found in both RA patients and CIA mice. Those CD4+CD28– OX40+ T cells showed high production of pro-inflammatory cytokines, including IFN-γ, TNF-α and IL-4, which may be correlated with clinical pathological features of RA patients and CIA mice. Blockade of the OX40–OX40L interaction resulted in inhibition of pro-inflammatory cytokine production in vitro, and also ameliorated arthritis progression in vivo55. A similar therapeutic benefit of OX40–OX40L blockade was found in another study, which resulted from a significant reduction in IL-17 from Th17 cells in peripheral synovial joints27. This evidence suggested that the regulatory role of OX40–OX40L signaling in autoimmune arthritis, which may represent an effective approach for immunomodulatory therapy.
5. Rationale for targeting OX40–OX40L interactions in cancer immunotherapy
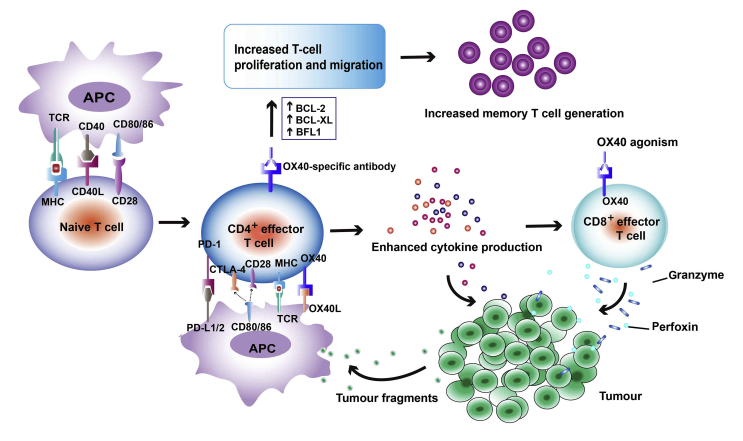
Cancer immunotherapy is an emerging cancer treatment that utilizes the immune system of the cancer host to destroy cancer cells. Many studies have demonstrated the rationale of targeting OX40–OX40L interactions in cancer immunotherapy, as ligation of OX40 signals can promote conventional (non-regulatory) CD4+ and CD8+ T cell survival, sustain anti-apoptotic protein expression like BCL-XL, BCL-2 and survivn, enhance cytokine production like IL-2, IL-4, IL-5 and IFN-γ, boost effector tumor-specific T cell immune responses, and augment tumor-specific memory T cell generation following antigen challenge, as summarized in Fig. 34, 10, 11. Therefore, OX40–OX40L interaction possesses potent immunological effects and fuels interest as a target to improve tumor immunotherapy, as summarized in Table 4.
Figure 3.
Schematic diagram of the rationale for targeting OX40–OX40L interactions in cancer immunotherapy. As ligation of OX40 signals can promote conventional (non-regulatory) CD4+ and CD8+ T cell survival, sustain anti-apoptotic protein expression (BCL-XL, BCL-2 and BFL1), enhance cytokine production of IL-2, IL-4, IL-5 and IFN-γ, boost effector tumor-specific T cell immune responses, and augment tumor-specific memory T cell generation following antigen challenge.
Table 4.
Summary of cancer immunotherapy targeting OX40–OX40L interaction alone or in combination.
| Combination type | OX40 therapy | Combo | Cancer model | Effect | Ref. |
|---|---|---|---|---|---|
| Immuno- therapy | OX86 | Anti-PD-1 (RMT3-2) | ID8 ovarian cancer murine model | Higher ratios of both effector CD4+ and CD8+ cells to Treg; enhanced cytolytic activity | 108 |
| OX86 | Anti-PD-1; HPV16-E7 peptide vaccine | TC-1 syngeneic mouse model | Addition of anti-PD-1 negated effect of anti-OX40/vaccine on tumor-growth inhibition | 109 | |
| OX86 | Anti-PD-1 (clone G4) | MMTV PyMT mammary cancer | Anti-PD-1 significantly attenuated the therapeutic effect of anti-OX40 | 110 | |
| OX86 | Anti-PD-L1, STING agonist | HER-2+ breast tumors | Primed immunity and reduced tumor growth | 111 | |
| OX86 | Anti-CTLA-4 (clone 9D9) | Carcinoma model | Enhanced T cell response, expansion, effector function, and memory of CD8 | 112 | |
| OX86 | CTLA-4 mAb (clone 9D9) | TRAMP-C1 prostate | 11/15 of tumor bearing mice rejected their tumors | 113 | |
| OX86 | CTLA-4 mAb (clone 9D9) | MCA-205 sarcoma tumors | 10/14 of tumor bearing mice rejected their tumors | 113 | |
| OX86 | Anti-CD40 and anti-CD137 mAb | 4T1 mammary carcinoma | Inhibited tumor growth when compared with anti-OX40 therapy alone | 114 | |
| OX86 | Anti-CD137 and anti-B7-H1 | Hepatocellular carcinoma (HCC) | Extension of survival and dense T cell infiltrates | 115 | |
| OX86 | Anti-4-1BB mAb (3H3) | MethA tumor murine model | Co-stimulation mediated CD8 T cell dependent rejection | 116 | |
| OX86 | Anti-4-1BB | Her-2/neu mice | Stimulating and enhancing both CD4+ and CD8+ T cells | 117 | |
| Radio-therapy (RT) | OX86 | Three times 20Gy SBRT | Lewis lung carcinoma (LLC) | Significant extended survival; long-term tumor free survivors; monotherapy ineffective | 118 |
| OX86 | A single dose of 20 Gy | LLC-OVA bearing mice | Enhanced therapeutic antitumor immunity; durable immunological memory | 119 | |
| OX86 | A single dose of 20 Gy | Murine colorectal carcinoma | One day following radiation is optimal to administration of anti-OX40 mAb | 120 | |
| OX86 | Total dose of 15, 24 or 36 Gy | Anti-PD1-resistant lung tumor model | Inhibited local and systemic antitumor growth; and improved survival rates | 121 | |
| Chemo-therapy | OX86 | A dose of 250 mg/kg CTX | B16 melanoma mice model | Significant extended survival; enhanced antitumor immunity | 122 |
| OX40 agonist | Radiation and CTX | Patients with prostatecancer | No effect on the degree of proliferation of peripheral blood lymphocytes | 123 | |
| Cytokine | OX86 | IL-2c | MCA-205 sarcoma mice model | Boosted tumor regression; enhanced long-term survival | 124 |
| OX86 | IL-2c | TRAMP-C1 prostate tumor mice model | Increased the survival of mice | 124 | |
| OX86 | IL-12 | Pulmonary metastases mice model (MCA205) | A significant reduction in pulmonary metastases; monotherapy ineffective | 125 | |
| OX86 | IL-12 | TRAMP-C1 prostate tumor mice model | A synergistic therapeutic effect; the enhanced anti-OX40-mediated tumor therapy by IL-12 | 125 | |
| Vaccine | OX86 | Anti-CTLA-4 andHER2 vaccination | Mammary carcinoma model | Extensive tumor destruction; restricted Th2-cytokine production by CD4 cells; enhanced IFN-γ production | 112 |
| OX86 | Anti-CTLA-4 andHER2 vaccination | Prostate adenocarcinoma spontaneous model | Reversed anergy; enhanced the expansion and function of CD8 T cells | 112 | |
| OX86 | GM-CSF expressing vaccine | FVB MMTV-neu (neu-N) mice | Breaking established CD8+ T cell tolerance, Prolonging the survival and effector function of CD8+ T cells | 126 | |
| OX86 | Anti4-1BB; vaccination | Her-2/neu transgenic mice | Retard tumor growth; enhanced the immune responses; improved antitumor effect | 117 | |
| OX86 | HPV16 E7 peptide vaccine | TC-1 syngeneic mouse model. | Significant (P<0.05) slowdown of tumor progression, and prolonged survival | 109 | |
| Others | OX86 | CD4+ lympho depletion | B16/F10 melanomas | Improved therapeutic efficacy for poorly immunogenic tumors | 127 |
| None | AdOX40L-modified DCs mOX-40L | B16/F10 tumors | Suppressed tumor growth; marked cytolytic activity | 128 | |
| Anti-OX-40R | B16/F10 melanoma | 25% treated mice survived tumor challenge. | 10 | ||
| mAbOX-86 | B16/F10 melanoma | Similar therapeutic efficacy as mOX-40L:Ig | 10 | ||
| mOX-40L | MCA303 sarcoma murine tumor model | Delayed tumor growth and 60% were free of tumor for >70 days | 10 | ||
| mOX-40L | SM1 breast cancer line | Enhanced antitumor activity | 10 | ||
| mOX-40L | CT26 tumor model | Enhanced tumor-free survival; resisted the tumor challenge | 10 | ||
| hOX-40L | CT26 tumor model | No significant anti-tumor effect | 10 | ||
| OX86 | MCA303 sarcoma tumor model | Improved tumor-free survival and delay tumor growth in younger mice but not older | 129 | ||
| OX86 | CT26 colon carcinoma murine model | Age-dependent efficacy in OX40-mediated tumor-free survival | 129 | ||
| OX86 | CT26 colon carcinoma murine model | Induced tumor rejection in 80% of mice | 130 | ||
| OX40L-Fc | MCA205 sarcoma murine model | 13% of treated animals resulted in the long-term survival | 131 | ||
| OX40L-Fc | CMS4(H-2d) sarcoma model | >80% of animals rejected their tumors | 131 | ||
| OX86 | CT26 colon carcinoma murine model | Inhibiting Treg-cell suppression and boosting effector T-cell activation | 132 | ||
| OX86 | CT26 tumor model | Facilitated systemic antitumor immunity | 133 | ||
| mOX40L fusion protein | CT26 tumor model | A reproducible inhibition of tumor growth | 134 | ||
| mOX40L fusion protein | 4T1 tumor model | Dose and route dependent anti-tumor effect | 134 | ||
| Fc-mOX40L | Colon 26-bearing mice | Produced complete remission | 135 | ||
| Fc-mOX40L | Renal cell carcinoma-bearing mice | Produced complete remission | 135 | ||
However, some studies have revealed that the antitumor activity of OX40 agonists may be altered according to the immunogenicity of different tumor types. More specifically, OX40 agonists are likely to enhance antitumor immunity against immunogenic tumors such as MCA303 sarcoma tumors, CT26 colon carcinoma tumors, or SM1 breast cancer10. However, they may have less immunological protection and be insufficient to induce tumor eradication with poorly immunogenic tumors, such as B16/F10 melanoma tumors10. Since poorly immunogenic tumors may not provide the priming required by OX40–XO40L signals. Novel combining strategies that employ OX40 agonists in conjunction with other therapeutic treatments have been widely investigated, including combining OX40 agonists with the immunotherapy checkpoint inhibitors PD-1 or CTLA-4, immunotherapy costimulatory molecules like 4-1BB, certain cytokines like IL-2 or IL-12, chemotherapy, or radiotherapy, as summarized in Table 4.
5.1. OX40 agonists and combination immunotherapy
The well-known “checkpoint inhibitor” programmed cell death protein 1 (PD-1) and CTL-associated antigen 4 (CTLA-4) can curtail immune responses and attenuate anti-tumor immunity. Administration of antagonist monoclonal antibodies (mAbs) targeting PD-1 or CTLA-4 show substantial promise to boost T cells anti-tumor immunity. Pembrolizumab is the first anti-PD-1 mAb approved by the U. S. Food and Drug Administration (FDA) to treat advanced metastatic melanomas, neck squamous cell carcinoma and metastatic non-small cell lung cancer. Two another anti-PD-1 mAbs (nivolumab and cemiplimab), and three anti-PD-L1 mAbs (atezolizumab, avelumab, and durvalumab) are also approved by the FDA for cancer treatment. On the other hand, ipilimumab is the first anti-CTLA-4 mAb for the treatment of melanoma and renal cell carcinoma. Indeed, blockade of “checkpoint inhibitors” PD-1 or CTLA-4 has demonstrated improved survival in both preclinical and clinical trials. However, clinical results also revealed that blockade PD-1or CTLA-4 alone was insufficient to induce tumor eradication and only a subset of patients showed objective clinical responses. In order to overcome the therapeutic limitations of checkpoint blockade monotherapy, many investigators studied the combination immunotherapy. OX40 plays a co-stimulatory role in T cell activation, and if combined with PD-1or CTLA-4 blockade it is supposed to further overcome tumor immune tolerance and ultimately generate a robust therapeutic immune response, as summarized in Table 4.
Indeed, combination of OX40 agonists with PD-1/PD-L1 blockade may have complementary immunological effects. As OX40–OX40L interaction can increase CD4+ and CD8+ T cells populations which express the PD-1 receptor, and enhance production of cytokines like IFN-γ that can up-regulate PD-L1 expression within the tumor microenvironment. Guo et al.108 demonstrated that a combination of PD-1 blockade and OX40 stimulation synergistically protects against poorly immunogenic ovarian tumor growth, whereas neither PD-1 blockade nor OX40 stimulation alone had an effect on tumor growth and ascites formation in an ID8 murine ovarian cancer model. However, combined anti-PD-1/anti-OX40 mAb treatment significantly increased the overall survival of mice and achieved 60% tumor-free mice. The combined treatment showed elevated ratios of both effector CD4+ and CD8+ T cells to Treg cells and myeloid-derived suppressor cells (MDSC), and elicitation of an antigen-specific CTL response owing to the formation of a local immunostimulatory microenvironment108.
In another study, Messenheimer et al.110 reported that timing and sequencing are crucial to optimize the therapeutic effect of PD-1 blockade combined with anti-OX40. Anti-OX40 treatment can result in a delayed tumor progression in a preclinical oncogene-driven mammary cancer model which is refractory to PD-1 blockade. While a significant reduction in survival and tumor control was obtained with concurrent combination of anti-OX40 and anti-PD-1 blockade, suggesting that PD-1 blockade may provide an adverse effect on anti-OX40-induced therapy. Otherwise, augmented therapeutic efficacy was achieved by sequential and delaying anti-PD-1 administration, as it is more effective to give anti-PD-1 antibody after the initial T-cell boost generated by anti-OX40110. Although much evidence has shown that OX40 agonists are likely to synergize with PD-1 blockade, a better understanding of the immunological interplay between them is still needed.
CTLA-4 serves as a “checkpoint inhibitor” through competitively binding to B7-1 or B7-2 to downregulate the CD28 co-stimulatory pathway of T cells11, 122. The therapeutic anti-tumor benefit of anti-CTLA-4 blockade is limited, especially with poorly immunogenic tumors. As OX40 and CTLA-4 are both induced to express on CD4+ and CD8+ T cells after TCR stimulation, many studies have been conducted to determine if combined anti-OX40 stimulation and anti-CTLA-4 blockade would augment therapeutic responses and generate broader tumor immunotherapy. Indeed, compared to monotherapy with anti-OX40 or anti-CTLA-4, the combined anti-OX40/anti-CTLA-4 immunotherapy improved tumor regression and survival of tumor-bearing hosts in some tumor models, such as the moderately immunogenic MCA-205 sarcoma tumors or the poorly immunogenic TRAMP-C1 prostate tumors11, 113. Specifically, the therapeutic efficacy of dual anti-OX40/anti-CTLA-4 therapy is associated with decreased inhibitory effects of Treg cells and increased number and function of effector of CD4+ and CD8+ T cells. No therapeutic efficacy of combined therapy was obtained with pretreatment of CD4 and/or CD8 T cell depletion, suggesting a T cell-dependent mechanism113. Dual anti-OX40/anti-CTLA-4 therapy also resulted in enhanced cytokine production, including both Th1 (IL-2 and IFN-γ) and Th2 (IL-4, IL-5, and IL-13) cytokines, which may help to drive potent polyclonal effector T cell responses113. Despite the enhanced anti-tumor ability that has been achieved with combination immunotherapy, tumor immunotherapy treatment still remains challenging because of tumor tolerance and poor immunogenicity11, 112.
5.2. Combination treatment of anti-OX40 with cytokines
The combination of cytokines with anti-OX40 immunotherapy is a popular strategy to enhance the immunogenicity of tumor cells and strengthen the antitumor immune response of the host. Many cytokines, such as IL-2, IL-12, IL-4, IL-15, TNF-α, or IFN-γ, have shown potent immunomodulatory and antitumor effects in various preclinical studies136, 137. Among them, two cytokines are of particular interest, IL-2 and IL-12. IL-2 has been approved by the FDA to treat metastatic melanoma and metastatic renal cell carcinoma. However, despite its great antitumor potency, the application of IL-2 in the clinic still faces several unavoidable drawbacks, such as short half-life and high dose-related toxicities136. IL-12 can exert potent anticancer effects through both innate and adaptive immune responses. Therefore, IL-12 often synergizes with other therapeutic agents to achieve immunomodulatory benefits.
Redmond et al.124 investigated the antitumor efficacy of a combination of anti-OX40 therapy with IL-2 therapy. According to their study, the expression of OX40 via TCR stimulation also required the involvement of the IL-2 receptor complex. Exogenous rIL-2 stimulation could strongly drive OX40 up-regulation in both murine and human T cells, suggesting the dual stimulation of TCR and IL-2R on OX40 expression, which was dependent upon the activation of JAK3 and the downstream transcription factors STAT3 and STAT5124. Using a murine pulmonary metastases (MCA205) tumor model they demonstrated that dual anti-OX40/IL-2c immunotherapy can boost antitumor immunity with the help of effector CD4 and CD8 T cells. Negligible anti-tumor efficacy was obtained with depletion of either CD4 or CD8 T cell subsets prior to combination treatment. Combination treatment did not affect the function of Treg cells124. In another study, Ruby et al.125 demonstrated the synergistic therapeutic efficacy of a combination of anti-OX40 and IL-12 in several different mouse tumor models, including a MCA205 tumor model, the TRAMP-C1 prostate tumor model, and the CT26 colon carcinoma. IL-12 signaling is quite critical for OX40-enhanced CD4 T cell survival, which is associated with STAT4-specific signaling.
5.3. Combining agonistic anti-OX40 mAb and RT
Radiotherapy (RT) is capable of anti-tumor effects and is widely used to treat loco-regional cancers, due to direct or indirect damage to the DNA of tumor cells138. RT is reported to induce bone marrow suppression and diminish T cell immunity, resulting in immunosuppressive effects to the cancer host. While RT can also be immunostimulatory by releasing tumor-associated antigens (TAAs), or increasing major histocompatibility complex (MHC) antigen expression to enhance tumor immunogenicity, this may depend on the radiation dose, type of tumor or irradiation site138. Many studies are underway to evaluate the efficacy of combining RT with agonistic anti-OX40 mAbs. Since RT mainly provides therapeutic applicability to local primary tumors, the agonistic anti-OX40 mAbs may act as an immunological partner to enhance the tumor eradication of RT, and meanwhile reduce its adverse effects.
Yokouchi et al.119 evaluated the therapeutic effect of an agonistic anti-OX40 mAb with RT to treat lung cancer in mice. Compared to monotherapy, the anti-OX40 mAb in combination with RT treatment showed an improved cure rate and prolonged survival. None of mice were cured by RT alone or anti-OX40 mAb alone, while about 75% of the mice showed complete remission after treatment with the combined therapeutic schedule, suggesting greater tumor elimination efficacy. They also found that irradiation can help augment the proportion of OX40+CD4+ and OX40+CD8+ T cells in draining lymph nodes (DLN), which may contribute to the synergistic effect of therapeutic immunity. Almost 89% of the mice treated with the anti-OX40 mAb in combination with RT remained disease free in a tumor rechallenge experiment, suggesting the durable tumor immunological memory of combination therapy119.
Consistent with those findings, MJ Gough et al.118 also confirmed the adjuvant role of anti-OX40 mAb to RT. They adopted the stereotactic body radiation therapy (SBRT) to a Lewis lung carcinoma (3LL)-established mouse cancer model. They found that RT initially decreased CD8 T cells within the radiation field compared to areas outside the radiation field, but later they gradually increased at the tumor site. Three treatments of RT can result in a significant increase in median survival but not durable tumor cures118. However, when RT was combined with a single dose of anti-OX40 mAb, not only the median survival was enhanced, tumor-free mice were obtained118. Anti-OX40 mAb facilitates the endogenous immune response to tumor cells mainly by increasing tumor antigen-specific CD8 T cell cytotoxic activity, which is definitely a valuable addition to RT.
In another study, Young et al.120 identified optimal timing of combination immunotherapy with RT in advanced malignancies. They found that the optimal combination timing of anti-OX40 mAb and anti-CTLA4 mAb with RT was different, owing to their different immunotherapy mechanisms. CTLA4 is a checkpoint inhibitor, and pretreatment with anti-CTLA4 mAb showed superior tumor control. As for anti-OX40 agonist mAb, which is co-stimulatory agonist, it was optimally delivered during the tumor antigen-presentation period post-radiation. One day following radiation was suggested, which coincided with the upregulation time of OX40 on T cells120.
5.4. OX40 engagement and chemotherapy combination
Cancer chemotherapy routinely used in the clinic often causes lymphopenia and is easily deemed to be immunosuppressive122. However, studies also revealed that chemotherapy drugs, if given at proper dose, may facilitate anti-tumor potency, since the tumor cells killed by chemotherapy may be immunologically active and capable of leading to antigen cross-presentation and furtherly promote an adaptive tumor-specific immune response139. It is promising to combine RT with agonistic anti-OX40 mAbs treatment, since antigen presentation provided by RT-killed tumor cells can enhance subsequent effector T cell function, with participation of co-stimulatory molecules140.
Cymerman et al.122 reported the potent antitumor immunity of a combination of the anti-OX40 agonist mAbs (OX86) with cyclophosphamide (CTX) in well-established, poorly immunogenic B16 melanoma tumors. Approximately 75% of tumor-bearing mice survived to Day 50 after being treated with a single dose of CTX on Day 6 followed by a single dose of OX86 on Day 7, owing to both direct antitumor effects and immune-enhancing properties. Neither CTX nor OX86 alone provided superior tumor protection. They also compared the efficacy of combination of CTX and OX86 with other immunotherapy monoclonal antibodies, such as anti-CD40 (FGK45), and anti-CTLA4 (9D9)122. They found that the combination of CTX with OX86 showed superior potency to any of the others in poorly immunogenic tumor models. Since CTX administration can induce partial destruction of the tumor, the antigen necessary to prime a tumor-specific response is available for OX40 to enhance the function of tumor-specific T cells, resulting in increased infiltration of CD8+ T cells and decrease of Treg cells. Clinical trials of combination anti-OX40 with RT or CTX have been conducted in patients with chemotherapy-resistant prostate cancer to evaluate the toxicity on peripheral blood lymphocytes (PBL)123. Results showed that the degree of proliferation of PBL was not affected by the administration of anti-OX40 with RT or CTX, suggesting a manageable safety and great tolerability profile for combination therapy.
5.5. Anti-OX40 cancer therapy in human clinical trials
Currently there are six different OX40-targeted molecules used in clinical trials against cancers (Table 5). The safety and efficacy of MEDI6469 (murine anti-OX40 agonist IgG1 antibody) is widely studied in patients with metastatic colorectal cancer (NCT02559024) and in head neck cancer (NCT02274155). Consistent with mouse tumor models, the combination strategies of agonist anti-OX40 antibody in clinic trails is currently ongoing in different tumors. As shown in Table 4, there are three different kinds of ongoing combination strategies. The first one combined anti-OX40 costimulation with radiation therapy. A phase 2 trial (NCT01862900) is designed to test the safety profile and combination efficacy of MEDI6469 with SBRT in patients with metastatic breast cancer. An amplified and directed immune response is expected. The combination of anti-OX40 with co-stimulatory molecules is also being developed. A phase 1 trial (NCT02315066) is being conducted to assess the safety and maximum tolerated dose of PF-04518600, a fully human anti-OX40 agonist IgG2 antibody, alone or in combination with PF-05082566 (anti-4-1BB agonist antibody) in select advanced or metastatic carcinoma patients, and to select a recommended dose for a phase 2 study. In addition, another combination trials aims to analyze the potential of combining anti-OX40 co-stimulatory with anti-CTLA4 or anti-PDL1 co-inhibitory. For example, a phase 1 trial (NCT02410512) is proceeding to assess the safety and pharmacokinetics of MOXR0916 (humanized agonist anti-OX40 monoclonal antibody) with MPDL3280A (engineered anti-PD-L1 antibody) in a dose-escalation manner in patients with local or metastatic solid tumors. Project (NCT03241173) will explore the safety and efficacy of INCAGN01949 (fully human anti-OX40 agonist antibody) in combination with nivolumab or ipilimumab in advanced or metastatic malignancies. There are many ongoing or completed phase 1 or phase 2 clinical trials of anti-OX40, and although phase 3 and 4 clinical trials have not yet been reported, they invariably demonstrate great promise in the development of anti-OX40 immunotherapy.
Table 5.
The ongoing (active, not recruiting) clinical trials with anti-OX40 mAbs (Date: 05/02/2019).
| NCT number and title of study | Intervention | Condition |
|---|---|---|
| NCT01862900—Stereotactic body radiation and monoclonal antibody to OX40 (MEDI6469) in breast cancer patients with metastatic lesions | MEDI6469 (murine anti-OX40 agonist IgG1) | Metastatic breast cancer; liver and lung metastases; |
| NCT02274155—Anti-OX40 antibody in head and neck cancer patients | MEDI6469 (murine anti-OX40 agonist IgG1) | Head and neck cancer |
| NCT02559024—Anti-OX40 antibody (MEDI6469) in patients with metastatic colorectal cancer | MEDI6469 (murine anti-OX40 agonist IgG1) | Colorectal neoplasms |
| NCT02315066—Study of OX40 agonist PF-04518600 alone and in combination with 4-1BB agonist PF-05082566 | PF-04518600 (fully human anti-OX40 agonist IgG2); PF-05082566 | Neoplasms |
| NCT02410512—A study to assess the safety and pharmacokinetics of MOXR0916 and atezolizumab in participants with locally advanced or metastatic solid tumors | MOXR0916 (humanized anti-OX40 agonist IgG1); atezolizumab (anti-PD-L1 mAb) | Neoplasms |
| NCT02221960—A phase 1 study to evaluate MEDI6383 alone and in combination with MEDI4736 in adult subjects with select advanced solid tumors | MEDI6383 (OX40L fusion protein); MEDI4736 (durvalumab) | Recurrent or metastatic solid tumors |
| NCT02705482—A study to evaluate MEDI0562 in combination with immune therapeutic agents in adult subjects with advanced solid tumors | MEDI0562 (humanized anti-OX40 agonist IgG4); tremelimumab; durvalumab | Select advanced solid tumors |
| NCT02923349—A phase 1/2, open-label, dose-escalation, safety study of INCAGN01949 in subjects with advanced or metastatic solid tumors | INCAGN01949 (fully human anti-OX40 agonist antibody, IgG1) | Advanced malignancies metastatic cancer |
| NCT03241173—A study exploring the safety and efficacy of INCAGN01949 in combination with immune therapies in advanced or metastatic malignancies | INCAGN01949 (fully human anti-OX40 agonist antibody, IgG1); nivolumab; ipilimumab | Advanced malignancies |
6. Conclusive remarks and future prospects
Targeting the OX40–OX40L interaction for therapeutic purposes is highly promising, as both agonistic and blockade therapies have shown great efficacy. Results from combination therapies also displayed impressive therapeutic benefits for OX40, especially OX40 agonists in combination cancer immunotherapy, in both preclinical experiments and clinical trials. However, despite these advantages, there are significant limitations to our understanding of the full spectrum of functions of the OX40–OX40L interactions and their related therapies, such as optimal timing of administration, off-target effects, the need of concurrent treatment with other therapy, and the possibility of translating therapy owing to the differences in OX40 expression between mouse and humans. Timing of OX40 agonist or blockade administration may be critical to success since OX40–OX40L interactions are a series of time-dependent checkpoints. Therefore, more investigation is urgently needed for the development of OX40-related drugs and therapies.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos. 81872824 and 81690261). The authors are grateful for the funding.
Yu Fu was responsible for writing the whole passage. Qing Lin, Zhirong Zhang, Ling Zhang were in charge of checking and revision. All figures and tables in the article were made by Yu Fu.
The authors have no conflicts of interest to declare.
Contributor Information
Zhirong Zhang, Email: zrzzl@vip.sina.com.
Ling Zhang, Email: femcivrogner@outlook.com.
References
- 1.Raphael I., Nalawade S., Eagar T.N., Forsthuber T.G. T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine. 2015;74:5–17. doi: 10.1016/j.cyto.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanmamed M.F., Pastor F., Rodriguez A., Perez-Gracia J.L., Rodriguez-Ruiz M.E., Jure-Kunkel M. Agonists of co-stimulation in cancer immunotherapy directed against CD137, OX40, GITR, CD27, CD28, and ICOS. Semin Oncol. 2015;42:640–655. doi: 10.1053/j.seminoncol.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 3.Meylan F., Siegel R.M. TNF superfamily cytokines in the promotion of Th9 differentiation and immunopathology. Semin Immunopathol. 2017;39:21–28. doi: 10.1007/s00281-016-0612-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ishii N., Takahashi T., Soroosh P., Sugamura K. OX40–OX40 ligand interaction in T-cell-mediated immunity and immunopathology. Adv Immunol. 2010;105:63–98. doi: 10.1016/S0065-2776(10)05003-0. [DOI] [PubMed] [Google Scholar]
- 5.Willoughby J., Griffiths J., Tews I., Cragg M.S. OX40: structure and function—what questions remain?. Mol Immunol. 2017;83:13–22. doi: 10.1016/j.molimm.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Fantini M.C., Becker C., Tubbe I., Nikolaev A., Lehr H.A., Galle P. Transforming growth factor β induced FoxP3+ regulatory T cells suppress Th1 mediated experimental colitis. Gut. 2006;55:671–680. doi: 10.1136/gut.2005.072801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Croft M. Control of immunity by the TNFR-related molecule OX40 (CD134) Annu Rev Immunol. 2010;28:57–78. doi: 10.1146/annurev-immunol-030409-101243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Webb G.J., Hirschfield G.M., Lane P.J. OX40, OX40L and autoimmunity: a comprehensive review. Clin Rev Allergy Immunol. 2016;50:312–332. doi: 10.1007/s12016-015-8498-3. [DOI] [PubMed] [Google Scholar]
- 9.Croft M., So T., Duan W., Soroosh P. The significance of OX40 and OX40L to T-cell biology and immune disease. Immunol Rev. 2009;229:173–191. doi: 10.1111/j.1600-065X.2009.00766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weinberg A.D., Rivera M.M., Prell R., Morris A., Ramstad T., Vetto J.T. Engagement of the OX-40 receptor in vivo enhances antitumor immunity. J Immunol. 2000;164:2160–2169. doi: 10.4049/jimmunol.164.4.2160. [DOI] [PubMed] [Google Scholar]
- 11.Linch S.N., McNamara M.J., Redmond W.L. OX40 agonists and combination immunotherapy: putting the pedal to the metal. Front Oncol. 2015;5:34. doi: 10.3389/fonc.2015.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rogers P.R., Song J., Gramaglia I., Killeen N., Croft M. OX40 promotes Bcl-xL and Bcl-2 expression and is essential for long-term survival of CD4 T cells. Immunity. 2001;15:445–455. doi: 10.1016/s1074-7613(01)00191-1. [DOI] [PubMed] [Google Scholar]
- 13.Song J., Salek-Ardakani S., Rogers P.R., Cheng M., van Parijs L., Croft M. The costimulation-regulated duration of PKB activation controls T cell longevity. Nat Immunol. 2004;5:150–158. doi: 10.1038/ni1030. [DOI] [PubMed] [Google Scholar]
- 14.Baum P.R., Gayle R.B., III, Ramsdell F., Srinivasan S., Sorensen R.A., Watson M.L. Molecular characterization of murine and human OX40/OX40 ligand systems: identification of a human OX40 ligand as the HTLV-1-regulated protein GP34. EMBO J. 1994;13:3992–4001. doi: 10.1002/j.1460-2075.1994.tb06715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soroosh P., Ine S., Sugamura K., Ishii N. OX40–OX40 ligand interaction through T cell-T cell contact contributes to CD4 T cell longevity. J Immunol. 2006;176:5975–5987. doi: 10.4049/jimmunol.176.10.5975. [DOI] [PubMed] [Google Scholar]
- 16.Linton P.J., Bautista B., Biederman E., Bradley E.S., Harbertson J., Kondrack R.M. Costimulation via OX40L expressed by B cells is sufficient to determine the extent of primary CD4 cell expansion and Th2 cytokine secretion in vivo. J Exp Med. 2003;197:875–883. doi: 10.1084/jem.20021290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakae S., Suto H., Iikura M., Kakurai M., Sedgwick J.D., Tsai M. Mast cells enhance T cell activation: importance of mast cell costimulatory molecules and secreted TNF. J Immunol. 2006;176:2238–2248. doi: 10.4049/jimmunol.176.4.2238. [DOI] [PubMed] [Google Scholar]
- 18.Zhang X., Xiao X., Lan P., Li J., Dou Y., Chen W. OX40 costimulation inhibits Foxp3 expression and Treg induction via BATF3-dependent and independent mechanisms. Cell Rep. 2018;24:607–618. doi: 10.1016/j.celrep.2018.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zander R.A., Obeng-Adjei N., Guthmiller J.J., Kulu D.I., Li J., Ongoiba A. PD-1 co-inhibitory and OX40 co-stimulatory crosstalk regulates helper T cell differentiation and anti-Plasmodium humoral immunity. Cell Host Microbe. 2015;17:628–641. doi: 10.1016/j.chom.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zander R.A., Vijay R., Pack A.D., Guthmiller J.J., Graham A.C., Lindner S.E. Th1-like Plasmodium-specific memory CD4+ T cells support humoral immunity. Cell Rep. 2017;21:1839–1852. doi: 10.1016/j.celrep.2017.10.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Q., Shi B.M., Xie F., Fu Z.Y., Chen Y.J., An J.N. Enhancement of CD4+ T cell response and survival via coexpressed OX40/OX40L in Graves' disease. Mol Cell Endocrinol. 2016;430:115–124. doi: 10.1016/j.mce.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 22.Sitrin J., Suto E., Wuster A., Eastham-Anderson J., Kim J.M., Austin C.D. The OX40/OX40 ligand pathway promotes pathogenic Th cell responses, plasmablast accumulation, and lupus nephritis in NZB/W F1 mice. J Immunol. 2017;199:1238–1249. doi: 10.4049/jimmunol.1700608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu B., Yu H., Sun G., Sun X., Jin H., Zhang C. OX40 promotes obesity-induced adipose inflammation and insulin resistance. Cell Mol Life Sci. 2017;74:3827–3840. doi: 10.1007/s00018-017-2552-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jahan N., Talat H., Curry W.T. Agonist OX40 immunotherapy improves survival in glioma-bearing mice and is complementary with vaccination with irradiated GM-CSF-expressing tumor cells. Neuro Oncol. 2018;20:44–54. doi: 10.1093/neuonc/nox125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lai C., August S., Albibas A., Behar R., Cho S.Y., Polak M.E. OX40+ regulatory T cells in cutaneous squamous cell carcinoma suppress effector T-cell responses and associate with metastatic potential. Clin Cancer Res. 2016;22:4236–4248. doi: 10.1158/1078-0432.CCR-15-2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murata K., Ishii N., Takano H., Miura S., Ndhlovu L.C., Nose M. Impairment of antigen-presenting cell function in mice lacking expression of OX40 ligand. J Exp Med. 2000;191:365–374. doi: 10.1084/jem.191.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dong W., Zhu P. Functional niche of inflamed synovium for Th17-cell expansion and activation in rheumatoid arthritis: implication to clinical therapeutics. Autoimmun Rev. 2012;11:844–851. doi: 10.1016/j.autrev.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 28.Hoshino A., Tanaka Y., Akiba H., Asakura Y., Mita Y., Sakurai T. Critical role for OX40 ligand in the development of pathogenic Th2 cells in a murine model of asthma. Eur J Immunol. 2003;33:861–869. doi: 10.1002/eji.200323455. [DOI] [PubMed] [Google Scholar]
- 29.Xiao X., Balasubramanian S., Liu W., Chu X., Wang H., Taparowsky E.J. OX40 signaling favors the induction of TH9 cells and airway inflammation. Nat Immunol. 2012;13:981–990. doi: 10.1038/ni.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiao X., Shi X., Fan Y., Wu C., Zhang X., Minze L. The costimulatory receptor OX40 inhibits interleukin-17 expression through activation of repressive chromatin remodeling pathways. Immunity. 2016;44:1271–1283. doi: 10.1016/j.immuni.2016.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Z., Zhong W., Hinrichs D., Wu X., Weinberg A., Hall M. Activation of OX40 augments Th17 cytokine expression and antigen-specific uveitis. Am J Pathol. 2010;177:2912–2920. doi: 10.2353/ajpath.2010.100353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boettler T., Choi Y.S., Salek-Ardakani S., Cheng Y., Moeckel F., Croft M. Exogenous OX40 stimulation during lymphocytic choriomeningitis virus infection impairs follicular Th cell differentiation and diverts CD4 T cells into the effector lineage by upregulating Blimp-1. J Immunol. 2013;191:5026–5035. doi: 10.4049/jimmunol.1300013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jacquemin C., Schmitt N., Contin-Bordes C., Liu Y., Narayanan P., Seneschal J. OX40 ligand contributes to human lupus pathogenesis by promoting T follicular helper response. Immunity. 2015;42:1159–1170. doi: 10.1016/j.immuni.2015.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tahiliani V., Hutchinson T.E., Abboud G., Croft M., Salek-Ardakani S. OX40 cooperates with ICOS to amplify follicular Th cell development and germinal center reactions during infection. J Immunol. 2017;198:218–228. doi: 10.4049/jimmunol.1601356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Montler R., Bell R.B., Thalhofer C., Leidner R., Feng Z., Fox B.A. OX40, PD-1 and CTLA-4 are selectively expressed on tumor-infiltrating T cells in head and neck cancer. Clin Transl Immunol. 2016;5:e70. doi: 10.1038/cti.2016.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Odobasic D., Ruth A.J., Oudin V., Kitching A.R., Holdsworth S.R. OX40 ligand is inhibitory during the effector phase of crescentic glomerulonephritis. Nephrol Dial Transplant. 2019;34:429–441. doi: 10.1093/ndt/gfy177. [DOI] [PubMed] [Google Scholar]
- 37.Gri G., Piconese S., Frossi B., Manfroi V., Merluzzi S., Tripodo C. CD4+CD25+ regulatory T cells suppress mast cell degranulation and allergic responses through OX40–OX40L interaction. Immunity. 2008;29:771–781. doi: 10.1016/j.immuni.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kitz A., Dominguez-Villar M. Molecular mechanisms underlying Th1-like Treg generation and function. Cell Mol Life Sci. 2017;74:4059–4075. doi: 10.1007/s00018-017-2569-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takeda I., Ine S., Killeen N., Ndhlovu L.C., Murata K., Satomi S. Distinct roles for the OX40–OX40 ligand interaction in regulatory and nonregulatory T cells. J Immunol. 2004;172:3580–3589. doi: 10.4049/jimmunol.172.6.3580. [DOI] [PubMed] [Google Scholar]
- 40.Valzasina B., Guiducci C., Dislich H., Killeen N., Weinberg A.D., Colombo M.P. Triggering of OX40 (CD134) on CD4+CD25+ T cells blocks their inhibitory activity: a novel regulatory role for OX40 and its comparison with GITR. Blood. 2005;105:2845–2851. doi: 10.1182/blood-2004-07-2959. [DOI] [PubMed] [Google Scholar]
- 41.Voo K.S., Bover L., Harline M.L., Vien L.T., Facchinetti V., Arima K. Antibodies targeting human OX40 expand effector T cells and block inducible and natural regulatory T cell function. J Immunol. 2013;191:3641–3650. doi: 10.4049/jimmunol.1202752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.So T., Croft M. Cutting edge: OX40 inhibits TGF-β- and antigen-driven conversion of naive CD4 T cells into CD25+Foxp3+ T cells. J Immunol. 2007;179:1427–1430. doi: 10.4049/jimmunol.179.3.1427. [DOI] [PubMed] [Google Scholar]
- 43.Ito T., Wang Y.H., Duramad O., Hanabuchi S., Perng O.A., Gilliet M. OX40 ligand shuts down IL-10-producing regulatory T cells. Proc Natl Acad Sci U S A. 2006;103:13138–13143. doi: 10.1073/pnas.0603107103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vu M.D., Xiao X., Gao W., Degauque N., Chen M., Kroemer A. OX40 costimulation turns off Foxp3+ Tregs. Blood. 2007;110:2501–2510. doi: 10.1182/blood-2007-01-070748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Griseri T., Asquith M., Thompson C., Powrie F. OX40 is required for regulatory T cell-mediated control of colitis. J Exp Med. 2010;207:699–709. doi: 10.1084/jem.20091618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Song A., Tang X., Harms K.M., Croft M. OX40 and Bcl-xL promote the persistence of CD8 T cells to recall tumor-associated antigen. J Immunol. 2005;175:3534–3541. doi: 10.4049/jimmunol.175.6.3534. [DOI] [PubMed] [Google Scholar]
- 47.Bansal-Pakala P., Halteman B.S., Cheng M.H.Y., Croft M. Costimulation of CD8 T cell responses by OX40. J Immunol. 2004;172:4821–4825. doi: 10.4049/jimmunol.172.8.4821. [DOI] [PubMed] [Google Scholar]
- 48.Song A., Song J., Tang X., Croft M. Cooperation between CD4 and CD8 T cells for anti-tumor activity is enhanced by OX40 signals. Eur J Immunol. 2007;37:1224–1232. doi: 10.1002/eji.200636957. [DOI] [PubMed] [Google Scholar]
- 49.Kopf M., Ruedl C., Schmitz N., Gallimore A., Lefrang K., Ecabert B. OX40-deficient mice are defective in Th cell proliferation but are competent in generating B cell and CTL responses after virus infection. Immunity. 1999;11:699–708. doi: 10.1016/s1074-7613(00)80144-2. [DOI] [PubMed] [Google Scholar]
- 50.Serghides L., Bukczynski J., Wen T., Wang C., Routy J.P., Boulassel M.R. Evaluation of OX40 ligand as a costimulator of human antiviral memory CD8 T cell responses: comparison with B7.1 and 4-1BBL. J Immunol. 2005;175:6368–6377. doi: 10.4049/jimmunol.175.10.6368. [DOI] [PubMed] [Google Scholar]
- 51.Fujita T., Ukyo N., Hori T., Uchiyama T. Functional characterization of OX40 expressed on human CD8+ T cells. Immunol Lett. 2006;106:27–33. doi: 10.1016/j.imlet.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 52.Buchan S.L., Manzo T., Flutter B., Rogel A., Edwards N., Zhang L. OX40- and CD27-mediated costimulation synergizes with anti-PD-L1 blockade by forcing exhausted CD8+ T cells to exit quiescence. J Immunol. 2015;194:125–133. doi: 10.4049/jimmunol.1401644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bassett J.D., Swift S.L., VanSeggelen H., Hammill J.A., McGray A.J., Evelegh C. Combined mTOR inhibition and OX40 agonism enhances CD8+ T cell memory and protective immunity produced by recombinant adenovirus vaccines. Mol Ther. 2012;20:860–869. doi: 10.1038/mt.2011.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sun G., Sun X., Li W., Liu K., Tian D., Dong Y. Critical role of OX40 in the expansion and survival of CD4 T-cell-derived double-negative T cells. Cell Death Dis. 2018;9:616. doi: 10.1038/s41419-018-0659-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jiang J., Liu C., Liu M., Shen Y., Hu X., Wang Q. OX40 signaling is involved in the autoactivation of CD4+CD28– T cells and contributes to the pathogenesis of autoimmune arthritis. Arthritis Res Ther. 2017;19:67. doi: 10.1186/s13075-017-1261-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lane P. Role of OX40 signals in coordinating Cd4 T cell selection, migration, and cytokine differentiation in T helper (Th)1 and Th2 cells. J Exp Med. 2000;191:201–205. doi: 10.1084/jem.191.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arestides R.S., He H., Westlake R.M., Chen A.I., Sharpe A.H., Perkins D.L. Costimulatory molecule OX40L is critical for both Th1 and Th2 responses in allergic inflammation. Eur J Immunol. 2002;32:2874–2880. doi: 10.1002/1521-4141(2002010)32:10<2874::AID-IMMU2874>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 58.Mendel I., Shevach E.M. Activated T cells express the OX40 ligand: requirements for induction and costimulatory function. Immunology. 2006;117:196–204. doi: 10.1111/j.1365-2567.2005.02279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Higgins L.M., McDonald S.A., Whittle N., Crockett N., Shields J.G., MacDonald T.T. Regulation of T cell activation in vitro and in vivo by targeting the OX40–OX40 ligand interaction: amelioration of ongoing inflammatory bowel disease with an OX40-IgG fusion protein, but not with an OX40 ligand-IgG fusion protein. J Immunol. 1999;162:486–493. [PubMed] [Google Scholar]
- 60.Ndhlovu L.C., Ishii N., Murata K., Sato T., Sugamura K. Critical involvement of OX40 ligand signals in the T cell priming events during experimental autoimmune encephalomyelitis. J Immunol. 2001;167:2991–2999. doi: 10.4049/jimmunol.167.5.2991. [DOI] [PubMed] [Google Scholar]
- 61.Yoshioka T., Nakajima A., Akiba H., Ishiwata T., Asano G., Yoshino S.I. Contribution of OX40/OX40 ligand interaction to the pathogenesis of rheumatoid arthritis. Eur J Immunol. 2000;30:2815–2823. doi: 10.1002/1521-4141(200010)30:10<2815::AID-IMMU2815>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 62.Malmström V., Shipton D., Singh B., Al-Shamkhani A., Puklavec M.J., Barclay A.N. CD134L expression on dendritic cells in the mesenteric lymph nodes drives colitis in T cell-restored SCID mice. J Immunol. 2001;166:6972–6981. doi: 10.4049/jimmunol.166.11.6972. [DOI] [PubMed] [Google Scholar]
- 63.Walker J.A., McKenzie A.N. TH2 cell development and function. Nat Rev Immunol. 2018;18:121–133. doi: 10.1038/nri.2017.118. [DOI] [PubMed] [Google Scholar]
- 64.Jember A.G., Zuberi R., Liu F.T., Croft M. Development of allergic inflammation in a murine model of asthma is dependent on the costimulatory receptor OX40. J Exp Med. 2001;193:387–392. doi: 10.1084/jem.193.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Akiba H., Miyahira Y., Atsuta M., Takeda K., Nohara C., Futagawa T. Critical contribution of OX40 ligand to T helper cell type 2 differentiation in experimental leishmaniasis. J Exp Med. 2000;191:375–380. doi: 10.1084/jem.191.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gramaglia I., Jember A., Pippig S.D., Weinberg A.D., Killeen N., Croft M. The OX40 costimulatory receptor determines the development of CD4 memory by regulating primary clonal expansion. J Immunol. 2000;165:3043–3050. doi: 10.4049/jimmunol.165.6.3043. [DOI] [PubMed] [Google Scholar]
- 67.Murata K., Nose M., Ndhlovu L.C., Sato T., Sugamura K., Ishii N. Constitutive OX40/OX40 ligand interaction induces autoimmune-like diseases. J Immunol. 2002;169:4628–4636. doi: 10.4049/jimmunol.169.8.4628. [DOI] [PubMed] [Google Scholar]
- 68.Xiao G., Harhaj E.W., Sun S.C. NF-κB-inducing kinase regulates the processing of NF-κB2 p100. Mol Cell. 2001;7:401–409. doi: 10.1016/s1097-2765(01)00187-3. [DOI] [PubMed] [Google Scholar]
- 69.Nakae S., Saijo S., Horai R., Sudo K., Mori S., Iwakura Y. IL-17 production from activated T cells is required for the spontaneous development of destructive arthritis in mice deficient in IL-1 receptor antagonist. Proc Natl Acad Sci U S A. 2003;100:5986–5990. doi: 10.1073/pnas.1035999100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li J., Li L., Shang X., Benson J., Merle Elloso M., Schantz A. Negative regulation of IL-17 production by OX40/OX40L interaction. Cell Immunol. 2008;253:31–37. doi: 10.1016/j.cellimm.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 71.Zhang N., Pan H.F., Ye D.Q. Th22 in inflammatory and autoimmune disease: prospects for therapeutic intervention. Mol Cell Biochem. 2011;353:41–46. doi: 10.1007/s11010-011-0772-y. [DOI] [PubMed] [Google Scholar]
- 72.Fujita H. The role of IL-22 and Th22 cells in human skin diseases. J Dermatol Sci. 2013;72:3–8. doi: 10.1016/j.jdermsci.2013.04.028. [DOI] [PubMed] [Google Scholar]
- 73.Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 74.Akiba H., Takeda K., Kojima Y., Usui Y., Harada N., Yamazaki T. The role of ICOS in the CXCR5+ follicular B helper T cell maintenance in vivo. J Immunol. 2005;175:2340–2348. doi: 10.4049/jimmunol.175.4.2340. [DOI] [PubMed] [Google Scholar]
- 75.Fehérvari Z., Sakaguchi S. CD4+ Tregs and immune control. J Clin Investig. 2004;114:1209–1217. doi: 10.1172/JCI23395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gavin M.A., Clarke S.R., Negrou E., Gallegos A., Rudensky A. Homeostasis and anergy of CD4+CD25+ suppressor T cells in vivo. Nat Immunol. 2001;3:33–41. doi: 10.1038/ni743. [DOI] [PubMed] [Google Scholar]
- 77.Tang Q., Henriksen K.J., Boden E.K., Tooley A.J., Ye J., Subudhi S.K. Cutting edge: CD28 controls peripheral homeostasis of CD4+CD25+ regulatory T cells. J Immunol. 2003;171:3348–3352. doi: 10.4049/jimmunol.171.7.3348. [DOI] [PubMed] [Google Scholar]
- 78.Humphreys I.R., Loewendorf A., de Trez C., Schneider K., Benedict C.A., Munks M.W. OX40 costimulation promotes persistence of cytomegalovirus-specific CD8 T cells: a CD4-dependent mechanism. J Immunol. 2007;179:2195–2202. doi: 10.4049/jimmunol.179.4.2195. [DOI] [PubMed] [Google Scholar]
- 79.Kjærgaard J., Tanaka J., Kim J.A., Rothchild K., Weinberg A., Shu S. Therapeutic efficacy of OX-40 receptor antibody depends on tumor immunogenicity and anatomic site of tumor growth. Cancer Res. 2000;60:5514–5521. [PubMed] [Google Scholar]
- 80.Nohara C., Akiba H., Nakajima A., Inoue A., Koh C.S., Ohshima H. Amelioration of experimental autoimmune encephalomyelitis with anti-OX40 ligand monoclonal antibody: a critical role for OX40 ligand in migration, but not development, of pathogenic T cells. J Immunol. 2001;166:2108–2115. doi: 10.4049/jimmunol.166.3.2108. [DOI] [PubMed] [Google Scholar]
- 81.Weinberg A.D., Bourdette D.N., Sullivan T.J., Lemon M., Wallin J.J., Maziarz R. Selective depletion of myelin-reactive T cells with the anti-OX-40 antibody ameliorates autoimmune encephalomyelitis. Nat Med. 1996;2:183–189. doi: 10.1038/nm0296-183. [DOI] [PubMed] [Google Scholar]
- 82.Weinberg A.D., Wegmann K.W., Funatake C., Whitham R.H. Blocking OX-40/OX-40 ligand interaction in vitro and in vivo leads to decreased T cell function and amelioration of experimental allergic encephalomyelitis. J Immunol. 1999;162:1818–1826. [PubMed] [Google Scholar]
- 83.Weinberg A.D., Lemon M., Jones A.J., Vainiene M., Celnik B., Buenafe A.C. OX-40 antibody enhances for autoantigen specific Vβ8.2+ T cells within the spinal cord of lewis rats with autoimmune encephalomyelitis. J Neurosci Res. 1996;43:42–49. doi: 10.1002/jnr.490430105. [DOI] [PubMed] [Google Scholar]
- 84.Patschan S., Dolff S., Kribben A., Dürig J., Patschan D., Wilde B. CD134 expression on CD4+ T cells is associated with nephritis and disease activity in patients with systemic lupus erythematosus. Clin Exp Immunol. 2006;145:235–242. doi: 10.1111/j.1365-2249.2006.03141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Farres M.N., Al-Zifzaf D.S., Aly A.A., Abd Raboh N.M. OX40/OX40L in systemic lupus erythematosus: association with disease activity and lupus nephritis. Ann Saudi Med. 2011;31:29–34. doi: 10.4103/0256-4947.75775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dolff S., Quandt D., Wilde B., Feldkamp T., Hua F., Cai X. Increased expression of costimulatory markers CD134 and CD80 on interleukin-17 producing T cells in patients with systemic lupus erythematosus. Arthritis Res Ther. 2010;12:R150. doi: 10.1186/ar3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li M., Yang Q., Zhang Y. Effects of CD134 monoclonal antibody on hemolysis activities and expression of perforin in peripheral blood mononuclear cells of systemic lupus erythematosus patients. Hybridoma. 2007;26:191–200. doi: 10.1089/hyb.2007.010. [DOI] [PubMed] [Google Scholar]
- 88.Manku H., Graham D.S., Vyse T.J. Association of the co-stimulator OX40L with systemic lupus erythematosus. J Mol Med. 2009;87:229–234. doi: 10.1007/s00109-008-0431-2. [DOI] [PubMed] [Google Scholar]
- 89.Saijo S., Asano M., Horai R., Yamamoto H., Iwakura Y. Suppression of autoimmune arthritis in interleukin-1-deficient mice in which T cell activation is impaired due to low levels of CD40 ligand and OX40 expression on T cells. Arthritis Rheum. 2002;46:533–544. doi: 10.1002/art.10172. [DOI] [PubMed] [Google Scholar]
- 90.Laustsen J.K., Rasmussen T.K., Stengaard-Pedersen K., Hørslev-Petersen K., Hetland M.L., Østergaard M. Soluble OX40L is associated with presence of autoantibodies in early rheumatoid arthritis. Arthritis Res Ther. 2014;16:474. doi: 10.1186/s13075-014-0474-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mattsson L., Lundberg K., Müssener E., Jansson A., Erlandsson Harris H., Larsson P. Antigen inhibition of collagen-induced arthritis is associated with up-regulation of IL-4 mRNA and induction of OX40 on T cells in draining lymph nodes. Clin Exp Immunol. 2003;131:241–247. doi: 10.1046/j.1365-2249.2003.02054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Obermeier F., Schwarz H., Dunger N., Strauch U.G., Grunwald N., Schölmerich J. OX40/OX40L interaction induces the expression of CXCR5 and contributes to chronic colitis induced by dextran sulfate sodium in mice. Eur J Immunol. 2003;33:3265–3274. doi: 10.1002/eji.200324124. [DOI] [PubMed] [Google Scholar]
- 93.Totsuka T., Kanai T., Uraushihara K., Iiyama R., Yamazaki M., Akiba H. Therapeutic effect of anti-OX40L and anti-TNF-α MAbs in a murine model of chronic colitis. Am J Physiol Gastrointest Liver Physiol. 2003;284:G595–G603. doi: 10.1152/ajpgi.00450.2002. [DOI] [PubMed] [Google Scholar]
- 94.Stüber E., Büschenfeld A., Lüttges J., von Freier A., Arendt T., Fölsch U.R. The expression of OX40 in immunologically mediated diseases of the gastrointestinal tract (celiac disease, Crohn's disease, ulcerative colitis) Eur J Clin Investig. 2000;30:594–599. doi: 10.1046/j.1365-2362.2000.00658.x. [DOI] [PubMed] [Google Scholar]
- 95.Wu X., Rosenbaum J.T., Adamus G., Zhang G.L., Duan J., Weinberg A. Activation of OX40 prolongs and exacerbates autoimmune experimental uveitis. Investig Ophthalmol Vis Sci. 2011;52:8520–8526. doi: 10.1167/iovs.11-7664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Endl J., Rosinger S., Schwarz B., Friedrich S.O., Rothe G., Karges W. Coexpression of CD25 and OX40 (CD134) receptors delineates autoreactive T-cells in type 1 diabetes. Diabetes. 2006;55:50–60. [PubMed] [Google Scholar]
- 97.Pakala S.V., Bansal-Pakala P., Halteman B.S., Croft M. Prevention of diabetes in NOD mice at a late stage by targeting OX40/OX40 ligand interactions. Eur J Immunol. 2004;34:3039–3046. doi: 10.1002/eji.200425141. [DOI] [PubMed] [Google Scholar]
- 98.Bresson D., Fousteri G., Manenkova Y., Croft M., von Herrath M. Antigen-specific prevention of type 1 diabetes in NOD mice is ameliorated by OX40 agonist treatment. J Autoimmun. 2011;37:342–351. doi: 10.1016/j.jaut.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Komura K., Yoshizaki A., Kodera M., Iwata Y., Ogawa F., Shimizu K. Increased serum soluble OX40 in patients with systemic sclerosis. J Rheumatol. 2008;35:2359–2362. [PubMed] [Google Scholar]
- 100.Hintzen R.Q., Pot K., Paty D., Oger J. Analysis of effector CD4 (OX-40+) and CD8 (CD45RA+ CD27–) T lymphocytes in active multiple sclerosis. Acta Neurol Scand. 2000;101:57–60. doi: 10.1034/j.1600-0404.2000.00011.x. [DOI] [PubMed] [Google Scholar]
- 101.Carboni S., Aboul-Enein F., Waltzinger C., Killeen N., Lassmann H., Peña-Rossi C. CD134 plays a crucial role in the pathogenesis of EAE and is upregulated in the CNS of patients with multiple sclerosis. J Neuroimmunol. 2003;145:1–11. doi: 10.1016/j.jneuroim.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 102.Bossini-Castillo L., Broen J.C., Simeon C.P., Beretta L., Vonk M.C., Ortego-Centeno N. A replication study confirms the association of TNFSF4 (OX40L) polymorphisms with systemic sclerosis in a large European cohort. J Transl Med. 2010;8:P5. doi: 10.1136/ard.2010.141838. [DOI] [PubMed] [Google Scholar]
- 103.Gourh P., Arnett F.C., Tan F.K., Assassi S., Divecha D., Paz G. Association of TNFSF4 (OX40L) polymorphisms with susceptibility to systemic sclerosis. Ann Rheum Dis. 2010;69:550–555. doi: 10.1136/ard.2009.116434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ferber I.A., Brocke S., Taylor-Edwards C., Ridgway W., Dinisco C., Steinman L. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE) J Immunol. 1996;156:5–7. [PubMed] [Google Scholar]
- 105.Glatigny S., Bettelli E. Experimental autoimmune encephalomyelitis (EAE) as animal models of multiple sclerosis (MS) Cold Spring Harb Perspect Med. 2018;8:a028977. doi: 10.1101/cshperspect.a028977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mok C.C., Lau C.S. Pathogenesis of systemic lupus erythematosus. J Clin Pathol. 2003;56:481–490. doi: 10.1136/jcp.56.7.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cunninghame Graham D.S., Graham R.R., Manku H., Wong A.K., Whittaker J.C., Gaffney P.M. Polymorphism at the TNF superfamily gene TNFSF4 confers susceptibility to systemic lupus erythematosus. Nat Genet. 2008;40:83–89. doi: 10.1038/ng.2007.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Guo Z., Wang X., Cheng D., Xia Z., Luan M., Zhang S. PD-1 blockade and OX40 triggering synergistically protects against tumor growth in a murine model of ovarian cancer. PLoS One. 2014;9 doi: 10.1371/journal.pone.0089350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shrimali R.K., Ahmad S., Verma V., Zeng P., Ananth S., Gaur P. Concurrent PD-1 blockade negates the effects of OX40 agonist antibody in combination immunotherapy through inducing T-cell apoptosis. Cancer Immunol Res. 2017;5:755–766. doi: 10.1158/2326-6066.CIR-17-0292. [DOI] [PubMed] [Google Scholar]
- 110.Messenheimer D.J., Jensen S.M., Afentoulis M.E., Wegmann K.W., Feng Z., Friedman D.J. Timing of PD-1 blockade is critical to effective combination immunotherapy with anti-OX40. Clin Cancer Res. 2017;23:6165–6177. doi: 10.1158/1078-0432.CCR-16-2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Foote J.B., Kok M., Leatherman J.M., Armstrong T.D., Marcinkowski B.C., Ojalvo L.S. A STING agonist given with OX40 receptor and PD-L1 modulators primes immunity and reduces tumor growth in tolerized mice. Cancer Immunol Res. 2017;5:468–479. doi: 10.1158/2326-6066.CIR-16-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Linch S.N., Kasiewicz M.J., McNamara M.J., Hilgart-Martiszus I.F., Farhad M., Redmond W.L. Combination OX40 agonism/CTLA-4 blockade with HER2 vaccination reverses T-cell anergy and promotes survival in tumor-bearing mice. Proc Natl Acad Sci U S A. 2016;113:E319–E327. doi: 10.1073/pnas.1510518113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Redmond W.L., Linch S.N., Kasiewicz M.J. Combined targeting of costimulatory (OX40) and coinhibitory (CTLA-4) pathways elicits potent effector T cells capable of driving robust antitumor immunity. Cancer Immunol Res. 2014;2:142–153. doi: 10.1158/2326-6066.CIR-13-0031-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Takeda K., Kojima Y., Uno T., Hayakawa Y., Teng M.W., Yoshizawa H. Combination therapy of established tumors by antibodies targeting immune activating and suppressing molecules. J Immunol. 2010;184:5493–5501. doi: 10.4049/jimmunol.0903033. [DOI] [PubMed] [Google Scholar]
- 115.Morales-Kastresana A., Sanmamed M.F., Rodriguez I., Palazon A., Martinez-Forero I., Labiano S. Combined immunostimulatory monoclonal antibodies extend survival in an aggressive transgenic hepatocellular carcinoma mouse model. Clin Cancer Res. 2013;19:6151–6162. doi: 10.1158/1078-0432.CCR-13-1189. [DOI] [PubMed] [Google Scholar]
- 116.Lee S.J., Myers L., Muralimohan G., Dai J., Qiao Y., Li Z. 4-1BB and OX40 dual costimulation synergistically stimulate primary specific CD8 T cells for robust effector function. J Immunol. 2004;173:3002–3012. doi: 10.4049/jimmunol.173.5.3002. [DOI] [PubMed] [Google Scholar]
- 117.Cuadros C., Dominguez A.L., Lollini P.L., Croft M., Mittler R.S., Borgström P. Vaccination with dendritic cells pulsed with apoptotic tumors in combination with anti-OX40 and anti-4-1BB monoclonal antibodies induces T cell-mediated protective immunity in Her-2/neu transgenic mice. Int J Cancer. 2005;116:934–943. doi: 10.1002/ijc.21098. [DOI] [PubMed] [Google Scholar]
- 118.Gough M.J., Crittenden M.R., Sarff M., Pang P., Seung S.K., Vetto J.T. Adjuvant therapy with agonistic antibodies to CD134 (OX40) increases local control after surgical or radiation therapy of cancer in mice. J Immunother. 2010;33:798–809. doi: 10.1097/CJI.0b013e3181ee7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yokouchi H., Yamazaki K., Chamoto K., Kikuchi E., Shinagawa N., Oizumi S. Anti-OX40 monoclonal antibody therapy in combination with radiotherapy results in therapeutic antitumor immunity to murine lung cancer. Cancer Sci. 2008;99:361–367. doi: 10.1111/j.1349-7006.2007.00664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Young K.H., Baird J.R., Savage T., Cottam B., Friedman D., Bambina S. Optimizing timing of immunotherapy improves control of tumors by hypofractionated radiation therapy. PLoS One. 2016;11 doi: 10.1371/journal.pone.0157164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Niknam S., Barsoumian H.B., Schoenhals J.E., Jackson H.L., Yanamandra N., Caetano M.S. Radiation followed by OX40 stimulation drives local and abscopal antitumor effects in an anti-PD1-resistant lung tumor model. Clin Cancer Res. 2018;24:5735–5743. doi: 10.1158/1078-0432.CCR-17-3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hirschhorn-Cymerman D., Rizzuto G.A., Merghoub T., Cohen A.D., Avogadri F., Lesokhin A.M. OX40 engagement and chemotherapy combination provides potent antitumor immunity with concomitant regulatory T cell apoptosis. J Exp Med. 2009;206:1103–1116. doi: 10.1084/jem.20082205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kovacsovics-Bankowski M., Chisholm L., Vercellini J., Crittenden M., Lary S., Curti B. Phase I/II clinical trial of anti-OX40, radiation and cyclophosphamide in patients with prostate cancer: immunological analysis. J ImmunoTher Cancer. 2013;1:P255. [Google Scholar]
- 124.Redmond W.L., Triplett T., Floyd K., Weinberg A.D. Dual anti-OX40/IL-2 therapy augments tumor immunotherapy via IL-2R-mediated regulation of OX40 expression. PLoS One. 2012;7 doi: 10.1371/journal.pone.0034467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ruby C.E., Montler R., Zheng R., Shu S., Weinberg A.D. IL-12 is required for anti-OX40-mediated CD4 T cell survival. J Immunol. 2008;180:2140–2148. doi: 10.4049/jimmunol.180.4.2140. [DOI] [PubMed] [Google Scholar]
- 126.Murata S., Ladle B.H., Kim P.S., Lutz E.R., Wolpoe M.E., Ivie S.E. OX40 Costimulation synergizes with GM-CSF whole-cell vaccination to overcome established CD8+ T cell tolerance to an endogenous tumor antigen. J Immunol. 2006;176:974–983. doi: 10.4049/jimmunol.176.2.974. [DOI] [PubMed] [Google Scholar]
- 127.Fujiwara S., Nagai H., Shimoura N., Oniki S., Yoshimoto T., Nishigori C. Intratumoral CD4+ T lymphodepletion sensitizes poorly immunogenic melanomas to immunotherapy with an OX40 agonist. J Investig Dermatol. 2014;134:1884–1892. doi: 10.1038/jid.2014.42. [DOI] [PubMed] [Google Scholar]
- 128.Zaini J., Andarini S., Tahara M., Saijo Y., Ishii N., Kawakami K. OX40 ligand expressed by DCs costimulates NKT and CD4+ Th cell antitumor immunity in mice. J Clin Investig. 2007;117:3330–3338. doi: 10.1172/JCI32693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ruby C.E., Weinberg A.D. OX40-enhanced tumor rejection and effector T cell differentiation decreases with age. J Immunol. 2009;182:1481–1489. doi: 10.4049/jimmunol.182.3.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Piconese S., Valzasina B., Colombo M.P. OX40 triggering blocks suppression by regulatory T cells and facilitates tumor rejection. J Exp Med. 2008;205:825–839. doi: 10.1084/jem.20071341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pardee A.D., McCurry D., Alber S., Hu P., Epstein A.L., Storkus W.J. A therapeutic OX40 agonist dynamically alters dendritic, endothelial, and T cell subsets within the established tumor microenvironment. Cancer Res. 2010;70:9041–9052. doi: 10.1158/0008-5472.CAN-10-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Burocchi A., Pittoni P., Gorzanelli A., Colombo M.P., Piconese S. Intratumor OX40 stimulation inhibits IRF1 expression and IL-10 production by Treg cells while enhancing CD40L expression by effector memory T cells. Eur J Immunol. 2011;41:3615–3626. doi: 10.1002/eji.201141700. [DOI] [PubMed] [Google Scholar]
- 133.Bulliard Y., Jolicoeur R., Zhang J., Dranoff G., Wilson N.S., Brogdon J.L. OX40 engagement depletes intratumoral Tregs via activating FcγRs, leading to antitumor efficacy. Immunol Cell Biol. 2014;92:475–480. doi: 10.1038/icb.2014.26. [DOI] [PubMed] [Google Scholar]
- 134.Ali S.A., Ahmad M., Lynam J., McLean C.S., Entwisle C., Loudon P. Anti-tumour therapeutic efficacy of OX40L in murine tumour model. Vaccine. 2004;22:3585–3594. doi: 10.1016/j.vaccine.2004.03.041. [DOI] [PubMed] [Google Scholar]
- 135.Sadun R.E., Hsu W.E., Zhang N., Nien Y.C., Bergfeld S.A., Sabzevari H. Fc-mOX40L fusion protein produces complete remission and enhanced survival in 2 murine tumor models. J Immunother. 2008;31:235–245. doi: 10.1097/CJI.0b013e31816a88e0. [DOI] [PubMed] [Google Scholar]
- 136.Sim G.C., Radvanyi L. The IL-2 cytokine family in cancer immunotherapy. Cytokine Growth Factor Rev. 2014;25:377–390. doi: 10.1016/j.cytogfr.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 137.Weiss J.M., Subleski J.J., Wigginton J.M., Wiltrout R.H. Immunotherapy of cancer by IL-12-based cytokine combinations. Expert Opin Biol Ther. 2007;7:1705–1721. doi: 10.1517/14712598.7.11.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Aliru M.L., Schoenhals J.E., Venkatesulu B.P., Anderson C.C., Barsoumian H.B., Younes A.I. Radiation therapy and immunotherapy: what is the optimal timing or sequencing?. Immunotherapy. 2018;10:299–316. doi: 10.2217/imt-2017-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chen G., Emens L.A. Chemoimmunotherapy: reengineering tumor immunity. Cancer Immunol Immunother. 2013;62:203–216. doi: 10.1007/s00262-012-1388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Aspeslagh S., Postel-Vinay S., Rusakiewicz S., Soria J.C., Zitvogel L., Marabelle A. Rationale for anti-OX40 cancer immunotherapy. Eur J Cancer. 2016;52:50–66. doi: 10.1016/j.ejca.2015.08.021. [DOI] [PubMed] [Google Scholar]